Abstract
Numerous cases of pneumonia from a novel coronavirus (SARS-CoV-2) emerged in Wuhan, China during December 2019.
We determined the correlations of patient parameters with disease severity in patients with COVID-19.
A total of 132 patients from Wuhan Fourth Hospital who had COVID-19 from February 1 to February 29 in 2020 were retrospectively analyzed.
Ninety patients had mild disease, 32 had severe disease, and 10 had critical disease. The severe/critical group was older (P < .05), had a higher proportion of males (P < .05), and had a greater mortality rate (0% vs 61.9%, P < .05). The main symptoms were fever (n = 112, 84.8%) and cough (n = 96, 72.7%). Patients were treated with antiviral agents (n = 94, 71.2%), antibiotics (n = 92, 69.7%), glucocorticoids (n = 46, 34.8%), intravenous immunoglobulin (n = 38, 27.3%), and/or traditional Chinese medicine (n = 40, 30.3%). Patients in the severe/critical group received mechanical ventilation (n = 22, 16.7%) or high-flow nasal can-nula oxygen therapy (n = 6, 4.5%). Chest computed tomography (CT) indicated bilateral pneumonia in all patients. Relative to the mild group, the severe/critical group had higher levels of leukocytes, C-reactive protein (CRP), procalcitonin (PCT), D-dimer, B-type natriuretic peptide (BNP), liver enzymes, and myocardial enzymes (P < .05), and decreased levels of lymphocytes and blood oxygen partial pressure (P < .05).
The main clinical symptoms of patients from Wuhan who had COVID-19 were fever and cough. Patients with severe/critical disease were more likely to be male and elderly. Disease severity correlated with increased leukocytes, CRP, PCT, BNP, D-dimer, liver enzymes, and myocardial enzymes, and with decreased lymphocytes and blood oxygen partial pressure.
Keywords: clinical characteristics, coronavirus, COVID-19, Treatment
1. Introduction
During December 2019, a series of pneumonia cases of unknown cause occurred in Wuhan (Hubei Province), China.[1] The clinical presentations and chest computed tomography (CT) images suggested this new disease was a viral pneumonia. Epidemiological investigations indicated that all of the initially diagnosed patients had previous exposure to the Huanan Seafood Wholesale Market. On January 7, 2020 NHC Key Laboratory of Systems Biology of Pathogens and Christophe Mérieux Laboratory in Beijing detected a novel coronavirus from a respiratory specimen, and the World Health Organization (WHO) subsequently named this virus SARS-CoV-2.[2] The genome sequence of the virus indicate its homology with SARS-CoV was 96.3%,[3] suggesting that bats may also be the natural host of this new virus.[4] Subsequent researchers documented person-to-person transmission of SARS-CoV-2.[5]
The resulting coronavirus disease 2019 (COVID-19) spread rapidly, and by March 1, 2020 there were 79,972 confirmed cases in China. COVID-19 spreads easily from person to person, and the early symptoms are non-specific, making diagnosis difficult. At present, there is incomplete information regarding the clinical features of patients with COVID-19. In this study, we retrospectively analyzed the epidemiological and clinical characteristics of 132 patients with real-time RT-PCR-confirmed SARS-CoV-2 infections in Wuhan Fourth Hospital during a 1 month period.
2. Methods
2.1. Patients
This study was approved by the Medical Ethics Committee of Taizhou Hospital of Zhejiang Province and conformed to the principles of the Helsinki Declaration. SARS-CoV-2 was isolated from respiratory tract specimens and identified by real-time RT-PCR in all patients. Diagnostic and identification procedures were according to the WHO[2] and the National Health Commission of the People's Republic of China.[6] This study examined 132 patients who were hospitalized at Wuhan Fourth Hospital from February 1 to 29, 2020 and were subsequently discharged or deceased.
According to the severity of COVID-19, the patients were classified as having critical, severe, or mild disease.[6] Critical disease was defined by the presence of any one of the following: respiratory failure requiring mechanical ventilation; shock; or multiple organ failure with the need for monitoring in an intensive care unit (ICU). Severe disease was defined by the presence of any one of the following: respiratory rate of 30 or more breaths per min; resting blood oxygen saturation of 93% or less; oxygenation index (PaO2/FiO2 ratio) below 300. Mild disease was defined as fever, respiratory symptoms, or mild pneumonia.
All patients were evaluated by lung CT on the morning after hospital admission. At that time, fasting venous blood was taken and sent to the laboratory for testing of blood cell counts, blood biochemistry, C-reactive protein (CRP), B-type natriuretic peptide (BNP), procalcitonin (PCT), myocardial enzymes, blood gas analysis, and D-Dimer.
2.2. Outcomes
The effects of demographics, signs, and symptoms at admission, comorbidities, laboratory results, CT findings, and treatment on overall survival were determined.
2.3. Statistical analysis
SPSS (version 23.0) was used for all analyses. Continuous measurements with normal distributions are expressed as means ± SDs; Student t test was used to compare the means of 2 samples and a variance test was used to compare more than 2 samples. Variables with non-normal distributions are presented as medians and compared using a rank sum test. Categorical variables as expressed count (%), and the χ2 test was used for comparisons. A P value below .05 was considered significant.
3. Results
We retrospectively examined 132 patients who had definite diagnoses of COVID-19 at Wuhan Fourth Hospital from February 1 to 29, 2020 (Table 1). Overall, there were more males than females (56.1 vs 43.9%), the mean age was 58.8 years (SD = 12.9), and 83 patients (62.9%) had one or more comorbidities. There were 52 patients (39.4%) with cardiovascular and cerebrovascular disease, 22 (16.7%) with endocrine system disease, 12 (9.1%) with digestive system disease, 7 (5.3%) with malignant tumors, 4 (3.1%) with respiratory system disease, 1 (0.8%) with chronic kidney disease, and 5 (3.8%) with blood system diseases. A total of 104 patients (78.8%) recovered and were discharged, 26 patients (19.7%) died, and 2 patients (1.5%) were transferred to another hospital. Among the 26 patients who died, 20 had severe disease and 6 had critical disease. Patients with severe/critical disease rather than mild disease were older, more likely to be male, have one or more comorbidities, and to die (P < .05 for all).
Table 1.
Demographics, comorbidities, and clinical outcomes of patients with COVID-19.
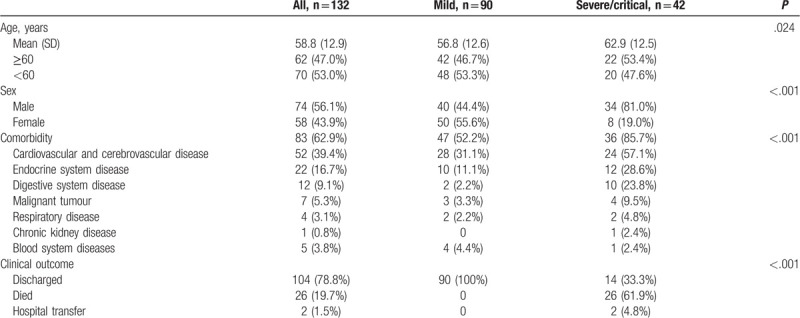
Analysis of symptoms at admission (Table 2) indicated that 112 patients (84.8%) had fever, 96 (72.7%) had cough, 46 (34.8%) had shortness of breath, 4 (3.0%) had chest pain, 34 (25.8%) had fatigue, 6 (4.5%) had sore throats, 2 (1.5%) had headaches, 2 (1.5%) had nausea, 4 (3.0%) had bloody sputum, 6 (4.5%) had diarrhea, 16 (12.1%) patients had poor appetite, and 120 (90.9%) had more than 1 sign or symptom. All patients had bilateral pneumonia based on chest CT exams.
Table 2.
Clinical characteristics and treatment of patients with COVID-19.
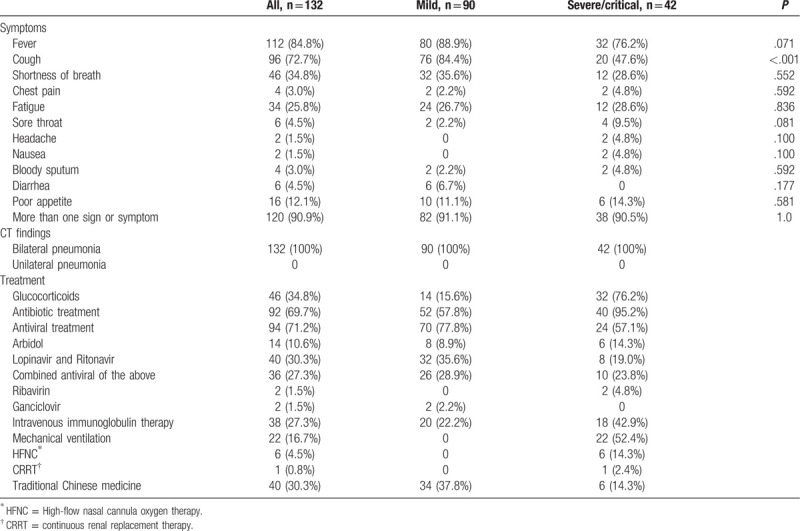
The blood test results (Table 3) indicated that 32 patients (24.2%) had low leukocyte counts and 12 (9.1%) had elevated leukocyte counts. Ninety two patients (69.7%) had decreased lymphocyte counts, 66 (50%) had elevated PCT, 116 (87.9%) had elevated C-reactive protein, 46 (34.8%) had increased BNP, 80 (60.6%) had increased D-dimer, 32 (24.2%) had elevated liver enzymes, 56 (42.4%) had elevated myocardial enzymes, and 50 (37.9%) had decreased blood oxygen partial pressure. Patients with severe/critical disease rather than mild disease had greater abnormalities in all these parameters (P < .05 for all)
Table 3.
Laboratory results of patients with COVID-19.
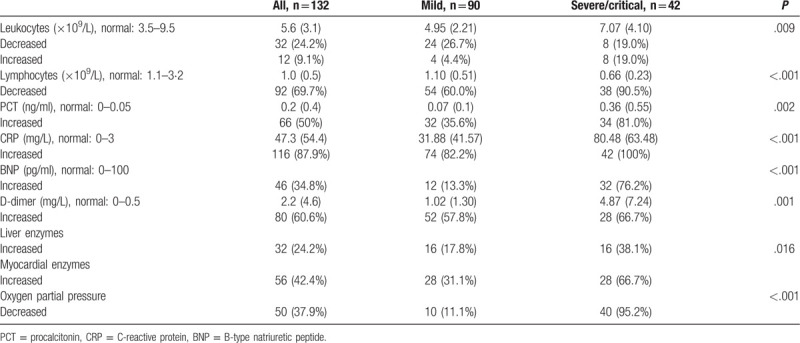
Analysis of the treatment regimens (Table 2) indicated that 46 patients (34.8%) received methylprednisone for 5 to 14 days (median: 8, interquartile range [IQR]: 7 to 10), 92 (69.7%) received an antibiotic, 88 (66.7%) received a single antibiotic, and 4 (3.0%) received multiple antibiotics. The antibiotics used were quinolones, cephalosporins, carbapenems, and semisynthetic penicillins. A total of 94 patients (71.2%) received one or more antiviral drugs, and 58 (43.9%) received a single antiviral agent. Fourteen patients (10.6%) received oral arbidol (200 mg thrice daily), 40 (30.3%) received oral lopinavir and ritonavir (500 mg twice daily), 2 (1.5%) received intravenous ribavirin (500 mg twice daily), 2 (1.5%) received intravenous ganciclovir (250 mg thrice daily), and 36 (27.3%) received oral arbidol, lopinavir, and ritonavir. In addition, 38 patients (27.3%) received intravenous immunoglobulin therapy. Twenty patients received non-invasive mechanical ventilation for 3 to 35 days (median: 6, IQR: 5 to 12), 2 received invasive ventilation for 6 to 10 days, 6 used high-flow nasal cannula oxygen therapy (HFNC) for 6 to 20 days (median: 8 IQR: 6 to 20), and 1 received continuous renal replacement therapy (CRRT) after admission. Forty patients (30.3%) used a traditional Chinese medicine.
Our analysis indicated significant relationships of leukocyte count, lymphocyte count, CRP, PCT, and D-dimer with disease severity (Table 4, P < .05 for all). In particular, patients with mild disease had a lower lymphocyte count, greater leukocyte count, and lower levels of CRP and D-dimer. The three groups had no statistical difference in age (P > .05), but patients with mild disease had a lower mortality rate (P < .05).
Table 4.
Inflammatory indicators in COVID-19 patients with mild, severe, and critical disease.

4. Discussion
SARS-CoV-2 is a novel coronavirus that contains a unique nucleocapsid (N) protein and spike (S) protein. The N protein envelops the RNA genome and can be used as a diagnostic antigen and the S protein can be used for virus typing.[7] There is definite human-to-human transmission of the virus,[8] the virus is highly infectious, and the mortality rate is high among those with multiple morbidities. COVID-19 patients are the main source of new infections. All patients in the present study had histories of exposure to other COVID-19 patients, and none of them were medical workers.
Our analysis of the clinical data of 132 patients with COVID-19 indicated that elderly patients were more likely to have severe/critical disease, especially those with one or more underlying diseases. In agreement, Wen et al[9] analyzed 46 patients with COVID-19 and found that the proportion of old patients with severe/critical disease was greater than the proportion of old patients with mild/common disease. In addition, most of our patients were male (56.1%), and there was a much higher proportion of males in the severe/critical group (81% P < .001), also in agreement with previous research.[10] Our study population had 90 patients with mild disease and 42 patients with severe/critical disease, and those with severe/critical disease had a greater mortality rate (P < .001).
Most of our patients had multiple symptoms, especially fever and respiratory symptoms (cough, chest pain, shortness of breath, sore throat, bloody and sputum). Fatigue and poor appetite were also common, and some patients had non-respiratory symptoms, such as headache, nausea, and diarrhea. These findings are similar to those of previous reports.[8,10] Interestingly, 84.4% of patients with mild disease had a cough, but only 47.6% of those with severe/critical disease had a cough (P < .001). The reason for this difference is unclear, and no previous studies reported this finding. All of our patients had evidence of bilateral pneumonia based on lung CT findings.
SARS-CoV-2 accumulates in the lungs and triggers a systemic inflammatory response, leading to pathological alterations of routine blood markers and ultimately to dysfunction of the heart, liver and kidneys. The changes of CRP, PCT, D-dimer, liver enzymes, and myocardial enzymes appear related to disease severity (P < .05).[11] In our patients, the leukocyte count was in the normal range for most patients, but was elevated in 24.2% of patients. There was also a positive correlation of leukocyte with disease severity (P = .009). Leukocytosis in COVID-19 patients may be related to bacterial co-infection. In particular, Guo et al[12] found that the mortality of patients with viral pneumonia increased when they had mixed bacterial infections. Similarly, leukocytosis was associated with mortality and disease severity in our study population. Our analysis of lymphocyte counts indicated lower levels in patients with more severe disease (P < .001), a finding that requires confirmation and further research.
The increased level of CRP in COVID-19 patients is likely a reflection of the “inflammatory storm” caused by this virus. We found that the CRP level was higher in the critical/severe group than in the mild group (P < .001), in agreement with Gao et al.[13] However, the level of PCT was also higher in our severe/critical group (P = .002), in contrast to previous reports.[14] It is possible that patients with severe/critical disease have higher levels of PCT because of bacterial co-infection, but the underlying mechanism is unclear and this topic needs further exploration.
When a pathogenic virus invades the body, an over-activation of the immune system leads to the over-production of numerous inflammatory factors. These inflammatory factors attack the host and can lead to organ dysfunction. Thus, we analyzed numerous indicators of organ dysfunction in our patients, including D-dimer, BNP, liver enzymes, myocardial enzymes, and oxygen partial pressure. All of these indicators were more abnormal in patients with critical/severe disease than mild disease (P < .05). Previous studies of patients infected with SARS-CoV indicated a slight to moderate increase of liver function and renal function index during the course of 2 to 3 weeks,[15] ultimately leading to lung inflammation and injury.[16] Therefore, abnormal elevations of these indicators indicate the severity of the disease caused by SARS-CoV and by SARS-CoV-2.
In summary, we retrospectively examined 132 patients from Wuhan who were diagnosed with COVID-19. The major clinical manifestations were fever and cough, and the proportions of elderly and male patients were greater among those with severe/critical disease than mild disease. Disease severity also correlated with increased leukocyte count, CRP, PCT, BNP, D-dimer, liver enzymes, and myocardial enzymes, and with decreased lymphocyte count and blood oxygen partial pressure. Due to our small sample size and the retrospective study design, the mechanism underlying the correlation between increased leukocyte count and PCT with COVID-19 severity is not clear, and requires further research.
Acknowledgments
We thank the Fourth Hospital of Wuhan for support and help.
Author contributions
HYL, JWW and LWX performed the data and statistical analysis, and wrote the manuscript. YZX and XLZ supported the fourth hospital of Wuhan to fight the epidemic of novel coronavirus pneumonia, contributed to patient data collection and treatment. YZX and JXF contributed to manuscript preparation and review. All of the authors have read and approved the manuscript.
Conceptualization: Hai-yan Li.
Data curation: Li-wei Xu.
Formal analysis: Hai-yan Li, Li-wei Xu.
Methodology: Hai-yan Li, Jin-wei Wang, Jia-xi Feng.
Project administration: Jin-wei Wang, You-zu Xu.
Resources: You-zu Xu.
Supervision: Jin-wei Wang, Xu-ling Zhao, Jia-xi Feng.
Validation: Xu-ling Zhao.
Writing – original draft: Hai-yan Li.
Writing – review & editing: Hai-yan Li.
Footnotes
Abbreviations: BNP = B-type natriuretic peptide, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein, CRRT = continuous renal replacement therapy, CT = Chest computed tomographyC-reactive, HFNC = high-flow nasal cannula oxygen therapy, ICU = intensive care unit, PCT = procalcitonin, WHO = World Health Organization.
How to cite this article: Li Hy, Wang Jw, Xu Lw, Zhao Xl, Feng Jx, Xu Yz. Clinical analysis of 132 cases COVID-19 from Wuhan. Medicine. 2020;99:44(e22847).
This project was supported by the Medjaden Academy & Research Foundation for Young Scientists (Grant No. COVID-19-MJA20200324).
Data availability the datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Hui DS, E IA, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. World Health Organization. 2020; https://apps.who.int/iris/handle/10665/330893. [Google Scholar]
- [3].Paraskevis D, Kostaki EG, Magiorkinis G, et al. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol 2020;79:104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dong NYX, Ye LW. Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. F1000 Research 2020;9:121. [Google Scholar]
- [5].Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].China NHCotpsRo. Diagnosis and treatment of pneumonia with new coronavirus infection (Trial Implementation version(5). http://www.nhc.gov.cn/xcs/zhengcwj/202002/3b09b894ac9b4204a79db5b8912d4440.shtml. 2020. [Google Scholar]
- [7].Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses 2020;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. NEng J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wen KLW, Zhang D, Zhang A, et al. Epidemiological and clinical characteristics of 46 newly-admitted coronavirus disease 2019 cases in Beijing (Chinese). Chin J Infect Dis 2020;38:150–4. [Google Scholar]
- [10].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Korppi M. Non-specific host response markers in the differentiation between pneumococcal and viral pneumonia: what is the most accurate combination? Pedia Int 2004;46:545–50. [DOI] [PubMed] [Google Scholar]
- [12].Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: The MuLBSTA Score. Frontiers Microbiol 2019;10:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gao L, Liu X, Zhang D, et al. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp Ther Med 2017;13:3479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen L, Liu H, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia (Chinese). Chin J Tuberc Respir Dis 2020;43:203–8. [DOI] [PubMed] [Google Scholar]
- [15].Wu VC, Hsueh PR, Lin WC, et al. Acute renal failure in SARS patients: more than rhabdomyolysis. Nephrol Dial Transplant 2004;19:3180–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and experimental immunology 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]


