Abstract
Artocarpus heterophyllus Lam. (AH) and Artocarpus lakoocha Roxb. (AL) are two endemic plants that grow on the Asian continent. To date, their applications have been aimed at using their fruit as a food source or for some of their therapeutic virtues. In this study, attention was given to the flowers of AH and AL. Initially, the cytotoxicity of the phytoextracts was assessed, and the content of minerals, phenols, and flavonoids was determined. Furthermore, some antioxidant components were identified by HPLC. Furthermore, the ability of AH and AL extracts to modulate the gene expression of some targets involved in the antioxidant response was studied. The results obtained highlighted the nutritional and antioxidant value of the AH and AL flower extracts. This study will contribute to enhancing the use of AH and AL flowers as potential supplements in human nutrition.
Keywords: Artocarpus lakoocha Roxb., Artocarpus heterophyllus Lam., antioxidant properties, DMPD radical, antimicrobial properties
1. Introduction
The genus Artocarpus consists of tropical plants of the Moraceae family that are mainly cultivated in Asian countries such as India, Bhutan, Sri Lanka, Thailand, Bangladesh, and Malaysia [1,2]. Artocarpus includes almost 60 genera and more than 1000 species. This genus is believed to produce a high yield along with an ample number of nutritional components. Its species are used as a source of food and also in traditional medicinal practices [3].
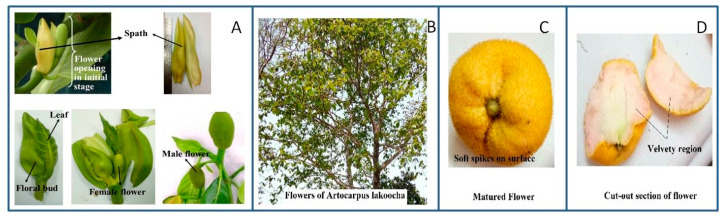
Artocarpus heterophyllus Lam. (AH), popularly known as Ceylon jack tree or jackfruit, is considered to be “poor man’s food”, as it is widely available in summer at an economical price when there is a shortage of agricultural produce in India [4,5]. In India, it is also called “Panasa”, “Atibruhatphala”, “Kantaphal”, and “Kanthal” due to its physical characteristics. The AH tree is also considered to be a source of flavonoids, sterols, volatile acids, carotenoids, and tannins, which vary depending on the stage of maturity [6,7,8,9,10]. Moreover, it also has a unique combination of iron, vitamin C, vitamin B complex (especially vitamin B6, niacin, folic acid, and riboflavin), and minerals (mainly calcium and potassium) [11,12]. The fruit of AH has been employed in value-added products such as fruit juice, dried chips, jam, candies, jelly, marmalades, leather, and ice cream, depending on the sensorial characteristics at different stages [11]. AH bears flowers after 6–8 years of planting, and flowers start to bloom from November to March. It is reported that, being a monoecious tree, the flowers are covered by two spathes of 5–10 cm in length [13] (Figure 1A). The central and peripheral regions bear male flowers, while footstalks from the main trunk bear female flowers (generally round in shape). The distinction between the flowers can be made based on surface characteristics: female flowers are generally large with a rough surface, whereas male flowers are small with a smooth surface (Figure 1A).
Figure 1.
(A) Artocarpus heterophyllus Lam. flowers; (B) tree of Artocarpus lakoocha Roxb. with flowers; (C) upside view of Artocarpus lakoocha Roxb. flower; (D) cut-out section of Artocarpus lakoocha flower.
Artocarpus lakoocha Roxb. (AL) is another important species of Artocarpus. It is commonly known as “lakuchi” in India, “lokhat” in Thailand, and “tampang” in Malaysia. The tree of AL is mostly known for its wood and edible fruits due to its excellent nutraceutical properties [14]. In the foothills of the Himalayas in Nepal, the leaves and flowers of this tree also act as fresh fodder for lactating animals due to its excellent source of bioactive compounds [15,16,17]. Traditionally, it is also taken as medicine for people suffering from liver-related diseases [18]. The edible fruits and flowers of AL are consumed as vegetables, pickles, and chutney due to its astringent and acidic flavor [14]. The extract and bioactive compounds from the bark, leaves, seeds, and pericarp of AL fruit have been shown to possess exceptional phytochemical, nutritional, and valuable pharmacological properties [16,19,20,21]. This fruit is capable of offering numerous inhibitory factors such as antibacterial, antitubercular, antiviral, antifungal, antiplatelet, antiarthritic, tyrosinase inhibitory, cytotoxicity, and H2O2 scavenging activity, as well as exceptional activities against herpes simplex virus (HSV) and human immunodeficiency virus (HIV) [1,22,23,24,25,26,27]. The flowering pattern of AL is similar to AH, where both male and female flowers appear on the same tree. The flowers are generally irregularly round with a yellow velvety surface. The male flowers are orange-yellow, while female flowers are reddish [28] (Figure 1B–D).
In general, in both the studied species, the male flowers appear first, and the female flowers come out after and are immediately fertilized. In order not to have the interference of pollination, in this work, it was decided to use only male flowers.
Most of the studies on this subject have explored the ripe fruit of both Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. [1,19]. However, scant attention has been paid to the flowers of these plants. As is known, the flowers can contain many secondary metabolites with high antioxidant and antimicrobial activities [29]. Therefore, the present study was undertaken to characterize and compare the flowers of Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. concerning their bioactive properties. Analyses of the content of flavonoids and phenolic compounds were performed on fresh starting material. This allowed us to establish the action of the entire plant phytocomplex. Indeed, the use of fresh extract allows one to keep the biological characteristics of the plant sample unaltered at the time of sampling [30]. Various environmental variables associated with the harvest or post-harvest can induce the plant to modify, i.e., increase or decrease, some biologically active secondary metabolites such as flavonoids and phenolic compounds [30]. Other tests, such as toxicity and antioxidant activity, were determined on a dry extract obtained with different solvents (water, ethanol, and methanol). In addition, the expression of some target genes involved in antioxidant pathways and the preliminary antimicrobial action were evaluated.
2. Results
2.1. Chemical Properties of Flowers
2.1.1. Mineral Composition of Flowers
The mineral content of the AL and AH flowers was analyzed by atomic absorption spectroscopy, and the most abundant minerals are presented in Table 1. Generally, the AH flower has a considerable amount of mineral content compared with the AL flower. The highest concentration of minerals in the AL flower was found to be copper (597.10 μg/100 g), zinc (421.06 μg/100 g), manganese (250 μg/100 g), and magnesium (13.40 mg/100 g), whereas calcium (25.35 mg/100 g), potassium (214.56 mg/100 g), and phosphorus (19.63 mg/100 g) were found to be higher in AH. Lower concentrations of phosphorous (11.85 mg/100 g), calcium (20.24 mg/100 g), iron (108.5 μg/100 g), and potassium (150 mg/100 g) were observed for AL, whereas magnesium (7.28 mg/100 g), copper (106.62 μg 100/g), manganese (185 μg/100 g), and zinc (320.22 μg/100 g) were found for the AH flower. These results show a significant difference between AL and AH at the level of mineral content. Indeed, both flowers contain appreciable amounts of essential minerals, which can contribute to the nutritional value of these flowers.
Table 1.
Antioxidant and mineral components of flowers.
| Parameters | Artocarpus lakoocha Roxb. | Artocarpus heterophyllus Lam. | p Value |
|---|---|---|---|
| Total phenol content (μg GAE/g) | 217.80 ± 1.25 | 883.20 ± 5.90 | <0.0001 |
| Total flavonoids content (μg QE/g) | 168.26 ± 1.50 | 658.52 ± 5.60 | <0.0001 |
| Total carotenoids (μg/g) | 16.96 ± 0.15 | 27.29 ± 0.25 | <0.0001 |
| Iron (μg/100 g) | 108.35 ± 3.65 | 196.36 ± 8.32 | <0.0001 |
| Copper (μg/100 g) | 597.10 ± 8.96 | 106.62 ± 5.21 | <0.0001 |
| Zinc (μg/100 g) | 421.06 ± 5.12 | 320.22 ± 6.85 | <0.0001 |
| Manganese (μg/100 g) | 250.05 ± 4.68 | 185.62 ± 4.55 | <0.0001 |
| Calcium (mg/100 g) | 20.24 ± 2.13 | 25.35 ± 0.81 | 0.0432 |
| Magnesium (mg/100 g) | 13.40 ± 0.52 | 7.28 ± 0.35 | 0.0074 |
| Potassium (mg/100 g) | 150.00 ± 8.10 | 214.56 ± 4.68 | <0.0001 |
| Phosphorus (mg/100 g) | 11.85 ± 0.06 | 19.63 ± 0.05 | 0.0002 |
Values are expressed as mean ± standard deviation (n = 15), a value of p ≤ 0.05 is considered statistically significant. The table shows the significant differences between AL and AH.
2.1.2. Phytochemical Composition of Flowers
The total phenolic content (TPC) and total flavonoids content (TFC) of both flowers are presented in Table 1. It is evident from the results that AH contains a higher amount of TPC and TFC compared with the AL flower. In general, the AH flower had 883.20 μg gallic acid equivalent (GAE)/g of phenolic content, whereas only 217.80 μg GAE/g of phenolic content was found in the AL flower. Furthermore, the total flavonoids content was 658.52 and 168.26 μg quercetin equivalent (QE)/g in AH and AL flowers, respectively. Particularly, phenols are synthesized in the plants in response to abiotic stress and injuries by insects or mammals. In a study performed by Li et al. [31], they reported the phenolic content of 51 flowers in the range of 34.14–1.11 mg GAE/g. The results of the present study are well within the range of phenolic content reported by previous authors. Many factors influence the phenolic content, which include, mainly, the botanical source and extraction methods [32]. From the results, it could be easily inferred that AH flowers exhibit higher antioxidant potential than AL flowers.
The total carotenoids content was found to be higher in the AH flower (27.29 μg/g) compared with the AL flower (16.96 μg/g). Carotenoids are metabolized in the liver into vitamin A, which helps protect the body from damage caused by oxidative stress and inflammation [33]. The TPC and TFC content of AL and AH flowers can vary depending on the solvent used for extraction and the origin of the products [34]. Indeed, the edible flower samples from China reported total phenols in terms of gallic acid (1177.8–27,717.2 μg/g), total flavonoids in terms of quercetin and hesperidin (49.9–14,576.6 μg/g), and quercetin and luteolin (8.8–480.0 μg/g) [35].
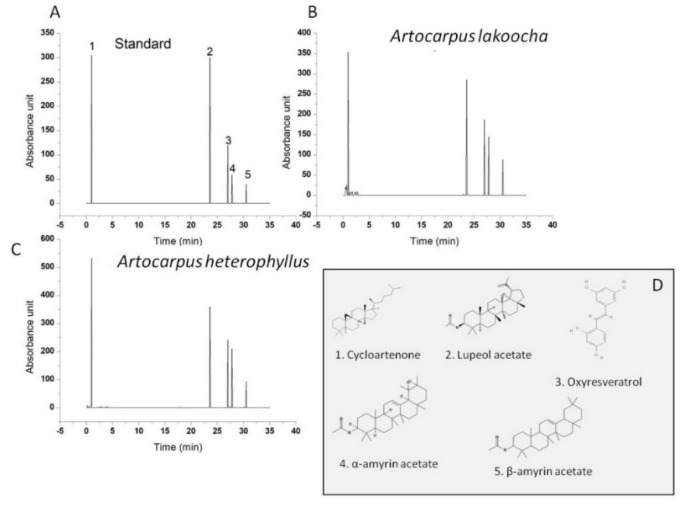
TPC and TFC analyses were performed on fresh extracts of the flower, while HPLC analysis was performed on a dry extract. Indeed, from previously reported studies, it was observed that oxyresveratrol, α and β amyrin acetate, lupeol acetate, and cycloartenone are major compounds found in different parts of the plant of both flowers, such as the stem, heartwood, pericarp, and bark [36]. The potential benefits of these compounds have been reviewed by several authors and include anti-hyperglycemic, hypolipidemic, antiatherosclerotic, anti-inflammatory, radical scavenging, and anti-hyperlipidemic activities [37,38,39]. In order to identify these compounds, aqueous extracts of both flowers were evaluated by HPLC analysis (Figure 2).
Figure 2.
(A) HPLC chromatogram of standards identified in Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam.: cycloartenone (1), lupeol acetate (2), oxyresveratrol (3), α-amyrin acetate (4), and β-amyrin acetate. (B) HPLC chromatogram of Artocarpus lakoocha flower extracts. (C) HPLC chromatogram of Artocarpus heterophyllus Lam. flower extracts. (D) The chemical structure of the main components.
2.2. Cytotoxicity Assay (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT))
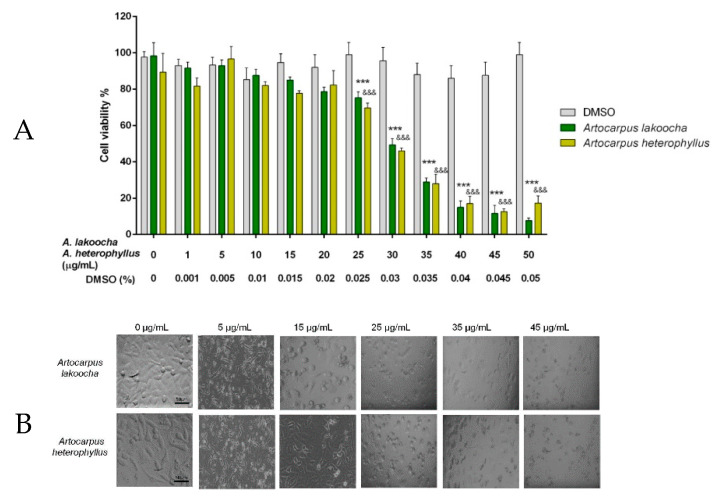
Cytotoxicity is a critical aspect to measure the therapeutic effect of a plant or any synthesized new material, which should produce effects only on the targeted cells without harming the host body. Thus, the biocompatibility of these flower extracts was evaluated in terms of the % viability of Caco-2 cells. Caco-2 cells were treated with the AL or AH flower extracts at different concentrations ranging from 0 to 50 μg/mL for 48 h. The results from the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Figure 3) showed that both extracts had some cytotoxic effect on Caco-2 cells in a concentration-dependent manner. The IC50 value of the AL and AH flower extracts was 28.43 and 29.37 μg/mL, respectively. All dosages above 35 μg/mL resulted in suffering and cell death. On the contrary, AL and AH flower extracts at concentrations below 20 μg/mL did not affect cell viability. DMSO (vehicle control) at all concentrations (0–0.05%) relevant to the treatment group showed no apparent cytotoxicity to Caco-2 cells. Since, in terms of cell viability, the floral extracts of AL and AH in ethanol, methanol, and water highlighted the same results (data not shown), only the results obtained from the extracts in aqueous solution are shown in Figure 3.
Figure 3.
Effects of flower aqueous extracts of Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. on Caco-2 cell viability. (A) The bars indicate percent cell viability of cells treated with one of the two floral extracts with the indicated concentrations (ranging from 0 to 50 μg/mL) for 48 h and subjected to a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data are representative of three replicates and shown as mean ± standard deviation; *** p < 0.001 vs. Artocarpus lakoocha Roxb. untreated group; &&& p < 0.001 vs. Artocarpus heterophyllus Lam. untreated group. (B) Effects on Caco-2 cell cytotoxicity after 48 h treatment with aqueous solution extracts of Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Only concentrations of 0, 5, 15, 25, 35, and 45 mg/mL are shown in the image.
2.3. Antioxidant Properties
There are many factors that influence the antioxidant potential of plant extracts, including the extraction method, the composition of the extract, and the procedure used to determine that [40]. There is no precise or standard extraction method, even for estimation of antioxidant potential. Hence, it is mandatory to evaluate the antioxidant potential by taking into account more than a single analytical method. Because the mechanism of antioxidant action differs by method, in the present study, the antioxidant potential of both flowers was measured by three different methods on the basis of free radical scavenging and ferric-reducing activity, which are 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP), and N, N-dimethyl-p-phenylenediamine (DMPD). Furthermore, the antioxidant action of the flower extracts was evaluated on the mRNA expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), heme oxygenase-1 (HO-1), and NAD(P)H quinone reductase (NQO1).
2.3.1. DPPH Free-Radical-Scavenging Activity
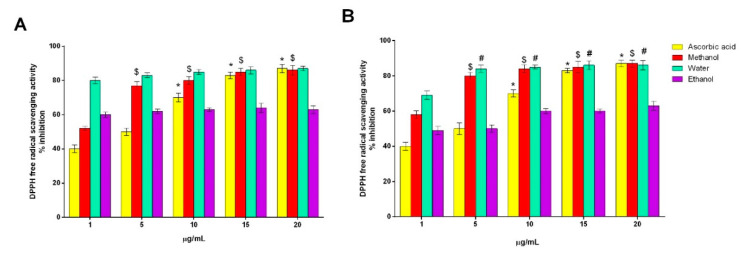
DPPH is a violet-colored dye that is scavenged by antioxidative compounds, and a color change is absorbed, which can be quantified spectroscopically at a wavelength of 515–528 nm. It is a widely used method for the quantification of free-radical-scavenging activity. However, this method is sensitive to light, oxygen, pH, and type of solvent used [5]. The DPPH scavenging activity of AH flower extracts and AL extracts in the concentration range of 1–20 µg/mL is presented in Figure 4A,B, respectively. All the assessed extracts were able to reduce the stable, purple-colored DPPH radical reaching 50% of reduction, except the ascorbic acid extract of both the samples at 1 µg/mL and the ethanolic extract of the AL extract at 1 µg/mL. A general trend was observed where increasing the concentration increased the scavenging activity in both the samples for all the solvents, except that for ethanolic extracts in AH flower extracts, which remained almost equal even after increasing the concentration. This antioxidative property can be attributed to several bioactive compounds present in the plants, such as carotenoids as well as phenolic and flavonoid compounds. From the above results, the aqueous and methanolic extracts at higher concentrations of both the samples proved their efficiency as an antioxidant. Similar results were observed in the aqueous and methanolic extracts of the fruits of AH and AL [5].
Figure 4.
2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical-scavenging activity: (A) Artocarpus heterophyllus Lam. flower extracts, (B) Artocarpus lakoocha Roxb. flower extracts. All measurements were performed in triplicate. Data are shown as the mean ± standard deviation, and one-way ANOVA with a Newman–Keuls post-test was used for statistical significance; * p < 0.05 vs. 1 µg/mL ascorbic acid; $ p < 0.05 vs. 1 µg/mL methanol extract; # p < 0.05 vs. 1 µg/mL water extract.
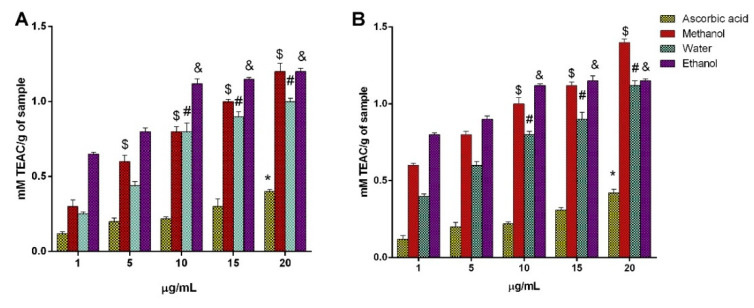
2.3.2. FRAP Assay
The FRAP assay, which is one of the simplest, rapidest, and most inexpensive tests, is very useful for routine analysis and is used to directly test the total antioxidant power of a sample [5]. It is based on the ferrous-to-ferric reduction potential and is considered to be an indicator of electron-donating activity [41]. The antioxidant activities of the AH and AL flower extracts using the FRAP assay in the concentration range of 1–20 mg/mL are shown in Figure 5A,B, respectively. The ferric-reducing power of both extracts increased as the concentration increased. The AH flower extract showed the highest ability to reduce Fe3+ to Fe2+ at a value of 1.4 mM Trolox equivalent (TEAC)/g for the methanolic extract at 20 µg/mL, and ethanolic extract of AL at 20 µg mL−1 reduced 1.4 mM TEAC/g. This revealed that a methanol soluble factor was most likely responsible for reducing the potential of the extract in the case of AH flower extracts and an ethanol soluble factor in that of AL. In general, AH flower extracts showed the maximum FRAP activity compared with AL flower extracts and reached 1.4 mM TEAC activity at the concentration of 20 µg/mL, while the other showed 1.2 mM TEAC activity. However, there was not much difference in both flowers in terms of activity at maximum concentration. This can be significantly correlated to the TPC and TFC content of both flowers. The antioxidant potential of plant products is significantly influenced by the amount of phenolic compounds and the carotenoids content. Indeed, dark-colored flowers exhibit stronger antioxidant potential than light-colored flowers due to the variation in the level of flavonoids [42]. From the present results, the carotenoids’ level significantly contributed to the antioxidant potential along with the phenolic and flavonoids content.
Figure 5.
Ferric-reducing antioxidant power (FRAP) assay: (A) Artocarpus heterophyllus Lam. flower extracts, (B) Artocarpus lakoocha Roxb. flower extracts. All measurements were performed in triplicate. Data are shown as the mean ± standard deviation, and one-way ANOVA with a Newman–Keuls post-test was used for statistical significance; * p < 0.05 vs. 1 µg/mL ascorbic acid; $ p < 0.05 vs. 1 µg/mL methanol extract; # p < 0.05 vs. 1 µg/mL water extract; & p < 0.05 vs. 1 µg/mL ethanol extract.
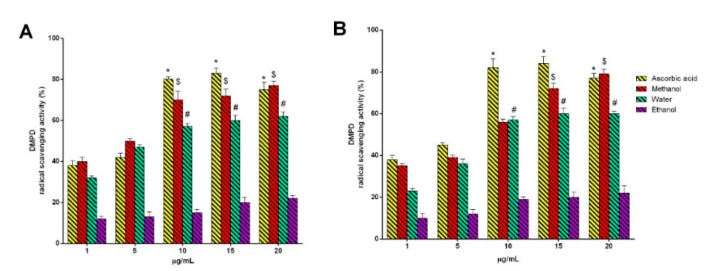
2.3.3. DMPD Radical Cation Decolorization Assay
Jagtap et al. [5] stated that the DMPD assay has some advantages due to the high stability of the endpoint, the quick reaction time, and it being cost effective and less cumbersome. In the presence of an oxidant solution (ferric chloride) at an acidic pH, DMPD is converted to a stable and colored DMPD radical cation (DMPD·+, absorption maxima 505 nm). The data in Figure 6A,B show the antioxidant activity (% RSA) of the AH and AL flower extracts in a concentration range of 1–20 µg/mL. The positive control, consisting of ascorbic acid, confirmed its antioxidant activity. Indeed, the results indicate that the antioxidative nature is dose dependent and is highest for the ascorbic acid extract in both the samples at 20 µg/mL. These data confirm the documented antioxidant action of ascorbic acid [43,44,45,46]. Ascorbic acid works as an antioxidant with many substrates such as reactive oxygen species, and its dose dependent activity has been demonstrated on many products of plant origin [43,45,47].
Figure 6.
DMPD (N, N-dimethyl-p-phenylenediamine) radical cation decolorization assay: (A) Artocarpus heterophyllus Lam. flower extracts, (B) Artocarpus lakoocha Roxb. flower extracts. All measurements were performed in triplicate. Data are shown as the mean ± standard deviation, and one-way ANOVA with a Newman–Keuls post-test was used for statistical significance; * p < 0.05 vs. 1 µg/mL ascorbic acid; $ p < 0.05 vs. 1 µg/mL methanol extract; # p < 0.05 vs. 1 µg/mL water extract.
From the results, it was revealed that DMPD activity was significantly affected by the method of extract, solvent type, and time of extraction. A similar observation was reported by Tai et al. [48].
2.3.4. HO-1, Nrf2, and NQO1 Gene Expression
Since in the enzyme systems described above, the two extracts showed some antioxidant activity, the aqueous extracts of AH and AL were used to evaluate the mRNA expression of Nrf2, HO-1 and NQO1 (Figure 7). In general, in both extracts, the dosages 15 and 20 µg/mL induce the mRNA expression of the three target genes compared to the control. In addition, both the extracts of AH and AL flowers increase the expression of HO-1 compared to the control starting from 10 µg/mL. Furthermore, it is interesting to note how the AL extracts increase the expression of Nrf2 compared to the control already at 5 µg/mL. Finally, only the AL extract increases the expression of NQO1 compared to the control at all dosages (1–20 µg/mL). Many authors have abundantly described the involvement of Nrf2, HO-1 and NQO1 in the processes that regulate the antioxidant response [49,50,51]. Nrf2 is a transcription factor that regulates the gene expression of many antioxidant cytoprotective and detoxifying enzymes through a promoter sequence known as the antioxidant response element (ARE) [52]. ARE is a promoter element found in many cytoprotective genes, and, therefore, Nrf2 plays a fundamental role in the cellular defense system against ARE-dependent environmental stresses as free radicals and/or reactive oxygen species (ROS) [52]. HO-1 and NQO1 are enzymes regulated by Nrf2/ARE signaling [53]. HO-1 has important antioxidant, anti-inflammatory, anti-apoptotic effects, and also appears to have an antiatherogenic action [54]. Indeed, HO-1 has been shown to be a critical gene in cellular protection and response against pro-oxidative stimuli such as ROS oxidized phospholipids [54]. At the same time, one of the major quinone reductases in mammals is NQO1. It plays multiple roles in cellular homeostasis to oxidative stress [55]. Established roles of NQO1 include the ability to enzymatically protect cellular quinones from free radical electron attacks in redox cycles. In particular, NQO1 would function as a component of a plasma membrane redox system generating antioxidant forms of ubiquinone, vitamin E, and superoxide reductase [55]. In Figure 7, the up-regulation of Nrf2, HO-1 and NQO1 mRNA confirm a direct action of the extracts of both flowers, at dosages higher than 15 μg/mL, towards the molecular signaling of cellular defense against oxidative stress also observed in other cell models and other plant extracts [49,53].
Figure 7.
mRNA expression in Caco-2 cells of heme oxygenase-1 (HO-1) (A and B), nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (C and D), and NAD(P)H quinone reductase (NQO1) (E and F) after treatment with flower extracts of Artocarpus heterophyllus Lam. (left) and Artocarpus lakoocha Roxb. (right), 0–20 µg/mL. Data are expressed as fold changes of the target gene (Nrf2 in A, HO-1 in B, and NQO1 in C) normalized to the internal standard control gene (β-actin). All measurements were performed in triplicate. Data are shown as the mean ± standard deviation, and one-way ANOVA with a Newman–Keuls post-test was used for statistical significance; ** p < 0.005; *** p < 0.0001 vs. 0 µg/mL.
2.4. Antimicrobial Properties
AH and AL flower extracts were evaluated against six food pathogenic bacterial strains. Table 2 presents the diameter of zone inhibition, where it was observed that both flower extracts exhibited strong antibacterial activities against all bacterial strains at the concentration of 200 μg/mL of flower extract. All the pathogenic strains were found to susceptible to AL and AH flower extracts with various zones of inhibition. However, both extracts showed strong antibacterial activity against Salmonella typhimurium at the concentration of 200 μg/mL, with a zone inhibition of 24 mm. The antibacterial action of the genus Artocarpus has also been observed in strains of periodontal bacteria that form biofilms as reported by Teanpaisan et al. [56]. Despite the antibacterial potential observed in these data, further in vivo studies are needed to confirm these results. In the case of Bacillus cereus, AH flower extracts were not found to be effective at both concentrations. The positive control (streptomycin) significantly affected the microbial growth.
Table 2.
Inhibition zones (mm) for common foodborne pathogens determined at 37 °C after 24 h of incubation.
| Artocarpus lakoocha Roxb. | Artocarpus heterophyllus Lam. | Streptomycin | ||||
|---|---|---|---|---|---|---|
| 100 μg/mL | 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | 200 μg/mL | |
| Staphylococcus aureus | 8 | 18 | 15 | 21 | 12 | 26 |
| Listeria monocytogenes | 11 | 19 | 12 | 19 | 13 | 23 |
| Salmonella typhimurium | 13 | 24 | 13 | 24 | 17 | 31 |
| Bacillus cereus | 7 | 12 | - | - | 10 | 18 |
| Escherichia coli MTCC 1568 | 12 | 22 | 15 | 22 | 15 | 26 |
| Bacillus subtilis ATCC 6633 | 6.5 | 12 | 5 | 10 | 8 | 15 |
Note: (-) = no activity observed.
3. Materials and Methods
3.1. Flowers and Chemicals
Matured male flowers of Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. were collected from the horticulture department of Tezpur University, Assam, India. All the reagents used for analysis were of analytical grade and procured from Merck-Sigma, India and Hymenia, Mumbai, India. Flowers were randomly harvested in November 2019 in the morning and divided into three equal batches, representing the replicates.
3.2. Preparation of Flower Extracts
In order to evaluate the biological effects of the flowers extracts of the two plants, extractions with three solvents—ethanol, methanol, and water—were performed. The flowers of AL and AH were air-dried in a shed at room temperature (26 °C) for 3 weeks, after which they were ground into a uniform powder. Three solvents (methanol, absolute ethanol, or water) were separately added to the powder in a ratio of 1:10 (powder:solvent). The maceration of the extracts was carried out at room temperature for 72 h in an incubator shaker. The solution was filtered using Whatman filter paper (0.42 μm) and stored in glass vials at −5 °C.
3.3. Mineral Composition
The mineral content was evaluated on the fresh extracts of the flowers of both plants. An atomic absorption spectrophotometric method was employed to evaluate the mineral composition in the fresh flower samples, where 0.5 g of powdered sample (tray dried at 40 °C for 4 h) was wet-digested using nitric acid and perchloric acid at 325 °C in an autodigester (KES 12L-VA, Pelican Equipment, Chennai, India). The digested sample was diluted and aspirated into the spectrophotometer. The amount of minerals present in the flower sample was estimated by atomic absorption spectrophotometry (Model-Ice 3500, Thermo Fischer Scientific, Mumbai, India). Flame photometry and the spectrophotometric method were used to measure the potassium and phosphorus content of the flower, respectively. A calibration curve for each mineral was prepared using the standard mineral procured from Merck-Sigma, Bangalore, India.
3.4. Spectrophotometric Analysis of Phytochemical Properties
The content of phenols, flavonoids, and carotenoids was determined on the fresh extract of the flowers of both plants. Total carotenoids in the flowers were estimated as per the method of Lee [17], where fresh sample (5 g) was mixed with 50 mL of a mixture of n-hexane/acetone/ethanol (v/v; 50:25:25) and placed in an incubator shaker (Excella E24, Eppendorf, Mumbai, India) at 25 °C for 10 min at 200 rpm. The mixture was centrifuged (Eppendorf 5430R, Hamburg, Germany) to separate the sediment and supernatant at 6500 rpm at 4 °C for 10 min. Then, the supernatant was carefully collected and made up to 50 mL with the solvent used for extraction. The absorbance of colored solution was measured at 450 nm using a precalibrated UV–Vis spectrophotometer (Cary 60, Agilent UNICO Products and Instruments Inc., Shanghai, China). The results were expressed in terms of the β-carotene equivalent.
The Folin–Ciocalteu assay was used for the determination of total phenolics present in the flower extracts and expressed in terms of gallic acid equivalent (GAE). The total flavonoids content of the flower extracts was measured and expressed in terms of quercetin equivalent (QE).
3.5. Cytotoxicity Study
The effects of the three plant extracts on cell viability were evaluated on human epithelial colorectal adenocarcinoma cells (Caco-2 cells). Caco-2 cells are a valid system for evaluating the biological effects of plant extracts for human use [57,58,59]. Caco-2 cells were obtained from the ATCC cell bank (Rockville, MD, USA) and were propagated in DMEM (Gibco BRL, Life Technologies, Mumbai, India) supplemented with 10% fetal calf serum, 1% sodium pyruvate, 1% L-glutamine solution, and 1% streptomycin/penicillin in a humidified atmosphere of 5% CO2 at 37 °C.
The effect of Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. flower extracts on cell viability was assayed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to obtain the range of toxic and nontoxic concentrations. Caco-2 cells were seeded in 96-well plates at a density of 5 × 104 cells per well for 24 h in complete medium. Cells were then treated with flower extracts at various concentrations (1–50 μg/mL) or with vehicle (DMSO at 0.001–0.05%) for 48 h; then, the cells were exposed to the MTT reagent (0.4 mg/mL in PBS) for 30 min at 37 °C, 5% CO2. The absorbance at 570 nm was measured using the microplate reader MPR A4i (Tosoh, Tokyo, Japan).
3.6. Antioxidant Potential
Based on the results obtained with the MTT test, the less toxic dosages (1–20 μg/mL) were used to evaluate the antioxidant power of the different extracts.
3.6.1. DPPH Free-Radical-Scavenging Activity
The total antioxidant properties of both flowers were estimated in terms of DPPH free radical scavenging following the method of Blois [20] with certain modifications. Different concentrations (1–15 µg/mL) of extracts were prepared in different solvents, such as acetone, aqueous, and methanol, and the extracts (0.5 mL) were mixed with 3 mL of DPPH solution in methanol (0.1 mM). The prepared mixture was allowed to react in the dark at 37 °C for 30 min, and absorbance was measured at 517 nm. Ascorbic acid served as a positive control.
| (1) |
where Ac = absorbance of control and As = absorbance of sample.
3.6.2. Ferric-Reducing Antioxidant Power (FRAP)
Flower extracts of various concentrations (1–20 µg/mL) were prepared in different solvents for the estimation of FRAP [21]. The flower extract was mixed with FRAP reagent (2.7 mL) and made up to 3 mL with distilled water. The mixture was allowed to react in the dark for 30 min at 37 °C. The absorbance of the colored mixture was recorded at 593 nm, and the results are expressed in terms of mM Trolox equivalent (TEAC)/g of the sample.
3.6.3. DMPD (N, N-Dimethyl-p-Phenylenediamine) Radical Cation Decolorization Assay
The DMPD assay was performed for the flower extracts (1–20 µg/mL), where the first DMPD solution (0.1 M) was prepared in distilled water. Then, the prepared solution (1 mL) was mixed with acetate buffer (100 mL) of 0.1 M concentration (pH 5 ± 0.2), followed by addition of ferric chloride solution (0.2 mL, 0.05 M), which resulted in the formation of purple cation radical (DMPD), and absorbance was measured at 505 nm. In a test tube, 1 μL of DMPD solution was mixed with 50 μL of extract, which was mixed uniformly at 25 °C for 10 min. The absorbance of the solution was measured at 505 nm, and the antioxidative potential was measured in a manner similar to the DPPH assay.
3.6.4. Expression of Antioxidant Genes
In order to evaluate the antioxidant effects of the AH and AL flower extracts in vitro, Caco-2 cells were treated for 48 h with extract in aqueous solution (1–20 µg/mL). The dose with the maximum effect observed in the antioxidant reactions described above was chosen. Total RNA was then extracted from 5 × 106 cells following a standard protocol [51,60,61,62,63]. RNA was quantified spectrophotometrically and 2 µg was back-transcribed using the SuperScript® VILO ™ cDNA synthesis kit (ThermoFisher, Mumbai, India). An aliquot (3.5 µL) of cDNA was then amplified by real-time PCR.
TaqMan® MGB (ThermoFisher) probes were used to evaluate the mRNA expression levels of genes involved in cellular oxidative processes. The probes were heme oxygenase-1 (HO-1) (Hs01110250_m1), nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (Hs00975961_g1), and NAD(P)H quinone reductase (NQO1) (Hs01045994_m1). The beta-actin gene (Hs01060665_g1) was used for housekeeping. All samples were amplified in triplicate in 96-well optical plates (ThermoFisher) by the ABI Prism 7000 Sequence Detection instrument (Applied Biosystems) following the instrument protocol. Quantification was performed using the comparative method C (T), also called method 2 (−ΔΔCT).
3.7. Antimicrobial Activity
The antibacterial activities of the flowers were measured against some food pathogenic strains (Staphylococcus aureus, Listeria monocytogenes, Salmonella typhimurium, Bacillus cereus, Escherichia coli (MTCC 1568), and Bacillus subtilis (ATCC 6633)). All bacteria were grown on blood agar plates. They were carried out in a plate assay as per the procedure outlined by Kiran et al. [64]. The swabbed plates were punched to create wells of 9 mm diameter, and the prepared flower extract (250 μL of different concentrations) was loaded in the well. Streptomycin was used as a positive control against food pathogens. The loaded plates were incubated for 24 h at 37 °C, and the zone of growth inhibition was used as the function of antimicrobial activity. The bacterial growth inhibition zone is expressed in millimeters.
3.8. HPLC Conditions
Some active components of the extract in aqueous solution were identified by HPLC. Oxyresveratrol, α and β-amyrin acetate, cycloartenone, and lupeol acetate were identified following procedures previously reported [65]. In particular, the main components of the AL and AH fresh flower extracts were separated by a Zorbax 300SB 4.6 × 150 mm C18 column, 5 μm, (Agilent, Palo Alto, CA, USA) with the Agilent 1200 system. The flower extracts were diluted with methanol at a ratio of 1:2, followed by filtration using a 0.45 μm PTFE membrane, and the filtered solution (10 μL) was injected. The mobile phase consisted of acetonitrile (A):acetic acid 0.1% (B) (v/v), using a gradient elution of 18–25% A at 0–10 min and 25–40% A at 10–25 min, and was pumped at a flow rate of 1.0 mL/min. This was followed by a 10 min equilibration period with initial conditions prior to injection of the next sample. The simultaneous detection of the active constituents was set at a wavelength of 200–400 nm. The standards were prepared following the same procedures for the samples.
3.9. Statistical Analysis
All experiments were performed in triplicate, all data are presented as the mean ± standard deviation, and a value of p ≤ 0.05 is considered statistically significant. The data were analyzed by two-way repeated measure ANOVA followed by a Holm–Sidak’s multiple test for the comparison of individual means. Gene expression data were analyzed by ordinary one-way ANOVA followed by a Holm–Sidak’s test. All the statistical analyses were performed using GraphPad Prism version 6.01 (GraphPad Software, San Diego, CA, USA).
4. Conclusions
AL and AH are plants with multiple uses in Asia. In particular, fruits are the most consumed and studied products to date. Few works have been focused on the characterization of the biological activity of phyto-extracts derived from AH and AL flowers. Here, the data collected highlight how both flowers are rich in mineral salts and antioxidant properties. In particular, iron, calcium and potassium predominate in AH, and copper, zinc, manganese and magnesium in AL. Given the difficulties in assimilating iron and calcium at the same time in the diet, these differences further enhance the plant extracts of AL. On the other hand, AH phytoextracts show a greater antioxidant component in the fresh extracts. These differences in content are not observed in the antioxidant enzymatic activity. Indeed, the phytoextracts of AH and AL flowers showed a strong antioxidant activity with the same order of magnitude. Furthermore, both phytoextracts increased the expression of both Nrf2 (promoter gene) and HO-1 and NQO1 (Nrf2-regulated genes). Therefore, the extracts of AH and AL flowers would actively modulate the genes involved in the expression of the redox enzymatic pathways. Finally, the antibacterial aspects of AH and AL phytoextracts cannot be excluded. These qualities need to be explored in further studies, but they indicate important potential in the microbiological field.
In conclusion, this study allowed us to evaluate the nutritional, mineral, and antioxidant potential of AH and AL flowers. Furthermore, a preliminary antimicrobial evaluation was carried out on the extracts of both flowers, which was necessary to investigate potential future applications in the microbiological field. In Asia, the fruits of AH and AL are best known for their nutritional and healthy virtues. This study showed that flowers also have important potential for human health. Furthermore, this study focused its attention only on the male flowers of both dioecious plants. Preliminary data collected on phyto-extracts of female flowers show a difference in the present chemical compounds and in biological activity.
Further studies are needed to exploit, in a sustainable way, the virtues of flowers derived from AH and AL as a supplement.
Author Contributions
Conceptualization, A.K.G. and M.A.R.; methodology, A.K.J.; software, A.K.G. and M.A.R.; validation, A.S., S.S. and M.S.; formal analysis, U.P.; investigation, D.S.; resources, A.M.; data curation, A.K.G.; writing—Original draft preparation, A.K.G.; writing—Review and editing, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Burlando B., Clericuzio M., Cornara L. Moraceae Plants with Tyrosinase Inhibitory Activity: A Review. Mini Rev. Med. Chem. 2017;17:108–121. doi: 10.2174/1389557516666160609071854. [DOI] [PubMed] [Google Scholar]
- 2.Arung E.T., Shimizu K., Kondo R. Artocarpus plants as a potential source of skin whitening agents. Nat. Prod. Commun. 2011;6:1397–1402. doi: 10.1177/1934578X1100600943. [DOI] [PubMed] [Google Scholar]
- 3.Jagtap U.B., Bapat V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010;129:142–166. doi: 10.1016/j.jep.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Tu Z.C., Xie X., Wang H., Wang H., Wang Z.X., Sha X.M., Lu Y. Jackfruit (Artocarpus heterophyllus Lam.) peel: A better source of antioxidants and a-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem. 2017;234:303–313. doi: 10.1016/j.foodchem.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Jagtap U.B., Panaskar S.N., Bapat V.A. Evaluation of antioxidant capacity and phenol content in jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Foods Hum. Nutr. 2010;65:99–104. doi: 10.1007/s11130-010-0155-7. [DOI] [PubMed] [Google Scholar]
- 6.Li J., Lin Z., Tang X., Liu G., Chen Y., Zhai X., Huang Q., Cao Y. Oxyresveratrol extracted from Artocarpus heterophyllus Lam. inhibits tyrosinase and age pigments in vitro and in vivo. Food Funct. 2020;11:6595–6607. doi: 10.1039/D0FO01193B. [DOI] [PubMed] [Google Scholar]
- 7.Riga R., Happyana N., Holisotan Hakim E. Sesquiterpenes produced by Pestalotiopsis microspora HF 12440 isolated from Artocarpus heterophyllus. Nat. Prod. Res. 2020;34:2229–2231. doi: 10.1080/14786419.2019.1578764. [DOI] [PubMed] [Google Scholar]
- 8.Ajiboye B.O., Ojo O.A., Oyinloye B.E., Okesola M.A., Oluwatosin A., Boligon A.A., Kappo A.P. Investigation of the In Vitro Antioxidant Potential Of Polyphenolic-Rich Extract of Artocarpus heterophyllus Lam Stem Bark and Its Antidiabetic Activity In Streptozotocin-Induced Diabetic Rats. J. Evid. Based Integr. Med. 2020;25:2515690X20916123. doi: 10.1177/2515690X20916123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Septama A.W., Jantan I., Panichayupakaranant P. Flavonoids of Artocarpus heterophyllus Lam. heartwood inhibit the innate immune responses of human phagocytes. J. Pharm. Pharmacol. 2018;70:1242–1252. doi: 10.1111/jphp.12952. [DOI] [PubMed] [Google Scholar]
- 10.Di X., Wang S., Wang B., Liu Y., Yuan H., Lou H., Wang X. New phenolic compounds from the twigs of Artocarpus heterophyllus. Drug Discov. Ther. 2013;7:24–28. doi: 10.5582/ddt.2013.v7.1.24. [DOI] [PubMed] [Google Scholar]
- 11.Hettiaratchi U.P., Ekanayake S., Welihinda J. Nutritional assessment of a jackfruit (Artocarpus heterophyllus) meal. Ceylon Med. J. 2011;56:54–58. doi: 10.4038/cmj.v56i2.3109. [DOI] [PubMed] [Google Scholar]
- 12.Ajayi I.A. Comparative study of the chemical composition and mineral element content of Artocarpus heterophyllus and Treculia africana seeds and seed oils. Bioresour. Technol. 2008;99:5125–5129. doi: 10.1016/j.biortech.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Shyamalamma S., Chandra S.B., Hegde M., Naryanswamy P. Evaluation of genetic diversity in jackfruit (Artocarpus heterophyllus Lam.) based on amplified fragment length polymorphism markers. Genet. Mol. Res. GMR. 2008;7:645–656. doi: 10.4238/vol7-3gmr457. [DOI] [PubMed] [Google Scholar]
- 14.Shailendra Kumar M.B., Rakesh Kumar M.C., Bharath A.C., Vinod Kumar H.R., Prashith Kekuda T.R., Nandini K.C., Rakshitha M.N., Raghavendra H.L. Screening of selected biological activities of artocarpus lakoocha roxb (moraceae) fruit pericarp. J. Basic Clin. Pharm. 2010;1:239–245. [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey A., Bhatnagar S.P. Preliminary Phytochemical screening and antimicrobial studies on Artocarpus lakoocha Roxb. Anc. Sci. Life. 2009;28:21–24. [PMC free article] [PubMed] [Google Scholar]
- 16.Maneechai S., De-Eknamkul W., Umehara K., Noguchi H., Likhitwitayawuid K. Flavonoid and stilbenoid production in callus cultures of Artocarpus lakoocha. Phytochemistry. 2012;81:42–49. doi: 10.1016/j.phytochem.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Boonyaketgoson S., Du Y., Valenciano Murillo A.L., Cassera M.B., Kingston D.G.I., Trisuwan K. Flavanones from the Twigs and Barks of Artocarpus lakoocha Having Antiplasmodial and Anti-TB Activities. Chem. Pharm. Bull. 2020;68:671–674. doi: 10.1248/cpb.c20-00080. [DOI] [PubMed] [Google Scholar]
- 18.Adewole S.O., Ojewole J.A. Artocarpus communis Forst. root-bark aqueous extract- and streptozotocin-induced ultrastructural and metabolic changes in hepatic tissues of Wistar rats. Afr. J. Tradit. Complementary Altern. Med. AJTCAM. 2007;4:397–410. [PMC free article] [PubMed] [Google Scholar]
- 19.Mamun S., Shaheen N., Basak T.A., Mohiduzzaman M., Banu C.P., Takano-Ishikawa Y. Hydrophilic antioxidant capacities and total phenol content of seasonal fruits of Bangladesh. Malays. J. Nutr. 2012;18:355–362. [PubMed] [Google Scholar]
- 20.Povichit N., Phrutivorapongkul A., Suttajit M., Chaiyasut C.C., Leelapornpisid P. Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pak. J. Pharm. Sci. 2010;23:403–408. [PubMed] [Google Scholar]
- 21.Maneechai S., Likhitwitayawuid K., Sritularak B., Palanuvej C., Ruangrungsi N., Sirisa-Ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and ‘Puag-Haad’. Med Princ. Pract. 2009;18:223–227. doi: 10.1159/000204354. [DOI] [PubMed] [Google Scholar]
- 22.Chuanasa T., Phromjai J., Lipipun V., Likhitwitayawuid K., Suzuki M., Pramyothin P., Hattori M., Shiraki K. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antivir. Res. 2008;80:62–70. doi: 10.1016/j.antiviral.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Sritularak B., Tantrakarnsakul K., Lipipun V., Likhitwitayawuid K. Flavonoids with anti-HSV activity from the root bark of Artocarpus lakoocha. Nat. Prod. Commun. 2013;8:1079–1080. doi: 10.1177/1934578X1300800811. [DOI] [PubMed] [Google Scholar]
- 24.Puntumchai A., Kittakoop P., Rajviroongit S., Vimuttipong S., Likhitwitayawuid K., Thebtaranonth Y. Lakoochins A and B, new antimycobacterial stilbene derivatives from Artocarpus lakoocha. J. Nat. Prod. 2004;67:485–486. doi: 10.1021/np030429e. [DOI] [PubMed] [Google Scholar]
- 25.Likhitwitayawuid K., Sritularak B., Benchanak K., Lipipun V., Mathew J., Schinazi R.F. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat. Prod. Res. 2005;19:177–182. doi: 10.1080/14786410410001704813. [DOI] [PubMed] [Google Scholar]
- 26.Saowakon N., Tansatit T., Wanichanon C., Chanakul W., Reutrakul V., Sobhon P. Fasciola gigantica: Anthelmintic effect of the aqueous extract of Artocarpus lakoocha. Exp. Parasitol. 2009;122:289–298. doi: 10.1016/j.exppara.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Wongon M., Limpeanchob N. Inhibitory effect of Artocarpus lakoocha Roxb and oxyresveratrol on alpha-glucosidase and sugar digestion in Caco-2 cells. Heliyon. 2020;6:e03458. doi: 10.1016/j.heliyon.2020.e03458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aneklaphakij C., Bunsupa S., Sirichamorn Y., Bongcheewin B., Satitpatipan V. Taxonomic Notes on the ’Mahat’ (Artocarpus lacucha and A. thailandicus, Moraceae) Species Complex in Thailand. Plants. 2020;9:391. doi: 10.3390/plants9030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevenson P.C., Nicolson S.W., Wright G.A., Manson J. Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Funct. Ecol. 2016;31:65–75. doi: 10.1111/1365-2435.12761. [DOI] [Google Scholar]
- 30.Esteban R., Balaguer L., Manrique E., Rubio de Casas R., Ochoa R., Fleck I., Pintó-Marijuan M., Casals I., Morales D., Jiménez M.S., et al. Alternative methods for sampling and preservation of photosynthetic pigments and tocopherols in plant material from remote locations. Photosynth. Res. 2009;101:77–88. doi: 10.1007/s11120-009-9468-5. [DOI] [PubMed] [Google Scholar]
- 31.Li A.-N., Li S., Li H.-B., Xu D.-P., Xu X.-R., Chen F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods. 2014;6:319–330. doi: 10.1016/j.jff.2013.10.022. [DOI] [Google Scholar]
- 32.González-Barrio R., Periago M.J., Luna-Recio C., Garcia-Alonso F.J., Navarro-González I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018;252:373–380. doi: 10.1016/j.foodchem.2018.01.102. [DOI] [PubMed] [Google Scholar]
- 33.Elvira-Torales L.I., García-Alonso J., Periago-Castón M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants. 2019;8:229. doi: 10.3390/antiox8070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2018;9:449–454. doi: 10.1016/j.jtusci.2014.11.001. [DOI] [Google Scholar]
- 35.Zheng J., Meenu M., Xu B. A systematic investigation on free phenolic acids and flavonoids profiles of commonly consumed edible flowers in China. J. Pharm. Biomed. Anal. 2019;172:268–277. doi: 10.1016/j.jpba.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Su B.-N., Cuendet M., Hawthorne M.E., Kardono L.B.S., Riswan S., Fong H.H.S., Mehta R.G., Pezzuto J.M., Kinghorn A.D. Constituents of the Bark and Twigs ofArtocarpusdadahwith Cyclooxygenase Inhibitory Activity. J. Nat. Prod. 2002;65:163–169. doi: 10.1021/np010451c. [DOI] [PubMed] [Google Scholar]
- 37.Akihisa T., Kojima N., Kikuchi T., Yasukawa K., Tokuda H., Masters E.T., Manosroi A., Manosroi J. Anti-Inflammatory and Chemopreventive Effects of Triterpene Cinnamates and Acetates from Shea Fat. J. Oleo Sci. 2010;59:273–280. doi: 10.5650/jos.59.273. [DOI] [PubMed] [Google Scholar]
- 38.Park J., Park J.H., Suh H.-J., Lee I.C., Koh J., Boo Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014;306:475–487. doi: 10.1007/s00403-014-1440-3. [DOI] [PubMed] [Google Scholar]
- 39.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frankel E.N., Meyer A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000;80:1925–1941. doi: 10.1002/1097-0010(200010)80:13<1925::AID-JSFA714>3.0.CO;2-4. [DOI] [Google Scholar]
- 41.Khaled-Khodja N., Boulekbache-Makhlouf L., Madani K. Phytochemical screening of antioxidant and antibacterial activities of methanolic extracts of some Lamiaceae. Ind. Crop. Prod. 2014;61:41–48. doi: 10.1016/j.indcrop.2014.06.037. [DOI] [Google Scholar]
- 42.Vukics V., Kery A., Guttman A. Analysis of Polar Antioxidants in Heartsease (Viola tricolor L.) and Garden Pansy (Viola x wittrockiana Gams.) J. Chromatogr. Sci. 2008;46:823–827. doi: 10.1093/chromsci/46.9.823. [DOI] [PubMed] [Google Scholar]
- 43.Njus D., Kelley P.M., Tu Y.J., Schlegel H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020;159:37–43. doi: 10.1016/j.freeradbiomed.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Kowalczyk D., Kazimierczak W., Zieba E., Mezynska M., Basiura-Cembala M., Lisiecki S., Karas M., Baraniak B. Ascorbic acid- and sodium ascorbate-loaded oxidized potato starch films: Comparative evaluation of physicochemical and antioxidant properties. Carbohydr. Polym. 2018;181:317–326. doi: 10.1016/j.carbpol.2017.10.063. [DOI] [PubMed] [Google Scholar]
- 45.Sogi D.S., Siddiq M., Roidoung S., Dolan K.D. Total phenolics, carotenoids, ascorbic acid, and antioxidant properties of fresh-cut mango (Mangifera indica L., cv. Tommy Atkin) as affected by infrared heat treatment. J. Food Sci. 2012;77:C1197–C1202. doi: 10.1111/j.1750-3841.2012.02933.x. [DOI] [PubMed] [Google Scholar]
- 46.Leonard S.S., Cutler D., Ding M., Vallyathan V., Castranova V., Shi X. Antioxidant properties of fruit and vegetable juices: More to the story than ascorbic acid. Ann. Clin. Lab. Sci. 2002;32:193–200. [PubMed] [Google Scholar]
- 47.Zadra M., Piana M., Brum T., Boligon A., Freitas R., Machado M., Stefanello S., Soares F., Athayde M. Antioxidant Activity and Phytochemical Composition of the Leaves of Solanum guaraniticum A. St.-Hil. Molecules. 2012;17:12560–12574. doi: 10.3390/molecules171112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai Z., Cai L., Dai L., Dong L., Wang M., Yang Y., Cao Q., Ding Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011;126:1648–1654. doi: 10.1016/j.foodchem.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 49.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mastinu A., Bonini S.A., Rungratanawanich W., Aria F., Marziano M., Maccarinelli G., Abate G., Premoli M., Memo M., Uberti D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients. 2019;11:728. doi: 10.3390/nu11040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhattacharjee S., Dashwood R.H. Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals. Antioxidants. 2020;9:865. doi: 10.3390/antiox9090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Dong H., Song E., Xu X., Liu L., Song Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014;209:56–67. doi: 10.1016/j.cbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Araujo J.A., Zhang M., Yin F. Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Front. Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross D., Siegel D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017;8:595. doi: 10.3389/fphys.2017.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teanpaisan R., Senapong S., Puripattanavong J. In vitro Antimicrobial and Antibiofilm Activity of Artocarpus Lakoocha (Moraceae) Extract against Some Oral Pathogens. Trop. J. Pharm. Res. 2014;13:1149–1155. doi: 10.4314/tjpr.v13i7.20. [DOI] [Google Scholar]
- 57.Aherne S.A., Kerry J.P., O’Brien N.M. Effects of plant extracts on antioxidant status and oxidant-induced stress in Caco-2 cells. Br. J. Nutr. 2007;97:321–328. doi: 10.1017/S0007114507250469. [DOI] [PubMed] [Google Scholar]
- 58.Pintus G., Jiménez S., Gascón S., Luquin A., Laguna M., Ancin-Azpilicueta C., Rodríguez-Yoldi M.J. Rosa canina Extracts Have Antiproliferative and Antioxidant Effects on Caco-2 Human Colon Cancer. PLoS ONE. 2016;11:e0159136. doi: 10.1371/journal.pone.0159136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nowak A., Sójka M., Klewicka E., Lipińska L., Klewicki R., Kołodziejczyk K. Ellagitannins from Rubus idaeus L. Exert Geno- and Cytotoxic Effects against Human Colon Adenocarcinoma Cell Line Caco-2. J. Agric. Food Chem. 2017;65:2947–2955. doi: 10.1021/acs.jafc.6b05387. [DOI] [PubMed] [Google Scholar]
- 60.Bonini S.A., Mastinu A., Maccarinelli G., Mitola S., Premoli M., La Rosa L.R., Ferrari-Toninelli G., Grilli M., Memo M. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cereb. Cortex. 2016;26:2832–2849. doi: 10.1093/cercor/bhw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gianoncelli A., Bonini S.A., Bertuzzi M., Guarienti M., Vezzoli S., Kumar R., Delbarba A., Mastinu A., Sigala S., Spano P., et al. An Integrated Approach for a Structural and Functional Evaluation of Biosimilars: Implications for Erythropoietin. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2015;29:285–300. doi: 10.1007/s40259-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazzari P., Pau A., Tambaro S., Asproni B., Ruiu S., Pinna G., Mastinu A., Curzu M.M., Reali R., Emilio Heiner Bottazzi M., et al. Synthesis and Pharmacological Evaluation of Novel 4-Alkyl-5-thien-2’-yl Pyrazole Carboxamides. Central Nerv. Syst. Agents Med. Chem. 2012;12:254–276. doi: 10.2174/187152412803760636. [DOI] [PubMed] [Google Scholar]
- 63.Mastinu A., Premoli M., Ferrari-Toninelli G., Tambaro S., Maccarinelli G., Memo M., Bonini S.A. Cannabinoids in health and disease: Pharmacological potential in metabolic syndrome and neuroinflammation. Horm. Mol. Biol. Clin. Investig. 2018;36:20180013. doi: 10.1515/hmbci-2018-0013. [DOI] [PubMed] [Google Scholar]
- 64.Kiran G.S., Priyadharshini S., Anitha K., Gnanamani E., Selvin J. Characterization of an exopolysaccharide from probiont Enterobacter faecalis MSI12 and its effect on the disruption of Candida albicans biofilm. RSC Adv. 2015;5:71573–71585. doi: 10.1039/C5RA10302A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y.-F., Ching C., Wu T.-S., Wu C.-R., Hsieh W.-T., Tsai H.-Y. Balanophora spicataand Lupeol Acetate Possess Antinociceptive and Anti-Inflammatory ActivitiesIn VivoandIn Vitro. Evid. Based Complement. Altern. Med. 2012;2012:371273. doi: 10.1155/2012/371273. [DOI] [PMC free article] [PubMed] [Google Scholar]