ABSTRACT
Importance
As the scientific community is in a marathon in finding out the cure for COVID-19, in this crisis, it is essential for the physicians not to forget about the basics. Due to the pandemic crisis, in many nursing homes and hospitals, there established new policies on decreasing unnecessary medications to minimize cross-contamination. Sometimes these policies are making providers avoid essential drugs such as Vitamins, including Vitamin D. In this paper, we try to emphasize the importance of Vitamin D in COVID-19 and respiratory viral patients.
Relevance
Vitamin D helps in decreasing the ‘pro-inflammatory cytokines’ in the lungs and acts in immunomodulatory function, and ‘also it will increase the anti-inflammatory, antiviral responses of the respiratory epithelial cells during infection.’
Conclusion
Due to the highly contagious nature of COVID-19 and the increased morbidity and mortality with no appropriate therapy and vaccine, one must be cautious and do everything to help COVID-19 patients. In hospitals and other health care settings to decrease cross-contamination, holding other non-essential medications is taking place. Discontinuing Vitamins could increase the mortality and morbidity of those affected, especially in deficient/insufficient individuals. Obtaining serum 25 (OH) D levels in all patients with viral respiratory infections, especially COVID-19, could help in the detection and treatment of Vitamin D deficiency and potentially decrease recovery time and improve outcome. Even though evidence suggests that vitamin D has the anti-inflammatory, antiviral properties, randomized double-blinded controlled trials are needed to verify this further, and to understand Vitamin D and COVID-19 better.
Abbreviations
Vitamin D receptor-VDR; 25(OH)D- 25 hydroxyvitamin D; 1,25 (OH)D-1,25 dihydroxy Vitamin D; 1α,25-dihydroxy Vitamin D-1,25[OH]2 D or calcitriol; IU- International Units; Interferons stimulated genes- ISG; ARI- acute respiratory infection; RSV- respiratory syncytial virus; RTI- Respiratory tract infections; COPD-Chronic obstructive pulmonary disease; BMI-Basal metabolic index; USA-USA.
KEYWORDS: 1,25(OH)2 D; calcitriol; COVID-19; SARS CoV2; respiratory tract infections; respiratory viruses; 25(OH) D; vitamin D
1. Introduction
1.1. Background
As the scientific community is in a marathon in finding out the cure for COVID-19, in this crisis, it is essential for the physicians not to forget about the basics. Due to the pandemic crisis, in many nursing homes and hospitals, there established new policies on decreasing unnecessary medications to minimize cross-contamination. Sometimes these policies are making providers avoid essential drugs such as Vitamins, including Vitamin D, especially in deficient and insufficient individuals. In this paper, we try to emphasize the importance of Vitamin D in COVID-19 and respiratory viral patients.
1.2. Objectives
The purpose of this review is to identify and summarise the importance of vitamin D and its role in protecting humans from COVID-19 and other respiratory viruses.
2. Discussion
COVID-19 was first described in Wuhan, China, in December 2019. As of 14 May 2020, there have been approximately 4,516,533 confirmed cases, with about 302,889 deaths (according to WHO). (https://covid19.who.int) The USA accounted for 32.5% of cases and 24.2% of total deaths around the globe. As per the data from residents treated in New York facilities, 73.6% of the individuals died are above 65 years of age as of May 13, which shows significant mortality in older adults[1]. COVID-19 pandemic is affecting every sector in the community, including, but not limited to, Information Technology, retail, and farming as well as manufacturing and health industries, impacting those involved financially, mentally, and physically. Several pharmaceutical companies are rushing to prepare target therapies and vaccines to combat this. Nevertheless, it is of utmost importance to employ a multidirectional approach to manage this pandemic.
Epidemiology and statistics among individuals with comorbid conditions have changed from Asian to the Western world. One significant difference between both across the hemisphere is obesity, which is an important comorbid condition in patients with COVID-19 in the Western population. According to the CDC, 2017–2018, age-adjusted data showed that 42.8% are obese in the USA (https://www.cdc.gov/obesity/data/adult.html). A positive correlation between Vitamin D deficiency and obesity is noted in studies[2]. It was posing that Vitamin D plays an integral part in those with an increased BMI. Now more than ever, treating patients with Vitamin D is vital due to various beneficial effects, including boosts immunity and innate immunity against viral diseases due to the potential role of Vitamin D in decreasing mortality associated with cytokine storm in COVID-19 patients [3–5]. Vitamin D can act as adjunctive therapy to treat ‘cytokine storm’ noted in patients of COVID-19[6]. Calcitriol was stressed in several infectious and inflammatory pulmonary diseases and was shown in in-vitro trials as being a protective mechanism in helping with the modulating and expression of ACE I/II from lipopolysaccharide caused lung injury[7].
The classic function of Vitamin D is bone homeostasis. Nevertheless, it also has a regulatory and modulates role in multiple domains such as inflammation, epithelial repair, host defense, and immunity. Deficiency in Vitamin D is more familiar in patients with respiratory disease, indicating that Vitamin D supplementation might be instrumental in this group of patients. Vitamin D receptor is expressed by respiratory macrophages/monocytes and epithelial cells[8]. Vitamin D and its receptor are considered to be vital in the protection against respiratory infections. Generally, upper respiratory infections are commonly associated with severe colds in the elderly and children as well as patients with chronic respiratory conditions such as chronic obstructive pulmonary disease and asthma, resulting in exacerbation of those conditions. The immunologically active metabolite of Vitamin D is one alpha 25 dihydroxy Vitamin D that interacts with the receptor found on respiratory macrophages/monocytes and epithelial cells and mediates epithelial cell differentiation, proliferation, and apoptosis. In vitro studies have shown that during respiratory viral infections, the antiviral potential of the immune cells is increased by Vitamin D through increased expression of the antimicrobial peptide just as defensin, innate interferons, and cathelicidin which are considered to be a vital component of airway responsiveness against viruses. Ultraviolet (UV) radiation exposure to the skin produces Vitamin D[8]. It is observed that for the population living in mid to high altitudes, the availability of Vitamin D is less in winter compared to the summer, leading to more chances of viral respiratory infections in the winter[9].
2.1. Mechanism of action
Sources of 25-(OH) D are intake of Vitamin D through diet and exposure to the sun. The immunologically functioning form of Vitamin D is Calcitriol and is formed (Figure 1) through sequential hydroxylation. 25-hydroxyvitamin D is produced by hydroxylation of Vitamin D3 (cholecalciferol) or Vitamin D2 (ergocalciferol) by the cytochrome P450 enzyme 25α hydroxylase (CYP27A1) in the liver. Mitochondrial 1α – hydroxylase enzyme (1α [OH] ase/CYP27B1) hydroxylates biologically inactive circulating metabolite 25-(OH) D intracellularly in the kidney, or extra renally into active form 1,25 (OH)2 D. There is evidence that many cells that contribute to innate immunity, such as macrophages, monocytes, dendritic and epithelial cells can synthesize 1,25 (OH)2 D from 25 (OH) D by 1α – hydroxylation. Pulmonary epithelial cells can express 1α hydroxylase and produce Vitamin D locally, which increases Vitamin D regulated gene expression, through which it exhibits immunomodulatory effects and host defense [10–12]. Serum levels are considered a more accurate indicator of Vitamin D status. 25(OH)D level equal to or greater than 30 ng/ml (75 nmol/L) is considered sufficient (Table 1) The Vitamin D levels between 20–29 ng/ml (50–70 nmol/l) are deemed insufficient, and the levels less than 20 ng/ml (50 nmol/L) as deficient [13]
Figure 1.
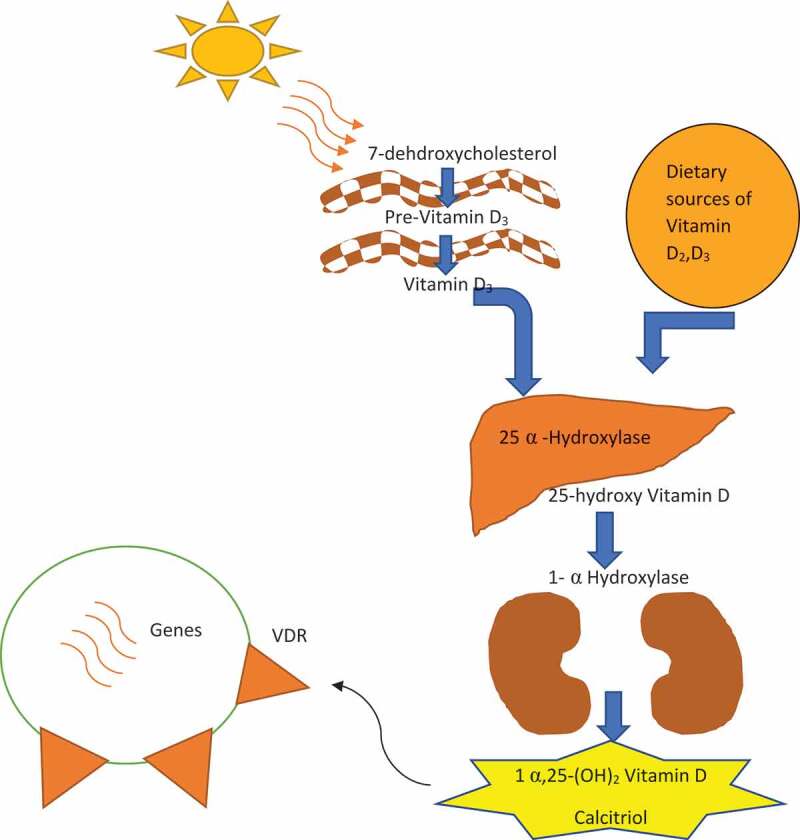
Vitamin D synthesis
Table 1.
Vitamin D levels
| Vitamin D status | Serum levels |
|---|---|
| Sufficient | Greater than or equals 30 ng/ml(75 nmol/l) |
| Insufficient | 20–29 ng/ml(50–74 nmol/l) |
| Deficient | Less than 20 ng/ml(50 nmol/L) |
Extracellular 25 hydroxyvitamin D binds to serum Vitamin D binding protein, which then is endocytically internalized, and synthesized intracellularly and 1, 25 dihydroxy Vitamin D tends to act on the nuclear Vitamin D receptor (VDR) [14]. Vitamin D-24-hydroxylase (24 [OH] ase/CYP24A1) catabolizes 1,25 (OH)2 D, and its precursor 25 (OH) D [15]. 1,25 (OH)2 D activates target gene expression by binding to the nuclear VDR. Moreover, based on co-regulatory proteins and the nature of the stimulus, there can be upregulation or downregulation of target genes. 1,25(OH)2 D by exerting negative feedback on the Vitamin D signaling system through decreasing CYP27B1 expression/1α (OH) ase transcription and also increasing CYP24A1 by increasing 24 (OH) ase catabolic function can limit its synthesis. Hansdottir et al. observed human respiratory epithelial cells in vitro showed increased levels of 1α-hydroxylase and decreased levels of inactivating 24-hydroxylase, hence enhancing the activation of Vitamin D [12]
Vitamin D shows its effect on both adaptive and innate immune responses. Various in vitro studies showed that 1, 25 (OH) D affected the development of Th1 mediated immunity by inhibiting it, which is essential for cellular response induction. Cytokines that are dependent on the activity of nuclear factor κB (NF-κB) in multiple cells, including macrophages, by blocking the activation of NF-κB p65 through upregulation of the NF-kB inhibitory protein 1κBα are also directly modulated by 1,25 (OH)2 D[16]. Toll-like receptors (TLRs) are transmembrane proteins that recognize molecular motifs of viral and bacterial origin and initiate innate immune responses. TLR3, which is mainly involved in defense against viruses, recognizes viral double-stranded RNA. The treatment with Vitamin D has shown to reduce double-stranded RNA-TLR3–induced expression of IL-8 in respiratory epithelial cells[12]. Both 25 (OH) D and 1, 25 (OH)2 D were shown to modulate T-cell adaptive immunity. The mechanism is by decreasing the pro-inflammatory type 1 cytokines such as IL-6, IL-8, IL-12, IFN-γ, as well as IL-17 and tumor necrosis factor-α) and also by increasing regulatory T cells and anti-inflammatory type 2 cytokines such as IL-4, IL-5, and IL-10 [17–19]. In the summer months, reduction in the pro-inflammatory levels of IL-1, IL-6, TNF-α, IFN-γ, and IL-10 are observed on TLR stimulation of human peripheral blood mononuclear cells when compared to the winter where the respiratory viral infections are at its peak[20].
In healthy donors, primary cd4 + T cells when cultured under Th17-polarizing conditions, it was observed that Vitamin D has reduced expression of pathogenic Th17 markers and their secretion of pro-inflammatory cytokines (IL-17A and IFN γ), which induced the expansion of CD25hi cells and has also increased the expression of CTLA-4 and Foxp3 regulatory markers[21]. A study of four healthy individuals supplemented with Vitamin D3 at a high dose (5000 to 10,000 IU/day) has increased production of suppressor cytokine IL-10 by non-CD 4+ and non CD8 + T cells[22]. In another randomized controlled trial on Vitamin D deficient patients, supplementing with high-dose (4000 IU/day) Vitamin D3 for two months has significantly reduced CD4 + T cell–nonspecific activation when compared to patients given with low dose (400 IU/day)[23]. Vitamin D generated by lung epithelium and widely expressed VDR in lung epithelium could lead to increased expression of antimicrobial peptides (such as cathelicidin and defensin β4) in adjacent macrophages and other innate immune cells [10,15,24]. Treating patients with 1,25 (OH)2 D has induced NF-κB inhibitor IκBα and, subsequently RSV induction of antiviral IFN-β and CXCL10 which are driven by NF-κB genes and antiviral IFN- ISG was downregulated[25].
Interestingly, it was identified that the levels of antiviral IFN-β and ISG15 decreased with 1,25 (OH)2 D treatment before influenza A exposure, but increased by treatment with 1,25 (OH)2 D after exposure thereby implying 1,25 (OH)2 D treatment efficacy during viral infection [26]. Vitamin D plays a vital role in the local respiratory homeostasis by directly affecting the replication of respiratory viruses, as shown in in vitro studies. It can also modulate the balance of Th1/Th2 or Tc1/Tc2 responses or induce the expression of antimicrobial peptides and also inhibit Th17 cytokine production [27,28].
Overall, Vitamin D helps in decreasing the replication of viruses by inducing defensins and cathelicidins and minimizes the congregation of pro-inflammatory cytokines that injure the lung lining by inflammation-causing pneumonia as well as helping to raise anti-inflammatory cytokines[6].
2.2. Effect of vitamin D on respiratory infections, including COVID-19
Respiratory infections, including influenza A and B, parainfluenza 1 and 2, and RSV infections, are prevalent in winter. All of these may be sensitive to antimicrobial peptides, and Vitamin D may play an essential role in the host’s defense against them. Data from epidemiological studies have linked a deficiency of Vitamin D to increased susceptibility to acute viral respiratory infections[29]. Several human and animal studies have supported a protective role of high maternal serum levels of Vitamin D against viral infection, suggesting that high Vitamin D serum levels in pregnancy might protect the infant from developing ARIs or viral-induced wheezing episodes. Camargo et al. reported high cord blood 25 (OH) D levels had been associated with a lower risk of respiratory infection and childhood wheezing[30]. Few studies also observed that increased Vitamin D plasma levels during the summer might be linked to a reduced prevalence of viral infections compared to winter when lower levels of Vitamin D are present [31]. Newborns who were having with 25 (OH) D levels higher than 30 ng/ml in cord blood were six times less likely to develop RSV respiratory infection in the first year of life as compared in those with lower than 20 ng/ml 25 (OH) D[32].
In a study conducted between December 2008 and January 2009 in 743 children (3–15-year-old), it was observed that low serum 25 (OH) Vitamin D level is associated with viral respiratory tract infection. At least one viral respiratory tract infection, which was confirmed by polymerase reaction from the nasopharyngeal specimen, was noted in 1/3 rd of participants. Serum 25 (OH) D level below 30 ng/ml (75 nmol/l) increased the risk of viral RTIs by 50%, and levels below 20 ng/ml (50 nmol/l) increased the risk by 70%[33]. It was observed that there was a linear relationship between Vitamin D plasma levels and respiratory infections; Every 10 nmol/L increase in 25 (OH) D was associated with a 7% lower risk of getting ARI, which was self-reported [34]. This association was confirmed in the USA in a large, nationally representative sample. A sample of National Health and Nutrition Examination Surveys from 2001–2006 involving 14,108 participants showed that 25 (OH) D levels less than 30 ng/ml were associated with having a 58% higher risk of developing ARI compared with participants with levels greater or equal to 30 ng/ml[29]. “ The plasma 25 (OH) D concentration is correlated positively with plasma cathelicidin concentration. As per several studies indicate, Vitamin D insufficiency, especially in children, is associated with asthma severity, inadequate control, extreme asthma exacerbations, and hospitalizations [35–38].
Ginde et al. Reported that in individuals with asthma and COPD, the association between low 25 (OH) D level and the incidence of URTI was stronger; people with circulating 25 (OH) D < 10 ng/ml always showed a higher risk than people with adequate circulating 25 (OH) D > 30 ng/ml)[39]. According to a study conducted recently in the USA, a group of older asthmatic adults who presented with Vitamin D deficiency had an increased number of hospitalizations and increased morbidity[40]. “Another study from Connecticut, the USA involving 195 patients, attempted to determine the impact of acute viral infections during autumn and winter in correlation with serum 25 (OH) D levels. It concluded that having a 25 (OH) D level more than or equal to 38 ng/ml reduced the infection risk factor by two folds[41]. A study conducted in the UK on a group of 6789 adults aged 45–47 concluded that for every 10 ng/ml increase in 25 (OH) D lowered the risk of respiratory infections by 7%. [34]
The majority of research available for review suggests that there is an increased risk for respiratory disease with Vitamin D deficiency. Supplementation with Vitamin D might improve the course and outcome of respiratory infections/diseases and provide benefits in the quality of life in affected patients. It is reasonably safe to take Vitamin D 1000 IU daily, which in most people should result in >20 ng/ml (50 nmol/l) to optimize nonspecific immunity and also to prevent infection. Cholecalciferol/VitaminD3 is the preferred treatment for the correction of Vitamin D deficiency due to its longer serum half-life, lower cost, and higher potency [42,43]. Studies have shown that 25 (OH) D levels <32 ng/ml has a positive correlation with baseline antimicrobial cathelicidin being elevated after patients receive high-dose ergocalciferol treatment (50,000 IU every other day for five days)[44]. Based on the available evidence, the supplementation of Vitamin D could be recommended for the prevention of viral infections, but its effectiveness depends on the baseline serum 25 (OH) D levels. It is dangerous to treat those with elevated 25 (OH) D levels and does not prove to add any benefit. The decision to initiate supplementation should include only people with low baseline plasma levels of 25 (OH) D [9,45,46]. It was also reported that intermittent high-dose Vitamin D supplementation does not work as well as a daily supplementation because of the short circulating half-life of Vitamin D [47–49]. Most studies correlate an improved respiratory outcome with maintaining serum 25 (OH) D levels above 30 ng/ml (75 nmol/l). Supplementing Vitamin D3 during pregnancy and infancy reduces visits to primary care for ARIs during early childhood. More than 462 studies have reported that preventive Vitamin D supplementation in healthy children has reduced the risk of respiratory infection [50,51]. In a group of 167 school children receiving 1200 IU/day VitaminD3, 10.8% developed influenza A, which was lower than the placebo group where 18.3% of children were infected with influenza A.51 In a study conducted by Bergman in adult patients with antibody deficiency, 4000 IU/d cholecalciferol reduced symptoms and antibiotic utilization[52]. Another study conducted in 5660 individuals with pooled data from 11 randomized controlled trials indicated that Vitamin D supplementation is beneficial in preventing respiratory tract infections[53].
Since sunlight is a significant source of Vitamin D, it is plausible to suggest that those who live in the northern hemisphere are likely to have a Vitamin D deficiency. As governing bodies enforce stay at home orders to prevent the spread of COVID-19, people are further limited to sun exposure[54]. COVID-19 started in December 2019 in Wuhan, China, and negatively impacted northern mid-latitude countries, where people tend to have low 25 (OH) D levels during winter months [55,56]. The activation of Vitamin D in the body is influenced by Magnesium resulting in phosphate and calcium homeostasis and is necessary for the growth and maintenance of bones. Magnesium is required by all enzymatic reactions occurring in the kidneys and liver[57]. The recommended dose of Magnesium is 250–500 mg/day and should be taken with 500–1000 mg/day of calcium. The standard recommended dose of Vitamin D is 600–800 IU/daily, but during the pandemic, 1000–2000 IU/daily in the form of multivitamin or supplement of Vitamin D is appropriate[3].
2.3. Conclusion
Vitamin D increases the anti-inflammatory and antiviral responses of epithelial cells in the respiratory system during respiratory viral infections in vitro. Several studies have been reviewed and showed that Vitamin D is an active bystander in various infectious diseases. As per recent studies, the use of daily or weekly intake of Vitamin D3 supplementation is highly recommended in the prevention of acute RTIs, in individuals with severe Vitamin D Deficiency. It is also suggested that Vitamin D should be taken along with Magnesium, as it is necessary for enzymatic reactions in the body. The prevalence of SARS CoV-2 in northern countries, including China, Italy, and the USA in winter months, correlates with the deficiency of 25 (OH) D levels.
Results from clinical trials show that supplementation of Vitamin D in patients with Vitamin D deficiency helps when exposed to viruses such as COVID-19, but has no added benefit to those with normal or high Vitamin D levels against viruses. Giving Vitamin D supplements in the autumn and winter months to patients at high risk could add an extra level of protection against the development of respiratory tract infections, especially during the COVID-19 pandemic. During the pandemic, 1000–2000 IU/daily in the form of a multivitamin or supplement of Vitamin D is appropriate. Daily supplementation of Vitamin D is recommended compared to intermittent dosing, due to short circulating half-life. Vitamin D can be an essential adjuvant therapy in treating patients affected with COVID-19 in Vitamin D deficient individuals. Due to the highly contagious nature of COVID-19 and the increased morbidity and mortality with no appropriate therapy and vaccine, one must be cautious and do everything to help COVID-19 patients. In hospitals and other health care settings to decrease cross-contamination, holding other non-essential medications is taking place. Discontinuing Vitamins could increase the mortality and morbidity of those affected, especially in deficient/insufficient individuals. Obtaining serum 25 (OH) D levels in all patients with viral respiratory infections, especially COVID-19, could help in the detection and treatment of Vitamin D deficiency and potentially decrease recovery time and improve outcome. It is highly essential to review every medication carefully to treat COVID-19 patients. In the end, all the clinician’s goal should be, decreasing patient’s susceptibility, morbidity, and mortality associated with SARS-CoV-2. More randomized, multicenter controlled trials are needed to understand better the dosage, timing, duration, and effectiveness of the therapy.
Acknowledgments
I thank Amber Thomas MSN, APRN, FNP-C, for help in editing this article and final proofreading. I appreciate all the authors who contributed to writing this review article.
Author contributions
MB, VMK, VS, and GPM contributed mechanism of action, abstract, conclusion, figure, and tables, MB, GPM, HK, MP, VS, VG, SA, SN, VMK, SVM contributed in writing an introduction, discussion, and conclusion. All authors contributed equally to the preparation of this manuscript, and all of the authors reviewed the manuscript and agreed with the findings and interpretation.
Data availability
from PUB MED, google scholar
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/
- [2].Yao Y, Zhu L, He L, et al. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med. 2015. September 15;8(9):14977‐14984. [PMC free article] [PubMed] [Google Scholar]
- [3]. https://www.medscape.com/viewarticle/930152
- [4].Balla M, Merugu GP, Patel M, et al. COVID-19, modern pandemic: a systematic review from front-line health care providers’ perspective. J Clin Med Res. 2020;12(4):215‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daneshkhah A, Agrawal V, Eshein A, et al. The Possible Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. medRxiv; 2020. DOI: 10.1101/2020.04.08.20058578. [DOI] [Google Scholar]
- [6].Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020. April 2;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu J, Yang J, Chen J, et al. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16(5):7432‐7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zdrenghea MT, Makrinioti H, Bagacean C, et al. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1):e1909. [DOI] [PubMed] [Google Scholar]
- [9].Hayes DP. Influenza pandemics, solar activity cycles, and vitamin D. Med Hypotheses. 2010;74(5):831‐834. [DOI] [PubMed] [Google Scholar]
- [10].Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770‐1773. [DOI] [PubMed] [Google Scholar]
- [11].Fritsche J, Mondal K, Ehrnsperger A, et al. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102(9):3314‐3316. [DOI] [PubMed] [Google Scholar]
- [12].Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090‐7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adams JS, Liu PT, Chun R, et al. Vitamin D in defense of the human immune response. Ann N Y Acad Sci. 2007;1117:94‐105. [DOI] [PubMed] [Google Scholar]
- [15].Prosser DE, Jones G.. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29(12):664‐673. [DOI] [PubMed] [Google Scholar]
- [16].Chen Y, Zhang J, Ge X, et al. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288(27):19450‐19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lemire JM, Archer DC, Beck L, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125(6Suppl):1704S‐1708S. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage pro-inflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458‐5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khoo AL, Chai LY, Koenen HJ, et al. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol. 2011;164(1):72‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fawaz L, Mrad MF, Kazan JM, et al. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016;166–167:59‐71. [DOI] [PubMed] [Google Scholar]
- [22].Allen AC, Kelly S, Basdeo SA, et al. A pilot study of the immunological effects of high-dose vitamin D in healthy volunteers. Mult Scler. 2012;18(12):1797‐1800. [DOI] [PubMed] [Google Scholar]
- [23].Konijeti GG, Arora P, Boylan MR, et al. Vitamin D supplementation modulates T cell-mediated immunity in humans: results from a randomized control trial. J Clin Endocrinol Metab. 2016;101(2):533‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kho AT, Sharma S, Qiu W, et al. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics. 2013;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hansdottir S, Monick MM, Lovan N, et al. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184(2):965‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khare D, Godbole NM, Pawar SD, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52(4):1405‐1415. [DOI] [PubMed] [Google Scholar]
- [27].Schedel M, Jia Y, Michel S, et al. 1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter. Nat Commun. 2016;7:10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nanzer AM, Chambers ES, Ryanna K, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132(2):297‐304.e3. [DOI] [PubMed] [Google Scholar]
- [29].Monlezun DJ, Bittner EA, Christopher KB, et al. Vitamin D status and acute respiratory infection: cross sectional results from the USA national health and nutrition examination survey, 2001–2006. Nutrients. 2015. March 13;7(3):1933‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Walker VP, Modlin RL.. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65(5Pt 2):106R‐113R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Camargo CA Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180‐e187. [DOI] [PubMed] [Google Scholar]
- [32].Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127(6):e1513‐e1520. [DOI] [PubMed] [Google Scholar]
- [33].Science M, Maguire JL, Russell ML, et al. Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents. Clin Infect Dis. 2013;57(3):392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Berry DJ, Hesketh K, Power C, et al. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106(9):1433‐1440. [DOI] [PubMed] [Google Scholar]
- [35].Brehm JM, Celedón JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the childhood asthma management program study. J Allergy Clin Immunol. 2010;126(1):52‐8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gupta A, Sjoukes A, Richards D, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184(12):1342‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brehm JM, Acosta-Pérez E, Klei L, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186(2):140‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ginde AA, Mansbach JM, Camargo CA Jr.. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. 2009;169(4):384‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tsai CL, Delclos GL, Huang JS, et al. Age-related differences in asthma outcomes in the USA, 1988–2006. Ann Allergy Asthma Immunol. 2013;110(4):240‐246.e1. [DOI] [PubMed] [Google Scholar]
- [41].Sabetta JR, DePetrillo P, Cipriani RJ, et al. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010. June 14;5(6):e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Heaney RP, Recker RR, Grote J, et al. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447‐E452. [DOI] [PubMed] [Google Scholar]
- [43].Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160‐169. [DOI] [PubMed] [Google Scholar]
- [44].Bhan I, Camargo CA Jr, Wenger J, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011;127(5):1302‐4.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol. 2012;4(2):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619‐4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martineau AR, Hanifa Y, Witt KD, et al. Double-blind randomized controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax. 2015;70(10):953‐960. [DOI] [PubMed] [Google Scholar]
- [49].Weiss ST, Litonjua AA. Vitamin D dosing for infectious and immune disorders. Thorax. 2015;70(10):919‐920. [DOI] [PubMed] [Google Scholar]
- [50].Urashima M, Segawa T, Okazaki M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255‐1260. [DOI] [PubMed] [Google Scholar]
- [51].Camargo CA Jr, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130(3):e561‐e567. [DOI] [PubMed] [Google Scholar]
- [52].Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomized and double-blind intervention study. BMJ Open. 2012. December 13;2(6):e001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bergman P, Lindh AU, Björkhem-Bergman L, et al. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013. June 19;8(6):e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Panarese A, Shahini E. Letter: covid-19, and vitamin D. Aliment Pharmacol Ther. 2020;51(10):993‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xie Z, W X, Zhang Z, et al. Prevalence of vitamin D inadequacy among Chinese postmenopausal women: a nationwide, multicenter, cross-sectional study. Front Endocrinol (Lausanne). 2019;9:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kroll MH, Bi C, Garber CC, et al. Temporal relationship between vitamin D status and parathyroid hormone in the USA. PLoS One. 2015. March 4;10(3):e0118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118(3):181‐189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
from PUB MED, google scholar


