ABSTRACT
Background: There are limited reports describing critically ill COVID-19 patients in the state of New York.
Methods: We conducted a retrospective analysis of 32 adult critically ill patients admitted to a community hospital in upstate New York, between 14 March and 12 April 2020. We collected demographic, laboratory, ventilator and treatment data, which were analyzed and clinical outcomes tabulated.
Results: 32 patients admitted to the intensive care unit (ICU) were included, with mean (±SD) follow-up duration 21 ± 7 days. Mean (±SD) age was 62.2 ± 11.2 years, and 62.5% were men. 27 (84.4%) of patients had one or more medical co-morbidities. The mean (±SD) duration of symptoms was 6.6 (±4.4) days before presentation, with cough (81.3%), dyspnea (68.7%), and fever (65.6%) being the most common. 23 (71.9%) patients received invasive mechanical ventilation. 5 (15.6%) died, 11 (34.4%) were discharged home, and 16 (50%) remained hospitalized, 8 (25%) of which were still in ICU. Mean (±SD) length of ICU stay was 10.2 (±7.7) days, and mean (±SD) length of hospital stay was 14.8 (±7.7) days.
Conclusion: Majority of patients were of older age and with medical comorbidities. With adequate resource utilization, mortality of critically ill COVID-19 patients may not be as high as previously suggested.
Abbreviations: ACE-i: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; ARDS: Acute Respiratory Distress Syndrome; BiPAP: Bilevel positive airway pressure; CABG: Coronary artery bypass graft; CFR: Case fatality rate; COVID-19: Coronavirus disease 19; CPAP: Continuous positive airway pressure; CRP: C – Reactive Protein; CT: Computed tomography; DVT: Deep vein thrombosis; ECMO: Extra Corporeal Membrane Oxygenation; ESICM: European Society of Intensive Care Medicine; FiO2: Fraction of inspired O2; HFNC: High Flow Nasal Cannula; HITF: Hypoxia-Inducible Transcription Factor; IBM: International Business Machines; ICU: Intensive Care Unit; IL: Interleukin; IMV: Invasive Mechanical Ventilation; IQR: Interquartile Range; ISTH: International Society of Thrombosis Hemostasis; NIV: Non Invasive Ventilation; NY: New York; PAI: Plasminogen activator inhibitor; PaO2: partial pressure of arterial oxygen; PCV: Pressure Control Ventilation; PEEP: Positive End Expiratory Pressure; RGH: Rochester General Hospital; RRH: Rochester Regional Health; RT-PCR: Reverse transcriptase polymerase chain reaction; RSV: Respiratory Syncytial virus; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SD: Standard Deviation; STEMI: ST segment elevation myocardial infarction; TNF: Tumor necrosis factor; USA: USA; VTE: Venous thromboembolism
KEYWORDS: SARS-CoV-2, COVID-19, ICU, ventilator, New York
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a novel coronavirus belonging to the Coronaviridae family that has gripped the world in a pandemic of proportions last seen over a century ago during the Spanish flu outbreak of 1918. Since the coronavirus disease 2019 (COVID-19) was first identified in late December 2019 in Wuhan, China, there are now more than 2,980,000 confirmed cases globally, with the United States (US) leading the global tally with more than 980,000 cases. In the US, the state of New York (NY) has emerged as the epicenter of the outbreak with disproportionately large cases, totaling at 293,000 as of 26 April 2020.
With high infectivity represented by an R0 of greater than 2 [1], human-to-human transmission and presence of a presymptomatic stage, SARS-CoV-2 led to an exponential growth of cases in a short period, overwhelming healthcare systems across the state and resulting in unprecedented effects on social, economic and healthcare sectors. This has galvanized hospital systems, including our own to come up with innovative means to handle the surge of cases at the peak of this pandemic, including expanding intensive care unit (ICU) teams, personal protective equipment conservation strategies, and grim conversations about resource allocation.
SARS-Cov-2 is an enveloped virus with a large plus-strand RNA genome, and acts primarily as a respiratory pathogen, infecting cells by attaching to ACE-2 receptors. The clinical spectrum of COVID-19 ranges from mild to critically ill cases [2] with reports indicating 5–9% of all cases are admitted to the ICU with severe respiratory failure [3,4].
Despite several observational studies and case series on COVID-19 patients from the inpatient and outpatient setting, there are currently limited reports describing critically ill patients in the US [5,6]. In this case series, we describe demographic characteristics, coexisting conditions, ventilation parameters, and clinical outcomes of patients admitted to the medical ICU at Rochester General Hospital (RGH), a tertiary community hospital in Monroe county, NY, which also functions as a safety net hospital for the area. We aim to help guide identification of those at greatest risk of deterioration, and improve decision making in managing this unique subset of patients.
2. Methods
2.1. Study population and institutional approval
We included adult patients, 18 years or older, with laboratory-confirmed COVID-19 infection who were admitted to the medical intensive care unit (ICU) at RGH or transferred to RGH from other community hospitals between 14 March and 12 April 2020. These patients were then followed up until 18 April 2020. We excluded pregnant or incarcerated patients, patients aged < 18 years of age, and patients requiring less than 6 L of supplemental oxygen. Rochester Regional Health (RRH) Institutional Review Board approved our case series (IRB:1982A), informed consent was waived and researchers analyzed only deidentified data.
2.2. Data collection
We collected demographic, clinical, laboratory, radiological, ventilator and treatment data by manual review of electronic medical records (EPIC). These were then analyzed to tabulate clinical outcomes. All documentation, investigations, and management of patients, had been performed at the discretion of the primary treatment team. A laboratory-confirmed case of COVID-19 was defined as a positive result on the SARS-CoV-2 real-time reverse transcriptase-polymerase-chain-reaction (RT-PCR) assay of nasopharyngeal or oropharyngeal swab or lower respiratory tract specimens. Specimens were obtained and processed according to CDC guidelines[7]. Until April 10th we utilized RRH laboratory-developed manual PCR assay with emergency use authorization from the CDC for inpatient use and kits from CDC at public health laboratories Buffalo or Wadsworth for outpatient use. After 10 April, we used the Cobas ® 6800 System by Roche for inpatient and out-patient tests.
2.3. Statistical analysis
We present categorical variables as counts and percentages. We present continuous variables as mean ± standard deviation (SD) or median and interquartile range (IQR), wherever appropriate. Data were analyzed using the following statistical tests: independent sample t-test, Wilcoxon-Mann Whitney test, Fisher Exact test, and Chi-square, as appropriate. The analysis was performed using SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, N.Y., USA). Two-tailed P values of < 0.05 were deemed statistically significant.
3. Results
3.1. Demographics and presenting features: (Table 1)
Table 1.
Baseline characteristics and presenting symptoms
| Characteristics | Patients (N = 32) |
|---|---|
| Age: years mean ±SD (range) | 62.2 ± 11.2 (37–84) |
| Male | 20 (62.5%) |
| Female | 12 (37.5%) |
| Ethnicity: number (percent) | |
| Hispanic | 12 (37.5%) |
| African American | 10 (31.2%) |
| Caucasian | 8 (25%) |
| Asian | 2 (6.25%) |
| Comorbidities: number (percent) | |
| Coronary artery disease/heart failure | 14 (43.7%) |
| Hypertension | 21 (65.6%) |
| COPD/asthma | 7 (21.9%) |
| Diabetes Mellitus | 15 (46.9%) |
| Chronic Kidney Disease | 5 (15.6%) |
| BMI>30 | 22 (68.8%) |
| Smoking History | 16 (50%) |
| Presenting Complaints: number (percent) | |
| Fever | 21 (65.6%) |
| Cough | 26 (81.3%) |
| Sputum production | 14 (43.8%) |
| Dyspnea | 22 (68.7%) |
| Sore throat | 1 (3.1%) |
| Headache | 7 (21.9%) |
| Fatigue | 22 (68.8%) |
| Myalgia | 14 (43.8%) |
| Syncope/pre-syncope | 5 (15.6%) |
| Diarrhea/GI symptoms | 13 (40.6%) |
| Chills | 18 (56.2%) |
| Altered mental status | 5 (15.6%) |
| Duration of symptoms: days mean (±SD), {range} | 6.6 (±4.4) {1-21} |
We identified 32 critically ill patients admitted to the RGH ICU between 14 March 2020 and 12 April 2020. The mean (±SD) follow-up duration was 21 days (±7 days), with a minimum of 7 and a maximum of 35 days. Demographic characteristics of these patients are detailed in Table 1. Mean (±SD) age was 62.2 ± 11.2 years, and 62.5% were men. 27 (84.4%) of patients had one or more medical comorbidities, of which obesity (68.8%) and hypertension (65.6%) were the most prevalent. 50% of them were either current or former smokers.
The mean (±SD) duration of symptoms was 6.6 (±4.4) days before presentation, with cough (81.3%), dyspnea (68.7%), and fever (65.6%) being the most common symptoms. Other symptoms included diarrhea, fatigue, and myalgia. The median (IQR) temperature on presentation was 102.2 degrees Fahrenheit (99.8–103.1); median (IQR) oxygen saturation by pulse oximetry was 89% (82–93%). 62.5% patients were hypoxic and 35% of these patients were hypoxic without reported dyspnea.
4. Laboratory, radiology and microbiological findings: (Table 2)
Table 2.
Presenting vitals, Laboratory data, Imaging findings and Microbiology data
| Parameter: | Patients (N = 32): | |
|---|---|---|
| Presenting vitals: Median (IQR) | ||
| Tmax – Fahrenheit | 102.2 (99.8–103.1) | |
| Systolic Blood Pressure – mmHg | 137.5 (119.3–158.8) | |
| Heart Rate – beats/minute | 95.5 (77.3–107) | |
| qSOFA | 1 (0–1) | |
| Oxygen saturation by pulse oximetry – % | 89 (82–93) | |
| Acute Hypoxia* (Percent) | 20/32 (62.5%) | |
| Silent hypoxia (Percent) |
7/20(35%) |
|
| Laboratory data: Median (IQR) |
Initial |
Lowest |
| Lymphocyte count – 103 cells/uL | 1.1 (0.6–1.4) | 0.6 (0.3–1.2) |
| Initial | Highest | |
| C Reactive Protein -mg/L | 137.4 (78.1–181.8) | 214.7 (152.3–305) |
| Procalcitonin – ng/mL | 0.2 (0.1–0.98) | 0.4 (0.17–4.1) |
| D-dimer – FEU ng/mL | 693 (479–1675.8) | 2020 (1094.5–7650) |
| Lactate Dehydrogenase – U/L | 391.5 (312.0–455.8) | 475 (429–610) |
| Ferritin – ng/mL | 757 (390.0–1209.0) | 1442 (747–2554) |
| Lactate – mmol/L | 1.6 (1.1–3.1) | |
| Troponin – ng/mL | 0.03 (0.01–0.15) | |
| Aspartate Transaminase – U/L | 72 (48–107.3) | |
| Alanine Transaminase – U/L | 53.5 (29–115.5) | |
| Alkaline Phosphatase – U/L | 88.5 (63.5–143.3) | |
| Total bilirubin – mg/dL | 0.6 (0.4–1.0) | |
| Coagulation parameters: Median (IQR) | ||
| Lowest Platelet count – 103 cells/uL | 166 (122–220) | |
| Highest INR | 1.2 (1.1–1.4) | |
| Highest PT – seconds | 13.7 (12.9–16.6) | |
| Highest APTT – seconds | 32.8 (29.8–56.9) | |
| Lowest Fibrinogen** – mg/dL | 599.5 (391.8–747.5) | |
| Imaging: | ||
| Abnormal Chest X-ray | 22 (66.6%) | |
| Abnormal CT chest | 6/6 (100%) | |
| Microbiology results: | ||
| Sputum culture | 12 (37.5%) | |
| Blood culture | 0 (0%) | |
| Rapid flu/RSV | 0/27 (0%) | |
| Positive repeat SARS-CoV-2 test after 14 days of initial test | 13/16 (81.25%) | |
*Acute hypoxia was defined as any new reading of SpO2 ≤ 90% on room air,
**Data available for eight patients
Table 2 shows both the initial and extreme laboratory values on ICU admission and during hospital stay. Lymphopenia was common, with median (IQR) Absolute lymphocyte count (ALC) of 1.1 (0.6–1.4) x 103 cells per cubic millimeter, and a third of the cases (34.4%) developed severe lymphopenia (with ALC ≤ 0.3 × 103 cells per cubic millimeter) during their ICU stay. The median (IQR) C-Reactive Protein (CRP) was 137.4 (78.1–181.8) mg/L and majority (68.75%) had initial CRP of ≥100 mg/L. Initial procalcitonin value of ≥0.5 was noted in 10 (31.25%) of the 32 patients, 5 of which had a positive sputum culture. D-dimer was elevated with a median initial D-dimer of 693 ng/mL, reaching extremely high values (>7650 ng/mL) in 11 (34.4%) of the 32 patients during hospital stay. Initial chest X-ray was abnormal in 22 (66.6%) patients. A CT chest was obtained in 6 patients, all of whom had abnormal findings.
All patients had laboratory-confirmed testing of COVID-19; of these 27 were also tested for Influenza A, Influenza B and Respiratory Syncytial Virus, all of which were negative for co-infections with these respiratory viruses. 16 of the 32 patients had follow up repeat SARS-CoV-2 RT PCR testing after 14 days of their initial test, of which 13 (81.25%) resulted positive.
4.1. ICU treatment characteristics: (Table 3)
Table 3.
Ventilatory data, other ICU therapies and medications used
| Parameter | Patient (N = 32^) |
|---|---|
| Respiratory support: | |
| High Flow Nasal Cannula | 5 (15.6%) |
| Intermediate Flow Nasal Cannula | 4 (12.5%) |
| Invasive Mechanical Ventilation (IMV) | 23 (71.9%) |
| Characteristics of IMV: Median (IQR) | |
| Lowest pre-intubation PaO2/FiO2 ratio * | 101 (75.8–183.8) |
| FiO2 4 hrs after intubation – percentage of oxygen | 70 (50–80) |
| PEEP max – cmH2O | 16 (14–20) |
| Plateau pressure 4 hrs after intubation – cmH2O | 26 (23–30.5) |
| Positive Inspiratory Pressure max – cmH2O | 29 (28–35) |
| Static compliance 4 hrs after intubation – cmH2O ** | 44 (31–59) |
| Driving pressures – cmH2O | 12 (0–14) |
| ICU therapies: | |
| Intravenous vasopressors | 12/32 (36.3%) |
| Neuromuscular blockade | 9/23 (39.1%) |
| Intravenous pulmonary vasodilators | 8/23 (34.8%) |
| Prone positioning | 7/23 (30.4%) |
| Extracorporeal membrane oxygenation (ECMO) | 3/23 (13.0%) |
| Peri-intubation intravenous fluids | 15/23 (65.2%) |
| Medication details: | |
| Hydroxychloroquine | 19 (59.4%) |
| Lopinavir-Ritonavir | 5 (15.6%) |
| Remdesivir | 0 (0%) |
| Tocilizumab | 3 (9.38%) |
| Azithromycin | 19 (59.4%) |
| Systemic Glucocorticoids | 15 (46.9%) |
| Statins | 19 (59.4%) |
| Concurrent antibiotics | 26 (8.1%) |
| ACE-i/ARB prior to hospitalization or during hospitalization | 10 (31.3%) |
| Thromboprophylaxis | 32 (100%) |
^N = 32 unless specified, *data available for 28 patients; we estimated PaO2 from SpO2 in 4 patients who did not have preventilation arterial blood gas values available. **Data available for 11 out of 23 patients intubated. ACE-I = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker
23 (71.9%) patients received endotracheal intubation and invasive mechanical ventilation (IMV) and the remaining 9 were managed with noninvasive ventilatory (NIV) support of which 4 received supplemental oxygen through high flow nasal cannula (HFNC). At that time, we did not use other modes of positive pressure ventilation such as bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP) in COVID-19 patients due to concerns of aerosolization.
For the 28 patients with data available, the median (IQR) ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) ratio was 101 (75.8–183.8) consistent with moderate to severe Acute Respiratory Distress Syndrome (ARDS); median (IQR) FiO2 of 70% (50–80%) at 4 hours post-intubation. Among the 23 patients who received IMV, Pressure Control Ventilation (PCV) was the most common ventilator mode selected (in 52.2%), mostly based on the preference of treating intensivist. Median (IQR) positive end expiratory pressure (PEEP) used was 16 cmH2O (14–20 cmH2O) and median (IQR) plateau pressure was relatively high at 26 cmH2O (23–30.5) cm H2O. Median (IQR) static lung compliance was 44 (31–59) cm H2O in 11 patients with data available; none were less than 10 cmH2O. Median (IQR) driving pressure was 12 cmH2O (0–14) cmH2O. 7 of the 23 patients (30.4%) underwent prone positioning.
Of the 32, approximately one third (34.8%) received intravenous pulmonary vasodilators (epoprostenol). 3 patients required extra corporeal membrane oxygenation (ECMO), 2 veno-venous and 1 veno-arterial). 12 (36.3%) required vasopressor support.
19 (59.4%) of the patients received Hydroxychloroquine and the same number received Azithromycin and statins during hospitalization. 5(15.6%) received lopinavir-ritonavir, while 3 (9.38%) received tocilizumab. None of the patients received Remdesivir. Just under a half (46.9%) received steroids during ICU stay. A third (31.3%) were on ACE-I/ARBs prior to admission.
4.2. Clinical Outcomes (Figure 1, Tables 4 and 5)
Figure 1.
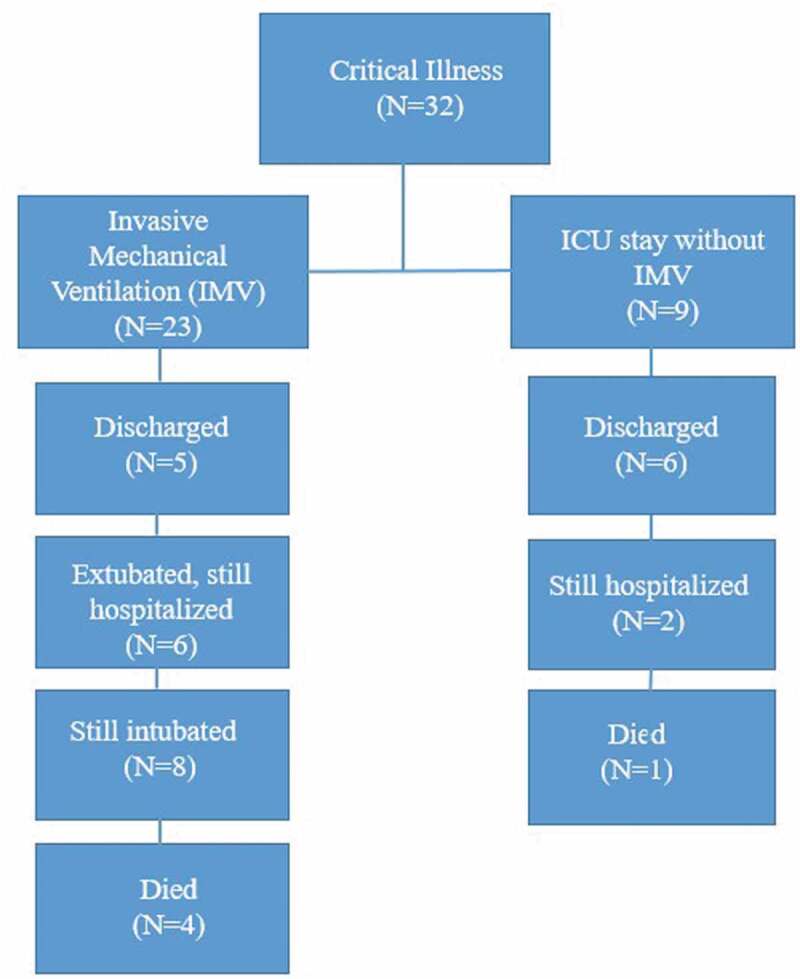
Disposition of patients at the time of analysis of results
Table 4.
Clinical outcomes, as per status of invasive mechanical ventilation
| Clinical Outcome: Mean (±SD) | All patients: | IMV (N = 23) | NIV (N = 9) | P value |
|---|---|---|---|---|
| Days on ventilator for those extubated; n = 11^ | 10(6.8) | |||
| Days on ventilator, for those still intubated; n = 8 | 14.9 (6.7) | |||
| Length of ICU stay – days | 10.2 (7.7) | 12.8 (7.4) | 3.4 (3.1) | <0.001 |
| Length of hospital stay – days | 14.8 (7.7) | 16.9 (7.9) | 9.2 (2.9) | 0.006 |
| Length of hospitalization in those still hospitalized – days | 18 (7.4) | 20.1 (6.9) | 10.5 (1.7) | 0.009 |
| Number discharged from ICU (excluding mortality); N = 32 (percent) | 16 (32%) | 9 (39.1%) | 7 (77.8%) | 0.12 |
| Number discharged from hospital (excluding mortality); N = 32 (percent) | 11 (34.4%) | 5 (21.7%) | 6 (66.7%) | 0.046 |
| Death; N = 32 (percent) | 5 (15.6%) | 4 (17.4%) | 1 (11.1%) | 1 |
| Duration of follow up – days | 20.8 (7.4) |
^This excludes one patient who underwent compassionate extubation, and three deaths
Table 5.
Clinical outcomes, as per age < or ≥60 years
| Clinical Outcome: Mean (±SD) | All patients: | Age <60 years | Age ≥60 years | P value |
|---|---|---|---|---|
| Days on ventilator for those extubated; n = 11^ | 10 (6.8) | 16.5 (6) | 6.3 (3.9) | 0.09 |
| Days on ventilator, for those still intubated; n = 8 | 14.9 (6.7) | 16 (6.6) | 14.2 (7.4) | 0.76 |
| Length of ICU stay – days | 10.2 (7.7) | 11.5 (9.5) | 9.2 (6.3) | 0.38 |
| Length of hospital stay – days | 14.8 (7.7) | 15.7 (8.5) | 14.2 (7.2) | 0.59 |
| Length of hospitalization in those still hospitalized – days | 18 (7.4) | 18.9 (7.8) | 17.3 (7.3) | 0.67 |
| Number discharged from ICU (excluding mortality); N = 32 (percent) | 16 (32%) | 6 (46.2%) | 10 (52.6%) | 1 |
| Number discharged from hospital (excluding mortality); N = 32 (percent) | 11 (34.4%) | 5 (38.5%) | 6 (31.6%) | 0.98 |
| Death; N = 32 (percent) | 5 (15.6%) | 1 (7.7%) | 4 (21%) | 0.63 |
| Duration of follow up – days | 20.8 (7.4) |
^This excludes one patient who underwent compassionate extubation, and three deaths
Follow up was complete until 18 April 2020, with mean (±SD) follow-up of 21 (±7) days, minimum of 7 days and a maximum of 35 days. Figure 1 outlines the disposition status of all 32 patients and Tables 4 and 5 list our clinical outcomes. Of the 32 patients, 5 (15.6%) died, 11 (34.4%) were discharged home, and 16 (50%) remained hospitalized, 8 (25%) of which were still in ICU. A greater percentage of patients aged 60 years or above died compared to patients under 60 years (21% vs. 7.7%). Of the 23 patients that received IMV, 11 (47.8%) were extubated during follow up, with mean (±SD) number of days of invasive mechanical ventilation of 10 (±6.85) days. 8 remained intubated, with mean (±SD) ventilator days of 14.9 (± 6.7) days. The mean (±SD) length of ICU stay among all patients was 10.2 (±7.7) days, and mean (±SD) length of hospital stay was 14.8 (±7.7) days. In those still hospitalized, mean (±SD) length of stay was 18 (±7.4) days which is likely to be an underestimate. The lengths of ICU stay and hospital stay were significantly higher in those who received IMV than those managed by NIV (12.8 vs. 3.4 days and 16.9 vs. 9.2 days) respectively.
4.3. Complications
5 (15.6%) patients had adverse venous thrombo embolic events (VTE) with 1 patient (3%) developing massive pulmonary embolism (PE), 2 patients (6.2%) developing deep vein thrombosis (DVT)-1 patient (3%) developing extensive clots in hemodialysis catheter requiring therapeutic anticoagulation, and 1 patient (3%) developed bilateral embolic strokes. All were on pharmacological VTE prophylaxis with weight-based heparin, or low molecular weight heparin (LMWH). 2 patients (6.2%) developed renal failure necessitating renal replacement therapy (RRT) and 1 patient (3%) developed an ST-segment elevation Myocardial infarction (STEMI) requiring coronary artery bypass graft (CABG) and subsequent VA-ECMO support. Transaminitis and myocarditis were uncommon.
5. Discussion
To our knowledge, this is the first case series exclusively on critically ill COVID-19 patients in the state of NY. Older patients, men and those with medical comorbidities were common in our series, suggesting that these patients may be at higher risk of severe illness and ICU admission. These findings were similar to other reports from New York City and Seattle [5,7]. Fever, cough and dyspnea were the most common symptoms. Since a majority of the patients had CRP ≥100, this could be used in creating risk calculators to identify patients at higher risk of critical illness. Interestingly, only half of our cases with elevated procalcitonin had bacterial infections, suggesting that procalcitonin is more likely a marker of sepsis in the critically ill than of bacterial infection, corresponding to prior published studies [8]. Initial chest X-ray was normal in a third (33.4%) of patients, therefore a normal chest X-ray cannot rule out infection with COVID-19. Secondary infection with positive sputum cultures were noted in a third (37.5%) of the patients, incidence similar to that in seasonal influenza [9]. Yet, coinfections with other respiratory viruses were not noted. As the protocol developed in our hospital for repeat testing of SARS-CoV-2, we noted that a very large number (13/16, 81.3%) of the patients tested positive after 2 weeks. Whether this implies continued infectivity, viral shedding, or mere inert viral RNA remains unclear.
Profound hypoxia seems to be the driving factor leading to intubation, as majority (62.5%) of the patients were hypoxic; interestingly, about a third [7/20, (35%)] of these patients were ‘silently’ hypoxic, with no reported dyspnea. To explain this, Conde et al., describe an interesting theory of the virus manifesting neurotropism [10], by involving the midbrain, respiratory and cardiovascular control centers, leading to decreased perception of dyspnea, despite hypoxia.
We noted low pre-ventilation PaO2/FiO2 ratios, consistent with ARDS definition by the Berlin criteria [11]. However, our median driving pressures and static compliance values suggest findings of near-normal compliance, which is unusual for ARDS. Higher PEEP was used early on but with more experience, intensivists started using lower PEEP. Prone positioning was attempted in a third of patients receiving IMV, to help improve oxygenation by increasing alveolar recruitment [12]. The threshold to prone was relaxed from strict PROSEVA study [13] indications to anyone with refractory hypoxemia; two patients on HFNC were tried on awake prone positioning, with improved oxygenation. The median FiO2 needs were high, with most patients requiring 50–60% FiO2 for long periods of time, underscoring prolonged profound hypoxia in this illness. Most patients were able to draw good tidal volumes despite low inspiratory pressures.
These findings raise the question about the factors involved in pathogenesis and response to treatment in COVID-19. The findings of severe hypoxemia with preserved compliance have been noted in recent literature [14]. Current evidence on the pathophysiology of hypoxia in COVID-19 is changing. The hypoxia is hypothesized to be from three mechanisms, dysregulation of pulmonary perfusion, pulmonary microthrombi, and ARDS [15]. Gatinnoni et al. [16] recently described two possible phenotypes of patients with respiratory failure from COVID-19; Type L – characterized by near normal pulmonary compliance and low recruitability, low PEEP response wherein the primary pathology is theorized to be loss of hypoxic vasoconstriction and possibly pulmonary microthrombi; Type H on the other hand behaves more like traditional ARDS with poor pulmonary compliance and hence better response to high PEEP ventilation and lung recruitment techniques. Type L is peculiar for hypoxia out of proportion to lung infiltrates on imaging [16]. As none of our deceased patients had autopsies (these being cancelled due to high risk of exposure), it is difficult for us to associate our findings with these theories [17]. Due to such variability in response, personalized ventilator settings and management have been advised for COVID-19 patients [18]. Figure 2 demonstrates the proposed ventilatory strategies for management of critically ill COVID-19 patients at RGH.
Figure 2.
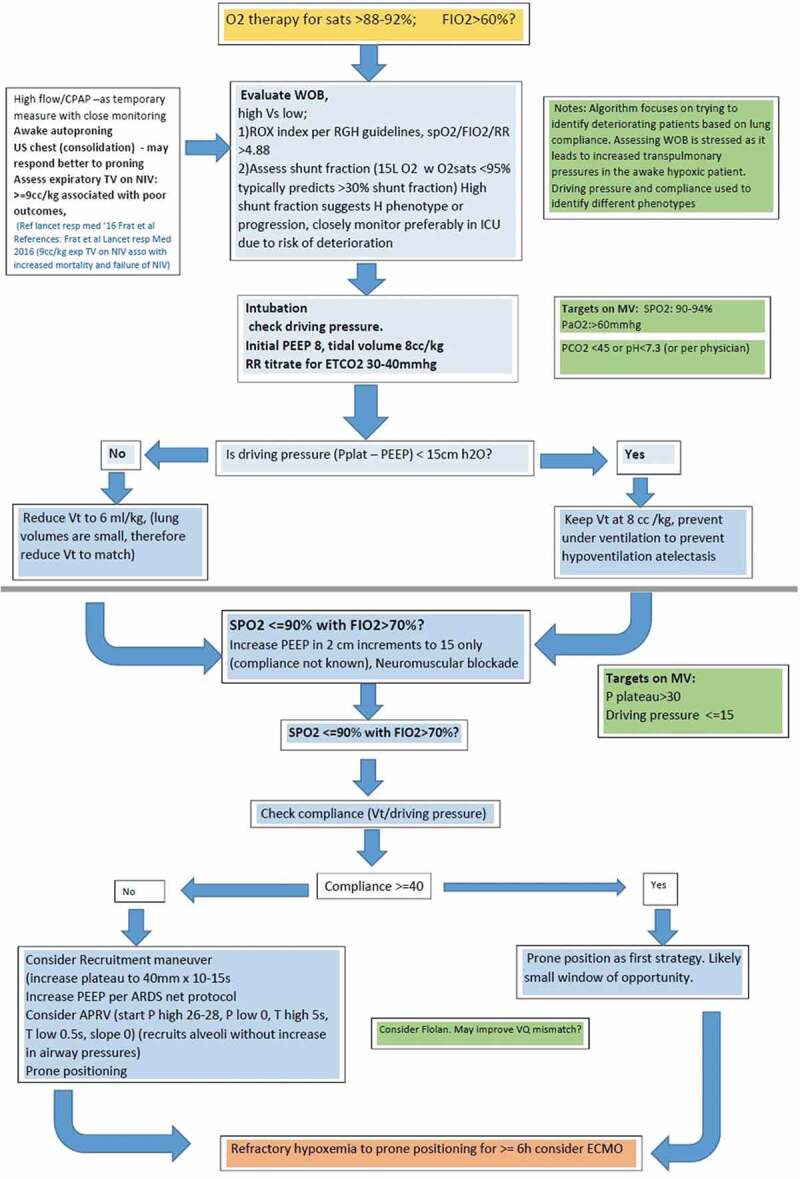
Proposed ventilation strategies for management of critically ill COVID-19 patients at RGH
^Inspired by ESICM webinar on ventilation strategies in COVID-19 [15]
Among other ICU therapies, epoprostenol was used in a third of the patients, and a variable response was perceived. It has been noted that in some cases of COVID-19, despite low PaO2/FiO2 ratio, perfusion is maintained, which along with atelectasis, leads to a right to left shunt phenomenon [19]. Therefore pulmonary vasodilators such as epoprostenol may not be useful in these cases. Only a third of the patients required vasopressor support, and shock and multiorgan failure were uncommon in our patient series. These findings are inconsistent with some prior reports [5].
The choice of COVID19-directed medication therapy was based on judgment of the infectious disease specialists and treating intensivists, and mostly based on an indigenous institutional algorithm (Figure 3(a,b)). Just above half the patients received Hydroxychloroquine or Azithromycin during their hospitalization. None of the patients received Remdesivir. When compared to the recent study of compassionate use of Remdesivir by Grien et al. [20], our patient population appeared to have had similar baseline characteristics and outcomes in terms of mortality, rate of extubation, ICU LOS, and hospital discharges. Yet, we have insufficient data to draw meaningful associations about these medications.
Figure 3.
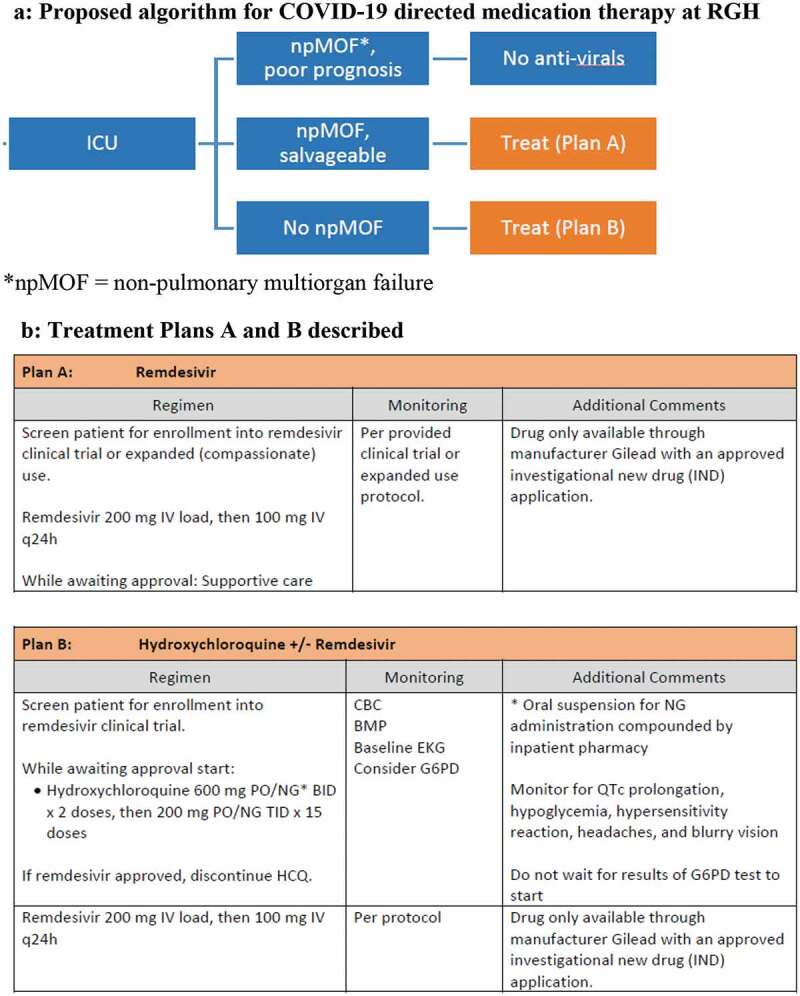
(a) Proposed algorithm for COVID-19 directed medication therapy at RGH. (b) Treatment Plans A and B described
*npMOF = nonpulmonary multiorgan failure
At 15.6%, our case fatality rate (CFR) appears to be lower than prior published literature internationally from Italy, and locally from NYC and Seattle [5,21,22]. This could be due to the fact that our hospital was not yet at overcapacity, and we had received timely information and guidance about the natural course and complications of the disease. This suggests that with adequate availability of health care resources, critically ill COVID-19 patients may experience lower morbidity and mortality than suggested by current data[22]. This re-enforces the concept of ‘flattening the curve’ to prevent strain on the healthcare system.
In our case series, there were no statistically significant differences in clinical outcomes in patients aged 60 years or older vs. patients younger than 60 years of age, which suggests that other prognostic factors, apart from age, play a role in morbidity and mortality in COVID-19. Longer duration of ICU and hospital stays were noted in patients who received IMV. This appears intuitive as patients who undergo IMV usually have more severe disease, more comorbidities and are at higher risk of developing complications during hospital stay (secondary infections, VTE), etc. These numbers need to be interpreted with caution, since they may be an underestimate for the 16 patients that remained in the hospital at time of data analysis.
Regarding complications, VTE events were the most common (15.6%) and additional non-pulmonary organ failure rate was low. Thrombotic complications in patients with severe COVID-19 is an area of debate, with some studies reporting rates of thrombotic complication as high as 31% despite adequate thromboprophylaxis[23]. The increased risk in thrombotic complications with COVID-19 is difficult to ascertain especially in critically ill patients where risk of clotting is ~20%[24].. It is hypothesized that with severe inflammatory state, there is increase in proinflammatory cytokines IL-1, IL-6, and TNF- α, leading to increased thrombin generation and stimulation of the coagulation pathway. Additionally, in hypoxia, there is upregulation of hypoxia-inducible transcription factor (HITF) which stimulates tissue factor and plasminogen activator inhibitor 1 (PAI-1) gene expression, predisposing to increased VTE complications [25]. Regardless of etiology, the importance of weight-adjusted, renal function-based thromboprophylaxis is underscored, as per latest International Society of Thrombosis Hemostasis (ISTH) consensus [26]. To preemptively treat these patients with full dose of anticoagulation seems premature, without stable epidemiological data, and efficacy and safety outcomes from randomized trials.
6. Limitations
The main limitation of our study is the small sample size, however, our study focused only on critically ill patients with the most severe disease. Second, due to the short follow-up, the final outcomes of the patients that remained in the hospital were not known, however, our aim was to report the in-hospital complications of these patients. Third, patients who had do-not-resuscitate (DNR) orders were included in the NIV group; these patients are likely to be older and sicker, and have higher likelihood of having worse clinical outcomes and complications. Nevertheless, the NIV group only had one death compared to four deaths in the IMV group. Fourth, we did not include the patients managed in the step-down unit of the hospital, but this came to our advantage as it served as an unbiased measure in selecting the sickest patients in the cohort who were then managed in the ICU. Finally being a retrospective, observational study, there are inherent biases including selection bias, confounding bias and the inability to attribute causation.
7. Conclusion
Our case series describes early experience of critically ill patients at a tertiary center in upstate NY. Our findings underscore the higher risk of severe illness in older patients and those with pre-existing medical conditions; and also inform us of the prolonged need of critical care resources in those critically ill with COVID-19. These findings can be used to screen patients at higher risk, and to guide resource allocation. Our case fatality rate (CFR) was lower than prior published data, rendering hope that with adequate medical infrastructure and timely resource allocation, mortality from COVID-19 may not be as high as currently reported in parts of the world where healthcare systems have become strained. Nevertheless, much remains unknown about the appropriate ventilation and management strategies of these patients. Large-scale prospective studies are needed to further elucidate the efficacy of novel treatments, and identify specific predictors of mortality and clinical outcomes in COVID-19 patients.
Author contributions
Authors JL and DSS had full access to all of the data in the study and take responsibility for the content of the manuscript, including the data and analysis. All the authors, JL, MSC, NS, BET, LKTB, SV, and DSS contributed substantially to the study design, data analysis and interpretation, writing, and revision of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Zhang S, Diao M, Yu W, et al. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Inter J Infect Dis. 2020;93:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020. February 24. (Epub ahead of print). DOI: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu Z, & McGoogan, J M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama.2020;323(13), 1239–1242. [DOI] [PubMed] [Google Scholar]
- [4].Grasselli G, Zangrillo A, Zanella A, et al. The COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. Published online April 6, 2020. DOI: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhatraju P, Ghassemieh B, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region — case Series. N Engl J Med. 2020. DOI: 10.1056/nejmoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020. DOI: 10.1101/2020.04.08.20057794 [DOI] [Google Scholar]
- [7].Center for Disease Control and Prevention (CDC) . 2019-nCoV Real-Time RT-PCR diagnostic panel instructions for use. Available from: https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-for-detection-instructions.pdf. https://www.fda.gov/media/134922/download
- [8].Harbarth S, Holeckova K, Froidevaux C, et al. Diagnostic Value of procalcitonin, Interleukin-6, and Interleukin-8 in critically Ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402. [DOI] [PubMed] [Google Scholar]
- [9].Chertow D, Memoli M.. Bacterial coinfection in influenza. JAMA. 2013;309(3):275. [DOI] [PubMed] [Google Scholar]
- [10].Conde Cardona G, Quintana Pájaro L, Quintero Marzola I, et al. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J Neurol Sci. 2020;412:116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].The ARDS Definition Task Force* . Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- [12].Galiatsou E, Kostanti E, Svarna E, et al. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med. 2006;174(2):187–197. [DOI] [PubMed] [Google Scholar]
- [13].Guérin C, Reignier J, Richard J, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. [DOI] [PubMed] [Google Scholar]
- [14].Gattinoni L, Coppola S, Cressoni M, et al. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020. DOI: 10.1164/rccm.202003-0817le [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Camporota L, Guerin C.. How to ventilate in COVID-19. ESICM Webinar; 2020. April 2. Available from: https://esicm-tv.org/webinar1_live_20-how-to-ventilate-in-covid-19.html. [Google Scholar]
- [16].Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020. DOI: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fox S, Akmatbekov A, Harbert J, et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from new orleans. medRxiv. 2020. DOI: 10.1101/2020.04.06.20050575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mauri T, Spinelli E, Scotti E, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020;1. DOI: 10.1097/ccm.0000000000004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luks A, Swenson E. COVID-19 lung injury and high altitude pulmonary edema: a false equation with dangerous implications. Ann Am Thorac Soc. 2020. DOI: 10.1513/annalsats.202004-327fr [DOI] [PubMed] [Google Scholar]
- [20].Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020. DOI: 10.1056/nejmoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Richardson S, Hirsch J, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020. DOI: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020. DOI: 10.1016/j.thromres.2020.04.01320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alhazzani W, Lim W, Jaeschke R, et al. Heparin thromboprophylaxis in medical-surgical critically Ill patients. Crit Care Med. 2013;41(9):2088–2098. [DOI] [PubMed] [Google Scholar]
- [25].Gralinski L, Bankhead A, Jeng S, et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4(4). DOI: 10.1128/mbio.00271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hunt B, Levi M. Thrombosis, Thromboprophylaxis & Coagulopathy in COVID- 19 Infections. ISTH webinar; 2020. April 9/14. Available from: https://academy.isth.org/isth/2020/covid-19/291581/marcel.levi.26.beverley.jane.hunt.thrombosis.thromboprophylaxis.26.coagulopathy.html?f=menu%3D8%2Abrowseby%3D8%2Asortby%3D2%2Alabel%3D19794 [Google Scholar]


