Abstract
Background:
Recent limited evidence suggests that the use of processed electroencephalographic (EEG) monitor to guide anesthetic management may influence postoperative cognitive outcomes, however, the mechanism is unclear.
Methods:
This exploratory single center, randomized clinical trial included patients who were ≥ 65 years of age undergoing elective non-cardiac surgery. The study aimed to determine whether monitoring the brain using a processed EEG monitor reduced EEG suppression and subsequent postoperative delirium. The interventional group received processed EEG-guided anesthetic management to keep the Patient State Index (PSI) above 35 computed by the SEDline Brain Function Monitor while the standard care group was also monitored but the EEG data were blinded from the clinicians. The primary outcome was intraoperative EEG suppression. A secondary outcome was incident postoperative delirium during the first three days after surgery.
Results:
All outcomes were analyzed using the intention-to-treat paradigm. 204 patients with a mean age of 72 ± 5 years were studied. Minutes of EEG suppression adjusted by the length of surgery was found to be less for the interventional group than the standard care group (median, interquartile range 1.4% (5.0%) and 2.5% (10.4%); Hodges-Lehmann estimated median difference (95% CI) of −0.8% (−2.1%, −0.000009%). The effect of the intervention on EEG suppression differed for those with and without preoperative cognitive impairment (interaction P=0.01), with the estimated incidence rate ratio and 95% CI of 0.39 (0.33, 0.44) for those with preoperative cognitive impairment and 0.48 (0.44, 0.51) for those without preoperative cognitive impairment. The incidence of delirium was not found to be different between the interventional (17%) and the standard care groups (20%), risk ratio = 0.85, 95% confidence interval (CI) = (0.47, 1.5).
Conclusions:
The use of processed EEG to maintain the PSI >35 was associated with less time spent in intraoperative EEG suppression. Preoperative cognitive impairment was associated with greater percent of surgical time spent in EEG suppression. A larger prospective cohort study to include more cognitively vulnerable patients is necessary to show whether an intervention to reduce EEG suppression is efficacious in reducing postoperative delirium.
Introduction
Postoperative delirium is a common yet serious geriatric syndrome that affects 10–60% of patients after major surgery. 1 Delirium is an acute confusional state defined by alterations in attention, consciousness, and disorganized thinking, and associated with increased morbidity and mortality. 2,3
Although prior studies have identified numerous patient-related factors associated with postoperative delirium, its pathophysiology is incompletely understood. 4 Recently, it has been proposed that use of a processed electroencephalogram (EEG) monitor during surgery may be associated with a lower rate of postoperative delirium. 5,6 Several studies speculated that patients who were monitored with processed EEG may have a lighter anesthetic depth (1) as estimated by processed EEG, which may lead to lower rates of postoperative delirium. 5-8 Subsequent studies that examined processed EEG more closely reported patients with more EEG suppression during surgery were more likely to experience delirium. 9 A burst suppression pattern on EEG indicates a severe reduction in the brain’s neuronal activity and metabolic rate. Although several cohort studies showed that intraoperative EEG suppression was associated with postoperative delirium, 9,10 it is unclear whether the use of a processed EEG resulted in a reduction of postoperative delirium directly through a reduction in EEG suppression. Furthermore, it is unclear whether the occurrence of EEG suppression can be reduced by modulating anesthetic doses. Prior studies also did not consider whether preoperative cognitive status influences the effects of anesthetic on the brain.
EEG suppression can be identified in real time by inspecting the raw EEG waveforms, but that is labor intensive and not practical in a busy intraoperative environment. In contrast, most processed EEG monitors display a continuous dimensionless number 11 which is calculated ranging from 0, no brain activity, to 100, awake. 12 BIS values between 40 and 60 allow sufficient anesthesia for surgery and prevention of intraoperative awareness. 13 Our pilot work (unpublished) showed that when the PSI falls below a certain value, EEG suppression is frequently seen. Hence, a more pragmatic way to reduce EEG suppression is through monitoring this processed EEG value.
The aims of this exploratory randomized control trial were: 1) to determine whether the use of processed EEG monitor to guide anesthesiologists to keep the dimensionless processed EEG value above a specified value (interventional group) reduces intraoperative EEG suppression when compared to standard anesthetic care (standard care group), and 2) to determine if incident postoperative delirium during the first three days after surgery was reduced in the interventional group when compared with the standard care group. We hypothesized that the intervention reduced the likelihood of EEG suppression during surgery, and in turn reduced the likelihood of postoperative delirium.
Materials and Methods
Study Design and Participants
This was a single center, exploratory randomized clinical trial. Patients aged ≥ 65 years undergoing major elective, non-cardiac surgery at the University of California San Francisco Medical Center (San Francisco, California) were recruited. Inclusion criteria were fluency in English and an anticipated length of hospital stay of at least two days after surgery. Exclusion criteria were preoperative delirium, inability to perform neurocognitive testing, history of intraoperative recall, contraindication to receiving light anesthesia (such as those with a history of heart failure, coronary artery disease or substance abuse), or undergoing surgery of the brain. The University of California Human Research Protection Program approved this study (approval number 14-14273). All patients provided written, informed consent. Registration of the clinical trial occurred prior to the start of the trial and any patient enrollment undertaken. The trial was registered with clinicaltrials.gov (NCT01983384, initial release 10-29-2013, Principal Investigator Jacqueline M Leung).
Intervention
Patients in the interventional group received processed EEG-guided anesthetic management while the standard care group received routine anesthetic care without the use of a processed EEG. Anesthesiologists for patients assigned to the interventional group were instructed to keep the Patient State Index® (PSI) above 35. The SEDline Brain Function Monitor (Masimo, Inc., Irvine CA), a processed EEG monitor computes anesthetic depth from digital EEG waves using a proprietary algorithm and displays it as a dimensionless parameter called the PSI (ranging 0 to 100). 14 In our prior pilot study, visual inspection of EEG by trained neurologists determined that EEG suppression occurred in less than 10% of patients when the PSI was greater than 35. 15 Accordingly, anesthesiologists caring for patients in the interventional group were asked to keep the PSI >35 by reduction of the dose of anesthetic agents (typically volatile agents first followed by intravenous infusion of propofol), as clinically permitted. As the main goal of the study was to reduce EEG suppression, no additional upper limit was set for the PSI. We chose a PSI level to be the threshold for the intervention rather than using EEG suppression waveforms as the former required little training of anesthesia providers in a large department. Furthermore, the PSI is frequently used as an indirect marker of anesthetic depth. Patients in the standard care group were also monitored with the Sedline monitors but the screen of the monitor was masked so all EEG data were blinded. Thus, anesthesiologists assigned to patients in the standard care group used conventional methods (e.g. vital signs, end-tidal anesthetic agent concentrations, etc.) to manage anesthesia. At our institution, processed EEG was not used routinely, hence the group not receiving the monitor was considered standard care.
Randomization and blinding
Patients were assigned to study arm before surgery by a computerized random number generator (Randomization) using a 1:1 randomization ratio, a seed was not specified and blocks were not used in randomization. Randomization occurred after consent for study participation was obtained during the preoperative interview. Randomization was communicated to the anesthesia team before the patient was transported to the operating room. The research assistants who conducted the delirium assessments were blinded to the processed EEG group assignment.
Preoperative Health and Functioning
Cognitive impairment was measured preoperatively because persons with cognitive impairment have abnormalities of resting state cortical EEG rhythms related to atrophy of cortical gray matter and cognition, 16 there is also evidence that pathologic delta activity exists during slow-wave sleep in patients with dementia. 17 We assessed cognitive impairment using the original Telephone Interview for Cognitive Status (TICS) which is scored from 0 to 41 points. 15T The TICS was adapted from the Mini Mental State Examination for use either in person or over the phone. All subjects were given the TICS by phone approximately one week prior to surgery, a TICS score of 31 or less was used to classify patients as having cognitive impairment. 18 Patients’ preoperative physical conditions were measured using the American Society of Anesthesiologists (ASA) physical classification, and comorbidity was measured using the Charlson comorbidity index. 19 Patients’ demographics, alcohol use, dependence in activities of daily living, and instrumental activities of daily living were measured during the preoperative interview.
Anesthetic Management
In this clinical trial, patients received a balanced general anesthetic consisting of inhaled and intravenous agents. Patients undergoing open abdominal procedures typically also had an epidural catheter placed for use in administering postoperative analgesia. Patients who underwent major spine surgery typically received an intravenous based anesthetic supplemented by low dose volatile agents to facilitate neuromonitoring. The anesthetic agents commonly included intravenous infusion of propofol, fentanyl, and lidocaine, used as a multi-modal regimen. Anesthesiologists were requested to maintain patients’ arterial blood pressure using vasoactive agents, as was done customarily per clinical practice. Patients received mechanical ventilation to maintain normocarbia. Intraoperative warming devices were used to keep body temperature between 36-37 °C. Oxygen saturation was maintained at >95%.
Outcomes
Measurement of EEG suppression
The raw EEG data, PSI, and burst suppression ratio (BSR) were retrieved from the Sedline monitor. Detailed methods were described in our previous work. 15 The EEG recordings started at anesthesia induction and concluded at the end of surgery. All EEG data were edited to include only the period after induction of anesthesia until 15 minutes before surgery end. We determined the cumulative duration of EEG suppression in minutes by converting a machine-derived EEG Burst Suppression Ratio (BSR) into minutes of EEG suppression. For the primary analysis of EEG suppression, we adjusted the minutes spent in EEG suppression by dividing the minutes spent in EEG suppression by the minutes of surgery because surgery times varied greatly even for the same type of surgery. For the secondary analysis of EEG suppression, the dependent variable was minutes of EEG suppression because the regression model included logarithm of time spent in surgery as an independent variable.
Measurement of Postoperative Delirium
Incident delirium present in any of the first three postoperative days was the secondary outcome. Delirium was measured preoperatively and daily on the first three postoperative days by trained research assistants using the Confusion Assessment Method (CAM), a screening instrument based on the Diagnostic and Statistical Manual-III-R criteria and was validated by health care providers. 20 Patients were considered to be delirious if the CAM assessment revealed acute onset or fluctuating course of mental status change, inattention, and either disorganized thinking or altered level of consciousness. If a CAM assessment was missing for any of the three days post-surgery, delirium was determined from the patient’s medical record in accordance with the chart-based CAM delirium identification method developed by Inouye et al. 21,22
In addition to measuring incident postoperative delirium on any of the first three days, we also measured the severity of delirium using the Memorial Delirium Assessment Scale (MDAS). 23
Measurement of Adherence to study protocol
We measured anesthesiologists’ perceived barrier to adhering to the assigned PSI range in the interventional group by a short self-report at the end of each surgery. The questions investigated whether the anesthesiologist had difficulty attaining the level of anesthetic depth as specified in the protocol and reasons why, such as hemodynamic instability or somatic responses such as patient movement, or patient-ventilator asynchrony, etc.
Measurement of Anesthetic Doses
In order to determine whether the intervention was associated with a reduction in doses of anesthetic administered, we calculated the cumulative doses of anesthetics and vasoactive agents which were abstracted from the electronic intraoperative record. End-tidal anesthetic concentration (ETAC) measurements of volatile agents were converted into age-adjusted minimum alveolar concentration (aaMAC) equivalents. 24
Measurement of Other Outcomes
Because light anesthetic depth may potentially increase the autonomic response, we recorded autonomic and somatic responses as estimates of depth of anesthesia. Details are described in the appendix. Other postoperative outcomes were recorded using pre-defined criteria described in our previous studies. 25 Length of hospital stay was also measured.
Statistical analysis and sample size calculation
All primary and secondary outcomes were analyzed according to the intention-to-treat paradigm. Plots were constructed to assess within-group distributions of variables to check for non-normality of continuous-valued variables. Variables deemed to be not normally distributed via visual inspection were analyzed using non-parametric tests.
Assessment of differences in preoperative patient related characteristics between the interventional and standard care groups were determined by computing standardized difference which is the difference between means or proportions divided by the pooled standard deviation. An absolute standardized difference of greater than or equal to 0.1 is often used to indicate non-trivial imbalance at baseline. The median and interquartile range of cumulative dose of intraoperative anesthetic drugs were computed for the interventional and the standard care groups. Differences in median doses were assessed using the Hodges-Lehman estimator and 95% confidence interval.
To assess the effect of the intervention on the primary outcome, EEG suppression, we estimated the median difference between groups in minutes spent in EEG suppression adjusted by minutes spent in surgery using the Hodges-Lehman estimator and 95% confidence interval. We adjusted for baseline differences between the intervention and standard care groups in preoperative cognitive impairment with the Stratified Wilcoxon Rank Sum test.
We evaluated whether the effect of the intervention in reducing in EEG suppression differed for those with and without preoperative cognitive impairment by computing a a Poisson regression model in which the dependent variable was minutes spent in EEG suppression and the independent variables were study group, preoperative cognitive impairment, the interaction between study groups and preoperative cognitive impairment and the logarithm of minutes spent in surgery.
For the secondary outcome, incident delirium, we computed the incidence of delirium occurring on any of the three postoperative days for each group and computed the relative risk and the 95% confidence interval.
We planned for the study to have sufficient power to detect a 50% difference in median adjusted minutes spent in EEG suppression between the treatment groups. We used G*Power 26 to estimate that a sample size of 99 per group (total 198) would provide greater than 80% power at the 0.05 significance level to detect at least a 50% difference in median adjusted minutes spent in EEG suppression using the Wilcoxon rank sum test. The values used to compute the power analysis were informed by pilot data in which the interventional group spent less percent time in EEG suppression than the control group (median, IQR 1.2% (5.4%) and 2.8% (13%). We planned for the study to have sufficient power to detect an absolute difference of at least 15% in incident delirium between the interventional and the standard care group. We used G*Power to estimate that a sample size of 99 subjects per group gave greater than 80% power at the 0.05 significance level. The values used to compute the sample size were informed by pilot data for which we found the incidence of delirium was 13% for the interventional group and 30% for the standard care group. An additional three subjects per group were recruited to address potential attrition after consent.
Data Safety Monitoring Board
We formed a Data and Safety Monitoring Board (DSMB) to monitor participant safety, data quality and to evaluate the progress of the study. Specific operating procedures were developed to track the safety data, design methods for measurement, and implement plan for adverse events reporting, and stopping rules. Details are shown in the appendix.
Results
Patient Recruitment
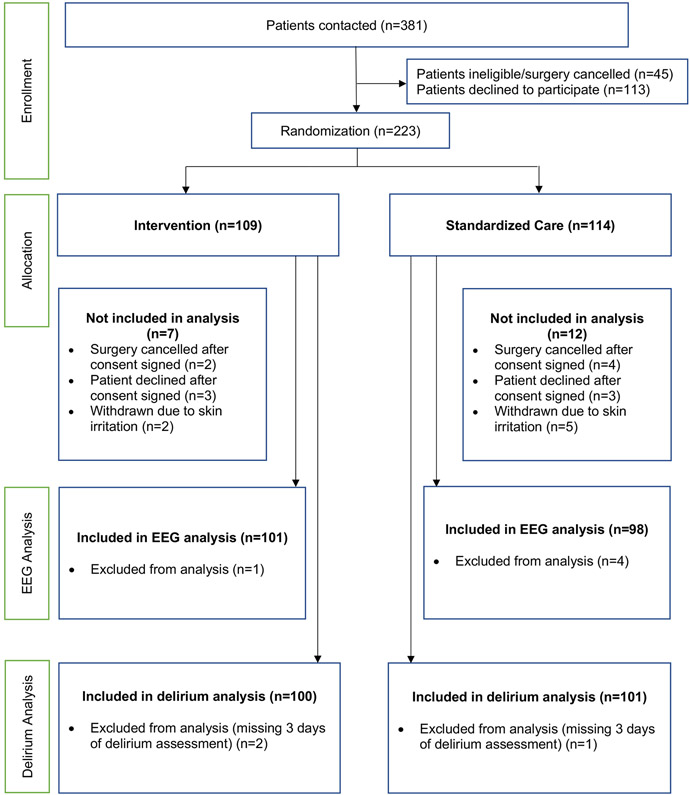
The study was conducted between June 2015 and September 2017. Overall, 204 patients were included in the intention-to-treat paradigm with 102 patients receiving processed-EEG guided anesthetic management and 102 patients receiving standard anesthetic management (Figure 1). Preoperative demographic and surgical variables for each interventional group are shown in table 1. Characteristics were not found to be different between the interventional and standard care groups with the exception of preoperative cognitive impairment where the standardized difference between the interventional and standard care groups exceeds 0.10, suggesting an imbalance between groups (Table 1).
Fig. 1.
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram depicting patient recruitment scheme for this clinical trial.
Table 1.
Comparison of patient and surgical characteristics between treatment groups
| Variable | Intervention (n=102) |
Standard Care (n=102) |
Difference Score |
|---|---|---|---|
| Age, mean ± SD, y | 72.1 ± 5.6 | 71.8 ± 5.3 | 0.06 |
| Sex, women | 53 (52.0%) | 53 (52.0%) | 0 |
| Race, white | 90 (88.2%) | 92 (90.2%) | −0.06 |
| Dependence in at least 1 of 5 ADLs | 23 (22.5%) | 26 (25.5%) | −0.07 |
| Dependence in at least 1 of 7 IADLs | 53 (52.0%) | 57 (55.9%) | −0.08 |
| Preoperative GDS >6 | 14 (13.7%) | 16 (15.7%) | −0.06 |
| Preoperative cognitive impairment (TICS score ≤ 31) | 13 (13%) | 8 (8%) | 0.16 |
| History of CNS disorder, yes | 44 (43.1%) | 40 (39.2%) | 0.08 |
| Charlson comorbidity index | |||
| 0-2 | 79 | 83 | −0.09 |
| 3+ | 23 | 19 | −0.09 |
| ASA Classification | |||
| I or II | 47 | 48 | 0.02 |
| III or IV | 55 | 54 | 0.02 |
| Type of surgery | |||
| Major spine procedures | 68 | 73 | −0.10 |
| Non-Spine procedures | 33 | 29 | 0.09 |
| Surgery duration (minutes), mean ± SD | 366 ± 134 | 358 ± 144 | 0.06 |
ADL = Activities of daily living
IADL = Instrumental activities of daily living
GDS = Geriatric depression scale
TICS = Telephone interview for cognitive status
CNS = Central nervous system
ASA = American Society of Anesthesiologists
Standardized Difference = the difference between mean or proportion divided by the pooled standard deviation. The criterion used to determine whether the baseline variables are imbalanced is a Standardized Difference >= 0.10.
Non-spine procedures included general = 42, vascular = 9, thoracic = 7, urologic = 4.
*Adherence to study protocol
Of the 102 patients assigned to the intervention, seven anesthesiologists reported that they were unable to adhere to the protocol. The primary reasons for non-compliance was discrepancy with vital signs (n=3, where patients appeared to be “light” by clinical observation, patient movement (n=2), no time to follow protocol (n=1), and other reason (n=1, the PSI had a delayed response and then increased rapidly).
Intraoperative EEG Suppression
Five patients were excluded from the EEG analysis because of machine malfunction (three due to Sedline battery failure and two due to corrupted data). The interventional group was found to have spent less minutes (adjusted by the duration of surgery) in EEG suppression than the standard care group (median, interquartile range 1.4% (5.0%) and 2.5% (10.4). Hodges Lehmann estimator and 95% CI, −0.8% (−2.1%, −0.000009%); Stratified Wilcoxon Rank Sum test=2.4; p=0.02 (Table 2).
Table 2.
Comparison of interventional and standard Care groups in EEG suppression and incident delirium
| Outcome Variable |
Intervention | Standard Care |
Estimate | Lower Bound 95% CI |
Upper Bound 95% CI |
P-Value |
|---|---|---|---|---|---|---|
| Median (IQR) |
Median (IQR) |
Hodges- Lehman |
||||
| Adjusted minutes in EEG Suppression* Median IQR | 1.4 (5.0) | 2.5 (10.4) | −0.8 | −2.1 | −0.000009 | 0.02 |
| Time(Minutes) in EEG Suppression | 4.2 (15.4) | 7.6 (28.3) | ||||
| N (%) | N (%) | Risk Ratio |
||||
| Incident Delirium | 17 (17%) | 20 (20%) | 0.85 | 0.47 | 1.5 | 0.53 |
Sample sizes for EEG Suppression: Intervention N=101, Standard Care N=98. Sample sizes for Delirium: Intervention N=100, Standard Care N=101.
The 95% confidence interval for EEG Suppression computed using the Hodges Lehman estimator. The p-value for the adjusted minutes spent in EEG suppression is from the Stratified Wilcoxon Rank Sum test; the p-value for incident delirium is from a Chi-square test.
The effect of the intervention on EEG suppression differed for those, with and without preoperative cognitive impairment (interaction P=0.01), with estimated Incidence rate ratio and 95% CI of 0.39 (0.33, 0.44) for those with preoperative cognitive impairment and 0.48 (0.44, 0.51) for those without preoperative cognitive impairment. The findings indicate that the intervention effect was quantitatively greater for the group with preoperative cognitive impairment compared to those without preoperative cognitive impairment.
Incident delirium
No patients were found to have delirium preoperatively. Of 201 patients assessed, 37 had delirium in, at least, one of the first three postoperative days. Three patients were excluded from delirium analysis because they had unexpected postoperative ventilatory support (one in the standard care and two in the interventional groups). The incidence of delirium was not found to be different between the interventional group (17%) and the standard care group (20%) (risk ratio = 0.85, 95% CI=(0.47, 1.5), p=0.53 (Table 2).
The severity of delirium as measured by the MDAS scores were not different between the standard care vs. the intervention groups. Hodge Lehmann estimates and 95% CI are −0.00005, (−0.99, 0.00005); −0.00007 (−0.00003, 0.99); −0.00008 (−0.99, 0.0000008) respectively respectively for the 1st, 2nd and 3rd postoperative days).
Intraoperative anesthetic doses and safety of the intervention
Intraoperative anesthetic doses are shown in Table 3. Overall, intraoperative anesthetic drugs were not found to be different between groups. 17% of patients experienced one or more postoperative adverse events (See table in the supplementary material). The incidence of any postoperative complication was not found to be significantly different between the interventional vs. the standard care the groups (18% vs 17%); risk ratio and 95% CI 1.07, (0.59, 1.95). The length of hospital stay was not found to be different between treatment groups. However, more patients in the interventional group were reported to have moved during surgery (n=4) compared to that in the standard care group (n=1), relative risk= 4.0, 95% CI (0.45, 31.2), though the width of the interval indicates that the study was underpowered for assessment of intraoperative movement.
Table 3.
Intraoperative anesthetic drugs
| Variable | Standard Care Median (IQR) n |
Intervention Median (IQR) n |
H-L Estimator |
Lower Bound 95% CI |
Upper Bound 95% CI |
P-value |
|---|---|---|---|---|---|---|
| ETAC (age-adjusted MAC equivalents) | 0.47 (0.46) n=102 |
0.44 (0.47) n=102 |
−0.06 | −0.02 | 0.13 | 0.14 |
| IV Fentanyl (mcg) | 599.3 (613.1) n=102 |
510.9 (569.3) n=102 |
32.3 | −62.7 | 140.5 | 0.51 |
| IV Propofol (mg) | 1600 (2239) n=102 |
1205 (1877) n=102 |
138.9 | −50.3 | 514.4 | 0.19 |
The main anesthetic doses between the interventional and standard care groups were being compared.
ETAC = End-tidal anesthetic concentration. For each patient, continuous measurements of the MAC were recorded, and a median value was calculated.
IV = intravenous
MAC = Mean alveolar concentration
Formula for age-adjusted MAC equivalents of volatile anesthetic agents (k): k = [ETACdesflurane/(6.6*10−0.00269(age-40))] + [ETACsevoflurane/(1.8*10−0.00269(age-40))] + [ETACisoflurane/(1.17*10−0.00269(age-40))]
Discussion
We reported results from an exploratory randomized clinical trial targeting a certain anesthetic depth as measured by a processed EEG to determine its feasibility, safety and potential ability to reduce intraoperative EEG suppression. Our study found that by targeting PSI above 35 reduced the percent of surgical time spent in EEG suppression. Our results also suggested a potential mechanistic insight into the relationship between EEG suppression and postoperative delirium. Specifically, preoperative cognitive impairment moderated the association between the intervention and EEG suppression.
Comparison with previous studies
Although two previous studies had a similar scheme of randomization – comparing controls with no monitor vs. a study group with a processed EEG, 5,6 anesthesiologists assigned to the interventional group were asked to keep the processed EEG values within a range {Bispectral (BIS) between 40-60, or 50-60}, values that were considered to be “adequate for surgery”. However, neither studies focused on EEG suppression. An earlier study by Sieber et al. similarly found that it was feasible to target two separate processed EEG levels in a small randomized control trials in hip fracture patients who were anesthetized with spinal anesthesia and receiving two different levels of propofol infusion for sedation. 7 However, a randomized study in hip fracture patients by the same group, an extension of their earlier work, reported that observer rated sedation levels did not influence postoperative delirium. 27 That study differed from our present report as they did not use processed EEG to guide sedation management. The Electroencephalography Guidance of Anesthesia (ENGAGES) trial recently reported the results of their clinical trial in unselected older adults undergoing either cardiac or noncardiac surgery and reported similar rates of postoperative delirium in those randomized to receive EEG-guided anesthetic management vs. those without the monitor. 28 Given that this trial included patients undergoing either cardiac or noncardiac surgery, the patient population was heterogeneous as the mechanisms for postoperative delirium may be quite different given the cardiopulmonary bypass effects on the brain and differences in anesthetic management in the two different surgical types. The inclusion of patients’ predisposing factors is central to investigations of postoperative delirium, a geriatric syndrome with multiple etiologies.
The intervention was associated with less surgical time spent in EEG suppression for those with and without cognitive impairment, but the effect was stronger for those with preoperative cognitive impairment. This novel finding suggests that patient vulnerability is an important consideration in evaluating for which patients processed EEG may be useful in monitoring brain function. It is possible that the effect size of the intervention might have been greater if more cognitively impaired patients were included. This important observation should guide future randomized trials on the use of processed EEG in delirium prevention.
Most prior investigators speculate that by using processed EEG, anesthesiologists can reduce the amount of anesthetics administered thereby resulting in reduced rates of postoperative delirium. The premise behind this speculation is that anesthetics by themselves are directly injurious to the brain, resulting in postoperative delirium. Our present results do not support this premise for two reasons: first, we did not find that the study groups differed significantly in anesthetic doses. Second, we found an interaction between study group and preoperative cognitive impairment on time spent in EEG suppression. Together these findings suggest that patients with preoperative cognitive impairment may benefit from EEG monitoring during surgery to reduce episodes of EEG suppression. Obviously, our present results need further validation in a larger study.
Potential limitations
Our finding that preoperative cognitive status moderated the effect of the intervention on intraoperative EEG suppression should be investigated in future studies given that most of our patients did not have preoperative cognitive impairment. Second, since the same pool of anesthesia providers participated in the management of patients in both standard care and interventional groups, potential problems in “contamination” may occur despite blinding of the EEG monitors which may decrease the impact of the intervention. However, despite this potential, we still observed a statistically significant difference in the proportion of time with EEG suppression between groups. Third, although we did not find that there was a difference in the doses of anesthetic agents between groups, our measurements were relatively crude, as we compared the median concentrations of the volatile anesthetics between groups and did not examine the moment to moment effect of the anesthetic concentrations on EEG suppression. Fourth, because this was an exploratory trial, our study was not designed, nor sufficiently powered to examine the impact of and the intervention on postoperative delirium or adverse events. Fifth, we had previously reported that the Burst Suppression Ratio may underestimate the amount of EEG suppression when compared with visual analysis. 15 However, despite this potential limitation, the intervention was still effective in lowering the time spent in EEG suppression by the intervention compared to the standard care group. We recommend further validation incorporating visual analysis of the EEG to better understand the effect of the intervention in reducing EEG suppression. Relying on processed EEG value alone may not prevent EEG suppression. However, it should be pointed out that in our prior investigation, the visual analysis of the raw EEG waveform was performed off-line by two neurologists. It is unclear whether real time evaluation of raw EEG waveform by anesthesia care providers was similarly accurate. Another consideration is that the standard care group had no access to processed EEG values. Therefore, the presence or absence of a processed EEG monitor rather than the actual level ‘targeted’ may be the factor, as has been demonstrated by others. However, our study was not designed to distinguish the exact cause of why EEG suppression was reduced other than the directions we gave to the anesthesiologists in the interventional group. In addition, more detailed preoperative neuropsychological testing to identify the type and severity of cognitive impairment would be helpful for risk stratification. To summarize, a larger study to target particularly more cognitively impaired patients would clearly be needed to validate the present results.
Clinical implications
Our exploratory randomized trial shows that an intervention to reduce EEG suppression by targeting a dimensionless processed EEG value was effective and that preoperative cognitive status moderated the association between the intervention and time spent in EEG suppression. This finding is novel and fills an important gap for understanding the mechanisms underlying the development of delirium after surgery. The proposition of using processed EEG to guide anesthetic depth in order to reduce postoperative delirium in unselected patients needs to be revised. Lastly, the relationship between anesthetic doses and postoperative delirium is not linear.
Summary
Results from this exploratory randomized clinical trial showed that the use of processed EEG and maintaining the anesthetic depth higher than a specified level was associated with less intraoperative EEG suppression, particularly in those with preoperative cognitive impairment. A larger prospective cohort study to especially include more cognitively vulnerable patients is necessary to confirm these results.
Supplementary Material
Key points summary.
Question
What is the relationship between EEG suppression and postoperative delirium?
Findings
An intervention to minimize deep anesthetic depth through processed EEG monitoring reduces EEG suppression; and preoperative cognitive impairment predicted EEG suppression.
Meaning
A clinical trial to reduce time spent in deep sedation was found to reduce time spend in EEG suppression, and cognitive impairment moderated the association.
Acknowledgements
The authors thank the anesthesiologists and surgeons at the University of California, San Francisco Medical Center for their cooperation and participation in making this clinical trial possible.
Funding: National Institute on Aging of the National Institutes of Health R21AG048456
Glossary of terms
- aaMAC
Age-adjusted minimum alveolar concentration
- ASA
American Society of Anesthesiologists
- BIS
Bispectral
- BSR
Burst Suppression Ratio
- CI
Confidence interval
- CAM
Confusion Assessment Method
- EEG
Electroencephalographic
- ETAC
End-tidal anesthetic concentration
- MDAS
Memorial Delirium Assessment Scale
- OR
Odds ratio
- RR
Risk ratio
- PSI
Patient State Index®
- TICS
Telephone Interview for Cognitive Status
Footnotes
Conflicts of interest: None
Clinical trial registry: The trial was registered with clinicaltrials.gov (NCT01983384)
There is currently no gold standard to measure anesthetic depth. For the purpose of the manuscript, the use of the term “anesthetic depth” refers to the processed EEG measurement as an estimate of anesthetic depth
References
- 1.Lipowski Z Delirium in the elderly patient. New Engl J Med 1989;320:578–82. [DOI] [PubMed] [Google Scholar]
- 2.Inouye S The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med 1994;97:278–88. [DOI] [PubMed] [Google Scholar]
- 3.Lipowski Z Delirium (acute confusional states). JAMA 1987;258:1789–92. [PubMed] [Google Scholar]
- 4.Siddiqi N Predicting delirium: time to use delirium risk scores in routine practice? Age Ageing 2016;45:9–10. [DOI] [PubMed] [Google Scholar]
- 5.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013;25:33–42. [DOI] [PubMed] [Google Scholar]
- 6.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. British journal of anaesthesia 2013;110 Suppl 1:i98–105. [DOI] [PubMed] [Google Scholar]
- 7.Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clinic proceedings Mayo Clinic 2010;85:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock EL, Torres BA, Lin N, Helsten DL, Nadelson MR, Mashour GA, Avidan MS. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesthesia and analgesia 2014;118:809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, Escallier KE, Ben Abdallah A, Lin N, Avidan MS. Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesthesia and analgesia 2016;122:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. 2015;BMC anesthesiology 15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soehle M, Ellerkmann RK, Grube M, Kuech M, Wirz S, Hoeft A, Bruhn J. Comparison between bispectral index and patient state index as measures of the electroencephalographic effects of sevoflurane. Anesthesiology 2008;109:799–805. [DOI] [PubMed] [Google Scholar]
- 12.Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C, Jacobsohn E, Evers AS. Anesthesia awareness and the bispectral index. The New England journal of medicine 2008;358:1097–108. [DOI] [PubMed] [Google Scholar]
- 13.Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev 2007:CD003843. [DOI] [PubMed] [Google Scholar]
- 14.Drover D, Ortega HR. Patient state index. Best practice & research Clinical anaesthesiology 2006;20:121–8. [DOI] [PubMed] [Google Scholar]
- 15.Muhlhofer WG, Zak R, Kamal T, Rizvi B, Sands LP, Yuan M, Zhang X, Leung JM. Burst-suppression ratio underestimates absolute duration of electroencephalogram suppression compared with visual analysis of intraoperative electroencephalogram. British journal of anaesthesia 2017;118:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babiloni C, Carducci F, Lizio R, Vecchio F, Baglieri A, Bernardini S, Cavedo E, Bozzao A, Buttinelli C, Esposito F, Giubilei F, Guizzaro A, Marino S, Montella P, Quattrocchi CC, Redolfi A, Soricelli A, Tedeschi G, Ferri R, Rossi-Fedele G, Ursini F, Scrascia F, Vernieri F, Pedersen TJ, Hardemark HG, Rossini PM, Frisoni GB. Resting state cortical electroencephalographic rhythms are related to gray matter volume in subjects with mild cognitive impairment and Alzheimer’s disease. Human brain mapping 2013;34:1427–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonanni E, Di Coscio E, Maestri M, Carnicelli L, Tsekou H, Economou NT, Paparrigopoulos T, Bonakis A, Papageorgiou SG, Vassilopoulos D, Soldatos CR, Murri L, Ktonas PY. Differences in EEG delta frequency characteristics and patterns in slow-wave sleep between dementia patients and controls: a pilot study. J Clin Neurophysiol 2012;29:50–4. [DOI] [PubMed] [Google Scholar]
- 18.Welsh KA. Detection of early dementia in the elderly. Exp Aging Res 1991;17:101. [PubMed] [Google Scholar]
- 19.American Society of Anesthesiologists. ASA PHYSICAL STATUS CLASSIFICATION SYSTEM: American Society of Anesthesiologists.
- 20.Inouye S, van Dyke C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method. Ann Intern Med 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr., Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. Journal of the American Geriatrics Society 2005;53:312–8. [DOI] [PubMed] [Google Scholar]
- 22.Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, Marcantonio ER, Wong B, Isaza I, Inouye SK. A tale of two methods: chart and interview methods for identifying delirium. Journal of the American Geriatrics Society 2014;62:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128–37. [DOI] [PubMed] [Google Scholar]
- 24.Nickalls R, Mapleson W. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. British journal of anaesthesia 2003;91:170–4. [DOI] [PubMed] [Google Scholar]
- 25.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does Preoperative Risk for Delirium Moderate the Effects of Postoperative Pain and Opiate Use on Postoperative Delirium? The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2013;10:946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- 27.Sieber FE, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JP, Jaberi M, Hasenboehler EA, Li T, Wang NY. Effect of Depth of Sedation in Older Patients Undergoing Hip Fracture Repair on Postoperative Delirium: The STRIDE Randomized Clinical Trial. JAMA Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildes TS, Mickle AM, Ben Abdallah A, Maybrier HR, Oberhaus J, Budelier TP, Kronzer A, McKinnon SL, Park D, Torres BA, Graetz TJ, Emmert DA, Palanca BJ, Goswami S, Jordan K, Lin N, Fritz BA, Stevens TW, Jacobsohn E, Schmitt EM, Inouye SK, Stark S, Lenze EJ, Avidan MS, Group ER. Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. JAMA 2019;321:473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.