Abstract
As a rare hereditary disease, congenital nephrogenic diabetes insipidus (NDI) is clinically characterized by polyuria with hyposthenuria and polydipsia. NDI results from collecting duct principal cell hyporesponsiveness or insensitivity to the antidiuretic action of arginine vasopressin (AVP). The principal cell-specific water channel aquaporin-2 (AQP2) plays an essential role in water reabsorption along osmotic gradients. The capacity to accumulate AQP2 in the apical plasma membrane in response to decreased fluid volume or increased plasma osmolality is critically regulated by the antidiuretic hormone AVP and its receptor 2 (AVPR2). Mutations in AVPR2 result in X-linked recessive NDI, the most common form of inherited NDI. Genetic defects in AQP2 cause autosomal recessive or dominant NDI. In this review, we provide an updated overview of the genetic and molecular mechanisms of congenital NDI, with a focus on the potential disease-causing mutations in AVPR2 and AQP2, the molecular defects in the AVPR2 and AQP2 mutants, post-translational modifications (i.e., phosphorylation, ubiquitination, and glycosylation) and various protein-protein interactions that regulate phosphorylation, ubiquitination, tetramerization, trafficking, stability, and degradation of AQP2.
Keywords: nephrogenic diabetes insipidus, AVPR2, AQP2, mutation, trafficking, phosphorylation, ubiquitination, glycosylation, protein-protein interaction
1. Introduction
There are two major forms of diabetes insipidus: central diabetes insipidus (CDI) and nephrogenic diabetes insipidus (NDI). CDI is caused by the failure of the hypothalamic-pituitary axis to produce or release the appropriate physiologic amounts of vasopressin. NDI results from vasopressin hyporesponsiveness or insensitivity of the collecting duct principal cells, which are responsible for water reabsorption through coordinated actions of apical AQP2 and basolateral AQP3 and AQP4. In both CDI and NDI, the kidney is unable to concentrate urine, leading to the production of excess urine or polyuria. NDI can be further classified into acquired and congenital forms. Compared to congenital NDI, acquired NDI is significantly more prevalent and occurs in various pathophysiologic conditions. The primary cause of acquired NDI is chronic administration of lithium, which is frequently used to treat a common chronic psychiatric disease, bipolar disorder. NDI develops as a result of long-term lithium therapy, although obstruction of the urinary tract, hypercalcemia, hypokalemia, and protein malnutrition can also cause NDI [1]. Congenital NDI is a genetic disorder, which we will discuss in detail below.
2. Signs and Symptoms
Symptoms often develop in patients with congenital NDI quickly after birth and the majority of newborns are diagnosed during the first year of life. The primary symptoms include polyuria, polydipsia, and nocturia. In addition, patients may exhibit a variety of other symptoms such as unexplained fever, poor feeding, constipation, diarrhea, vomiting, lethargy, irritability, and retching. Therefore, some infants may not be able to grow or gain weight at the normal rate. Adult patients gradually develop orthostatic hypotension, megacystis hydronephrosis, and hydroureter because of persistent polyuria (reviewed in [2]).
3. Genetic Basis of Congenital NDI
There are two forms of congenital NDI: primary inherited NDI and secondary inherited NDI. The vast majority of the primary inherited NDI cases (90%) show an X-linked recessive mode of inheritance. This disorder results from mutations in the antidiuretic hormone arginine vasopressin receptor 2 gene (AVPR2) on the long arm of the X chromosome (Xq28) [3]. To date, a total of 287 potential disease-causing mutations in AVPR2 have been described, with 177 (62%) being missense mutations (The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff; http://www.hgmd.cf.ac.uk). The second major type involves small deletions, with an incidence of approximately 18%. Splicing mutations, small insertions, and gross deletions have been also reported, although they occur less frequently.
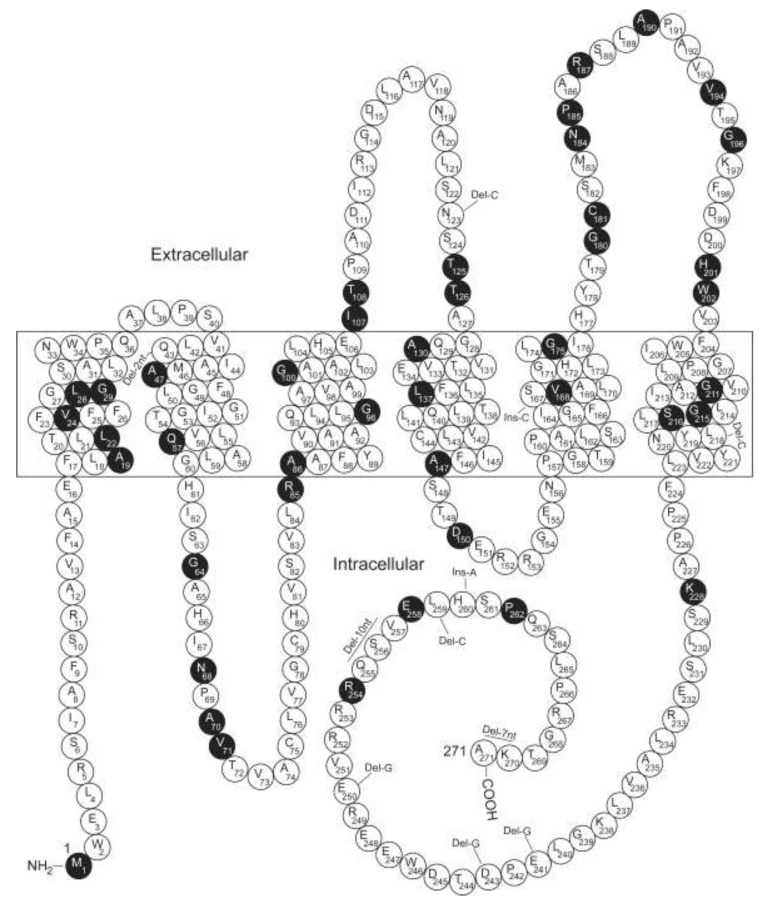
About 10% and 1% of cases of the primary inherited NDI exhibit an autosomal recessive or dominant mode of inheritance, respectively. In both scenarios, the disorder is associated with defects in AQP2 on the long arm of chromosome 12 (12q13). The first such NDI patient described was a male, who was determined to be a compound heterozygote for two AQP2 missense mutations (R187C and S217P) [4]. Since then, at least 70 putative disease-causing AQP2 mutations have been reported in 74 families (Figure 1 and Table 1). Among them are 54 missense mutations, 4 splicing mutations, 9 small deletions, 1 gross mutation, and 2 small insertions (The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff; http://www.hgmd.cf.ac.uk). In brief, inactivation of AVPR2 or AQP2 is the genetic basis of congenital NDI.
Figure 1.
Diagram of the AQP2 protein and the locations of the affected residues by the putative disease-causing AQP2 mutations. AQP2 is depicted with six transmembrane domains. The extracellular, transmembrane and cytoplasmic domains are represented as reported [4]. Solid symbols indicate the affected residues because of the mutations (Table 1).
Table 1.
Potential disease-causing mutations in AQP2.
| No. of Family | Accession Number | Name of Mutation | Domain | Nucleotide Change | Predicted Change | Ref | |
|---|---|---|---|---|---|---|---|
| Mis-sense | 1 | CM086773 | M1I | NH2 | ATG-to-ATT | Met-to-Ile | [5] |
| 1 | CM137423 | A19V | TMI | GCC-to-GTC | Ala-to-Val | [6] | |
| 1 | CM970097 | L22V | TMI | CTC-to-GTC | Leu-to-Val | [7] | |
| 1 | CM106380 | V24A | TMI | GTC-to-GCC | Val-to-Ala | [8] | |
| 1 | CM024085 | L28P | TMI | CTC-to-CCC | Leu-to-Pro | [9] | |
| 1 | CM086774 | G29S | TM1 | GGC-to-AGC | Gly-to-Ser | [5] | |
| 2 | CM024086 | A47V | TMII | GCG-to-GTG | Ala-to-Val | [9] | |
| 2 | CM021246 | Q57P | TMII | CAG-to-CCG | Glu-to-Pro | [10] | |
| 1 | CM940082 | G64R | CII | GGG-to-AGG | Gly-to-Arg | [11] | |
| 1 | CM970098 | N68S | CII | AAC-to-AGC | Asn-to-Ser | [12] | |
| 1 | CM056523 | A70D | CII | GCC-to-GAC | Ala-to-Asp | [13] | |
| 2 | CM950080 | V71M | CII | GTG-to-ATG | Val-to-Met | [14] | |
| 1 | CM153550 | A86V | TMIII | GCC-GTC | Ala-to-Val | [15] | |
| 1 | CM138317 | G96Q | TMIII | GGG-GAG | Gly-to-Glu | [16] | |
| 2 | CM021247 | G100V | TMIII | GGA-to-GTA | Gly-to-Val | [10] | |
| 1 | CM062427 | G100R | TMIII | GGA-to-AGA | Gly-to-Arg | [17] | |
| 1 | CM068766 | I107N | EII | ATC-to-AAC | Ile-to-Asn | [18] | |
| 1 | CM146787 | T108M | EII | ACG-ATG | Thr-to Met | [19] | |
| 1 | CM980100 | T125M | EII | ACG-to-ATG | Thr-to-Met | [20] | |
| 1 | CM970100 | T126M | EII | ACG-to-ATG | Thr-to-Met | [12] | |
| 1 | CM1411333 | A130V | TMIV | GCG-to-GTG | Ala-to-Val | [21] | |
| 1 | CM1210558 | L137P | TMIV | CTG-to-CCG | Leu-to-Pro | [22] | |
| 1 | CM970101 | A147T | TMIV | GCC-to-ACC | Ala-to-Thr | [12] | |
| 2 | CM071560 | D150E | ICII | GAT-to-GAA | Asp-to-Glu | [23] | |
| 1 | CM973106 | V168M | TMV | GTG-to-ATG | Val-to-Met | [24] | |
| 1 | CM980101 | G175R | TMV | GGG-to-AGG | Gly-to-Arg | [20] | |
| 1 | CM062428 | G180S | EIII | GGC-to-AGC | Gly-to-Ser | [17] | |
| 1 | CM970102 | C181W | EIII | TGC-to-TGG | Cys-to-Trp | [7] | |
| 1 | CM1411334 | N184H | EIII | AAT-to-CAT | Asn-to-His | [21] | |
| 1 | CM950081 | P185A | EIII | CCT-to-GCT | Pro-to-Ala | [14] | |
| 3 | CM940083 | R187C | EIII | CGC-to-TGC | Arg-to-Cys | [11] | |
| 1 | CM056522 | R187H | EIII | CGC-to-CAC | Arg-to-His | [13] | |
| 1 | CM950082 | A190T | EIII | GCT-to-ACT | Ala-to-Thr | [14] | |
| 1 | CM024087 | V194I | EIII | GTC-to-ATC | Val-to-Ile | [9] | |
| 1 | CM087015 | G196D | EIII | GGC-to-GAC | Gly-to-Asp | [25] | |
| 1 | CM120677 | H201Y | EIII | CAC-to-TAC | His-to-Tyr | [26] | |
| 1 | CM960073 | W202C | EIII | TGG-to-TGT | Trp-to-Cys | [27] | |
| 1 | CM120678 | G211R | TMVI | GGC-to-CGC | Gly-to-Arg | [26] | |
| 1 | CM156813 | G215S | TMVI | GGC-to-AGC | Gly-to-Ser | [28] | |
| 1 | CM071561 | G215C | TMVI | GGC-to-TGC | Gly-to-Cys | [23] | |
| 2 | CM940084 | S216P | TMVI | TCC-to-CCC | Ser-to-Pro | [11] | |
| 1 | CM099886 | S216F | TMVI | TCC-to-TTC | Ser-to-Phe | [29] | |
| 1 | CM106381 | K228E | CIV | AAG-to-GAG | Lys-to-Glu | [8] | |
| 1 | CM096039 | R254Q | CIV | CGG-to-CAG | Arg-to-Gln | [30] | |
| 1 | CM056296 | R254L | CIV | CGG-to-CTG | Arg-to-Leu | [31] | |
| 1 | CM1514252 | R254W | CIV | CGG-to-TGG | Arg-to-Trp | [32] | |
| 1 | CM980102 | E258K | CIV | GAG-to-AAG | Glu-to-Lys | [33] | |
| 2 | CM950083 | P262L | CIV | CCG-to-CTG | Pro-to-Leu | [14] | |
| Non-sense | 2 | CM972978 | R85X | CII | CGA-TGA | Arg-to-Stop | [24] |
| 1 | CM970099 | G100X | TMIII | GGA-to-TGA | Gly-to-Stop | [34] | |
| Frame-shift | 1 | CD034849 | 127–128del | TMII | 2bp deletion | Post-elongation | [35] |
| 1 | CD941595 | 369delC | EII | 1bp deletion | Stop at Codon 131 | [11] | |
| 1 | CD024169 | 652delC | TMVI | 1bp deletion | Post-elongation | [9] | |
| 1 | CD014628 | 721delG | CIV | 1 bp deletion | Post-elongation | [36] | |
| 1 | CD024654 | 727delG | CIV | 1 bp deletion | Post-elongation | [37] | |
| 1 | CD137424 | 750delG | CIV | 1 bp deletion | Post-elongation | [6] | |
| 1 | CD014629 | 763–772del | CIV | 10 bp deletion | Post-elongation | [36] | |
| 1 | CD137425 | 775delC | CIV | 1 bp deletion | Post-elongation | [6] | |
| 1 | CI156810 | 501–502insC | CIV | 1 bp insertion | Post-elongation | [28] | |
| 1 | CI034768 | 779–780insA | CIV | 1 bp insertion | Post-elongation | [38] | |
| 1 | CD983834 | 812–818del | CIV | 7 bp deletion | Post-elongation | [36] | |
| Splicing site | 1 | CS1213334 | IVS2-1G>A | NA | G-to-A | NA | [14] |
| 1 | CS024161 | IVS3+1G>A | NA | G-to-A | NA | [9] | |
| 1 | CS034835 | IVS3-1G>A | NA | G-to-A | NA | [35] |
Note: All mutations listed in http://www.hgmd.cf.ac.uk as of 09/20/2020 are presented.
The secondary inherited form refers to NDI as a complication associated with inherited human diseases, such as Bartter syndrome, apparent mineralocorticoid excess, Renal Fanconi syndrome, cystic kidney disorders, familial hypomagnesemia with hypercalciuria and nephrocalcinosis, and distal renal tubular acidosis (reviewed in [39]).
4. The AVP-AVPR2-AQP2 Signaling Pathway and Beyond
The general mechanisms involved in the AVP-AVPR2-AQP2 signaling pathway have been reviewed in detail elsewhere [40,41], only a succinct overview is given here. In response to decreased plasma volume or increased plasma osmolality, AVP is generated in the hypothalamus and secreted from the pituitary gland. AVPR2 is the cognate G protein-coupled receptor for AVP. In the principal cells of the collecting duct, binding of AVP to AVPR2 triggers a signaling cascade including involvement of a number of kinases, changes in the activities of phosphatases and other enzymes, and reorganization of the cytoskeleton. Collectively, these signaling events facilitate AQP2 routing to the apical plasma membrane and inhibit endocytosis-mediated AQP2 internalization. Consequently, there is an accumulation of AQP2 in the apical plasma membrane permitting water to move down its osmotic gradient through the membrane into the interstitium and subsequently into the circulation. AVP stimulation of AVPR2 also activates the adenylyl cyclases AC3 and AC6, promoting conversion of ATP to cyclic adenosine monophosphate (cAMP) mobilizing cAMP-dependent kinases such as PKA to function in AQP2 trafficking. AQP2 translocation can also take place in a cAMP-independent manner (reviewed in [2]).
The short-term effect of AVP on AQP2 is the movement of AQP2 from the intracellular vesicles to the apical membrane. A sustained increase of circulating AVP for 24 h or more can induce a long-term effect on water reabsorption by increasing the abundance of AQP2 [42,43]. This increase is considered as a consequence of enhanced AQP2 transcription [42,44].
Apart from the AVP-AVPR2-AQP2 signaling pathway, AVP affects other targets including the urea transporter UT-A1 [45], and the epithelial sodium channel [46]. Hence, AVP selectively increases the permeabilities of the apical membrane to water, urea, and sodium.
AQP2 is also controlled by multiple endogenous signaling molecules/hormones. They modulate AQP2 trafficking primarily through G protein-coupled receptors. Important among them are purines, calcitonin, secretin, glucagon, angiotensin II, serotonin, and prostaglandins (reviewed in [47]). Various alternative receptors also appear to impact AQP2 as well (reviewed in [48]). These include the farnesoid X receptor [49], the peroxisome proliferator-activated receptor-γ [50], angiotensin II receptor type I [51], the bile acid receptor TGR5 [49], the glucocorticoid and mineralocorticoid receptors [52], the estrogen receptor-α [53], and the liver X receptor-β [48].
5. Molecular Defects of AVPR2 Mutants
Genetic defects in AVPR2 may result in: (1) improperly processed or unstable mRNA like promoter alterations, exon skipping, aberrant splicing, frame-shift or non-sense mutations, which result in truncated proteins, (2) decreased binding capacity of the receptor to AVP, or (3) mis-folding of the receptor that is retained intracellularly and incapable of routing to the basolateral membrane to bind AVP (reviewed in [54,55]). The vast majority of the AVPR2 mutations lead to mis-folding of the receptor [56]. The mis-folded receptors concentrate in the Golgi apparatus and are subsequently degraded by the ubiquitin-proteasome pathway, conferring loss-of-function phenotypes on AVPR2 [57]. Rescuing the normal function of these mis-folded proteins, therefore, may provide new therapeutically useful strategies for the management of NDI patients. The key step in any such approach is to facilitate the freeing of the trapped receptor from the intracellular compartments. Nonpeptide inhibitors could be explored to rescue the mis-folded AVPR2 variants by acting as pharmacologic chaperons rather than endocytosis antagonists [58]. Indeed, administration of the vasopressin inhibitor SR49059 significantly ameliorated the symptoms of polyuria and polydipsia without affecting other renal physiological parameters such as creatinine excretion and electrolyte measurements [59]. These findings support the concept that pharmacologic chaperons might have clinical utility in the management of some forms of congenital NDI.
6. Molecular Defects of Recessive AQP2 Mutants
Patients with congenital autosomal recessive NDI are homozygous for a mutation in AQP2 or carry two different AQP2 mutations. Currently, all AQP2 mutations causing recessive NDI are located throughout the six transmembrane domains and five connecting loops of AQP2. With a few exceptions that possess a residual water transport capacity, these mutants are non-functional [9,12,60] and are retained in the endoplasmic reticulum (ER) as evidenced by their scattered ‘reticular’ expression pattern, by their unglycosylated (29 kDa) or high mannose glycosylated (32 kDa) variants, and by their decreased stability in comparison with WT AQP2 [60,61,62]. Consistent with the recessive nature of inheritance and possibly because of their mis-folding, these AQP2 mutants exist as monomers and are incapable of heteroligomerizing with and impeding the routing and maturation of WT AQP2 in its pathway to the apical membrane. Typical examples showing all these features are the AQP2-R187C and AQP2-A190T mutants [38,60,63,64]. R187 and A190 reside in one of the two most highly conserved fragments of the major intrinsic protein family. The signature motif consisting of three invariant amino acids (Asn-Pro-Ala) in this region is positioned just proximal to the mutated R187 and A190. Both R187 and A190 themselves are also strongly conserved in the major intrinsic protein family.
7. Molecular Defects of Dominant AQP2 Mutants
Unlike recessive AQP2 mutants, the dominant AQP2 mutants are functional water channels. However, they are mis-routed to other subcellular compartments rather than to the apical membrane [33,36,37,38]. Because these AQP2 dominant mutants are correctly folded in the ER, they interact with WT-AQP2 to form heterotetramers and alter intracellular trafficking of the WT and mutant AQP2 complexes [65]. Consequently, the apical membrane of the renal collecting duct principal cells lacks sufficient levels of WT-AQP2, leading to dominant NDI. In contrast to the recessive mutants, all AQP2 mutations identified in dominant NDI are detected in the carboxyl terminus of AQP2, highlighting the crucial role of this domain in regulating the AQP2 sorting. AQP2-P262L, nevertheless, is a unique mutation, which was identified in two families with recessive NDI [64]. These patients are compound heterozygotes for AQP2-P262L together with AQP2-A190T or AQP2-R187C. Functional analyses in oocytes revealed that AQP2-P262L was a properly folded and functional aquaporin. AQP2-P262L localized to intracellular vesicles and was not retained in the ER when expressed in polarized cells. Upon co-expression, AQP2-P262L formed heteroteramers with WT-AQP2, but not with AQP2-R187C, leading to a rescued apical membrane localization of AQP2-P262L [64]. Hence, AQP2-P262L exhibited a cellular phenotype clearly distinct from other AQP2 mutants in recessive NDI. Instead, and in line with the molecular location of the mutation, it showed all the characteristics observed for AQP2 mutants in dominant NDI. Nevertheless, the mis-sorting of AQP2-P262L, unlike other AQP2 dominant mutants, is overruled by apical sorting of WT-AQP2, resulting in the carriers of the P262L mutation being asymptomatic as are those of AQP2 recessive mutations [64].
8. Regulation of AQP2 by Post-Translational Modifications
Post-translational modifications play a crucial role in signaling transduction, protein maturation and folding by altering the cellular distribution, stability, function, and binding partners of their substrate proteins. Recent studies suggest that phosphorylation, ubiquitination, and glycosylation are major post-translational modifications that regulate AQP2 function, routing, stability, and protein–protein interactions.
8.1. Phosphorylation
Bioinformatic analysis identified a number of putative phosphorylation sites in AQP2 for various kinases including protein kinases A (PKA) and casein kinase II [66], with the C-terminal S256 residue perhaps being the best characterized. As discussed above, binding of AVP to AVPR2 triggers a signaling cascade that leads to increased cAMP levels. One of the cAMP effectors is PKA. The PKA holoenzyme is a heterotetramer comprised of two inactive catalytic subunits and two regulatory subunits. Each regulatory domain binds one catalytic subunit. Upon interaction of cAMP with the PKA regulatory elements, the catalytic subunits are released from the heterotetramer, allowing them to function as active serine-threonine kinases, phosphorylating several substrates including AQP2. PKA-catalyzed AQP2 phosphorylation at S256 increases the rate of exocytosis [66]. Altering cAMP abundance and/or PKA enzymatic activity with calcitonin or prostaglandin E2 changes both AQP2 phosphorylation and trafficking [67,68]. Nevertheless, the AQP2 S256 residue can be phosphorylated and, thereby, regulated in response to vasopressin even in PKA-null cells, indicating that AVPR2-mediated AVP signaling in collecting duct cells is not entirely PKA-dependent [69].
Casein kinase II also phosphorylates S256, which is necessary for transition of AQP2 through the Golgi [70]. Vasopressin or forskolin was unable to stimulate translocation of the AQP2-S256A mutant when expressed in LLC-PK1 cells [71,72]. Since the AQP2-S256L mutation also prevents phosphorylation at S256 and the subsequent accumulation of AQP2 on the apical membrane of the collecting duct principal cells, mice homozygous for this mutation had severe urine concentration defects and developed congenital progressive hydronephrosis [73]. These studies illustrate a critical role of S256 phosphorylation in the apical targeting of AQP2 and thus water transport.
Large-scale phospho-proteomic analysis later revealed that AQP2 S261, S264, and S269 are additional AVP-mediated phosphorylation targets [74,75]. Phosphorylation at these sites also impacts AQP2 trafficking. S261 phosphorylation may stabilize ubiquitinated AQP2 and intracellular localization, since it is primarily found in the intracellular vesicles after ubiquitination and endocytosis and S261 phosphorylation is reduced in response to AVP treatment [76]. S269 phosphorylation has been detected exclusively on the plasma membrane [75], however, suggesting a role in promoting AQP2 plasma membrane targeting and/or in inhibiting AQP2 endocytosis [77]. Phosphorylation at S256 appears unnecessary for AQP2 recycling because substitution of S256 into alanine has little effect since AQP2-S256A recycles rapidly and constitutively. Nevertheless, S256 phosphorylation is essential for subsequent phosphorylation of other C-terminal serines [75] since phosphorylated forms of AQP2 at S264 and S269 are not detected in cells expressing the AQP2 S256 mutant or in kidneys from AQP2 S256 mutant mice [75].
8.2. Ubiquitination
The ubiquitin proteasome and lysosomal proteolysis pathways are two primary mechanisms for protein degradation in mammalian cells. Ubiquitination is a complex post-translational modification in which ubiquitin is covalently added to a target protein through a system consisting of E1 activation, E2 conjugation, and E3 ligation enzymes. Ubiquitination facilitates protein endocytosis and lysosomal degradation as well as impacting the interaction of the target protein with its binding partners. AQP2 possesses three potential ubiquitination sites (K228, K238, and K270) [78]. Mutagenesis analyses revealed, however, that ubiquitination occurs only at K270 whereby a K63-linked chain comprising generally two to three ubiquitin moieties is attached [78]. This modification at the plasma membrane enhances AQP2 endocytosis, routing to intraendosomal vesicles and subsequent degradation [78]. Cullin-5 is a vasopressin-activated calcium-mobilizing receptor and a member of the Cullin family of scaffold proteins of the E3 complex. It may be involved in the attachment of ubiquitin to AQP2, leading to ubiquitination, internalization, and lysosomal and/or proteosomal degradation of AQP2 after 1-desamino-8-d-arginine vasopressin withdrawal [79]. Analyses of several large-scale transcriptomic and proteomic datasets defined a panel of E3 ligases that are most likely to form complexes with AQP2, with NEDD4 and NEDD4L being the most prominent [80], although there are other required effectors. Indeed, while NEDD4 and NEDD4L mediate ubiquitination and degradation of AQP2, NDFIP is required to link NEDD4 and NEDD4L to AQP2 [81].
It appears that AQP2 phosphorylation at S256, S261, S264, S269 and ubiquitination at K270 are coordinately regulated to fine tune AQP2 intracellular distribution. Phosphorylation usually takes place as a priming event for ubiquitination which, in turn, affects protein phosphorylation by regulating kinase activities [82]. Moreover, AQP2 phosphorylation can nullify the dominant endocytic signal of K63-linked polyubiquitination. Specifically, phosphorylation at S269 and ubiquitination at K270 of AQP2 can occur simultaneously, with elevated S269 phosphorylation and lowered AQP2 endocytosis occurring in the presence of the maximal K270 polyubiquitination levels [83]. Hence, AQP2 phosphorylation is apparently able to counterbalance polyubiquitination and governs its final localization.
8.3. Glycosylation
Glycosylation is the enzymatic process in which oligosaccharides (also referred as to glycans) are attached to proteins. N-linked glycans are almost always attached to the nitrogen atom of an asparagine (Asn) side chain. This particular Asn appears as a part of the Asn-X-Ser/Thr consensus sequence, where X is any amino acid excluding proline [84]. The sequence Asn123-Ser124-Thr125 in the second extracellular loop of AQP2 is a consensus N-linked glycosylation site.
Glycosylation induces a complex effect on AQP2 function. In an AQP2-transfected Madin–Darby canine kidney cell line (clone WT10), 34% of the AQP2 molecules were glycosylated, which was reduced to 2% after treatment with the glycosylation inhibitor tunicamycin. Moreover, in tunicamycin-treated WT10 cells, all the AQP2 in the apical membrane was unglycosylated, whereas in untreated cells 30% of AQP2 in the apical membrane was glycosylated. These observations suggest that glycosylation is not involved in the routing of AQP2 in Madin–Darby canine kidney cells [85]. Analyses of the AQP2 N123Q mutant, which carries the disrupted N-linked glycosylation consensus site, revealed that this mutation still allowed for generation of the nonglycosylated 29-kDa precursor, but neither the 31-kDa nor the mature glycosylated 42-46-kDa variants. The mutant protein, however, had a shorter half-life, compared to WT-AQP2 [86]. A shorter half-life was also observed for the AQP2-S256D and AQP2-E258K mutants [86], although their glycosylation was similar to WT-AQP2 [86]. These findings indicate that glycosylation per se is not crucial for AQP2 folding and that AQP2 folding is independent of the primary calnexin/calreticulin ER quality control mechanisms [86,87]. The AQP2-T125M mutant identified in patients with recessive NDI [9,20], nevertheless, is not glycosylated due to the loss of the consensus N-linked glycosylation sequence. This mutant is not localized to the plasma membrane [9], implying that it is not correctly folded and retained in the ER, or because of other unknown mechanisms, does not translocate to the cell surface. Further analyses of the AQP2-N123Q revealed that glycosylation is neither required for tetramerization in the ER nor for trafficking from the ER to the Golgi complex. Addition of the N-linked glycan, instead, is critical for exit from the Golgi complex and routing of AQP2 to the plasma membrane [86], conflicting with what was reported in WT10 cells [85].
9. Regulation of AQP2 by Protein–Protein Interactions
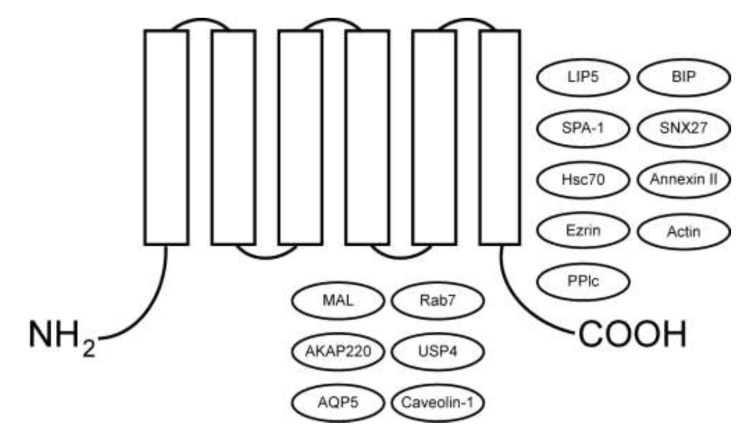
Multiple proteins interact with AQP2 and regulate AQP2 degradation, post-translational modification, and trafficking (Figure 2 and Table 2).
Figure 2.
AQP2-interacting proteins. AQP2 binding partners are indicated, as well as the location for the interaction in those cases where it has been mapped (Table 2).
Table 2.
AQP2-interacting proteins.
| Target | Interacting Protein | Proposed Function | Method Applied | Ref | ||||
|---|---|---|---|---|---|---|---|---|
| YTH | PD | IP | IHC | MS | ||||
| hAQP2 | LIP5 | Lysosomal degradation | Xa | Xa | X | X | [88] | |
| hAQP2 | Caveolin-1 | AQP2 internalization | X | X | [89] | |||
| hAQP2 | MAL | Increases apical surface expression | X | X | [90] | |||
| rAQP2 | SPA-1 | Regulates trafficking to the apical membrane | Xa | X | X | X | [91,92] | |
| rAQP2 | Hsc70 | Co-localized in the apical membrane: involved in trafficking | Xac | X | X | X | [93,94] | |
| hAQP2 | AKAP220 | Phosphorylation of AQP2 triggering trafficking | Xd | Xb | [95] | |||
| rAQP2 | Ezrin | Endocytosis | Xd | Xd | X | [96] | ||
| hAQP2 | hAQP5 | Impairing AQP2 membrane localization | X | X | [97] | |||
| rAQP2 | Annexin II | Phosphorylation dependent binding to the C-terminus | X | X | [94] | |||
| rAQP2 | PP1c | Phosphorylation dependent binding to the C-terminus | Xd | X | X | [94] | ||
| rAQP2 | Bip | Phosphorylation dependent binding to the C-terminus | Xd | X | X | [94] | ||
| rAQP2 | Actin | Trafficking | X | X | [94,98] | |||
| mAQP2 | Rab7 | Trafficking | X | X | X | [94,99] | ||
| mAQP2 | USP4 | Deubiquitination | X | X | [100] | |||
| rAQP2 | SNX27 | Lysosomal degradation | X | X | X | [101] | ||
Note: a: using AQP2 C-terminus; b: using rat kidney; c: using human kidney cDNA library; d: using full-length AQP2. YTH: yeast two-hybrid; PD: glutathione S-transferase pull-down; IP: immunoprecipitation; IHC: immunohistochemistry; MS: mass spectroscopy.
One of the AQP2-binding proteins is lysosomal trafficking regulator interacting protein 5 (LIP5) [88]. LIP5 functions in multivesicular body formation. The complex formation between LIP5 and the AQP2 C-terminal tail promotes AQP2 lysosomal degradation and occurs regardless of the state of phosphorylation at S256 and ubiquitination at K270 [88]. This interaction was identified by yeast two-hybrid and confirmed by glutathione S-transferase pull-down, co-immunoprecipitation, and colocalization assays. Knockdown of LIP5 in mpkCCD cells attenuated phorbol ester-induced AQP2 degradation [88]. LIP5, therefore, appears important in the regulation of AQP2 degradation, possibly by inhibiting the formation of late endosomes [88].
Dynamic intracellular mobilization of AQP2 depends on its specific interactions with the components of the trafficking machinery and the actin cytoskeleton. These include Caveolin-1 [89], the myelin and lymphocyte-associated protein (MAL) [90], the signal-induced proliferation-associated gene-1 (SPA-1) [91], the 70-kDa heat shock proteins hsc70 and hsp70 [93], A-kinase anchoring protein 220 (AKAP220) [95], 14-3-3 [102], Ezrin [96], and AQP5 [97]. Each of these AQP2-intercating proteins is co-expressed and colocalized with AQP2 in the collecting duct cells [89,90,91,93,95,96,97,102].
AQP2-Caveolin-1 interaction is believed to regulate the internalization of AQP2 through a caveolin-1-dependent pathway in MDCK cells [89]. MAL is a detergent-resistant membrane-associated protein involved in apical sorting events and AQP2 phosphorylation at S256 appears to enhance its binding affinity with MAL. While MAL does not affect apical delivery of AQP2 or its detergent-resistant association with membrane in renal epithelial cells, it promotes the S256 phosphorylation and apical localization of AQP2 by inhibiting its internalization [90].
SPA-1, a PDZ-domain containing GTPase-activating protein for Rap1 was identified in a biochemical search for AQP2-interacting proteins [91]. The physiological relevance and significance of this interaction is highlighted by the coincided distribution of SPA-1 with that of AQP2 in renal collecting ducts, the concomitant co-relocation by hydration status, and the impaired AQP2 trafficking to the cell surface membrane in MDCK cells expressing a SPA-1 mutant lacking Rap1 GTPase-activating protein activity or the constitutively active mutant of Rap1 [91]. Moreover, AQP2 apical localization was reduced in SPA-1-deficient mice. These studies illustrate that SPA-1 interacts with AQP2 and promotes at least partially AQP2 trafficking [91].
The 70-kDa heat shock proteins were uncovered as proteins interacting with the AQP2 C-terminus in a yeast two-hybrid screening [93]. The interaction was found to be direct, partially inhibited by ATP, and critically affected by the Ser-256 residue in the AQP2 C terminus. Vasopressin enhances their interaction, as evidenced by increased binding in immunoprecipitation assays and by an increased co-localization of the two proteins on the apical membrane of principal cells in rat collecting ducts. Hsc70 knockdown increased membrane accumulation of AQP2 and reduced endocytosis of rhodamine-transferrin. Hence, the interaction influences AQP2 trafficking [93].
Structurally diverse A-Kinase Anchoring Proteins (AKAPs) share a common function: acting as scaffold proteins binding to PKA and other signaling proteins and physically recruiting these protein complexes to distinct cellular locations. This enables regulation of specific substrates through phosphorylation by PKA and dephosphorylation by phosphatases. In response to dehydration, PKA phosphorylates AQP2 at S256 to promote its apical membrane sorting. AKAP220 interacts with AQP2 in yeast two-hybrid assays and co-localizes with AQP2 in the cytosol of the inner medullary collecting ducts [95]. AKAP220 co-expression increases forskolin-stimulated phosphorylation of AQP2 expressed in transiently transfected COS cells. Based on these results, AKAP220 is thought to enhance AQP2 trafficking by recruiting PKA to phosphorylate AQP2 [95]. The low molecular weight compound 3,3′-diamino-4,4′-dihydroxydiphenylmethane (FMP-API-1) and its derivatives are disruptors of the AKAP-PKA complex. These drugs elevated AQP2 membrane activity to the same extent as vasopressin in mpkCCD cells and robustly activated AQP2 in an AVPR2-inhibited NDI mouse model [103]. Direct activation of PKA by dissociating AKAP led to the phosphorylation of a very specific group of proteins compared to G protein-coupled receptor agonists or adenylyl cyclase activators such as forskolin, which induced phosphorylation of many more PKA substrates (reviewed in [47]). FMP-API-1/27 appears to be a renal-specific AKAP-PKA disruptor, which acted mainly in collecting ducts without phosphorylating most of the PKA substrates in the whole kidney and heart [103]. This specificity would lower the potential adverse side effects in other cells and organs. Hence, FMP-API-1/27 may represent a promising lead compound for the treatment of congenital NDI caused by AVPR2 mutations [103].
Proteins that differentially bind to phosphorylated versus non-phosphorylated peptides of the AQP2 C-terminus were identified by mass spectroscopy and validated by co-immunoprecipitation [94]. This approach not only confirmed previously documented hsc70 and hsp70 as AQP2 binding partners, but also revealed a higher affinity of these proteins to the nonphosphorylated form rather than to the S256-phosphorylated AQP2. Contrarily, the interaction of hsp70-5 (BiP/grp78), another heat shock protein, with phosphorylated AQP2 was found to be stronger when compared to the nonphosphorylated protein [94]. In aggregate, these data support the concept that S256 phosphorylation governs AQP2 trafficking by modulating interactions of hsp70 family members with the AQP2 C-terminus [94].
Phosphorylation at S256 and other sites in the AQP2 C-terminus also modulates AQP2 interaction with 14-3-3ζ and -θ [102]. 14-3-3ζ silencing increased AQP2 ubiquitination, reduced AQP2 protein half-life, and decreased AQP2 levels while 14-3-3θ knockdown had an opposite effect [102]. The binding of 14-3-3 proteins to AQP2, therefore, apparently impacts AQP2 phosphorylation, trafficking, ubiquitination, and degradation [102].
Ezrin is an actin-binding protein that also interacts with AQP2 [96]. Knockdown of Ezrin elevated AQP2 membrane accumulation and decreased AQP2 endocytosis. Direct binding of Ezrin to AQP2 promotes AQP2 endocytosis, linking AQP2 trafficking to the dynamic actin cytoskeletal network [96].
We reported that AQP5 interacts with Aqp2 in multiple assays [97]. AQP2 and AQP5 are close homologs and possess 66% sequence identity. AQP5 is normally synthesized in the eyes, salivary glands, lung, and sweat glands [104,105,106]. Impaired AQP5 trafficking in the lacrimal gland results in Sjögren’s syndrome, featured by dry eye and mouth [107]. While Aqp5 is undetectable in the normal mouse kidney [108], it is readily detectable and co-localizes with Aqp2 in Dot1lAC mice in which the histone H3 K79 methyltransferase Dot1l is specifically inactivated in the epithelial cells of the connecting tubule/collecting duct. Overexpression of AQP5 reduced AQP2 cell surface expression. AQP5 is also expressed and co-localizes with AQP2 at the perinuclear region of the collecting duct cells in patients with diabetic nephropathy [97]. These discoveries demonstrate that AQP5 may regulate AQP2 trafficking, possibly by forming heterotetramers with AQP2, and that the impaired apical localization of AQP2 because of abnormal AQP5 expression may contribute to the deterioration of glucosuria-induced polyuria in diabetic patients as well as to the development of hypoosmotic polyuria in Dot1lAC mice [97,109].
Most recently, an AQP2 interactome listing 139 AQP2-interacting proteins was reported (https://hpcwebapps.cit.nih.gov/ESBL/Database/AQP2-interactome/) [110], including the 70-kDa heat shock proteins and 14-3-3 proteins discussed previously. This interactome offers an overall picture of a dynamic biological process in which AQP2 is synthesized in the rough ER, matures via the Golgi apparatus, transported to endosomes that move into or out of the plasma membrane, and regulated in the plasma membrane [110].
10. Summary and Perspectives
AQP2-mediated water reabsorption in the collecting duct principal cells is tightly controlled by the molecular action of AVP through its receptor AVPR2. AVP binding to AVPR2 triggers a signaling cascade, leading to increased apical localization of AQP2 and, thus, increased osmotic water permeability of the apical membrane in collecting duct principal cells. To date, a total of 287 (in AVPR2) and 70 (in AQP2) potential disease-causing mutations have been identified in patients with hereditary NDI. These mutations cause or are suggested to cause mis-folding, mis-routing, impaired binding to AVP (for AVPR2 mutants), and/or impaired function as a water channel (for AQP2 mutants). Phosphorylation, ubiquitination, and glycosylation are the major post-translational modifications that regulate AQP2 trafficking and degradation. Numerous AQP2 binding partners promote or inhibit AQP2 post-translational modifications, translocation to the plasma membrane, and/or degradation. AQP2 appears to be a “model membrane protein” for elucidating protein trafficking in epithelial cells, and an excellent candidate to evaluate the role of post-translational controls on protein function and the highly interactive hormone-regulated signaling networks that coordinate exocytic and endocytic processes. As more data regarding the sophisticated signaling and regulatory mechanisms regulating AQP2 trafficking is gained, novel potential therapeutic strategies for NDI will likely emerge [47]. Successful development of these strategies may hold promise for future management of the disease.
Abbreviations
CDI: central diabetes insipidus; NDI: nephrogenic diabetes insipidus; AVP: arginine vasopressin; AVPR2: arginine vasopressin receptor 2; cAMP: cyclic adenosine monophosphate; AKAP: diverse A-Kinase Anchoring Protein; PKA: protein kinases A; LIP5: lysosomal trafficking regulator interacting protein 5; MAL: myelin and lymphocyte-associated protein, SPA-1: signal-induced proliferation-associated gene-1, Hsp: heat shock protein. ER: endoplasmic reticulum.
Author Contributions
Writing, W.Z.; Review and Editing, C.G., P.J.H. and W.Z.; Supervision, W.Z. All authors have read and agreed to the published verison of the manuscript.
Funding
This work was supported by the following grants: National Institutes of Health Grants DK080236 (to W.Z.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Khanna A. Acquired nephrogenic diabetes insipidus. Semin. Nephrol. 2006;26:244–248. doi: 10.1016/j.semnephrol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Higgins P.J., Zhang W. Development and Diseases of the Collecting Duct System. Results Probl. Cell Differ. 2017;60:165–203. doi: 10.1007/978-3-319-51436-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Ouweland A.M., Dreesen J.C., Verdijk M., Knoers N.V., Monnens L.A., Rocchi M., van Oost B.A. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat. Genet. 1992;2:99–102. doi: 10.1038/ng1092-99. [DOI] [PubMed] [Google Scholar]
- 4.Deen P.M., Verdijk M.A., Knoers N.V., Wieringa B., Monnens L.A., van Os C.H., van Oost B.A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 5.Sahakitrungruang T., Wacharasindhu S., Sinthuwiwat T., Supornsilchai V., Suphapeetiporn K., Shotelersuk V. Identification of two novel aquaporin-2 mutations in a Thai girl with congenital nephrogenic diabetes insipidus. Endocrine. 2008;33:210–214. doi: 10.1007/s12020-008-9074-x. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki S., Chiga M., Kikuchi E., Rai T., Uchida S. Hereditary nephrogenic diabetes insipidus in Japanese patients: Analysis of 78 families and report of 22 new mutations in AVPR2 and AQP2. Clin. Exp. Nephrol. 2013;17:338–344. doi: 10.1007/s10157-012-0726-z. [DOI] [PubMed] [Google Scholar]
- 7.Canfield M.C., Tamarappoo B.K., Moses A.M., Verkman A.S., Holtzman E.J. Identification and characterization of aquaporin-2 water channel mutations causing nephrogenic diabetes insipidus with partial vasopressin response. Hum. Mol. Genet. 1997;6:1865–1871. doi: 10.1093/hmg/6.11.1865. [DOI] [PubMed] [Google Scholar]
- 8.Leduc-Nadeau A., Lussier Y., Arthus M.F., Lonergan M., Martinez-Aguayo A., Riveira-Munoz E., Devuyst O., Bissonnette P., Bichet D.G. New autosomal recessive mutations in aquaporin-2 causing nephrogenic diabetes insipidus through deficient targeting display normal expression in Xenopus oocytes. J. Physiol. 2010;588:2205–2218. doi: 10.1113/jphysiol.2010.187674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marr N., Bichet D.G., Hoefs S., Savelkoul P.J., Konings I.B., De Mattia F., Graat M.P., Arthus M.F., Lonergan M., Fujiwara T.M., et al. Cell-biologic and functional analyses of five new Aquaporin-2 missense mutations that cause recessive nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2002;13:2267–2277. doi: 10.1097/01.ASN.0000027355.41663.14. [DOI] [PubMed] [Google Scholar]
- 10.Lin S.H., Bichet D.G., Sasaki S., Kuwahara M., Arthus M.F., Lonergan M., Lin Y.F. Two novel aquaporin-2 mutations responsible for congenital nephrogenic diabetes insipidus in Chinese families. J. Clin. Endocrinol. Metab. 2002;87:2694–2700. doi: 10.1210/jcem.87.6.8617. [DOI] [PubMed] [Google Scholar]
- 11.Van Lieburg A.F., Verdijk M.A., Knoers V.V., van Essen A.J., Proesmans W., Mallmann R., Monnens L.A., van Oost B.A., van Os C.H., Deen P.M. Patients with autosomal nephrogenic diabetes insipidus homozygous for mutations in the aquaporin 2 water-channel gene. Am. J. Hum. Genet. 1994;55:648–652. [PMC free article] [PubMed] [Google Scholar]
- 12.Mulders S.M., Knoers N.V., Van Lieburg A.F., Monnens L.A., Leumann E., Wuhl E., Schober E., Rijss J.P., Van Os C.H., Deen P.M. New mutations in the AQP2 gene in nephrogenic diabetes insipidus resulting in functional but misrouted water channels. J. Am. Soc. Nephrol. 1997;8:242–248. doi: 10.1681/ASN.V82242. [DOI] [PubMed] [Google Scholar]
- 13.Cheong H.I., Cho S.J., Zheng S.H., Cho H.Y., Ha I.S., Choi Y. Two novel mutations in the aquaporin 2 gene in a girl with congenital nephrogenic diabetes insipidus. J. Korean Med. Sci. 2005;20:1076–1078. doi: 10.3346/jkms.2005.20.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bichet D.G., El Tarazi A., Matar J., Lussier Y., Arthus M.F., Lonergan M., Bockenhauer D., Bissonnette P. Aquaporin-2: New mutations responsible for autosomal-recessive nephrogenic diabetes insipidus-update and epidemiology. Clin. Kidney J. 2012;5:195–202. doi: 10.1093/ckj/sfs029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia Castano A., Perez de Nanclares G., Madariaga L., Aguirre M., Chocron S., Madrid A., Lafita Tejedor F.J., Gil Campos M., Sanchez Del Pozo J., Ruiz Cano R., et al. Novel mutations associated with nephrogenic diabetes insipidus. A clinical-genetic study. Eur. J. Pediatr. 2015;174:1373–1385. doi: 10.1007/s00431-015-2534-4. [DOI] [PubMed] [Google Scholar]
- 16.Rugpolmuang R., Deeb A., Hassan Y., Deekajorndech T., Shotelersuk V., Sahakitrungruang T. Novel AQP2 mutation causing congenital nephrogenic diabetes insipidus: Challenges in management during infancy. J. Pediatric Endocrinol. Metab. 2014;27:193–197. doi: 10.1515/jpem-2013-0097. [DOI] [PubMed] [Google Scholar]
- 17.Carroll P., Al-Mojalli H., Al-Abbad A., Al-Hassoun I., Al-Hamed M., Al-Amr R., Butt A.I., Meyer B.F. Novel mutations underlying nephrogenic diabetes insipidus in Arab families. Genet. Med. 2006;8:443–447. doi: 10.1097/01.gim.0000223554.46981.7a. [DOI] [PubMed] [Google Scholar]
- 18.Zaki M., Schoneberg T., Al Ajrawi T., Al Said A.N., Sangkuhl K., Rompler H. Nephrogenic diabetes insipidus, thiazide treatment and renal cell carcinoma. Nephrol. Dial. Transplant. 2006;21:1082–1086. doi: 10.1093/ndt/gfk024. [DOI] [PubMed] [Google Scholar]
- 19.Park Y.J., Baik H.W., Cheong H.I., Kang J.H. Congenital nephrogenic diabetes insipidus with a novel mutation in the aquaporin 2 gene. Biomed. Rep. 2014;2:596–598. doi: 10.3892/br.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goji K., Kuwahara M., Gu Y., Matsuo M., Marumo F., Sasaki S. Novel mutations in aquaporin-2 gene in female siblings with nephrogenic diabetes insipidus: Evidence of disrupted water channel function. J. Clin. Endocrinol. Metab. 1998;83:3205–3209. doi: 10.1210/jc.83.9.3205. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto M., Okada S., Kawashima Y., Nishimura R., Miyahara N., Kawaba Y., Hanaki K., Nanba E., Kondo Y., Igarashi T., et al. Clinical overview of nephrogenic diabetes insipidus based on a nationwide survey in Japan. Yonago Acta Med. 2014;57:85–91. [PMC free article] [PubMed] [Google Scholar]
- 22.Duzenli D., Saglar E., Deniz F., Azal O., Erdem B., Mergen H. Mutations in the AVPR2, AVP-NPII, and AQP2 genes in Turkish patients with diabetes insipidus. Endocrine. 2012;42:664–669. doi: 10.1007/s12020-012-9704-1. [DOI] [PubMed] [Google Scholar]
- 23.Iolascon A., Aglio V., Tamma G., D’Apolito M., Addabbo F., Procino G., Simonetti M.C., Montini G., Gesualdo L., Debler E.W., et al. Characterization of two novel missense mutations in the AQP2 gene causing nephrogenic diabetes insipidus. Nephron Physiol. 2007;105:p33–p41. doi: 10.1159/000098136. [DOI] [PubMed] [Google Scholar]
- 24.Vargas-Poussou R., Forestier L., Dautzenberg M.D., Niaudet P., Dechaux M., Antignac C. Mutations in the vasopressin V2 receptor and aquaporin-2 genes in 12 families with congenital nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 1997;8:1855–1862. doi: 10.1681/ASN.V8121855. [DOI] [PubMed] [Google Scholar]
- 25.Loonen A.J., Knoers N.V., van Os C.H., Deen P.M. Aquaporin 2 mutations in nephrogenic diabetes insipidus. Semin. Nephrol. 2008;28:252–265. doi: 10.1016/j.semnephrol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Liberatore Junior R.D., Carneiro J.G., Leidenz F.B., Melilo-Carolino R., Sarubi H.C., De Marco L. Novel compound aquaporin 2 mutations in nephrogenic diabetes insipidus. Clin. (Sao Paulo) 2012;67:79–82. doi: 10.6061/clinics/2012(01)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oksche A., Moller A., Dickson J., Rosendahl W., Rascher W., Bichet D.G., Rosenthal W. Two novel mutations in the aquaporin-2 and the vasopressin V2 receptor genes in patients with congenital nephrogenic diabetes insipidus. Hum. Genet. 1996;98:587–589. doi: 10.1007/s004390050264. [DOI] [PubMed] [Google Scholar]
- 28.Cen J., Nie M., Duan L., Gu F. Novel autosomal recessive gene mutations in aquaporin-2 in two Chinese congenital nephrogenic diabetes insipidus pedigrees. Int. J. Clin. Exp. Med. 2015;8:3629–3639. [PMC free article] [PubMed] [Google Scholar]
- 29.Moon S.S., Kim H.J., Choi Y.K., Seo H.A., Jeon J.H., Lee J.E., Lee J.Y., Kwon T.H., Kim J.G., Kim B.W., et al. Novel mutation of aquaporin-2 gene in a patient with congenital nephrogenic diabetes insipidus. Endocr. J. 2009;56:905–910. doi: 10.1507/endocrj.K09E-078. [DOI] [PubMed] [Google Scholar]
- 30.Savelkoul P.J., De Mattia F., Li Y., Kamsteeg E.J., Konings I.B., van der Sluijs P., Deen P.M. p.R254Q mutation in the aquaporin-2 water channel causing dominant nephrogenic diabetes insipidus is due to a lack of arginine vasopressin-induced phosphorylation. Hum. Mutat. 2009;30:E891–E903. doi: 10.1002/humu.21082. [DOI] [PubMed] [Google Scholar]
- 31.De Mattia F., Savelkoul P.J., Kamsteeg E.J., Konings I.B., van der Sluijs P., Mallmann R., Oksche A., Deen P.M. Lack of arginine vasopressin-induced phosphorylation of aquaporin-2 mutant AQP2-R254L explains dominant nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2005;16:2872–2880. doi: 10.1681/ASN.2005010104. [DOI] [PubMed] [Google Scholar]
- 32.Dollerup P., Thomsen T.M., Nejsum L.N., Faerch M., Osterbrand M., Gregersen N., Rittig S., Christensen J.H., Corydon T.J. Partial nephrogenic diabetes insipidus caused by a novel AQP2 variation impairing trafficking of the aquaporin-2 water channel. BMC Nephrol. 2015;16:217. doi: 10.1186/s12882-015-0213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulders S.M., Bichet D.G., Rijss J.P., Kamsteeg E.J., Arthus M.F., Lonergan M., Fujiwara M., Morgan K., Leijendekker R., van der Sluijs P., et al. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J. Clin. Investig. 1998;102:57–66. doi: 10.1172/JCI2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochberg Z., Van Lieburg A., Even L., Brenner B., Lanir N., Van Oost B.A., Knoers N.V. Autosomal recessive nephrogenic diabetes insipidus caused by an aquaporin-2 mutation. J. Clin. Endocrinol. Metab. 1997;82:686–689. doi: 10.1210/jcem.82.2.3781. [DOI] [PubMed] [Google Scholar]
- 35.Tajima T., Okuhara K., Satoh K., Nakae J., Fujieda K. Two novel aquaporin-2 mutations in a sporadic Japanese patient with autosomal recessive nephrogenic diabetes insipidus. Endocr. J. 2003;50:473–476. doi: 10.1507/endocrj.50.473. [DOI] [PubMed] [Google Scholar]
- 36.Kuwahara M., Iwai K., Ooeda T., Igarashi T., Ogawa E., Katsushima Y., Shinbo I., Uchida S., Terada Y., Arthus M.F., et al. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am. J. Hum. Genet. 2001;69:738–748. doi: 10.1086/323643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marr N., Bichet D.G., Lonergan M., Arthus M.F., Jeck N., Seyberth H.W., Rosenthal W., van Os C.H., Oksche A., Deen P.M. Heteroligomerization of an Aquaporin-2 mutant with wild-type Aquaporin-2 and their misrouting to late endosomes/lysosomes explains dominant nephrogenic diabetes insipidus. Hum. Mol. Genet. 2002;11:779–789. doi: 10.1093/hmg/11.7.779. [DOI] [PubMed] [Google Scholar]
- 38.Kamsteeg E.J., Bichet D.G., Konings I.B., Nivet H., Lonergan M., Arthus M.F., van Os C.H., Deen P.M. Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus. J Cell Biol. 2003;163:1099–1109. doi: 10.1083/jcb.200309017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bockenhauer D., Bichet D.G. Inherited secondary nephrogenic diabetes insipidus: Concentrating on humans. Am. J. Physiol. Ren. Physiol. 2013;304:F1037–F1042. doi: 10.1152/ajprenal.00639.2012. [DOI] [PubMed] [Google Scholar]
- 40.Moeller H.B., Fuglsang C.H., Fenton R.A. Renal aquaporins and water balance disorders. Best Pr. Res. Clin. Endocrinol. Metab. 2016;30:277–288. doi: 10.1016/j.beem.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Bichet D.G. GENETICS IN ENDOCRINOLOGY Pathophysiology, diagnosis and treatment of familial nephrogenic diabetes insipidus. Eur. J. Endocrinol. 2020;183:R29–R40. doi: 10.1530/EJE-20-0114. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen S., DiGiovanni S.R., Christensen E.I., Knepper M.A., Harris H.W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl. Acad. Sci. USA. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasui M., Marples D., Belusa R., Eklof A.C., Celsi G., Nielsen S., Aperia A. Development of urinary concentrating capacity: Role of aquaporin-2. Am. J. Physiol. 1996;271:F461–F468. doi: 10.1152/ajprenal.1996.271.2.F461. [DOI] [PubMed] [Google Scholar]
- 44.Saito T., Higashiyama M., Nagasaka S., Sasaki S., Saito T., Ishikawa S.E. Role of aquaporin-2 gene expression in hyponatremic rats with chronic vasopressin-induced antidiuresis. Kidney Int. 2001;60:1266–1276. doi: 10.1046/j.1523-1755.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang B., Bankir L. Urea and urine concentrating ability: New insights from studies in mice. Am. J. Physiol. Ren. Physiol. 2005;288:F881–F896. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]
- 46.Bankir L., Fernandes S., Bardoux P., Bouby N., Bichet D.G. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J. Am. Soc. Nephrol. 2005;16:1920–1928. doi: 10.1681/ASN.2004121079. [DOI] [PubMed] [Google Scholar]
- 47.Cheung P.W., Bouley R., Brown D. Targeting the Trafficking of Kidney Water Channels for Therapeutic Benefit. Annu. Rev. Pharm. Toxicol. 2020;60:175–194. doi: 10.1146/annurev-pharmtox-010919-023654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su W., Cao R., Zhang X.Y., Guan Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Ren. Physiol. 2020;318:F193–F203. doi: 10.1152/ajprenal.00304.2019. [DOI] [PubMed] [Google Scholar]
- 49.Li S., Qiu M., Kong Y., Zhao X., Choi H.J., Reich M., Bunkelman B.H., Liu Q., Hu S., Han M., et al. Bile Acid G Protein-Coupled Membrane Receptor TGR5 Modulates Aquaporin 2-Mediated Water Homeostasis. J. Am. Soc. Nephrol. 2018;29:2658–2670. doi: 10.1681/ASN.2018030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song J., Knepper M.A., Hu X., Verbalis J.G., Ecelbarger C.A. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J. Pharmacol. Exp. Ther. 2004;308:426–433. doi: 10.1124/jpet.103.058008. [DOI] [PubMed] [Google Scholar]
- 51.Procino G., Gerbino A., Milano S., Nicoletti M.C., Mastrofrancesco L., Carmosino M., Svelto M. Rosiglitazone promotes AQP2 plasma membrane expression in renal cells via a Ca-dependent/cAMP-independent mechanism. Cell. Physiol. Biochem. 2015;35:1070–1085. doi: 10.1159/000373933. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X.Y., Wang B., Guan Y.F. Nuclear Receptor Regulation of Aquaporin-2 in the Kidney. Int. J. Mol. Sci. 2016;17:1105. doi: 10.3390/ijms17071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheema M.U., Irsik D.L., Wang Y., Miller-Little W., Hyndman K.A., Marks E.S., Frokiaer J., Boesen E.I., Norregaard R. Estradiol regulates AQP2 expression in the collecting duct: A novel inhibitory role for estrogen receptor alpha. Am. J. Physiol. Ren. Physiol. 2015;309:F305–F317. doi: 10.1152/ajprenal.00685.2014. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwara T.M., Bichet D.G. Molecular biology of hereditary diabetes insipidus. J. Am. Soc. Nephrol. 2005;16:2836–2846. doi: 10.1681/ASN.2005040371. [DOI] [PubMed] [Google Scholar]
- 55.Robben J.H., Knoers N.V., Deen P.M. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am. J. Physiol. Ren. Physiol. 2006;291:F257–F270. doi: 10.1152/ajprenal.00491.2005. [DOI] [PubMed] [Google Scholar]
- 56.Hermosilla R., Oueslati M., Donalies U., Schonenberger E., Krause E., Oksche A., Rosenthal W., Schulein R. Disease-causing V(2) vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5:993–1005. doi: 10.1111/j.1600-0854.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 57.Romisch K. A cure for traffic jams: Small molecule chaperones in the endoplasmic reticulum. Traffic. 2004;5:815–820. doi: 10.1111/j.1600-0854.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 58.Morello J.P., Salahpour A., Laperriere A., Bernier V., Arthus M.F., Lonergan M., Petaja-Repo U., Angers S., Morin D., Bichet D.G., et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Investig. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernier V., Morello J.P., Zarruk A., Debrand N., Salahpour A., Lonergan M., Arthus M.F., Laperriere A., Brouard R., Bouvier M., et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 60.Deen P.M., Croes H., van Aubel R.A., Ginsel L.A., van Os C.H. Water channels encoded by mutant aquaporin-2 genes in nephrogenic diabetes insipidus are impaired in their cellular routing. J. Clin. Investig. 1995;95:2291–2296. doi: 10.1172/JCI117920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deen P.M., van Aubel R.A., van Lieburg A.F., van Os C.H. Urinary content of aquaporin 1 and 2 in nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 1996;7:836–841. doi: 10.1681/ASN.V76836. [DOI] [PubMed] [Google Scholar]
- 62.Tamarappoo B.K., Verkman A.S. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J. Clin. Investig. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuwahara M. Aquaporin-2, a vasopressin-sensitive water channel, and nephrogenic diabetes insipidus. Intern. Med. 1998;37:215–217. doi: 10.2169/internalmedicine.37.215. [DOI] [PubMed] [Google Scholar]
- 64.De Mattia F., Savelkoul P.J., Bichet D.G., Kamsteeg E.J., Konings I.B., Marr N., Arthus M.F., Lonergan M., van Os C.H., van der Sluijs P., et al. A novel mechanism in recessive nephrogenic diabetes insipidus: Wild-type aquaporin-2 rescues the apical membrane expression of intracellularly retained AQP2-P262L. Hum. Mol. Genet. 2004;13:3045–3056. doi: 10.1093/hmg/ddh339. [DOI] [PubMed] [Google Scholar]
- 65.Kamsteeg E.J., Wormhoudt T.A., Rijss J.P., van Os C.H., Deen P.M. An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J. 1999;18:2394–2400. doi: 10.1093/emboj/18.9.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown D. The ins and outs of aquaporin-2 trafficking. Am. J. Physiol. Ren. Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- 67.Bouley R., Lu H.A., Nunes P., Da Silva N., McLaughlin M., Chen Y., Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J. Am. Soc. Nephrol. 2011;22:59–72. doi: 10.1681/ASN.2009121267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olesen E.T., Rutzler M.R., Moeller H.B., Praetorius H.A., Fenton R.A. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc. Natl. Acad. Sci. USA. 2011;108:12949–12954. doi: 10.1073/pnas.1104691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Datta A., Yang C.R., Limbutara K., Chou C.L., Rinschen M.M., Raghuram V., Knepper M.A. PKA-independent vasopressin signaling in renal collecting duct. FASEB J. 2020;34:6129–6146. doi: 10.1096/fj.201902982R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Procino G., Carmosino M., Marin O., Brunati A.M., Contri A., Pinna L.A., Mannucci R., Nielsen S., Kwon T.H., Svelto M., et al. Ser-256 phosphorylation dynamics of Aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. FASEB J. 2003;17:1886–1888. doi: 10.1096/fj.02-0870fje. [DOI] [PubMed] [Google Scholar]
- 71.Fushimi K., Sasaki S., Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 72.Katsura T., Gustafson C.E., Ausiello D.A., Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am. J. Physiol. 1997;272:F817–F822. doi: 10.1152/ajprenal.1997.272.6.F816. [DOI] [PubMed] [Google Scholar]
- 73.McDill B.W., Li S.Z., Kovach P.A., Ding L., Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl. Acad. Sci. USA. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffert J.D., Pisitkun T., Wang G., Shen R.F., Knepper M.A. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc. Natl. Acad. Sci. USA. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffert J.D., Fenton R.A., Moeller H.B., Simons B., Tchapyjnikov D., McDill B.W., Yu M.J., Pisitkun T., Chen F., Knepper M.A. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J. Biol. Chem. 2008;283:24617–24627. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoffert J.D., Nielsen J., Yu M.J., Pisitkun T., Schleicher S.M., Nielsen S., Knepper M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am. J. Physiol. Ren. Physiol. 2007;292:F691–F700. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 77.Moeller H.B., Praetorius J., Rutzler M.R., Fenton R.A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc. Natl. Acad. Sci. USA. 2010;107:424–429. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamsteeg E.J., Hendriks G., Boone M., Konings I.B., Oorschot V., van der Sluijs P., Klumperman J., Deen P.M. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. USA. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee Y.J., Lee J.E., Choi H.J., Lim J.S., Jung H.J., Baek M.C., Frokiaer J., Nielsen S., Kwon T.H. E3 ubiquitin-protein ligases in rat kidney collecting duct: Response to vasopressin stimulation and withdrawal. Am. J. Physiol. Ren. Physiol. 2011;301:F883–F896. doi: 10.1152/ajprenal.00117.2011. [DOI] [PubMed] [Google Scholar]
- 80.Medvar B., Raghuram V., Pisitkun T., Sarkar A., Knepper M.A. Comprehensive database of human E3 ubiquitin ligases: Application to aquaporin-2 regulation. Physiol Genom. 2016;48:502–512. doi: 10.1152/physiolgenomics.00031.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trimpert C., Wesche D., de Groot T., Pimentel Rodriguez M.M., Wong V., van den Berg D.T.M., Cheval L., Ariza C.A., Doucet A., Stagljar I., et al. NDFIP allows NEDD4/NEDD4L-induced AQP2 ubiquitination and degradation. PLoS ONE. 2017;12:e0183774. doi: 10.1371/journal.pone.0183774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunter T. The age of crosstalk: Phosphorylation, ubiquitination, and beyond. Mol. Cell. 2007;28:730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 83.Moeller H.B., Aroankins T.S., Slengerik-Hansen J., Pisitkun T., Fenton R.A. Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J. Cell Sci. 2014;127:3174–3183. doi: 10.1242/jcs.150680. [DOI] [PubMed] [Google Scholar]
- 84.Mellquist J.L., Kasturi L., Spitalnik S.L., Shakin-Eshleman S.H. The amino acid following an asn-X-Ser/Thr sequon is an important determinant of N-linked core glycosylation efficiency. Biochemistry. 1998;37:6833–6837. doi: 10.1021/bi972217k. [DOI] [PubMed] [Google Scholar]
- 85.Baumgarten R., Van De Pol M.H., Wetzels J.F., Van Os C.H., Deen P.M. Glycosylation is not essential for vasopressin-dependent routing of aquaporin-2 in transfected Madin-Darby canine kidney cells. J. Am. Soc. Nephrol. 1998;9:1553–1559. doi: 10.1681/ASN.V991553. [DOI] [PubMed] [Google Scholar]
- 86.Hendriks G., Koudijs M., van Balkom B.W., Oorschot V., Klumperman J., Deen P.M., van der Sluijs P. Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J. Biol. Chem. 2004;279:2975–2983. doi: 10.1074/jbc.M310767200. [DOI] [PubMed] [Google Scholar]
- 87.Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 88.Van Balkom B.W., Boone M., Hendriks G., Kamsteeg E.J., Robben J.H., Stronks H.C., van der Voorde A., van Herp F., van der Sluijs P., Deen P.M. LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J. Am. Soc. Nephrol. 2009;20:990–1001. doi: 10.1681/ASN.2008060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aoki T., Suzuki T., Hagiwara H., Kuwahara M., Sasaki S., Takata K., Matsuzaki T. Close association of aquaporin-2 internalization with caveolin-1. Acta Histochem. Et Cytochem. 2012;45:139–146. doi: 10.1267/ahc.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamsteeg E.J., Duffield A.S., Konings I.B., Spencer J., Pagel P., Deen P.M., Caplan M.J. MAL decreases the internalization of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. USA. 2007;104:16696–16701. doi: 10.1073/pnas.0708023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noda Y., Horikawa S., Furukawa T., Hirai K., Katayama Y., Asai T., Kuwahara M., Katagiri K., Kinashi T., Hattori M., et al. Aquaporin-2 trafficking is regulated by PDZ-domain containing protein SPA-1. Febs Lett. 2004;568:139–145. doi: 10.1016/j.febslet.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 92.Noda Y., Horikawa S., Katayama Y., Sasaki S. Identification of a multiprotein "motor" complex binding to water channel aquaporin-2. Biochem. Biophys. Res. Commun. 2005;330:1041–1047. doi: 10.1016/j.bbrc.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 93.Lu H.A., Sun T.X., Matsuzaki T., Yi X.H., Eswara J., Bouley R., McKee M., Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J. Biol. Chem. 2007;282:28721–28732. doi: 10.1074/jbc.M611101200. [DOI] [PubMed] [Google Scholar]
- 94.Zwang N.A., Hoffert J.D., Pisitkun T., Moeller H.B., Fenton R.A., Knepper M.A. Identification of phosphorylation-dependent binding partners of aquaporin-2 using protein mass spectrometry. J. Proteome Res. 2009;8:1540–1554. doi: 10.1021/pr800894p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okutsu R., Rai T., Kikuchi A., Ohno M., Uchida K., Sasaki S., Uchida S. AKAP220 colocalizes with AQP2 in the inner medullary collecting ducts. Kidney Int. 2008;74:1429–1433. doi: 10.1038/ki.2008.402. [DOI] [PubMed] [Google Scholar]
- 96.Li W., Jin W.W., Tsuji K., Chen Y., Nomura N., Su L., Yui N., Arthur J., Cotecchia S., Paunescu T.G., et al. Ezrin directly interacts with AQP2 and promotes its endocytosis. J. Cell Sci. 2017;130:2914–2925. doi: 10.1242/jcs.204842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu H., Chen L., Zhang X., Zhou Q., Li J.M., Berger S., Borok Z., Zhou B., Xiao Z., Yin H., et al. Aqp5 is a new transcriptional target of dot1a and a regulator of aqp2. PLoS ONE. 2013;8:e53342. doi: 10.1371/journal.pone.0053342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noda Y., Horikawa S., Katayama Y., Sasaki S. Water channel aquaporin-2 directly binds to actin. Biochem. Biophys. Res. Commun. 2004;322:740–745. doi: 10.1016/j.bbrc.2004.07.195. [DOI] [PubMed] [Google Scholar]
- 99.Wang W.L., Su S.H., Wong K.Y., Yang C.W., Liu C.F., Yu M.J. Rab7 involves Vps35 to mediate AQP2 sorting and apical trafficking in collecting duct cells. Am. J. Physiol. Ren. Physiol. 2020;318:F956–F970. doi: 10.1152/ajprenal.00297.2019. [DOI] [PubMed] [Google Scholar]
- 100.Murali S.K., Aroankins T.S., Moeller H.B., Fenton R.A. The Deubiquitylase USP4 Interacts with the Water Channel AQP2 to Modulate Its Apical Membrane Accumulation and Cellular Abundance. Cells. 2019;8:265. doi: 10.3390/cells8030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi H.J., Jang H.J., Park E., Tingskov S.J., Norregaard R., Jung H.J., Kwon T.H. Sorting Nexin 27 Regulates the Lysosomal Degradation of Aquaporin-2 Protein in the Kidney Collecting Duct. Cells. 2020;9:1208. doi: 10.3390/cells9051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moeller H.B., Slengerik-Hansen J., Aroankins T., Assentoft M., MacAulay N., Moestrup S.K., Bhalla V., Fenton R.A. Regulation of the Water Channel Aquaporin-2 via 14-3-3theta and -zeta. J. Biol. Chem. 2016;291:2469–2484. doi: 10.1074/jbc.M115.691121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ando F., Mori S., Yui N., Morimoto T., Nomura N., Sohara E., Rai T., Sasaki S., Kondo Y., Kagechika H., et al. AKAPs-PKA disruptors increase AQP2 activity independently of vasopressin in a model of nephrogenic diabetes insipidus. Nat. Commun. 2018;9:1411. doi: 10.1038/s41467-018-03771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.King L.S., Kozono D., Agre P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 105.Krane C.M., Goldstein D.L. Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm. Genome. 2007;18:452–462. doi: 10.1007/s00335-007-9041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raina S., Preston G.M., Guggino W.B., Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J. Biol. Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 107.Tsubota K., Hirai S., King L.S., Agre P., Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- 108.Krane C.M., Towne J.E., Menon A.G. Cloning and characterization of murine Aqp5: Evidence for a conserved aquaporin gene cluster. Mamm. Genome. 1999;10:498–505. doi: 10.1007/s003359901030. [DOI] [PubMed] [Google Scholar]
- 109.Wu H., Chen L., Zhou Q., Zhang X., Berger S., Bi J., Lewis D.E., Xia Y., Zhang W. Aqp2-expressing cells give rise to renal intercalated cells. J. Am. Soc. Nephrol. 2013;24:243–252. doi: 10.1681/ASN.2012080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chou C.L., Hwang G., Hageman D.J., Han L., Agrawal P., Pisitkun T., Knepper M.A. Identification of UT-A1- and AQP2-interacting proteins in rat inner medullary collecting duct. Am. J. Physiol. Cell Physiol. 2018;314:C99–C117. doi: 10.1152/ajpcell.00082.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]