Abstract
Prolactin (PRL) has both stimulatory and inhibitory effects on testicular function, a finding we hypothesized may be related in some part to the form of the hormone present or administered. In the analysis of the pituitary secretion profiles of early pubescent vs. mature male rats, we found PRL released from early pubescent pituitaries had about twice the degree of phosphorylation. Treatment of mature males with either unmodified PRL (U-PRL) or phosphorylated PRL (via the molecular mimic S179D PRL) for a period of 4 wk (circulating level of ~50 ng/ml) showed serum testosterone decreased by ~35% only by treatment with the phosphomimic S179D PRL. Given the specificity of this effect, it was initially surprising that both forms of PRL decreased testicular expression of 3β-hydroxysteroid dehydrogenase and steroidogenic acute regulatory protein. Both forms also increased expression of the luteinizing hormone receptor, but only S179D PRL increased the ratio of short to long PRL receptors. Endogenous PRL and luteinizing hormone levels were unchanged in all groups in this time frame, suggesting that effects on steroidogenic gene expression were directly on the testis. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling analysis combined with staining for 3β-hydroxysteroid dehydrogenase and morphometric analysis showed that S179D PRL, but not U-PRL, increased apoptosis of Leydig cells, a finding supported by increased staining for Fas and Fas ligand in the testicular interstitium, providing an explanation for the specific effect on testosterone. S179D PRL, but not U-PRL, also increased apoptosis of primary spermatogonia, and U-PRL, but not S179D PRL, decreased apoptosis of elongating spermatids. Thus, in mature males, hyperprolactinemic levels of both forms of PRL have common effects on steroidogenic proteins, but specific effects on the apoptosis of Leydig and germ cells.
Keywords: phosphorylated prolactin, S179D prolactin, apoptosis, Leydig cells, testosterone, 3β-hydroxysteroid dehydrogenase, steroidogenic acute regulatory protein, luteinizing hormone receptor, luteinizing hormone, short prolactin receptor
Prolactin (PRL) is a 23-kDa polypeptide hormone produced and secreted in largest quantity by lactotropes of the anterior pituitary (reviewed in Ref. 13). It has a broad range of functions in the male reproductive system, but its role in testes remains poorly understood and is somewhat species specific (reviewed in Ref. 1).
In studies by other investigators, PRL has been shown to be responsible for the induction of Leydig cell proliferation and differentiation in prepubertal hypophysectomized rats (10) and the maintenance of Leydig cell morphology, upregulation of luteinizing hormone receptor (LHR) expression, and potentiation of luteinizing hormone (LH)-induced steroidogenesis in hypophysectomized rats (2, 10, 32, 36, 54). In vitro approaches, utilizing murine Leydig tumor cell lines and isolated rodent primary Leydig cell cultures, have concluded that PRL exerts trophic effects on Leydig cell steroidogenic function, mostly through an effect on LH-induced testosterone production (23, 32, 33, 45), although some of these effects have been shown to have biphasic dose-response curves, with high concentrations actually exerting an inhibitory effect (33). Hyper-prolactinemia in adult animals has a negative effect on testosterone production, a result until now considered to be largely a consequence of reduced LH secretion (5, 16, 18, 23, 34, 42).
Several posttranslationally modified forms of PRL exist (reviewed in Ref. 31); however, in the rat, phosphorylated PRL and unmodified PRL (U-PRL) make up most of the PRL released by the pituitary (20, 21). The relative proportion of U-PRL to phosphorylated PRL has been shown to be physiologically regulated in females (20, 21), but not yet in males, and these two forms of PRL have been shown to have distinct biological activities (28, 44). For investigations in vivo (8, 26, 29, 50–53), a molecular mimic of phosphorylated PRL has been used to both produce the large quantities necessary for in vivo experiments by recombinant technology and to circumvent the potential problem of conversion of phosphorylated PRL into U-PRL during the course of an experiment. The mimic was produced by substituting an aspartate residue for the normally phosphorylated serine, thereby producing S179D PRL (4). This mimic reproduces the growth antagonist properties of phosphorylated PRL (4, 44), and intracellular signals generated by mixed preparations of U-PRL and phosphorylated PRL lie in between those of recombinant U-PRL and S179D PRL (7, 47). In previous experiments in multiple systems, U-PRL has been shown to promote cell proliferation (29, 49–52) and some cell differentiation (47). Phosphorylated PRL/S179D PRL on the other hand is an antagonist to U-PRL-mediated growth (4, 29, 38, 47, 49–52), although it is one that nevertheless promotes differentiation (29, 47, 51) and/or apoptosis (40, 41, 52, 53) through the generation of alternate intracellular signals (40, 41, 47–49). The exact outcome of the effect of pituitary PRL therefore depends on both the total amount and the ratio of U-PRL to phosphorylated PRL released (reviewed in Ref. 43).
Until recently, all experimentation with PRL used material extracted from pituitaries, which contain a variable mixture of U-PRL and phosphorylated PRL, regardless of the species of origin (reviewed in Ref. 31). Given that PRL’s effects in the testis have been reported to involve both proliferation and differentiation, the potential existed for the two forms of PRL to have distinct effects on testicular function. We report physiological regulation of PRL phosphorylation in male animals and both common and distinct effects of each form of PRL in the testis.
MATERIALS AND METHODS
Animal Experiments
All animal procedures were approved by the University of California, Riverside, Campus Committee on Laboratory Animal Care and performed in accordance with National Institutes of Health guidelines. Animals were housed in standard cages and kept under 12:12-h light-dark environmental conditions throughout the course of the experiment. Food and water were provided ad libitum.
PRL secretion profile
The PRL secretion profiles of pituitaries from 25-day-old and sexually mature male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were compared by two-dimensional gel electrophoresis, performed and quantified as described previously (20, 21). Briefly, pituitaries were harvested and cut into 1-mm square pieces after the posterior lobe was removed. The pieces were washed three times in DMEM and then incubated for 2 h in fresh medium. After we harvested the 2-h-conditioned medium, isoelectric point and molecular weight standards were added before the addition of 2 vol of −20°C acetone and precipitation of all proteins overnight at −20°C. Pelleting of the precipitate was followed by dissolution in urea lysis buffer (21). Pituitary pieces were homogenized in urea lysis buffer, and once again isoelectric point and molecular weight standards were added before two-dimensional gel electrophoresis. PRL isoforms were identified by reference to the triangulated standards and were quantified by densitometry (20, 21). Each pituitary and secretion were separately analyzed, and six pituitaries were used for each age group.
Administration of PRL to sexually mature males
In three separate experiments, sexually mature males were randomly divided into three groups (control, U-PRL, and S179D PRL). For all of the data presented, Alzet osmotic pumps (Alza, Palo Alto, CA) delivering 24 μg/kg of the appropriate recombinant hormone or saline (diluent for the hormones) daily were implanted subcutaneously interscapularly under local anesthesia. The hormones were stable throughout the treatment period as evidenced by both concentration and bioactivity testing at the end of the 4-wk period. This rate of delivery resulted in a circulating concentration of the administered PRLs of ~50 ng/ml (8, 29). After the appropriate time frame for full analysis (see RESULTS) was established, the animals were killed 4 wk after implantation, at the same time of day and under low-stress conditions. Trunk blood was collected, and whole testes were dissected. For each testis harvested, the organ was decapsulated and cut into halves. One half was immediately snap frozen in liquid nitrogen for later RNA extraction. The other half was immediately fixed in periodate-lysine-paraformaldehyde fixative for immunohistochemical (IHC) examination.
Recombinant PRLs
The recombinant human U-PRL and phosphorylated PRL (S179D PRL) preparations utilized in this study were produced as previously described by Chen et al. (4). This procedure resulted in a well-folded preparation concentrated to ~1 mg/ml in saline. Both recombinant hormones were expressed in Escherichia coli and therefore were not posttranslationally modified, as they would have been in a eukaryotic cell. The resulting PRL preparations were tested for stimulatory or antagonistic biological activity in the rat lymphoma Nb2 cell proliferation bioassay (4). Human forms of administered PRL were used so that the rats’ endogenous PRL levels could be assessed in response to treatment.
Hormone Assays
Trunk blood serum preparations were used for measurement of total testosterone, rat LH (rLH), and rat PRL (rPRL). The concentrations of each were measured by commercial enzyme immunosorbent assays (EIA) purchased from ALPCO Diagnostics, Salem, NH (testosterone and rPRL) and Endocrine Technologies, Newark, CA (rLH). All samples were measured at least in duplicate, and all were measured in the same assay, repeated twice. The coefficients of intra-assay variation were 2.8% for testosterone, 2.2% for rLH, and 2.1% for rPRL. The minimal reproducible detectable limit for rLH was determined to be 0.5 ng/ml. A separate control analysis excluded cross reactivity of the human U-PRL and S179D PRL recombinant hormones with the anti-rPRL antibody utilized in the rPRL EIA.
RNA Extraction and Production of cDNA
Using TRI reagent RNA isolation (Sigma-Aldrich, St. Louis, MO), we extracted total RNA from each frozen testis sample, essentially according to the method of Chomzynski and Mackey (6). Five micrograms of total RNA were added to a mix containing 1 μl of oligo(dT) (18–25 mer) and 8 μl of double distilled H2O. The mixture was denatured for 10 min at 70°C, after which it was quickly chilled on ice for 5 min. Nine microliters of a mixture containing 4 μl of 5× first-strand buffer, 2 μl of DTT (0.1 M), 1 μl of dNTPs (10 mM/dNTPs), 1 μl of RNase Out (ribonuclease inhibitor), and 1 μl of murine Moloney leukemia virus reverse transcriptase were added. The solutions were mixed gently by pipetting and incubated for 1 h at 37°C. This was followed by a 10-min incubation at 70°C, after which time the samples were again chilled on ice for 1 min. The resulting cDNA was stored at −20°C until real-time RT-PCR analysis. All reagents utilized for reverse transcription were purchased from Invitrogen (Carlsbad, CA).
Real-Time Quantitative RT-PCR
Quantitative PCRs (qPCR) for LHR, 3β-hydroxysteroid dehydrogenase (3β-HSD), and steroidogenic acute regulatory (StAR) protein mRNA were performed. For qPCR, SYBR green-based technology was employed, using the ABI Prism SDS 7700 sequence detection system (Applied Biosystems, Foster City, CA). Power SYBR green Mastermix (12.5 μl) containing SYBR green 1 dye, AmpliTaq Gold DNA polymerase, dNTPs containing a mixture of dUTP and dTTP, ROX passive reference dye, and buffer was mixed along with 1 μl each of the sense and anti-sense primers (10 mM/each) for each gene of interest, 2.5 μl of the template (50 ng/ml), and 8.0 μl of double distilled H2O. All samples were run simultaneously and in triplicate on the same 96-well optical microtiter plate. qPCR runs for individual genes were conducted separately and repeated twice. The mRNA expression level of the GAPDH housekeeping gene was also assessed concurrently during each run for normalization purposes. The following amplification parameters were chosen for each gene: amplification erase hold at 55°C for 5 min, denaturation at 94°C for 10 min, annealing at 55°C for 15 s, and extension at 72°C for 1 min. A separate melting curve analysis was also run to characterize the fluorescent intensities of the amplified products and to verify the absence of contamination (data not shown). The relative quantification method was utilized to calculate the difference (in fold) in the expression of each gene of interest in all treatment groups, using the following equation (3): ΔΔCt = 2− (experimental ΔCt − control ΔCt), where Ct is threshold cycle.
The amplification plots for all samples were analyzed for amplification efficiency. Nontemplate controls were used as negative controls.
Oligonucleotide primers for all the genes of interest were purchased from Sigma-Genosys (The Woodlands, TX). All were designed using the Primer Premier software program (Premier Biosoft International, Palo Alto, CA) in accordance with conditions specified in the Applied Biosystems Primer Express technical guide. The sense and anti-sense primers were as follows: for rat LHR, 5′-CTGTTCA-CCCAAGACACTCCAATG-3′ (sense) and 5′-CGACTGGTCAG-GAGAACAAAGAGG-3′ (antisense) (predicted size = 159 bp); for rat StAR, 5′-ATGCCTGAGCAAAGCGGTGTC-3′ (sense) and 5′-CAAGTGGCTGGCGAACTCTATCTG-3′ (antisense) (predicted size = 189 bp); for rat 3β-HSD, 5′-CCAGTGTATGTAGGCAAT-GTGGC-3′ (sense) and 5′-CCATTCCTTGCTCAGGGTGC-3′ (anti-sense) (predicted size = 162 bp); for rat GAPDH, 5′-CCATG-GAGAAGGCTGGGG-3′ (sense) and 5′-CAAAGTTGTCATGGAT-GACC-3′ (antisense) (predicted size = 195 bp); for rat long PRL receptor, 5′-GATGACAATGAGGACGAG-3′ (sense) and 5′-TGTT-GAAGATTTGGGTG-3′ (antisense) (predicted size: 285 bp); for rat short PRL receptor, 5′-GGGGAACTTGACTACTG-3′ (sense) and 5′-CGTGAGACTGAGGGAT-3′ (antisense) (predicted size = 260 bp).
Tissue Processing
After dissection, the testes were immediately fixed in periodate-lysine-paraformaldehyde overnight at 4°C, after which time they were washed in PBS. The organs were infiltrated with a 30% sucrose-PBS solution for 3 days. After sucrose infiltration, the testis halves from each animal were embedded in OCT compound (Sakura Finetek USA, Torrance, CA) and stored at −80°C until sectioning.
Combined TUNEL and IHC Analysis
Analysis of Leydig cell apoptosis was carried out by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) using a commercial ApopTag kit (Chemicon International, Temecula, CA). Two 20-μm frozen sections from the decapsulated midtestis halves were cut and mounted on each slide. Two nonconsecutive slides per animal were chosen for analysis. The TUNEL technique was performed according to the manufacturer’s instructions with the following modifications. First, the slides were brought to room temperature for 30 min after which time they were fixed in ice-cold acetone for 10 min. This was followed by two washes with 0.01% (vol/vol) Triton X-100-PBS (PBS-T) for 5 min each and postfixation in a 1% paraformaldehyde-PBS solution for an additional 10 min. Two more washes in PBS-T were then followed by another fixation in a 2:1 ethanol-acetic acid solution at −20°C for 5 min. The sections were again washed twice (5 min/wash) and then treated with proteinase K (20 mg/ml) (Chemicon International) for 15 min at room temperature. This was followed by incubation in equilibration buffer for 10 min at room temperature and a 1-h incubation at 37°C with the digoxigenin-conjugated terminal deoxynucleotidyl transferase enzyme. The reaction was stopped with a stop-wash buffer, and the sections were again washed twice in PBS-T. Incubation in a fluorescein-conjugated sheep anti-digoxigenin antibody was performed for 30 min at room temperature, protected from light.
TUNEL analysis was also combined with staining for the PRL receptor. After TUNEL staining, sections were blocked with 10% goat serum (Zymed Laboratories, San Francisco, CA) for 30 min and then incubated in rabbit anti-rPRL receptor antibody at 1:200 dilution (kindly provided by Dr. Patricia Ingleton, University of Sheffield Medical School, Sheffield, UK) raised against the common extracellular domain of the PRL receptor. After sections were washed in PBS-T, they were incubated in goat anti-rabbit IgG conjugated with Alexa fluor 594 (1:1,000) (Molecular Probes, Eugene, OR) for 1 h at 37C. A final wash sequence was followed by mounting in ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (Molecular Probes), and the slides were stored at −20°C until confocal fluorescence microscopy. This dual TUNEL and PRL receptor analysis was repeated on two separate occasions.
To positively identify Leydig cells undergoing apoptosis, some TUNEL staining was followed by additional staining with rabbit anti-rat 3β-HSD (1:4,000) for 1 h at 37°C. 3β-HSD is a key steroidogenic enzyme that serves as a specific marker for Leydig cells. The antibody was raised using a peptide specific to rat 3β-HSD (CTLVEQHRETLDTKSQ; custom synthesis by Sigma Genesis, Tokyo, Japan). Before they were stained with this antibody, sections were blocked with 10% goat serum, as above. After primary antibody incubation, sections were again washed and subsequently incubated with a goat anti-rabbit IgG Alexa fluor 594-conjugated antibody (1:1,000) for an additional 1 h at 37°C (Molecular Probes). After the final antibody incubation and washing, the sections were mounted as above. This dual analysis was repeated on three separate occasions for a total of six slides per animal.
Morphometric Analysis
In each of the TUNEL-IHC trials, two slides per animal were visualized with the Pathway HT confocal fluorescence microscope (BD Biosciences, Rockville, MD). For 3β-HSD, 30 random images of testicular interstitium lying in between two to three seminiferous tubules per animal were photographed. TUNEL-positive cells were visualized under the FITC channel. The cytosol of Leydig cells stained with the anti-3β-HSD-Alexa fluor 594 antibodies was visualized under the Texas red channel. Merging of images from both channels resulted in some red cells having green nuclei and some cells having yellow regions, resulting from a breakdown of the boundary between the nucleus and cytoplasm. Only the cells with yellow regions were counted as apoptotic. Nuclei were also stained with DAPI and visualized under the UV channel. With the use of both color-merged images and images from individual channels, TUNEL-positive Leydig cells were tabulated. Leydig cell number and relative 3β-HSD protein levels used analyses of the images photographed in the Texas red channel only. With the use of the Simple PCI image analysis software system (Compix, Sewickley, PA), a gray-scale threshold value of 300 was set to eliminate any background fluorescence. This allowed only for areas of the interstitium that were positive for 3β-HSD to be chosen for analysis. Once the threshold was set, the following parameters were measured: 1) total area (which measures the number of pixels in a selected object, which in this case is the number of pixels per 3β-HSD-positive Leydig cell), 2) total gray level (the sum intensity of each pixel within the object at or above the set threshold), 3) total mass (the total fluorescence intensity detected), 4) object count (the total number of objects measured at or above the set threshold), and 5) region of interest area (area of the field of view of the image; this value remained constant from image to image).
Primary spermatogonia and elongating spermatids were identified morphologically because staining with anti c-Kit produced anomalous results. Morphometric analysis was again conducted by analyzing 30 images per animal.
Fas and Fas Ligand Staining
As with the TUNEL-IHC analysis, two 20-μm frozen sections from midtestis halves were cut and mounted on each slide. Two slides per animal were chosen for both analyses with the individual antibodies. The slides were brought to room temperature for 30 min, fixed in ice-cold acetone for 10 min, and then washed twice with PBS-T for 5 min each. To control for nonspecific binding, the sections were blocked with 10% (vol/vol) goat serum for 30 min and then incubated with the goat anti-mouse Fas and goat anti-mouse Fas ligand antibodies (both at dilutions of 1:50) (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 37°C. This step was followed by two washes in PBS-T for 5 min and a final incubation with Texas red-conjugated mouse anti-goat IgG (Molecular Probes) (1:1,000) also for 1 h at 37°C. The sections were washed twice with PBS-T, air dried, and mounted, using ProLong Gold antifade reagent with DAPI. As with the TUNEL-IHC analysis, two slides per animal from each group were visualized by confocal microscopy, but 20 random images of testicular interstitium lying in between two to three seminiferous tubules per animal were photographed.
Statistical Analysis
Data from the two-dimensional gels, the EIAs, and multiple qPCR analyses were analyzed by one-way ANOVA, followed by the Tukey-Kramer multiple comparisons posttest (GraphPad Instat Statistics software, San Diego, CA). Data analyzed for the multiple TUNEL analyses were evaluated with an unpaired t-test with Welch correction. All values are expressed as means ± SE (graphical data and tables) with total cells analyzed given in Table 2. P < 0.05 was considered statistically significant.
Table 2.
Rat hormone levels in response to the 4-wk treatment
| Hormone | Control | U-PRL | S179D PRL |
|---|---|---|---|
| Rat LH, ng/ml | 1.54±0.123 | 1.53±0.124 | 1.52±0.124 |
| Rat PRL, ng/ml | 6.0±0.92 | 7.1±1.34 | 5.2±1.09 |
Values are means ± SE from 2 separate analyses, each with 8 values. Errors were rounded to 2 decimal places. No significant differences were observed.
RESULTS
PRL Secretion Profile
Table 1 shows the result of the PRL isoform analysis. Consistent with previous publications where female animals (20, 21) or primary cells in culture (17) were used, we found the isoform profile of secreted PRL to be different from that inside the cells, a result of intragranular phosphorylation just before exocytosis (17). As can be seen, the percentage of total PRL that was phosphorylated in the secretion from the early pubescent tissue was twice that from the sexually mature tissue (P < 0.001), showing for the first time that the degree of PRL phosphorylation is physiologically regulated in male animals.
Table 1.
Intracellular and secreted isoforms of PRL from early pubertal and mature male rats
| Isoform 1 | U-PRL | Phospho-PRL | Diphospho-PRL | Total Phosphorylated | |
|---|---|---|---|---|---|
| Early puberty | |||||
| Pituitary | 2.55±0.54 | 80.32±1.05 | 15.07±1.43 | 2.06±0.16 | |
| Secretion | 0 | 55.32±1.39* | 33.0±0.7* | 11.69±0.7* | 44.68±1.4* |
| Mature | |||||
| Pituitary | 6.82±2.69 | 83.25±3.38 | 9.9±0.69 | 0 | |
| Secretion | 0 | 77.82±1.61 | 19.99±0.97 | 2.19±0.69 | 22.18±1.66 |
Values are percentage of total ± SE and are derived from 6 animals in each category. Monophosphorylated (phospho) prolactin (PRL) is phosphorylated on the equivalent serine to that mimicked by an aspartate in S179D PRL. Diphosphorylated (diphospho) PRL contains this phosphoserine and an additional threonine phosphorylation (43). Isoform 1 is an as yet unidentified posttranslational modification with a less acidic isoelectric point than unmodified PRL (U-PRL).
P < 0.001 compared with mature male secretion.
Effect of the Recombinant PRL Preparations on Testosterone, LH, and Endogenous PRL Levels
At 3 wk of treatment, S179D PRL reduced and U-PRL increased the release of endogenous PRL [data not shown as previously published (51)]. By 5.5 wk, production of endogenous PRL normalized and was not different from control with either form of administered PRL [data not shown as previously published (51)]. At the same time, testosterone was reduced at both time points (51). For the present study, we wanted to further analyze a time point where endogenous PRL secretion had normalized, but one where there was still a likelihood of determining the ongoing mechanism of the effect on testosterone production. Results of the rPRL assay at 4 wk showed levels of endogenous rPRL to be unchanged in all treatment groups (Table 2). As noted previously in MATERIALS AND METHODS, the administered human U-PRL and S179D PRL do not cross-react in the rPRL assay. The effect of the administered PRLs on circulating total testosterone after 4 wk is illustrated in Fig. 1. U-PRL was without effect, but total testosterone was 35% lower in animals treated with S179D PRL. Despite expected feedback from lower testosterone levels in the S179D PRL-treated group, LH levels were also unchanged as a result of treatment, regardless of the form of PRL used (Table 2). The level of LH measured was threefold the limit of detection in the assay and hence it should have been possible to measure a decrease in response to S179D PRL and, even more readily, to measure an increase in response to lowered testosterone levels. Thus the 4-wk time point was chosen for further analysis because the lack of effect on pituitary hormones allowed us to eliminate an acute hypothalamic or pituitary mechanism for lowered circulating testosterone.
Fig. 1.
Circulating total testosterone. Animals were treated with S179D prolactin (PRL) or unmodified PRL (U-PRL) for 4 wk. CTRL, control. **P < 0.01 (ANOVA followed by Tukey-Kramer multiple comparisons posttest).
qPCR Analysis of Steroidogenic Proteins
Because no changes were detected in levels of endogenous rPRL and rLH, the reduction in total testosterone may have been the result of a direct effect of S179D PRL on the testis and more specifically the interstitial Leydig cells responsible for producing testosterone. IHC staining for PRL receptors confirmed the findings of others (19, 22, 25), demonstrating that PRL receptors were expressed on Leydig cells and stages of germ cell development ranging from primary spermatogonia to elongating spermatids (data not shown). Therefore, qPCR was performed to assess the effects of treatment with each PRL on key components of the biosynthetic pathway. qPCR analysis of the LHR showed the expression of this receptor to be upregulated in response to treatment with both U-PRL and S179D PRL (Fig. 2A). At the same time, analysis of StAR protein and 3β-HSD revealed statistically significant decreases when treated with either U-PRL or S179D PRL (Fig. 2, B and C, respectively).
Fig. 2.
Quantitative PCR (qPCR) of luteinizing hormone receptor (LHR), steroidogenic acute regulatory (StAR) protein, and 3β-hydroxysteroid dehydrogenase (3β-HSD) mRNA expression. A: LHR. B: StAR protein. C: 3β-HSD. Value of the CTRL group ββCt (where Ct is threshold cycle) was normalized to 1. GAPDH was used to normalize per cell. **P < 0.01; *P < 0.05; ***P < 0.001 (ANOVA followed by Tukey-Kramer multiple comparisons posttest).
TUNEL-IHC Analysis
Preliminary histological morphometric analyses on hematoxylin and eosin-stained sections from the 3- and 5.5-wk-treated animals suggested a decrease in Leydig cell number in response to S179D PRL (data not shown). To test whether Leydig cells were actually reduced in number in the full analysis of the 4-wk samples, Leydig cells were identified by staining for 3β-HSD and the number of TUNEL-positive Leydig cells was assessed. This assessment was of advanced apoptosis where there was no clear distinction between the nuclear and cytoplasmic compartments, and hence merging of the FITC (TUNEL) and Texas red (3β-HSD) channels caused apoptotic Leydig cells to have yellow regions. As shown in Table 3, S179D PRL caused a statistically significant increase in the number of apoptotic Leydig cells. In addition, S179D PRL was shown to decrease the total number of Leydig cells, as assessed by the number of cells staining positively for 3β-HSD (Table 3), a result consistent with the TUNEL analysis. U-PRL, by comparison, had no significant effect on either apoptosis or the number of Leydig cells. Because U-PRL produced an equivalent decrease in the amount of 3β-HSD per cell (see below), the reduced number of Leydig cells in response to S179D PRL was not due to an inability to recognize them.
Table 3.
Apoptotic cells after 4 wk of treatment
| Control | U-PRL | S179D PRL | |
|---|---|---|---|
| Apoptotic Leydig cells | 2±0.88 (28) | 4±1.09 (52) | 10±1.6*(115) |
| Number of Leydig cells | 296±13.4 (3,551) | 334±35.8 (4,002) | 234±12.2*(2,803) |
| Apoptotic primary spermatogonia | 1,070±7.6 | 1,075±66.7 | 1,242±13.4* |
| Apoptotic elongating spermatids | 283±7.6 | 98±5.6† | 250±15.5 |
Values are means ± SE and are given as cells per animal sampling. Numbers in parentheses are total counted in 3 analyses by examination of 1,080 images.
P < 0.05 vs. control;
P < 0.003 vs. control.
IHC staining for Fas expression showed S179D PRL to markedly increase fluorescence intensity (Fig. 3C), whereas U-PRL had only a minor effect (Fig. 3B). Because Fas is not functional without Fas ligand, we also stained for expression of the ligand. S179D PRL markedly increased expression of Fas ligand (Fig. 3F), but U-PRL had no effect (Fig. 3E). The images presented are representative of 40 sections per group, not subjected to morphometric analysis because the result was so conclusive.
Fig. 3.
Analysis of Fas and Fas ligand (FasL) expression. Confocal montage images of testis sections stained with anti-Fas (A–C) or anti-FasL (D–F) (green color). A and D: controls. B and E: treatment with U-PRL. C and F: treatment with S179D PRL. 4′,6-Diamidino-2-phenylindole (DAPI) was used as a nuclear stain (blue color). Forty sections were examined for each treatment. Scale bar = 50 μm.
Morphometric analysis also showed that S179D PRL increased the number of apoptotic primary spermatogonia, whereas U-PRL was without effect (Table 3). At the same time, U-PRL decreased the number of apoptotic elongating spermatids, whereas S179D PRL was without effect (Table 3).
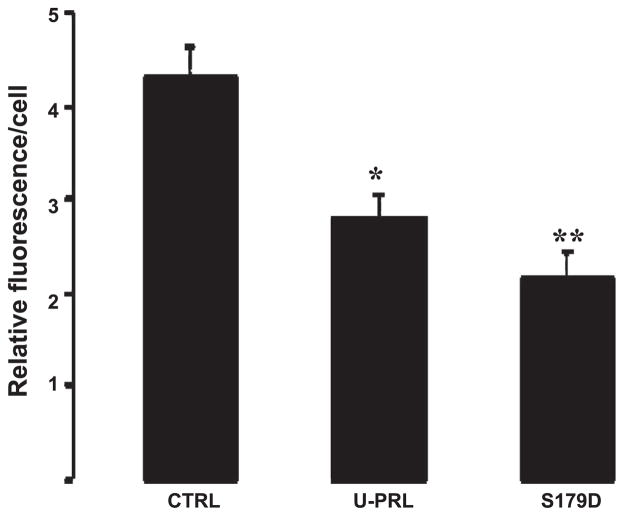
Quantification of the immunofluorescence intensity of 3β-HSD per cell revealed both U-PRL and S179D PRL to cause a statistically significant decrease in fluorescence compared with the CTRL group (Fig. 4), an effect similar to that observed for mRNA expression (Fig. 2C).
Fig. 4.
Quantification of 3β-HSD immunofluorescence intensity (1,080 images were examined). *P < 0.01; **P < 0.001 compared with control (ANOVA followed by Tukey-Kramer multiple comparisons posttest).
qPCR for Long and Short PRL Receptors
Normal rat tissues only express a long form and one short form of the PRL receptor (35). qPCR was used to quantify the relative amounts of mRNA for the long and short PRL receptor. Figure 5 shows that treatment with S179D PRL increased the ratio of short to long receptor in the testis, whereas U-PRL was without significant effect.
Fig. 5.
Effect of treatment on the ratio of short to long PRL receptors. qPCR analysis with normalization to GAPDH. **Different from control with P < 0.002 (Mann-Whitney unpaired nonparametric test).
DISCUSSION
The results presented show that the ratio of U-PRL to phosphorylated PRL changes between early puberty, prepuberty, and adulthood in the male rat and hence that the degree of phosphorylation of released PRL is physiologically regulated in males, as it is in females (20, 21). The inference therefore is that the different forms of PRL have different functions and hence that the form of PRL administered may differentially affect testicular function. To our knowledge, all previous analyses of the role of PRL in testicular function have used preparations of PRL extracted from pituitaries and hence these preparations have contained variable ratios of unmodified to phosphorylated hormone, depending on the method of extraction and physiological status of the donor animals. In this study, we have separately analyzed the effect of U-PRL and phosphorylated PRL by the administration of each to normal adult males. This administered PRL was over and above the animal’s own PRL and in the short term (3 wk) affected endogenous PRL secretion (present study and Ref. 51). However, by 4 wk of treatment, endogenous PRL release had normalized. The amount of administered PRL results in a constant circulating concentration of ~50 ng/ml (8, 29), an amount that would be considered hyperprolactinemic for a male animal and a concentration likely to affect gonadotrope function. However, at the 4 wk time point, there was no evidence of altered LH release. This was not surprising for the U-PRL-treated animals because testosterone was normal; however, it was surprising for those treated with S179D PRL because testosterone was lower and one would have expected this to increase LH secretion to compensate. We have no explanation of this at present, although we speculate that the phosphomimic, S179D PRL, can alter the set point of feedback regulation at the hypothalamus or pituitary level to allow for lower testosterone levels. This may be one reason for the higher proportion of phosphorylated PRL in immature males. Saidi et al. (37) and Huang et al. (23) have also documented states of elevated PRL and suppressed testosterone associated with unchanged LH levels. Also, Klemcke and Bartke (27) have reported chronic hyperprolactinemia in mice to significantly elevate LH with unchanged testosterone. Thus the relationships among PRL, LH, and testosterone are clearly influenced by additional factors, one of which may be the form of PRL elevated.
With normal LH and endogenous PRL levels, both forms of administered PRL reduced expression of 3β-HSD and StAR at the mRNA level. The effect on 3β-HSD was confirmed at the protein level by IHC and morphometric analysis. 3β-HSD is the rate-limiting enzyme in the production of testosterone, but the degree of change was apparently insufficient to significantly affect testosterone production by itself because U-PRL, which was similarly effective on these parameters, did not affect circulating total testosterone levels. Although the variability in basal levels of testosterone is such that small changes are hard to discern (9), the lack of change in testosterone with U-PRL and lowering of testosterone with S179D PRL have been observed in two separate studies and with all time points within those studies (Ref. 51 and present work). Because all of the qPCR results and 3β-HSD IHC fluorescence were normalized, they represent expression per cell. Only when reduced expression per cell was combined with a reduced number of cells was there a significant effect on circulating testosterone. Thus S179D PRL reduced the number of Leydig cells by ~20% in addition to reducing 3β-HSD and StAR per cell, thereby producing a distinct and negative effect on circulating testosterone.
The relatively small number of Leydig cells observed to be undergoing apoptosis at the 4-wk time point is most likely the result of both of the criterion chosen, since only advanced apoptosis was recorded, and timing, since preliminary experiments had also suggested increased apoptosis at the earlier 3-wk time point. The cumulative effect at 4 wk was sufficient to produce a reduction in the number of 3β-HSD-positive cells. Because Leydig cell number has been considered stable in the adult (39), any significant apoptosis of these cells, even if minor, is critical. A similar result was described by Taylor et al. (39) and Gao et al. (14, 15) studying the mechanism by which ethane-1,2-dimethane sulfonate and elevated corticosterone-lowered testosterone levels. U-PRL did not have a significant effect on the number of cells staining positively for 3β-HSD, but there was a trend toward an increase. This could be indicative of the role that the unmodified form of the hormone has as a growth or anti-apoptotic factor (30, 46) and perhaps could explain how U-PRL could decrease 3β-HSD expression when normalized per cell but have no significant effect on circulating testosterone. In keeping with this idea is the evidence for an anti-apoptotic effect of U-PRL on elongating spermatids. As described by others (19, 22, 25), we also determined that primary spermatogonia and elongating spermatids expressed PRL receptors and hence this may be a direct effect of U-PRL. Treatment with S179D PRL also promoted apoptosis of primary spermatogonia; however, because of the effect on circulating testosterone, it is unclear whether this is a direct or indirect effect. A higher proportion of phosphorylated PRL in the immature animals may promote apoptosis or inhibit the anti-apoptotic effect of U-PRL on Leydig cells and primary spermatogonia, thereby keeping function low until the full onset of puberty and a reduction in the degree of PRL phosphorylation with maturity.
Because the majority of previous studies conducted on Leydig cell apoptosis have shown the process to most commonly occur via the extrinsic pathway (e.g., Refs. 12, 15), IHC analysis of Fas and Fas ligand was performed. Staining for both Fas and Fas ligand was increased in the testicular interstitium in response to S179D PRL. U-PRL produced a small increase in Fas staining but had no effect on staining for Fas ligand, and both are necessary to induce apoptosis. This observation lends support to the observed increase in Leydig cell apoptosis by S179D PRL.
The LHR is only expressed on Leydig cells in the testis (reviewed in Ref. 11). Both forms of PRL increased expression of the LHR, a result consistent with positive effects of PRL administration in prepubescent and hypophysectomized animals (2, 10, 32, 36, 54) because this would be expected to increase sensitivity to LH. Making the assumption that the mRNA was translated into protein displayed on the plasma membrane, one might also have expected this to normalize 3β-HSD by increasing sensitivity to LH when LH levels were normal in the adult animals, but this was not the case in the presence of either form of PRL. However, the positive effect in prepubescent animals was insufficient to elevate testosterone to normal adult levels (10); therefore, a positive effect of administered PRL (which mainly contains U-PRL because it is an extract of cells and not secretion) may only manifest itself when it is counteracting the effects of high endogenous phosphorylated PRL or when there is essentially no endogenous PRL.
Although S179D PRL and U-PRL can signal through the same receptor (7), extended exposure to S179D PRL has been shown to alter splicing of the PRL receptor mRNA in other tissues and species to produce more of the short PRL receptor forms (40, 47–49). The same is true in the rat testis. Increased expression of the short PRL receptor(s) and signaling therefrom in other cell types and species have been shown to result in increased apoptosis (40, 49), and the same may be true for Leydig cells. However, given that PRL receptors are not confined to Leydig cells in the testis, it will not be possible to be definitive in this regard until antibodies specific to the different receptor forms become available. Increased apoptosis of primary spermatogonia could be a direct or indirect effect, the latter via reduced testosterone. The anti-apoptotic effect of U-PRL on elongated spermatids may be direct because testosterone was unchanged with this treatment. The net effect of hyperprolactinemia is therefore complex and dependent on the ratio of the two major forms of PRL.
The findings of this study are consistent with a direct effect of both recombinant forms of PRL on the testis in vivo. The negative effect of hyperprolactinemia on testosterone production in adult animals therefore is in part direct and not only a consequence of reduced LH secretion. This conclusion is consistent with the work of Huang et al. (24). These investigators set out to explain the apparent paradox that PRL decreased testosterone in vivo while increasing its production from isolated Leydig cells in vitro, a paradox they were able to attribute to the intermediary role of interstitial macrophages and TNF-α production (24). Thus it is possible that both PRLs exert their common effects on testosterone biosynthesis via the testicular macrophage but their differential effects on apoptosis via PRL receptors on Leydig cells, spermatogonia, and elongated spermatids.
Acknowledgments
We thank Sally Scott for expert animal care.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-61005 to A. M. Walker, with a minority supplement to V. L. Williams, and by a grant from the Prostate Cancer Foundation.
References
- 1.Bartke A. Prolactin in the male: 25 years later. J Androl. 2004;25:661–666. doi: 10.1002/j.1939-4640.2004.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 2.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 3.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 4.Chen TJ, Kuo CB, Tsai KF, Liu JW, Chen DY, Walker AM. Development of recombinant human prolactin receptor antagonists by molecular mimicry of the phosphorylated hormone. Endocrinology. 1998;139:609–616. doi: 10.1210/endo.139.2.5758. [DOI] [PubMed] [Google Scholar]
- 5.Cheung CY. Prolactin suppresses luteinizing hormone secretion, and pituitary responsiveness to luteinizing hormone-releasing hormone by a direct action at the anterior pituitary. Endocrinology. 1983;113:632–638. doi: 10.1210/endo-113-2-632. [DOI] [PubMed] [Google Scholar]
- 6.Chomzynski P, Mackey K. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- 7.Coss D, Kuo CY, Yang L, Ingleton P, Luben R, Walker AM. Dissociation of Janus kinase 2 and signal transducer and activator of transcription 5 activation after treatment of Nb2 cells with a molecular mimic of phosphorylated prolactin. Endocrinology. 1999;140:5087–5094. doi: 10.1210/endo.140.11.7104. [DOI] [PubMed] [Google Scholar]
- 8.Coss D, Yang L, Kuo CB, Xu X, Luben RA, Walker AM. Effects of prolactin on osteoblast alkaline phosphatase, and bone formation in the developing rat. Am J Physiol Endocrinol Metab. 2000;279:E1216–E1225. doi: 10.1152/ajpendo.2000.279.6.E1216. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins C. Endocrine signaling, and male reproduction. Biol Reprod. 1981;24:1–2. doi: 10.1095/biolreprod24.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Dombrowicz D, Sente B, Closset J, Hennen G. Dose-dependent effects of human prolactin on the immature hypophysectomized rat testis. Endocrinology. 1992;130:695–700. doi: 10.1210/endo.130.2.1733717. [DOI] [PubMed] [Google Scholar]
- 11.Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol. 1998;60:461–490. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- 12.Francavilla S, D’Abrizio P, Rucci N, Silvano G, Properzi G, Straface E, Cordeschi G, Necozione S, Gnessi L, Arizzi M, Ulisse S. Fas and Fas ligand expression in fetal and adult human testis with normal or deranged spermatogenesis. J Clin Endocrinol Metab. 2000;85:2692–2700. doi: 10.1210/jcem.85.8.6723. [DOI] [PubMed] [Google Scholar]
- 13.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 14.Gao HB, Tong MH, Hu YQ, You HY, Guo QS, Ge R, Hardy MP. Glucocorticoid induces apoptosis in rat Leydig cells. Endocrinology. 2002;143:130–138. doi: 10.1210/endo.143.1.8604. [DOI] [PubMed] [Google Scholar]
- 15.Gao HB, Tong MH, Hu YQ, You HY, Guo QS, Ge R, Hardy MP. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol. 2003;199:153–163. doi: 10.1016/s0303-7207(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 16.Garcia A, Herbon L, Barkan A, Papavasiliou S, Marshall JC. Hyperprolactinemia inhibits gonadotropin-releasing hormone (GnRH) stimulation of the number of pituitary GnRH receptors. Endocrinology. 1985;117:954–959. doi: 10.1210/endo-117-3-954. [DOI] [PubMed] [Google Scholar]
- 17.Greenan JR, Balden E, Ho TWC, Walker AM. Biosynthesis of the secreted 24 kd isoforms of prolactin. Endocrinology. 1989;125:2041–2048. doi: 10.1210/endo-125-4-2041. [DOI] [PubMed] [Google Scholar]
- 18.Gregory SJ, Townsend J, McNeilly AS, Tortonese DJ. Effects of prolactin on the luteinizing hormone response to gonadotropin-releasing hormone in primary pituitary cell cultures during the ovine annual reproductive cycle. Biol Reprod. 2004;70:1299–1305. doi: 10.1095/biolreprod.103.022806. [DOI] [PubMed] [Google Scholar]
- 19.Hair WM, Gubbay O, Jabbour HN, Lincoln GA. Prolactin receptor expression in human testis, and accessory tissues: localization and function. Mol Human Reprod. 2002;3:606–611. doi: 10.1093/molehr/8.7.606. [DOI] [PubMed] [Google Scholar]
- 20.Ho TWC, Kawaminami M, Walker AM. Secretion of phosphorylated, and nonphosphorylated monomer prolactin isoforms during rat pregnancy and pseudopregnancy. Endocrine. 1993;1:435–439. [Google Scholar]
- 21.Ho TWC, Leong FS, Olaso CH, Walker AM. Secretion of specific nonphosphorylated, and phosphorylated rat prolactin isoforms at different stages of the estrous cycle. Neuroendocrinology. 1993;58:160–165. doi: 10.1159/000126528. [DOI] [PubMed] [Google Scholar]
- 22.Hondo E, Kurohmaru M, Sakai S, Ogawa K, Hayashi Y. Prolactin receptor expression in rat spermatogenic cells. Biol Reprod. 1995;52:1284–1290. doi: 10.1095/biolreprod52.6.1284. [DOI] [PubMed] [Google Scholar]
- 23.Huang WJ, Yeh JY, Tsai SC, Chiao YC, Chen JJ, Lu CC, Hwang SW, Wang SW, Chang LS, Wang PS. Regulation of testosterone secretion by prolactin in male rats. J Cell Biochem. 1999;74:111–118. [PubMed] [Google Scholar]
- 24.Huang WJ, Yeh JY, Kan SF, Chang LS, Wang PS. Role of testicular interstitial macrophages in regulating testosterone release in hyperprolactinemia. J Cell Biochem. 2003;88:766–773. doi: 10.1002/jcb.10412. [DOI] [PubMed] [Google Scholar]
- 25.Jabbour HN, Lincoln GA. Prolactin receptor expression in the testis of the ram: localization, functional activation, and the influence of gonadotrophins. Mol Cell Endocrinol. 1999;148:151–161. doi: 10.1016/s0303-7207(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 26.Johnson TE, Vue M, Brekhus S, Khong A, Ho TW, Walker AM. Unmodified prolactin (PRL) promotes PRL secretion and acidophil hypertrophy and is associated with pituitary hyperplasia in female rats. Endocrine. 2003;20:101–109. doi: 10.1385/ENDO:20:1-2:101. [DOI] [PubMed] [Google Scholar]
- 27.Klemcke HG, Bartke A. Effects of chronic hyperprolactinemia in mice on plasma gonadotropin concentrations, and testicular human chorionic gonadotropin binding sites. Endocrinology. 1981;108:1763–1768. doi: 10.1210/endo-108-5-1763. [DOI] [PubMed] [Google Scholar]
- 28.Krown KA, Wang YF, Ho TW, Kelly PA, Walker AM. Prolactin isoform 2 as an autocrine growth factor for GH3 cells. Endocrinology. 1992;131:595–602. doi: 10.1210/endo.131.2.1639009. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CB, Wu W, Xu X, Yang L, Chen C, Coss D, Birdsall B, Nasseri D, Walker AM. Pseudophosphorylated prolactin (S179DPRL) inhibits growth, and promotes β-casein gene expression in the rat mammary gland. Cell Tissue Res. 2002;309:429–437. doi: 10.1007/s00441-002-0598-8. [DOI] [PubMed] [Google Scholar]
- 30.Leff MA, Buckley DJ, Krumenacker JS, Reed JC, Miyashita T, Buckley AR. Rapid modulation of the apoptosis regulatory genes, bcl-2, and bax by prolactin in rat Nb2 lymphoma cells. Endocrinology. 1996;137:5456–5462. doi: 10.1210/endo.137.12.8940371. [DOI] [PubMed] [Google Scholar]
- 31.Lorenson MY, Walker AM. Structure function relationships in prolactin. In: Horseman ND, editor. Prolactin. Norwell, MA: Kluwer Academic; 2000. pp. 188–217. [Google Scholar]
- 32.Manna PR, El-Hefnawy T, Kero J, Huhtaniemi IT. Biphasic action of prolactin in the regulation of murine Leydig tumor cell functions. Endocrinology. 2001;142:308–318. doi: 10.1210/endo.142.1.7899. [DOI] [PubMed] [Google Scholar]
- 33.Maran RRM, Arunakaran J, Aruldhas MM. Prolactin, and Leydig cells: biphasic effects of prolactin on LH-, T3-, and GH-induced testosterone/oestradiol secretion by Leydig cells in pubertal rats. Int J Androl. 2001;24:48–55. doi: 10.1046/j.1365-2605.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 34.McNeilly AS, Sharpe RM, Fraser HM. Increased sensitivity to the negative feedback effects of testosterone induced by hyperprolactinemia in the adult male rat. Endocrinology. 1983;112:22–28. doi: 10.1210/endo-112-1-22. [DOI] [PubMed] [Google Scholar]
- 35.Nagano M, Kelly PA. Tissue distribution, and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J Biol Chem. 1994;269:13337–13345. [PubMed] [Google Scholar]
- 36.Purvis K, Clausen OPF, Olsen A, Hang F, Hansson V. Prolactin and Leydig cell responsiveness to LH/hCG. Arch Androl. 1979;3:219–230. doi: 10.3109/01485017908988408. [DOI] [PubMed] [Google Scholar]
- 37.Saidi K, Wenn RV, Sharif F. Bromocriptine for male infertility. Lancet. 1977;29:250–251. doi: 10.1016/s0140-6736(77)91038-8. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder MD, Brockman JL, Walker AM, Schuler LA. Inhibition of prolactin (PRL)-induced proliferative signals in breast cancer cells by a molecular mimic of phosphorylated PRL, S179D PRL. Endocrinology. 2003;144:5399–5407. doi: 10.1210/en.2003-0826. [DOI] [PubMed] [Google Scholar]
- 39.Taylor MF, de Boer-Brouwer M, Woolveridge I, Teerds KJ, Morris ID. Leydig cell apoptosis after the administration of ethane dimethansulfonate to the adult male rat is a Fas-mediated process. Endocrinology. 1999;140:3797–3804. doi: 10.1210/endo.140.8.6919. [DOI] [PubMed] [Google Scholar]
- 40.Ueda EK, Lo HL, Bartolini P Walker AMS179D. Prolactin uses the extrinsic pathway, and mitogen-activated protein kinase signaling to induce apoptosis in human endothelial cells. Endocrinology. 2006;147:4627–4637. doi: 10.1210/en.2006-0348. [DOI] [PubMed] [Google Scholar]
- 41.Ueda E, Ozerdem U, Chen YH, Yao M, Huang KT, Sun H, Martins-Green M, Bartolini P, Walker AM. A molecular mimic demonstrates that phosphorylated human prolactin is a potent anti-angiogenic hormone. Endo Relat Cancer. 2006;13:95–111. doi: 10.1677/erc.1.01076. [DOI] [PubMed] [Google Scholar]
- 42.Waeber C, Reymond O, Reymond M, Lemarchand-Beraud T. Effects of hyper-, and hypoprolactinemia on gonadotropin secretion, rat testicular luteinizing hormone/human chorionic gonadotropin receptors and testosterone production by isolated Leydig cells. Biol Reprod. 1983;28:167–177. doi: 10.1095/biolreprod28.1.167. [DOI] [PubMed] [Google Scholar]
- 43.Walker AM. S179D prolactin: antagonistic agony! Mol Cell Endocrinol. 2007;276:1–9. doi: 10.1016/j.mce.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YF, Walker AM. Dephosphorylation of standard prolactin produces a more biologically active molecule. Evidence for antagonism between non-phosphorylated and phosphorylated prolactin in the stimulation of Nb2 cell proliferation. Endocrinology. 1993;133:2156–2160. doi: 10.1210/endo.133.5.8404666. [DOI] [PubMed] [Google Scholar]
- 45.Weiss-Messer E, Ber R, Barkey RJ. Prolactin MA10 Leydig cell steroidogenesis: biphasic effects of prolactin and signal transduction. Endocrinology. 1996;137:5509–5518. doi: 10.1210/endo.137.12.8940378. [DOI] [PubMed] [Google Scholar]
- 46.Witorsch RJ, Day EB, Lavoie HA, Hashemi N, Taylor JK. Comparison of glucocorticoid-induced effects in prolactin-dependent, and autonomous rat Nb2 lymphoma cells. Proc Soc Exp Biol Med. 1993;203:454–460. doi: 10.3181/00379727-203-43622. [DOI] [PubMed] [Google Scholar]
- 47.Wu W, Coss D, Lorenson MY, Kuo CB, Xu X, Walker AM. Different biological effects of unmodified prolactin, and a molecular mimic of phosphorylated prolactin involve different signaling pathways. Biochemistry. 2003;42:7561–7570. doi: 10.1021/bi034217s. [DOI] [PubMed] [Google Scholar]
- 48.Wu W, Ginsburg E, Vonderhaar BK, Walker AM. S179D prolactin increases vitamin D receptor, and p21 through upregulation of short 1b prolactin receptor in human prostate cancer cells. Cancer Res. 2005;65:7509–7515. doi: 10.1158/0008-5472.CAN-04-3350. [DOI] [PubMed] [Google Scholar]
- 49.Wu W, Chen YH, Ueda E, Tan D, Bartolini P, Walker AM. Different forms of prolactin have opposing effects on the expression of cell cycle regulatory proteins in differentiated mammary epithelial cells. Oncol Res. 2006;16:75–84. doi: 10.3727/000000006783981233. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Kreye E, Kuo CB, Walker AM. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence, and size when D.U145 human prostate cancer cells were grown in nude mice. Cancer Res. 2001;61:6098–6104. [PubMed] [Google Scholar]
- 51.Xu X, Wu W, Williams V, Khong A, Chen YH, Deng C, Walker AM. Opposite effects of unmodified prolactin, and a molecular mimic of phosphorylated prolactin on morphology and the expression of prostate specific genes in the normal rat prostate. Prostate. 2003;54:25–33. doi: 10.1002/pros.10168. [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Kuo CB, Liu Y, Coss D, Xu X, Chen C, Oster-Granite ML, Walker AM. Administration of unmodified prolactin (U-PRL) and a molecular mimic of phosphorylated prolactin (PP-PRL) during rat pregnancy provides evidence that the U-PRL:PP-PRL ratio is crucial to the normal development of pup tissues. J Endocrinol. 2001;168:227–238. doi: 10.1677/joe.0.1680227. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Lii S, Kuo B, Buckley A, Buckley D, Chen C, Xu X, Coss D, Walker AM. Maternal prolactin composition can permanently affect epidermal γδT cell function in the offspring. Dev Comp Immunol. 2002;26:849–860. doi: 10.1016/s0145-305x(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 54.Zipf WB, Payne AH, Kelch RP. Prolactin, growth hormone, and luteinizing hormone in the maintenance of testicular luteinizing receptors. Endocrinology. 1978;103:595–600. doi: 10.1210/endo-103-2-595. [DOI] [PubMed] [Google Scholar]