Abstract
Purpose of Review
Concerning adverse neuromuscular effects, there are quite a few reports about the incidence and prevalence of chloroquine (CQ) and hydroxychloroquine (HCQ) myopathy. Given the above, I decided to explore the relationships of these drugs with skeletal muscle in an attempt to clarify how they affect the muscle now and in the future, as millions of people are using CQ and HCQ.
Recent Findings
The literature review identified 28 publications about CQ/HCQ myopathy, totaling 56 patients, from 1963 to 2020. A compilation of all patients was carried out by computing demographic features, clinical aspects, laboratory exams, and clinical evolution. All articles but two represented a large series about incidence and prevalence of the myopathy. Fifty-nine percent used QC, mean daily dose was 393 mg per day, and mean duration of treatment was 37 months. The predominant underlying diseases were rheumatoid arthritis (42.8%) and lupus erythematosus (26.8%). Respiratory distress was present in 12.5% in patients with proximal muscle weakness (87.2%). Dysphagia and cervical and axial weakness were observed in a smaller percentage. Creatine kinase was elevated in 60.7%, and EMG showed a myopathic pattern in 54%. Muscle biopsy showed a vacuolar pattern in 53.7%, and curvilinear bodies (CB) were the predominant ultrastructural finding (86.8%). After drug withdrawal, 85.4% of patients improved, and 12.7% died from other causes than myopathy.
Summary
CQ and HCQ myopathy has been known for a long time, but the incidence is low, being described only with long-term use. The use of these drugs for a short period has not been reported, although a prolonged elimination half-life of these drugs actually exists.
Keywords: Chloroquine, Hydroxychloroquine, Myopathy, Myotoxicity
Introduction
The world faces the deadly COVID-19 pandemic, the most challenging situation in a century that we have confronted. Currently, emerging therapies and repurposing of old drugs have been considered therapeutic strategies, including chloroquine (CQ) and hydroxychloroquine (HCQ) [1, 2], both drugs showing activity against COVID-19 in vitro [3]; however, the level of preclinical and clinical data is not strong and must be approved by a higher level of evidence [4, 5].
Specifically, at the beginning of 1943, the incidental repurposing of antimalarial drugs (quinacrine and CQ) was demonstrated after a crucial improvement of cutaneous rashes and arthritis in soldiers on malaria prophylaxis. Sometime later, in 1951, the first trial showed the efficacy of mepacrine in lupus erythematosus from 18 cases. However, nine of the results were very dramatic, and the other nine showed good-to-slight improvement [6].
CQ and HCQ are disease-modifying anti-rheumatic drugs (DMARDs) that suppress the clinical progression of several autoimmune diseases (e.g., systemic lupus erythematosus, rheumatoid arthritis, antiphospholipid syndrome, primary Sjögren’s syndrome, and sarcoidosis) [7].
Over the past few decades, these two compounds have also drawn attention as potential antiviral agents [8].
The suggested mechanism of action is raising the pH of the cell membrane, thus making it difficult for the virus to enter cells and interfering in the final stages of virus replication [3]. Also, they have an immunomodulatory effect and block the cascade of events that lead to acute respiratory distress syndrome [9]. Regardless of promising experimental studies, clinical trials have shown controversial effects [10, 11].
Peripheral nervous system manifestations caused by COVID-19 are rare, with the most common ones being hypogeusia (5.6%) and hyposmia (5.1%), while skeletal muscle complaints were reported in 10.3% of patients; however, electromyography, muscle MRI, or histopathologic findings were not mentioned [12]. Two recently published studies of COVID-19 in China reported myalgia or fatigue in 44–70% of hospitalized patients and increased creatine kinase(CK) in up to 33% of admitted patients [13, 14]. Additionally, a third of patients infected with other coronavirus infections manifested with myalgias and elevated CKs [15] and rhabdomyolysis [16]. So, it is possible that coronavirus infections may cause a viral myositis [17].
Although the good tolerability of CQ and HCQ is well-proven and considered safe even during pregnancy [18], they can cause side effects, some of which are classified as “non-serious” and do not impede the continuation of treatment. In contrast, others are considered “serious” and require drug suspension, which, unfortunately, does not always lead to their complete resolution. The first group is represented by gastrointestinal and cutaneous manifestations, while retinal, neuromuscular, and cardiac toxicities are part of the second group [19].
Concerning adverse neuromuscular effects, there are quite a few reports about the incidence and prevalence of CQ and HCQ myopathy. Given the above, I decided to explore the relationships of these drugs with skeletal muscle in an attempt to clarify how they affect the muscle now and in the future, as millions of people are using CQ and HCQ.
The review of the literature using PubMed let me identify 28 publications about antimalarial myopathy (only case reports or case series), totaling 56 patients, from 1963 to April 2020 [20–41]. A compilation of data from these studies was carried out and analyzed in a general context by computing demographic features (age, gender, previous diagnosis, duration of CQ/HCQ treatment), clinical aspects (muscle weakness, respiratory distress), laboratory tests (creatine kinase (CK) levels, morphological and ultrastructural findings from skeletal muscle biopsy, electromyography), and clinical evolution (improvement or death). Mean and median values were obtained for age, daily dose, treatment duration, and CK values, and percentage values in relation to gender, underlying disease, the drug used, clinical symptoms, CK elevation rates, respiratory distress, EMG features, muscle biopsy findings, and evolution were also analyzed.
Results
Two articles presented large studies of the incidence and prevalence of antimalarial myopathy, the others being case reports. These findings are detailed in Table 1 and Table 2.
Table 1.
Compilation of all patients’ data
| N | G | Age | Underlying disease | Drug | Dur treat (M) | DD (mg) | CK (IU/l) | MW | EMG | MB | EM | Dysp/dys | FW | CW/AW | RD | Improv/death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whisnant et al., 1963 [19] | 1 | F | 44 | DLE | CQ | 48 | 625 | Y | VM | ND | No | No | No | No | Y | ||
| 2 | F | 47 | DLE | CQ | 11 | 500 | Y | No VM | ND | No | No | No | No | Y | |||
| 3 | M | 51 | Knee arthritis | CQ | 6 | 250 | Y | No VM | ND | No | No | No | No | Y | |||
| 4 | F | 55 | DLE | CQ | 36 | 750 | Y | No VM | ND | No | No | No | No | Y | |||
| Begg et al., 1964 [20] | 5 | M | 28 | RA | CQ | 12 | 500 | Y, P limbs | Neurop | No SF | ND | No | No | No | No | Y | |
| Ebringer and Coville, 1967 [21] | 6 | F | 49 | Pain shoulder | CQ | 42 | 250 | 231 | Y, P limbs | VM | ND | No | No | Y | No | Y | |
| Rewcastle et al., 1965 [22] | 7 | M | 33 | SLE | CQ | 60 | 500 | ND | Y, P limbs | Myop | VM | Coiled material electron-dense membrane (CB?) | Y | Y | Y/Y | No | Y |
| Hicklin et al., 1965 [23] | 8 | F | 72 | RA | CQ | 24 | 400 | nl | Y, P limbs | Neurop | VM | ND | No | No | Y | No | Y |
| Eddie et al., 1966 [24] | 9 | M | 54 | Lumbar spondylosis | CQ | 7 | 240 | nl | Y, P limbs | Neurop | VM | ND | No | No | No | No | Y |
| Chapman et al., 1969 [25] | 10 | F | 64 | Non-specific papular rush | CQ | 66 | 400 | Y, P limbs | Myop | VM | ND | Y | No | No | No | Y | |
| Gerard et al., 1973 [26] | 11 | F | 57 | DLE | CQ | 36 | 700 | 2230 | Y, P L limbs | Neurop | VM | Membrane vermicular structure shape (CB?) | No | No | No | No | Y |
| Mastaglia et al., 1977 [27] | 12 | F | 55 | SLE/thymoma | CQ | 5 | 450 | 170 | Y, P limbs | Myopathic | VM | CB | No | Y | No | No | Y |
| Parodia et al.,1985 [28] | 13 | F | 28 | SLE | CQ | 24 | 500 | nl | Y, P limbs | Myop | VM | ND | No | No | No | No | Y |
| Estes et al., 1987 [29] | 14 | F | 58 | DLE | CQ | 72 | 500 | 206 | Y, P limbs | Neurop | NSF | CB | No | No | No | No | Y |
| 15 | F | 47 | SLE | CQ | 24 | 500 | 617 | Y, P limbs | Myop | VM | CB | No | No | Y | No | Y | |
| 16 | F | 54 | RA | HCQ | 24 | 200 | Y, P L limbs | Myop | No SF | CB | No | No | No | No | Y | ||
| 17 | M | 67 | RA | HCQ | 36 | 200 | 110 | Y, PL limbs | Myop | No SF | CB | No | No | No | No | Y | |
| 18 | F | 69 | RA | HCQ | 7 | 400 | 235 | Y, P limbs | Myop | No SF | CB | No | No | No | No | no | |
| 19 | M | 59 | SLE | HCQ | 24 | 400 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Seguin et al., 1995 [30] | 20 | F | 61 | RA | HCQ | 60 | 600 | nl | Y, P limbs | Myop | VM | CB | No | No | No | Y | Y |
| Avina-Zubieta et al., 1995 [31] | 21 | M | 40 | RA | CQ | 12 | 250 | nl | Y, P L limbs | ND | No SF | ND | No | No | No | No | Y |
| 22 | M | 71 | RA | CQ | 12 | 250 | nl | Y, P L limbs | Myop | VM | ND | No | No | No | No | Y | |
| 23 | F | 49 | RA | CQ | 12 | 250 | nl | Y, P limbs | ND | No SF | ND | No | No | No | No | Y | |
| Richards, 1998 [32] | 24 | F | 65 | SLE | HCQ | 6 | 400 | nl | Y | Myop | No SF | No CB | No | No | No | Y | Y |
| Nucci et al., 1996 [33] | 25 | F | 59 | RA | CQ | 10 | 250 | nl | Y, P limbs | Myop | VM | CB | No | No | No | No | Death |
| Stein et al., 2000 [34] | 26 | F | 72 | RA | HCQ | 24 | 400 | 336 | Y, P limbs | Neurop | VM | CB | Y | No | No | No | Y |
| Bolanos-Meade, 2005 [35] | 27 | M | 51 | Chronic graft-versus-host disease | HCQ | 2 | 800 | nl | Y | Myop | VM | ND | Y | No | Y | Y | Y |
| Casado et al., 2006 [36] | 28 | M | 68 | RA | CQ | 31 | 250 | NA | Y | Myop | VM | CB/MB | No | No | No | No | Death |
| 29 | F | 73 | RA | CQ | 23 | 250 | 1497 | Y | Myop | VM | MB | No | No | No | No | Y | |
| 30 | F | 77 | RA | CQ | 26 | 250 | NA | Y | Myop | VM | CB/MB | No | No | No | No | Y | |
| 31 | M | 77 | Psoriatic arthritis | HCQ | 74 | 400 | NA | Y | Myop | No SF | CB/MB | No | No | No | No | Death | |
| 32 | F | 78 | RA | CQ | 28 | 250 | NA | Y | Myop | No SF | CB/MB | No | No | No | No | Death | |
| 33 | F | 28 | SLE | HCQ | 12 | 400 | 207 | nl | nl | No SF | CB | No | No | No | No | Y | |
| 34 | F | 74 | RA | CQ | 62 | 250 | NA | Y | Neurop | No SF | CB | No | No | No | No | Y | |
| 35 | M | 57 | RA | CQ | 76 | 250 | 276.3 | nl | nl | No SF | CB/MB | No | No | No | No | Y | |
| 36 | F | 65 | RA | CQ | 26 | 250 | NA | nl | nl | No SF | MB | No | No | No | No | Y | |
| 37 | F | 61 | RA | CQ | 104 | 250 | NA | nl | nl | No SF | CB/MB | No | No | No | No | Y | |
| 38 | F | 59 | RA | CQ | 70 | 250 | 255.5 | Y | Myop | No SF | CB/MB | No | No | No | No | Y | |
| 39 | M | 39 | Pol R | CQ | 11 | 250 | NA | nl | nl | No SF | CB/MB | No | No | No | No | Y | |
| 40 | F | 53 | SS | CQ | 6 | 250 | NA | nl | nl | No SF | CB/MB | No | No | No | No | Y | |
| 41 | F | 64 | SS | CQ | 68 | 250 | 319.7 | nl | nl | No SF | CB | No | No | No | No | Y | |
| 42 | F | 62 | RA | CQ | 30 | 250 | NA | Y | Myop | No SF | CB | No | No | No | No | Y | |
| Mateen et al., 2007 [37] | 43 | M | 89 | Morphea | HCQ | 216 | 400 | 557 | Y, P U L | Myop | VM | ND | Y | No | Y | No | Y |
| Siddiqui et al., 2007 [38] | 44 | F | 88 | RA | HCQ | 60 | 600 | nl | Y, P limbs | Myop | VM | CB | No | No | No | Y | Death |
| Abdel-Hamid et al., 2008 [39] | 45 | F | 56 | SLE/SS/autoimmune hepatitis/cirrhosis | HCQ | 6 | 400 | 146 | Y, P limbs | Myop, myotonia | VM | CB | No | No | Y | Y | Death |
| 46 | F | 64 | PSS/ILD | HCQ | 48 | 400 | nl | Y, P limbs | Fib, myotonia | VM | CB | Y | No | Y | Y | Death | |
| Lonesky et al., 2009 [40] | 47 | F | 66 | RA | HCQ | 144 | 200 | 343 | Y, P limbs/Y | Myotonia | VM | CB | No | No | No | No | Y |
| Posada et al., 2010 [41] | 48 | F | 59 | DEL | HCQ | 7 | 500 | 218 | Y, P limbs | Myop | VM | CB | No | No | No | No | Y |
| Kwon et al., 2010 [42] | 49 | F | 70 | CTD | HCQ | 60 | 400 | 609 | Y, P/D limbs | Myop | VM | ND | No | No | Y | Y | Y |
| Vinciguerra et al., 2015 [43] | 50 | F | 63 | RA | HCQ | 2 | 400 | nl | Y, P limbs | Myop | NSF | Lipofucsin-like material | No | No | No | No | Y |
| Takizawa et al., 2018 [44] | 51 | F | 59 | SLE | HCQ | 8 | 300 | 437 | Y, P limbs | ND | ND | ND | No | No | No | No | Y |
| Khosa et a, 2018 [45] | 52 | F | 52 | SLE/PM | HCQ | NA | NA | Y | NA | VM | No CB | No | No | No | No | Y | |
| 53 | F | 76 | PM/CREST/SS/PBC | HCQ | NA | NA | Y | SM neurop | nl | No CB | No | No | No | No | Y | ||
| 54 | M | 59 | SLE | HCQ | NA | 1683 | Y, P L limbs | Neurop | VM | CB | No | No | No | No | Y | ||
| 55 | F | 62 | NA | HCQ | NA | NA | Y, P L limbs/Y | NA | VM | CB | No | No | No | No | Y | ||
| Shukla et al., 2019 [46] | 56 | F | 65 | CTD | HCQ | 36 | 200 | 1203 | Y, P/D limbs | Myop | VM | No CB | No | No | Y | No | Y |
N, number of patients; G, gender; CM, comorbity; RD, respiratory distress; MW, muscle weakness; FW, facial weakness; CW, cervical weakness; CK, creatine kinase (ref. value < 200 IU); EMG, electromyogram; MB, muscle biopsy; EM, electron microscopy; Myop, myopathic; Neuro, neuromyop; M, months; VM, vacuolar myopathy; SF, specific findings; L, lower; P, proximal; RA, rheumatoid arthritis, SS, Sjögren syndrome; DLE, discoid lupus erythematosus; SLE, systemic lupus erythematosus; PSS, progressive systemic sclerosis; ILD, interstitial lung disease; CTD, connective tissue disease; ND, not done; NA, not available; Dysp, dysphagia; Dys, dysarthria; Y, yes; CB curvilinear bodies; MB, myeloid bodies; nl, normal
Table 2.
Data compilation of CQ and HCQ myopathy based in the literature review from 1963 to April/2020
| Data | Values | n |
|---|---|---|
| Age (year) | 59(M) | 56 |
| 59 (Med) | ||
| 28–89 | ||
| G (F)% | 73.2 | 56 |
| Underlying disease (%) | ||
| RA | 42.8 | 56 |
| SLE | 26.8 | |
| ORD | 17.8 | |
| Misc | 12.6 | |
| Drug % | ||
| CQ | 58.9 | 56 |
| HQC | 41 | |
| DD(mg/d) | 393(M) | 56 |
| 400(Med) | ||
| DT(m) | 37(QC) | 56 |
| 24(HCQ) | ||
| PW/CW/Dys (%) | 87.2/17.8/8.9 | 56 |
| Elevated CK %/M/Med | 60.7/613/339 | 34 |
| RD % | 12.5 | 56 |
| EMG % | ||
| Myop | 54 | 50 |
| Neuromyo | 16 | |
| N | 2 | |
| Muscle biopsy % | ||
| Vacuolar | 53.7 | 54 |
| NSF | 46.2 | |
| MB (EM) % | ||
| CB | 86.8 | 38 |
| MB | 8 | |
| Impr/death (%) | 85.4/12.7 | 55 |
M, mean; Med, median; y, year; G, gender; F, female; CQ, chloroquine; HCQ, hydroxychloroquine; DD, daily dose; DT, duration treatment; m, months; PW, proximal weakness; CW, cervical weakness; Dys, dysphagia; OM, optic microscopy; EM, electron microscopy; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; ORD, other rheumatic diseases; Misc, miscellaneous; CQ, chloroquine; HQC ORD, other rheumatic disorders; CK, creatine kinase; RD, respiratory distress; Myop, myopathic; Neuromyo, neuromyopathic; N, neuropathy; NSF, no specific findings; CB, curvilinear bodies; MB, myeloid bodies; Impr, improvement
A previous and elegant study [26] selected 214 patients from a retrospective review of 4405 patients who initiated antimalarial therapy from January 1987 to April 1993 due to different rheumatic disorders. From these, only three patients presented with CQ myopathy from a total of 303 patient-years with an estimated incidence of 1 in 100 patient-years [26]. After 10 years, the second series of patients was published as a prospective study, which evaluated over 3 years all patients with rheumatic diseases who were taking antimalarial drugs. A total of 119 patients were included, 15 being detected with CQ or HCQ myopathy. The annual incidence was 1.2%, with a prevalence of 6.7% [31].
The mean age of affected patients was 59 years (median 59, varying from 28 to 89 years), with a clear predominance of females (73.2%). The preference for the use of CQ or HCQ was not explained in those previous reports: 58.9% used CQ and 41% used HCQ. The median daily dose was 400 mg per day (mean 393 mg), and the mean duration of treatment was 37 months (median 24 months). The underlying diseases treated with these drugs were rheumatoid arthritis, 42.8%; systemic and discoid lupus erythematosus (SLE/DLE), 26.8%; other rheumatic disorders (progressive systemic sclerosis (PSS), connective tissue disorder, psoriatic arthritis, polymyositis/calcinosis/Raynaud’s phenomenon, esophageal dysmotility/sclerodactyly/telangiectasia, Sjögren’s syndrome/primary biliary cirrhosis, PSS/ interstitial lung disorder), 17.8%; and a miscellaneous group (chronic graft-versus-host disease, shoulder pain, lumbar spondylosis, knee arthritis, morphea, polymyalgia rheumatica), 12.6% (Table 1).
Respiratory distress was present in 12.5% of patients as an initial symptom and associated with proximal muscle weakness (87.2%). Dysphagia, cervical weakness, and axial weakness were also observed in smaller percentages: 8.9%, 17.8%, and 1.8%, respectively.
In laboratory tests, 34 (60.7%) patients underwent measurement of CK level that was elevated in 60.7% (mean value, 339.5 IU; median value, 613 IU; ref. value < 130 to 170 IU). EMG was performed for 50 patients with a predominance of a myopathic pattern (54%), followed by a neuromyopathic pattern in 16% and neurogenic in only one (2%).
Muscle biopsy, analyzed by optical microscopy (OM), was performed in 54 (96.4%) patients showing a vacuolar pattern in 53.7% and non-specific findings in 46.2% (Fig. 1). A total of 38 (67.8%) muscle samples were processed for electron microscopy (EM); the predominant ultrastructural finding was the presence of CB in 38 (86.8%) patients, while myeloid bodies and non-specific findings were present only in three cases (8.0%). From 16 patients with a non-specific finding by OM, 15 (93.7%) demonstrated the presence of CB and myeloid bodies on ultrastructural examination. After withdrawing the drug (CQ or HCQ), 85.4% showed an improvement, and 12.7%% died (Table 2 and Table 3).
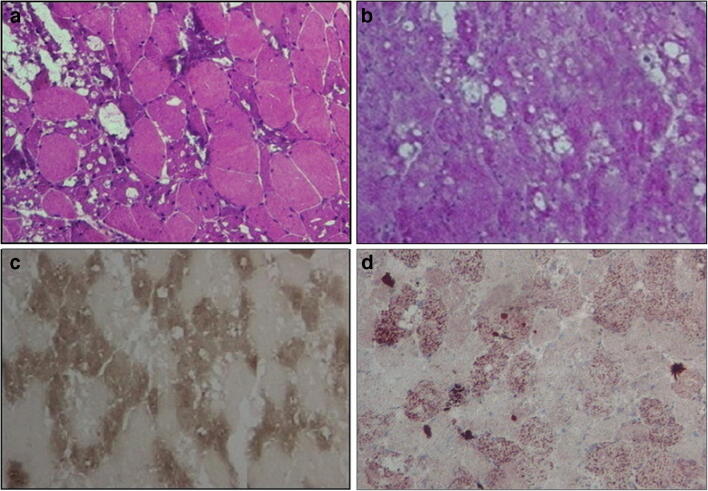
Fig. 1.
Muscle biopsy findings from patient with dermatomyositis after chloroquine use for 6 months. a H&E: fiber-size variation and multiple intracytoplasmic vacuoles. b PAS: vacuoles without glycogen. c ATPase 4.35: presence of vacuoles in type 1 and 2 fibers. d Oil Red O: vacuoles without lipides (personal archive from author)
Table 3.
Data from patients that evolved with death
| Patient | Gender | Age | Underlying disease | Drug | Dur treat (M) | DD (mg/d) | RD | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 25 | F | 59 | RA | CQ | 10 | 250 | No | Cardiac insufficiency and broncopneumonia |
| 29 | M | 68 | RA | CQ | 31 | 250 | No | Myocardial infarction |
| 31 | M | 77 | Psoriatic arthritis | HQC | 74 | 400 | No | Sepsis |
| 32 | F | 78 | RA | CQ | 28 | 250 | No | Heart failure |
| 44 | F | 88 | RA | HQC | 60 | 600 | Y | Obstructive airway disease |
| 45 | F | 56 | SLE/SS/autoimmune hepatitis | HQC | 6 | 400 | Y | Gallstones and sepsis |
| 46 | F | 64 | PSS/ILD | HQC | 48 | 400 | Y | Pulmonary fibrosis/Nocardia/Aspergillus |
CQ, chloroquine; HQC, hydroxichloroquine; Dur treat (M), duration of treatment; DD, daily dose; RD, respiratory distress; M, months; m/d, milligrams per day; Y, yes
Discussion
The muscle toxicity of CQ and HCQ is often unrecognized and is likely to be more common than described in the literature since the diagnosis of muscle toxicity attributable to these drugs is quite challenging. This is mainly because the underlying diseases and associated medications mask the symptoms of possible harmful effects on skeletal, cardiac, and smooth muscle, and also because of a lack of classic symptoms and/or morphological abnormalities of muscle. CQ has a large volume of distribution in the human body with an elimination half-life of 20–60 days and a tendency to accumulate in metabolically active tissues like the brain, muscle, skin, heart, and liver than in blood [9, 13]. Based on that, the recommended dose of 500 mg twice per day can approach dangerous thresholds with prolonged treatment compared with a lethal dose of chloroquine (5 g) in adults.
The pathways of antimalarial drugs involved in muscle toxicity, besides being complex, are far from being fully explained, and available data are scanty with no long-term monitoring of experimental models or detailed molecular analysis. Even now, it is difficult to relate the composite action mechanisms of these drugs to their efficacy in different autoimmune and infectious disorders.
HCQ and CQ belong to a class of drugs known as 4-aminoquinolines (containing an amino group attached to a quinoline ring). They are most notable for their roles as antimalarial drugs [9, 13]. Both drugs have a flat aromatic core structure. They are classified as weak bases due to the presence of a basic side chain, which contributes to the accumulation of these drugs in intracellular compartments, especially lysosomal organelles. Their amphiphilic properties elevate intralysosomal pH causing specific lysosomal disarrangement and autophagic dysfunctions, which result in vacuolar changes in muscle. They also specifically inhibit the lysosomal proteinase, cathepsin B, responsible for intracellular proteolysis [42].
The rimmed vacuolar changes found in muscle have been considered the most representative aspect in muscle biopsies of patients with myopathy induced by antimalarials; however, the absence of these vacuoles in some cases does not exclude the diagnosis [25]. This study demonstrated by OM the absence of vacuoles in half the sample (53.7%), which could be partially explained by the interval between the interruption of therapy and timing of the muscle biopsy beyond the technical difficulties in interpreting the biopsies. On the other side, their presence has also been described in other neuromuscular disorders, such as sporadic and familial inclusion body myositis, myofibrillary myopathy, oculopharyngeal muscular dystrophy, and some other myopathies [43–46].
Considering the high percentage of CB (86.8%) found on ultrastructural exam makes us think that EM is more sensitive for the diagnosis of CQ or HCQ induced myopathy than OM. Also, two previous reports [17, 21] observed the presence of coiled- and vermicular-shaped material very similar to CB on EM. So, considering both cases, the percentage of CB would increase to 92% of cases. By contrast, the literature described CB as a rare finding on muscle tissue seen in some but not all patients who have received treatment with antimalarials [29, 37]. CB has been described for the first time in different tissues, including muscle, of patients with late infantile and juvenile forms of Batten disease. They appear as tightly packed, short, curved linear bodies under an electron microscope [47–50].
Another relevant aspect is that the consequences of using CQ and HCQ are not restricted to the peripheral organelles of the cell and seem to involve other pathways. Nrf2 is a basic leucine zipper (bZIP) protein that regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation [51]. In vacuolar muscle disorders, such as autophagic vacuolar myopathy induced by CQ/HCQ, Nrf2 is persistently activated with negative consequences on organ functions. The chronic activation of Nrf2 in skeletal muscle results in changes in cellular redox potential, a response that contributes to muscle pathologies [52–54].
Looking at some of the results, I was initially surprised not to find seemingly typical clinical symptoms and biopsy changes in this compilation. A study conducted by Casado and colleagues suggested serial muscle enzyme screening of patients on these therapies as a way to identify patients at risk. All 15 patients with myopathy presented increased levels, mild to moderate, of CK or lactic dehydrogenase. By contrast, Kalajian and Callen [55] did not find an association between elevated serum muscle enzymes and underlying antimalarial-induced myopathy in patients taking CQ or HCQ.
In this review, seven asymptomatic patients with mildly elevated muscle enzymes (CK or DHL) and normal EMG presented curvilinear and myeloid bodies on EM as a unique finding. The isolated presence of this specific ultrastructural finding in skeletal muscle may not always signify a muscle disorder. However, it could be a muscular accumulation of CQ/HCQ or their metabolites, as demonstrated by Kumamoto et al., who observed dense membranous structures (CB) in soleus muscle fibers by EM in CQ-treated rats [31, 56].
Experimental studies have suggested that the absolute tissue levels of CQ are 2.5 times higher than those of HCQ. Thus, the depositing of the drug in several tissues with subsequent enzyme inactivation, which is the proposed mechanism for toxicity, might be more likely to occur with CQ [26]. Nevertheless, we did not observe crucial differences between compounds in terms of symptoms, morphological analyses, or clinical evolution. The number of patients using CQ or HCQ, 58.9% and 41%, respectively, was not very different; specifically, in relation to morphological findings, we identified almost the same number of cases with vacuolar myopathy in the group using CQ [15] and HCQ [17].
Concerning outcomes, prompt recovery was usual. Improvement after discontinuation of therapy occurred in 85.4% of cases, and seven deaths (12.7%) were reported. Apparently, five of the deaths were not related to the antimalarial drug (dose, treatment duration), but as a result of underlying disease complications, and the other two deaths occurred due to cardiac complications. Despite major drug interactions of CQ and HCQ with other medications leading to a greater risk of arrhythmia, there were no reports of this as being as the cause of death (Table 3).
Conclusions
CQ and HCQ myopathy has been known for a long time, but the reports are sporadic, and the incidence is low, being described only with long-term use. The use of these drugs even for a short period requires attention as a prolonged elimination half-life of these drugs can be harmful.
Data Availability Statement
We declare that the supplementary material is available as a supplementary table.
Compliance with Ethical Standards
Conflict of Interest
The author declares no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines and according to the Declaration of Helsinki.
Footnotes
This article is part of the Topical Collection on Clinical Pharmacology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease. Pharmacotherapy. 2020. 10.1002/phar.2398. [DOI] [PMC free article] [PubMed]
- 2.Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis ciaa237. 2020. 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- 4.Shukla AM, Archibald LK, Wagle Shukla A, Mehta HJ, Cherabuddi K. Chloroquine and hydroxychloroquine in the context of COVID-19. Drugs Context. 2020;9:2020-4-5. doi: 10.7573/dic.2020-4-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geleris J, Sun Y, Platt J, et al. Observational study of Hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taherian E, Rao A, Malemud C, Askari A. The biological and clinical activity of anti-malarial drugs in autoimmune disorders. Curr Rheumatol Rev. 2013;9:45–62. doi: 10.2174/1573397111309010010. [DOI] [PubMed] [Google Scholar]
- 8.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: na old drug against today’s diseases. Lancet Infection Diseases. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 10.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial. Int J Antimicrob Agents. 2020. 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed]
- 11.Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020:e201127. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- 13.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus- infected pneumonia in Wuhan, China. JAMA Epub. 2020 Feb 7. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 15.Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 16.Chen LL, Hsu CW, Tian YC, Fang JT. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int J Clin Pract. 2005;59(10):1162–1166. doi: 10.1111/j.1368-5031.2005.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020;94(22):959–969. doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- 18.Divala TH, Mungwira RG, Mawindo PM, et al. Chloroquine as weekly chemoprophylaxis or intermittent treatment to prevent malaria in pregnancy in Malawi: a randomized controlled trial. Lancet Infect Dis. 2018;18(10):1097–1107. doi: 10.1016/S1473-3099(18)30415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plantone D, Koudriavtseva T. Current and future use of Chloroquine and Hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: A mini-review. Clin Drug Investig. 2018;38(8):653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 20.Whisnant JP, Espinosa RE, Kierland RR, et al. Chloroquine neuromyopathy. Mayo Clin Proc. 1963;38:1–13. [PubMed] [Google Scholar]
- 21.Begg TB, Simpson JA. Chloroquine neuromyopathy. Brit med J. 1964;1:770. doi: 10.1136/bmj.1.5385.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebringer A, Colville P. Chloroquine Neuromyopathy associated with Keratopathy and retinopathy. Brit Med J. 1967;2:219–220. doi: 10.1136/bmj.2.5546.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rewcastle NB, Humphrey JG. Vacuolar myopathy: clinical, histochemical and microscopic study. Arch Neurol. 1965;12:570–582. doi: 10.1001/archneur.1965.00460300018003. [DOI] [PubMed] [Google Scholar]
- 24.Hicklin JA. Chloroquine neuromyopathy. Ann phys Med. 1968;9:189–192. doi: 10.1093/rheumatology/9.5.189. [DOI] [PubMed] [Google Scholar]
- 25.Eddie J, Ferrier M. Chloroquine myopathy. J Neurol Neurosurg Psychiatry. 1966;29:331–337. doi: 10.1136/jnnp.29.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman S, Ewen W. Chloroquine-induced myopathy. Br J Dermatol. 1969;81:217–219. doi: 10.1111/j.1365-2133.1969.tb16011.x. [DOI] [PubMed] [Google Scholar]
- 27.Gerard JM, Stoupel N, A Colher et AL. Morphologlc study of a neuromyopathy caused by prolonged chloroqume treatment. Eur Neurol. 1973;9:363–379. doi: 10.1159/000114244. [DOI] [PubMed] [Google Scholar]
- 28.Mastaglia FL, Papadimitriou JM, Dawkins RL et AL. Vacuolar myopathy associated with chloroquine, lupus erythematosus and thymoma. J Neurol Sci. 1977;34:315–328. doi: 10.1016/0022-510x(77)90149-6. [DOI] [PubMed] [Google Scholar]
- 29.Parodia A, Regestab G, Reboraa A. Chloroquine-induced Neuromyopathy. Dermatologica. 1985;171:203–205. [PubMed] [Google Scholar]
- 30.Estes ML, Ewing-Wilson D, Chou SM, et al. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med. 1987;82:447–455. doi: 10.1016/0002-9343(87)90444-x. [DOI] [PubMed] [Google Scholar]
- 31.Seguin P, Camus C, Leroy JP, et al. Respiratory failure associated with hydroxychloroquine neuromyopathy [letter] Eur Neurol. 1995;35:236–237. doi: 10.1159/000117135. [DOI] [PubMed] [Google Scholar]
- 32.Avina-Zubieta JA, Johnson ES, Suarez-Almazor ME, et al. Incidence of myopathy in patients treated with antimalarials. A report of three cases and a review of the literature. Br J Rheumatol. 1995;34:166–170. doi: 10.1093/rheumatology/34.2.166. [DOI] [PubMed] [Google Scholar]
- 33.Richards AJ. Hydroxychloroquine myopathy. J Rheumatol. 1998;25:1642–1643. [PubMed] [Google Scholar]
- 34.Nucci A, Queiroz LS, Samara AM. Chloroquine neuromyopathy. Clin Neuropathol. 1996;15(5):256–258. [PubMed] [Google Scholar]
- 35.Stein M, Bell MJ, Ang LC. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27:2927–2931. [PubMed] [Google Scholar]
- 36.Bolaños-Meade J, Zhou L, Hoke A, et al. Hydroxychloroquine causes severe vacuolar myopathy in a patient with chronic graft-versus-host disease. Am J Hematol. 2005;78(4):306–309. doi: 10.1002/ajh.20294. [DOI] [PubMed] [Google Scholar]
- 37.Casado E, Gratacos J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385–390. doi: 10.1136/ard.2004.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mateen J, Keegan M. Severe, reversible dysphagia from chloroquine and hydroxychloroquine myopathy. Can J Neurol Sci. 2007;34:377–379. doi: 10.1017/s0317167100006880. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui K, Huberfeld I, Weidenheim M, et al. Hydroxychloroquine-induced toxic myopathy causing respiratory failure. Chest. 2007;131:588–590. doi: 10.1378/chest.06-1146. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Hamid H, Oddis CV, Lacomis D. Severe hydroxychloroquine myopathy. Muscle Nerve. 2008;38(3):1206–1210. doi: 10.1002/mus.21091. [DOI] [PubMed] [Google Scholar]
- 40.Lonesky TA, Kreuter JD, Wortmann RL, et al. Hydroxychloroquine and colchicine induced myopathy. J Rheumatol. 2009;36(11):2617–2618. doi: 10.3899/jrheum.081315. [DOI] [PubMed] [Google Scholar]
- 42.Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20(7):773–774. doi: 10.1177/0961203310385553. [DOI] [PubMed] [Google Scholar]
- 43.Kwon JB, Kleiner A, Ishida K, et al. Hydroxychloroquine-induced myopathy. J Clin Rheumatol. 2010;16:28–31. doi: 10.1097/RHU.0b013e3181c47ec8. [DOI] [PubMed] [Google Scholar]
- 44.Vinciguerra C, Sicurelli F, Fioravanti A, et al. Hydroxychloroquine neuromyotoxicity: a case with rapid course and complete recovery. Neurol Sci. 2015;36:2293–2294. doi: 10.1007/s10072-015-2355-2. [DOI] [PubMed] [Google Scholar]
- 45.Takizawa N, Fujita Y. A rare but reversible cause of myopathy: Hydroxychloroquine induced myopathy. Arc Cas Rep C Med. 2018;3(3):158. [Google Scholar]
- 46.Khosa S, Khanlou N, Khosa S, et al. Hydroxychloroquine- induced autophagic vacuolar myopathy with mitochondrial abnormalities. Neuropathol. 2018;38:646–652. doi: 10.1111/neup.12520. [DOI] [PubMed] [Google Scholar]
- 47.Shukla S, Gultekin SH, Saporta M. Pearls & Oy-sters: Hydroxychloroquine-induced toxic myopathy mimics Pompe disease: critical role of genetic test. Neurology. 2019;92(7):e742–e745. doi: 10.1212/WNL.0000000000006914. [DOI] [PubMed] [Google Scholar]
- 48.Stauber WT, Hedge AM, Trout JT, et al. Inhibition of lysosomal function in red and white skeletal muscles by chloroquine. Exp Neurol. 1981;71:295–306. doi: 10.1016/0014-4886(81)90090-x. [DOI] [PubMed] [Google Scholar]
- 49.Dubowitz V, Sewry CA. Oldfors A. Fourth ed. Philadelphia: Saunders Elsevier Press; 2013. p. 592. [Google Scholar]
- 50.Neville E, Maunder-Sewry A, McDougall J, Sewell JR, et al. Chloroquine-induced cytosomes with curvilinear profiles in muscle. Muscle Nerve. 1979;2(5):376–381. doi: 10.1002/mus.880020509. [DOI] [PubMed] [Google Scholar]
- 51.Tebay LE, Robertson H, Durant ST, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues and energy status, and pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88:108. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khelfi A, Azzouzc M, Abtrouna R, et al. Direct mechanism of action in toxic myopathies. Ann Pharm Fr. 2017;75(5):323–343. doi: 10.1016/j.pharma.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Duleh S, Wang X, Komirenko A, et al. Activation of the Keap1/Nrf2 stress response pathway in autophagic vacuolar myopathies. Acta Neuropathol Commun. 2016;31;4(1):115. doi: 10.1186/s40478-016-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellezzaa I, Giambancoa I, Minellia A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress. (review) BBA - Molecular Cell Research. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Kalajian AH, Callen JP. Myopathy induced by antimalarial agents: the relevance of screening muscle enzyme levels. Arch Dermatol. 2009;145:597–600. doi: 10.1001/archdermatol.2009.60. [DOI] [PubMed] [Google Scholar]
- 56.Kumamoto T, Araki S, Watanabe S, Ikebe N, Fukuhara N. Experimental chloroquine myopathy: morphological and biochemical studies. Eur Neurol. 1989;29(4):202–207. doi: 10.1159/000116412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We declare that the supplementary material is available as a supplementary table.