Abstract
The two primary molecular regulators of lifespan are sirtuin-1 (SIRT1) and mammalian target of rapamycin complex 1 (mTORC1). Each plays a central role in two highly interconnected pathways that modulate the balance between cellular growth and survival. The activation of SIRT1 [along with peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α) and adenosine monophosphate-activated protein kinase (AMPK)] and the suppression of mTORC1 (along with its upstream regulator, Akt) act to prolong organismal longevity and retard cardiac ageing. Both activation of SIRT1/PGC-1α and inhibition of mTORC1 shifts the balance of cellular priorities so as to promote cardiomyocyte survival over growth, leading to cardioprotective effects in experimental models. These benefits may be related to direct actions to modulate oxidative stress, organellar function, proinflammatory pathways, and maladaptive hypertrophy. In addition, a primary shared benefit of both SIRT1/PGC-1α/AMPK activation and Akt/mTORC1 inhibition is the enhancement of autophagy, a lysosome-dependent degradative pathway, which clears the cytosol of dysfunctional organelles and misfolded proteins that drive the ageing process by increasing oxidative and endoplasmic reticulum stress. Autophagy underlies the ability of SIRT1/PGC-1α/AMPK activation and Akt/mTORC1 suppression to extend lifespan, mitigate cardiac ageing, alleviate cellular stress, and ameliorate the development and progression of cardiomyopathy; silencing of autophagy genes abolishes these benefits. Loss of SIRT1/PGC-1α/AMPK function or hyperactivation of Akt/mTORC1 is a consistent feature of experimental cardiomyopathy, and reversal of these abnormalities mitigates the development of heart failure. Interestingly, most treatments that have been shown to be clinically effective in the treatment of chronic heart failure with a reduced ejection fraction have been reported experimentally to exert favourable effects to activate SIRT1/PGC-1α/AMPK and/or suppress Akt/mTORC1, and thereby, to promote autophagic flux. Therefore, the impairment of autophagy resulting from derangements in longevity gene signalling is likely to represent a seminal event in the evolution and progression of cardiomyopathy.
Keywords: Heart failure, Sirtuin-1, Akt/mTOR pathway, Adenosine monophophase-activated protein kinase, Cardiac ageing
The two primary molecular regulators of lifespan identified to date are sirtuin-1 (SIRT1) and mammalian target of rapamycin (mTOR).1 Each gene represents the cornerstone of two interconnected pathways that regulate the balance between cellular growth and survival. When nutrients are plentiful, organisms prioritize the utilization of fuels to expand the cell mass, and mTOR signalling is central to this process. In contrast, when nutrients are in short supply, organisms minimize the utilization of anabolic pathways and adopt a safe and sheltered set of biological conditions that preserve the structural and functional integrity of existing cells; SIRT1 is critical to this response.
SIRT1 and Akt/mTOR signalling in the regulation of organismal longevity, cardiac ageing, and cardiomyocyte survival
The counterbalancing effects of SIRT1 and mTOR control the set point between cellular growth and cellular homeostasis. The positioning of this set point is exquisitely sensitive to the environmental energy supply and the redox state.2 , 3
Role of SIRT1 in organismal longevity and cardiac ageing
SIRT1 is one of a family of redox-sensitive nicotinamide adenine dinucleotide-dependent deacetylases that catalyse the post-translational modification of hundreds of proteins that are involved in metabolism and cellular homeostasis. The yeast orthologue of SIRT1 is Sir2 (silent information regulator 2). Overexpression of Sir2 extends lifespan,4 and the ability of caloric restriction to prolong survival in yeast is dependent on the action of Sir2 to produce cytoprotective effects.5 Interestingly, in mammals, the organ that is critically involved in the longevity effects of Sir2 is the heart. The mammalian orthologue of Sir2 plays an essential role in mediating cell survival in cardiac myocytes,6 and mice that are deficient in Sir2α (the murine orthologue of Sir2) exhibit developmental abnormalities in the heart7 and develop early-onset heart failure.8 , 9
The expression of SIRT1 in most organs diminishes following birth, but it normally persists at high levels in the healthy heart,10 unless the myocardium exhibits the effects of ageing or shows evidence of a cardiomyopathic process.11 , 12 Mild-to-moderate up-regulation of SIRT1 prevents ageing in the heart,13 and SIRT1 has cardioprotective effects in a broad range of experimental models (Figure 1). Activation of SIRT1 activates antioxidant mechanisms and reduces oxidative stress, promotes mitochondrial health and biogenesis, and diminishes proinflammatory pathways in cardiomyocytes in order to promote cell survival.14–16 SIRT1 also mediates the ability of redox modulators and inflammasome suppressors to attenuate cardiac hypertrophy and to reduce cell senescence and death following cardiac injury.17 , 18 Cardiac-specific deletion of SIRT1 in mice augments mitochondrial production of reactive oxygen species, enhances oxidative and endoplasmic reticulum stress, and sensitizes the heart to pressure overload and ischaemia/reperfusion injury, leading to cardiac dysfunction and cardiomyopathy.19–21 Conversely, SIRT1 enrichment or activation improves cardiac function and prevents adverse ventricular remodelling following experimental infarction22 , 23; mitigates cardiac injury and mitochondrial dysfunction produced by diverse cellular stresses24–26; and ameliorates fibrosis produced by pressure overload.27 In experimental models of heart failure, activation of SIRT1 restores the functionality of sarco-endoplasmic reticulum Ca2+-ATPase and improves cardiac function,28 , 29 whereas suppression of SIRT1 decreases angiogenesis and leads to systolic and diastolic abnormalities.19 , 21 , 30
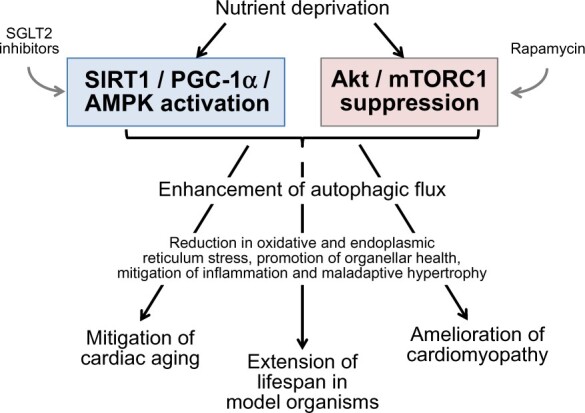
Figure 1.
Effects of nutrient sensor signalling on the cellular mechanisms that underlie cardioprotection. Cellular mechanisms are shown in blue, and cardiac responses are shown in red. Akt, protein kinase B; mTORC1, mammalian target of rapamycin complex 1; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1alpha; SIRT1, sirtuin-1.
Many of the adaptive effects of SIRT1 signalling on organellar health and cellular stress are mediated or facilitated by its action to deacetylate peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α), a member of a family of transcription coactivators that play a central role in the regulation of cellular energy metabolism. Like SIRT1, PGC-1α exerts cardioprotective effects in numerous experimental models as a result of its actions to promote mitochondrial biogenesis and antioxidant mechanisms, while suppressing inflammation (Figure 1).31–34 Loss of PGC-1α signalling is accompanied by an accelerated transition from hypertrophy to heart failure.35 Cardiac-specific deletion of PGC-1α leads to impaired oxidative metabolism, increased oxidative stress and the development of dilated cardiomyopathy.36 , 37 Interestingly, both cardiac ageing and heart failure are characterized by a decline in the expression and activity of PGC-1α,37–40 and activation of PGC-1α leads to attenuation of the ageing process in the myocardium and amelioration of the development of heart failure.41 , 42 PGC-1α hypomorphic mice show a vascular senescence phenotype that is associated with increased reactive oxygen species, mitochondrial abnormalities, and reduced telomerase activity.43 Suppression of PGC-1α recapitulates age-related changes in mitochondrial gene expression, whereas up-regulation prevents senescence-related changes in the myocardium.41
These experimental observations supporting an important cardioprotective effect of SIRT1/PGC-1α signalling are consistent with studies showing a linkage between SIRT1/PGC-1α activity and cardiac disorders (including heart failure) in the clinical setting. Polymorphisms of SIRT1 in humans are associated with cardiac developmental abnormalities44 and an increased predisposition to cardiac injury45 , 46 and cardiac hypoperfusion syndromes.47 Conversely, gain of function polymorphisms in the gene for PGC-1α have been linked with longer lifespans in clinical cohorts.48 Down-regulation of SIRT1 is accompanied by increase in oxidative stress and inflammatory signalling in human cardiomyocytes.11 Circulating levels of SIRT1 are inversely related to levels of proinflammatory cytokines in patients with coronary artery disease; low SIRT1 levels are accompanied by increased telomere attrition.49 SIRT1 expression is decreased in peripheral blood monocytes in patients with Type 2 diabetes50 and in patients with obesity with increased epicardial adipose tissue volume.51 The expression of SIRT1 is suppressed both in peripheral leucocytes and cardiomyocytes of patients with chronic cardiomyopathy.52 , 53 Similarly, the expression of PGC-1α is depressed in the myocardium of patients with heart failure and a reduced ejection fraction54–56 and is accompanied by defective mitochondrial replication and antioxidant defence mechanisms.57
Role of Akt/mTOR in organismal longevity and cardiac ageing
Both Akt and mTOR are serine/threonine protein kinases that function as critical promoters of cell growth and proliferation. mTOR exists in two complexes, mTOR complex 1 (mTORC1) and mTOR complex 2, and Akt potentiates the activation of mTORC1, which is preferentially inhibited by rapamycin.1 Akt/mTORC1 signalling influences hundreds of downstream effectors that promote anabolic pathways, drives mitochondrial production of reactive oxygen species to facilitate cellular replication and innate immunity, and enhances the expression of the senescence-associated secretory phenotype that is essential to the cellular disposal required for effective organ growth.58 Inhibition of mTOR redirects the priorities of the cell away from growth towards homeostasis and survival. mTOR suppression in yeast extends lifespan and is critical to the ability of caloric restriction to prolong survival in model organisms59–61; interestingly, the effect of mTOR on longevity in yeast is independent of the effects of Sir2. Mice with genetically-driven hypomorphic mTOR expression have an increased lifespan, an effect that is mimicked when mTOR activity is suppressed by rapamycin.62
The action of mTOR activity to promote anabolic pathways is required for cardiomyocyte replication during foetal development and adaptive hypertrophy during pressure overload,63 but it contributes to maladaptive cardiac hypertrophy when hearts are stressed or injured in adulthood.64 Specifically, complete cardiac-specific deletion of mTOR during embryonic development promotes lethality63 and undermines the ability of the heart to tolerate states of rapid-onset pressure overload.64 , 65 In contrast, partial mTORC1 suppression (produced by heterozygous deletion of mTORC1 or by rapamycin) in states of cardiac stress or injury ameliorates maladaptive hypertrophy and fibrosis and retards the development of heart failure (Figure 1).66 , 67 The cardiac ageing that results from inflammasome activation is related to activation of the Akt/mTOR pathway,20 and inhibition of the immunoproteasome system in the heart by rapamycin attenuates both inflammation and sympathetically-mediated hypertrophy.68 Increases in oxidative stress in cardiomyocytes may cause premature senescence as a result of aberrantly increased Akt/mTOR signalling.69 Sustained activation of Akt disrupts mitochondrial energetics and accentuates ageing-induced cardiac hypertrophy and myocardial contractile dysfunction70 , 71; mitochondrial function is normalized following mTOR inhibition.72 The totality of these experimental observations explains why cardiac-specific overactivation of the Akt/mTOR pathway induces heart failure,73 whereas suppression of Akt signalling ameliorates heart failure in experimental models.74
These findings supporting an effect of AKT/mTOR to promote cardiomyopathy are consistent with similar observations in the clinical setting. The myocardium in patients with a non-ischaemic cardiomyopathy shows aberrant activation of mTORC1; the intensity of this activation is associated with the severity of cardiac fibrosis and a poor prognosis.75 In hypertensive patients with heart failure, there is an inverse relation between the degree of Akt activation and measures of cardiomyocyte senescence.76 Akt activation may help to explain the insulin resistance that is characteristic of patients with chronic heart failure,77 and mTORC1 up-regulation impairs cardiac function in obesity-related heart failure.78 Activation of Akt in the human myocardium distinguishes the transition from well-compensated left ventricular hypertrophy to decompensated heart failure.79
Interplay of SIRT1/PGC-1α and Akt/mTOR and the intermediary role of adenosine monophosphate-activated protein kinase in modulating cardiomyocyte survival
The SIRT1/PGC-1α and Akt/mTOR pathways are highly interconnected, both at a molecular and physiological level (Figure 2). SIRT1 can modulate the transcription of Akt and mTOR as a result of its deacetylase activity,80 and additionally, SIRT1 and PGC-1α can negatively regulate the transcription of Akt and directly interfere with Akt and mTOR.81–84 At the same time, up-regulation of Akt leads to suppression of PGC-1α,85 whereas inhibition of mTOR by rapamycin or Akt down-regulation leads to activation of SIRT1 and PGC-1α.86–90 Interventions that retard organ-level ageing (e.g. glucose deprivation, cytoprotective drugs and genetic suppression of inflammasome activity) act to simultaneously up-regulate SIRT1/PGC-1α and suppresses the Akt/mTOR pathway.20 , 91–93 Furthermore, drugs that act to directly up-regulate SIRT1 (e.g. resveratrol and SIRT1 activators) also serve to inhibit Akt/mTOR,85 , 94–98 and conversely, suppression of SIRT1 leads to up-regulation of Akt/mTOR.98 The interplay between SIRT1/PGC-1α and Akt/mTOR is greatly enhanced by the fact that both SIRT1 and Akt/mTOR influence common downstream targets.99 , 100 Activators of SIRT1/PGC-1α and suppressors of Akt/mTOR can act synergistically or competitively to influence both lifespan as well as the cardiac response to ageing.71 , 101 The set point for the interplay of pathways that regulate growth and survival in cardiomyocytes is sensitive to both nutrients and the redox state.2 , 3
Figure 2.
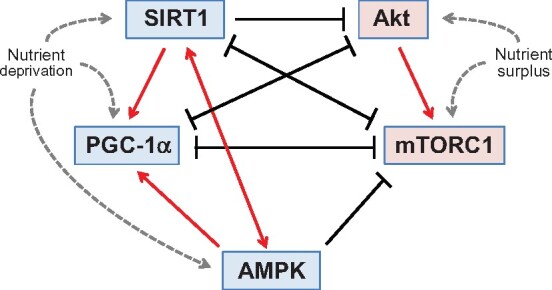
Mutual enhancement and antagonism of nutrient sensor signalling in the regulation of autophagic flux in cardiomyocytes. Nutrient deprivation sensors that promote autophagic flux are shown in blue, whereas the nutrient surplus sensors that suppress autophagy are shown in red. Akt, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1 alpha; SIRT1, sirtuin-1.
An important mediator of the interconnectivity between SIRT1 and Akt/mTOR is adenosine monophosphate-activated protein kinase (AMPK). AMPK discerns the balance between cytosolic levels of ATP and AMP, and it acts to promote ATP synthesis. Ageing is accompanied by suppression of AMPK,102 and in turn, up-regulation of AMPK ameliorates the effects of cardiac ageing by mitigating fibrosis,103 promoting ischaemic tolerance in the myocardium,104 , 105 and reversing ageing-related impairment of angiogenesis and regenerative repair.106 , 107 In general, caloric restriction activates both SIRT1, PGC-1α, and AMPK in parallel, and the molecular actions of AMPK support those of SIRT1/PGC-1α and oppose those of Akt/mTOR with respect to cellular homeostasis and survival (Figure 2). In addition, the actions of AMPK and SIRT1/PGC-1α reinforce each other93; the effect of AMPK to promote NAD+ leads to SIRT1 activation,108 and AMPK can activate PGC-1α by phosphorylation.109 Simultaneously, SIRT1 can augment the activity of upstream regulators of AMPK,110 while inhibition of AMPK leads to suppression of PGC-1α.93 In addition, AMPK can inhibit mTOR by an action on its upstream regulators as well as through a direct effect on components of the mTORC1 complex.111 , 112 As a result of the interplay of these effects, AMPK augments the ability of SIRT1/PGC-1α signalling to oppose the actions of the Akt/mTOR pathway.
Mechanisms underlying the effects of SIRT1, AMPK, and Akt/mTOR on longevity and cardiac ageing and their role in the development of cardiomyopathy
What cellular mechanism underlies the ability of SIRT1/AMPK activation and Akt/mTORC1 suppression to prolong lifespan, slow cardiac ageing and mitigate the development of cardiomyopathy and heart failure? The accumulation of dysfunctional organelles and misfolded proteins drives the ageing process by increasing oxidative and endoplasmic reticulum stress, typically with secondary activation of proinflammatory pathways.113 , 114 SIRT1/PGC-1α and AMPK signalling and Akt/mTORC1 inhibition can act directly to maintain organellar integrity, to promote antioxidant mechanisms and to interfere with activation of the inflammasome.20 , 72 , 115–119 Akt/mTORC1 can also directly modulate the functions of the senescence-associated secretory phenotype.58
Role of autophagy in promoting longevity and cardiomyocyte survival
Yet, the most important mechanism by which SIRT1/PGC-1α/AMPK and Akt/mTORC1 prevents cellular stress and ageing is the disposal and neutralization of unwanted and injurious cytosolic constituents by the cellular housekeeping process of autophagy. Autophagy is an evolutionarily-conserved degradative pathway, which involves the encircling of dangerous cellular components by a double-membrane vesicle; its fusion with the lysosome allows degradative enzymes to destroy the vesicle’s contents.120 The process not only negates the effects of the injurious constituent, but it allows for recycling of the breakdown products, thus boosting cellular ATP.
Autophagic flux is the most important determinant of lifespan and cardiac ageing.86 , 121–123 Normal and pathological ageing is accompanied by a reduced capacity for autophagy.122 , 124–126 Mutation of essential autophagy genes induces degenerative changes in tissues that closely resemble those of ageing,98 and inhibition of autophagy compromises the longevity effects of caloric restriction.122 , 127 , 128 Loss of autophagy allows for the accumulation of deranged organelles and misfolded proteins, which are the major source of oxidative and endoplasmic reticulum stress in cardiomyocytes.114 , 129 Conversely, enhancement of autophagic flux prevents the molecular and cellular features of ageing in the myocardium.130 Pharmacological or genetic interventions that increase lifespan in model organisms act through stimulation of autophagy.122 , 127
How does autophagy delay ageing and promote cellular survival? The formation of autophagic vacuoles and their fusion with lysosomes disposes of misfolded proteins (as well as glucose and lipid intermediates), thus reducing endoplasmic reticulum stress. Furthermore, the autophagic clearance of deranged mitochondria and peroxisomes (referred to as mitophagy and pexophagy, respectively) is critical to the mitigation of oxidative stress.131 (Cardiomyocytes are replete with mitochondria and peroxisomes, which underlie their enormous capacity to consume oxygen and generate reactive oxygen species.) Amelioration of oxidative and endoplasmic reticulum stress is essential to cardiomyocytes, since non-proliferating cells cannot utilize cell division to mediate dilution of intracellular debris or replace cells that have died.122
Role of SIRT1/AMPK and Akt/mTOR signalling in the modulation of autophagy
SIRT1/PGC-1α/AMPK and Akt/mTORC1 are the primary mediators of the ability of autophagy to prolong organismal longevity (Figure 1). The most important inducer of autophagy is caloric restriction, which acts to prolong organismal survival by signalling through both SIRT1/PGC-1α/AMPK as well as Akt/mTORC1.122 , 132
In states of glucose deprivation, AMPK promotes autophagy by directly activating several autophagy genes, including Ulk1,133–136 whereas in states of nutrient surplus, mTOR prevents Ulk1 activation and disrupts the interaction between Ulk1 and AMPK.108 Starvation does not prolong longevity if mTOR signalling is already suppressed,128 and conversely, mTOR inhibition with rapamycin does not favourably affect survival if autophagy genes are already knocked down or out.136 Conversely, SIRT1 deacetylases (and thereby activates) several autophagy genes137; SIRT1-mediated deacetylation of beclin 1 promotes autophagic flux138; and PGC-1α interacts with the E3 ubiquitin ligase Parkin to mediate mitophagy.139 , 140 Importantly, the longevity effects of SIRT1 are mediated by its actions to promote autophagy,141 and caloric restriction does not induce mitochondrial autophagy in aged animals if SIRT1 is absent.142 The actions of SIRT1 to extend lifespan by promoting autophagy can be attenuated by activation of Akt.71 Knockdown or knockout of autophagy genes abolishes the lifespan-prolonging effects of caloric restriction, resveratrol, or Sir2 overexpression.143 These observations, when considered collectively, strongly support the critical role of autophagy in mediating the ability of SIRT1/PGC-1α/AMPK and Akt/mTOR signalling to influence organismal survival.
SIRT1/PGC-1α/AMPK and Akt/mTORC1 are also the primary mediators of the ability of autophagy to retard cardiac ageing (Figure 1).144 The effects of inflammasome suppression to retard age-related deleterious changes in the heart are related to inhibition of Akt/mTOR and activation of SIRT1, leading to enhanced autophagic flux.20 Ageing-related cardiomyocyte contractile dysfunction and loss of mitophagy are accompanied by suppression of PGC-1α and are ameliorated by mTOR inhibition with rapamycin and with direct Sirt1 activators.71 , 102 AMPK activation restores autophagy in aged hearts,145 and knockout of AMPK promotes cardiac ageing by suppressing autophagy, an action that is not alleviated by concurrent inhibition of Akt.102 The effects of Akt to exacerbate cardiac ageing are dependent on its actions to suppress autophagy,126 , 127 and the effects of mTOR inhibition with rapamycin to mitigate oxidative stress and ageing are mediated though enhanced mitophagy.131 Similarly, the actions of caloric restriction to mitigate cardiac ageing are accompanied by simultaneous suppression of mTOR and enhanced autophagic flux.146 These findings demonstrate the importance of autophagy in mediating the effects of SIRT1/PGC-1α/AMPK and Akt/mTOR on cardiomyocyte senescence.
Importance of longevity gene signalling and autophagy modulation in the development and treatment of chronic heart failure
As a result of its critical role in maintaining cardiomyocyte health, autophagy plays a major role in the evolution and progression of heart failure. Diseases that lead to heart failure (as well as the heart failure state itself) mimic the ageing process, in that they are characterized by an increase in oxidative and endoplasmic reticulum stress, which is exacerbated by a striking impairment in the capacity of the heart to stimulate autophagy. Autophagic flux of cardiomyocytes is markedly impaired in cardiomyocytes derived from injured or failing hearts147–149; in return, pharmacological stimulation of autophagic flux can directly ameliorate oxidative stress and organellar dysfunction, thereby preventing or reversing cardiomyocyte dysfunction and mitigating the development of cardiomyopathy.150–152 This deficiency in autophagic capacity in heart failure is related to the simultaneous impairment of SIRT1/PGC-1α and AMPK signalling52 , 53 , 153 and enhanced activation of the Akt/mTORC1 pathway in cardiomyocytes.147 , 151 , 154 These derangements in longevity gene signalling is seen both experimentally and clinically.
Interestingly, most treatments for heart failure and a reduced ejection fraction have been reported to exert favourable effects on SIRT1/AMPK and Akt/mTOR signalling, thereby, on autophagic flux. The action of angiotensin-converting enzyme inhibitors to mitigate the effects of angiotensin II may involve signalling through SIRT1155 , 156 and enhancement of PGC-1α.157 Angiotensin receptor blockers have been noted to promote autophagy,158 effects that have been attributed to their effects to activate AMPK and inhibit Akt/mTOR.159 , 160 Beta-blockade is accompanied by up-regulation of AMPK,161 , 162 and carvedilol up-regulates PGC-1α163 and appears to enhance autophagic flux through SIRT1 stimulation164 and by mTOR inhibition.165 Spironolactone activates SIRT1/AMPK in the heart,166 and its action to inhibit Akt/mTOR signalling has been linked to its effect to promote autophagic flux.167 , 168 PGC-1α activation can interfere with the deleterious actions of mineralocorticoid receptor activation.169 Natriuretic peptides may activate AMPK,170 , 171 and neprilysin may stimulate Akt/mTOR signalling and suppress PGC-1α.172–174 Hydralazine up-regulates both AMPK and SIRT1, and thus, prolongs longevity in model organisms.175 Digitalis glycosides induce autophagy (potentially by activating AMPK),176 but they also activate Akt, which may limit the positive inotropic effect of these drugs.177–180 The effect of cardiac resynchronization to effect reverse remodelling is accompanied by activation of autophagic flux and improvement in mitochondrial function.181 Therefore, currently available treatments for heart failure appear to exert a consistently favourable influence on the interplay of SIRT1/AMPK and Akt/mTOR in a manner that promotes autophagy.
SGLT2 inhibitors have recently been shown to have favourable effects on the evolution and progression of heart failure in the presence and absence of Type 2 diabetes.182 When the actions of SGLT2 are inhibited, the urinary loss of calories triggers systemic transcriptional reprogramming that closely mimics that seen during states of nutrient deprivation.183 , 184 The depletion of tissue nutrients that follows glycosuria leads to activation of SIRT1 and AMPK and the suppression of Akt and mTOR.183 , 184 It is therefore noteworthy that several SGLT2 inhibitors up-regulate SIRT1, PGC-1α and AMPK, while simultaneously inhibiting the Akt/mTOR pathway,118 , 183–191 thus potentially explaining the action of these drugs to promote autophagy in diverse organs, including the heart.192 , 193 The induction of autophagy may underlies the ability of SGLT2 inhibitors to mute oxidative stress, promote organellar integrity, suppress proinflammatory pathways, and ameliorate the course of experimental cardiomyopathy.118 , 188 , 193–197 Importantly, because nutrient deprivation elicits a system-wide response, SGLT2 inhibitors can exert cardioprotective effects, even though SGLT2 is not expressed in the heart.183 , 184 In addition, SGLT2 inhibitors may be able to bind directly to SIRT1 to activate its functions (Figure 1).198
Drugs that are well-characterized agonists and antagonists of SIRT1/AMPK and Akt/mTOR signalling may also prove to have favourable effects in the treatment of chronic heart failure. Metformin is an established agonist of AMPK (which also up-regulates PGC-1α), and it promotes autophagy and ameliorates the development of experimental diabetic and non-diabetic cardiomyopathy.26 , 199–201 Epidemiological studies have suggested that the use of metformin may be accompanied by a reduced risk of heart failure, but these reports have been difficult to interpret, given the observational nature of these analyses and concerns that the reported benefits may have been related to an adverse effect of the comparator drugs rather than a favourable action of metformin.202 Resveratrol (an activator of SIRT1)18 , 29 , 45 , 203–208 and rapamycin and its analogues (inhibitors of mTORC1)151 , 209–211 have also been shown to enhance autophagic flux and to ameliorate cardiomyopathy in experimental models. However, clinical trial evidence to support a benefit of metformin, resveratrol and rapamycin in patients with chronic heart failure is lacking.
Conclusions
Genes that modulate lifespan in model organisms play a crucial role in the regulation of cellular growth and survival as a result of their effects on the cellular housekeeping process of autophagy. Autophagic flux is exquisitely controlled by the interplay of the SIRT1/AMPK and Akt/mTOR pathways, which underlies the ability of caloric restriction and the redox state to modulate ageing (Take home figure). The interaction of these longevity genes is particularly important in cardiomyocytes, since these cells readily produce reactive oxygen species and their non-proliferating state impairs the dilution of cellular stress and the replenishment of senescent cells from stem cell niches. The impairment of autophagy that results from derangements in longevity gene signalling is likely to represent a seminal event in the evolution and progression of cardiomyopathy. Enhancement of autophagic flux may be an important feature of current and future treatments for heart failure.
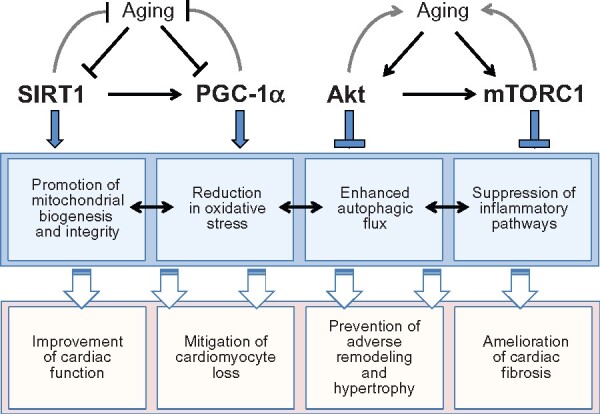
Take home figure.

Pathways that mediate and influence the interplay of lifespan extension, cardiac ageing, and the development of cardiomyopathy. Akt, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1 alpha; SIRT1, sirtuin-1.
Conflict of interest: M.P. has consulted for Abbvie, Actavis, Akcea, Amgen, AstraZeneca, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, NovoNordisk, Pfizer, Sanofi, Synthetic Biologics, and Theravance.
References
- 1. Pan H, Finkel T. Key proteins and pathways that regulate lifespan. J Biol Chem 2017;292:6452–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, Zhao Z, Wang Y. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull 2015;116:67–72. [DOI] [PubMed] [Google Scholar]
- 3. Meijles DN, Zoumpoulidou G, Markou T, Rostron KA, Patel R, Lay K, Handa BS, Wong B, Sugden PH, Clerk A. The cardiomyocyte “redox rheostat”: redox signalling via the AMPK-mTOR axis and regulation of gene and protein expression balancing survival and death. J Mol Cell Cardiol 2019;129:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 1999;13:2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000;289:2126–2128. [DOI] [PubMed] [Google Scholar]
- 6. Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res 2004;95:971–980. [DOI] [PubMed] [Google Scholar]
- 7. Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 2003;100:10794–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mu W, Zhang Q, Tang X, Fu W, Zheng W, Lu Y, Li H, Wei Y, Li L, She Z, Chen H, Liu D. Overexpression of a dominant-negative mutant of SIRT1 in mouse heart causes cardiomyocyte apoptosis and early-onset heart failure. Sci China Life Sci 2014;57:915–924. [DOI] [PubMed] [Google Scholar]
- 9. Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 2005;280:43121–43130. [DOI] [PubMed] [Google Scholar]
- 10. Ogawa T, Wakai C, Saito T, Murayama A, Mimura Y, Youfu S, Nakamachi T, Kuwagata M, Satoh K, Shioda S. Distribution of the longevity gene product, SIRT1, in developing mouse organs. Congenit Anom (Kyoto) 2011;51:70–79. [DOI] [PubMed] [Google Scholar]
- 11. Barcena de Arellano ML, Pozdniakova S, Kühl AA, Baczko I, Ladilov Y, Regitz-Zagrosek V. Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging (Albany NY) 2019;11:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J 2013;27:4332–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 2007;100:1512–1521. [DOI] [PubMed] [Google Scholar]
- 14. Ruan Y, Dong C, Patel J, Duan C, Wang X, Wu X, Cao Y, Pu L, Lu D, Shen T, Li J. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell Physiol Biochem 2015;35:1116–1124. [DOI] [PubMed] [Google Scholar]
- 15. Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, Deng W, Tang QZ. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol 2018;114:38–47. [DOI] [PubMed] [Google Scholar]
- 16. Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem 2010;285:8375–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 2010;285:3133–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marín-Aguilar F, Lechuga-Vieco AV, Alcocer-Gómez E, Castejón-Vega B, Lucas J, Garrido C, Peralta-Garcia A, Pérez-Pulido AJ, Varela-López A, Quiles JL, Ryffel B, Flores I, Bullón P, Ruiz-Cabello J, Cordero MD. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 2020;19:e13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanz MN, Grimbert L, Moulin M, Gressette M, Rucker-Martin C, Lemaire C, Mericskay M, Veksler V, Ventura-Clapier R, Garnier A, Piquereau J. Inducible cardiac-specific deletion of Sirt1 in male mice reveals progressive cardiac dysfunction and sensitization of the heart to pressure overload. Int J Mol Sci 2019;20:E5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu YJ, Hsu SC, Hsu CP, Chen YH, Chang YL, Sadoshima J, Huang SM, Tsai CS, Lin CY. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int J Cardiol 2017;228:543–552. [DOI] [PubMed] [Google Scholar]
- 21. Planavila A, Dominguez E, Navarro M, Vinciguerra M, Iglesias R, Giralt M, Lope-Piedrafita S, Ruberte J, Villarroya F. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice: a role for Sirt1-Mef2 in adult heart. J Mol Cell Cardiol 2012;53:521–531. [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Zhou Y, Wang K, Wang L, Chen P, Hu H, Wang C, Zhang N, Ma Q, Huang M, Hu D, Zhang L, Wu R, Wang Y, Xu Q, Yu H, Wang J. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant 2014;33:1083–1092. [DOI] [PubMed] [Google Scholar]
- 23. Kanamori H, Takemura G, Goto K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Morishita K, Kawasaki M, Mikami A, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. Resveratrol reverses remodeling in hearts with large, old myocardial infarctions through enhanced autophagy-activating AMP kinase pathway. Am J Pathol 2013;182:701–713. [DOI] [PubMed] [Google Scholar]
- 24. Prola A, Pires Da Silva J, Guilbert A, Lecru L, Piquereau J, Ribeiro M, Mateo P, Gressette M, Fortin D, Boursier C, Gallerne C, Caillard A, Samuel JL, François H, Sinclair DA, Eid P, Ventura-Clapier R, Garnier A, Lemaire C. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ 2017;24:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Liang X, Chen Y, Zhao X. Screening SIRT1 activators from medicinal plants as bioactive compounds against oxidative damage in mitochondrial function. Oxid Med Cell Longev 2016;2016:4206392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma S, Feng J, Zhang R, Chen J, Han D, Li X, Yang B, Li X, Fan M, Li C, Tian Z, Wang Y, Cao F. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev 2017;2017:4602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bugyei-Twum A, Ford C, Civitarese R, Seegobin J, Advani SL, Desjardins JF, Kabir G, Zhang Y, Mitchell M, Switzer J, Thai K, Shen V, Abadeh A, Singh KK, Billia F, Advani A, Gilbert RE, Connelly KA. Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc Res 2018;114:1629–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gorski PA, Jang SP, Jeong D, Lee A, Lee P, Oh JG, Chepurko V, Yang DK, Kwak TH, Eom SH, Park ZY, Yoo YJ, Kim DH, Kook H, Sunagawa Y, Morimoto T, Hasegawa K, Sadoshima J, Vangheluwe P, Hajjar RJ, Park WJ, Kho C. Role of SIRT1 in modulating acetylation of the sarco-endoplasmic reticulum Ca2+-ATPase in heart failure. Circ Res 2019;124:e63–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gu XS, Wang ZB, Ye Z, Lei JP, Li L, Su DF, Zheng X. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet Mol Res 2014;13:323–335. [DOI] [PubMed] [Google Scholar]
- 30. Maizel J, Xavier S, Chen J, Lin CH, Vasko R, Goligorsky MS. Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction. Am J Physiol Heart Circ Physiol 2014;307:H1691–H1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang CL, Feng H, Li L, Wang JY, Wu D, Hao YT, Wang Z, Zhang Y, Wu LL. Globular CTRP3 promotes mitochondrial biogenesis in cardiomyocytes through AMPK/PGC-1α pathway. Biochim Biophys Acta Gen Subj 2017;1861:3085–3094. [DOI] [PubMed] [Google Scholar]
- 32. Geng T, Li P, Yin X, Yan Z. PGC-1α promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am J Pathol 2011;178:1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waldman M, Nudelman V, Shainberg A, Abraham NG, Kornwoski R, Aravot D, Arad M, Hochhauser E. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1α axis. Exp Cell Res 2018;373:112–118. [DOI] [PubMed] [Google Scholar]
- 34. Palomer X, Salvadó L, Barroso E, Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol 2013;168:3160–3172. [DOI] [PubMed] [Google Scholar]
- 35. Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA 2006;103:10086–10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kärkkäinen O, Tuomainen T, Mutikainen M, Lehtonen M, Ruas JL, Hanhineva K, Tavi P. Heart specific PGC-1α deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc Res 2019;115:107–118. [DOI] [PubMed] [Google Scholar]
- 37. Faerber G, Barreto-Perreia F, Schoepe M, Gilsbach R, Schrepper A, Schwarzer M, Mohr FW, Hein L, Doenst T. Induction of heart failure by minimally invasive aortic constriction in mice: reduced peroxisome proliferator-activated receptor γ coactivator levels and mitochondrial dysfunction. J Thorac Cardiovasc Surg 2011;141:492–500. [DOI] [PubMed] [Google Scholar]
- 38. Zhao L, Zou X, Feng Z, Luo C, Liu J, Li H, Chang L, Wang H, Li Y, Long J, Gao F, Liu J. Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp Gerontol 2014;56:3–12. [DOI] [PubMed] [Google Scholar]
- 39. Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM, Yellon DM. Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res 2013;99:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Derbré F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JA, Fuentes T, Gratas-Delamarche A, Monsalve M, Viña J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age (Dordr) 2012;34:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whitehead N, Gill JF, Brink M, Handschin C. Moderate modulation of cardiac PGC-1α expression partially affects age-associated transcriptional remodeling of the heart. Front Physiol 2018;9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD, Liu SK. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin 2018;39:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong S, Salazar G, Patrushev N, Ma M, Forouzandeh F, Hilenski L, Alexander RW. Peroxisome proliferator-activated receptor γ coactivator-1α is a central negative regulator of vascular senescence. Arterioscler Thromb Vasc Biol 2013;33:988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shan J, Pang S, Wanyan H, Xie W, Qin X, Yan B. Genetic analysis of the SIRT1 gene promoter in ventricular septal defects. Biochem Biophys Res Commun 2012;425:741–745. [DOI] [PubMed] [Google Scholar]
- 45. Yamac AH, Uysal O, Ismailoglu Z, Ertürk M, Celikten M, Bacaksiz A, Kilic U. Premature myocardial infarction: genetic variations in SIRT1 affect disease susceptibility. Cardiol Res Pract 2019;2019:8921806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rahimi E, Ahmadi A, Boroumand MA, Mohammad Soltani B, Behmanesh M. Nutrient sensing pathway genes expression dysregulated in patients with T2DM and coronary artery disease. Diabetes Res Clin Pract 2019;151:39–45. [DOI] [PubMed] [Google Scholar]
- 47. Hou J, Xie X, Tu Q, Li J, Ding J, Shao G, Jiang Q, Yuan L, Lai X. SIRT1 gene polymorphisms are associated with nondiabetic type 1 cardiorenal syndrome. Ann Hum Genet 2019;83:445–453. [DOI] [PubMed] [Google Scholar]
- 48. Clark J, Reddy S, Zheng K, Betensky RA, Simon DK. Association of PGC-1alpha polymorphisms with age of onset and risk of Parkinson’s disease. BMC Med Genet 2011;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Opstad TB, Kalstad AA, Pettersen AÅ, Arnesen H, Seljeflot I. Novel biomolecules of ageing, sex differences and potential underlying mechanisms of telomere shortening in coronary artery disease. Exp Gerontol 2019;119:53–60. [DOI] [PubMed] [Google Scholar]
- 50. Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, Tanese L, Russo G, Pitocco D, Lanza GA, Volpe M, Lüscher TF, Cosentino F. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 2017;66:2472–2482. [DOI] [PubMed] [Google Scholar]
- 51. Mariani S, Costantini D, Lubrano C, Basciani S, Caldaroni C, Barbaro G, Poggiogalle E, Donini LM, Lenzi A, Gnessi L. Circulating SIRT1 inversely correlates with epicardial fat thickness in patients with obesity. Nutr Metab Cardiovasc Dis 2016;26:1033–1038. [DOI] [PubMed] [Google Scholar]
- 52. Akkafa F, Halil Altiparmak I, Erkus ME, Aksoy N, Kaya C, Ozer A, Sezen H, Oztuzcu S, Koyuncu I, Umurhan B. Reduced SIRT1 expression correlates with enhanced oxidative stress in compensated and decompensated heart failure. Redox Biol 2015;6:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, Shih CC, Hsu C. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci 2014;21:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, Klaus K, Nair KS, Hajjar RJ, Redfield MM. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ Heart Fail 2019;12:e005131. [DOI] [PubMed] [Google Scholar]
- 55. Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res 2010;106:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol 2009;46:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wan X, Gupta S, Zago MP, Davidson MM, Dousset P, Amoroso A, Garg NJ. Defects of mtDNA replication impaired mitochondrial biogenesis during Trypanosoma cruzi infection in human cardiomyocytes and chagasic patients: the role of Nrf1/2 and antioxidant response. J Am Heart Assoc 2012;1:e003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, Georgilis A, Montoya A, Wolter K, Dharmalingam G, Faull P, Carroll T, Martínez-Barbera JP, Cutillas P, Reisinger F, Heikenwalder M, Miller RA, Withers D, Zender L, Thomas GJ, Gil J. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 2015;17:1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005;310:1193–1196. [DOI] [PubMed] [Google Scholar]
- 60. Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 2003;426:620. [DOI] [PubMed] [Google Scholar]
- 61. Garratt M, Nakagawa S, Simons MJ. Comparative idiosyncrasies in life extension by reduced mTOR signalling and its distinctiveness from dietary restriction. Aging Cell 2016;15:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 2013;4:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 2004;24:9508–9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest 2010;120:2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Rüegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation 2011;123:1073–1082. [DOI] [PubMed] [Google Scholar]
- 66. Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens 2006;24:1663–1670. [DOI] [PubMed] [Google Scholar]
- 67. Dai DF, Liu Y, Basisty N, Karunadharma P, Dastidar SG, Chiao YA, Chen T, Beyer RP, Chin MT, Maccoss M, La Spada AR, Rabinovitch PS. Differential effects of various genetic mouse models of the mechanistic target of rapamycin complex I inhibition on heart failure. Geroscience 2019;41:847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang HM, Fu J, Hamilton R, Diaz V, Zhang Y. The mammalian target of rapamycin modulates the immunoproteasome system in the heart. J Mol Cell Cardiol 2015;86:158–167. [DOI] [PubMed] [Google Scholar]
- 69. Nacarelli T, Azar A, Sell C. Aberrant mTOR activation in senescence and aging: a mitochondrial stress response? Exp Gerontol 2015;68:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 2011;106:1173–1191. [DOI] [PubMed] [Google Scholar]
- 71. Ren J, Yang L, Zhu L, Xu X, Ceylan AF, Guo W, Yang J, Zhang Y. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell 2017;16:976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wende AR, O'Neill BT, Bugger H, Riehle C, Tuinei J, Buchanan J, Tsushima K, Wang L, Caro P, Guo A, Sloan C, Kim BJ, Wang X, Pereira RO, McCrory MA, Nye BG, Benavides GA, Darley-Usmar VM, Shioi T, Weimer BC, Abel ED. Enhanced cardiac Akt/protein kinase B signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Mol Cell Biol 2015;35:831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Su M, Chen Z, Wang C, Song L, Zou Y, Zhang L, Hui R, Wang J. Cardiac-specific overexpression of miR-222 induces heart failure and inhibits autophagy in mice. Cell Physiol Biochem 2016;39:1503–1511. [DOI] [PubMed] [Google Scholar]
- 74. Sang HQ, Jiang ZM, Zhao QP, Xin F. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomed Pharmacother 2015;71:185–189. [DOI] [PubMed] [Google Scholar]
- 75. Yano T, Shimoshige S, Miki T, Tanno M, Mochizuki A, Fujito T, Yuda S, Muranaka A, Ogasawara M, Hashimoto A, Tsuchihashi K, Miura T. Clinical impact of myocardial mTORC1 activation in nonischemic dilated cardiomyopathy. J Mol Cell Cardiol 2016;91:6–9. [DOI] [PubMed] [Google Scholar]
- 76. González A, Ravassa S, Loperena I, López B, Beaumont J, Querejeta R, Larman M, Díez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J Hypertens 2007;25:2148–2157. [DOI] [PubMed] [Google Scholar]
- 77. Kemppainen J, Tsuchida H, Stolen K, Karlsson H, Björnholm M, Heinonen OJ, Nuutila P, Krook A, Knuuti J, Zierath JR. Insulin signalling and resistance in patients with chronic heart failure. J Physiol 2003;550:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Völkers M, Doroudgar S, Nguyen N, Konstandin MH, Quijada P, Din S, Ornelas L, Thuerauf DJ, Gude N, Friedrich K, Herzig S, Glembotski CC, Sussman MA. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med 2014;6:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 2001;103:670–677. [DOI] [PubMed] [Google Scholar]
- 80. Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res 2014;114:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu Z, Gan L, Liu G, Chen Y, Wu T, Feng F, Sun C. Sirt1 decreased adipose inflammation by interacting with Akt2 and inhibiting mTOR/S6K1 pathway in mice. J Lipid Res 2016;57:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang T, Du X, Zhao L, He M, Lin L, Guo C, Zhang X, Han J, Yan H, Huang K, Sun G, Yan L, Zhou B, Xia G, Qin Y, Wang C. SIRT1 facilitates primordial follicle recruitment independent of deacetylase activity through directly modulating Akt1 and mTOR transcription. FASEB J 2019;33:14703–14716. [DOI] [PubMed] [Google Scholar]
- 83. Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 2010;5:e9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown EL, Foletta VC, Wright CR, Sepulveda PV, Konstantopoulos N, Sanigorski A, Della Gatta P, Cameron-Smith D, Kralli A, Russell AP. PGC-1α and PGC-1β increase protein synthesis via ERRα in C2C12 myotubes. Front Physiol 2018;9:1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alayev A, Berger SM, Holz MK. Resveratrol as a novel treatment for diseases with mTOR pathway hyperactivation. Ann N Y Acad Sci 2015;1348:116–123. [DOI] [PubMed] [Google Scholar]
- 86. Zheng H, Fu Y, Huang Y, Zheng X, Yu W, Wang W. mTOR signaling promotes foam cell formation and inhibits foam cell egress through suppressing the SIRT1 signaling pathway. Mol Med Rep 2017;16:3315–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Y, Li X, He Z, Chen W, Lu J. Rapamycin attenuates palmitate-induced lipid aggregation by up-regulating sirt-1 signaling in AML12 hepatocytes. Pharmazie 2016;71:733–737. [DOI] [PubMed] [Google Scholar]
- 88. Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 2013;523:82–87. [DOI] [PubMed] [Google Scholar]
- 89. Houde VP, Brûlé S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 2010;59:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Corum DG, Tsichlis PN, Muise-Helmericks RC. AKT3 controls mitochondrial biogenesis and autophagy via regulation of the major nuclear export protein CRM-1. FASEB J 2014;28:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen P, Chen F, Lei J, Li Q, Zhou B. Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin a attenuates d-galactose-induced brain aging in mice. Neurotherapeutics 2019;16:1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F, Chen X. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev 2012;133:387–400. [DOI] [PubMed] [Google Scholar]
- 93. Ma L, Wang R, Wang H, Zhang Y, Zhao Z. Long-term caloric restriction activates the myocardial SIRT1/AMPK/PGC-1α pathway in C57BL/6J male mice. Food Nutr Res 2020;64. doi:10.29219/fnr.v64.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM, Liu WJ, Luo LL, Fu YC. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res 2014;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem 2010;285:36387–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guan P, Sun ZM, Wang N, Zhou J, Luo LF, Zhao YS, Ji ES. Resveratrol prevents chronic intermittent hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR pathway. Life Sci 2019;233:116748. [DOI] [PubMed] [Google Scholar]
- 97. Park D, Jeong H, Lee MN, Koh A, Kwon O, Yang YR, Noh J, Suh PG, Park H, Ryu SH. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci Rep 2016;6:21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang WR, Li TT, Jing T, Li YX, Yang XF, He YH, Zhang W, Lin R, Zhang JY. SIRT1 regulates the inflammatory response of vascular adventitial fibroblasts through autophagy and related signaling pathway. Cell Physiol Biochem 2017;41:569–582. [DOI] [PubMed] [Google Scholar]
- 99. Hong S, Zhao B, Lombard DB, Fingar DC, Inoki K. Cross-talk between sirtuin and mammalian target of rapamycin complex 1 (mTORC1) signaling in the regulation of S6 kinase 1 (S6K1) phosphorylation. J Biol Chem 2014;289:13132–13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007;450:736–740. [DOI] [PubMed] [Google Scholar]
- 101. Alayev A, Sun Y, Snyder RB, Berger SM, Yu JJ, Holz MK. Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle 2014;13:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang S, Kandadi MR, Ren J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: role of autophagy and mitophagy. Biochim Biophys Acta Mol Basis Dis 2019;1865:1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: actions beyond metabolic regulation. J Mol Cell Cardiol 2016;91:188–200. [DOI] [PubMed] [Google Scholar]
- 104. Slámová K, Papoušek F, Janovská P, Kopecký J, Kolář F. Adverse effects of AMP-activated protein kinase alpha2-subunit deletion and high-fat diet on heart function and ischemic tolerance in aged female mice. Physiol Res 2016;65:33–42. [DOI] [PubMed] [Google Scholar]
- 105. Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev 2010;131:739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ahluwalia A, Tarnawski AS. Activation of the metabolic sensor-AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J Physiol Pharmacol 2011;62:583–587. [PubMed] [Google Scholar]
- 107. Cieslik KA, Trial J, Entman ML. Defective myofibroblast formation from mesenchymal stem cells in the aging murine heart rescue by activation of the AMPK pathway. Am J Pathol 2011;179:1792–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007;104:12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 2008;283:27628–27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006;126:955–968. [DOI] [PubMed] [Google Scholar]
- 112. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008;30:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J. Aging and autophagy in the heart. Circ Res 2016;118:1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martín-Fernández B, Gredilla R. Mitochondria and oxidative stress in heart aging. Age (Dordr). 2016;38:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tang BL. Sirt1 and the mitochondria. Mol Cells 2016;39:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Reho JJ, Guo DF, Rahmouni K. Mechanistic target of rapamycin complex 1 signaling modulates vascular endothelial function through reactive oxygen species. J Am Heart Assoc 2019;8:e010662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Morita M, Gravel SP, Chénard V, Sikström K, Zheng L, Alain T, Gandin V, Avizonis D, Arguello M, Zakaria C, McLaughlan S, Nouet Y, Pause A, Pollak M, Gottlieb E, Larsson O, St-Pierre J, Topisirovic I, Sonenberg N. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab 2013;18:698–711. [DOI] [PubMed] [Google Scholar]
- 118. Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 2018;15:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baldelli S, Aquilano K, Ciriolo MR. PGC-1α buffers ROS-mediated removal of mitochondria during myogenesis. Cell Death Dis 2014;5:e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest 2015;125:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Leon LJ, Gustafsson ÅB. Staying young at heart: autophagy and adaptation to cardiac aging. J Mol Cell Cardiol 2016;95:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell 2011;146:682–695. [DOI] [PubMed] [Google Scholar]
- 123. Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular aging. Circ Res 2018;123:803–824. [DOI] [PubMed] [Google Scholar]
- 124. Meléndez A, Tallóczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for Dauer development and life-span extension in C. elegans. Science 2003;301:1387–1391. [DOI] [PubMed] [Google Scholar]
- 125. Tóth ML, Sigmond T, Borsos E, Barna J, Erdélyi P, Takács-Vellai K, Orosz L, Kovács AL, Csikós G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008;4:330–338. [DOI] [PubMed] [Google Scholar]
- 126. Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 2010;327:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009;462:1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhu X, Shen W, Yao K, Wang H, Liu B, Li T, Song L, Diao D, Mao G, Huang P, Li C, Zhang H, Zou Y, Qiu Y, Zhao Y, Wang W, Yang Y, Hu Z, Auwerx J, Loscalzo J, Zhou Y, Ju Z. Fine-tuning of PGC1α expression regulates cardiac function and longevity. Circ Res 2019;125:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, Leeuwenburgh C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol 2018;15:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dutta D, Xu J, Kim JS, Dunn WA Jr, Leeuwenburgh C. Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy 2013;9:328–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lempiäinen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf) 2013;208:410–421. [DOI] [PubMed] [Google Scholar]
- 133. Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011;331:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011;13:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Seo AY, Lau PW, Feliciano D, Sengupta P, Gros MAL, Cinquin B, Larabell CA, Lippincott-Schwartz J. AMPK and vacuole-associated Atg14p orchestrate μ-lipophagy for energy production and long-term survival under glucose starvation. Elife 2017;6:e21690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 2010;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 2008;105:3374–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Esteves AR, Filipe F, Magalhães JD, Silva DF, Cardoso SM. The role of Beclin-1 acetylation on autophagic flux in Alzheimer’s disease. Mol Neurobiol 2019;56:5654–5670. [DOI] [PubMed] [Google Scholar]
- 139. Dethlefsen MM, Kristensen CM, Tøndering AS, Lassen SB, Ringholm S, Pilegaard H. Impact of liver PGC-1α on exercise and exercise training-induced regulation of hepatic autophagy and mitophagy in mice on HFF. Physiol Rep 2018;6:e13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ivankovic D, Chau KY, Schapira AH, Gegg ME. Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J Neurochem 2016;136:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy 2010;6:186–188. [DOI] [PubMed] [Google Scholar]
- 142. Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 2010;120:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, Djukovic D, Beyer RP, Raftery D, MacCoss M, Tian R, Rabinovitch PS. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8:314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Salazar G, Cullen A, Huang J, Zhao Y, Serino A, Hilenski L, Patrushev N, Forouzandeh F, Hwang HS. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy 2019. ;doi:10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Li C, Yu L, Xue H, Yang Z, Yin Y, Zhang B, Chen M, Ma H. Nuclear AMPK regulated CARM1 stabilization impacts autophagy in aged heart. Biochem Biophys Res Commun 2017;486:398–405. [DOI] [PubMed] [Google Scholar]
- 146. Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 2011;50:117–127. [DOI] [PubMed] [Google Scholar]
- 147. Caragnano A, Aleksova A, Bulfoni M, Cervellin C, Rolle IG, Veneziano C, Barchiesi A, Mimmi MC8, Vascotto C, Finato N, Sponga S, Livi U, Isola M, Di Loreto C, Bussani R, Sinagra G, Cesselli D, Beltrami AP. Autophagy and inflammasome activation in dilated cardiomyopathy. J Clin Med 2019;8:E1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Saito T, Asai K, Sato S, Hayashi M, Adachi A, Sasaki Y, Takano H, Mizuno K, Shimizu W. Autophagic vacuoles in cardiomyocytes of dilated cardiomyopathy with initially decompensated heart failure predict improved prognosis. Autophagy 2016;12:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bartlett JJ, Trivedi PC, Pulinilkunnil T. Autophagic dysregulation in doxorubicin cardiomyopathy. J Mol Cell Cardiol 2017;104:1–8. [DOI] [PubMed] [Google Scholar]
- 150. Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest 2013;123:5284–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Singh SR, Zech ATL, Geertz B, Reischmann-Düsener S, Osinska H, Prondzynski M, Krämer E, Meng Q, Redwood C, van der Velden J, Robbins J, Schlossarek S, Carrier L. Activation of autophagy ameliorates cardiomyopathy in mybpc3-targeted knockin mice. Circ Heart Fail 2017;10:e004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, Ogino A, Tsujimoto A, Goto K, Maruyama R, Kawasaki M, Mikami A, Fujiwara T, Fujiwara H, Minatoguchi S. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res 2012;96:456–465. [DOI] [PubMed] [Google Scholar]
- 153. Sung MM, Zordoky BN, Bujak AL, Lally JS, Fung D, Young ME, Horman S, Miller EJ, Light PE, Kemp BE, Steinberg GR, Dyck JR. AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovasc Res 2015;107:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ramos FJ, Kaeberlein M, Kennedy BK. Elevated MTORC1 signaling and impaired autophagy. Autophagy 2013;9:108–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Marampon F, Gravina GL, Scarsella L, Festuccia C, Lovat F, Ciccarelli C, Zani BM, Polidoro L, Grassi D, Desideri G, Evangelista S, Ferri C. Angiotensin-converting-enzyme inhibition counteracts angiotensin II-mediated endothelial cell dysfunction by modulating the p38/SirT1 axis. J Hypertens 2013;31:1972–1983. [DOI] [PubMed] [Google Scholar]
- 156. Clarke NE, Belyaev ND, Lambert DW, Turner AJ. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond) 2014;126:507–516. [DOI] [PubMed] [Google Scholar]
- 157. Zoll J, Monassier L, Garnier A, N'Guessan B, Mettauer B, Veksler V, Piquard F, Ventura-Clapier R, Geny B. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol (1985) 2006;101:385–391. [DOI] [PubMed] [Google Scholar]
- 158. Wu X, He L, Cai Y, Zhang G, He Y, Zhang Z, He X, He Y, Zhang G, Luo J. Induction of autophagy contributes to the myocardial protection of valsartan against ischemia-reperfusion injury. Mol Med Rep 2013;8:1824–1830. [DOI] [PubMed] [Google Scholar]
- 159. Hernández JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med 2014;18:709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mavroeidi V, Petrakis I, Stylianou K, Katsarou T, Giannakakis K, Perakis K, Vardaki E, Stratigis S, Ganotakis E, Papavasiliou S, Daphnis E. Losartan affects glomerular AKT and mTOR phosphorylation in an experimental model of type 1 diabetic nephropathy. J Histochem Cytochem 2013;61:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ma L, Gul R, Habibi J, Yang M, Pulakat L, Whaley-Connell A, Ferrario CM, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. Am J Physiol Heart Circ Physiol 2012;302:H2341–H2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hu H, Li X, Ren D, Tan Y, Chen J, Yang L, Chen R, Li J, Zhu P. The cardioprotective effects of carvedilol on ischemia and reperfusion injury by AMPK signaling pathway. Biomed Pharmacother 2019;117:109106. [DOI] [PubMed] [Google Scholar]
- 163. Yao K, Zhang WW, Yao L, Yang S, Nie W, Huang F. Carvedilol promotes mitochondrial biogenesis by regulating the PGC-1/TFAM pathway in human umbilical vein endothelial cells (HUVECs). Biochem Biophys Res Commun 2016;470:961–966. [DOI] [PubMed] [Google Scholar]
- 164. Wong WT, Li LH, Rao YK, Yang SP, Cheng SM, Lin WY, Cheng CC, Chen A, Hua KF. Repositioning of the β-Blocker carvedilol as a novel autophagy inducer that inhibits the NLRP3 inflammasome. Front Immunol 2018;9:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Allen SA, Tomilov A, Cortopassi GA. Small molecules bind human mTOR protein and inhibit mTORC1 specifically. Biochem Pharmacol 2018;155:298–304. [DOI] [PubMed] [Google Scholar]
- 166. Liu GZ, Zhang S, Li YY, Liu YW, Zhang Y, Zhao XB, Yuan Y, Zhang JW, Khannanova Z, Li Y. Aldosterone stimulation mediates cardiac metabolism remodeling via Sirt1/AMPK signaling in canine model. Naunyn Schmiedebergs Arch Pharmacol 2019;392:851–863. [DOI] [PubMed] [Google Scholar]
- 167. Long HD, Lin YE, Liu MJ, Liang LY, Zeng ZH. Spironolactone prevents dietary-induced metabolic syndrome by inhibiting PI3-K/Akt and p38MAPK signaling pathways. J Endocrinol Invest 2013;36:923–930. [DOI] [PubMed] [Google Scholar]
- 168. Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S, Yan T. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci Rep 2016;36:e00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yuan Y, Chen Y, Zhang P, Huang S, Zhu C, Ding G, Liu B, Yang T, Zhang A. Mitochondrial dysfunction accounts for aldosterone-induced epithelial-to-mesenchymal transition of renal proximal tubular epithelial cells. Free Radic Biol Med 2012;53:30–43. [DOI] [PubMed] [Google Scholar]
- 170. Plante E, Menaouar A, Danalache BA, Broderick TL, Jankowski M, Gutkowska J. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia 2014;57:1257–1267. [DOI] [PubMed] [Google Scholar]
- 171. Ruiz-Ojeda FJ, Aguilera CM, Rupérez AI, Gil Á, Gomez-Llorente C. An analogue of atrial natriuretic peptide (C-ANP4-23) modulates glucose metabolism in human differentiated adipocytes. Mol Cell Endocrinol 2016;431:101–108. [DOI] [PubMed] [Google Scholar]
- 172. Kim J, Han D, Byun SH, Kwon M, Cho SJ, Koh YH, Yoon K. Neprilysin facilitates adipogenesis through potentiation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Mol Cell Biochem 2017;430:1–9. [DOI] [PubMed] [Google Scholar]
- 173. Siepmann M, Kumar S, Mayer G, Walter J. Casein kinase 2 dependent phosphorylation of neprilysin regulates receptor tyrosine kinase signaling to Akt. PLoS One 2010;5:e13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dietl A, Winkel I, Pietrzyk G, Paulus M, Bruckmann A, Schröder JA, Sossalla S, Luchner A, Maier LS, Birner C. Skeletal muscle alterations in tachycardia-induced heart failure are linked to deficient natriuretic peptide signalling and are attenuated by RAS-/NEP-inhibition. PLoS One 2019;14:e0225937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Dehghan E, Goodarzi M, Saremi B, Lin R, Mirzaei H. Hydralazine targets cAMP-dependent protein kinase leading to sirtuin1/5 activation and lifespan extension in C. elegans. Nat Commun 2019;10:4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hundeshagen P, Hamacher-Brady A, Eils R, Brady NR. Concurrent detection of autolysosome formation and lysosomal degradation by flow cytometry in a high-content screen for inducers of autophagy. BMC Biol 2011;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wang Y, Qiu Q, Shen JJ, Li DD, Jiang XJ, Si SY, Shao RG, Wang Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int J Biochem Cell Biol 2012;44:1813–1824. [DOI] [PubMed] [Google Scholar]
- 178. Buzaglo N, Golomb M, Rosen H, Beeri R, Ami HC, Langane F, Pierre S, Lichtstein D. Augmentation of ouabain-induced increase in heart muscle contractility by Akt inhibitor MK-2206. J Cardiovasc Pharmacol Ther 2019;24:78–89. [DOI] [PubMed] [Google Scholar]
- 179. Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 2007;293:C509–C536. [DOI] [PubMed] [Google Scholar]
- 180. Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 2007;293:C1489–C1497. [DOI] [PubMed] [Google Scholar]
- 181. Yu Z, Gong X, Yu Y, Li M, Liang Y, Qin S, Fulati Z, Zhou N, Shu X, Nie Z, Dai S, Chen X, Wang J, Chen R, Su Y, Ge J. The mechanical effects of CRT promoting autophagy via mitochondrial calcium uniporter down-regulation and mitochondrial dynamics alteration. J Cell Mol Med 2019;23:3833–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. McMurray JJV, Solomon SD, Docherty KF, Jhund PS. The Dapagliflozin And Prevention of Adverse outcomes in Heart Failure trial (DAPA-HF) in context. Eur Heart J 2020;doi:10.1093/eurheartj/ehz916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, Gerszten R, Patti ME. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 2019;4:123130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond) 2019;133:2415–2430. [DOI] [PubMed] [Google Scholar]
- 185. Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 2016;65:2784–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kim JW, Lee YJ, You YH, Moon MK, Yoon KH, Ahn YB, Ko SH. Effect of sodium-glucose cotransporter 2 inhibitor, empagliflozin, and α-glucosidase inhibitor, voglibose, on hepatic steatosis in an animal model of type 2 diabetes. J Cell Biochem 2018;120:8534–8546. [DOI] [PubMed] [Google Scholar]
- 187. Mohamed HE, Asker ME, Keshawy MM, Hasan RA, Mahmoud YK. Inhibition of tumor necrosis factor-α enhanced the antifibrotic effect of empagliflozin in an animal model with renal insulin resistance. Mol Cell Biochem 2020;466:45–54. [DOI] [PubMed] [Google Scholar]
- 188. Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, Nakata K, Kimura Y, Tanno M, Miura T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep 2018;6:e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wei D, Liao L, Wang H, Zhang W, Wang T, Xu Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci 2020;247:117414. [DOI] [PubMed] [Google Scholar]
- 190. Kim JH, Ko HY, Wang HJ, Lee H, Yun M, Kang ES. Effect of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on gluconeogenesis in proximal renal tubules. Diabetes Obes Metab 2020;22:373–382. [DOI] [PubMed] [Google Scholar]
- 191. Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol 2019;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Xu C, Wang W, Zhong J, Lei F, Xu N, Zhang Y, Xie W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol 2018;152:45–59. [DOI] [PubMed] [Google Scholar]
- 193. Aragón-Herrera A, Feijóo-Bandín S, Otero Santiago M, Barral L, Campos-Toimil M, Gil-Longo J, Costa Pereira TM, García-Caballero T, Rodríguez-Segade S, Rodríguez J, Tarazón E, Roselló-Lletí E, Portolés M, Gualillo O, González-Juanatey JR, Lago F. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol 2019;170:113677. [DOI] [PubMed] [Google Scholar]
- 194. Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 2019;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther 2017;31:119–132. [DOI] [PubMed] [Google Scholar]
- 196. Yurista SR, Silljé HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail 2019;21:862–873. [DOI] [PubMed] [Google Scholar]
- 197. Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med 2017;104:298–310. [DOI] [PubMed] [Google Scholar]
- 198. Ying Y, Jiang C, Zhang M, Jin J, Ge S, Wang X. Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging (Albany NY) 2019;11:2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 2011;60:1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sun D, Yang F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem Biophys Res Commun 2017;486:329–335. [DOI] [PubMed] [Google Scholar]
- 201. Yin M, van der Horst IC, van Melle JP, Qian C, van Gilst WH, Silljé HH, de Boer RA. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol 2011;301:H459–H468. [DOI] [PubMed] [Google Scholar]
- 202. Packer M. Is metformin beneficial for heart failure in patients with type 2 diabetes? Diabetes Res Clin Pract 2018;136:168–170. [DOI] [PubMed] [Google Scholar]
- 203. Wang G, Song X, Zhao L, Li Z, Liu B. Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. Biomed Res Int 2018;2018:2150218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Das SK, Zhabyeyev P, Basu R, Patel VB, Dyck JRB, Kassiri Z, Oudit GY. Advanced iron-overload cardiomyopathy in a genetic murine model is rescued by resveratrol therapy. Biosci Rep 2018;38. pii: BSR20171302. doi: 10.1042/BSR20171302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lu Y, Lu X, Wang L, Yang W. Resveratrol attenuates high fat diet-induced mouse cardiomyopathy through upregulation of estrogen related receptor-α. Eur J Pharmacol 2019;843:88–95. [DOI] [PubMed] [Google Scholar]
- 206. Wan X, Wen JJ, Koo SJ, Liang LY, Garg NJ. SIRT1-PGC1α-NFκB pathway of oxidative and inflammatory stress during Trypanosoma cruzi infection: benefits of SIRT1-targeted therapy in improving heart function in Chagas disease. PLoS Pathog 2016;12:e1005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Kuno A, Hori YS, Hosoda R, Tanno M, Miura T, Shimamoto K, Horio Y. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. J Biol Chem 2013;288:5963–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Riba A, Deres L, Sumegi B, Toth K, Szabados E, Halmosi R. Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid Med Cell Longev 2017;2017:6819281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Pei Z, Deng Q, Babcock SA, He EY, Ren J, Zhang Y. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: role of autophagy and ER stress. Toxicol Lett 2018;284:10–20. [DOI] [PubMed] [Google Scholar]
- 210. Flynn JM, O'Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 2013;12:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Choi JC, Worman HJ. Reactivation of autophagy ameliorates LMNA cardiomyopathy. Autophagy 2013;9:110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


