Abstract
The RNA interference (RNAi) pathway possesses immense potential in silencing any gene in human cells. Small interfering RNA (siRNA) can efficiently trigger RNAi silencing of specific genes. FDA Approval of siRNA therapeutics in recent years garnered a new hope in siRNA therapeutics. However, their therapeutic use is limited by several challenges. siRNAs, being negatively charged, are membrane-impermeable and highly unstable in the systemic circulation. In this review, we have comprehensively discussed the extracellular barriers, including enzymatic degradation of siRNAs by serum endonucleases and RNAases, rapid renal clearance, membrane impermeability, and activation of the immune system. Besides, we have thoroughly described the intracellular barriers such as endosomal trap and off-target effects of siRNAs. Moreover, we have reported most of the strategies and techniques in overcoming these barriers, followed by critical comments in translating these molecules from bench to bedside.
Keywords: siRNA delivery systems, barriers to siRNA delivery, endosomal escape, reticuloendothelial system entrapment, renal clearance, off-target effects, immune system activation, chemical modifications, membrane impermeability
1. Introduction
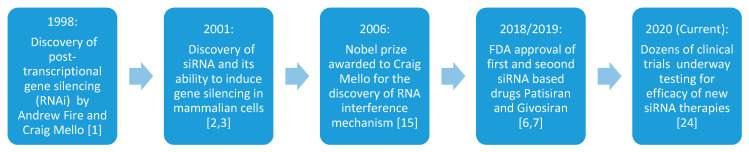
In 1998, Andrew Fire and Craig Mello published a seminal paper in which they discovered the phenomenon of post-transcriptional gene silencing (PTGS) and termed it as RNA interference (RNAi) [1]. Later, in 2001, two research groups reported that 21 to 22 nucleotide(nt) double-stranded RNAs, popularly known as small interfering RNAs (siRNAs), can induce gene silencing in mammalian cells without causing nonspecific interferon response [2,3]. siRNAs, composed of a duplex of 20 to 24 nucleotide base pairs, work by interacting with the RNAi pathway enzyme dicer for cleavage. The single-stranded antisense strand complementary to the target mRNA is handed to the RNA- induced silencing complex (RISC) loading complex (RLC), which destroys the target mRNA, hence silencing the target gene or, in other words, inhibit the formation of the target protein [4]. Section 2 further explains the mechanism of siRNA in detail. These siRNAs later kicked off a revolution in biology due to their potential to inhibit virtually all genes by a base sequence of target mRNA alone, which gives RNAi several advantages over small-molecule drugs as a therapeutic strategy [5]. Approval of the first siRNA-based drug patisiran (Onpattro™) by the U.S. Food and Drug Administration (FDA) in 2018 for polyneuropathy of hereditary transthyretin-mediated amyloidosis has fostered a new interest by the pharmaceutical as well as academic research groups in RNAi based drugs [6]. Recently in 2019, givosiran (Givlaari™) has been approved by the US FDA for acute hepatic porphyria [7], which has further reinforced the confidence in siRNA based products. A timeline of these events is shown below in Figure 1.
Figure 1.
Timeline highlighting the major historical events leading up to siRNA therapeutics.
Furthermore, dozens of siRNA-based products are already in different stages of clinical trials [8]. For instance, an investigational RNAi product targeting glycolate oxidase, termed lumasiran, is currently in phase 3 clinical trial for the treatment of primary hyperoxaluria type 1 (PH1), in which calcium oxalate crystals are formed in the kidney and urinary tract, leading to recurrent kidney stones and nephrocalcinosis [9]. Lumisiran has shown promising results with encouraging safety and tolerability profile with no serious adverse event during the study time [10].
Similarly, fitusiran, another RNAi therapy, targets antithrombin (AT) in the liver, silencing AT gene expression, and prevents AT synthesis. Fitusiran is undergoing phase III clinical trial as a prophylactic treatment of hemophilia [11,12]. Also, vutrisiran is undergoing a phase-III clinical trial for the therapy of transthyretin amyloidosis (ATTR) with cardiomyopathy [13]. See Section 4 for more details on current clinical trials on siRNA therapeutics. Although several studies have shown the significant potential of siRNA therapeutics in-vitro, yet systemic siRNA therapy faces several extracellular as well as intracellular barriers for translation of siRNA therapeutics from bench to bedside. Several reviews have been published to address this topic comprehensively, particularly on the applications of siRNA therapeutics [14,15,16]. However, there is an urgent need for a thorough investigation of barriers reported in the scientific literature. Also, there have been several studies that describe the methods and techniques in overcoming these barriers for the translation of siRNA therapeutics to the clinics. Briefly, the major extracellular barriers are enzymatic degradation of siRNAs by serum endonucleases and RNAases, rapid renal clearance of siRNA delivery system, impermeability of siRNA to the biological membranes, activation of the immune system, plasma protein sequestration, and capillary endothelium crossing. In contrast, the intracellular obstacles to siRNA action are the endosomal trap, arrival at the correct intracellular site of action (cytosol), and off-target effects. In this review, we attempted to describe comprehensively these barriers and the milestones achieved in addressing the obstacles followed by critical commentary on innovation and prospects.
2. Mechanism of Action of siRNA Induced RNA Interference (RNAi)
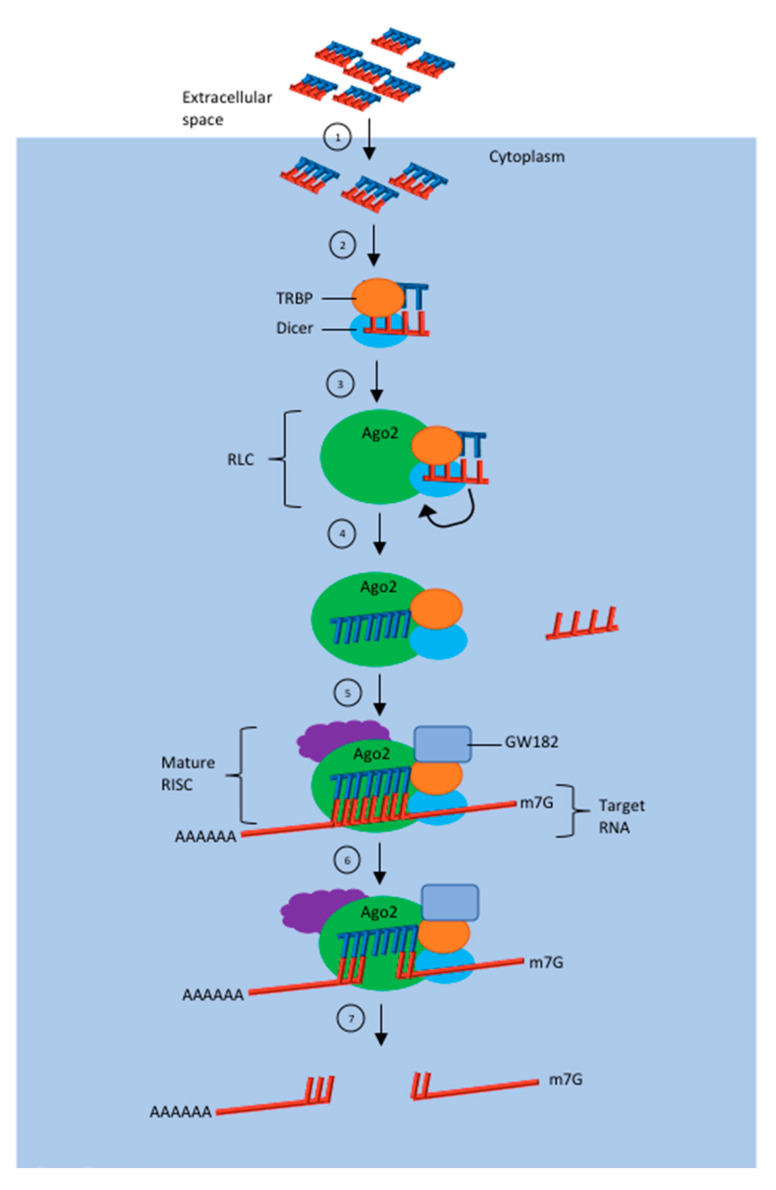
siRNA therapies take advantage of a naturally occurring phenomenon known as RNAi. The mechanism of action begins with the synthesis of a generally perfectly base-paired dsRNA ranging from 15 to 30 bp in length [15]. The length of dsRNA plays a crucial role as siRNA smaller than 15 bp does not incorporate itself with the RNAi machinery. Additionally, siRNA larger than 30 bp can lead to cytotoxicity and nonspecific interactions via the protein kinase R (PKR) pathway [17]. Following the successful entrance into the cytosol via endocytosis, synthetically produced siRNAs can interact directly with cytosolic RNAi enzymes known as Dicer and Tar RNA Binding Protein (TRBP) [18]. siRNA larger than 21 bp will interact with the Dicer enzyme that cleaves and hands it off to the RNA-induced silencing complex (RISC) loading complex (RLC) [4,19]. In comparison, siRNA shorter than 21 bp can bypass Dicer cleavage, and through interactions mediated by TAR RNA-binding protein, it can successfully enter RISC. Prior to mature RISC production, the complex must successfully select the guide strand between the antisense and sense strand. The antisense strand is the guide strand of choice, and through chemical modifications, biases towards its selection are possible. The siRNA guide strands, or antisense strands usually have exact 100% complementarity to a single target mRNA which allows for the potent and successful silencing of the gene. Following the successful loading of the guiding stand, TRBP and Dicer have the ability to dissociate from RISC. Ago2, which is an enzyme that joins and associates itself with the RLC, plays an important role for gene silencing as it has intrinsic silencer activity to efficiently cleave the mRNA targets [20]. Gene expression is regulated through the inhibition of mRNA translation in the form of inducing mRNA sequestration in cytoplasmic processing bodies (P bodies) and/or GW bodies, which ultimately promotes mRNA degradation and directing transcriptional gene silencing of the target gene [21,22,23]. Figure 2 illustrates the mechanism of siRNA action.
Figure 2.
General mechanism of action for siRNA induced gene silencing. (1) Synthetic siRNA successfully enters the cell via endocytosis. (2) siRNAs will then travel in the cytosol until it comes into contact with cytosolic RNAi enzymes–dicer and Tar RNA Binding Protein (TRBP) to form RISC Loading Complex (RLC). (3) Strand selection is done. (4) Production of mature RISC following successful guiding strand selection. (5) siRNA guiding strand with full complementarity to a single target mRNA induces potent and targeted gene silencing. (6) Mature RISC can regulate gene expression via inhibition of mRNA translation, inducing mRNA sequestration in cytoplasmic P bodies and or GW bodies to promote mRNA degradation. (7) Degraded mRNA is no longer useful and cannot go through translation to produce protein.
3. Advantages of siRNA over Other RNAi Therapeutics
There are several types of RNAi therapeutics: micro RNA (miRNA), short hairpin (shRNA), and siRNA. miRNA is a single-stranded RNA that has the stem-loop structure and binds imperfectly to mRNA. Due to this characteristic, miRNA can degrade many sets of similar mRNAs, causing toxicity [24]. shRNA has a tight hairpin structure, making it more complicated than other RNAi therapeutics. It requires the promoter to be expressed and located in the nucleus to act appropriately [25]. Lastly, siRNA is the short stretch double-stranded RNA that can degrade the complementary mRNA. siRNA has high transfection efficacy and fewer obstacles, making the best fit for RNAi therapeutics [26]. Table 1 illustrates the different types of RNAi therapeutics.
Table 1.
Types of RNAi therapeutics.
| Type of RNAi | Silent Feature of RNAi Therapeutics | References |
|---|---|---|
| miRNA | Stem-loop structure Binds imperfectly to mRNA and degrades many sets of similar target mRNA |
[24] |
| shRNA | RNA with a tight hairpin turn Requires promoter to be expressed and must be located in the nucleus to act |
[25] |
| siRNA | Short stretch dsRNA capable of degrading complementary mRNA Higher transfection efficiency and fewer obstacles. |
[26] |
4. siRNA-Based Therapeutics in Clinical Trials
Many siRNA-based therapeutics are making their way onto the clinical trial stage for a multitude of diseases. The following clinical trials are specific to therapeutics to treat cancer. Although many of these therapeutics did not make their way to Phase II trials, some have been more recently modified, making its way back to the clinical stage. An investigational siRNA therapeutic is CALAA-01 from Calando Pharmaceuticals [18]. This therapeutic is a four-component polymer-based nanoparticle siRNA delivery system around 75 nm in diameter [27,28]. The complex consists of a linear, cationic cyclodextrin-based polymer known as adamantane polyethylene glycol or PEG, which is incorporated to provide steric stabilization, along with a human transferrin protein-targeting agent and a siRNA that targets the reduction in the M2 subunit of ribonucleotide reductase (RRM2) expression [18]. Existing data reveals that RRM2 plays a large role during nucleic acid metabolism and is upregulated in many tumor types [18,27,28,29]. It is proposed that the suppression of RRM2 will lead to cell cycle arrest and cell death [30].
Another therapeutic investigation was from Alnylam Pharmaceuticals named ALN-VSP. This siRNA-based therapeutic is a nearly neutral nanoparticle formulation about 80-100 nm in diameter, with two different chemically modified siRNAs targeting different proteins, in a 1:1 molar ratio [31,32]. The siRNAs individually target vascular endothelial growth factor A (VEGFA) and kinesin spindle protein (KSP). Both KSP and VEGFA are highly expressed in a variety of tumor types [31], where VEGF is proposed to modulate tumor angiogenesis or the growth of blood vessels to supply nutrients to tumors, and KSP is essential for mitotic spindle formation in proliferative cells [31]. Targeting of VEGF is proposed to decrease blood supply to the tumor, and KSP is targeted to stop mitotic spindle formation [31].
Atu027, developed by Silence Therapeutics, is a cationic lipoplex-based siRNA delivery system made up of a blunt-ended, chemically modified 23-mer RNA oligonucleotide along with three cationic lipids. The siRNA is created to target protein kinase N3 (PKN3). It is proposed that PKN3 suppression stabilizes vessel integrity and attenuate inflammatory responses in the vasculature of tumors and secondary organ sites via modulation of actin and adherin junction dynamics [18,33,34]. Furthermore, this will ultimately lead to inhibition of mobilization and engraftment of metastatic tumor cells due to the reduction of both tumor and host vascular permeability [18].
Other therapeutics termed TKM-PLK1 from Tekmira carried a siRNA targeting polo-like kinase 1 (PLK1). It is a lipid nanoparticle about 80 nm in diameter targeting PLK1. It is thought to be a key protein during multiple steps in cell division, DNA stability, and DNA repair. It has also been found to be upregulated in many human tumors. It is proposed that the silencing of PKL1 may induce apoptosis and tumor cell death [35].
Another reported therapeutic is PN2258 from ProNAi Therapeutics, which is a liposomal formulation around 130 nm in diameter. It comprises a 24-base chemically unmodified DNA oligonucleotide and four lipid molecules, making the therapeutic unique as it does not contain siRNA [36]. Since it does not contain siRNA, the RNAi mechanism at the RISC complex is not induced. It was included with the other clinical trials because it is systematically administered and is a nucleic acid formulation for anticancer treatment. The nucleic acid portion, also known as PNT100, follows a mechanism where it binds to the 5′-untranscribed regulatory regions of the BCL2 protein gene and blocks transcription via DNA interference rather than RNA interference.
5. Barriers to siRNA Delivery
As described earlier, small double-stranded RNA (ds RNA) molecules can efficiently trigger the post-transcriptional gene silencing; still, their delivery has been the major challenge to applying siRNA-based therapeutics in humans [37,38]. Barriers depend on the target organ and the desired route of administration of siRNA. The affluence of siRNA delivery depends on the accessibility of target tissue or organ inside the body. Broadly two administration routes, localized administration, and systemic administration, are used. Localized siRNA delivery has fewer barriers than systemic delivery. In localized delivery of siRNA, therapy is applied directly to the target organ or tissues, offers high bioavailability at the target site, and avoids the barriers that are shown by the systemic administration [39]. Several organs are compliant to local or topical siRNA delivery, including eyes, mucous membranes, localized tumors, and skin [40,41]. Lungs infections can be treated with local siRNA delivery; direct installation of siRNA through intranasal or intra-tracheal routes permits direct contact with epithelial cells of the lungs [42]. Readers are encouraged to read excellent reviews about physical and immunologic barriers of local delivery of siRNA [43,44].
In contrast, the siRNA systemic delivery has various challenges that limit the siRNA bioavailability at the target site [45]. Upon intravenous injection, unmodified naked siRNA is degraded by endogenous enzymes. Also, due to their small size (MW 13 kDa), siRNA is eliminated through kidneys. The siRNA is a negatively charged molecule, and it is almost impossible for siRNA to cross the biological membrane. The half-life of most naked or unmodified siRNA is between 5–10 min [46]. Table 2 illustrates the barriers faced by siRNA to reach its site of action. These barriers limit the application of siRNA-based therapeutics in humans and warrant specific chemical modifications of either siRNA or the delivery system for their widespread use in clinical conditions.
Table 2.
Barriers faced by siRNA to reaching site of Action.
| Extracellular Barriers | Intracellular Barriers |
|---|---|
| Endogenous Nucleases | Endosomal trap |
| Elimination through kidneys due to small size | Arrival at the correct intracellular site of action (cytosol), |
| Impermeability to biological membranes | Off-target effects. |
| Entrapment by Reticuloendothelial System | |
| Plasma protein sequestration | |
| Capillary Endothelium Crossing |
6. Intravascular Degradation and Renal Clearance
The first biological barrier after injection is intravascular degradation of siRNA by nucleases enzyme in plasma. The naked or unmodified siRNA is unstable in the systemic circulation and more susceptible to A-type nucleases, which are ubiquitous in intracellular and extracellular space [47]. Besides, fast renal clearance results in the very short half-life of siRNA, ranging from 5–10 min [48,49,50]. Nucleases in plasma or tissues degrade the unmodified siRNA in few minutes to 1 h, potentially limiting the use of siRNA-based therapeutics [51,52]. The reason for these challenges is attributed to their small size, approximately 7 nm in length and smaller molecular weight, i.e., approximately 13 kDa [53]. To this end, siRNA modification is necessary to resist nuclease mediated degradation, reducing siRNA susceptibility towards nucleases and improving their in-vivo properties. However, siRNA modification alone may not be enough to achieve the therapeutic activity. In this way, physical encapsulation of siRNA and chemical modification promotes proper therapeutic activity [54,55].
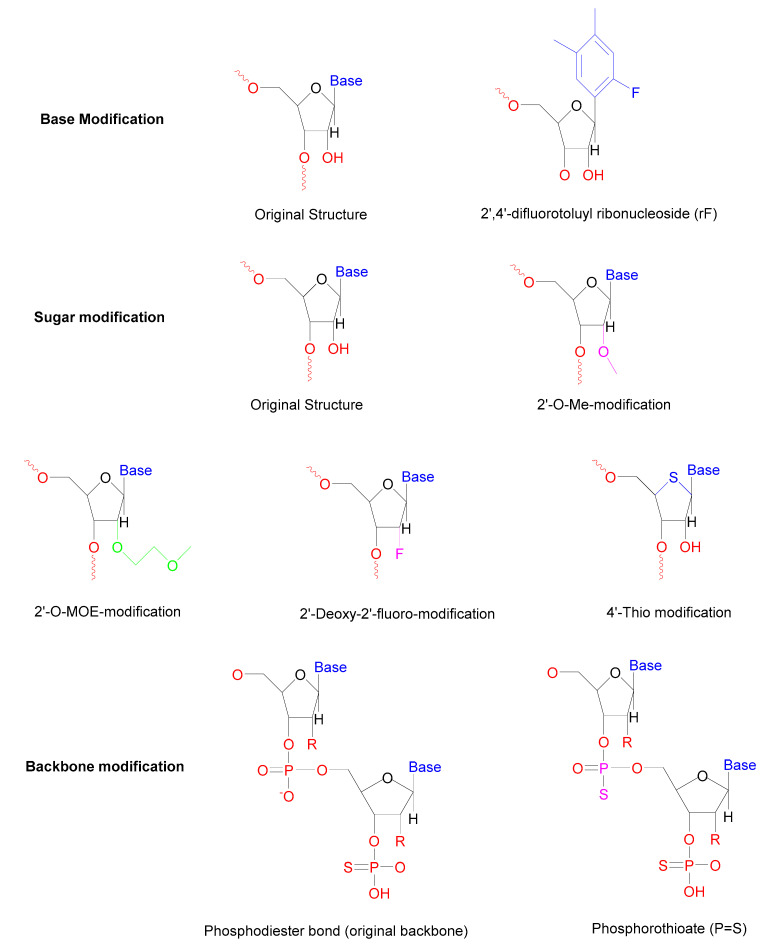
Researchers have turned on modifying the sugars, backbone, or bases of oligoribonucleotides for stabilization to overcome intravascular degradation [56]. The modification of base with internal uridine to rF (2,4-difluorotoluyl ribonucleoside) substitutions shows better resistance towards intravascular degradation by nucleases in the serum [57]. Modification of both the sense and antisense strand enhances the nuclease resistance in the serum [58]. In the case of sugar modifications, 4′-thioribonucleosides modification shows higher resistance towards serum nucleases [59]. If ribose 2′-OH group modified with a CH3 group or fluoride atom within both the sense as well as antisense strand, enhanced their resistance towards endonucleases and also improved therapeutic potency of siRNA [60]. A study demonstrated that the siRNA modified with the 2′fluoro group significantly enhanced resistance towards nucleases in plasma but unable to generate prolonged silencing in mice and cell cultures [61]. Figure 3 illustrates these modifications. It has been suggested that modification of siRNA with small molecules such as 2,4-dinitrophenol (DNP) not only increases their ability to resist nucleases, but it also enhances their membrane impermeability [62]. Some reputed modifications of siRNA are presented in Table 3.
Figure 3.
Chemical modifications of siRNA.
Table 3.
Characteristics of siRNA modifications.
| Modification | Type of Modification | Outcome | Reference |
|---|---|---|---|
| Base | Internal uridine to rF (2,4-difluorotoluyl ribonucleoside) substitutions | Enhanced resistance towards serum nucleases | [57] |
| Sugar | 2′-Deoxy-2′-fluoro-β-D-arabino, 2′-O-MOE modification, 2′-O-Me modification | Increase the half-life in the serum | [63] |
| Locked nucleic acids | Longer half-life reduces immune activation | [64] | |
| 4′-Thio modified the ring oxygen | Enhanced resistance towards nucleases | [65] | |
| Backbone | Phosphorothioate (PS) modifications Morpholino oligomers |
The longer half-life of duplex More potent |
[66] |
Extensive chemical modification of siRNA showed high in-vivo efficacy. For instance, siRNA modification of the sense strand comprised of 2’F-pyrimidines, 2’-deoxypurines with 5’- and 3’-terminal inverted a basic end caps. While, the antisense strand comprised of 2’-fluoro-substituted pyrimidines, 2’-methoxy purines with a single phosphorothioate (PS) linkage at the 3’-terminus, gives prolonged half-life in the serum, reaching 2 to 3 days as compared to naked unmodified siRNA with half-life minutes to less than 1 h [67]. However, the extended chemical modification screening process elaborated that improved serum stability can be achieved by thermodynamic stabilization selectively of some positions within the duplex compared to the thorough substitutions of siRNA with several analogs [68,69].
One of the main mechanisms of removing the siRNA therapeutics from the bloodstream is through urine by glomerular filtration in the kidneys. The pore size of the glomerular filtration barrier is about 8 nm [70], so if the nanoparticles are designed to have a particle size of about 20 nm, this barrier challenge can be addressed efficiently [70]. There are variable reports about the half-life of unmodified siRNA in serum, several studies suggest that chemically modified siRNA lasts longer in nuclease rich environment than the unmodified siRNA [71]. Furthermore, increasing its molecular weight by attachment of ligands, incorporating larger particles, or binding to plasma proteins efficiently saves siRNA from elimination [72].
In this context, researchers at Alnylam Pharmaceutical Inc. conjugated the cholesterol with the 3’ end of the siRNA sense strand by a short trans-4-hydroxylprolinol linker. This cholesterol-siRNA conjugation caused the avid binding of modified siRNA with plasma proteins and increased the plasma retention time with the half-life of 90 min [73]. Cholesterol-siRNA conjugation also provided higher resistance to nucleases with greater serum stability and prolonged circulation time [72]. In another study, it observed that cholesterol-siRNA conjugate resulted in greater stability and silenced the endogenous gene expression of apo B protein, which regulates cholesterol metabolism [74]. On the other hand, naked siRNA without cholesterol conjugation did not show any silencing activity inside the cell [74].
Conjugation with polymer, especially PEG, is a common concept to increase the half-life of siRNA in blood and enhance the pharmacokinetic profiles [46,75]. A study demonstrated that conjugating siRNA with 20k PEG resulted in a slower rate of renal clearance (50% remained 1 h after injection) as compared to unmodified siRNA (<10% remained 15 min after injection) [76]. Similarly, the conjugation of PEG at 2-nucleotide 3’-overhang significantly increased the downregulation of PSMA expressing tumors [77].
Besides, siRNA-cationic polymer showed 100-fold more retention time in blood than naked siRNA [78]. The cationic RNAi nanoparticles (NPs) were also shown to be excreted through kidneys. The primary reason may be their interaction with anionic proteins on the capillary wall of the glomerulus; the resulting neutralization may have resulted in the ready release of siRNA on the capillary bed of glomerulus with subsequent excretion. To overcome this barrier, cationic RNAi NPs may also be PEGylated to reduce renal clearance and increase the circulation time of siRNA [55].
Another study showed that cationic comb-type copolymers (CCC), having a considerably higher density of hydrophilic graft chains, increased the stability of siRNA against nuclease and plasma components. The siRNA complexed with CCC acquired considerably longer blood circulation than naked siRNA or complexed with PEI (jetPEI™) [78].
7. Activation of the Innate Immune System
The function of innate immunity is to identify the pathogens, eradicate them, and contribute to adaptive immunity. Host cells depend on the pattern recognition receptors that detect infectious and non-self-agents, including lipopolysaccharide, viral RNAs, bacterial DNA, viral glycoproteins, and peptidoglycan [79].
Previously, it was thought that siRNA shorter than 30 nucleotides were small enough to evade the immune system and avoid nonspecific stimulation of interferon response. However, subsequent experiments with short synthetic siRNAs showed that siRNA could activate the immune response and trigger the production of cytokines in-vivo and in-vitro [80]. This innate immune response can be triggered by the siRNA or by the vehicles, including cationic lipids used to deliver siRNA in-vivo [81].
Mammalian cells possess immune cells that express pattern recognition receptors named as Toll-like receptors (TLR). These receptors recognize foreign pathogen-associated structural patterns. This immune response can be activated either by TLR dependent or TLR independent pathways [79].
7.1. TLR Dependent Pathway
In TLR dependent pathway, thirteen TLR receptors have been recognized in humans as well as the mouse. Among these receptors, three TLR receptors, including TLR1, TLR2, and TLR3, have been identified to be triggered in response to siRNA [82]. TLR3 recognizes the duplex siRNA in a sequence-independent manner. Meanwhile, TLR7/8 recognizes the siRNA in a sequence-dependent manner. The example of sequence-dependent immune response by TLR7/8, the uridine, and guanosine-rich sequences with either UG dinucleotide or 5′-UGU-3′ potently trigger the innate immune response [82]. Avoidance of such sequences decreases the immune response [80]. Also, AU rich patterns have shown greater influence on TLR8 response [83]. Signaling by these intracellular TLR receptors requires acidification of endosomes along with maturation. Therefore, this endosomal acidification can be inhibited by the use of chloroquine or by buffering agents [84].
TLR3 possesses a specific gene structure among the TLR receptors and linked to its subfamily dissimilar from TLR7/8. At the cellular level, TLR3 expression in human leukocytes is vastly restricted to mature dendritic cells (mDCs). While in mice, TLR3 is found in a vast variety of immune cells. Recently, TLR3 expression on the cell surface has been noted in primary human endothelial cells such as lung, aorta, dermis, choroidal, and umbilical vein. TLR3 can also be present in several other cells, including epithelial, endothelial, and fibroblast lines [85]. Alexopoulou et al. demonstrated that dsRNA act as a TLR3 agonist by examining the response of splenocytes, macrophages, or bone marrow-derived DCs in TLR3 knockout mice to polyinosinic–polycytidylic acid (polyIC), a long synthetic dsRNA homolog [86]. TLR3 receptors on the surface of endothelial cells can also be activated by exogenous siRNA of 21 nucleotide base pairs in-vivo, resulting in the release of cytokines such as IL-12 and IFNγ. Unlike the various nucleic acid-sensing TLR receptors, that signal through the MyD88, TLR3 receptor signals through the TRIF adaptor protein [87]. TRIF signal is generated for the production of IFNβ along with IFNα4 by TANK-binding kinase-1 (TBK1), leading to the activation of transcription factor IRF3, which results in a positive feedback phenomenon to cause TLR3 signaling. This mechanism leads to the production of IFNβ and IFNα4 signals back to the cell, causing the up-regulation of IRF7 and other IFN-inducible genes. TLR3 activation via TRIF also activates RIP1 and TRAF6, leading to the activation of ATF, NF-κB, and c-Jun transcription factors along with the induction of inflammatory cytokines such as IL-6 and TNFα [88].
As discussed earlier, TLR7 and TLR8 recognize the siRNA in a sequence-dependent manner. It has been observed that plasmacytoid dendritic cells (pDCs), B cells, and monocytes express TLR7. At the same time, TLR8 is expressed by myeloid DC (mDC) and macrophages in humans [89]. TLR7 has been shown to detect single-stranded RNA (ssRNA), polyuridine, as well as synthetic ssRNA rich in uridine along with guanosine [90]. Similarly, TLR8 recognizes AU-rich and GU-rich ssRNA, and RNA viruses also trigger it. siRNA duplexes with GU-rich sequences or identified 5′-UGU-3′ motifs within particular siRNAs confer this highly immunostimulatory activity [81]. According to studies, it has been stated that TLR7/8 recognizes the siRNA in a sequence-specific manner [91]. Several immune-stimulatory signaling mechanisms are stated in Table 4.
Table 4.
TLR-dependent signaling in response to siRNA sequences.
Avoiding uridine and guanosine motifs may reduce a sequence-specific immune response. The replacement of siRNA motifs with adenosines may also abrogate the immune activation [97]. Interestingly, replacement of 2′-hydroxyl uridines with 2′-fluoro, 2′-O-methyl, or 2′-deoxy uridines blocks the immune activation. Thus, RNA immune recognition by TLRs can be prevented using 2′-ribose modifications of uridines [82]. The addition of 2′-O-Me uridine and guanosine into the sense strands of siRNA forms non-inflammatory siRNA without reducing their activity, and also protect the 5’ end of the guide strand that is vulnerable towards exonucleases [98]. As discussed earlier, chloroquine and bafilomycin A1 inhibit endosomal acidification and block the TLR3, TLR7, and TLR8 of siRNA without interfering with their RNAi activity [99].
7.2. TLR Independent Pathway
In TLR independent pathway, cytoplasmic RNA sensors such as retinoic acid-inducible gene 1 (RIG-1, also known as DDX58), melanoma differentiation-associated gene 5 (MDA-5), and dsRNA-binding protein kinase (PKR) are included [88]. It has been reported that RIG-1 binds to either ssRNA or dsRNA with uncapped 5’triphosphates, leading to the production of IFN. Therefore RIG-1 expression is readily upregulated in the para or autocrine manner, following the interferon response [100]. RIG-I and MDA5 activate the common downstream adaptor molecule MAVS. Activated MAVS then recruits multiple signaling molecules, including TRAFs, TBK1, and IRF3/7, leading to the transcriptional upregulation of type I interferons and other proinflammatory cytokines [101]. Finally, dsRNA-dependent PKR phosphorylates the serine along with threonine residues of the target protein. In humans, most of the cells express a low level of PKR and thus remain inactive. However, PKR binding to dsRNA leads to the formation of the dimer that ultimately undergoes autophosphorylation and subsequent activation of the pathway [102]. It has been suggested that the detection of dsRNA by PKR is a sequence-independent manner and interferon presence upregulates its expression [103].
8. Protein Binding
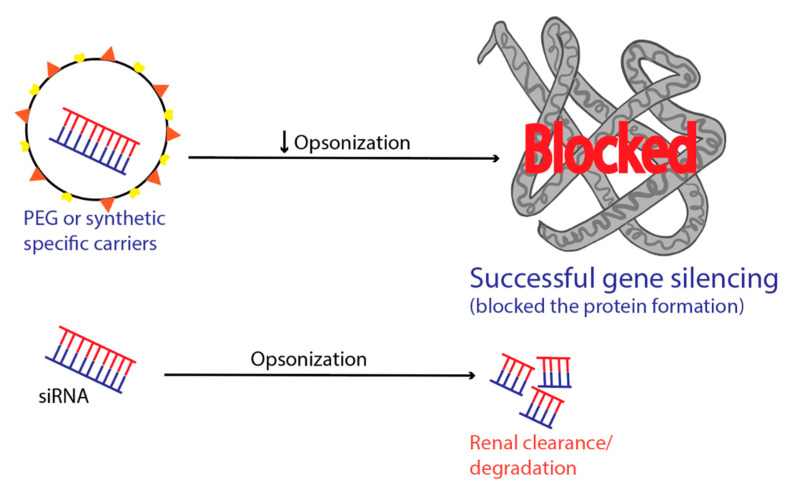
To achieve cell permeation of siRNA for effective concentration within the cell, a positively charged carrier is an essential requirement. Blood complement proteins and cell membrane proteins are usually negatively charged in the systemic circulation. The surface of the siRNA loaded carrier is positively charged, and it can be readily opsonized by opsonin proteins or may cause un-specific interaction with the cell membrane proteins [104]. Opsonins are essentially blood complement proteins or immunoglobulins. These blood complement proteins bind with the positively charged siRNA carriers through electrostatic interaction. The opsonization mechanism can occur anywhere in the blood circulation, completing from seconds to days. The opsonized carriers then undergo reticuloendothelial system (RES) filtration, and their cell surface binding leads to inflammation. The opsonization process makes siRNA carriers more susceptible to RES filtration and results in fast renal clearance (Figure 4) [55]. Opsonized carriers loaded RNA drugs accumulate in the liver and spleen and may cause toxic effects [105]. Thus, ultimately opsonization process reduces the therapeutic concentration needed for their efficient RNAi activity [55].
Figure 4.
Surface modification of siRNA carriers to decrease opsonization, renal clearance, and degradation.
Surface modifications are the primary strategies to reduce the interaction between opsonin proteins adsorption and RNAi carriers. Coating of positively charge carriers with hydrophilic polymers such as PEG or specific synthetic polymers significantly decreases the opsonization along with unspecific binding [105,106]. For example, palmitate-avidin containing PLGA carrier adsorbed PEG-biotin on their surface, and the 10 kDa PEG-coated particle reduced protein adsorption by 75% [107].
9. RES Entrapment
As listed earlier, a major problem is the uptake of nucleic acid drugs by the RES. RES/mononuclear phagocytic system containing organs, including the liver, spleen, lung, and bone marrow, are saturated with the fenestrated capillaries along with phagocytic cells, macrophages, and monocytes [108]. It is thought that nanoparticles greater than 100 nm are trapped by RES in the liver, bone marrow, spleen, and lung, leading to degradation by activated monocytes and macrophages [108]. siRNA loaded nanoparticles undergoing the opsonization process are readily removed by the macrophages in RES [55]. Once the siRNA therapeutics reach the bloodstream, they have to be protected from the phagocytic cells of the mononuclear phagocyte system (MPS) [70]. The uptake of siRNA-nanoparticles from blood circulation to RES organs is quite a fast process. Still, these carriers are processed and eliminated slowly, resulting in prolonged retention of these particles in the filtering organs [55]. Somehow, the RES filtration process of specially siRNA-based therapeutics becomes helpful sometimes when the desired organ is a RES-rich organ [39].
Numerous factors, such as surface features, charge, and size of the carrier, may interfere with RES uptake and bio-distribution. It is thought that a surface with a negative charge is more susceptible to clearance from blood as compared to positive or neutral charged carriers. Surface modification is the primary strategy to bypass this barrier [39]. For example, siRNA carriers can be grafted with hydrophilic polymers such as PEG and other surfactant copolymers such as polyethylene oxide and poloxamers that give them stealth properties and cause them to remain in the blood for a prolonged time [109,110]. These modifications resist the protein adsorption, thereby protect the siRNA against opsonization and ensure cargo stability. These stealth properties are efficient for particles ranging from 70–200 nm. It is interesting to note that more pegylation may also neutralize the positive charge of the surface needed for the siRNA uptake into the cells [39]. Hence customized pegylation is required that causes the carrier to evade RES, but meantime does not compromise the cellular uptake of siRNA. For instance, if pegylation of siRNA-lipoplexes is increased from 1–2 to 5 mol% of PEG2000, it completely eradicates the siRNA-mediated gene silencing against PTEN protein in-vitro [111]. It is noteworthy that the pegylation strategy needs further exploration to identify the size and molecular weight of the PEG for designing an optimum siRNA delivery system.
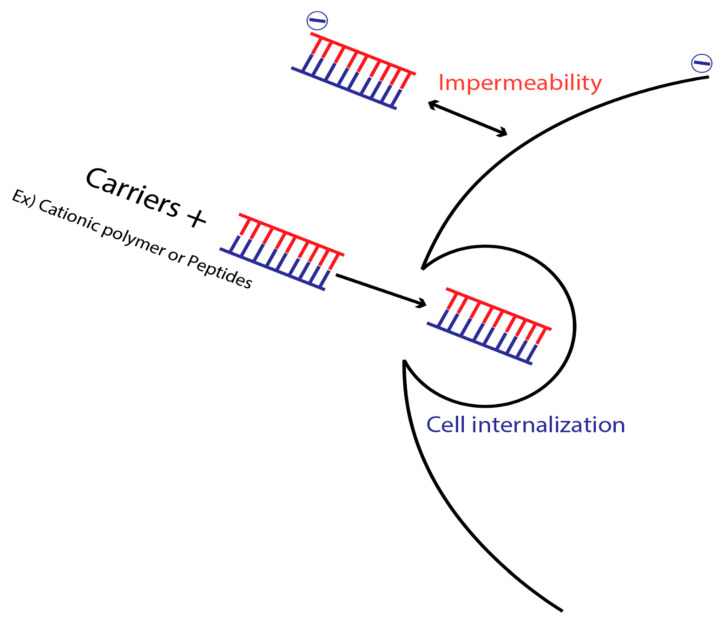
10. Membrane Impermeability
As discussed earlier, naked siRNA cannot cross the plasma membrane because of its negative charge. Despite its small size, the negative charge and high hydrophilicity prevent siRNA from passing through the biological membrane. Hence, efficient delivery of siRNA needs modification to overcome this barrier [112]. In this context, carriers that enable efficient siRNA delivery are required. Complexation of siRNA with cationic polymers or lipids can mask the net negative charge of siRNA. Furthermore, these nanoparticles with positive charge interact with the negatively charged biological membrane, thus causing internalization [113]. Alternatively, efficient delivery of siRNA can be achieved by conjugating the siRNA with ligands, immunoglobulins, or aptamers that recognize specific antigens on target cells. When these conjugates bind to the cell, the siRNA uptake is done by receptor-mediated endocytosis resulting in endosomes formation with subsequent siRNA delivery into the cytoplasm [112]. Figure 5 illustrates this concept.
Figure 5.
Carriers to overcome the impermeability of the siRNA.
Due to the hydrophilicity and negative charge of the siRNA, various carriers are used, including cationic polymers and peptides. Peptides, in particular, have received specific attention because they show great promise as siRNA carriers, based on the diversity of their physiochemical properties and functions. Based on the function, several classes of peptides, including cell-penetrating peptides (CPPs), endosome-disrupting peptides, and non-covalent multifunctional peptide complexes, can be used [114]. CPPs, also referred to as protein transduction domains (PTD), can penetrate the plasma membrane of a cell and facilitate the delivery of various cargoes. Moreover, the cytotoxicity of CPPs is relatively low [115]. For instance, Tanaka et al. reported stearic acid conjugated a CPP demonstrated that injecting STR-CH2R4H2C peptide with siVEGF suppressed the tumor growth efficiently compared with naked siRNA or controlled group [116].
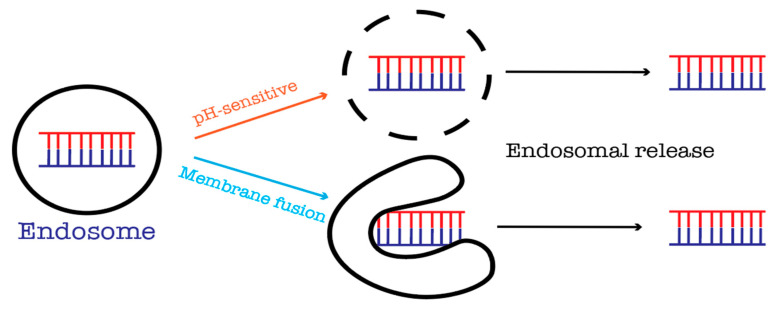
11. Endosomal Escape
Following the internalization of the siRNA, a major barrier remains to be its inability to escape endosomes. Thus, a carrier or modification that allows for the disruption of the endosomal membrane is essential for efficient endosomal escape and gene silencing by siRNA. Many recent delivery vehicles have taken advantage of the change from extracellular to the endosomal environment. Among the many differences, the change in pH is often utilized for efficient endosomal disruption and further release of siRNA once inside the target cell [117]. Protonable cationic polymers have been shown to increase delivery efficiency due to their high buffering capacity [45].
Several endosomolytic agents such as polymers, proteins, peptides, and small molecules like chloroquine can also be employed in siRNA carrier formulations to avoid the endosomal trap [118]. These carrier increases the counter-ion concentrations, leading to osmotic swelling, endosomal membrane rupture, and the subsequent escape into the cytosol [119]. A prime example of a positively charged polymer is polyethyleneimine (PEI), which can act as a “proton sponge” and facilitate the endosomal release of siRNA. The proton sponge effect facilitates the chloride ion influx and destroys the endosomes with subsequent release of contents into the cytoplasm [119]. The “proton sponge” effect or proton absorbance by buffering polymers such as PEI is known to increase the ATPase-mediated influx of protons and counter ions credited to their ability to prevent acidification [120]. As a result, the increased counter ion concentration inside the endosome leads to osmotic swelling, and ultimately endosomal membrane rupture, releasing the carrier and siRNA into the cytosol [121]. Although the PEI has this capability, one of the major drawbacks is its cytotoxicity, known to increase along with its physical size [121]. Another reported agent for causing effective endosomal escape is poly(lactic-co-glycolic acid) (PLGA) [122]. PLGA is a copolymer, biodegradable, biocompatible, and helps in RNAi activity of the siRNA. PLGA provides a sustained release to the siRNA with increased cellular uptake [122]. Especially, the layer-by-layer complex (nanoparticles) with PEI and PLGA increases the endosomal escape efficiency [122]. Another strategy for endosomal escape is protonation; two amino acids arginine and lysine get protonated in low pH that lead to destabilizing of cellular membranes. Lysine- and arginine-based polymers covalently linked to siRNA formulation have been used extensively to induce the release of siRNA from endosomes into the cytoplasm [123].
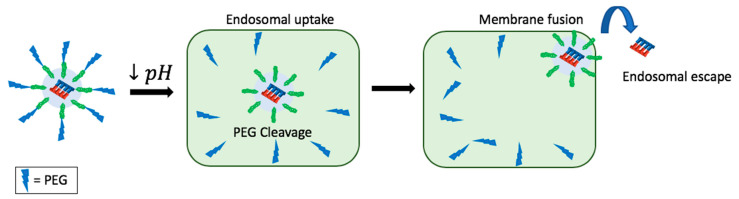
Additionally, recent reports have cited the development of a PEG-based cleavable polymeric system, composed of nonlamellar highly fusogenic lipid nanoparticles that are further stabilized by surface-exposed PEG-conjugated vinyl ether lipids [124]. Its mechanism of action involves the hydrolysis of the vinyl ether bond at low pH inside of the endosomal environment, which results in the destabilization of the liposome and removal of PEG (Figure 6) [45]. This destabilization allows for the fusion between the liposome and endosomal membrane, ultimately releasing the siRNA in the cytosol efficiently, escaping the endosome [124].
Figure 6.
Mechanism of action of PEG-based cleavable polymeric system that responds to decreased pH in the endosomal environment.
Another strategy for promoting endosomal escape is the incorporation of fusogenic molecules that allows the carriers to fuse with the biological membrane following the release of endosomal contents into the cytoplasm [125]. For instance, a fusogenic lipid, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), has been extensively used in lipid-based siRNA carriers, that have a greater impact on endosomal escape. At low pH, DOPE undergoes a phase transition from the lamellar phase to the inverted hexagonal phase, leading to the destabilization of the endosomal membrane [126]. DOPE-PEG combination can also be used in the endosomal escape strategy [127].
Besides, fusogenic protein-based carriers have also been employed for rendering endosomal escape such as HA2 peptide, and a pH-sensitive fusogenic GALA peptide that changes their conformations in response to acidification of the endosome and triggers the endosomal release (Figure 7) [45].
Figure 7.
Ways to overcome endosomal entrapment.
12. Off-Target Effects
In terms of siRNA formulation to accumulate in tumors, specifically, it is only around 20 to 40% higher than that of normal tissue [128]. The enhanced permeation and retention (EPR) effect, which stems from leaky blood vessels in tumors, allows for the preferential uptake of the formulation in tumors compared to normal tissue but is not significant enough and may ultimately cause more harm than good in the long run [128]. Thus, to overcome these off-target effects, the use of surface-ligand modifications for siRNA formulations have recently been utilized for more targeted delivery of cargo. Many tumors and other diseases overproduce cell-surface receptors that can be preferentially targeted through the modification of a carrier with surface ligand modifications. To overcome the off-target effects, the receptor-targeted-delivery, conjugation with antibody, and chemical modification have been used.
First, receptor-targeted-delivery targets the specific receptors that are expressed in the specific type of tissue. This approach can directly deliver to the specific tissue and overcome the off-target effects. For example, researchers conjugated GalNAc ligand onto the siRNA to treat liver disease [129]. The drug based on GalNAc conjugation, givosiran, was approved by the FDA, as mentioned earlier in the introduction. GalNAc is one of the examples where modification was done at 3’ terminus of the sense strand. GalNAc conjugation bind to a receptor called ASGPR, also known as the asialogycoprotein receptor, which is responsible for uptake and clearance of circulation glycoprotein through a process of receptor binding to terminal galactose moieties followed by endocytosis [129]. This conjugate increased about 5-fold RNAi activity in vivo. During the experiment of targeting TTR mRNA, also known as transthyretin mRNA, the significant amounts of TTR mRNA were reduced in the liver cells, hepatocytes [130]. Besides, the PS (phosphorothioate) linkage modification of GalNAc conjugate improved the protection of 5’-exonuclease and required the lower dose of siRNA. Further research showed that not only triantennary but also the monovalent GalNAc conjugation improved the binding affinity and gene silencing efficiency [130]. The chemical modification may also prevent off-target effects [131]. Table 5 illustrates these chemical modifications. Particularly incorporating 2’-O-methyl modification of position 2 into one strand of the siRNA reduced the off-target effects and off-target mRNA [131]. As discussed earlier in Section 4, the use of transferrin protein to target the transferrin receptor, which is upregulated in various human tumor cells, is common [132]. Specifically, CALAA-01 made it onto the clinical stage and was tested in a cancer clinical trial [18]. Although the trials were discontinued after Stage I, fluorescence-based microscopy utilizing a CALAA-01 specific stain revealed the accumulation of the therapeutic within tumors but not in adjacent healthy tissue [18].
Table 5.
GalNAc conjugation to overcome off-target effects.
| GalNAc Conjugation | Modifications | Results | References |
|---|---|---|---|
| Triantennary | -Added PS linkages for the protection against the 5’-exonuclease. | -Reduced the off-target effects. -silenced mTTR in the liver cell (hepatocytes) |
[131] |
| Monovalent | -2’ and 4’of the RNA’s ribose sugar backbone for GalNAc linker conjugation. | -Similar or better binding affinity and gene silencing efficiency compared to Triantennary GalNAc. | [130] |
Additionally, in various tumors, it is possible to target the blood vessels supplying nutrients to the tumor through surface modification with peptide ligands. Among the many peptides, cyclic RGD (cRGD) is among the most commonly used modifications. cRGD can be isolated from in vivo tumor phage selection display and preferentially binds to avβ3 and avβ5 integrins. It is overexpressed on angiogenic endothelial cells of various tumor types and even on the tumors themselves [128]. cRGD is known to bind to integrin receptors 100-fold more tightly than linear RGD, making it the peptide of choice for surface modification [133]. A functionalized chitosan nanoparticle targeting PLXDC1 (a receptor highly expressed in tumor vasculature) inhibited ovarian tumor growth by about 90%. The modified cRGD nanoparticle showed to be 60% more effective than non-modified nanoparticles [134]. It is important to note that these peptide modifications to target blood vessel endothelial cells are yet to make it onto the clinical stage and is currently mainly being tested in murine models.
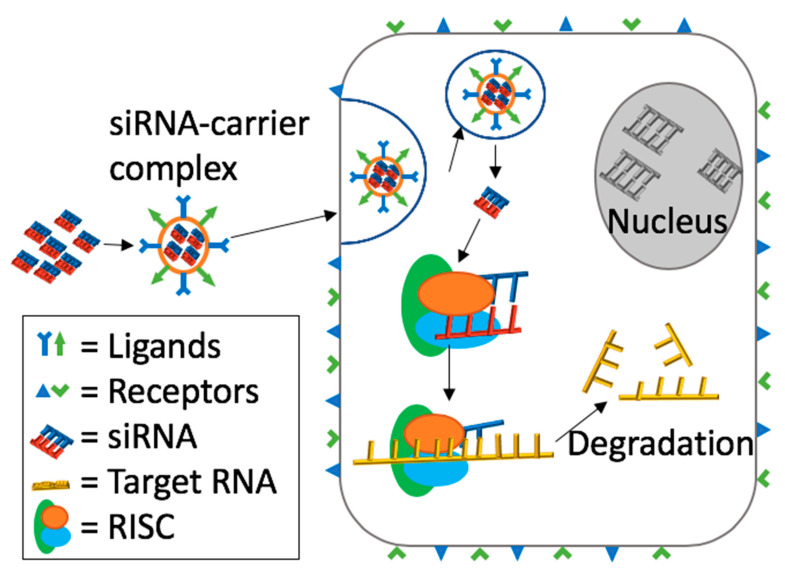
Finally, the antibody conjugation with siRNA may also minimize the off-target effects. During the experiment, antibody complex F105-p-siRNA was specifically delivered into HIV-infected Jurkat cells without triggering any interferon responses and delivering to adjacent cells [135]. Besides, another experiment of inhibiting the growth of B16 tumors, antibody-siRNA complex, was able to successfully prevent the growth, which explains that the antibody-siRNA complex has great therapeutic potentials [135]. Figure 8 explains general mechanism of ligand-receptor mediated targeting and siRNA delivery.
Figure 8.
General mechanism of ligand-receptor mediated targeting and siRNA delivery. Nanoparticle carriers holding siRNA will be surface modified with complementary ligands of receptors overexpressed on the damaged or diseased cell. Once internalized, it will incorporate with the RNAi pathway and efficiently silence the gene of interest.
13. Other Strategies to Improve siRNA Effectiveness:
13.1. Aptamer-siRNA Conjugation
The aptamer is a single-stranded oligonucleotide with a three-dimensional structure that can be used to target cells. Their high affinity as well as specificity towards specific molecules, make it similar to monoclonal antibodies. However, aptamer shows several advantages over antibodies as they possess little-to-no toxicity and immunogenicity [136]. Both aptamer and the siRNA are nucleic acids, so both can be combined with simple covalent linkage or complementation (annealing). This combination of two has been mentioned as aptamer-siRNA conjugates [137]. Researchers can identify aptamers from libraries (>1014 shapes per library) by using a technique termed as SELEX (Systematic Evolution of Ligands by Exponential Enrichment) proposed by Gold and Szostak in 1990 [138]. Two different research groups first defined aptamer mediated siRNAs delivery. In both types of research, prostate-specific membrane antigen (PSMA) targeting RNA aptamers were used. PSMA is the first cancer cell-specific marker protein used for siRNA delivery with the help of aptamers. For critical metastatic cancer, an aptamer identifying alpha V and beta 3 (αVβ3) integrin were selected and linked to a siRNA against eukaryotic elongation factor 2, inhibiting proliferation and inducing apoptosis in target cells [139]. The last decade has seen several aptamers that bind to cell surface receptors, and upon ligand binding, it causes active internalization of cargoes, especially siRNA [140]. Table 6 illustrates the application of aptamers for delivery applications of nucleic acids.
Table 6.
Aptamers based on cell surface protein along with their delivery applications.
| Receptor | Selection method | RNA/DNA | Delivery Applications | References |
|---|---|---|---|---|
| Transferrin receptor (TfR) | The extracellular purview of mouse TfR | RNA/DNA | Protein involved targeting lysosome | [141] |
| Nucleolin | N/A | DNA | Imaging variety of tumors | [142] |
| Tenascin C (TN-C) | Purified TN-C | RNA | Imaging variety of tumors | [139] |
| Prostate-specific membrane antigen (PSMA) | The extracellular purview of PSMA | RNA | Cellular imaging, siRNA delivery along with anticancer drug delivery | [143,144] |
| Epidermal growth factor receptor (EGFR) | The extracellular purview of EGFR | RNA | Delivery of nanoparticles | [145] |
13.2. Exosomes for siRNA Delivery
Another evolving innovative siRNA delivery vehicle is exosomes. Exosomes are naturally occurring RNA carriers that regulate the gene expression of recipient cells. Exosomes have been exploited for their use in delivering the siRNA within the cells [146]. They can bypass the several barriers encountered by other delivery vehicles for siRNA. Exosomes possess several advantages over other carriers, including greater delivery efficiency, membrane crossing capacity, biocompatibility, and non-immunogenicity [147,148]. Exosomes also show less toxicity and better safety while delivering the siRNA into the cells [149]. Besides, exosomes comprise of a specific protein and lipid composition that makes them suitable in delivering various cargoes directly to the cytosol through fusing with biological membranes [147]. Exosomes are nano-sized (40–100 nm) extracellular vesicles, and their design is based on endosome [150]. Once exosomes reach the desired target cells, their uptake can be mediated either endocytosis or fusion with biological membrane followed by internalization of siRNA in the cytosol [151]. Exosomes possess a slightly negative charge that is the reason for their stability in the blood. Besides, exosomes are more compatible with the immune system and do not trigger the immune response and have been reported to avoid off-target effecrs [152,153,154]. One of the most promising ability of exosome is to cross several biological barriers especially blood brain barrier (BBB), that warrant their potential use in several neurological disorders [155].
14. Innovations and Prospects
Besides its effectiveness, siRNA-based therapeutics face several challenges that limit their potential from bench to bedside. Nevertheless, numerous successful in-vitro studies and several investigations aimed at addressing the challenges for clinical application of siRNA therapeutics engenders great hope in this field. RNAi gains popularity in the scientific community quickly due to its potential to cause targeted knockdown of gene expression. Also, a great advancement in siRNA experiment protocols, easy availability of siRNA reagent kits, fast and efficient means to tailor siRNAs specific to desired sequences, latest bioinformatics software, and availability of the whole of the genome has made siRNA a lucrative tool for the cancer therapy as well as other intractable ailments [156]. A recent review delineates in detail the use of bioinformatics tools, software packages, and protocols in helping to design precise siRNA sequences for silencing desired genes and avoiding off-target effects [157]. Clinical evaluation of siRNA therapeutics began as early as 2004, but it was mostly for local administration. Now, the availability of FDA approved siRNA therapeutics bolstered hope in siRNA therapeutics [14]. Several approaches have been investigated to solve these challenges that have been thoroughly described in this review. For instance, chemical modification of the dsRNA has shown in numerous studies to attenuate immune response [158], permit resistance to endogenous endonucleases and exonucleases [159], improve sequence selectivity to reduce off-target RNAi activity [160], enhanced cellular permeability [161], and improved antisense strand selectivity by the RISC complex [162]. Another important factor in enhancing the effectiveness of siRNA therapy is the sequence selection. The antisense strand in dsRNA is the guide strand for the activation of RISC binding to the target mRNA. The sequences of the antisense strand is the single most important determinant of siRNA effectiveness. The sequence selection is not only important on-target potency but also it can profoundly minimize the off-target RNAi activity [163]. Avoiding the endosomal trap of siRNA and enhancing its escape for its cytoplasmic activity is a major challenge in RNAi based therapy [164]. Mostly, the siRNA, along with the carriers, enters the cells via endocytosis by interacting with anionic proteoglycans to form endocytic vesicles. After entry into the cell, the siRNA is entrapped in the endosomal vesicle, in case, the siRNA delivery system stays trapped in the vesicle, the late endosomal vesicle becomes acidified by membrane proton pump ATPase. It is relocated to the lysosome, where it is further acidified, resulting in degradation of the siRNA. Several carriers have been investigated to enhance the endosomal escape and increased transfection efficiency [45].
Keeping in view the progress made so far in addressing the barriers to siRNA therapy, numerous ongoing clinical trials in different stages, improved protocols for the systemic administration of siRNA treatment for targeted therapy, more mature clinical research and development, and the Good Manufacturing Practices, it is hoped that numerous siRNA based therapies will get approval from FDA in the years to come. Beyond these novel techniques and modifications in siRNA delivery systems for enabling the success of siRNA therapeutics, it is much likely that RNAi therapy has several paths for impactful innovations in challenging diseases over the next decade.
15. Conclusions
Most of the traditional drugs interfere with or modulate the disease-causing proteins. However, siRNA can silence the expression of the target proteins by interfering with the expression of target genes. Although it is almost two decades since the discovery of siRNA therapeutics, several challenges have limited the usefulness of siRNA therapeutics. With a lot of research in addressing those challenges, and recent emergence of siRNA drugs in the market has garnered a new hope in siRNA therapy. In this review, we have comprehensively described almost all the challenges pertaining to the success of siRNA therapy and the innovations and techniques that have been discovered to address those challenges. Many of the hurdles that were limiting the use of siRNA as an effective tool for the therapy of intractable disease has been overcome, several clinical trials and great interest by the Pharmaceutical as well as Biological Companies reveal that we will see some great breakthroughs in the therapy of cancer and other challenging diseases in the next decade.
Acknowledgments
M.I.S. acknowledges for Fulbright fellowship from the United States Education Foundation in Pakistan and the Institute of International Education. S.K. and K.Y.C. also acknowledge the support for Summer Undergraduate Fellowship (SURF) from the Center for Undergraduate Excellence (CUE), Chapman University, Orange, CA, USA.
Author Contributions
Conceptualization, M.I.S.; R.K.T.; Manuscript was written and reviewed by all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from the Chapman University School of Pharmacy, Irvine, CA, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Caplen N.J., Parrish S., Imani F., Fire A., Morgan R.A. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA. 2001;98:9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 4.Snead N.M., Wu X., Li A., Cui Q., Sakurai K., Burnett J.C., Rossi J.J. Molecular basis for improved gene silencing by Dicer substrate interfering RNA compared with other siRNA variants. Nucleic Acids Res. 2013;41:6209–6221. doi: 10.1093/nar/gkt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Draz M.S., Fang B.A., Zhang P., Hu Z., Gu S., Weng K.C., Gray J.W., Chen F.F. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4:872. doi: 10.7150/thno.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng Y., Xiao H., Zhang J., Liang X.-J., Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 2019;37:801–825. doi: 10.1016/j.biotechadv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Scott L.J. Givosiran: First Approval. Drugs. 2020;80:335–339. doi: 10.1007/s40265-020-01269-0. [DOI] [PubMed] [Google Scholar]
- 8.O’Driscoll C.M., Bernkop-Schnürch A., Friedl J.D., Préat V., Jannin V. Oral delivery of non-viral nucleic acid-based therapeutics-do we have the guts for this? Eur. J. Pharm. Sci. 2019;133:190–204. doi: 10.1016/j.ejps.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 9.AJMC Lumasiran Meets Primary End Point in Phase 3 Study in Patients With PH1. [(accessed on 25 December 2019)]; Available online: https://www.ajmc.com/newsroom/lumasiran-meets-primary-endpoint-in-phase-3-study-in-patients-with-ph1.
- 10.ClinicalTrials.gov A Study to Evaluate Lumasiran in Children and Adults With Primary Hyperoxaluria Type 1 (ILLUMINATE-A) [(accessed on 15 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03681184.
- 11.Machin N., Ragni M.V. An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B. J. Blood Med. 2018;9:135–140. doi: 10.2147/JBM.S159297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ClinicalTrials.gov A Study of Fitusiran (ALN-AT3SC) in Severe Hemophilia A and B Patients Without Inhibitors. [(accessed on 3 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03417245.
- 13.ClinicalTrials.gov HELIOS-B: A Study to Evaluate Vutrisiran in Patients With Transthyretin Amyloidosis With Cardiomyopathy. [(accessed on 15 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04153149.
- 14.Wang J., Lu Z., Wientjes M.G., Au J.L.-S. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setten R.L., Rossi J.J., Han S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P., An K., Duan X., Xu H., Li F., Xu F. Recent advances in siRNA delivery for cancer therapy using smart nanocarriers. Drug Discov. Today. 2018;23:900–911. doi: 10.1016/j.drudis.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.-H., Behlke M.A., Rose S.D., Chang M.-S., Choi S., Rossi J.J. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 19.Cheloufi S., Dos Santos C.O., Chong M.M., Hannon G.J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Carmell M.A., Rivas F.V., Marsden C.G., Thomson J.M., Song J.-J., Hammond S.M., Joshua-Tor L., Hannon G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 21.Morris K.V., Chan S.W.-L., Jacobsen S.E., Looney D.J. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 22.Eystathioy T., Chan E.K., Tenenbaum S.A., Keene J.D., Griffith K., Fritzler M.J. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell. 2002;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dijk E., Cougot N., Meyer S., Babajko S., Wahle E., Séraphin B. Human Dcp2: A catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002;21:6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esau C.C., Monia B.P. Therapeutic potential for microRNAs. Adv. Drug Deliv. Rev. 2007;59:101–114. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.McAnuff M.A., Rettig G.R., Rice K.G. Potency of siRNA versus shRNA mediated knockdown in vivo. J. Pharm. Sci. 2007;96:2922–2930. doi: 10.1002/jps.20968. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y., Yamaoka K., Nishikawa M., Takakura Y. Quantitative and temporal analysis of gene silencing in tumor cells induced by small interfering RNA or short hairpin RNA expressed from plasmid vectors. J. Pharm. Sci. 2009;98:74–80. doi: 10.1002/jps.21398. [DOI] [PubMed] [Google Scholar]
- 27.Davis M.E., Zuckerman J.E., Choi C.H.J., Seligson D., Tolcher A., Alabi C.A., Yen Y., Heidel J.D., Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 30.Heidel J.D., Liu J.Y.-C., Yen Y., Zhou B., Heale B.S., Rossi J.J., Bartlett D.W., Davis M.E. Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin. Cancer Res. 2007;13:2207–2215. doi: 10.1158/1078-0432.CCR-06-2218. [DOI] [PubMed] [Google Scholar]
- 31.Tabernero J., Shapiro G.I., LoRusso P.M., Cervantes A., Schwartz G.K., Weiss G.J., Paz-Ares L., Cho D.C., Infante J.R., Alsina M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 32.Barros S.A., Gollob J.A. Safety profile of RNAi nanomedicines. Adv. Drug Deliv. Rev. 2012;64:1730–1737. doi: 10.1016/j.addr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Strumberg D., Schultheis B., Traugott U., Vank C., Santel A., Keil O., Giese K., Kaufmann J., Drevs J. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int. J. Clin. Pharmacol. Ther. 2012;50:76. doi: 10.5414/CPP50076. [DOI] [PubMed] [Google Scholar]
- 34.Schultheis B., Strumberg D., Santel A., Vank C., Gebhardt F., Keil O., Lange C., Giese K., Kaufmann J., Khan M. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014;32:4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 35.Northfelt D.W., Hamburg S.I., Borad M.J., Seetharam M., Curtis K.K., Lee P., Crowell B., Vocila L., Fredlund P., Gilbert M.J. A phase I dose-escalation study of TKM-080301, a RNAi therapeutic directed against polo-like kinase 1 (PLK1), in patients with advanced solid tumors: Expansion cohort evaluation of biopsy samples for evidence of pharmacodynamic effects of PLK1 inhibition. J. Clin. Oncol. Am. Soc. Clin. Oncol. 2013 doi: 10.1200/jco.2013.31.15_suppl.tps2621. [DOI] [Google Scholar]
- 36.Tolcher A.W., Rodrigueza W.V., Rasco D.W., Patnaik A., Papadopoulos K.P., Amaya A., Moore T.D., Gaylor S.K., Bisgaier C.L., Sooch M.P. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014;73:363–371. doi: 10.1007/s00280-013-2361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y., Aimetti A.A., Langer R., Gu Z. Bioresponsive materials. Nat. Rev. Mater. 2016;2:1–17. doi: 10.1038/natrevmats.2016.75. [DOI] [Google Scholar]
- 38.Sioud M. RNA Interference. Springer; Berlin/Heidelberg, Germany: 2015. RNA interference: Mechanisms, technical challenges, and therapeutic opportunities; pp. 1–15. [DOI] [PubMed] [Google Scholar]
- 39.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grzelinski M., Urban-Klein B., Martens T., Lamszus K., Bakowsky U., Höbel S., Czubayko F., Aigner A. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum. Gene Ther. 2006;17:751–766. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- 41.Palliser D., Chowdhury D., Wang Q.-Y., Lee S.J., Bronson R.T., Knipe D.M., Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 42.Ballarín-González B., Thomsen T.B., Howard K.A. Clinical translation of RNAi-based treatments for respiratory diseases. Drug Deliv. Transl. Res. 2013;3:84–99. doi: 10.1007/s13346-012-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geusens B., Sanders N., Prow T., Van Gele M., Lambert J. Cutaneous short-interfering RNA therapy. Expert Opin. Drug Deliv. 2009;6:1333–1349. doi: 10.1517/17425240903304032. [DOI] [PubMed] [Google Scholar]
- 44.Thomas M., Lu J.J., Chen J., Klibanov A.M. Non-viral siRNA delivery to the lung. Adv. Drug Deliv. Rev. 2007;59:124–133. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subhan M.A., Torchilin V. siRNA based drug design, quality, delivery and clinical translation. Nanomed. Nanotechnol. Biol. Med. 2020;29:102239. doi: 10.1016/j.nano.2020.102239. [DOI] [PubMed] [Google Scholar]
- 46.Tai W. Current aspects of siRNA bioconjugate for in vitro and in vivo delivery. Molecules. 2019;24:2211. doi: 10.3390/molecules24122211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shegokar R., Al Shaal L., Mishra P. SiRNA delivery: Challenges and role of carrier systems. Die Pharm. Int. J. Pharm. Sci. 2011;66:313–318. [PubMed] [Google Scholar]
- 48.Bartlett D.W., Davis M.E. Effect of siRNA nuclease stability on the in vitro and in vivo kinetics of siRNA-mediated gene silencing. Biotechnol. Bioeng. 2007;97:909–921. doi: 10.1002/bit.21285. [DOI] [PubMed] [Google Scholar]
- 49.Jackson A.L., Linsley P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 50.Zahir-Jouzdani F., Mottaghitalab F., Dinarvand M., Atyabi F. siRNA delivery for treatment of degenerative diseases, new hopes and challenges. J. Drug Deliv. Sci. Technol. 2018;45:428–441. doi: 10.1016/j.jddst.2018.04.001. [DOI] [Google Scholar]
- 51.Rana T.M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 52.Volkov A.A., Kruglova N.Y.S., Meschaninova M.I., Venyaminova A.G., Zenkova M.A., Vlassov V.V., Chernolovskaya E.L. Selective protection of nuclease-sensitive sites in siRNA prolongs silencing effect. Oligonucleotides. 2009;19:191–202. doi: 10.1089/oli.2008.0162. [DOI] [PubMed] [Google Scholar]
- 53.Kim S.-S., Garg H., Joshi A., Manjunath N. Strategies for targeted nonviral delivery of siRNAs in vivo. Trends Mol. Med. 2009;15:491–500. doi: 10.1016/j.molmed.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis D.L., Wolff J.A. Methods in Enzymology. Volume 392. Elsevier; Amsterdam, The Netherlands: 2005. Delivery of siRNA and siRNA expression constructs to adult mammals by hydrodynamic intravascular injection; pp. 336–350. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y., Zhang C., Liang W. Development of RNAi technology for targeted therapy—A track of siRNA based agents to RNAi therapeutics. J. Control. Release. 2014;193:270–281. doi: 10.1016/j.jconrel.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 56.Czauderna F., Fechtner M., Dames S., Aygün H., Klippel A., Pronk G.J., Giese K., Kaufmann J. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003;31:2705–2716. doi: 10.1093/nar/gkg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia J., Noronha A., Toudjarska I., Li F., Akinc A., Braich R., Frank-Kamenetsky M., Rajeev K.G., Egli M., Manoharan M. Gene silencing activity of siRNAs with a ribo-difluorotoluyl nucleotide. ACS Chem. Biol. 2006;1:176–183. doi: 10.1021/cb600063p. [DOI] [PubMed] [Google Scholar]
- 58.Potenza N., Moggio L., Milano G., Salvatore V., Di Blasio B., Russo A., Messere A. RNA interference in mammalia cells by RNA-3′-PNA chimeras. Int. J. Mol. Sci. 2008;9:299–315. doi: 10.3390/ijms9030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoshika S., Minakawa N., Kamiya H., Harashima H., Matsuda A. RNA interference induced by siRNAs modified with 4′-thioribonucleosides in cultured mammalian cells. FEBS Lett. 2005;579:3115–3118. doi: 10.1016/j.febslet.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 60.Choung S., Kim Y.J., Kim S., Park H.-O., Choi Y.-C. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006;342:919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 61.Layzer J.M., McCaffrey A.P., Tanner A.K., Huang Z., Kay M.A., Sullenger B.A. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao H., Wang J.H. Biomembrane-permeable and ribonuclease-resistant siRNA with enhanced activity. Oligonucleotides. 2005;15:196–205. doi: 10.1089/oli.2005.15.196. [DOI] [PubMed] [Google Scholar]
- 63.Dowler T., Bergeron D., Tedeschi A.-L., Paquet L., Ferrari N., Damha M.J. Improvements in siRNA properties mediated by 2′-deoxy-2′-fluoro-β-D-arabinonucleic acid (FANA) Nucleic Acids Res. 2006;34:1669–1675. doi: 10.1093/nar/gkl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao K., Huang L. Achieving efficient RNAi therapy: Progress and challenges. Acta Pharm. Sin. B. 2013;3:213–225. doi: 10.1016/j.apsb.2013.06.005. [DOI] [Google Scholar]
- 65.Hoshika S., Minakawa N., Shionoya A., Imada K., Ogawa N., Matsuda A. Study of Modification Pattern–RNAi Activity Relationships by Using siRNAs Modified with 4′-Thioribonucleosides. ChemBioChem. 2007;8:2133–2138. doi: 10.1002/cbic.200700342. [DOI] [PubMed] [Google Scholar]
- 66.Amarzguioui M., Holen T., Babaie E., Prydz H. Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Res. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrissey D.V., Blanchard K., Shaw L., Jensen K., Lockridge J.A., Dickinson B., McSwiggen J.A., Vargeese C., Bowman K., Shaffer C.S. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41:1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- 68.Chernolovskaya E.L., Zenkova M.A. Chemical modification of siRNA. Curr. Opin. Mol. Ther. 2010;12:158–167. [PubMed] [Google Scholar]
- 69.Bramsen J.B., Laursen M.B., Damgaard C.K., Lena S.W., Ravindra Babu B., Wengel J., Kjems J. Improved silencing properties using small internally segmented interfering RNAs. Nucleic Acids Res. 2007;35:5886–5897. doi: 10.1093/nar/gkm548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tatiparti K., Sau S., Kashaw S.K., Iyer A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials. 2017;7:77. doi: 10.3390/nano7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Behlke M.A. Progress towards in vivo use of siRNAs. Mol. Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chernikov I.V., Gladkikh D.V., Meschaninova M.I., Ven’yaminova A.G., Zenkova M.A., Vlassov V.V., Chernolovskaya E.L. Cholesterol-containing nuclease-resistant siRNA accumulates in tumors in a carrier-free mode and silences MDR1 gene. Mol. Ther. Nucleic Acids. 2017;6:209–220. doi: 10.1016/j.omtn.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfrum C., Shi S., Jayaprakash K.N., Jayaraman M., Wang G., Pandey R.K., Rajeev K.G., Nakayama T., Charrise K., Ndungo E.M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 74.Soutschek J., Akinc A., Bramlage B., Charisse K., Constien R., Donoghue M., Elbashir S., Geick A., Hadwiger P., Harborth J. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 75.Gaziova Z., Baumann V., Winkler A.-M., Winkler J. Chemically defined polyethylene glycol siRNA conjugates with enhanced gene silencing effect. Bioorg. Med. Chem. 2014;22:2320–2326. doi: 10.1016/j.bmc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iversen F., Yang C., Dagnæs-Hansen F., Schaffert D.H., Kjems J., Gao S. Optimized siRNA-PEG conjugates for extended blood circulation and reduced urine excretion in mice. Theranostics. 2013;3:201. doi: 10.7150/thno.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dassie J.P., Liu X.-Y., Thomas G.S., Whitaker R.M., Thiel K.W., Stockdale K.R., Meyerholz D.K., McCaffrey A.P., McNamara J.O., Giangrande P.H. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat. Biotechnol. 2009;27:839–846. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sato A., Choi S.W., Hirai M., Yamayoshi A., Moriyama R., Yamano T., Takagi M., Kano A., Shimamoto A., Maruyama A. Polymer brush-stabilized polyplex for a siRNA carrier with long circulatory half-life. J. Control. Release. 2007;122:209–216. doi: 10.1016/j.jconrel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 79.Judge A., Maclachlan I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 2008;19:111–124. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- 80.Judge A.D., Sood V., Shaw J.R., Fang D., McClintock K., MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 81.Robbins M., Judge A., MacLachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19:89–102. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
- 82.Meng Z., Lu M. RNA interference-induced innate immunity, off-target effect, or immune adjuvant? Front. Immunol. 2017;8:331. doi: 10.3389/fimmu.2017.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forsbach A., Nemorin J.-G., Montino C., Müller C., Samulowitz U., Vicari A.P., Jurk M., Mutwiri G.K., Krieg A.M., Lipford G.B. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 2008;180:3729–3738. doi: 10.4049/jimmunol.180.6.3729. [DOI] [PubMed] [Google Scholar]
- 84.Yasuda H., Leelahavanichkul A., Tsunoda S., Dear J.W., Takahashi Y., Ito S., Hu X., Zhou H., Doi K., Childs R. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008;294:F1050–F1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kleinman M.E., Yamada K., Takeda A., Chandrasekaran V., Nozaki M., Baffi J.Z., Albuquerque R.J., Yamasaki S., Itaya M., Pan Y. Sequence-and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 87.Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 88.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 89.Bender A.T., Tzvetkov E., Pereira A., Wu Y., Kasar S., Przetak M.M., Vlach J., Niewold T.B., Jensen M.A., Okitsu S.L. TLR7 and TLR8 Differentially Activate the IRF and NF-κB Pathways in Specific Cell Types to Promote Inflammation. ImmunoHorizons. 2020;4:93–107. doi: 10.4049/immunohorizons.2000002. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z., Ohto U., Shibata T., Taoka M., Yamauchi Y., Sato R., Shukla N.M., David S.A., Isobe T., Miyake K. Structural analyses of Toll-like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 2018;25:3371–3381. doi: 10.1016/j.celrep.2018.11.081. [DOI] [PubMed] [Google Scholar]
- 91.Hornung V., Guenthner-Biller M., Bourquin C., Ablasser A., Schlee M., Uematsu S., Noronha A., Manoharan M., Akira S., de Fougerolles A. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 92.Tan X., Jia F., Wang P., Zhang K. Nucleic acid-based drug delivery strategies. J. Control. Release. 2020;323:240–252. doi: 10.1016/j.jconrel.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dermani F.K., Jalilian F.A., Hossienkhani H., Ezati R., Amini R. SiRNA delivery technology for cancer therapy: Promise and challenges. Acta Medica Iranica. 2019 doi: 10.18502/acta.v57i2.1760. [DOI] [Google Scholar]
- 94.Kim S.j., Chen Z., Essani A.B., Elshabrawy H.A., Volin M.V., Volkov S., Swedler W., Arami S., Sweiss N., Shahrara S. Identification of a novel toll-like receptor 7 endogenous ligand in rheumatoid arthritis synovial fluid that can provoke arthritic joint inflammation. Arthritis Rheumatol. 2016;68:1099–1110. doi: 10.1002/art.39544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khan A.A., Pluta L., Yousefi B., Damania B. Endosomal TLR-8 Senses microRNA-1294 Resulting in the Production of NFḱB Dependent Cytokines Running title: miRNA1294 activates TLR-8. Front. Immunol. 2019;10:2860. doi: 10.3389/fimmu.2019.02860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta K., Saldanha M., Parasnis M., Devarajan P.V., Jain R., Dandekar P. Targeted Intracellular Drug Delivery by Receptor Mediated Endocytosis. Springer; Berlin/Heidelberg, Germany: 2019. Toll-like receptor-mediated endocytosis in infectious disease; pp. 323–349. [Google Scholar]
- 97.Imaeda A., Tomoike F., Hayakawa M., Nakamoto K., Kimura Y., Abe N., Abe H. N 6-methyl adenosine in siRNA evades immune response without reducing RNAi activity. Nucleosides Nucleotides Nucleic Acids. 2019;38:972–979. doi: 10.1080/15257770.2019.1641205. [DOI] [PubMed] [Google Scholar]
- 98.Hoerter J.A., Walter N.G. Chemical modification resolves the asymmetry of siRNA strand degradation in human blood serum. RNA. 2007;13:1887–1893. doi: 10.1261/rna.602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J., Brutkiewicz R.R. The Toll-like receptor 9 signalling pathway regulates MR 1-mediated bacterial antigen presentation in B cells. Immunology. 2017;152:232–242. doi: 10.1111/imm.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sioud M. Recent advances in small interfering RNA sensing by the immune system. New Biotechnol. 2010;27:236–242. doi: 10.1016/j.nbt.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 101.Hur S. Double-stranded RNA sensors and modulators in innate immunity. Annu. Rev. Immunol. 2019;37:349–375. doi: 10.1146/annurev-immunol-042718-041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hull C.M., Bevilacqua P.C. Discriminating self and non-self by RNA: Roles for RNA structure, misfolding, and modification in regulating the innate immune sensor PKR. Acc. Chem. Res. 2016;49:1242–1249. doi: 10.1021/acs.accounts.6b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Safran S.A., Eckert D.M., Leslie E.A., Bass B.L. PKR activation by noncanonical ligands: A 5′-triphosphate requirement versus antisense contamination. RNA. 2019;25:1192–1201. doi: 10.1261/rna.071910.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Owens D.E., III, Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Sun Y., Zhao Y., Zhao X., Lee R.J., Teng L., Zhou C. Enhancing the therapeutic delivery of oligonucleotides by chemical modification and nanoparticle encapsulation. Molecules. 2017;22:1724. doi: 10.3390/molecules22101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Auguste D.T., Furman K., Wong A., Fuller J., Armes S.P., Deming T.J., Langer R. Triggered release of siRNA from poly (ethylene glycol)-protected, pH-dependent liposomes. J. Control. Release. 2008;130:266–274. doi: 10.1016/j.jconrel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salmaso S., Caliceti P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013;2013 doi: 10.1155/2013/374252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peer D., Lieberman J. Special delivery: Targeted therapy with small RNAs. Gene Ther. 2011;18:1127–1133. doi: 10.1038/gt.2011.56. [DOI] [PubMed] [Google Scholar]
- 109.Du J., Sun Y., Shi Q.-S., Liu P.-F., Zhu M.-J., Wang C.-H., Du L.-F., Duan Y.-R. Biodegradable nanoparticles of mPEG-PLGA-PLL triblock copolymers as novel non-viral vectors for improving siRNA delivery and gene silencing. Int. J. Mol. Sci. 2012;13:516–533. doi: 10.3390/ijms13010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo G., Jin C., Long J., Fu D., Yang F., Xu J., Yu X., Chen W., Ni Q. RNA interference of MBD1 in BxPC-3 human pancreatic cancer cells delivered by PLGA-poloxamer nanoparticles. Cancer Biol. Ther. 2009;8:594–598. doi: 10.4161/cbt.8.7.7790. [DOI] [PubMed] [Google Scholar]
- 111.Santel A., Aleku M., Keil O., Endruschat J., Esche V., Fisch G., Dames S., Löffler K., Fechtner M., Arnold W. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 112.Musacchio T., Torchilin V.P. siRNA delivery: From basics to therapeutic applications. Front. Biosci. (Landmark Ed.) 2013;18:58–79. doi: 10.2741/4087. [DOI] [PubMed] [Google Scholar]
- 113.Liu Y., Song Z., Zheng N., Nagasaka K., Yin L., Cheng J. Systemic siRNA delivery to tumors by cell-penetrating α-helical polypeptide-based metastable nanoparticles. Nanoscale. 2018;10:15339–15349. doi: 10.1039/C8NR03976C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cummings J.C., Zhang H., Jakymiw A. Peptide carriers to the rescue: Overcoming the barriers to siRNA delivery for cancer treatment. Transl. Res. 2019;214:92–104. doi: 10.1016/j.trsl.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rathnayake P., Gunathunge B., Wimalasiri P., Karunaratne D., Ranatunga R. Trends in the binding of cell penetrating peptides to siRNA: A molecular docking study. J. Biophys. 2017;2017 doi: 10.1155/2017/1059216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanaka K., Kanazawa T., Ogawa T., Takashima Y., Fukuda T., Okada H. Disulfide crosslinked stearoyl carrier peptides containing arginine and histidine enhance siRNA uptake and gene silencing. Int. J. Pharm. 2010;398:219–224. doi: 10.1016/j.ijpharm.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 117.Mainini F., Eccles M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules. 2020;25:2692. doi: 10.3390/molecules25112692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Varkouhi A.K., Scholte M., Storm G., Haisma H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 119.Lu S., Morris V.B., Labhasetwar V. Effectiveness of small interfering RNA delivery via arginine-rich polyethylenimine-based polyplex in metastatic and doxorubicin-resistant breast cancer cells. J. Pharmacol. Exp. Ther. 2019;370:902–910. doi: 10.1124/jpet.119.256909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Urban-Klein B., Werth S., Abuharbeid S., Czubayko F., Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 121.Sonawane N.D., Szoka F.C., Verkman A. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 122.Choi K.Y., Correa S., Min J., Li J., Roy S., Laccetti K.H., Dreaden E., Kong S., Heo R., Roh Y.H. Binary targeting of siRNA to hematologic cancer cells in vivo using layer-by-layer nanoparticles. Adv. Funct. Mater. 2019;29:1900018. doi: 10.1002/adfm.201900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang H., Yuan S., Ma Z., Ji P., Ma X., Wu Z., Qi X. Genetic recombination of poly (l-lysine) functionalized apoferritin nanocages that resemble viral capsid nanometer-sized platforms for gene therapy. Biomater. Sci. 2020;8:1759–1770. doi: 10.1039/C9BM01822K. [DOI] [PubMed] [Google Scholar]
- 124.Shin J., Shum P., Thompson D.H. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG–lipids. J. Control. Release. 2003;91:187–200. doi: 10.1016/S0168-3659(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 125.Cupic K.I., Rennick J.J., Johnston A.P., Such G.K. Controlling endosomal escape using nanoparticle composition: Current progress and future perspectives. Nanomedicine. 2019;14:215–223. doi: 10.2217/nnm-2018-0326. [DOI] [PubMed] [Google Scholar]
- 126.Koynova R., Wang L., MacDonald R.C. An intracellular lamellar–nonlamellar phase transition rationalizes the superior performance of some cationic lipid transfection agents. Proc. Natl. Acad. Sci. USA. 2006;103:14373–14378. doi: 10.1073/pnas.0603085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hatakeyama H., Akita H., Kogure K., Oishi M., Nagasaki Y., Kihira Y., Ueno M., Kobayashi H., Kikuchi H., Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 128.Leng Q., Woodle M.C., Mixson A.J. Targeted delivery of siRNA therapeutics to malignant tumors. J. Drug Deliv. 2017;2017 doi: 10.1155/2017/6971297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsuda S., Keiser K., Nair J.K., Charisse K., Manoharan R.M., Kretschmer P., Peng C.G., Kel’in V.A., Kandasamy P., Willoughby J.L. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 130.Nair J.K., Willoughby J.L., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 131.Jackson A.L., Burchard J., Schelter J., Chau B.N., Cleary M., Lim L., Linsley P.S. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walker R.A., Day S.J. Transferrin receptor expression in non-malignant and malignant human breast tissue. J. Pathol. 1986;148:217–224. doi: 10.1002/path.1711480305. [DOI] [PubMed] [Google Scholar]
- 133.Gurrath M., Müller G., Kessler H., Aumailley M., Timpl R. Conformation/activity studies of rationally designed potent anti-adhesive RGD peptides. Eur. J. Biochem. 1992;210:911–921. doi: 10.1111/j.1432-1033.1992.tb17495.x. [DOI] [PubMed] [Google Scholar]
- 134.Han H.D., Mangala L.S., Lee J.W., Shahzad M.M., Kim H.S., Shen D., Nam E.J., Mora E.M., Stone R.L., Lu C. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin. Cancer Res. 2010;16:3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song E., Zhu P., Lee S.-K., Chowdhury D., Kussman S., Dykxhoorn D.M., Feng Y., Palliser D., Weiner D.B., Shankar P. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 136.Foy J.W.-D., Rittenhouse K., Modi M., Patel M. Local tolerance and systemic safety of pegaptanib sodium in the dog and rabbit. J. Ocul. Pharmacol. Ther. 2007;23:452–466. doi: 10.1089/jop.2006.0149. [DOI] [PubMed] [Google Scholar]
- 137.Kruspe S., Giangrande P.H. Aptamer-siRNA chimeras: Discovery, progress, and future prospects. Biomedicines. 2017;5:45. doi: 10.3390/biomedicines5030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 139.Hicke B.J., Stephens A.W., Gould T., Chang Y.-F., Lynott C.K., Heil J., Borkowski S., Hilger C.-S., Cook G., Warren S. Tumor targeting by an aptamer. J. Nucl. Med. 2006;47:668–678. [PubMed] [Google Scholar]
- 140.Thiel K.W., Giangrande P.H. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides. 2009;19:209–222. doi: 10.1089/oli.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chi-Hong B.C., Dellamaggiore K.R., Ouellette C.P., Sedano C.D., Lizadjohry M., Chernis G.A., Gonzales M., Baltasar F.E., Fan A.L., Myerowitz R. Aptamer-based endocytosis of a lysosomal enzyme. Proc. Natl. Acad. Sci. USA. 2008;105:15908–15913. doi: 10.1073/pnas.0808360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ko H.Y., Lee J.H., Kang H., Ryu S.H., Song I.C., Lee D.S., Kim S. A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. J. Nucl. Med. 2010;51:98–105. doi: 10.2967/jnumed.109.069880. [DOI] [PubMed] [Google Scholar]
- 143.Wullner U., Neef I., Eller A., Kleines M., Tur M.K., Barth S. Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2. Curr. Cancer Drug Targets. 2008;8:554–565. doi: 10.2174/156800908786241078. [DOI] [PubMed] [Google Scholar]
- 144.McNamara J.O., Andrechek E.R., Wang Y., Viles K.D., Rempel R.E., Gilboa E., Sullenger B.A., Giangrande P.H. Cell type–specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 145.Li N., Larson T., Nguyen H.H., Sokolov K.V., Ellington A.D. Directed evolution of gold nanoparticle delivery to cells. Chem. Commun. 2010;46:392–394. doi: 10.1039/B920865H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 147.Van den Boorn J.G., Schlee M., Coch C., Hartmann G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 148.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013;11:1–10. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 150.Théry C., Zitvogel L., Amigorena S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 151.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kooijmans S.A., Vader P., van Dommelen S.M., van Solinge W.W., Schiffelers R.M. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012;7:1525. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H., Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu M., Xing H., Xun Z., Yang T., Ding P., Cai C., Wang D., Zhao X. Exosome-based small RNA delivery: Progress and prospects. Asian J. Pharm. Sci. 2018;13:1–11. doi: 10.1016/j.ajps.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sekhar G.N., Watson C.P., Fidanboylu M., Sanderson L., Thomas S.A. Advances in Pharmacology. Volume 71. Elsevier; Amsterdam, The Netherlands: 2014. Delivery of antihuman African trypanosomiasis drugs across the blood–brain and blood–CSF barriers; pp. 245–275. [DOI] [PubMed] [Google Scholar]
- 156.Dominska M., Dykxhoorn D.M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 2010;123:1183–1189. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 157.Fakhr E., Zare F., Teimoori-Toolabi L. Precise and efficient siRNA design: A key point in competent gene silencing. Cancer Gene Ther. 2016;23:73–82. doi: 10.1038/cgt.2016.4. [DOI] [PubMed] [Google Scholar]
- 158.Robbins M., Judge A., Liang L., McClintock K., Yaworski E., MacLachlan I. 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol. Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 159.Bramsen J.B., Laursen M.B., Nielsen A.F., Hansen T.B., Bus C., Langkjær N., Babu B.R., Højland T., Abramov M., Van Aerschot A. A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity. Nucleic Acids Res. 2009;37:2867–2881. doi: 10.1093/nar/gkp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Janas M.M., Schlegel M.K., Harbison C.E., Yilmaz V.O., Jiang Y., Parmar R., Zlatev I., Castoreno A., Xu H., Shulga-Morskaya S. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-02989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shum K., Rossi J. siRNA Delivery Methods. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 162.Snead N.M., Escamilla-Powers J.R., Rossi J.J., McCaffrey A.P. 5′ Unlocked nucleic acid modification improves siRNA targeting. Mol. Ther. Nucleic Acids. 2013;2:e103. doi: 10.1038/mtna.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W.S., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 164.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]