Abstract
Objective
To examine the impact of fasting and glucose tolerance on selected metabolic variables in children with spinal muscular atrophy (SMA) type II in a well state because of reports of glucose regulation abnormalities in SMA.
Study design
In this prospective pilot study, 6 children with SMA type II ages 7–11 years participated in an oral glucose tolerance test and a supervised medical fast during two overnight visits at the University of Utah. At baseline, a dual energy x-ray absorptiometry (DXA) scan was performed to determine body composition. Labs were obtained at baseline and in response to the respective interventions. Data analysis was descriptive. Pre- and post-fasting data were evaluated using Wilcoxon signed ranks.
Results
All children were variably obese at baseline, based on DXA scan. All 6 participants demonstrated hyperinsulinemia, and 3 of 6 participants met formal American Diabetes Association criteria for impaired glucose tolerance. Homeostatic insulin resistance calculations indicated that 5 of 6 participants were insulin resistant. All participants tolerated a monitored fast for 20 hours without hypoglycemia (blood glucose<54 mg/dL). Free fatty acids significantly increased between pre- and post-fasting, while several plasma amino acids significantly decreased during fasting.
Conclusions
Children with SMA type II, determined to be obese using objective variables, are at increased risk for impaired glucose tolerance, whether or not they visually appear obese. Further studies are needed to determine the prevalence of impaired glucose tolerance and tolerance for fasting within the broader heterogeneous SMA population and to develop appropriate guidelines for intervention.
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disease resulting in progressive muscular weakness and atrophy. SMA is classified into clinical subtypes based on maximum achieved motor milestones.1–3 Children with SMA type II typically present between 6–18 months of age; they achieve the ability to sit but never walk independently.3,4 Bulbar, feeding, and respiratory insufficiency occur at some point in the majority of patients with type II.5,6 Despite advances in life expectancy, attributed to advances and standardization of medical care 7–9, there is limited knowledge of altered metabolism and nutrition in SMA.
Patients with SMA have documented decreased lean muscle mass and increased fat mass in comparison with healthy peers regardless of body mass index.10,11 Increased visceral fat mass is a risk factor for insulin resistance and decreased glucose sensitivity in adults and children12,13 and has been associated with peripheral neuropathy.14–17 Healthy individuals rely on glycogen stores in the liver and muscle for short-term energy needs.18,19 Little is known about how diminished lean mass affects fat lipolysis and protein catabolism during periods of fasting in children and adults with severe neuromuscular disease. Impaired fatty acid metabolism has been observed in children with SMA during fasting.20,21 Poor tolerance for fasting (hypoglycemia, coma) has been observed in adults with various neuromuscular diseases including SMA.22,23 SMA and survival motor neuron (SMN) gene depleted mice demonstrated pancreatic defects and altered glucose metabolism affecting glucose sensitivity.24,25 Pancreatic tissue from infants with SMA type I recapitulated some of these findings.24 Given the convergence of such data, further study is warranted. The primary aims for this study were to explore whether children with SMA type II demonstrate impaired glucose tolerance after glucose loading or intolerance of fasting in a well state.
METHODS
Participants were admitted to the University of Utah Center for Clinical and Translational Science (CCTS) in Salt Lake City, Utah, for two overnight inpatient visits. These visits consisted of an oral glucose tolerance test (OGTT) visit and a fasting visit, separated by 8 weeks.
Participants were 7–11 years of age, consumed at least 50% of their caloric intake by mouth, and met clinical diagnostic criteria for SMA type II. Homozygous SMN1 deletion was documented in all participants; SMN2 dosage of 3 copies was confirmed at the Ohio State University Molecular Diagnostic Laboratory for all 6 participants. Participants were excluded if they were acutely ill, taking oral hypoglycemic agents, or diagnosed previously with impaired glucose tolerance. Parental consents and assents were obtained for all participants under University of Utah IRB (64793).
Oral Glucose Tolerance Test Visit
The first visit consisted of body composition analysis using dual energy x-ray absorptiometry (DXA) and a formal oral glucose tolerance test (OGTT). The evening prior, participants consumed a standardized meal and snack (14% protein, 54% carbohydrate, and 32% fat).
Following a 10-hour overnight fast, baseline blood samples were collected for: hemoglobin A1c, Insulin Growth Factor (IGF)-1, blood glucose, insulin, glucagon, plasma quantitative amino acids (PQAA), and cortisol. Samples were analyzed by ARUP Laboratories (Salt Lake City, UT) using standardized clinical protocols. For safety purposes, baseline and final glucose labs were also analyzed at bedside (YSI 2300 STAT PLUS, YSI Incorporated, Yellow Springs, Ohio). Participants consumed an oral glucose load of 1.75g glucose/kg body weight (maximum dose 75 g) following baseline lab collection. Subsequent blood samples were collected at 30, 60, 90, 120, and 180 minutes and analyzed for: glucose, insulin, glucagon, PQAA, and cortisol. The first and last urine voids were collected and assessed for urinary ketones using Ketostix reagent strips (Bayer HealthCare, Mishawaka, Indiana). OGTT test results were evaluated using American Diabetes Association guidelines and reference values for non-obese children.26, 27
DXA and Anthropometrics
Norland DXA (XR-36 software version 3.3.1, Fort Atkinson, Wisconsin) for small subjects was used to assess whole body composition (percent body fat). Body fat percentiles developed for 8–11 year olds using 1999–2004 National Health and Nutrition examination Survey (NHANES) data from whole body DXA scans (Hologic; Bedford, Massachussetts) were used to determine over fat (>85th percentile) and obese (greater than 95th percentile) classifications based on sex.28
Additional anthropometric measures included: segmental length, arm span, weight, abdominal circumference, chest circumference, ulnar length, mid-arm circumference, and triceps skinfold thickness. Length and circumference measures were obtained using a nonstretchable tape measure to the nearest mm. Triceps skinfold thickness was measured using the Lange skinfold caliper (Santa Cruz, CA) to the nearest mm on the right side. Measurements were obtained by trained study staff using standard assessment methods.29
Body mass index (BMI) for age percentiles were determined using Center for Disease Control (CDC) growth charts.
Per CDC criteria, a BMI for age greater than the 85th percentile was considered overweight; a BMI greater than the 95th percentile was considered obese.
Three-day dietary record
A three-day dietary record for two weekdays and one weekend day was obtained from participants prior to their OGTT visit. Diet records were analyzed using Food Processor nutrition analysis software (version 10.5.2, 2009 ESHA Research, Salem, Oregon).
Insulin resistance and hyperinsulinemia standards
Homeostatic model assessment for insulin resistance (HOMA-IR) was used to evaluate insulin resistance. Insulin resistance was calculated as equaling fasting glucose (mg/dL) x fasting insulin (μU/mL)/ 405.30 Insulin resistance levels were compared with HOMA-IR cut-off values in obese children and adolescents (2.67 for prepubertal children, 3.82 for pubertal females, and 5.22 for pubertal males).31 Insulin levels at 0, 30, 60, 90, and 120 minutes during the OGTT were totaled.32 Hyperinsulinemia was defined as an insulin sum greater than 300 μU/mL.31
Fasting Visit
During the second visit, participants underwent a medically supervised 20 hour fast after receiving the same standardized evening meal with snack. Initial fasting lab samples were analyzed for: insulin, glucose, epinephrine, norepinephrine, cortisol, glucagon, free fatty acids, and PQAA. Blood samples were collected every two hours for glucose, insulin, and cortisol. Additional samples were collected every 4 hours for glucagon, free fatty acids, and PQAA. Epinephrine and norepinephrine samples were collected at 4 hours, 12 hours, 16 hours, and at 20 hours after beginning the fast. Glucose labs were analyzed at the bedside using YSI monitors. All other labs were analyzed by ARUP Laboratories. Each void was tested for urinary ketones using Ketostix. For initial and final voids, urine samples were collected and sent to ARUP Laboratories for complete urinalysis.
Data Collection and Statistical Methods
Study data were collected and managed using REDCap (Research Electronic Data Capture) .33 Descriptive statistics were used to evaluate pilot data using Microsoft Excel (Version 14). Pre- and post-fasting data were evaluated using Wilcoxon sign ranks. Significance was set at P<0.05 for all comparisons; no adjustments were made for multiple testing as this analysis was considered to be exploratory in a relatively small sample size.
RESULTS
Sex, ethnicity, race, and age were reported on the parental consent form for each participant. Six participants were enrolled in the study: 4 males and 2 females. All six participants completed both study visits. Participants were between 7 to 11 years old (mean age 8.9±1.7 years). Ethnicity and race included: 5 Non-Hispanic Caucasian, and 1 Hispanic African American. Four participants (3 males, 1 female) were considered prepubertal. One female and one male were considered pubertal based on age and Tanner stage. Only one patient had a gastrostomy tube. Tube placement was for supplemental nighttime feeds and consisted of less than 50% of total caloric intake. Per patient and parental report, participants did not have issues with swallowing, with one exception after an illness. However, a videofluoroscopic swallow study performed prior to study enrollment for this participant was normal. Three of 6 participants were taking valproic acid and l-carnitine as a putative disease- modifying therapy for SMA.
Several participants employed the use of respiratory therapies. Two participants, A and B, used BiPAP 6–12 hours daily. The other 4 participants reported never using BiPAP. Participant B used cough assist daily. All 6 participants reported the use of cough assist or vest therapy as needed during illness.
Frequency and duration of physical activity was reported. At the first visit, 5 participants reported at least 30 minutes of range of motion activity daily. All participants spent at least 30 minutes in a stander at least twice a week. Other physical activity included swimming 1–2 times/week for at least an hour for two participants. One innovative physical activity reported by one participant included a supported harness system attached to a treadmill for supported walking. Only one participant, F, reported at least an hour of physical activity daily.
DXA and Anthropometrics
DXA results, BMI, and BMI classification for each participant are included in Table I. Five of 6 participants were classified as overweight or obese based on BMI for age percentile greater than the 85th percentile. DXA scan results presented average lean body mass of 10.44 ± 6.93 kg. Average total fat mass was 24.82 ± 4.65 kg. Percent body fat, calculated using DXA scan results, averaged 71.6±13.1% body fat. Using NHANES DXA scan comparative data from children 8–11 years of age for body composition reference range criteria, all study participants’ percent body fat exceeded the 95th percentile and thus are considered obese.
Table 1.
Baseline labs, body composition and insulin resistance measures for SMA type II pilot study(n=6)
| Participant | BMI(kg/m2) | BMI for age classification | Body fat (%) | HgA1c (%) | IGF-1 (ng/mL) | HOMA- IR OGTT | HOMA IR Fasting | 120min Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| A | 15.6 | Normal | 56.66 | 5.3 | 477 | 3.42B | 1.65B | 169 |
| B | 18.9 | Overweight | 83.43 | 5.7 | 261 | 7.89C | 4.45C | 127 |
| CA | 23.3 | Obese | 76.10 | 5.1 | 147 | 9.38C | 8.94C | 185 |
| DA | 21.3 | Obese | 82.14 | 4.9 | 127 | 3.78C | 8.50C | 155 |
| EA | 23.0 | Obese | 77.54 | 5.4 | 198 | 6.23C | 6.48C | 115 |
| F | 20.8 | Overweight | 53.52 | 4.9 | 234 | 4.94B | 6.20B | 127 |
Oral Glucose Tolerance
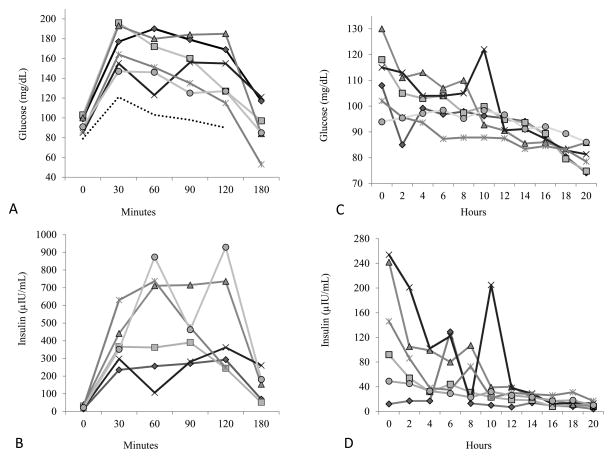
Baseline hemoglobin A1c and IGF-1 values (Table I) were within normal ranges with the exception of participant B. This participant’s hemoglobin A1c level was slightly elevated and considered to be in the pre-diabetic range. Participant D reached laboratory criteria for hypoglycemia following completion of the OGTT with a YSI glucose level of 52.4 mg/dL (2.9 mmol/L), but remained clinically asymptomatic. However, an increased amplitude of hand tremor (polyminimyoclus) was evident as she fed herself her lunch. Interestingly, this participant was the only child unable to consume the prescribed amount of glucose drink (leaving 5 ml). Glucose ranges during the OGTT are summarized in Figure 1. Glucose values were above documented normal blood glucose reference values in normal children during OGTT. Oral glucose tolerance testing indicated that 3 of 6 participants exhibited impaired glucose tolerance. Two of the 3 participants with OGTT determined impaired glucose tolerance had been on stable doses of valproic acid for several years. None of these children had a family history of diabetes.
Figure 1.
(A) Individual plasma blood glucose levels during the oral glucose tolerance test in 6 children with SMA type II. The dashed bottom line represents the mean value for healthy children.27 The dotted line at 140 mg/dL represents the cut-off for impaired glucose tolerance at 120 minutes. (B) Individual plasma insulin levels during the oral glucose tolerance test for the same 6 children. (C, D) Individual plasma blood glucose and insulin levels, respectively during the medically supervised 20-hour fast in 6 well children with SMA type II.
Basal glucose and insulin levels were analyzed using the HOMA-IR assessment model. Values for determining prepubertal and pubertal insulin resistance for obese children were used for comparison in Table I. Four of 6 and 5 of 6 participants were considered insulin resistant based on this model for basal glucose and insulin levels at the OGTT and fasting visits, respectively.
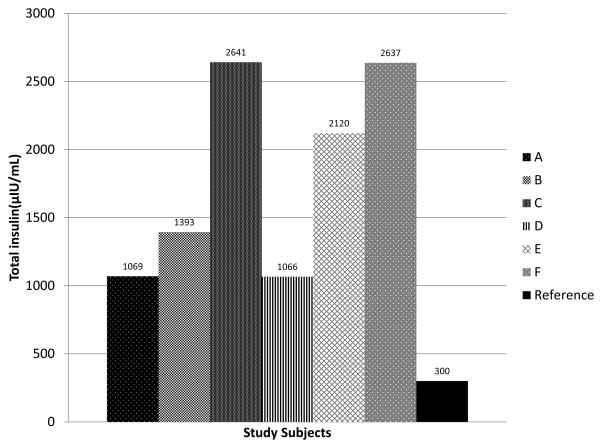
All of the participants demonstrated hyperinsulinemia during the OGTT. The sum of insulin levels at 0, 30, 60, 90, and 120 minutes were calculated for each participant (Figure 2).
Figure 2.
The sum of insulin levels during the oral glucose tolerance test (at 0, 30, 60, 90, and 120 minutes) for 6 children with SMA type II. All of the participants exceeded established cut- off values for children for hyperinsulinemia.31
Three-Day Dietary Records
Five participants submitted dietary records for review. The mean and standard deviation of caloric intake for patients was 1328.2±90.4 calories (39.9±10.8 kcal/kg body weight and 9.8 ±1.1 kcal/cm height). Protein intake was 55.3± 13.8 g (1.6± 0.4 g protein/kg body weight). Percent energy intake of fat and carbohydrates were 35.9± 2.4% and 47.9±6.0%, respectively. Fiber intake was 12.1±4.2 g.
Fasting
All participants completed 20 hours of medically supervised fasting without glucose levels falling below 54 mg/dL (3.0 mmol/L). Symptoms were monitored and recorded at least every 2 hours. The most commonly reported symptom was hunger/stomach ache for 4 participants. One participant complained of a mild headache at 16 hours that resolved by the 18 hour mark; one participant reported being tired at 20 hours. After the fast concluded, a noticeable increase in polyminimyoclonus amplitude was observed in one participant. Group mean insulin levels fell to within normative fasting insulin values after 14 hours of fasting. Figure 1 depicts insulin and glucose levels during OGTT and Fasting visits.
Many plasma metabolites and hormones changed significantly before and after fasting. (Pre- and post-fasting values are summarized in Table II; available at www.jpeds.com). At the end of fasting, insulin values were significantly decreased (P<0.05). Alanine, branched chain amino acids (BCAA), methionine, and phenylalanine were among the amino acids significantly decreased after fasting (P<0.05). Free fatty acids were significantly increased after fasting (P<0.05). Glutamine, cortisol, norepinephrine, and epinephrine values remained relatively unchanged. Mean and standard deviation values of epinephrine pre- and post-fast and norepinephrine pre-and post-fast were 56.0±6.5 pg/mL (n=3) and 69.0±24.2 pg/mL (n=4), 199.5±116.9 pg/mL (n=6) and 222.6 ±130.0 pg/mL (n=5), respectively. Ketones were present in the urine of 3 of the 6 participants at the end of the fasting visit.
Table 2.
Summary of pre and post fast plasma metabolites and hormones in 6 children with type II SMA after a 20 hour fast
| Fed State (Pre Fast) | Fasted State (Post Fast) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Metabolite/hormone | median | Q1 | Q3 | Min | Max | median | Q1 | Q3 | Min | Max | P valueA |
| Glucose (mg/dL) | 111.5 | 102 | 118 | 98.9 | 130 | 79.9 | 74.8 | 85.8 | 74.1 | 86 | 0.03* |
| Insulin (uIU/mL) | 119 | 49 | 242 | 12 | 254 | 9.5 | 8 | 13 | 4 | 17 | 0.03* |
| Cortisol (ug/dL) | 6.9 | 1.8 | 12 | 1.6 | 14.1 | 4.7 | 4.5 | 4.9 | 3.6 | 9.7 | 0.56 |
| Free fatty acids(mmol/L) | 0.24 | 0.14 | 0.37 | 0.12 | 0.65 | 0.94 | 0.93 | 1.07 | 0.91 | 1.39 | 0.03* |
| Glutamine (umol/L) | 421.5 | 348 | 500 | 273 | 562 | 397 | 356 | 519 | 317 | 531 | 1 |
| Alanine (umol/L) | 508 | 378 | 723 | 314 | 773 | 185 | 167 | 240 | 151 | 275 | 0.03* |
| Arginine (umol/L) | 97 | 87 | 104 | 84 | 134 | 52.5 | 49 | 66 | 39 | 70 | 0.03* |
| Asparagine (umol/L) | 44 | 39 | 63 | 34 | 67 | 26.5 | 23 | 34 | 23 | 39 | 0.03* |
| Citrulline (umol/L) | 25.5 | 15 | 35 | 14 | 39 | 17.5 | 17 | 21 | 16 | 26 | 0.1 |
| Cystine (umol/L) | 29.5 | 23 | 34 | 19 | 42 | 35.5 | 32 | 39 | 24 | 41 | 0.31 |
| Glutamic acid (umol/L) | 69 | 40 | 93 | 26 | 110 | 44 | 19 | 84 | 13 | 85 | 0.03* |
| Glycine (umol/L) | 197 | 172 | 245 | 158 | 257 | 183.5 | 174 | 272 | 159 | 309 | 0.68 |
| Histidine (umol/L) | 72.5 | 67 | 77 | 57 | 87 | 62.5 | 59 | 69 | 58 | 88 | 0.43 |
| Isoleucine (umol/L) | 104 | 94 | 122 | 43 | 211 | 51.5 | 41 | 64 | 35 | 67 | 0.03* |
| Methionine (umol/L) | 38.5 | 21 | 42 | 17 | 43 | 14 | 14 | 15 | 12 | 20 | 0.03* |
| Leucine (umol/L) | 171 | 146 | 208 | 73 | 335 | 95 | 87 | 103 | 59 | 110 | 0.03* |
| Lysine (umol/L) | 204.5 | 153 | 226 | 123 | 289 | 107 | 97 | 111 | 85 | 148 | 0.03* |
| Ornithine (umol/L) | 57 | 52 | 77 | 36 | 88 | 29 | 25 | 35 | 21 | 37 | 0.03* |
| Phenylalanine (umol/L) | 80 | 58 | 87 | 52 | 88 | 40.5 | 38 | 50 | 30 | 51 | 0.03* |
| Proline (umol/L) | 331 | 241 | 444 | 213 | 533 | 99.5 | 96 | 119 | 86 | 126 | 0.03* |
| Serine (umol/L) | 134.5 | 121 | 144 | 109 | 147 | 117 | 106 | 128 | 100 | 130 | 0.07 |
| Taurine (umol/L) | 39 | 34 | 51 | 33 | 54 | 45.5 | 34 | 57 | 33 | 58 | 0.32 |
| Threonine (umol/L) | 135 | 111 | 165 | 93 | 182 | 64.5 | 59 | 85 | 45 | 104 | 0.03* |
| Tryptophan (umol/L) | 54.5 | 43 | 69 | 39 | 79 | 42 | 31 | 45 | 24 | 46 | 0.06 |
| Tyrosine (umol/L) | 113.5 | 65 | 125 | 52 | 127 | 35 | 33 | 43 | 29 | 52 | 0.03* |
| Valine (umol/L) | 376.5 | 276 | 415 | 156 | 602 | 171.5 | 158 | 206 | 114 | 249 | 0.03* |
Wilcoxon Sign Rank test pre and post fasting
P <0.05
Most glucagon levels obtained during the OGTT and fasting visits remained below detectable limits, <25 ng/L. Those glucagon values that were detectable did not demonstrate recognizable trends or associations with other study data.
DISCUSSION
Our pilot cohort study results suggest that obese children with SMA type II are at increased risk for insulin resistance and impaired glucose tolerance and demonstrate abnormal glucose metabolism in a well state. Specifically, the children in our study demonstrated hyperinsulinemia during the OGTT and insulin resistance based on HOMA-IR modeling. Half of the participants met diagnostic criteria for impaired glucose tolerance. Such findings emphasize the importance of obesity assessment and future glucose metabolism research in patients with SMA.
Hyperinsulinemia in children with SMA has serious potential long-term health implications. Hyperinsulinemia may precede the glucose intolerance exhibited in diabetes by 10 years.34,35 The development of type 2 diabetes often begins with hyperinsulinemia and normal or slightly elevated glucose that ultimately leads to impaired glucose tolerance and insulin resistance.36 In the United States, approximately 1 in 433 youth have diabetes; type 2 diabetes comprises 0.24/1000.37,38 Clinically, we are aware of 3 of 153 individuals with SMA type II in our natural history study database with documented type 2 diabetes. Two of them presented in childhood; one, an obese Hispanic girl, presented in diabetic ketoacidosis. LaMarca et al reported a 29-year- old male who likewise presented in diabetic ketoacidosis.39 Several other children or adolescents have manifested hyper and hypoglycemia and/or metabolic acidosis in a catabolic setting, but have not been formally evaluated.
In our study, hemoglobin A1c levels did not accurately reflect the degree of glucose metabolism abnormalities discovered with OGTT and do not appear to be a sensitive screening measure to assess patients with SMA for glucose intolerance in this setting.
The 6 participants in this study were all considered to be obese based on DXA determined body composition. Alternatively, using BMI for age percentiles on the CDC growth chart, only half were considered obese. Obesity is the largest determinant for insulin resistance in youth40 and the prevalence of metabolic syndrome rises with increased obesity.41 Previous research has indicated that children with SMA have increased fat mass and diminished lean mass when compared with healthy peers regardless of BMI.10,11 However, determining obesity in patients with SMA is difficult, because they may appear normal on growth charts and in person even in the setting of obesity. Based on previously published and unpublished research and clinical data, we suggest that children with SMA type II should be evaluated for obesity using appropriate anthropometrics/body composition analysis annually and especially with a BMI for age greater than the 25th percentile, gynecomastia, or increased abdominal fat and referred appropriately by their primary care provider.
Diet record analysis indicates that our patients are not eating excessive amounts of carbohydrates or calories in comparison with dietary reference intake (DRI) guidelines.42 However, our cohort’s dietary fiber intake comprised half the amount of fiber recommendations (25–31 g). Decreased fiber intake is associated with increased obesity risk and impaired glucose metabolism in adolescents.43 Decreased physical activity caused by decreased mobility and muscular weakness also puts patients with SMA and other neuromuscular diseases at increased risk of obesity. Only one of our participants met the Physical Activity Guidelines for Americans of one hour of physical activity daily.44 All participants in this study were confined to an electric wheelchair for the majority of each day, with limited opportunities for exercise especially during school days. As they get bigger, the significant assistance and equipment required to safely permit regular daily physical activity becomes an increasing obstacle.
We suspect that being overly fat plays a role in the observed glucose metabolism abnormalities. However, we cannot preclude developmental pancreatic defects, as implicated in mouse model studies. Bowerman et al demonstrated abnormalities of glucose metabolism in a SMA and SMN-depleted mouse model that may be potentially relevant to disease pathogenesis in humans.24,25 Their SMN 2B/− intermediate SMA mice were glucose intolerant; intolerance increased with age. These mice exhibited hyperglucagonemia, increased insulin sensitivity, and fasting hyperglycemia. Pancreatic islet cells in the SMA mice demonstrated a markedly increased proportion of glucagon-producing α cells and decreased number of insulin-producing β cells. This phenomenon was also observed in samples from 6 deceased human infants with SMA type I from our natural history cohort.24 Aged SMN-depleted mice (SMN+/−), which may be similar to milder forms of SMA, demonstrated weight gain, hyperinsulinemia, increased number of β cells, increased hepatic insulin and glucagon sensitivity, and fasting hyperglycemia.25
In contrast, the children in this pilot study did not exhibit hyperglucagonemia, glucagon sensitivity, or increased insulin sensitivity. Baseline insulin and glucose values were sufficiently high to indicate insulin resistance and half of the OGTT results indicated impaired glucose tolerance. Similar to aged SMN-depleted mice, our cohort demonstrated increased weight gain and hyperinsulinemia. We hypothesize that the SMN-depleted mice more closely mimic milder variants of SMA and we could potentially see more pronounced abnormalities with age. Pancreatic abnormalities may also exist, but this has not been evaluated.
Prior published research indicates a poor tolerance for fasting in patients with neuromuscular disease. Orngreen et al examined this issue in adults with various neuromuscular diseases including 4 adult patients with SMA type II who developed hypoglycemia within 14–23 hours of fasting.22 Bruce and Jacobsen studied two females with SMA type II with a history of hypoglycemia and coma. Both developed ketonuria during the course of a 12-hour fast, and one developed hypoglycemia.23 The authors of both studies concluded that neuromuscular patients with lower lean body mass were at increased risk for hypoglycemia.
Surprisingly, all participants in our study successfully completed the 20-hour fast without exhibiting laboratory evidence of hypoglycemia. One participant’s increased hand tremor may indicate subclinical symptoms/effects of fasting not been captured in the laboratory data. Age of participants may have been a factor because lean muscle mass decreases over time in SMA.10 Thus, a longer period of fasting may have been necessary in this younger age group to demonstrate fasting issues. We observed a significant drop in alanine, BCAAs, and phenylalanine, and increases in plasma free fatty acids and urinary ketones during the fast. BCAA concentrations in healthy adults become elevated during fasting due to transamination of BCAA in the muscle.43 We hypothesize that the decrease in BCAA in our cohort was due to the significantly reduced lean muscle mass in SMA. We also observed a significant decrease in alanine during fasting, a marker of energy metabolism. This decrease has also been noted by Orngreen for adult neuromuscular patients compared with healthy age-matched controls.22 Interestingly, the insulin levels in neuromuscular patients in their study were significantly higher in the fed versus the fasted state, an increase not observed in healthy adult controls. In our present study, insulin levels prior to fasting were also significantly higher in the fed versus fasted state.
Limitations of this study include the small number of participants in this pilot study which precluded recruiting a broad spectrum of patients with SMA type II. Patients with SMA type II requiring greater than 50% of calories via gastrostomy tube were specifically excluded to standardize intake. Due to this exclusion criterion and concerns of increased risks of decompensation in the setting of the prolonged fasting associated with this study, patients who were underfed or cachectic were less likely to participate. Thus, we cannot extend our observations to those patients. Additionally, study observations do not predict how our obese patients would fare with prolonged fasting in the setting of a catabolic state during illness or associated with several days of inadequate nutrition. DXA data obtained in this study used different DXA equipment than the NHANES data. We cannot preclude a potential contributing effect of valproic acid on weight and glucose metabolism abnormalities in 3 of 6 participants. Because all participants were considered obese by DXA determined body fat composition compared with healthy peers, implications from this study should be restricted to this cohort. The lack of appropriate age- matched controls limits our conclusions as to whether the metabolite changes observed in patients with SMA type II during fasting are clinically significant compared with healthy children. We were unable to obtain historical fasting reference data for these metabolites in healthy children for comparison.
In conclusion, all six children with SMA type II participating in this pilot study demonstrated hyperinsulinemia with insulin resistance and/or impaired glucose metabolism. Obesity is a growing problem in general, but especially in children with neuromuscular diseases including SMA, many of whom do not appear clinically obese. More research is needed to determine the extent of glucose metabolism and fasting abnormalities in children across the heterogeneous SMA population along with other neuromuscular diseases. Further studies are needed to determine the modifying impact that body composition may play in response to fasting and glucose loading in neuromuscular patients. Appropriate strategies for diagnosis, referrals for dietary counseling and clinical management of metabolism issues in children with SMA and other neuromuscular diseases warrant more attention to help ensure the best outcomes for our patients well into their adult years.
Acknowledgments
Funded by CureSMA, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD69045), and the National Center for Advancing Translational Sciences of the National Institutes of Health (1ULTR001067). K.S. has received additional clinical trial contract support from ISIS pharmaceuticals.
The authors wish to acknowledge Yue Zhang, PhD (University of Utah), for his assistance with statistical questions, and Thomas Prior, PhD (Ohio State University) for SMN2 dosage.
ABBREVIATIONS AND ACRONYMS
- SMA
Spinal muscular atrophy
- DXA
Dual energy x-ray absorptiometry
- OGTT
Oral glucose tolerance test
- SMN
Survival motor neuron
- HOMA-IR
Homeostatic model assessment for insulin resistance
- BMI
Body mass index
- PQAA
Plasma quantitative amino acids
- BCAA
Branched chain amino acids
Footnotes
Portions of the study were presented at the meeting of Families of Spinal Muscular Atrophy, <city>, <state>, June <days>, 2014.
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebecca Hurst Davis, Email: becky.hurst@utah.edu, Originally affiliated with University of Utah, Department of Neurology Pediatric Motor Disorders Research Program and Division of Nutrition; University of Utah, Division of Nutrition, 30 North 1900 East SOM 3R149, Salt Lake City, UT 84132, Phone: 801-585-1499, Fax: 801-587-9346, Currently affiliated with Intermountain Healthcare, Salt Lake City, UT.
Elizabeth A. Miller, Email: e.a.miller@utah.edu, Originally affiliated with University of Utah, Department of Neurology Pediatric Motor Disorders Research Program, Salt Lake City, UT, Currently affiliated with Shriner’s Children’s Hospital, Salt Lake City, UT.
Ren Zhe Zhang, Originally affiliated with University of Utah, Department of Neurology Pediatric Motor Disorders Research Program, Salt Lake City, UT., Currently affiliated with Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA.
Kathryn J. Swoboda, Email: kswoboda@mgh.harvard.edu, Center for Human Genetics Research, Department of Neurology, Massachusetts General Hospital, 185 Cambridge, Simches 5-238, Boston, MA 02114.
References
- 1.Munsat TL. International SMA Collaboration. Neuromuscul Disord. 1991;1:81. doi: 10.1016/s0960-8966(06)80015-5. [DOI] [PubMed] [Google Scholar]
- 2.Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord. 1995;5:3–5. doi: 10.1016/0960-8966(94)00075-k. [DOI] [PubMed] [Google Scholar]
- 3.Russman BS. Spinal muscular atrophy: clinical classification and disease heterogeneity. J Child Neurol. 2007;22:946. doi: 10.1177/0883073807305673. [DOI] [PubMed] [Google Scholar]
- 4.Pearn J. Classification of spinal muscular atrophies. Lancet. 1980;1:919–922. doi: 10.1016/s0140-6736(80)90847-8. [DOI] [PubMed] [Google Scholar]
- 5.Messina S, Pane M, De Rose P, Vasta I, Sorleti D, Aloysius A, Sciarra F, Mangiola F, Kinali M, Bertini E, Mercuri E. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. 2008;18:389–393. doi: 10.1016/j.nmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Chen YS, Shih HH, Chen TH, Kuo CH, Jong YJ. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J Pediatr. 2012;160:447–451. doi: 10.1016/j.jpeds.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Lemoine TJ, Swoboda KJ, Bratton SL, Holubkov R, Mundorff M, Srivastava R. Spinal muscular atrophy type 1: are proactive respiratory interventions associated with longer survival? Pediatr Crit Care Med. 2012 May;13(3):e161–5. doi: 10.1097/PCC.0b013e3182388ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oskoui M, Levy G, Garland CJ, Gray JM, O’Hagen J, DeVivo DC, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 9.Mannaa MM, Kalra M, Wong B, Cohen AP, Amin RS. Survival probabilities of patients with childhood spinal muscle atrophy. J Clin Neuromuscul Dis. 2009;10:85–89. doi: 10.1097/CND.0b013e318190310f. [DOI] [PubMed] [Google Scholar]
- 10.Poruk KE, Hurst Davis R, Smart AL, Chisum BS, LaSalle BA, Chan GM, et al. Observational study of caloric and nutrient intake, bone density, and body composition in infants and children with spinal muscular atrophy type I. Neuromuscul Disord. 2012;22:966–973. doi: 10.1016/j.nmd.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sproule DM, Montes J, Montgomery M, Battista V, Koenigsberger D, Martens B, et al. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscul Disord. 2009;19:391–396. doi: 10.1016/j.nmd.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 13.Weiss R, Dufour S, Taksall SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripheral Nerve System. 2012;17(Supplement):15–21. [Google Scholar]
- 15.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol. 2006;63:1075–1079. doi: 10.1001/archneur.63.8.noc50336. [DOI] [PubMed] [Google Scholar]
- 17.Bongaerts BWC, Rathmann W, Kowall B, Herder C, Stöckl D, Meisinger C, Ziegler D. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy. Diabetes Care. 2012;35:1891–1893. doi: 10.2337/dc11-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahill GF., Jr Starvation in man. Endocrinol Metab. 1976;5:397–415. doi: 10.1016/s0300-595x(76)80028-x. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PJ, Shah SD, Cryer PE. Insulin, glucagon, and catecholamines in prevention of hypoglycemia during fasting. Am J Physiol Endocrinol Metab. 1989;256:E651–E661. doi: 10.1152/ajpendo.1989.256.5.E651. [DOI] [PubMed] [Google Scholar]
- 20.Crawford TO, Sladkey JT, Hurko O, Besner-Johnson A, Kelley RI. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45:337–343. doi: 10.1002/1531-8249(199903)45:3<337::aid-ana9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Tein I, Sloane AE, Donner EJ, Lehotay DC, Millington DS, Kelley RI. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12:21–30. doi: 10.1016/0887-8994(94)00100-g. [DOI] [PubMed] [Google Scholar]
- 22.Orngreen MC, Zacho M, Hebert A, Laub M, Vissing J. Patients with severe muscle wasting are prone to develop hypoglycemia during fasting. Neurology. 2003;61:997–1000. doi: 10.1212/01.wnl.0000086813.59722.72. [DOI] [PubMed] [Google Scholar]
- 23.Bruce AK, Jacobsen E. Hypoglycaemia in spinal muscular atrophy. Lancet. 1995;346:609–610. doi: 10.1016/s0140-6736(95)91439-0. [DOI] [PubMed] [Google Scholar]
- 24.Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A, et al. Glucose Metabolism and Pancreatic Defects in Spinal Muscular Atrophy. Ann Neurol. 2012 Aug;72:256–68. doi: 10.1002/ana.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowerman M, Michalski JP, Beauvais A, Murray LM, DeRepentigny Y, Kothary R. Defects in pancreatic development and glucose metabolism in SMN-depleted mice independent of canonical spinal muscular atrophy neuromuscular pathology. Hum Mol Genet. 2014:1–13. doi: 10.1093/hmg/ddu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diagnosis and classification of diabetes mellitus. American Diabetes Association. Diabetes Care. 2012 Jan;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopf CF, Cresto JC, Dujovne IL, Ramos O, deMajo SF. Oral glucose tolerance test in 100 normal children. Acta Diabetologia Latina. 1977;14:95–103. doi: 10.1007/BF02581396. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Li Y, Freedman DS, Borrud LG, Flegal KM. National Health Statistics Reports. 43. Hyattsville, MD: National Center for Health Statistics; 2011. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999–2004. [PubMed] [Google Scholar]
- 29.Lee RD, Nieman DC. Nutritional Assessment. 4. Dubuque IA: McGraw Hill; 2007. pp. 191–230. [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Kurtoğlu S, Hatipoğlu N, Mazicioğlu M, Kendirici M, Keskin M, Kondolot M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J Clin Res Ped Endo. 2010;2:100–106. doi: 10.4274/jcrpe.v2i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruhama Y, Abe R. A familial form of obesity without hyperinsulinism at the outset. Diabetes. 1981;30:14–18. doi: 10.2337/diab.30.1.14. [DOI] [PubMed] [Google Scholar]
- 33.Harris Paul A, Taylor Robert, Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25 year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 35.Zimmet PZ, Collins VR, Dowse GK, Knight LT. Hyperinsulinaemia in youth is a predictor of type 2 (non-insulin dependent) mellitus. Diabetologia. 1992;35:534–541. doi: 10.1007/BF00400481. [DOI] [PubMed] [Google Scholar]
- 36.Beck-Nielsen H, Groop LC. Metabolic and genetic characterization of prediabetic states: sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:1714–1721. doi: 10.1172/JCI117518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 38.Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Larence JM, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37:402–408. doi: 10.2337/dc13-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaMarca NH, Golden L, John RM, Naini A, DeVivo DC, Sproule DM. Diabetic ketoacidosis in a adult patient with spinal muscular atrophy type II: further evidence of extra neural pathology due to survival motor neuron 1 mutation? J Child Neurol. 2013;28:1517–1520. doi: 10.1177/0883073812460096. [DOI] [PubMed] [Google Scholar]
- 40.Lee JM, Hermann WH, Okumara MJ, Gurney JG, Davis MM. Prevalence and determinants of insulin resistance among US adolescents. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 41.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Tanaksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and metabolic syndrome in children and adolescents. N Eng J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington (DC): The National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 43.Brauchla M, Juan WY, Story J, Kranz S. Sources of dietary fiber and the association of fiber intake with childhood obesity risk (in 2–18 year olds) and diabetes risk of adolescents 12–18 year olds: NHANES 2003–2006. J of Nutr and Metab. 2012 doi: 10.115/2012/736258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008 homepage on the Internet. cited 2015 Feb 12. Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf.
- 45.Adibi SA. Metabolism of branched-chain amino acids in altered nutrition. Metabolism. 1976;25:1287–1302. doi: 10.1016/s0026-0495(76)80012-1. [DOI] [PubMed] [Google Scholar]