Abstract
Simple Summary
Many alterations specific to cancer cells have been investigated as targets for targeted therapies. Chromosomal instability is a characteristic of nearly all cancers that can limit response to targeted therapies by ensuring the tumor population is not genetically homogenous. Polo-like Kinase 1 (PLK1) is often up regulated in cancers and it regulates chromosomal instability extensively. PLK1 has been the subject of much pre-clinical and clinical studies, but thus far, PLK1 inhibitors have not shown significant improvement in cancer patients. We discuss the numerous roles and interactions of PLK1 in regulating chromosomal instability, and how these may provide an avenue for identifying targets for targeted therapies. As selective inhibitors of PLK1 showed limited clinical success, we also highlight how genetic interactions of PLK1 may be exploited to tackle these challenges.
Abstract
Polo-like kinase 1 (PLK1) is overexpressed near ubiquitously across all cancer types and dysregulation of this enzyme is closely tied to increased chromosomal instability and tumor heterogeneity. PLK1 is a mitotic kinase with a critical role in maintaining chromosomal integrity through its function in processes ranging from the mitotic checkpoint, centrosome biogenesis, bipolar spindle formation, chromosome segregation, DNA replication licensing, DNA damage repair, and cytokinesis. The relation between dysregulated PLK1 and chromosomal instability (CIN) makes it an attractive target for cancer therapy. However, clinical trials with PLK1 inhibitors as cancer drugs have generally displayed poor responses or adverse side-effects. This is in part because targeting CIN regulators, including PLK1, can elevate CIN to lethal levels in normal cells, affecting normal physiology. Nevertheless, aiming at related genetic interactions, such as synthetic dosage lethal (SDL) interactions of PLK1 instead of PLK1 itself, can help to avoid the detrimental side effects associated with increased levels of CIN. Since PLK1 overexpression contributes to tumor heterogeneity, targeting SDL interactions may also provide an effective strategy to suppressing this malignant phenotype in a personalized fashion.
Keywords: Polo-like kinase 1, chromosomal instability, DNA damage repair, synthetic dosage lethality
1. Introduction
Tumor heterogeneity and an increased rate of genetic mutations are prevalent features of cancer that need to be addressed in therapy to prevent treatment resistance and improve patient outcomes. Tumor heterogeneity refers to the presence of multiple distinct genotypes and phenotypes within the tumor cell population. Heterogeneity can exist within a single tumor, referred to as intra-tumor heterogeneity, or between patients of the same cancer type, defined as inter-tumor heterogeneity [1]. It contributes to drug resistance, tumor relapse and overall worse prognosis [1,2], and presents a hurdle for successful application of precision medicine and targeted therapies. One of the key driving force of tumor heterogeneity is chromosomal instability (CIN), a form of genomic instability that is frequent in many cancers, affecting up to 80% of solid tumors [3,4]. Addressing the source of the plasticity and resistance itself, such as targeting CIN, may prove to be a more efficient approach over targeting specific alterations, individually and in combination, as they arise. Targeting CIN in this way may allow to suppress tumor evolution, which usually complicates treatment and increases heterogeneity.
Chromosomal integrity is monitored carefully by cells throughout the cell cycle and there are multiple checkpoints dedicated to ensuring mitosis results in a faithful distribution of the genetic material to daughter cells. Normally, these checkpoints ensure cell division results in two healthy daughter cells with the complete intact genetic material, however in cancer, the checkpoints can become compromised, resulting in genetic defects of the daughter cells. Checkpoint defects promote mishaps in DNA replication and cell division, leading to CIN and aneuploidy. Processes coordinated throughout the cell cycle that, when dysregulated, can contribute to CIN include: double strand DNA break repair [5], DNA replication fork resolution [6], kinetochore-microtubule (MT) attachment formation [7], centrosome maturation and positioning [8], bipolar spindle formation [9], sister chromatid alignment and segregation [10], cleavage furrow formation [11], and telomere maintenance [12].
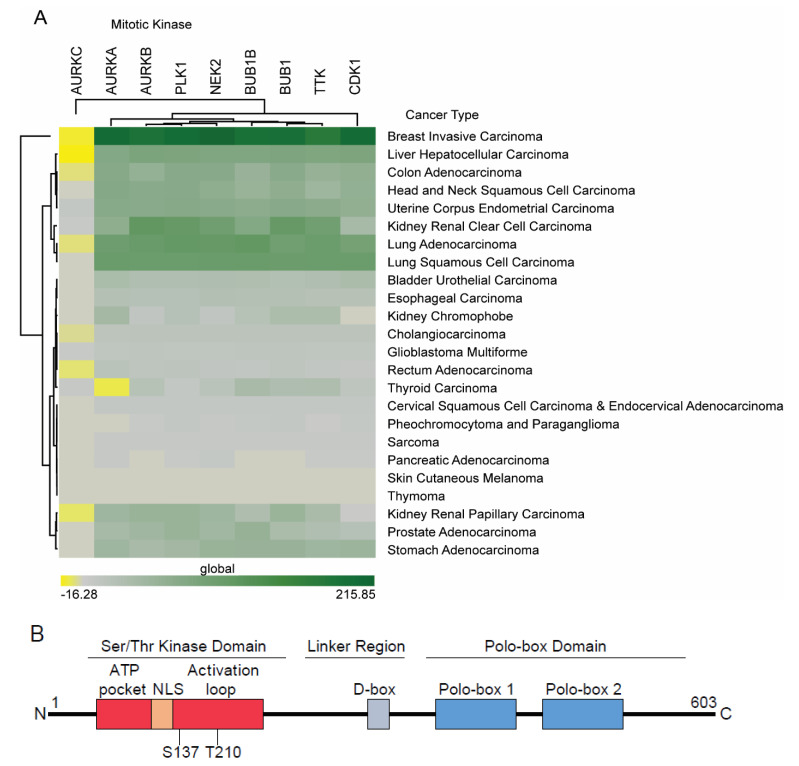
The key players regulating the cell cycle, and therefore CIN, are the multiple mitotic kinases within the cell. These kinases drive regulatory feedback and signaling loops that either arrest the cell cycle, or drive progression through the cell cycle checkpoints. Additionally, these kinases are often act as parts of regulatory feedback loops downstream of the cell cycle checkpoints to ensure commitment to cell cycle progression once checkpoint requirements have been cleared. These kinases include: cyclin dependent kinase 1 (CDK1 or CDC2), polo-like kinase 1 (PLK1), the Aurora kinases A, B, and C (AURKA/B/C), NIMA related kinase 2 (NEK2), Bub1, BubR1 (or Bub1B), and TTK (or MPS1) [13]. Wee1 and Myt1 (or PKMYT1) kinases oppose the phosphatase activity of cell division cycle 25C phosphatase (CDC25C) to activate Cyclin/CDK1 complexes, but are not considered mitotic kinases themselves, as they play a signal transduction role [13]. PLK1 and CDK1 with Cyclin B are required for entering into mitosis [14]. NEK2 activity drives centrosome dissociation, initiating the centrosome cycle [15]. AURKA, AURKB, and PLK1 are important for regulating spindle dynamics and chromosome attachments [16], with AURKA activity also regulating central spindle microtubule dynamics [17]. AURKB also regulates centrosome clustering for bipolar spindle formation [18]. TTK and BubR1 are critical components of the spindle assembly checkpoint (SAC) [19]. CDK1 and PLK1 work at the SAC to control anaphase promoting cyclosome proteasome complex (APC/C) activation [20,21]. Lastly, PLK1 and AURKB signaling initiates cytokinesis [22]. Working in concert, these kinases regulate a multitude of cell cycle checkpoints, effector proteins, and each other and their deregulation is associated with multiple malignancies [13,23]. Many of these molecules have been found to be overexpressed in tumor cells and have been put forward as potential therapeutic targets, including CDK1 [24], NEK2 [25], AURKA [26], Bub1 [27], and TTK [28]. In fact, our own analyses of the TCGA data across multiple cancers show their abnormal expression in various tumor types (Figure 1A). A precise understanding of their role in malignancy should provide novel opportunities to exploit them for treatment purposes without affecting normal cells that have intact cell cycle checkpoints. PLK1 in particular, is a key player that has been well-characterized and with further study may provide valuable options for developing novel targeted therapies. The extensive crosstalk between PLK1 and the Aurora kinases has been well studied and PLK1 carries out many of its functions in cooperation with the Aurora kinases, but the complexities of this relationship is reviewed elsewhere, and the functions of PLK1 will be the focus of this text [29]. The processing and timing of key cell cycle events can become dysregulated, leading to CIN through abnormal PLK1 activities, and in this review, we discuss therapeutic avenues that may arise from near-ubiquitous PLK1 deregulation in cancer cells.
Figure 1.
Mitotic kinases that are involved in cancer. (A). Differential expression of mitotic kinases across cancer types as analyzed from TCGA data. Green color indicates higher expression in cancer. (B). A schematic representation of PLK1 structure. This includes the Ser/Thr kinase domain, the linker region, and PBD. Activating phosphorylation at residues T210 and S137 is required catalytic activity. The D-box is the site for signaling for proteasome degradation. NLS targets PLK1 into the nucleus, while PBD targets PLK1 to the centrosome, substrates and functional partners.
2. Regulation and Activity of PLK1 in the Cell Cycle
PLK1 is a serine/threonine (Ser/Thr) protein kinase belonging to the PLK family, which overall consists of 5 kinases, PLK1 to PLK5 [29]. The structure of PLK1 includes the kinase domain, and two polo-box motifs composing the polo-box domain (PBD) (Figure 1B). The PBD of PLK1 is the hallmark of the PLK family. This PBD primarily recognizes and binds substrates that contain a consensus PLK1 phosphorylation motif, although regulation of some PLK1 partners do not seem to be reliant on their phosphorylation [30,31,32]. Many molecules interacting with PLK1 PBD are controlled through priming phosphorylation by cyclin B/CDK1 to generate the consensus PDB-binding phospho-motif, which allows PLK1 and cyclin B/CDK1 to cooperatively regulate the cell cycle [20,33,34,35].
PLK1 is primarily expressed in actively dividing cells and is barely detectable in non-proliferating cell types, which is consistent with its role in cell cycle progression [36,37,38]. PLK1-null mice are embryonic lethal, with embryos arrested after the eight-cell stage [39], suggesting a critical role for this kinase in early dividing cells and embryo development. PLK1 expression is regulated at the mRNA level by a cell cycle gene homology region in the promoter that represses PLK1 mRNA production during the G1 phase [40]. PLK1 protein levels peak in G2 and remains high until cytokinesis and mitotic exit [41,42]. PLK1 protein degradation is mediated through the destruction box (D-box) motif and subsequent proteasome targeting in G1/S to keep PLK1 levels low [43]. Heat shock protein 90 (Hsp90) helps stabilize and accumulate PLK1 protein prior to mitotic entry [44]. PLK1 is also regulated at the post-translational level, with the activating loop within the PLK1 kinase domain only being phosphorylated upon entry to mitosis [41,45]. This phosphorylation event releases PLK1 from an autoinhibitory interaction between its catalytic and PBD domains, and has been shown to be carried out, at least in some cases, by BORA and AURKA kinases [30,42,46]. Interestingly, phosphorylation at two sites in the activation loop, S137 and T210, increases PLK1 kinase activity with different timing kinetics [47]. While either phosphorylation event triggers partial PLK1 activation, phosphorylation at both sites synergistically increases its catalytic action [47].
In addition to the modulation of its kinase activity, PLK1 localization changes throughout the cell cycle, moving from the cytosol to the centrosome and spindle poles in early mitosis to kinetochores in metaphase cells to central spindle at anaphase and midbody in cytokinesis [41,48,49]. This dynamic localization pattern is partially directed by interactions of its PBD with cyclin B/CDK1-primed phospho-substrates. PLK1 also localizes to the nucleus prior to entering mitosis through its nuclear localization signal (NLS). Disrupting the PLK1 NLS causes cells to arrest in the G2 phase [50]. It is interesting to note that kinase-defective PLK1 stably associates with centrosomes and this centrosome associated PLK1 prevents mitotic re-entry of cells with DNA damage [51]. Thus, it appears that PLK1 drives cells through distinct stages of the cell cycle by acting within different cellular compartments. Below, we discuss how PLK1 associates with different cellular compartments to regulate normal cell cycle progression without compromising the integrity of the genome.
3. The Role of PLK1 in Driving Cell Cycle Progression
3.1. PLK1 and DNA Replication
Premature cell cycle progression can drive replication stress and lead to double strand DNA breaks at unresolved single strand DNA replication forks. This can be driven by overactive mitotic kinases, including PLK1 [52]. To prevent this replication stress, the S-phase ATR checkpoint suppresses forkhead box protein M1 (FoxM1) until the G2 phase to inhibit mitotic entry and control expression of PLK1 and Cyclin B [53]. Accumulation and activation of PLK1 begins at the completion of S-phase [54]. Recent evidence shows that PLK1 is suppressed by active DNA replication and the completion of DNA replication releases this suppression, leading to increased activation of PLK1 through phosphorylation and triggering mitotic progression. This notion is supported by the finding that inhibitors of CHEK1, the key ATR effector, uncouples the sequential activation of PLK1 until after DNA replication is complete [52].
PLK1 can promote DNA replication in some contexts such as DNA damage-induced stress. Origin recognition complex subunit 2 (ORC2) phosphorylation by PLK1 helps promote DNA replication initiation and formation of the pre-replicative complex under stress, such as DNA-damaging ultraviolet (UV) irradiation [55]. PLK1 may also promote DNA replication through regulation of the DNA replication inhibitor geminin, as depletion of PLK1 was shown to slow DNA synthesis and stabilize geminin [56]. This suggests PLK1 can promote the progression of DNA replication, potentially even in the presence of replication stress.
3.2. PLK1 and Mitotic Entry
Cyclins and their CDKs are the drivers of the cell cycle, moving the cells forward towards successful cell division through signaling the transition through checkpoints via positive feedback loops. Cyclin B/CDK1 is the main kinase for mitotic entry following satisfaction of the G2/M phase checkpoint along with PLK1. PLK1 phosphorylates cyclin B in its nuclear export sequence, sequestering it in the nucleus prior to mitotic entry [57] and also phosphorylates CDC25C, which in turn activates cyclin B [58]. PLK1 and Cyclin B/CDK1 cooperatively act to positively regulate FoxM1. FoxM1 is a transcriptional regulator of G2/M phase genes. FoxM1 expression begins to increase in S phase and is phosphorylated by Cyclin B/CDK1 and PLK1 to increase its transcriptional activity of mitotic targets, such as AURKB, cyclin B, and PLK1 itself, in a positive feedback loop in preparation to irreversibly commit cells to mitotic entry [33].
3.3. PLK1 and Mitotic Entry Following DNA Damage
PLK1 is also a main component of the mitotic re-entry mechanism following cell cycle arrest. After prolonged DNA damage and subsequent cell cycle arrest, cells can adapt by silencing the checkpoint signaling to continue dividing despite the continued presence of DNA damage. This is referred to as checkpoint adaptation often found in tumor cells and results in increased genomic instability [59]. Phosphorylation by active PLK1 helps drive G2 checkpoint recovery by attenuating the ATM/ATR response and allowing mitotic entry despite DNA damage [60]. PLK1 acts on the DNA damage sensing Mre11/Rad50/Nibrin (MRN) complex, upstream of the ATM/ATR DNA damage checkpoint. Phosphorylation of the Mre11 subunit of the complex by PLK1 blocks activation of the checkpoint, or stops checkpoint signaling to allow re-entry into the cell cycle [61]. PLK1 kinase activity also drives G2 checkpoint adaptation following DNA damage by promoting the movement of p53 out of the nucleus and by promoting p53 degradation through ubiquitination [62,63]. PLK1 is also able to inhibit p53 transcription [64]. Thus, the increasing activity of PLK1 seems to be a mechanism by which cancer cells can continue cell division despite DNA damage, resulting in increased levels of CIN.
4. Role of PLK1 during Mitosis and Cytokinesis
4.1. PLK1 and Centrosome Function
Accurate chromosome segregation requires coordination of centrosome positioning, chromosome condensation, nuclear envelope breakdown, kinetochore-MT attachment, and spindle tension. In the normal centrosome cycle, centrioles disengage and duplicate to form two separate centrosomes, the centrosomes undergo elongation and maturation where they recruit components required for MT nucleation, and finally, separately migrate to opposite poles to form the bipolar spindle required for chromosome segregation in coordination with the cell cycle. In this context, CIN can arise from two mechanistically distinct scenarios: centrosome amplification, meaning the centrioles duplicate more than one time, or errors in centrosome positioning. Increased PLK1 expression is correlated with the presence of multiple centrosomes, suggesting it regulates centriole duplication early on in the centrosome cycle [2,65]. PLK1 and Separase activities are both required for cleavage of cohesin between centrioles, resulting in disengagement of centrioles in anaphase to allow for centriole duplication in the following interphase [66]. Centrosome duplication occurs once per cell cycle, so increased expression and activity of PLK1 could perceivably allow this process to occur inordinately. And indeed, PLK1 activity has been shown to drive centriole disengagement, allowing centriole reduplication outside of the typical centriole duplication [67]. Constitutively active PLK1 has been shown to protect against DNA damage-induced centrosome amplification in BRCA1-deficient cells [68]. Additionally, loss of Liver Kinase B1 (or STK11) leads to centrosome amplification through increased phosphorylation and activation of PLK1 [69], and BubR1 expression prevents centrosome amplification through inhibition of PLK1 [70]. The specific role of PLK1 in centrosome amplification requires more investigation, but PLK1 seems to be an important player in regulating centrosome number.
In addition to centrosome duplication, PLK1 also regulates centrosome maturation through recruitment of the pericentriolar material including the γ-tubulin ring complex (γ-TuRC). The γ-TuRC allows for MT nucleation, which forms the actual spindle fibers. The centrosomes and newly forming spindles separate to form the bipolar spindle essential for proper chromosome segregation. PLK1 and a PP1 subunit, MYPT1, work antagonistically to recruit γ-tubulin to the centrosomes required for MT nucleation, with PLK1 activity promoting recruitment [71,72]. Another way PLK1 promotes centrosome maturation for bipolar spindle formation is through the displacement of ninein-like protein (Nlp or NINL), an important component of the centrosome which promotes non-mitotic spindle MT nucleation during interphase by recruiting γ-TuRC, in later cell cycle [73]. PLK1 additionally regulates recruitment of γ-TuRC to the centrosome through the γ-TuRC targeting factor, NEDD1, by direct phosphorylation and indirectly through HAUS augmin-like complex subunit 6 (HAUS6 or FAM29A), which regulates NEDD1 localization [74,75]. PLK1 also interacts with and helps localize the MT-associated protein 9 (MAP9 or ASAP) to the centrosome, which again has a role in recruiting γ-TuRC [76]. PLK1 further stabilizes the centrosome structure for spindle formation through phosphorylation of the centrosome protein, Kizuna, and loss of Kizuna results in fragmented pericentriolar material and diffuse defective spindles [77]. All functions together highlight the importance of PLK1 in coordinating the number and formation of correct spindles originating from the centrosomes for equal bipolar cell division.
Centrosome clustering is a process that prompts centrosome-amplified cancer cells to form a pseudo-bipolar spindle that is mitosis competent, as multipolar cell divisions are often inviable [8]. Although chromosomal passenger complex and kinetochore tension sensing components have been proposed to be essential for centrosome clustering [18], PLK1 along with Stat3 and Stathmin, has also been directly linked to centrosome clustering by regulating γ-tubulin levels [78] as well as in the recruitment of Eg5 motor proteins [79,80], to promote cells through mitosis. Disruption of this process in particular is expected to have potent anticancer activity [81], and PLK1 may provide a mechanism to target centrosome clustering.
4.2. PLK1 and Chromosome Alignment
Sister chromatid cohesion and condensation are essential for proper diploid daughter cell formation. This is regulated by condensin I and II complexes, which both contain structural maintenance of chromosomes (SMC) proteins that form a ring structure and bind linear chromatin. Condensin I is present in interphase, while condensin II is active in mitotic cells. PLK1 regulates the levels of the CAP-H2 component in condensin II in early mitosis by phosphorylating CAP-H2 and protects condensin II from APC/C degradation [82]. Dysregulation of condensin II through PLK1 inhibition leads to anaphase segregation defects such as lagging chromosomes [82]. PLK1 also binds to condensin II through CAP-D3, which is primed by the initial CDK1-dependent phosphorylation, to promote further phosphorylation and condensin II activity [83]. PLK1 also phosphorylates the histone kinase Haspin, which in turn generates phospho-histone marks, like phosphoH3T3 to induce activation of AURKB and other chromosomal passenger proteins, which guide chromosome structure in mitosis [84,85].
Apart from regulating the condensin complex, PLK1 also regulates cohesin on the chromosome arms by phosphorylating this protein to promote its dissociation and thereby, dictates chromosome separation [86,87]. PLK1 also works with the PLK1-interacting checkpoint helicase (PICH or ERCC6L) to colocalize at the kinetochores with cohesion and loss of PICH leads to chromosome disorganization [88]. PLK1 acts to drive chromosome segregation forward, and one of the main functions of the SAC is to counteract PLK1-driven cohesin dissociation through PP2A antagonistic activity until all requirements for correct chromosome segregation are satisfied [87,89].
Paradoxically, while the SAC inhibits PLK1 activity, PLK1 has a role in establishing the SAC complex. PLK1 works with the dual specificity TTK protein kinase to recruit SAC components, Mad1 and Mad2. Dual inhibition of PLK1 and TTK leads to mitotic slippage or premature cell cycle progression, indicative of a weakened checkpoint response [90]. Normally, TTK signals to activate the SAC in response to unattached kinetochores, which signals Bub1 to recruit other SAC components, including PP2A, shugoshin 1 (SGO1) and interestingly, also PLK1, to the centromere [21,89]. PLK1 also acts upstream of TTK, where it needed for the full activation of this kinase [21]. PLK1 recruited by Bub1 phosphorylates Met-Glu-Leu-Thr (MELT) repeats of a kinetochore scaffold protein Knl1 [21], and these phospho-motifs act as scaffolding to recruit additional SAC components [91]. Defects in SAC function result in defective chromosome segregation, and this is another mechanism through which PLK1 dysregulation contributes to CIN.
4.3. PLK1 and Kinetochore-Microtubule Dynamics
In addition to its role in chromosome alignment, PLK1 has also been implicated in the maintenance of Kinetochore-MT attachments. The cytoplasmic linker protein CLIP-170 recruits PLK1 to kinetochores and loss of this interaction promotes kinetochore attachment defects [92]. PLK1 also recruits the MIS12 kinetochore complex assembly cochaperone protein, SUGT1 (or Sgt1) to kinetochores, which initiates the process of forming the kinetochore-MT attachments [93]. PLK1 activity at the kinetochores directly decreases MT dynamics, thereby stabilizing the kinetochore-MT attachments [94], and overly strong stabilization of MTs by overactive PLK1, promotes mis-attachments leading to CIN [95]. However, too dynamic or lax an attachment is also detrimental to cells if it results in insufficient tension to evenly pull apart sister chromatids. MYPT1 destabilizes kinetochore-MT attachments and this action is, at least in part, through dephosphorylation of PLK1 at kinetochores [96]. Cyclin A/CDK1 phosphorylates and recruits phosphatase MYPT1, a PP1 complex subunit, to antagonize and dephosphorylate PLK1, and increase the dynamic nature of the kinetochore-MT attachments [96]. Dysregulation of PLK1 can also contribute to CIN by prematurely generating kinetochore-MT attachments, leading to errors in chromosome segregation. Therefore, it can be concluded that dysregulation of PLK1 in either direction is sufficient to drive defects in the kinetochore attachments, thereby increasing chromosome segregation defects ultimately resulting in CIN.
Phosporylation of the kinetochore components is maintained by SAC signaling until sufficient spindle tension is present in the kinetochore-MT attachments for successful chromosome segregation at which time, the phosphorylation is removed and the signaling cascade activates APC/C [97]. PLK1 knockdown cells are still able to form kinetochore-MT attachments [98], however, PLK1 is required for maintenance of the inhibitory kinetochore tension-sensing phosphorylation epitope known as 3F3/2, which signals inhibition of APC/C by the SAC [97,99]. PLK1 also phosphorylates nuclear distribution protein C (NudC), causing its accumulation in early mitosis or when tension at the kinetochores is lost, which helps to maintain chromosome alignment until tension is achieved [100]. Interestingly, phosphorylation of PLK1 at serine 137 (S137) has been shown to bypass the constraints of the SAC [47,94]. This would further promote CIN in the context of PLK1 overexpression.
4.4. PLK1 and Cytokinesis
Just after the chromosomes successfully segregate during anaphase, interpolar spindles begin to accumulate and overlap with opposing directionality in the equatorial plane between the two spindle poles to form the central spindle. The central spindles are able to push against one another through the action of MT-associated proteins, such as PRC1 and centrosome protein CEP55, and kinesin motor proteins, such as kinesin family member 20A (KIF20A or MKlp2) [101]. PLK1 localizes to the central spindle with KIF20A following metaphase [102], but is not strictly required for central spindle formation [49]. PLK1 also interacts with other kinesin family members, such as KIF2C (or MCAK) and KIF23 (or MKlp1), which are important for the movement of the spindles between daughter cells and maintaining chromosome stability [103,104,105]. Prolonged PLK1 activity caused by blocking either dephosphorylation or degradation delays mitotic exit [106], while overexpression of PLK1 leads to abscission defects [107]. PLK1 inhibition can lead to cell division without cytokinesis through mis-localization of anillin and myosin II and the small GTPase Ras homolog gene family, member A (RhoA), which leads to contractile ring defects and consequently, binucleate cells [49]. This suggests PLK1 helps to ready the cell for the formation of the contractile ring and correct abscission.
The timing and location of the contractile ring and cleavage furrow ingression is critical for even distribution of the daughter cell chromosomes and organelles. Cytokinesis in cells with incompletely separated chromosomes can result in aneuploid daughter cells, as seen with PLK1 dysregulation through inhibition of PLK1 activity and through PLK1 overexpression, both resulting in binucleate and polyploid cells [49,107]. Relevant to this, PLK1 regulates the timing and location of RhoA signaling to form the contractile ring and initiate cleavage furrow ingression driven by the MT motor complex centralspindlin [108]. PLK1 regulates recruitment of centralspindlin through PRC1 [108]. PLK1-produced phosphorylation forms the docking site in the central spindle for epithelial cell transforming 2 (Ect2), a Rho-specific guanine nucleotide exchange factor important for cleavage furrow formation [109,110]. Correct localization of Ect2 to the central spindle is required for correct positioning of the RhoA signaling cascade and the resulting cleavage furrow ingression [49]. PLK1 also stabilizes CEP55 and causes it to dissociate from the centrosome, allowing it to re-localize to the midbody just prior to abscission [111]. These movements are tightly regulated to prevent an early initiation of cytokinesis [112,113]. Dysregulation through PLK1 overexpression leads to mis-localization of CEP55 and disrupts abscission, also resulting in binucleate cells [107]. Timing of cytokinesis until after chromosome segregation is important to prevent CIN and aneuploidy and is partially regulated by PLK1.
5. PLK1 and Maintenance of DNA Integrity
5.1. PLK1 and DNA Damage Checkpoint Function
Before cells enter mitosis, the DNA damage checkpoint monitors any injury to DNA molecules to prevent mitotic entry, while DNA damage is present. ATR, ATM, and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) are the main mediators that activate the signaling cascade to arrest cells in the presence of DNA damage and work in concert with the mitotic kinases, including PLK1. Inhibiting ATM-induced arrest leads to increased PLK1 kinase activity, suggesting ATM inhibits PLK1 [114,115]. Conversely, ATM activity in response to DNA damage inhibits activation of PLK1 by phosphorylation and promotes PLK1 dephosphorylation by PP2A [116,117]. ATR-mediated checkpoint response has not been shown to induce the same effect in all cases [116], but ATR has been shown to be capable of inhibiting PLK1 in ATM-deficient cells [115]. Another cell cycle kinase, AURKA, and its activator, BORA, phosphorylate PLK1 at T210 to activate PLK1, and this phosphorylation is inhibited in response to DNA damage [118]. Increased PLK1 activity opposes checkpoint signaling and is required for re-entry into mitosis following DNA damage induced G2/M phase arrest resulting from UV irradiation [119]. DNA-PKcs physically interacts with PLK1 and promotes its activation through phosphorylation. PLK1 also phosphorylates DNA-PKcs in return, in preparation for mitotic entry [120,121]. Additionally, PLK1 opposes activation of a cell cycle arrest effector, the CHEK2 kinase, which acts downstream of ATM and DNA-PKcs [119]. One of CHEK1 upstream regulator proteins, Claspin, which is an adaptor protein that binds both BRCA and DNA, is also negatively regulated by PLK1 [122]. Mitotic re-entry following DNA damage and inhibition of ATM can also be driven by increased phosphorylation of PLK1 by AURKA [123]. These checkpoints function to control cell cycle arrest through regulating PLK1 activity in response to DNA damage, thereby preventing mitotic progression. PLK1 also interacts directly with sensors of DNA damage to regulate cell cycle arrest. PLK1 phosphorylates the Rad9 component of the Rad9A-Hus1-Rad1 (9-1-1) protein complex that localizes to sites of DNA damage and promotes checkpoint-mediated arrest through ATR and double strand DNA break (DSB) repair mechanisms. The phosphorylation of Rad9 by PLK1 suppresses this checkpoint activation [124]. DNA damage during mitosis has also been found to inhibit PLK1, although this mechanism was found to be both ATM and p53 independent [125].
5.2. PLK1 and DNA Damage Repair Pathways
PLK1 is a modulator of the homologous recombination (HR) pathway, acting on both the proteins that comprise the MRN complex and the Rad51 recombinase. Within the MRN complex, PLK1 phosphorylates Mre11 at residue S649, which can inhibit MRN complex localization and recruitment of both HR and non-homologous end-joining (NHEJ) repair proteins to sites of DNA damage [61]. This allows for premature checkpoint termination, and consequently re-entry into the cell cycle despite unrepaired lesions [61]. Conversely, PLK1 directly phosphorylates the Rad51 recombinase, stimulating a cascade of events that facilitate further phosphorylation of Rad51 by casein kinase 2 (CK2) and resulting in binding to the MRN complex componentNbs1 [126]. This response increases the presence of Rad51 at sites of DNA damage and enhances the overall responsiveness of the HR pathway, contributing to cell proliferation and tumorigenesis. PLK1 also phosphorylates BRCA2, a stabilizer of Rad51 that promotes HR, and this phosphorylation is inhibited by DNA damage to promote HR-mediated repair [127]. Aberrant PLK1 phosphorylation of BRCA2 can help override this DNA damage checkpoint to enhance the transition to mitosis, regardless of whether or not DNA damage has been successfully repaired [127]. This represents another mechanism, where elevated PLK1 activity has an essential function in tumor cells and could provide a targetable vulnerability.
While cells in S and G2 phases look to HR for DSB repair, those in G1 and M phases may depend on the more error prone NHEJ repair network for resolution. Unlike HR, NHEJ does not require sister chromatids to serve as template DNA and can therefore, be called upon at any point during the cell cycle. Interestingly, PLK1 has been shown to inhibit double strand DNA break repair through NHEJ during mitosis as a mechanism to prevent telomeric fusions and genomic instability [128]. PLK1 does this jointly with CDK1 through phosphorylation of 53BP1 and X-ray cross complementing (XRCC4) to inhibit their localization to DNA, thereby inhibiting NHEJ [128,129,130]. XRCC4 is a critical partner of DNA ligase IV in the completion of NHEJ [128]. PLK1-mediated phosphorylation of 53BP1 prevents binding of ubiquitinated histones and localization to DNA damage foci for signaling [129]. While PLK1 prevents erroneous telomeric fusions in mitosis, dysregulation of PLK1 could also disturb necessary double strand DNA break repairs at other stages of the cell cycle.
5.3. PLK1 and Telomerase
One unique DNA structure that is important to the linear chromatin maintenance is the telomere. Telomeres are repetitive DNA sequences that protect the chromosome ends from replicative degradation and erroneous recognition and ligation as DSBs [131]. Telomeres work in concert with the shelterin complex which is composed of telomeric repeat binding factor 1 and 2 (TERF1, TERF2), protection of telomeres protein 1 (POT1), TERF1 interacting nuclear factor 2 (TINF2), tripeptidyl-peptidase 1 (TPP1), and repressor activator protein 1 (RAP1) [132]. Chromosome ends are prone to dysfunction and degradation leading to CIN, particularly when telomerase action is suboptimal relative to the cell division activity [133]. When chromosome ends go uncapped by the shelterin complex, they are prone to fusion through recognition by the double strand DNA damage repair pathways, such as NHEJ, and these fusions can drastically alter both chromatin structure and sequence [133]. Human telomerase reverse transcriptase (hTERT) replicates and lengthens the telomere sequence on chromosome ends to prevent telomere shortening, which leads to cellular senescence and apoptosis. Adult human tissues do not continually express hTERT, with low or limited activity being observed at each cell division, which effectively limits the number of the allowed cell divisions. This mechanism, however, is bypassed in cancer, as activating mutations of the promoter region in the hTERT gene is one of the most frequently observed alteration contributing to oncogenesis [134,135].
In regards to this, PLK1 interacts with hTERT, and hTERT activity positively correlates with PLK1 activity, suggesting enhanced PLK1 action leads to increased telomere maintenance [131]. PLK1 inhibition results in increased telomeric fusions, suggesting a loss of the protective telomere function [136]. In this context, PLK1 could act through the stabilization of hTERT by inhibiting its ubiquitination-mediated degradation via PLK1-provided phosphorylation [131]. PLK1 also indirectly regulates hTERT activity by the stabilizing phosphorylation of tankyrase (TNKS), a poly-ADP-ribose polymerase (PARP) that enhances hTERT activity [136]. Phosphorylation of TNKS by PLK1 inhibits binding of a negative regulator of telomere length, TERF1, to telomeric DNA, allowing telomerase elongation [137]. Conversely, PLK1 also phosphorylates TERF1 and increases its affinity for the telomeres [138]. PLK1 also regulates TERF1 via PINX1 [139]. PLK1 overexpression promotes PINX1 proteasomal degradation through phosphorylation of PINX1, allowing telomerase access to the telomeres [140]. Telomere lengthening activity is often observed in cancer cells as a mechanism used to limit DNA damage caused by increased proliferation [133]. Telomere lengthening activity is in part upregulated by PLK1, providing another potential PLK1-induced vulnerability.
Telomerase inhibition in anticancer therapies has been widely pursued and trialed, as it is aimed for a target that, similarly to PLK1, is distinguished in cancer cells from normal cells almost ubiquitously [141]. However, in vitro results have been largely more promising than in vivo, with little success observed at clinical stages [142,143]. The arising issues with telomerase inhibitors have varied from induction of high levels of genomic instability in surviving cells, which actually increases tumor aggressiveness, to resistance provided by alternative telomere lengthening mechanisms [144]. Nonetheless, some drugs proved to be more successful. Thus, Imetelstat (GRN163L), a telomerase inhibitor, is about to enter Phase III clinical trials for hematologic myeloid malignancies, having previously demonstrated effectiveness in esophageal squamous cell carcinoma [145], lung cancer [146,147], and blood cancers [148,149,150,151,152] by decreasing tumor growth and increasing tumor sensitivity to radiation. A detailed dissection of the interplay between PLK1 and telomerase could provide an effective strategy for further improvements in this direction.
6. Targeting Cancer Cells through PLK1
PLK1 itself is an attractive target for the development of cancer therapeutics because there is a mass of evidence indicating that it is highly overexpressed selectively in tumor cells compared to the healthy adult tissues. This pattern of increased expression is reflective of a common trend of the amplification of proto-oncogenes [153]. Studies revealing PLK1 overexpression have been done in melanoma [154], non-small cell lung cancer [155], head and neck squamous cell carcinoma [156], ovarian and endometrial cancers [157,158], esophageal squamous cell carcinoma [159], hepatocellular carcinoma [160,161], hepatoblastoma [162], colorectal cancers [163], bladder cancers [2], gastric carcinoma [164], pancreatic cancer and cancer cell lines [165], gliomas [166], breast cancers [167], including triple-negative breast cancer [168], and castration-resistant prostate cancer cell lines [169]. In many cases, overexpression of PLK1 correlates with poor prognosis [155,156], tumorigenicity and aggressiveness [170], and tumor-initiating cell (TIC) propagation [171,172]. This consistency across tumor types combined with minimal expression in normal tissues and its extensive role in chromosomal instability highlights strong PLK1 potential as a target for a tumor-agnostic cancer therapy.
6.1. PLK1 Inhibitors as Therapeutic Agents and Associated Challenges
The combination of PLK1s role in CIN and its widespread overexpression across cancer types has led to the development and intensive study of PLK1 inhibitors as possible cancer therapeutics. The first assessment of inhibiting PLK1 as an anti-cancer strategy was done with anti-sense oligonucleotides in 2002 [173]. The first chemical inhibitors gained attention for their anti-cancer effects shortly after [174], albeit the first PLK1 inhibitors were of a broader specificity. Later, more specific inhibitors have been developed, but overall clinical outcomes are still being modest at best. This is despite many pre-clinical studies showing PLK1 inhibition reduces tumor growth in various cell line-based models [165,175,176] and accumulated evidence, showing that PLK1 inhibition can cause lethality in TICs [171,177,178]. Since PLK1 is a part of a very intricate cell cycle signaling network, the type and timing of inhibition may be one of the factors contributing to poor or inconsistent clinical results. Indeed, one study found PLK1 inhibition to cause both radiosensitization and radioresistance, depending on the timing of treatment [179], and another work found inhibition causing either metaphase arrest or G2 phase arrest depending on inhibitor doses applied [180].
ON 01910.Na is a non-competitive multi-kinase inhibitor and was one of the first PLK1 inhibitors introduced [174]. This compound also targets other kinases, including PI3K. ON 01910.Na was tested in clinical trials up to phase III in combination with gemcitabine, but the outcome of the combination therapy was found to be 19% of patients with partial response, in comparison with 13% of patients with partial response for gemcitabine alone, with an overall increase of median survival of 3 months [181]. This poor improvement in patients, along with other studies reporting relatively low specific activity of ON 01910.Na towards PLK1 compared to newer agents, such as the ATP-binding competitor BI 2536 [182] has led to this compound being considered mostly unsuccessful.
A large group of other PLK1 inhibitors are the ATP-binding competitors based on the dihydropteridinone class of compounds, which act to inhibit PLK1 kinase activity. The most well-known members of this group are BI 6727 (or volasertib) and BI 2536. BI 2536 showed a low response rate in clinical trials [183], but is still widely used in pre-clinical work for studying the effects of PLK1 inhibition in cell line models [123,169,171,182]. Volasertib is based on the same prototype as BI 2536 but has been more successful. It shows the most promise as an anti-cancer agent in acute myeloid leukemia (AML) and it was granted the FDA Breakthrough Therapy status in 2013 in order to expedite the clinical trial process in critical illnesses [184]. However, it is yet to be FDA-approved. Of the Phase II volasertib trials that have been completed, one study showed a modest increase of 5.6 versus 2.3 months with cytarabine combination therapy in AML, one study showed insufficient improvement in metastatic urothelial cancer [185], and another study showed insufficient improvement in non-small cell lung cancer [186].
A group of thymoquinone derivative inhibitors, poloxin and poloxin-2, target the PBD motif of PLK1 [187,188]. These compounds disrupt PLK1 localization and show a phenotype of chromosome segregation defects and activation of the SAC [187,189], but these compounds are still early in development. TAK960, a pyrimidodiazepinone-based PLK1 inhibitor, acts as an ATP-competing reagent and is also in early developmental stage [190,191]. NMS-P937 is yet another new generation ATP-competitive PLK1 inhibitor, identified using structure-driven drug design on the pyrazoloquinazoline scaffold [192,193]. GSK461364, an older ATP-competing inhibitor, has entered phase I clinical trials for safety. GSK461364 is less potent towards PLK2 and PLK3 over PLK1 by 300 fold [180], but was found to cause a high incidence of side-effects such as venous thrombotic emboli [194]. Most recently, highly selective ATP competitive inhibitors, PCM-075 (onvansertib) and CYC140, entered into early clinical trials (clinicaltrials.gov identifiers NCT03884829 and NCT03414034). Overall, PLK1 inhibition has not had success as a targeted cancer therapy despite high anticipation arising from promising pre-clinical investigations. This is somewhat surprising, since the rationale for targeting PLK1 is strongly supported in theory by the accumulated preclinical data and our understanding of its role in cancer cells. The lack of response to PLK1 inhibitors could be explained through either non-specific effects of the PLK1 inhibitors, or to resistance to PLK1 inhibition. In particular, adaptive resistance is displayed by CIN tumor cells [195]. For example, cancer cells adapt to decreased microtubule-kinetochore dynamics and suppressed CIN induced by treatment with KIF2C/MCAK inhibitors by adjusting AURKB levels, ultimately returning the cancer cells to their original level microtubule-kinetochore dynamics and chromosome mis-segregation [195]. As a regulator of CIN and microtubule-kinetochore dynamics, it is plausible PLK1 inhibition could elicit similar acquired resistance.
The structural similarity of the kinase and PBD domains in other PLK family members and non-specific targeting may be another source of challenges in PLK1-based therapies. The other PLK family proteins have diverse cellular roles [29]. PLK2 has a biological role in G1 phase progression and centriole duplication [196,197]. It is a p53 target gene and might function as a tumor suppressor as it is often downregulated in cancer [198,199,200]. Of interest in regard to toxicities resulting from non-specific PLK inhibition, PLK2 has a well-established role in neural plasticity and in this context, inhibitors targeting PLK2 may do more harm than help [201,202]. PLK3 has a role in Golgi fragmentation [203]. It is also suggested as a tumor suppressor due to its upregulation following p53-mediated DNA damage response and oxidative stress response, and its downregulation in some malignancies [204,205,206]. It has also been shown to promote tumor initiation in vivo [207]. PLK4 and PLK5 are structurally more divergent than PLKs 1, 2, and 3 [208] and therefore, should be to a lesser extent non-specifically affected by PLK1 inhibitors. Overall, the functions of the other PLK family proteins are much less studied in comparison to PLK1, they may have currently unknown functions critical to healthy cell biology and thus, caution is required, when using PLK inhibitors in treatment protocols [29]. This is further confounded by issues associated with the diverse functions of PLK1 itself associated with its complex contribution to CIN.
Targeting CIN to address the source of tumor heterogeneity is an appealing idea but must be approached carefully. While CIN can promote tumorigenesis in some cases such as when the increased rate of mutations gives rise to a growth advantage, it also can inhibit tumorigenesis in other cases where CIN-driven aneuploidy causes a growth defect or is incompatible with cell division [209]. The paradoxical aspect of CIN and its role in tumorigenesis is an emerging area of study in cancer. Most studies attribute this to very complex regulatory mechanisms dedicated to ensuring genomic stability, where too big or too small shift in either direction might produce undesirable effects. On the tumorigenic side, elevated CIN leads to an increased array of mutations and phenotypes in the heterogenous tumor cell population, some of which will likely confer a growth advantage or drug resistance in the altered tumor microenvironment [210]. However within the tumor suppressive response, extremely high levels of CIN are just not compatible with cell viability, as eventually, the accumulation of DNA defects will render cancer cells unable to complete mitosis or carry out other vital cell processes, causing their elimination [210]. Consistent with these ideas, it has been shown that CIN levels dictate whether it acts in a tumor suppressive or pro-oncogenic manner [209,211], and the worst prognoses are associated with low to intermediate CIN rather than its higher levels [212]. One explanation for this paradoxical behavior, CIN itself can impede tumor growth due to the increased defect burden, and so reducing this CIN burden contributes to increased cell growth, This could explain inconsistent results regarding the effect of PLK1 on tumor initiation versus tumor inhibition, with some studies showing PLK1 driving oncogenic transformation and increased growth advantage but others showing PLK1 overexpression decreasing tumor initiation rates [107,213,214,215]. Because the level of CIN determines its impact in cancer, inhibiting high CIN to intermediate tumorigenic levels is a real risk and altering PLK1 activity could produce unpredictable outcomes. Indeed, PLK1 haplo-insufficient mice displayed widespread aneuploidy accompanied by a higher incidence of tumor formation [39], and inhibition of PLK1 in an adenomatous polyposis coli protein (APC)-truncated context impaired SAC function and led to increased CIN and tumor formation [214], reminiscent of a tumor suppressor-like function in tumorigenesis. However, it should be noted that results generated in in vitro cell line work may not accurately reflect the tumor environment and selective pressures exerted on CIN cells that exist in animal models and human cancers. Inhibition of PLK1 might increase rather than decrease tumor development in some cases. These mechanisms of this context-dependent tumorigenesis versus tumor suppression may not be unique to PLK1, and may be a result of CIN regulatory feedback loops that aid cellular response to insult [195], but nonetheless, these conflicting results show the importance of the complex role of CIN and PLK1 and using a less conventional approaches to indirect targeting PLK1 overexpression in cancer. One of these strategies could be based on targeting genetic interactions of PLK1 instead of the direct inhibition of its activity.
6.2. Genetic Interactions of PLK1 as Therapeutic Targets
Genetic interactions represent a concept pioneered in yeast studies and have become popular in cancer research with the advent of large-scale genomic studies. Genomics and associated technologies have allowed complex genetic interaction networks to be more easily identified in comprehensive unbiased ways [216,217]. Genetic interactions are defined as an unexpected phenotype resulting from the combination of mutations whose individual mutation phenotypes are not additive, but rather synergistic [218]. These genetic interactions are grouped into two main classes, negative and positive. Negative genetic interactions result in synergistic decreases in cell viability or cell death, whereas positive genetic interactions provide a growth advantage to cells [219]. Negative genetic interactions are the focus in target discovery in cancer research, since cancer is accompanied by array of deleterious genetic alterations that provide a route for identifying a matching synergistic negative interaction to provide valuable potential targets revealed by high through-put screening. These screens allow to generate comprehensive gene interaction maps for multiple cancer-related mutations [220,221,222]. The two main types of negative genetic interactions are synthetic lethality (SL) and synthetic dosage lethality (SDL). The main difference between these two interactions is the nature of gene alterations involved. SL refers to the combination of two loss-of-function alterations, and SDL refers to the combination of a gain-of-function alteration and a loss-of-function alteration [220,221]. In case of PLK1, the aim should be to identify its SDL partners loss-of-function or inhibition of which results in cell lethality only when PLK1 overexpression is present.
It is important to reliably identify effective therapeutic targets to improve treatments, but often, as in the case of PLK1, directly targeting a seemingly promising cancer-related molecule produces strong harmful side effects or unpredictable outcomes. That is where the SDL strategy comes into play, as it provides an alternative approach to targeting overexpressed or overactivated molecules that does not rely on their direct inhibition. Instead, this method aims to inhibit their SDL partners, which can be proteins with less varied functions, which is expected to widen the therapeutic window for safe successful treatment. In particular, in the case of PLK1 and PLK1-related CIN, where too much and too little activity are both highly detrimental to cells, using an SDL approach should circumvent any risks associated with the induction of oncogenic CIN levels.
Studies proposing SL or SDL strategies as a way to indirectly target CIN seem to show more promise than approaches to directly target CIN-controlling molecules. An example of the success of this strategy is targeting the SL interaction between PARP and the breast cancer susceptibility gene (BRCA) in cancer [223,224]. PARP and BRCA both have roles in DNA damage repair, PARP through the resolution of single strand breaks and BRCA through homologous repair, and therefore they both individually control CIN. In addition to displaying SL with each other, PARP and BRCA also show SL with other targets within CIN pathways [210]. Emerging evidence has extended the list of SL of PARP to include a variety of DNA damage genes, such as MRE11 [225], ATR [226], ATM [227], and RAD54B [228]. It might be also possible to map the SL interactions between the DNA damage pathways rather than the individual genes and use this information for treatment development [210]. As shown in Figure 1A, many mitotic kinases in addition to PLK1 are elevated across different cancer types. Regardless of whether this overexpression of components regulating CIN is a mechanism of cancer cells to facilitate tumorigenicity or is a result of increased CIN and the presence of an increased number of chromosomes, as often seen in cancers, is a question for future studies, these mitotic kinases represent a source of cancer-specific alterations for SDL target study. The identification of SDL interactions of PLK1 in the processes promoting CIN, as discussed in this review, is expected to provide novel options for developing effective anti-cancer therapies that would bypass dangers associated with its direct inhibition.
7. Conclusions
PLK1 is a mitotic kinase near-ubiquitously overexpressed in cancer, with a key multifaceted role in the maintenance of chromosomal stability. It is also a highly attractive therapeutic target for cancer based on these characteristics and a mass of pre-clinical studies. However, PLK1 action is extremely complex and varied, and is far from being sufficiently understood. PLK1 can contribute to CIN and therefore tumor heterogeneity through the dysregulation of mitotic entry by overriding mitotic checkpoints to drive cancer cells to mitosis before DNA replication has been properly resolved or DNA damage repaired, through dysregulation of centrosome duplication, maturation, and the formation of the mitotic spindle, through premature chromosome segregation by overriding the SAC, through improper chromosome segregation due to altered kinetochore dynamics, and through altered telomere function. Using PLK1 as a therapeutic target needs to be approached expertly to avoid undesirable outcomes due to the complex role of the level of CIN and the role of PLK1 in essential cell processes. Identifying the SDL interactions of PLK1 will provide a valuable information for developing effective precision therapies for treating PLK1-overexpressing malignancies.
Acknowledgments
We thank members of the Vizeacoumar and Freywald laboratories for their critical comments and insights.
Abbreviations
| PLK1 | Polo-like kinase 1 |
| CIN | Chromosomal instability |
| SDL | Synthetic dosage lethalality |
| MT | Microtubule |
| CDK1 | Cyclin dependent kinase 1 |
| AURKA/B/C | Aurora kinases A, B, C |
| NEK2 | NIMA related kinase 2 |
| CDC25C | Cell division cycle 25C |
| Bub1 | Budding uninhibited by benzimidazoles 1 |
| BubR | Budding uninhibited by benzimidazole-related |
| SAC | Spindle assembly checkpoint |
| APC/C | Anaphase promoting cyclosome proteasome complex |
| PBD | Polo-box domain |
| Hsp90 | Heat shock protein 90 |
| NLS | Nuclear localization signal |
| ATR | Ataxia-telangiectasia and Rad3 related |
| FoxM1 | Forkhead box protein M1 |
| CHEK1/2 | Checkpoint kinase 1/2 |
| ORC2 | Origin recognition complex subunit 2 |
| MRN | Mre11/Rad50/Nibrin |
| ATM | Ataxia-telangiectasia mutated |
| STK11 | Liver kinase B1 |
| γ-TuRC | γ-tubulin ring complex |
| Nlp or NINL | Ninein-like protein |
| NEDD1 | Neural precursor cell expressed developmentally down-regulated protein 1 |
| HAUS6 | HAUS augmin-like complex subunit 6 |
| MAP9 | MT-associated protein 9 |
| SMC | Structural maintenance of chromosomes |
| PICH | PLK1-interacting checkpoint helicase |
| PP2A | Protein phosphotase 2A |
| SGO1 | Shugoshin 1 |
| MELT | Met-Glu-Leu-Thr |
| CLIP-170 | Cytoplasmic linker protein |
| SGT1 | Suppressor of G2 allele of SKP1 |
| NudC | Nuclear distribution protein C |
| PRC1 | Protein regulator of cytokinesis 1 |
| CEP55 | Centrosomal protein 55 |
| KIF20A | Kinesin family member 20A |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| BRCA1/2 | Hereditary breast cancer type 1/2 susceptibility protein |
| HR | Homologous recombination |
| NHEJ | Non-homologous end-joining |
| CK2 | Casein kinase 2 |
| DSB | Double strand DNA break |
| XRCC4 | X-ray cross complementing 4 |
| 53BP1 | TP53 binding protein |
| TERF1 | Telomeric repeat binding factor 1 |
| POT1 | Protection of telomeres protein 1 |
| TINF2 | TERF1 interacting nuclear factor 2 |
| TPP1 | Tripeptidyl-peptidase 1 |
| RAP1 | Repressor activator protein 1 |
| hTERT | Human telomerase reverse transcriptase |
| TNKS | Tankyrase |
| PARP | Poly-ADP-ribose polymerase |
| PINX1 | PIN2 Interacting Telomerase Inhibitor 1 |
| TIC | Tumor-initiating cell |
| AML | Acute myeloid leukemia |
| APC | Adenomatous polyposis coli protein |
| SL | Synthetic lethality |
| RAD54B | RAD54 homolog B |
Author Contributions
Conceptualization F.J.V., A.F. and C.E.C.; writing—draft preparation C.E.C. and M.J.M.; formal analysis F.S.V.; visualization C.E.C.; writing—review C.E.C., M.J.M., O.A., F.S.V., A.F., and F.J.V.; project administration and funding acquisition A.F. and F.J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the operating grants from Canadian Institute of Health Research (PJT-156309; PJT-156401) and Cancer Research Society (22493) to F.J.V. and A.F. C.E.C. is supported by Lisa-Rendall Fellowship from the Saskatchewan Cancer Agency. M.J.M. is supported by CoMGRAD award from the University of Saskatchewan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y., Matsuyama H., Kawauchi S., Matsumoto H., Nagao K., Ohmi C., Sakano S., Furuya T., Oga A., Naito K., et al. Overexpression of polo-like kinase 1 (PLK1) and chromosomal instability in bladder cancer. Oncology. 2006;70:231–237. doi: 10.1159/000094416. [DOI] [PubMed] [Google Scholar]
- 3.Lengauer C., Kinzler K.W., Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;10:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 4.Weaver B.A., Cleveland D.W. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Smiraldo P.G., Gruver A.M., Osborn J.C., Pittman D.L. Extensive chromosomal instability in Rad51d-deficient mouse cells. Cancer Res. 2005;65:2089–2096. doi: 10.1158/0008-5472.CAN-04-2079. [DOI] [PubMed] [Google Scholar]
- 6.Burrell R.A., McClelland S.E., Endesfelder D., Groth P., Weller M.-C., Shaikh N., Domingo E., Kanu N., Dewhurst S.M., Gronross E., et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494:492–496. doi: 10.1038/nature11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H., Lampson M., Efimov A., Yen T.J. Chromosome instability in tumor cells due to defects in Aurora B mediated error correction at kinetochores. Cell Cycle. 2018;17:2622–2636. doi: 10.1080/15384101.2018.1553340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganem N.J., Godinho S.A., Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silkworth W.T., Cimini D. Transient defects of mitotic spindle geometry and chromosome segregation errors. Cell Div. 2012;7:19. doi: 10.1186/1747-1028-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber T.D., McManus K., Yuen K.W.Y., Reis M., Parmigiani G., Shen D., Barrett I., Nouhi Y., Spencer F., Markowitz S. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc. Natl. Acad. Sci. USA. 2008;105:3443–3448. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama Y., Soeda S., Ikeuchi M., Kakae K., Yamaguchi N. Cytokinesis failure leading to chromosome instability in v-SRC-induced oncogenesis. Int. J. Mol. Sci. 2017;18:811. doi: 10.3390/ijms18040811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boukamp P., Popp S., Krunic D. Telomere-dependent chromosomal instability. J. Investig. Dermatol. Symp. Proc. 2005;10:89–94. doi: 10.1111/j.1087-0024.2005.200401.x. [DOI] [PubMed] [Google Scholar]
- 13.Nigg E.A. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 14.Gheghiani L., Loew D., Lombard B., Mansfeld J., Gavet O. PLK1 activation in late G2 sets up commitment to mitosis. Cell Rep. 2017;19:2060–2073. doi: 10.1016/j.celrep.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Helps N.R., Luo X., Barker H.M., Cohen P.T. NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem. J. 2000;349:509–518. doi: 10.1042/bj3490509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Luca M., Lavia P., Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle. 2006;5:296–303. doi: 10.4161/cc.5.3.2392. [DOI] [PubMed] [Google Scholar]
- 17.Courthéoux T., Reboutier D., Vazeille T., Cremet J.-Y., Benaud C., Vernos I., Prigent C. Microtubule nucleation during central spindle assembly requires NEDD1 phosphorylation on serine 405 by Aurora, A. [(accessed on 18 September 2020)];J. Cell Sci. 2019 132 doi: 10.1242/jcs.231118. Available online: http://jcs.biologists.org/content/132/10/jcs231118. [DOI] [PubMed] [Google Scholar]
- 18.Leber B., Maier B., Fuchs F., Chi J., Riffel P., Anderhub S., Wagner L., Ho A.D., Salisbury J.L., Boutros M., et al. Proteins required for centrosome clustering in cancer cells. Sci. Transl. Med. 2010;2:33–38. doi: 10.1126/scitranslmed.3000915. [DOI] [PubMed] [Google Scholar]
- 19.Tipton A.R., Ji W., Sturt-Gillespie B., Bekier M.E., Wang K., Taylor W.R., Liu S.T. Monopolar spindle 1 (MPS1) kinase promotes production of closed MAD2 (C-MAD2) conformer and assembly of the mitotic checkpoint complex. J. Biol. Chem. 2013;288:35149–35158. doi: 10.1074/jbc.M113.522375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golan A., Yudkovsky Y., Hershko A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J. Biol. Chem. 2002;277:15552–15557. doi: 10.1074/jbc.M111476200. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda M., Tanaka K. Plk1 bound to Bub1 contributes to spindle assembly checkpoint activity during mitosis. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-09114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira J.G., Pereira A.J., Akhmanova A., Maiato H. Aurora B spatially regulates EB3 phosphorylation to coordinate daughter cell adhesion with cytokinesis. J. Cell Biol. 2013;201:709–724. doi: 10.1083/jcb.201301131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao W. The mitotic checkpoint in cancer therapy. Cell Cycle. 2005;4:1495–14999. doi: 10.4161/cc.4.11.2130. [DOI] [PubMed] [Google Scholar]
- 24.Prevo R., Pirovano G., Puliyadi R., Herbert K.J., Rodriguez-Berriguete G., O’Docherty A., Greaves W., McKenna W.G., Higgins G.S. CDK1 inhibition sensitizes normal cells to DNA damage in a cell cycle dependent manner. Cell Cycle. 2018;17:1513–1523. doi: 10.1080/15384101.2018.1491236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokuryo T., Yokoyama Y., Yamaguchi J., Tsunoda N., Ebata T., Nagino M. NEK2 Is an effective target for cancer therapy with potential to induce regression of multiple human malignancies. Anticancer Res. 2019;39:2251–2258. doi: 10.21873/anticanres.13341. [DOI] [PubMed] [Google Scholar]
- 26.Tang A., Gao K., Chu L., Zhang R., Yang J., Zheng J. Aurora kinases: Novel therapy targets in cancers. Oncotarget. 2017;8:23937–23954. doi: 10.18632/oncotarget.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricke R.M., Jeganathan K.B., van Deursen J.M. Bub1 overexpression induces aneuploidy and tumor formation through Aurora B kinase hyperactivation. J. Cell Biol. 2011;193:1049–1064. doi: 10.1083/jcb.201012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y., Wang A., Lin J., Wu L., Zhang H., Yang X., Wan X., Miao R., Sang X., Zhao H. Mps1/TTK: A novel target and biomarker for cancer. J. Drug Target. 2017;25:112–118. doi: 10.1080/1061186X.2016.1258568. [DOI] [PubMed] [Google Scholar]
- 29.Lens S.M.A., Voest E.E., Medema R.H. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 30.Cheng K.-Y., Lowe E.D., Sinclair J., Nigg E.A., Johnson L.N. The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 2003;22:5757–5768. doi: 10.1093/emboj/cdg558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elia A.E.H., Cantley L.C., Yaffe M.B. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 32.Seong Y.-S., Kamijo K., Lee J.-S., Fernandez E., Kuriyama R., Miki T., Lee K.S. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 2002;277:32282–32293. doi: 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- 33.Fu Z., Malureanu L., Huang J., Wang W., Li H., van Deursen J.M., Tindall D.J., Chen J. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat. Cell Biol. 2008;10:1076–1082. doi: 10.1038/ncb1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraft C., Herzog F., Gieffers C., Mechtler K., Hagting A., Pines J., Peters J.-M. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan E.H.Y., Santamaria A., Silljé H.H.W., Nigg E.A. Plk1 regulates mitotic Aurora A function through βTrCP-dependent degradation of hBora. Chromosoma. 2008;117:457–469. doi: 10.1007/s00412-008-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golsteyn R.M., Schultz S.J., Bartek J., Ziemiecki A., Ried T., Nigg E.A. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J. Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 37.Holtrich U., Wolf G., Bräuninger A., Karn T., Böhme B., Rübsamen-Waigmann H., Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. USA. 1994;91:1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan J., Hörlin A., Hock B., Stutte H.J., Rübsamen-Waigmann H., Strebhardt K. Polo-like kinase, a novel marker for cellular proliferation. Am. J. Pathol. 1997;150:1165–1172. [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L.-Y., Wood J.L., Minter-Dykhouse K., Ye L., Saunders T.L., Yu X., Chen J. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol. Cell Biol. 2008;28:6870–6876. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchiumi T., Longo D.L., Ferris D.K. Cell cycle regulation of the human polo-like kinase (PLK) promoter. J. Biol. Chem. 1997;272:9166–9174. doi: 10.1074/jbc.272.14.9166. [DOI] [PubMed] [Google Scholar]
- 41.Golsteyn R.M., Mundt K.E., Fry A.M., Nigg E.A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seki A., Coppinger J.A., Jang C.-Y., Yates J.R., Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giráldez S., Galindo-Moreno M., Limón-Mortés M.C., Rivas A.C., Herrero-Ruiz J., Mora-Santos M., Saez C., Japon M.A., Tortolero M., Romero F. G1/S phase progression is regulated by PLK1 degradation through the CDK1/βTrCP axis. FASEB J. 2017;31:2925–2936. doi: 10.1096/fj.201601108R. [DOI] [PubMed] [Google Scholar]
- 44.De Cárcer G. Heat shock protein 90 regulates the metaphase-anaphase transition in a polo-like kinase-dependent manner. Cancer Res. 2004;64:5106–5112. doi: 10.1158/0008-5472.CAN-03-2214. [DOI] [PubMed] [Google Scholar]
- 45.Mundt K.E., Golsteyn R.M., Lane H.A., Nigg E.A. On the regulation and function of human polo-like kinase 1 (PLK1): Effects of overexpression on cell cycle progression. Biochem. Biophys. Res. Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 46.Jang Y.-J., Ma S., Terada Y., Erikson R.L. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem. 2002;277:44115–44120. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 47.Van de Weerdt B.C.M., van Vugt M.A.T.M., Lindon C., Kauw J.J.W., Rozendaal M.J., Klompmaker R., Wolthuis R.M.F., Medema R.H. Uncoupling anaphase-promoting complex/cyclosome activity from spindle assembly checkpoint control by deregulating polo-like kinase 1. Mol. Cell Biol. 2005;25:2031–2044. doi: 10.1128/MCB.25.5.2031-2044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang Y.-J., Lin C.-Y., Ma S., Erikson R.L. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc. Natl. Acad. Sci. USA. 2002;99:1984–1989. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petronczki M., Glotzer M., Kraut N., Peters J.-M. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Taniguchi E., Toyoshima-Morimoto F., Nishida E. Nuclear translocation of Plk1 mediated by its bipartite nuclear localization signal. J. Biol. Chem. 2002;277:48884–48888. doi: 10.1074/jbc.M206307200. [DOI] [PubMed] [Google Scholar]
- 51.Kishi K., van Vugt M.A.T.M., Okamoto K., Hayashi Y., Yaffe M.B. Functional dynamics of polo-like kinase 1 at the centrosome. Mol. Cell Biol. 2009;29:3134–3150. doi: 10.1128/MCB.01663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemmens B., Hegarat N., Akopyan K., Sala-Gaston J., Bartek J., Hochegger H., Lindqvist A. DNA Replication Determines Timing of Mitosis by Restricting CDK1 and PLK1 Activation. Mol. Cell. 2018;71:117–128. doi: 10.1016/j.molcel.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saldivar J.C., Hamperl S., Bocek M.J., Chung M., Bass T.E., Cisneros-Soberanis F., Samejina K., Xie L., Paulson J.R., Earnshaw W.C., et al. An intrinsic S/G2 checkpoint enforced by ATR. Science. 2018;24:806–810. doi: 10.1126/science.aap9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akopyan K., Silva Cascales H., Hukasova E., Saurin A.T., Müllers E., Jaiswal H., Hollamn D.A.A., Kops G.J.P.L., Medema R.H., Lindqvist A. Assessing kinetics from fixed cells reveals activation of the mitotic entry network at the S/G2 transition. Mol Cell. 2014;6:843–853. doi: 10.1016/j.molcel.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 55.Song B., Liu X.S., Davis K., Liu X. Plk1 phosphorylation of Orc2 promotes DNA replication under conditions of stress. Mol. Cell Biol. 2011;31:4844–4856. doi: 10.1128/MCB.06110-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yim H., Erikson R.L. Polo-like kinase 1 depletion induces DNA damage in early S prior to caspase activation. Mol. Cell Biol. 2009;29:2609–2621. doi: 10.1128/MCB.01277-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyoshima-Morimoto F., Taniguchi E., Shinya N., Iwamatsu A., Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215–220. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- 58.Toyoshima-Morimoto F., Taniguchi E., Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis C.W., Golsteyn R.M. Cancer cells that survive checkpoint adaptation contain micronuclei that harbor damaged DNA. Cell Cycle. 2016;15:3131–3145. doi: 10.1080/15384101.2016.1231287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syljuåsen R.G., Jensen S., Bartek J., Lukas J. Adaptation to the ionizing radiation-induced G2 checkpoint occurs in human cells and depends on checkpoint kinase 1 and Polo-like kinase 1 kinases. Cancer Res. 2006;66:10253–10257. doi: 10.1158/0008-5472.CAN-06-2144. [DOI] [PubMed] [Google Scholar]
- 61.Li Z., Li J., Kong Y., Yan S., Ahmad N., Liu X. Plk1 phosphorylation of Mre11 antagonizes the DNA damage response. Cancer Res. 2017;15:3169–3180. doi: 10.1158/0008-5472.CAN-16-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X.S., Li H., Song B., Liu X. Polo-like kinase 1 phosphorylation of G2 and S-phase-expressed 1 protein is essential for p53 inactivation during G2 checkpoint recovery. EMBO Rep. 2010;11:626–632. doi: 10.1038/embor.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X., Li H., Zhou Z., Wang W.-H., Deng A., Andrisani O., Liu X.S. Plk1-mediated Phosphorylation of Topors Regulates p53 Stability. J. Biol. Chem. 2009;284:18588–18592. doi: 10.1074/jbc.C109.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ando K., Ozaki T., Yamamoto H., Furuya K., Hosoda M., Hayashi S., Fukuzawa M., Nakagawara A. Polo-like Kinase 1 (Plk1) Inhibits p53 Function by Physical Interaction and Phosphorylation. J. Biol. Chem. 2004;279:25549–25561. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- 65.Liu X., Erikson R.L. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc. Natl. Acad. Sci. USA. 2002;99:8672–8676. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsou M.-F.B., Wang W.-J., George K.A., Uryu K., Stearns T., Jallepalli P.V. Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev. Cell. 2009;17:344–354. doi: 10.1016/j.devcel.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shukla A., Kong D., Sharma M., Magidson V., Loncarek J. Plk1 relieves centriole block to reduplication by promoting daughter centriole maturation. Nat. Commun. 2015;6:8077. doi: 10.1038/ncomms9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou J., Zhang D., Qin G., Chen X., Wang H., Zhang D. BRCA1 and FancJ cooperatively promote interstrand crosslinker induced centrosome amplification through the activation of polo-like kinase 1. Cell Cycle. 2014;13:3685–3697. doi: 10.4161/15384101.2014.964973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werle K., Chen J., Xu H.-G., Zhao R.-X., He Q., Lu C., Cui R., Liang J., Li Y.-L., Xu Z.-X. Liver kinase B1 regulates the centrosome via PLK1. Cell Death Dis. 2014;5:e1157. doi: 10.1038/cddis.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Izumi H., Matsumoto Y., Ikeuchi T., Saya H., Kajii T., Matsuura S. BubR1 localizes to centrosomes and uppresses centrosome amplification via regulating Plk1 activity in interphase cells. Oncogene. 2009;28:2806–2820. doi: 10.1038/onc.2009.141. [DOI] [PubMed] [Google Scholar]
- 71.Yamashiro S., Yamakita Y., Totsukawa G., Goto H., Kaibuchi K., Ito M., Hartshorne D.J., Matsumura F. Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1. Dev. Cell. 2008;14:787–797. doi: 10.1016/j.devcel.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nai S., Shi Y., Ru H., Ding Y., Geng Q., Li Z., Dong M.-Q., Xu X., Li J. Chk2-dependent phosphorylation of myosin phosphatase targeting subunit 1 (MYPT1) regulates centrosome maturation. Cell Cycle. 2019;18:2651–2659. doi: 10.1080/15384101.2019.1654795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casenghi M., Meraldi P., Weinhart U., Duncan P.I., Körner R., Nigg E.A. Polo-like Kinase 1 Regulates Nlp, a Centrosome Protein Involved in Microtubule Nucleation. Dev. Cell. 2003;5:113–125. doi: 10.1016/S1534-5807(03)00193-X. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X., Chen Q., Feng J., Hou J., Yang F., Liu J., Jiang Q., Zhang C. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the TuRC to the centrosome. J. Cell Sci. 2009;122:2240–2251. doi: 10.1242/jcs.042747. [DOI] [PubMed] [Google Scholar]
- 75.Zhu H., Fang K., Fang G. FAM29A, a target of Plk1 regulation, controls the partitioning of NEDD1 between the mitotic spindle and the centrosomes. J. Cell Sci. 2009;122:2750–2759. doi: 10.1242/jcs.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eot-Houllier G., Venoux M., Vidal-Eychenié S., Hoang M.-T., Giorgi D., Rouquier S. Plk1 Regulates Both ASAP Localization and Its Role in Spindle Pole Integrity. J. Biol. Chem. 2010;285:29556–29568. doi: 10.1074/jbc.M110.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oshimori N., Ohsugi M., Yamamoto T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 2006;8:1095–1101. doi: 10.1038/ncb1474. [DOI] [PubMed] [Google Scholar]
- 78.Morris E.J., Kawamura E., Gillespie J.A., Balgi A., Kannan N., Muller W.J., Roberge M., Dedhar S. Stat3 regulates centrosome clustering in cancer cells via Stathmin/PLK1. Nat. Commun. 2017;8:15289. doi: 10.1038/ncomms15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith E., Hégarat N., Vesely C., Roseboom I., Larch C., Streicher H., Straatman K., Flynn H., Skehel M., Hirota T., et al. Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. EMBO J. 2011;30:2233–2245. doi: 10.1038/emboj.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertran M.T., Sdelci S., Regué L., Avruch J., Caelles C., Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 2011;30:2634–2647. doi: 10.1038/emboj.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marthiens V., Piel M., Basto R. Never tear us apart—The importance of centrosome clustering. J. Cell Sci. 2012;125:3281–3292. doi: 10.1242/jcs.094797. [DOI] [PubMed] [Google Scholar]
- 82.Kagami Y., Ono M., Yoshida K. Plk1 phosphorylation of CAP-H2 triggers chromosome condensation by condensin II at the early phase of mitosis. Sci. Rep. 2017;7:5583. doi: 10.1038/s41598-017-05986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abe S., Nagasaka K., Hirayama Y., Kozuka-Hata H., Oyama M., Aoyagi Y., Obuse C., Hirota T. The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II. Genes Dev. 2011;25:863–874. doi: 10.1101/gad.2016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai J., Sullivan B.A., Higgins J.M.G. Regulation of mitotic chromosome cohesion by Haspin and Aurora, B. Dev. Cell. 2006;11:741–750. doi: 10.1016/j.devcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 85.Zhou L., Tian X., Zhu C., Wang F., Higgins J.M.G. Polo-like kinase-1 triggers histone phosphorylation by Haspin in mitosis. EMBO Rep. 2014;15:273–281. doi: 10.1002/embr.201338080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang Y.H., Park J.-E., Yu L.-R., Soung N.-K., Yun S.-M., Bang J.K., Seong Y.-S., Yu H., Garfield S., Veenstra T., et al. Self-regulated Plk1 recruitment to kinetochores by the Plk1-PBIP1 interaction is critical for proper chromosome segregation. Mol. Cell. 2006;24:409–422. doi: 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Sumara I., Vorlaufer E., Stukenberg P., Kelm O., Redemann N., Nigg E.A., Peters J.-M. The dissociation of cohesin from chromosomes in prophase is regulated by polo-like kinase. Mol. Cell. 2002;9:515–525. doi: 10.1016/S1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 88.Kurasawa Y., Yu-Lee L.-Y. PICH and Cotargeted Plk1 Coordinately Maintain Prometaphase Chromosome Arm Architecture. Mol. Biol. Cell. 2010;21:1188–1199. doi: 10.1091/mbc.e09-11-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang Z., Shu H., Qi W., Mahmood N.A., Mumby M.C., Yu H. PP2A Is Required for Centromeric Localization of Sgo1 and Proper Chromosome Segregation. Dev. Cell. 2006;10:575–585. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Von Schubert C., Cubizolles F., Bracher J.M., Sliedrecht T., Kops G.J., Nigg E.A. Plk1 and Mps1 Cooperatively Regulate the Spindle Assembly Checkpoint in Human Cells. Cell Rep. 2015;12:66–78. doi: 10.1016/j.celrep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 91.Bollen M. Kinetochore signalling: The KIss that MELTs Knl1. Curr. Biol. 2014;24:R68–R70. doi: 10.1016/j.cub.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 92.Li H., Liu X.S., Yang X., Wang Y., Wang Y., Turner J.R., Liu X. Phosphorylation of CLIP-170 by Plk1 and CK2 promotes timely formation of kinetochore–microtubule attachments. EMBO J. 2010;29:2953–2965. doi: 10.1038/emboj.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X.S., Song B., Tang J., Liu W., Kuang S., Liu X. Plk1 Phosphorylates Sgt1 at the Kinetochores to Promote Timely Kinetochore-Microtubule Attachment. Mol. Cell. Biol. 2012;32:4053–4067. doi: 10.1128/MCB.00516-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu D., Davydenko O., Lampson M.A. Polo-like kinase-1 regulates kinetochore–microtubule dynamics and spindle checkpoint silencing. J. Cell Biol. 2012;198:491–499. doi: 10.1083/jcb.201205090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bakhoum S.F., Thompson S.L., Manning A.L., Compton D.A. Genome stability is ensured by temporal control of kinetochore–microtubule dynamics. Nat. Cell Biol. 2008;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dumitru A.M.G., Rusin S.F., Clark A.E.M., Kettenbach A.N., Compton D.A. Cyclin A/Cdk1 modulates Plk1 activity in prometaphase to regulate kinetochore-microtubule attachment stability. ELife. 2017;6:e29303. doi: 10.7554/eLife.29303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahonen L.J., Kallio M.J., Daum J.R., Bolton M., Manke I.A., Yaffe M.B., Stukenberg P.T., Gorbsky G.J. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 2005;15:1078–1089. doi: 10.1016/j.cub.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 98.Van Vugt M.A.T.M., Van De Weerdt B.C.M., Vader G., Janssen H., Calafat J., Klompmaker R., Wolthuis R.M.F., Medema R.H., Yakunin A.F., Proudfoot M., et al. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/cdc20 Activation and Initiation of cytokinesis. J. Biol. Chem. 2004;279:36841–368540. doi: 10.1074/jbc.M313681200. [DOI] [PubMed] [Google Scholar]
- 99.Daum J.R., Tugendreich S., Topper L.M., Jorgensen P.M., Hoog C., Hieter P., Gorbsky G.J. The 3F3/2 anti-phosphoepitope antibody binds the mitotically phosphorylated anaphase-promoting complex/cyclosome. Curr. Biol. 2000;10:R850–R852. doi: 10.1016/S0960-9822(00)00836-8. [DOI] [PubMed] [Google Scholar]
- 100.Nishino M., Kurasawa Y., Evans R., Lin S.-H., Brinkley B.R., Yu-Lee L.-Y. NudC Is Required for Plk1 Targeting to the Kinetochore and Chromosome Congression. Curr. Biol. 2006;16:1414–1421. doi: 10.1016/j.cub.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 101.Glotzer M. The 3Ms of central spindle assembly: Microtubules, motors and MAPs. Nat. Rev. Mol. Cell Biol. 2009;10:9–209. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neef R., Preisinger C., Sutcliffe J., Kopajtich R., Nigg E.A., Mayer T.U., Barr F.A. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 2003;162:863–876. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu X., Zhou T., Kuriyama R., Erikson R.L. Molecular interactions of Polo-like-kinase 1 with the mitotic kinesin-like protein CHO1/MKLP-1. J. Cell Sci. 2004;117:3233–3246. doi: 10.1242/jcs.01173. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L., Shao H., Huang Y., Yan F., Chu Y., Hou H., Zhu M., Fu C., Aikhionbare F., Fang G., et al. PLK1 phosphorylates mitotic centromere-associated kinesin and promotes its depolymerase activity. J. Biol. Chem. 2010;286:3033–3046. doi: 10.1074/jbc.M110.165340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bakhoum S.F., Genovese G., Compton D.A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindon C., Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Cárcer G., Venkateswaran S.V., Salgueiro L., El Bakkali A., Somogyi K., Rowald K., Montañés P., Sanclemente M., Escobar B., De Martino A., et al. Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat. Commun. 2018;9:3012. doi: 10.1038/s41467-018-05429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adriaans I.E., Basant A., Ponsioen B., Glotzer M., Lens S.M.A. PLK1 plays dual roles in centralspindlin regulation during cytokinesis. J. Cell Biol. 2019;218:1250–1264. doi: 10.1083/jcb.201805036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burkard M.E., Maciejowski J., Rodríguez-Bravo V., Repka M., Lowery E.M., Clauser K.R., Zhang C., Shokat K.M., Carr S.A., Yaffe M.B., et al. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009;7:e1000111. doi: 10.1371/journal.pbio.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burkard M.E., Randall C.L., LaRochelle S., Zhang C., Shokat K.M., Fisher R.P., Jallepalli P.V. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl. Acad. Sci. USA. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang Y.-C., Wu C.-H., Yen T.-C., Ouyang P. centrosomal protein 55 (Cep55) stability is negatively regulated by p53 protein through polo-like kinase 1 (Plk1) J. Biol. Chem. 2011;287:4376–4385. doi: 10.1074/jbc.M111.289108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bastos R.N., Barr F.A. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J. Cell Biol. 2010;191:751–760. doi: 10.1083/jcb.201008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fabbro M., Zhou B.-B., Takahashi M., Sarcevic B., Lal P., Graham M.E., Gabrielli B.G., Robinson P.J., Nigg E.A., Ono Y., et al. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev. Cell. 2005;9:477–488. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 114.Smits V.A.J., Klompmaker R., Arnaud L., Rijksen G., Nigg E.A., Medema R.H. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 115.Van Vugt M.A.T.M., Smits V.A.J., Klompmaker R., Medema R.H. Inhibition of Polo-like Kinase-1 by DNA Damage Occurs in an ATM- or ATR-dependent Fashion. J. Biol. Chem. 2001;276:41656–41660. doi: 10.1074/jbc.M101831200. [DOI] [PubMed] [Google Scholar]
- 116.Lee H.-J., Hwang H.-I., Jang Y.-J. Mitotic DNA damage response: Polo-like Kinase-1 is dephosphorylated through ATM-Chk1 pathway. Cell Cycle. 2010;9:2389–2398. doi: 10.4161/cc.9.12.11904. [DOI] [PubMed] [Google Scholar]
- 117.Tsvetkov L., Stern D.F. Phosphorylation of Plk1 at S137 and T210 is Inhibited in Response to DNA Damage. Cell Cycle. 2004;4:166–171. doi: 10.4161/cc.4.1.1348. [DOI] [PubMed] [Google Scholar]
- 118.Bruinsma W., Aprelia M., García-Santisteban I., Kool J., Xu Y.J., Medema R.H. Inhibition of Polo-like kinase 1 during the DNA damage response is mediated through loss of Aurora A recruitment by Bora. Oncogene. 2016;36:1840–1848. doi: 10.1038/onc.2016.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Vugt M.A.T.M., Gardino A.K., Linding R., Ostheimer G.J., Reinhardt H.C., Ong S.-E., Tan C.S., Miao H., Keezer S.M., Li J., et al. A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to Inactivate the G2/M DNA Damage Checkpoint. PLoS Biol. 2010;8:e1000287. doi: 10.1371/journal.pbio.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Douglas P., Ye R., Trinkle-Mulcahy L., Neal J.A., De Wever V., Morrice N.A., Meek K., Lees-Miller S.P. Polo-like kinase 1 (PLK1) and protein phosphatase 6 (PP6) regulate DNA-dependent protein kinase catalytic subunit (DNA-PKcs) phosphorylation in mitosis. Biosci. Rep. 2014;34:257–271. doi: 10.1042/BSR20140051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee K.-J., Shang Z.-F., Lin Y.-F., Sun J., Morotomi-Yano K., Saha D., Chen B.P. The Catalytic Subunit of DNA-Dependent Protein Kinase Coordinates with Polo-Like Kinase 1 to Facilitate Mitotic Entry. Neoplasia. 2015;17:329–338. doi: 10.1016/j.neo.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mamely I., Van Vugt M.A., Smits V.A.J., Semple J.I., Lemmens B., Perrakis A., Medema R.H., Freire R. Polo-like Kinase-1 Controls Proteasome-Dependent Degradation of Claspin during Checkpoint Recovery. Curr. Biol. 2006;16:1950–1955. doi: 10.1016/j.cub.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 123.Macůrek L., Lindqvist A., Lim D., Lampson M.A., Klompmaker R., Freire R., Clouin C., Taylor S.S., Yaffe M.B., Medema R.H. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nat. Cell Biol. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 124.Wakida T., Ikura M., Kuriya K., Ito S., Shiroiwa Y., Habu T., Kawamoto T., Okumura K., Ikura T., Furuya K. The CDK-PLK1 axis targets the DNA damage checkpoint sensor protein RAD9 to promote cell proliferation and tolerance to genotoxic stress. ELife. 2017;6 doi: 10.7554/elife.29953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuan J.-H., Feng Y., Fisher R.H., Maloid S., Longo D.L., Ferris D.K. Polo-Like Kinase 1 inactivation following mitotic dna damaging treatments is independent of ataxia telangiectasia mutated kinase11federal funds from the National Cancer Institute, NIH, under contract NO1-CO-12400.Note: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Mol Cancer Res. 2004;2:417–426. [PubMed] [Google Scholar]
- 126.Yata K., Lloyd J., Maslen S., Bleuyard J.-Y., Skehel M., Smerdon S.J., Esashi F. Plk1 and CK2 Act in Concert to Regulate Rad51 during DNA Double Strand Break Repair. Mol. Cell. 2012;45:371–383. doi: 10.1016/j.molcel.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee M., Daniels M.J., Venkitaraman A.R. Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene. 2003;23:865–872. doi: 10.1038/sj.onc.1207223. [DOI] [PubMed] [Google Scholar]
- 128.Terasawa M., Shinohara A., Shinohara M. Canonical Non-Homologous End Joining in Mitosis Induces Genome Instability and Is Suppressed by M-phase-Specific Phosphorylation of XRCC4. PLoS Genet. 2014;10:e10045633. doi: 10.1371/journal.pgen.1004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benada J., Burdová K., Lidak T., Von Morgen P., Macurek L. Polo-like kinase 1 inhibits DNA damage response during mitosis. Cell Cycle. 2015;14:219–231. doi: 10.4161/15384101.2014.977067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orthwein A., Fradet-Turcotte A., Noordermeer S.M., Canny M.D., Brun C.M., Strecker J., Escribano-Diaz C., Durocher D. Mitosis Inhibits DNA Double-Strand Break Repair to Guard Against Telomere Fusions. Science. 2014;344:189–193. doi: 10.1126/science.1248024. [DOI] [PubMed] [Google Scholar]
- 131.Huang Y., Sun L., Liu N., Wei Q., Jiang L., Tong X., Ye X. Polo-like Kinase 1 (Plk1) Up-regulates Telomerase Activity by Affecting Human Telomerase Reverse Transcriptase (hTERT) Stability. J. Biol. Chem. 2015;290:18865–18873. doi: 10.1074/jbc.M114.635375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 133.Martínez P., Thanasoula M., Muñoz P., Liao C., Tejera A., McNees C., Flores J.M., Fernández-Capetillo O., Tarsounas M., Blasco M.A. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shay J., Bacchetti S. A survey of telomerase activity in human cancer. Eur. J. Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 135.Leão R.R., Apolónio J.D., Lee D., Figueiredo A., Tabori U., Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: Clinical impacts in cancer. J. Biomed. Sci. 2018;25:22. doi: 10.1186/s12929-018-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ha G.-H., Kim H.-S., Go H., Lee H., Seimiya H., Chung D.H., Lee C.-W. Tankyrase-1 function at telomeres and during mitosis is regulated by Polo-like kinase-1-mediated phosphorylation. Cell Death Differ. 2011;19:321–332. doi: 10.1038/cdd.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith S. Tankyrase, a Poly(ADP-Ribose) Polymerase at Human Telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 138.Wu Z.-Q., Yang X., Weber G., Liu X.S. Plk1 Phosphorylation of TRF1 Is Essential for Its Binding to Telomeres. J. Biol. Chem. 2008;283:25503–25513. doi: 10.1074/jbc.M803304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pang Q., Qian D., Cheng J., Ding X., Chen X., Chen X., Guan Y., Zhang B., Wang J., Er P., et al. PinX1 suppresses tumorigenesis by negatively regulating telomerase/telomeres in colorectal carcinoma cells and is a promising molecular marker for patient prognosis. OncoTargets Ther. 2016;9:4821–4831. doi: 10.2147/OTT.S103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang C., Yu J., Yuan K., Lan J., Jin C., Huang H. Plk1-mediated mitotic phosphorylation of PinX1 regulates its stability. Eur. J. Cell Biol. 2010;89:748–756. doi: 10.1016/j.ejcb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 141.Yuan X., Larsson C., Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene. 2019;38:6172–6183. doi: 10.1038/s41388-019-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Z., Koeneman K.S., Corey D.R. Consequences of telomerase inhibition and combination treatments for the proliferation of cancer cells. Cancer Res. 2003;63:5917–5925. [PubMed] [Google Scholar]
- 143.Falchetti M.L., Pallini R., Levi A. Telomerase and Cancer. Am. J. Cancer. 2004;3:1–11. doi: 10.2165/00024669-200403010-00001. [DOI] [Google Scholar]
- 144.Bentham Science Publisher. Cunningham A., Love W., Zhang R., Andrews L., Tollefsbol T. Telomerase inhibition in cancer therapeutics: Molecular-based approaches. Curr. Med. Chem. 2006;13:2875–2888. doi: 10.2174/092986706778521887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu X., Zhang J., Yang S., Kuang Z., Tan G., Yang G., Wei Q., Guo Z. Telomerase antagonist imetelstat increases radiation sensitivity in esophageal squamous cell carcinoma. Oncotarget. 2017;8:13600–13610. doi: 10.18632/oncotarget.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dikmen Z.G., Gellert G.C., Jackson S., Gryaznov S., Tressler R., Dogan P., Wright W.E., Shay J.W. In vivoInhibition of Lung Cancer by GRN163L: A Novel Human Telomerase Inhibitor. Cancer Res. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 147.Frink R.E., Peyton M., Schiller J.H., Gazdar A.F., Shay J.W., Minna J.D. Telomerase inhibitor imetelstat has preclinical activity across the spectrum of non-small cell lung cancer oncogenotypes in a telomere length dependent manner. Oncotarget. 2016;7:31639–31651. doi: 10.18632/oncotarget.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang E.S., Wu K., Chin A.C., Chen-Kiang S., Pongracz K., Gryaznov S., Moore M.A.S. Telomerase inhibition with an oligonucleotide telomerase template antagonist: In vitro and in vivo studies in multiple myeloma and lymphoma. Blood. 2004;103:258–266. doi: 10.1182/blood-2003-02-0546. [DOI] [PubMed] [Google Scholar]
- 149.Akiyama M., Hideshima T., Shammas M.A., Hayashi T., Hamasaki M., Tai Y.T., Richardson P., Gryaznov S., Munshi N.C., Anderson K.C. Effects of oligonucleotide N3′→P5′ thio-phosphoramidate (GRN163) targeting telomerase RNA in human multiple myeloma cells. Cancer Res. 2003;63:6187–6194. [PubMed] [Google Scholar]
- 150.Wang X., Hu C.S., Petersen B., Qiu J., Ye F., Houldsworth J., Eng K., Huang F., Hoffman R. Imetelstat, a telomerase inhibitor, is capable of depleting myelofibrosis stem and progenitor cells. Blood Adv. 2018;2:2378–2388. doi: 10.1182/bloodadvances.2018022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bruedigam C., Porter A.H., Wackrow B., Straube J., Cooper L.T., Song A., Lee S.C.-W., Abdel-Wahab O., Hill G.R., Lane S.W. Integrated molecular analysis identifies replicative stress as sensitizer to imetelstat therapy in AML. Blood. 2017;130:798. [Google Scholar]
- 152.Thompson C.A.H., Gu A., Yang S.Y., Mathew V., Fleisig H.B., Wong J.M. Transient Telomerase Inhibition with Imetelstat Impacts DNA Damage Signals and Cell-Cycle Kinetics. Mol. Cancer Res. 2018;16:1215–1225. doi: 10.1158/1541-7786.MCR-17-0772. [DOI] [PubMed] [Google Scholar]
- 153.Du J., Hannon G.J. The centrosomal kinase Aurora-A/STK15 interacts with a putative tumor suppressor NM23-H1. Nucleic Acids Res. 2002;30:5465–5475. doi: 10.1093/nar/gkf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Strebhardt K. Prognostic Value of Pololike Kinase Expression in Melanomas. JAMA. 2000;283:479–480. doi: 10.1001/jama.283.4.479. [DOI] [PubMed] [Google Scholar]
- 155.Wolf G., Elez R., Doermer A., Holtrich U., Ackermann H., Stutte H.J., Altmannsberger H.-M., Rübsamen-Waigmann H., Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–549. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 156.Knecht R., Elez R., Oechler M., Solbach C., Von Ilberg C., Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–2797. [PubMed] [Google Scholar]
- 157.Takai N., Miyazaki T., Fujisawa K., Nasu K., Hamanaka R., Miyakawa I. Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett. 2001;164:41–49. doi: 10.1016/S0304-3835(00)00703-5. [DOI] [PubMed] [Google Scholar]
- 158.Takai N., Miyazaki T., Fujisawa K., Nasu K., Hamanaka R., Miyakawa I. Polo-like kinase (PLK) expression in endometrial carcinoma. Cancer Lett. 2001;169:41–49. doi: 10.1016/S0304-3835(01)00522-5. [DOI] [PubMed] [Google Scholar]
- 159.Zhao C., Gong L., Li W., Chen L. Overexpression of Plk1 promotes malignant progress in human esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2009;136:9–16. doi: 10.1007/s00432-009-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mok W.C. Polo-like kinase 1, a new therapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 2012;18:3527–3536. doi: 10.3748/wjg.v18.i27.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun W., Su Q., Cao X., Shang B., Chen A., Yin H., Liu B. High Expression of Polo-Like Kinase 1 Is Associated with Early Development of Hepatocellular Carcinoma. Int. J. Genom. 2014;2014:1–9. doi: 10.1155/2014/312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamada S.-I., Ohira M., Horie H., Ando K., Takayasu H., Suzuki Y., Sugano S., Hirata T., Goto T., Matsunaga T., et al. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: Identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23:5901–5911. doi: 10.1038/sj.onc.1207782. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi T., Sano B., Nagata T., Kato H., Sugiyama Y., Kunieda K., Kimura M., Okano Y., Saji S. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci. 2003;94:148–152. doi: 10.1111/j.1349-7006.2003.tb01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cai X.P., Chen L.D., Bin Song H., Zhang C.X., Yuan Z.W., Xiang Z.X. PLK1 promotes epithelial-mesenchymal transition and metastasis of gastric carcinoma cells. Am. J. Transl. Res. 2016;8:4172–4183. [PMC free article] [PubMed] [Google Scholar]
- 165.Gray P.J., Bearss D., Han H., Nagle R., Tsao M.-S., Dean N., Von Hoff D.D. Identification of human polo-like kinase 1 as a potential therapeutic target in pancreatic cancer. Mol. Cancer Ther. 2004;3:641–646. [PubMed] [Google Scholar]
- 166.Dietzmann K., Kirches E., Jachau K., Mawrin C., Von Bossanyi P. Increased Human Polo-Like Kinase-1 Expression in Gliomas. J. Neuro Oncol. 2001;53:1–11. doi: 10.1023/A:1011808200978. [DOI] [PubMed] [Google Scholar]
- 167.King S.I., Purdie C.A., Bray S.E., Quinlan P.R., Jordan L.B., Thompson A.M., Meek D.W. Immunohistochemical detection of Polo-like kinase-1 (PLK1) in primary breast cancer is associated with TP53mutation and poor clinical outcome. Breast Cancer Res. 2012;14:40. doi: 10.1186/bcr3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maire V., Nemati F., Richardson M., Vincent-Salomon A., Tesson B., Rigaill G., Gravier E., Marty-Prouvost B., De Koning L., Lang G., et al. Polo-like Kinase 1: A Potential Therapeutic Option in Combination with Conventional Chemotherapy for the Management of Patients with Triple-Negative Breast Cancer. Cancer Res. 2012;73:813–823. doi: 10.1158/0008-5472.CAN-12-2633. [DOI] [PubMed] [Google Scholar]
- 169.Deeraksa A., Pan J., Sha Y., Liu X.-D., Eissa N.T., Lin S.-H., Yu-Lee L.-Y. Plk1 is upregulated in androgen-insensitive prostate cancer cells and its inhibition leads to necroptosis. Oncogene. 2012;32:2973–2983. doi: 10.1038/onc.2012.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Z., Chen L., Wang H., Ahmad N., Liu X.S. Inhibition of Plk1 represses androgen signaling pathway in castration-resistant prostate cancer. Cell Cycle. 2015;14:2142–2148. doi: 10.1080/15384101.2015.1041689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu K., Law J.H., Fotovati A., Dunn S.E. Small interfering RNA library screen identified polo-like kinase-1 (PLK1) as a potential therapeutic target for breast cancer that uniquely eliminates tumor-initiating cells. Breast Cancer Res. 2012;14:22. doi: 10.1186/bcr3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lerner R.G., Grossauer S., Kadkhodaei B., Meyers I., Sidorov M., Koeck K., Hashizume R., Ozawa T., Phillips J.J., Berger M.S., et al. Targeting a Plk1-Controlled Polarity Checkpoint in Therapy-Resistant Glioblastoma-Propagating Cells. Cancer Res. 2015;75:5355–5366. doi: 10.1158/0008-5472.CAN-14-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spänkuch-Schmitt B., Wolf G., Solbach C., Loibl S., Knecht R., Stegmüller M., Von Minckwitz G., Kaufmann M., Strebhardt K. Downregulation of human polo-like kinase activity by antisense oligonucleotides induces growth inhibition in cancer cells. Oncogene. 2002;21:3162–3171. doi: 10.1038/sj.onc.1205412. [DOI] [PubMed] [Google Scholar]
- 174.Gumireddy K., Reddy M.R., Cosenza S.C., Boominathan R., Baker S.J., Papathi N., Jiang J., Holland J., Reddy E.P. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:497. doi: 10.1016/j.ccr.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 175.Gleixner K.V., Ferenc V., Peter B., Gruze A., Meyer R.A., Hadzijusufovic E., Cerny-Reiterer S., Mayerhofer M., Pickl W.F., Sillaber C., et al. Polo-like Kinase 1 (Plk1) as a Novel Drug Target in Chronic Myeloid Leukemia: Overriding Imatinib Resistance with the Plk1 Inhibitor BI 2536. Cancer Res. 2010;70:1513–1523. doi: 10.1158/0008-5472.CAN-09-2181. [DOI] [PubMed] [Google Scholar]
- 176.Liu M., Lei M., Erikson R.L. Normal Cells, but Not Cancer Cells, Survive Severe Plk1 Depletion. Mol. Cell. Biol. 2006;26:2093–2108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Francescangeli F., Patrizii M., Signore M., Federici G., Di Franco S., Pagliuca A., Baiocchi M., Biffoni M., Ricci-Vitiani L., Todaro M., et al. Proliferation State and Polo-Like Kinase1 Dependence of Tumorigenic Colon Cancer Cells. Stem Cells. 2012;30:1819–1830. doi: 10.1002/stem.1163. [DOI] [PubMed] [Google Scholar]
- 178.Grinshtein N., Datti A., Fujitani M., Uehling D., Prakesch M., Isaac M., Irwin M.S., Wrana J.L., Al-Awar R., Kaplan D.R. Small Molecule Kinase Inhibitor Screen Identifies Polo-Like Kinase 1 as a Target for Neuroblastoma Tumor-Initiating Cells. Cancer Res. 2011;71:1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 179.Lund-Andersen C., Patzke S., Nahse-Kumpf V., Syljuåsen R.G. PLK1-inhibition can cause radiosensitization or radioresistance dependent on the treatment schedule. Radiother. Oncol. 2014;110:355–361. doi: 10.1016/j.radonc.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 180.Gilmartin A.G., Bleam M.R., Richter M.C., Erskine S.G., Kruger R.G., Madden L., Hassler D.F., Smith G.K., Gontarek R.R., Courtney M.P., et al. Distinct concentration-dependent effects of the polo-like kinase 1-specific inhibitor GSK461364A, including differential effect on apoptosis. Cancer Res. 2009;69:6969–6977. doi: 10.1158/0008-5472.CAN-09-0945. [DOI] [PubMed] [Google Scholar]
- 181.O’Neil B.H., Scott A.J., Ma W.W., Cohen S.J., Leichman L., Aisner D.L., Menter A.R., Tejani M.A., Cho J.K., Granfortuna J., et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann. Oncol. 2015;26:1923–1929. doi: 10.1093/annonc/mdv264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Steegmaier M., Hoffmann M., Baum A., Lénárt P., Petronczki M., Krššák M., Gürtler U., Garin-Chesa P., Lieb S., Quant J., et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 183.Mross K., Dittrich C.E., Aulitzky W., Strumberg D., Schutte J., Schmid R.M., Hollerbach S., Merger M., Munzert G., Fleischer F., et al. A randomised phase II trial of the Polo-like kinase inhibitor BI 2536 in chemo-naïve patients with unresectable exocrine adenocarcinoma of the pancreas—A study within the Central European Society Anticancer Drug Research (CESAR) collaborative network. Br. J. Cancer. 2012;107:2800–2867. doi: 10.1038/bjc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boehringer Ingelheim’s Investigational Volasertib Receives FDA Breakthrough Therapy Designation. [(accessed on 13 September 2019)]; Available online: https://www.boehringer-ingelheim.us/press-release/boehringer-ingelheims-investigational-volasertib-receives-fda-breakthrough-therapy.
- 185.Stadler W.M., Vaughn D.J., Sonpavde G., Vogelzang N.J., Tagawa S.T., Petrylak D.P., Rosen P., Lin C.-C., Mahoney J., Modi S., et al. An open-label, single-arm, phase 2 trial of the Polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer. 2013;120:976–982. doi: 10.1002/cncr.28519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ellis P.M., Leighl N.B., Hirsh V., Reaume M.N., Blais N., Wierzbicki R., Sadrolhefazi B., Gu Y., Liu D., Pilz K., et al. A Randomized, Open-Label Phase II Trial of Volasertib as Monotherapy and in Combination With Standard-Dose Pemetrexed Compared With Pemetrexed Monotherapy in Second-Line Treatment for Non–Small-Cell Lung Cancer. Clin. Lung Cancer. 2015;16:457–465. doi: 10.1016/j.cllc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 187.Reindl W., Yuan J., Krämer A., Strebhardt K., Berg T. Inhibition of Polo-like Kinase 1 by Blocking Polo-Box Domain-Dependent Protein-Protein Interactions. Chem. Biol. 2008;15:459–466. doi: 10.1016/j.chembiol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 188.Scharow A., Raab M., Saxena K., Sreeramulu S., Kudlinzki D., Gande S., Dötsch V., Kurunci-Csacsko E., Klaeger S., Kuster B., et al. Optimized Plk1 PBD inhibitors based on poloxin induce mitotic arrest and apoptosis in tumor cells. ACS Chem. Biol. 2015;10:2570–2579. doi: 10.1021/acschembio.5b00565. [DOI] [PubMed] [Google Scholar]
- 189.Yuan J., Sanhaji M., Krämer A., Reindl W., Hofmann M., Kreis N.-N., Zimmer B., Berg T., Strebhardt K. Polo-box domain inhibitor poloxin activates the spindle assembly checkpoint and inhibits tumor growth in vivo. Am. J. Pathol. 2011;179:2091–2099. doi: 10.1016/j.ajpath.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hikichi Y., Honda K., Hikami K., Miyashita H., Kaieda I., Murai S., Uchiyama N., Hasegawa M., Kawamoto T., Sato T., et al. TAK-960, a novel, orally available, selective inhibitor of polo-like kinase 1, shows broad-spectrum preclinical antitumor activity in multiple dosing regimens. Mol. Cancer Ther. 2011;11:700–709. doi: 10.1158/1535-7163.MCT-11-0762. [DOI] [PubMed] [Google Scholar]
- 191.Klauck P.J., Bagby S.M., Capasso A., Bradshaw-Pierce E.L., Selby H.M., Spreafico A., Tentler J.J., Tan A.C., Kim J., Arcaroli J.J., et al. Antitumor activity of the polo-like kinase inhibitor, TAK-960, against preclinical models of colorectal cancer. BMC Cancer. 2018;18:136. doi: 10.1186/s12885-018-4036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Beria I., Ballinari D., Bertrand J.A., Borghi D., Bossi R.T., Brasca M.G., Cappella P., Caruso M., Ceccarelli W., Ciavolella A., et al. Identification of 4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline derivatives as a new class of orally and selective polo-like kinase 1 inhibitors. J. Med. Chem. 2010;53:3532–3551. doi: 10.1021/jm901713n. [DOI] [PubMed] [Google Scholar]
- 193.Sero V., Tavanti E., Vella S., Hattinger C.M., Fanelli M., Michelacci F., Versteeg R., Valsasina B., Gudeman B., Picci P., et al. Targeting polo-like kinase 1 by NMS-P937 in osteosarcoma cell lines inhibits tumor cell growth and partially overcomes drug resistance. Investig. New Drugs. 2014;32:1167–1180. doi: 10.1007/s10637-014-0158-6. [DOI] [PubMed] [Google Scholar]
- 194.Olmos D., Barker D., Sharma R., Brunetto A.T., Yap T.A., Taegtmeyer A.B., Barriuso J., Medani H., Degenhardt Y.Y., Allred A.J., et al. Phase I Study of GSK461364, a Specific and Competitive Polo-like Kinase 1 Inhibitor, in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2011;17:3420–3430. doi: 10.1158/1078-0432.CCR-10-2946. [DOI] [PubMed] [Google Scholar]
- 195.Orr B., Talje L., Liu Z., Kwok B.H., Compton D.A. Adaptive Resistance to an Inhibitor of Chromosomal Instability in Human Cancer Cells. Cell Rep. 2016;17:1755–1763. doi: 10.1016/j.celrep.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma S., Charron J., Erikson R.L. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol. Cell. Biol. 2003;23:6936–6943. doi: 10.1128/MCB.23.19.6936-6943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Warnke S., Kemmler S., Hames R., Tsai H.-L., Hoffmann-Rohrer U., Fry A.M., Hoffmann I. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr. Biol. 2004;14:1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 198.Matthew E.M., Yang Z., Peri S., Andrake M., Dunbrack R.L., Ross E., El-Deiry W.S. Plk2 loss commonly occurs in colorectal carcinomas but not adenomas: Relationship to mTOR signaling. Neoplasia. 2018;20:244–255. doi: 10.1016/j.neo.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Syed N., Smith P., Sullivan A., Spender L.C., Dyer M.J., Karran L., O’Nions J., Allday M., Hoffmann I., Crawford D., et al. Transcriptional silencing of Polo-like kinase 2(SNK/PLK2)is a frequent event in B-cell malignancies. Blood. 2005;107:250–256. doi: 10.1182/blood-2005-03-1194. [DOI] [PubMed] [Google Scholar]
- 200.Burns T.F., Fei P., Scata K.A., Dicker D.T., El-Deiry W.S. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol. Cell Biol. 2003;23:5556–5571. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ang X.L., Seeburg D.P., Sheng M., Harper J.W. Regulation of postsynaptic RapGAP SPAR by Polo-like kinase 2 and the SCFbeta-TRCP ubiquitin ligase in hippocampal neurons. J. Biol. Chem. 2008;283:29424–29432. doi: 10.1074/jbc.M802475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Inglis K.J., Chereau D., Brigham E.F., Chiou S.-S., Schöbel S., Frigon N.L., Yu M., Caccavello R.J., Nelson S., Motter R., et al. Polo-like kinase 2 (PLK2) phosphorylates α-synuclein at serine 129 in central nervous system. J. Biol. Chem. 2008;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ruan Q., Wang Q., Xie S., Fang Y., Darzynkiewicz Z., Guan K., Jhanwar-Uniyal M., Dai W. Polo-like kinase 3 is Golgi localized and involved in regulating Golgi fragmentation during the cell cycle. Exp. Cell Res. 2004;294:51–59. doi: 10.1016/j.yexcr.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 204.Xie S. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 2001;276:43305–43312. doi: 10.1074/jbc.M106050200. [DOI] [PubMed] [Google Scholar]
- 205.Dai W., Li Y., Ouyang B., Pan H., Reissmann P., Li J., Wiest J., Stambrook P., Gluckman J.L., Noffsinger A., et al. PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Genes Chromosom. Cancer. 2000;27:332–336. doi: 10.1002/(sici)1098-2264(200003)27:3<332::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 206.Li B., Ouyang B., Pan H., Reissmann P.T., Slamon D.J., Arceci R., Lu L., Dai W. prk, a Cytokine-inducible Human Protein Serine/Threonine Kinase Whose Expression Appears to be Down-regulated in Lung Carcinomas. J. Biol. Chem. 1996;271:19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 207.Yang Y., Bai J., Shen R., Brown S.A., Komissarova E.V., Huang Y., Jiang N., Alberts G.F., Costa M., Lu L., et al. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077–4085. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu X.S. Targeting Polo-Like Kinases: A Promising Therapeutic Approach for Cancer Treatment. Transl. Oncol. 2015;8:185–195. doi: 10.1016/j.tranon.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Silk A.D., Zasadil L.M., Holland A.J., Vitre B., Cleveland D.W., Weaver B.A. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc. Natl. Acad. Sci. USA. 2013;110:E4134–E4141. doi: 10.1073/pnas.1317042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cunningham C.E., Macauley M.J., Yadav G., Vizeacoumar F.S., Freywald A., Vizeacoumar F.J. Targeting the CINful genome: Strategies to overcome tumor heterogeneity. Prog. Biophys. Mol. Biol. 2019;147:77–91. doi: 10.1016/j.pbiomolbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 211.Zasadil L.M., Britigan E.M.C., Ryan S.D., Kaur C., Guckenberger D.J., Beebe D.J., Moser A.R., Weaver B.A. High rates of chromosome missegregation suppress tumor progression but do not inhibit tumor initiation. Mol. Biol. Cell. 2016;27:1981–1989. doi: 10.1091/mbc.E15-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Birkbak N.J., Eklund A.C., Li Q., McClelland S.E., Endesfelder D., Tan P., Tan I.B., Richardson A.L., Szallasi Z., Swanton C. Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res. 2011;71:3447–3452. doi: 10.1158/0008-5472.CAN-10-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smith M.R., Wilson M.L., Hamanaka R., Chase D., Kung H.-F., Longo D.L., Ferris D.K. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem. Biophys. Res. Commun. 1997;234:397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 214.Raab M., Sanhaji M., Matthess Y., Hörlin A., Lorenz I., Dötsch C., Habbe N., Waidmann O., Kurunci-Csacsko E., Firestein R., et al. PLK1 has tumor-suppressive potential in APC-truncated colon cancer cells. Nat. Commun. 2018;9:1106. doi: 10.1038/s41467-018-03494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li Z., Liu J., Li J., Kong Y., Sandusky G., Rao X., Liu Y., Wan J., Liu X. Polo-like kinase 1 (Plk1) overexpression enhances ionizing radiation-induced cancer formation in mice. J. Biol. Chem. 2017;292:17461–17472. doi: 10.1074/jbc.M117.810960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boone C., Bussey H., Andrews B.J. Exploring genetic interactions and networks with yeast. Nat. Rev. Genet. 2007;8:437–449. doi: 10.1038/nrg2085. [DOI] [PubMed] [Google Scholar]
- 217.Davierwala A.P., Haynes J., Li Z., Brost R.L., Robinson M.D., Yu L., Mnaimneh S., Ding H., Zhu H., Chen Y., et al. The synthetic genetic interaction spectrum of essential genes. Nat. Genet. 2005;37:1147–1152. doi: 10.1038/ng1640. [DOI] [PubMed] [Google Scholar]
- 218.Mani R., St. Onge R.P., Hartman J.L., Giaever G., Roth F.P. Defining genetic interaction. Proc. Natl. Acad. Sci. USA. 2008;105:3461–3466. doi: 10.1073/pnas.0712255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Eboucher B., Ejenna S. Genetic interaction networks: Better understand to better predict. Front Genet. 2013;4:290. doi: 10.3389/fgene.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nair S.P., Kundapur D., Vizeacoumar F.S., Freywald A., Uppalapati M., Vizeacoumar F.J. A road map to personalizing targeted cancer therapies using synthetic lethality. Trends Cancer. 2019;5:11–29. doi: 10.1016/j.trecan.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 221.Paul J.M., Templeton S.D., Baharani A., Freywald A., Vizeacoumar F.J. Building high-resolution synthetic lethal networks: A ‘Google map’ of the cancer cell. Trends Mol. Med. 2014;20:704–715. doi: 10.1016/j.molmed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 222.Vizeacoumar F.J., Arnold R., Vizeacoumar F.S., Chandrashekhar M., Buzina A., Young J.T.F., Kwan J.H.M., Sayad A., Mero P., Lawo S., et al. A negative genetic interaction map in isogenic cancer cell lines reveals cancer cell vulnerabilities. Mol. Syst. Biol. 2013;9:696. doi: 10.1038/msb.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N.J., Helelday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 224.Farmer H., McCabe N., Lord C.J., Tutt A.N.J., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nat. Cell Biol. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 225.Koppensteiner R., Samartzis E.P., Noske A., Von Teichman A., Dedes I., Gwerder M., Imesch P., Ikenberg K., Moch H., Fink D., et al. Effect of MRE11 loss on PARP-inhibitor sensitivity in endometrial cancer in vitro. PLoS ONE. 2014;9:e100041. doi: 10.1371/journal.pone.0100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mohni K.N., Thompson P.S., Luzwick J.W., Glick G.G., Pendleton C.S., Lehmann B.D., Pietenpol J.A., Cortez D. A Synthetic lethal screen identifies DNA Repair pathways that sensitize cancer cells to combined ATR inhibition and cisplatin treatments. PLoS ONE. 2015;10:e0125482. doi: 10.1371/journal.pone.0125482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Subhash V.V., Tan S.H., Yeo M.S., Yan F.L., Peethala P.C., Liem N., Krishnan V., Yong W.P. ATM Expression predicts veliparib and irinotecan sensitivity in gastric cancer by mediating P53-independent regulation of cell cycle and apoptosis. Mol. Cancer Ther. 2016;15:3087–3096. doi: 10.1158/1535-7163.MCT-15-1002. [DOI] [PubMed] [Google Scholar]
- 228.McAndrew E.N., Lepage C.C., McManus K.J. The synthetic lethal killing of RAD54B-deficient colorectal cancer cells by PARP1 inhibition is enhanced with SOD1 inhibition. Oncotarget. 2016;7:87417–87430. doi: 10.18632/oncotarget.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]