Abstract
The World Health Organization (WHO) declared COVID-19 (novel coronavirus) as a global pandemic in the middle of March 2020, after the disease spread to more than 150 countries and territories leading to tens of thousands of cases within a couple of months. To date, there are no effective pharmaceutical treatments available. As well as that, the novel vaccines have not yet been approved as establishing their efficacy will take time. This study aims to summarize the evidence regarding corticosteroids such as dexamethasone for the treatment of COVID-19. Electronic searches were conducted on 7 September 2020 on Google Scholar database, MEDLINE and PubMed. A further search was conducted on the World Health Organization’s COVID-19 research article database. The findings of recent investigations that proved, both, the in vitro and in vivo activity of corticosteroids against COVID-19 and other coronavirus-related pneumonia were discussed. Low doses of corticosteroids (dexamethasone) could reduce the mortality in patients with severe COVID-19 disease; however, they had no effect on the mortality rate of those patients with a mild form of the condition. Moreover, the liberal use of corticosteroids was not advocated for, as high doses of the drug can cause more harm than good.
Keywords: Coronaviruses, SARS-CoV-2, Corticosteroids, COVID-19, Dexamethasone, In vivo
Introduction
In December 2019, a new pneumonia-like disease emerged in Wuhan, China. The clinical features of the patients, who were confirmed to be infected with the novel coronavirus (SARS-CoV-2), included a dry cough, fever and dyspnoea with lower respiratory tract involvement [1]. Subsequently, person-to-person transmission occurred [2]. The virus then rapidly spread to over 200 countries around the world, resulting in 27 million confirmed cases and 883,000 deaths globally according to a report issued by WHO on the 7th of September 2020.
The COVID-19 pandemic is considered the most severe global public health crisis since the influenza outbreak in 1918. Coronaviruses (CoV) are enveloped viruses that possess a single-stranded, positive-sense RNA with a genome ranging from 26 to 32 kb, the largest among all recognized RNA viruses [3]. Phylogenetically, these viruses can be classified into four genera: α-coronavirus, β-coronavirus, γ-coronavirus, and δ-coronavirus. Among these genera, the α-coronaviruses and β-coronaviruses mainly infect mammals, and generally lead to respiratory disease in humans and gastritis in animals. Contrastingly, the γ-coronaviruses and δ-coronaviruses are able to infect birds [4]. Currently, there are many strains of coronaviruses that infect humans: HCoVHKU1, HCoV-NL63, HCoV-229E, SARS-CoV and MERS-CoV [5]. SARS-CoV-2, which is also a strain, has recently been investigated in 2019.
Results have noted that SARS-CoV-2 could transmit more rapidly from person to person. Due to this, in March 2020, the World Health Organization (WHO) declared the outbreak of the novel coronavirus disease, SARS-CoV-2, to be a pandemic.
Methods
Electronic searches were conducted on the 7th of September 2020 on Google Scholar database, MEDLINE and PubMed. A further search was conducted on the World Health Organization’s COVID-19 research article database. The search items included coronavirus, SARS-CoV-2, corticosteroids and dexamethasone. This review was performed to analyze the recent literature which showed the effect of dexamethasone in the treatment of patients with coronavirus disease.
Results and Discussion
Characteristics of the Novel Coronavirus (SARS-CoV-2)
When analyzing the structure of the SARS-CoV-2 spike protein (the main antigenic component that is responsible for inducing host immune responses), it was found to be extremely similar to that of SARS. However, the affinity of SARS-CoV-2 spike glycoprotein (S protein) for the angiotensin-converting enzyme 2 (ACE-2) receptor, found on the surface of human cells, is approximately 20 times higher than that of the SARS’ S protein [6, 7].
The SARS-CoV-2 S protein component is a trimeric glycoprotein that consists of two subunits: S1 and S2. S1 is responsible for receptor binding, whilst the S2 subunit is responsible for membrane fusion. Additionally, the S1 subunit, which has two independent domains, the N- and C-terminals, shows much higher variability than S2 [8].
Hamming et al. identified the immune localization of ACE-2 protein, the functional receptor for the SARS-CoV-2, in human tissues. The most significant outcome of their study was that there was surface expression of ACE-2 on the lung alveolar epithelial cells and in the enterocytes of the small intestine. In addition to that, ACE-2 protein was identified in venous and arterial endothelial cells and in arterial smooth muscle cells in some organs [9]. The physiological role of ACE-2 protein in most organs has not been clarified but it is assumed to be an important regulator of cardiac function and of blood pressure [10]. Following entry into target cells, coronaviruses activate aryl hydrocarbon receptors (AhR) via an indoleamine 2,3-dioxygenase (IDO1)–independent mechanism. The activated AhR induces the upregulation of a number of AhR-dependent downstream effectors, resulting in a “Systemic AhR Activation Syndrome” (SAAS). The SAAS is comprised of inflammation, thromboembolism and fibrosis which consequently cause injury to many organs, and potentially death [11, 12].
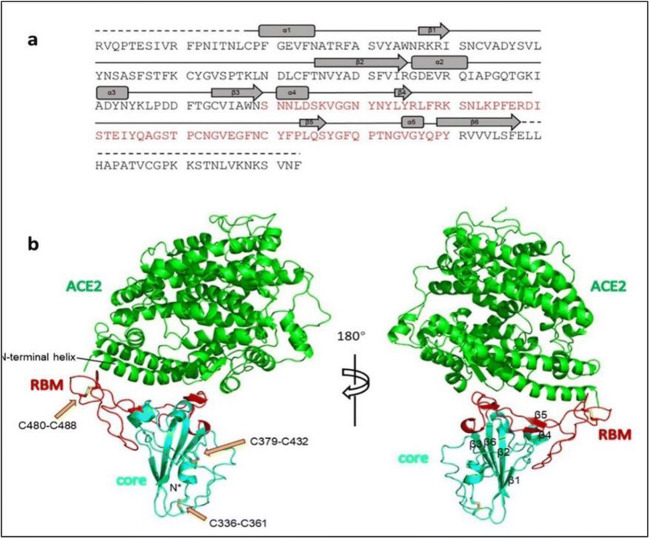
The atomic details of the interaction between the receptor-binding domain (RBD) and the peptidase domain of human ACE-2 protein (Fig. 1) clarify the significance of residual differences that enable effective cross-species infection and in addition human-to-human transmission [14]. Therefore, the soluble SARS-CoV-2 RBD is of probable use as an immunogen which is capable of neutralizing antibodies against SARS-CoV-2. This is considered a significant target site for developing vaccines and immunotherapeutic agents [14, 15]. Recently, Hoffmann et al. [16] documented the same fact that SARS-CoV-2 uses the SARS-CoV receptor ACE-2 for entrance and the serine protease TMPRSS2 for S protein priming. This reality was documented in China by Lu et al. [17], who also pointed out the similarity of the receptor-binding domain in both SARS-CoV and SARS-CoV-2 despite the amino acid mutations at several receptor-binding domains of the SARS-CoV-2 [18].
Fig. 1.
Sequence and secondary structures of SARS-CoV-2 RBD (a). The RBM is coloured red. Overall structure of SARS-CoV-2 RBD bound with ACE-2 (b). The N-terminal helix of ACE-2 responsible for binding is labelled [18]
The incubation time of the new coronavirus is between 3 and 7 days but may be up to 14 days in some cases [19]. The most common symptoms of SARS-CoV-2 infection are dry cough, fever, weakness and anosmia. The main laboratory findings include a raised white cell count with lymphopenia, elevated CRP, ferritin and increased d-dimers [20]. A dysregulated host immune response to SARS-CoV-2 lung infection leading to exuberant cytokine release (such as IL-1, IL-6 and TNF-α) and immune-mediated lung injury has been postulated as a critical pathogenetic factor in the progression to adult respiratory distress syndrome (ARDS) [21].
ARDS starts to develop about after 7 days of the disease, because of an explosive host immune response due to uncontrolled viral replication. The SARS-CoV-2 infection has sequential stages, with the progression, from one stage to the next, causing the deterioration in the health of the patient.
In phase I, which occurs at the time of inoculation and the initial introduction of the disease, patients begin to show non-specific symptoms: most commonly, a dry cough and fever. During this period, the virus multiplies and establishes residence in the host tissues by binding to the ACE-2 receptor in cells, with the respiratory system being primarily affected. As proliferation of the virus occurs, the immune system is simultaneously attempting to expel it from the lungs, and, in some cases, causing immune-mediated damage of the pulmonary structures, in the process [22].
Phase II is caused by the uncontrolled replication of the virus. This process is driven by the direct cytotoxicity of ACE-2 which acts as a catalyst for further activation of the immune system and therefore worsens the hyper-inflammatory state [23]. In addition to the other symptoms, the patient begins to demonstrate severe hypoxemia with a PaO2/FiO2 ratio (the ratio between arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2)) of less than 300 mmHg [24].
In phase III, granulocyte colony-stimulating factor (G-CSF); inflammatory cytokines and biomarkers such as IL-2, IL-6, IL-7 and TNF-α (tumour necrosis factor-α); macrophage inflammatory protein 1-α; d-dimer; C-reactive protein (CRP); and ferritin are remarkedly elevated in patients who are critically ill. During this phase, patients are susceptible to developing shock, respiratory failure and even cardiopulmonary collapse [25].
Corticosteroids/Dexamethasone
Corticosteroid drugs are a class of synthetic steroid hormones that are produced in the adrenal cortex in healthy individuals. Corticosteroids include glucocorticoids and mineralocorticoids; they are used to treat a wide range of diseases and symptoms [26]. Dexamethasone is a steroid compound, belonging to the corticosteroid class (more precisely a glucocorticoid). It is used in the treatment of numerous conditions, including chronic obstructive lung disease, severe allergies, rheumatic problems, asthma, several skin conditions, brain swelling and alongside antibiotics in tuberculosis (https://pubchem.ncbi.nlm.nih.gov/compound/Dexamethasone).
Chemical and Physical Properties of Dexamethasone
Dexamethasone is a white, odourless crystalline powder. It is stable when exposed to air. It is practically insoluble in water (≤ 0.1 mg/mL) [27]. The molecular formula is C22H29FO5. The molecular weight is 392.47 Da and is also chemically known as 1-dehydro-9α-fluoro-16α-methyl hydrocortisone, and the structural formula is shown in Fig. 2:
Fig. 2.
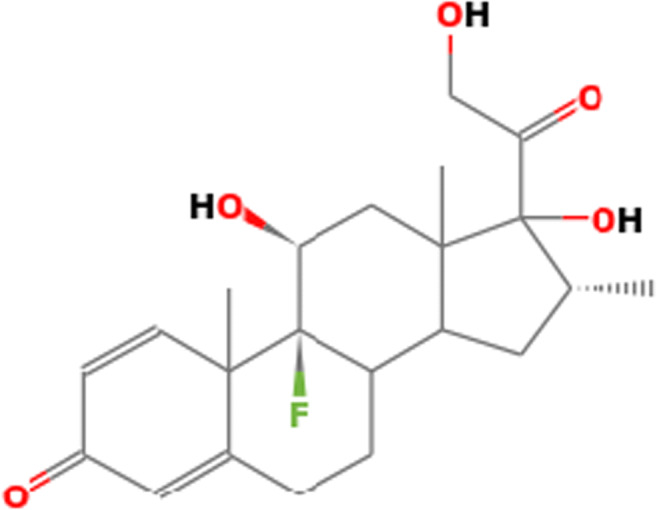
The chemical structure of dexamethasone (https://pubchem.ncbi.nlm.nih.gov/compound/Dexamethasone)
Posology
Dexamethasone is given in doses, ranging from 0.5 to 10 mg daily, with the dose being dependent on the disease being treated. In more acute conditions, a dose higher than (10 mg/day) may be required. The dose, also, depends on the patient’s response. In order to reduce the side effects, the lowest effective dose should be used [28].
Dexamethasone exerts a good inhibitory effect on inflammatory factors and is predominantly used as an auxiliary treatment for viral pneumonia. The action of dexamethasone mimics the action of the compounds the body produces to quell inflammation, naturally. It is about 25 times more active than other corticosteroid compounds [29], and this higher potency might be one of the reasons as to why dexamethasone has been shown to be effective in treating SARS-CoV-2 patients.
Moreover, dexamethasone is also stronger than nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen and aspirin. Dexamethasone is, both, anti-inflammatory and immunosuppressive, whilst NSAIDs only inhibit the vascular stage of inflammation [30].
The main anti-inflammatory effect of dexamethasone is to inhibit a pro-inflammatory gene that encodes for chemokines, cytokines, cell adhesion molecules (CAM) and the acute inflammatory response [31]. Dexamethasone possesses strong anti-inflammatory effects with weak mineralocorticoid property compared with other corticosteroid compounds [32]. Dexamethasone produces its anti-inflammatory effects by affecting two aspects: chemotaxis and vasodilation. Additionally, as aforementioned, following entry into cells, coronaviruses result in SAAS and medications, like dexamethasone, that are currently being researched appear to downregulate both the AhR and IDO1 genes and so further diminishing inflammation [11].
Mechanism of Action
The mechanism of action of dexamethasone depends on the dose used: the genomic (in the case of low doses) and non-genomic mechanisms (with high doses of dexamethasone). Most effects of dexamethasone are via the genomic mechanism which require a longer period, whereas dexamethasone effects through the non-genomic mechanism occur more rapidly, at the risk of more side effects [33, 34].
I—Genomic Mechanisms
Being small, lipophilic substances, dexamethasone can easily pass through the cell membrane by diffusion and enter the cytoplasm of the target cells and proceed by binding to glucocorticoid receptors in the cytoplasm.
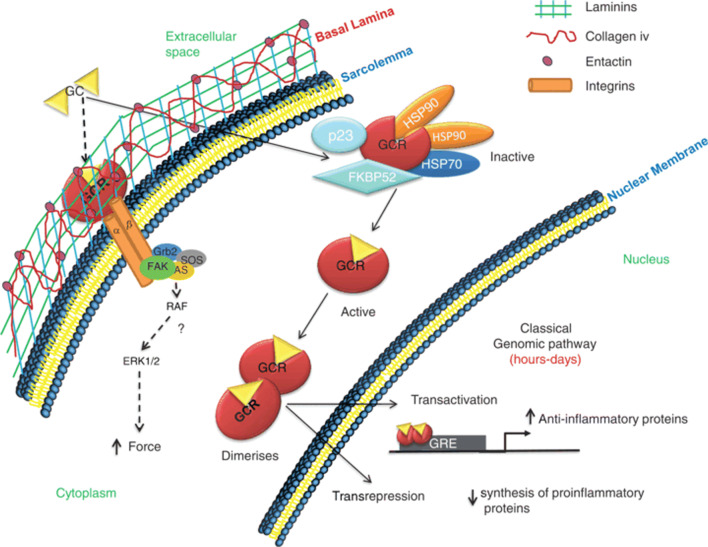
Dexamethasone binds to the glucocorticoid receptor (GR) on the cell membrane (Fig. 3), and the formation of this complex leads to translocation of the corticosteroid into the cell, where it travels to the nucleus. Here, it reversibly binds to several specific DNA sites resulting in stimulation (transactivation) and suppression (trans-repression) of a large variety of gene transcription [36]. It can inhibit the production of pro-inflammatory cytokines such as interleukin IL-1, IL-2, IL-6, IL-8, TNF, IFN-gamma, VEGF and prostaglandins [33, 37]. Importantly, five of these are linked to SARS-CoV-2 severity [38]. At the same time, it can also induce the synthesis of glucocorticoid response element resulting in the activation of anti-inflammatory cytokine synthesis, notably IL-10 and lipocortin-1.
Fig. 3.
A schematic diagram mechanism mediating the genomic (continuous arrows) and non-genomic (dashed arrows) effects of corticosteroids (in general) in mammalian skeletal muscle fibres [35]. p23: protein associated with progesterone receptor (23 kDa), HSP70 (70 kDa) and HSP90 (90 kDa): are heat shock proteins, SOS: Son of Sevenless, Grb2: growth factor receptor-bound protein 2
II—Non-Genomic Mechanisms
At high doses of the medication, dexamethasone binds to the membrane-associated GR on cells, such as T lymphocytes, resulting in the impairment of receptor signalling and a T lymphocyte–mediated immune response [39]. The glucocorticoid receptor combines to integrins, leading to the activation of FAK (focal adhesion kinase) (Fig. 3). As well as that, a high dose of dexamethasone also interacts with the movement of Ca+2 and Na+1 across the cell membrane, resulting in a rapid decrease in inflammation [40].
Dexamethasone as a COVID-19 Treatment
As SARS-CoV-2 is a recent virus, there are, currently, no specific vaccines nor anti-viral drugs which have been proven to treat or prevent COVID-19 infection. At the moment, medical researchers and front-line doctors have been focusing on some anti-viral, anti-malarial and anti-inflammatory drugs which have been used for many years and are widely available, such as hydroxychloroquine, chloroquine, remdesivir, lopinavir and corticosteroids. However, not all the outcome data of treatment with such agents were statistically significant; some of them helped to speed up the recovery time of patients with SARS-CoV-2 disease and some of them did not have any positive outcome. This might be because immune systems are unique and may respond differently to different drugs and the fact that confounding factors such as underlying health conditions were not adjusted for.
Most of the adverse outcomes of coronavirus disease are associated with severe inflammation, lung injury secondary to ARDS and thus diffuse alveolar damage [41]. Therefore, to control the immune-mediated damage of lung tissue, corticosteroids drugs have been given in severe cases of coronavirus such as MERS, SARS and SARS-CoV-2 [42, 43].
Due to their quick anti-inflammatory and immunosuppressive impact, corticosteroid medications are broadly used to treat hyper-inflammatory conditions, including the previous coronavirus diseases such as Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) [44, 45]. There are, however, only a few clinical studies where corticosteroids have been used to treat patients with SARS-CoV-2 and most of these studies have a high heterogeneity regarding the dose, the type of corticosteroid drug, the course for which the medication was given and which patients are suitable for the drug [46].
One of the main roles of glucocorticoids, to consider, is the fact that they cause immunosuppression and are anti-inflammatory [31, 47]. As they suppress the adaptive immune response, glucocorticoids play an important role in the modulation of several biological functions in immune cells and in different organs and tissues in the human body [48]. The latest research suggests that glucocorticoids could have both stimulatory and inhibitory impacts on the immune response depending on their concentration in the blood and how long it is taken [23]. Clinically, the primary reason for the use of glucocorticoids is that it might be beneficial in preventing damage of structures, like pulmonary in the case of SARS-CoV-2, by inhibiting cytokine production [49]. A number of studies have been conducted on the use of this drug for treating hyper-inflammatory states secondary to viral infections caused by respiratory syncytial virus (RSV); MERS; influenza; and, now, SARS-CoV-2.
In the primaeval stage of inflammation, glucocorticoids decrease inflammatory cell exudation, phagocytosis and capillary dilation. Whilst in the severe inflammatory stage, it can inhibit the fibroblasts and its excessive proliferation, which are normally responsible for fibrosis [50]. In vitro studies showed that corticosteroids are able to inhibit respiratory syncytial virus and rhinovirus-induced cytokine release [51]. An in vitro study found that the addition of 0.1 μM of dexamethasone to a cultured sample of human alveolar epithelial cells, H441 and A549, could delay cell proliferation and also resulted in a shorter recuperation time when compared to the untreated cultured cells [52].
Moreover, earlier studies showed that low doses of dexamethasone (0.4 mg/kg per day, given in four doses over 48 h) had a positive impact in patients with lung infections caused by the RSV [53]. Furthermore, a similar dose of dexamethasone (0.6 mg/kg day) was given to patients with bronchiolitis, again, caused by RSV, and results were approved of in terms of having positive benefits.
Contrastingly, other studies, however, have shown that corticosteroids may impair anti-viral innate immune responses, which would further exacerbate the severity of the condition [54, 55], and McGee et al. concluded there is no evidence highlighting the benefits of using corticosteroids for RSV in clinical trials carried out on children [56].
In terms of SARS, Zhong et al. suggested that the high dose of corticosteroids can be used in patients with this condition, if symptomatic for 3 days and/or more, and if medical investigation results are suggestive of progressive lung involvement, as this would reduce the risk of pulmonary fibrosis [57]. However, despite the fact it advocated for a low-to-moderate dose of corticosteroids in confirmed severe cases of SARS, another study produced results that were in concordance with Zhong et al. as it, too, reported a lowered mortality among patients and a shorter hospital stay [58]. It has, also, been concluded that low doses of methylprednisolone (1 to 2 mg per kg per a day) for a more prolonged period of time might be associated with significant improvement in pulmonary results and decrease in the number of days on mechanical ventilation [59].
On the other hand, when therapeutic systematic corticosteroids were used on patients who had a severe pneumonia-like infection caused by SARS-CoV and MERS-CoV, it resulted in the acceleration of virus replication. This is perhaps because steroids suppress the innate immune system which, in turn, then means it cannot eradicate the pathogens as efficiently [60]. Moreover, in a retrospective observational study of MERS patients, the result showed that patients who were given corticosteroids were more likely to require mechanical ventilation, vasopressors and renal replacement therapy [61].
Recently, a systematic review and meta-analysis was performed to assess the impact of corticosteroid treatment on the outcomes of 6458 patients affected by influenza. The review specified that corticosteroids were associated with a higher mortality rate when compared with the placebo group [62]. Again, this may be due to the fact that steroids cause a patient to become immunocompromised. Moreover, 0.4 mg per kg per day for 5 days of dexamethasone was administrated to treat acute respiratory distress syndrome (ARDS) induced by H5N1 in patients in Vietnam [63]. The study only demonstrated negative results. Conversely, Steinberg et al. found that administrating corticosteroids to patients with ARDS improved oxygen saturation, inflammatory markers and length of ICU stay [64].
A group of researchers from Spain carried out a RCT investigation, between March 2013 and December 2018, which included 277 patients; they found out that early administration of dexamethasone was able to decrease the mechanical ventilation time and reduce the overall mortality rate in patients with moderate-to-severe ARDS [65]. However, glucocorticoids showed to cause hyperglycaemia as it increases gluconeogenesis secondary to decreased hepatic insulin sensitivity [66]. Nevertheless, it was, interestingly, noted that there was no significant difference between the control group and the dexamethasone group regarding the adverse effects, for instance hyperglycaemia which occurred in 70% of the control group and 76% in the dexamethasone group, with both groups being in ICU [65].
Hazbun et al. used methylprednisolone (125 mg every 6 h for 24 h followed by 60 mg every 12 h for 10 days) for patients with ARDS caused by SARS-CoV-2. In the 48 h after the initiation of the methylprednisolone treatment, there was a reduction in the alveolar-arterial (A-a) O2 gradient from 455 to 228 mmHg, highlighting that there was an improvement in oxygen transfer for the lungs into the circulation. Moreover, the study demonstrated that 95% of patients were liberated from mechanical ventilation after 8 days of treatment. However, all the patients showed hyperglycaemia following the administration of methylprednisolone, and this was corrected with insulin [67].
Another study reported that the administration of standard does of corticosteroids (methylprednisolone) (without mentioning the quantity) in patients with pneumonia due to coronavirus significantly reduced the risk of death (62% of 201 patients) [68].
In further investigations, glucocorticoids have been given in combination with other medical compounds, like anti-malarial drugs, serine protease inhibitors, anti-viral and IL blockers, to estimate if those medications, together, have a synergistic effect [49]. Recently, a clinical trial carried out in France compared the use of hydroxychloroquine alone, with the use of hydroxychloroquine (600 mg/day for 10 days) in combination with dexamethasone (20 mg/day for 5 days and 10 mg/day for the next subsequent 5 days) in the treatment of patients with ARDS caused by SARS-CoV-2 (phase III). The primary outcome report showed that the patients who took the combination of hydroxychloroquine and dexamethasone had a mortality rate of 46%. The report also presented the mortality rate to be 61.8% in patients who received hydroxychloroquine alone [69].
A team of front-line physicians from China stated that the use of corticosteroids (methylprednisolone or equivalent) for patients who were critically ill was beneficial. They, however, were against the liberal use of the drug and only recommended short courses (less than 7 days) of corticosteroids (0.5–1 mg per kg per day), used prudently, for critical patients with SARS-CoV-2 pneumonia [70].
On the other hand, Russel and his co-workers in February 2020, based on results of previous studies on the use of steroids in MERS, SARS and influenza patients, support that corticosteroid treatment should not be used for the treatment of SARS-CoV-2-induced lung injury or shock, unless it is for a clinical trial due to the lack of substantial clinical evidence proving their efficacy [71].
As well as that, Ling et al. carried out a study on 66 patients out of the 292 patients who had tested positive for SARS-CoV-2, in January 2020, in Shanghai. The authors compared the presence of RNA in various secretions and excreta in a group receiving glucocorticoid treatment to a group receiving standard supportive treatment. The results demonstrated that the throat swabs were negative for the viral RNA after a median time of 9.5 days (6.0–11.0 days), whereas the stool samples showed to be clear of the viral RNA after 11.0 days (9.0–16.0) of onset of symptoms. The study also reported that viral RNA detection in both oropharyngeal and faecal samples was longer in the glucocorticoid group than in the non-glucocorticoid group. Consequently, the authors concluded that glucocorticoids are not a suitable for the treatment of COVID-19, especially in those with mild symptoms [72]. This may be due to dexamethasone suppressing the cytokine storm [73, 74].
The findings of clinical trials as reported in the Henry Ford Health System (HFHS)–centralized clinical microbiology laboratory indicated that an early short course of methylprednisolone (0.5 to 1 mg per kg per day in two divided doses for 3 days) in patients with moderate-to-severe COVID-19 reduced the need for escalation of care and improved clinical outcomes [75].
The latest recovery trial of using dexamethasone has been performed in the UK by a team of researchers at Oxford University. Six milligrammes of dexamethasone once daily for up to 10 days was given to 2104 patients, and the results were compared with those of 4321 patients who were not given dexamethasone. The average age of the participants was 66.1 years, and 36% of patients were female. The preliminary findings demonstrate that dexamethasone reduced the 28-day mortality by 35% in patients receiving invasive mechanical ventilation and by 20% in patients receiving supplementary oxygen. As the treatment was only used at a low-to-medium dose for up to10 days, no side effects have been noted thus far. The benefits of the drug were clearer in patients who were treated for more than 7 days after the initial onset of symptoms, when inflammatory lung damage is likely to have been more common. On the other hand, it was observed that this medication had no benefit on the outcomes of patients who had mild symptoms and so corticosteroids is only suitable for patients who are in hospital, under mechanical ventilation (severe situation) [76]. In addition to reducing the mortality ratio of patients with a severe form of COVID-19, using corticosteroids has prohibited the worsening of ventilator parameters, and subsequent ventilation; it has also reduced the duration of hospital stay and improved oxygenation status [77].
High doses of corticosteroids may cause more harm than good, especially if the treatment is given at a time when there is uncontrolled viral replication but with a low level of inflammation. Although there are potential hazards associated with high doses of corticosteroids when treating patients with SARS-CoV-2 pneumonia, including secondary infections and prolonged virus shedding, but in severely ill patients, if the hyper-inflammatory state is not controlled, cytokine-related lung injury could cause rapidly progressive pneumonia, the outcomes of which can be long-term and irreversible [70].
According to these latest findings, the WHO has welcomed the preliminary results regarding the use of dexamethasone in the treatment of SARS-CoV-2 patients, as this drug treatment was proven to save lives [78].
Side Effects
The common side effects of dexamethasone include an increase in appetite, mood changes, agitation and headache. Sometimes, it causes blurred vison with dizziness, and in the long term (more than a week), it could lead to arrhythmias. Therefore, in people with chronic diseases like heart disease and diabetes, high doses of corticosteroid should be used with caution [79].
Conclusion
The outcome data regarding the use of corticosteroids, especially dexamethasone, for SARS-CoV-2 so far, although not conclusive, are promising with some findings suggesting that low-to-moderate doses of corticosteroids (dexamethasone and methylprednisolone) could lower the mortality rate in patients with a severe form of the condition. It is, however, not recommended for patients with mild symptoms.
To further improve our understanding of the parameters and the effect of glucocorticoids on patients with SARS-CoV-2 infection, more randomized clinical trials on this treatment are necessary.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
This article is part of the Topical Collection on Covid-19
Impact Statements
• This review characterizes the mechanism of COVID-19 infections and the mechanism of action of corticosteroid compounds.
• There are several in vivo trials that have evaluated the efficacy of corticosteroids focusing on dexamethasone, for the treatment of COVID-19 and other coronaviruses.
• The available articles displayed the efficacy of corticosteroids as an anti-inflammatory and immunosuppressive agent.
• These results are promising and should encourage others to carry out larger scale studies to further examine the potential efficacy of corticosteroids, especially dexamethasone, in treating COVID-19.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3):e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuk-Woo Chan J, Yuan S, Kok K-H, To K-WK, Ch H, Yang J, Xing F, Liu J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghinai I, McPherson TD, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan J. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong N, Yang X, Ye L, et al. Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. bioRxiv. 2020;10.1101/2020.01.20.913368.
- 8.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 12.Turski WA, Wnorowski A, Turski GN, Turski CA, Turski L, et al. Restor Neurol Neurosci. 2020. 10.3233/RNN-201042. [DOI] [PMC free article] [PubMed]
- 13.Lan J, Ge J, Yu J, et al. Crystal structure of the 2019-nCoV spike receptor-binding domain bound with the ACE2 receptor. bioRxiv. 2020;10.1101/2020.02.19.956235.
- 14.Jin HL, Choi Y, Jeong KW. Crosstalk between aryl hydrocarbon receptor and glucocorticoid receptor in human retinal pigment epithelial cells. Int J Endocrinol. 2017;2017:5679517. doi: 10.1155/2017/5679517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, Lu H, Siddiqui P, Zhou Y, Jiang S. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol. 2005;174(8):4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D, Zhu C, Ai L, et al. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.She J, Jiang J, Ye L, Hu L, Bai C, Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Trans Med. 2020;9:19–17. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–e00120. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a clinician’s perspective. Diabetes Metab Syndr. 2020;14(5):971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression [e-pub ahead of print]. Lancet. 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed]
- 26.Ramamoorthy S, Cidlowski JA. Corticosteroids-mechanisms of action in health and disease. Rheum Dis Clin N Am. 2016;42(1):15–31. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dexamethasone product package inserts. In: Daily Med. Bethesda (MD): National Library of Medicine (US). https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=537b424a-3e07-4c81-978c-1ad99014032a.
- 28.electronic medicine compendium- Dexamethasone, https://www.medicines.org.uk/emc/
- 29.Zoorob RJ, Cender D. A different look at corticosteroids. Am Fam Physician. 1998;58(2):443–450. [PubMed] [Google Scholar]
- 30.Becker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesth Prog. 2013;60(1):25–32. doi: 10.2344/0003-3006-60.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saraya MA, Amal Abd El-Azeem I. Dexamethasone as adjunctive therapy for treatment of varicella pneumonia. Egyptian J Chest Dis Tuberculosis. 2012;61(3):9–13. doi: 10.1016/j.ejcdt.2012.10.019. [DOI] [Google Scholar]
- 33.Chikanza IC. Mechanisms of corticosteroid resistance in rheumatoid arthritis: a putative role for the corticosteroid receptor beta isoform. Ann N Y Acad Sci. 2002;966:39–48. doi: 10.1111/j.1749-6632.2002.tb04200.x. [DOI] [PubMed] [Google Scholar]
- 34.Lecoq L, Vincent P, Lavoie-Lamoureux A, Lavoie JP. Genomic and non-genomic effects of dexamethasone on equine peripheral blood neutrophils. Vet Immunol Immunopathol. 2009;128(1–3):126–131. doi: 10.1016/j.vetimm.2008.10.303. [DOI] [PubMed] [Google Scholar]
- 35.Pérez MH-A, Cormack J, Mallinson D, Mutungi G. A membrane glucocorticoid receptor mediatesthe rapid/non-genomic actions of glucocorticoidsin mammalian skeletal muscle fibres. J Physiol. 2013;591(20):5171–5185. doi: 10.1113/jphysiol.2013.256586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signaling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman SP, Flower RJ, Croxtall JD. Dexamethasone suppression of IL-1β induced cyclooxygenase 2 expression is not mediated by lipocortin-1 in A549 cells. Biochem Biophys Res Commun. 1994;202:931–939. doi: 10.1006/bbrc.1994.2019. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitre-Aguilar IB, Cabrera-Quintero AJ, Zentella-Dehesa A. Genomic and non-genomic effects of glucocorticoids: implications for breast cancer. Int J Clin Exp Pathol. 2015;8(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 40.Grzanka A, Misiołek M, Golusiński W, Jarząb J. Molecular mechanisms of glucocorticoids action: implications for treatment of rhinosinusitis and nasal polyposis. Eur Arch Otorhinolaryngol. 2011;268:247–253. doi: 10.1007/s00405-010-1330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arabi YM, Balkhy HH, Hayden FG, et al. Middle East respiratory syndrome. N Engl J Med. 2017;376(6):584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohadian MS. A review on currently available potential therapeutic options for COVID-19. Int J Gen Med. 2020;13:443–467. doi: 10.2147/IJGM.S263666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu K, Fang Y, Liu W, Wang MF, et al. Clinical characteristics of novel corona virus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020. 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed]
- 47.Montón C, Ewig S, Torres A, El-Ebiary M, Filella X, Rañó A, et al. Role of glucocorticoids on inflammatory response in non-immunosuppressed patients with pneumonia: a pilot study. Eur Respir J. 1999;14(July (1)):218–220. doi: 10.1034/j.1399-3003.1999.14a37.x. [DOI] [PubMed] [Google Scholar]
- 48.Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solinas C, Perra L, Aiello M, Migliori E, Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev. 2020;S 1359–6101(20):30161. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Inf Secur. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliver BGG, Robinson P, Peters M, Black J. Viral infections and asthma: an inflammatory interface? Eur Respir J. 2014;44(6):1666. doi: 10.1183/09031936.00047714. [DOI] [PubMed] [Google Scholar]
- 52.Nalayanda DD, Fulton WB, Colombani PM, Wang T-H, Abdullah F. Pressure induced lung injury in a novel in vitro model of the alveolar interface: Protective effect of dexamethasone. J Pediatr Surg. 49(2014):61–5. 10.1016/j.jpedsurg.2013.09.030. [DOI] [PubMed]
- 53.Van Woensel JB, Lutter R, Biezeveld M, et al. Effect of dexamethasone on tracheal viral load and interleukin-8 tracheal concentration in children with respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;22(8):721–726. doi: 10.1097/01.inf.0000078165.62923.15. [DOI] [PubMed] [Google Scholar]
- 54.Simpson JL, Carroll M, Yang IA, et al. Reduced antiviral interferon production in poorly controlled asthma is associated with neutrophilic inflammation and high-dose inhaled corticosteroids. Chest. 2016;149:704–713. doi: 10.1016/j.chest.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Davies JM, Carroll ML, Li H, et al. Budesonide and formoterol reduce early innate anti-viral immune responses in vitro. PLoS One. 2011;6:e27898. doi: 10.1371/journal.pone.0027898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGee S, Hirschmann J. Use of corticosteroids in treating infectious diseases. Arch Intern Med. 2008;168:1034–1046. doi: 10.1001/archinte.168.10.1034. [DOI] [PubMed] [Google Scholar]
- 57.Zhong NS, Zeng GQ. Our strategies for fighting severe acute respiratory syndrome (SARS) Am J Respir Crit Care Med. 2003;168:7–9. doi: 10.1164/rccm.200305-707OE. [DOI] [PubMed] [Google Scholar]
- 58.Chen RC, Tang XP, Tan SY. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 60.Matsuyama S, Kawase M, Nao N, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. 2020; 10.1101/2020.03.11.987016.
- 61.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 62.Ni Y, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY, Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5 Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 64.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 65.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM, dexamethasone in ARDS network Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 66.Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hazbun ME, Faust AC, Ortegon AL, et al. The combination of tocilizumab and methylprednisolone along with initial lung recruitment strategy in coronavirus disease 2019 Patients requiring mechanical ventilation: a series of 21 consecutive cases. Crit Care Explor. 2020;2(6):e0145. doi: 10.1097/CCE.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 69.Stephan F, Lamrani L, Dexamethasone treatment for severe acute respiratory distress syndrome induced by COVID-19 (DHYSCO), ClinicalTrials.gov Identifier: NCT04347980, https://clinicaltrials.gov/ct2/show/NCT04347980.
- 70.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-n-CoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ling Y, Xu S-B, Lin Y-X, Tian D, Zhu Z-Q. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siordia JA, Bernaba M, Yoshino K, et al. Systematic and Statistical Review of Coronavirus Disease 19 Treatment Trials. SN Compr Clin Med. 2020;2:1120–1131. 10.1007/s42399-020-00399-6 [DOI] [PMC free article] [PubMed]
- 75.Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, Miller J, Kenney RM, Alangaden G, Ramesh MS, Ford H. COVID-19 Management Task Force, Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis ciaa601. 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed]
- 76.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19: recovery trial. NEJM. 2020. 10.1056/NEJMoa2021436.
- 77.Wang Y, Jiang W, He Q, Liu B, Dong N, et al. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China, medRxiv preprint 10.1101/2020.03.06.20032342
- 78.WHO report 16 June 2020, https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients.
- 79.Medicine Net Dexamethasone: https://www.medicinenet.com/dexamethasone-decadron-dexpak/article.htm#what_is_dexamethasone_and_how_does_it_work_mechanism_of_action 5.