Abstract
Background
Soil-transmitted helminths (STHs) infect almost 1·5 billion people worldwide. The control of STH infections is based on preventive chemotherapy using either albendazole or mebendazole. Before being widely used, a sufficient body of evidence on efficacy, safety and acceptability is warranted for the new chewable child-friendly formulation of mebendazole that was recently developed.
Methods
We conducted a randomised controlled superiority trial in four primary schools and kindergartens on Pemba Island, Tanzania. We considered eligible children aged 3 to 12 years with a hookworm infection intensity of at least 50 eggs per gram (EPG) of stool and no chronic diseases. Participants were allocated to treatment arms (ratio 1:1) using a computer generated random sequence. Our primary outcome was geometric mean based egg reduction rate (ERR) against hookworm assessed 14–21 days post-treatment. This trial complete and is registered with ClinicalTrials.gov, number NCT03995680 (June 24, 2019).
Findings
397 children were eligible and randomised into the solid (198) or chewable (199) tablet arms, of whom 393 were analysed. We found no significant difference between both formulations in terms of ERR (solid 70·8% versus chewable 68·5%, difference in ERRgeometric mean 2·3%-points, 95% CI -7·8 to 12·6, p = 0.65) and CR (11·2 versus 12·7%, 95% CI -4·9 to 7·9, p = 0.65) against hookworm infections. Adverse events were mild in both treatment arms.
Interpretations
Though we could not demonstrate superiority in terms of efficacy of the new formulation, the difference between arms was small and therefore, the chewable formulation could be safely used as an alternative to swallowable tablets, in particular in young children who may have swallowing difficulties. This might help increase compliance and, consequently, enhance the effect of preventive chemotherapy.
Research in context.
Evidence before this study
Soil-transmitted helminths infect almost one quarter of the world's population. Children are at highest risk of morbidity caused by these parasites. The main control strategy for soil-transmitted helminth infections is preventive chemotherapy, which consists of the mass administration of either albendazole or mebendazole to at-risk populations. A recently registered new child-friendly formulation of mebendazole is planned to be used globally in preventive chemotherapy programs. The only previous trial published in the literature (as of 15th of September 2020) testing this chewable tablet versus placebo found cure rates of 84 and 34% against Ascaris lumbricoides and Trichuris trichiura, respectively.
Added value of this study
We evaluated for the first time the safety and efficacy of the new formulation against hookworm and concomitant soil-transmitted helminth infections using the swallowable, standard mebendazole as a side-by side comparator. Additionally, for the first time, we observed and interviewed participants to assess whether they were satisfied with the formulation they were given.
Implications of all the available evidence
The new chewable mebendazole formulation has a similar safety and efficacy profile as the solid formulation; its efficacy is high against A. lumbricoides, moderate against hookworm, but poor against T. trichiura infections. Thus, in areas where T. trichiura and hookworm infections are common, multiple doses, combination chemotherapy or albendazole based treatments should be considered to achieve an increased and broad spectrum of activity. The simplicity of the new chewable tablet's administration and its better taste will ease treatment of young children in preventive chemotherapy programs. The method of administration should be improved by providing children a glass of water during and or after having chewed the tablets.
Alt-text: Unlabelled box
1. Introduction
Soil-transmitted helminthiases are caused by one of the four soil-transmitted helminths (STHs): Ascaris lumbricoides, Trichuris trichiura, and hookworm (Ancylostoma duodenale and Necator americanus). Currently, these parasites infect almost 1·5 billion people worldwide, mostly in the tropics and subtropics [1]. Annually, STH infections lead to an estimated 1·9 million disability-adjusted life years [2]. The health burden of STH includes nutritional deficiencies, anaemia, physical and cognitive impairment in children and reduction in work performance in adulthood [3], [4], [5], [6], [7], [8]. Currently, STH infections are mainly controlled through preventive chemotherapy, the regular administration of mebendazole or albendazole to at-risk populations [9]. However, the mebendazole tablets currently used are solid (non-chewable and hard to crush), which might be problematic for young children. The WHO's call for a more child-friendly formulation of mebendazole resulted in the development of a chewable strawberry-flavoured tablet by Johnson&Johnson (Vermox™) to effectively treat children >1 years of age [10]. A randomised, placebo-controlled trial testing the efficacy and safety of the chewable mebendazole reported good tolerability and cure rates (CRs) of 84% and 34% against A. lumbricoides and T. trichiura, respectively, but could not draw any conclusion on the efficacy against hookworm [11,12]. Moreover, the children's acceptability of this formulation has not yet been explored. Finally, to date, no head-to-head comparison with the current standard solid formulation of mebendazole has been conducted. Before the new formulation is provided to millions of children every year, solid evidence on its performance is required.
Formulation factors affecting dissolution rates and hence absorption and bioavailability are known to influence clinical outcomes [13]. Indeed, a CR of 100% was reported for the chewable mebendazole in four hookworm infected children in the above mentioned Phase 2 study [11]. We hypothesized that the chewable formulation of mebendazole has a higher efficacy against hookworm infections than the solid one, which shows a poor performance [14]. The aim of this study was to compare the efficacy, safety, acceptability and age-appropriateness of the two mebendazole formulations.
2. Methods
2.1. Study design
This randomised superiority parallel trial took place on Pemba Island, Tanzania. The study was conducted in four kindergarten and primary schools. Ethical approval was obtained from the Zanzibar Heath Research Ethics Committee (ZAHREC, no. ZAHREC/02/APR/2019/19) and from the Ethics Committee of Northern and Central Switzerland (EKNZ, no. 2019–00,351).
The trial protocol and supporting CONSORT checklist are available as supporting information.
2.2. Participants
We recorded the name, age, sex and school grade of potentially eligible children. Caregivers of children attending the schools and aged 3–12 years were invited for information sessions where a study staff member orally communicated the purpose, procedures, benefits and potential risks of participating in our study. Caregivers were encouraged to ask any questions and those who decided to have their child participate were asked to sign a written informed consent form. Caregivers who could not read provided a thumbprint and an impartial witness signed to confirm that all the information had been appropriately communicated.
Children with caregiver consent were invited for a clinical and physical examination if they provided two baseline stool samples and these were found to be positive for hookworm (initially set to a minimum of ≥ 100 eggs per gram [EPG] of stool, and then lowered to ≥ 50 EPG due to difficulties in finding enough children with EPG ≥ 100; an amendment to the protocol was submitted and approved) and at least two of the four Kato-Katz thick smears with more than one hookworm egg. We excluded children who had severe anaemia (Hb < 80 g/l), had currently or in the past a major systemic or chronic illness self-reported or assessed by the physician, had received any anthelmintic drug in the past four weeks, were participating in another clinical trial, or were pregnant.
2.3. Randomisation and masking
A computer-generated random allocation sequence with varying random blocks of four or eight was provided by the trial statistician (JH). The randomisation code was stratified by baseline infection intensities (light or moderate/heavy, according to WHO thresholds) [15]. Participants were allocated 1:1 to one of the treatment arms: chewable mebendazole or solid mebendazole. Both types of tablets were the product of Johnson&Johnson. Treatment allocation was concealed for the personnel administering the drugs using sealed opaque envelopes labelled with the treatment identification code. Due to the nature of the trial, only the outcome assessing laboratory personnel could be blinded.
2.4. Procedures
After consenting, each caregiver received an empty container labelled with their child's unique identification number (ID) for the first stool sample. The following morning when the child handed in the stool sample it received a second empty container for the second stool sample, which was collected the next day. When possible, the two stool samples were collected on consecutive days. Most samples were collected from participants themselves at schools. In a few cases where participants were ill or did not come to school, we collected the samples from their households. All samples were transported to the laboratory where trained technicians prepared duplicate Kato-Katz thick smears from each sample. Under a light microscope, hookworm, A. lumbricoides and T. trichiura eggs were counted and recorded separately within one hour after slide preparation, to avoid clearing of hookworm eggs. Quality control was performed on 10% of stool samples. For hookworm quality control, a piece of each sample was transferred to a new container, labelled with a new ID making it impossible for technicians to identify the sample. For A. lumbricoides and T. trichiura we selected 10% of the Kato-Katz slides, removed the original ID and labelled them with a new ID.
Potentially eligible children were invited for the physical and clinical examinations and treatment. Each participant's haemoglobin levels were measured from a finger prick (HemoCue® 301), and females 10 years of age and above provided a urine sample to perform a rapid pregnancy test. Weight, height and temperature were recorded. Eligible children were enroled by MSP who then administered the study drug. Children were either allocated to receive the solid (500 mg) or the chewable (500 mg) tablet. The two types of tablet had similar sizes but the chewable tablet had a pleasant taste (strawberry), whereas the swallowable tablet did not (no taste). Furthermore, the chewable tablet easily dissolved in water, but the solid one was hard and needed to be crushed before mixing with water. The method of administration depended on the formulation and the participant's age: in the solid tablet arm 3–5 year old children were given a crushed tablet [16], and mixed with a small amount of water according to WHO [17], while 6–12 year old children were given the whole tablet and asked to swallow it with a glass of water. In the chewable tablet arm all children (3–12 year old) were encouraged to chew the tablet and swallow it without water. After chewing the tablets, participants were asked whether they would like some water. All participants received a package of biscuits together with treatment. Three and 24 h after treatment, physicians and nurses actively questioned each child for adverse events using a questionnaire. At follow-up, between 14 and 21 days after treatment, every participant was asked to deliver two more stool samples [18]. Follow-up samples were also collected at school, if possible on two consecutive days, and underwent the same procedures as described for baseline samples. All participants who were still found infected with any STH at follow-up were treated with albendazole (400 mg)and ivermectin (200 µg/kg).
2.5. Observation and satisfaction questionnaire
To better understand whether one of the formulations was deemed more acceptable by children than the other, we designed a short questionnaire (Table 5), and captured the answers using tablet computers. The questionnaire consisted of three questions that every participant answered right before he/she received treatment and three or four questions (depending on the treatment arm) he/she answered right after treatment. Children were interviewed privately, i.e. other children could not overhear their answers to the questionnaire. Moreover, the interviewers observed children while they ingested the tablet and recorded how the child took the tablet and whether the child had difficulties swallowing the drug. The questionnaire was applied to children of all ages.
Table 5.
Questionnaire and observation results by treatment arm.
| Questions | Answers | Solid (n = 181) | Chewable (n = 184) | Total (n = 365) |
|---|---|---|---|---|
| Baseline questions | ||||
| Have you ever taken a tablet for belly worms? | Yes | 130 (72) | 128 (70) | 258 (71) |
| No | 51 (28) | 56 (30) | 107 (29) | |
| If yes, how did you eat it? | Swallowed whole with water | 26 (20) | 36 (28) | 62 (24) |
| Chewed it and drank water | 97 (75) | 86 (67) | 183 (71) | |
| Chewed it without water | 7 (5) | 5 (4) | 12 (5) | |
| Cannot respond | 0 | 1 | 1 | |
| Do you like swallowing a pill whole with water? | No | 21 (12) | 21 (12) | 42 (12) |
| Yes | 160 (88) | 162 (88) | 322 (88) | |
| Observations | ||||
| How did the child eat the tablet? | Swallowed whole with water | 168 (93) | – | – |
| Crushed it and added water | 8 (4) | – | – | |
| Chewed tablet | 5 (2) | – | – | |
| Chewed without water | – | 23 (13) | – | |
| Added water to form paste | – | 0 | – | |
| Drank water after chewing | – | 161 (87) | – | |
| Did anything happen during treatment? | Difficulty swallowing | 4 (2) | 1 | 5 (1) |
| Difficulty swallowing and resisted to take the tablet | 1 | 0 | 1 | |
| Resisted to take the tablet | 0 | 1 | 1 | |
| No problem | 176 (97) | 182 (99) | 358 (98) | |
| After treatment questions | ||||
| Did you like the taste of the tablet? | No | – | 9 (5) | – |
| Yes | – | 175 (95) | – | |
| Was the tablet too big, too small or good? | Small | 30 (16) | 20 (11) | 50 (14) |
| Big | 59 (33) | 80 (43) | 139 (38) | |
| Good | 85 (47) | 82 (45) | 167 (46) | |
| No answer | 0 | 1 | 1 | |
| Crushed | 7 (4) | 1 | 8 (2) | |
| Would it be alright for you take this tablet again? | No | 47 (26) | 66 (36) | 113 (31) |
| Yes | 134 (74) | 118 (64) | 252 (69) | |
| Do you prefer to chew or swallow the tablet? | Chew | 32 (18) | 116 (63) | 148 (41) |
| Swallow | 149 (82) | 67 (36) | 216 (59) | |
| No answer | 0 | 1 | 1 |
Data are n (%).
2.6. Outcomes
The primary outcome of this study was the egg reduction rate (ERR) against hookworm assessed 14 to 21 days post-treatment with mebendazole using the quadruplicate Kato-Katz thick smear method. As secondary outcomes we assessed the safety (number of adverse events), the ERRs of mebendazole against A. lumbricoides and T. trichiura and the CR against all three parasite infections. Results for these co-infections were obtained in the same manner as for hookworm. Additionally, we assessed the acceptability of each type of tablet in each study arm.
2.7. Sample size
Because ERRs do not follow a known distribution we ran a series of conservative simulations using data from Speich et al. [19] to determine the required sample size. An initial round of simulations showed that differences in ERRs are associated with substantial higher power compared to CRs. A panel of experts expected the CR in the standard treatment arm of 10% and in the chewable treatment arm of 20%. We used Monte Carlo resampling techniques to draw samples with weighted sampling weights to simulate two trial arms with CR of 10 and 20%, respectively. The simulations revealed that the assumed CR translate roughly into ERRs of 38% and 64% and that a sample size of 160 patients per arm are required to detect a statistical significant difference with 80% power. To account for potential loss to follow up (which was low in our previous studies in this setting) of 10% and to include a safety margin of 14% we aim to recruit in total 200 participants per trial arm.
2.8. Statistical analysis
Data were double entered into a database (Access 2003, Microsoft) by two individual team members using EpiInfo 3.5.4 and crosschecked using its Data Compare tool. Discrepancies between both entries were clarified by consulting the original laboratory sheets. Statistical analysis were performed on STATA version 16 (StataCorp) and R version 3.5.1. The analysis included all children who provided two baseline stool samples and at least one follow-up sample (available case analysis).
The primary analysis used the full analysis set (available case population) defined as all randomised children who provide any follow-up data with trial arm comparisons based on the initial treatment assignment. Children who were identified as non-eligible after randomisation (negative at baseline) were excluded from the analysis. Palatability and ease of swallowing are critical attributes in children; therefore, a per-protocol analysis was conducted additionally to assess drug efficacy only in adherent participants.
Geometric mean egg counts (calculated from all four Kato-Katz thick smears) before and after treatment were used to assess the corresponding ERRs, calculated as:
Arithmetic means were calculated as:
The 95% confidence intervals (CIs) for ERRs and the difference between ERRs were estimated via bootstrap resampling. Superiority was claimed if the 95% CI of the difference in ERRs did not include zero. Logistic regression models were used to assess efficacy in terms of CRs. In a subsequent analysis an adjusted logistic regression (adjustment for age, sex, weight and baseline infection intensity) was performed. Adverse events were evaluated descriptively as the difference in the proportion of children reporting adverse events in each arm before and after treatment. To check whether the tablet formulation influenced participants’ answers to the questions they were asked after receiving the tablets, we used a two-sample test of proportions.
2.9. Role of funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
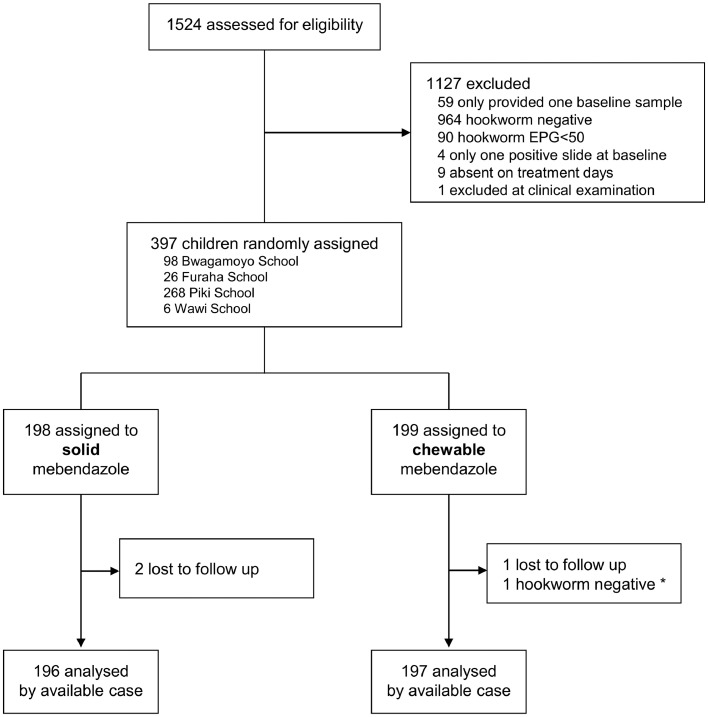
This trial took place between July 12, 2019, and October 9, 2019. We screened a total of 1524 children for hookworm infections. 1465 provided two stool samples and, of these, 500 were positive for hookworm. 81% (407/500) fulfilled the minimum parasitological eligibility criteria (EPG >50 and more than one egg positive slide). Nine children did not show up for treatment and one was excluded due to the presence of sickle cell anaemia. A total of 397 children were randomised, including one hookworm negative child erroneously randomised. 199 and 198 children were assigned to the chewable and solid tablet arm, respectively. Three children were lost to follow-up, as they were absent from school. Of note, two children provided one stool sample delayed (up to 28 days after treatment), and two other children only provided one follow-up sample. There were no protocol deviations from the treatment protocol; therefore, the per-protocol and available case population are identical. 393 children were included in the analysis (the per protocol and available case population were identical) (Fig. 1).
Fig. 1.
Trial profile. *hookworm-negative participant who was wrongly randomised and treated.
Participants in both treatment arms were similar in terms of age, sex, weight, height and hookworm baseline infection intensity. At baseline, 98% of participants were co-infected with T. trichiura and 49.8% with A. lumbricoides. Only seven participants (2%) did not suffer from a co-infection with other STHs. Moderate and heavy infection intensities were rare in the case of hookworm (3%) but common in the case of T. trichiura (38%) and A. lumbricoides (62%) (Table 1).
Table 1.
Baseline characteristics of all randomized children for each treatment arm.
| Solid N = 198 | Chewable N = 198 | |
|---|---|---|
| Mean age [years] | 9.3 (2.1) | 9.4 (2.1) |
| Girls | 80 (40.4) | 88 (44.2) |
| Mean weight [kg] | 26.0 (6.6) | 25.4 (6.3) |
| Mean height [cm] | 130.8 (11.6) | 130.5 (11.6) |
| Children aged ≤ 5 | 10 (5.1) | 7 (3.5) |
| Children aged >5 | 188 (94.9) | 191 (94.5) |
| Hookworm | ||
| EPG median | 192 (102–402) | 165 (96–402) |
| EPG geometric mean | 224.9 | 218.0 |
| EPG arithmetic mean | 427.0 | 441.2 |
| Minimum EPG | 54 | 54 |
| Maximum EPG | 9180 | 7524 |
| Infection intensity | ||
| Light (1–1999 EPG) | 192 (97) | 191 (96) |
| Moderate (2000–3999 EPG) | 4 (2) | 5 (3) |
| Heavy (≥4000 EPG) | 2 (1) | 2 (1) |
| Trichuris trichiura | ||
| Infected children | 195 | 195 |
| EPG median | 540 (273–1338) | 600 (234–1446) |
| EPG geometric mean | 633.5 | 584.6 |
| EPG arithmetic mean | 1,040.0 | 1,257.0 |
| Minimum EPG | 0 | 0 |
| Maximum EPG | 19,362 | 15,894 |
| Infection intensity | ||
| Light (1–999 EPG) | 126 (64) | 118 (61) |
| Moderate (1000–9999 EPG) | 68 (35) | 75 (38) |
| Heavy (≥10,000 EPG) | 1 (1) | 2 (1) |
| Ascaris lumbricoides | ||
| Infected children | 91 | 107 |
| EPG median | 0 (0–5,766) | 522 (0–8,586) |
| EPG geometric mean | 7,564.9 | 6,787.5 |
| EPG arithmetic mean | 7,865.5 | 9,559.8 |
| Minimum EPG | 0 | 0 |
| Maximum EPG | 92,244 | 101.382 |
| Infection intensity | ||
| Light (1–4999 EPG) | 38 (42) | 36 (34) |
| Moderate (5000–49,999 EPG) | 43 (47) | 57 (53) |
| Heavy (≥50,000 EPG) | 10 (11) | 14 (13) |
Data are n (%), median (IQR), mean (SD). EPG = eggs per gram of stool. One child hookworm-negative at baseline excluded from the chewable tablet arm.
We observed no statistically significant difference in the ERR with 70·8% in the solid tablet arm versus 68·5% in the chewable tablet arm (difference in ERRgeometric mean 2·3%-points, 95% CI −7·8 to 12·6, p = 0·65) (Table 2). Likewise, both groups were comparable with respect to CRs with 11·2% in the solid arm and 12·7% in the chewable arm (difference in CR 1·5%-points, 95% CI −4·9 to 7·9, p = 0.65). Adjusting for age, sex and weight did not noteworthy change the point or interval estimate.
Table 2.
Cure rates (CRs) and egg reduction rates (ERRs) against hookworm, Trichuris trichiura and Ascaris lumbricoides 14 to 21 days after treatment.
| Solid | Chewable | |
|---|---|---|
| Hookworm | ||
| Children positive before treatment | 196 | 197 |
| Children cured after treatment | 22 | 25 |
| EPG geometric mean | ||
| Before treatment | 226.7 | 219.0 |
| After treatment | 66.2 | 69.0 |
| ERR (95% CI) | 70.8 (63.5–76.7) | 68.5 (60.4–75.3) |
| EPG arithmetic mean | ||
| Before treatment | 430.1 | 443.0 |
| After treatment | 309.3 | 273.9 |
| ERR (95% CI) | 28.1 (8.5–44.3) | 38.2 (26.6–47.8) |
| CR (95% CI) | 11.2% (7.2–16.5) | 12.7% (8.4–18.2) |
| Trichuris trichiura | ||
| Children positive before treatment | 193 | 193 |
| Children cured after treatment | 14 | 19 |
| EPG geometric mean | ||
| Before treatment | 576.7 | 631.5 |
| After treatment | 149.1 | 168.7 |
| ERR (95% CI) | 74.2 (67.2–79.8) | 73.3 (65.7–79.4) |
| EPG arithmetic mean | ||
| Before treatment | 1,030.6 | 1,283.9 |
| After treatment | 506.1 | 605.1 |
| ERR | 50.9 (67.0–79.8) | 52.9 (65.6–79.7) |
| CR | 7.3 (4.0–11.9) | 9.8 (6.0–14.9) |
| Ascaris lumbricoides | ||
| Children positive before treatment | 89 | 106 |
| Children cured after treatment | 87 | 101 |
| EPG geometric mean | ||
| Before treatment | 7,352.4 | 6,811.6 |
| After treatment | 0.2 | 0.3 |
| ERR | > 99.9 | > 99.9 |
| EPG arithmetic mean | ||
| Before treatment | 16,635.0 | 17,857.0 |
| After treatment | 29.0 | 226.8 |
| ERR | 99.8 | 98.7 |
| CR | 97.8 (92.1–99.7) | 95.3 (89.3–98.5) |
Two children who provided the second stool sample beyond the 14-21 days post-treatment window.
Data are n (%) and mean (SD). CR = cure rate, CI = confidence interval, EPG = eggs per gram of stool, ERR = egg reduction rate.
For the remaining parasites there was also no significant difference in efficacy between both treatment arms. For T. trichiura geometric mean ERRs were 74·2 and 73·3% (difference in ERRgeometric mean 0·9%-points, 95% CI −8·3 to 10·2), for the solid and chewable tablet, respectively and for A. lumbricoides in both arms ERRs above 99·9% (difference in ERRgeometric mean 0·0%-points, CI −0·011 to 0·004) were observed. The solid tablet cured 7·3% of children with T. trichiura and 97·8% of children with A. lumbricoides; the chewable tablet cured 9·8% of those infected with T. trichiura and 95·3% of those with A. lumbricoides (Table 2).
CRs and ERRs excluding children with any protocol deviation, i.e. excluding two children with only one follow up sample and two children who provided the second stool sample beyond the 14–21 days post-treatment window are shown in supplementary Table 1.
3.1. Safety
Adverse event/symptom data were collected for all 397 participants at all time points by different and blinded personnel. All adverse events were mild and the number of adverse events was comparable in both treatment arms (Table 3). At baseline, 47 (12%) children reported at least one clinical symptom. Three hours and 24 h post-treatment, 8% and 15% of participants reported adverse events, respectively. At all three time points the most common symptoms or adverse events were fever, headache and abdominal pain (Table 4).
Table 3.
Number of symptoms or adverse events (AEs) reported and number of children reporting symptoms or adverse events at each adverse event assessment time point by treatment arm.
| Time point | Number of | Solid (n = 196) | Chewable (n = 197) | Total (N = 393) |
|---|---|---|---|---|
| Baseline clinical exam | Symptoms | 29 | 27 | 56 |
| Children | 26 (13) | 21 (11) | 47 (12) | |
| 3 h after treatment | AEs | 18 | 17 | 35 |
| Children | 17 (9) | 16 (8) | 33 (8) | |
| 24 h after treatment | AEs | 38 | 34 | 72 |
| Children | 31(16) | 28 (14) | 59 (15) |
Table 4.
Number of participants reporting each symptom before and adverse event after treatment, by treatment arm.
| Time point | Symptom | Solid (n = 196) | Chewable (n = 197) | Total (N = 393) |
|---|---|---|---|---|
| Clinical examination before treatment | Headache | 6 | 4 | 10 |
| Abdominal pain | 3 | 6 | 9 | |
| Itching | 0 | 0 | 0 | |
| Nausea | 1 | 3 | 4 | |
| Vomiting | 1 | 2 | 3 | |
| diarrhoea | 1 | 1 | 2 | |
| Fever (>37.5 °C) | 16 | 11 | 27 | |
| Other | 1 | 0 | 1 | |
| 3 h after treatment | Headache | 4 | 2 | 6 |
| Abdominal pain | 6 | 8 | 14 | |
| Itching | 0 | 0 | 0 | |
| Nausea | 2 | 1 | 3 | |
| Vomiting | 0 | 0 | 0 | |
| diarrhoea | 0 | 0 | 0 | |
| Fever (>37.5 °C) | 5 | 6 | 11 | |
| Other | 1 | 0 | 1 | |
| 24 h after treatment | Headache | 10 | 9 | 19 |
| Abdominal pain | 15 | 14 | 29 | |
| Itching | 0 | 1 | 1 | |
| Nausea | 0 | 2 | 2 | |
| Vomiting | 1 | 2 | 3 | |
| diarrhoea | 3 | 2 | 5 | |
| Fever (>37.5 °C) | 8 | 4 | 12 | |
| Other | 1 | 0 | 1 |
3.2. Acceptability of the two drug formulations
The questionnaire answers and observations made during treatment were obtained from 365 (92%) of children (Table 5). Not all children responded to the questionnaire and underwent the observation due to technical issues with the tablets used to collect this data. Overall, 70% of children reported to have already taken anthelmintics, and of those 71% said they chewed it and drank water. When asked if they like to swallow a tablet with water 88% of children responded yes with no difference in the responses amongst age. Interviewers only observed seven children who had difficulty while taking the tablet: five in the solid and two in the chewable tablet arm. Five children chewed the solid tablet although they were asked not to. Only eight children in this treatment arm were aged five or below and, therefore, required the tablet to be crushed with some added water. In the chewable tablet arm, when asked whether they would like some water after chewing the tablet, 87% of children said yes. After receiving the chewable tablet, 95% of children reported to have liked its taste. In terms of size more children in the chewable arm said the tablet was too big, compared to the solid arm (43 vs 33%, difference: 10·0%-points, 95% CI 0·0 to 20·1, p = 0·05). Children in the chewable arm were significantly less willing to take this tablet again (64 vs 74%, difference: 9·9%-points, 95% CI 0·5 to 19·3, p = 0·04). Also, there were more children in the chewable arm who stated they would have preferred to receive the other formulation (36 vs 18%, difference: 18·9%-points, 95% CI 10·0 to 23·2, p < 0·001), compared to the solid arm.
4. Discussion
We evaluated, for the first time, the safety and efficacy of the new mebendazole formulation, which will be used in millions of children, against hookworm and concomitant STH infections using the current standard mebendazole as a side-by side comparator. Additionally, for the first time, we observed and interviewed participants to assess whether one of the formulations was more accepted than the other.
The chewable and solid tablets performed similarly with respect to efficacy and safety. Regarding safety, our results were similar to those of a safety trial which also took place on Pemba Island, Tanzania [12]. Surprisingly, the new product specifications such as dissolution or disintegration seem to have, therefore, no influence on efficacy [13]. The only other published study testing the efficacy of the chewable formulation versus placebo found a slightly lower CR against A. lumbricoides (84%, vs 95% in our study) than we did and a considerably higher CR against T. trichiura (34%, vs 10% in our study) [11]. These discrepancies could be due to the higher proportion of participants with moderate/heavy infections in our study, which are more refractory to treatment [20]. In the previous study, only four participants were infected with hookworm in the active treatment arm, and all four were cured (in contrast to a CR of 13% in our study), which could be due to their low sample size. Nonetheless, the efficacy observed in our study is in line with a recent meta-analysis, [14] and reconfirms that mebendazole shows only a poor performance against T. trichiura and hookworm infections.
Children in the solid tablet arm seemed to be more satisfied, but we also found reasons to believe the use of chewable tablets is, overall, more advantageous. Before treatment, most (71%) of those reporting to have already taken anthelmintics said they chewed the tablet and drank water. During treatment with the chewable tablet, we observed that many children had difficulties in swallowing the chewed pieces of the tablet and 87% accepted the offered glass of water. It seemed that the chewed tablet absorbed their saliva and dried up their mouth, making it difficult to swallow the drug. This might have led children to be more accepting of the non-chewable tablet, but this issue might be avoided by providing a glass of water together with the chewable tablet. Moreover, 95% of those in the chewable tablet arm reported to like their strawberry flavour and over half said it is either too small or the right size.
One limitation of our study is that we did not evaluate the performance of the chewable tablet when it is mixed in water, which is recommended by WHO for children below the age of three years (not included in our study) [17]. A second important limitation is that, since this was a school-based trial, we only included 17 preschoolers (3–5 years old). The chewable mebendazole is more likely to represent an advantage for younger children. We believe the results from the questionnaire and observation might have been different had there been more young children included in the trial.
In conclusion, both mebendazole formulations show an equal performance in efficacy and safety. The only differences observed were the children's responses and satisfaction to the tablets. Given that most children reported to like the taste of the tablet and that, before receiving our treatment, said they preferred chewing instead of swallowing tablets, we believe that the new formulation will facilitate the treatment of all children, particularly the younger ones. Using these tablets will avoid the time consuming crushing of the solid tablets and could increase compliance, since their taste is pleasant. Treatment recommendations might consider recommending giving children a glass of water while chewing the tablets. Future studies should further explore the efficacy, safety and acceptability of the new chewable tablet in a larger number of preschoolers. However, to provide a more efficacious, broad spectrum of activity treatment multiple doses [21], or combination chemotherapy should be considered [22].
5. Contributors
MSP, Said MA, Shaali MA, JH, and JK planned and designed the study; MSP, FB, Said MA, Shaali MA conducted the study; MSP, JH and JK analysed and interpreted the trial data; MSP and JK wrote the first draft and JH revised the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
Acknowledgments
We are grateful to all the children who participated in our study as well as for their teachers precious support. We also thank all other local field assistants, laboratory technicians and drivers from the Public Health Laboratory Ivo de Carneri for their dedicated and hard work during this clinical trial. Finally, we thank Johnson & Johnson for providing the chewable mebendazole tablets used in this study
Funding statement
We are grateful for the Swiss National Science Foundation (grant number 320030_175585) for financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data sharing statement
The study protocol is available with this publication. Individual deidentified participant data that underlie the results reported in this manuscript are available upon request beginning directly after publication and ending 1 year after. Supporting clinical documents, including approval of the proposal, and the informed consent form plan are further made available upon request immediately following publication for at least 1 year. Access will be granted to researchers who provide a scientifically sound proposal. The sponsor, investigator, and collaborators will approve the proposals on the basis of scientific merit. Requests should be directed to the corresponding author (jennifer.keiser@swisstph.ch). Data requests will need to sign a data access agreement prior to granted access.
Funding
This work was supported by the Swiss National Foundation (grant number 320030175585)
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100556.
Appendix. Supplementary materials
References
- 1.WHO. Soil-transmitted helminth infections. 2020. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections.
- 2.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethony J., Brooker S., Albonico M. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 4.Hotez P.J., Brooker S., Bethony J.M., Bottazzi M.E., Loukas A., Xiao S. Hookworm infection. Engl J Med. 2004;351(8):799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar R., Rose A., Mohan V.R. Study design and baseline results of an open-label cluster randomized community-intervention trial to assess the effectiveness of a modified mass deworming program in reducing hookworm infection in a tribal population in southern India. Contemp Clin Trials Commun. 2017;5:49–55. doi: 10.1016/j.conctc.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kattula D., Sarkar R., Rao Ajjampur S.S. Prevalence & risk factors for soil transmitted helminth infection among school children in South India. Indian J Med Res. 2014;139(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- 7.Hall A., Hewitt G., Tuffrey V., De Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4(s1):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bethony J., Brooker S., Albonico M. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 9.WHO . World Health Organization; Geneva: 2017. Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: 2019. Mebendazole 500mg chewable tablets - WHO-PQ recommended summary of product characteristics. [Google Scholar]
- 11.Silber S.A., Diro E., Workneh N. Efficacy and safety of a single-dose mebendazole 500mg chewable, rapidly-disintegrating tablet for Ascaris lumbricoides and Trichuris trichiura infection treatment in pediatric patients: a double-blind, randomized, placebo-controlled, phase 3 study. Am J Trop Med Hyg. 2017;97(6):1851–1856. doi: 10.4269/ajtmh.17-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman A.J., Ali S.M., Albonico M. Safety of a new chewable formulation of mebendazole for preventive chemotherapy interventions to treat young children in countries with moderate-to-high prevalence of soil transmitted helminth infections. J Trop Med. 2012;2012 doi: 10.1155/2012/590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrigan O.I., Timoney R.F. Some aspects of the influence of formulation on the bioavailability of drugs from solid dosage forms. Ir J Med Sci. 1974;143(4):197–207. doi: 10.1007/BF03004763. [DOI] [PubMed] [Google Scholar]
- 14.Moser W., Schindler C., Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ (Clin Res ed) 2017;358:j4307. doi: 10.1136/bmj.j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . World Health Organization; Geneva: 2012. Eliminating soil-transmitted helminthiasis as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. [Google Scholar]
- 16.Vitamin Angels. Global Impact. 2016 https://www.vitaminangels.org/global-impact-toreduce-malnutrition [Google Scholar]
- 17.WHO . World Health Organization; Geneva: 2007. Action against worms. [Google Scholar]
- 18.WHO . World Health Organization; Geneva: 2013. Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminths. [Google Scholar]
- 19.Speich B., Ame S.M., Ali S.M. Oxantel pamoate-albendazole for Trichuris trichiura infection. Engl J Med. 2014;370(7):610–620. doi: 10.1056/NEJMoa1301956. [DOI] [PubMed] [Google Scholar]
- 20.Levecke B., Montresor A., Albonico M. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2014;8(10):e3204. doi: 10.1371/journal.pntd.0003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmeirim M.S., Ame S.M., Ali S.M., Hattendorf J., Keiser J. Efficacy and safety of a single dose versus a multiple dose regimen of mebendazole against hookworm infections in children: a randomised, double-blind trial. EClinicalMedicine. 2018;1:7–13. doi: 10.1016/j.eclinm.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser W., Schindler C., Keiser J. Drug combinations against soil-transmitted helminth infections. Adv Parasitol. 2019;103:91–115. doi: 10.1016/bs.apar.2018.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.