Abstract
It is difficult to identify suspected cases of atypical patients with coronavirus disease 2019 (COVID-19), and data on severe or critical patients are scanty. This retrospective study presents the clinical, laboratory, and radiological profiles, treatments, and outcomes of atypical COVID-19 patients without respiratory symptoms or fever at onset. The study examined ten atypical patients out of 909 severe or critical patients diagnosed with COVID-19 in Wuhan Union Hospital West Campus between 25 January 2020 and 10 February 2020. Data were obtained from the electronic medical records of severe or critical patients without respiratory symptoms or fever at onset. Outcomes were followed up to discharge or death. Among 943 COVID-19 patients, 909 (96.4%) were severe or critical type. Of the severe or critical patients, ten (1.1%) presented without respiratory symptoms or fever at admission. The median age of the ten participants was 63 years (interquartile range (IQR): 57–72), and seven participants were men. The median time from symptom onset to admission was 14 d (IQR: 7–20). Eight of the ten patients had chronic diseases. The patients had fatigue (n = 5), headache or dizziness (n = 4), diarrhea (n = 5), anorexia (n = 3), nausea or vomiting (n = 3), and eye discomfort (n = 1). Four patients were found to have lymphopenia. Imaging examination revealed that nine patients had bilateral pneumonia and one had unilateral pneumonia. Eventually, two patients died and eight were discharged. In the discharged patients, the median time from admission to discharge lasted 24 d (IQR: 13–43). In summary, some severe or critical COVID-19 patients were found to have no respiratory symptoms or fever at onset. All such atypical cases should be identified and quarantined as early as possible, since they tend to have a prolonged hospital stay or fatal outcomes. Chest computed tomography (CT) scan and nucleic acid detection should be performed immediately on close contacts of COVID-19 patients to screen out those with atypical infections, even if the contacts present without respiratory symptoms or fever at onset.
Keywords: SARS-CoV-2, COVID-19, Atypical clinical characteristics
1. Introduction
In December 2019, a new virus, now named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), triggered a massive epidemic [1]. As of 13 September 2020, 28 677 773 confirmed cases and 918 118 deaths have been reported around the world [2]. The rapidly growing number of cases and the quick spread of the disease further confirm that this virus can swiftly transmit from person to person [3], [4], [5], [6]. The basic reproductive number (R0) of SARS-CoV-2 infection has been estimated to be 2.2 [7]. Household clusters [3], transmission within the community, and infection among healthcare workers [6] have been reported as three major forms of transmission via congregation. The reported case fatality rate (CFR) of COVID-19 is 4.0% [8], which is lower than that of severe acute respiratory syndrome (SARS; 9.2%) and that of Middle East respiratory syndrome (MERS; 34.4%) [9]. Higher CFR was associated with advanced age, higher Sequential Organ Failure Assessment (SOFA) score, greater level of D-dimer [10]. The CFR of critical cases was 61.5% [11].
Human coronaviruses, such as SARS coronavirus (SARS-CoV) and MERS coronavirus (MERS-CoV), affect multiple systems, but particularly target respiratory tracts [12], [13], [14]. Fever, dry cough, dyspnea, and fatigue have been the most common symptoms observed in confirmed COVID-19 patients [1]. Less common symptoms include headache and diarrhea, among others [4]. Few patients infected with SARS-CoV-2 have had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhea, sneezing, or sore throat), indicating that COVID-19 is not a typical upper respiratory tract infection. Cases of insidious transmission of COVID-19 via asymptomatic persons have also been reported [15]. The viral load detected in asymptomatic patients is similar to that in their symptomatic counterparts, suggesting that transmission potential is independent of the symptoms presented [16]. The existence of asymptomatic cases poses a serious threat to public health; however, data on asymptomatic patients with SARS-CoV-2 infection are scanty. Similar to asymptomatic patients, COVID-19 patients with atypical symptoms might not be aware of their illness and, as a result, might not be properly quarantined. Moreover, they might fail to seek medical attention or might be neglected by medical professionals, and thus unwittingly transmit the virus to others.
In this study, we aimed to describe the clinical, laboratory, and radiological characteristics, treatments, and outcomes of severe or critical COVID-19 patients who presented no respiratory symptoms or fever at onset. We hope that our study findings will provide useful information to clinicians who are currently fighting on the front lines of the battle with the COVID-19.
2. Methods
2.1. Study design and participants
Wuhan Union Hospital West Campus is a designated hospital for COVID-19 patients. We collected clinical data of all patients with confirmed COVID-19 admitted to Wuhan Union Hospital West Campus from 25 January 2020 to 10 February 2020. Included in this study were severe or critical patients who had no fever and/or respiratory symptoms before a definite diagnosis was made or they were admitted. Patients who had fever and/or respiratory discomfort and cases whose clinical symptoms were incomplete or missing were excluded from this study. By the cutoff day of the study, all enrolled patients had been discharged or died. No patients or medical staff involved in patient care took part in the study design and statistical analyses. This retrospective study was approved by the institutional ethics board of Wuhan Union Hospital, Huazhong University of Science and Technology (No. 0036), Wuhan, China. Written informed consent was waived due to the critical situation of an emerging infectious disease.
2.2. Data collection
Data were collected concerning demographics, clinical symptoms, medical history, underlying comorbidities, laboratory findings, chest computed tomography (CT) findings, therapeutic measures (e.g., antiviral therapy and respiratory support), and clinical outcomes. Diagnoses and condition classifications of COVID-19 were based on the Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 3) [17]. Laboratory tests—all performed according to the clinical care needs of the patients—basically consisted of a complete blood count, blood biochemical analysis (assessment of liver and renal function), coagulation testing, and tests for C-reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase (LDH), muscle hemoglobin, hypersensitive troponin, and creatine kinase (CK) levels. Radiological abnormalities were determined based on the documentation and were reviewed by two physicians in respiratory medicine.
2.3. Definitions
An atypical patient was defined as a patient with laboratory-confirmed COVID-19 but without characteristic fever or respiratory symptoms before hospital admission. Fever was defined as an axillary temperature of 37.3 °C or higher. Respiratory symptoms mainly consisted of cough, expectoration hemoptysis, chest tightness, and dyspnea. Low blood oxygen saturation (SpO2) was defined as SpO2 ≤ 93% at rest [17]. The date of disease onset was defined as the day on which the symptoms were first noticed. The date of definite diagnosis of SARS-CoV-2 infection was defined as the day on which a positive specimen was obtained from the patient. Shock and acute respiratory distress syndrome (ARDS) was defined in accordance with the World Health Organization (WHO) interim guidance. Acute kidney injury (AKI) was defined based on the highest serum creatinine level and urine output criteria [18]. Acute cardiac injury (ACI) was diagnosed if the serum levels of cardiac biomarkers (e.g., troponin I (TnI)) were above the 99th percentile of the upper reference limit or if new abnormalities were electrocardiographically and echocardiographically identified [19]. Secondary infection was established if a patient had symptoms of nosocomial pneumonia or bacteremia and the culture showed that specimens from the lower respiratory tract or blood, taken ≥ 48 h after admission, were positive for a new pathogen [20]. The criteria for discharge were as follows: ① Body temperature returned to normal for more than 3 d; ② there was significant improvement or disappearance of respiratory symptoms; ③ imaging examination showed a marked improvement in exudative lesions in the lungs; and ④ two consecutive respiratory specimens, taken more than 24 h apart, were negative for viral nucleic acid. The patient needs to meet the above four conditions at the same time to be discharged.
2.4. Statistical analysis
Categorical variables were expressed as counts and percentages; continuous variables were presented as median and interquartile range (IQR) values. All statistical analyses were performed by using the Statistical Package for the Social Sciences (SPSS) version 23.0 software package (IBM, USA). The analyses were deemed to be exploratory and descriptive, since the small number of patients in this study was not randomly selected.
3. Results
3.1. Patient characteristics
As of 10 February 2020, a total of 943 confirmed COVID-19 patients had been admitted into Wuhan Union Hospital West Campus. Of these, 34 (3.6%) were of moderate type, and 909 (96.4%) were of severe or critical type. Of the severe or critical patients, 899 who had no relevant information in their medical records or who presented with respiratory symptoms or fever upon admission were excluded. Eventually, ten (1.11%) COVID-19 patients were included in this study (Table 1 ). Over the entire study period, the median age of the participants was 63 years (IQR: 57–72), and seven were men. Eight patients had one or more chronic disease(s), including hypertension (n = 4), cardiovascular disease (n = 3), diabetes (n = 1), cerebrovascular diseases (n = 1), respiratory system disease (n = 1), and carcinoma (n = 1).
Table 1.
Baseline characteristics of the ten studied atypical COVID-19 patients.
| Variable | Value |
|---|---|
| Age (year), median (IQR) | 63 (57–72) |
| Gender | |
| Male | 7 |
| Female | 3 |
| Comorbidities | |
| Hypertension | 4 |
| Cardiovascular disease | 3 |
| Diabetes | 1 |
| Chronic pulmonary disease | 1 |
| Cerebrovascular disease | 1 |
| Malignancy | 1 |
| Symptoms and signs on admission to hospital | |
| Fatigue | 5 |
| Headache/dizziness | 4 |
| Anorexia | 3 |
| Nausea/vomiting | 3 |
| Diarrhea | 5 |
| Eye discomfort | 1 |
| Axillary temperature (°C), median (IQR) | 36.5 (36.3–36.7) |
| Heart rate (bpm), median (IQR) | 80 (73–90) |
| Respiratory rate (tpm), median (IQR) | 18 (17–19) |
| SpO2 (%), median (IQR) | 97 (95–99) |
| Onset of initial symptom to (d), median (IQR) | |
| Hospital admission | 14 (9–21) |
| Typical symptoms | 16 (11–22) |
| Discharge or death | 42 (28–56) |
| Onset of hospital admission to (d), median (IQR) | |
| Appearance of fever | 3 (2–7) |
| Appearance of cough and expectoration | 2 (1–4) |
| Appearance of dyspnea | 3 (2–6) |
| Length of stay (d), median (IQR) | |
| All patients | 21 (11–33) |
| Discharged patients | 24 (13–43) |
bpm: beats per minute; tpm: times per minute.
The median time from symptom onset to admission was 14 d (IQR: 9–21). On admission, five patients had fatigue, four had headache or dizziness, five had diarrhea, three had anorexia, three had nausea or vomiting, and one had eye discomfort. Vital signs, including heart rate, respiratory rate, and axillary temperature, were normal in all patients. Five patients showed abnormal systolic pressure. And the SpO2 was > 93% in all patients at their admission during rest state. Follow-up study revealed that two patients died and eight were discharged. In the two deceased patients, the time from admission to death was 3 d (Patient 1) and 10 d (Patient 2), respectively. In the discharged patients, the median time from admission to discharge was 24 d (IQR: 13–43) (Table 1).
3.2. Vital signs and symptoms after admission
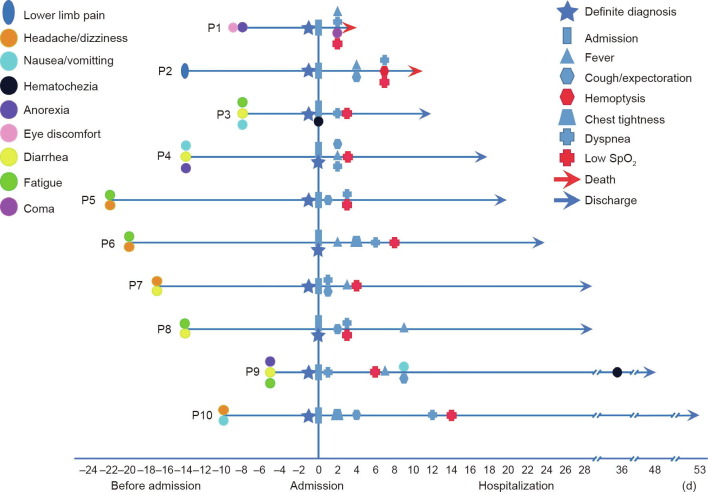
Vital signs and clinical symptoms were monitored in all patients on a daily basis. Fig. 1 lists the abnormal vital signs and symptoms of the ten patients during their disease course. After admission, seven patients developed fever and all ten developed respiratory symptoms. All ten patients experienced a decrease in SpO2 (≤ 93%) at rest during hospitalization.
Fig. 1.
Changes in vital signs and clinical symptoms in the ten studied patients over the entire course of COVID-19. P1–P10 refer to the ten patients, respectively. The origin point represents the date of admission.
3.3. Laboratory findings
All patients were diagnosed using a positive nucleic acid test before or on admission. At admission, the white blood cell (WBC) count was below the normal range (4 × 109–1 × 1010 L−1) in two patients and above the normal range in one patient. Three patients had neutrophils above the normal range (> 6.3 × 109 L−1). Four patients suffered from lymphopenia (lymphocyte count < 1.1 × 109 L−1). Red blood cell (RBC) count was below the normal range (< 3.5 × 1012 L−1) in two patients. Platelets were above the normal range (100 × 1012–300 × 1012 L−1) in three patients. D-dimer level was above the normal range (0–0.5 mg·L−1) in seven patients. Two patients had abnormal myocardial zymogram: One of these patients had elevated creatine kinase-myocardial band isoenzyme (CK-MB) (> 6.6 ng·mL−1) and muscle hemoglobin (> 147 ng·mL−1), while the other patient had increased muscle hemoglobin (> 147 ng·mL−1) and hypersensitive Tn I (> 28 pg·mL−1). In five patients, the liver function was abnormal, to various degrees, with alanine aminotransferase (ALT) (n = 5) or aspartate aminotransferase (AST) (n = 2) above the normal range (5–40 U·L−1). Two patients had renal impairment of different degrees, with elevated blood urea nitrogen (BUN) (> 8.2 mmol·L−1) or serum creatinine (> 111 μmol·L−1). CRP was above the normal range (0–8 g·L−1) in five patients. Seven patients had elevated serum levels of PCT (> 0.05 ng·mL−1). In five patients, glucose was increased (> 6.1 mmol·L−1) (Table 2 ).
Table 2.
Laboratory findings of the ten studied atypical COVID-19 patients on admission.
| Variables | No. | Variables | No. | Variables | No. |
|---|---|---|---|---|---|
| Blood routines | Coagulation function | Albumin/globin | |||
| WBC count (× 109 L−1) | PT (s) | < 1.0 | 3 | ||
| < 4 | 2 | 11–16 | 10 | < 1.5 | 7 |
| 4–10 | 7 | INR | Creatinine (μmol·L−1) | ||
| > 10 | 1 | 0.83–1.36 | 10 | < 57 | 2 |
| Neutrophil count (× 109 L−1) | D-dimer (mg·L−1) | 57–111 | 7 | ||
| < 1.82 | 2 | 0–0.5 | 3 | > 111 | 1 |
| 1.82–6.30 | 5 | > 0.5 | 1 | Blood urea nitrogen (mmol·L−1) | |
| > 6.30 | 3 | > 1.0 | 6 | 2.9–8.2 | 9 |
| Lymphocyte count (× 109 L−1) | APTT (s) | > 8.2 | 1 | ||
| < 1.1 | 4 | < 27 | 1 | Uric acid (μmol·L−1) | |
| 1.1–3.2 | 6 | 27–45 | 8 | < 208 | 4 |
| Monocyte count (× 109 L−1) | > 45 | 1 | 208–428 | 6 | |
| 0.1–0.6 | 8 | Fibrinogen (g·L−1) | Glucose (mmol·L−1) | ||
| > 0.6 | 2 | 2–4 | 6 | 3.9–6.1 | 5 |
| RBC count (× 1012 L−1) | > 4 | 4 | > 6.1 | 5 | |
| < 3.5 | 2 | Liver and kidney function | Index of myocardial injury | ||
| 3.5–5.0 | 8 | ALT (U·L−1) | LDH (U·L−1) | ||
| Platelet count (× 109 L−1) | 5–40 | 5 | 109–245 | 4 | |
| 100–300 | 7 | > 40 | 5 | > 245 | 6 |
| > 300 | 3 | AST (U·L−1) | CK (U·L−1) | ||
| Inflammatory indicators | 8–40 | 8 | ≤ 185 | 10 | |
| CRP (g·L−1) | > 40 | 2 | CK-MB (ng·mL−1) | ||
| 0–8 | 5 | Albumin (g·L−1) | > 6.6 | 1 | |
| > 8 | 5 | < 33 | 5 | Hypersensitive TnI (pg·mL−1) | |
| PCT (ng·mL−1) | 33–55 | 5 | > 28 | 1 | |
| < 0.05 | 3 | — | — | Muscle hemoglobin (ng·mL−1) | |
| > 0.05 | 7 | — | — | > 147 | 2 |
INR: international normalized ratio; PT: prothrombin time; APTT: activated partial thromboplastin time.
3.4. Imaging findings
All patients received a chest CT scan, and abnormalities were detected in all ten patients. Nine patients had bilateral pneumonia, and only one had unilateral pneumonia. Seven patients showed ground-glass opacity and multiple patchy shadows. Two patients exhibited ground-glass opacity. One patient showed multiple patchy shadows (Table 3 ). In addition, two patients had emphysema. Finally, pulmonary lesions improved in eight discharged patients. CT images before and after the treatment of two representative patients are shown in Fig. 2 .
Table 3.
Chest CT findings of the ten studied atypical COVID-19 patients.
| Chest CT | No. |
|---|---|
| Distribution of lesions | |
| Bilateral | 9 |
| Unilateral | 1 |
| Features of lesions | |
| Patchy shadows alone | 1 |
| Ground-glass opacity alone | 2 |
| Both patchy shadows and ground-glass opacity | 7 |
| Consolidation | 2 |
Fig. 2.
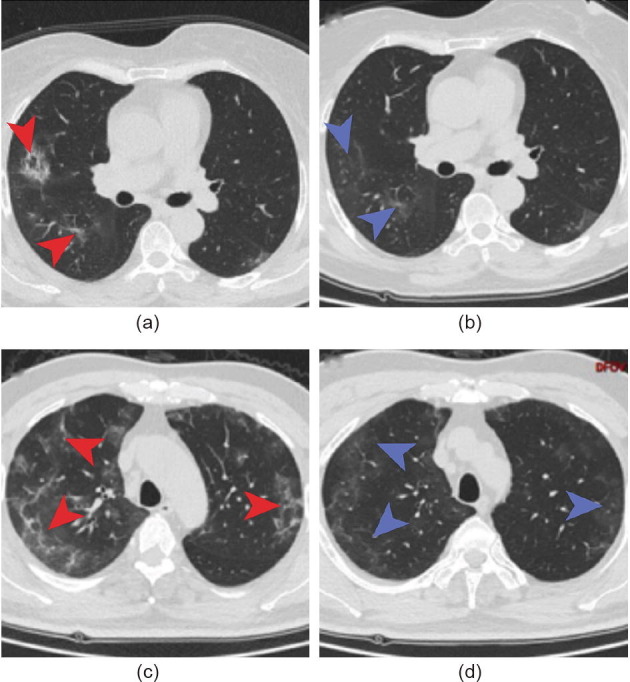
Chest CT images. (a) Chest CT image from a 66-year-old woman showing ground-glass opacity and subsegmental consolidation at admission; (b) chest CT image 24 d after admission showing that consolidation was absorbed; (c) chest CT image from a 57-year-old man exhibiting bilateral ground-glass opacity and multiple subsegmental consolidation after admission; (d) chest CT image 26 d after admission showing that consolidation was absorbed.
3.5. Treatments and outcomes
Each patient was given antiviral treatment. The median time of antiviral treatment lasted for 18 d (IQR: 10–29). Seven patients were on antibiotic treatment for 10 d (IQR: 6–14), with the anti-bacterial spectrum covering common pathogens and some atypical pathogens. One patient developed fungal infection and was treated by antifungal therapy. Three patients received glucocorticoid therapy. Five patients used nasal cannulation, and three patients were put on noninvasive mechanical ventilation or high-flow nasal cannulation. Two patients were placed on an invasive ventilator (Table 4 ).
Table 4.
Treatments and outcomes of the ten studied atypical COVID-19 patients.
| Variable | No. | Median time of treatment (IQR) (d) |
|---|---|---|
| Complications | ||
| Shock | 2 | — |
| ACI | 2 | — |
| ARDS | 4 | — |
| AKI | 2 | — |
| Secondary infection | 1 | — |
| Treatment | ||
| Antiviral therapy | 10 | 18 (10–29) |
| Abidor | 10 | — |
| Interferon-γ | 3 | — |
| Lopinavir and ritonavir | 1 | — |
| Ribavirin | 2 | — |
| Chloroquine phosphate | 2 | — |
| Traditional Chinese medicine | 2 | — |
| Antibiotic therapy | 7 | 10 (6–14) |
| Antifungal therapy | 1 | 9 |
| Enhanced immune therapy | 7 | 17 (8–25) |
| Glucocorticoid therapy | 3 | 9 |
| Oxygen support | 17 (8–28) | |
| Nasal cannulation | 5 | — |
| Noninvasive ventilation or high-flow nasal cannulation | 3 | — |
| Invasive mechanical ventilation | 2 | — |
| Nutritional support | 5 | 4 (2–5) |
| Clinical outcomes | ||
| Discharged | 8 | — |
| Death | 2 | — |
Of the ten studied patients, two patients died and eight patients were discharged. The two deceased patients were a 70-year-old man (Patient 1) and a 78-year-old man (Patient 2), both of which had pre-existing chronic diseases. Patient 1 was diagnosed as having COVID-19, hypertension, and cardiac pre-excitation syndrome, and was given symptomatic treatment after admission. Later, he developed hypoxemia and acute myocardial injury and was given ventilator-assisted breathing. The hypoxemia remained unresolved. Having developed severe hypoxemia, hypotension, and confusion, the patient experienced a sudden cardiac arrest on Day 3 after admission and was pronounced dead. Patient 2 was transferred to Wuhan Union Hospital West Campus and diagnosed with severe pneumonia, vein thrombosis, and necrosis of the right lower extremity. He was immediately operated on for limb amputation. He was then transferred to the intensive care unit (ICU) and intubated for ventilator-assisted breathing. Later, his symptoms improved and the patient was removed from the ICU. On Day 7 after admission, the patient experienced hypoxemia and was subjected to intubation again. On Day 10 after admission, the patient died of acute myocardial infarction, respiratory failure, and shock.
4. Discussion
The clinical spectrum of SARS-CoV-2 infection appears to be broad, ranging from atypical infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure, to death [5], [6], [21]. The existence of atypical cases presents a serious threat to public health, but information on atypical patients with COVID-19 is extremely limited. Here, we described a total of ten severe or critical patients (1.1%) who initially presented with atypical symptoms without respiratory symptoms or fever at onset.
SARS-CoV-2 mainly attacks the lungs, causing typical symptoms including respiratory discomfort and fever. In addition, atypical presentations including gastrointestinal symptoms (anorexia, diarrhea, nausea, vomiting, and abdominal pain), fatigue, rhinorrhea, nasal stuffiness, testicular pain, non-specific neurological symptoms (dizziness, headache, neuralgia, and myalgia), anosmia, ageusia, and ocular and cutaneous manifestations have been reported in COVID-19 patients [22], [23], [24]. The underlying pathological mechanisms might be ascribed to the comprehensive expression of the angiotensin-converting enzyme 2 (ACE2) receptors, the host cell entry receptor of SARS-CoV-2, on various organs [25]. Recent studies have shown that infected infants, children, young people, and frail older people might present with atypical clinical symptoms. Regretfully, it is still unknown whether there is a certain group of people who are prone to atypical symptoms.
The severe or critical patients in this study were older than their counterparts with common symptoms who had been included in previous studies [5], [21], and 70% of the patients were male. Published studies have suggested that older male patients are more vulnerable to SARS-CoV-2 infection [5]. Of the ten patients, eight were discharged and two died. The median time from onset to hospital admission was 14 d (IQR: 9–21), which was much longer than that of typical patients (7 d (IQR: 4–8)) who presented with respiratory symptoms or fever at onset [6]. For the eight discharged patients, the median hospital stay lasted for 24 d (IQR: 13–43), indicating that the hospital stay time was longer in the atypical patients than in the typical COVID-19 patients (10 d (IQR: 7–14)) reported in other study [6]. In the later stages of the disease, all of the studied atypical patients eventually developed respiratory symptoms or fever (Fig. 1). A time window (IQR: 11–22 d) between the initial atypical symptoms and the eventual typical symptoms (i.e., fever and/or respiratory presentations) was observed in the ten patients. It is worth noting that a couple of these patients (two of ten) went to non-fever or non-emergency clinics for medical attention, so their admission might well have been delayed. These atypical cases suffered from more delays from onset to admission and a longer waiting time for hospital admission compared with typical patients with onset fever, as previously reported by Wang et al. [6]. These atypical symptoms might have been ignored by either doctors or the patients themselves, and the resultant delays might have affected timely diagnosis or treatment, and even have led to the prolonged hospital stay or poor prognosis.
In this study, we found that the most common laboratory abnormalities included depressed total lymphocytes, increased D-dimer level, elevated serum glucose, and liver function impairment. Moreover, the two deceased patients had laboratory abnormalities, mainly involving coagulative activation and myocardial injury, suffered from comorbidities, and were of advanced age. These findings were similar to those previously observed in patients with typical onset symptoms [6]. Limited by the small sample size in our study, we cannot analyze the risk factors for critical illness in these atypical patients. A prior study showed that age, hemoptysis, dyspnea, comorbidities, neutrophil-to-lymphocyte ratio, LDH, and direct bilirubin were independent predictive factors for the occurrence of critical illness in COVID-19 patients, and these findings were used to develop a web-based risk calculator [26]. When using this calculator, four (including the two deceased cases) of the ten patients in our study were scored as being in the high-risk group, and the other six cases were all scored as being in the medium-risk group; these results aligned with the severe or critical condition of the ten patients in our study. Therefore, the prognostic risk indicators of atypical patients might be similar to those of all COVID-19 patients, although this possibility needs to be verified in future studies with large sample sizes.
In this study, all individuals tested positive for SARS-CoV-2 infection with quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Radiologically, CT scans showed bilateral ground-glass or patchy opacity in 100% of the patients, which is consistent with prior findings [6], [11], [21], [27]. Therefore, chest CT scans and nucleic acid detection should be performed immediately on close contacts of COVID-19 patients to identify atypical infections, even if these contacts present without respiratory symptoms or fever.
Since SARS-CoV-2 is a newly emerging virus, no antiviral treatment or vaccine has been proven to be effective as yet for SARS-CoV-2 infection. The current management of the disease mainly includes early diagnosis, isolation, and supportive treatments. Based on the Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 3) [17], the principal treatment for the ten patients in our study involved antiviral therapy and supportive care, nasal cannulation, immunity adjustment, nutritional support, and so forth. Aggressive treatments such as intensive care and invasive mechanical ventilation were needed when patients’ condition worsened. In general, the treatments for atypical infection were essentially similar to those for typical patients [11], [22].
To address the massive COVID-19 pandemic, the State Council of the People’s Republic of China is employing both traditional strategies and some unprecedentedly drastic measures, such as personal or family isolation, social distancing, city and community lockdown, and the shutdown of non-essential outbound or inbound flights, among others [28], [29]. At present, China’s efforts have effectively contained the epidemic, and the whole country, including Wuhan, is largely free from infection. Nonetheless, atypical patients infected with SARS-CoV-2 may cause a resurgence of the epidemic in China, so they pose a significant infection-control challenge. Our study showed that it is extremely important for public health to effectively isolate patients and trace and quarantine contacts as early as possible. Since atypical infection can lead to disastrous outcomes, the public should be informed of the danger of such hidden transmission. Furthermore, mounting evidence shows that pre-symptomatic and asymptomatic cases can be highly contagious to a susceptible population [29], [30], [31], and thus present a substantial challenge to epidemic control. Due to the varied range of atypical symptoms in COVID-19 patients, it is difficult to diagnose atypical COVID-19 patients simply according to symptoms. Non-specific laboratory results between COVID-19 patients and other patients with influenza virus infections also made differential diagnosis difficult. However, in a SARS-CoV-2 endemic area, these symptoms and laboratory tests can provide a warning. Overall consideration of epidemiology history, symptoms, chest CT scans, laboratory tests, and, above all, SARS-CoV-2 nucleic acid detection can make it possible to diagnose atypical COVID-19 patients early on.
Our study has some limitations. First, only ten patients were included in the study, so the interpretation of our findings might be limited due to sample size. Second, as the Union West Hospital is a designated hospital for severe and critical cases, moderate and mild patients who presented with atypical symptoms were lacking. Further larger and multicentric studies are warranted to explore the frequency of atypical cases and to better characterize this special group of patients. Finally, the role of atypical cases in the transmission of the virus is not fully understood and needs further studies to determine the relevant dynamics and transmission mechanism.
5. Conclusions
In conclusion, patients with confirmed COVID-19 who presented without respiratory symptoms or fever at onset account for a certain proportion of severe or critical patients. All such cases should be identified and quarantined as early as possible, since atypical infection tended to result in a protracted hospital stay and fatal outcome. Chest CT scan and nucleic acid detection should be performed immediately among close contacts of COVID-19 patients to identify those with atypical infection, even if the contacts present without respiratory symptoms or fever at onset.
Acknowledgments
Acknowledgments
This research was supported by the National Natural Science Foundation of China (82041018, 81770096, 81700091, and 81800094), the National Science and Technology Major Project of the Ministry of Science and Technology of China (2019ZX09301001), the National Key Research and Development Program of the Ministry of Science and Technology of China (2020YFC0844300), and the Fundamental Research Funds for the Central Universities (China) (2020kfyXGYJ011).
Authors’ contribution
YJ, JC, and JX designed the study. YL, ZY, SW, LD, AY, and TL collected the demographic and clinical data and information on medical resources. JX, YL, and ZY summarized all data. Statistical analysis was made by JX and ZY. The manuscript was drafted by JX, YL, ZY, SW, and JF. YJ and JC critically revised the manuscript.
Compliance with ethics guidelines
The present protocol was evaluated and approved by the institutional ethics board of Wuhan Union Hospital, Huazhong University of Science and Technology (No. 0036), Wuhan, China. Written informed consent was waived for the critical situation of an emerging infectious disease.
Juanjuan Xu, Zhengrong Yin, Yu Liu, Sufei Wang, Limin Duan, Yi An, Jinshuo Fan, Tingting Liao, Yang Jin, and Jianguo Chen declare that they have no conflict of interest or financial conflicts to disclose.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO coronavirus disease (COVID-19) dashboard [Internet]. Geneva: WHO; c2020 [updated 2020 Sep 14; cited 2020 Sep 14]. Available from: https://covid19.who.int/.
- 3.Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Du H., Qin Y., Roberts J., Cummings O.W., Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67(18):8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 9.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia: MERS, SARS and coronaviruses. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 14.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 15.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Health Commission of the People’s Republic of China; National Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 3) [Internet]. Beijing: The State Council of the People’s Republic of China; 2020 Jan 22 [cited 2020 Apr 5]. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202001/f492c9153ea9437bb587ce2ffcbee1fa/files/39e7578d85964dbe81117736dd789d8f.pdf. Chinese.
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:124–38.
- 19.Gao C., Wang Y., Gu X., Shen X., Zhou D., Zhou S., et al. The Community-Acquired Pneumonia–China Network. Association between cardiac injury and mortality in hospitalized patients infected with avian influenza A (H7N9) virus. Crit Care Med. 2020;48(4):451–458. doi: 10.1097/CCM.0000000000004207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garner J.S., Jarvis W.R., Emori T.G., Horan T.C., Hughes J.M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J., Thomsen T., Sell N., Goldsmith A.J. Abdominal and testicular pain: an atypical presentation of COVID-19. Am J Emerg Med. 2020;38(7) doi: 10.1016/j.ajem.2020.03.052. 1542.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noh J.Y., Yoon J.G., Seong H., Choi W.S., Sohn J.W., Cheong H.J., et al. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect. 2020;81(5):816–846. doi: 10.1016/j.jinf.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abobaker A., Raba A.A., Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol. 2020;92(11):2458–2464. doi: 10.1002/jmv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Z., Wang L., Cauchemez S., Xu X., Wang X., Cowling B.J., et al. Risk for transportation of coronavirus disease from Wuhan to other cities in China. Emerg Infect Dis. 2020;26(5):1049–1052. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27(2):taaa020. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kam K.Q., Yung C.F., Cui L., Lin Tzer Pin R., Mak T.M., Maiwald M., et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. 2020;71(15):847–849. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Z.D., Tang A., Li K.F., Li P., Wang H.L., Yi J.P., et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052–1054. doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]