Abstract
Transmission of infectious respiratory diseases starts from pathogen-laden respiratory droplets released during coughing, sneezing, or speaking. Here we report an on-mask chemical modulation strategy, whereby droplets escaping a masking layer are chemically contaminated with antipathogen molecules (e.g., mineral acids or copper salts) preloaded on polyaniline-coated fabrics. A colorimetric method based on the color change of polyaniline and a fluorometric method utilizing fluorescence quenching microscopy are developed for visualizing the degree of modification of the escaped droplets by H+ and Cu2+, respectively. It is found that even fabrics with low fiber-packing densities (e.g., 19%) can readily modify 49% of the escaped droplets by number, which accounts for about 82% by volume. The chemical modulation strategy could offer additional public health benefits to the use of face covering to make the sources less infectious, helping to strengthen the response to the current pandemic or future outbreaks of infectious respiratory diseases.
Material Advancement Progression: MAP4: Demonstrate
Graphical Abstract
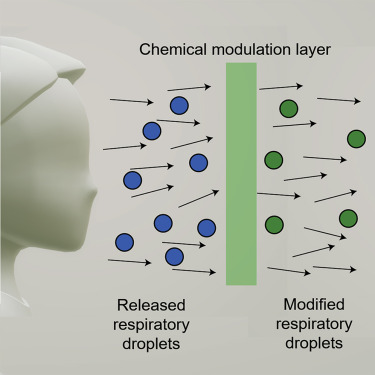
Highlights
-
•
Polyaniline-coated, nonwoven fabrics serve as a reservoir to load antipathogen agents
-
•
Antipathogen agents are dissolved in escaped droplets and eventually concentrated
-
•
The degree of modification of the droplets is evaluated by microscopy imaging methods
-
•
Low-fiber-density fabrics can readily modify a significant fraction of escaped droplets
Progress and Potential
Mask wearing has become a new norm in many parts of the world in the COVID-19 pandemic. There has been much interest in enhanced masks that can better protect the wearers. However, a mask or face covering is much more effective in protecting others because it can block and reroute a large portion of the virus-laden respiratory droplets from symptomatic or asymptomatic infected wearers. Here, we propose an on-mask chemical modulation strategy to enhance this function by making the escaped droplets less infectious. As a proof of concept, antipathogen agents (e.g., mineral acid and copper salt) preloaded on nonwoven fabrics are shown to transfer to and are concentrated in escaped droplets to the level capable of deactivating pathogens. We hope that this approach leads to additional work, which, if eventually adopted, can help to cut down the sources of transmission and strengthen the public health response to control and mitigate the outbreak of infectious respiratory diseases.
On-mask chemical modulation of model respiratory droplets is demonstrated, whereby a nonwoven mask layer is preloaded with antipathogen agents with the purpose of chemically contaminating escaped droplets to make them less infectious. Materials selection focuses on releasing these agents into the droplets in simulated sneezing and coughing, but not during simulated inhalation. It is found that even a low-fiber-density fabric can modify a significant fraction of escaped droplets.
Introduction
Viral respiratory diseases with epidemic potentials, such as influenza, SARS, MERS, and the current COVID-19 pandemic, represent a major global challenge that requires collaborative, holistic, and timely response.1 For infection to occur, respiratory viruses must be transmitted from one person to another through the physical space. There should be many ways to set up physicochemical barriers to drastically reduce the number and viability of the viruses before they reach the host and breach the last line of biological and immunological defenses.2 Public health measures to control and mitigate any infectious diseases must address one or more of the three necessary steps required for transmission (Figure 1 ). This includes identifying and isolating the source, breaking the chain of transmission, and protecting the susceptible hosts.3, 4, 5 Transmission of infectious respiratory diseases starts from pathogen-laden respiratory fluid droplets released by an infected source during coughing, sneezing, singing, or even speaking, which can then propagate through several direct and indirect pathways to infect others.6 , 7 Therefore, intervention methods that can reduce the amount (e.g., through the use of face covering) or the infectiousness of newly released droplets should be most useful, as they help to cut down the source and greatly reduce the burden of other control measures at later steps along the transmission pathways.8
Figure 1.
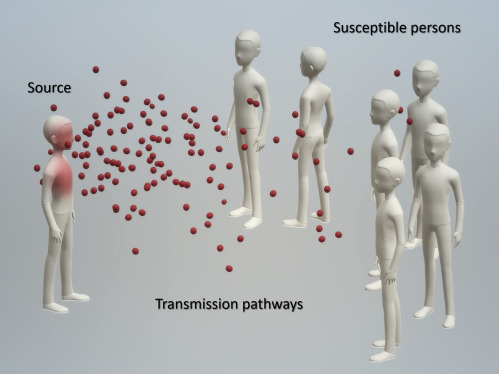
Three Necessary Steps for the Transmission of an Infectious Disease
A source that can release the pathogens, possible transmission pathways along which the pathogens remain structurally intact and infectious before eventually reaching other hosts, and susceptible persons that the pathogens can successfully infect to duplicate themselves and start a new cycle. Mitigation strategies must address one or more of these steps. Isolating the source or making it less infectious should be most effective, as it cuts down the source of the spread chain and greatly reduces the burden at the later stages along the transmission pathways.
Sneezing and coughing are violent expiratory activities accompanied by dynamic and complex liquid fragmentation, droplet ejection, and water evaporation, releasing a much higher flux of respiratory fluid compared with normal breathing. The ejected droplets are typically in the range of micrometers to millimeters in size, and can travel at a speed of 10–20 m/s7 , 9 , 10 Therefore, they can reach a distance of well over 4–6 m. Although the droplets are relatively large and can settle via gravity, after evaporation a certain fraction can turn into submicron-sized nuclei (i.e., dried or semidried mass of respiratory droplets) that can remain suspended in ambient air flow for an extended period of time.11 The use of a face covering can drastically reduce the number of droplets and their forward traveling distance.12, 13, 14 for this reason, in many parts of the world wearing a mask is strongly recommended and even mandated in public areas to prevent and slow down the spread of COVID-19.15 There are a few types of masks commonly available: respirators (such as N95), surgical masks, and face coverings using common fabrics. Among these masks, respirators are designed to have the highest filtration efficiency and are best for protecting wearers in potentially high-viral-dosage environments, such as for healthcare workers. Surgical or so-called medical masks have a relatively lower filtration efficiency for ultrafine particles or droplets and are more effective in blocking outgoing droplets.12 Recent reports showed that some common fabrics also have good performance16 , 17 and may reduce the droplet transmission when widely implemented by the public, making them as possible substitutes when and where professional grades of masks are not widely available. In general, masks with higher fiber-packing density are more effective in blocking the droplets. However, they also make breathing more difficult. Moreover, more efficient droplet trapping can lead to accumulation of respiratory fluid in the masks, which not only decreases their efficacy but also turns them into a secondary source of virus-laden mist in exhalation cycles.18
Here, we report an alternative strategy that focuses on chemically altering respiratory droplets when they escape a masking layer to make them less infectious rather than blocking them completely. There has been increasingly high interest in chemically modified masks that can deactivate trapped pathogens on the outer surfaces, and these are usually designed to better protect wearers.19, 20, 21, 22, 23, 24, 25 However, to the best of our knowledge, much less attention has been paid to improve or enhance face coverings for the purpose of better protecting others from the wearers, and chemical modulation of respiratory droplets has yet to be explored. A mask filters air both inward (i.e., during inhalation) and outward (i.e., during exhalation). Enhanced masks for better self-protection have been very appealing from the perspective of individual users. However, mask concepts for better protection of others from the wearer helps to address larger-scale public health needs to control and mitigate an outbreak of infectious respiratory diseases.
As shown in Figure 2 A, a chemical modulation layer loaded with antipathogen agents can be inserted, such as an add-on or insert of common face-covering fabrics, to leach antipathogen agents into the outgoing droplets. Eventually, these solutes are drastically concentrated when droplets evaporate to yield dried or semidried respiratory nuclei, helping to deactivate pathogens. Proofs of concept are demonstrated using pneumatically generated droplets of model respiratory fluid to simulate coughing and sneezing. The droplets fly through a piece of low-density, nonwoven fabric (e.g., a medical gauze or lint-free wipe) coated with polyaniline doped with mineral acid or copper salt, and eventually land on a detector film for microscopy imaging. Colorimetric and fluorometric imaging methods are developed to visually differentiate modified and unmodified droplets received by the detector film. It is found that a single layer of gauze mask that barely hinders air-flow (i.e., with undetectable pressure drop) can readily modify about 19% of the penetrating droplets by number, which accounts for about 28% by volume. Using a lint-free wipe with low pressure drop comparable with that of a commercial protection mask, the number and volume fractions of modified droplets increase to about 49% and 82%, respectively. Importantly, air flows without the droplets, which reassemble the inhalation process, do not cause these agents to desorb. These results suggest that the chemical modulation of respiratory droplets, when used in conjunction with face covering, could bring significant additional benefits to mitigate the spread of infectious respiratory diseases, especially for those transmittable through presymptomatic or asymptomatic carriers such as COVID-19.
Figure 2.
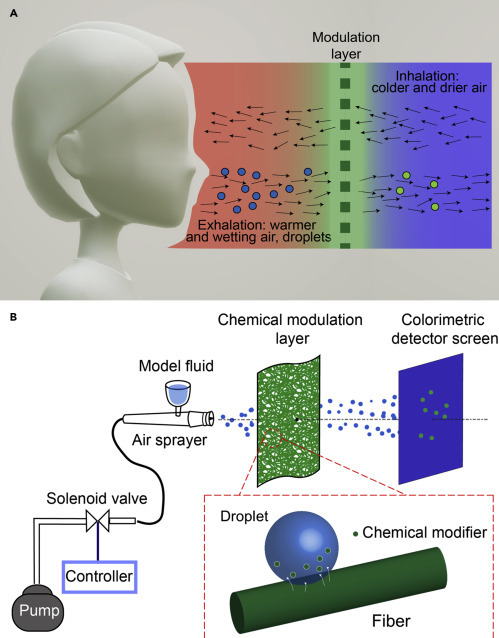
On-Mask Chemical Modulation of Respiratory Droplets: Hypothesis and Approach
(A) Exhalation pushes warmer and wetter air outward, along with respiratory droplets through the mask to the environment. In contrast, inhalation draws colder and drier air from the environment, but without droplets. A chemical modulation layer can be inserted to leach antipathogen agents into the outgoing droplets. To avoid inhaling antipathogen agents (e.g., chemicals or particles), their release mechanism should be activated by the droplets.
(B) In the experimental design, droplets of model respiratory fluids were pneumatically pulsed to simulate those released during coughing and sneezing with comparable range of size distribution, number density, and velocity. The droplets were directed toward a modulation layer (e.g., a nonwoven fabric loaded with chemical modifiers). A detector film was placed at the downstream to collect the escaped droplets, so that their degrees of chemical modification could be visualized directly.
Results and Discussion
Materials Selection
Release of antipathogen agents (e.g., chemicals or particles) should not be triggered during inhalation to avoid being transported to the respiratory tracts and lungs. Note that exhalation blows warm and wet air with droplets outward, while inhalation draws drier and colder air from the environment inward without droplets (Figure 2A). This excludes volatile or particulate modifiers that could desorb or detach from the fibers, which become a potential inhalation hazard. Therefore, nonvolatile molecular agents such as phosphoric acids and copper salts are selected because they can readily dissolve in droplets. A low pH environment (pH < 3) is generally suitable for disinfection because it denatures proteins and disrupts the lipid membrane in pathogens.26 , 27 Ionic copper is a well-known broad-spectrum antimicrobial agent.28 , 29 To enable loading of acid and copper ions on the nonwoven fabrics, the fibers are coated with a layer of conducting polymer polyaniline by in situ polymerization. Polyaniline is chosen for its ease of synthesis and its capability to noncovalently and reversibly bind mineral acids and metal cations.30, 31, 32, 33 Two types of nonwoven fabrics are tested as the model masking layer. One is a medical gauze cloth with around 11% of volumetric fiber density and nearly zero pressure drop. The other is a lint-free wipe commonly used for removing dust from surfaces in laboratories with around 19% of volumetric fiber density, which results in a pressure drop comparable with that of the commercial protection mask (Figure S1). These low-density fabrics do not hinder air flow and breathing significantly if used alone or with other types of face covering.
Experimental Setup to Simulate Coughing and Sneezing
As shown in Figure 2B, an air sprayer operated in a pulsed mode was used to simulate the process of coughing and sneezing. Studies related to respiratory droplets34 , 35 often use a mixture of biopolymers, NaCl, and sometimes surfactants as artificial respiratory liquid. In this work, an aqueous solution of polyvinyl alcohol (PVA) and NaCl was poured into the sprayer as the model fluid. The solutes simulate the hygroscopic components in respiratory fluids and leave drying stains on the detector screen for location of the droplets and measurement of their sizes. The air-flow speed during pulsed spraying was set to 20 m/s, and the resulting droplets (collected 6 cm away from the nozzle) were found to be around 16–160 μm in diameter. The air-flow speed and the size distribution of the droplets are comparable with those generated by coughing and sneezing (Table S1). These droplets were blown toward a chemical modulation layer placed close to the nozzle. A colorimetric or fluorometric detector film was placed downstream to capture escaped droplets for analysis of their sizes and concentrations of chemical modifiers.
Preparation of the Chemical Modulation Layer
Polyaniline was coated on common fabrics as illustrated in Figure 3 A. A roll of gauze or wipe was dipped into a monomer solution and then squeezed to remove the excess before being immersed in an oxidant solution to trigger the in situ polymerization, similar to the rapidly mixed reactions often used for synthesizing polyaniline dispersions.31 , 36 The fibers serve as heterogeneous nucleation sites, facilitating the growth of polymer on their surface. After polymerization, the fabrics were rinsed with deionized water to remove by-products and weakly bonded polyaniline. To load phosphoric acid or copper sulfate, polyaniline-coated fabrics were soaked in the corresponding solutions for 30 min before rinsing with ethanol and air-drying.
Figure 3.
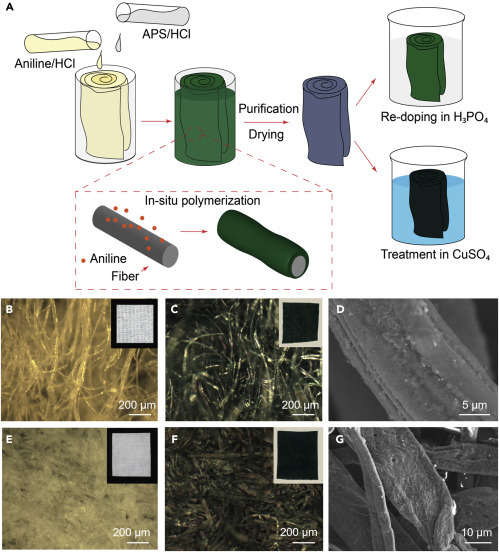
Preparation of the Chemical Modulation Layer
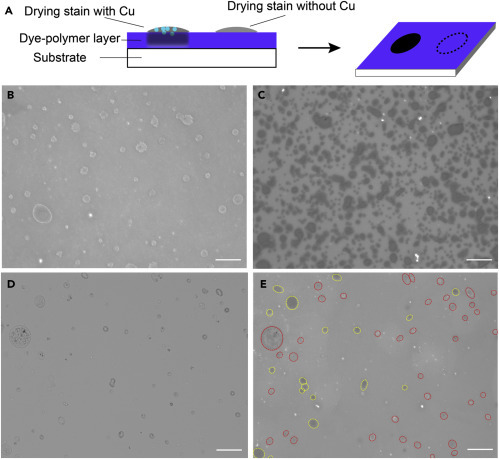
(A) A roll of nonwoven fabric was coated with polyaniline by in situ polymerization of aniline. The fabric was then washed with deionized water and dried in air. The polyaniline coating can be doped with molecular antipathogen agents such as mineral acids or Cu ions by soaking in H3PO4 or CuSO4 solutions, respectively.
(B–G) Optical microscopy images showing the gauze fibers (B) before and (C) after polyaniline growth. The insets are photos of the gauze fabrics (2 cm × 2 cm). (D) SEM image of coated gauze fibers. Corresponding images of a lint-free wipe are shown in (E), (F), and (G).
Figures 3B and 3E are optical microscopy images of the pristine gauze and lint-free wipe taken under reflection mode, respectively, exhibiting nonwoven fiber mat structures. The difference in fiber-packing density is more distinguishable under transmission mode (Figure S2). Figures 3C and 3F are images of the gauze and wipe coated with acid-doped polyaniline, respectively. Both fabrics maintained their nonwoven structures after coating, indicating that the in situ polymerization process does not alter the microstructures of the fabrics. The color of the fabrics turned green, which is characteristic for the doped emeraldine salt form of polyaniline. Scanning electron microscopy (SEM) images of the coated gauze (Figure 3D) and wipe (Figure 3G) show that polyaniline indeed coated individual fibers rather than forming particles trapped in between the fibers.
Preparation of the Colorimetric Detector Film
Although the size distribution of fine droplets can be measured by microscopy and particle size analyzers,37 simultaneous determination of the size and concentration of specific chemicals has been difficult. In this work, the concentration of phosphoric acids (i.e., pH) in the modified droplet was evaluated by a colorimetric detector film. Polyaniline was chosen again as the colorimetric indicator because it can be processed into insoluble films with high spatial uniformity and high color intensity. The color of polyaniline switches reversibly between green and blue upon doping by the acid and dedoping by the base, respectively. Therefore, droplets modified with a sufficiently high concentration of acid should induce a color change on the undoped detector film.
Figure 4 A illustrates how the detector film was prepared. A white-colored smooth plastic film, such as the separator membrane used in batteries, was floated on the surface of a polymerizing bath made of the monomer and the oxidant in HCl. After 10 min of reaction under magnetic stirring, a film of polyaniline was formed on the side in contact with the reactants (Figure 4B). The green film turned blue when rinsed with deionized water and dedoped in ammonium hydroxide (Figure 4C). The dedoped film exhibited high spatial uniformity even when observed under an optical microscope (Figure 4D), making it suitable for colorimetric differentiation of acid-modified and unmodified droplets. Figure 4E is an atomic force microscopy (AFM) image of the detector film, showing the edge of the film, where part of it was removed using Scotch tape. The film had a uniform thickness of about 100 nm throughout the scanned region (Figure 4F).
Figure 4.
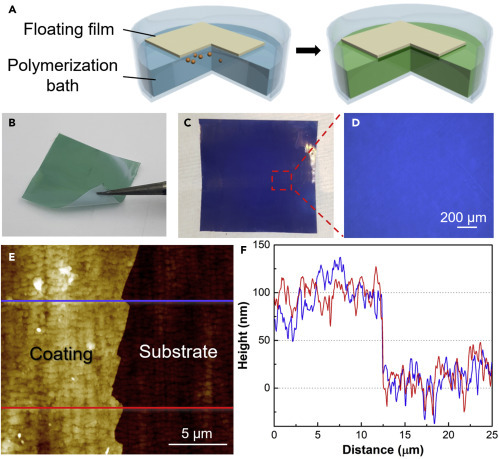
Preparation of the Colorimetric Detector Film
(A) A layer of polyaniline was grown on one side of a smooth plastic film floating on the surface of the polymerization bath.
(B–D) (B) The as-grown green film (5 cm × 5 cm) turned (C) blue after dedoping, which yielded (D) a highly uniform blue field of view under an optical microscope.
(E) AFM image showing a torn edge of the polyaniline layer.
(F) Height profiles taken from (E) show that the coating thickness is around 100 nm.
Colorimetric Visualization of Droplet Stains
The schematic drawings in Figures 5A and 5D explain how the degree of chemical modification of each droplet can be visualized. Since the model fluid contains PVA and NaCl, all the droplets landing on the detector film will leave topologically rough stains on the smooth detector film. Under the reflection mode of optical microscopy, these stains should appear as dark spots on a bright background due to a reduced level of reflection. Therefore, reflection mode can be used to locate all the droplets that landed on the detector film regardless of their pH values. The transmission mode is relatively insensitive to the roughness of the stains but can differentiate colors. Therefore, under transmission mode, stains of unmodified droplets should be barely visible (Figure 5A, right), but stains of modified droplets (Figure 5D, right) should appear as green dots on a blue background.
Figure 5.
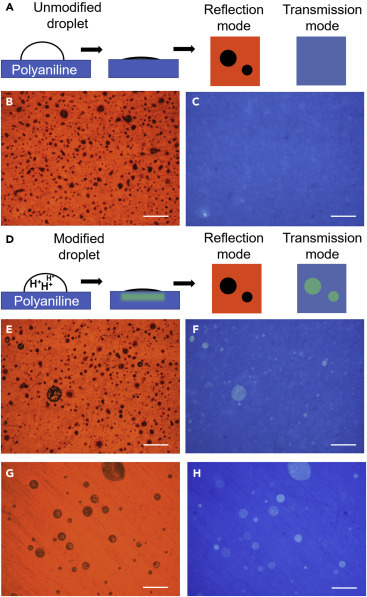
Colorimetric Visualization of Modified Droplets
(A–C) (A) Unmodified, nearly neutral droplets leave drying stains made of polymer and salts that are highly visible under (B) the reflection mode of imaging, but not under (C) the transmission mode.
(D) In contrast, since modified droplets contain acid and can dope polyaniline, they leave green stains that are visible under both modes of imaging.
(E–H) (E and F) and (G and H) are representative images of stains of droplets passing through a gauze cloth and a lint-free wipe, respectively. Note that not all the droplets observed under the reflection mode (E and G) can be seen under the transmission mode, due to insufficient level of acid modification. All scale bars represent 200 μm.
The colorimetric detector film was first tested with droplets sprayed through an uncoated gauze cloth. Indeed, dry stains of these unmodified droplets are highly visible under the reflection mode (Figure 5B). Since the undoped polyaniline is hydrophobic, it allows the landed droplets to bead up (see Figure S3 for contact angles), preventing them from spreading out. In conjunction with the viscosity of the fluid, this results in a well-defined contact line and eventually a clear edge of dried stain for each droplet, making it possible to calculate the size of droplets based on the size of their drying marks. Despite the large number of stains observed in the reflection mode, none of them can be clearly identified in the transmission mode (Figure 5C). In contrast, for droplets passing through a gauze (Figures 5E and 5F) or a wipe (Figures 5G and 5H) modified with H3PO4, many green dots are clearly visible in the transmission images. By comparing the images taken under reflection mode (Figures 5E and 5G) and transmission mode (Figures 5F and 5H), droplets can be differentiated based on their degrees of acid modification, which can be used to determine the number and volumetric percentages of modified droplets.
Quantitative Analysis of Microscopy Images
The reflection and transmission images of the same area of view are used in conjunction to quantify the degree of acid modification for each droplet. Intuitively this includes two steps: locate all the droplets that land on the detector film based on the reflection image and then quantify the degree of modification based on the color change in the transmission image. Overlaying the two images would have been a straightforward way to achieve this, but is distorted by some unwanted features such as rolling marks on the plastic film or accidental scratches. Therefore, the droplet marks in the reflection images are processed by the algorithm of Hough circle transformation38 (Figure S4) and converted into a circular mask, which is then applied to the transmission images to locate the droplets. As illustrated in Figures 6A–6C, a color image under the reflection mode underwent several steps of preprocessing, including conversion to grayscale, Gaussian blur, color inversion, and binarization, before Hough circle transformation. The circular outlines were then overlapped on the green channel (Figures 6D and 6E) of the transmission image to evaluate the brightness (i.e., pixel intensity) inside each circle. The green channel was chosen because the modified droplets created green marks on the blue background, which best represented the degree of acid doping of the polyaniline detector film. This created a composite image (Figure 6F) in which the size and pixel intensity of each circle were readily accessible.
Figure 6.
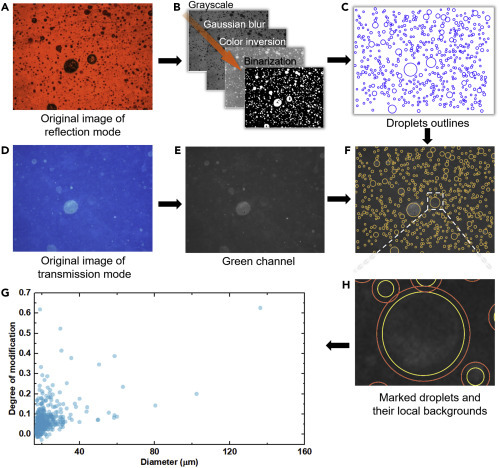
Quantitative Analysis of Colorimetric Microscopy Images
(A) Since both unmodified and modified droplets are visible in the reflection mode of image, it was used to create marks to identify the positions of all droplets in (D) the corresponding transmission mode of image, in which only modified droplets are visible.
(B) The reflection image underwent a few steps of processing to yield a noise-reduced, binarized image to prepare for recognition using Hough circle transformation.
(C) Eventually the droplets were approximated as circular marks, which were used to identify the locations and sizes of the droplets in the transmission image.
(D and E) (E) The green channel of (D) was extracted to calculate pixel intensity values.
(F–H) (F) By overlapping the marking patterns (C) and (E), the pixel intensity inside each droplet, as illustrated by the yellow circles in (H), can be measured, together with the pixel intensity of local background around each droplet (red circle). Pixel intensities inside and outside a droplet were used to calculate its degree of modification based on Equation 1 (see also Supplemental Information). Such analysis produced the calculated degree of modification of all droplets and their corresponding radii based on optical microscopy images from (A) and (D), which are plotted in (G). Each dot represents one droplet.
In digital images, the brightness of each pixel corresponds to an intensity value between 0 and 255. The brightness of a droplet can then be calculated by averaging the pixel intensities of thousands of pixels. Therefore, the contrast in brightness, which is the greenness of the droplet stain, reflects the doping level of the underlying polyaniline spot and the degree of acid modification of the droplets by the chemical modulation layer. The contrast is defined as
| (Equation 1) |
where I d is the average value of all pixels within the top 50% of pixel intensity and I b is the mode value of all pixels in the surrounding background (Figure 6H). The detailed processes are available in Supplemental Information and Figure S5.
Calibrating Degree of Modification Based on pH Values
Next, how the degree of modification is related to the pH value of the droplets was experimentally defined. In equilibrium, the onset of acid doping of polyaniline is normally around pH 4.39 However, for volume-limited microscale droplets, lower pH values (i.e., higher concentrations of acid) are needed to dope a polyaniline film of finite thickness. As shown in Figure 7 A, model fluids with pH values adjusted to 1.3, 1.6, 1.9, 2.1, 2.3, and 2.5 were sprayed onto the detector film to generate droplet marks with known pH values. As expected, the contrast of the droplet marks indeed decreased with increasing pH and eventually diminished at pH 2.5. The quantification process described in Figure 6 was then applied to analyze the microscopy images and yielded a distribution of degree of modification for droplets of each pH value (Figure 7B). The peak position of the control fluid with a pH around 6.0 (control) is around zero modification, and the degree of modification shifts to higher values with decreasing pH values. Model fluid with pH 2.5 can dope the polyaniline detector film at equilibrium, but the corresponding droplets did not produce sufficiently visible contrast, which is attributed to an insufficient amount of acid in the droplets and insufficient diffusion time before the droplets dried. Since the highest pH value of the droplets that can produce a detectable color change was 2.3, the threshold of acid modification was set at the peak of the pH 2.3 curve (Figure 7B, red dashed line), corresponding to a value of a degree of modification of 0.075. Therefore, only droplets with a degree of modification larger than 0.075 are counted as being modified hereafter. Since the pH value of the initial fluid is around 6.0, this threshold of modification represents an increase in acid concentration by at least 3.7 orders of magnitude for modified droplets. This is drawn rather conservatively to avoid over-representing the fraction of modified droplets.
Figure 7.
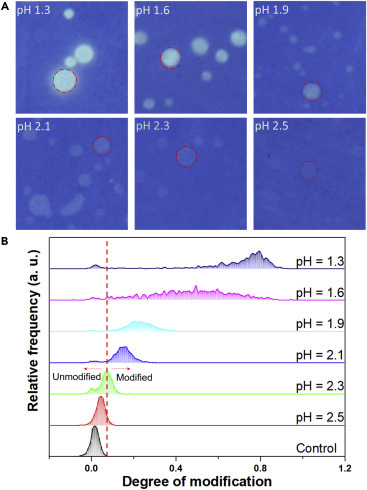
Calibration of the Degree of Modification
Droplets from stock solutions of six known pH values (1.3, 1.6, 1.9, 2.1, 2.3, and 2.5) were sprayed onto the colorimetric screen, and (A) the resulting images taken under transmission mode were used to calibrate (B) how the color changes (i.e., greener dot means higher degree of modification) correlate with the pH values of the droplets. Microscopy observation in (A) shows that for a droplet to produce a distinguishable green dot on the blue polyaniline film, its pH value needs to be ≤2.3. All images in (A) are 200 μm × 200 μm.
Evaluation of Chemical Modification Efficiency by Nonwoven Fabrics
The droplet modification efficiency can be defined based on number () and volume () fractions:
| (Equation 2) |
| (Equation 3) |
For η n, the denominator is obtained by counting the number of all received droplets based on their dried marks on the colorimetric detector. The numerator is defined as the number of droplets with a degree of modification larger than 0.075. η v can be regarded as η n weighted by volume. Polyaniline-coated gauzes and wipes were tested, together with a control experiment in which the model fluid was sprayed through a piece of pristine gauze. The size and degree of modification for each collected droplet were measured and plotted in Figure 8 . Each dot in Figures 8A–8C represents one droplet, and each plot includes results obtained from over 4,000 droplets (Table S2). For each plot, the distribution of droplet size and the degree of modification are also shown at the horizontal and vertical axes, respectively. The horizontal red dashed lines denote the modification threshold, and only droplets above this line were counted as modified. The volumetric densities of the gauze (Figure 8B) and lint-free wipe (Figure 8C) are only 11% and 19%, respectively, but both can modify significant fractions of droplets. η n for the gauze and the wipe were calculated to be about 19% and 49%, respectively. The corresponding η v values were 28% and 82%, respectively. Since the value of η v is more sensitive to the large droplets, higher η v values suggest that larger droplets are more likely to be modified by the fabrics. Figures 8B and 8C show that a higher number of large droplets (>50 μm in diameter) escaped from the wipe than from the gauze. This can be attributed to the coalescence of entrapped droplets within the fiber mat, which is more pronounced in higher-density fabrics. The above results show that a denser, thicker, or multilayer fabric with higher areal fiber density will increase the efficiency of droplet modification but will also lead to a larger pressure drop, making it more difficult to breathe. Therefore, to achieve optimized modifying efficiency, one would need to select a suitable range of fiber density based on the acceptable level of pressure drop during the entirety of application.
Figure 8.
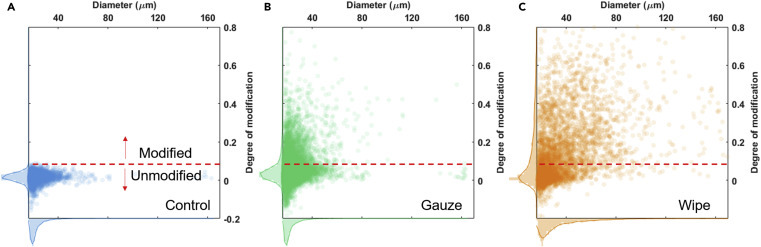
Effect of Droplet Modulation by the Gauze and Wipe
The histograms show the distributions of the size and the corresponding degree of modification of droplets, after they passed through (A) a blank gauze, (B) a gauze, and (C) a lint-free wipe coated with acid-doped polyaniline, respectively. Each dot represents one droplet on the detector screen, and each histogram consists of over 4,000 droplets. The red dashed lines denote the threshold of modification chosen to calculate modulation efficiency, which corresponds to the peak value of pH 2.3 curve in Figure 7B.
Droplet-Fiber Interactions
Droplets can escape from a nonwoven fiber network in three ways (Figure 9 ): (1) some small droplets can be carried through the network by air stream without colliding with any fiber; (2) some droplets collide with fibers without losing much kinetic energy and eventually bounce their way out of the network; (3) some droplets are caught by the fibers upon collision, and can then glide along the fibers and grow larger via coalescence with other droplets and/or condensation of water vapor.40 , 41 Indeed, large droplets of around 100 μm in diameter trapped in between the fibers can often be observed under the optical microscope (Figure S6). Entrapped droplets can be released again, especially in higher-speed air flows (e.g., during coughing or sneezing) either as enlarged individual droplets or many smaller ones due to splitting, which is known as re-entrainment. Types 1–3 would lead to unmodified droplets, slightly modified droplets, and significantly modified droplets, respectively, based on the length of the retention time of droplet-fiber interactions. With thicker and denser layers of nonwoven fabrics, types 1 and 2 would diminish, but type 3 becomes the dominating factor. For virus-laden respiratory droplets, type-3 mechanism reduces the efficacy of face covering as it nebulizes entrapped fluid in the mask, regenerating fine mists of virus-containing droplets into the air.
Figure 9.
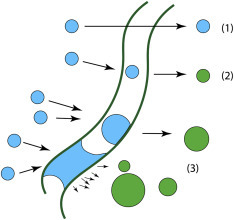
Possible Ways for Droplets to Escape the Modulation Layer
Schematic drawings showing that (1) droplets can escape the layer unmodified due to lack of any or significant interaction with the fibers, the fraction of which is largely determined by the packing density of the fabric; (2) droplets colliding with the fibers have higher chances of being modified; and (3) droplets can get caught on the fibers and grow larger through coalescence and/or condensation of water vapor, which leads to longer retention time (i.e., higher degree of modification), before they break apart via the air flow and escape.
The results obtained with fabrics with different fiber densities are consistent with the above analysis. The gauze layer allowed a larger number of droplets to pass through and resulted in a lower fraction of modified droplets. The wipe has nearly doubled fiber density and allowed much fewer and larger droplets to pass through, resulting in a much higher fraction of modified droplets. These observations suggest that droplets escaping from the lint-free wipe were dominated by the type-3 mechanism, which has the longest retention time of droplets for mass transfer and thus a higher degree of modification (Figure 8C).
The above analysis is intended to guide the materials selection of the fabric layer for the chemical modulation of droplets. Using a few layers of low-density fiber network or a single denser layer can drastically reduce type-1 and type-2 escaped droplets but increase type-3 droplets. This would lead to an entrapment problem for unmodified thick masks, but with the chemical modifier the entrapped fluids become less infectious either on the mask or after re-emission (type 3) by strong air flows during sneezing or coughing.
Droplets Modified by Copper-Loaded Fabrics
The methodology demonstrated for acid modification can be adopted to study the chemical modification of other agents. As a proof of concept, Figure 10 demonstrates chemical modulation of droplets by copper ions as well as fluorometric differentiation of droplets by their copper contents under a fluorescence microscope. Copper ion is a well-studied broad-spectrum antipathogen agent,28 , 29 which can also be loaded on the polyaniline-coated fabrics to dissolve in the passing droplets.32 The small volume of the collected droplets and the presence of PVA and NaCl in the model fluids, which are of higher concentrations than copper salt, make it difficult to differentiate their copper content by mapping techniques based on elemental contrast. Therefore, a fluorometric detector mechanism was developed, which is inspired by our earlier work of fluorescence quenching microscopy (FQM).42 , 43 As shown in Figure 10A, the detector film is now a fluorescence dye/polymer layer spin-coated on a glass substrate. Neocuproine (Nc) is often used in quantitative analysis of copper content in solution based on colorimetric response.44 Unfortunately, direct colorimetric response from the molecular dye was too weak to generate sufficient contrast in optical images. Alternatively, the fluorescence of Nc, which can be quenched in the presence of Cu2+ (see Figure S7), is employed for microscopy imaging. Under fluorescence mode, droplets modified with Cu should generate dark spots on a bright background due to fluorescence quenching, while unmodified droplets should not. Figure 10B is an FQM image showing the dry marks of unmodified droplets sprayed through a pristine gauze without any modifiers. Although the dry marks are slightly visible, they do not quench the fluorescence. Figure 10C is an FQM image of the dry marks of copper-modified droplets sprayed directly from Cu-containing model fluid. Indeed, all the droplets appear as dark spots. By comparing the contrasts of these dark spots with those generated from droplets with known copper concentrations (Figure S8), one can conclude that the concentration of Cu2+ in the modified droplets is >10−4 M, which is above the threshold needed for deactivating influenza viruses.45 Note that the concentration of copper can become even higher as these droplets dry and shrink further.
Figure 10.
Modulation of Droplets by Cu Ions and Fluorometric Visualization
(A) Droplets passing through polyaniline-coated fabrics doped with CuSO4 can dissolve Cu ions. A Nc/PMMA film spin-coated on a coverglass was used as a fluorometric detector to capture the droplets, which was then imaged by FQM. The Cu-modified droplets can quench the fluorescence of their underlying Nc dye and thus appear as dark spots under FQM.
(B) Fluorescence image of a control sample of Cu-free droplets on the detector film, showing drying marks of droplets, which did not quench the fluorescence.
(C) Fluorescence image of another control sample of Cu-containing droplets on the detector film. The stains quenched fluorescence strongly and appeared as dark spots.
(D) Transmission mode of image of a sample collected after they passed a Cu-loaded gauze, which clearly shows the drying marks of the droplets, but cannot differentiate which droplets were modified with Cu.
(E) Under fluorescence mode, Cu-modified droplets are clearly visible as dark spots. The yellow circles highlight modified droplets and the red circles mark the unmodified ones. A total of 18 out of 58 droplets were modified. All scale bars represent 50 μm.
Gauze cloths coated with polyaniline doped with CuSO4 were tested in the same way as shown in Figure 2B. Droplets collected and dried on the detector film were imaged under both transmission (Figure 10D) and fluorescence (Figure 10E) modes. In the transmission mode, all droplet marks are visible. In FQM mode, Cu-containing droplets can be differentiated by their characteristic dark appearance. In Figure 10E, modified and unmodified droplets are circled by red and yellows lines, respectively. By visual observation, 18 out of 58 spots appear dark, suggesting a of 31%. Note that this is higher than the for H3PO4 due to the much more conservative threshold to categorize modified droplets.
Control Experiments Simulating Inhalation
The results shown above demonstrate the promise of using a chemical modifying layer to contaminate outgoing respiratory droplets with soluble molecular species for the purpose of inactivating released pathogens. This may help to enhance public health response to reduce the spread of infectious respiratory diseases. Since people also inhale air through face covers, it is crucial to avoid the release of particles or chemicals from the modifying layer. As illustrated in Figure 2A, one of the major differences between exhalation and inhalation is the presence of droplets. Ambient air without droplets should not trigger the release. Therefore, volatile species or nanoparticle-based droplet modifiers were intentionally avoided due to the potential risk of desorption or detachment, which could lead to direct delivery into the respiratory tracts or even the lungs through inhalation. Therefore, the selection of antipathogen agents is narrowed down to nonvolatile and water-soluble species, as they can be selectively dissolved by droplets but not released in air flow.
Indeed, passing ambient air through a dry, unused Cu-loaded fabric releases a barely detectable level of copper ions in the downstream air (see Supplemental Information). However, in practice a nonwoven fabric can often be dampened when entrapped respiratory droplets accumulate, which increases the humidify of inhaled air. It is also important that the mask modifiers should not be readily released in high-humidity air or through a damp mask during normal inhalation. Therefore, control experiments simulating harsher and wetter inhalation conditions were carried out to examine whether copper ions or acids can be released from the polyaniline-coated fibers. As illustrated in Figure S9, high-humidity air (23°C, 99% relative humidity) was drawn through a lint-free wipe sandwiched between two chambers, one of which was connected to a house vacuum. The wipe was first dampened by spraying the model respiratory liquid for 1 s to simulate accumulated entrapped fluid. Two air-flow speeds (0.2 m/s and 1 m/s) comparable with the low and high ends of typical rates of inhalation were tested. The air “inhaled” through the CuSO4- or H3PO4-loaded wipe was then bubbled through a 10-mL deionized water reservoir to dissolve any released copper salt or acid. After 1 h of continuous “inhalation,” the concentrations of copper and proton in the 10-mL water reservoir were measured by inductively coupled plasma mass spectrometry (ICP-MS) and a pH meter, respectively. The results (Figure S9 and Table S3) showed no significant increase in the proton or copper concentration in the reservoir, even when the wipe was subjected to an extreme air-flow rate of 6 m/s for 10 min. For all the flow rates tested, the copper concentrations in the reservoir were below the mean level in natural water, and orders of magnitude lower than the upper limit of copper contaminant level in drinkable water.46
Conclusion and Outlook
Due to the limited experimental capabilities, the droplets characterized in this work are mostly larger than 10 μm in diameter. Note that these large droplets should make up a significant majority in the overall volume of respiratory fluids released in expiratory activities. Although these droplets usually settle down easily, they contain high loadings of viruses due to their larger volumes, turning objects into infectious fomites. They can also turn into smaller-sized nuclei after evaporation,47, 48, 49 which may stay suspended in air flow for an extended period of time. Viruses in the nuclei are surrounded and protected by a myriad of other respiratory masses and could stay infectious for a long time. An on-mask chemical modulation strategy could help to reduce the viability of viruses and other pathogens of infectious respiratory diseases released from patients.
Nonwoven fabrics are known to be effective in trapping droplets, and here we show that they are also an effective material platform for chemically modulating respiratory droplets. Low packing density (11%) fabric such as a medical gauze does not cause measurable pressure drop, but can readily modify 19% of the escaped droplet by number and 28% by volume. Increasing the fiber density to 19% leads to 49% of outgoing droplets being modified by number and 82% by volume. In practice, the modulation layer can be applied as an add-on at the outer surface of the common masks or as an insert. Although polyaniline is used as a model coating material here, it does offer an additional benefit in that it changes color when acid or metal ions are depleted from the modifying layer. Depleted coatings can also be easily regenerated by redoping (Figure S10). It is worth noting that there should be many other combinations of antipathogen molecules (e.g., quaternary ammonium compounds) and polymer coatings (e.g., sulfonated polyaniline, poly(styrene sulfonate), and polypeptides) suitable for achieving droplet modification, as long as they do not easily desorb or detach during inhalation. The polymer should be able to reversibly load and release those chemical modifier compounds and must strongly adhere to the nonwoven fabrics, which may be achieved by covalent grafting or even being directly incorporated as a component in the fibers.
A mask blocks and interacts with particulate matter in both incoming and outgoing directions. Thus, it offers protection for both the wearer and other people around the wearer, the latter of which turns out to be crucial in the public health response to the current COVID-19 pandemic and has been mandated in many regions. In this regard, a mask is not only “personal protective” equipment, but more importantly “public heath” equipment. The work presented here highlights this aspect of mask wearing and enhances its protection function of others around potential carriers.8 , Much work remains to be done to make this on-mask chemical modulation approach a disease-control tool for public health. It may also be useful for better protecting heathcare workers in patient wards or those large temporary shelter hospitals for triaging, isolating, and caring for patients with non-critical conditions.50 We believe it is a worthwhile effort because reducing the infectiousness of the sources cuts down direct person-to-person transmission, reduces indirect transmission through fomites, and lowers the burden of other measures along the transmission pathways of infectious respiratory diseases.
Experimental Procedures
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jiaxing Huang (jiaxing-huang@northwestern.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Source data and MATLAB codes for figures in the paper are available upon request.
Preparation of Chemical Modulation Layer
In a typical experiment, a piece of nonwoven fabric with a width of 5 cm and a length of 60 cm was immersed in a premixed solution of 5 mL of aniline in 30 mL of 1 M HCl. The fabric was manually rubbed to remove the bubble and ensure wetting. Ammonium persulfate (APS; 0.5 g) was dissolved in 30 mL of 1 M HCl and added into the aniline solution after a few minutes. The mixture was rubbed and stirred slightly by hand and left with sealing overnight. The color of the fabric usually started to darken after a few minutes. The next day, the fabric was rinsed with deionized water three times to remove by-products and loosely bonded polyaniline. Before doping with acids or copper salts, the fabric was squeezed to remove water and tap dried with paper towels. It was then immersed in phosphoric acid solution (pH 1) for 30 min, followed by rinsing in ethyl alcohol twice to remove the excessive acid solution. For copper treatment, instead of acids, the coated fabric was put into 0.1 M CuSO4 solution for 30 min and washed with ethyl alcohol. After drying in air, the fabric was blown with ambient air at about 20 m/s for 30 s using an air sprayer (Paasche, VLS0316) without loading any fluid to further remove weakly bonded polyaniline, if any. All chemicals were purchased from Sigma-Aldrich. The medical gauzes were purchased from Walgreens (premium rolled gauze, item code 150014). Lint-free wipes were purchased from Fisher Scientific (Technicloth #TX606). See Table S4 for detailed information of the fabrics.
Preparation of Colorimetric Detector Film
Aniline (0.5 mL) was dissolved in 20 mL of 1 M HCl solution. APS (0.5 g) was dissolved in another 20 mL of 1 M HCl solution. The two solutions were mixed under stirring. The substrate (Celgard Li-ion battery separator film), typically 5 cm × 5 cm, was immediately put on the surface of the solution with continuous stirring. Caution was taken to avoid trapping air bubbles underneath the film. After 20 min, the green film was removed from the solution and rinsed in deionized water. After drying it was placed on the surface of 0.1 wt % of ammonia solution for 30 min to be dedoped. Finally, it was rinsed with ethyl alcohol to remove excess ammonia solution.
Preparation of Model Respiratory Fluid
PVA with a molecular weight of 143,000 was dissolved in water at 80°C and 2.5 mg/mL. NaCl was then added to the solution after cooling to room temperature at a concentration of 10 mg/mL. Model fluid with known pH was made by adding a known volume of phosphoric acid into PVA/NaCl solution. The pH was further confirmed by a pH meter (Fisher Scientific AE150). PVA and NaCl were purchased from Sigma-Aldrich.
Preparation of Fluorometric Detector Film
Poly(methyl methacrylate) (PMMA; molecular weight ∼350,000) at a concentration of 10 mg/mL was dissolved in anisole, followed by dissolving neocuproine hemihydrate (99%) at a concentration of 25 mg/mL. The solution was spin-coated on a coverglass by a spin coater (model #WS-400BZ-6NPP/LITE, Laurell Technologies, North Wales, PA, USA) at 2,000 rpm for 1 min. The chemicals were purchased from Sigma-Aldrich.
Simulation of Coughing and Sneezing
An air sprayer (Paasche, VLS0316) was connected to an air compressor set to 40 psi. A solenoid valve with a working voltage of 12 V was placed at the outlet of the compressor and also connected to a programmable source meter (Tekpower TP3005P), which was connected to a laptop computer. Spraying time was controlled by setting the duration of voltage output. The flux of the sprayer was adjusted so that 1 s of spraying yielded a sufficient number of droplets on the detection screen. A home-made channel was used to fix the fabric and the detection screen. The sprayer was placed 2 cm away from the fabric and pointed to it to simulate the situation of a person wearing a mask. The sprayed area on the fabric was approximately 2.5 cm × 2.5 cm. For lint-free wipes with lower penetrating rate, the distance was adjusted to 0.5 cm to increase the flux so that enough droplets could be received on the screen. The distance from the fabric layer to the detection film was 4 cm for both cases. For each spraying test, the time was set to 1 s. Longer spraying time would lead to water-layer formation on the fabric and bias the result. The fabric was dried in air before the next spray. Each fabric was sprayed three times at the same spot. The detection screen was cut to a size of 2 cm × 2 cm to receive escaped droplets.
Optical Microscopy
All detection screens were examined a few minutes after spraying using an optical microscope (Nikon Eclipse E600 POL upright microscope; Nikon Instruments, Melville, NY, USA) equipped with a CCD camera (2,048 × 1,536 pixels; Teledyne QImaging, MicroPublisher 3.3 RTV, Surrey, BC, Canada) under reflection (bright-field) and transmission mode (dark-field). Offset, exposure time, and gain of the camera were to set to 0, 40 ms, and 2.56, respectively. Regions with sparse droplets or dirty background were discarded.
Fluorescence Quenching Microscopy
FQM images were taken on a Nikon Eclipse TE2000-U inverted fluorescence microscope (Nikon Instruments) equipped with an X-cite 120 PC illumination system (EXFO Photonic Solutions, Mississauga, ON, Canada) as the light source. An ET-GFP filter cube (FITC/Cy2; Chroma Technology, Bellows Falls, VT, USA) was used to select light in the UV range. Exposure time was 10 s.
Characterization Methods
SEM images were obtained by a field-emission scanning electron microscope (SU8300; Hitachi). The thickness of colorimetric detection layer was measured using an atomic force microscope (XE-100; Park Systems) under non-contact mode. Elemental analysis was conducted on a Thermo iCAP Q inductively coupled plasma mass spectrometer.
Control Experiment Simulating Inhalation
Cu-loaded lint-free wipes and acid-loaded wipes were sprayed by model respiratory fluid for 1 s to simulate dampened fabrics. An ultrasonic humidifier was used to create highly humid upstream air (99% relative humidity), which was drawn through the wipes at air-flow speeds of 0.2 m/s and 1 m/s for 1 h. The downstream air was then guided into a tube containing 10 mL of deionized water. The same process was repeated at an extreme flow rate of 6 m/s for 10 min. The concentration of Cu and protons from the masking layer in the deionized water reservoir were analyzed by ICP-MS and a pH meter (Fisher Scientific AE150). See Supplemental Information for more details.
Image Processing
Readers are referred to Supplemental Information for detailed processes.
Acknowledgments
The work is mainly supported by a National Science Foundation (RAPID DMR-2026944 managed by SSMC subdivision). H.H. thanks the Ryan Fellowship and the Northwestern University International Institute for Nanotechnology for encouragement. Y.L. thanks his parents for funding his study at Northwestern University. Additional sources of support include Dean's support from the McCormick School of Engineering to support J.H. and an earlier gift donation to J.H.'s lab, and personal donations from some of the authors in purchasing small supplies and consumables to expedite the work. The work made use of the IMSERC and the Keck-II facility of the NUANCE Center at Northwestern University, which has received support from the NSF (CHE-1048773); Soft and Hybrid Nanotechnology Experimental Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the Keck Foundation; the State of Illinois and International Institute for Nanotechnology. The authors also thank Profs. L. Jiang (Beijing), Q. Cheng (Beijing), and W. Zhang (Nanjing), and Ms. X. Xiao (Chicago) for their gifts of various types of masks and supplies, some of which were used to better equip the authors to perform on-site research during the period of stay-home order of the State of Illinois, and Dr. M. Kadir, Dr. Z. Yu, L. Prestowitz, L.J. Bichelmeir, S.G. Fine, Mr. J. Suerth (Feynlab), and many others for helpful discussions and encouragement.
Author Contributions
J.H. conceptualized the work and oversaw the research. H.H. and H.P. designed the experiments, developed the colorimetric and fluorometric detection methods, and drafted the manuscript. H.H. and Y.L. designed the image-processing protocol; Y.L. wrote the codes to extract and analyze data from the optical microscopy images.
Declaration of Interests
Northwestern University has filed a patent application including discoveries made in this work.
Published: October 29, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.matt.2020.10.012.
Supplemental Information
References
- 1.Gates B. Responding to Covid-19—a once-in-a-century pandemic? N. Engl. J. Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 2.Huang H., Fan C., Li M., Nie H.-L., Wang F.-B., Wang H., Wang R., Xia J., Zheng X., Zuo X. COVID-19: a call for physical scientists and engineers. ACS Nano. 2020;14:3747–3754. doi: 10.1021/acsnano.0c02618. [DOI] [PubMed] [Google Scholar]
- 3.Flint J., Racaniello V.R., Rall G.F., Skalka A.M. John Wiley & Sons; 2015. Principles of Virology. [Google Scholar]
- 4.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Practices H.C.I.C. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- 6.Xie X., Li Y., Sun H., Liu L. Exhaled droplets due to talking and coughing. J. R. Soc. Interfaces. 2009;6(Suppl 6):S703–S714. doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal R., Ni R., Seo J.-H. The flow physics of COVID-19. J. Fluid Mech. 2020;894:F2. [Google Scholar]
- 8.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.H., McDevitt J.J., Hau B.J.P., Yen H.L., Li Y., Ip D.K.M., Peiris J.S.M. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharfman B.E., Techet A.H., Bush J.W.M., Bourouiba L. Visualization of sneeze ejecta: steps of fluid fragmentation leading to respiratory droplets. Exp. Fluids. 2016;57:24. doi: 10.1007/s00348-015-2078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Z.Y., Weng W.G., Huang Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J. R. Soc. Interfaces. 2013;10:20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourouiba L., Dehandschoewercker E., Bush J.W.M. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. [Google Scholar]
- 12.Cook T.M. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic—a narrative review. Anaesthesia. 2020;75:920–927. doi: 10.1111/anae.15071. [DOI] [PubMed] [Google Scholar]
- 13.Li X.P., Niu J.L., Gao N.P. Characteristics of physical blocking on co-occupant's exposure to respiratory droplet residuals. J. Cent. South Univ. 2012;19:645–650. doi: 10.1007/s11771-012-1051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S., Dhanak M., Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys. Fluids (1994) 2020;32:061708. doi: 10.1063/5.0016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention CDC Calls on Americans to Wear Masks to Prevent COVID-19 Spread. https://www.cdc.gov/media/releases/2020/p0714-americans-to-wear-masks.html
- 16.Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M., Liao L., Xiao W., Yu X.Z., Wang H.T., Wang Q.Q., Lin Y.L., Kilinc-Balci F.S., Price A., Chu L. Household materials selection for homemade cloth face coverings and their filtration efficiency enhancement with triboelectric charging. Nano Lett. 2020;20:5544–5552. doi: 10.1021/acs.nanolett.0c02211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberge R.J. Effect of surgical masks worn concurrently over N95 filtering facepiece respirators: extended service life versus increased user burden. J. Public Health Manag. Pract. 2008;14:E19–E26. doi: 10.1097/01.PHH.0000311904.41691.fd. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.H., Wu C.Y., Lee C.N., Anwar D., Wysocki K.M., Lundgren D.A., Farrah S., Wander J., Heimbuch B.K. Assessment of iodine-treated filter media for removal and inactivation of MS2 bacteriophage aerosols. J. Appl. Microbiol. 2009;107:1912–1923. doi: 10.1111/j.1365-2672.2009.04375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan F.S., Rubino I., Lee S.H., Koch B., Choi H.J. Universal and reusable virus deactivation system for respiratory protection. Sci. Rep. 2017;7:39956. doi: 10.1038/srep39956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Si Y., Zhang Z., Wu W., Fu Q., Huang K., Nitin N., Ding B., Sun G. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 2018;4:eaar5931. doi: 10.1126/sciadv.aar5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Cao C.Y., Huang J.Y., Chen Z., Chen G.Q., Lai Y.K. Progress on particulate matter filtration technology: basic concepts, advanced materials, and performances. Nanoscale. 2020;12:437–453. doi: 10.1039/c9nr08851b. [DOI] [PubMed] [Google Scholar]
- 23.Rubino I., Choi H.J. Respiratory protection against pandemic and epidemic diseases. Trends Biotechnol. 2017;35:907–910. doi: 10.1016/j.tibtech.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J., Hu Z., Zabihi F., Chen Z., Zhu M. Progress and perspective of antiviral protective material. Adv. Fiber Mater. 2020;2:123–139. doi: 10.1007/s42765-020-00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balagna C., Perero S., Percivalle E., Nepita E.V., Ferraris M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020;1:100006. [Google Scholar]
- 26.Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheller C., Krebs F., Minkner R., Astner I., Gil-Moles M., Watzig H. Physicochemical properties of SARS-CoV-2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control. Electrophoresis. 2020;41:1137–1151. doi: 10.1002/elps.202000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent M., Hartemann P., Engels-Deutsch M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health. 2016;219:585–591. doi: 10.1016/j.ijheh.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Mitra D., Kang E.T., Neoh K.G. Antimicrobial copper-based materials and coatings: potential multifaceted biomedical applications. ACS Appl. Mater. Interfaces. 2020;12:21159–21182. doi: 10.1021/acsami.9b17815. [DOI] [PubMed] [Google Scholar]
- 30.Dimitriev O.P. Doping of polyaniline by transition-metal salts. Macromolecules. 2004;37:3388–3395. [Google Scholar]
- 31.Huang J.X. Syntheses and applications of conducting polymer polyaniline nanofibers. Pure Appl. Chem. 2006;78:15–27. [Google Scholar]
- 32.Virji S., Fowler J.D., Baker C.O., Huang J., Kaner R.B., Weiller B.H. Polyaniline nanofiber composites with metal salts: chemical sensors for hydrogen sulfide. Small. 2005;1:624–627. doi: 10.1002/smll.200400155. [DOI] [PubMed] [Google Scholar]
- 33.Chiang J.C., Macdiarmid A.G. Polyaniline-protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986;13:193–205. [Google Scholar]
- 34.Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W., Elankumaran S., Marr L.C. Relationship between humidity and influenza A viability in droplets and implications for influenza's seasonality. PLoS One. 2012;7:e46789. doi: 10.1371/journal.pone.0046789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D., Huang J.X., Kaner R.B. Polyaniline nanofibers: a unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009;42:135–145. doi: 10.1021/ar800080n. [DOI] [PubMed] [Google Scholar]
- 37.Gralton J., Tovey E., McLaws M.L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimme C., Ballard D., Sklansky J. Finding circles by an array of accumulators. Commun. ACM. 1975;18:120–122. [Google Scholar]
- 39.Jin Z., Su Y.X., Duan Y.X. An improved optical pH sensor based on polyaniline. Sens. Actuators B. 2000;71:118–122. [Google Scholar]
- 40.Zhang R.F., Liu B.F., Yang A.K., Zhu Y.Y., Liu C., Zhou G.M., Sun J., Hsu P.C., Zhao W.T., Lin D.C. In situ investigation on the nanoscale capture and evolution of aerosols on nanofibers. Nano Lett. 2018;18:1130–1138. doi: 10.1021/acs.nanolett.7b04673. [DOI] [PubMed] [Google Scholar]
- 41.Gac J.M., Gradon L. Analytical investigation and numerical modeling of collisions between a droplet and a fiber. J. Colloid Interface Sci. 2012;369:419–425. doi: 10.1016/j.jcis.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Kim J., Cote L.J., Kim F., Huang J.X. Visualizing graphene based sheets by fluorescence quenching microscopy. J. Am. Chem. Soc. 2010;132:260–267. doi: 10.1021/ja906730d. [DOI] [PubMed] [Google Scholar]
- 43.Kong Z.Z., Daab M., Yano H., Huang H.Y., Breu J., Sasaki T., Nguyen S.T., Huang J.X. Visualizing transparent 2D sheets by fluorescence quenching microscopy. Small Methods. 2020;4:2000036. [Google Scholar]
- 44.Larsen E.R. Spectrophotometric determination of copper in fertilizer with neocuproine. Anal. Chem. 1974;46:1131–1132. [Google Scholar]
- 45.Imai K., Ogawa H., Bui V.N., Inoue H., Fukuda J., Ohba M., Yamamoto Y., Nakamura K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir. Res. 2012;93:225–233. doi: 10.1016/j.antiviral.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Gaetke L.M., Chow C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 47.Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U S A. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber T.P., Stilianakis N.I. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L., Wei J., Li Y., Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z., Chen S., Yang J., Wang J., Zhai X., Barnighausen T. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data and MATLAB codes for figures in the paper are available upon request.


