Abstract
Background
High patient turnover presents challenges and opportunity to provide hepatitis C virus (HCV) care in US jails (remand facilities). This study describes the HCV care cascade in the New York City (NYC) jail system during the direct-acting antiviral (DAA) treatment era.
Methods
Patients admitted to the NYC jail system from January 2014 through December 2017 were included in this retrospective cohort analysis. We describe rates of screening, diagnosis, linkage to jail-based care, and treatment among the overall cohort, and among subgroups with long jail stays (≥120 days) or frequent stays (≥10 admissions). The study protocol was approved by a third-party institutional review board (BRANY, Lake Success, NY).
Findings
Among the 121,371 patients in our analysis, HCV screening was performed in 40,219 (33%), 4665 (12%) of whom were viremic, 1813 (39%) seen by an HCV clinician in jail, and 248 (5% of viremic patients) started on treatment in jail. Having a long stay (adjusted risk ratio [aRR] 8·11, 95% confidence interval [CI] 6·98, 9·42) or frequent stays (aRR 1·51, 95% CI 1·04, 2·18) were significantly associated with being seen by an HCV clinician. Patients with long stays had a higher rate of treatment (14% of viremic patients). Sustained virologic response at 12 weeks was achieved in 147/164 (90%) of patients with available virologic data.
Interpretation
Jail health systems can reach large numbers of HCV-infected individuals. The high burden of HCV argues for universal screening in jail settings. Length of stay was strongly associated with being seen by an HCV clinician in jail. Treatment is feasible among those with longer lengths of stay.
Funding
None.
Keywords: Hepatitis C virus, Direct-acting antiviral, Jail, Incarcerated, Care cascade
Research in context.
Evidence before this study
The published data on HCV in US jail settings, which are primarily remand facilities, suggest high prevalence of infection. We are not aware of any description of a full care cascade during the direct-acting antiviral (DAA) treatment era in large US jail systems. We searched the literature on PubMed and Google Scholar from January 2014 to December 2019, beginning at the start of the DAA treatment era, including all published studies related to correctional populations. Search terms used include hepatitis C, cascade, direct-acting antiviral, jail, remand, correctional, and incarcerated.
Added value of this study
This is the first descriptive study of a full cascade of HCV care, including screening, treatment, and confirmation of cure for a large cohort (N = 121,371) from a US jail system where DAA treatment was available. It provides current estimates of HCV prevalence among those screened, including key demographic and risk subgroups. It demonstrates feasibility of treatment among underserved groups in jail, and highlights challenges of serving those with short or frequent incarcerations.
Implications of all the available evidence
These data provide further impetus to urgently scale up HCV screening and treatment in US jails. With rapid turnover of a population with high HCV prevalence, jails provide opportunities to treat and prevent transmission. We highlight the importance of developing better models of HCV care suited for short and unpredictable lengths of stay in remand settings.
Alt-text: Unlabelled box
1. Introduction
Incarcerated populations have a higher hepatitis C virus (HCV) prevalence than general populations. With HCV risk factor- and 1945–1965 birth cohort-based screening, there was a seroprevalence of 21% among those tested in 2013–2014 (19% of patients were tested) in New York City (NYC) jails [1]. This compares to an estimated 16% seroprevalence in the overall United States (US) incarcerated population, and 1·7% seroprevalence among the total US adult population during 2013–2016 [2]. Despite the high prevalence among incarcerated persons, testing and treatment activity in correctional facilities remains low [3]. US guidelines recommend routine opt-out HCV testing and treatment while incarcerated if the length of stay permits [4]. The HCV “care cascade,” representing steps from diagnosis to treatment, will require attention particularly among correctional populations in order to reach HCV elimination goals [5].
In the community, the HCV care cascade has improved during the direct-acting antiviral (DAA) treatment era [6,7], but incarcerated populations still face barriers to diagnosis and care [8], [9], [10]. With 10.7 million admissions nationally in 2018, US jails are primarily remand settings with high turnover (mean length of stay was 25 days) [11]. Little data exist on the care cascade in US jail settings. High turnover presents opportunities to screen and treat many individuals, but restricted patient movement within jails can delay patient care, and short stays [12] can preclude completion of treatment while in custody. These dynamics make it critical to understand the gaps in the care cascade that are amenable to health services quality improvement.
Our study aimed to describe the cascade of HCV care for individuals incarcerated in the NYC jail system during 2014–2017, the initial years of DAA treatment. Duration and frequency of jail exposure influences the feasibility of HCV care provided in jail settings, given concerns about treatment outcomes with discharge to the community mid-treatment [13]. Therefore, we analyzed subgroups with significant exposure to our jails: those with long jail stays and those with frequent incarceration. Those with long jail stay were hypothesized to have a higher likelihood of completing screening and treatment while in jail. Those with frequent jail stays have a high burden of homelessness, mental illness, and substance use disorders [14]. We hypothesize that they have a higher prevalence of HCV compared to the overall jail population, but have low rates of treatment in jail due to short stays. Understanding the gaps in these cascades will inform efforts to improve HCV care for individuals detained in jails.
2. Methods
2.1. Patients
We performed a retrospective observational cohort analysis of all individuals with ≥1 admission to the NYC jail system from January 1, 2014, through December 31, 2017. We excluded patients who did not undergo a jail admission medical intake evaluation, those previously cured with interferon therapy in NYC jails, and those who entered jail on HCV DAA treatment from the community.
2.2. HCV care
Correctional Health Services (CHS) of NYC Health + Hospitals, the city's public health care system, is responsible for providing health care and discharge planning services to individuals incarcerated in the NYC jail system. During our analysis time frame, the system consisted of eleven jail facilities for men and one for women. CHS transitioned from risk-factor- and 1945–1965 birth cohort-based HCV screening as recommended by the Centers for Diseases Control and Prevention (CDC) [15,16] to universal opt-out screening in a staged fashion by facility from 2014 through early 2019. At the two facilities with universal testing during the study time period, HCV rapid testing was offered on admission. A positive antibody test result or positive self report of HCV diagnosis prompted an HCV RNA test. Those with detectable HCV RNA were referred for jail-based treatment assessment by a CHS physician who treated HIV and HCV infection. Treatment was started in jail if estimated length of stay was sufficient to complete treatment in jail, or if there was medical urgency, such as advanced liver disease.
2.3. Care cascade
Our cascade of care included 1) screening for exposure to HCV, 2) detection of HCV viremia, 3) linkage to an HCV clinician in jail, 4) treatment initiation, and 5) confirmation of sustained virologic response at 12 weeks (SVR12). We report a care cascade for (a) the overall cohort, (b) patients with at least one jail stay that was ≥120 days during 2014–2017 (long stay cohort), and (c) patients with frequent jail admissions, defined as ≥10 incarcerations during 2014–2017 (frequent stay cohort). A duration of ≥120 days was considered a “long stay” as it would allow sufficient time for workup and completion of most DAA treatment courses.
Patients were “screened for HCV” if they self-reported a positive history of HCV on admission, had an antibody test via rapid immunoassay test (OraQuick HCV Rapid Antibody Test, OraSure Technologies, Inc, Bethlehem, PA) or standard immunoassay test (Abbott EIA 2·0 HCV antibody assay, Abbott Laboratories, Abbott Park, IL), or had an HCV RNA or genotype assay done from April 2011 to April 2018. All laboratory data in this period were included in the analysis since some of our cohort were diagnosed in our system prior to 2014. They may not have needed repeat testing after 2014, but were still considered screened. We performed a validation of self-reported history of HCV, and found a 94% concordance with positive HCV antibody testing in jail. “HCV RNA detectable” was determined by a positive result, based on the test manufacturer's reference range, of the most recent RNA test sent by CHS through April 30, 2018. This approach was used to exclude those who were cured in the community or spontaneously cleared the infection. Those who received HCV treatment in jail were all classified as having had “detectable HCV RNA.” Being “seen by an HCV clinician” was defined as being seen by a CHS physician (including general internists and infectious diseases physicians) who treats HCV in the jail after a test result showing detectable HCV RNA. “Started HCV treatment” was defined as starting treatment in jail.
SVR12 lab tests to confirm cure were defined as an HCV viral load checked ≥64 days after the projected end date of the treatment course. The 64-day time point was chosen to allow for a window around usual clinical care, a definition used in recent real-world treatment outcomes studies [17,18].
2.4. Data sources and definitions
We obtained data through April 30, 2018 for our cohort admitted between January 1, 2014 to December 31, 2017 to allow at least four months for screening and treatment to occur. Variables including age, race/ethnicity, sex, history of homelessness, HIV infection status, alcohol and opioid use disorders, and serious mental illness (SMI) were obtained from CHS electronic health records (EHR), which records primary data collected from all clinical encounters in jail. Age categories (born before 1945, born 1945–1965, and born after 1965) were chosen to examine differences between patients within and outside of the birth cohort (born during 1945–1965) included in the 2012 CDC HCV screening recommendations [16]. Race/ethnicity were collapsed into categories: Non-Hispanic White, Non-Hispanic Black, Hispanic, and Other/Unknown. History of homelessness was captured by patient self-report. Jail lengths of stay were extracted from the CHS EHR with data transferred from NYC Department of Correction (DOC) records.
Given that patients with mental health or substance use comorbidities have lower rates of accessing DAA therapy [19], we included these variables in our analysis. Patients were designated to have SMI according to CHS policy, which changed during the study period but comprised mainly patients diagnosed with psychotic disorders, bipolar disorder, depressive disorders and, in the last year of the study period, post-traumatic stress disorder. Probable alcohol use disorder was defined as patient self-report or the presence of an International Statistical Classification of Diseases and Related Health Problems (ICD) 9/10 diagnosis code [20]. Probable opioid use disorder was defined based on patient self-report, positive urine toxicology screening on medical intake, any methadone or buprenorphine prescription, or presence of a related ICD 9/10 diagnosis code.
HIV-positive status was defined by either self-reported status at intake or history of a positive HIV antibody or viral load test in CHS EHR. HCV antibody results were either from rapid antibody test results recorded in CHS EHR or immunoassay-based antibody testing processed at BioReference Laboratories (Elmwood Park, NJ), which processes all CHS lab testing. Treatment initiation information was extracted from the CHS HCV treatment tracking database. To determine SVR12 status, aggregate HCV viral load data were obtained from NYC Department of Health and Mental Hygiene (DOHMH) surveillance data. In NYC, all laboratories are legally mandated to report to the DOHMH all positive HCV antibody, positive and negative HCV viral load, and genotype results processed in NYC lab facilities, including tests from NYC jails. Data extraction from the DOHMH surveillance registry was finalized in July 2019.
All data were de-identified after linking different sources in the electronic health records and the DOHMH surveillance registry. The de-identified data were stored in a secured folder on password-protected servers maintained by CHS. The files were encrypted and no one but the study investigators had access.
2.5. Outcome of interest
While treatment initiation is an important outcome, the decision to start treatment was primarily influenced by the patient's estimated length of stay in jail, and less dependent on other patient characteristics. Therefore, we decided to do further analysis to identify factors significantly associated with being “seen by an HCV clinician”, since this is the terminal step of the cascade that is under CHS control and a necessary step before starting treatment.
2.6. Statistical analysis
We used descriptive statistics, Mann Whitney U test, and Chi-squared analysis to explore differences between patients who were in the long stay cohort or the frequent stay cohort, relative to the rest of the overall cohort. We used log-binomial regression to calculate unadjusted and adjusted risk ratios (aRR) and 95% confidence intervals (95% CI) for the outcome of being seen by an HCV clinician among those with detectable HCV RNA. To calculate aRRs, predictors hypothesized to be significant were included in the final multivariable model, including age, sex, race/ethnicity, HIV co-infection status, having a long jail stay, having frequent jail stays, SMI, and opioid use disorder. Individuals with missing data (N = 22) were exclude from the regression analysis. We defined statistical significance as a two-sided p-value of <0.05. We used SPSS version 24 (IBM, Somers, NY) for statistical analysis.
2.7. Study oversight
This study protocol was reviewed by a third-party institutional review board (IRB) (BRANY, Lake Success, NY) and approved under BRANY File #18- PRS-175-419(HHC). BRANY IRB granted a full waiver of informed consent, based on satisfying the waiver criteria set forth in 45 CFR 46.116(d).
Role of funding: None.
3. Results
3.1. Cohort characteristics
We included 121,371 patients admitted from January 1, 2014 through December 31, 2017, after excluding individuals who either had no medical intake evaluations (N = 12,121), were cured previously with interferon-based therapy in jail (N = 18), or had entered jail while on HCV treatment from the community (N = 88). These patients had a median age of 33 (IQR 25–44), 88·8% were male, and 3·5% were HIV-positive (Table 1). Most of the cohort (84·3%) were born after 1965. There were 8·8% with SMI, 16·7% with a history of homelessness, and 17·3% with probable opioid use disorder. Median cumulative length of stay in jail per patient during the cohort time period was 27 days (IQR 4–144), and median number of jail admissions per patient was 1 (IQR 1–2). There were 28,925 (23·8%) patients who had at least one jail stay lasting ≥120 days, and 961 (0·8%) patients with ≥10 jail admissions. Relative to the rest of the overall cohort population, the frequent stay cohort were older and had a significantly higher prevalence of HIV, SMI, history of homelessness, alcohol use disorder, and opioid use disorder (Table 1). The most common treatment regimens included sofosbuvir + velpatasvir for 12 weeks (N = 88), elbasvir + grazoprevir for 12 weeks (N = 84), ledipasvir + sofosbuvir for 12 weeks (N = 52), and glecaprevir + pibrentasvir for 8 weeks (N = 14).
Table 1.
Overall cohort and subgroup characteristics, patients admitted to NYC jails 2014–2017.
| Total N = 121,371 | Long Stay N = 28,925 | p value* | Frequent Stay N = 961 | p value* | ||||
|---|---|---|---|---|---|---|---|---|
| Age, median (IQR)a | 33 (25–44) | 43 (35–51) |
<0·0001 |
41 (34–49) | 0·047 | |||
| N | Column% | N | Column% | N | Column% | |||
|
Birth cohort (year)b Before 1945 1945–1965 After 1965 |
270 18,768 102,316 |
0·2 15·5 84·3 |
75 4388 24,457 |
0·3 15·2 84·6 |
0·095 |
1 195 765 |
0·1 20·3 79·6 |
<0·0001 |
|
Sexc Male Female |
107,881 13,485 |
88·9 11·1 |
27,040 1885 |
93·5 6·5 |
<0·0001 |
878 83 |
91·4 8·6 |
0·015 |
|
Race/ethnicity Hispanic Non-Hispanic Black Non-Hispanic White Other or unknown |
40,949 62,093 11,573 6756 |
33·7 51·2 9·5 5·6 |
10,092 15,393 2114 1326 |
34·9 53·2 7·3 4·6 |
<0·0001 |
269 570 95 27 |
28·0 59·3 9·9 2·8 |
<0·0001 |
|
HIV Yes No |
4252 117,119 |
3·5 96·5 |
1085 27,840 |
3·8 96·2 |
0·0090 |
75 886 |
7·8 92·2 |
<0·0001 |
|
Serious mental illness (SMI) Yes No |
10,654 110,717 |
8·8 91·2 |
4130 24,795 |
14·3 85·7 |
<0·0001 |
253 708 |
26·3 73·7 |
<0·0001 |
|
Ever Homeless Yes No |
20,233 101,138 |
16·7 83·3 |
7661 21,264 |
26·5 73·5 |
<0·0001 |
644 317 |
67·0 33·0 |
<0·0001 |
|
Alcohol Use Disorder Yes No |
14,148 107,223 |
11·7 88·3 |
4912 24,013 |
17·0 83·0 |
<0·0001 |
482 479 |
50·2 49·8 |
<0·0001 |
|
Opioid use disorder* Yes No |
20,946 100,425 |
17·3 82·7 |
6679 22,246 |
23·1 76·9 |
<0·0001 |
536 425 |
55·8 44·2 |
<0·0001 |
| Length of stay in days, median (IQR)d | 12 (3–53) | 129 (46–240) |
<0·0001 |
8 (4–14) |
<0·0001 |
|||
| Number of incarcerations, median (IQR)e | 1 (1–2) | 2 (1–3) |
<0·0001 |
13 (10–14) |
<0·0001 |
|||
Age calculated at admission date of last incarceration.
Missing data for 17 individuals.
Missing data for 5 individuals.
Median of each individual's median length of stay during the cohort time frame, including all jail stays from January 1, 2014 to December 31, 2017.
Median of each individual's cumulative number of incarcerations during the cohort time frame. This includes all jail admissions from January 1, 2014 to December 31, 2017.
Chi-square testing was done for categorical variables, and Mann Whitney U testing was done for continuous variables. Comparison was between the subgroup and the rest of total cohort.
3.2. Care cascades
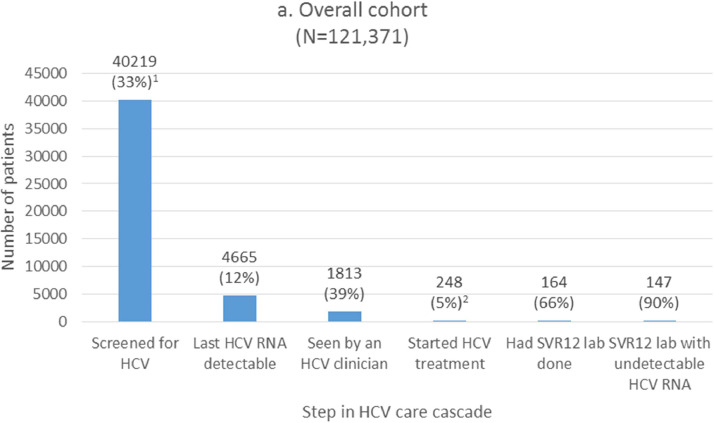
In the overall cohort (N = 121,371), 40,219 (33%) patients were screened for HCV (Fig. 1a). HCV RNA was detectable in 4665 (12%) of screened patients. Of viremic patients, 1813 (39%) were seen by an HCV clinician, and 248 (5%) started on treatment (14% among those seen by an HCV clinician). Treatment regimen durations were 8 weeks (6%), 12 weeks (93%), and 24 weeks (1%). There were 225 (91%) who completed treatment in jail, 19 (8%) who were discharged from jail on treatment, and 4 (2%) who discontinued treatment. Among the 164 persons with RNA testing done at the SVR12 time point, 147 (90%) had an undetectable HCV RNA at this time point.
Fig. 1.
Hepatitis C virus (HCV) cascades of care for patients incarcerated during January 1, 2014–December 31, 2017 a) overall, b) long stay, c) and frequent stay.
NOTE: Percentages are calculated using the previous step as the denominator, unless otherwise indicated.
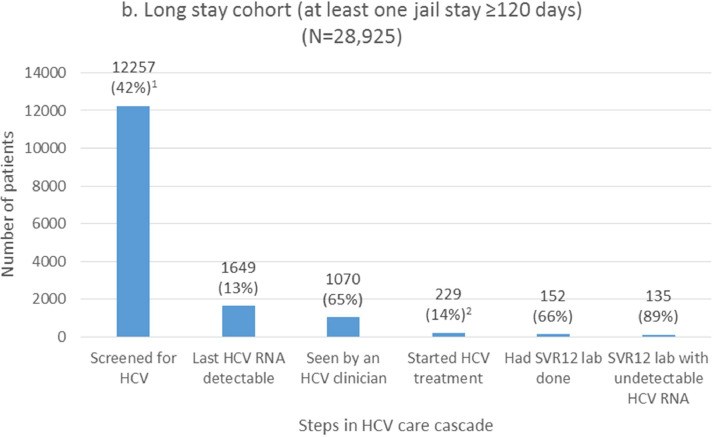
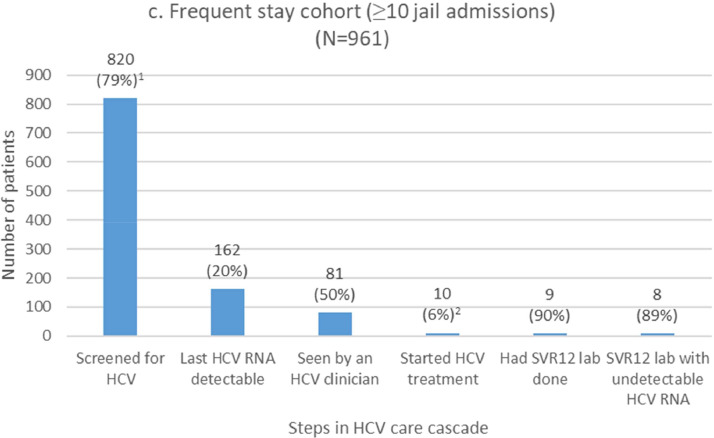
We report cascade outcomes among subgroups with long or frequent jail exposures, respectively (Fig. 1b–c). These groups are not mutually exclusive. HCV screening rates for the long stay and frequent stay cohorts were 42% and 79%, respectively. Detectable HCV RNA was present in 13% (long stay cohort) and 20% (frequent stay cohort) of these screened individuals. Among those with detectable HCV RNA infection, the proportion that were seen by an HCV clinician was 65% and 50%, and HCV treatment was started in 14% and 6% of viremic patients in the long stay and frequent stay cohorts, respectively (21% and 12% among those seen by an HCV clinician). Among those with virologic results available, SVR12 rates were 89%.
Fig. 1.
Continued.
We also characterized cascade stages among basic demographic groups and patient groups who are either at risk for accelerated HCV disease progression or underserved for HCV care (Table 2). During a period when universal opt-out screening was being gradually implemented by CHS, there were high rates of screening among patients born in the 1945–1965 birth cohort (72%), those with HIV infection (90%), and those with opioid use disorder (73%) (Table 2). These groups were the focus of birth cohort- and risk factor-based screening. Women in our cohort had higher screening rates than men (55% versus 30%). Proportion of detectable HCV RNA among those who screened positive for HCV exposure was particularly high among the 1945–1965 birth cohort (15%), non-Hispanic White cohort (17%), HIV cohort (21%), and those with a history of homelessness (17%), alcohol use disorder (18%), and opioid use disorder (23%). Among HCV-viremic patients, the group with the highest rate of being seen by an HCV clinician was the HIV co-infected group (76%). However, treatment rates were low across all subgroups, ranging from 0 to 7% (Table 2).
Table 2.
Cascade outcomes by demographics and selected priority groups
NOTE: Percentages are calculated using the previous step as the denominator, unless otherwise indicated.
| Total N = 121,371 |
Screened for HCV N = 40,219 (33·1%) |
Last HCV RNA detectable N = 4665 (11·6%) |
Seen by HCV clinician N = 1813 (38·9%) |
Treated N = 248 (5·3%)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Column% | N (row%) | Row% | N | Row% | N | Row% | N | Row%a | ||
|
Birth cohort (year)b Before 1945 1945–1965 After 1965 |
270 18,768 102,316 |
0·2 15·5 84·3 |
148 13,435 26,633 |
54·8 71·6 26·0 |
18 2023 2624 |
12·2 15·1 9·9 |
5 813 995 |
27·8 40·2 37·9 |
0 106 142 |
0·0 5·2 5·4 |
|
|
Sexc Male Female |
107,881 13,485 |
88·9 11·1 |
32,818 7401 |
30·4 54·9 |
3985 680 |
12·1 9·2 |
1542 271 |
38·7 39·9 |
231 17 |
5·8 2·5 |
|
|
Race/ethnicity Hispanic Non-Hispanic Black Non-Hispanic White Other or unknown |
40,949 62,093 11,573 6756 |
33·7 51·2 9·5 5·6 |
13,417 19,691 5468 1643 |
32·8 31·7 47·2 24·3 |
2258 1352 944 111 |
16·8 6·9 17·3 6·8 |
874 577 315 47 |
38·7 42·7 33·4 42·3 |
122 69 51 6 |
5·4 5·1 5·4 5·4 |
|
|
HIV Yes No |
4252 117,119 |
3·5 96·5 |
3814 36,405 |
89·7 31·1 |
810 3855 |
21·2 10·6 |
612 1201 |
75·6 31·2 |
48 200 |
5·9 5·2 |
|
|
Serious mental illness Yes No |
10,654 110,717 |
8·8 91·2 |
5983 34,236 |
56·2 30.9 |
687 3978 |
11·5 11·6 |
313 1500 |
45·6 37·7 |
47 201 |
6·8 5·1 |
|
|
Ever homeless Yes No |
20,233 101,138 |
16·7 83·3 |
10,829 29,390 |
53·5 29·1 |
1854 2811 |
17·1 9·6 |
806 1007 |
43·5 35·8 |
119 129 |
6·4 4·6 |
|
|
Alcohol use disorder Yes No |
14,148 107,223 |
11·7 88·3 |
8257 31,962 |
58·4 29·8 |
1470 3195 |
17·8 10·0 |
655 1158 |
44·6 36·2 |
107 141 |
7·3 4·4 |
|
|
Opioid use disorder Yes No |
20,946 100,425 |
17.3 82.7 |
15,365 24,854 |
73.4 24.7 |
3603 1062 |
23·4 4·3 |
1372 441 |
38·1 41·5 |
203 45 |
5·6 4·2 |
|
| Length of stay in days, median (IQR)d |
27 (4–144) |
62 (11–216) |
NA |
225 (105–358) |
384 (250–557) |
||||||
| Number of incarcerations, median (IQR)e |
1 (1–2) |
2 (1–3) |
NA |
3 (1–4) |
3 (1–5) |
||||||
Percentage out of those who had last HCV RNA detectable.
Missing data for 17 individuals.
Missing data for 5 individuals.
Median of each individual's cumulative length of stay during the cohort time frame (January 1, 2014 to December 31, 2017).
Median of each individual's cumulative number of incarcerations during the cohort time frame (January 1, 2014 to December 31, 2017).
3.3. Factors associated with linkage to jail-based HCV clinician
In our multivariable regression models, female sex (vs. male sex) (aRR 1·41, 95% CI 1·15–1·72), being HIV co-infected (vs. HIV negative) (aRR 11·05, 95% CI 9·07–13·46), having a long stay (vs. not having a long stay) (aRR 8·11, 95% CI 6·98–9·42), and having frequent stays (vs. not having frequent stays) (aRR 1·51, 95% CI 1·04–2·18) were signficantly associated with being seen by an HCV clinician among those with detectable HCV RNA (Table 3).
Table 3.
Factors associated with being seen by an HCV clinician while in jail among those with detectable HCV RNA.
| Patient characteristics | Seen by an HCV clinician while in jail N (Row%a) | Unadjusted RR (95% CI) | Adjusted RR (95% CI)b |
|---|---|---|---|
|
Age (birth year) Before 1945 1945–1965 After 1965 |
5 (27·8) 813 (40·2) 995 (37·9) |
– 1·75 (0·62, 4·92) 1·59 (0·56, 4·46) |
Not significant |
|
Sex Male Female |
1542 (38·7) 271 (39·9) |
– 1·05 (0·89, 1·24) |
– 1.41 (1.15, 1.72) |
|
Race / ethnicity Hispanic Non-Hispanic Black Non-Hispanic White Other or unknown |
874 (38·7) 577 (42·7) 315 (33·4) 47 (42·3) |
– 1·18 (1·03, 1·35) 0·79 (0·68, 0·93) 1·16 (0·79, 1·71) |
Not significant |
|
HIV Positive Not-Positive |
612 (75·6) 1201 (31·2) |
6·83 (5·74, 8·13) – |
11.05 (9.07, 13.46) – |
|
Long stayc Yes No |
1070 (64·9) 743 (24·6) |
5·65 (4·96, 6·44) – |
8.11 (6.98, 9.42) – |
|
Frequent stayd Yes No |
81 (50·0) 1732 (38·5) |
1·60 (1·17, 2·19) – |
1.51 (1.04, 2.18) – |
|
Serious mental illness Yes No |
313 (45·6) 1500 (37·7) |
1·38 (1·17, 1·63) – |
Not significant |
|
Opioid use disorder Yes No |
1372 (38·1) 441 (41·5) |
0·87 (0·75, 1·00) – |
Not significant |
RR = risk ratio.
Denominators are those with detectable HCV RNA.
Final multivariable model included age, sex, race/ethnicity, HIV status, long stay status, and frequent stay status, serious mental illness, and opioid use disorder.
All patients with ≥1 jail stay that was ≥120 days during the cohort time frame (January 1, 2014 to December 31, 2017).
All patients with ≥10 incarcerations during the cohort time frame (January 1, 2014 to December 31, 2017).
4. Discussion
This study is the first description of a complete HCV care cascade in a large US jail system during the DAA treatment era. We report a high prevalence of HCV among those screened in the NYC jail system and identified opportunities to improve rates of screening, linkage to a jail-based HCV clinician, and treatment initiation. Our findings indicate that we screened one third of our patients, 39% of those with detectable HCV RNA saw a CHS HCV clinician, and 5% of viremic patients started treatment in jail. Patients who had at least one long jail stay were more likely to be seen by an HCV clinician and start treatment, compared with the rest of the cohort. This suggests that short duration of jail stay was a barrier to care for some of our patients. After confirming a diagnosis on admission, patients should be triaged for treatment in jail or linkage to treatment in the community if length of stay is short.
Given these data, our quality improvement initiatives should focus on effective implementation of universal screening and increasing treatment activity in jail. Since universal screening was gradually implemented during the cohort time period, our overall screening rate of 33% is expected to improve. Our study showed the highest rate of detectable HCV RNA among screened individuals in the 1945–1965 birth cohort (15·1%), but also high among those born after 1965 (9·9%) (Table 2). These rates among the younger group are approximately 10-fold the estimated prevalence for the general US population [2], arguing for universal screening in our jail population. Focusing on the 1945–1965 birth cohort is not sufficient as incidence of HCV among young adults is rising. Many young adults with recent HCV infection experience incarceration [21]. Consequently, HCV prevalence is shifting to a younger age over time in US correctional settings [22]. Risk factor-based screening is associated with missed opportunities for diagnosis as risk factors are not always accurately assessed and documented [23]. Taken together, universal opt-out screening is the preferred strategy for the incarcerated population, a practice recommended by current guidelines [4]. As of 2019, CHS offers universal opt-out screening at all admission facilities.
Our data indicate a higher uptake of HCV screening among women than men, but a higher RNA positivity rate among screened men than women. These results are likely because universal HCV screening was offered to all women on admission from June 2015 onward, while in men, it was only launched at one of the four male admission facilities during the study period. A higher proportion of women were screened on a routine basis, leading to a lower test positivity rate among women than men. Women were also more likely than men to see an HCV clinician (Table 3), likely because the sole female facility offered more efficient care to women. While men could be transferred between eleven jail facilities, women remained at one facility, which likely reduced interruptions in care. NYC's decarceration efforts will reduce the number of jail facilities, allowing for more consolidated, efficient care [24].
Since individuals in US correctional settings are all entitled to basic health care, HCV care in jail settings can alleviate some disparities in access to treatment in the community. While patients with mental health and substance use disorders are less likely to access DAA treatment in the community [19], those with SMI, history of homelessness, alcohol use disorder, and opioid use disorder in our cohort had similar or higher rates of being seen by an HCV clinician and starting treatment, compared to the overall cohort (Table 2). This is not surprising since the barriers to initiating care in jail are primarily security restrictions on patient movement, patients’ willingness to engage in care, and short lengths of stay.
We need to better understand and address barriers to treatment that exist beyond short length of stay, since 1420 (86%) of individuals in the long stay cohort did not receive treatment. These barriers may include delays in diagnosis, linkage to HCV clinician, and determination of length of stay, which can all result in lost time for treatment. Our transition to universal HCV rapid immunoassay testing on all admissions may reduce time to RNA testing. Linkage to an HCV clinician in jail can be delayed due to restrictions on patient movement due to security issues. CHS leverages telehealth visits when appropriate to alleviate the need for patient transport, and has increased the pool of non-specialist clinicians (including MD and PA) involved in managing HCV. It can be difficult to ascertain length of stay for patients on remand, so time may be lost waiting for legal decisions. We are developing data models to predict length of stay on admission so time for treatment can be maximized. HIV-HCV co-infected patients may face additional barriers to treatment. Despite high rates of linkage to jail-based care which may be due to clinicians prioritizing care for all patients with HIV (Table 2), clinicians may initially be focusing on HIV control before considering HCV treatment, losing time required for HCV treatment in jail. However, level of HIV control should not serve as a barrier to treating HCV. Real world experience has demonstrated successful HCV treatment for HIV-HCV co-infected individuals not on HIV antiretroviral therapy, those with CD4 counts <200 cells/mL, and those with detectable HIV viremia [25]. Therefore, HCV treatment can be offered to co-infected individuals without requiring HIV control as a prerequisite.
Our frequently incarcerated cohort warrant additional focus for quality improvement given a higher proportion with HCV viremia among those screened than the overall cohort (20% versus 12%) (Fig. 1a and 1c). This may be due to higher HCV prevalence, more testing opportunities with frequent incarceration, and poor access to HCV treatment in the community among the frequently incarcerated group. The high prevalence of homelessness may lead to competing priorities of accessing food and shelter. Therefore, correctional settings can offer them opportunities to access HCV treatment and care coordination. However, treatment initiation in jail was rare in the frequently incarcerated cohort (6%), likely due to short lengths of stay for individual jail admissions (Table 1). We have scaled up discharge planning efforts to link patients to community-based care after a pilot study in our jail showed feasibility [26]. Furthermore, efforts to alleviate homelessness and poor access to substance use disorder treatment may help break the cycle of frequent incarceration [27].
Access to HCV treatment in the community has improved in the DAA era, with 52% of those with a positive HCV RNA test in NYC between 2014 and 2017 initiating treatment [7]. However, ongoing barriers to care limit progression through the HCV care cascade for many incarcerated individuals [3,[28], [29]]. These barriers include insufficient screening activity, with one prison in Canada screening only 7% of individuals where testing is prompted by patients’ request [29]. Universal testing results in higher rates of screening compared to testing only on clinician request [30]. Short stays in correctional facilities also impede the level of HCV care provided. The Canadian study [29] found that patients with a prison length of stay ≥1 month had a higher HCV screening rate relative to the overall population (17% v. 7%), which is consistent with our data showing higher screening rates in the long stay cohort. Rapid point-of-care (POC) testing can improve the entire cascade of care, as one study at a large prison in the United Kingdom found that use of a POC antibody and RNA testing strategy resulted in a higher frequency of screening and treatment initiation compared to conventional dry blood spot testing [31]. Use of POC testing was also associated with shorter time between the steps of patient admission, testing, clinical assessment, and treatment initiation. Finally, barriers to treatment initiation can be improved by employing nurse-led models of HCV care to reduce the need for specialist physicians [32], [33]. One nurse-led treatment model involving 14 prisons in Australia resulted in a 96% SVR12 rate, while requiring specialist consultation in only 18% of cases [32]. Additionally, use of telemedicine clinics [30] can reduce the need to transport patients between facilities for care. Therefore, the HCV care cascade in correctional settings can be improved by applying more efficient diagnostic workflows and updating models of care.
Our study has limitations. This HCV care cascade represents a dynamic time period where CHS scaled up screening and treatment activity. Since we transitioned from risk factor-based screening to universal screening in a sequential fashion during the study period, patients were exposed to different screening practices based on their admission facility and time of admission. Therefore, we were unable to report screening rates for each policy (risk factor-based versus universal). The 33% screening rate helps us understand the baseline screening rate before universal screening was fully implemented. There may be residual confounding factors that influence our outcome of interest (being seen by an HCV clinician), including patients being housed in areas more difficult for clinicians to access (i.e. solitary confinement). Retaining marginalized populations in care after HCV treatment is challenging [34], and our rate of SVR12 confirmation was suboptimal at 66%. Our reported SVR12 rate of 90% could be biased toward those who were more engaged in health care. We are working on measures to improve rates of SVR12 confirmation by improving linkage to care after discharge from jail. We published a detailed analysis of our treatment outcomes [35]. While DOHMH surveillance data are presumed to capture all people tested for HCV in NYC, and the data have been used for other epidemiological studies of HCV in NYC [36], there could be reporting or deduplication errors that could not be ascertained. Finally, we cannot generalize our results to other US jail systems. Others may have different care cascades due to available local resources. Nevertheless, our data describe what is feasible in a large jail population and highlight opportunities for improvement.
With >10 million admissions in 201811 and a high prevalence of HCV in this population, U.S. jails represent a key opportunity to treat HCV in a population that may be less like to engage in community-based care. Our data support universal screening and increased HCV treatment activity in jails, along with improved linkage to community follow-up for those with short stays as strategies to advance HCV elimination efforts. Future studies should evaluate strategies to expedite treatment while in jail.
Declaration of Competing Interest
The authors of this study have no conflicts of interest to disclose.
Acknowledgments
Author contributions
JC and RM conceptualized the study. JC, FK, and RM contributed to the literature search and study design. JC, FK, JS, and AB contributed to data collection. JC, FK, JS, AB, and MJA contributed to data analysis. JC, FK, JS, AB, MJA, AW, PY, ZR, and RM contributed to data interpretation. JC, FK, JS, and AB contributed to the figures. JC, FK, JS, AB, MJA, ZR, AW, PY, and RM contributed to writing of the report. JC, PY, and RM contributed to supervision of the project.
Data sharing statement
Because this study contains sensitive information from a vulnerable population, the data cannot be shared.
Funding
This study had no funding.
Acknowledgments
We thank Ruth Leibowitz and the CHS Information Technology team for data retrieval from CHS electronic health records. We thank Monica Katyal and Janet Wiersema for critical review of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100567.
Contributor Information
Justin Chan, Email: chanj15@nychhc.org.
Ross MacDonald, Email: rmacdonald@nychhc.org.
Appendix. Supplementary materials
References
- 1.Akiyama M.J., Kaba F., Rosner Z., Alper H., Holzman R.S., MacDonald R. Hepatitis C screening of the “Birth Cohort” (Born 1945-1965) and younger inmates of New York City jails. Am J Public Health. 2016;106(7):1276–1277. doi: 10.2105/AJPH.2016.303163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmeister M.G., Rosenthal E.M., Barker L.K. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–1031. doi: 10.1002/hep.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HepCorrections. Available at:http://www.hepcorrections.org/. Accessed March 6, 2019.
- 4.AASLD-IDSA. HCV testing and treatment in correctional settings. Recommendations for testing, managing, and treating hepatitis C. Available at:https://www.hcvguidelines.org/unique-populations/correctional. Accessed March 6, 2019.
- 5.Scott N., Doyle J.S., Wilson D.P. Reaching hepatitis C elimination targets requires health system interventions to enhance the care cascade. Int J Drug Policy. 2018;47:107–116. doi: 10.1016/j.drugpo.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman A., Douglas A., Nwosu S., Choi L., Chastain C. Increasing success and evolving barriers in the hepatitis C care cascade during the direct acting antiviral era. PLoS One. 2018 Jun 18;13(6) doi: 10.1371/journal.pone.0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore M.S., Bocour A., Laraque F., Winters A. A surveillance-based hepatitis C care cascade, New York City, 2017. Public Health Rep. 2018;133(4):497–501. doi: 10.1177/0033354918776641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochstatter K.R., Stockman L.J., Holzmacher R. The continuum of hepatitis C care for criminal justice involved adults in the DAA era: a retrospective cohort study demonstrating limited treatment uptake and inconsistent linkage to community-based care. Health Just. 2017;5(1):10. doi: 10.1186/s40352-017-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawks L., Norton B.L., Cunningham C.O., Fox A.D. The hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23(6):473–478. doi: 10.1111/jvh.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kronfli N., Nitulescu R., Cox J. Previous incarceration impacts access to hepatitis C virus (HCV) treatment among HIV-HCV co-infected patients in Canada. J Int AIDS Soc. 2018;21(11):e25197. doi: 10.1002/jia2.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jail Inmates in 2018. Bureau of Justice Statistics (BJS). Available at:https://www.bjs.gov/index.cfm?ty=pbdetail&iid=6826. Accessed May 14, 2020.
- 12.DOC Statistics. Department of Correction. Available at:www1.nyc.gov/site/doc/about/doc-statistics.page. Accessed July 24, 2019.
- 13.Aspinall E.J., Mitchell W., Schofield J. A matched comparison study of hepatitis C treatment outcomes in the prison and community setting, and an analysis of the impact of prison release or transfer during therapy. J Viral Hepat. 2016;23:1009–1016. doi: 10.1111/jvh.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald R., Kaba F., Rosner Z. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262–2268. doi: 10.2105/AJPH.2015.302785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cdc.gov. (2015). Testing Recommendations for Hepatitis C Virus Infection | HCV | Division of Viral Hepatitis | CDC. Available at:https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm. Accessed 8 Jan. 2020.
- 16.Smith B.D., Morgan R.L., Beckett G.A. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR–4):1–32. [PubMed] [Google Scholar]
- 17.Reddy K.R., Lim J.K., Kuo A. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: observations through HCV-target database. Aliment Pharmacol Ther. 2017;45:115–126. doi: 10.1111/apt.13823. [DOI] [PubMed] [Google Scholar]
- 18.Welzel T.M., Nelson D.R., Morelli G. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66(10):1844–1852. doi: 10.1136/gutjnl-2016-311609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain M.K., Thamer M., Therapondos G. Has access to hepatitis C virus therapy changed for patients with mental health or substance use disorders in the direct-acting antiviral period? Hepatology. 2019;69(1):51–63. doi: 10.1002/hep.30171. [DOI] [PubMed] [Google Scholar]
- 20.Classification of Diseases. 2019. Available at:https://www.who.int/classifications/icd/en/Accessed November 6, 2019.
- 21.Suryaprasad A.G., White J.Z., Xu F. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59(10):1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 22.Larney S., Mahowald M.K., Scharff N., Flanigan T.P., Beckwith C.G., Zaller N.D. Epidemiology of hepatitis C virus in Pennsylvania state prisons, 2004-2012: limitations of 1945–1965 birth cohort screening in correctional settings. Am J Public Health. 2014;104(6):e69–e74. doi: 10.2105/AJPH.2014.301943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuncio D.E., Newbern E.C., Fernandez-Vina M.H., Herdman B., Johnson C.C., Viner K.M. Comparison of risk-based hepatitis C screening and the true seroprevalence in an urban prison system. J Urban Health. 2015;92(2):379–386. doi: 10.1007/s11524-015-9945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A Roadmap to Closing Rikers. Available at:https://rikers.cityofnewyork.us/. Accessed December 5, 2019.
- 25.Bhattacharya D., Belperio, Shahoumian T. Effectiveness of all-oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus genotype 1-coinfected patients treated in routine practice. Clin Infect Dis. 2017;64(12):1711–1720. doi: 10.1093/cid/cix111. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama M.J., Columbus D., MacDonald R. Linkage to hepatitis C care after incarceration in jail: a prospective, single arm clinical trial. BMC Infect Dis. 2019;19(1):703. doi: 10.1186/s12879-019-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall G., Walters S., Gould H., Lim S. Housing versus treatment first for supportive housing participants with substance use disorders: a comparison of housing and public service use outcomes. Subst Abus. 2020;41(1):70–76. doi: 10.1080/08897077.2018.1449049. [DOI] [PubMed] [Google Scholar]
- 28.Papaluca T., Hellard M.E., Thompson A.J.V., Lloyd A.R. Scale-up of hepatitis C treatment in prisons is key to national elimination. Med J Aust. 2019;210(9):391–393. doi: 10.5694/mja2.50140. [DOI] [PubMed] [Google Scholar]
- 29.Kronfli N., Dussault C., Klein M.B., Lebouché B., Sebastiani G., Cox J. The hepatitis C virus cascade of care in a Quebec provincial prison: a retrospective cohort study. CMAJ Open. 2019;7(4):E674–E679. doi: 10.9778/cmajo.20190068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morey S., Hamoodi A., Jones D. Increased diagnosis and treatment of hepatitis C in prison by universal offer of testing and use of telemedicine. J Viral Hepat. 2019;26(1):101–108. doi: 10.1111/jvh.13017. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed Z., Al-Kurdi D., Nelson M. Time matters: point of care screening and streamlined linkage to care dramatically improves hepatitis C treatment uptake in prisoners in England. Int J Drug Policy. 2020;75 doi: 10.1016/j.drugpo.2019.102608. [DOI] [PubMed] [Google Scholar]
- 32.Papaluca T., McDonald L., Craigie A. Outcomes of treatment for hepatitis C in prisoners using a nurse-led, statewide model of care. J Hepatol. 2019;70(5):839–846. doi: 10.1016/j.jhep.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Overton K., Clegg J., Pekin F. Outcomes of a nurse-led model of care for hepatitis C assessment and treatment with direct-acting antivirals in the custodial setting. Int J Drug Policy. 2019;72:123–128. doi: 10.1016/j.drugpo.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Morris L., Smirnov A., Kvassay A. Initial outcomes of integrated community-based hepatitis C treatment for people who inject drugs: findings from the Queensland Injectors’ Health Network. Int J Drug Policy. 2017;47:216–220. doi: 10.1016/j.drugpo.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 35.Chan J., Schwartz J., Kaba F. Outcomes of hepatitis C virus treatment in the New York City jail population: successes and challenges facing scale up of care. Open Forum Infect Dis. 2020 doi: 10.1093/ofid/ofaa263. (accepted, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocour A., Greene S.K., Laraque F., Winters A. Estimating the prevalence of chronic hepatitis C virus infection in New York City, 2015. Epidemiol Infect. 2018;146(12):1537–1542. doi: 10.1017/S095026881800170X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.