Abstract
Herbaceous peony (Paeonia lactiflora Pall.) is a new high-end cut flower, but a large number of lateral branches often appear in some excellent cultivars, which is inconvenient for cut flower production. In the present study, we analyzed the effects of paclobutrazol (PBZ) on the lateral branches of P. lactiflora and adopted a next-generation sequencing approach to identify miRNAs and mRNAs that were differentially expressed involved in the PBZ response. Our results indicate that PBZ may inhibit the production of lateral branches on P. lactiflora. There were 827 differentially expressed genes (DEGs) and 104 differentially expressed miRNAs (DEMs). Integrative analysis revealed 29 miRNA–mRNA interactions related to PBZ stress. Our results provided a wealth of genetic information and data on metabolic pathways for revealing the regulatory mechanism of PBZ inhibition of the development of lateral branches in P. lactiflora and provided a new possibility for reducing lateral branch formation in the production of herbaceous peony cut flowers.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02489-7) contains supplementary material, which is available to authorized users.
Keywords: Herbaceous peony, Paclobutrazol application, miRNA, Transcriptome sequencing, Integrated analyses
Introduction
Herbaceous peony (Paeonia lactiflora Pall.) belongs to the Paeoniaceae family and is a traditional Chinese flower with a history of more than 3900 years of cultivation. It is considered “the flower phase” in China’s colorful gardens (Yuan and Yu 2011; Shen et al. 2012). In recent years, P. lactiflora has been used in wedding bouquets and large floral arrangements and has very broad market prospects in Euro-American countries (Yu et al. 2011). The herbaceous peony cut flower industry standard not only requires a specific flower shape, flower color, flower aroma, petal texture, inflorescence stem and vase life but also requires no or few lateral branches (Lu and Yu 2009; Zhao et al. 2019). More lateral branches not only endlessly consume the nutrients in the plants but also affect the growth and development of the top buds and ultimately affect the quality of the cut flowers (Zhao et al. 2015). In actual production, it is often necessary to manually remove the side branches and side buds. This process can not only easily cause damage to the top bud but also increase the time and effort. Therefore, it is of great significance to explore ways to inhibit the growth of lateral branches.
Paclobutrazol (PBZ) is a triazoles compound that has been used as a plant growth retardant in horticultural and agronomic crops in recent decades (Hua et al. 2014; Mohammadi et al. 2017; Yooyongwech et al. 2017; Dorairaj and Ismail 2017). PBZ regulates the growth of plants by regulating changes in the balance of important plant hormones, such as the gibberellins (MacDonald 2017), abscisic acid and cytokinins. Many studies have confirmed that PBZ mainly and effectively reduces plant height, leaf area and internode elongation (Carver et al. 2014; Eiasu et al. 2017). Furthermore, we also found that the growth of plant lateral branches could be significantly inhibited by PBZ, which has rarely been described in the existing literature.
Previous studies have demonstrated that the development of plant lateral branches can be divided into two stages: one is the formation of axillary meristems and the other is the growth of axillary buds (Liu et al. 2012). To date, phytohormones, including auxin (Ongaro and Leyser 2008), cytokinin (Dierck et al. 2016) and strigolactone (Tan et al. 2019), have been reported to play a critical role in mediating branching. For example, during the development of the vegetative and inflorescence phases, axillary meristems require auxin to initiate, and the movement of auxin from the stem end to the base inhibits the growth of axillary buds (McSteen 2009; Shimizu-Sato et al. 2009). Several genes related to the development of plant shoot branches were regulated by exogenous auxin, such as dwarf10 (d10) (Arite et al. 2007; Zhang et al. 2010), More axillary branching 4 (MAX4) (Otori et al. 2017), branched1 (BRC1) (Chen et al. 2013) and barren stalk1 (ba1) (Woods et al. 2011). However, the formation of plant traits is controlled by multiple genes, and it is very difficult to elucidate its intrinsic regulatory mechanisms only from the perspective of phytohormones. The combined analysis of mRNA and miRNA has been shown to be an important method for exploring transcriptional regulation mechanisms at the transcriptional and post-transcriptional levels (Zheng et al. 2015; Yang et al. 2016; Wu et al. 2016; Sarkar et al. 2017; Guo et al. 2017; Zhang et al. 2018). However, the comprehensive analysis of mRNA and miRNA in the growth development of herbaceous peony lateral branches has rarely been performed. Combined with mRNA and small RNA sequencing, this study further provided plentiful genetic information and data for exogenous PBZ inhibition of the growth of lateral branches in herbaceous peony. The results of our research provided an important theoretical basis for the cultivation of herbaceous peony cut flowers with no or few lateral branches.
Materials and methods
Plant materials
A 5-year-old Paeonia lactiflora Pall. cultivar ‘Zi feng yu’ was selected as the plant material and was grown under field conditions in the herbaceous peony germplasm nursery at Yangzhou University, Jiangsu Province, China (Latitude, 32° 39′ N; Longitude, 119° 42′ E). When the buds of the plants were exposed to the ground in March, they were sprayed weekly with 100 mg/ml PBZ (Solarbio, Beijing, China) until the flower withered; the control group was sprayed with deionized water at the same time. The length and diameter of the lateral branches were measured every 7 days as a stage (S1: 45 days after germination; S2: 52 days after germination; S3: 59 days after germination; S4: 66 days after germination). Three biological replicates with each 30 plants in PBZ application and control. The lateral branches of the herbaceous peony were collected at the S4 stage, frozen in liquid nitrogen and then stored at − 80 °C for RNA extraction.
RNA extraction, library construction and sequencing
Total RNA was extracted from the lateral branches of the control and PBZ application at the S4 stage using the RNAprep Pure kit (Tiangen, Beijing, China), and its purity was checked using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Sequencing libraries were generated using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. First-strand cDNA was synthesized using a random hexamer primer and M-MuLV Reverse Transcriptase (RNaseH). Second-strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Then, PCR was performed with phusion high-fidelity DNA polymerase, universal PCR primers and index (X) primer. The clustering of the index-coded samples was performed on a cBot cluster generation system using a TruSeq PE cluster kit v3-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq 2500 platform.
Functional annotation and characterization of unigenes and differentially expressed mRNAs
First, the raw reads (which have been filed in the NCBI Sequence Read Archive, SRR8196949, SRR8196951) were filtered by deleting the adaptor sequences, reads containing 10% or more unknown nucleotides (Ns) and low-quality reads containing 50% or more low-quality (Q-value ≤ 10) bases. The rest of the high-quality reads were assembled de novo to use trinity (https://github.com/trinityrnaseq/trinityrnaseq/wiki). In this study, the longest transcript (from alternative splicing transcripts) was selected as the unigene. The gene annotations for the entire all-unigenes were performed via BLAST search against Nr (NCBI non-redundant protein sequences), Nt (NCBI non-redundant nucleotide sequences), Swiss-Prot (a manually annotated and reviewed protein sequence database), Pfam (Protein family), GO (Gene Ontology), KO (KEGG Ortholog database), and KOG/COG (Clusters of Orthologous Groups of proteins), with an E-value of < 0.00001. The expression level of the unigene was calculated utilizing the fragments per kilobase per million fragments (FPKM) method. Differential expression analysis of two samples was performed using the DEGseq (2010) R package. The P-value was adjusted using q value. Q value < 0.005 & |log2-fold change (FC)|> 1 was set as the threshold for significantly differential expression. Those differentially expressed genes (DEGs) with the maximum absolute value of fold change were considered to be extremely important genes related to the growth and development of lateral branches.
Small RNA sequencing, analysis and prediction of miRNA targets
Small RNA sequencing libraries were generated from lateral branching samples using NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (NEB, USA.) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA), and then, the libraries were sequenced on an Illumina Hiseq 2500 platform. The assembled sequence data for these raw reads have been uploaded to NCBI Sequence Read Archive (SRA) under the accession numbers SRR8196948 and SRR8196950. Mapped small RNA tags were used to look for known miRNAs. Using miRBase 20.0 as a reference, modified software mirdeep2 and srna-tools-cli were used to acquire the potential miRNA and draw the secondary structures. The novel miRNA can be predicted by using the structural characteristics of the hairpin of the miRNA precursor. Using the DEGseq (Wang et al. 2010) R package to perform differential expression analysis of two samples, the P-value was adjusted using q-value. The q-value < 0.01 and |log2 FC|> 1 were set as the threshold for significantly differential expression by default. The target gene of miRNA in plant psRobot was predicted by using psRobot_tar.
Comprehensive analysis of miRNA and mRNA expression profiles
To predict the interaction between miRNA and mRNA, correlation expression analysis was performed based on the negative regulation standard of miRNA and corresponding mRNA. Target genes for differentially expressed miRNAs (DEMs) were predicted by psRobot_tar. Predicted target genes were then compared to DEGs to find overlapping genes between predicted target genes of up-or downregulated DEMs and up-or downregulated DEGs. Functional classifications of overlapping genes were analyzed by means of GO and the KEGG pathway.
Real-time quantitative PCR validation (qRT-PCR)
To verify the results of RNA sequencing, ten intersecting genes were randomly selected for qRT-PCR reactions. The primers were designed using Primer Premier 5.0 software. All the primer sequences used in this research are shown in Table S1. The P. lactiflora actin gene was used as an internal control. qRT-PCR was performed using the SYBR® Premix Ex TaqTM (Perfect Real Time) (TaKaRa, Japan) and contained 12.5 μL 2 × SYBR Premix Ex TaqTM buffer, 2 μL cDNA solution, 2 μL mix solution of target gene primers (10 μM) and 8.5 μL ddH2O in a final volume of 25 μL. The amplification was carried out under the following conditions: 95 °C for 30 s, 40 cycles at 95 °C for 5 s, 52 °C for 30 s and 72 °C for 30 s. The relative expression of the gene to be tested was calculated using the 2−ΔΔCT algorithm. Three biological replicates were performed for each gene.
Results
Morphological indices
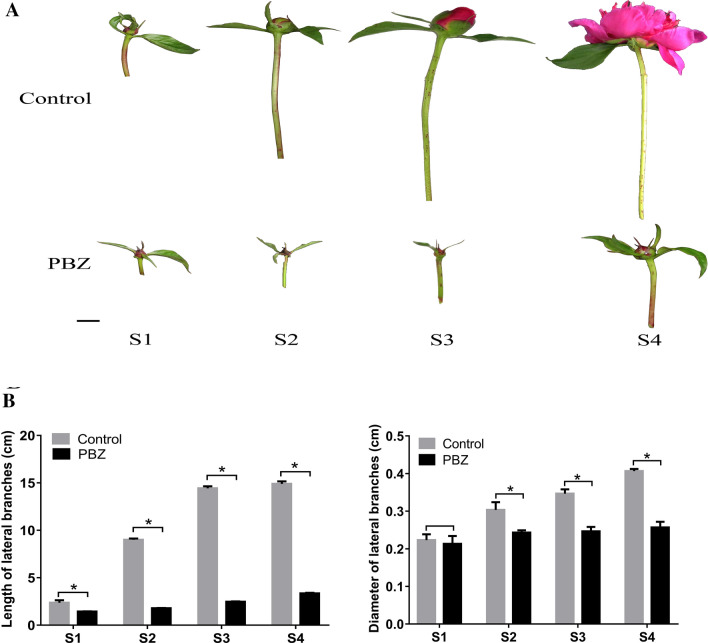
In this study, after spraying with PBZ, the growth and development of the lateral branches of P. lactiflora were significantly affected (Fig. 1). During the four development stages of the stem, the length of lateral branches of control plants ranged from 2.37 to 14.90 cm, and after PBZ application, the length of lateral branches ranged from only 1.44 to 3.36 cm. In addition, the diameter of lateral branches in the control gradually increased to 0.41 cm, while the diameter of lateral branches only increased to 0.26 cm after PBZ application.
Fig. 1.
The effect of paclobutrazol application on morphology of lateral branches of Paeonia lactiflora. a Morphology of lateral branches of Paeonia lactiflora. b Statistics analysis of morphological indices of Paeonia lactiflora lateral branches. The length and diameter of lateral branches are presented as the mean values ± SD, and asterisks indicate values that were significantly different between control and PBZ application at P < 0.05 (Student’s t-test). Scale bars = 1 cm
De novo assembly and functional annotation
Illumina HiSeqTM2500 sequencing yielded over 29 and 27 million high-quality transcriptome reads in the control and PBZ application groups, respectively. The raw reads were assembled from the beginning to obtain 63,067 unigenes with an average contig length of 714 bp. The majority of unigenes were 200–500 bp (61.36%), followed by 500–1000 bp (16.20%), 1000–2000 bp (15.22%) and > 2000 bp (7.22%) in length. To determine the hypothetical function of unigenes, they were contrasted to the non-redundant protein database and NCBI nucleotide sequences according to the priority order of the Gene Ontology (GO) database, the Cluster of Orthologous Groups (COG) database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. A total of 28,183 (44.68%) of the unigenes were annotated in the NR database; the other unannotated unigenes represent new genes whose function has not yet been determined. There were 20,572 (32.61%) unigenes assigned to the GO database, which could be divided into 46 functional groups (Fig. S1), including 14 cellular components, 12 molecular functions and 20 biological processes. A total of 10,598 (16.80%) unigenes were assigned to the KOG database and classified into 26 functional categories (Fig. S2a). According to the annotation information of the KEGG database, a total of 9140 (14.49%) unigenes were mapped to 32 metabolic pathways, mainly involved in translation, signal transduction and carbohydrate metabolism (Fig. S2b).
MicroRNA expression profiling and screening for DEMs
Small RNA deep sequencing generated 11,603,914 and 12,707,443 raw reads in the control and PBZ application, respectively, with lengths ranging from 18 to 30 nt. After removing reads containing adaptor, insert, poly(A) contamination and reads of low quality, 11,366,515 clean reads from the control and 12,232,441 from the PBZ application were obtained, among which 4,822,771 (42.43%) and 2,376,141 (19.42%) unique reads, respectively, mapped to our transcriptome data set. The mapped small RNA sequences were clustered into 9 RNA classes (Table S2). Approximately 5.34% and 3.49% of the small RNA from the control and PBZ application, respectively, were annotated as miRNA. All small RNA reads mapped in the transcriptome were used as queries against known miRNAs in the database. In all, 1,111 conserved miRNAs representing 32 known miRNA families were obtained. In this study, after the PBZ application, there were 104 differentially expressed miRNAs, of which 28 miRNAs were upregulated and 76 miRNAs were downregulated (Fig. 2).
Fig. 2.
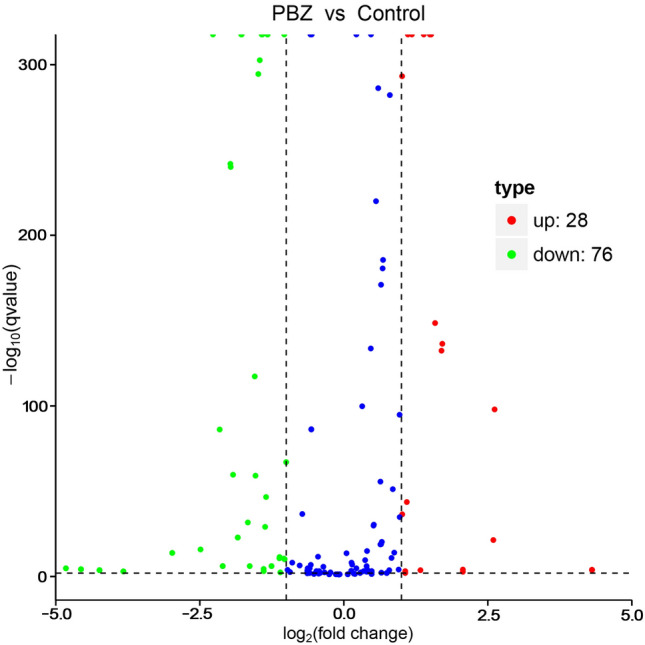
Comparison of expression patterns of differentially expressed miRNAs identified between paclobutrazol application and control. The red dots mean significantly up-regulated DEMs and the green dots represent significantly down-regulated DEMs. The blue dots represent non-DEMs
mRNA expression profiling and screening for DEGs
A total of 1988 genes which were targeted by miRNA among PBZ application and the control (Table S3), and 827 genes were differentially expressed based on the comparison of mRNA expression profiles of the PBZ application and the control (Fig. 3), in which 460 genes showed decreased abundance, while 367 genes exhibited increased expression. These DEGs could be used as important branch-related potential candidate genes for the development of lateral branches in P. lactiflora. Then, the unigene expression (FPKM) data were calculated between control and PBZ application with DEGSeq and revealed that 41,864 genes were expressed in both the control and PBZ, as well as 9713 genes that were specifically expressed in the control. With PBZ application, 7786 genes were expressed (Fig. S3).
Fig. 3.

Comparison of expression patterns of differentially expressed genes identified between paclobutrazol application and control. The red dots mean significantly up-regulated DEGs and the green dots represent significantly down-regulated DEGs. The blue dots represent non-DEGs
DEGs found in the lateral branch of PBZ processing showed only minor expression changes, and 62% of all DEGs were assigned a |log2 FC| less than 2.0. In addition, 31% of all DEGs showed a |log2 FC| greater than 2.0, and 7% of all DEGs showed a |log2 FC | greater than 4.0.
Integrative miRNA–mRNA expression and function analysis
Mapping the DEMs and their targeted mRNA yielded 827 targeted mRNAs. Among these target genes, we were concerned with the corresponding target genes that are opposite to miRNA expression. A miRNA target gene was defined as a consistent target gene when the expression pattern was opposite to that of the miRNA. In the present study, 104 DEMs were predicted possessing consistent target genes in lateral branches. Among these intersection genes, we noticed that the number of downregulated intersection genes were significantly higher than that of the upregulated intersection genes (Fig. 4a). In total, we discovered 6 target genes upregulated by 18 miRNAs; among them, c35685_g1 may potentially be regulated by 6 miRNAs (miR156b, miR156a, miR157a-5p, miR156a-5p, miR156f-5p, miR156), and 9 target genes were downregulated by 11 miRNAs (Table S4). In addition, the KEGG pathway cluster analysis of the intersection genes identified 10 pathways (Fig. 4b).
Fig. 4.
Hierarchical cluster and function analysis of significant intersection genes. a Hierarchical cluster analysis of significant intersection genes between paclobutrazol application and control. Colors indicate the log2(fold change) from high (red) to low (blue), as indicated by the color scale. The names of intersection genes are shown on the right side of the panel. b Enrichment scatter plot of the intersection genes. The Y-axis indicates the pathway name, and the X-axis indicates the enrichment factor corresponding to the pathway. The q-value is represented by the color of the dot
To evaluate the validity and reliability of sequencing data and verify the expression profiles of genes in two libraries, ten intersection genes were randomly selected for qRT-PCR analysis among the miRNA–mRNA interaction network groups (Fig. 5). The qRT-PCR results of genes were basically consistent with the RNA-Seq data, showing that the sequencing data obtained in this study were reliable and could be further analyzed.
Fig. 5.
The qRT-PCR analysis of significant intersection genes. The qRT-PCR results are represented as mean ± SD values of three biological replicates, asterisks indicate values that were significantly different between the control and paclobutrazol application at P < 0.05 (Student’s t-test)
Discussion
The adaptation of plants to abiotic stress is mainly through rapid signal cascades and gene transcription networks to initiate the regulation of plant development and metabolic responses under stress, especially at the transcriptional and post-transcriptional levels (Wani et al. 2018). miRNAs not only control the post-transcriptional regulation of their targets but also interact with each other in regulatory networks affecting development and responses to biotic and abiotic stresses (Garg et al. 2019). In the present research, 340,945 miRNAs were identified in the two miRNA libraries based on differential expression analysis, of which 78,232 novel miRNAs were not reported in any species. Integrated miRNA and mRNA analysis showed that miR156, miR393a, and miR396c were strongly downregulated in the branch, compared to PBZ application and control. These three miRNAs have been found to be related to plant growth and development in a wide range of species across the plant kingdom (Lin et al. 2013; Peng et al. 2014). In the present study, higher expression levels of miR156, miR393a, and miR396c in the branch might mean that these three miRNAs play important roles in lateral branch development. Sixteen DEGs were predicted to be target genes of miR156, miR393a, and miR396c. These DEGs might be important candidate genes associated with lateral branch development in P. lactiflora, on the basis of potential functions of these miRNAs in lateral branch development and growth. Therefore, further research is suggested in the future for verifying this prediction.
Through the classification between GO and KEGG, the functional and pathway assignments of the differentially expressed mRNAs showed a variety of enzymatic, metabolic, physiological, and developmental responses, which might play important roles in response to PBZ inhibition of lateral branch growth in herbaceous peony. Such alterations might include the following: (i) carbohydrate metabolism, (ii) phenylpropanoid biosynthesis, (iii) cell growth and death, (iv) plant hormone signal transduction, (v) hydrolase activity, and (vi) external encapsulating structure. To the best of our knowledge, it is believed that the growth and development of lateral branches are linked with phytohormones. In barley and tomato, it has been reported that paclobutrazol can remarkably affect plant growth and development by altering the photosynthetic rate and changing the levels of plant hormones (Kim et al. 2012). Plant hormone signal transduction is important in the initiation, growth and development of the lateral buds (Durbak et al. 2012), and these signals were integrated to regulate the formation of the lateral branches (Domagalska and Leyser 2011; Janssen et al. 2014). In our study, the ‘plant hormone signal transduction’ pathway was significantly enriched in PBZ application samples, playing a role in the early response to PBZ stress. Detailed information for the ‘plant hormone signal transduction’ pathway showed that several phytohormone signal-related genes were quickly induced by PBZ application, whereas most phytohormone signal-related genes remained constant or were downregulated in control samples. In addition, enrichment of GO terms such as the ‘transmembrane receptor protein serine/threonine kinase activity’ pathway implied that the disruption of phosphorylation had an important influence on extracellular signal transduction under PBZ stress, and both kinases and phosphatases for reversible phosphorylation have been reported, and their involvement in response to abiotic stress tolerance has been demonstrated (Gahlaut et al. 2016).
Based on the integrated analysis of miRNA and mRNA expression profiles, this study aimed to recognize the miRNA–mRNA pairs regulated in the lateral branch during PBZ application. To validate some of the predicted targets, it was found that 15 mRNAs are cleaved at the exact position as they are supposed to be in our analysis among 827 common predicted targets. Further inspection of the transcriptomic data revealed that 15 miRNAs produced 29 miRNA–mRNA interaction pairs and they had opposite regulation. The majority of 15 oppositely expressed targets with their cognate miRNA could be clustered into such specific groups, such as probable receptor protein kinase, fructokinaseand mitochondrial arginine transporter. In the present investigation, miR166p target transcripts encoded receptor-like protein kinases involved in the negative regulation of cell communication, phosphorylation and carbohydrate derivative metabolic process. The miR166p target gene (c32052_g1) may be involved in the signal network regulation of PBZ inhibiting lateral branch growth by means of receptor-like protein kinases. Previous studies have also confirmed similar viewpoints that both the miR166 group and its target genes regulate plant growth, including apical and lateral meristem formation, stunted growth, and vascular development (Kim et al. 2005; Jung and Park 2007; Li et al. 2017).
Conclusion
In summary, this study provided a wealth of genetic information and data on metabolic pathways to reveal the regulatory mechanism of PBZ inhibition of the development of lateral branches in P. lactiflora. There were 29 negative miRNA–mRNA interactions between 104 differentially expressed miRNAs and 827 differentially expressed mRNAs that were related to PBZ stress. This present study provided a theoretical basis for the use of PBZ to remove lateral branches of herbaceous peony.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Gene ontology functional classification of unigenes. The Y-axis indicates the number of a specific category of genes existed in main category (JPG 1218 kb)
(a) EuKaryotic Ortholog Groups (KOG) and (b) Kyoto Encyclopedia of Genes and Genomes (KEGG) functional classifications of unigenes (JPG 1400 kb)
Venn diagram of differentially expressed genes (DEGs) in different comparisons (PDF 34 kb)
Gene-specific primers sequence for detection by qRT-PCR (DOC 23 kb)
The RNA classification statistics table (DOCX 13 kb)
Target genes of miRNAs identified in this study (XLSX 40 kb)
List of oppositely regulated miRNA–mRNA pairs in small RNA and transcriptome sequencing (XLSX 10 kb)
Acknowledgements
This study was supported by the Agricultural Science & Technology Independent Innovation Fund of Jiangsu Province (CX[14]2023), the Scientific Research Foundation for high level talents of Yangzhou University (137010420) and the Priority Academic Program Development from the Jiangsu Government.
Author contributions
DZ and JT conceived the study and designed the experiments; LL performed the experiments; LL and YW were responsible for data analysis and manuscript writing, with suggestions offered by JT. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare there are no competing interests.
References
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007;51(6):1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- Carver ST, Arnold MA, Byrne DH, Armitage AR, Lineberger RD, King AR. Growth and flowering responses of sea marigold to daminozide, paclobutrazol, or uniconazole applied as drenches or sprays. J Plant Growth Regul. 2014;33(3):626–631. [Google Scholar]
- Chen XL, Zhou XY, Xi L, Li JX, Zhao RY, Ma N, Zhao LJ. Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema × grandiflora cv. Jinba) PLoS One. 2013;8(4):e61717. doi: 10.1371/journal.pone.0061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierck R, De Keyser E, De Riek J, Dhooghe E, Van Huylenbroeck J, Prinsen E, Van Der Straeten D. Change in auxin and cytokinin levels coincides with altered expression of branching genes during axillary bud outgrowth in chrysanthemum. PLoS One. 2016;11(8):e0161732. doi: 10.1371/journal.pone.0161732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Bio. 2011;12(4):211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- Dorairaj D, Ismail MR. Distribution of silicified microstructures, regulation of cinnamyl alcohol dehydrogenase and lodging resistance in silicon and paclobutrazol mediated Oryza sativa. Front Physiol. 2017;8:491. doi: 10.3389/fphys.2017.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbak A, Yao H, McSteen P. Hormone signaling in plant development. CurrOpin Plant Biol. 2012;15(1):92–96. doi: 10.1016/j.pbi.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Eiasu BK, Matafeni N, Dyafta V, Chigwaya K. Rose-scented geranium (Pelargonium spp.) plant growth, and essential oil yield and composition as affected by paclobutrazol application. HortScience. 2017;52(7):991–995. [Google Scholar]
- Gahlaut V, Jaiswal V, Kumar A, Gupta PK. Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticum aestivum L.) TheorAppl Genet. 2016;129(11):2019–2042. doi: 10.1007/s00122-016-2794-z. [DOI] [PubMed] [Google Scholar]
- Garg V, Khan AW, Kudapa H, Kale SM, Chitikineni A, Sun QW, Sharma M, Li CY, Zhang BH, Liu X, Kishor PK, Varshney RK. Integrated transcriptome, small RNA and degradome sequencing approaches provide insights into Ascochyta blight resistance in chickpea. Plant Biotechnol J. 2019;17(5):914–931. doi: 10.1111/pbi.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YS, Jia MA, Yang YM, Zhan LL, Cheng XF, Cai JY, Zhang J, Yang J, Liu T, Fu Q, Zhao JH, Shamsi IH. Integrated analysis of tobacco miRNA and mRNA expression profiles under PVY infection provids insight into tobacco-PVY interactions. Sci Rep. 2017;7:4895. doi: 10.1038/s41598-017-05155-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua SJ, Zhang YF, Yu HS, Lin BG, Ding HD, Zhang DQ, Ren Y, Fang ZG. Paclobutrazol application effects on plant height seed yield and carbohydrate metabolism in canola. Int J AgricBiol. 2014;16(3):471–479. [Google Scholar]
- Janssen BJ, Drummond RS, Snowden KC. Regulation of axillary shoot development. CurrOpin Plant Biol. 2014;17:28–35. doi: 10.1016/j.pbi.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Jung JH, Park CM. MIR166/165 genes exhibit dynamic expression patterns in regulating shoot apical meristem and floral development in Arabidopsis. Planta. 2007;225(6):1327–1338. doi: 10.1007/s00425-006-0439-1. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, Chung KS, Kim JA, Lee M, Lee Y, Kim VN, Chua NH, Park CM. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005;42(1):84–94. doi: 10.1111/j.1365-313X.2005.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wilson RL, Case JB, Binder BM. A comparative study of ethylene growth response kinetics in eudicots and monocots reveals a role for gibberellin in growth inhibition and recovery. Plant Physiol. 2012;160(3):1567–1580. doi: 10.1104/pp.112.205799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Xie X, Li J, Cui YH, Hou YM, Zhai LL, Wang X, Fu YL, Liu RR, Bian SM. Conservation and diversification of the miR166 family in soybean and potential roles of newly identified miR166s. BMC Plant Biol. 2017;17:32. doi: 10.1186/s12870-017-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DB, Yang YW, Khalil R, Xian ZQ, Hu GJ, Li ZG. SlmiR393 controls the auxin receptor homologous genes expression, and regulates sensitivity to auxin in tomato root growth. SciHortic. 2013;162:90–99. [Google Scholar]
- Liu YH, Yu L, Ding JH, Wang RZ, Hang ZG, Xiao LT. Research progress in synergistic regulatory roles of phytohormones in shoot branching. J Plant Physiol. 2012;48(10):941–948. [Google Scholar]
- Lu GP, Yu XN. An analysis of the american peony society’s gold medal. J Cent S U For Technol. 2009;29(5):191–194. [Google Scholar]
- MacDonald JE. Applying paclobutrazol at dormancy induction inhibits shoot apical meristem activity during terminal bud development in Picea mariana seedlings. Trees. 2017;31(1):229–235. [Google Scholar]
- McSteen P. Hormonal regulation of branching in grasses. Plant Physiol. 2009;149(1):46–55. doi: 10.1104/pp.108.129056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi MHS, Etemadi N, Arab MM, Aalifar M, Arab M, Pessarakli M. Molecular and physiological responses of iranian perennial ryegrass as affected by trinexapac ethyl, paclobutrazol and abscisic acid under drought stress. Plant PhysiolBioch. 2017;111:129–143. doi: 10.1016/j.plaphy.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Ongaro V, Leyser O. Hormonal control of shoot branching. J Exp Bot. 2008;59(1):67–74. doi: 10.1093/jxb/erm134. [DOI] [PubMed] [Google Scholar]
- Otori K, Tamoi M, Tanabe N, Shigeoka S. Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci Biotech Bioch. 2017;81(8):1470–1477. doi: 10.1080/09168451.2017.1321954. [DOI] [PubMed] [Google Scholar]
- Peng T, Sun HZ, Qiao MM, Zhao YF, Du YX, Zhang J, Li JZ, Tang GL, Zhao QZ. Differentially expressed microRNA cohorts in seed development may contribute to poor grain filling of inferior spikelets in rice. BMC Plant Biol. 2014;14:196. doi: 10.1186/s12870-014-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Maji RK, Dey S, Sarkar A, Ghosh Z, Kundu P. Integrated miRNA and mRNA expression profiling reveals the response regulators of a susceptible tomato cultivar to early blight disease. DNA Res. 2017;24(3):235–250. doi: 10.1093/dnares/dsx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MM, Wang Q, Yu XN, da Silva JAT. Micropropagation of herbaceous peony (Paeonia lactiflora Pall.) SciHortic. 2012;148:30–38. [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H. Auxin-cytokinin interactions in the control of shoot branching. Plant MolBiol. 2009;69(4):429–435. doi: 10.1007/s11103-008-9416-3. [DOI] [PubMed] [Google Scholar]
- Tan M, Li GF, Chen XL, Xing LB, Ma JJ, Zhang D, Ge HJ, Han MY, Sha GL, An N. Role of cytokinin, strigolactone, and auxin export on outgrowth of axillary buds in apple. Front Plant Sci. 2019;10:616. doi: 10.3389/fpls.2019.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26(1):136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- Wani SH, Tripathi P, Zaid A, Challa GS, Kumar A, Kumar V, Upadhyay J, Joshi R, Bhatt M. Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.) Plant MolBiol. 2018;97(6):469–487. doi: 10.1007/s11103-018-0761-6. [DOI] [PubMed] [Google Scholar]
- Woods DP, Hope CL, Malcomber ST. Phylogenomic analyses of the BARREN STALK1/LAX PANICLE1 (BA1/LAX1) genes and evidence for their roles during axillary meristem development. MolBiolEvol. 2011;28(7):2147–2159. doi: 10.1093/molbev/msr036. [DOI] [PubMed] [Google Scholar]
- Wu C, Li XY, Guo S, Wong SM. Analyses of RNA-Seq and sRNA-Seq data reveal a complex network of anti-viral defense in TCV-infected Arabidopsis thaliana. Sci Rep. 2016;6:36007. doi: 10.1038/srep36007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang F, Li J, Chen JP, Zhang HM. Integrative analysis of the microRNAome and transcriptome illuminates the response of susceptible rice plants to rice stripe virus. PLoS One. 2016;11(1):e0146946. doi: 10.1371/journal.pone.0146946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yooyongwech S, Samphumphuang T, Tisarum R, Theerawitaya C, Cha-um S. Water-deficit tolerance in sweet potato [Ipomoea batatas (L.) Lam.] by foliar application of paclobutrazol: role of soluble sugar and free proline. Front Plant Sci. 2017;8:1400. doi: 10.3389/fpls.2017.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XN, Guo PP, Lu GP, Zhang QX. Optimum harvesting time of herbaceous peony buds for cutting flowers. J For Res. 2011;22(1):137–140. [Google Scholar]
- Yuan QL, Yu XN. Flower culture of peony and chinese herbaceous peony with landscape garden in china. J Beijing For U. 2011;10(3):53–57. [Google Scholar]
- Zhang SY, Li G, Fang J, Chen WQ, Jiang HP, Zou JH, Liu X, Zhao XF, Li XB, Chu CC, Xie Q, Jiang XN, Zhu LH. The interactions among DWARF10, auxin and cytokinin underlie lateral bud outgrowth in rice. J Integr Plant Biol. 2010;52(7):626–638. doi: 10.1111/j.1744-7909.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Li KC, Xing RG, Liu S, Chen XL, Yang HY, Li PC. miRNA and mRNA expression profiles reveal insight into chitosan-mediated regulation of plant growth. J Agr Food Chem. 2018;66(15):3810–3822. doi: 10.1021/acs.jafc.7b06081. [DOI] [PubMed] [Google Scholar]
- Zhao DQ, Gong SJ, Hao ZJ, Meng JS, Tao J. Quantitative proteomics analysis of herbaceous peony in response to paclobutrazol inhibition of lateral branching. Int J MolSci. 2015;16(10):24332–24352. doi: 10.3390/ijms161024332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DQ, Tang YH, Xia X, Sun J, Meng JS, Shang JL, Tao J. Integration of transcriptome, proteome, and metabolome provides insights into how calcium enhances the mechanical strength of herbaceous peony inflorescence stems. Cells. 2019;8(2):102. doi: 10.3390/cells8020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Zhao L, Wang Y, Shen JZ, Zhang YF, Jia SS, Li YS, Ding ZT. Integrated RNA-Seq and sRNA-Seq analysis identifies chilling and freezing responsive key molecular players and pathways in tea plant (Camellia sinensis) PLoS One. 2015;10(4):e0125031. doi: 10.1371/journal.pone.0125031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene ontology functional classification of unigenes. The Y-axis indicates the number of a specific category of genes existed in main category (JPG 1218 kb)
(a) EuKaryotic Ortholog Groups (KOG) and (b) Kyoto Encyclopedia of Genes and Genomes (KEGG) functional classifications of unigenes (JPG 1400 kb)
Venn diagram of differentially expressed genes (DEGs) in different comparisons (PDF 34 kb)
Gene-specific primers sequence for detection by qRT-PCR (DOC 23 kb)
The RNA classification statistics table (DOCX 13 kb)
Target genes of miRNAs identified in this study (XLSX 40 kb)
List of oppositely regulated miRNA–mRNA pairs in small RNA and transcriptome sequencing (XLSX 10 kb)