Abstract
The Artery of Percheron (AOP) is an uncommon anatomic variant that provides arterial supply to the paramedian region of the thalami and bilaterally to the rostral part of the midbrain; it is a solitary arterial trunk that branches from a proximal segment of the posterior cerebral artery (PCA). Although AOP infarction results in a characteristic pattern of ischemia, namely bilateral paramedian thalamic infarct with or without midbrain involvement, it may cause diagnostic difficulties due to the variety of its clinical presentations and wide differentials, as well as its small diameter and the difficulty of obtaining visualization through diagnostic imaging. Early neuroimaging of AOP infarction and correct diagnosis are mandatory for early initiation of the appropriate treatment and better patient outcomes. This study discusses the imaging patterns and imaging differentials of AOP infarction and its clinical presentation. A 55-year-old man presented to the emergency department unconscious with Glasgow Coma Scale score of 4. Pupillary light reflex on both eyes was poorly reactive with dilatated right pupil. The patient flexed his arm and extended his leg on painful stimulus. Laboratory tests and electrocardiogram were unremarkable. Emergency cerebral CT scan and transcranial Doppler ultrasound were normal. He gradually regained consciousness with residual somnolence, ptosis, and vertical gaze palsy. Second CT scan showed bilateral paramedian thalamic areas of hypodensity, CT angiography (CTA) was unremarkable. MRI showed bilateral high-signal intensity on paramedian thalami fast spin echo T2, FLAIR, and diffusion-weighted sequences, low signal on apparent diffusion coefficient sequence. MR angiography (MRA) revealed an abnormal tiny vessel arising from the P1 segment of the left posterior cerebral artery. Imaging findings were consistent with AOP infarction. Aspirin was started, 4 hours after admission the patient regained consciousness, and gradually improved on the following days till he was discharged on the 15th day, with mild neurologic deficit. AOP must be considered whenever paramedian thalamic infarction is noted in neuroimaging. The difficulty in visualizing the AOP using diagnostic imaging is due to its small diameter, leading to the limited abilities of MRA and CTA to diagnose AOP infarction. An absence of evidence of AOP infarction in MRA or even CTA does not exclude its diagnosis. Good knowledge of the imaging characteristics of AOP infarction will help in early diagnosis and the achievement of good patient outcomes.
Keywords: Artery of Percheron, Infraction, Paramedian, Thalamus, Midbrain, Posterior cerebral artery
Introduction
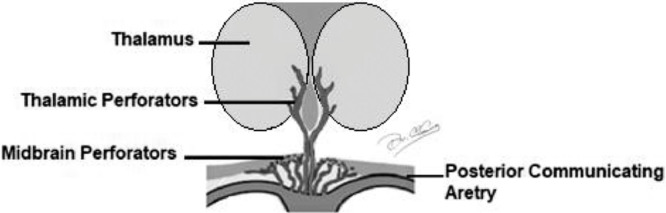
The thalamus represents a relay center for both sensory and motor mechanisms, and regulates consciousness, sleep, and alertness. The thalamus and midbrain have a complex blood supply with a great number of feeding arteries [1], [2], [3]. The arterial supply is provided by perforating branches from the posterior cerebral artery (PCA) and the posterior communicating artery (PComA) [4]. Arteries supplying the thalamus are predominantly small vessels originating from PComA and segments P1 and P2 of PCA. Although there is a significant variation and overlap, thalamic vascular supply is classically categorized into 4 territories: anterior, paramedian, inferolateral, and posterior. The anterior territory is supplied by the polar artery or thalamotuberal arteries, which arise from the PComA [2,3,5]. The inferolateral territory is supplied by the thalamogeniculate arteries, which arise from the P2 segment of the PCA [3,6]. The posterior territory is supplied by the posterior choroidal arteries, which also arise from the P2 segment of the PCA [7,8]. The paramedian territory of the thalami is supplied by the perforating thalamic arteries, also called paramedian arteries [6,8]. The paramedian thalamic arteries arise from the P1 segment of the PCA and may share a common origin with the superior paramedian mesencephalic arteries that supply the medial areas of the upper brainstem [4,8,9]. The paramedian thalamic and rostral midbrain territories are supplied by thalamoperforators, anterior branches of the P1 segments of the PCA [10]. Regarding the arterial configuration of the paramedian arteries, there are several normal variants[2,4], which have great variability with respect to number, size, and territorial contribution [8,11] (Fig. 1).
Fig 1.
Blood supply of the thalamus.
According to Percheron, there are 4 normal variants of the neurovascular anatomy of the thalami and midbrain [6]:
-
•
Variant I: it is the most common variant, where each perforating artery arises from each left and right PCA [6,7] (Fig. 2).
-
•
Variant II: it is less common, asymmetrical variant, where perforating arteries arise directly from the proximal segment of one of the PCAs [4,7] (Fig. 3).
-
•
Variant II b: in this variant, the bilateral perforating thalamic arteries arise from a single arterial trunk called the artery of Percheron, which arises from the P1 segment of one PCA. It supplies the paramedian thalami and the rostral midbrain bilaterally [3,7] (Fig. 4).
-
•
Variant III: this variant is an arcade variant with several small perforating branches arising from a single arterial arc that bridges the P1 segments of both PCAs [4,7] (Fig. 5).
Fig. 2.
Variant I, where each perforating artery arises from each left and right PComA.
Fig. 3.
Variant II a, where perforating arteries arise directly from the proximal segment of one of the PComA.
Fig. 4.
Variant II b, where bilateral perforating thalamic arteries arise from a single arterial trunk called the artery of Percheron.
Fig. 5.
Variant III, several small perforating branches arising from a single arterial arc that bridges the P1 segments of both PComA.
Case report
A 55-year-old man was presented to an emergency department after he was found unconscious in front of his home. He had no significant past medical history or long-term use of medications. On physical examination, he was unconscious with a Glasgow Coma Scale score of 4, he was afebrile (32.5°C), his pulse was 45-55 beats/min, his respiratory rate was 14 breaths/min, and his blood pressure was 100/55 mm Hg. His pupillary light reflex for both eyes was poorly reactive, with a dilatated right pupil. The patient flexed his arm and extended his leg on painful stimulus. Laboratory tests were all unremarkable; his complete blood-cell count was normal, his urea, creatinine, electrolytes, and liver enzyme levels were within the normal range, and he had normal arterial blood-gas values. A cardiovascular examination demonstrated a regular rhythm with no murmur and an electrocardiogram turned out to be normal. Neurologic examination of this patient was misleading and unhelpful because his clinical signs and symptoms were unspecific. An emergency cerebral CT scan without contrast was performed and was found to be normal, with no significant signs of acute hemorrhage or ischemia. A transcranial Doppler was performed and showed complete patency of the carotid, basilar, and vertebral arteries. The patient was referred to the intensive care unit for respiratory support and close observation. Four hours after admission, he gradually regained consciousness but remained somnolent. At that time, he was noted to have ptosis and ocular movement examination revealed vertical gaze palsy. Another CT scan was performed on the second day and showed bilateral paramedian thalamic areas of hypodensity measuring approximately 15 × 11 mm (Fig. 6), but CT angiography (CTA) was unremarkable. MRI was done, fast spin echo T2 and FLAIR sequences showed bilateral high-signal intensity on the paramedian thalami and rostral midbrain (Fig. 7). Diffusion-weighted sequence and apparent diffusion coefficient showed high-signal intensity and low-signal intensity in corresponding regions, respectively (Fig. 8). Due to previous findings, it was decided that a CT angiography (MRA) should be done; it revealed an abnormal tiny vessel arising from the P1 segment of the left PCA, consistent with AOP (Fig. 9). Treatment with aspirin was started, and a hypercoagulation workup was performed; the prothrombin and partial thromboplastin times were normal. The factor II, factor V, antithrombin III, and C and S proteins were within normal limits. In the following days, the patient's clinical status gradually improved; he was then transferred to the regular nursing ward, where he received physical therapy. By the 7th day of hospitalization, he was awake and alert but still suffered from a few neurologic deficits, such as mild memory impairment, slight vertical gaze palsy, and ptosis. On the 15th day, the patient was discharged with outpatient follow-ups and aspirin to be continued at home.
Fig. 6.

Noncontrast CT scan image showing bilateral paramedian thalamic areas of hypodensity measuring about 15 × 11 mm (white arrows).
Fig. 7.
Axial MRI images showing bilateral high-signal intensity on paramedian thalami in (A) fast spin echo T2 sequence (white arrows) and (B) FLAIR sequence (black arrows).
Fig. 8.
(A) DW axial image demonstrates bilateral paramedian thalamic hyperintense signal due to restricted diffusion (arrows), and (B) apparent diffusion coefficient demonstrates hypointense signal (arrows).
Fig. 9.

MRA of the brain showed single artery arising from P1 segment of the left PSA (white arrow).
Discussion
AOP is a rare vascular variant in which bilateral perforating thalamic arteries branching from a single arterial trunk arises from the P1 segment of 1 PCA and supplies both paramedian thalami. The specific prevalence of AOP is not identified, it has been roughly estimated that it may occur in one-third of the population, but due to its small diameter it is difficult to visualize; it may remain unrecognized [8,12,13]. If occluded, the AOP is the only variant that results in bilateral paramedian thalamic infarcts, with or without midbrain involvement [8,14,15]. Although rare, the AOP has been demonstrated on MRA before [15].
Although thalamic infarcts were reported long ago, they remain a rare presentation of stroke and account for only 11% of all vertebrobasilar infarcts [16]. The complicated anatomical structure of the thalamus and its vascular variability are responsible for the diversity of the clinical features that show up due its damage by ischemic infarctions; moreover, these clinical features may involve the midbrain due the vascular overlap. Paramedian thalamic infarction associated with midbrain due to occlusion of AOP is difficult to diagnose clinically due to the variability involvement of thalamus and midbrain, and ambiguous clinical presentations [9]. Bilateral involvement has been reported in a limited number of cases and results from a combination of predisposing factors and anatomic variations [8]. Two large studies have been done about the prevalence of AOP infarction among all ischemic stroke patients, they reported that it occurs in a prevalence of 0.1% and 0.3% [17,18]. Another study done by Hiratsuka et al. concluded that about 20, 5% of the study sample had an AOP [19]. An ischemic stroke in the territory of an AOP usually presents with 3 main symptoms that are found in patients with bilateral paramedian thalamic strokes [6,8,11]:
-
•
Vertical gaze palsy
-
•
Memory impairment
-
•
Coma
The most frequent symptom is impaired consciousness and it may present as drowsiness rising to coma [20]. Bilateral paramedian thalamic strokes may also be associated with rostral midbrain lesions leading to "thalamopeduncular" syndrome, which is characterized by [8,11]:
-
•
Hemiplegia
-
•
Cerebellar ataxia
-
•
Movement disorders
-
•
Oculomotor disturbances
Disrupted connection with the hippocampus may lead to impaired memory and confabulation. “Thalamic dementia” (behavioral and cognitive consequences of thalamic involvement) may be a late complication [8,10,21]. It has been reported that seizure may be the initial manifestation as colonic movements [22,23]. Psychiatric symptoms could manifest as personality changes, loss of initiative, delusional jealousy, hypersexuality, disinhibition, emotional lability, or delirium [13,24,25].
The most common causes of bilateral paramedian thalamic stroke are small artery occlusion and cardio embolism [26,27]. Risk factors include hypertension, diabetes mellitus, atrial fibrillation, systemic hypotension, vertebral artery dissection, inflammation, neoplasm, coagulopathy and basilar apex aneurysms [28].
Imaging patterns of AOP infarction
Diagnosing an artery of Percheron infarction is critical for directing the appropriate time sensitive management and preventing additional unnecessary procedures [8,29]. The diagnosis of AOP infarction is uncommon, because of its variable anatomical presence and due to variation of P1 segment size. Diffusion-weighted imaging (DWI) and FLAIR MRI sequences are the examinations of choice for early diagnosis of AOP infarction. On MRI infarction of the AOP appears as hyperintense signal on T2 and FLAIR sequences and hypointense signal on T1sequence, restricted bilateral ventromedial thalamic regions, and high-signal intensity on DWI and hypointensity on apparent diffusion coefficient images indicating diffusion restriction, which is consistent with infarction.
On MRA of the brain AOP appears as a single common artery arising from P1 segment of one PCA and divides into 2 branches distally supplying bilateral thalami.
MRI normally allows visualization of the initial infarct in cases of acute cerebral ischemia and is usually used in stroke centers as the primary or early secondly imaging modality [6]. Early diagnosis is best made by a DWI sequence [29].
On CT infarction of AOP appears as a region of decreased attenuation representing cytotoxic edema in bilateral paramedian thalamic region. CT brain does not always confirm AOP infarcts, many studies report normal imaging results in early CT and MR (DWI) brain scan in symptomatic and even comatose patients with AOP ischemic infarction [9]. The absence of evidence of AOP in CTA or MRA does not exclude its presence [30]. It is recommended to repeat CT or MRI at a later stage [6].
Imaging differentials of AOP infarction
Many differentials are supposed to be kept in mind in case of bilateral paramedian thalamic lesions. Top of the basilar syndrome, in which bilateral thalamic infarct occur due to embolic occlusion of posterior cerebral, superior cerebellar and pontine arteries is one of the differentials. Patients with the top of the basilar syndrome show a classic triad includes impaired consciousness, long tract neurologic signs besides complex ocular symptoms. Venous thrombosis leading to occlusion of internal cerebral veins such as Vein of Galen and Straight Sinus can cause isolated bilateral thalamic infarct. Patients with cerebral venous thrombosis may present with symptoms of increased intracranial pressure such as headache, papilledema, vomiting, convulsions, aphasia, and focal neurologic deficits. Chronic hypertensive encephalopathy in rare cases may lead to bilateral thalamic lacunar infarcts and microbleedings.
Nonvascular differentials may also be considered in case of bilateral paramedian thalamic lesions, such as inflammatory conditions including tuberculosis, malaria, meningioencephalitis, and viral infections including Flavi virus and West Nile Virus encephalitis, in such conditions patient history, specific imaging characteristics and the presence or absence of lesions outside of the thalami aid in narrowing the differential diagnosis [28]. Neoplastic conditions such as bilateral thalamic Glioma, a rare neoplastic condition causes bilateral thalamic expansion and hydrocephalus; it appears as hyperintense signal on T2 and FLAIR sequences, which is similar to bilateral thalamic infarcts, but absence of restricted diffusion and correlation with clinical symptoms, will lead to exclusion of infarction and lead to the correct diagnosis. Demyelinating diseases such as Acute Demyelinating Encephalomyelitis and Multiple Sclerosis may cause bilateral thalamic lesions that appear hyperintense in T2‐weighted images and enhance after administration of gadolinium. Extrapontine myelinolysis, due to rapid correction of hypernatremia, in patients with diabetes, chronic renal diseases and inappropriate antidiuretic hormone syndrome, can rarely appear as bilateral thalamic lesions. In this condition, patients may present with reduced level of consciousness or even coma, spastic hemiparesis, and pseudobulbar palsy. Isolated bilateral thalamic involvement in extrapontine myelinolysis is rare. Typical lesions from extrapontine myelinolysis will involve internal and external capsules, basal ganglia such as the putamen, caudate nucleus and lateral geniculate bodies, these lesions appear hyperintense on both T2-weighted and FLAIR sequences and hypointense on T1 sequence, they possibly demonstrate restricted diffusion. Involvement of these structures beside bilateral thalamic lesions my help in the differentiation of extrapontine myelinolysis from bilateral thalamic infarcts.
Spongiform encephalopathy due to the presence of prion protein such as Creutzfeldt-Jakob disease could be one of the differentials; it is characterized by neurologic dysfunction, rapid progressive dementia, and myoclonus. Creutzfeldt-Jakob lesions appear as hyperintense lesions on T2-weighted and FLAIR sequences in bilateral thalamic region, putamen, caudate nuclei, periaqueductal region, occipital cortex, and white matter. These findings may be associated with cerebral atrophy.
Thiamine (vitamin B1) deficiency due to malnutrition, malabsorption, or chronic alcohol consumption, will lead to Wernicke’s encephalopathy which is characterized by altered level of consciousness, ataxia, ocular dysfunction, and anterograde amnesia. It may appear hyperintense on both T2-weighted and FLAIR sequences and hypointense on T1-weighted sequence with contrast enhancement within the medial thalami. These thalamic lesions may show restricted diffusion similar to infarction. However, similar MR signals appear around the third ventricle, periaqueductal region, mamillary bodies and tectal plate. MRI findings, patient’s history and physical examination are crucial to differentiate between Wernicke’s encephalopathy and bilateral thalamic infarctions.
Metabolic disorder such as Leigh Syndrome (mitrochondropathy), Gangliosidoses (Lysosomal disorders), Krabbe's disease, and Wilson's disease may cause bilateral thalamic lesions. In Wilson's disease, a rare autosomal recessive metabolic disorder of copper metabolism affecting multiple systems, hyperintense lesions are seen in T2 and FLAIR sequences and hypointense on T1-weighted sequence. Clinically, symptoms of Wilson’s disease are usually detected in young adults and may include dysarthria, dystonia, tremors, ataxia, Parkinsonism, and psychiatric manifestations [31]. Paramagnetic properties of deposited copper will lead to the characteristic MRI signals of Wilson's disease which is seen in the lentiform nucleus, thalami, globus pallidus, caudate nuclei, putamen, beside atrophy of the caudate nuclei, and brainstem. Extrathalamic changes seen on MRI as well as patient’s history and clinical findings will exclude Wilson's disease. Fahr's disease is another differential of bilateral thalamic lesions; it is a rare, genetically dominant, inherited neurologic disorder, characterized by abnormal deposits of calcium in special areas of the brain, such as caudate and dentate nuclei, putamen, globus pallidus, and thalami (Tables 1 and 2).
Table 1.
| Differentials | Nonacute presentations |
|---|---|
| Vascular | Chronic hypertensive encephalopathy leading to lacunar infarcts/microbleeds |
| Tumors | Astrocytoma, glioblastoma multiforme, germinoma, lymphomas (1% of central nervous system tumors) |
| Congenital metabolic syndrome | Leigh syndrome (mitrochondropathy), Gangliosidoses (Lysosomal disorders), Krabbe's disease, Wilson's Disease |
| Cerebrovascular Ferrocalcinosis | Fahr's disease |
Table 2.
| Differentials | Acute/subacute presentations |
|---|---|
| Arterial occlusion | Stroke-infarct-top of basilar artery syndrome, PoA occlusion |
| Venous thrombosis | Cerebral venous thrombosis such as vein of Galen or straight sinus |
| Viral infection | Flavi virus infections, Japanese encephalitis and West Nile Virus encephalitis) |
| Other infections | Tuberculous, meningioencephalitis, fungal infection, malaria, toxoplasmosis |
| Demyelination | Acute demyelinating encephalomyelitis, multiple sclerosis |
| Spongiform encephalopathy | Variant Creutzfeldt-Jakob disease, Creutzfeldt-Jakob disease |
| Thiamine deficiency | Wernicke's encephalopathy |
| Hypoxic injury | Profound hypoxia of the newborn |
Conclusion
Although it is a rare anatomical variant, AOP must be considered whenever paramedian thalamic infarction is noted in neuroimaging. Occlusion of AOP results in complex clinical manifestations, varying from motor dysfunction to sensory symptoms and behavioral abnormalities, beside altered mental status, cognitive impairment and oculomotor defect, this variety of clinical manifestations will complicate diagnosis of AOP infarction. In case of bilateral thalamic infarction, many differentials must be kept in mind such as top of the basilar artery syndrome, cerebral venous thrombosis, and many nonvascular causes. Difficulty of visualization of AOP by diagnostic imaging is due to its small diameter leading to limited abilities of MRA and CTA in diagnosing AOP infarction. Absence of evidence of AOP infarction in MRA or even CTA does not exclude its diagnosis. Good knowledge of the characteristic pattern of ischemia in case of AOP infarction and well understanding of the variety of its clinical presentation will help in early diagnosis, optimal therapy, and good patient outcome.
Learning points
-
•
AOP is an uncommon anatomic variant that provides arterial supply to the paramedian region of thalami and the rostral part of midbrain bilaterally; it is a solitary arterial trunk that branches from a proximal segment of PCA.
-
•
Although Infarction of AOP results in a characteristic pattern of ischemia, bilateral paramedian thalamic infarct with or without midbrain involvement, it may cause diagnostic difficulties due to the verity of its clinical presentations and wide differentials, beside its small diameter and difficult visualization by diagnostic imaging.
-
•
Early neuroimaging of AOP infarction and correct diagnosis is mandatory for early initiation of appropriate treatment and better patient outcomes.
Imam Abdulrahman Bin Faisal University consent for a case report
Dear respected participant
You are requested to take your condition as a part in this case report.
This form contains information that will help you decide whether to be part of this study.
The purpose of the study is to discuss the imaging patterns and imaging differentials of your condition.
It is a rare medical condition known as Artery of Percheron infarction. The direct benefits of your participation are to improve the knowledge about imaging characteristics of AOP infarction, which will help in early diagnosis of similar cases and achievement of good patient outcomes.
Taking part in this research project is voluntary. You do not have to participate. Please take time to read this entire form and ask questions before deciding whether to take part in this research project.
In this case report, we will never disclose any information that may identify you, even by a court subpoena, in any federal, state, or local civil, criminal, administrative, legislative, or other proceedings. We will disclose your information for any purpose to which you have consented, as described in this informed consent document.
The results of this study will be published in an article or presentation but will not include any information that would let others know who you are. We may use or share this case study information for future research studies, which will not ask for your additional informed consent for these studies.
If you have questions about your rights as a research participant, or wish to obtain information, ask questions, or discuss any concerns about this study, please contact the principal investigator.
Email: 
Phone: 
By signing this document, you are agreeing to be in this study. Make sure you understand what the study is about before you sign. I will give you a copy of this document for your records and I will keep a copy with the study records. If you have any questions about the study after you sign this document, you can contact the study team.
I understand what the study is about, and my questions so far have been answered. I agree to take part in this study.
Name: 
Signature: 
Date of Signature (dd/mm/yy): 
Footnotes
Competing interests: The author has no affiliations with or involvement in any organization or entity with any financial interest, or nonfinancial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Lamot U, Ribaric I, Popovic KS. Artery of Percheron infarction: review of literature with a case report. Radiol Oncol. 2015;49(2):141–146. doi: 10.2478/raon-2014-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krampla W, Schmidbauer B, Hruby W. Ischaemic stroke of the artery of Percheron (2007: 10b) Eur Radiol. 2008;18(1):192–194. doi: 10.1007/s00330-007-0615-0. [DOI] [PubMed] [Google Scholar]
- 3.Matheus MG, Castillo M. Imaging of acute bilateral paramedian thalamic and mesencephalic infarcts. Am J Neuroradiol. 2003;24(10):2005–2008. [PMC free article] [PubMed] [Google Scholar]
- 4.Godani M, Auci A, Torri T, Jensen S, Del Sette M. Coma with vertical gaze palsy: relevance of angio-CT in acute Percheron artery syndrome. Case Rep Neurol. 2010;2(2):74–79. doi: 10.1159/000315835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamot U, Popovic KS, Ribarič I, Vrabec M, Lovric D, editors. Artery of Percheron: basic knowledge and tips on how to diagnose an ischemic infarction of this usually overlooked neurovascular variant in emergency settings, Eur Congress Radiol. 2014. [Google Scholar]
- 6.Cassourret G, Prunet B, Sbardella F, Bordes J, Maurin O, Boret H. Ischemic stroke of the artery of Percheron with normal initial MRI: a case report. Case Rep Med. 2010;2010(425734) doi: 10.1155/2010/425734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin OSM, Zangana HM, Hussein EMH, Ameen NA. Bilateral infarction of paramedian thalami: a report of two cases of artery of Percheron occlusion and review of the literature. Case Rep. 2011;2011 doi: 10.1136/bcr.09.2010.3304. bcr0920103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzaro NA, Wright B, Castillo M, Fischbein N, Glastonbury C, Hildenbrand P. Artery of Percheron infarction: imaging patterns and clinical spectrum. Am J Neuroradiol. 2010;31(7):1283–1289. doi: 10.3174/ajnr.A2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke. 2004;35(12):2826–2831. doi: 10.1161/01.STR.0000147039.49252.2f. [DOI] [PubMed] [Google Scholar]
- 10.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 11.Perren F, Clarke S, Bogousslavsky J. The syndrome of combined polar and paramedian thalamic infarction. Arch Neurol. 2005;62(8):1212–1216. doi: 10.1001/archneur.62.8.1212. [DOI] [PubMed] [Google Scholar]
- 12.Percheron G. Arteries of the human thalamus. II. Arteries and paramedian thalamic territory of the communicating basilar artery. Revue Neurol. 1976;132(5):309–324. [PubMed] [Google Scholar]
- 13.López-Serna R, González-Carmona P, López-Martínez M. Bilateral thalamic stroke due to occlusion of the artery of Percheron in a patient with patent foramen ovale: a case report. J Med Case Rep. 2009;3(1):7392. doi: 10.4076/1752-1947-3-7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teoh HL, Ahmad A, Yeo LL, Hsu E, Chan BP, Sharma VK. Bilateral thalamic infarctions due to occlusion of artery of Percheron. J Neurol Sci. 2010;293(1-2):110–111. doi: 10.1016/j.jns.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19(9):1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 16.Ben SL, Jemaa H, Benammou S, Tlili-Graiess K. Occlusion of the artery of Percheron: clinical and neuroimaging correlation. J Neuroradiol. 2008;35(4):244. doi: 10.1016/j.neurad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Kumral E, Evyapan D, Balkır K, Kutluhan S. Bilateral thalamic infarction: clinical, etiological and MRI correlates. Acta Neurol Scand. 2001;103(1):35–42. doi: 10.1034/j.1600-0404.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 18.Evaluation for detectability of the paramedian artery using 3D TOF MRA on 3tesla MRI2012. Hiratsuka Y, Kikuchi K, Miki H, Mochizuki T, editors. Evaluation for detectability of the paramedian artery using 3D TOF MRA on 3tesla MRI2012Eur Congress Radiol. 2012 [Google Scholar]
- 19.Arauz A, Patiño-Rodríguez HM, Vargas-González JC, Arguelles-Morales N, Silos H, Ruiz-Franco A. Clinical spectrum of artery of Percheron infarct: clinical–radiological correlations. J Stroke Cerebrovasc Dis. 2014;23(5):1083–1088. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Alaicescu M, Nistorescu A, Popa G, Botezatu L, Dediu A, Ifrim E. Anatomic, clinical and pathophysiologic correlates in acute bilateral paramedian thalamic infarcts. Rev Romana Neurol. 2007;6:88–91. [Google Scholar]
- 21.Wang J, Fu X, Jiang C, Liu H, Zhao Y, Han W. Bilateral paramedian thalamic infarction initially presenting as a convulsive seizure. Case Rep Neurol Med. 2013;2013 doi: 10.1155/2013/704952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashiro K, Furuya T, Noda K, Urabe T, Hattori N, Okuma Y. Convulsive movements in bilateral paramedian Thalamic and Midbrain infarction. Case Rep Neurol. 2011;3(3):289–293. doi: 10.1159/000334754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann DM, Siccoli M, Brugger P, Wachter K, Mathis J, Achermann P. Evolution of neurological, neuropsychological and sleep-wake disturbances after paramedian thalamic stroke. Stroke. 2008;39(1):62–68. doi: 10.1161/STROKEAHA.107.494955. [DOI] [PubMed] [Google Scholar]
- 24.Aravinthan S, Muthalagan J, Murugappan M, Ponnudurai R. Bilateral thalamic infarct with neuropsychiatric manifestations. Indian J Psychiatry. 2003;45(2):59. [PMC free article] [PubMed] [Google Scholar]
- 25.Mutarelli EG, Omuro AM, Adoni T. Hypersexuality following bilateral thalamic infarction: case report. Arquivos de Neuro-Psiquiatria. 2006;64(1):146–148. doi: 10.1590/s0004-282x2006000100032. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal N, Tolia A, Hansberry DR, Duffis EJ, Barrese JC, Gandhi CD. Current differential diagnoses and treatment options of vascular occlusions presenting as bilateral thalamic infarcts: a review of the literature. J Neurointerven Surg. 2013;5(5):419–425. doi: 10.1136/neurintsurg-2012-010352. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal N, Chaudhari A, Hansberry DR, Prestigiacomo CJ. Redefining thalamic vascularization vicariously through Gerald Percheron: a historical vignette. World Neurosurg. 2014;81(1):198–201. doi: 10.1016/j.wneu.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Smith AB, Smirniotopoulos JG, Rushing EJ, Goldstein SJ. Bilateral Thalamic lesions. Am J Roentgenol. 2009;192(2):W53–W62. doi: 10.2214/AJR.08.1585. [DOI] [PubMed] [Google Scholar]
- 29.Shea YF, Lin OY, Chang RS, Luk JK. Artery of Percheron infarction. Hong Kong Med J. 2012;18(5):446. e1-2. [PubMed] [Google Scholar]
- 30.Chang YM, Fan YK. Artery of Percheron occlusion in an elderly male: a case report. J Clin Med Res. 2015;7(2):126–128. doi: 10.14740/jocmr2009w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegde AN, Mohan S, Lath N, Lim CC. Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics. 2011;31(1):5–30. doi: 10.1148/rg.311105041. [DOI] [PubMed] [Google Scholar]
- 32.Linn J, Hoffmann LA, Danek A, Brückmann H. Differential diagnosis of bilateral thalamic lesions. Rofo. 2007;179(3):234–245. doi: 10.1055/s-2007-962857. [DOI] [PubMed] [Google Scholar]