Abstract
The present study was conducted to evaluate the prevalence of the signaling pathways mutation rate in the Gastrointestinal (GI) tract cancers in a systematic review and meta-analysis study. The study was performed based on the PRISMA criteria. Random models by confidence interval (CI: 95%) were used to calculate the pooled estimate of prevalence via Metaprop command. The pooled prevalence indices of signal transduction pathway mutations in gastric cancer, liver cancer, colorectal cancer, and pancreatic cancer were 5% (95% CI: 3–8%), 12% (95% CI: 8–18%), 17% (95% CI: 14–20%), and 20% (95% CI: 5–41%), respectively. Also, the mutation rates for Wnt pathway and MAPK pathway were calculated to be 23% (95% CI, 14–33%) and 20% (95% CI, 17–24%), respectively. Moreover, the most popular genes were APC (in Wnt pathway), KRAS (in MAPK pathway) and PIK3CA (in PI3K pathway) in the colorectal cancer, pancreatic cancer, and gastric cancer while they were beta-catenin and CTNNB1 in liver cancer. The most altered pathway was Wnt pathway followed by the MAPK pathway. In addition, pancreatic cancer was found to be higher under the pressure of mutation compared with others based on pooled prevalence analysis. Finally, APC mutations in colorectal cancer, KRAS in gastric cancer, and pancreatic cancer were mostly associated gene alterations.
Subject terms: Cancer, Genetics, Molecular biology
Introduction
Cell signaling is a communication process of cell activities mediated by downstream genes and proteins. Distraction of signaling process induce disturbance in cellular mechanisms and may cause diseases, such as cancer, autoimmunity, and diabetes. In the major category, the signaling pathways are divided into intracellular activating signaling pathways, such as Hippo signaling and Notch signaling pathways or the extracellular activating pathways, for instance, Mitogen-activated protein kinase (MAPK) signaling, Nuclear factor κB (NF-κB), Janus kinase1/signal transducer and activator of transcription (STAT) signaling pathway, Wnt signaling pathways, Hedgehog, Smad signaling pathway, and PtdIns 3-kinase (PI3) signaling pathways. The Smad signaling is critical in TGF-β signaling, which controls the transcription. MAPK signaling pathway makes use of three different downstream effectors, including Extracellular-signal-regulated kinase pathway, c-Jun N-terminal kinase (JNK) pathway, and p38 pathway. Also, the Wnt signaling pathway is important in cell differentiation and proliferation. In Wnt signaling, the Wnt/β-catenin signaling pathway is the only canonical pathway2. The p53 signaling is not a canonical signaling pathway but due to the p53 non-transcriptional functions, the role of this pathway in generating cancer and its interaction with other signaling pathways, p53 can be considered as an individual pathway3.
Gastrointestinal (GI) cancers are a group of cancers that affect the digestive system and its accessory organs. The most prevalent cancers related to GI tract are colorectal, gastric, and esophageal cancers, respectively4. Mutations in signaling pathways, such as signal transduction systems, are the basic triggering mechanisms in different types of cancers5. The role of MAPK signaling pathway, Wnt, TGF beta, and JAK-STAT signaling pathways are more common in cancer induction. The Wnt signaling pathway, which include genes like PTEN (phosphatase and tensin homolog deleted on chromosome 10), WISP3 (Wnt1-inducible signaling protein 3), APC (Adenomatous polyposis coli), β-catenin, AXIN, and TCF4 (T-cell factor 4), has significant role in carcinogenesis. Thus, its microsatellite instability (MSI), among other carcinogenesis processes, has been a hot topic, especially in the studies of colorectal cancer6–8. APC mutation and promoter hypermethylation are two important mechanisms in carcinogenesis and colorectal cancer (CRC) progression9–11. Two AXIN genes, AXIN1 and AXIN2, could be prone to mutation in some CRC cases12,13. PIK3CA (phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha) and PTEN are two important genes in the PI3K/AKT signal pathway and previous studies have put emphasis on them as important genes in the CRC development by altering the proliferation and cell death patterns14,15. Moreover, CTNNB1 (catenin beta 1) transformation via β-catenin alteration as another mediators of the Wnt/β-catenin pathway have been found in some of the liver tumors16. Liver carcinogenesis process is related to the interactions of three major pathways: the p53/p21, the p16/cyclin D1/pRB, and the Wnt/wingless17,18. Also, numerous factors such as TNFα (tumour necrosis factor alpha), TGFβ (transforming growth factor beta), c-myc, IGF2R (insulin like growth factor 2 receptor), SMAD2, SMAD4, DLC-1, and HIC1 (HIC ZBTB transcriptional repressor 1) could initiate liver tumorogenesis17,18.
Mutation analysis of signaling pathway mediators could have prognostic impact on tumor development. Transformation of the epidermal growth factor receptor (EGFR) and its downstream pathway mediators could lead to development of human tumors19. Two vital intracellular pathways affected by EGFR are the RAS/RAF/MAPK and the PIK3CA/PTEN/AKT signaling pathways. These pathways mediate activation of transcription factors like ERK (extracellular regulated MAP kinase) and p38 and lead to cell transformation reactions like the up-regulation of proliferation, relocation, mesenchymal separation induction, and apoptosis reduction. As EGFR has been a target for anti-tumor drugs, its mutations and related downstream signaling pathway mutations have become important20.
Indeed, interaction of various signaling pathway mediator mutations and their behavior in cancer development has been a hot topic. These alterations could include susceptibility, resistant or non-sense for treatment management or tumorogenesis in different individuals geographically. By considering the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) criteria21, we made an attempt to evaluate the prevalence of the signaling pathways mutation rate in the GI tract cancers in a systematic review and meta-analysis setting.
Results
Search results
A total of 10,808 records were detected using the search strategy keywords. After screening by the title and abstracts, 414 articles were included for further analysis. Next, the full-text assessment resulted in selecting 121 eligible records including 65 studies on colorectal cancer (CRC), 21 on liver cancer (LC), 16 on Gastric Cancer (GC), 9 on pancreatic cancer1, and 15 on other gastrointestinal cancers, namely esophagus, bile duct, rectal cancer, gall bladder, and ampullary adenocarcinomas. The details of screened data based on PRISMA guideline are provided in Fig. 1. The numbers of participants for the assessment of the GI cancer mutations induced 17,269, 1056, 2500, 378, 1080 individuals for CRC, LC, GC, PC, and other GI cancers, respectively.
Figure 1.
PRISMA Flow Diagram of our study population, the diagram indicates the primary search item frequencies, duplicates, Studies included in qualitative synthesis and Studies included in quantitative synthesis.
Bias assessment
The risk of bias assessment is given in Table 1. Also, the RTI tool for the risk of bias determined one study with high risk of Selection Bias. Also, the Selection Bias, Performance Bias, Detection Bias, and Selective Outcome bias indicated 25, 3, 4, and 33 studies with unclear risk of bias, respectively. Furthermore, high risks of Selection Bias and Selective Outcome Bias were evaluated in 3 and 2 references, respectively.
Table 1.
Key: + : Low risk of bias, − High risk of bias ?, Unclear risk of bias, *: Non-applicable in non RCT by RTI.
| Author | Year | Country | Selection bias | Performance bias | Detection bias | Attrition bias | Selective outcome | Confounding | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Müller | 1998 | Germany | ? | ? | + | * | + | + | 22 |
| 2 | Sparks | 1998 | USA | − | ? | + | * | + | + | 23 |
| 3 | Kondo | 1999 | Japan | − | + | + | * | + | + | 16 |
| 4 | Koyama | 1999 | Japan | ? | + | + | * | ? | + | 24 |
| 5 | Shitara | 1999 | Japan | + | + | + | * | ? | + | 25 |
| 6 | Mirabelli | 1999 | Canada | + | + | + | * | + | + | 26 |
| 7 | Huang | 1999 | France | + | + | + | * | + | + | 27 |
| 8 | Wong | 2001 | China | + | + | + | * | + | + | 28 |
| 9 | Fujimori | 2001 | Japan | + | + | + | * | + | + | 29 |
| 10 | Kawate | 2001 | Japan | ? | + | + | * | ? | + | 30 |
| 11 | Rashid | 2001 | China | + | + | + | * | + | + | 31 |
| 12 | Shitoh | 2001 | Japan | + | + | + | * | + | + | 32 |
| 13 | Chen | 2002 | Taiwan | ? | + | + | * | ? | + | 33 |
| 14 | Taniguchi | 2002 | United States | + | + | + | * | + | + | 34 |
| 15 | Clements | 2002 | USA | + | + | + | * | ? | + | 35 |
| 16 | Engeland | 2002 | Netherlands | + | + | + | * | + | + | 36 |
| 17 | Yuen | 2002 | UK | ? | + | + | * | + | + | 37 |
| 18 | Abraham | 2002 | United States | ? | + | + | * | + | + | 38 |
| 19 | Yoo | 2002 | South Korea | + | + | + | * | + | + | 39 |
| 20 | Tannapfel | 2003 | Germany | ? | + | + | * | + | + | 40 |
| 21 | Jass | 2003 | Australia | + | + | + | * | + | + | 41 |
| 22 | Zhang | 2003 | Japan | + | + | + | * | + | + | 42 |
| 23 | Sakamoto | 2004 | Japan | + | + | + | * | ? | + | 43 |
| 24 | Bläker | 2004 | Germany | ? | + | + | * | ? | + | 44 |
| 25 | Fransén | 2004 | Sweden | + | + | + | * | + | + | 45 |
| 26 | Li | 2005 | China | + | + | + | * | + | + | 46 |
| 27 | Immervoll | 2005 | Norway | + | + | + | * | − | + | 47 |
| 28 | Pasche, | 2005 | USA | + | + | + | * | + | + | 48 |
| 29 | Thorstensen | 2005 | Norway | + | + | + | * | + | + | 49 |
| 30 | Noda | 2006 | Japan | + | + | ? | * | ? | + | 50 |
| 31 | Mikami | 2006 | Japan | + | + | + | * | + | + | 51 |
| 32 | Schönleben | 2008 | USA | + | + | + | * | ? | + | 52 |
| 33 | Ching-Shian Leong, | 2008 | Malaysia | + | ? | ? | * | ? | + | 53 |
| 34 | Nomoto | 2008 | Japan | ? | + | + | * | + | + | 54 |
| 35 | Schonleben | 2008 | Germany | ? | + | + | * | + | + | 55 |
| 36 | Pan | 2008 | China | + | + | + | * | + | + | 56 |
| 37 | Kim | 2008 | Korea | + | + | + | * | + | + | 57 |
| 38 | Xie | 2009 | Korea | + | + | + | * | + | + | 58 |
| 39 | Seth | 2009 | UK | − | + | + | * | + | + | 59 |
| 40 | Cieply | 2009 | USA | + | + | + | * | + | + | 60 |
| 41 | Dahse | 2009 | Germany | + | + | + | * | + | + | 61 |
| 42 | Kim | 2009 | South Korea | + | + | + | * | + | + | 62 |
| 43 | Packham | 2009 | Australia | + | + | + | * | ? | + | 63 |
| 44 | Baldus | 2010 | Germany | + | + | + | * | + | + | 64 |
| 45 | Irahara | 2010 | USA | + | + | + | * | + | + | 65 |
| 46 | Smith | 2010 | UK | + | + | + | * | ? | + | 66 |
| 47 | Liao | 2010 | China | ? | + | + | * | ? | + | 67 |
| 48 | Catenacci | 2011 | USA | + | + | + | * | + | + | 68 |
| 49 | Watanabe | 2011 | Japan | + | + | + | * | + | + | 69 |
| 50 | Metzger | 2011 | Luxembourg | + | + | + | * | ? | + | 70 |
| 51 | Naghibalhossaini | 2011 | Iran | + | + | + | * | − | + | 71 |
| 52 | Sameer | 2011 | India | + | + | + | * | + | + | 72 |
| 53 | Purcell | 2011 | New Zealand | + | + | + | ? | + | + | 73 |
| 54 | Ueda | 2011 | Japan | + | + | + | * | + | + | 74 |
| 55 | Mohri | 2012 | Japan | ? | + | + | * | + | + | 75 |
| 56 | Sukawa | 2012 | Japan | + | + | + | * | + | + | 76 |
| 57 | Bond | 2012 | Australia | + | + | + | ? | + | + | 77 |
| 58 | Laghi | 2012 | Italy | + | + | + | * | + | + | 78 |
| 59 | Levidou | 2012 | Greece | + | + | + | * | + | + | 79 |
| 60 | Lee | 2012 | Korea | + | + | + | * | + | + | 80 |
| 61 | Li | 2012 | China | + | + | + | * | ? | + | 81 |
| 62 | Paliga | 2012 | Canada | + | + | + | * | ? | + | 82 |
| 63 | Voorham | 2012 | Netherlands | + | + | + | * | + | + | 83 |
| 64 | Whitehall | 2012 | Australia | + | + | + | * | + | + | 84 |
| 65 | Khiari | 2012 | Tunisia | + | + | + | * | ? | + | 85 |
| 66 | Tai | 2012 | Taiwan | + | + | + | * | + | + | 86 |
| 67 | Ree | 2012 | Norway | + | + | + | * | + | + | 87 |
| 68 | Chen | 2013 | Taiwan | + | + | + | * | ? | + | 88 |
| 69 | Garcia-Carracedo | 2013 | USA | ? | + | + | * | + | + | 89 |
| 70 | Hidaka | 2013 | Japan | + | + | + | * | ? | + | 90 |
| 71 | Kan | 2013 | USA | + | + | + | * | + | + | 91 |
| 72 | Saigusa | 2013 | Japan | + | + | + | * | + | + | 92 |
| 73 | Shi | 2013 | China | ? | + | + | * | ? | + | 93 |
| 74 | Aissi | 2013 | Tunisia | + | + | + | * | ? | + | 94 |
| 75 | Fleming | 2013 | USA | + | + | + | * | + | + | 95 |
| 76 | Long | 2013 | China | + | + | + | * | + | + | 96 |
| 77 | Van Grieken | 2013 | UK, Japan, Singapore | + | + | + | * | ? | + | 97 |
| 78 | Gurzu | 2013 | Romania | + | + | + | * | + | + | 98 |
| 79 | Wang | 2013 | USA | + | + | + | * | + | + | 99 |
| 80 | Han | 2013 | Korea | + | + | + | * | ? | + | 100 |
| 81 | Neumann | 2013 | Germany | + | + | + | * | + | + | 101 |
| 82 | Shen | 2013 | China | + | + | + | * | + | + | 102 |
| 83 | Yip | 2013 | Malaysia | ? | + | + | * | + | + | 103 |
| 84 | Zhang | 2014 | China | + | + | + | * | + | + | 104 |
| 85 | Mohammadi asl | 2014 | Iran | + | + | + | * | ? | + | 105 |
| 86 | Chen | 2014 | China | + | + | + | * | + | + | 106 |
| 87 | Lee | 2014 | Korea | + | + | + | * | ? | + | 107 |
| 88 | Ahn | 2014 | Korea | + | + | + | * | ? | + | 108 |
| 89 | Chang | 2014 | Taiwan | ? | + | + | * | + | + | 109 |
| 90 | Jia | 2014 | China | ? | + | ? | * | ? | + | 110 |
| 91 | Wang | 2014 | USA, China | + | + | + | * | + | + | 111 |
| 92 | Zhu | 2014 | China | + | + | + | * | + | + | 112 |
| 93 | Tong | 2014 | PR China | + | + | + | * | + | + | 113 |
| 94 | Gao | 2014 | China | + | + | + | * | ? | + | 114 |
| 95 | Li | 2014 | China | ? | + | + | * | + | + | 115 |
| 96 | Saito | 2014 | Japan | ? | + | + | * | + | + | 116 |
| 97 | Schlitter | 2014 | Germany | ? | + | + | ? | + | + | 117 |
| 98 | Marchio | 2014 | Peru | + | + | + | * | + | + | 118 |
| 99 | Mikhitarian | 2014 | USA | ? | + | + | * | + | + | 119 |
| 100 | Yoda | 2015 | Japan | ? | + | + | * | + | + | 120 |
| 101 | Zaitsu | 2015 | Japan | + | + | + | * | + | + | 121 |
| 102 | Lu | 2015 | China | ? | + | + | * | ? | + | 122 |
| 103 | Kawamata | 2015 | Japan | + | + | + | * | ? | + | 123 |
| 104 | Lan | 2015 | Taiwan | + | + | + | * | + | + | 124 |
| 105 | Samara | 2015 | Greek | + | + | + | * | + | + | 125 |
| 106 | Abdelmaksoud Damak | 2015 | Tunisia | + | + | + | * | ? | + | 126 |
| 107 | Kawazoe | 2015 | Japan | + | + | + | * | + | + | 127 |
| 108 | Lin | 2015 | USA | + | + | + | * | + | + | 128 |
| 109 | Suarez | 2015 | France | + | + | + | * | ? | + | 129 |
| 110 | Witkiewicz | 2015 | USA | + | + | + | * | + | + | 130 |
| 111 | Okabe | 2016 | USA | + | + | + | * | + | + | 131 |
| 112 | Grellety | 2016 | France | + | + | + | * | ? | + | 132 |
| 113 | Jauhri | 2016 | India | + | + | + | * | ? | + | 133 |
| 114 | Nam | 2016 | Republic of Korea | + | + | + | * | + | + | 134 |
| 115 | Dallol | 2016 | Saudi Arabia | + | + | + | * | + | + | 135 |
| 116 | Yuan | 2016 | China | ? | + | + | * | + | + | 136 |
| 117 | Ziv | 2017 | New York | ? | + | ? | * | + | + | 137 |
| 118 | Ho | 2017 | Hong Kong | + | + | + | * | + | + | 138 |
| 119 | Hänninen | 2018 | Finland | + | + | + | * | + | + | 139 |
| 120 | Mizuno | 2018 | USA | + | + | + | * | + | + | 140 |
| 121 | Yang | 2018 | China | + | + | + | * | + | + | 141 |
Signaling pathways mutations in gastric cancer
From among 16 studies on GC, mostly the MAPK and PI3 pathways were analyzed in 2489 participants. The most evaluated gene in MAPK was KRAS and mutations ranged from 0 to 20%. Also, the PI3K mutations in the PI3 pathway were 3 to 8.7% and CTNNB1 mutations ranged from 1.7% to 7%. The detailed data are listed in Table 2 and supplementary Table 2.
Table 2.
GI tract cancer signaling pathway mutations based on genes and exon (n = 121).
| Cancer type (number of studies) | Pathway (number of studies) | Gene (number of studies) | Exon | Mutant% | Sample No | Reference(s) |
|---|---|---|---|---|---|---|
|
CRC (n = 65) |
MAPK (n = 43) | KRAS (n = 46) | 1 | 24 | 86 | 142 |
| 1, 2 | 14.6 | 48 | 43 | |||
| 2 | 34–44.9 | 1167 | 64,101,106,125,127,141 | |||
| 2, 3, 4 | 49 | 37 | 59 | |||
| 3, 4 | 3.8 | 264 | 127 | |||
| NR | 2.5–75 | 11,561 | 36,42,45,50,51,63,65–67,69,71,77,79,83,84,86,92,94,98,99,102,103,107–109,112,113,123,124,128,132,134,135 | |||
| BRAF (n = 33) | NR | 0–78 | 8146 | 37,45,50,51,63,65,67,71,77–79,83,84,93,98,108,109,112,127,128,132,134 | ||
| 11, 13–15 | 10 | 37 | 59 | |||
| 11, 15 | 6.9 | 676 | 102 | |||
| 15 | 2.3–46.2 | 982 | 64,79,101,103,105,106,125,141 | |||
| Wnt (n = 18) | beta-catenin (n = 6) | 3 | 3–37.5 | 491 | 26,29,32,51 | |
| NR | 4–27 | 97 | 22,42 | |||
| APC (n = 10) | NR | 28–73 | 750 | 41,83,88,99,107,128,135 | ||
| 15 | 50–52 | 180 | 32,126 | |||
| AXIN2 (n = 2) | 7, 8 | 1.4–20 | 381 | 49,62 | ||
| CTNNB1 (n = 7) | 3 | 1.3–16 | 274 | 85,126 | ||
| NR | 1–48 | 387 | 23,83,128,133 | |||
| PI3 (n = 15) | PIK3CA (n = 17) | 9, 22 | 0–21 | 1556 | 51,53,64,67,101,102,106 | |
| NR | 0–34 | 3634 | 65,83,84,107,109,112,124,127,128,134,135 | |||
| PTEN (n = 7) | 1–9 | 0 | 49 | 103 | ||
| 8 | 17 | 310 | 49 | |||
| NR | 0–28 | 459 | 83,128,133,135 | |||
| P53 (n = 5) | P53 (n = 5) | NR | 18–63 | 1589 | 49,77,99,128,135 | |
|
LC (n = 21) |
MAPK (n = 3) | KRAS (n = 3) | 2–18 | 0 | 25 | 40 |
| NR | 4–16 | 92 | 118,122 | |||
| BRAF (n = 2) | NR | 0 | 105 | 40,118 | ||
| Wnt (n = 15) | beta-catenin (n = 8) | NR | 15–70 | 225 | 33,34,91,129 | |
| 3 | 2.8–41 | 156 | 16,27,28,57 | |||
| AXIN (n = 3) | 3–5 | 25 | 36 | 57 | ||
| NR | 2–12.5 | 153 | 34,118 | |||
| CTNNB1 (n = 7) | 3 | 12–75 | 370 | 34,60,73,74,131 | ||
| NR | 15–31 | 86 | 110,118 | |||
| P53 (n = 4) | TP 53 (n = 4) | NR | 1.2–61 | 296 | 91,96,118,122 | |
|
PC (n = 9) |
MAPK (n = 5) | KRAS (n = 6) | 1 | 47–67 | 79 | 47,55 |
| 2 | 27 | 11 | 75 | |||
| NR | 42–92 | 199 | 52,119,130 | |||
| BRAF (n = 4) | 5, 11, 15 | 0–2.7 | 79 | 47,55 | ||
| NR | 0–2.7 | 90 | 52,119 | |||
| Wnt (n = 2) | beta-catenin (n = 1) | 3 | 23 | 21 | 38 | |
| AXIN (n = 1) | NR | 5 | 109 | 130 | ||
| PI3 (n = 4) | PIK3CA (n = 5) | All | 11 | 36 | 55 | |
| NR | 4–11 | 147 | 52,130 | |||
| 9 | 12 | 52 | 119 | |||
| 9, 20 | 2.7 | 36 | 89 | |||
|
GC (n = 16) |
MAPK (n = 5) | KRAS (n = 4) | 1 | 14 | 104 | 39 |
| 2 | 0 | 34 | 141 | |||
| NR | 4.2–20 | 767 | 97,120 | |||
| Wnt (n = 6) | AXIN1 (n = 2) | NR | 3.8–7.1 | 200 | 56,90 | |
| AXIN2 (n = 3) | NR | 4.6–9.8 | 292 | 56,62,90 | ||
| APC (n = 1) | NR | 2.5 | 237 | 80 | ||
| CTNNB1 (n = 4) | NR | 1.7–3.6 | 322 | 80,90,120 | ||
| 3 | 7.1 | 70 | 56 | |||
| PI3 (n = 5) | PIK3CA (n = 5) | NR | 5.1–7.2 | 292 | 80,120 | |
| 1, 9, 20 | 4.3–8.7 | 325 | 46,76 | |||
| 18 | 3 | 100 | 104 | |||
| PTEN (n = 1) | NR | 20 | 221 | 121 | ||
| AKT (n = 1) | 6 | 2 | 100 | 104 |
NR not reported.
The results of meta-analysis revealed that pooled prevalence index of signal transduction pathway mutations in GC was 5% (95% CI: 3–8%) and there was high heterogeneity between these studies in estimating the prevalence (I-squared = 91.25%, P = 0.001) (Fig. 2). Also, since the CI of the test (Egger’s test) does not include zero, there is no bias in our results (Egger's test = 3.51, P = 0.0001, 95% CI: 2.49 to 4.53). The pooled prevalence funnel plot in GC signal transduction pathway mutations is illustrated in Fig. 2. Furthermore, the Subgroup analyses of pooled prevalence Signal Transduction Pathway Mutations in GC are summarized in Table 3.
Figure 2.
Heterogeneity and pooled prevalence funnel plot of the included studies for GC signal transduction pathway mutations.
Table 3.
Subgroup analysis of pooled prevalence of Signal Transduction Pathway Mutations in GC, CRC, HCC, and PC based on gene, pathway, and method of diagnosis.
| Outcome | Subgroup | No. of studies | Summery Odds Ratio (95% CI) | Between studies | ||
|---|---|---|---|---|---|---|
| I2 | P heterogeneity | Q | ||||
| GC | Gene | |||||
|
AXIN2 CTNNB1 KRAS BRAF PIK3C |
2 3 4 2 4 |
6% (3– 9%) 2% (1–4%) 14% (2–34%) 0% (0–0%) 5% (3–8%) |
7.7% 0.0% 96.3% 39.2% 41.43% |
0.298 0.592 0.001 0.200 0.160 |
3.78 3.19 8.15 1.42 6.38 |
|
| Pathway | ||||||
|
Wnt MAPK PI3 |
8 5 6 |
5% (2–9%) 7% (1–17%) 6% (2–12%) |
83.4% 95.3% 88.7% |
0.0001 0.0001 0.0001 |
5.03 2.84 4.50 |
|
| Method for detection | ||||||
|
PCR, SS Array ARMS-PCR PCR-SSCP |
13 4 2 2 |
8% (4–14%) 3% (2–5%) 1% (0–6%) 4% (1–9%) |
94.7% 40.0% 29.0% 40.43% |
0.0001 0.170 0.130 0.345 |
5.33 7.37 1.00 3.65 |
|
| CRC | Gene | |||||
|
Beta-Catenin CTNNB1 APC KRAS BRAF NRAS SMAD4 PTEN PIK3C |
4 5 7 41 27 6 6 5 17 |
17% (4–36%) 9% (1–22%) 44% (33–55%) 32% (29–36%) 9% (6–12%) 7% (0–23%) 7% (3–12%) 5% (0–14%) 9% (6–12%) |
92.97% 93.35% 89.18% 94.24% 95.83% 99.17% 90.65% 90.97% 92.65% |
0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 |
3.30 2.94 11.68 29.60 9.22 5.24 5.03 10.48 14.07 |
|
| Pathway | ||||||
|
Wnt MAPK/ERK Smad (TGF-β) PI3 |
18 73 9 21 |
23% (14–33%) 20% (17–24%) 7% (4–10%) 9% (6–12%) |
96.25% 97.74% 86.69% 91.29% |
0.001 0.001 0.001 0.001 |
7.69 19.68 7.51 10.58 |
|
| Method for detection | ||||||
|
PCR, SS High-throughput Genotyping NGS PCR, Pyrosequencing |
67 9 18 12 |
17% (14–21%) 4% (0–12%) 28% (22–35%) 17% (11–25%) |
97.21% 95.90% 94.90% 96.95% |
0.001 0.001 0.001 0.001 |
16.90 2.44 1.96 13.69 |
|
| LC (HCC) | Gene | |||||
| Beta-Catenin | 7 | 20% (10–31%) | 77.20% | 0.001 | 6.06 | |
| Pathway | ||||||
| Wnt | 13 | 17% (11–23%) | 72.34% | 0.001 | 9.11 | |
| Method for detection | 2.56 | |||||
|
SSCP, SS PCR, SS |
5 16 |
14% (1–34%) 11% (6–17%) |
92.16% 79.51% |
0.001 0.001 |
6.04 4.22 |
|
| PC | Gene | |||||
| KRAS | 5 | 58% (31–83%) | 93.64% | 0.001 | 5.60 | |
| PIK3C | 4 | 6% (3–10%) | 14.84% | 0.320 | 5.13 | |
| Pathway | ||||||
| MAPK | 8 | 31% (5–66%) | 97.66% | 0.001 | 4.75 | |
| PI3 | 4 | 6% (3–10%) | 14.84% | 0.320 | 5.13 | |
| Method for detection | ||||||
| PCR, SS | 11 | 31% (5–66%) | 92.05% | 0.001 | 3.84 | |
GC: gastric cancer; CRC: colorectal cancer; HCC: hepatocellular carcinoma; PC: pancreatic cancer. SS: Sanger Sequencing, SSCP: Single-stranded conformation polymorphism; HPLC: High-performance liquid chromatography, NGS: next-generation sequencer, ARMS-PCR: amplification refractory mutation system polymerase chain reaction.
Signaling pathways mutations in CRC
CRC related signaling pathway mutation was found in 65 studies. A majority of study samples had the mean age > 60 years and male/female ratios of CRC incidence in most of the evaluated studies were reported more than 2:1. The most prevalent mutation analysis was taken from KRAS exon 2, BRAF exon 15, PIK3CA exon 9 and 20, and APC and beta-Catenin exon 3. Most of the studies were cross-sectional and total CRC patients included 17,269 cases. These studies reported different mutation rates based on the sample size, selected gene, and method of use. The results showed a wide range of mutation in different pathways and related genes as listed in supplementary Table 3. The KRAS mutations in the MAPK pathway were 2.5 to 75% and the BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations ranged from 0 to 78.6%. The Wnt signaling mediator mutations, such as beta-catenin, were reported from 3 to 37.5% and APC mutations ranged from 28.4 to 73%. The p53 was assessed in 5 studies and its mutation rate was reported 18–65% (Table 2).
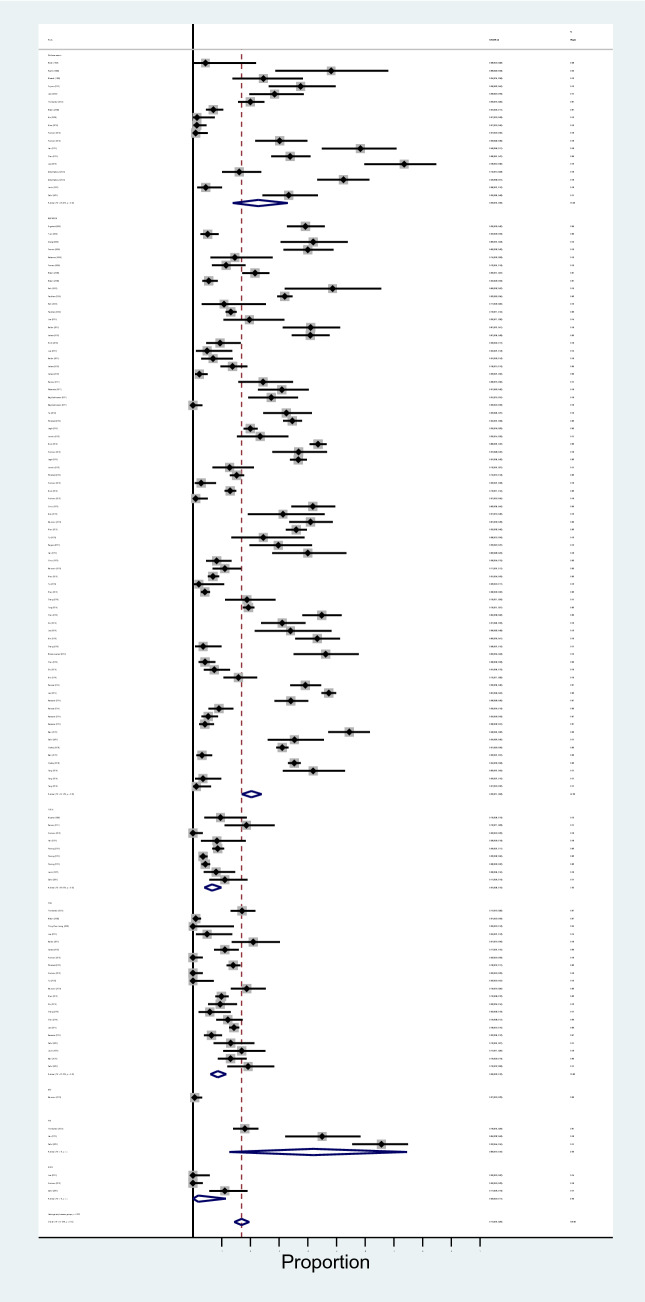
The results of meta-analysis revealed that pooled prevalence of signal transduction pathway mutations in CRC was 17% (95% CI: 14%, 20%) and there was a high heterogeneity between these studies in estimating the prevalence (I-squared = 97.63%, P = 0.0001) (Fig. 3). Also, the subgroup analysis for heterogeneity was performed in CRC included studies based on the different pathways (heterogeneity plot in Fig. 4), detection method (heterogeneity plot in Fig. 5), and involved genes (heterogeneity plot in Fig. 6). The CI of test (Egger’s test) included zero, thus there was no significant bias in the results (Egger's test = − 0.692, P = 0.109, 95% CI: − 1.54 to 0.156). The pooled prevalence funnel plot in CRC signal transduction pathway mutations is illustrated in Fig. 7 and the Subgroup analyses of pooled prevalence signal transduction pathway mutations in CRC are summarized in Table 3.
Figure 3.
Heterogeneity plot of the included studies for CRC signal transduction pathway mutations.
Figure 4.
Subgroup analysis for heterogeneity based on the different pathways for CRC signal transduction pathway mutations.
Figure 5.

Subgroup analysis for heterogeneity based on the detection method for CRC signal transduction pathway mutations.
Figure 6.

Subgroup analysis for heterogeneity based on involved genes for CRC signal transduction pathway mutations.
Figure 7.

Pooled prevalence funnel plot in CRC signal transduction pathway mutations.
Signaling pathway mutations in liver cancer (LC)
The search on liver cancer resulted in a total of 1056 hepatocellular carcinoma (HCC) and 174 hepatoblastoma participants in 21 studies. There were different ranges of mutations in these studies, which are listed in supplementary Table 4. The Wnt signaling was the most evaluated pathway in which the CTNNB1 gene mutation ranges were evaluated to be 12–75% and the beta-catenin genes had the mutation ranges of 2.8–41%. In addition, the mutation ranges in p53 were 1.2 to 61% and the JAKs in the JAK signaling pathway were observed to be 1.2 to 16%.
The results of meta-analysis showed that pooled prevalence of signal transduction pathway mutations in LC was 12% (95% CI: 8–18%) and there was a high heterogeneity between these studies in estimating the prevalence (I-squared = 85.34%, P = 0.0001) (Fig. 8). Also, since the CI of the test (Egger’s test) included zero, there was no significant bias in the results (Egger's test = − 0.442, P = 0.411, 95% CI: − 0.65 to 1.53). The pooled prevalence funnel plot in LC signal transduction pathway mutations is illustrated in Fig. 8. Furthermore, the Subgroup analyses of pooled prevalence signal transduction pathway mutations in LC are summarized in Table 3.
Figure 8.
Heterogeneity and pooled prevalence funnel plot of the included studies for liver cancer signal transduction pathway mutations.
Signaling pathways mutations in pancreatic cancer1
In a total of 9 studies, 392 PC patients were studied with the KRAS and PIK3CA mutations reported 42 to 92% and 2.7 to 12%, respectively. More data are shown in supplementary Table 5.
The results of meta-analysis showed that pooled prevalence of signal transduction pathway mutations in pancreatic cancer was 20% (95% CI: 5–41%) and there was a high heterogeneity between these studies in estimating the prevalence (I-squared = 97.14%, P = 0.0001) (Fig. 9). Also, the CI of the test (Egger’s test) included zero, s no significant bias was present in the results (Egger's test = − 1.351, P = 0.568, 95% CI: − 6.37 to 3.66). The pooled prevalence funnel plot in PC signal transduction pathway mutations is illustrated in Fig. 9. Furthermore, the Subgroup analyses of pooled prevalence signal transduction pathway mutations in pancreatic cancer are summarized in Table 3.
Figure 9.
Heterogeneity and pooled prevalence funnel plot of the included studies for pancreatic cancer (PC) signal transduction pathway mutations.
Signaling pathways mutations in other GI cancers
The other GI cancers included gastro-esophageal cancer, rectal cancer, esophageal squamous cell carcinoma, gallbladder carcinoma, and cholangiocarcinoma. Different signaling pathways in these GI cancers are listed in supplementary Table 6. Briefly, KRAS was the popular gene for mutation analysis ranging from 0% mutation in squamous cell anal carcinoma to 57% in small intestinal adenocarcinoma. BRAF was analyzed in 6 studies with its mutation reported to be 0–45%. Moreover, APC mutations were reported between 9.5 and 47% in different malignancies.
Signaling pathway mutation association with clinic-pathological features and patients survival
The extracted data about clinic-pathological features and patients survival were listed in supplement Tables 2 to 6. As glimpse, the clinic-pathological features statistically significant in association with signaling pathway mutations that they were mentioned in 2 individual studies for gastric cancer and 30, 6, 1 and 2 individual studies for CRC, LC, PC and other GI cancers, respectively.
Survival rate assessment in association with signaling pathway mutations were listed in supplement Tables 2 to 6. The survival rate or prognostic feature in association with signaling pathway mutations were mentioned in 1, 6, 1, 1, 0 and 1 included studies for CRC, LC, PC and other GI cancers, respectively.
Discussion
The aim of the current study was to evaluate the prevalence of the signaling pathway mutation rate in GI tract cancers in a systematic review and meta-analysis setting. It should note that, the signaling pathway mutations were comprehensively studied by The Cancer Genome Atlas (TCGA)1. Furthermore, the inclusion criteria for the current study were different with TCGA assessments. Also, this study could be a lead for further investigations in the field of the signaling pathway mutations prevalence and might be useful for further TCGA comprehensive updates. Appropriate keywords were used for search strategy in popular academic databases. Data were screened and eligibility of the studies was evaluated according to the inclusion criteria. PRISMA guideline was used as the study protocol. Through the search strategy, we found that GI malignancies included CRC, LC, PC, GC, esophageal cancer, rectal cancer, and bile duct neoplasm or cholangiocarcinoma. The results obtained in the current study showed that most alterations in CRC patients were in the KRAS gene in MAPK pathway within the range of 3.8 to 54.5%. These differences could be due to the study population or the methodology in different studies although the cancer stage and other risk factors could also play major roles. Furthermore, the pooled prevalence indices of signal transduction pathway mutations in GC, CRC, LC, and PC were 5% (95% CI: 3–8%), 17% (95% CI: 14–20%), 12% (95% CI: 8–18%), and 20% (95% CI: 5–41%), respectively. The higher rates in pooled prevalence could suggest more association between the signal transduction mutations and GI cancers incidence. The subgroup analysis for CRC shows that KRAS and APC are the most mutant genes with 32% (95% CI, 29–36%) and 44% (95% CI, 33–55%) mutation rates, respectively. Also, the most altered pathway was Wnt (23%) (95% CI, 14–33%), followed by MAPK (20%) (95% CI, 17–24%) pathway.
The CRC carcinogenesis is firstly initiated by the mild colon polyps and gradually progresses to the cancerous lesions. The adenocarcinoma is globally the most prevalent type of the CRC143,144. Recently, different studies have been reported focusing on the cost-effectiveness of the CRC screening programs indicating the importance of the CRC diagnosis145,146. Signaling pathways have crucial impacts on the development of different cancers5. Although the nucleotide alterations have critical impacts on cancer initiation, the environmental factors are predisposing elements in cancer induction and are affecting the signaling pathways mutations147,148. As an example, smoking affects CRC cancers generation and mortality149–151. In this regard, lung cancer investigations revealed that smoking could increase the EGFR and its downstream elements, such as KRAS and BRAF mutations148. Moreover, studies on CRC and smoking showed that TGFβ signaling pathway mutations have significant roles in carcinogenesis147. Inflammation is another key player in generation of cancer152,153. TLR2 alterations associated with inflammation could lead to the signaling pathways related ERK (extracellular-regulated kinase) and PI3K/AKT mutations. The importance of the inflammation in the CRC were illustrated by Liu and et al.154. These substitutions might be due to the microbiome disturbance, too155.
The MAPK/ERK signaling was analyzed in the study reported by Sameer et al.156 who found KRAS mutation to be 24% in 86 CRC patients. Meanwhile, Tong et al.113 reported the highest rate (75%) of the KRAS mutations in CRC patients in codon 12 in 1506 individuals. Tong’s study showed different mutation rates between the separate codons of the KRAS gene with the highest in codon 12 and the lowest (2.5%) in codon 61. Also, in the study conducted by Kawazoe et al.127 on 264 metastatic colorectal cancers (mCRC), the KRAS exon 2 mutation was calculated to be 34%, as the highest mutation rate. In this study, BRAF mutation rate was reported to be 5.4%. The highest prevalence for the BRAF mutation reported in other studies was 78%88. This huge difference in the BRAF mutation rate could be due to the differences in the sample size and the method used for analysis.
The Wnt/beta-catenin signaling and PI3K/AKT signal have been assessed in a variety of studies. The Wnt/beta-catenin was assessed in 18 different studies and the most evaluated genes were APC, beta-catenin, and CTNNB1. Fujimori et al.26 showed that 37.5% of the 73 CRC patients had mutations in the exon 3 of the beta-catenin gene. Also, Shitoh et al.32 reported the rate of 3% for beta-catenin mutation in exon 3, and 27% in the high-frequency microsatellite instability (MSI-H). Furthermore, the APC gene mutations were assessed in 10 different studies with the lowest reported to be 33% in the study by Chen88 study and the highest as 73% reported by Lee et al.107. The previous studies showed that the MSI could be associated with the in/del substitutions in genome hot spots which can initiate CRC tumorogenesis by increasing the mismatches indiscriminately157–159. Investigation on Wnt/beta-catenin signaling was firstly introduced by the association between APC gene and beta-catenin160,161. Other studies found the interactions of these genes with beta-catenin-Tcf (T-cell factor) complex suggesting the association of these genes with CRC omplication 162. The role of APC gene in causing cancer was initially introduced in the familial adenomatous polyposis (FAP)163. This gene facilitates beta-catenin distorting. APC gene mutations influence beta-catenin and AXIN protein binding sites164,165. Moreover, they could maximize the protein stability and life cycle166. Thus, the carcinogenesis process is accelerated by altered signal transduction and cell cycle167.
From among the studies which assessed the PI3 signal transduction pathway, the mutation of PIK3CA gene was reported in 20 studies ranging between 0 and 34%. Meanwhile, Thorstensen et al.49 found p53 gene mutation rate to be about 18% in CRC patients.
There are variable reports in the matter of clinic-pathological association with mutations in the current study. In the conducted study by Sameer et al.156 the clinic-pathological assessment indicated that, the SMAD4 mutations are more frequent in colon tumors and statistically associated with tumor grade and lymph nodes involvement. Tong and colleagues113 reports the KRAS mutations are in association with gender and tumor site. Also, Kawazoe et al.127 points out the BRAF mutations are associated with tumor location, site of metastasis and differentiation pattern. Meanwhile, Yang and colleagues168 reports the association of the KRAS mutations with tumor location, type of tumor, differentiation pattern and gender of the patients. Furthermore, there were limited data about the association of the mutations in signaling pathways with survival rate in patients. Some studies suggested BRAF mutations169 and SMAD4 mutations140 are association with poor prognosis and survival rate. Highly variable and limited data about clinic-pathological features, survival and prognosis in association with signaling pathway mutations were extracted. The clinic-pathological features and patients survival association with signaling pathway mutations is one of the current study limitations and needs further investigation.
HCC is the fifth cause of death worldwide and is mostly inducted by the chronic liver disorders, such as viral hepatitis170,171. In LC patients, the Wnt signaling was the top research interest and the CTNNB1 was the most assessed gene. The CTNNB1 mutation was also investigated in HCC patients in different studies118,129,131. Purcell et al.73 reported CTNNB1 mutations in 15% of hepatoblastoma patients while the reported prevalence in Ueda’s study was 75%74. Our study subgroup analysis for liver cancer145 studies showed that beta-catenin has higher mutation rate (20% (95% CI, 10–31%)) and the most altered pathway was Wnt (17% (95% CI, 11–23%)). It has been indicated that the CTNNB1 and P53 genes are the most involved genes in the HCC172,173. Moreover, the conducted studies showed that the P53 mutations were mostly associated with the Asian and African countries, while the CTNNB1 mutations were mostly associated with HCC in the Western countries172,173.
The pancreatic cancer is known as the forth cause of cancer mortality in the US with only 10% of the cases living more than 5 years174. Witkiewicz et al.130 assessed different genes in MAPK/ERK, PI3K/AKT, and Wnt/beta-catenin signaling pathways in pancreatic ductal adenocarcinoma patients. They showed that the AXIN1, KRAS, and PI3CA mutations rate were 5%, 92%, and 4%, respectively. Moreover, the high rate of KRAS mutations in pancreatic cancer patients was confirmed by the other studies55,119,175. Our study showed that in the subgroup analysis for pancreatic cancer, the KRAS was the most mutated gene (58% (95% CI: 31–83%)) and MAPK was the most altered pathway (31% (95% CI: 5–66%)). In GC, mutations were 14% (95% CI: 2–34%) for KRAS, 7% (95% CI: 1–17%) for MAPK, and 6% (95% CI: 2–12%) for PI3 pathways. In the pancreatic and gastric cancers, the most evaluated pathways were PI3 and MAPK. The KRAS gene generates a GTPase protein which is critical in regulating the cell proliferation and metabolism176. The mutations in KRAS leads to impaired cells activity enhancement and malignancy progression177.
Gastric Cancer (GC), as another invasive GI cancer, has significant mortality rate worldwide178. Zhang et al.104 studied 100 advanced primary GC cases for the purpose of evaluating PI3K/AKT signaling pathway mutations. They suggested that the MAPK/ERK and PI3K/AKT pathways could be potential therapeutic targets for GC treatment179,180. The AKT gene produced a protein in the PI3K/Akt pathway which could play a role in tumorogenesis80. The mutations in the PIK3CA and AKT in PI3K/AKT pathway could affect downstream signaling pathway genes, like mTOR (mechanistic target of rapamycin kinase) and caspase 9, which are important in GC progression104,181,182. Wang et al.99 investigated hedgehog pathway in GC patients and showed that the PTCH1 (patched 1) and SMO (smoothened) genes were mutated in 51.2% and 25.6% of the cases. Alterations in PTCH1 gene were associated with the basal cell carcinoma and basal cell nevus syndrome183,184.
Moreover, most of the studies included used PCR followed by the Sanger sequencing, as the method of choice. However, some studies used SSCP-PCR (single-strand conformational polymorphism PCR) to detect mutation. The method used the least was the NGS (next generation sequencing) as a preferred method in the recent years. The NGS can be used to analyze numerous samples at the same time and thus reduce the cost and the time required185. But the Sanger sequencing is an accurate and sensitive method for mutation analysis and it has been suggested for the confirmation of the NGS results186. Also, in the subgroup analysis for the GC, the method of detection could be mentioned as a potent source of the heterogeneity in the current study (Table 3).
The major limitation in the current study was the extent of subject; it is suggested that further investigations use more narrowing strategies. Also, we aimed at minimizing the author bias in data extraction and screening biases using different authors and double check strategies. Also, it should be mentioned that the p53 signaling is not a canonical signaling pathway but due to the p53 non-transcriptional functions, the importance of this pathway in cancer generation, and its interaction with other signaling pathways, in the present study, we assessed p53 as individual pathway3.
In conclusion, progression of GI cancers is affected by signaling pathway mediators. Different studies have shown diverse results based on their population, method, and target gene. Our study concluded that the most important genes that are under mutation pressure include KRAS and PI3CA in the CRC, PC, and GC while beta-catenin and CTNNB1 are genes under mutation pressure for liver malignancies. Subgroup analysis and heterogeneity of the studies could illustrate more valid data between different studies for screening strategies. In this regard, signal transduction pathway mutations pooled prevalence was higher in PC and lower in GC (20% vs. 5%). Thus, PC is the most common cancer involved by signal transduction mediator’s mutations. Among studied genes, KRAS in GC and pancreatic cancer and APC in CRC had the most association with cancer outcome. Moreover, MAPK had higher mutation rate among the studied pathways. Furthermore, PCR-SS method had the highest popularity among different methods. Future studies should be carried out to focus on cancer progression and patient’s survival assessments.
Methods
Search strategy
In the present comprehensive study, we assessed all relevant original research studies via the electronic literature search in Web of Science (SCIE), PubMed (Including MEDLINE), Science Direct, Scopus, EMBASE, and Google Scholar databases using the keywords including Polymorphism, Mutation, Mutation Rate, Mutation Prevalence, Silent Mutation, Point Mutation, Missense Mutation, INDEL Mutation, Frameshift Mutation, Synonymous Mutation, Non-synonymous Mutation, Transversion Mutation, Transition Mutation, Insertion Mutation, Deletion Mutation, Digestive System Diseases, Gastrointestinal Neoplasms, Digestive System Abnormalities, Biliary Tract Diseases, Biliary Tract Neoplasms, Gallbladder Diseases, Anorectal Malformations, Colorectal Neoplasms, Pancreatic Neoplasms, Hepatocellular carcinoma, Esophageal cancer, Intestinal Diseases, Stomach Diseases, Stomach cancer, Gastric cancer, Liver Diseases, Liver Neoplasms, Pancreatic Diseases, Signaling Pathways, Signal Transduction, Wnt Signaling Pathway, and MAP Kinase Signaling System between January 1998 and September 28, 2019. Also, the reference lists of the screened studies were reviewed so as to find relevant studies (the exact search strategy is available in the supplement data of supplementary Table 1).
Inclusion and exclusion criteria
The studies were screened by two independent authors and all the studies meeting the inclusion criteria were included. Any discrepancy between the two reviewer authors were sorted out by a third expert. Inclusion criteria were the English language writing, publication up to the date of the search, the study setting of cross-sectional or cohort, and the data eligibility for the study. Furthermore, the meta-analysis, conference seminars, and review articles were excluded from the search results.
Data extraction
Selected studies were listed in EndNote software (EndNote X7, Thomson Reuters) and were reviewed by two authors of the study independently; disagreements between them were settled by a third expert. All the relevant studies were screened considering the inclusion criteria and the data were extracted. The extracted data included the first author’s name, the publication date (based on year), country, design of the study, type of the cancer, sample size, mutation pathway, gene name, mean age, gender, mutation positive population, and method of detection.
Risk bias assessment
The risk bias for the non-randomized controlled trials (RCT) was assessed making use of the 13 items in the Research Triangle Institute (RTI), Evidence-based Practice Center187.
Meta-analysis
In this study, to compute of the pooled estimate of prevalence we used the Metaprop command and random models with confidence interval of CI = 95%. The prevalence estimation performed by random effects models in some analyses due to statistically significant of the heterogeneity test. In the present study, for the evaluation of statistical heterogeneity between studies we used Cochran’s Q test and I2 statistics. In addition, for the assessment of the source of heterogeneity among studies we used subgroup analysis. Also, funnel plot and Egger test used for the publication bias assessment. For the statistical analysis in this study STATA 16.0 (Stata Corp, College Station, TX, USA) were used by setting the statistical significant value at p < 0.05.
Supplementary information
Acknowledgements
Authors acknowledge the support provided by Systematic Review Network, Iran University of Medical Sciences, Tehran, Iran. The study was funded by the grant awarded from Iran University of Medical Sciences, Tehran, Iran (number: 97-275-12664).
Abbreviations
- ARMS-PCR
Amplification refractory mutation system polymerase chain reaction
- APC
Adenomatous polyposis coli
- ARID2
AT-rich interactive domain
- ACVR2A
Activin A receptor type 2A
- ADAMTS17
A disintegrin-like and metalloprotease
- BCLAF1
BCL2 associated transcription factor 1
- BTN3A2
Butyrophilin subfamily 3 member A2
- BOK
Bcl-2 related ovarian killer
- CRC
Colorectal cancer
- CDKN2A
Cyclin-dependent kinase inhibitor 2A
- CCND1
Cyclin D1
- CDHR1
Cadherin related family member 1
- CTNNB1
Catenin beta 1
- CGH
Comparative genomic hybridization
- CISH
Chromogenic in situ hybridization
- CAPRIN2
Caprin family member 2
- DPC4
Deleted in pancreatic cancer-4
- DHPLC
Denaturing high pressure liquid chromatography
- ESCC
Esophageal squamous cell carcinoma
- EGFR
Epidermal growth factor receptor
- FLT3
FMS-like tyrosine kinase 3
- FBXW7
F-box and WD repeat domain containing 7
- FLG
Human filaggrin gene
- GC
Gastric cancer
- GI
Gastrointestinal
- GBC
Gallbladder carcinoma
- GNAS
Guanine nucleotide binding protein, alpha stimulating
- CGH
Comparative genomic hybridization
- GLTSCR1
Glioma tumor suppressor candidate region gene 1
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HCC
Hepatocellular carcinoma
- HPLC
High-performance liquid chromatography
- HRM
High resolution melt
- IHC
Immunohistochemistry
- ISH
In situ hybridization
- IPMN/IPMNC
Intraductal papillary mucinous neoplasm/carcinoma
- ICC
Intrahepatic cholangiocarcinoma
- IGF2R
Insulin like growth factor 2 receptor
- JAK1
Janus kinase 1
- KDR
Kinase insert domain receptor
- KLHL22
Kelch like family member 22
- LOH
Loss of heterozygosit
- LM
Liver malignancy
- mCRC
Metastatic colorectal cancer
- MAPK
Mitogen-activated protein kinase
- MSS
Microsatellite stable
- MSI
Microsatellite instability
- mTOR
Mechanistic target of rapamycin kinase
- MSI-L
Microsatellite instability low
- MSI-H
Microsatellite instability high
- NGS
Next-generation sequencing
- NOTCH1
Notch receptor 1
- PC
Pancreatic cancer
- PCR-SS
Polymerase chain reaction-sanger sequencing
- PCR-SSCP
Single strand conformation polymorphism polymerase chain reaction
- PCR-RFLP
Restriction fragment length polymorphism
- PTEN
Phosphatase and tensin homolog
- PTP
Protein tyrosine phosphatase
- PTPN11
Protein tyrosine phosphatase non-receptor Type 11
- PI3K
Phosphatidylinositol 3-kinase
- PDGFRA
Platelet derived growth factor receptor alpha
- PIK3CA
Phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha
- PMN
Papillary mucinous neoplasm
- qRTPCR
Quantitative real-time polymerase chain reaction
- RHOA
Ras homolog family member A
- RNF169
Ring finger protein 169
- RUNX1
Runt-related transcription factor 1
- STK11
Serine/threonine kinase 11
- SMO
Smoothened, frizzled class receptor
- SOX9
SRY-box transcription factor 9
- SMAD2
SMAD family member 2
- SSCA
Single strand confirmation analysis
- SSA/Ps
Sessile serrated adenoma/polyps
- SNP
Single-nucleotide polymorphism
- TGF-B
Transforming growth factor beta
- TRPC4AP
Transient receptor potential cation channel subfamily C member 4 associated protein
- VHL
Von Hippel–Lindau
- VCAM1
Vascular cell adhesion molecule 1
- WISP3
Wntl-inducible signaling pathway protein 3
- WGS
Whole genome sequencing
- WES
Whole-exome sequencing
Author contributions
Study concept and design: M. H. K. N. and A. T.; analysis and interpretation of data: Y. M., S. S., F. Z., and N. M.; drafting of the manuscript: F. S. T., G. H. A. and M. P.; critical revision of the manuscript for important intellectual content: M. H. K. N., H. A., A. M. J., M. M. L. and S. A. M.; statistical analysis: Y. M., N. M., M. P., M. E., S. S. and A. T.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73770-1.
References
- 1.Sanchez-Vega F, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173:321–337.e310. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge MJ. Module 2: cell signalling pathways. Cell Signal. Biol. 2014;6:csb0001002. doi: 10.1042/csb0001002. [DOI] [Google Scholar]
- 3.Stegh AH. Targeting the p53 signaling pathway in cancer therapy–the promises, challenges and perils. Expert Opin. Ther. Targets. 2012;16:67–83. doi: 10.1517/14728222.2011.643299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 6.Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Can. Res. 2002;62:2447–2454. [PubMed] [Google Scholar]
- 7.Thorstensen L, et al. WNT1 inducible signaling pathway protein 3, WISP-3, a novel target gene in colorectal carcinomas with microsatellite instability. Gastroenterology. 2001;121:1275–1280. doi: 10.1053/gast.2001.29570. [DOI] [PubMed] [Google Scholar]
- 8.Guanti G, et al. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum. Mol. Genet. 2000;9:283–287. doi: 10.1093/hmg/9.2.283. [DOI] [PubMed] [Google Scholar]
- 9.Giles, R. H., Van Esa, J. H. & Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer1653, 1–24 (2003). [DOI] [PubMed]
- 10.Narayan S, Roy D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol. Cancer. 2003;2:41. doi: 10.1186/1476-4598-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiltunen MO, et al. Hypermethylation of the APC (adenomatous polyposis coli) gene promoter region in human colorectal carcinoma. Int. J. Cancer. 1997;70:644–648. doi: 10.1002/(SICI)1097-0215(19970317)70:6<644::AID-IJC3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Leung JY, et al. Activation of AXIN2 expression by β-Catenin-T cell factor a feedback repressor pathway regulating Wnt signaling. J. Biol. Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 13.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frattini M, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br. J. Cancer. 2007;97:1139. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrone F, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann. Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 16.Kondo Y, et al. β-Catenin accumulation and mutation of exon 3 of the β-catenin gene in hepatocellular carcinoma. Jpn. J. Cancer Res. 1999;90:1301–1309. doi: 10.1111/j.1349-7006.1999.tb00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makuuchi A-MH. M. Molecular basis of multistep hepatocarcinogenesis: genetic and epigenetic events. Scand. J. Gastroenterol. 1999;34:737–742. doi: 10.1080/003655299750025642. [DOI] [PubMed] [Google Scholar]
- 18.Buendia, M. A. in Seminars in Cancer Biology.185–200 (Elsevier). [DOI] [PubMed]
- 19.Fearon ER. Molecular genetics of colorectal cancer. Ann. Rev. Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 20.El Zouhairi M, Charabaty A, Pishvaian MJ. Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest. Cancer Res. GCR. 2011;4:15–21. [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller O, Nimmrich I, Finke U, Friedl W, Hoffmann I. A β-catenin mutation in a sporadic colorectal tumor of the RER phenotype and absence of β-catenin germline mutations in FAP patients. Genes Chromosom. Cancer. 1998;22:37–41. doi: 10.1002/(SICI)1098-2264(199805)22:1<37::AID-GCC5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Can. Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 24.Koyama M, Ito M, Nagai H, Emi M, Moriyama Y. Inactivation of both alleles of the DPC4/SMAD4 gene in advanced colorectal cancers: Identification of seven novel somatic mutations in tumors from Japanese patients. Mutat. Res. Mutat. Res. Genom. 1999;406:71–77. doi: 10.1016/S1383-5726(99)00003-5. [DOI] [PubMed] [Google Scholar]
- 25.Shitara Y, et al. No mutations of the Smad2 gene in human sporadic gastric carcinomas. Jpn. J. Clin. Oncol. 1999;29:3–7. doi: 10.1093/jjco/29.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Mirabelli-Primdahl L, et al. Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999;59:3346–3351. [PubMed] [Google Scholar]
- 27.Huang H, et al. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am. J. Pathol. 1999;155:1795–1801. doi: 10.1016/S0002-9440(10)65496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::AID-CNCR1301>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor. Cancer Res. 2001;61:6656–6659. [PubMed] [Google Scholar]
- 30.Kawate S, et al. Mutational analysis of the Smad6 and Smad7 genes in hepatocellular carcinoma. Int. J. Mol. Med. 2001;8:49–52. [PubMed] [Google Scholar]
- 31.Rashid A, et al. β-Catenin mutations in biliary tract cancers: a population-based study in China. Can. Res. 2001;61:3406–3409. [PubMed] [Google Scholar]
- 32.Shitoh K, et al. Frequent activation of the beta-catenin-Tcf signaling pathway in nonfamilial colorectal carcinomas with microsatellite instability. Genes Chromosom. Cancer. 2001;30:32–37. doi: 10.1002/1098-2264(2000)9999:9999<::AID-GCC1065>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Chen YW, Jeng YM, Yeh SH, Chen PJ. p53 gene and Wnt signaling in benign neoplasms: β-catenin mutations in hepatic adenoma but not in focal nodular hyperplasia. Hepatology. 2002;36:927–935. doi: 10.1016/S0270-9139(02)00099-X. [DOI] [PubMed] [Google Scholar]
- 34.Taniguchi K, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- 35.Clements WM, et al. β-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Can. Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- 36.Engeland M, et al. K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene. 2002;21:3792–3795. doi: 10.1038/sj/onc/1205466. [DOI] [PubMed] [Google Scholar]
- 37.Yuen ST, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Can. Res. 2002;62:6451–6455. [PubMed] [Google Scholar]
- 38.Abraham SC, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am. J. Pathol. 2002;160:953–962. doi: 10.1016/S0002-9440(10)64917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo J, et al. ras Gene mutations and expression of Ras signal transduction mediators in gastric adenocarcinomas. Arch. Pathol. Lab. Med. 2002;126:1096–1100. doi: 10.1043/0003-9985(2002)126<1096:rgmaeo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Tannapfel A, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jass JR, et al. APC mutation and tumour budding in colorectal cancer. J. Clin. Pathol. 2003;56:69–73. doi: 10.1136/jcp.56.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, et al. beta-Catenin and ras oncogenes detect most human colorectal cancer. Clin. Cancer Res. 2003;9:3073–3079. [PubMed] [Google Scholar]
- 43.Sakamoto N, et al. Frequent hypermethylation of RASSF1A in early flat-type colorectal tumors. Oncogene. 2004;23:8900–8907. doi: 10.1038/sj.onc.1207993. [DOI] [PubMed] [Google Scholar]
- 44.Bläker H, et al. Mutational activation of the RAS-RAF-MAPK and the wnt pathway in small intestinal adenocarcinomas. Scand. J. Gastroenterol. 2004;39:748–753. doi: 10.1080/00365520410005847. [DOI] [PubMed] [Google Scholar]
- 45.Fransén K, et al. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 46.Li VSW, et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005 doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Immervoll H, Hoem D, Kugarajh K, Steine SJ, Molven A. Molecular analysis of the EGFR-RAS-RAF pathway in pancreatic ductal adenocarcinomas: lack of mutations in the BRAF and EGFR genes. Virchows Arch. 2006;448:788–796. doi: 10.1007/s00428-006-0191-8. [DOI] [PubMed] [Google Scholar]
- 48.Pasche B, et al. Somatic acquisition and signaling of TGFBR1*6A in cancer. JAMA. 2005;294:1634–1646. doi: 10.1001/jama.294.13.1634. [DOI] [PubMed] [Google Scholar]
- 49.Thorstensen L, et al. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia (New York, N. Y.) 2005;7:99–108. doi: 10.1593/neo.04448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noda H, et al. Frequent involvement of ras-signalling pathways in both polypoid-type and flat-type early-stage colorectal cancers. J. Exp. Clin. Cancer Res. CR. 2006;25:235–242. [PubMed] [Google Scholar]
- 51.Mikami M, et al. Mutational analysis of β-catenin and the RAS-RAF signalling pathway in early flat-type colorectal tumours. Eur. J. Cancer. 2006;42:3065–3072. doi: 10.1016/j.ejca.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 52.Schönleben F, et al. Mutational analyses of multiple oncogenic pathways in intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2008;36:168–172. doi: 10.1097/MPA.0b013e318158a4d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ching-Shian Leong V, et al. PIK3CA gene mutations in breast carcinoma in Malaysian patients. Cancer Genet. Cytogenetics. 2008;187:74–79. doi: 10.1016/j.cancergencyto.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Nomoto S, et al. Adverse prognosis of epigenetic inactivation in RUNX3 gene at 1p36 in human pancreatic cancer. Br. J. Cancer. 2008;98:1690–1695. doi: 10.1038/sj.bjc.6604333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schonleben F, Qiu W, Remotti HE, Hohenberger W, Su GH. PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbeck's Arch. Surg. 2008;393:289–296. doi: 10.1007/s00423-008-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan KF, Liu WG, Zhang L, You WC, Lu YY. Mutations in components of the Wnt signaling pathway in gastric cancer. World J. Gastroenterol. 2008;14:1570–1574. doi: 10.3748/wjg.14.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim YD, et al. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2008;23:110–118. doi: 10.1111/j.1440-1746.2007.05250.x. [DOI] [PubMed] [Google Scholar]
- 58.Xie HJ, et al. Mutational analysis of JAK1 gene in human hepatocellular carcinoma. Neoplasma. 2009;56:136–140. doi: 10.4149/neo_2009_02_136. [DOI] [PubMed] [Google Scholar]
- 59.Seth R, et al. Concomitant mutations and splice variants in KRAS and BRAF demonstrate complex perturbation of the Ras/Raf signalling pathway in advanced colorectal cancer. Gut. 2009;58:1234–1241. doi: 10.1136/gut.2008.159137. [DOI] [PubMed] [Google Scholar]
- 60.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SPS. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahse R, Kromeyer-Hauschild K, Berndt A, Kosmehl H. No incidence of BRAF mutations in salivary gland carcinomasImplications for anti-EGFR Therapies. J. Biomed. Biotechnol. 2009 doi: 10.1155/2009/501736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MS, Kim SS, Ahn CH, Yoo NJ, Lee SH. Frameshift mutations of Wnt pathway genes AXIN2 and TCF7L2 in gastric carcinomas with high microsatellite instability. Hum. Pathol. 2009;40:58–64. doi: 10.1016/j.humpath.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Packham D, Ward RL, Ap Lin V, Hawkins NJ, Hitchins MP. Implementation of novel pyrosequencing assays to screen for common mutations of BRAF and KRAS in a cohort of sporadic colorectal cancers. Diagn. Mol. Pathol. 2009;18:62–71. doi: 10.1097/PDM.0b013e318182af52. [DOI] [PubMed] [Google Scholar]
- 64.Baldus SE, et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin. Cancer Res. 2010;16:790–799. doi: 10.1158/1078-0432.CCR-09-2446. [DOI] [PubMed] [Google Scholar]
- 65.Irahara N, et al. NRAS mutations are rare in colorectal cancer. Diagn. Mol. Pathol. 2010;19:157–163. doi: 10.1097/PDM.0b013e3181c93fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith G, et al. Activating K-Ras mutations outwith hotspot codons in sporadic colorectal tumours-implications for personalised cancer medicine. Br. J. Cancer. 2010;102:693–703. doi: 10.1038/sj.bjc.6605534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao, W. et al. Gene mutations in epidermal growth factor receptor signaling network and their association with survival in Chinese patients with metastatic colorectal cancers. Anatomical record (Hoboken, N.J. : 2007)293, 1506–1511. 10.1002/ar.21202 (2010). [DOI] [PubMed]
- 68.Catenacci DV, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol. Ther. 2011;12:9–46. doi: 10.4161/cbt.12.1.15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe T, et al. Differential gene expression signatures between colorectal cancers with and without KRAS mutations: crosstalk between the KRAS pathway and other signalling pathways. Eur. J. Cancer (Oxford, England: 1990) 2011;47:1946–1954. doi: 10.1016/j.ejca.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 70.Metzger B, et al. The human epidermal growth factor receptor (EGFR) gene in European patients with advanced colorectal cancer harbors infrequent mutations in its tyrosine kinase domain. BMC Med. Genet. 2011 doi: 10.1186/1471-2350-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naghibalhossaini F, Hosseini HM, Mokarram P, Zamani M. High frequency of genes' promoter methylation, but lack Of BRAF V600E Mutation among Iranian colorectal cancer patients. Pathol. Oncol. Res. 2011;17:819–825. doi: 10.1007/s12253-011-9388-5. [DOI] [PubMed] [Google Scholar]
- 72.Syed Sameer A, Shah ZA, Abdullah S, Chowdri NA, Siddiqi MA. Analysis of molecular aberrations of Wnt pathway gladiators in colorectal cancer in the Kashmiri population. Hum. Genom. 2011;5:441–452. doi: 10.1186/1479-7364-5-5-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Purcell R, et al. HGF/c-Met related activation of beta-catenin in hepatoblastoma. J. Exp. Clin. Cancer Res. CR. 2011;30:96. doi: 10.1186/1756-9966-30-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ueda Y, et al. Wnt signaling and telomerase activation of hepatoblastoma: correlation with chemosensitivity and surgical resectability. J. Pediatr. Surg. 2011;46:2221–2227. doi: 10.1016/j.jpedsurg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Mohri D, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J. Gastroenterol. 2012;47:203–213. doi: 10.1007/s00535-011-0482-y. [DOI] [PubMed] [Google Scholar]
- 76.Sukawa Y, et al. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J. Gastroenterol. 2012;18:6577–6586. doi: 10.3748/wjg.v18.i45.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bond CE, et al. p53 mutation is common in microsatellite stable, BRAF mutant colorectal cancers. Int. J. Cancer. 2012;130:1567–1576. doi: 10.1002/ijc.26175. [DOI] [PubMed] [Google Scholar]
- 78.Laghi L, et al. MSH3 protein expression and nodal status in MLH1-deficient colorectal cancers. Clin. Cancer Res. 2012;18:3142–3153. doi: 10.1158/1078-0432.ccr-12-0175. [DOI] [PubMed] [Google Scholar]
- 79.Levidou G, et al. ERK/pERK expression and B-raf mutations in colon adenocarcinomas: correlation with clinicopathological characteristics. World J. Surg. Oncol. 2012;10:47. doi: 10.1186/1477-7819-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J, et al. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS ONE. 2012;7:e38892. doi: 10.1371/journal.pone.0038892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, et al. Low frequency of PIK3CA gene mutations in hepatocellular carcinoma in Chinese population. Pathol. Oncol. Res. 2012;18:57–60. doi: 10.1007/s12253-011-9416-5. [DOI] [PubMed] [Google Scholar]
- 82.Paliga A, et al. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: a role for concurrent radiation and EGFR inhibitors. Br. J. Cancer. 2012;107:1864–1868. doi: 10.1038/bjc.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voorham QJM, et al. Comprehensive mutation analysis in colorectal flat adenomas. PLoS ONE. 2012 doi: 10.1371/journal.pone.0041963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whitehall VLJ, et al. Oncogenic PIK3CA mutations in colorectal cancers and polyps. Int. J. Cancer. 2012;131:813–820. doi: 10.1002/ijc.26440. [DOI] [PubMed] [Google Scholar]
- 85.Khiari M, et al. The prognostic value of the immunohistochemical expression and mutational pattern of the key mediator of Wnt signaling: beta-catenin in Tunisian patients with colorectal carcinoma. Appl. Immunohistochemistry Mol. Morphol. AIMM. 2012;20:62–70. doi: 10.1097/PAI.0b013e31821a20c2. [DOI] [PubMed] [Google Scholar]
- 86.Tai CJ, et al. Clinical-pathological correlation of K-Ras mutation and ERK phosphorylation in colorectal cancer. Pol. J. Pathol. 2012;63:93–100. [PubMed] [Google Scholar]
- 87.Ree AH, et al. Tumor phosphatidylinositol-3-kinase signaling and development of metastatic disease in locally advanced rectal cancer. PLoS ONE. 2012;7:e50806. doi: 10.1371/journal.pone.0050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen TH, et al. The prognostic significance of APC gene mutation and miR-21 expression in advanced-stage colorectal cancer. Colorectal Dis. 2013;15:1367–1374. doi: 10.1111/codi.12318. [DOI] [PubMed] [Google Scholar]
- 89.Garcia-Carracedo D, et al. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin. Cancer Res. 2013;19:6830–6841. doi: 10.1158/1078-0432.ccr-13-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hidaka Y, et al. Alteration in the Wnt/beta-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum. Pathol. 2013;44:2438–2448. doi: 10.1016/j.humpath.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Kan Z, et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422–1433. doi: 10.1101/gr.154492.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saigusa, S. et al. Decreased expression of DUSP4 is associated with liver and lung metastases in colorectal cancer. Medi. Oncol. Northwood, London, England)30, 620. 10.1007/s12032-013-0620-x (2013). [DOI] [PubMed]
- 93.Shi Y, Li J, Wu SY, Qin L, Jiao YF. BRAF mutation is associated with the unique morphology of traditional serrated adenoma of the colorectum. Int. J. Surg. Pathol. 2013;21:442–448. doi: 10.1177/1066896913499628. [DOI] [PubMed] [Google Scholar]
- 94.Aissi S, et al. KRAS mutations in colorectal cancer from Tunisia: Relationships with clinicopathologic variables and data on TP53 mutations and microsatellite instability. Mol. Biol. Rep. 2013;40:6107–6112. doi: 10.1007/s11033-013-2722-0. [DOI] [PubMed] [Google Scholar]
- 95.Fleming NI, et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Can. Res. 2013;73:725–735. doi: 10.1158/0008-5472.CAN-12-2706. [DOI] [PubMed] [Google Scholar]
- 96.Long J, et al. Correlation of TP53 mutations with HCV positivity in hepatocarcinogenesis: Identification of a novel TP53 microindel in hepatocellular carcinoma with HCV infection. Oncol. Rep. 2013;30:119–124. doi: 10.3892/or.2013.2430. [DOI] [PubMed] [Google Scholar]
- 97.Van Grieken NCT, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: Results from a large international multicentre study. Br. J. Cancer. 2013;108:1495–1501. doi: 10.1038/bjc.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gurzu S, et al. Serrated pathway adenocarcinomas: molecular and immunohistochemical insights into their recognition. PLoS ONE. 2013;8:e57699. doi: 10.1371/journal.pone.0057699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang XD, et al. Mutations in the hedgehog pathway genes SMO and PTCH1 in human gastric tumors. PLoS ONE. 2013;8:e54415. doi: 10.1371/journal.pone.0054415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han SW, et al. Targeted sequencing of cancer-related genes in colorectal cancer using next-generation sequencing. PLoS ONE. 2013;8:e64271. doi: 10.1371/journal.pone.0064271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neumann J, et al. Alterations in the EGFR pathway coincide in colorectal cancer and impact on prognosis. Virchows Archiv. Int. J. Pathol. 2013;463:509–523. doi: 10.1007/s00428-013-1450-0. [DOI] [PubMed] [Google Scholar]
- 102.Shen Y, et al. Effectors of epidermal growth factor receptor pathway: the genetic profiling ofKRAS, BRAF, PIK3CA, NRAS mutations in colorectal cancer characteristics and personalized medicine. PLoS ONE. 2013;8:e81628. doi: 10.1371/journal.pone.0081628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yip WK, et al. Molecular alterations of Ras-Raf-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt signaling pathways in colorectal cancers from a tertiary hospital at Kuala Lumpur, Malaysia. APMIS: Acta Pathologica, microbiologica, et immunologica Scandinavica. 2013;121:954–966. doi: 10.1111/apm.12152. [DOI] [PubMed] [Google Scholar]
- 104.Zhang QY, Cheng WX, Li WM, Au W, Lu YY. Occurrence of low frequency PIK3CA and AKT2 mutations in gastric cancer. Mutat. Res. 2014;769:108–112. doi: 10.1016/j.mrfmmm.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 105.Asl JM, Almasi S, Tabatabaiefar MA. High frequency of BRAF proto-oncogene hot spot mutation V600E in cohort of colorectal cancer patients from Ahvaz City, southwest Iran. Pak. J. Biol. Sci. PJBS. 2014;17:565–569. doi: 10.3923/pjbs.2014.565.569. [DOI] [PubMed] [Google Scholar]
- 106.Chen J, et al. BRAF V600E mutation and KRAS codon 13 mutations predict poor survival in Chinese colorectal cancer patients. BMC Cancer. 2014;14:802. doi: 10.1186/1471-2407-14-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SY, et al. Comparative genomic analysis of primary and synchronous metastatic colorectal cancers. PLoS ONE. 2014;9:e90459. doi: 10.1371/journal.pone.0090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahn TS, et al. The BRAF mutation is associated with the prognosis in colorectal cancer. J. Cancer Res. Clin. Oncol. 2014;140:1863–1871. doi: 10.1007/s00432-014-1735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang LC, et al. Mutational profiles of different macroscopic subtypes of colorectal adenoma reveal distinct pathogenetic roles for KRAS, BRAF and PIK3CA. BMC Gastroenterol. 2014 doi: 10.1186/s12876-014-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jia D, et al. Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology (Baltimore, MD) 2014;60:1686–1696. doi: 10.1002/hep.27243. [DOI] [PubMed] [Google Scholar]
- 111.Wang K, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 112.Zhu K, et al. Mutations of KRAS and PIK3CA as independent predictors of distant metastases in colorectal cancer. Med. Oncol. 2014 doi: 10.1007/s12032-014-0016-6. [DOI] [PubMed] [Google Scholar]
- 113.Tong JH, et al. Characterization of rare transforming KRAS mutations in sporadic colorectal cancer. Cancer Biol. Ther. 2014;15:768–776. doi: 10.4161/cbt.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao YB, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat. Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 115.Li M, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat. Genet. 2014;46:872–876. doi: 10.1038/ng.3030. [DOI] [PubMed] [Google Scholar]
- 116.Saito T, et al. Downregulation of sFRP-2 by epigenetic silencing activates the beta-catenin/Wnt signaling pathway in esophageal basaloid squamous cell carcinoma. Virchows Archiv Int. J. Pathol. 2014;464:135–143. doi: 10.1007/s00428-014-1538-1. [DOI] [PubMed] [Google Scholar]
- 117.Schlitter AM, et al. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod. Pathol. 2014;27:73–86. doi: 10.1038/modpathol.2013.112. [DOI] [PubMed] [Google Scholar]
- 118.Marchio A, et al. A peculiar mutation spectrum emerging from young peruvian patients with hepatocellular carcinoma. PLoS ONE. 2014;9:e114912. doi: 10.1371/journal.pone.0114912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mikhitarian K, et al. Epidermal growth factor receptor signaling pathway is frequently altered in ampullary carcinoma at protein and genetic levels. Mod. Pathol. 2014;27:665–674. doi: 10.1038/modpathol.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoda Y, et al. Integrated analysis of cancer-related pathways affected by genetic and epigenetic alterations in gastric cancer. Gastric Cancer. 2015;18:65–76. doi: 10.1007/s10120-014-0348-0. [DOI] [PubMed] [Google Scholar]
- 121.Zaitsu Y, et al. Loss of heterozygosity of PTEN (encoding phosphate and tensin homolog) associated with elevated HER2 expression is an adverse prognostic indicator in gastric cancer. Oncology. 2015;88:189–194. doi: 10.1159/000368984. [DOI] [PubMed] [Google Scholar]
- 122.Lu J, et al. Targeted sequencing of cancer-associated genes in hepatocellular carcinoma using next generation sequencing. Mol. Med. Rep. 2015;12:4678–4682. doi: 10.3892/mmr.2015.3952. [DOI] [PubMed] [Google Scholar]
- 123.Kawamata H, et al. Discrepancies between the K-ras mutational status of primary colorectal cancers and corresponding liver metastases are found in codon 13. Genomics. 2015;106:71–75. doi: 10.1016/j.ygeno.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 124.Lan YT, et al. Mutations in the RAS and PI3K pathways are associated with metastatic location in colorectal cancers. J. Surg. Oncol. 2015;111:905–910. doi: 10.1002/jso.23895. [DOI] [PubMed] [Google Scholar]
- 125.Samara M, et al. Mutation profile of KRAS and BRAF genes in patients with colorectal cancer: Association with morphological and prognostic criteria. Genet. Mol. Res. 2015;14:16793–16802. doi: 10.4238/2015.December.14.6. [DOI] [PubMed] [Google Scholar]
- 126.Abdelmaksoud-Damak R, et al. Expression and mutation pattern of beta-catenin and adenomatous polyposis coli in colorectal cancer patients. Arch. Med. Res. 2015;46:54–62. doi: 10.1016/j.arcmed.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 127.Kawazoe A, et al. A retrospective observational study of clinicopathological features of KRAS, NRAS, BRAF and PIK3CA mutations in Japanese patients with metastatic colorectal cancer. BMC Cancer. 2015;15:258. doi: 10.1186/s12885-015-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin EI, et al. Mutational profiling of colorectal cancers with microsatellite instability. Oncotarget. 2015;6:42334–42344. doi: 10.18632/oncotarget.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suarez MI, et al. Wnt/beta-catenin signaling pathway in hepatocellular carcinomas cases from Colombia. Ann. Hepatol. 2015;14:64–74. doi: 10.18632/oncotarget.2945. [DOI] [PubMed] [Google Scholar]
- 130.Witkiewicz AK, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015 doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okabe H, et al. Diverse basis of beta-catenin activation in human hepatocellular carcinoma: implications in biology and prognosis. PLoS ONE. 2016;11:e0152695. doi: 10.1371/journal.pone.0152695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grellety T, et al. Challenging a dogma: co-mutations exist in MAPK pathway genes in colorectal cancer. Virchows Archiv Int. J. Pathol. 2016;469:459–464. doi: 10.1007/s00428-016-1991-0. [DOI] [PubMed] [Google Scholar]
- 133.Jauhri M, et al. Targeted molecular profiling of rare genetic alterations in colorectal cancer using next-generation sequencing. Med. Oncol. (Northwood, London, England) 2016;33:106. doi: 10.1007/s12032-016-0820-2. [DOI] [PubMed] [Google Scholar]
- 134.Nam SK, et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to kras mutation status in advanced colorectal cancers with distant metastasis. PLoS ONE. 2016;11:e0151865. doi: 10.1002/cncr.299741371/journal.pone.0151865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dallol A, et al. Clinical significance of frequent somatic mutations detected by high-throughput targeted sequencing in archived colorectal cancer samples. J. Transl. Med. 2016 doi: 10.1186/s12967-016-0878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan W, et al. Whole-exome sequencing of duodenal adenocarcinoma identifies recurrent Wnt/β-catenin signaling pathway mutations. Cancer. 2016;122:1689–1696. doi: 10.1002/cncr.29974. [DOI] [PubMed] [Google Scholar]
- 137.Ziv E, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget. 2017;8:23529–23538. doi: 10.18632/oncotarget.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ho DW, et al. TSC1/2 mutations define a molecular subset of HCC with aggressive behaviour and treatment implication. Gut. 2017;66:1496–1506. doi: 10.1136/gutjnl-2016-312734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hänninen UA, et al. Exome-wide somatic mutation characterization of small bowel adenocarcinoma. PLoS Genet. 2018 doi: 10.1371/journal.pgen.1007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mizuno T, et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2018;44:684–692. doi: 10.1016/j.ejso.2018.02.247. [DOI] [PubMed] [Google Scholar]
- 141.Yang Q, et al. Mutation status and immunohistochemical correlation of KRAS, NRAS, and BRAF in 260 Chinese colorectal and gastric cancers. Front. Oncol. 2018 doi: 10.3389/fonc.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Samara M, et al. Mutation profile of KRAS and BRAF genes in patients with colorectal cancer: association with morphological and prognostic criteria. Genet. Mol. Res. 2015;14:16793–16802. doi: 10.4238/2015.December.14.6. [DOI] [PubMed] [Google Scholar]
- 143.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marley AR, Nan H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016;7:105–114. [PMC free article] [PubMed] [Google Scholar]
- 145.Tappenden P, et al. Option appraisal of population-based colorectal cancer screening programmes in England. Gut. 2007;56:677–684. doi: 10.1136/gut.2006.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pickhardt PJ, et al. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–2221. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 147.Zhong R, et al. Genetic variations in the TGFβ signaling pathway, smoking and risk of colorectal cancer in a Chinese population. Carcinogenesis. 2012;34:936–942. doi: 10.1093/carcin/bgs395. [DOI] [PubMed] [Google Scholar]
- 148.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int. J. Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 149.Botteri E, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 150.Japuntich, S. J. et al. Smoking status and survival among a national cohort of lung and colorectal cancer patients. Nicotine & Tobacco Research (2018). [DOI] [PMC free article] [PubMed]
- 151.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int. J. Cancer. 2009;124:2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 152.Correa P, Piazuelo MB. Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol. Hepatol. Rev. 2011;7:59. [PMC free article] [PubMed] [Google Scholar]
- 153.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu YD, et al. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways. Int. Immunopharmacol. 2018;59:375–383. doi: 10.1016/j.intimp.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 155.Schwabe RF, Jobin C. The microbiome and cancer. Nat. Rev. Cancer. 2013;13:800. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sameer AS, et al. SMAD4–molecular gladiator of the TGF-beta signaling is trampled upon by mutational insufficiency in colorectal carcinoma of Kashmiri population: an analysis with relation to KRAS proto-oncogene. BMC Cancer. 2010;10:300. doi: 10.1186/1471-2407-10-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 158.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science (New York, N. Y.) 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 159.Aaltonen LA, et al. Clues to the pathogenesis of familial colorectal cancer. Science (New York, N. Y.) 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 160.Su L-K, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science (New York, N. Y.) 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 161.Rubinfeld B, et al. Association of the APC gene product with beta-catenin. Science (New York, N. Y.) 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 162.Korinek V, et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science (New York, N. Y.) 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 163.Nishisho I, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science (New York, N. Y.) 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 164.Marvin ML, et al. AXIN2-associated autosomal dominant ectodermal dysplasia and neoplastic syndrome. Am. J. Med. Genet. Part A. 2011;155:898–902. doi: 10.1002/ajmg.a.33927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Salahshor S, Woodgett J. The links between axin and carcinogenesis. J. Clin. Pathol. 2005;58:225–236. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Polakis P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 167.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 168.Yang Q, et al. Mutation status and immunohistochemical correlation of KRAS, NRAS, and BRAF in 260 chinese colorectal and gastric cancers. Front. Oncol. 2018;8:487. doi: 10.3389/fonc.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nam SK, et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS ONE. 2016;11:e0151865. doi: 10.1371/journal.pone.0151865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 171.Nault JC, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013;4:2218. doi: 10.1038/ncomms3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guichard C, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boyault S, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology (Baltimore, MD) 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 174.Kleeff J, et al. Pancreatic cancer. Nat. Rev. Dis. Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 175.Bailey P, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 176.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 2008;9:517. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging ras back in the ring. Cancer Cell. 2014;25:272–281. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 178.Herrero R, Park JY, Forman D. The fight against gastric cancer - the IARC Working Group report. Best Pract. Res. Clin. Gastroenterol. 2014;28:1107–1114. doi: 10.1016/j.bpg.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 179.Soutto M, et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64:1028–1039. doi: 10.1136/gutjnl-2014-307191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 182.Parsons, R. in Seminars in Cell & Developmental Biology.171–176 (Elsevier).
- 183.Gailani MR, et al. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992;69:111–117. doi: 10.1016/0092-8674(92)90122-S. [DOI] [PubMed] [Google Scholar]
- 184.Hahn H, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/S0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 185.Sikkema-Raddatz B, et al. Targeted next-generation sequencing can replace Sanger sequencing in clinical diagnostics. Hum. Mutat. 2013;34:1035–1042. doi: 10.1002/humu.22332. [DOI] [PubMed] [Google Scholar]
- 186.Mu W, Lu HM, Chen J, Li S, Elliott AM. Sanger confirmation is required to achieve optimal sensitivity and specificity in next-generation sequencing panel testing. J. Mol. Diagn. JMD. 2016;18:923–932. doi: 10.1016/j.jmoldx.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 187.Viswanathan, M., Berkman, N. D., Dryden, D. M. & Hartling, L. Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI item bank. (2013). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.