Abstract
With aging, pressure ulcers become a common health problem causing significant morbidity and mortality for physically limited or bedridden elderly persons. Here, we present our strategy for such patients. Between August 2010 and March 2019, 117 patients were enrolled. Patient age, etiology, defect size and location, flap reconstruction, outcome, and follow-up period were reviewed. Of these patients, 64 were female and 53 were male, with an age range of 21 to 96 years (mean 75.6). The mean area of defect was 61.5 cm2. The most common etiology was dementia (33.3%), and ulcers were most frequently caused by sacral pressure (70.3%). The commonest surgical treatment was a V–Y advancement flap (50%). The complication rate was 27.5%, including dehiscence and late recurrence. Negative pressure wound therapy could be used if the initial defect was large. V–Y advancement flap is the most frequent surgical treatment for sacral pressure ulcers because it is simple and available for most types of defect. Primary closure may be considered as the simplest method if the defective area is <16 cm2. Intraoperative indocyanine green angiography can help avoid secondary flap revisions. Our protocol ensures a short surgery time, little bleeding, and a low complication rate.
Keywords: surgical flaps, pressure sore, indocyanine green, negative-pressure wound therapy, vacuum-assisted closure
1. Introduction
Pressure injuries entail localized injury to the skin and underlying tissues, usually over a bony prominence as a result of pressure or pressure in combination with shear.[1] The epidemiology of pressure ulcers varies considerably according to clinical setting, with incidence rates ranging from 0.4% to 38% in acute care, 2.2% to 23.9% in long-term care, and 0% to 17% in home care.[2] Gusenoff et al reported that 50% of patients with pressure sores are aged 70 years or older; in an acute care hospital setting, the prevalence of pressure sores reaches 3% to 4%, and the incidence ranges from 1% to 8%.[3]
Factors associated with pressure sores in adults living in acute care hospitals are pressure, shear, friction, moisture, infection, ischemia, anemia, male gender, loss of sensory perception, hypoalbuminemia, diabetes mellitus, incontinence, and frailty with aging.[3,4] The most common sites of pressure sore formation include the ischium, femoral trochanter, sacrum, and heel.[3]
Conservative management is ineffective for stage III or IV pressure sores, and plastic surgery to create flap coverage of the sore becomes inevitable.[3,5] The optimal approach to high-grade pressure sores involves collaboration among physiotherapists, specialist nurses, social workers, and plastic surgeons. Surgical reconstruction combined with patient rehabilitation and education effectively reduces the postoperative recurrence rate of such sores.[3]
Here, we report our experience of treatment using plastic surgery and caring strategies for patients with pressure sores.
2. Patients and methods
We report on 117 patients seen in the Tri-Service General Hospital, Taiwan between August 2010 and March 2019, with stage III or IV pressure sores. All patients were admitted by or had consulted with a single plastic surgeon who specializes in pressure sores.[6–10] Patients and their families were well informed of the operative risks, and they all agreed with the proposed treatment. All reconstructive strategies were guided by our treatment protocol (Fig. 1).[6] There are no clear criteria for selecting patients for pressure sore surgery, but decision guidelines have been developed.[11–13] Patients with inadequate home facilities or lack of family involvement, or those who were not fit for anesthesia, were excluded. Patient characteristics, including age, gender, cause of the defect, location of any comorbidities, wound cultures, lesion size, flap size, hospital stay, and follow-up time were recorded (Table 1). Of these 117 patients, 64 were female and 53 were male. Their ages ranged from 21 to 96 years (mean 75.6). The choice of flap was based on the experience and preference of the surgical team. Generally, we use a V–Y flap for small sacral sores, a superior gluteal artery perforator (SGAP) flap for larger sacral sores, a tensor fascia lata flap for small trochanteric sores and a pedicled anterolateral thigh (pALT) flap for larger trochanteric sores.
Figure 1.
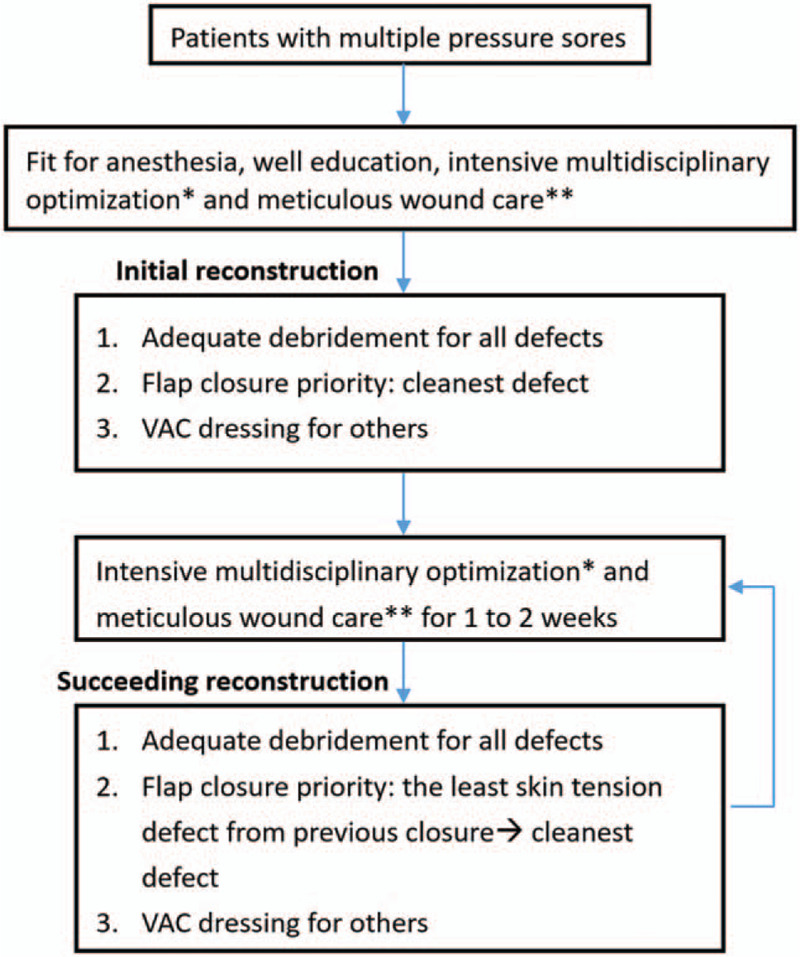
Algorithm used for performing reconstructive surgery. ∗Intensive multidisciplinary optimization: all patients were treated by a team comprising well-trained nursing staff, aides, a physician, a dietician, physical therapists, and a social worker. This team provides comprehensive care, including medication, surgery, nursing, nutrition, rehabilitation and social support. ∗∗Meticulous wound care: wound cleaning and using wet-gauze dressing with saline-diluted iodine twice a day. Bowel and bladder control was used to prevent wound contamination. Treatment avoided weight bearing on the wounds, and used low-air-loss beds with turning every 2 hours.
Table 1.
Patient data.
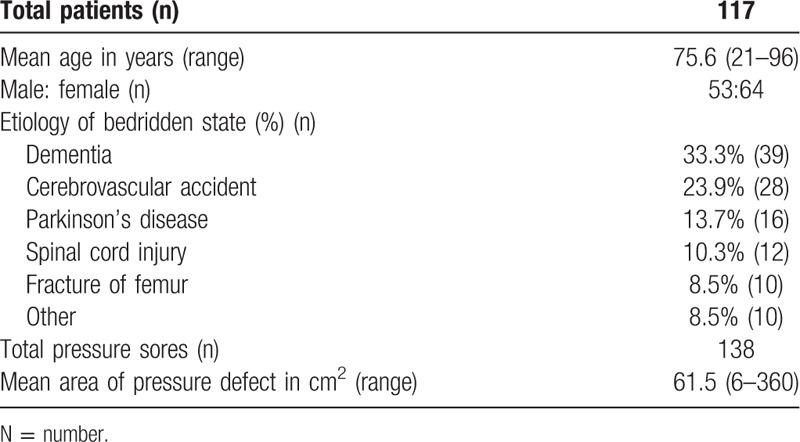
In our treatment protocol, we performed vacuum-assisted closure (VAC) therapy, also known as negative pressure wound therapy (NPWT), after wound debridement as a bridge to final reconstruction[6] (Fig. 2).
Figure 2.
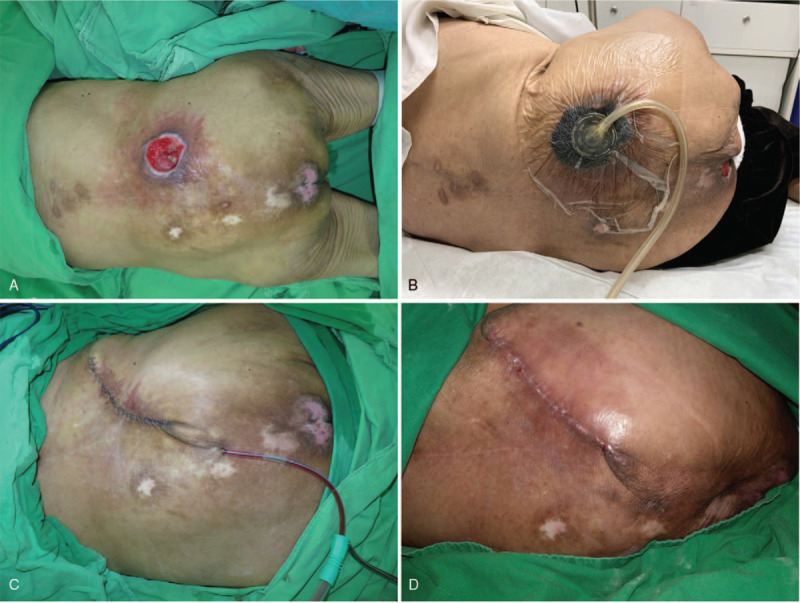
(A) This 89-year-old female patient had a pressure sore over the sacral region. (B) Vacuum-assisted closure therapy was performed after debridement. (C) Primary closure was performed after the wound become stable and clean. (D) A well-healed lesion was noted after 2 weeks.
This study was approved by the Institutional Review Board of the Tri-Service General Hospital (Taipei, Taiwan). All data were analyzed anonymously and according to the principles in the Declaration of Helsinki.
3. Results
The mean area of the defect was 61.5 cm2. The most common etiology of the patients bedridden state was dementia (33.3%) and the second was a cerebrovascular accident (23.9%). The most common comorbidities were hypertension (55.7%) and diabetes (39.1%). A total of 138 pressure sores was recorded (Fig. 3), including 97 on the sacrum (70.3%), 23 on the trochanter (16.7%), 6 on the back (4.3%), 5 on the heels (3.6%), 5 on the ischium (3.6%), 1 on the chest wall (0.7%), and 1 on the shoulder (0.7%).
Figure 3.
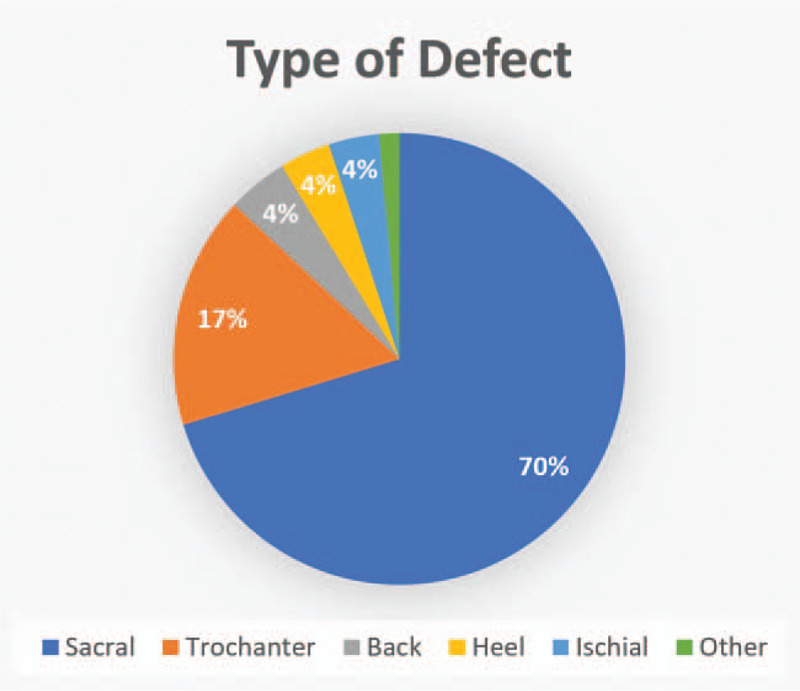
Type of defect.
The most common wound culture result demonstrated mixed flora (23.5%), followed by Enterococcus sp. (20%), no growth (19.1%), and Pseudomonas aeruginosa (11.3%).
The most frequently used plastic surgery intervention (Fig. 4) was a V–Y advancement flap (50%), then primary closure (24.6%), followed by a SGAP flap (10.1%). For the 97 pressure sores on the sacrum, the most frequently utilized approach for reconstruction was the V–Y flap (67 patients, 69.1%), followed by delayed primary closure (16 patients, 16.5%), and a SGAP flap (14 patients, 14.4%) (Table 2; Fig. 5). For all defects, the flap areas ranged from 32 to 201 cm2 (mean 102.6). The mean amount of debridement was 2.75 times the lesional area (range 1–9) with a mean of 43.9 days of hospital stay (range 10–439). The mean follow-up time was 5.3 months (range 3–24).
Figure 4.
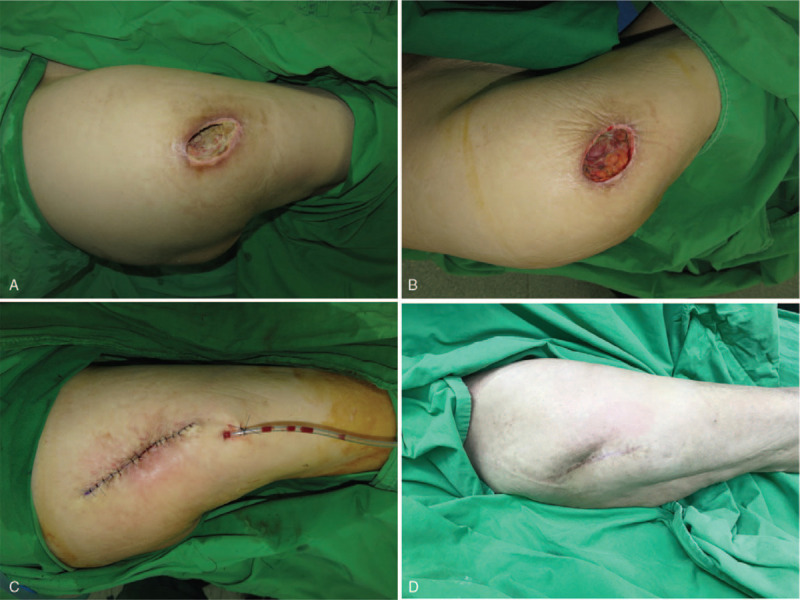
(A) This 85-year-old female patient had a pressure sore over the right trochanteric region. (B) After debridement. (C) Primary closure was performed. (D) A well-healed lesion with no recurrence was noted after 2 years.
Table 2.
Flap reconstruction for pressure sores.
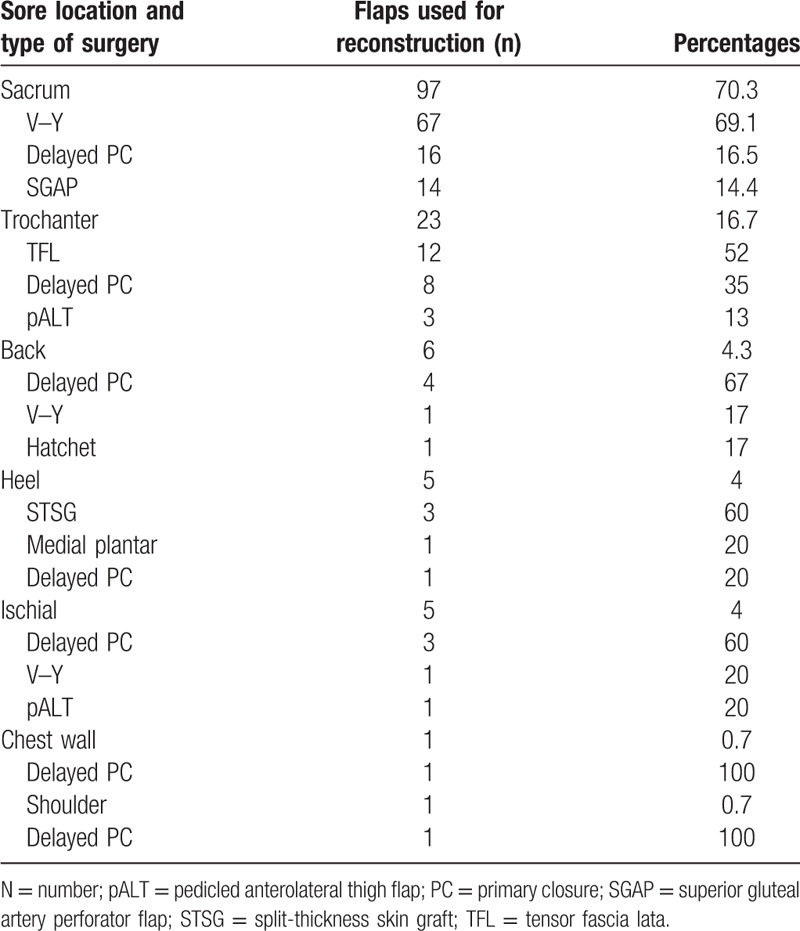
Figure 5.
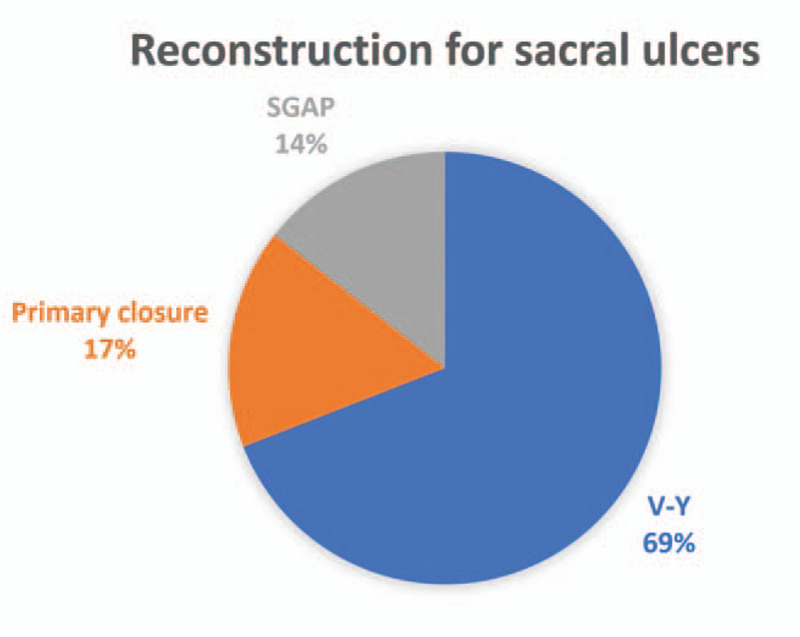
Distribution of reconstruction procedures used for treating sacral ulcers. SGAP, superior gluteal artery perforator flap; V–Y, V–Y advancement flap.
The minor complication rate for each ulcer was 20.3% (28 patients), including partial dehiscence and necrosis. The major complication rate was 7.2% (10 patients), including flap failure and dehiscence. There were no significant differences among the age or comorbidity groups in terms of postoperative complication rates (P > .05). However, flaps with complications were significantly larger than those without complications (P = .032; Table 3). The mortality rate was 9.4% (11 patients; Table 4). The causes of mortality included 6 cases of hospital-acquired pneumonia, 2 of respiratory failure, one of renal failure, one of upper gastrointestinal bleeding and one of fungemia.
Table 3.
Complications.
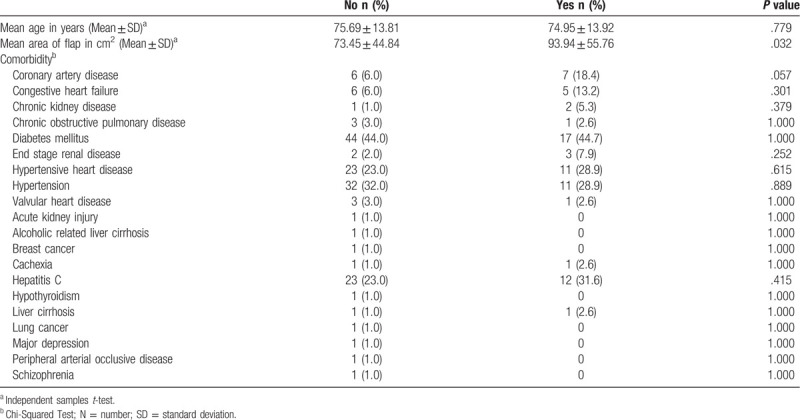
Table 4.
Outcomes.

In cases of primary closure, the mean defect area was 16 cm2. This was used for 34 wounds (24.6%). There were 2 minor complications (partial dehiscence with secondary healing) (5.9%) and 5 major complications (total dehiscence) (14.7%).
Because of occasional partial compromise of the flaps, we also used intraoperative indocyanine green (ICG) angiography with the SPY Elite fluorescence imaging system (Stryker Corp., Kalamazoo, MI, USA) on pALT and SGAP flaps.[7] Using ICG angiography we could identify the compromised area and resect it simultaneously during surgery (Fig. 6). This helped avoid secondary revision of the flap.
Figure 6.
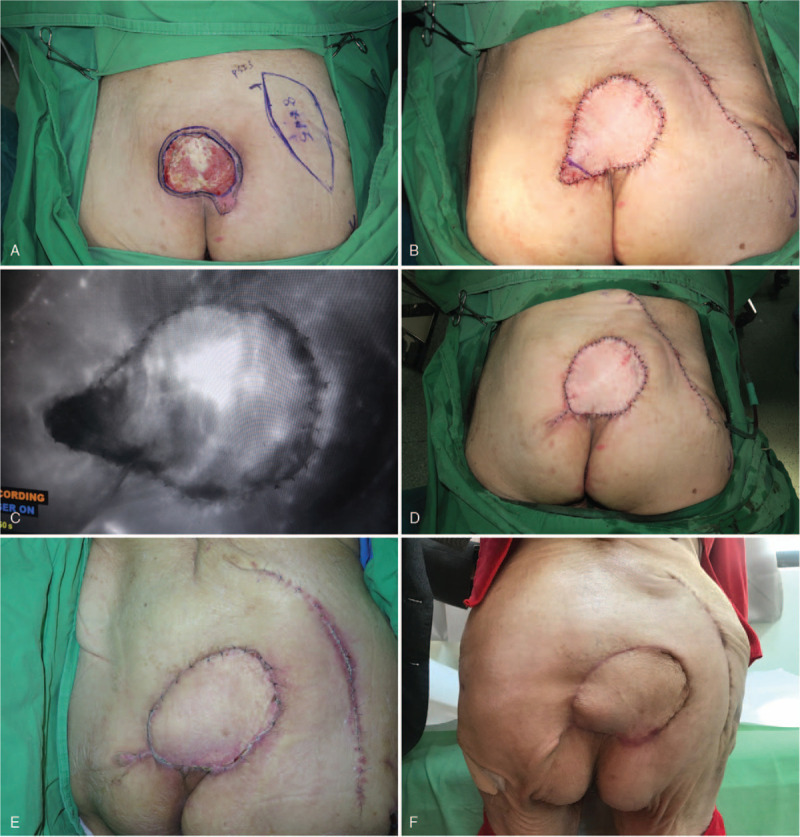
This 89-year-old male patient had a sacral pressure sore. (A) We designed and applied a SGAP flap for coverage. (B) We used intraoperative ICG, but partial poor perfusion was noted. (C, D) We identified and removed the compromised area immediately. (E) Good flap survival with no necrosis was noted after 2 weeks. (F) A well-healed lesion with no recurrence was noted after 2 years.
4. Discussion
The basic surgical principles for the treatment of pressure sores include complete excision of all devitalized tissue in the wound and covering the defect with a durable and well-perfused tissue flap using plastic surgery. Local flaps are the first choice for such coverage. These can be musculocutaneous, fasciocutaneous, or perforator flaps and can be applied as rotation, advancement, or island flaps.[6]
In our management strategy for elderly patients with multiple pressure sores, we prefer treatments with a short operative time, minimal bleeding, and short anesthesia time. Therefore, a multistaged operation for a patient is inevitable. The skin in elderly patients is lax, and delayed primary closure layer by layer could possibly obliterate the cavity without tension and wound dehiscence.[6] Plastic surgeons are educated not to resurface pressure sores by primary closure methods because of the high complication rates. In a previous study of primary closure in cases of pressure ulcers, the dehiscence rate was high at 34%.[14] In our cases, primary closure was used in 24.6% of the wounds. The rate of minor complications (partial dehiscence with secondary healing) was 5.9% and for major complication (total dehiscence) it was 14.7%. These results were better than our initial expectations. Therefore, we advocate that under adequate screening with pressure sores sized <16 cm2 and given the laxity of elderly patients skin, primary closure methods should be considered for resurfacing pressure sores after debridement because of the benefits they provide: a short operative time, minimal bleeding, and brief anesthesia.
In managing pressure sores on the sacrum, we preferred to utilize primary closure for a smaller defect, V–Y advancement flaps for moderate defects, and SGAP for larger defects.[6] With the help of intraoperative ICG-assisted angiography, we can identify the compromised areas in pALT and SGAP flaps and resect them simultaneously during surgery. This helps avoid secondary revision of the flap.
Most patients with pressure sores are old and wound healing takes longer times. This may explain why the complication rate is higher than following general surgery. Statistical analysis showed that there were no significant differences among the age or comorbidity groups in terms of postoperative complication rates. Larger defects need larger flaps for coverage and wound tension might be higher than with small defects. In addition, the relatively low blood supply in large flaps may cause higher complication rates. This would be why the flaps with complications were larger than those without.
One limitation of this study is that there were many confounding factors, which could have interfered with the results of surgery. Therefore, we excluded those patients with inadequate home facilities or lack of family involvement, or those who were not fit for anesthesia. Another limitation was that our treatment protocol (Fig. 1) was not standardized for all patients. To date, there are no clear criteria for selecting patients for pressure sore surgery. Although we followed published guidelines,[11–13] these limitations make the evaluation of surgical techniques for pressure sores difficult.
5. Conclusion
After adequate debridement, NPWT can be used if the initial defect is large. V–Y advancement flaps were most frequently used in treating sacral pressure ulcers in our series because this method is simple and available for most defects. Primary closure may be considered as the simplest method if the defects are smaller than 16 cm2. Intraoperative ICG angiography can help avoid secondary flap revisions. Treating pressure ulcers requires careful family education, intensive multidisciplinary optimization, and meticulous wound care. Our treatment protocol for elderly patients with multiple pressure sores ensures a short surgery time, low amounts of bleeding, and a low complication rate. Moreover, the patients quality of life increases after control of any sepsis, and the burden of caregivers is alleviated. Patient selection is critical, and a multidisciplinary management team is required to ensure stringent care.
Acknowledgments
I would like to thank all my tutors and colleagues who contributed to this study. I am also very grateful to my family and friends for their encouragement and spiritual support during my study.
Author contributions
C.-Y.C. contributed to the literature search, data collection, data analysis, data interpretation, and writing. I.-H.C. contributed to the literature searching, data interpretation, and critical revision. C.-K.C., C.-J.W., T.-S.C., and H.-H.L. contributed to data analysis and data interpretation. K.-F.H. and D.-W.H. contributed to figure collection and formatting. Y.-S.T. Contributed to the studys conception, design, data analysis, data interpretation, and editing.
Conceptualization: Yuan-Sheng Tzeng.
Data curation: Chun Yu Chen, Chien-Ju Wu.
Formal analysis: Yu-Lung Chiu, Chun-Kai Chang, Tzi-Shiang Chu.
Funding acquisition: Yuan-Sheng Tzeng.
Investigation: Hung-Hui Liu, Dun-Wei Huang.
Methodology: I-Han Chiang.
Software: Yu-Lung Chiu, Kuo-Feng Hsu.
Supervision: I-Han Chiang, Kuang-Ling Ou, Yuan-Sheng Tzeng.
Writing – original draft: Chun Yu Chen.
Writing – review & editing: Kuang-Ling Ou, Yuan-Sheng Tzeng.
Footnotes
Abbreviations: ICG = indocyanine green, NPWT = negative pressure wound therapy, pALT = pedicled anterolateral thigh, SGAP = superior gluteal artery perforator, VAC = vacuum-assisted closure.
How to cite this article: Chen CY, Chiang IH, Ou KL, Chiu YL, Liu HH, Chang CK, Wu CJ, Chu TS, Hsu KF, Huang DW, Tzeng YS. Surgical treatment and strategy in patients with pressure sores: a single-surgeon experience. Medicine. 2020;99:44(e23022).
This study was supported by a grant, MOST 108-2314-B-016-041, from the Ministry of Science and Technology, TSGH-C107-117 from Tri-Service General Hospital, Taiwan, ROC.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].National Pressure Ulcer Advisory Panel. Support Surface Standards Initiative: Terms and definitions related to support surfaces. 2007:1-10. [Google Scholar]
- [2].Lyder CH. Pressure ulcer prevention and management. JAMA 2003;289:223–6. [DOI] [PubMed] [Google Scholar]
- [3].Gusenoff JA, Redett RJ, Nahabedian MY. Outcomes for surgical coverage of pressure sores in nonambulatory, nonparaplegic, elderly patients. Ann Plast Surg 2002;48:633–40. [DOI] [PubMed] [Google Scholar]
- [4].Fisher AR, Wells G, Harrison MB. Factors associated with pressure ulcers in adults in acute care hospitals. Adv Skin Wound Care 2004;17:80–90. [DOI] [PubMed] [Google Scholar]
- [5].Lemaire V, Boulanger K, Heymans O. Free flaps for pressure sore coverage. Ann Plast Surg 2008;60:631–4. [DOI] [PubMed] [Google Scholar]
- [6].Chiang IH, Wang CH, Tzeng YS. Surgical treatment and strategy in patients with multiple pressure sores. Int Wound J 2018;15:900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chang CK, Wu CJ, Chen CY, et al. Intraoperative indocyanine green fluorescent angiography-assisted modified superior gluteal artery perforator flap for reconstruction of sacral pressure sores. Int Wound J 2017;14:1170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin CT, Chang SC, Chen SG, et al. Modification of the superior gluteal artery perforator flap for reconstruction of sacral sores. J Plast Reconstr Aesthet Surg 2014;67:526–32. [DOI] [PubMed] [Google Scholar]
- [9].Wang CY, Shih YJ, Chou CY, et al. Successful pedicled anterolateral thigh flap reconstruction for a recurrent ischial pressure ulcer: a case with multiple recurrences over a 7-year follow-up. Wounds 2015;27:E12–5. [PubMed] [Google Scholar]
- [10].Wang CY, Wu CJ, Shih YJ, et al. Modification of the pedicled anterolateral thigh myocutaneous flap for the reconstruction of ischial pressure ulcers: a retrospective case study of 21 patients. Wounds 2019;31:75–80. [PubMed] [Google Scholar]
- [11].National Pressure Ulcer Advisory Panel Pressure ulcers’ prevalence, cost and risk assessment: consensus development conference statement. Decubitus 1989;2:24–8. [PubMed] [Google Scholar]
- [12].Linder RM, Morris D. The surgical management of pressure ulcers: a systematic approach based on staging. Decubitus 1990;3:32–8. [PubMed] [Google Scholar]
- [13].Foster RD, Anthony JP, Mathes SJ, et al. Ischial pressure sore coverage: a rationale for flap selection. Br J Plast Surg 1997;50:374–9. [DOI] [PubMed] [Google Scholar]
- [14].Schryvers OI, Stranc MF, Nance PW. Surgical treatment of pressure ulcers: 20-year experience. Arch Phys Med Rehabil 2000;81:1556–62. [DOI] [PubMed] [Google Scholar]


