Abstract
One year of adjuvant trastuzumab is considered the standard treatment for patients with HER2 positive breast cancer. However, a shorter duration of trastuzumab may be associated with reduced costs and side effects. Results from randomized trials with diverse non-inferiority margins comparing one year to a shorter duration of adjuvant trastuzumab are not consistent and have not been systematically reviewed using a non-inferiority meta-analysis approach.
We conducted a systematic review and meta-analysis of randomized trials to assess whether a shorter duration of adjuvant trastuzumab was non-inferior to one year of treatment or not. The non-inferiority margin for the meta-analysis was pre-defined as the median of the margins of all the trials included. Data of 11,376 patients from 5 trials were analyzed. Non-inferiority margins in included studies varied from 1.15 to 1.53 with median of 1.29 for HR of DFS. A shorter duration of trastuzumab was non-inferior to one year of therapy for DFS (HR 1.13, 95%CI 1.03–1.24) but inconclusive for OS (HR 1.14, 95%CI 1.00–1.30). In a subgroup analysis for DFS outcome, shorter therapy was non-inferior in patients with ER positive disease (HR 1.10, 95%CI 0.95–1.28) and those with sequential therapy (HR 0.97, 95%CI 0.75–1.27) and when the duration of treatment was 6 months (HR 1.09, 95%CI 0.98–1.22). Although a shorter duration of adjuvant trastuzumab was non-inferior to one year of therapy for DFS in patients with HER2 positive breast cancer based on our HR margin of 1.29, any benefit of a shorter duration comes at a loss of efficacy with an increase in absolute risk up to 3.9% for 5 year DFS. Whether the potential increased risk is clinically acceptable for the benefits of a shorter duration remains debatable.
Keywords: Adjuvant, HER2, Breast, Trastuzumab
Highlights
-
•
Shorter duration of adjuvant trastuzumab in HER2 positive breast cancer is attractive.
-
•
Inconsistent results seen from the PHARE and PERSEPHONE trials.
-
•
Meta-analysis suggests shorter duration of trastuzumab is non-inferior.
-
•
Highlights need for appropriately chosen and applied non-inferiority margin.
1. Introduction
Trastuzumab is an established part of adjuvant HER2 positive breast cancer treatment. In a disease that is associated with aggressive biology and previously portended poor prognosis, trastuzumab has significantly improved outcomes [1]. Adjuvant trastuzumab therapy has been shown to significantly improve survival outcomes for patients in multiple trials, with a durable effect that has been confirmed in long term follow up [[2], [3], [4], [5], [6]]. One year of adjuvant trastuzumab, chosen by expert consensus, is considered the standard since the HERA trial’s initial results in 2005 which also demonstrated that extending trastuzumab to two years does not improve survival outcomes over one year but does increase side effects, namely cardiotoxicity [7,8]. From recent long term follow up of HERA, out to 11 years, the rates of cardiotoxicity were 7.3% in the two year trastuzumab duration cohort versus 4.4% in the one year cohort [2,9].
Debate continues regarding the optimal duration of adjuvant trastuzumab in breast cancer treatment, especially with the recent discordant results from the PERSEPHONE trial and final results from the PHARE and Short-HER trials [[10], [11], [12]]. Several previous meta-analyses have examined whether one year duration is superior to a shorter duration but no previous non-inferiority meta-analysis has been performed, so whether a shorter duration of trastuzumab is non-inferior to the current one year standard remains unknown [[13], [14], [15], [16], [17]]. We therefore performed a non-inferiority meta-analysis to determine whether a shorter duration is non-inferior or not.
Efforts to de-escalate treatment have been ongoing to decrease side effects such as cardiotoxicity but also cost associated with treatment. The FinHER trial in 2006 showed excellent recurrence free survival results (HR 0.29, 95%CI 0.13–0.64) and low rates of cardiotoxicity with a 9 week course of trastuzumab, leading to several large non-inferiority trials with shorter durations of trastuzumab using heterogeneous non-inferiority margins, with inconsistent results [18]. PHARE, published in 2013, used a shorter duration of 6 months compared with one year did not meet its non-inferiority margin of 2% at 2 years for DFS [12]. The most recently published PERSEPHONE trial, a similarly well-designed trial, also used 6 months of adjuvant trastuzumab, did meet its non-inferiority margin of 3% at 4 years for DFS, suggesting a shorter duration is adequate [11].
A shorter duration of trastuzumab is attractive in terms of decreasing cardiotoxicity, costs, resources, and alleviating treatment burden on patients. Previous meta-analyses have shown cardiotoxicity rates can be approximately halved by using a shorter duration of trastuzumab, but none have employed a non-inferiority approach to analyse survival outcomes [[13], [14], [15], [16], [17]]. All previous meta-analyses have tested for superiority of one year over a shorter duration, which does not best address the clinical question. We therefore aimed to conduct a systematic review and meta-analysis to answer whether a shorter duration of adjuvant trastuzumab is truly non-inferior and we did this using a non-inferiority meta-analysis approach.
2. Methods
We report this systematic review in accordance with standards defined by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [19,20].
2.1. Study population and trial eligibility
Defined population included patients with HER2 positive breast cancer treated in the (neo)adjuvant setting, using a one year duration of trastuzumab as our control arm and a shorter duration as our experimental arm. We included non-inferiority RCTs comparing 6-months or shorter duration of adjuvant trastuzumab treatment with one year treatment, in patients with HER2 positive breast cancer, that used DFS as their primary endpoint. Papers, abstracts, and presentations were included if they provided sufficient data. Case studies and trials involving advanced, metastatic disease, or other treatment combinations were excluded.
2.2. Search methods and study selection
A comprehensive literature search was performed including PubMed, EMBASE, and The Cochrane Library without restriction for language or publication status. We also searched for abstracts and presentations from the American Society of Clinical Oncology’s (ASCO) Annual Meeting, ASCO Breast Cancer Symposium, San Antonio Breast Cancer Symposium (SABCS), and European Society for Medical Oncology (ESMO) and reviewed citation lists. The search was done using the terms trastuzumab or Herceptin, breast cancer or breast tumor or breast neoplasm, duration, adjuvant, and clinical trial, from January 2000 to June 2019. Search terms were the combination of 1) Trastuzumab or Herceptin and 2) breast cancer or breast tumor or breast neoplasm and 3) clinical trial and 4) adjuvant.
2.3. Primary outcome
DFS was defined as our primary outcome. DFS was defined as the time from randomization or the date of the first treatment dose until the first occurrence of disease recurrence or death from any cause.
2.4. Secondary outcome
Secondary outcomes included OS for the entire population.
2.5. Subgroup analysis
Subgroup analysis for DFS based on ER status (positive or negative), lymph node status (positive or negative), duration of trastuzumab (6 months or 9 weeks), timing of trastuzumab with chemotherapy (concurrent or sequential) and age (<50 or ≥50).
2.6. Data extraction
Two reviewers (PS, PB) independently extracted data from each included study. Discordance was resolved by discussion and third reviewer (JR). Trial name, phase, year published, number of patients, median follow up, duration and timing of trastuzumab, nodal status, ER status, non-inferiority margin, and hazard ratios (HR) with respective 95% confidence intervals (CI) for disease-free survival (DFS) and overall survival (OS) were extracted from each trial.
2.7. Risk of bias assessment
The risk of bias in the included studies was assessed by two reviewers (PS, JR) using the Cochrane Collaboration risk of bias tool that assesses risk of bias in six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential threats to validity [21,22].
2.8. Quality of evidence
The level of evidence for the pre-specified outcomes of interest was assessed and reported as low, moderate or high based on the GRADE approach developed by the Grading of Recommendations, Assessment, Development, and Evaluations working group using GradePro [23].
2.9. Statistical analysis
The study population, including patient characteristics, interventions and risk of bias were summarized descriptively. The intention-to-treat data reported in each individual trial was used for non-inferiority meta-analysis. Pooled HRs (shorter duration treatment vs 1 year treatment) with two sided 95%CI for DFS and OS were estimated using meta-analysis with random-effects models approach to account for between-study heterogeneity. Heterogeneity was assessed using Cochran Q test, quantified using the I2 statistic and considered mild if I2 < 30% and notable if I2 ≥ 50% [24]. The calculated median of non-inferiority margins for DFS from each trial was used to predefine the non-inferiority margin of HR 1.29 for the pooled analysis, corresponding to an absolute risk increase of 3.9%, assuming a 5 year DFS of 85% and exponential distribution [25]. The shorter duration of treatment was considered non-inferiority to the 1 year treatment if the upper bound of the 95% CI of HR was below our predefined non-inferiority margin for the meta-analysis. Subgroup analyses were also conducted using similar methods to compare DFS outcomes for sub-population stratified by ER status, nodal status, length and timing of trastuzumab treatment and age [26]. The meta-analysis was conducted using R. 3.6.1 and RevMan 5.3 analysis software (Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Eligible studies and characteristics
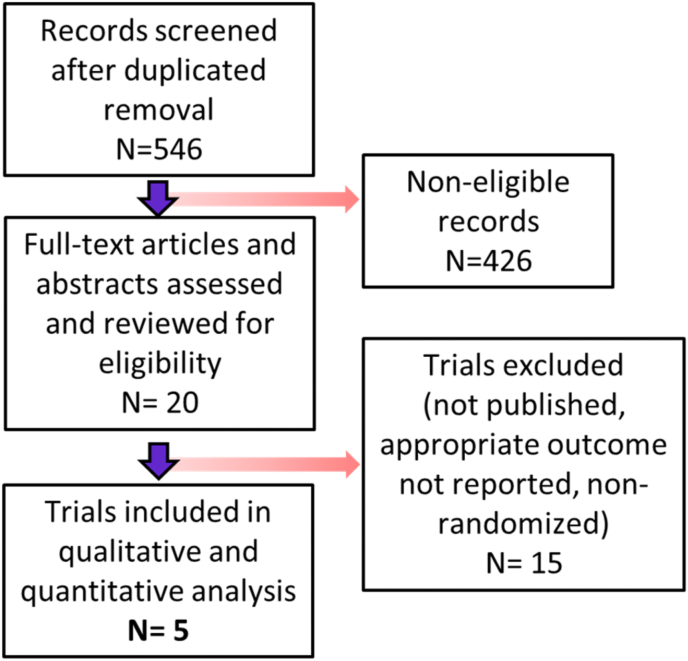
Following removal of duplicates, the systematic literature search, identified 546 records which were screened for inclusion of which 426 records were deemed non-eligible. 20 full text articles and abstracts were reviewed, of which 15 were excluded, leaving 5 trials included in the qualitative and quantitative analysis. Fig. 1 shows flowchart of selection and exclusion process.
Fig. 1.
Study selection flowchart.
Non-inferiority margins varied across the included studies. PHARE had the most stringent non-inferiority margin HR 1.15 or absolute increase of 2% at 2 years for DFS while HORG had the largest non-inferiority margin HR 1.53 or absolute increase of 8% at 3 years for DFS [12,27,28]. PERSEPHONE, PHARE, and HORG used 6 months of trastuzumab, while Short-HER and SOLD used 9 weeks, to compare with the standard one year duration [[10], [11], [12],27,29]. Most patients received chemotherapy with anthracycline backbone. PHARE and PERSEPHONE used both concomitant and sequential delivery of trastuzumab [11,12]. Approximately two-thirds of patients had ER positive disease. The characteristics of the 5 included trials are summarized in Table 1.
Table 1.
Characteristics of trials included.
| First Author | Year Published | Study Name | MedianFollow-up (m) | Duration ofTrastuzumab | Number ofpatients | PrimaryOutcome | Non-inferiorityMargin | OutcomeMet | Chemotherapy | ConcomitantTrastuzumab | NodeNegative | ER Positive |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Earl [11] | 2019 | PERSEPHONE | 64.8 | 6 m | 4088 | DFS | 3% 4yr DFS | Yes | Anthracycline and/or taxane regimen | 47% | 59% | 69% |
| HR 1.316 | (90% received anthracycline) | |||||||||||
| Pivot [12] | 2013/2019 | PHARE | 90 | 6 m | 3380 | DFS | 2% 2yr DFS | No | Investigator choice/multiagent | 57% | 54% | 58% |
| HR 1.15 | (89% received anthracycline) | |||||||||||
| Mavroudis [27,28] | 2015 | HORG | 47/51 | 6 m | 481 | DFS | 8% 3yr DFS | No | FECq2w x4 then Dq2w x4 | 100% | 21% | 67% |
| HR 1.53 | ||||||||||||
| Conte [10] | 2018 | Short-HER | 72 | 9 w | 1253 | DFS, OS | 5 yr DFS | No | long: AC/EC q3w x4 then T/D q3w x4 | 100% | 54% | 68% |
| HR 1.29 | short: Dq3w x3 then FECq3w x3 | |||||||||||
| Joensuu [29] | 2018 | SOLD | 62.4 | 9 w | 2174 | DFS | 4% 5 yr DFS | No | Dq3w x3 then FECq3w x3 | 100% | 60% | 66% |
| HR 1.3 | ||||||||||||
| A = doxorubicin, C = cyclophosphamide, D = docetaxel, E = epirubicin, F = fluorouracil, T = paclitaxel, w = week, m = month | ||||||||||||
No significant bias was detected, results shown in Table 2, although PHARE did not report on blinding of outcome assessment and Short-HER did not report on allocation concealment, blinding of personnel or blinding of assessment [10,12].
Table 2.
Risk of bias.
4. Survival outcomes
4.1. Primary outcome
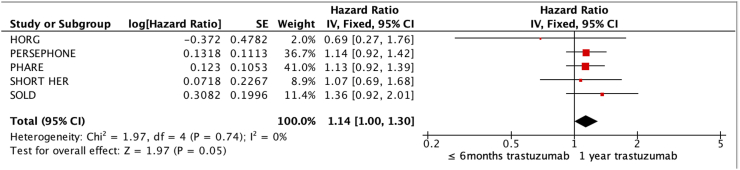
Data of 11,376 patients from 5 trials were analyzed. All 5 trials used DFS as their primary outcome (Short-HER used a co-primary outcome of DFS and OS). A shorter duration of trastuzumab was non-inferior to one year of therapy for DFS (HR 1.13, 95%CI 1.03–1.24, I2 0%) as the upper bound of 95%CI (1.24) was below our non-inferiority margin of HR 1.29 (Fig. 2). GRADE recommendations, summarized in Table 3, show moderate certainty for DFS.
Fig. 2.
Forest plot for DFS.
Table 3.
GRADE recommendation.
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Certainty | Importance |
|---|---|---|---|---|---|---|---|---|
| DFS | ||||||||
| 5 | randomized trials | not serious | seriousa | not serious | not serious | none | ⊕⊕⊕◯ MODERATE | Important |
| OS | ||||||||
| 5 | randomized trials | not serious | not serious | not serious | seriousb | none | ⊕⊕⊕◯. MODERATE | Important |
CI: Confidence interval
Explanations
a. Only one trial was able to show non-inferiority
b. Wide confidence intervals in OS results
4.2. Secondary outcome
All five trials included OS outcomes, using the corrected OS results from HORG [28]. Applying the same predefined non-inferiority margin calculated using DFS, non-inferiority is inconclusive for OS (HR 1.14, 95%CI 1.00–1.30, I2 0%) as the upper bound of 95%CI (1.30) was above our non-inferiority margin of HR 1.29 (Fig. 3). GRADE recommendations also show moderate certainty OS.
Fig. 3.
Forest plot for OS.
4.3. Subgroup analysis
For DFS subgroup analysis, five trials reported DFS results based on ER status. Four trials reported DFS results based on nodal status. Two trials used 9 weeks duration and three trials 6 months. All five trials included DFS results based on timing of trastuzumab, with all five having concomitant use and two also with sequential use. Only three trials were able to be included in DFS analysis based on age.
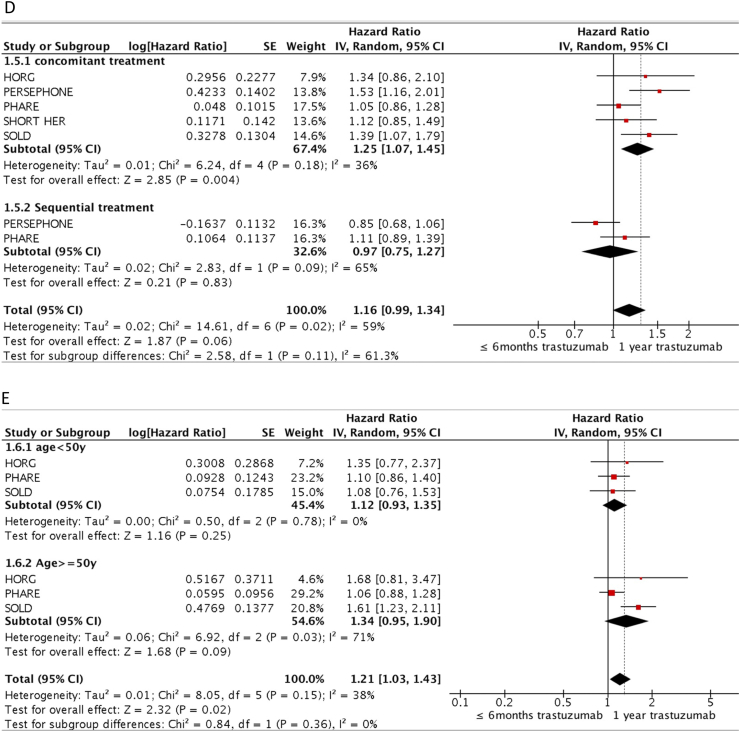
Subgroup analysis for DFS (Fig. 4) show that non-inferiority for DFS was met for patients with ER positive disease (HR 1.10, 95%CI 0.95–1.28, I2 20%) but was inconclusive for patients with ER negative disease (HR 1.22, 95%CI 1.06–1.41, I2 0%). Nodal status did not seem to affect results as node negative (HR 1.12, 95% 0.93–1.35, I2 6%) and positive (HR 1.16, 95%CI 0.99–1.36, I2 0%) subgroups did not meet non-inferiority. Duration of trastuzumab within the experimental arm did influence results as patients treated with 6 months met non-inferiority (HR 1.09, 95%CI 0.98–1.22, I2 0%) while those treated with the even shorter duration of 9 weeks did not meet non-inferiority (HR 1.26, 95%CI 1.02–1.55, I2 16%). Patients who received sequential trastuzumab did meet non-inferiority (HR 0.97, 95%CI 0.75–1.27, I2 65%), while those treated with concomitant trastuzumab did not (HR 1.25, 95%CI 1.07–1.45, I2 36%). Stratifying by age showed neither groups under 50 years old (HR 1.12, 95%CI 0.93–1.35, I2 0%) or 50 years and older (HR 1.34, 95%CI 0.95–1.90, I2 71%) met non-inferiority.
Fig. 4.
DFS Subgroup by A) ER status (positive or negative), B) lymph node status (positive or negative), C) duration of trastuzumab (6 months or 9 weeks), D) timing of trastuzumab with chemotherapy (concurrent or sequential) and E) age (<50 or ≥50).
5. Discussion
Results from our meta-analysis suggest that a shorter duration of trastuzumab is non-inferior in terms of DFS for the treatment of adjuvant HER2 positive breast cancer. Based on our calculated, predefined non-inferiority margin of HR 1.29, a shorter duration is at least no worse than 3.9% in terms of absolute risk increase for DFS. GRADE recommendation for DFS results is moderate based on serious inconsistency in results of included trials, as only PERSEPHONE was able to meet non-inferiority threshold.
OS in the included trials was a secondary endpoint, apart from the Short-HER trial which included DFS and OS as primary outcome. No included trial had separate non-inferiority margin for OS and in applying our same predefined DFS non-inferiority margin to OS results we are assuming we accept the same difference, which is debatable. While OS in our analysis did not meet our calculated non-inferiority threshold, DFS has been shown to be a good surrogate for OS in adjuvant HER2 positive breast cancer and longer follow up is needed to confirm [30]. GRADE recommendation for OS results is moderate due to serious imprecision of these results evidenced by wide 95%CI margins in the included trials.
In our subgroup analysis, non-inferiority was met in patients with ER positive disease and for those treated with at least 6 months of trastuzumab, and these results may help guide selection of patients in future de-escalation trials. Lower risk patients with negative lymph nodes did not meet our non-inferiority margin; however, these results are limited by lack of lymph node status data from PERSEPHONE that carries substantial weight (29%) which has yet to be published and was not made available for this analysis.
The main strength of our study is the use of a non-inferiority methodological approach. Our analysis is the first to our knowledge to examine whether a shorter duration of adjuvant trastuzumab is non-inferior to the standard one-year duration in terms of DFS. The trials included in our analysis were all designed as non-inferiority trials, yet previous meta-analyses, summarized in Table 4, have examined whether one year is superior to a shorter duration [[13], [14], [15], [16], [17]]. We feel the non-inferiority meta-analysis is more appropriate in terms of methodological approach and it has been used in previous unrelated non-inferiority meta-analysis [31]. Our analysis demonstrates the use of a non-inferiority meta-analysis approach which may be useful in other scenarios as treatment outcomes across different types of cancer are improving, patients are living longer, and emphasis on reducing toxicity and costs continues to grow. Limitation of the study include not having individual patient level data, variable non-inferiority margins across included studies, incomplete subgroup data, and different durations of trastuzumab within the experimental arm for which we used random effects model to help address these limitations.
Table 4.
Summary of previous meta-analyses.
| First Author | Year | Journal | Reported | DFS | OS | Cardiotoxicity | Trial Included |
|---|---|---|---|---|---|---|---|
| Gyawali [15] | 2017 | Cancer Treat Reviews | Superioritya | 1.24 (1.07–1.44) | 1.28 (1.02–1.63) | 2.65b(2.00–3.50) | 4 (not SOLD) |
| Niraula [17] | 2018 | Breast Cancer Res Treat | Superioritya | 1.21 (1.09–1.36) | 1.23 (1.07–1.42) | 2.48b(1.94–3.17) | 5 |
| Inno [16] | 2018 | Breast Cancer Res Treat | Superioritya | 1.19 (1.08–1.30) | 1.22 (1.07–1.39) | 0.4c(0.32–0.49) | 5 |
| Chen [13] | 2019 | Cancer Treat Reviews | Superioritya | 1.13 (1.03–1.25) | 1.16 (1.01–1.32) | 0.52c(0.43–0.62) | 6 (included E2198) [48] |
| Goldvaser [14] | 2019 | JNCI Cancer Spectrum | Superioritya | 1.14 (1.05–1.25) | 1.15 (1.02–1.29) | 0.67c(0.55–0.81) | 6 (included E2198) [48] |
Reported superiority of 12 months of trastuzumab vs ≤ 6 months.
Reported as 12 months of trastuzumab vs ≤ 6 months.
Reported as ≤6 months vs 12 months of trastuzumab.
Whether these results are directly applicable in clinical practice remain debatable. The interpretation and application of results from non-inferiority trials needs to be done cautiously. The selection of a non-inferiority margin is reliant on what is deemed a clinically acceptable loss of efficacy for the benefits gained by de-escalation and are inherently subjective. Given the lower bound of the 95% CI for DFS is above 1.00, but the upper bound still below the predefined non-inferiority margin, a shorter duration can be considered inferior than the standard yet still non-inferior based on the margin used. This unusual result of a non-inferiority study can be seen with large sample sizes or from too wide a non-inferiority margin [32]. Variation in what is deemed clinically acceptable is evidenced by the heterogeneity of non-inferiority margins used in the included trials of this analysis. The PHARE trial used the most stringent margin of 2% in terms of DFS while HORG defined a loss of up to 8% in DFS as clinically acceptable. PERSEPHONE, the only trial included to meet non-inferiority, used a non-inferiority margin of 3% at 4 year for DFS, but whether this margin or the calculated margin used in this analysis is acceptable in practice will vary amongst physicians, patients, and regions. If a non-inferiority margin HR of 1.15, as used in PHARE, or a HR of 1.20, as in the original PERSEPHONE design, were used instead of our pre-defined HR of 1.29, our results would not have met non-inferiority.
The trials included in this analysis began in a different era of adjuvant HER2 positive breast cancer treatment, their results now arriving in a rapidly evolving treatment landscape with ongoing efforts to improve outcomes in higher risk patients and de-escalate therapy in lower risk patients. The addition of pertuzumab for dual HER2 blockade has been shown to improve DFS in higher risk patients in the adjuvant setting and improve pathological complete response rate in the neoadjuvant setting [[33], [34], [35], [36]]. For patients with residual disease following neoadjuvant therapy, substituting trastuzumab-emtansine for trastuzumab significantly decreases the risk of recurrence [37]. Marginal benefits have also been shown with the addition of neratinib following completion of trastuzumab therapy [38,39]. Most patients included in our analysis received anthracyclines as part of their chemotherapy backbone, but excellent survival outcomes and side effect profiles have been demonstrated with anthracycline sparing regimens in low risk patients, with some results confirmed in longer term follow up [18,[40], [41], [42], [43]]. Anthracycline sparing regimens for low risk patients significantly decrease the risk of cardiotoxicity but no trial has yet to investigate de-escalating both the chemotherapy backbone and trastuzumab duration or to compare these two strategies.
We did not include an analysis of cardiotoxicity as previous meta-analyses done have all shown similar results of significantly decreased cardiotoxicity with a shorter trastuzumab duration. The most recent meta-analysis by Goldvaser et al. examining cardiac toxicity showed a decreased risk of cardiac dysfunction (OR 0.67, 95%CI 0.55–0.81) and congestive heart failure (OR 0.66, 95%CI 0.50–0.86). However, the overall risk of high-grade CHF in patients treated with trastuzumab versus placebo is only 1.44% (95%CI, 0.79%–2.64%) and related cardiotoxicity is typically reversible [44,45]. For patients with access to treatment who are tolerating trastuzumab without complication the benefit of a shorter duration in terms of reducing cardiotoxicity may not be seen as meaningful. However, for those at high risk of cardiotoxicity or those experiencing cardiotoxicity on treatment, the results from our analysis may be helpful in framing discussion around risk-benefit of a shorter duration of trastuzumab.
Whether a shorter duration of trastuzumab is adopted more broadly, or further trials at de-escalation with shorter duration are attempted, will likely be with an emphasis on pharmacoeconomic outcomes as discussed by the authors of the PHARE trial and PERSEPONE commentary [12,46]. The drug costs associated with trastuzumab therapy will improve with the introduction of biosimilars, but a shorter duration may still improve patient’s quality of life and decrease the indirect costs associated with treatment such as cardiac monitoring, management of toxicity, and help free up resources for the ever increasing and large current demand for cancer care services. Analyses done have shown favorable cost-benefit for reducing the duration of trastuzumab specific to the region studied and this could have significant implications for improving access to care, especially in resource limited areas [[47], [48], [49], [50]]. Additionally, the development of biomarkers to identify lower risk patients who may be ideal candidates for shorter durations of trastuzumab are needed.
6. Conclusion
A shorter duration of adjuvant trastuzumab appears non-inferior to one year for DFS, particularly in patients with ER positive disease and patients treated with 6 months of trastuzumab. GRADE recommendation is moderate but results from this analysis will hopefully help guide further trials with appropriately chosen non-inferiority margins to confirm optimal duration of trastuzumab in low risk patients. A shorter duration may be safer in terms of risk of cardiac toxicity and has potential cost and resource savings, especially in resource limited regions. Application of these results remain a matter of clinical judgment and shared decision making with patients.
Declaration of competing interest
Author RF has accepted honoraria from Bayer, Pzifer, BMS and Novartis as well as travel grant from Janssen. JR has accepted honorarium and was on advisory board for Roche and Lilly.
References
- 1.Slamon D. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Cameron D. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez E.A. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romond E.H. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith I. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 7.Goldhirsch A. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart M.J. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 9.Gianni L.P. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12(3):236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 10.Conte P. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER studydouble dagger. Ann Oncol. 2018;29(12):2328–2333. doi: 10.1093/annonc/mdy414. [DOI] [PubMed] [Google Scholar]
- 11.Earl H.M. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393(10191):2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pivot X. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393(10191):2591–2598. doi: 10.1016/S0140-6736(19)30653-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen L. Short-duration versus 1-year adjuvant trastuzumab in early HER2 positive breast cancer: a meta-analysis of randomized controlled trials. Canc Treat Rev. 2019;75:12–19. doi: 10.1016/j.ctrv.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Goldvaser H. Deescalating adjuvant trastuzumab in HER2-positive early-stage breast cancer: a systemic review and meta-analysis. JNCI Cancer Spectr. 2019;3(2) doi: 10.1093/jncics/pkz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyawali B., Niraula S. Duration of adjuvant trastuzumab in HER2 positive breast cancer: overall and disease free survival results from meta-analyses of randomized controlled trials. Canc Treat Rev. 2017;60:18–23. doi: 10.1016/j.ctrv.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Inno A. One year versus a shorter duration of adjuvant trastuzumab for HER2-positive early breast cancer: a systematic review and meta-analysis. Breast Canc Res Treat. 2019;173(2):247–254. doi: 10.1007/s10549-018-5001-x. [DOI] [PubMed] [Google Scholar]
- 17.Niraula S., Gyawali B. Optimal duration of adjuvant trastuzumab in treatment of early breast cancer: a meta-analysis of randomized controlled trials. Breast Canc Res Treat. 2019;173(1):103–109. doi: 10.1007/s10549-018-4967-8. [DOI] [PubMed] [Google Scholar]
- 18.Joensuu H. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 19.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7716):332–336. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamseer L. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ Br Med J (Clin Res Ed) 2015;349(jan02 1) doi: 10.1136/bmj.g7647. g7647-g7647. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT T.J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane handbook for systematic reviews of interventions version 6.0 (updated july 2019). Cochrane. 2019. www.training.cochrane.org/handbook Available from: [Google Scholar]
- 22.Julian P.T.H. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Br Med J (Clin Res Ed) 2011;343(7829):889–893. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schünemann H.B.J., Guyatt G., Oxman A., editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group; 2013. 2013. [Google Scholar]
- 24.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Weir I.R., Trinquart L. Design of non-inferiority randomized trials using the difference in restricted mean survival times. Clin Trials. 2018;15(5):499–508. doi: 10.1177/1740774518792259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeks J.J. Systematic reviews in health care: systematic reviews of Evaluations of diagnostic and screening tests. BMJ Br Med J (Clin Res Ed) 2001;323(7305):157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavroudis D. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG) Ann Oncol. 2015;26(7):1333–1340. doi: 10.1093/annonc/mdv213. [DOI] [PubMed] [Google Scholar]
- 28.Mavroudis D. Corrigendum to Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG) Ann Oncol. July 2015;26(Issue 7):444–445. doi: 10.1093/annonc/mdv213. Pages 1333-1340. Ann Oncol, 2020. 31(3) [DOI] [PubMed] [Google Scholar]
- 29.Joensuu H. Effect of adjuvant trastuzumab for a duration of 9 Weeks vs 1 Year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4(9):1199–1206. doi: 10.1001/jamaoncol.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad E.D. Disease-free survival as a surrogate for overall survival in patients with HER2-positive, early breast cancer in trials of adjuvant trastuzumab for up to 1 year: a systematic review and meta-analysis. Lancet Oncol. 2019;20(3):361–370. doi: 10.1016/S1470-2045(18)30750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acuna S.A. Laparoscopic versus open resection for rectal cancer: a noninferiority meta-analysis of quality of surgical resection outcomes. Ann Surg. 2019;269(5):849–855. doi: 10.1097/SLA.0000000000003072. [DOI] [PubMed] [Google Scholar]
- 32.Piaggio G. Reporting of noninferiority and equivalence randomized TrialsAn extension of the CONSORT statement. J Am Med Assoc. 2006;295(10):1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 33.Gianni L.D. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 34.Schneeweiss A. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol : official journal of the European Society for Medical Oncology. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 35.Schneeweiss A. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Canc. 2018;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 36.von Minckwitz G. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Minckwitz G. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2018;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 38.Chan A.P. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 39.Martin M. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 40.Jones S.E.D. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2 -amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol. 2013;14(11):1121–1128. doi: 10.1016/S1470-2045(13)70384-X. [DOI] [PubMed] [Google Scholar]
- 41.Tolaney S.M. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolaney S.M. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2019;37(22):1868–1875. doi: 10.1200/JCO.19.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Ramshorst M.S. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630–1640. doi: 10.1016/S1470-2045(18)30570-9. [DOI] [PubMed] [Google Scholar]
- 44.de Azambuja E. Trastuzumab-associated cardiac events at 8 Years of median follow-up in the Herceptin adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32(20):2159–+. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 45.Long H.D. Risk of congestive heart failure in early breast cancer patients undergoing adjuvant treatment with trastuzumab: a meta-analysis. Oncol. 2016;21(5):547–554. doi: 10.1634/theoncologist.2015-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurvitz S.A. Is the duration of adjuvant trastuzumab debate still clinically relevant? Lancet. 2019;393(10191):2565–2567. doi: 10.1016/S0140-6736(19)30946-8. [DOI] [PubMed] [Google Scholar]
- 47.Ansaripour A., Uyl-de Groot C.A., Redekop W.K. Adjuvant trastuzumab therapy for early HER2-positive breast cancer in Iran: a cost-effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics. 2018;36(1):91–103. doi: 10.1007/s40273-017-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dedes K.J. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a model-based analysis of the HERA and FinHer trial. Ann Oncol. 2007;18(9):1493–1499. doi: 10.1093/annonc/mdm185. [DOI] [PubMed] [Google Scholar]
- 49.Hulme C. LBA12_PRPERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): cost effectiveness analysis results. Ann Oncol. 2018;29(suppl_8) [Google Scholar]
- 50.Purmonen T.T. Short-course adjuvant trastuzumab therapy in early stage breast cancer in Finland: cost-effectiveness and value of information analysis based on the 5-year follow-up results of the FinHer Trial. Acta Oncol. 2011;50(3):344–352. doi: 10.3109/0284186X.2011.553841. [DOI] [PubMed] [Google Scholar]