Abstract
Background:
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate that retains the antitumor effects of trastuzumab while also delivering the cytotoxic antimicrotubule agent, DM1, directly to tumor cells that overexpress human epidermal growth factor receptor 2. The pharmacokinetic (PK) profile of T-DM1 has been well characterized in Western, Asian, and Japanese patients; this single-center, phase I study (NCT03153163) examined the PK of T-DM1 and safety specifically in Chinese patients.
Methods:
Patients with locally advanced or metastatic breast cancer, previously treated with trastuzumab and a taxane, received open-label T-DM1 at 3.6 mg/kg every 3 weeks. Serum T-DM1 and total trastuzumab, and plasma DM1 were evaluated, and PK parameters were calculated using standard noncompartmental approaches. Adverse events (AEs) were assessed, and immunogenicity was evaluated by measuring antidrug antibodies to T-DM1.
Results:
Among 11 Chinese patients, mean (±standard deviation) PK parameters (maximum serum concentration, 77.6 ± 17.4 μg/mL; clearance 11.0 ± 2.6 mL/d/kg; terminal half-life 3.8 ± 1.0 days) were similar to those previously reported in Western and Japanese patients. One patient transiently developed antidrug antibodies, which did not appear to influence safety or PK. T-DM1 was generally well tolerated. Grade 3–4 AEs occurred in 7 patients (63.6%) and serious AEs occurred in 4 patients (36.4%). Platelet count decrease was the most common all-grade AE (10/11; 90.9%), grade 3–4 AE (5/11; 45.5%), and serious AE (3/11; 27.3%), but did not appear to be associated with any clinically significant bleeding events.
Conclusions:
T-DM1 PK in Chinese patients was consistent with those in global and Asian populations, supporting its use in patients with advanced human epidermal growth factor receptor 2-positive breast cancer following progression on trastuzumab and a taxane. The safety profile of T-DM1 was consistent with prior experience.
Keywords: antibody–drug conjugate, ethnic sensitivity, human epidermal growth factor receptor 2, metastatic breast cancer, pharmacokinetics, trastuzumab emtansine, trastuzumab emtansine
1. Introduction
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 20% of primary invasive breast cancers.[1–4] Untreated, HER2-positive breast cancer represents an aggressive form of the disease with a shorter time to relapse after initial treatment and shorter overall survival (OS).[5,6] The addition of the humanized anti-HER2 antibody trastuzumab to chemotherapy provided patients with HER2-overexpressing tumors a markedly better outcome than was possible with chemotherapy alone.[7] Subsequently, dual targeting of HER2 with trastuzumab plus pertuzumab further improved outcomes in patients with metastatic breast cancer (MBC)–with a median OS of 57.1 months and an 8-year OS rate of 37% reported with first-line trastuzumab plus pertuzumab and a taxane[8]–as well as in patients with early breast cancer.[9–11]
Nonetheless, virtually all patients with HER2-positive MBC develop progressive disease,[12] with tumors continuing to express high levels of HER2.[13] Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate comprising trastuzumab conjugated to a cytotoxic antimicrotubule agent derived from maytansine (DM1) via a thioether linker molecule (4-[N-maleimidomethyl]cyclohexane-1-carboxylate).[14–16] T-DM1 retains the antitumor effects of trastuzumab and also delivers DM1 directly to HER2-overexpressing cells,[17] with clinical studies demonstrating the efficacy and safety of single-agent T-DM1 in patients with HER2-positive metastatic breast cancer[18–21] and those with residual invasive disease after neoadjuvant treatment and surgery.[22] While T-DM1 is approved for use in many countries for the treatment of HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane (separately or in combination), it is not yet approved for use in China, with the only approved second-line treatment for HER2-positive MBC being lapatinib plus capecitabine[23] and more recently pyrotinib plus capecitabine.[24] Therefore, new therapeutic options are needed.
The pharmacokinetic (PK) profile of T-DM1 has been well characterized in Western, Asian, and Japanese patients with HER2-positive MBC in 8 phase I–III clinical trials of single-agent T-DM1.[25,26] Overall, PK data were consistent in Japanese patients.[26] PK data were also consistent between Asian, white, and non-Asian, non-white patients, but the specific ethnic background of the patients comprising the Asian population was not reported.[26] Thus, it is necessary to evaluate the PK of T-DM1 in the Chinese population to evaluate the potential impact of factors that vary among Asian subpopulations, such as diet and genetic background, on the variability of T-DM1 PK.
The aim of this open-label phase I study was to characterize the PK of T-DM1 and its relevant analytes and to evaluate the safety of T-DM1 in Chinese patients with HER2-positive locally advanced breast cancer or MBC.
2. Methods
2.1. Study design
This open-label, single-center, phase I study (NCT03153163) was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice. Prior to study initiation, approval was obtained from the independent ethics committee at the Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China, where the study was conducted. All patients provided written informed consent.
2.2. Patients
The study included Chinese patients aged ≥18 years with HER2-positive locally advanced breast cancer or MBC. HER2 status was prospectively and centrally assessed on archival paraffin-embedded tumor tissue, with HER2-positive status defined as immunohistochemistry 3+ and/or gene amplified (ie, HER2 to chromosome 17 [CEP 17] ratio ≥2) by in situ hybridization. Patients were to have received prior breast cancer treatment with trastuzumab and a taxane either in the adjuvant or advanced settings, with documented progression during or after the most recent advanced breast cancer treatment or within 6 months after completing adjuvant therapy. In addition, patients had baseline left ventricular ejection fraction (LVEF) ≥50% measured by either echocardiogram or multiple gated acquisition scan, adequate organ function, and an Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were excluded from the study if they had received prior treatment with T-DM1, lapatinib, or capecitabine, or if the last dose of their prior chemotherapy or trastuzumab, hormonal therapy, or radiation therapy was within 21, 7, or 14 days, respectively, of the first dose of study treatment. Patients with brain metastases that were untreated, symptomatic, progressive, or required therapy (eg, radiation, surgery) ≤28 days before the first dose of study treatment were also excluded, as were patients with grade ≥3 peripheral neuropathy at baseline; cumulative anthracycline exposures of doxorubicin > 500 mg/m2, epirubicin > 720 mg/m2, or mitoxantrone > 120 mg/m2; cardiopulmonary dysfunction, including uncontrolled hypertension; unstable angina or serious cardiac arrhythmia not controlled by medication; baseline LVEF < 50%, or a history of LVEF < 40% during prior trastuzumab treatment; a history of symptomatic CHF; myocardial infarction ≤6 months prior; current dyspnea at rest; current severe, uncontrolled systemic disease; or who were pregnant or lactating.
2.3. Treatment
All patients received single-agent T-DM1 3.6 mg/kg administered by intravenous infusion every 3 weeks, with study treatment continued until death, disease progression, or unmanageable toxicity.
2.4. End points and assessments
The primary objective of the study was to characterize the PK of T-DM1 (including T-DM1, total trastuzumab, and DM1) in Chinese patients with HER2-positive advanced breast cancer who had received prior trastuzumab and taxane. Safety and tolerability were secondary end points, and the characterization of antidrug antibodies (ADAs) to T-DM1 in Chinese patients with HER2-positive advanced breast cancer was an exploratory end point.
Given the structural complexity of T-DM1, comprising both monoclonal antibody and small-molecule components, the PK of T-DM1 was characterized by primary PK analyte serum T-DM1 and other PK analytes, including serum total trastuzumab and plasma DM1. T-DM1 conjugate and total trastuzumab concentrations in serum were quantified using validated enzyme-linked immunosorbent assays.[27] The T-DM1 conjugate assay measured all conjugated trastuzumab containing 1 or more covalently bound DM1 molecules, while excluding unconjugated trastuzumab. The total trastuzumab assay quantified all forms of conjugated and fully unconjugated trastuzumab. Plasma samples were assayed for DM1 concentrations using a validated liquid chromatography tandem mass spectrometry method. PK matrices of serum and plasma were used for detection of large- and small-molecule components of T-DM1, respectively, based on assay performance (eg., accuracy and precision in each matrix). T-DM1 and total trastuzumab serum concentrations and DM1 plasma concentrations were assessed in all patients during cycles 1, 2, 3, and 4 and at treatment discontinuation. This sampling schedule allowed description of the full concentration–time curves for T-DM1, total trastuzumab, and DM1. During cycle 1, sampling was conducted on days 1 (pre- and 30 minute post-infusion), 2, 3, 4, 8, 11, 15, and 18; during cycles 2 and 3, sampling was conducted pre-and 30 minutes post-infusion on day 1; and during cycle 4, sampling was conducted pre-infusion only.
ADAs to T-DM1 were evaluated using a validated enzyme-linked immunosorbent assay. The numbers and proportions of ADA-positive and ADA-negative patients were evaluated at baseline (pre-infusion on day 1 of cycle 1), during treatment (pre-infusion on day 1 of cycles 3 and 4), and at treatment discontinuation. In addition, adverse events (AEs), laboratory parameters, and LVEF measurements were evaluated throughout treatment and during post-treatment follow-up, for at least 28 days after the final dose of study treatment. AEs were mapped to the Medical Dictionary for Regulatory Activities thesaurus terms and graded according to the National Cancer Institute Common Toxicity Criteria for AE v4.03. Prespecified selected AEs, known to be related to T-DM1, were also assessed; these were based on prior experience with trastuzumab, maytansine, and/or T-DM1.
2.5. Statistical analyses
The study was expected to enroll at least 8 PK-evaluable patients. This number of patients was selected based on the experience of similar studies, which supported that 8 patients would allow adequate PK characterization. Patient baseline demographics and clinical characteristics were summarized for all enrolled patients, using descriptive statistics (eg, mean, median, standard deviation [SD], and range) for continuous variables (eg, age, body weight), and frequency counts for categorical variables (eg, sex, age category, and Eastern Cooperative Oncology Group performance status). The PK parameters of T-DM1 and relevant analytes (ie, total trastuzumab, DM1) were determined with the use of non-compartmental analysis using Phoenix WinNonlin 8.1 software, and mean (± SD) PK parameters reported. Incidences of AEs and serious AE (SAEs) and those leading to treatment discontinuation or modification were calculated.
Safety and PK analyses were performed in the safety-evaluable and PK-evaluable populations, respectively. The safety-evaluable population was defined as all patients who received at least 1 dose of T-DM1 and the PK-evaluable patients were defined as those who completed protocol-specified treatment and blood sampling for adequate PK parameter estimation.
3. Results
3.1. Patients
A total of 18 patients were screened, of whom 11 were eligible and were enrolled in the study between June 20 and December 26, 2017 (Fig. 1). Enrollment of patients into the study continued until it was confirmed there were 8 PK-evaluable patients; thus, a total of 11 patients were enrolled. The most common reason for screening failure was the presence of brain metastases (n = 3). Nine patients completed the study, including follow-up and at that time the study was terminated by the sponsor because the study end points had been reached. At that time 2 additional patients were receiving benefit from T-DM1 on-study. These patients were withdrawn and enrolled in the T-DM1 extension study (TDM4529 g/BO25430) so that they could continue to receive T-DM1. Reasons for treatment discontinuation among the 9 patients who completed the study were AEs in 1 patient and disease progression in 8 patients (see Fig. 1). All 11 patients had discontinued study treatment by the clinical cutoff date of September 27, 2018, with a median treatment duration of 4.9 months (or 8 cycles). Patient demographics and clinical characteristics are shown in Table 1. All patients were female and Chinese. The median age was 50 years (range: 29 to 65). All patients except 1 were aged < 65 years. Per the inclusion criteria, all patients had a diagnosis of late, locally advanced breast cancer or MBC. All patients had prior cancer therapy; only 1 patient had prior treatment in the metastatic-only setting, and 6 patients had prior treatment in the neoadjuvant/adjuvant-only setting. Four patients had treatment in both the neoadjuvant/adjuvant and metastatic settings. All patients had received prior trastuzumab therapy; the median duration of prior treatment was 9.9 months (range: 3.7 to 32.2).
Figure 1.

Patient disposition. AST = aspartate transaminase, HER2 = human epidermal growth factor receptor 2, ULN = upper limit of normal.
Table 1.
Patient demographics and clinical characteristics.

3.2. PKs
All 11 enrolled patients completed at least 1 cycle of T-DM1 treatment and provided planned blood samples for PK analysis. Following the first dose of T-DM1, maximum serum concentration (Cmax) of serum T-DM1 was reached at 30 minutes post end of infusion, with a mean ± SD of 77.6 ± 17.4 μg/mL (Table 2). Systemic clearance of T-DM1 was more rapid than clearance of total trastuzumab: at a T-DM1 dose of 3.6 mg/kg, clearance of serum T-DM1 was 11.0 ± 2.6 mL/day/kg and clearance of total trastuzumab was 5.9 ± 2.4 mL/day/kg (Table 2). Mean concentration–time curves of T-DM1 in serum, total trastuzumab in serum, and DM1 in plasma after the first dose of T-DM1 are shown in Figure 2. The terminal half-life of serum T-DM1 was 3.8 ± 1.0 days, and the terminal half-life of serum total trastuzumab was 7.8 ± 3.6 days (Table 2). Following the first dose of T-DM1, the peak plasma concentration of DM1 was 16.5 ± 3.4 ng/mL.
Table 2.
Cycle 1 pharmacokinetic parameters of T-DM1 and its analytes in Chinese patients (n = 11) with locally advanced inoperable or metastatic HER2-positive breast cancer treated with 3.6 mg/kg.
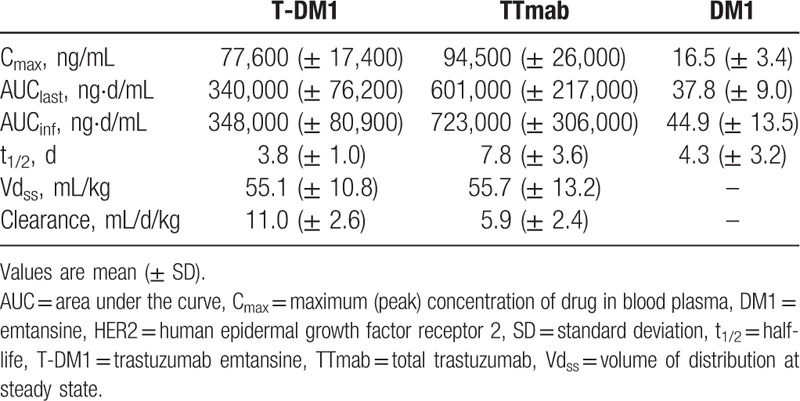
Figure 2.

Mean concentration–time curves of serum trastuzumab emtansine (T-DM1), serum total trastuzumab, and plasma emtansine (DM1) in patients (n = 11) with locally advanced inoperable or metastatic human epidermal growth factor receptor 2-positive breast cancer after the first dose of T-DM1 at 3.6 mg/kg every 3 weeks. Error bars are standard deviation.
3.3. Immunogenicity
All eleven patients were evaluable for immunogenicity. One patient tested positive for treatment-induced ADAs to T-DM1. With a time to onset of 9 weeks, the ADA response was transient. There was no impact on safety or PK: the patient did not experience any immune-related AEs or hypersensitivity reactions, and their PK parameters were within the PK parameter ranges for the overall study.
3.4. Safety
The safety-evaluable population comprised 11 patients, and the median number of treatment cycles received was 8 (range 2–21). There were no deaths in the study, and no AEs led to study withdrawal; 1 AE (grade 3 dysphagia, serious) led to withdrawal of study drug in 1 patient (Table 3).
Table 3.
Safety summary and selected adverse events (ie, related to the known safety profile of T-DM1) in Chinese patients with locally advanced inoperable or metastatic HER2-positive breast cancer.

While all patients experienced ≥1 treatment-related AE (Table 3), there were no new safety signals identified. The most commonly reported all-grade AEs were platelet count decrease (10/11; 90.9% of patients), alanine transaminase (ALT) increase (6/11; 54.5%), and aspartate transaminase (AST) increase, nausea, influenza-like illness, pyrexia, and decreased appetite (all 5/11; 45.5%). Among the 5 patients who experienced bleeding events, all were grade 1 to 2 in severity and resolved.
Seven patients (63.6%) experienced grade 3/4 AEs; no grade 5 AEs were reported. The most commonly reported grade ≥3 AEs were platelet count decrease (5/11; 45.5%) and hepatic function abnormal (2/11; 18.2%); all other grade ≥3 AEs were reported in single patients only. Four patients (36.4%) experienced a total of 16 SAEs (Table 3). The majority of SAEs (13/16) were laboratory abnormalities (ie, platelet count decrease, ALT increase, and AST increase).
Selected AEs are shown in Table 3. Thrombocytopenia, reported in 10 patients (90.9%), was the most common selected AE. All except 2 events of thrombocytopenia resolved, with 5 patients (45.5%) experiencing grade ≥3 events (grade 3 in 2 patients; grade 4 in 3 patients). Hepatotoxicity was reported in 8 patients (72.7%), most of which (29/34 events; 85.3%) resolved. The majority of the hepatotoxicity events were ALT increase, AST increase, and blood bilirubin increase. Two patients (18.2%) experienced grade 3 events (both hepatic function abnormal presenting with an increase in ALT and AST), 1 of which was reported as serious. Also, 5 patients (45.5%) reported hemorrhagic AEs, all of which were grade 1 to 2 in severity and all resolved. Two patients (18.2%) reported grade 1 infusion-related reaction/hypersensitivity (both pyrexia), all of which resolved, and four patients (36.4%) reported grade 1 to 2 peripheral sensory neuropathy (non-serious) of which 2 events resolved and 2 events did not resolve by the clinical cutoff date. There were no events of cardiotoxicity or pulmonary toxicity.
4. Discussion
In this study, T-DM1 PKs in Chinese patients were consistent with those in Western[25] and Japanese patients,[29] supporting that the T-DM1 PKs are comparable across ethnic groups.[26] In Chinese patients with HER2-positive advanced breast cancer, T-DM1 had a mean (±SD) Cmax of 77.6 ± 17.4 μg/mL, mean clearance of 11.0 ± 2.6 mL/d/kg, and mean terminal half-life of 3.8 ± 1.0 days, supporting its use as a second-line therapy in Chinese patients with HER2-positive locally advanced breast cancer or MBC following progression on trastuzumab in combination with cytotoxic agents. One patient transiently developed ADAs, which did not appear to impact safety or PK, and T-DM1 was generally well tolerated. All patients reported at least 1 AE, but this was in line with expectations.
The safety and tolerability of T-DM1 was also evaluated in this study. The most common all-grade AEs were platelet count decrease (10 patients, 90.9%), followed by ALT increase (6 patients, 54.5%) and AST increase, nausea, influenza-like illness, pyrexia, and decreased appetite (all 5 patients each, 45.5%). The overall rate of grade 3–4 AEs (63.6%) was greater than that reported in the phase 3 studies of T-DM1 in patients with MBC (32% to 48%).[18–21] However, as in the phase 3 studies, this was largely due to decreases in platelet counts. Platelet count decrease was the most common SAE (3 patients) and grade 3 or 4 AE (5 patients) in our study. The incidence of grade ≥3 thrombocytopenia (45.5%) was similar to that (44.4%) observed in Asian patients in a pooled safety analysis of T-DM1 studies in MBC.[28] The increased risk of grade ≥3 thrombocytopenia did not appear to result in serious clinical consequences. No events of grade ≥3 hemorrhage were reported in our study; all were grade 1 to 2 in severity and resolved. This is consistent with the known low incidence of grade ≥3 hemorrhagic events in previous studies of T-DM1.[28]
Overall, the PK parameters of T-DM1 in Chinese patients were similar to those previously reported in global and Asian patients, on the basis of PK data of the primary PK analyte T-DM1 and total trastuzumab.[25,26,29] However, the reported mean plasma DM1 Cmax in the current study appeared to be higher than values reported by Girish and colleagues (16.5 ng/mL vs 4.6–5.4 ng/mL).[25] A potential reason for this variability could be minor differences between studies in sample handling, given the known limited stability of the analyte. However, it is important to note that this difference did not seem to have an effect on safety, since the safety profile in the current study was consistent with that of previous studies. In addition, all reportable plasma DM1 concentrations in the current study were below 59.7 ng/mL, the highest concentration observed across all clinical studies (data on file).
While the prospective study design is a strength of the current study, interpretation of the study results needs to be made with caution due to the small size of the study (N = 11).
5. Conclusions
These results support the use of T-DM1 in Chinese patients with HER2-positive locally advanced breast cancer or MBC previously treated with trastuzumab and a taxane. The PK of T-DM1 in Chinese patients was similar to historical data in other patients with advanced breast cancer. The safety profile of T-DM1 was consistent with prior experience and no new safety signals were detected.
Author contributions
Conceptualization: Zao Li.
Data curation: Dongmei Ji, Weina Shen, Jian Zhang, Junning Cao, Wenhua Li, Xichun Hu.
Formal analysis: Lisa H. Lam, Fan Wu, Bei Wang, Zao Li, Guofang Sun, Shang-Chiung Chen.
Investigation: Dongmei Ji, Weina Shen, Jian Zhang, Junning Cao, Wenhua Li, Xichun Hu.
Methodology: Shang-Chiung Chen.
Project administration: Dongmei Ji, Weina Shen, Jian Zhang, Junning Cao, Wenhua Li, Lisa H. Lam, Fan Wu, Bei Wang, Zao Li, Guofang Sun, Xichun Hu, Shang-Chiung Chen.
Resources: Xichun Hu.
Writing – review & editing: Dongmei Ji, Weina Shen, Jian Zhang, Junning Cao, Wenhua Li, Lisa H. Lam, Fan Wu, Bei Wang, Zao Li, Guofang Sun, Xichun Hu, Shang-Chiung Chen.
Footnotes
Abbreviations: ADA = antidrug antibodies, AE = adverse event, ALT = alanine transaminase, AST = aspartate transaminase, Cmax = maximum serum concentration, DM1 = derivative of maytansine 1, HER2 = human epidermal growth factor receptor 2, LVEF = left ventricular ejection fraction, MBC = metastatic breast cancer, OS = overall survival, PK = pharmacokinetic, SAE = serious adverse event, SD = standard deviation, T-DM1 = trastuzumab emtansine.
How to cite this article: Ji D, Shen W, Zhang J, Cao J, Li W, Lam LH, Wu F, Wang B, Li Z, Sun G, Hu X, Chen SC. A phase I study of pharmacokinetics of trastuzumab emtansine in Chinese patients with locally advanced inoperable or metastatic human epidermal growth factor receptor 2-positive breast cancer who have received prior trastuzumab-based therapy. Medicine. 2020;99:44(e22886).
DJ and WS Co-first authors; XH and S-CC contributed equally to the manuscript.
Funded by F. Hoffmann-La Roche Ltd.
The datasets generated during and/or analyzed during the current study are publicly available.
Qualified researchers may request access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Please be aware, however, that due to additional commitments with co-development partners these data will not be available for general request until after July 31, 2020. Further details on Roche's criteria for eligible studies are available here: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
DJ, WS, JZ, JC, WL, and XH report no conflicts of interest. LL, BW, ZL, and S-CC. are employees of and have ownership interest (stock) in F. Hoffmann-La Roche Ltd.; F.W. and G.S. are employees of Roche (China) Holding Ltd. This trial was sponsored by F. Hoffmann-La Roche Ltd. Medical writing support, under the direction of the authors, was provided by Twist Medical, with funding provided by F. Hoffmann-La Roche Ltd.
References
- [1].Reese DM, Slamon DJ. HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells 1997;15:1–8. [DOI] [PubMed] [Google Scholar]
- [2].Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 2003;8:307–25. [DOI] [PubMed] [Google Scholar]
- [3].Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004;5:63–9. [DOI] [PubMed] [Google Scholar]
- [4].Zhang H, Zhang S, Wang Y, et al. Re-evaluation of HER2 status in 1 501 invasive breast cancers according to the 2013 American Society of Clinical Oncology/College of American Pathology guidelines. Zhonghua Bing Li Xue Za Zhi 2015;44:42–7. [PubMed] [Google Scholar]
- [5].Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. [DOI] [PubMed] [Google Scholar]
- [6].Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–12. [DOI] [PubMed] [Google Scholar]
- [7].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- [8].Swain SM, Miles D, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519–30. [DOI] [PubMed] [Google Scholar]
- [9].Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. [DOI] [PubMed] [Google Scholar]
- [10].von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018;89:27–35. [DOI] [PubMed] [Google Scholar]
- [12].Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019;11:1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Spector NL, Xia W, Burris H, III, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 2005;23:2502–12. [DOI] [PubMed] [Google Scholar]
- [14].Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280–90. [DOI] [PubMed] [Google Scholar]
- [15].Feld J, Barta SK, Schinke C, et al. Linked-in: design and efficacy of antibody drug conjugates in oncology. Oncotarget 2013;4:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim SB, Wildiers H, Krop IE, et al. Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III study of trastuzumab emtansine (T-DM1) vs. treatment of physician's choice in previously treated HER2-positive advanced breast cancer. Int J Cancer 2016;139:2336–42. [DOI] [PubMed] [Google Scholar]
- [17].Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128:347–56. [DOI] [PubMed] [Google Scholar]
- [18].Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Krop IE, Kim SB, González-Martin A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689–99. [DOI] [PubMed] [Google Scholar]
- [20].Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 2017;18:743–54. [DOI] [PubMed] [Google Scholar]
- [22].von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019;380:617–28. [DOI] [PubMed] [Google Scholar]
- [23].Xu B, Hu X, Jiang Z, et al. National consensus in China on diagnosis and treatment of patients with advanced breast cancer. Ann Transl Med 2015;3:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blair HA. Pyrotinib: first global approval. Drugs 2018;78:1751–5. [DOI] [PubMed] [Google Scholar]
- [25].Girish S, Gupta M, Wang B, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 2012;69:1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li C, Wang B, Lu D, et al. Ethnic sensitivity assessment of the antibody-drug conjugate trastuzumab emtansine (T-DM1) in patients with HER2-positive locally advanced or metastatic breast cancer. Cancer Chemother Pharmacol 2016;78:547–58. [DOI] [PubMed] [Google Scholar]
- [27].Dere R, Yi JH, Lei C, et al. PK assays for antibody-drug conjugates: case study with ado-trastuzumab emtansine. Bioanalysis 2013;5:1025–40. [DOI] [PubMed] [Google Scholar]
- [28].Diéras V, Harbeck N, Budd GT, et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. J Clin Oncol 2014;32:2750–7. [DOI] [PubMed] [Google Scholar]
- [29].Yamamoto H, Ando M, Aogi K, et al. Phase I and pharmacokinetic study of trastuzumab emtansine in Japanese patients with HER2-positive metastatic breast cancer. Jpn J Clin Oncol 2015;45:12–8. [DOI] [PubMed] [Google Scholar]


