Abstract
Alpha(α)-thalassemia is a blood disorder caused by many types of inheritable α-globin gene mutations which causes no-to-severe clinical symptoms, such as Hb Bart’s hydrops fetalis that leads to early foetal death. Therefore, the aim of this meta-analysis was to provide an update from year 2010 to 2020 on the prevalence of α-thalassemia in Southeast Asia. A systematic literature search was performed using PubMed and SCOPUS databases for related studies published from 2010 to 2020, based on specified inclusion and exclusion criteria. Heterogeneity of included studies was examined with the I2 index and Q-test. Funnel plots and Egger’s tests were performed in order to determine publication bias in this meta-analysis. Twenty-nine studies with 83,674 subjects were included and pooled prevalence rates in this meta-analysis were calculated using random effect models based on high observed heterogeneity (I2 > 99.5, p-value < 0.1). Overall, the prevalence of α-thalassemia is 22.6%. The highest α-thalassemia prevalence was observed in Vietnam (51.5%) followed by Cambodia (39.5%), Laos (26.8%), Thailand (20.1%), and Malaysia (17.3%). No publication bias was detected. Conclusions: This meta-analysis suggested that a high prevalence of α-thalassemia occurred in selected Southeast Asia countries. This meta-analysis data are useful for designing thalassemia screening programs and improve the disease management.
Keywords: prevalence, α-thalassemia, Southeast Asia, meta-analysis, haematological disorder
1. Introduction
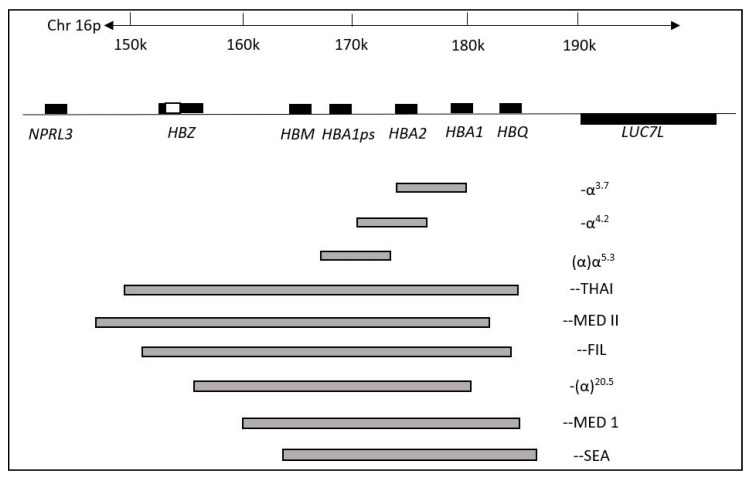
Thalassemia is the most common hereditary red blood cell disorder which causes anemias due to defective genes that code for globin proteins synthesis [1]. The inheritance of the thalassemia genotype could result in the individual being either a carrier or a patient. There are two major types of thalassemia: (1) alpha (α) and (2) beta (β), in which the former is the most common form of thalassemia worldwide especially in Southeast Asia populations [2,3]. Both α- and β-thalassemia arise from genetic defects in α and/or β-globin genes, which regulate the number of globin chains in red blood cells. Genetic defects in either α and/or β-globin genes can cause imbalance in numbers of α and β chains in red blood cells. This leads to the manifestation of clinical conditions known as α- or β-thalassemia. The most common genetic defect in α-thalassemia is a deletion in the α-globin gene involving one or both globin genes such as -α3.7, -α4.2, --SEA, --THAI, -αCD59, -α20.5, -αIVS I-1 and others (Figure 1) [4,5,6]. A highly severe form of deletional α-thalassemia, known as Haemoglobin (Hb) Bart’s hydrops fetalis, is a homozygous α0-thalassemia deletion with a complete loss of functional α-globin that leads to foetal death or death shortly after birth. Currently, there is no effective treatment for this disease [7,8].
Figure 1.
Some common changes in α-thalassemia in Southeast Asia. The genes are shown in boxes with a scale in kilobases (kb). The most common deletions of α-thalassemia mutations are indicated by grey bars indicating the length of deletion. (Adapted from Farashi & Harteveld, 2018 [6]).
There are also cases of non-deletional mutations in the α-globin gene such as Hb Constant Spring (CS), which is caused by a nucleotide substitution in the termination codon TAA→CAA and also Hb Pakse (-α4PS), which is caused by the termination codon (UAA→CAA). This results in the elongation of the α-globin chain protein and causes severe anemia with serious complications that include liver impairment, cardiac disease and endocrine disorder [9,10]. Some cases of Hb CS require red blood cell transfusions when Hb drop to dangerously low levels [11]. Most importantly, an individual can carry a single or multiple type form of the mutation (deletional/non-deletional), which give rise to different clinical manifestations and complicates diagnosis as well as treatments for α-thalassemia [12]. Both deletional and non-deletional α-thalassemia prevalence rates are highly important in determining the overall severity of clinical symptoms in a region or a specific population.
Despite the detrimental effects of thalassemia, evidence shows that thalassemia confers a protective effect against hyperparasitemia due to malaria infection [13]. Plasmodium vivax parasitemia were two to three times lower in thalassemia patients as compared to malaria cases in people without thalassemia. However, the protective effect of thalassemia against parasitemia was not observed in a study conducted in Papua New Guinea in children aged 3–21 months [14]. Therefore, there is a natural selection force which leads to the prevalence of thalassemia cases in malaria-endemic areas [15].
Most of the α-thalassemia cases reveal some abnormalities in their red cell index. According to the British Committee for Standards in Haematology, a value of <27 pg in the average amount of Hb found in red blood cells (also referred as Mean Corpuscular Hemoglobin) is the primary screening threshold to quantify Hb subtypes [16]. However, subjects with a single gene deletion or carriers of the mutation in the non-severe form of α-thalassemia may present a normal Hb level. Different heterozygosity or homozygosity of gene deletions or mutations in the α-thalassemia gene gives different phenotypes, which complicates treatment [12]. Therefore, diagnosing of non-severe forms of α-thalassemia is a highly challenging task.
Several countries in the Southeast Asia region have reported the prevalence of α-thalassemia in different ethnic groups independently, revealing that the prevalence of α-thalassemia differs from country to country with different ethnicities [4,5,17,18]. However, no studies have systematically meta-analyzed the prevalence and epidemiology of α-thalassemia—where the results of these similar studies are quantitatively combined—for this region. Therefore, the aim of this meta-analysis was to provide an update (from 2010 until 2020) using data concerning α-thalassemia prevalence in Southeast Asia, focusing on Cambodia, Laos, Malaysia, Thailand, and Vietnam. The study outcome on the perspective of α-thalassemia prevalence in Southeast Asia could aid in designing healthcare policies for α-thalassemia screening in large populations and provide better genetic counselling programs.
2. Materials and Methods
2.1. Study Guidelines and Literature Search
PRISMA guidelines were followed for conducting and reporting the results of this meta-analysis (Table S1) [19]. PubMed (Table S2) and SCOPUS (Table S3) databases were searched up to March 2020 with the lower limit set to January 2010 using related terms, including alpha, thalassemia, southeast, and Asian.
2.2. Selection of Studies and Data Extraction
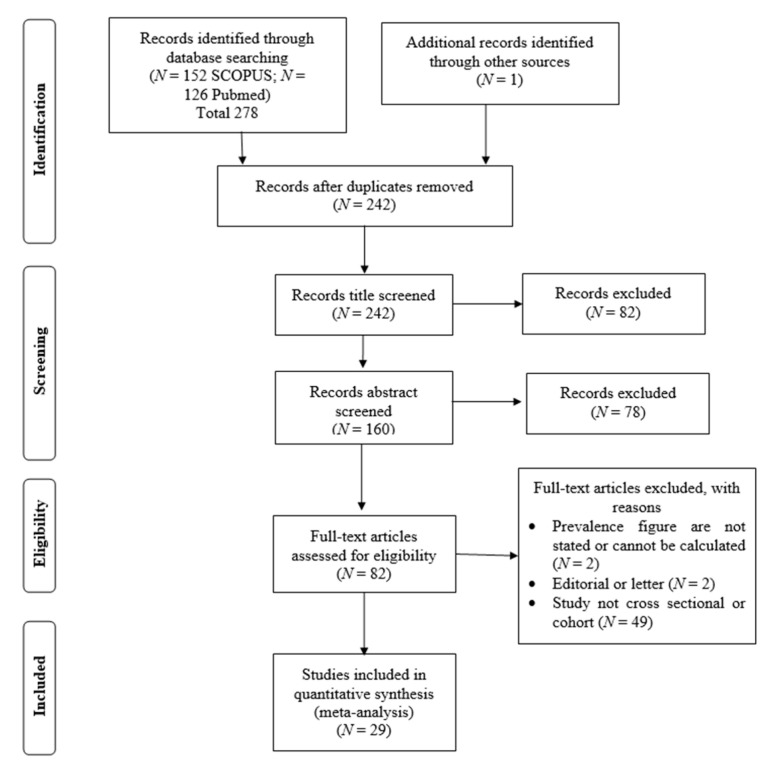
All search results were screened by two investigators, and all potential studies were independently reviewed to be included in this meta-analysis. The main inclusion criteria were: (1) studies published in English in which the prevalence of α-thalassemia (including all deletional and non-deletional mutations) in Southeast Asian countries were reported; and (2) the study was a peer-reviewed publication. Studies that did not report cross-sectional, observational, cohort, or prevalence of α-thalassemia were excluded. We identified additional eligible studies based on references that were cited in the relevant articles. When publications overlapped, only the study with the largest or the most recent data was included in this meta-analysis. Data, including first author’s name, publication year, country, sample size, and prevalence of α-thalassemia of the included studies, were extracted and documented by the reviewing investigators. A total number of 278 articles were included in this meta-analysis (Tables S4 and S5). The study selection and review process are illustrated in Figure 2.
Figure 2.
Flow diagram of the systemic literature search in this study.
2.3. Statistical Analyses
The prevalence of α-thalassemia was calculated for each study with the number of reported α-thalassemia cases as the numerator and the total sample size as the denominator. Homogeneity across studies was investigated with the I2 index (represented as percentage) and Q-test (represented as a p-value) that indicated heterogeneity between studies. It was reported that an I2 value > 75% and Q-test with a p-value < 0.1 was regarded as high heterogeneity [20,21]. A random effects model was used to combine individual effect sizes to create pooled α-thalassemia prevalence if a significantly high heterogeneity was observed. If other results were obtained, a fixed effects model was utilized. A forest plot was generated to illustrate the prevalence of each study with a 95% confidence interval (95% CI) that contributed to the analysis along with the combined prevalence rate. A subsequent meta-analysis was also performed based on each respective country. Funnel plots and Egger’s tests of asymmetry were performed to identify any bias within the results [22,23]. All analyses were performed with Comprehensive Meta-Analysis version 2 software (Biostat, Inc., New Jersey, USA) [24].
3. Results
3.1. Study Characteristics
Twenty-nine studies with a total number of 83,674 subjects were included in this meta-analysis after a detailed assessment of records obtained from the database and additional searching. These studies were published between January 2010 and October 2019. Among all included studies, two were carried out in Cambodia, three in Laos, five in Malaysia, 20 in Thailand, and two in Vietnam (Table 1). The main characteristics of the studies included in the meta-analysis were recorded and are shown in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
| Author [Reference] | α-Thalassemia Genotyping Method | Genotypes Found in the Study | Country | Specific Ethnic 1 | Events 2 | Total 3 |
|---|---|---|---|---|---|---|
| Munkongdee et al., 2016 [25] | Polymerase chain reaction (PCR) | -α3.7, -α4.2, --SEA, αCS, αPs | Cambodia | N/A | 646 | 1631 |
| Jomoui et al., 2017 [26] | PCR | --SEA | Cambodia | N/A | 7 | 21 |
| Wongprachum et al., 2012 [27] | PCR | -α3.7, -α4.2, --SEA, --THAI, αCS, αPs, αQ-Thailand | Laos | N/A | 130 | 411 |
| Jomoui et al., 2017 [26] | PCR | --SEA | Laos | N/A | 28 | 52 |
| Tritipsombut et al., 2012 [28] | PCR | -α3.7, -α4.2, --SEA, αCS, αPs | Laos | N/A | 30 | 349 |
| Azma et al., 2012 [29] | PCR | Malaysia | N/A | 14 | 400 | |
| Azma et al., 2014 [4] | PCR | -α3.7, -α4.2, --SEA, αCS, αCD59, αIVS I-1 | Malaysia | Malay, Chinese, Indian, Other | 736 | 1623 |
| Jameela et al., 2011 [30] | PCR | -α3.7, -α4.2, --SEA, --FIL, α125 | Malaysia | Malay, Chinese, Indian, Sikh, Iban | 10 | 310 |
| Mohd Yatim et al., 2014 [31] | PCR | -α3.7, --SEA, αCS, αCD59, | Malaysia | Malay | 28 | 68 |
| Tan et al., 2010 [3] | PCR | -α3.7, -α4.2, --SEA, --THAI, --FIL, αCS, α125, | Malaysia | Kadazandusun | 42 | 125 |
| Charoenkwan et al., 2010 [32] | PCR | -α3.7, -α4.2, --SEA, -αQ-Thailand, αCS | Thailand | N/A | 142 | 566 |
| Lithanatudom et al., 2016 [17] | PCR | -α3.7, -α4.2, --SEA, --THAI, αCS, αPs | Thailand | Yong, Yuan, Lue, Khuen, Blang, Mon, Paluang, Lawa | 33 | 141 |
| Nillakupt et al., 2012 [33] | PCR | -α3.7, --SEA, αCS, αPs | Thailand | N/A | 47 | 266 |
| Pongjantharasatien et al., 2016 [34] | PCR | --SEA, --THAI, --FIL, -αthal-1 | Thailand | N/A | 4555 | 31,632 |
| Pichanun et al., 2010 [35] | PCR | -α3.7, αCS, αPs | Thailand | N/A | 36 | 587 |
| Pharephan et al., 2016 [36] | PCR | -α3.7, -α4.2, --SEA, αCS | Thailand | N/A | 229 | 638 |
| Panyasai et al., 2016 [37] | PCR | -α3.7, -αQT, --SEA, | Thailand | N/A | 51 | 23,914 |
| Panomai et al., 2010 [38] | PCR | -α3.7, -α4.2, --SEA, --THAI, αCS, αPs | Thailand | N/A | 40 | 190 |
| Prayalaw et al., 2014 [39] | PCR | -α3.7, -α4.2, --SEA, αCS, -αQ-Thailand | Thailand | N/A | 75 | 300 |
| Seeratanachot et al., 2015 [40] | Realtime-PCR | -α3.7, -α4.2, --SEA | Thailand | N/A | 62 | 250 |
| Wisedpanichkij et al., 2015 [41] | PCR | -α3.7, -α4.2, --SEA, αCS | Thailand | N/A | 409 | 578 |
| Uaprasert et al., 2013 [42] | PCR | -α3.7, -α4.2, αCS | Thailand | N/A | 67 | 241 |
| Srivorakun et al., 2011 [43] | PCR | -α3.7, --SEA, αCS | Thailand | N/A | 44 | 226 |
| Tritipsombut et al., 2012 [28] | PCR | -α3.7, -α4.2, --SEA, αCS, αPs | Thailand | N/A | 85 | 1460 |
| Chaibunruang et al., 2013 [44] | PCR | --SEA, --THAI | Thailand | N/A | 1874 | 12,525 |
| Kulaphisit et al., 2017 [5] | PCR | -α3.7, -α4.2, --SEA, --THAI, αCS, αPs | Thailand | Yong, Lue, Yuan, Shan, Khuen, Htin, Paluang, Blang, Lawa, Mon, Skaw Karen, Pwo Karen, Padong Karen | 124 | 668 |
| Thanyaornwanya et al., 2019 [45] | PCR | -α3.7, -α4.2, αCS, αPs | Thailand | N/A | 676 | 1192 |
| Jomoui et al., 2017 [26] | PCR | --SEA | Thailand | N/A | 66 | 96 |
| Mankhenthong et al., 2019 [46] | PCR | -α3.7, -α4.2, --SEA, --THAI, αCS | Thailand | N/A | 118 | 1290 |
| Pata et al., 2019 [47] | PCR | -α3.7, -α4.2, --SEA, --THAI, αCS | Thailand | N/A | 82 | 195 |
| O’Riordan et al., 2010 [18] | PCR | -α3.7, -α4.2, --SEA, --THAI, --FIL, αCS | Vietnam | Kinh, Dao, Tay, Nung, S’Tieng, M’Nong, Rac Iay, E De | 996 | 1431 |
| Hoa Nguyen et al., 2014 [48] | PCR | -α3.7, -α4.2, --SEA, --THAI, --SEA, αCS, αPs | Vietnam | Cό-Tu | 98 | 298 |
| Total | 11,580 | 83,674 | ||||
1 N/A: Not available due to ethnicities were not reported by the study. 2 Events: Number of subjects carrying alpha-thalassemia. 3 Total: Total number of subjects.
3.2. Meta-Analysis Outcomes
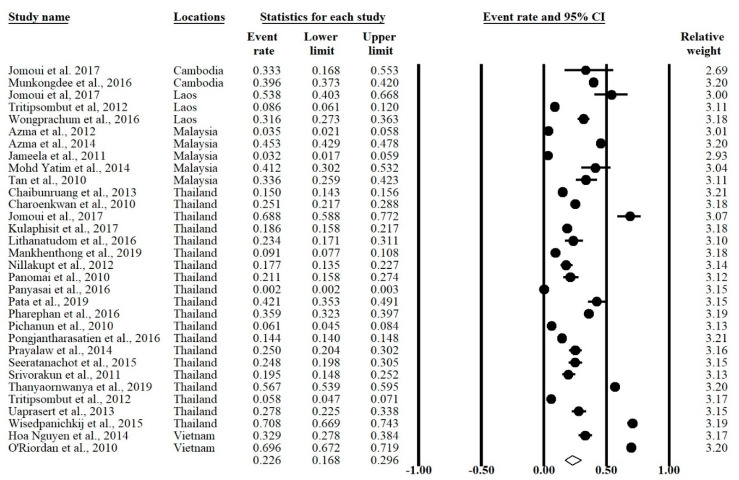
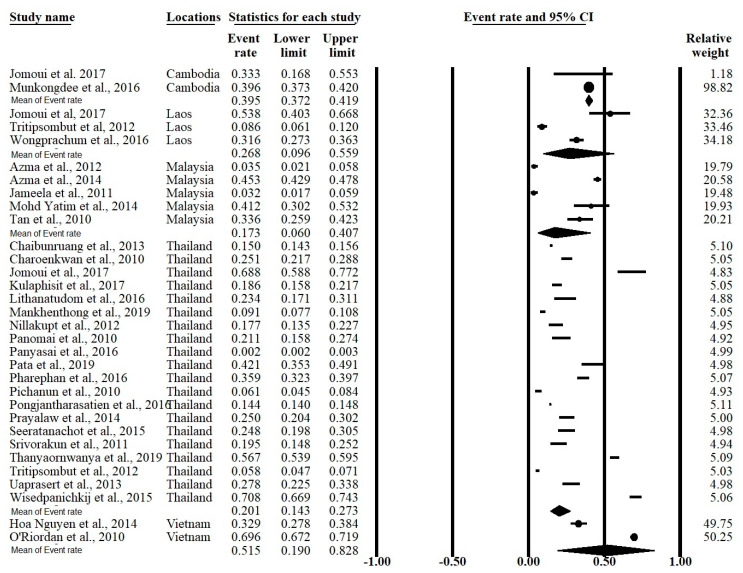
The meta-analysis was conducted using a random effects model found significant heterogeneity showed an I2 > 99.5% and p-value < 0.001 in overall and all subgroups except Cambodia (Table 2). The forest plot showed that the overall prevalence rate of α-thalassemia occurrence in this meta-analysis was 0.226 (95% CI = 0.168–0.296; I2 = 99.5%; p-value < 0.1) (Figure 3). In the subgroup analysis based on country, Vietnam had the highest prevalence rate (51.5%) of α-thalassemia followed by Cambodia (39.5%) Laos (26.8%), Thailand (20.1%), and Malaysia (17.3%) (Figure 4).
Table 2.
Prevalence rate and heterogeneity of α-thalassemia in overall and subgroups of the study.
| Heterogeneity | Prevalence Rate (95% CI) | Sample Size (N) | No. of Studies (N) | Subgroups | |
|---|---|---|---|---|---|
| I2 (%) | p-Value | ||||
| 99.53 | <0.001 | 0.226 (0.168–0.296) | 83,674 | 32 | Overall |
| 0 | 0.560 | 0.395 (0.372–0.419) | 1652 | 2 | Cambodia |
| 97.26 | <0.001 | 0.268 (0.096–0.559) | 812 | 3 | Laos |
| 98.20 | <0.001 | 0.173 (0.060–0.407) | 2526 | 5 | Malaysia |
| 99.47 | <0.001 | 0.201 (0.143–0.273) | 76,955 | 20 | Thailand |
| 99.22 | <0.001 | 0.515 (0.190–0.828) | 1729 | 2 | Vietnam |
Figure 3.
Forest plot of α-thalassemia overall prevalence using random effects model.
Figure 4.
Forest plot of the α-thalassemia prevalence grouped according to country.
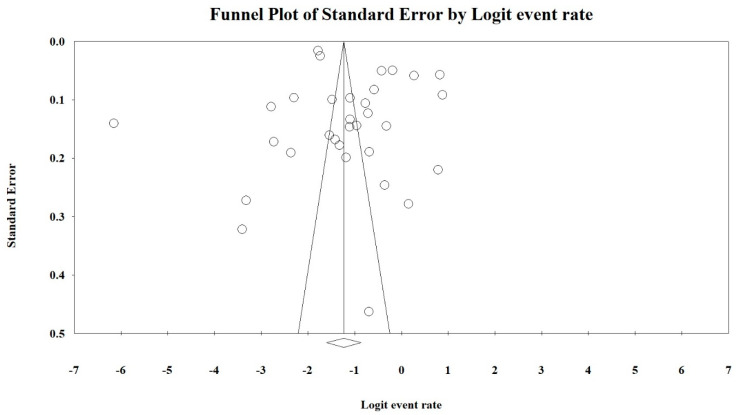
3.3. Publication Bias
Funnel plots and Egger’s tests were performed to estimate the publication bias of the included literature. The shape of the funnel plot revealed obvious evidence of symmetry (Figure 5). The value for Egger’s test was t-value = 1.24 with a p-value = 0.112.
Figure 5.
Funnel plot of the overall prevalence of α-thalassemia in this study.
4. Discussion
This study is the first to report the prevalence of α-thalassemia in the Southeast Asia region over the past 10 years (2010–2020). We did not obtain any α-thalassemia-related studies that fulfilled our inclusion and exclusion criteria from other Southeast Asian countries, including Brunei, Indonesia, Myanmar, Philippines, Singapore, and Timor-Leste. Using a random effects model, the overall prevalence rate of α-thalassemia in the included countries was 22.6%, which indicated a significant reduction of ~50% of the prevalence in the Southeast Asia region since 2008. The World Health Organization had reported the α-thalassemia prevalence as 44.6% in 2008 [49]. India and Brazil, were reported at about 12% and 19.2%, respectively [50,51]. The prevalence rates were high in countries such as UAE, Oman and Saudi Arabia at 50% [52].
The high prevalence of α-thalassemia in the past was due to the lack of knowledge regarding the seriousness of this disease among the populations in these countries, especially those living in rural areas with limited access to education and those who could not afford to obtain the proper education similar to that found in urban areas. α-thalassemia is an inherited disease and the mutations may pass from parent to child, affecting the haemoglobin production. Hence, the educational and screening campaigns regarding this disease conducted by the representative bodies (either government or non-government organization) have successfully reduced the prevalence of α-thalassemia in the Southeast Asia region.
Random effects models were used, which are based on the assumption that the true effect could vary between studies [51]. The existence of publication bias in this meta-analysis was determined using a funnel plot and Egger’s tests. The shape of the funnel plot in this meta-analysis showed an obvious symmetry, indicating the risk of publication bias is significantly low. This hypothesis was also supported by statistical evidence from Egger’s test (t-value = 1.24; p-value = 0.112) in which the publication bias did not significantly exist in this meta-analysis. Therefore, we concluded that there was no publication bias detected in this meta-analysis.
When stratified according to country, Vietnam has the leading α-thalassemia prevalence rate of 51.5%. The high prevalence rate is probably due to one of the observational studies conducted in Vietnam focusing on the country’s minority ethnic groups and thus, this likely skewed the actual prevalence rate [51]. The prevalence of α-thalassemia in Cambodia was the second highest (39.5%) when compared to other countries included in this meta-analysis. However, since there was only two studies that reported the prevalence of α-thalassemia Cambodia and Vietnam, more data are required to estimate the actual prevalence rate of this disease in both of these countries. The prevalence of α-thalassemia in Laos, Malaysia, and Thailand was quite similar, ranging from 17.3% to 26.8%. Alpha thalassemia is an inheritable disease where the presence of multiple deletional and non-deletional mutations can cause severe clinical complications. The presence of α-thalassemia major causes hydrops fetalis and prenatal deaths [4,5]. Therefore, the low prevalence of α-thalassemia in these countries and this region indicates that genetic screening for α-thalassemia mutations in the parents could be done in a population focused approach. Allele frequency and genetic diversity amongst the different populations provide information that can be used effectively in designing thalassemia prevention programs [53].
Thalassemia patients suffers from anemia caused by the imbalance of globin chains and impairment of haemoglobin solubility of erythrocytes. The reduced globin chains were shown to impair the cytoadherence of Plasmodium [54,55]. These abnormalities of erythrocytes have been shown to confer a protective effect against malaria infection [12,56]. Hence, there is a natural selection pressure which causes thalassemia becoming prevalent in countries with high incidence of malaria. Our meta-analysis was not exhaustive, however it shows that Vietnam had the highest prevalence rate (51.5%) and the lowest malaria cases (5794 cases) among the countries included in our study which supported the protective factor of thalassemia [57].
There are several limitations that should be addressed in this meta-analysis. Firstly, only data from certain Southeast Asian countries were available to be included in this meta-analysis; therefore, the calculated α-thalassemia-related prevalence rate in this study was specific to selected Southeast Asian regions. Besides, only studies from 2010 to 2020 were included in this meta-analysis, and it is possible for studies published before the year 2010 that meet the inclusion criteria but were not included in this meta-analysis, as this study focused on the prevalence rates from recent past 10 years only. We also did not include ethnic stratification because the majority of the studies included in this analysis did not report the ethnic group in the subject population. Alpha thalassemia genotype stratification was not be performed due to inconsistencies in the reporting of genotypes in the studies included in this analysis.
5. Conclusions
This is the first meta-analysis that investigated α-thalassemia prevalence in Southeast Asian countries, and the findings suggest high prevalence of α-thalassemia events in certain countries which warrants attention as α-thalassemia major could cause severe health complications and impose a substantial burden to the health authority and families. The data in this meta-analysis may be beneficial to the representative bodies in designing educational and screening campaigns regarding this disease in order to further reduce α-thalassemia rates in these countries.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/20/7354/s1, Table S1: PRISMA checklist of items to include when reporting a systematic review or meta-analysis, Table S2: Initial literature search from Pubmed, Table S3: Initial literature search from SCOPUS, Table S4: The SCOPUS literature (N = 152) included in the analysis, Table S5: The Pubmed literature (N = 126) included in the analysis.
Author Contributions
Conceptualization, L.P.W.G. and P.-C.L.; methodology, L.P.W.G. and E.T.J.C.; validation, E.T.J.C.; investigation, L.P.W.G. and E.T.J.C; writing—original draft, L.P.W.G.; writing—reviewing and editing, L.P.W.G. and P.-C.L.; resources, P.-C.L.; supervision, P.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Higgs D.R., Engel J.D., Stamatoyannopoulos G. Thalassemia. Lancet. 2012;379:73–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 2.Rosnah B., Rosline H., Zaidah A.W., Noor Haslina M.N., Marini R., Shafini M.Y., Nurul Ain F.A. Detection of common deletional alpha-thalassemia spectrum by molecular technique in Kelantan, Northeastern Malaysia. ISRN Hematol. 2010;2012:462969. doi: 10.5402/2012/462969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan J.I.M.A., Lee P.C., Wee Y.C., Tan K.L., Mahali N.F., George E., Chua K.H. High prevalence of alpha- and beta-thalassemia in the Kadazadusuns in East Malaysia: Challenges in providing effective health care for an indigenous group. J. Biomed. Biotechnol. 2010;2010:706872. doi: 10.1155/2010/706872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azma R.Z., Ainoon O., Hafiza A., Azlin I., Noor Farisah A.R., Nor Hidayati S., Noor Hamidah H. Molecular characteristic of alpha thalassemia among patients diagnosed in UKM medical centre. Malays. J. Pathol. 2014;36:27–32. [PubMed] [Google Scholar]
- 5.Kulaphisit M., Kampuansai J., Leecharoenkiat K., Wathikthinnakon M., Kangwanpong D., Munkongdee T., Svasti S., Fucharoen S., Smith D.R., Lithanatudom P. A comprehensive ethnic-based analysis of alpha thalassemia allele frequency in northern Thailand. Sci. Rep. 2017;7:4690. doi: 10.1038/s41598-017-04957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farashi S., Harteveld C.L. Molecular basis of α-thalassemia. Blood Cells Mol. Dis. 2018;70:43–53. doi: 10.1016/j.bcmd.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Chui D.H.K. Alpha-Thalassemia: Hb H disease and Hb Bart’s hydrops fetalis. Ann. N. Y. Acad. Sci. 2005;1054:25–32. doi: 10.1196/annals.1345.004. [DOI] [PubMed] [Google Scholar]
- 8.Weatherall D.J., Clegg J.B. The Thalassemia Syndrome. 4th ed. Blackwell Scientific Publication; Oxford, UK: 2011. [Google Scholar]
- 9.Casale M., Meloni A., Filosa A., Cuccia L., Caruso V., Palazzi G., Rita Gamberini M., Pitrolo L., Caterina Putti M., Giuseppe D’Ascola D., et al. Multiparametric Cardiac Magnetic Resonance Survey in Children with Thalassemia Major. Circ. Cardiovasc. Imaging. 2015;8:e003230. doi: 10.1161/CIRCIMAGING.115.003230. [DOI] [PubMed] [Google Scholar]
- 10.Kurtoglu A.U., Kurtoglu E., Temizkan A.K. Effect of iron overload on endocrinopathies in patients with beta-thalassaemia major and intermedia. Endokrynol. Pol. 2012;63:260–263. [PubMed] [Google Scholar]
- 11.Suthat F., Pranee W. Haemoglobinopathies in Southeast Asian. Indian J. Med. Res. 2011;134:498–506. [PMC free article] [PubMed] [Google Scholar]
- 12.Galanello R., Cao A. Alpha-thalassemia. Gen. Med. 2011;13:83–88. doi: 10.1097/GIM.0b013e3181fcb468. [DOI] [PubMed] [Google Scholar]
- 13.Kuesap J., Chaijaroenkul W., Rungsihirunrat K., Pongjantharasatien K., Na-Bangchang K. Coexistance of Malaria and Thalassemia in malaria endemic areas of Thailand. Korean J. Parasitol. 2015;53:265–270. doi: 10.3347/kjp.2015.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosanas-Uegell A., Senn N., Raru P., Aponte J.J., Reeder J.C., Siba P.M., Michon P., Mueller I. Lack of associations of α(+)-thalassemia with the risk of Plasmodium falciparum and Plasmodium vivax infection and disease in a cohort of children aged 3-21 months from Papua New Guinea. Int. J. Parasitol. 2012;42:e1000097. doi: 10.1016/j.ijpara.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Vento S., Cainelli F., Cesario F. Infections and thalassemia. Lancet Infect. Dis. 2006;6:226–233. doi: 10.1016/S1473-3099(06)70437-6. [DOI] [PubMed] [Google Scholar]
- 16.Ryan K., Bain B.J., Worthington D., James J., Plews D., Mason A., Roper D., Rees D.C., de la Salle B., Streetly A., et al. Significant haemoglobinopathies: Guidelines for screening and diagnosis. Br. J. Haematol. 2010;149:35–49. doi: 10.1111/j.1365-2141.2009.08054.x. [DOI] [PubMed] [Google Scholar]
- 17.Lithanatudom P., Khampan P., Smith D.R., Svasti S., Fucharoen S., Kangwanpong D., Kampuansai J. The prevalence of alpha-thalssemia amongst Tai and Mon-Khmer ethnic groups residing in northern Thailand: A population-based study. Hematology. 2016;21:480–485. doi: 10.1080/10245332.2016.1148374. [DOI] [PubMed] [Google Scholar]
- 18.O’Riordan S., Hien T.T., Miles K., Allen A., Quyen N.N., Hung N.Q., Anh D.Q., Tuyen L.N., Khia D.B., Thai C.Q., et al. Large scale screening for haemoglobin disorders in southern Vietnam: Implications for avoidance and management. Br. J. Haematol. 2010;150:359–364. doi: 10.1111/j.1365-2141.2010.08237.x. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Loberati A., Tetzlaff J., Altman D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dersimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Light R.J., Pillemer D.B. Summing up: The Science of Reviewing Research. Harvard University Press; Cambridge, MA, USA: 1984. [Google Scholar]
- 24.Borenstein M., Hedges L., Higgins J., Rothstein H.R. Comprehensive meta-analysis version 2. Englewood. 2005;104:188–191. [Google Scholar]
- 25.Munkongdee T., Tanakulmas J., Butthep P., Winichagoon P., Main B., Yiannakis M., George J., Devenish R., Fucharoen S., Svasti S. Molecular epidemiology of hemoglobinpathies in Cambodia. Hemoglobin. 2016;40:163–167. doi: 10.3109/03630269.2016.1158723. [DOI] [PubMed] [Google Scholar]
- 26.Jomoui W., Fucharoen G., Sanchaisuriya K., Charoenwijitkul P., Maneesarn J., Xu X., Fucharoen S. Genetic origin of α0-thalassemia (SEA deletion) in Southeast Asian populations and application to accurate prenatal diagnosis of Hb Bart’s hydrops fetalis syndrome. J. Hum. Gen. 2017;62:747–754. doi: 10.1038/jhg.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wongprachum K., Sanchaisuriya K., Dethvongphanh M., Norcharoen B., Vidamaly V., Sanchaisuriya P., Fucharoen S., Fucharoen G., Schelp F.P. Molecular heterogeneity of thalassemia among pregnant Laotian women. Acta Hematol. 2016;135:65–69. doi: 10.1159/000438739. [DOI] [PubMed] [Google Scholar]
- 28.Tritipsombut J., Sanchaisuriya K., Phollarp P., Bouakhasith D., Sanchaisuriya P., Fucharoen G., Fucharoen S., Schelp F.P. Micromapping of thalassemia and hemoglobinopathies in different regions of northeast Thailand and Vientaine, Laos people’s democratic republic. Hemoglobin. 2012;36:47–56. doi: 10.3109/03630269.2011.637149. [DOI] [PubMed] [Google Scholar]
- 29.Azma R.Z., Ainoon O., Azlin I., Hamenuddin H., Hadi N.A., Tatt W.K., Syazana I.N., Asmaliza A.M., Das S., Hamidah N.H. Prevalence of iron deficiency anaemia and thalassemia trait among undergraduate medical students. Clin. Ter. 2012;163:287–291. [PubMed] [Google Scholar]
- 30.Jameela S., Sharifah Sabirah S.O., Babam J., Phan C.L., Visalachy P., Chang K.M., Salwana M.A., Zuridah A., Subramanian Y., Rahimah A. Thalassemia screening among students in a secondary school in Ampang, Malaysia. Med. J. Malays. 2011;66:522–524. [PubMed] [Google Scholar]
- 31.Mohd Yatim N.F., Abd Rahim M., Menon K., Al-Hassan F.M., Ahmad R., Manocha A.B., Saleem M., Yahaya B.H. Molecular characterization of α and β-thalassaemia among malay patients. Int. J. Mol. Sci. 2014;15:8835–8845. doi: 10.3390/ijms15058835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charoenkwan P., Taweephol R., Sirichotiyakul S., Tantiprabha W., Sae-Tung R., Suanta S., Sakdasirisathaporn P., Sanguansermsri T. Cord blood screening for α-thalassemia and hemoglobin variants by isoelectric focusing in northern Thai neonates: Correlation with genotypes and hematologic parameters. Blood Cells Mol. Dis. 2010;45:53–57. doi: 10.1016/j.bcmd.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Nillakupt K., Nathalang O., Arnutti P., Jindadamrongwech S., Boonsiri T., Panichkul S., Areekul W. Prevalence and hematological parameters of thalassemia in the Kradarn subdistrict Chachoengsao province, Thailand. J. Med. Assoc. Thai. 2011;95:S124–S132. [PubMed] [Google Scholar]
- 34.Pongjantharasatien K., Banyatsuppasin W., Pounsawat S., Jindadamrongwech S. Occurrence of the --SEA, --THAI, and --FIL α-thalassemia-1 carriers from a 7-year study at Ramathibodi hospital, Bangkok, Thailand. Hemoglobin. 2016;40:283–284. doi: 10.1080/03630269.2016.1189932. [DOI] [PubMed] [Google Scholar]
- 35.Pichanun D., Munkongdee T., Klamchuen S., Butthep P., Winichagoon P., Fucharoen S., Svasti S. Molecular screening of the Hbs constant spring (codon 142, TAA>CAA, α2) and paksé (codon 142, TAA>TAT, α2) mutations in Thailand. Hemoglobin. 2010;34:582–586. doi: 10.3109/03630269.2010.526914. [DOI] [PubMed] [Google Scholar]
- 36.Pharephan S., Sirivatanapa P., Makonkawkeyoon S., Tuntiwechapikul W., Makonkawkeyoon L. Prevalence of α-thalassemia genotypes in pregnant women in northern Thailand. Indian J. Med. Res. 2016;143:315–322. doi: 10.4103/0971-5916.182622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panyasai S., Fucharoen G., Fucharoen S. Hemoglobin variants in Northern Thailand: Prevalence, heterogeneity and molecular characteristics. Genet. Test Mol. Biomark. 2016;20:37–43. doi: 10.1089/gtmb.2015.0182. [DOI] [PubMed] [Google Scholar]
- 38.Panomai N., Sanchaisuriya K., Yamsri S., Sanchaisuriya P., Fucharoen S., Schelp F.P. Thalassemia and iron deficiency in a group of northeast Thai school children: Relationship to the occurrence of anaemia. Eur. J. Pediatr. 2010;169:1317–1322. doi: 10.1007/s00431-010-1218-3. [DOI] [PubMed] [Google Scholar]
- 39.Prayalaw P., Fuchafoen G., Fucharoen S. Routine screening for α-thalassemia using an immunochromatographic strip assay for haemoglobin Bart’s. J. Med. Screen. 2014;21:120–125. doi: 10.1177/0969141314538611. [DOI] [PubMed] [Google Scholar]
- 40.Seeratanachot T., Shimbhu D., Charoenkwan P., Sanguansermsri T. Detection of deletion α+-thalassemia mutation [-α (3.7), -α (4.2)] by quantitative PCR assay. Southeast Asian J. Trop. Med. Public Health. 2015;46:110–115. [PubMed] [Google Scholar]
- 41.Wisedpanichkij R., Jindadamrongwech S., Butthep P. Identification of Hb constant spring (HBA2: c.427T>C) by an automated high performance liquid chromatography method. Hemoglobin. 2015;39:190–195. doi: 10.3109/03630269.2015.1027828. [DOI] [PubMed] [Google Scholar]
- 42.Uaprasert N., Settapiboon R., Amomsiriwat S., Sarnthammakul P., Thanapat T., Rojnuckarin P., Sutcharitchan P. Diagnostic utility of isoelectric focusing and high performance liquid chromatography in neonatal cord blood screening for thalassemia and non-sickling hemoglobinopathies. Clin. Chim. Acta. 2013;427:23–26. doi: 10.1016/j.cca.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Srivorakun H., Fucharoen G., Changtrakul Y., Komwilaisak P., Fucharoen S. Thalassemia and hemoglobinopathies in South East Asia newborns: Diagnostic assessment using capillary electrophoresis system. Clin. Biochem. 2011;44:406–411. doi: 10.1016/j.clinbiochem.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Chaibunruang A., Prommetta S., Yamsri S., Fucharoen G., Sae-Ung N., Sanchaisuriya K., Fucharoen S. Molecular and hematological studies in a large cohort of α0-thalassemia in northeast Thailand: Data from a single referral centre. Blood Cells Mol. Dis. 2013;51:89–93. doi: 10.1016/j.bcmd.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Thanyaornwanya C., Singha K., Fucharoen G., Sanchaisuriya K., Thepphitak P., Wintachai P., Karnpean R., Fucharoen S. Molecular characteristics of α+-thalassemia (3.7 kb deletion) in Southeast Asia: Molecular subtypes, haplotypic heterogeneity, multiple founder effects and laboratory diagnosis. Clin. Biochem. 2019;71:31–37. doi: 10.1016/j.clinbiochem.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Mankhenthong K., Phusua A., Suantan S., Srisittipoj P., Charoenkwan P., Sanguansermsri T. Molecular characteristics of thalassemia and haemoglobin variants in prenatal diagnosis program in northern Thailand. Int. J. Hematol. 2019;110:474–481. doi: 10.1007/s12185-019-02694-y. [DOI] [PubMed] [Google Scholar]
- 47.Pata S., Laopajon W., Pongpaiboon M., Thongkum W., Polpong N., Munkongdee T., Paiboonsukwong K., Fucharoen S., Tayapiwatana C., Kasinrerk W. Impact of the detection of ζ-globin chains and haemoglobin Bart’s using immunochromatographic strip test for α0-thalassemia (--SEA) differential diagnosis. PLoS ONE. 2019;14:e0223996. doi: 10.1371/journal.pone.0223996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoa Nguyen V., Sanchaisuriya K., Wongprachum K., Nguyen M.D., Phan T.T., Vo V.T., Sanchaisuriya P., Fucharoen S., Schelp F.P. Hemoglobin constant spring is markedly high in women of an ethnic minority group in Vietnam: A community-based survey and hematologic features. Blood Cell Mol. Dis. 2014;52:161–165. doi: 10.1016/j.bcmd.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Modell B., Darlison M. Global epidemiology in haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadkarni A., Phanasgaonkar S., Colah R., Mohanty D., Ghosh K. Prevalence and Molecular Characterization of α-Thalassemia Syndromes among Indians. Genet. Test. 2008;12:177–180. doi: 10.1089/gte.2007.0080. [DOI] [PubMed] [Google Scholar]
- 51.Souza A.E.S., Cardoso G.L., Takanashi S.Y.L., Guerreiro J.F. α-Thalassemia (3.7 kb deletion) in a population from the Brazilian Amazon region: Santarém, Pará State. Genet. Mol. Res. 2009;8:477–481. doi: 10.4238/vol8-2gmr601. [DOI] [PubMed] [Google Scholar]
- 52.AL-Awamy B.H. Thalassemia syndromes in Saudi Arabia. Meta-analysis of local studies. Saudi Med. J. 2000;21:8–17. [PubMed] [Google Scholar]
- 53.Kee B.P., Lian L.H., Lee P.C., Lai T.X., Chua K.H. Genetic data for 15 STR loci in a Kadazan-Dusun population from East Malaysia. Genet. Mol. Res. 2011;10:739–743. doi: 10.4238/vol10-2gmr1064. [DOI] [PubMed] [Google Scholar]
- 54.Forget B.G., Bunn H.F. Classification of the disorders of haemoglobin. Cold Spring Harb. Perspect. Med. 2013;3:a011684. doi: 10.1101/cshperspect.a011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krause M.A., Diakite S.A.S., Lopera-Mesa T.M., Amaratunga C., Arie T., Traore K., Doumbia S., Konate D., Keefer J.R., Diakite M., et al. α-thalassemia impairs the cytoadherence of Plasmodium falciparum-infected erythrocytes. PLoS ONE. 2012:e37214. doi: 10.1371/journal.pone.0037214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gundula M.-O., Gros P. Erthrocyte variants and the nature of their malaria protective effect. Cell. Microbiol. 2005;7:753–763. doi: 10.1111/j.1462-5822.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization . World Malaria Report 2019. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.