Highlights
-
•
The primary treatment for stage III achalasia is esophagectomy which is an invasive procedure.
-
•
Here a patient with stage III achalasia in whom a laparoscopic esophago-gastrostomy was successfully performed.
-
•
Laparoscopic esophagogastrostomy is less invasive, less morbid and has less complications than esophagectomy.
-
•
The evaluation of long term results of laparoscopic esophagogastrostomy helps to consider it as an effective minimally invasive procedure alternative to esophagectomy.
Keywords: Achalasia cardia, Laparoscopic esophago-gastrostomy, Endoscopy, Heller myotomy, Dysphagia
Abstract
Introduction
Achalasia is a rare primary motor disorder of the esophagus presenting with a classical triad of symptoms comprising dysphagia, regurgitation and weight loss. It is diagnosed from esophagogram which needs medical and surgical intervention.
Presentation of case
A 63-year-old woman with dysphagia was admitted to our hospital. Endoscopy revealed a dilated distal and middle oesophagus with constriction of GE junction. Barium swallow revealed narrowing of GE junction and gross dilatation of oesophagus, thus diagnosed Stage III achalasia. It was treated with a laparoscopic oesophagogastrostomy using five-port technique. The gastrohepatic omentum was opened. Followed by division of the gastrophrenic attachments over the anterior aspect of the left crus. Then anterior wall of stomach was incised using a cautery. Endostapler was introduced through the gastrostomy, one blade introduced at the fundus and other at the lower end of esophagus, all confirmed endoscopically. Anterior surface of lower end of esophagus was approximated with fundus of stomach by endostapler creating new Gastroesophageal junction. Port site closure was done using PDS. There were no postoperative complications. Follow-up after 32 months did not reveal any structural changes in upper GI endoscopy and the patient, on PPIs and prokinetic drugs has been free from symptoms upto date.
Discussion
The surgical treatment for stage III achalasia is a matter of controversy. Here a patient with stage III achalasia in whom laparoscopic esophago-gastrostomy was successfully performed.
Conclusion
The primary treatment for stage III achalasia is esophagectomy. Laparoscopic esophagogastrostomy which is less invasive approach represents an alternative to esophagectomy and laparoscopic Heller Myotomy.
1. Introduction
The work has been reported in line with SCARE guidelines [1]. Achalasia is a primary motility disorder of the esophagus characterized by lack of peristalsis and failure of the lower esophageal sphincter (LES) to relax appropriately in response to swallowing [2]. Although a relatively rare disease, with an estimated prevalence of new cases of 0.5–1 per 100,000 population a year and with no clear age predilection, achalasia is one of the major spastic motor disorders of the esophagus [3]. Sir Thomas Willis provided the first documented case report of achalasia in 1674, when he described his experience with esophageal dilation with a whalebone in a patient who had dysphagia and a dilated esophagus [4]. Its pathogenesis is presumed to be idiopathic or infectious neurogenic degeneration, several emotional stress, trauma, drastic weight reduction, and Chagas have also been implicated. Achalasia is also known to be a premalignant condition of esophagus. The classical triad of presenting symptoms consists of dysphagia, regurgitation and weight loss. The dysphagia that patients experience begins with liquids and progress to solids. Pneumonia, lung abscess and bronchiectasis often results from long standing achalasia. Esophagogram will show a dilated esophagus with a distal narrowing referred to as the classic bird’s beak appearance of the barium filled esophagus. Non surgical treatment includes medications like nitroglycerine, nitrates, calcium channel blockers and injection of botulinum toxin directly into LES [5]. Surgical treatment includes pneumatic dilatation, Heller’s myotomy and esophagectomy [6]. Laparoscopic Heller myotomy is now the operation of choice. Achalasia stage III requires esophagectomy to be treated successfully. Here a patient with stage III achalasia, laparoscopic oesophagogastrostomy was successfully performed with no recurrence of symptoms in two years with regular follow up.
2. Presentation of case
A 63-year-old woman was admitted to our hospital complaining of dysphagia, regurgitation of undigested food, retrosternal chest pain and weight loss of 6 kgs in 6 months. The dysphagia progressively worsened to involve both fluids and solids. No significant Drug, family, psychosocial, medical or surgical history was noted.
General physical examination revealed moderately built, poorly nourished, fair general condition, with blood pressure 100/60 mmHg and pulse rate 86 bpm. No abnormalities were detected clinically in systemic examination. Laboratory investigations showed Hb: 13.1 g/dl, RFT: urea-12 mg/dl, creatinine-0.4 mg/dl, LFT: albumin-2.6 gm/dl. USG abdomen and ECG were normal, Echocardiogram was normal with ejection fraction 58%. Endoscopy showed dilated distal and middle esophagus with esophageal ulcer, constriction of GE junction. Barium swallow revealed narrowing of GE junction, gross dilatation of oesophagus with pooling of contrast (See Fig. 1). All these investigations led to the diagnosis of Stage III achalasia.
Fig. 1.
Barium swallow showing narrowing of GE junction.
Patient was put on endoscopy guided nasojejunostomy tube for a period of two months for enteral nutrition. After two months, patient was posted for surgery. Under GA, patient was put in a supine split-legged position. A five-port technique was followed: three 5-mm and two 10-mm ports were used. A 10 mm, 30-degree laparoscope was introduced through 10-mm port placed in the supraumbilical region. All secondary ports were placed under laparoscopic visualization. The second port (5 mm), to be used for the surgeon’s right hand instruments, was placed 2 cm below the left costal margin along the mid clavicular line. The third port (10 mm) was placed in left hypochondrium along the left anterior axillary line. The fourth port (5 mm) was placed in the right hypochondrium along the mid clavicular line to be used for the surgeons left hand instruments. The fifth port (5 mm) for the liver retractor used to expose the hepatic ligament and hiatic ligament was placed in the right hypochondrium along the anterior axillary line. The gastrohepatic omentum was opened and then extended to the right and left crura to expose the esophagus circumferentially at the hiatus. The gastrophrenic attachments over the anterior aspect of the left crus were divided and this dissection was extended so as to mobilize the angle of His. Further the highest short gastric vessels were divided. The phrenoesophageal ligament was then opened at the right and the dissection between the crus and esophagus was carried anteriorly during this maneuver. Both anterior and posterior vagus nerves were identified. Followed by incision of the anterior wall of stomach using a cautery (See Fig. 2). Endostapler was introduced through the gastrostomy, one blade introduced at the fundus and other at the lower end of esophagus, all confirmed endoscopically. Anterior surface of lower end of esophagus was approximated with fundus of stomach using an endostapler, thus creating new Gastroesophageal junction. A twenty eight size abdominal drain was placed at the site of anastomosis. Hemostasis was confirmed. Gastrostomy wound was closed in two layers. Port site closure was achieved using PDS. Skin closure was performed with the aid of stapler. Patient withstood the procedure well. The operation time was 3 h. After 2 h observation in post-operative recovery, patient was shifted to ward with the instructions of nil per mouth for one day. During post operative period, patient was put on feeding jejunostomy along with intravenous analgesics, antibiotics, PPIs and fluids. No immediate post operative complications were seen. Oral liquid diet was started on 7th postoperative day after oral contrast X-ray showed rapid passage of contrast (See Fig. 3). As the patient tolerated the liquids well, she was started on semisolid food followed by solids. Patient was discharged from the hospital on post-operative day 12 with instructions to report immediately if she develops any new symptoms. Surgical gastroenterologist performed and junior resident assisted the procedure. Feeding jejunostomy tube was removed after fifteen days of surgery. The patient came back with GERD symptoms after 1 month of surgery. She was advised to take PPI (Pantoprazole 40 mg) OD and prokinetic drug (Domperidone 10 mg) TID for the same. No adverse effects of above mentioned drugs are noted till date. Endoscopic examination of upper GI did not reveal any structural changes at 32 month of follow-up. Patient has been asymptomatic with above mentioned two drugs till date. Apart from this patient is stable after surgery and leading a normal life without any significant complaints.
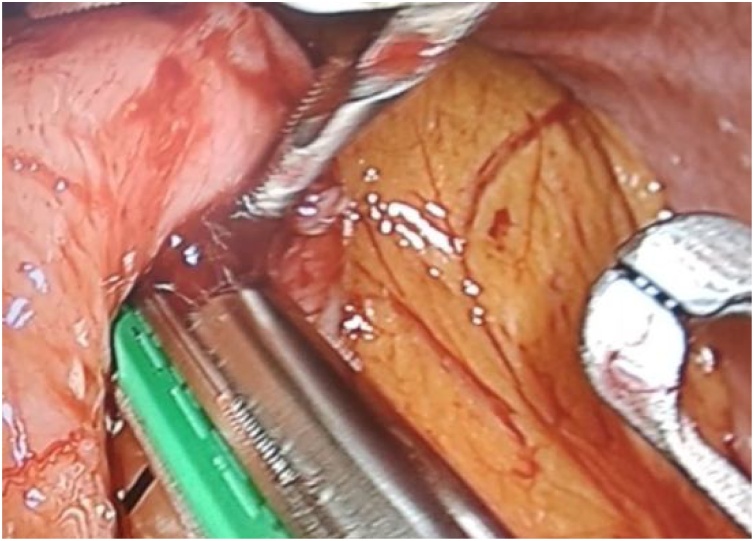
Fig. 2.
Passing of endostapler through the gastrostomy.
Fig. 3.
Barium swallow showing rapid passage of oral contrast through GE junction.
3. Discussion
The surgical treatment for stage III achalasia with markedly dilated and sigmoid-shaped esophagus is a matter of controversy. Some authors recommend esophagectomy as the primary treatment, because of the belief that Heller myotomy cannot improve dysphagia in such cases. An article [7] has been published on laparoscopic esophagogastrotomy with posterior semifundoplication to prevent gastro esophageal reflux in the management of stage III achalasia. Here we present a female patient with a stage III achalasia in whom laparoscopic esophagogastrostomy was performed. The patient presented with GERD symptoms after 1 month of surgery for which she was started on proton pump inhibitors and prokinetic drugs. The patient is asymptomatic with above mentioned drugs upto date.
4. Conclusion
The primary treatment for stage III achalasia is esophagectomy which is an invasive procedure. Laparoscopic esophagogastrostomy is less invasive, less morbid and has less complications than esophagectomy. The evaluation of long term results of laparoscopic esophagogastrostomy helps to consider it as an effective minimally invasive procedure alternative to esophagectomy.
Conflicts of interest
The author discloses no conflicts of interest.
Sources of funding
No funding for research.
Ethical approval
None.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Nagamallesh C S, contributed to design, data collection, critical editing and final approval of the version to be published.
Dharmendra. B L contributed to final approval of the version to be published.
Registration of research studies
N/A.
Guarantor
Nagamallesh C S. MS (General surgery).
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Patti M.G., Fisichella P.M., Perretta S. Impact of minimally invasive surgery on the treatment of esophageal achalasia: a decade of change. J. Am. Coll. Surg. 2003;196(698) doi: 10.1016/S1072-7515(02)01837-9. [DOI] [PubMed] [Google Scholar]
- 3.Mayberry J.F. Epidemiology and demographics of achalasia. Gastrointest. Endosc. Clin. North Am. 2001;11(235) [PubMed] [Google Scholar]
- 4.Langerman Alexander. Shackelford’s surgery of the alimentary tract. In: Yeo Charles J., editor. Epidemiology, Pathophysiology and Clinical Features of Achalasia. 7th ed. Elsevier Saunders; Philadelphia, PA: 2013. p. 349. (Chapter 28) [Google Scholar]
- 5.Courtney M., Townsend J.R., Daniel Beauchamp R., Mark Evers B., KennethMattox L., editors. The Biological Basis of Modern Surgical Practice. 19th ed. Elsevier India Private Limited; New Delhi: 2014. Section 9. Sabiston textbook of surgery; pp. 1025–1028. [Google Scholar]
- 6.Zaninotto Giovanni, Costantini Mario. Shackelford’s surgery of the alimentary tract. In: Yeo Charles J., editor. Laparoscopic Esophageal Myotomy: Techniques and Results. 7th ed. Elsevier Saunders; Philadelphia, PA: 2013. pp. 354–360. (Chapter 29) [Google Scholar]
- 7.Ablassmaier B., Jacobi C., Stoesslein R. Laparoscopic esophagogastrostomy: an alternative minimally invasive treatment for achalasia stage III. Surg. Endosc. 2002;16(216) doi: 10.1007/s004640042006. cited on 31.07.2020. [DOI] [PubMed] [Google Scholar]