Abstract
This cross-sectional study examines trends in test result turnaround rates for COVID-19 testing nursing facility residents and staff in hot spot counties in the US.
Skilled nursing facility (SNF) residents comprise over 40% of coronavirus disease 2019 (COVID-19) deaths nationally.1 Surveillance testing is critical for controlling asymptomatic and presymptomatic viral transmission in these high-risk settings.2 For surveillance testing in SNFs to effectively guide infection control, results need to be obtained in less than 1 day.3 To facilitate such rapid testing,4 Medicare began distributing point-of-care severe acute respiratory syndrome coronavirus 2 antigen test instruments in July 2020, focused on SNFs in COVID-19 hot spot counties.5 Little is known about the adequacy of test result turnaround in SNFs.
Methods
We performed a cross-sectional study using the Medicare COVID-19 Nursing Home Database, a federally mandated weekly survey of all Medicare-certified SNFs, to examine facility-reported test result turnaround time. Beginning on August 16, 2020, the survey included 2 questions on test result turnaround: “During the past 2 weeks, on average how long did it take your long-term care facility to receive COVID-19 viral (nucleic acid or antigen) test results of residents?” or “staff and/or facility personnel?” with possible answers of less than 1 day, 1 to 2 days, 3 to 7 days, more than 7 days, or, for residents only, “no testing in the past 2 weeks” (eAppendix in the Supplement). We combined these data with SNF characteristics from the National Institute on Aging–funded LTCFocus.org database and the 2020 Medicare Nursing Home Compare database.
Per institutional policy, institutional review board approval and written informed consent were not required for research using publicly available data. Using surveys from the weeks ending August 16 to September 6, 2020, compared with September 13 to September 27, 2020, we examined test result turnaround for SNF staff and residents nationally and in Medicare-designated hot spot counties (eAppendix in the Supplement).5 We used multivariable linear probability models to estimate the association between SNF characteristics and result turnaround time longer than 2 days, controlling for SNF characteristics and state fixed effects, with county-level clustered standard errors (eAppendix in the Supplement). Analyses were performed using Stata statistical software (version 16, Stata Corp).
Results
Among the 15 065 respondents (98% of 15 355 Medicare-certified SNFs included in the data set), test result turnaround time was less than 1 day for 960 (6.2%) and 713 (4.8%) SNFs testing staff and residents respectively by September 7, 2020 (Figure, A and C). Rates rose to 2188 (13.5%) and 1516 (9.5%) by the week ending September 27. In hot spot counties, 167 (10.4%) and 125 (8.5%) SNFs testing staff and residents had less than 1 day turnaround by September 7, increasing to 248 (16.4%) and 196 (13.2%) by the week ending September 27.
Figure. National Distribution of Staff and Resident Testing Turnaround Times, August 16 to September 27, 2020.
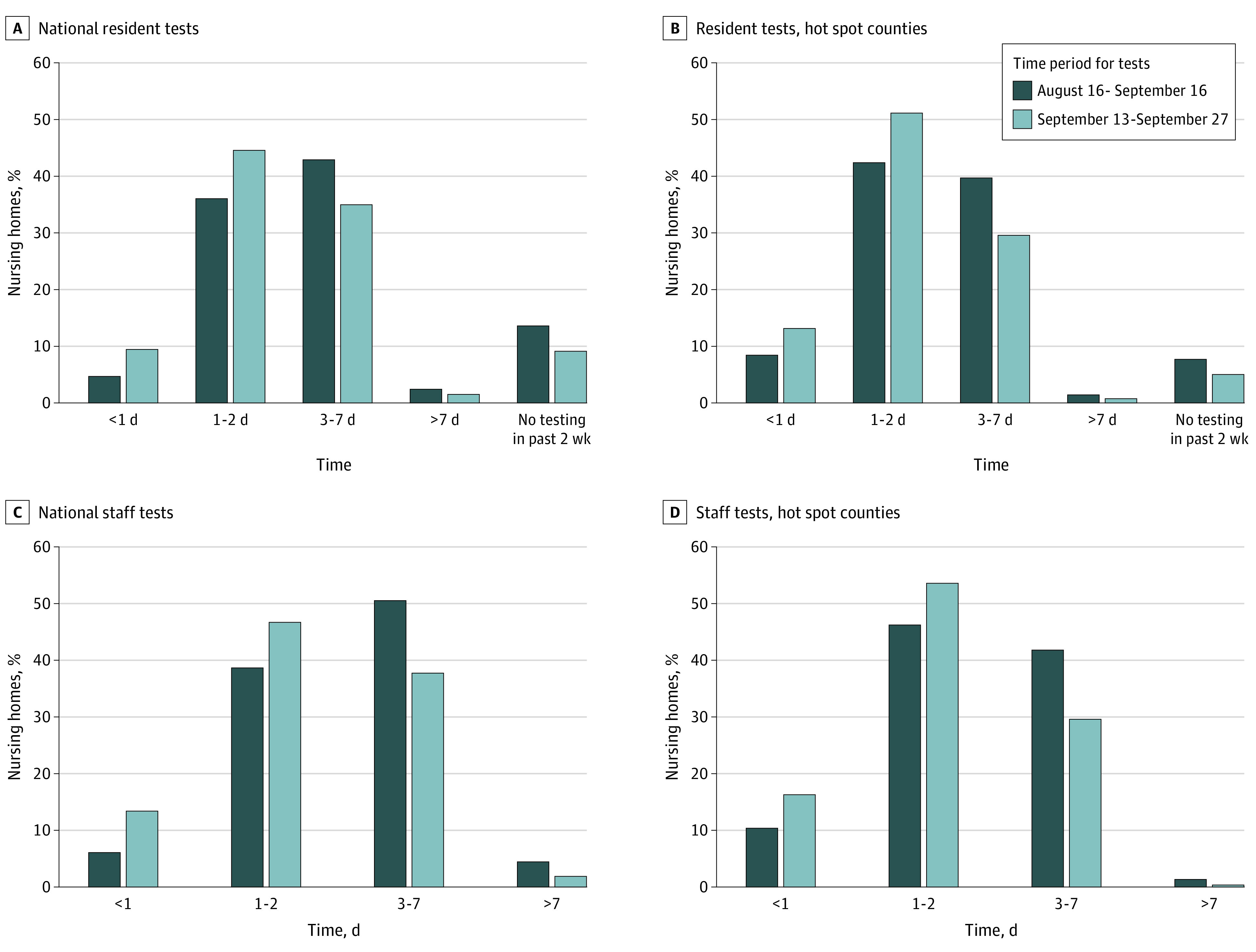
Distribution of test result turnaround times by survey response category for all skilled nursing facilities (SNFs) nationally for (A) residents (14 972 SNFs for time period 1 and 15 036 for time period 2) and (B) staff (14 967 SNFs for time period 1 and 14 988 for time period 2). There were 15 065 SNFs that submitted a nonmissing response to either the resident or staff testing question in time period 2. C and D, The same data for SNFs in 62 hot spot counties (1524 SNFs for resident testing in time period 1 and 1532 for time period 2; 1523 SNFs for staff testing in time period 1 and 1522 for time period 2). The difference in sample size between staff and resident categories within a time period is because, by design, the SNFs that reported that they did not perform resident tests in the preceding 2 weeks did not provide testing result turnaround answers; also, in some time periods, up to 2.5% of 15 355 total SNFs had missing data. There was no option for SNFs to indicate that they did not perform staff testing in the preceding 2 weeks. All estimates are weighted by facility bed size.
Nationally, test result turnaround time was 3 days or longer for 8117 (55.1%) and 6394 (45.5%) SNFs testing staff and residents and 642 (43.3%) and 621 (41.3%) in hot spot counties by September 7, 2020 (Figure). By September 27, this decreased to 5768 (39.8%) and 5145 (36.6%) of SNFs testing staff and residents nationally and 459 (29.9%) and 469 (30.4%) in hot spot counties.
There were statistically significant differences in the proportion of SNFs with test result turnaround times longer than 2 days for staff or residents across different characteristics, but they were mostly small in magnitude (Table). Turnaround time of more than 2 days was weakly correlated with new county-level COVID-19 cases that week ending September 27, after adjustment.
Table. Association of Skilled Nursing Facility Characteristics and COVID-19 Testing Turnaround Times of More Than 2 Days for the Week Ending September 27, 2020.
| Characteristic | Overall sample characteristics (%)a | Residents | Staff | ||
|---|---|---|---|---|---|
| Turnaround time >2 d (%) | Adjusted difference, percentage points (95% CI)b | Turnaround time >2 d (%) | Adjusted difference, percentage points (95% CI)b | ||
| Ownership type | |||||
| Nonprofit | 23.6 | 35.2 | 1 [Reference] | 37.6 | 1 [Reference] |
| Government owned | 6.4 | 32.5 | −2.2 (−6.0 to 1.7) | 32.7 | −2.9 (−6.4 to 0.7) |
| For profit | 69.8 | 40.5 | 4.2 (1.8 to 6.6) | 39.2 | 0.5 (−1.7 to 2.8) |
| Bed size, No | |||||
| 1-50 | 14.0 | 34.4 | 1 [Reference] | 36.0 | 1 [Reference] |
| 51-100 | 38.6 | 37.8 | 1.2 (−1.7 to 4.2) | 37.9 | 0.1 (−2.5 to 2.7) |
| 101-150 | 31.8 | 39.3 | 2.5 (−0.7 to 5.8) | 37.9 | 1.0 (−1.9 to 3.9) |
| 151-200 | 10.2 | 42.3 | 2.8 (−1.1 to 6.6) | 41.9 | 0.7 (−2.9 to 4.3) |
| ≥201 | 5.2 | 44.8 | 2.3 (−2.2 to 6.9) | 45.1 | −0.5 (−4.8 to 3.8) |
| Chain affiliation | |||||
| No | 39.2 | 37.3 | 1 [Reference] | 38.0 | 1 [Reference] |
| Yes | 54.6 | 40.2 | 2.5 (0.6 to 4.5) | 39.1 | 2.2 (0.4 to 3.9) |
| Missing | 6.2 | 34.2 | 6.0 (0.3 to 11.7) | 34.7 | 6.2 (0.7 to 11.6) |
| Quartile of Medicaid revenue share | |||||
| 1 (lowest) | 23.5 | 36.3 | 1 [Reference] | 36.7 | 1 [Reference] |
| 2 | 23.4 | 39.0 | 0.9 (−1.5 to 3.4) | 38.7 | 0.7 (−1.5 to 3.0) |
| 3 | 23.5 | 39.7 | 1.9 (−0.6 to 4.4) | 39.8 | 2.4 (0.0 to 4.7) |
| 4 (highest) | 23.4 | 41.2 | 3.7 (1.2 to 6.1) | 39.4 | 2.7 (0.4 to 5.1) |
| Quartile of non-White resident share | |||||
| 1 (lowest) | 23.0 | 36.9 | 1 [Reference] | 38.2 | 1 [Reference] |
| 2 | 22.5 | 37.4 | −0.7 (−3.3 to 1.8) | 37.3 | −1.1 (−3.5 to 1.3) |
| 3 | 22.6 | 39.1 | −0.4 (−3.1 to 2.4) | 38.2 | −0.5 (−3.2 to 2.1) |
| 4 (highest) | 22.6 | 43.5 | 1.2 (−1.9 to 4.3) | 41.9 | 0.4 (−2.6 to 3.4) |
| Missing | 9.3 | 33.1 | −6.3 (−11.1 to −1.4) | 33.9 | −5.3 (−9.9 to −0.7) |
| Overall quality score | |||||
| 1 | 16.5 | 42.3 | 1 [Reference] | 40.9 | 1 [Reference] |
| 2 | 19.4 | 40.4 | −1.0 (−3.8 to 1.7) | 39.4 | −1.7 (−4.3 to 0.9) |
| 3 | 17.5 | 37.9 | −3.6 (−6.4 to −0.9) | 38.2 | −3.2 (−5.9 to −0.6) |
| 4 | 21.1 | 37.7 | −2.5 (−5.2 to 0.1) | 36.5 | −4.3 (−6.8 to −1.7) |
| 5 | 24.2 | 36.2 | −3.4 (−6.2 to −0.5) | 37.5 | −3.8 (−6.5 to −1.0) |
| Quartile of county new COVID-19 case ratec | |||||
| 1 (lowest) | 25.1 | 42.6 | 1 [Reference] | 44.4 | 1 [Reference] |
| 2 | 25.2 | 39.4 | 0.3 (−2.9 to 3.5) | 39.5 | −2.1 (−5.1 to 0.8) |
| 3 | 24.8 | 37.2 | 1.7 (−1.7 to 5.1) | 35.9 | −0.6 (−4.3 to 3.1) |
| 4 (highest) | 24.9 | 35.8 | 3.2 (−0.5 to 6.9) | 33.8 | −1.9 (−5.5 to 1.6) |
| Any resident COVID-19 casesd | |||||
| No | 20.4 | 38.6 | 1 [Reference] | 38.1 | 1 [Reference] |
| Yes | 79.6 | 38.7 | −0.6 (−2.9 to 1.7) | 38.5 | 0.8 (−1.3 to 2.8) |
| Any staff COVID-19 casesd | |||||
| No | 6.3 | 38.1 | 1 [Reference] | 37.9 | 1 [Reference] |
| Yes | 93.7 | 38.8 | 1.8 (−2.0 to 5.7) | 38.5 | 2.4 (−0.9 to 5.6) |
| Hot spot countye | |||||
| No | 89.8 | 39.5 | 1 [Reference] | 39.4 | 1 [Reference] |
| Yes | 10.2 | 32.5 | −4.0 (−7.6 to −0.4) | 30.2 | −3.7 (−7.6 to 0.1) |
Abbreviation: COVID-19, coronavirus disease 2019.
A total of 15 065 skilled nursing facilities.
Adjusted differences in the probability of reporting a test result turnaround time longer than 2 days were estimated using linear probability regressions that contained all the facility and county characteristics included in the table, state fixed effects, the weekly rate of new resident and staff cases in the facility, and indicators for the type of lab used for test processing (private, state health department, other). Standard errors were clustered at the county level.
New county case rate refers to the 7-day average of new daily COVID-19 cases for the same week in which test result turnaround times were reported. County case rates obtained from the publicly available New York Times Coronavirus (COVID-19) Data in the United States repository.
COVID-19 cases defined as either suspected or confirmed cases since January 1, 2020, as reported by the skilled nursing facility.
Hot spot counties are those designated by the US Centers for Medicare Services to receive point-of-care testing kits during the first wave of distribution based on community rates of COVID-19.
Discussion
In a comprehensive federal survey, only a small fraction of SNFs had less than 1 day turnaround for staff or resident testing by late September 2020. Although testing delays improved over time, the state of testing is far behind the less than 24-hour turnaround that epidemiological modeling suggests is essential to prevent COVID-19 outbreaks in SNFs.2,3
Unfortunately, even in hot spot counties where all facilities should have received point-of-care instruments by mid-August, less than 17% of SNFs had a turnaround of less than 1 day. Conflicting regulations and testing supply shortages may be hampering efforts to take advantage of these devices.6
Limitations of this study include reliance on facility-reported test result turnaround times, an inability to differentiate between turnaround times of 1 and 2 days owing to survey design, and lack of data on the type of testing used by SNFs.
eAppendix
References
- 1.Kaiser Family Foundation . State Data and Policy Actions to Address Coronavirus. KFF. 2020. Accessed September 15, 2020. https://www.kff.org/coronavirus-covid-19/issue-brief/state-data-and-policy-actions-to-address-coronavirus/)
- 2.Coronavirus Commission on Safety and Quality in Nursing Homes : Commission Final Report. 2020. Accessed October 23, 2020. https://edit.cms.gov/files/document/covid-final-nh-commission-report.pdf)
- 3.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. medRxiv 2020. .Accessed September 15, 2020. http://medrxiv.org/lookup/doi/10.1101/2020.06.22.20136309) [DOI] [PMC free article] [PubMed]
- 4.Interim Final Rule (IFC) , CMS-3401-IFC, Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency related to Long-Term Care (LTC) Facility Testing Requirements and Revised COVID-19 Focused Survey Tool | CMS. Accessed September 15, 2020. https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and-memos-states-and/interim-final-rule-ifc-cms-3401-ifc-additional-policy-and-regulatory-revisions-response-covid-19)
- 5.Nursing Home Data - Point of Care Device Allocation | Data.CMS.gov. Accessed September 17, 2020. https://data.cms.gov/Special-Programs-Initiatives-COVID-19-Nursing-Home/Nursing-Home-Data-Point-of-Care-Device-Allocation/jbvf-tb74)
- 6.Nursing homes fret over Trump’s testing mandate - POLITICO. Accessed September 17, 2020. https://www.politico.com/news/2020/09/08/nursing-homes-coronavirus-testing-mandate-410229)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix


