Abstract
Introduction
A sufficient dietary intake of the vitamin niacin is essential for normal cellular function. Niacin is transported into the cells by the monocarboxylate transporters: sodium-dependent monocarboxylate transporter (SMCT1 and SMCT2) and monocarboxylate transporter (MCT1). Despite the importance of niacin in biological systems, surprisingly, its in vivo biodistribution and trafficking in living organisms has not been reported. The availability of niacin radiolabelled with the short-lived positron emitting radionuclide carbon-11 ([11C]niacin) would enable the quantitative in vivo study of this endogenous micronutrient trafficking using in vivo PET molecular imaging.
Methods
[11C]Niacin was synthesised via a simple one-step, one-pot reaction in a fully automated system using cyclotron-produced carbon dioxide ([11C]CO2) and 3-pyridineboronic acid ester via a copper-mediated reaction. [11C]Niacin was administered intravenously in healthy anaesthetised mice placed in a high-resolution nanoScan PET/CT scanner. To further characterize in vivo [11C]niacin distribution in vivo, mice were challenged with either niacin or AZD3965, a potent and selective MCT1 inhibitor. To examine niacin gastrointestinal absorption and body distribution in vivo, no-carrier-added (NCA) and carrier-added (CA) [11C]niacin formulations were administered orally.
Results
Total synthesis time including HPLC purification was 25 ± 1 min from end of [11C]CO2 delivery. [11C]Niacin was obtained with a decay corrected radiochemical yield of 17 ± 2%.
We report a rapid radioactivity accumulation in the kidney, heart, eyes and liver of intravenously administered [11C]niacin which is consistent with the known in vivo SMCTs and MCT1 transporter tissue expression. Pre-administration of non-radioactive niacin decreased kidney-, heart-, ocular- and liver-uptake and increased urinary excretion of [11C]niacin. Pre-administration of AZD3965 selectively decreased [11C]niacin uptake in MCT1-expressing organs such as heart and retina.
Following oral administration of NCA [11C]niacin, a high level of radioactivity accumulated in the intestines. CA abolished the intestinal accumulation of [11C]niacin resulting in a preferential distribution to all tissues expressing niacin transporters and the excretory organs.
Conclusions
Here, we describe the efficient preparation of [11C]niacin as PET imaging agent for probing the trafficking of nutrient demand in healthy rodents by intravenous and oral administration, providing a translatable technique to enable the future exploration of niacin trafficking in humans and to assess its application as a research tool for metabolic disorders (dyslipidaemia) and cancer.
1. Introduction
Water-soluble niacin, also known as nicotinic acid (a form of vitamin B3), is the precursor of nicotinamide adenine dinucleotide (NAD) and nicotinamide-adenine-dinucleotide-phosphate (NADP), two coenzymes involved in various metabolic pathways, fatty acid synthesis and protection against oxidative cell damage [1].
Niacin is ingested from a variety of plant- and animal-derived foods, but most commonly obtained from fortified foods, such as wheat flour and cereals. Niacin can also be biosynthesised in the liver from the essential amino acid tryptophan, however this proceeds with low efficiency.
Niacin is taken up by cells via the sodium-dependent monocarboxylate transporter (SMCT1 and SMCT2) and monocarboxylate transporter-1 (MCT1) expressed in the plasma membrane [[2], [3], [4], [5], [6], [7]]. SMCT1 and SMCT2 co-transport niacin and one Na+ with high (Km = 310–230 μM) and low (Km = 3.7 mM) affinity processes, respectively [[2], [3], [4], [5], [6], [7]]. SMCTs are present in the digestive tract, liver and cortical regional of kidneys [[2], [3], [4], [5], [6], [7]]. MCT1 co-transports niacin and one H+ via a low-affinity process
(Km = millimolar range) [[8], [9], [10], [11], [12], [13]]. MCT1 transporters are ubiquitous with the highest expression in the heart [14]. MCT1 is also expressed in retina which explains the incidence of ocular side effects (niacin maculopathy) at high administered doses of niacin [5,[15], [16], [17]].
SMCT1 and MCT1 transport the vitamin in the form of nicotinate, however nicotinate structural analogues (e.g. 2-picolinate) are not recognized as substrates. Niacin's transport is inhibited by short chain fatty acids (e.g. acetate, priopionate), monocarboxylate drugs (e.g. salicylates), lactate and pyruvate [2,3].
The recommended daily allowance (RDA) for niacin is 14–16 mg/day for adults. In addition to a regular baseline dietary niacin intake, increases in vitamin intake is required in patients affected by gastrointestinal conditions (chronic colitis, coeliac disease, Crohn's disease, severe ulcerative colitis, gastroenterostomy), cancer and HIV infection, alcoholism, and anorexia nervosa [18,19]. Niacin deficiency results in pellagra, a disease which is characterized by the appearance of symptoms affecting the skin (dermatitis), the gastro-intestinal system (diarrhea) and the nervous system (dementia).
Besides being an essential vitamin, high doses of niacin (0.5–3 g/day) have been used to lower plasma cholesterol levels, preventing atherosclerotic events and delaying the progression of cardiovascular disease [18,20]. The anti-lipolytic effect involves the G-protein-coupled receptor (GPR109A) expressed in white adipose tissue (WAT), spleen and lungs. The activation of GPR109A in the dermal dendritic cells mediates cutaneous flushing which is a severe side effect affecting compliance, reducing its clinical usefulness [21].
In vivo positron emission tomography (PET) imaging has not yet been adopted for the systematic study of niacin. However, to date, tritium (3H) and carbon-14 (14C) isotopologous radiolabelled niacin have been used in cell-based assays and in laboratory animal studies [[22], [23], [24], [25], [26]]. The tissue distribution of [3H]niacin after post-mortem analysis in mice showed distribution of radioactivity in the kidneys, heart, liver, retina, intestine with a rapid and transient uptake in adipose tissue [25]. However, the use of 3H and 14C radiotracers precludes the assessment of the radiolabelled vitamin in vivo for human translational studies. Their low beta energies and long radioactive half-lives (12.5 and 5700 years, respectively) hamper their use due to the associated risks of long-term radioactivity exposure and the need for post-mortem tissue analysis.
Our aim was to characterize the whole-body pharmacokinetics and tissue-distribution of niacin in vivo. To achieve this, we have developed an efficient method to produce niacin autologously radiolabelled with the short-lived positron-emitting radionuclide carbon-11 ([11C]niacin). Carbon-11 labelling, maintains the chemical structure and biological properties of the vitamin, allowing its straightforward translation from preclinical to clinical research, without toxicological assessment, and reduces risks associated with radioactive waste management and patient radiation exposure due to the short half-life of 11C (half-life = 20.4 min).
The development of [11C]niacin and its preclinical evaluation will have a significant value in: 1) understanding of the mechanisms of intestinal absorption and renal excretion/reabsorption of this essential vitamin under physiological conditions as well as the identification of factors that negatively affect vitamin homeostasis causing pathophysiological conditions; 2) targeting MCT1, a transporter which is overexpressed in tumours; and 3) optimising/assessing treatment regimen where niacin is used as a drug for lowering plasma cholesterol levels to prevent atherosclerotic events. In this study, we have developed a method to produce [11C]niacin from a boronic ester derivative and advanced our understanding of the biodistribution and kinetics of this vitamin in mice. The trafficking of [11C]niacin in vivo was studied comparing the effects of routes of administration (intravenous (IV) vs. oral gavage (OG)), gender, the effect of carrier niacin and MCT1 inhibition.
2. Methods
2.1. [11C]CO2 production
[11C]CO2 was produced using a Siemens RD112 cyclotron by 11 MeV proton bombardment of nitrogen (+0.5% O2) gas via the 14N(p,α)11C reaction. The cyclotron-produced [11C]CO2 was bubbled in a stream of helium gas with a flow rate of 60 mL/min post target depressurisation directly into a reaction vial A (time from end of bombardment (EOB) to end of delivery (EOD) = 1 min and 50 s). The standard parameters for preclinical 11C productions were 10 μA for 10 min, with an estimated yield at the EOD of approximately 3.3 ± 0.2 GBq of [11C]CO2.
2.2. Radiosynthesis of [11C]niacin – manual system
[11C]Niacin was produced from 3-pyridineboronic acid ester (1, Fig. 1A) and [11C]CO2 following an adapted protocol reported by Pike V. et al. [27]. Aiming to produce [11C]niacin in high radiochemical yield (RCY, Table 1), we optimized fluoride sources (CsF and potassium fluoride (KF)/kryptofix (K2.2.2) complex), copper catalysts (copper(I) thiophene-2-carboxylate (CuTC), copper iodide (CuI)), and bases (N,N,N′,N′-tetramethylethylenediamine (TMEDA) and 1,8diazabicyclo[5.4.0]undec-7-ene (DBU)). Oven-dried vials (KX Microwave Vials, 5 mL) and crimp caps (Fisherbrand, centre hole with 3.0 mm PTFE seal aluminium silver 20 mm, part # 10132712) were used. The vials were prepared in a glovebox (Plas-Labs, Inc. 815 PGB Series) under nitrogen atmosphere and controlled CO2 levels (lower than 30 ppm).
Fig. 1.
Scheme for [11C]niacin radiolabeling and diagram of the automated synthesis to produce [11C]niacin.
Reagents and conditions: [11C]CO2, 1 (1 equiv., 60 μmol), CuI (10 μmol) KF (33.3 μmol), K2.2.2 (33.3 μmol), TMEDA (500 μmol), DMF (400 μL), 5 min at 100 °C. Vial A contains the reaction mixture. Vial B is an empty vial connected to vial A. Vial C contains 2.2 mL of a solution composed by water/ethanol/sulfuric acid (80:20:0.05).
Table 1.
Optimization of reaction conditions for the synthesis of [11C]niacin (manual system).
| Entrya | CuI (μmol) | KF (μmol) | K2.2.2 (μmol) | TMEDA (μmol) | DMF (μL) | Non-isolated RCYb (%) | TEc (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 33.3 | 33.3 | 500 | 400 | 0 | 0 |
| 2 | 10 | 33.3 | 33.3 | 500 | 400 | 99 | 37 |
| 3 | 100 | 33.3 | 33.3 | 500 | 400 | 84 | 33 |
| 4 | 10 | 33.3 | 33.3 | 5 | 400 | 82 | 5 |
| 5d | 10 | 33.3 | 33.3 | 500 | 400 | 60 | 3 |
| 6e | 10 | 33.3 | 33.3 | 500 | 400 | 100 | 1 |
| 7 | 10 | 33.3 | 33.3 | 500 | 800 | 88 | 5 |
| 8 | 10 | 10 | 10 | 500 | 400 | 83 | 19 |
| 9f | 10 | – | – | 500 | 400 | 76 | 19 |
1 (60 μmol).
Non-isolated radiochemical yield (RCY) of the crude product has been determined by analytical radio-HPLC.
The trapping efficiency (TE) has been calculated as a ratio of the decay corrected radioactivity in the vial and the total radioactivity produced by the cyclotron.
Addition of DBU to the reaction mixture (3 μmol).
Addition of DBU to the reaction mixture (10 μmol).
CsF (150 μmol).
2.3. Radiosynthesis of [11C]niacin – automatic system
The optimal condition for [11C]niacin production following the manual system was implemented in a fully automated system on an Eckert & Ziegler (E&Z) Modular Lab system (Fig. 1B). Prior to production, an automated “flow test” sequence in the Eckert & Ziegler (E&Z) software was performed by applying helium pressure into the system to check that the flow of gases was not obstructed, and the system was gas tight. In the first step, cyclotron-produced [11C]CO2 was bubbled into a reaction vial (Vial A). Vial A contains 1 (12.3 mg, 60 μmol, 1 equiv.), TMEDA (8.3 equiv.), CuI (0.2 equiv.), K2.2.2 (0.6 equiv.), KF (0.6 equiv.) in DMF (400 μL) at 0 °C for 1.75 min. At the end of [11C]CO2 delivery, the was vial was heated at 110 °C for 5 min (Fig. 1B) then a helium flow at 60 mL/min was applied for 5 min and the solvent from Vial A was distilled into Vial B kept at 20 °C. Vial A was cooled at 25 °C and 2.1 mL of acidic mobile phase (Vial C) was transferred with helium (60 mL/min) to Vial A. The crude mixture was transferred to an HPLC injection loop through a vent filter. The reaction purified by semipreparative HPLC using a mobile phase composed by 0.05% H2SO4 in ethanol:water solution (20:80). The mixture was transferred to an HPLC loop (2 mL) for subsequent semipreparative HPLC purification using a Primesep 100 HPLC column (5 μm particle size, L × I.D. 15 cm × 10 mm) equipped with a radioactivity detector (radio-RP-HPLC) and eluted with a mobile phase composed of 0.05% H2SO4 in ethanol:water solution (5:95) at a flow rate of 4 mL/min (retention time: 9 min). The [11C]niacin peak was collected. A final volume of 1.3 ± 0.1 mL of [11C]niacin was formulated with PBS with 4% ethanol in injectable solution and passed through a vented sterile 0.22 μm filter. Analytical HPLC analysis for the quality control (QC) of the final tracer product was carried out on an HPLC analytical Primesep 100 column, 5 μm particle size, L × I.D. 15 cm × 4.6 mm. Isolated RCY, radiochemical purity (RCP), molar activity (Am) and pH have been determined.
2.4. Quality control (QC) of [11C]niacin
Analytical HPLC analysis for the QC of the final tracer product was carried out on an Agilent 1200 HPLC system equipped with a UV detector (λ = 250 nm) and a β+-flow detector coupled in series. The samples were injected on to an analytical Primesep 100 column (5 μm particle size, L × I.D. 15 cm × 4.6 mm), which was eluted with a mobile phase of 0.05% H2SO4 in ethanol:water solution (20:80). The column flow rate is 1.5 mL/min and was kept at 25 °C (Fig. S1). The typical retention of niacin is in between 4:08 min for the UV absorbance (the radioactivity detector is 15 s at 1.5 mL/min further downstream from the UV detector, Fig. S1). A calibration curve is determined from UV absorbance peak areas of niacin standards. Then the UV peak area of the [11C]niacin formulation is fit on the calibration curve to determine the niacin concentration in the formulation.
2.5. Preclinical experiments
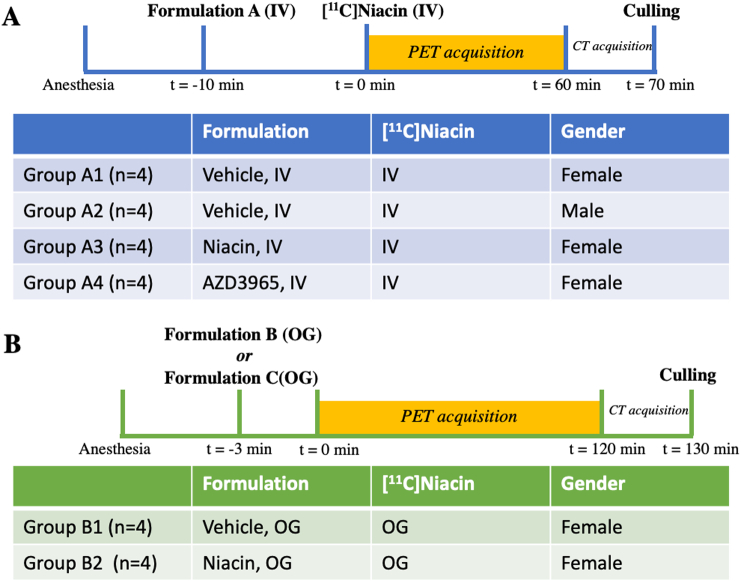
In vivo studies were carried out in male and female mice (Balb/C, Charles River UK Ltd) fed with standard mouse diet (VRF1 (P), SDS, UK) and given drinking water ad libitum. All animal studies were carried out in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. Experiments complied with UK Research Councils' and Medical Research Charities' guidelines on responsibility in the use of animals in bioscience research, under UK Home Office project and personal licenses. The reporting of this study complied with the Animal Research: Reporting in vivo experiments (ARRIVE) guidelines (https://www.nc3rs.org.uk/arrive-guidelines). 56–66-day old mice were selected because they have an ideal size to perform a total body imaging in our small-animal PET scanner. PET/CT scans were taken once on each mouse using a single administration route (Fig. 2).
Fig. 2.
Illustration of the PET/CT image acquisition of intravenous (IV) and orogastric gavage (OG) administration of [11C]niacin.
Formulation A is composed of 4% ethanol in 10 mM PBS in groups A1 and A2, 5 mg/kg niacin dissolved in 4% ethanol in 10 mM in group A3, 1.1 mg/kg AZD3965 dissolved in 4% ethanol in 10 mM PBS in group A4. Formulation B is composed of non-carrier added (NCA) [11C]niacin (26 ± 4 μg/kg) in 4% ethanol in 10 mM PBS (group B1). Formulation C is composed of carrier added (CA) [11C]niacin (5 mg/kg) in 4% ethanol in 10 mM PBS (group B2).
2.6. [11C]niacin administration following protocol A (intravenous administration)
Sixteen mice were divided into four groups (Fig. 2A and Table S2). Group A1: female mice (n = 4) receiving IV injection of vehicle (4% ethanol in 10 mM PBS, 30 μL) 10 min before [11C]niacin IV injection (126 ± 14 μL, 3.4 ± 1.0 MBq, 20 g); group A2: male mice (n = 4) receiving IV injection of vehicle (30 μL) 10 min before [11C]niacin IV injection (150 ± 0 μL, 2.4 ± 0.2 MBq, 25 ± 1 g); group A3: female mice (n = 4) receiving IV injection of niacin 5 mg/kg (30 μL) 10 min before [11C]niacin IV injection (111 ± 7 μL, 2.1 ± 0.3 MBq, 17 ± 1 g); and group A4: female mice (n = 4) receiving IV injection of AZD3695 1.1 mg/kg (30 μL) 10 min before [11C]niacin IV injection (104 ± 4 μL, 2.0 ± 0.4 MBq, 20 ± 1 g). Mice were anaesthetised and placed in a high-resolution nanoPET/CT scanner (Mediso, Budapest, Hungary) and the IV administrations were carried out via the tail vein cannula. Dynamic PET image data were acquired for 60 min. Subsequently, a CT scan was acquired for attenuation correction and anatomical reference.
2.7. [11C]niacin administration following protocol B (oral gavage administration)
Eight female mice were divided into two groups (Fig. 2B and Table S2). Group B1 (no-carrier-added (NCA) group, 19 ± 1 g): four mice receiving by OG 165 μL of a solution composed of [11C]niacin (2.9 ± 0.6 MBq) and vehicle (30 μL, 4% ethanol in PBS, NCA group). Group B2 (carrier-added (CA) group, 19 ± 1 g): four mice receiving by OG 165 μL of a solution composed of [11C]niacin (3.4 ± 1.3 MBq) and niacin (30 μL, 5 mg/kg, 4% ethanol in PBS). For dynamic microPET studies, anaesthetized animals were placed on the PET-CT holder immediately after OG of [11C]niacin formulation and a 120 min PET scan started; a delay of approximately 2.5–3 min between tracer delivery due to the time taken to administered [11C]niacin by OG and position the animal in the scanner. The start of the PET scan has been set at 0 min for the x-axes of Fig. 3, Fig. 6.
Fig. 3.
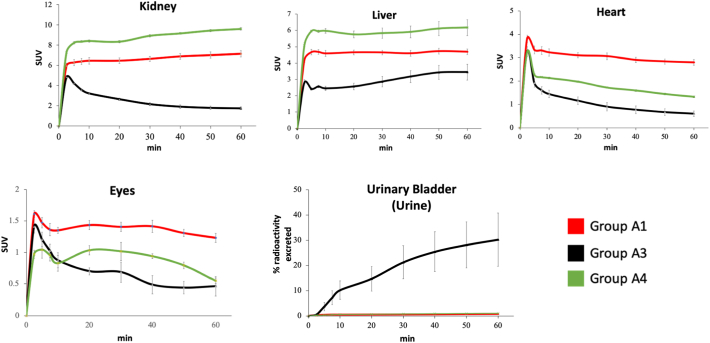
Time Activity curves of mice receiving [11C]niacin IV.
Time-SUV profile (0–60 min) of kidney, liver, heart and eyes in no-niacin-added (red line, group A1, vehicle IV), niacin-challenged mice (black line, group A3, niacin IV) and AZD3965-challenged mice (green line, group A4, AZD3965 IV). Data are the mean ± SEM. Note that the y-scale (SUV) varies between the different tissues.
Fig. 6.
Time Activity curves of mice receiving [11C]niacin OG.
Time (0–120 min)-% gastric emptying profile (A), time-% intestinal absorption profile (B), time% excretion profile (C), and time-SUVOG profile for kidneys liver, heart in mice receiving NCA [11C]niacin (red line, group B1, vehicle OG) and CA [11C]niacin (black line, group B2, niacin OG). Data are the mean ± SEM. Note that the y-scale (SUV) varies between the different tissues.
2.8. PET/CT imaging in mice
Dynamic PET scans (1:5 coincidence mode; 5-ns coincidence time window) were performed on a nanoScan PET/CT 8 W scanner (Mediso Ltd., Budapest, Hungary) over 60 (groups A1-A4) or 120 min (groups B1-B2) followed by CT (180 projections, 55 kVp X-ray source, 600-ms exposure time, 1:4 binning and semi-circular acquisition) using their proprietary acquisition software (Nucline 1.07). Ten minutes before the in vivo protocol was scheduled to start, mice were anaesthetised in a heated induction box by inhalation of 3% isoflurane in 100% oxygen.
After completion of the PET data acquisition, computed tomography (CT) scans were performed to provide anatomical information. CT images were acquired over 7 min. After the PET/CT scans, animals were culled (70- and 130-minutes post radiotracer IV injection and OG administration, respectively).
Whole-body Tera-Tomo (Mediso) 3-dimensional reconstruction was performed (400–600-keV energy window, 1–3 coincidence mode, 4 iterations and 6 subsets) using an isotropic voxel size of 0.4 mm3. Images were corrected for attenuation, scatter, and decay.
For dynamic scans of the anaesthetized whole mouse, the acquired data were binned into nine image frames (4 × 150 and 5 × 600 s) for Fig. 3, Fig. 4 and ten image frames (5 × 300 and 5 × 1200 s) for Fig. 6, Fig. 7. VivoQuant® software (http://www.vivoquant.com/) was used for image display and volume-of-interest (VOI) analysis.
Fig. 4.

PET images of mice receiving [11C]niacin IV.
Maximum intensity projections of PET images from: no-niacin-added mice at 0–2.5 min and 1020 min (group A1, A–B, vehicle IV), niacin-challenged mice at 0–2.5 min and 10–20 min (group A3, C-D, niacin IV), AZD3965-challenged mice at 0–2.5 min and 10–20 min (group A4, E–F, AZD3965 IV). Radioactivity of 2.5 ± 0.3 MBq was injected into mice for dynamic PET/CT imaging. PET images are displayed according to the intensity scale for tracer activity, from white (highest), through red (intermediate) to purple (lowest).
Fig. 7.
PET images of mice receiving [11C]niacin OG.
Maximum intensity projections of PET images from mice receiving NCA [11C]niacin (upper row, group B1, vehicle OG) and CA [11C]niacin (lower row, group B2, niacin OG) at 0–3 min (A and D), 5–10 min (B and E) and 60–80 min (C and F) after start of PET imaging. Radioactivity of 3.2 ± 0.7 MBq was administered into mice for dynamic PET/CT imaging. PET images are displayed according to the intensity scale for tracer activity, from white (highest), through red (intermediate) to purple (lowest).
For animals receiving [11C]niacin IV, standardized uptake value (SUV) = [decay-corrected mean tissue radioactivity concentration (Bq/ml)/injected dose (Bq)] × body weight (g).
For animals receiving [11C]niacin by OG, SUVOG = [decay-corrected mean tissue activity concentration (Bq/ml) / (administered dose (Bq) minus decay-corrected radioactivity left in the stomach (Bq))] × body weight (g). In each experiment, volumes of interest (VOI) for the whole mouse, stomach, liver, intestine, kidneys, brain, heart, muscle and eyes, urinary bladder were drawn manually, and the radioactivity (Bq) in each VOI was estimated using VivoQuant software.
Gastric emptying and intestinal absorption in animals receiving [11C]niacin by OG were estimated by determining the radioactivity amount in stomach and small intestine, respectively, as a function of time [28]. Gastric emptying was estimated from the ratio between the [11C]niacin in the stomach and the amount in the whole-body (administered dose). Intestinal absorption was estimated from the ratio between the radioactivity of [11C]niacin in the small intestine versus the radioactivity in the whole-body minus the radioactivity detected in the stomach.
2.9. Biodistribution studies
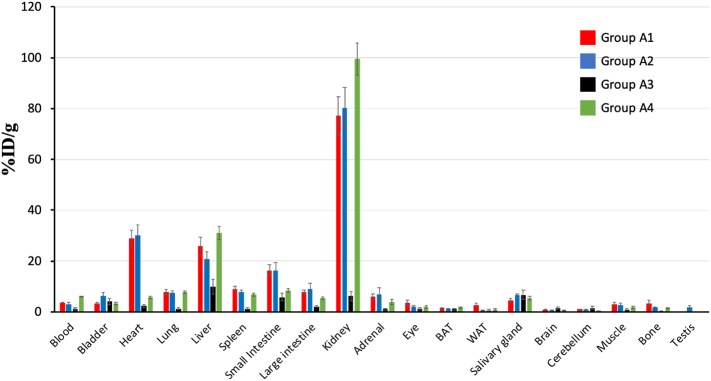
Biodistribution studies were performed on the group of animals receiving [11C]niacin IV (Groups A1A4). Tissues including bladder, heart, brain, lungs, liver, spleen, small intestine, large intestine, kidneys, adrenal gland, eyes, interscapular brown adipose tissue (BAT), posterior subcutaneous white adipose tissue (WAT), stomach, kidneys, salivary gland, brain, cerebellum, muscle, thigh bone, testis were excised. Blood was also collected. All samples were weighed, and radioactivity content measured using an automated well counter with a standard dilution of [11C]niacin. Counts were decaycorrected and the percent injected dose per gram of tissue (%ID/g) calculated (Fig. 5).
Fig. 5.
Biodistribution of radioactivity after IV injection of [11C]niacin in groups A1-A4. Biodistribution results at 70 min post injection of: A) [11C]niacin IV administration in female mice pre-treated IV with vehicle (red, no-niacin-added group, vehicle IV); [11C]niacin IV administration in male mice pre-treated IV with niacin-free vehicle (blue, no-niacin-added group, vehicle IV); [11C]niacin IV administration in female mice pre-treated IV with niacin (black, niacin-challenged group, niacin IV); [11C]niacin IV administration in female mice pre-treated IV with AZD3965 (green, AZD3965-challenged group, AZD3965 IV). Data are percentage injected dose per gram (%ID/g), mean ± SEM.
2.10. Statistical analysis
Quantitative data were expressed as mean ± SEM. Organ SUV at various time points were compared between the groups using a repeated measures mixed-effect model with an auto-regressive covariance structure. Post-hoc analyses were corrected for multiple comparisons (IBM SPSS Statistics. Version 24.0). Differences at the 95% confidence level (p < 0.05) were considered significant. Tables S3–S9:
*p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant.
3. Results
3.1. Optimization of the [11C]niacin radiosynthesis (manual system)
The cyclotron-produced [11C]CO2 was bubbled into a reaction vial containing CuI, KF, K2.2.2, and 1 in DMF at 0 °C (Fig. 1). Then the reaction mixture was heated a 110 °C for 5 min. The reaction was subsequently flushed with helium for 5 min, cooled at room temperature and quenched an acidic mobile phase solution (0.05% H2SO4 in ethanol:water 5:95). [11C]Niacin crude mixture was analysed by analytical radioHPLC. In order to obtain [11C]niacin with high non-isolated RCY and trapping efficiency (TE), an optimization process was performed by modifying: i) the amount of copper catalyst, ii) the amount of trapping reagent, iii) the amount of the solvent, iv) the amount of fluoride source. As initial experiments, the amount of the CuI was varied from 1 to 100 μmol (Table 1, entries 1–3). Using 10 μmol of CuI [11C]niacin was obtained with a non-isolated RCY of 99% and a TE of 37% (entry 2). Decreasing the amount of the trapping agent TMEDA from 500 to 5 μmol the non-isolated RCY of [11C]niacin did not change (entries 3–4). However, a detrimental decrease of TE was observed using 5 μmol of TMEDA (entry 4). The addition of a [11C]CO2-trapping agent such as DBU did not favour the TE either (entries 5–6). Increasing the content of DMF from 400 to 800 μL (entry 7) or decreasing the content of KF/K222 from 33.3 to 10 μmol (entry 8) or replacing the KF/K222 complex with CsF (entry 9) did not affect the non-isolated RCY but the TE efficient halved.
3.2. Radiosynthesis of [11C]niacin (automatic system)
The results presented in Table 1 show that the production [11C]niacin is maximized when 60 μmol of 1 is reacted with 10 μmol of CuI, 33.3 μmol of KF–K2.2.2 and 500 μmol of TMEDA in DMF for 5 min at 110 °C. Using this condition, the fully automated synthesis time to produce [11C]niacin including HPLC purification was 25 ± 1 min (Table S1, n = 27) from end of [11C]CO2 delivery (EOD). The amount of [11C]niacin obtained was 239 ± 20 MBq in 1.3 ± 0.1 mL PBS with 4% ethanol in injectable solution starting from 3.5 ± 0.2 GBq of cyclotron produced [11C]CO2 with isolated RCY of 37 ± 2%, Am of 6.8 ± 0.6 GBq/μmol at EOD and RCP of >99% (Table S1, n = 27).
3.3. PET image analysis in mice receiving [11C]niacin following protocol A (intravenous administration)
To examine [11C]niacin trafficking in vivo, [11C]niacin was administered intravenously in healthy anaesthetised mice placed in a high-resolution nanoScan PET/CT scanner. In the no-niacin-added group (group A1), PET images results demonstrated [11C]niacin uptake in kidneys, liver, heart, eyes (Fig. 3, Fig. 4A and B). The kidney/heart and kidney/brain uptake ratios, derived from the dynamic PET images between 5 and 60 min, were 2.2 and 30, respectively. Similar results were observed in mice receiving [14C]niacin (IP, 110 μg) with kidney/heart and kidney/brain ratios of 2.8 and 19.3 between 10 and 60 min [29]. Time–activity curves of [11C]niacin in liver, brain and eyes were generally nongender specific (group A1 versus A2), with the exception of higher kidney uptake in male mice. No difference in urine clearance was observed between groups A1 and A2.
Mice challenged with niacin (group A3) prior to [11C]niacin PET showed a significant decrease of [11C]niacin uptake in kidneys (4-folds), heart (5-folds) and eyes (3-folds) compared to the no-niacin-added control group A1 (Fig. 3, Fig. 4C and D). At 10 min post-radiotracer injection a remarkable 2-fold decrease in [11C]niacin uptake in the kidneys and 20-fold increase in urinary excretion was observed in group A3 versus group A1 (Fig. 3). From 10 to 60 min in group A3 the radioactivity in the kidney decreased with a concomitant increase in the urinary bladder.
To further examine the role of MCT1 on [11C]niacin uptake, mice were challenged with a selective potent selective MCT1 inhibitor AZD3965 (Ki = 3.2 nM, group A4).
A significant decrease of [11C]niacin uptake in the AZD3965-challenged group (group A4) compared to the no-niacin-added group (group A1) was observed in heart and eyes (2-fold) (Fig. 3, Fig. 4E and F) with a concomitant statistically significant increase of kidney radioactivity uptake (1.3-fold).
3.4. Biodistribution study in mice receiving [11C]niacin following protocol A (intravenous administration, groups A1-A4)
Ex vivo biodistribution analysis conducted at 70 min post-[11C]niacin administration in groups A1-A4 showed radioactivity uptake >20% of the injected dose per gram (%ID/g) in kidney, heart, and liver (Fig. 5). Kidney uptake was three-fold higher than heart and liver uptake in group A1 (Fig. S3A). No gender differences (groups A1 and A2) were observed in the ex vivo biodistribution (Figs. 5, S3A and Table S9). Biodistribution analysis of niacin-challenge mice showed a decreased kidney/liver ratio uptake from 3 to 1 (Fig. S3A) compared to group A1 and no changes on kidney/heart ratio between the two groups. Biodistribution analysis of AZD3965-challenged group showed an increase of kidney/heart ratio from 3 to 17 (Fig. S3A) compared to group A1 and no changes on kidney/liver ratio between the two groups. Ex vivo biodistribution profile of kidney/liver and kidney/heart ratios were in agreement with those obtained from last PET imaging frame (50–60 min, Fig. S3B).
3.5. PET image analysis in mice receiving [11C]niacin following protocol B (OG administration, groups B1-B2)
To establish the relative contribution of intestinal transporters toward niacin absorption, we investigated the gastric emptying, intestinal absorption and tissue distribution of OG delivered [11C]niacin (Fig. 6A–C) in group B1 (no-carrier-added (NCA) [11C]niacin) and group B2 (carrier-added (CA) [11C]niacin - Fig. 2B).
Gastric emptying by 1 h was ~20% (Fig. 6A) with no significant differences between groups. Dynamic PET studies showed that intestinal absorption was a limiting factor in determining the whole body [11C]niacin distribution. In the NCA group (group B1), once the radioactivity was delivered into the intestine, ~40% administered radioactivity was trapped by the intestine (Fig. 7A–C) and low radioactivity was observed in the systemic organs.
The OG administration of CA [11C]niacin (group B2) showed a reduced intestinal trapping of only 3% of the total administered radioactivity (Figs. 6B and 7D-F). CA [11C]niacin entering in the systemic circulation was distributed through the mouse body. Renal, liver and heart uptake in group B2 was between 1.5- and 4-fold higher than B1 group from 20 to 120 min. Among those organs, the liver showed the highest uptake with a rapid accumulation in the first 20 min followed by a plateau up to 120 min (Fig. 6). The kidney and hearts showed a rapid uptake in the first 10 min followed by a quick washout.
As expected, group B2 showed a significantly higher excretion of [11C]niacin than B1 group with 20% versus 2% of the total administered radioactivity excreted in 120 min (Fig. 6C). This suggests that the high concentration of niacin doses administered to mice resulted in a faster elimination through the kidneys to the urinary bladder. These results demonstrated that the ADME of [11C]niacin administered orally is influenced by the content of niacin in the formulation.
4. Discussion
In developing our carbon-11 labelling strategy to produce [11C]niacin, we initially considered two previously described [14C]niacin labelling procedures: a) the carboxylation of the 3-lithiopyridine, produced in situ by reacting t-butyl‑lithium and 3-bromopyridine, with [14C]CO2 [30] and b) the cyanation of 3-bromopyridine with [14C]KCN followed by acid hydrolysis [31,32].
The first strategy uses air and moisture sensitive organolithium reagents that are inconvenient for a clinical setting use, as the reagents need to be handled with great care to avoid contamination by atmospheric carbon dioxide and moisture resulting in poor yields and lower molar activities of 11Cradiotracers. By applying the same approach to a commercially available 3-pyridylmagnesium bromide (0.25 M, THF), [11C]niacin was not obtained. The alternative strategy to obtain [11C]niacin from [11C]KCN has not been pursued as its production is impractical, requiring long synthesis times (15–25 min), multiple steps from cyclotron-produced [11C]CO2 and dedicated infrastructure, only available in a few radiochemistry laboratories world-wide, thereby limiting its use. Inspired by the recent developments in 11C-carboxilic labelling methods via boronic ester derivatives [27], we have applied this quick and efficient chemistry to the 11C isotopologous labelling of niacin directly from the easily accessible synthon: [11C]CO2.
IV administration of [11C]niacin to female mice revealed a high accumulation in heart, liver and kidneys. The radioactivity of these organs increased rapidly post-IV injection followed by a very slow wash out, indicative of trapping of the radiotracer. The uptake of [11C]niacin observed in vivo mirrors the known distribution of SMCT and MCT1 transporters in these organs. Among these tissues, we observed that the kidneys showed the highest uptake which is consistent with the high SMCT mRNA expression levels reported for the renal cortex and the outer stripe of outer medulla [7,33].
SMCT gene expression in mouse kidneys show that males have higher SMCT gene expression level than females [34]. Comparing groups A1 (female) and A2 (male), the renal uptake in our study showed a significant gender-difference, with male uptake being 1.3-fold higher than female mice (Fig. S2).
To examine the relationship between [11C]niacin distribution and monocarboxylate transporters, mice were challenged with niacin (group A3) prior to [11C]niacin PET. A significant decrease of [11C]niacin uptake in the niacin-challenge mice (group A3) was observed in kidneys, heart and eyes compared to the no-niacin-added control group A1. We postulate this phenomenon may be a consequence of saturation of niacin transporters, facilitating the clearance and excretion of [11C]niacin via the kidney and bladder. In niacin-challenge group the urinary excretion at 60 min was ~40 times higher than that in the no-niacin-added group confirming a fast elimination of [11C]niacin. These studies demonstrated that the amount of [11C]niacin excreted is dose dependent. In line with these results, the urinary excretion of IP injection of [14C]niacin (5 mg/kg) in rats reached 27% within 2 h and the challenge with niacin (500 mg/kg) increased urine excretion by 2.5-fold [35].
Based on these results, [11C]niacin might be a valuable tool for imaging renal, cardiac and liver function in humans, particularly in light of clinical studies suggesting a benefit of niacin use in pathologies such as chronic kidney [36,37] and liver diseases [38] and atherosclerosis [39,40].
Furthermore, overexpression of the niacin-transporter MCT1 has been shown in common types of cancer, including lymphoma, ovarian and breast cancer and MCTs are considered as promising drug targets for cancer treatment. Indeed, the selective MCT1-inhibitor AZD3965 has demonstrated a potent therapeutic activity across a number of lymphoma cell [41,42]. In the current study, AZD3965-challenged mice showed a decreased radiotracer uptake in MCT1-expressing organs such as heart and eyes and an increased renal uptake. We postulate that the increase in kidneys may be a consequence of saturation of MCT1 transporters in the body and that more [11C]niacin is available for uptake by renal SMCTs [7,33]. The urinary excretion of [11C]niacin in AZD3965-challenged group (group A4) was low (1% of ID at 60 min) and within the same range of no-niacin-added group (group A1) confirming that the renal uptake is controlled by only SMCT transporters. Thus, the assessment of [11C]niacin in cancer animal models could establish the role of MCT1 in tumour proliferation and malignancy.
A limitation of our preclinical study is that there are no data available of [11C]niacin's metabolites. Future studies focusing on the identification of plasma/blood/urine metabolites in rodents and humans will be crucial to further clarify the full in vivo profile of this novel radiotracer.
Humans and other mammals obtain niacin via the gastrointestinal absorption to maintain normophysiological function. Ingested niacin is absorbed by the intestinal epithelium and enters in the blood to be distributed into the body. Thus, to examine niacin gastrointestinal absorption and systemic biodistribution in vivo, [11C]niacin was orally delivered in mice. In the intestine, SMCT1 is predominantly expressed in terminal jejunum, ileum and the large bowel while SMCT2 is expressed in the jejunum with little or no expression in the duodenum or large intestine [43]. The expression of MCT1 is most intense in the cecum and colon, but low in the stomach and small intestine [6]. PET analysis of OG [11C]niacin revealed that once in the intestine, [11C]niacin was trapped in the small intestine and the body distribution was notably low. These results are supported by the in vitro findings of niacin uptake by intestinal epithelial cells whose function is dysregulated during niacin deficiency (pellagra), as evidenced by intestinal inflammation and diarrhea [24].
Instead, co-administration of macroscopic amounts of non-radioactive niacin together with [11C]niacin (CA group) led to a low intestinal trapping and high absorption within minutes. This is in line with the in vitro studies using human intestinal epithelial cells showing that unlabelled niacin causes a significant decrease in [3H]niacin uptake [24]. The CA [11C]niacin absorbed by the intestine entered the systemic circulation and was distributed throughout the body in liver, heart, kidneys. OG studies demonstrated that the amount of niacin excreted is dependent on the niacin concentration in the administered dose. This implies that the homeostasis of niacin in mice is finely-tuned, and the administration of high concentrations of niacin increases its trafficking and excretion.
5. Conclusions
Although niacin has been recognized as important for cellular growth, development and well-being, no data exists regarding its trafficking in vivo. Here, we have for the first time succeeded in applying the non-invasive PET imaging technique to the qualitative and semiquantitative evaluation of the whole-body pharmacokinetics and tissue-distribution of radiolabelled niacin in living mice. The isotopologous radiosynthesis of niacin using the short-lived radioactive carbon-11 was achieved using cyclotron-produced [11C]CO2 starting from commercially available precursor and reagents in a fully automated radiolabelling procedure. Then, we have advanced our understanding of the in vivo biodistribution and kinetics of [11C]niacin and the molecular mechanisms of niacin absorption via two routes of administration (IV versus OG), compared gender differences and analysed the effect of niacin- or AZD3965-challenge.
The current work lays the foundation for studying [11C]niacin in vivo, and the development of a new translational tool to study niacin trafficking in physiological and pathological conditions (e.g. dyslipidemia, cancer) in humans and understand adverse effect profile such as flushing, gastrointestinal and metabolic and ocular side effects. Additionally, it establishes a model system that can be used to investigate further factors such as age, vitamin supplements, drugs, food, physiological and pathological conditions that may differentially affect niacin's ADME.
Acknowledgements
This work was supported by Medical Research Council [MRC, MR/K022733/1], European Commission, FP7-PEOPLE-2012-ITN [316882, RADIOMI] and the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z]. This work was supported by Wellcome Trust Multi-User Equipment grant [212885/Z/18Z] and by EPSRC Programme Grant [EP/S032789/1]. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nucmedbio.2020.07.002.
Contributor Information
Salvatore Bongarzone, Email: salvatore.bongarzone@kcl.ac.uk.
Antony Gee, Email: antony.gee@kcl.ac.uk.
Appendix A. Supplementary data
Supplementary material
References
- 1.Bogan K.L., Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 2.Gopal E., Fei Y.J., Miyauchi S., Zhuang L., Prasad P.D., Ganapathy V. Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochem J. 2005;388:309–316. doi: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopal E., Miyauchi S., Martin P.M., Ananth S., Roon P., Smith S.B. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 4.Srinivas S.R., Gopal E., Zhuang L., Itagaki S., Martin P.M., Fei Y.J. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2) Biochem J. 2005;392:655–664. doi: 10.1042/BJ20050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tachikawa M., Murakami K., Martin P.M., Hosoya K., Ganapathy V. Retinal transfer of nicotinate by H+ -monocarboxylate transporter at the inner blood-retinal barrier. Microvasc Res. 2011;82:385–390. doi: 10.1016/j.mvr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwanaga T., Takebe K., Kato I., Karaki S., Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- 7.Yanase H., Takebe K., Nio-Kobayashi J., Takahashi-Iwanaga H., Iwanaga T. Cellular expression of a sodium-dependent monocarboxylate transporter (Slc5a8) and the MCT family in the mouse kidney. Histochem Cell Biol. 2008;130:957–966. doi: 10.1007/s00418-008-0490-z. [DOI] [PubMed] [Google Scholar]
- 8.Chidlow G., Wood J.P., Graham M., Osborne N.N. Expression of monocarboxylate transporters in rat ocular tissues. Am J Phys Cell Phys. 2005;288:C416–C428. doi: 10.1152/ajpcell.00037.2004. [DOI] [PubMed] [Google Scholar]
- 9.Pellerin L., Pellegri G., Bittar P.G., Charnay Y., Bouras C., Martin J.L. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 10.Bergersen L., Rafiki A., Ottersen O.P. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. 2002;27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- 11.Park S.J., Smith C.P., Wilbur R.R., Cain C.P., Kallu S.R., Valasapalli S. An overview of MCT1 and MCT4 in GBM: small molecule transporters with large implications. Am J Cancer Res. 2018;8:1967–1976. [PMC free article] [PubMed] [Google Scholar]
- 12.Simanjuntak M.T., Tamai I., Terasaki T., Tsuji A. Carrier-mediated uptake of nicotinic acid by rat intestinal brush-border membrane vesicles and relation to monocarboxylic acid transport. J Pharmacobiodyn. 1990;13:301–309. doi: 10.1248/bpb1978.13.301. [DOI] [PubMed] [Google Scholar]
- 13.Takanaga H., Maeda H., Yabuuchi H., Tamai I., Higashida H., Tsuji A. Nicotinic acid transport mediated by pH-dependent anion antiporter and proton cotransporter in rabbit intestinal brush-border membrane. J Pharm Pharmacol. 1996;48:1073–1077. doi: 10.1111/j.2042-7158.1996.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 14.Fishbein W.N., Merezhinskaya N., Foellmer J.W. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve. 2002;26:101–112. doi: 10.1002/mus.10168. [DOI] [PubMed] [Google Scholar]
- 15.Gass J.D. Nicotinic acid maculopathy. 1973. Retina. 2003;23:500–510. [PubMed] [Google Scholar]
- 16.Jampol L.M. Niacin maculopathy. Ophthalmology. 1988;95:1704–1705. doi: 10.1016/s0161-6420(88)32955-6. [DOI] [PubMed] [Google Scholar]
- 17.Domanico D., Verboschi F., Altimari S., Zompatori L., Vingolo E.M. Ocular effects of niacin: a review of the literature. Med Hypothesis Discov Innov Ophthalmol. 2015;4:64–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Prousky J., Millman C.G., Kirkland J.B. Pharmacologic use of niacin. J Evid Based Complement Alternat Med. 2011;16:91–101. doi: 10.1177/2156587211399579. [DOI] [Google Scholar]
- 19.Badawy A.A. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49:238–250. doi: 10.1093/alcalc/agu010. [DOI] [PubMed] [Google Scholar]
- 20.Boden W.E., Sidhu M.S., Toth P.P. The therapeutic role of niacin in dyslipidemia management. J Cardiovasc Pharmacol Ther. 2014;19:141–158. doi: 10.1177/1074248413514481. [DOI] [PubMed] [Google Scholar]
- 21.Vosper H. Niacin: a re-emerging pharmaceutical for the treatment of dyslipidaemia. Br J Pharmacol. 2009;158:429–441. doi: 10.1111/j.1476-5381.2009.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector R. Niacin and niacinamide transport in the central nervous system. In vivo studies. J Neurochem. 1979;33:895–904. doi: 10.1111/j.1471-4159.1979.tb09919.x. [DOI] [PubMed] [Google Scholar]
- 23.Henderson L.M., Gross C.J. Transport of niacin and niacinamide in perfused rat intestine. J Nutr. 1979;109:646–653. doi: 10.1093/jn/109.4.646. [DOI] [PubMed] [Google Scholar]
- 24.Nabokina S.M., Kashyap M.L., Said H.M. Mechanism and regulation of human intestinal niacin uptake. Am J Phys Cell Phys. 2005;289:C97–103. doi: 10.1152/ajpcell.00009.2005. [DOI] [PubMed] [Google Scholar]
- 25.Carlson L.A., Hanngren A. Initial distribution in mice of 3h-labeled nicotinic acid studied with autoradiography. Life Sci. 1962;3(1964):867–871. doi: 10.1016/0024-3205(64)90149-3. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzen A., Stannek C., Lang H., Andrianov V., Kalvinsh I., Schwabe U. Characterization of a G protein-coupled receptor for nicotinic acid. Mol Pharmacol. 2001;59:349–357. doi: 10.1124/mol.59.2.349. [DOI] [PubMed] [Google Scholar]
- 27.Riss P.J., Lu S., Telu S., Aigbirhio F.I., Pike V.W. Cu(I)-catalyzed (11)C carboxylation of boronic acid esters: a rapid and convenient entry to (11)C-labeled carboxylic acids, esters, and amides. Angew Chem Int Ed Eng. 2012;51:2698–2702. doi: 10.1002/anie.201107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sala-Rabanal M., Ghezzi C., Hirayama B.A., Kepe V., Liu J., Barrio J.R. Intestinal absorption of glucose in mice as determined by positron emission tomography. J Physiol. 2018;596:2473–2489. doi: 10.1113/JP275934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins P.B., Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem. 1972;247:778–783. [PubMed] [Google Scholar]
- 30.Machulla H.-J., Dutschka K. 11C-Labelled radiopharmaceuticals: synthesis and high pressure liquid chromatography of nicotinic-11C acid amide. J Label Compd Radiopharm. 1979;16:287–293. doi: 10.1002/jlcr.2580160208. [DOI] [Google Scholar]
- 31.Murray A., 3rd, Foreman W.W., Langham W. The halogen-metal Interconversion reaction and its application to the synthesis of nicotinic acid labeled with isotopic carbon. Science. 1947;106:277. doi: 10.1126/science.106.2751.277. [DOI] [PubMed] [Google Scholar]
- 32.Ravi S., Mathew K.M., Sivaprasad N. A rapid microwave induced synthesis of [carboxyl-14C]-nicotinic acid (vitamin B3) and [carbonyl-14C]-nicotinamide using K14CN. J Radioanal Nucl Chem. 2008;275:441–444. [Google Scholar]
- 33.Becker H.M., Mohebbi N., Perna A., Ganapathy V., Capasso G., Wagner C.A. Localization of members of MCT monocarboxylate transporter family Slc16 in the kidney and regulation during metabolic acidosis. Am J Physiol Ren Physiol. 2010;299:F141–F154. doi: 10.1152/ajprenal.00488.2009. [DOI] [PubMed] [Google Scholar]
- 34.Si H., Banga R.S., Kapitsinou P., Ramaiah M., Lawrence J., Kambhampati G. Human and murine kidneys show gender- and species-specific gene expression differences in response to injury. PLoS One. 2009;4:e4802. doi: 10.1371/journal.pone.0004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrack B., Greengard P., Kalinsky H. On the relative efficacy of nicotinamide and nicotinic acid as precursors of nicotinamide adenine dinucleotide. J Biol Chem. 1966;241:2367–2372. [PubMed] [Google Scholar]
- 36.Streja E., Kovesdy C.P., Streja D.A., Moradi H., Kalantar-Zadeh K., Kashyap M.L. Niacin and progression of CKD. Am J Kidney Dis. 2015;65:785–798. doi: 10.1053/j.ajkd.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Taketani Y., Masuda M., Yamanaka-Okumura H., Tatsumi S., Segawa H., Miyamoto K. Niacin and chronic kidney disease. J Nutr Sci Vitaminol (Tokyo) 2015;(61 Suppl):S173–S175. doi: 10.3177/jnsv.61.S173. [DOI] [PubMed] [Google Scholar]
- 38.Kashyap M.L., Ganji S., Nakra N.K., Kamanna V.S. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): novel use for an old drug? J Clin Lipidol. 2019;13:873–879. doi: 10.1016/j.jacl.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Tavintharan S., Kashyap M.L. The benefits of niacin in atherosclerosis. Curr Atheroscler Rep. 2001;3:74–82. doi: 10.1007/s11883-001-0014-y. [DOI] [PubMed] [Google Scholar]
- 40.Ruparelia N., Digby J.E., Choudhury R.P. Effects of niacin on atherosclerosis and vascular function. Curr Opin Cardiol. 2011;26:66–70. doi: 10.1097/HCO.0b013e3283410c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble R.A., Bell N., Blair H., Sikka A., Thomas H., Phillips N. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2017;102:1247–1257. doi: 10.3324/haematol.2016.163030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtis N.J., Mooney L., Hopcroft L., Michopoulos F., Whalley N., Zhong H. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget. 2017;8:69219–69236. doi: 10.18632/oncotarget.18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teramae H., Yoshikawa T., Inoue R., Ushida K., Takebe K., Nio-Kobayashi J. The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed Res. 2010;31:239–249. doi: 10.2220/biomedres.31.239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material