ABSTRACT
The electronic cigarette (EC), was initially introduced as a safe alternative to conventional cigarette smoking While initially seemingly innocuous, over 2800 E-cigarette, or Vaping, product use-associated lung injury (EVALI) cases have been reported in the USA, with a spectrum of clinical severity ranging from mild dyspnea to overt respiratory failure In this report we highlight three EVALI cases whom presented with dyspnea and a variety of non-specific symptoms. Diagnostic imaging demonstrated bilateral reticular infiltrates and ground-glass opacities with lymphadenopathy. Clinically, patients failed to respond to empiric antibiotics but improved after initiating steroids. Consistent with prior case series, our patients reported exposure to EC liquids containing tetrahydrocannabinol (THC)/cannabidiols (CBD) additives, suggesting Vitamin E acetate as the potentially harmful constituent. In this case series and review, we not only summarize prior clinical studies that have evaluated the effects of vaping on cardiopulmonary function as well as case reports on EVALI, but also discuss the pathophysiology of vaping and EVALI. It remains unclear not only why some individuals develop EVALI, but why the clinical and pathological presentations vary. EVALI remains a significant public health concern and clinicians must maintain a high index of suspicion for this novel phenomenon.
KEYWORDS: Electronic cigarette, vaping, vitamin E Acetate, surfactant, vaping-associated lung injury, e-cigarette associated lung injury, VALI, EVALI
1. Introduction
Electronic cigarettes (EC) produce an aerosol by heating liquid that contains a glycerin and propylene glycol base along with additives such as nicotine, flavorings, THC, or CBD. This process, vaping, was introduced as an alternative to conventional cigarette smoking and over the past decade popularity has increased [1]. While initially marketed as a safe, tobacco-cessation product, numerous scientific studies have suggested that a host of factors could predispose to the development of cardiopulmonary disease – heavy metals, flavorings, toxic byproducts from vaporizing – yet, the actual concentrations at which these substances are deposited onto the respiratory epithelium, alveolar-capillary interface, or within the blood are low, and conflicting studies suggest that vaping is a ‘safe alternative’ compared to tobacco use. More recently, however, these studies have been underscored by an entirely new disease entity, E-cigarette, or Vaping, product use-associated lung injury (EVALI), a heterogeneous collection of respiratory pathology with symptoms ranging from mild dyspnea to overt respiratory failure [2–8]. In cases of EVALI, Vitamin E acetate, a contaminant found exclusively in THC/CBD-containing compounds, has been suggested as the culprit [9]. Here, we present 3 cases of EVALI that presented to our facility and provide a literature review not only on the direct physiological effects of vaping, but expound on its potential pathophysiological mechanisms.
2. Case presentation
Three patients reported a history of vaping with THC-only or THC/Nicotine-containing liquids (length of time ranging from four months to two years) (Table 1). All patients presented with dyspnea and a variety of non-specific constitutional symptoms (fever, headache, nausea, vomiting, and diarrhea), with a duration of five days to two months (Table 2). Laboratory workup demonstrated neutrophil-predominant leukocytosis and elevated inflammatory markers. Imaging demonstrated bilateral reticular infiltrates and ground-glass opacities with occasional lymphadenopathy (Figures 1 and 2). Empiric antibiotics were administered for sepsis of presumed pulmonary etiology. Workup for bacterial, viral, and fungal etiologies were negative. One patient underwent bronchoscopy with bronchoalveolar lavage and biopsy that showed no acute pathology (Figure 3). Antibiotic therapy was discontinued in the setting of lack of clinical improvement and high-dose steroids initiated, leading to clinical and radiological improvement.
Table 1.
Patient demographics, smoking, and vaping history
| Case Number | 1 | 2 | 3 |
|---|---|---|---|
| Age | 50 | 33 | 35 |
| Gender | Female | Male | Male |
| Race | White | White | African American |
| Marital status | Single | Single | Single |
| Employment status | Unemployed | Full-time | Unemployed |
| Smoking history | Former smoker, about 35PY, quit 4 months ago and started E-cigarettes; no marijuana. | Never smoker; no marijuana. | Former smoker, about 0.25pack/day, quit 1.5 years ago; smokes marijuana daily since then. |
| Length of vaping history | 4 months | 2 years | One episode of vaping 2 months before admission, with dyspnea thereafter |
| Product | Unknown | THC | Marijuana |
| Last day of vaping | Unknown | One day prior to admission | The day before admission |
| Brand | Unknown | Changed supplier recently | Unknown |
Table 2. All three patients were relatively young (age < 50), of mixed gender (2/3 male, 1/3 female), and mixed race (2/3 Caucasian, 1/3 African American). All had a prior vaping history utilizing non-THC and THC-containing products for a range of 4 months to 2 years. The onset of the symptoms consistently occurred following the vaping of products of unknown origin purchased illicitly. Abbreviations: PY – pack years; THC – tetrahydrocannabinol.
Table 2.
Clinical characteristics of vaping cases
| Case Number | 1 | 2 | 3 |
|---|---|---|---|
| Smoking History | Former smoker, 35 PY | Never smoker | Former smoker: cigarettes 0.25packs per day, quit 1.5 years ago; smoke marijuana daily. |
| History of lung diseases | No | No | No |
| Constitutional symptoms | Fever, hot flashes, sweaty, | Fever and chills, significant night sweats, unintentionally weight loss of 18–20lbs over the past week. | Some night sweats, unintentional weight loss of 10lb |
| Duration of disease onset before admission | 5 days | 2 months | 2 months |
| Vital signs | Maximum Temperature 39.1C | Maximum Temperature 38C, Maximum Heart Rate 133 bpm, Maximum Respiratory Rate 53 b/m | Unremarkable |
| Respiratory symptoms | SOB, dry cough, Pleural chest pain (L > R) | Cough, dyspnea | Exertional dyspnea, cough, chest pain when coughing |
| Non-respiratory symptoms | None | Nausea, projectile vomiting, and watery diarrhea | None |
| GI symptoms | None | Yes | None |
| Physical exam | Bibasilar cackles | Diffuse crackles, more pronounced bibasilar, L > R | Diffuse crackles bilaterally, no wheeze |
| Significant Labs | WBC 16.4 k, Na 131 | Na 133, K 3.0, WBC 9.2->17.8 k, | PaO2 68 mmHg, CRP 12 |
| Transaminitis | No LFT results available | AST 35, ALT 53 | Normal AST 19, ALT 22 |
| CXR on admission | Hazy bilateral basilar opacities | Punctate high density over the left upper chest, possible minimal infiltrate at posterior lower lung | Extensive opacities seen throughout bilateral lungs, predominantly throughout the upper lobes and peripheral distribution. Patchy changes seen within the perihilar lower lobes. |
| Chest CT | Enlarged prevascular node (1.2 × 1.8 cm), prominent precarinal LN, prominent Rt hilar LN. Diffuse bilateral GGO | Bilateral infiltrates of somewhat ground-glass in appearance with interstitial and septae, and some precarinal and subcarinal adenopathy. | Scattered ground-glass attenuation with superimposed interlobular septal thickening and areas of confluent airspace opacities bilaterally |
| Infectious Disease Workup | Negative | Negative | Negative |
| Bronchoscopy | Yes | No | No |
| BAL | Negative for malignancy and infection | No | No |
| Biopsy | EBUS: S7: negative for tumor and granuloma. Lingula biopsy: negative bacterial and fungal cultures | No | No |
| Treatment | Antibiotics: ceftriaxone, doxycycline; Steroids: Prednisone 60 mg once a day for 4 days | Antibiotics: Ceftriaxone; Steroids: Methylprednisolone 50 mg twice a day for 4 days, then 40 mg once a day for 1 day; | Antibiotics: Ceftriaxone and azithromycin; Steroids: methylprednisolone 60 mg three times a day for 2 days, prednisone 40 mg with 4 weeks taper |
| Response to treatment | Oxygen demands decreased after steroids X 2d | One day after initiated steroids | One days after initiated steroids |
| Length of hospital stay | 4 days | 6 days | 3 days |
| Primary Dx on admission | Sepsis | Nausea and vomiting due to gastroenteritis | Dyspnea on exertion |
| Primary Dx on discharge | Vaping induced lung injury | Vaping induced lung injury | Pulmonary infiltrates |
Table 1. All three patients had no previous lung disease. Onset of acute symptoms started between 5 days to 2 months prior to admission. One of three patients had neutrophil-dominant leukocytosis. All three patients demonstrated radiologic abnormalities as above. One patient underwent bronchoscopy, bronchoalveolar lavage, and lung biopsy; while macrophages and other inflammatory cells were seen on BAL, no specific underlying pathology was identified. All patients demonstrated symptomatic and radiographic improvement following steroid administration. Abbreviations: BAL - Bronchoalveolar lavage; bpm – beats per minute; b/m - breaths per minute; CT – computed tomography; EBUS - Endobronchial ultrasound; GGO – ground glass opacities; LN – lymphadenopathy; PY – pack years.
Figure 1.
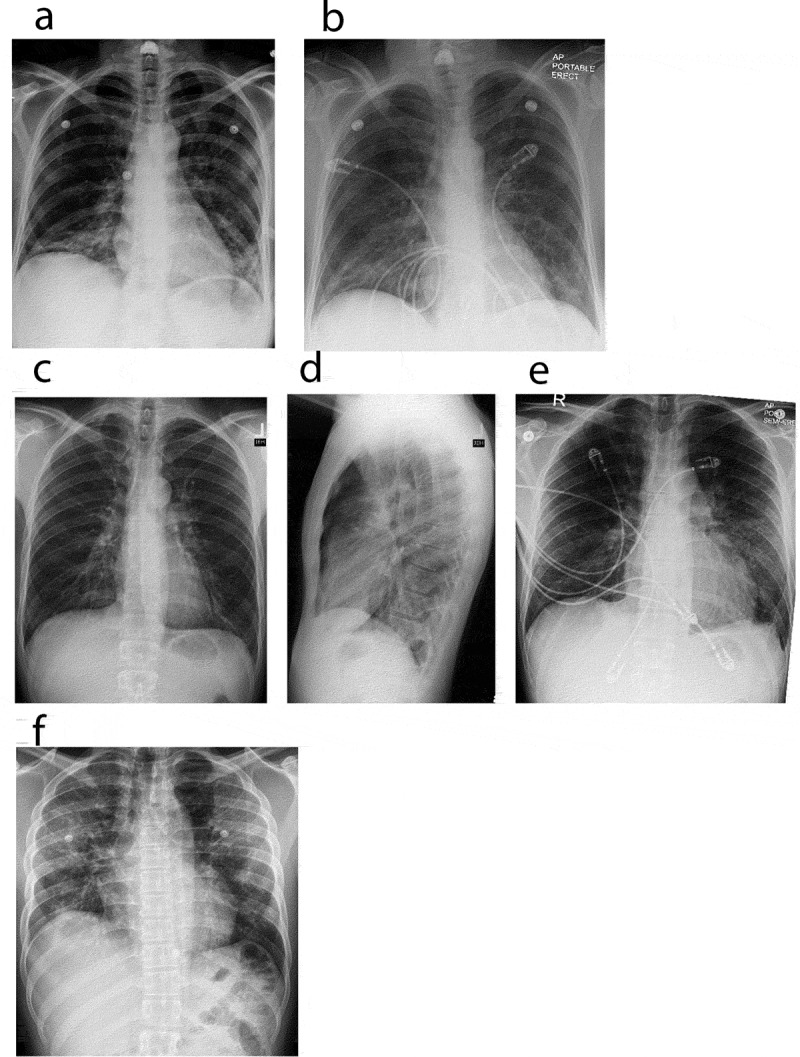
Chest X ray. Chest radiographs depicting a spectrum of EVALI. (a) and (b) Chest radiographs of case 1. (a) (Day 1 of admission, pre-treatment), revealed bi-basilar haziness whereas (b) (steroid day 2), demonstrated mild improvement of left lower lobe infiltrates. (c–e) Chest radiographs of case 2. (c, d) (Day 1 of admission, pre-treatment), revealed minimal infiltrates bilaterally. Punctate density of the left upper chest is suggestive of a calcified granuloma (c), with lateral view (d) showing minimal infiltrate. (e) (steroid day 3), demonstrates clinical improvement in bilateral infiltrates. (f) Chest radiograph of case 3. (f) (Day 1 of admission, pre-treatment), revealed patchy infiltrates bilaterally
Figure 2.
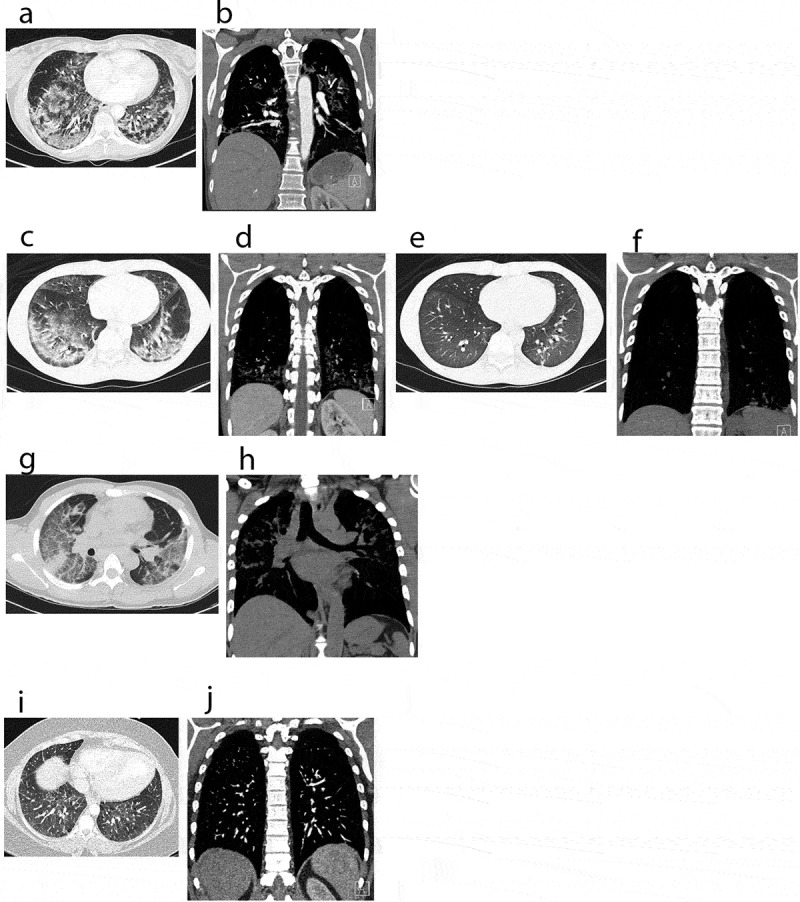
CT scans: computerized tomography of chest, showing a spectrum of EVALI in axial and coronal views. (a, b) Chest CT images of case 1 on admission, pre-treatment. (a) revealed bilateral ground glass opacities and interstitial infiltrates. (b) revealed prominent right hilar lymph nodes and an enlarged intrathoracic node. (c–f) Chest CT images of case 2. (c, d) (on admission, pre-treatment) demonstrating bilateral ground glass infiltrates. (e, f) (steroid day 4) showing significant improvement of lung infiltrates bilaterally. (g, h) Chest CT images of case 3 on admission, pre-treatment. (g) demonstrating diffuse patchy ground-glass opacities bilaterally. (h) showing mediastinal lymphadenopathy and interstitial infiltrates bilaterally. (i, j) Chest CT images of an asymptomatic patient with vaping history. CT images showed mild diffuse ground-glass opacities bilaterally
Figure 3.
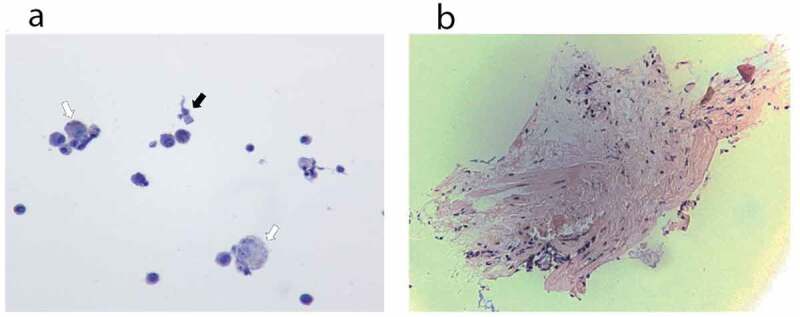
Histopathology. (a) Cytology from bronchoalveolar lavage fluid of case 1. The hollow arrows point to few non-vacuolated foamy macrophages, without droplet deposition. The black arrow points to one bronchial cell. (b) Pathology from lung biopsy of case 1. Minimal tissue. There is no alveolar damage observed
3. Discussion
3.1. Vaping: to vape or not to vape
Despite the early reputation of EC as a safe smoking cessation aid, major scientific communities including the WHO and US-FDA, noted not only that the EC is ‘not considered to be a legitimate therapy for smokers trying to quit and not a proven nicotine-replacement therapy,’ but also that there existed ‘no scientific evidence to confirm the product’s safety’ [10]. Borne out of this uncertainty, a variety of in vitro, in vivo, and clinical studies were conducted to evaluate the safety and potential toxicity profiles of EC and various EC-liquids on a cellular, organismal, and functional perspective, respectively. In vitro studies utilized a variety of designs and focused on a few critical areas – EC-liquids (direct liquid, vapor condensate, and vapor), flavorings, toxic metals, and coil power – in two main model systems – direct application in cell culture or an air-liquid interface cell-based system. Sassano utilized high-throughput to evaluate large amounts of EC-liquids simultaneously, highlighting that not only are EC-liquids extremely heterogenous, but that increased concentrations of the base EC-liquid, resulted in increased cellular toxicity [11]. Furthermore, multiple in vitro studies have confirmed that certain flavor additives, vanillin and cinnamaldehyde, were associated with higher toxicity [11–15].
Functional human studies have demonstrated conflicting and controversial data regarding the physiological effects on cardiopulmonary function. Results demonstrated that compared to EC, a return to tobacco for as little as one week demonstrated not only significantly increased levels of carbon monoxide and carboxyhemoglobin, but also significantly reduced FEV1 and FVC [16]. The authors furthermore highlighted significantly reduced alveolocapillary membrane damage when compared to traditional tobacco use, suggesting an increased safety profile of EC use [16].
3.2. E-cigarettes, or vaping, product use-associated lung injury
Multiple cases of EVALI were reported not only in the Unities States, with a heterogenous distribution of pathology – hypersensitivity pneumonitis, eosinophilic pneumonia, organizing pneumonia – but also in a variety of different countries – Japan (acute lung injury with prominent lipid-laden macrophages), Guam (diffuse alveolar hemorrhage), Australia, and the UK (lipoid pneumonia) [17–23]. Similar to initial reports, all individuals presented with similar symptomatology – dyspnea and fever – similar laboratory and imaging diagnostics – leukocytosis and bilateral ground-glass opacities – and demonstrated symptomatic, functional, and radiological improvement following steroids. In addition, all cases shared an underlying history of vaping and pathological findings of lipid-laden macrophages, though no data existed on the type of EC nor EC-liquid. Scattered reports did highlight cases that specifically identified antecedent vaping of THC-containing EC-liquids, suggesting a possible association of THC-containing EC-liquids and EVALI [24,25].
Of the published case series on EVALI, a set of common characteristics were noted: (1) History of several days of pulmonary symptoms (shortness of breath and cough, less commonly hemoptysis) temporally associated with vaping [3,26–31], (2) Exposure to EC-liquids containing THC oil, with frequent co-exposure to nicotine, however some cases have been identified in those who reported vaping exclusively nicotine products [3], (3) Concomitant gastrointestinal symptoms [3,25,27]. Typical objective findings on presentation were tachypnea and hypoxia, while characteristic laboratory diagnostics demonstrated leukocytosis with neutrophil predominance, mild transaminase elevation, and an elevated ESR [3]. On diagnostic imaging, the presence of bilateral infiltrates was practically universal with CT revealing bilateral ground grass opacities with a predominantly basilar distribution and subpleural sparing [32]. Taken together, these preliminary studies afforded the CDC to opportunity to provide interim diagnostic criteria for the identification of probable and confirmed cases of pulmonary disease associated with E-cigarette use, or EVALI, as well as guidance on the management of such cases, with specific instruction to avoid purchasing illicit THC-containing products, and moreover to avoid THC-containing products altogether [3,26,29,33]. Consistent with these previous reports, all patients presented in this case series reported a similar constellation of presenting symptoms – dyspnea, productive cough, pleuritic chest discomfort, and intermittent fever – that was temporally associated with vaping, specifically of THC-containing products. Diagnostics demonstrated leukocytosis with associated neutrophilic predominance, mild transaminase elevation, and the absence of concomitant infection. Imaging confirmed the presence of bibasilar ground glass opacities. While one patient presented in this case series noted predominantly gastrointestinal symptomatology, symptoms were felt to be a confounding factor, as they predated vaping history. Nonetheless, patients with vaping history may present with no symptoms at all but have ground-glass opacities on CT (Figure 2(i,j)).
3.3. Pathobiology of vaping-associated lung injury
During the evaluation of EVALI-mediated hypoxic respiratory failure, all patients underwent CT imaging which demonstrated the presence of bilateral ground glass opacities, and several patients underwent bronchoscopy, bronchoalveolar lavage, and lung biopsy to further characterize the specific type of lung-injury. Unfortunately, there appeared to be no single underlying pathology with a wide range of reported patterns of pneumonitis – acute eosinophilic pneumonia, eosinophilic pneumonitis, organizing pneumonia, lipoid pneumonia, diffuse alveolar hemorrhage, and hypersensitivity pneumonitis [17–22,34]. Despite the varied gross pathological descriptions, a common feature was the presence of ground glass opacities, lipid-laden macrophages, and pneumocyte vacuolization [3,27,29,34,35]. Multiple reports suggested that the presence of lipid-laden macrophages within the lung is a common pathologic feature [3,21], suggesting pathogenic similarity to lipoid pneumonia [3,21,36,37]. In our series, similar to others, BAL demonstrated scattered reactive bronchial cells with a background of pigmented macrophages and mixed inflammatory cells. While Oil Red or Sudan staining was not performed, macrophages were not noted to be vacuolated, suggesting the absence of lipid-laden macrophages. Lingular biopsy was negative for tumor and granuloma. Currently, our understanding of the pathophysiology of the cellular and molecular mechanisms underlying EVALI continues to evolve and it we hypothesize that EVALI actually represents an umbrella term to describe a variety of pathologies that result in airway damage and hypoxemia.
Lipoid pneumonia has been well documented in the literature, first formally described by Laughlen [38]. In this landmark work, Laughen described ‘an unusual microscopic picture in autopsy sections from cases of pneumonia, that were found in all lobes of the lung except the left upper lobe’ [38]. Of the examined specimens, alveoli contained ‘almost exclusively, large vacuolated mononuclear cells, that contained so many droplets that they were distorted in shape and considerably swollen’. The droplets were noted to be exclusively of oil with the ‘oil containing cells appearing healthy, even those loaded with droplets’. Retrospective analysis noted that all patients had received various lipophilic compounds via nasopharyngeal administration – paraffin or menthol/abalone (a ‘customary practice to treat nose and throat infections’) – that was temporally associated with the development of fever and hypoxia, quite similar to EVALI patients who inhale a lipid-rich aerosol base composed of vegetable glycerin and propylene glycol [38]. He complimented his observation with direct application of menthol/aboline to live rabbits and subsequently observed the ‘presence of vacuolated mononuclear cells of the same type and character as the clinical cases’ as well as the presence of ‘red blood corpuscles’ [38]. Later studies also highlighted the complicated clinical presentation in lipoid pneumonia noting the presence not only of lipid-laden macrophages, but also the presence of alveolar hemorrhage [39,40]. For such cases of lipoid pneumonia, steroids appear to be a well-documented treatment modality [41].
We hypothesize that the presence of lipid-laden macrophages is not pathognomonic for EVALI, but rather reflects the vaporization and subsequent inhalation of a liquid aerosol that is primarily composed of a lipophilic base. Indeed, a radiologic hallmark of both lipoid pneumonia as well as EVALI appears to be areas of low attenuation (−30 to −150 Hounsfield Units (HU)) on computed tomography, suggesting fatty infiltration within the airways and parenchyma [21,37,39,42]. The vaporization of such lipophilic bases, as well as their associated byproducts generated from their pyrolysis, in addition to multiple other potentially toxic compounds – flavorings, heavy metals – may result in bronchiole plugging, inciting an inflammatory response or exerting local changes with respect to surfactant homeostasis [43–46].
3.4. Pathobiological mechanisms of vaping-induced lung injury: a focus on surfactant biology
While the pathophysiological process of EVALI has yet to be identified, mounting evidence suggests vitamin E acetate additives in illicitly prepared and distributed EC-cartridges. Several reports describe analyses of BAL fluid and vaping products that support a possible role for vitamin E acetate in EVALI [28,47–49]. The Minnesota DOH performed chemical analyses of EC-products obtained from EVALI patients and found that among the toxicants assayed for, vitamin E acetate was most commonly identified and furthermore was not present in THC-containing liquids confiscated one year prior to the EVALI outbreak [47]. Consistent with this finding, a laboratory associated with the New York State DOH reported that of a group of individuals identified with EVALI who used THC-containing EC-products and developed pulmonary illnesses, all used a product that ultimately tested positive for vitamin E acetate [49]. Furthermore, Anresco Laboratories highlighted that THC-containing EC-liquids obtained from reputable and regulated California-based manufacturers contained no vitamin E acetate, but that a high concentration of vitamin E acetate was found in illicit samples [50]. In a comparative analysis of BAL fluid from 51 EVALI patients juxtaposed with samples obtained from a healthy comparator group (comprised of nonusers, exclusive cigarette smokers and exclusive users of nicotine-containing e-cigarette products), vitamin E acetate was detected in 94% of BAL fluid samples of patients with EVALI, whereas the contaminant was not once demonstrated in the convenience sample [9,48]. Indeed at least one state has already banned vitamin E acetate as a constituent of EC-liquids, although it appears to be exclusively found in illicitly manufactured THC-containing EC-liquids [50,51].
4. Conclusion
While initially touted as safe, in vitro, in vivo, and human studies from a molecular, cellular, and functional perspective have called into question the safety profile of EC and their respective EC-liquids. Compared to traditional cigarette use, vaping of appropriately manufactured and unadulterated liquids appears to be associated with less deleterious effects on respiratory parameters such as FEV1, FVC, carbon monoxide, and carboxyhemoglobin. Vaping does however, carry a significantly increased risk compared to healthy non-smokers/non-vapers. While it appears that these effects are mediated by a repertoire of potential toxins and toxicities that affect the alveolar-capillary membrane, the bronchi/bronchioles, immune responses, as well as surfactant homeostasis, the exact etiology and mechanism of EVALI remains unclear. Moreover, EVALI itself likely represents a spectrum of clinical disease ranging from asymptomatic to severe respiratory failure, much as it represents a diverse umbrella of histopathological findings. Indeed, the asymptomatic EVALI may manifest merely as the presence of ground-glass opacities on chest imaging without associated symptomatology or abrogation of pulmonary function tests. It appears that the EVALI epidemic stems from the presence of illicit EC-liquids containing vitamin E acetate, however numerous reports, prior to the introduction of THC-containing EC-liquids or the presence of vitamin E acetate, highlighted early EVALI cases, suggesting that in addition to the potential vaping-associated toxicities, there may exist patient specific predisposing factors [52–54]. This may help to explain the presence of EVALI in early reports as well as the few EVALI cases without evidence of vitamin E acetate on lipidomic analysis.
EVALI represents a spectrum of clinical disease ranging from asymptomatic to acute respiratory failure. Recognition remains challenging not only due to the presence of asymptomatic cases, but that EVALI remains a clinical diagnosis that is predicated on the absence of an underlying infectious or rheumatological pathology and an associated temporal relationship between vaping and the onset of symptoms. Diagnostic criteria for the identification of presumed cases of EVALI have been released and cessation of EC use and initiation of steroid therapy, appear to be cornerstones of management.
Acknowledgments
We appreciate our colleagues Bill McAllister (Multi-site Imaging Informatics Administrator, MedStar Health Franklin Square Medical Center) and Dr. Jeffery Iding (Pathologist, MedStar Health Franklin Square Medical Center) who provided great assistance with obtaining images that greatly improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–637. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention . Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Published 2020. [Updated 2020 January28]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#latest-outbreak-information
- [3].Layden, Jennifer E., et al. “Pulmonary illness related to e-cigarette use in Illinois and Wisconsin.” New England Journal of Medicine 382.10 (2020): 903-916. [DOI] [PubMed] [Google Scholar]
- [4].Meo SA, Ansary MA, Barayan FR, et al. Electronic cigarettes: impact on lung function and fractional exhaled nitric oxide among healthy adults. Am J Mens Health. 2019;13(1):1557988318806073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chaumont M, van de Borne P, Bernard A, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L705–L719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Staudt MR, Salit J, Kaner RJ, et al. Altered lung biology of healthy never smokers following acute inhalation of e-cigarettes. Respir Res. 2018;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lappas AS, Tzortzi AS, Konstantinidi EM, et al. Short-term respiratory effects of e-cigarettes in healthy individuals and smokers with asthma. Respirology. 2018;23(3):291–297. [DOI] [PubMed] [Google Scholar]
- [8].Kales SN, Christiani DC.. Acute chemical emergencies. N Engl J Med. 2004;350(8):800–808. [DOI] [PubMed] [Google Scholar]
- [9].Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use-associated lung injury - 10 states, August-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(45):1040–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].World Health Organization . Marketers of electronic cigarettes should halt unproved therapy claims. World Health Organization. Published 2008. [cited 2020 February8]. New Release 2008 Web site. Available from: https://www.who.int/mediacentre/news/releases/2008/pr34/en [Google Scholar]
- [11].Sassano MF, Davis ES, Keating JE, et al. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018;16(3):e2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barrington-Trimis JL, Samet JM, McConnell R.. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Holden VK, Hines SE.. Update on flavoring-induced lung disease. Curr Opin Pulm Med. 2016;22(2):158–164. [DOI] [PubMed] [Google Scholar]
- [14].Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leigh NJ, Lawton RI, Hershberger PA, et al. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control. 2016;25(Suppl 2):ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barna S, Rozsa D, Varga J, et al. First comparative results about the direct effect of traditional cigarette and e-cigarette smoking on lung alveolocapillary membrane using dynamic ventilation scintigraphy. Nucl Med Commun. 2019;40(2):153–158. [DOI] [PubMed] [Google Scholar]
- [17].Arter ZL, Wiggins A, Hudspath C, et al. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin Respir J. 2018;12(3):1295–1299. [DOI] [PubMed] [Google Scholar]
- [19].Itoh M, Aoshiba K, Herai Y, et al. Lung injury associated with electronic cigarettes inhalation diagnosed by transbronchial lung biopsy. Respirol Case Rep. 2018;6(1):e00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Agustin M, Yamamoto M, Cabrera F, et al. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Viswam D, Trotter S, Burge PS, et al. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. Case Reports 2018;2018:bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sommerfeld CG, Weiner DJ, Nowalk A, et al. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018;141:6. [DOI] [PubMed] [Google Scholar]
- [23].Flower M, Nandakumar L, Singh M, et al. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. 2017;5(3):e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].He T, Oks M, Esposito M, et al. “Tree-in-bloom”: severe acute lung injury induced by vaping cannabis oil. Ann Am Thorac Soc. 2017;14(3):468–470. [DOI] [PubMed] [Google Scholar]
- [25].Triantafyllou GA, Tiberio PJ, Zou RH, et al. Vaping-associated acute lung injury: a case series. Am J Respir Crit Care Med. 2019;200(11):1430–1431. [DOI] [PubMed] [Google Scholar]
- [26].Perrine CG, Pickens CM, Boehmer TK, et al. Characteristics of a multistate outbreak of lung injury associated with e-cigarette use, or vaping - USA, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(39):860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia - North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lewis N, McCaffrey K, Sage K, et al. E-cigarette use, or vaping, practices and characteristics among persons with associated lung injury - Utah, April–October 2019. Morbidity and Mortality Weekly Report (MMWR) Website. Published 2019. [cited 2020 February8]. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6842e1.htm [DOI] [PMC free article] [PubMed]
- [29].Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use - interim guidance. MMWR Morb Mortal Wkly Rep. 2019;68(36):787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Christiani, David C. “Vaping-induced acute lung injury.” (2020): 960-962. [DOI] [PubMed] [Google Scholar]
- [31].Hooper, Randol W., and Jamie L. Garfield. “An emerging crisis: vaping-associated pulmonary injury.” (2020): 57-58. [DOI] [PubMed] [Google Scholar]
- [32].Gilbert HA, Inventor. Smokeless non-tobacco cigarette. 1963.
- [33].Siegel D, Jatlaoui T, Koumans E, et al. Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury - USA, October 2019. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report (MMWR) Website. Published 2019. [cited 2020 February8]. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6841e3.htm?s_cid=mm6841e3_w [DOI] [PMC free article] [PubMed]
- [34].Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780–1781. [DOI] [PubMed] [Google Scholar]
- [35].Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488–1489. [DOI] [PubMed] [Google Scholar]
- [36].Hadda V, Khilnani GC. Lipoid pneumonia: an overview. Expert Rev Respir Med. 2010;4(6):799–807. [DOI] [PubMed] [Google Scholar]
- [37].Betancourt SL, Martinez-Jimenez S, Rossi SE, et al. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010;194(1):103–109. [DOI] [PubMed] [Google Scholar]
- [38].Laughlen GF. Studies on pneumonia following naso-pharyngeal injections of oil. Am J Pathol. 1925;1(4):407–414.401. [PMC free article] [PubMed] [Google Scholar]
- [39].Bréchot JM, Buy JN, Laaban JP, et al. Computed tomography and magnetic resonance findings in lipoid pneumonia. Thorax. 1991;46(10):738–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Marchiori E, Zanetti G, Mano CM, Hochhegger B. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med. 2011;105(5):659-666. [DOI] [PubMed] [Google Scholar]
- [41].Shaikh AY, Oliveira PJ. Exogenous lipoid pneumonia (fire-eater’s lung). Am J Med. 2014;127(2):e3–4. [DOI] [PubMed] [Google Scholar]
- [42].Marchiori E, Zanetti G, Mano CM, et al. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med. 2011;105(5):659–666. [DOI] [PubMed] [Google Scholar]
- [43].Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129(10):4290–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Singanayagam A, Snelgrove RJ. Less burn, more fat: electronic cigarettes and pulmonary lipid homeostasis. J Clin Invest. 2019;129(10):4077–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sosnowski TR, Jabłczyńska K, Odziomek M, et al. Physicochemical studies of direct interactions between lung surfactant and components of electronic cigarettes liquid mixtures. Inhal Toxicol. 2018;30(4–5):159–168. [DOI] [PubMed] [Google Scholar]
- [46].Balakrishnan S. Lipoid pneumonia in infants and children in South India. Br Med J. 1973;4(5888):329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement - Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68(47):1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Blount, Benjamin C., et al. “Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI.” New England Journal of Medicine 382.8 (2020): 697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].New York State Department of Health . New York state department of health announces update on investigation into vaping-associated pulmonary illnesses. Published 2019. [cited 2020 February7]. Available from: https://www.health.ny.gov/press/releases/2019/2019-09-05_vaping.htm
- [50].Eisenberg Z, Moy D, Lam V, et al. Contaminant analysis of illicit vs regulated market extracts. Published 2019. [cited 2020 February8]. Available from: https://cannabis.anresco.com/analysis-of-illicit-vs-regulated-market-extracts/
- [51].Fields A. Washington state bans vape products containing vitamin e acetate, thought to be linked to illness. Published 2019. [cited 2020 February8]. Available from: https://www.seattletimes.com/seattle-news/Washington-state-bans-vape-products-containing-vitamin-e-acetate-thought-to-be-linked-to-illness
- [52].McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141(4):1110–1113. [DOI] [PubMed] [Google Scholar]
- [53].Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47(1):15–17. [DOI] [PubMed] [Google Scholar]
- [54].Atkins G, Drescher F. Acute inhalational lung injury related to the use of electronic nicotine delivery system (ENDS). CHEST. 2015;148(4):83A. [Google Scholar]


