Abstract
Supervised exercise dietary programs are recommended to relieve cancer-related fatigue and weight increase induced by adjuvant treatment of early breast cancer (EBC). As this recommendation lacks a high level of evidence, we designed a multicenter randomized trial to evaluate the impact of an Adapted Physical Activity Diet (APAD) education program on fatigue. We randomized 360 women with EBC who were receiving adjuvant chemotherapy and radiotherapy to APAD or usual care at eight French cancer institutions. Data were collected at baseline, end of chemotherapy, end of radiotherapy, and 6 months post-treatment. The primary endpoint was the general cancer-related fatigue score using the MFI-20 questionnaire. Fatigue correlated with the level of precariousness, but we found no significant difference between the two groups in terms of general fatigue (p = 0.274). The APAD arm has a smaller proportion of patients with confirmed depression at the end of follow-up (p = 0.052). A transient modification in physical activity levels and dietary intake was reported in the experimental arm. However, a mixed hospital- and home-based APAD education program is not enough to improve fatigue caused by adjuvant treatment of EBC. Cancer care centers should consider integrating more proactive diet–exercise supportive care in this population, focusing on precarious patients.
Keywords: breast cancer, exercise, diet, education, fatigue, weight, quality of life
1. Introduction
Cancer-related fatigue is the most distressing and common symptom reported by patients undergoing adjuvant therapy for breast cancer (BC) [1,2,3,4]. Fatigue provokes an increase in sedentary behaviors, modification of dietary intake, metabolic changes, fat mass increase, depression, and anxiety [5], and can alter cancer prognosis and treatment [6,7,8]. Exercise and nutrition programs are recommended by experts and medical societies to relieve cancer-related fatigue during active treatment and to prevent an increase in weight [9,10]. Exercise for cancer patients must include both moderate-intensity aerobic exercise and muscle-strengthening exercises [9], and be regular, frequent (at least 2 h per week), and progressive. Nutrition must aim to maintain a healthy weight, promote eating more plant-based foods, limit red and processed meat, energy-dense foods, salt, sugary drinks, and alcohol, and not rely on dietary supplements [11]. This is a particularly relevant effect in the clinical context of BC, as body mass index (BMI) before and after BC diagnosis, and weight gain after diagnosis were associated with increased mortality in recent meta-analyses [7,8]. Nutritional consultations help manage nutritional disorders that worsen fatigue, such as anemia, diarrhea, nausea, and vomiting [10,12].
The combination of exercise and dietary support has been reported to induce significant weight loss in survivors of early BC (EBC) after adjuvant chemotherapy/radiotherapy [13,14,15]. Four randomized controlled trials (RCTs) have assessed the combination of exercise and diet in EBC patients undergoing adjuvant chemotherapy and/or radiotherapy, but they were designed as pilot trials with less than 30 patients in each randomization group [16,17,18,19]. Therefore, the benefits of an exercise-diet intervention during adjuvant chemotherapy and radiotherapy need to be evaluated in a well-powered and multicenter RCT. We previously reported a monocentric RCT evaluating an adapted physical activity and diet (APAD) intervention during adjuvant treatment of EBC [18,20] and found a beneficial effect on patient-reported outcomes (PROs). However, these results need to be validated in larger, multicenter cohorts in order to evaluate the impact of the heterogeneity of organizations and sociocultural parameters on the results of the intervention.
As social deprivation has been reported to significantly impact cancer risk and program efficacy, this variable needs to be addressed in order to identify the impact of social inequalities on the performance of a given intervention. For example, vulnerable individuals identified using the validated Evaluation of Deprivation and Health Inequalities in Public Health Centers (EPICES) index are more likely already to have cancer, a higher mean BMI, greater prevalence of current smoking, lower adherence to screening programs, and greater standardized mortality ratio compared to non-vulnerable individuals [21,22,23]. Thus, this social grading variable is expected to impact the adherence of patients to supportive care programs and ultimately induce differences in treatment-induced fatigue in this population.
We designed the present multicenter RCT to assess the effect of a combined exercise and diet intervention delivered during a six-cycle adjuvant chemotherapy regimen followed by radiotherapy on cancer-related fatigue in EBC patients. We hypothesized that the combined supervised program would yield beneficial effects compared to usual care on cancer-related fatigue as a primary outcome, especially in patients considered to be more vulnerable in terms of social deprivation. Secondary outcomes include BMI, nutritional parameters, physical abilities, anxiety-depressive symptoms, and health-related quality of life.
2. Materials and Methods
2.1. Design
The present study was a two-arm, multicenter, randomized, controlled, prospective trial. The APAD2 trial was designed and implemented to evaluate the impact of an exercise and nutrition-based supportive care intervention during 6 months of chemotherapy and radiotherapy on fatigue, evaluated using the MFI-20 questionnaire in EBC patients treated in eight French cancer centers. The APAD intervention was compared to usual care without specific exercise and/or nutrition care (control arm). The primary hypothesis was the possibility of obtaining a 4-point reduction in the mean score on the General fatigue subscale in the intervention group with respect to the control group.
2.2. Participants
Eligible participants were women over 18 years of age with histologically proven and newly (<6 months) affected by EBC accessible to initial surgery, without consideration of their baseline physical activity level or dietary intake. Patients were enrolled after undergoing curative surgery. All patients were to receive six cycles of adjuvant chemotherapy (three cycles of epirubicin/cyclophosphamide/5-fluorouracil every 3 weeks (FEC100 protocol), followed by three taxane-based cycles, either docetaxel every 3 weeks [24] or paclitaxel weekly for 9 weeks), followed by 6 weeks of radiotherapy. Patients affected by HER2-positive tumors also received adjuvant trastuzumab for a total of 52 weeks, starting at the initiation of taxane chemotherapy. Exclusion criteria were metastatic disease, any other primary tumor, medical contra-indications to moderate-intensity physical activity, inability to attend intervention sessions or assessments, and a difficulty or disability preventing the patient from correctly understanding the trial information or requirements. The study was approved by the local ethics committee (NCT04109326) and conducted in accordance with the Declaration of Helsinki and principles of good clinical practice.
Before chemotherapy, potential participants were identified by the hospital medical oncologists. All participants were informed of the goal of the study and the potential benefits of diet and exercise on fatigue during adjuvant therapy. The patients who provided written informed consent and completed baseline assessments were randomly assigned (1:1 ratio) to the APAD experimental arm or control arm, stratified by center and precariousness level as assessed by the EPICES score [22] using the minimization method. Randomization was performed at the Montpellier ICM Biometric Unit using a computer program generated with Stata software version 12 (StatCorp, LLC, College Station, TX, USA). Participants, interventionists, and assessors were not masked to group assignment. The control group was a usual care group without any diet or exercise intervention. No particular material was delivered to the control group during the intervention, and these patients were not asked to limit exercise practice or eat/avoid specific foods during the intervention period.
2.3. Intervention
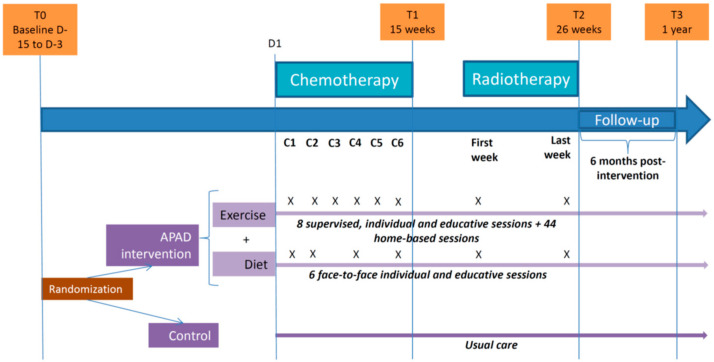
The APAD education program, which was based on the previously published trial method [18], was implemented during chemotherapy and radiotherapy (26 weeks). The intervention included twice-weekly exercise sessions and six individual nutritional therapeutic education sessions. The exercise sessions were individually supervised hospital-based exercise sessions and non-supervised home-based sessions combining one muscle strength session and one aerobic session each week (Figure 1). The nutritional education sessions targeted body weight control and the modification of feeding behaviors according to the WCRF recommendations [25]. The nutritional sessions were planned on the same days as supervised hospital-based exercise sessions. The intervention was tailored to maintain the patients’ exercise level and dietary intake in accordance with the guidelines throughout the intervention period.
Figure 1.
Flow diagram of the Adapted Physical Activity Diet 2 (APAD2) trial. C indicates chemotherapy cycle time points. X indicates intervention times in the associated randomization arm. Number of participating patients: T0 (Baseline): Control (n = 180), Adapted Physical Activity Diet (APAD) (n = 180). T2 (completed Chemotherapy and Radiotherapy sessions): Control (n = 170), APAD (n = 160). T3 (1 year after inclusion): Control (n = 157), APAD (n = 144).
2.3.1. Exercise
The exercise program delivered by trained professionals combined aerobic and muscle strengthening exercises according to international recommendations: 120 min of moderate to vigorous physical activity per week associated with strength training [9,26,27,28]. One muscle strength session and one aerobic session were scheduled each week (10 min of warm-up, at least 30 min of exercise, 10 min of stretching and 10 min of relaxation time with a goal of muscle recovery and well-being). Strength sessions targeted six main muscle groups (hamstrings, quadriceps, buttocks, abdominal, back, shoulders/arms), and each skill was performed for 2 to 5 sets with 6 to 12 repetitions with individual adaptation and progression. Aerobic exercise was performed at moderate intensity and adapted to the patient’s physical condition and progression, with a target of 50–75% of the maximum heart rate for 30 to 45 min (adapted to the patient’s physical condition). The initial exercise intensity was individualized but generally began at 50–55% of the maximum heart rate and progressed to 65–75% of the maximum heart rate by weeks 20 to 26. Supervised hospital-based sessions were achieved on a cycloergometer. For home-based practice, patients were given the option of various modalities of aerobic exercise (e.g., walking, jogging, cycling, dancing/fitness, swimming) to sustain adherence to the program and promote enjoyment. Hospital-based supervised exercise sessions aimed to provide the patients with relevant instructions that allow reproducibility at home and increased autonomy. Every supervised session was based on theory-based behavioral targets and techniques to improve behavioral change and patient adherence. Hospital-based supervised exercise sessions were scheduled on the same day as chemotherapy and during radiotherapy every 3 weeks to avoid additional cost. A total of eight hospital-based supervised exercise sessions and 44 at-home sessions were planned during the course of the intervention. Non-supervised, home-based sessions were planned at least twice per week, except only one home-based session was scheduled for the weeks that included one supervised hospital-based exercise session. Precise written instructions were given to patients in the educational and personable APAD-Moving workbook, which included information on their disease and reasons for being physically active during active treatment for cancer, illustrated instructions on performing the home exercises, the schedule for planned home-based sessions, and a patient log to evaluate adherence. Patients were asked to fill in the adherence log at home with whether planned sessions were achieved, the number of achieved muscular exercises, duration of each session, rating of perceived exertion (on scale of 1 to 10), reason for missed sessions, and anything else that they would like to discuss with the exercise specialist at the next supervised session.
2.3.2. Diet
Patients in the intervention arm received diet counselling with therapeutic education from a dietician at six individual face-to-face sessions. Each session lasted approximately 30 min. During chemotherapy, four diet sessions were scheduled to achieve balanced dietary intake, advising patients on controlling weight and managing with the potential toxicities and side effects of chemotherapy. Two more sessions were planned at the beginning and end of radiotherapy for all intervention groups. Weight control was pursued in patients with BMI < 30 kg/m2; weight normalization was targeted in patients with BMI ≥ 30 kg/m2 (i.e., to decrease BMI to less than 30 kg/m2 by the end of adjuvant therapy). Each consultation involved an evaluation of nutritional status, nutrition care tailored to the patient’s caloric needs and potential toxicities related to treatment, and nutritional education. The purpose of these consultations was to teach the principles of a well-balanced diet, foster weight control during treatment, and induce appropriate feeding behaviors after treatment.
2.3.3. Evaluation of Nutritional Status
At the first session, nutritional status was evaluated based on the patient’s usual weight, current weight, and their weight measured 1 to 6 months prior to study enrollment according to the French National Authority for Health criteria. The dietician assessed the patient’s daily energy requirement by computing their basal metabolic rate (BMR) [29] according to the corrected formula of Harris and Benedict. Dietary intake was prospectively measured by asking patients to fill out a questionnaire on 3 consecutive days of food intake at the first and last session. For the other sessions, dietary intake was measured by a 24-h recall food survey and a 10-point visual analogue scale.
2.3.4. Nutrition Care
Nutrition care aimed for weight control through balanced dietary intake tailored to the patient’s energy needs and potential toxicities related to the cancer treatment. The dietician verified the patient’s intake utilizing the following guidelines: daily energy intake was compared to the estimated daily energy needs, patients were guided to regularly distribute their dietary intake into three main meals with an optional snack in the afternoon, macronutrient distribution was compared to the French dietary reference intakes for a balanced diet (i.e., 30–35% lipids, 50–55% carbohydrates, and 10–15% protein) [30], and food group intake was guided to meet the recommendations of the WCRF [25]. If the patient’s habits did not correspond with these guidelines, or their daily energy intake was higher or lower than 10% of the estimated daily energy needs, the dietician counselled the patient on modifications regarding foods, nutrients, meals, and calorie distribution. If the patient’s BMI was >30 kg/m2 by the end of chemotherapy, a new weight goal was set to decrease the patient’s BMI to the range of 25 to 30 kg/m2. A new range of daily energy needs was then estimated with a corresponding distribution according to food group balance and the WCRF guidelines [25]. Patients were given a printed example of food groups, servings, and distribution that they may eat on a typical day. In the following sessions, the dietician computed the patient’s intake again and adapted the advice to the evolution of the patient’s intake. Specific advice was given to patients on the management of potential toxicities and side effects of chemotherapy.
2.3.5. Nutritional Education
Nutritional education aimed to teach the patients the principles of a well-balanced and healthy diet based on WCRF guidelines [25], inform them of industrial food packaging, and fight preconceived ideas by using practical applications and educational games. Nutritional education was tailored to the patients’ habits and means, precariousness level, and cultural and social environment.
Session 1: Presentation of detailed well-balanced menus.
Session 2: Identify the nature and specific roles of food groups—food balance education based on the food pyramid presentation.
Session 3: Food balance education based on the “APAD fridge game” consisting of the elaboration of three balanced meals with the food provided on the picture (food and dish choices from proposed menus to obtain balanced meals in special contexts, such as picnic, fast food, or restaurant).
Session 4: Teach to read labels and food packaging—food balance education through examples of complex mixed dishes (e.g., lasagna).
Session 5: Evidence-based information on the relationship between nutrition and cancer using a quiz game pointing out preconceived ideas.
Session 6: Post-treatment diet benefits and recommendations, and delivery of a booklet summarizing dietary WCRF recommendations.
2.3.6. Missed Sessions
Missed supervised exercise or diet counseling sessions at the hospital during chemotherapy could not be rescheduled, as the patients only came to the hospital once every 3 weeks during chemotherapy. In the case of a missed supervised session, a phone call was made to the patient by the exercise and/or diet specialists. The discussion focused on reasons for not attending the session, patient adherence in the last 3 weeks, encouraging the patient to attend future exercise or diet counseling sessions, taking into account the difficulties, and delivering education targets and content of the missed session if possible.
In contrast, missed supervised hospital-based sessions during radiotherapy were rescheduled as soon as possible because most of the patients came to the hospital every weekday during radiation therapy.
2.4. Outcomes and Assessments
The endpoints and assessments were concordant with those of the previous APAD1 trial [18,20]. Endpoints included subjective PROs and objective outcomes. Assessments were conducted at each site at baseline, just before the start of adjuvant chemotherapy (T0); the end of chemotherapy (T1); end of radiotherapy (T2); and the 6-month follow-up (T3). The primary endpoint was self-reported cancer-related fatigue assessed by the General fatigue subscale of the Multidimensional Fatigue Inventory (MFI-20) [31,32], a 20-item self-report instrument that covers five dimensions. The other four subscales (Physical fatigue, Mental fatigue, Reduced activity, and Reduced motivation) and the total score were considered secondary outcomes.
An objective measure of physical fatigue, lower limb muscle endurance, was measured using the sit-to-stand test at 15 and 30 s. The adherence to chemotherapy was evaluated using the relative dose intensity (RDI), which was calculated as the ratio of the cumulative dose intensity (mg/m2/week) to the dose intensity planned in the chemotherapy protocol. In addition, anxiety and depression symptomatology was evaluated using the 14-item Hospital Anxiety Depression Scale (HADS) self-report questionnaire [33]. Quality of life (QoL) was assessed by the EORTC QLQ-C30 questionnaire, a validated cancer-specific instrument [34] evaluating five functions (physical, role, cognitive, emotional, and social), nine symptoms (fatigue, pain, nausea and vomiting, dyspnea, loss of appetite, insomnia, constipation, diarrhea, and financial difficulties), and global health status. Physical activity was assessed using the 16-item Global Physical Activity Questionnaire (GPAQ) developed by the World Health Organization (WHO) [35,36]. The GPAQ assesses the intensity, duration, and frequency of physical activity in a usual week in three domains: activity at work, travel to and from places, and recreational activities. Physical activity is then expressed in terms of metabolic equivalent (MET), which is the ratio between the speed of metabolism during physical activity and the speed of metabolism at rest [37]. MET values are applied to vigorous and moderate intensity variables in work and recreational settings. One MET is defined as 1 kcal/kg/h and is equivalent to the energy cost of sitting quietly. We attribute 4 MET and 8 MET to the time spent on moderately intense and vigorous physical activity, respectively. Outcomes considered were average METs (MET/min/day) from activities of moderate and vigorous intensity (work and recreational); average METs from moderate intensity transport (cycling and walking), total physical activity (MET-minutes/week), and sedentary time (min/day). We also calculated the proportion of patients with a low level of total physical activity and the proportion of patients that failed to meet the WHO recommendations. Anthropometric measures (body weight, height, BMI, and waist circumference) were used to describe weight gain. Dietary intake was evaluated using a food record [38] of the foods and beverages consumed for 3 consecutive days (including one weekend day); the data were entered into nutritional analysis software and calories and nutrient intake computed (Nutritional Analysis Software, release 8, Villiers-les-Nancy: MICRO 6, 2007). All endpoints, except the nutritional evaluation, were assessed at four time points: pre-intervention, baseline assessment before the start of adjuvant chemotherapy (T0); end of chemotherapy (T1); end of radiotherapy (T2; i.e., immediately post-intervention); and 6 months after the end of treatment (i.e., 1 year after inclusion in the study). The nutritional evaluation was conducted at three time points: T0, T2, and T3.
2.5. Statistical Considerations
2.5.1. Sample Size Calculation and Randomization
The sample size calculation was based on the primary endpoint, the General fatigue subscale of the MFI-20 questionnaire. Smets et al. [31] estimated a mean score of 16 (standard deviation [SD] 8) for the General fatigue subscale in cancer patients undergoing radiotherapy; therefore, we considered this the reference value and the main time point of the evaluation was T2. In order to detect a 4-point reduction in the mean score on the General fatigue subscale in the intervention group with respect to the control group (i.e., reduction of 25% of the reference), the sample size calculation was based on a global risk alpha of 1% (bilateral situation), 90% (1-β) power, SD of 9, and the consideration of a repeated measures design (one pre-intervention measure and roughly three post-intervention measures, with a hypothetical correlation coefficient between measures of 0.2). The sample size was estimated to be 161 patients per arm in Stata Statistical Software, Release 10 (StataCorp, College Station, TX, USA). Considering a 12% loss to follow-up, 180 patients were required per group, a total of 360 patients overall in the trial. Randomization (1:1) was achieved using the minimization method and patients stratified according to two factors: the level of socioeconomic deprivation assessed by the French EPICES score [21,22] and recruiting center. Randomization was centralized at the Biometrics Units of the ICM, Montpellier.
2.5.2. Statistical Analysis
All analyses were performed in the intention-to-treat population. Analyses related to the impact of the program were also performed in the per-protocol population. This population considers all eligible patients treated and evaluated, which in the case of the intervention arm was defined as all patients who completed at least one supervised or unsupervised exercise session. An initial descriptive analysis of the baseline variables was performed and balance between treatment groups checked for the main demographic, socio-professional, clinical, and PRO variables.
The efficacy of the program was evaluated by the relative difference in the General fatigue score at each time point (T1, T2, and T3) with respect to T0 and compared between two groups using the Kruskal–Wallis test. A model approach was also used; the evolution of the General fatigue score over time was assessed using a linear mixed model (LMM). Random intercepts and random slopes were included to take into account the time effect. Interaction terms were also considered. The model coefficients were estimated by maximum likelihood; coefficients are presented as β1 for arm effect (APAD with respect to control) and as β0 for time effect. Separate models were adjusted for each other of the MFI-20 questionnaire subscales and for all other secondary endpoints.
The EORTC QLQ-C30 was analyzed following the EORTC guidelines [39], with scores for each functional and symptom subscale. HADS scores assessing anxiety and depression were categorized according to Zigmond classification (absence of disorder, suspected disorder, disorder).
Physical activity data recorded with the GPAQ were analyzed according to the WHO guidelines and using the STEPS analysis syntax program for cleaning and analyses.
Categorical and ordinal variables were compared using the Pearson chi-squared or Fisher’s exact tests. Continuous variables were presented as mean and standard deviation (SD) and were compared using the non-parametric Kruskal–Wallis test.
In the case of missing values, no imputation method was used. All reported p-values are two-sided, and a significance threshold of 0.05 was considered. Statistical analyses were performed using the STATA 13 software (StataCorp LP, College Station, TX, USA).
2.6. Ethics Approval, Consent to Participate and Trial Registration
The study was approved by the French institutional review board (i.e., Comité de Protection des Personnes Sud-Méditerranée III; N°ID-RCB: 2012-A01648-35), the Agence Nationale de Sécurité du Médicament et des produits de santé (N°ANSM: 130313B-12), and the Commission nationale de l’informatique et des libertés. Written informed consent was obtained from all participants. The trial was registered under the identification number NCT04109326.
3. Results
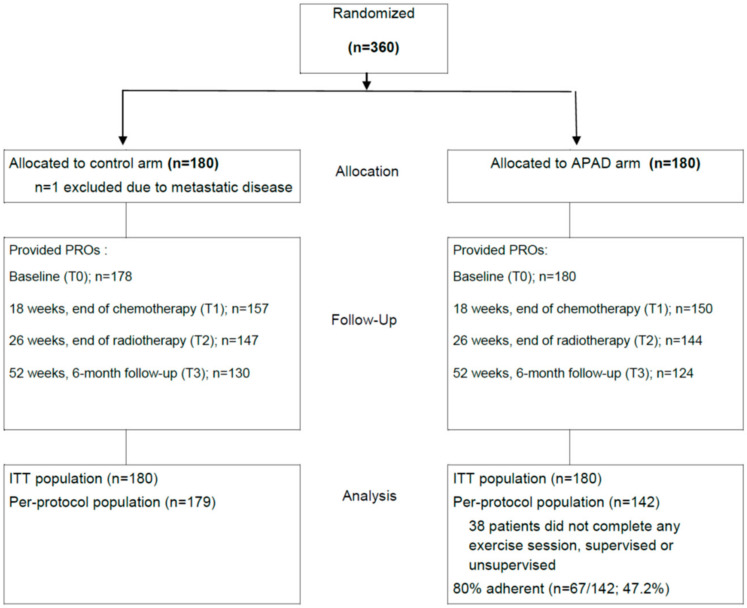
From May 2013 to December 2014, 360 patients were randomized at eight centers to the APAD (n = 180) and control (n = 180) arms. PROs were collected from 99%, 85%, 81%, and 71% of the 360 randomized patients at T0, T1, T2, and T3, respectively (Figure 2). Thirty-eight patients in the APAD arm (21%) did not complete any of the supervised or unsupervised exercise sessions. Among the per-protocol APAD population (n = 142), 67 patients (47.2%) had 80% adherence to the exercise program (defined as the completion of at least 80% of supervised and 80% of unsupervised sessions); 93 patients (65.5%) had global 80% adherence (both modalities confounded).
Figure 2.
CONSORT diagram of the study.
The baseline characteristics of the patients are presented in Table 1. Most of the patients completed the study (80% in the APAD arm, 87.7% in the control arm). The study was discontinued early for 17% and 7.2% of the patients in the APAD and control arm, respectively, due to patient withdrawal from the study (p = 0.014).
Table 1.
Baseline characteristics of patients in the intention-to-treat population.
| Control n = 180 |
APAD n = 180 |
Total n = 360 |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 52.35 | 10.09 | 52.66 | 9.69 | 52.51 | 9.88 |
| Weight (kg) | 67.00 | 14.13 | 68.41 | 14.60 | 67.71 | 14.36 |
| BMI (kg/m2) | 25.22 | 5.30 | 25.72 | 5.14 | 25.47 | 5.22 |
| BMI categories | n | % | n | % | n | % |
| <18.5 kg/m2 | 6 | 3.33 | 2 | 1.12 | 8 | 2.23 |
| 18.5–24.9 kg/m2 | 99 | 55.00 | 95 | 53.07 | 194 | 54.04 |
| 25–29.9 kg/m2 | 45 | 25.00 | 45 | 25.14 | 90 | 25.07 |
| ≥30 kg/m2 | 30 | 16.67 | 37 | 20.67 | 67 | 18.66 |
| Post-menopausal | 88 | 48.89 | 88 | 48.89 | 176 | 48.89 |
| Tobacco smoking | ||||||
| Non-smoker | 102 | 56.67 | 86 | 47.78 | 188 | 52.22 |
| Smoker | 29 | 16.11 | 34 | 18.89 | 63 | 17.50 |
| Ex-smoker | 49 | 27.22 | 60 | 33.33 | 109 | 30.28 |
| Marital status | ||||||
| Single/divorced/widowed, no child | 16 | 8.99 | 10 | 5.56 | 26 | 7.26 |
| Single/divorced/widowed, with child | 23 | 12.92 | 37 | 20.56 | 60 | 16.76 |
| Married/living together, no child | 21 | 11.80 | 26 | 14.44 | 47 | 13.13 |
| Married/living together, with child | 118 | 66.29 | 107 | 59.44 | 225 | 62.85 |
| Education level | ||||||
| No qualifications | 29 | 16.57 | 24 | 13.56 | 53 | 15.06 |
| Secondary level | 43 | 24.57 | 31 | 17.51 | 74 | 21.02 |
| Completed high school | 29 | 16.57 | 43 | 24.29 | 72 | 20.45 |
| Completed ≥ 2 years at university | 74 | 42.29 | 79 | 44.64 | 153 | 43.47 |
| Usual professional status | ||||||
| Full or part-time employed | 97 | 53.89 | 103 | 57.22 | 200 | 55.56 |
| Retired | 42 | 23.33 | 41 | 22.78 | 83 | 23.06 |
| Unemployed/medical leave | 41 | 22.78 | 36 | 20.00 | 77 | 21.38 |
| EPICES precariousness (or deprivation) level | ||||||
| Non-precarious | 109 | 60.56 | 109 | 60.56 | 218 | 60.56 |
| Intermediate | 60 | 33.33 | 60 | 33.33 | 120 | 33.33 |
| Precarious | 11 | 6.11 | 11 | 6.11 | 22 | 6.11 |
| Surgery type | n | % | n | % | n | % |
| Lumpectomy | 89 | 49.44 | 88 | 48.89 | 177 | 49.17 |
| Quadrantectomy | 37 | 20.56 | 45 | 25.00 | 82 | 22.78 |
| Mastectomy | 54 | 30.00 | 46 | 25.56 | 100 | 27.78 |
| T stage | ||||||
| T1 | 91 | 50.56 | 97 | 53.89 | 188 | 52.22 |
| T2 | 74 | 41.11 | 73 | 40.56 | 147 | 40.83 |
| T3 | 11 | 6.11 | 8 | 4.44 | 19 | 5.28 |
| T3 | 3 | 1.67 | 1 | 0.56 | 4 | 1.11 |
| T4 | 1 | 0.56 | 0 | 0 | 1 | 0.28 |
| Tis | 0 | 0 | 1 | 0.56 | 1 | 0.28 |
| T stage | ||||||
| N0 | 71 | 39.66 | 79 | 44.63 | 150 | 42.13 |
| N1 | 86 | 48.04 | 83 | 46.89 | 169 | 47.47 |
| N2 | 14 | 7.82 | 11 | 6.21 | 25 | 7.02 |
| N3 | 7 | 3.91 | 3 | 1.69 | 10 | 2.81 |
| NX | 1 | 0.56 | 1 | 0.56 | 2 | 0.56 |
| Breast cancer subtype | ||||||
| Triple negative | 17 | 18.48 | 17 | 18.89 | 34 | 18.68 |
| HER2+, ER+, and/or PR+ | 29 | 31.52 | 35 | 38.89 | 64 | 35.16 |
| HER2+, ER−, and PR− | 9 | 9.78 | 10 | 11.11 | 19 | 10.44 |
| HER2−, ER+, and/or PR+ | 37 | 40.22 | 28 | 31.11 | 65 | 35.71 |
3.1. Fatigue
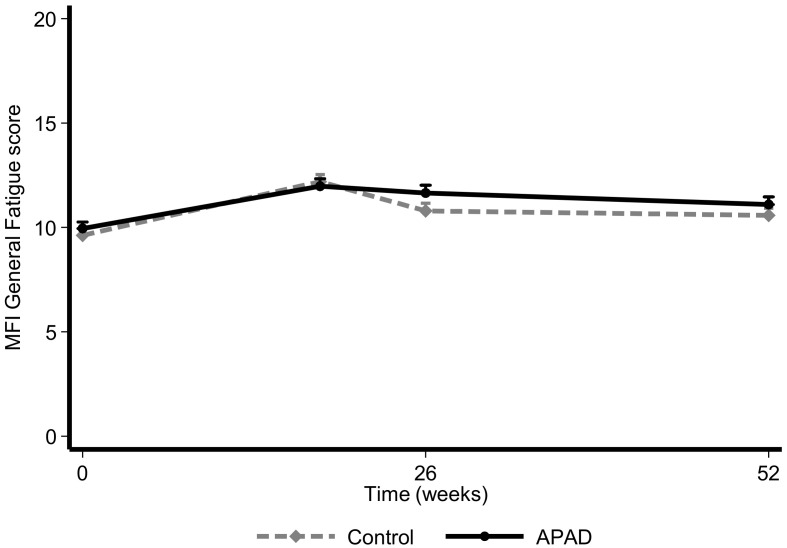
Compared to T0, the median relative difference in General fatigue scores at T1 was 25% in the control arm and 21% in the APAD arm, which is not a significant difference between the two arms (p = 0.274; Table 2). At T2, the increase in fatigue was greater in the APAD arm (20%) than in the control arm (8%), but the difference was still not significant (p = 0.157). However, at T3, 1 year after inclusion, the increase in fatigue in the APAD arm (15%) was lower than that of the control arm (20%), though it was not statistically or clinically significant (p = 0.933, Figure 3). According to the adjusted model, general fatigue tends to increase over time (β0 = 0.024 [95% CI 0.01; 0.034]; p < 005) without an observable effect of the intervention in terms of a reduction in general fatigue over time (β1 = 0.33, p = 0.374; Table 2).
Table 2.
Fatigue sub-scales of the MFI20 and quality of life (EORTC QLQ-C30) in the intention-to-treat population.
| Baseline (T0) | End of CT (T1) | End of RT (T2) | 1 Year after Inclusion (T3) | LMM Coefficients 1 (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | Mean | SD | p | Mean | SD | p | |||
| General fatigue (Endpoint) | Control | 9.63 | 4.2 | 12.19 | 4.21 | 0.683 | 10.79 | 4.44 | 0.107 | 10.58 | 3.84 | 0.231 | β1 = 0.33 [−0.40; 1.07], p = 0.374 |
| APAD | 9.95 | 4.18 | 11.97 | 4.37 | 11.65 | 4.44 | 11.1 | 4.07 | β0 = 0.24 [0.014; 0.034], p < 0.05 | ||||
| Median (range) of the relative difference 2 | Control | 0.25 (−0.67; 3.0) | 0.083 (−0.60; 3.50) | 0.20 (−0.69; 3.5) | |||||||||
| APAD | 0.21 (−0.5; 2.75) | 0.20 (−0.58; 3.25) | 0.15 (−0.6; 3.0) | ||||||||||
| p = 0.274 | p = 0.157 | p = 0.933 | |||||||||||
| Physical fatigue | Control | 9.55 | 3.72 | 11.28 | 4.05 | 0.896 | 10.61 | 3.96 | 0.985 | 10.1 | 3.63 | 0.742 | β1 = −0.15 [−0.81; 0.52], p = 0.670 |
| APAD | 9.11 | 3.85 | 11.33 | 4.19 | 10.64 | 4.25 | 10.06 | 3.77 | β0 = 0.02 [0.007; 0.025], p < 0.05 | ||||
| Mental fatigue 3 | Control | 7.66 | 3.81 | 9.19 | 4.26 | 1 | 8.9 | 4.31 | 1 | 8.82 | 4.26 | 0.872 | β2 = −0.015 [−0.035; 0.005], p = 0.152 |
| APAD | 8.57 | 4.07 | 9.15 | 4.24 | 9.06 | 4.71 | 9.06 | 4.65 | β0 = 0.03 [0.013; 0.042], p < 0.05 | ||||
| Reduced activities | Control | 8.34 | 3.64 | 10.12 | 4.26 | 0.466 | 9.14 | 3.92 | 0.433 | 8.75 | 4.02 | 0.743 | β1 = 0.30 [−0.40; 0.99], p = 0.399 |
| APAD | 9.01 | 4.01 | 9.84 | 4.64 | 9.67 | 4.49 | 8.9 | 4 | β0 = 0.004 [−0.005; 0.014], p = 0.402 | ||||
| Reduced motivation 3 | Control | 7.73 | 3.5 | 8.22 | 3.92 | 0.587 | 7.84 | 3.46 | 0.651 | 8.07 | 3.19 | 0.572 | β1 = −0.30 [−0.34; 0.93], p = 0.360 |
| APAD | 8.54 | 3.73 | 8.31 | 3.7 | 8.15 | 3.77 | 8.1 | 3.83 | β0 = 0.002 [−0.006; 0.009], p = 0.696 | ||||
| EORTC QLQ-C30 | |||||||||||||
| Global health status | Control | 69.94 | 18.67 | 59.39 | 21.22 | 0.516 | 66.55 | 19.53 | 0.429 | 67.44 | 19.3 | 0.537 | β1 = −0.0026 [−0.071; 0.065], p = 0.940 |
| APAD | 68.45 | 19.55 | 60.83 | 21.34 | 64.82 | 19.1 | 69.17 | 17.76 | β0 = 0.001 [−0.001; 0.001], p = 0.824 | ||||
| Physical functioning | Control | 87.17 | 14.59 | 79.79 | 19.1 | 0.219 | 84.4 | 15.38 | 0.083 | 85.45 | 17.26 | 0.194 | β1 = −0.020 [−0.034; 0.075], p = 0.466 |
| APAD | 88.48 | 14.64 | 82 | 19.58 | 86.49 | 17.11 | 89.46 | 12.95 | β0 = −0.0001 [−0.0007; 0.0006], p = 0.873 | ||||
| Role functioning | Control | 84.46 | 22.16 | 77.99 | 23.34 | 0.257 | 84.35 | 21.03 | 0.966 | 87.05 | 18.48 | 0.309 | β1 = 0.026 [−0.073; 0.125], p = 0.607 |
| APAD | 86.57 | 20.4 | 80.63 | 23.25 | 83.8 | 22.01 | 90.05 | 16.86 | β0 = 0.0009 [−0.0005; 0.0022], p = 0.220 | ||||
| Emotional functioning | Control | 63.7 | 23.52 | 72.75 | 24.83 | 0.211 | 75.51 | 23.09 | 0.309 | 73.26 | 20.1 | 0.679 | β1 = 0.007 [−0.106; 0.119], p = 0.910 |
| APAD | 63.66 | 23.95 | 70.5 | 22.93 | 73.1 | 23.28 | 73.21 | 23.33 | β0 = 0.004 [0.002; 0.005], p < 0.01 | ||||
| Cognitive functioning | Control | 85.21 | 20.5 | 79.3 | 25.14 | 0.657 | 80.16 | 21.75 | 0.849 | 79.97 | 23.42 | 0.957 | β1 = −0.019 [−0.114; 0.076], p = 0.695 |
| APAD | 84.17 | 20.32 | 78.56 | 24.37 | 79.58 | 22.4 | 80.24 | 23.12 | β0 = −0.002 [−0.004; −0.001], p < 0.01 | ||||
| Social functioning | Control | 82.58 | 24.15 | 66.77 | 30.63 | 0.827 | 73.58 | 27.71 | 0.23 | 82.04 | 23.99 | 0.849 | β1 = 0.003 [−0.128; 0.134], p = 0.964 |
| APAD | 84.45 | 21.97 | 66.44 | 29.73 | 69.84 | 28.42 | 83.6 | 21.78 | β0 = −0.00008 [−0.002; 0.002], p = 0.942 | ||||
| Fatigue symptom | Control | 28.54 | 21.86 | 44.87 | 27.24 | 0.934 | 34.62 | 25.73 | 0.152 | 32.26 | 21.96 | 0.935 | β1 = −0.042 [−0.248; 0.164], p = 0.687 |
| APAD | 28.49 | 23.35 | 45.38 | 29.13 | 37.4 | 23.8 | 31.63 | 21.15 | β0 = 0.004 [0.001; 0.007], p = 0.018 | ||||
1 In the linear mixed model (LMM; log transformed variables): β1 is the coefficient of the variable ‘arm’ (interpreted as APAD effect with respect to Control) noted as β2 when interaction (arm by time) was significant. β0 is the coefficient of the variable time (weeks) variable. 2 For each patient, the relative difference (RD) with respect to the baseline value of the General fatigue subscale at the end of chemotherapy (CT), end of radiotherapy (RT; end of the oncological treatment), and 1 year after inclusion was calculated as: (GFS_endRT − GFS_Inclusion)/GFS_Inclusion. A smaller RD indicates a greater reduction in general fatigue. 3 A baseline imbalance between arms was observed for Mental fatigue (p = 0.019) and Reduced motivations (p = 0.025). No other baseline imbalance was observed.
Figure 3.
Evolution of the MFI General Fatigue score according to randomization arm in the intention-to-treat population. Data are presented as mean + SD.
No significant benefit of the APAD program was observed when the analysis was conducted by precariousness level (EPICES score). However, we observed a clear gradient in the baseline fatigue according to the stratum of precariousness, with the fatigue level being higher for the most precarious stratum throughout the study (Figures S1 and S2).
For all other fatigue subs-scales of the MFI20, no difference was observed between treatment arms with respect to their evolution over time. Overall, physical and mental fatigue tended to increase across time in both arms (β0 = 0.02 and β0 = 0.03, p < 0.05; Table 2). Notably, a baseline imbalance disfavoring the APAD arm was observed for Mental fatigue (p = 0.019) and Reduced motivations (p = 0.025).
When the fatigue sub-scales were analyzed in the per-protocol population (Table S1), we observed that the APAD program had a positive impact, enhancing motivation over time despite the initial imbalance (β2 = −0.023 [95% CI −0.39; −0.008]; p = 0.003), and reducing mental fatigue (β2 = −0.020 [−0.04; 0.002], p = 0.069).
In a sub-group analysis considering the 80% adherent population in the APAD and control arms, we found no significant difference in the relative differences in General fatigue scores (primary endpoint) (data not shown).
3.2. QoL and Psychological Distress
Quality of life and psychological distress scores on the EORTC QLQ-C30 and HADS, respectively, at baseline and during follow-up are described in Table 2 and Table 3. At the end of radiotherapy, we found no significant difference between arms in terms of QoL (Table 2, Figure S3). Overall, emotional function (both arms together) increased (LMM coefficient β0 = 0.004 [0.002; 0.005], p< 0.01) and cognitive function decreased (β0 = −0.002 [−0.004; −0.001], p < 0.01) over time. The symptom fatigue also increased (β0 = 0.004 [0.001; 0.007], p = 0.018), which is consistent with the results observed in the MFI score analysis. No impact of the APAD program was observed in regard to improving QoL dimensions global health status, functional dimensions, and fatigue symptoms.
Table 3.
Anxiety and depression disorders in the intention-to-treat population.
| Control | APAD | ||||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| Baseline (T0) | |||||
| Anxiety | 0.662 | ||||
| Absence (<7) | 0 | 0.00 | 0 | 0.00 | |
| Suspected (8–10) | 2 | 1.12 | 3 | 1.67 | |
| Confirmed (>10) | 176 | 98.88 | 177 | 98.33 | |
| Mean anxiety (SD) | 11.85 (2.56) | 11.92(2.69) | |||
| Depression | 0.368 | ||||
| Absence (<7) | 0 | 0.00 | 2 | 1.11 | |
| Suspected (8–10) | 70 | 39.33 | 71 | 39.44 | |
| Confirmed (>10) | 108 | 60.67 | 107 | 59.44 | |
| Mean depression (SD) | 18.93 (3.34) | 18.78 (3.54) | |||
| End of chemotherapy (T1) | |||||
| Anxiety | 0.974 | ||||
| Absence (<7) | 0 | 0.00 | 0 | 0.00 | |
| Suspected (8–10) | 1 | 0.64 | 1 | 0.67 | |
| Confirmed (>10) | 156 | 99.36 | 149 | 99.33 | |
| Mean anxiety (SD) | 12.29 (3.21) | 11.99 (3.03) | |||
| Depression | 0.163 | ||||
| Absence (<7) | 0 | 0.00 | 3 | 2.00 | |
| Suspected (8–10) | 55 | 35.03 | 57 | 38.00 | |
| Confirmed (>10) | 102 | 64.97 | 90 | 60.00 | |
| Mean depression (SD) | 20.25 (3.26) | 20.46 (3.22) | |||
| End of radiotherapy (T2) | |||||
| Anxiety | |||||
| Absence (<7) | 0 | 0.00 | 0 | 0.00 | |
| Suspected (8–10) | 0 | 0.00 | 0 | 0.00 | |
| Confirmed (>10) | 147 | 100.00 | 142 | 100.00 | |
| Mean anxiety (SD) | 11.69 (3.04) | 11.97 (3.04) | |||
| Depression | 0.576 | ||||
| Absence (<7) | 1 | 0.68 | 1 | 0.70 | |
| Suspected (8–10) | 68 | 46.26 | 57 | 40.14 | |
| Confirmed (>10) | 78 | 53.06 | 84 | 59.15 | |
| Mean depression (SD) | 20.38 (3.06) | 20.08 (3.34) | |||
| 1 year after inclusion (T3) | |||||
| Anxiety | 0.367 | ||||
| Absence (<7) | 0 | 0.00 | 1 | 0.81 | |
| Suspected (8–10) | 1 | 0.77 | 0 | 0.00 | |
| Confirmed (>10) | 129 | 99.23 | 123 | 99.19 | |
| Mean anxiety (SD) | 11.85 (2.42) | 11.81 (3.11) | |||
| Depression | 0.052 | ||||
| Absence (<7) | 0 | 0.00 | 2 | 1.61 | |
| Suspected (8–10) | 43 | 33.08 | 55 | 44.35 | |
| Confirmed (>10) | 87 | 66.92 | 67 | 54.03 | |
| Mean depression (SD) | 20.26 (2.87) | 19.87 (3.62) | |||
When the analysis was conducted on the per-protocol population (Table S1 and Figure S4), we observed slightly better physical function at the end of radiotherapy (p = 0.043), but the LMM did not show a global trend over time for an impact of the APAD program on this function (β1 = 0.025 [−0.034; 0.084]; p = 0.800).
In a sub-group analysis considering the 80% adherent population in the APAD and control arms, we observed significantly better physical function in the APAD subgroup at T2 (p = 0.003) and T3 (p = 0.017). However, in that subgroup analysis, a baseline imbalance favored the 80% adherent APAD subgroup, which exhibited better physical function (p = 0.028) and lesser pain symptom (p = 0.002) at T0. This may be explained, in part, to better adherence to the program.
Regarding psychological distress (Table 3), a lower proportion of patients with confirmed depression (score >10) was observed in the APAD arm (54.03%) with respect to control (66.92%) at T3 (p = 0.052). A similar result was observed in a per-protocol analysis (p = 0.026; Table S2).
3.3. Physical Activity
Dimensions of the GPAQ and sit-to-stand test are described in Table 4.
Table 4.
Physical activity according to the GPAQ and muscular test in the intention-to-treat population.
| Baseline (T0) | End of CT (T1) | End of RT (T2) | 1 Year after Inclusion (T3) (n = 113; n = 97) | LMM Coefficients 1 (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (158/168) | (n = 139; n = 129) | (n = 134; n = 128) | |||||||||||
| Mean | SD | Mean | SD | p | Mean | SD | p | Mean | SD | p | |||
| Total MET (MET.min/wk) | Control | 1998.63 | 2632.06 | 2339.37 | 4181.16 | 0.03 | 1522.69 | 1591.27 | 0.048 | 2157.1 | 2902.02 | 0.03 | β1 = 0.74 [0.37; 1.10], p < 0.001 |
| APAD | 2133.07 | 3206.8 | 2023.1 | 1729.05 | 1760.78 | 1464.05 | 2363.96 | 2386.1 | β0 = −0.005 [−0.007; 0.007], p = 0.864 | ||||
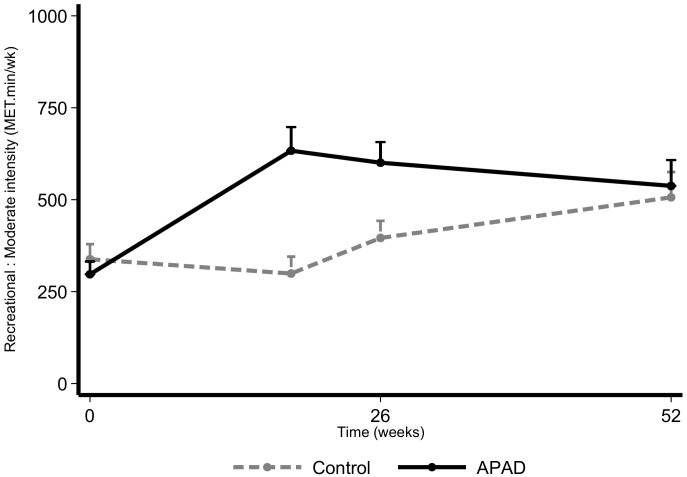
| Recreational–Moderate intensity (MET.min/wk) | Control | 338 | 511.97 | 299.08 | 539.7 | <0.001 | 395.82 | 538.71 | 0.001 | 506.27 | 728.95 | 0.349 | β1 = 0.95 [0.46; 1.43], p < 0.001 |
| APAD | 296.98 | 454.64 | 632.87 | 730.3 | 600.56 | 630.25 | 537.2 | 694.62 | β0 = 0.021 [0.012; 0.030], p < 0.001 | ||||
| Recreational–Vigorous intensity (MET.min/wk) | Control | 122.78 | 461.56 | 219.86 | 829.16 | 0.433 | 173.73 | 618.49 | 0.636 | 216.28 | 777.94 | 0.353 | β1 = 0.27 [−0.13; 0.67], p = 0.184 |
| APAD | 135 | 450.99 | 187.6 | 538.19 | 220.31 | 767.45 | 274.06 | 654.44 | β0 = 0.008 [0.001; 0.015], p = 0.019 | ||||
| Work—Moderate intensity (MET.min/wk) | Control | 816.46 | 1479.06 | 515.11 | 1419.7 | 0.006 | 429.28 | 849.11 | 0.674 | 667.65 | 1423.57 | 0.743 | β1 = 0.34 [−0.19; 0.86], p = 0.212 |
| APAD | 824.4 | 1638.35 | 576.59 | 1001.46 | 508.78 | 998.82 | 546.1 | 963.79 | β0 = −0.012 [0.022; −0.002], p = 0.014 | ||||
| Work—Vigorous intensity (MET.min/wk) | Control | 212.41 | 1378.38 | 523.17 | 2778.69 | 0.935 | 53.73 | 316.49 | 0.813 | 120.35 | 804.99 | 0.721 | β1 = 0.10 [−0.16; 0.36], p = 0.454 |
| APAD | 374.29 | 1906.61 | 78.76 | 420.47 | 79.38 | 556.03 | 225.15 | 1119.72 | β0 = 0.00003 [−0.005; 0.005], p = 0.989 | ||||
| Travel—Moderate intensity (MET.min/wk) | Control | 508.99 | 816.59 | 782.16 | 1117.32 | 0.071 | 470.12 | 702.81 | 0.277 | 646.55 | 1307.34 | 0.141 | β1 = 0.19 [−0.35; 0.72], p = 0.494 |
| APAD | 502.4 | 805.68 | 547.29 | 964.76 | 465.23 | 542.61 | 781.44 | 1734.93 | β0 = 0.01 [0.01; 0.02], p = 0.022 | ||||
| Sitting or reclining time (min/day) | Control | 372.41 | 175.26 | 355.29 | 197.46 | 0.041 | 357.07 | 171.3 | 0.84 | 337.12 | 164.13 | 0.946 | β1 = 0.18 [0.02; 0.33], p = 0.023 |
| APAD | 400.85 | 178.62 | 391.05 | 177.33 | 369.2 | 177.26 | 353.76 | 183.5 | β0 = −0.004 [−0.008; −0.0004], p = 0.029 | ||||
| Muscular test | |||||||||||||
| Sit-to-stand 30 s | Control | 18.09 | 4.67 | 17.3 | 5.51 | 0.013 | 18.86 | 5.32 | 0.39 | 19.42 | 6.02 | 0.615 | β1 = 0.001 [−0.002; 0.002], p = 0.094 |
| β0 = 0.001 [0.0002; 0.002], p = 0.017 | |||||||||||||
| APAD | 17.72 | 4.87 | 18.61 | 4.77 | 19.68 | 5.64 | 20.04 | 6.16 | |||||
| Sit-to-stand ratio | Control | 1.97 | 0.38 | 1.93 | 0.2 | 0.341 | 1.94 | 0.16 | 0.59 | 1.93 | 0.16 | 0.844 | β1 = −0.002 [−0.014; 0.01], p = 0.694 |
| (30 s/15 s) | β0 = −0.0001 [−0.0004; 0.0002], p = 0.431 | ||||||||||||
| APAD | 1.93 | 0.18 | 1.94 | 0.17 | 1.95 | 0.13 | 1.93 | 0.15 | |||||
1 In the linear mixed model (LMM; log transformed variables): β1 is the coefficient of the variable ‘arm’ (interpreted as APAD effect with respect to Control) noted as β2 when interaction arm by time was significant. β0 is the coefficient of the variable time (weeks) variable. Note: No baseline imbalance was observed for the studied variables (for sitting or reclining time (GPAQ), p = 0.136). CT: chemotherapy, RT: radiotherapy.
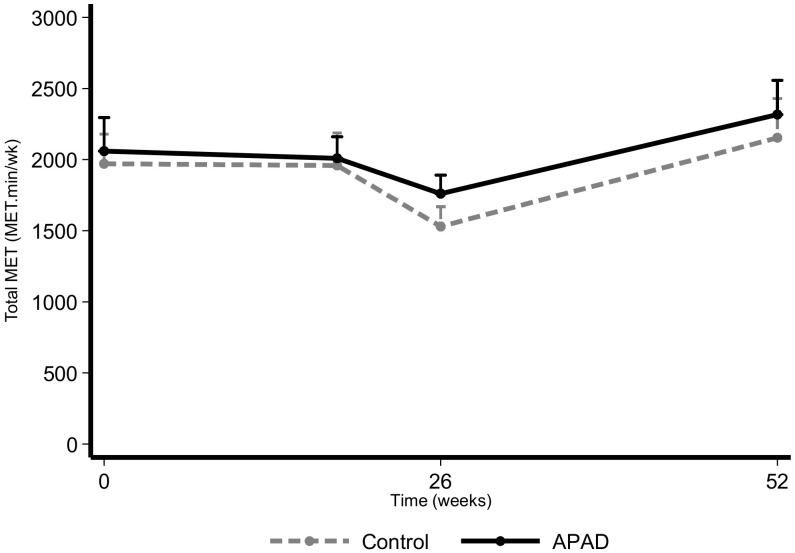
The intervention had a positive impact on the total MET (Figure 4) and on the moderate intensity recreational activities (Figure 5), as they were significantly higher in the APAD arm with respect to control (β1 = 0.74 [0.37; 1.10]; p < 0.001 and β1 = 0.96 [0.46; 1.43]; p < 0.001). However, the sitting or reclining time per day appeared to be slightly higher in the APAD arm (β1 = 0.18 [0.02; 0.33]; p = 0.023; Figure S5).
Figure 4.
Evolution of the total MET based on the GPAQ according to randomization arm in the intention-to-treat population. Data are presented as mean + SD.
Figure 5.
Moderate intensity recreational MET over time based on the GPAQ according to randomization arm in the intention-to-treat population. Data are presented as mean + SD.
Regarding compliance with WHO recommendations (Table 5), patients in the APAD arm more frequently met the recommendations compared to the control arm at T1 (81.40% vs. 61.87%, p < 0.001) and T3 (86.60% vs. 68.14%, p = 0.002).
Table 5.
Compliance with the WHO Stepwise 1 recommendations for physical activity (GPAQ) in the intention-to-treat population.
| Control | APAD | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p | ||
| Baseline (T0) | ||||||||
| Low activity | 0.084 | |||||||
| No | 105 | 66.46 | 96 | 57.14 | 201 | 61.66 | ||
| Yes | 53 | 33.54 | 72 | 42.86 | 125 | 38.34 | ||
| Failed to meet WHO recommendations | 0.568 | |||||||
| No | 118 | 74.68 | 130 | 77.38 | 248 | 76.07 | ||
| Yes | 40 | 25.32 | 38 | 22.62 | 78 | 23.93 | ||
| End of chemotherapy (T1) | ||||||||
| Low activity | 0.004 | |||||||
| No | 71 | 51.08 | 88 | 68.22 | 159 | 59.33 | ||
| Yes | 68 | 48.92 | 41 | 31.78 | 109 | 40.67 | ||
| Failed to meet WHO recommendations | 0.000 | |||||||
| No | 86 | 61.87 | 105 | 81.40 | 191 | 71.27 | ||
| Yes | 53 | 38.13 | 24 | 18.60 | 77 | 28.73 | ||
| End of radiotherapy (T2) | ||||||||
| Low activity | 0.094 | |||||||
| No | 81 | 60.45 | 90 | 70.31 | 171 | 65.27 | ||
| Yes | 53 | 39.55 | 38 | 29.69 | 91 | 34.73 | ||
| Failed to meet WHO recommendations | 0.071 | |||||||
| No | 95 | 70.90 | 103 | 80.47 | 198 | 75.57 | ||
| Yes | 39 | 29.10 | 25 | 19.53 | 64 | 24.43 | ||
| 1 year after inclusion (T3) | ||||||||
| Low activity | 0.003 | |||||||
| No | 64 | 56.64 | 74 | 76.29 | 138 | 65.71 | ||
| Yes | 49 | 43.36 | 23 | 23.71 | 72 | 34.29 | ||
| Failed to meet WHO recommendations | 0.002 | |||||||
| No | 77 | 68.14 | 84 | 86.60 | 161 | 76.67 | ||
| Yes | 36 | 31.86 | 13 | 13.40 | 49 | 23.33 | ||
1 STEPS analysis program.
3.4. Dietary Intake
The evolution of dietary intake and weight control by randomization arm is summarized in Table 6 and Figure S6. At T2, a significant increase in the consumption of fiber was observed in the APAD arm (p = 0.020), as well as a reduction in the consumption of animal proteins and alcohol (p = 0.003 and p = 0.019, respectively). However, both reductions seem to have been only temporary, as 6 months later the effect was diminished. These results were confirmed when we analyzed the evolution of these parameters over time in an LMM.
Table 6.
Dietary intake per day (3-day record) and weight control variables in the intention-to-treat population.
| Baseline (T0) | End of Radiotherapy (T2) | 1 Year after Inclusion (T3) | LMM Coefficients 1 [95% CI] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | p | Mean | SD | p | Mean | SD | p | |||
| Total energy (Kcal) | Control | 1507.46 | 362.87 | 0.312 | 1402.41 | 386.27 | 0.637 | 1382.97 | 384.02 | 0.16 | β1 = −0.0020 [−0.054; 0.050], p = 0.947 |
| APAD | 1463.76 | 434.09 | 1377.56 | 373.81 | 1452.03 | 372.93 | β0 = −0.0010 [−0.002; −0.0002], p = 0.013 | ||||
| Animal proteins (g) | Control | 19.64 | 17.92 | 0.233 | 10.38 | 10.04 | 0.003 | 8.35 | 8.99 | 0.233 | β1 = −0.26 [−0.55; 0.017], p = 0.066 |
| APAD | 17.29 | 17.03 | 7.95 | 9.94 | 7.42 | 8.55 | β0 = −0.017 [−0.022; −0.011], p < 0.001 | ||||
| Vegetal proteins (g) | Control | 8.85 | 5.93 | 1 | 5.61 | 4.29 | 0.415 | 5.49 | 4.3 | 0.836 | β1 = −0.084 [−0.23; 0.067], p = 0.276 |
| APAD | 9.2 | 6.87 | 5.45 | 5.59 | 5.53 | 5.07 | β0 = −0.010 [−0.013; −0.007], p < 0.01 | ||||
| Lipids (g) | Control | 61.43 | 21.98 | 0.147 | 57 | 22.9 | 0.22 | 54.82 | 24.05 | 0.407 | β1 = −0.037 [−0.11; 0.034], p = 0.306 |
| APAD | 57.72 | 23.49 | 52.32 | 18.73 | 56.34 | 19.54 | β0 = −0.001 [−0.003; −0.0002], p = 0.021 | ||||
| Monounsaturated lipids (g) | Control | 22 | 9.64 | 0.207 | 19.39 | 9.95 | 0.805 | 19.73 | 12.88 | 0.538 | β1 = −0.024 [−0.11; 0.057], p = 0.558 |
| APAD | 20.55 | 9.27 | 18.37 | 7.15 | 19.62 | 7.51 | β0 = −0.002 [−0.003; 0.0004], p = 0.009 | ||||
| Polyunsaturated lipids (g) | Control | 7.86 | 5.35 | 0.549 | 7.92 | 5.32 | 0.791 | 7.26 | 4.51 | 0.085 | β1 = 0.022 [−0.078; 0.12], p = 0.671 |
| APAD | 7.4 | 4.31 | 7.35 | 3.97 | 8.11 | 4.42 | β0 = 0.0002 [−0.001; 0.002], p = 0.773 | ||||
| Simple sugars (g) | Control | 69.97 | 28.08 | 0.926 | 61.93 | 23.26 | 0.273 | 61.26 | 24.46 | 0.504 | β1 = 0.023 [−0.058; 0.10], p = 0.582 |
| APAD | 70.25 | 30.15 | 65.66 | 24.96 | 64.03 | 26.17 | β0 = −0.002 [−0.004; 0.001], p < 0.001 | ||||
| Alcohol (g) | Control | 4.18 | 6.58 | 0.742 | 4.11 | 5.95 | 0.019 | 4.15 | 6.83 | 0.055 | β1 = −0.091 [−0.010; −0.0009], p = 0.549 |
| APAD | 4.44 | 7.31 | 2.25 | 4.18 | 2.98 | 5.67 | β0 = −0.005 [−0.010; -0.0009], p = 0.020 | ||||
| Fiber (g) | Control | 15.54 | 5.56 | 0.277 | 15.35 | 5.73 | 0.02 | 15.35 | 6.03 | 0.003 | β1 = 0.096 [0.026; 0.17], p = 0.007 |
| APAD | 16.28 | 5.87 | 17.37 | 6.49 | 17.58 | 5.9 | β0 = 0.0005 [−0.0006; 0.001], p = 0.389 | ||||
| Weight control | |||||||||||
| Weight (kg) | Control | 67 | 14.13 | 0.44 | 66.23 | 12.93 | 0.571 | 67.13 | 13.98 | 0.576 | β1 = 0.020 [−0.020; 0.060], p = 0.334 |
| APAD | 68.41 | 14.6 | 67.57 | 13.56 | 68.28 | 14.19 | β0 = 0.0001 [−0.0001; 0.0002], p = 0.389 | ||||
| BMI (kg/m2) | Control | 25.22 | 5.3 | 0.317 | 24.95 | 5.01 | 0.51 | 25.23 | 5.28 | 0.655 | β1 = 0.020 [−0.019; 0.059], p = 0.320 |
| APAD | 25.72 | 5.14 | 25.29 | 4.79 | 25.47 | 5.01 | β0 = 0.0001 [−0.0001; 0.0002], p = 0.337 | ||||
| Waist size (cm) | Control | 163.08 | 6.49 | 0.717 | 87.09 | 13.05 | 0.827 | 87.26 | 15.74 | 0.344 | β1 = 0.0034 [−0.027; 0.034], p = 0.827 |
| APAD | 163.06 | 6.32 | 86.87 | 11.72 | 88.65 | 13.48 | β0 = 0.0002 [−0.0001; 0.0004], p = 0.133 | ||||
1 In the linear mixed model (LMM; log transformed variables): β1 is the coefficient of the variable ‘arm’ (interpreted as APAD effect with respect to Control) noted as β2 when interaction arm by time was significant. β0 is the coefficient of the variable time (weeks) variable. Note: No baseline imbalance was observed for the studied variables.
According to the adjusted LMM, the intervention had a positive significant impact on increasing fiber consumption over time (β1 = 0.096 [0.026; 0.17]; p = 0.007). A trend of reduced consumption of animal proteins was also observed and was lower in the APAD arm than the control arm (β1 = −0.26 [−0.55; 0.017]; p = 0.066). No change in weight over time was observed.
3.5. Chemotherapy Completion Rates
The RDI was high overall, with 90.7% and 80% of the patients having an RDI > 80% and 90%, respectively, in the intention-to-treat population. No difference was found between the two arms (80.79% vs. 79.21%, p = 0.71 for RDI > 90%; 91.53% vs. 89.89%, p = 0.595 for RDI > 80% in APAD and control arms, respectively).
4. Discussion
In the present study, we reported the results of a large, multicenter, randomized trial evaluating the impact of an APAD education program on fatigue, fat mass, and health-related QoL. The results do not support our previous conclusions [18,20] and the main hypothesis of the efficacy of the exercise and diet education program to relieve cancer-related fatigue in EBC patients receiving adjuvant treatments. One of the main pitfalls could be linked to patient adherence to the protocol. The drop-out rate in the present study was higher in the APAD arm, with 17% of the population discontinuing the program early. In addition, the intensity of physical activity appears to have insufficient autonomy, with low adherence to the intervention. Twenty-one percent of the patients did not perform the complete supervised exercise sessions, and only 65% had completed at least 80% of the home sessions. Our intervention appears to change low activity only. Thus, this education program appears to be more of a lifestyle change, in terms of adherence and clinical impact, to more active practice, as confirmed by the high proportion of patients achieving the WHO targets without achieving a level of physical activity high enough to induce a clinically significant reduction in fatigue. The design of our program, with discontinuous supervision, may explain the ambiguous results for fatigue during follow-up [40,41,42,43,44,45].
As reported in recent meta-analyses and guidelines, supervised exercise interventions appear to have significantly greater effects on fatigue than unsupervised exercise interventions, and shorter supervised interventions with a duration ≤12 weeks appear to induce greater effects on fatigue than supervised interventions with a longer duration [9,46]. However, the results indicate significant benefits on depressive symptoms. This impact supports a reported decrease in psychological distress and an improvement in self-esteem with exercise [9].
In contrast to our hypothesis, we found no significant effect of the intervention on BMI and the chemotherapy completion rates. Previous reports on combined diet and exercise interventions delivered during chemotherapy [16,17] failed to find any benefits on hip circumference and BMI. One explanation could be based on the significant, but transient, modification of nutritional intake, specifically animal proteins, alcohol, and fiber, without persistent marked behavioral modifications, as highlighted by the trends in protein and lipid consumption in the LMM. Evaluation of the impact of continued education programs, with later-time supervised sessions, could help define the best method of sustaining early changes.
A meta-analysis of 32 randomized studies comprising 2626 EBC patients evaluated the impact of supervised aerobic or resistance exercise interventions during adjuvant treatment and reported a pooled significant improvement in strength [47]. High-dose training or a focus on resistance training appears to be associated with better effects on strength in this patient population [41,45,48]. At the same time, health education interventions appear to be associated with a lesser impact on cancer-related fatigue compared to exercise training [49].
Regarding behavioral outcomes after combined interventions including diet and exercise components delivered during chemotherapy, two studies [16,17] yielded significant changes in dietary intake and one presented significant changes in total physical activity in the intervention group (post- vs. pre-intervention) [17]. Our APAD intervention had a significantly favorable impact on leisure time physical activity at the end of chemotherapy and the end of radiotherapy, but improvements in total physical activity were not significant. Physical activity done in the framework of the APAD intervention was reported in the leisure category, which explains the enhancement of that physical activity type in our study. Regarding dietary intake, no significant changes were observed in the APAD arm of our study versus usual care. The 3-day record method and food to nutrition conversion software we used generated large standard deviations (see Table 4, baseline values for nutrients) that possibly impaired the statistical power to detect a between-group difference. The two previous studies [16,17] that demonstrated dietary changes analyzed dietary data that were collected by food frequency questionnaires.
Few studies evaluating a diet and/or exercise intervention have included follow-up measures [40,41,42,43,44]. Most of them, such as our present study, failed to show that significant effects were maintained after the end of the intervention [40,42,44,45]. One study reported improvements in the 6-min walk test 6 months post-intervention [43]. Difficulties could be related to the weak supervision and support over time, making patients unable to maintain the initially induced changes in behavior. The impact of the intervention seems to be limited in time, even for trials evaluating different doses of exercise in this context [50]. This finding may promote the necessity of setting longer supervised intervention models that can include, for example, the present “in-treatment” module during the chemotherapy part of the treatment plan, followed by additional supervised sessions during radiotherapy and a 6-month internet-based “survivor” module designed to maintain behavioral changes and support autonomy with limited cost. Several telephone- or internet-based diet–exercise interventions have been tested in BC survivors and yielded health benefits [51,52,53] with moderate to good adherence rates (from 41–87% of adherent patients) [54]. However, these adherence rates pinpoint the need for enduring strategies to maintain the motivation of this “survivor” population. In the study by Stone et al., adherence was significantly associated with moderate levels of exercise interventions (similar to the activity levels selected in our present study), BMI, physical health, and employment status, with reduced adherence in women working full-time compared to those who do not work full-time [55]. These findings, combined with the difference in general fatigue found in the present study, encompass the impact of social inequalities on the global impact of cancer and cancer treatments, as well as access and adherence to supportive care programs in these populations.
Our study was designed primarily to validate our previous results by assessing, in a pragmatic context, the effectiveness of an education program on exercise and diet compared to the standard of care in France (the usual care control arm). Though pragmatic and comprehensive, the main drawback of this design is that it does not allow disentanglement of the independent effects of exercise and dietary components. Another limitation is the differential drop-out rate in the APAD vs. usual care groups. Although many outcomes improved significantly in the APAD arm, and an increase was observed in the declared leisure physical activity, the between-group difference was not significant for objectively measured physical activity. The spontaneous physical activity level of the usual care group may have partly diluted the effects of the APAD intervention. Given the number of comparisons we made at each time point for the secondary outcomes without adjusting for multiple testing, we would expect a few false discoveries by chance.
Finally, based on the publication of Smets et al. [31], we hypothesized that our population would be affected by significant levels of cancer- and treatment-induced fatigue. However, our population was affected by much lower levels of fatigue than the expected mean Global fatigue score of 16, as the mean General fatigue scores were 9.95 and 9.63 for the APAD and control arms, respectively. These relatively low levels of fatigue could explain some of the negative results. Buffart et al. recently reported in a meta-analysis that, even if exercise induced a benefit for all patients during treatment, only patients affected by the worse levels of fatigue experienced a persistent impact of exercise post-treatment [56]. Thus, targeting specific subgroups of patients with higher scores for fatigue or variables associated with a greater level of fatigue during treatment may be more beneficial and cost-effective. In this regard, the observation of a gradient in the level of fatigue throughout the treatment period according to the stratum of precariousness confirms that the level of precariousness evaluated using the EPICES score is a stratification factor and fully justifies its inclusion as a stratification factor in this study. It seems important to consider this social parameter in the design of future studies evaluating the impact of supportive care interventions during the treatment of EBC.
5. Conclusions
In French patients receiving adjuvant treatment for EBC, a mixed hospital- and home-based diet and exercise education program during adjuvant chemotherapy and radiation therapy inducing a transient modification in the level of physical activity and dietary intake is not sufficient to demonstrate improvements in fatigue during and after treatment. We found no impact on the dose intensity of chemotherapy. Fatigue appears to correlate with the level of precariousness. Cancer care centers should consider integrating more proactive diet–exercise supportive care into the management of patients with BC who are receiving chemotherapy and/or radiotherapy, focusing on fatigued and/or precarious patients. Additional information is needed in order to identify the optimal intervention timing to induce persistent lifestyle changes and reduce long term alterations of patients’ quality of life in this setting.
Abbreviations
| APAD | adapted physical activity and diet |
| EBC | early breast cancer |
| BMI | body mass index |
| GPAQ | Global Physical Activity Questionnaire |
| MFI | Multidimensional Fatigue Inventory |
| PRO | patient-reported outcome |
| QoL | quality-of-life |
| RCT | randomized controlled trial |
| RDI | relative dose index |
| SD | standard deviation |
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/10/3081/s1, Figure S1: General Fatigue subscale of the MFI20 according to EPICE strata (precariousness level), Figure S2: General Fatigue subscale of the MFI20 according to randomization arms stratified by EPICE score (precariousness level), Figure S3: Evolution of quality of life (EORTC QLQ-C30) in the intention-to-treat population, Figure S4: Evolution of quality of life (EORTC QLQ-C30) in the per protocol population, Figure S5: Evolution of the different physical activity domains of the GPAQ questionnaire in the intention-to-treat population, Figure S6: Evolution of nutrient intake and weight control variables (weight, BMI, waist size) by randomization arm in the intention-to-treat population, Table S1: Fatigue sub-scales of the MFI20 over time in the per protocol population, Table S2: Anxiety and depression disorders in the per protocol population.
Author Contributions
Conceptualization, W.J., P.S., M.C., J.-P.B., S.G., G.R. and G.N.; Data curation, M.J., S.G., C.J. and S.L.; Formal analysis, W.J. and M.J.; Funding acquisition, J.-P.B. and G.R.; Investigation, W.J., A.A. (Antoine Arnaud), C.L.-P., P.D., A.A. (Ahmed Azzedine), O.T., S.S.-L., S.M., V.D., G.L., J.G., G.R. and L.V.; Methodology, M.J., P.S., M.C., J.-P.B., S.G., G.R. and G.N.; Project administration, J.-P.B., C.J., S.L. and G.R.; Resources, J.-P.B.; Supervision, W.J., S.M., J.-P.B., S.G., C.J., G.R. and G.N.; Validation, W.J., A.A. (Antoine Arnaud), M.J., C.L.-P., P.D., P.S., A.A. (Ahmed Azzedine), O.T., S.S.-L., S.M., M.C., J.-P.B., S.G., C.J., S.L., V.D., G.L., J.G., G.R., G.N. and L.V.; Writing–original draft, W.J., A.A. (Antoine Arnaud), M.J., C.L.-P., P.D., P.S., A.A. (Ahmed Azzedine), O.T., S.S.-L., S.M., M.C., J.-P.B., S.G., C.J., S.L., V.D., G.L., J.G., G.R., G.N. and L.V.; Writing–review & editing, W.J., A.A. (Antoine Arnaud), M.J., C.L.-P., P.D., P.S., A.A. (Antoine Arnaud), O.T., S.S.-L., S.M., M.C., J.-P.B., S.G., C.J., S.L., V.D., G.L., J.G., G.R., G.N. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the INCa-DGOS (Grant SHP-ESF 2012-079) and by a Montpellier Cancer SIRIC grant (INCa_Inserm_DGOS_12553).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Henry D.H., Viswanathan H.N., Elkin E.P., Traina S., Wade S., Cella D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 2.Fabi A., Falcicchio C., Giannarelli D., Maggi G., Cognetti F., Pugliese P. The course of cancer related fatigue up to ten years in early breast cancer patients: What impact in clinical practice? Breast. 2017;34:44–52. doi: 10.1016/j.breast.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Williams L.A., Bohac C., Hunter S., Cella D. Patient and health care provider perceptions of cancer-related fatigue and pain. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2016;24:4357–4363. doi: 10.1007/s00520-016-3275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelzang N.J., Breitbart W., Cella D., Curt G.A., Groopman J.E., Horning S.J., Itri L.M., Johnson D.H., Scherr S.L., Portenoy R.K. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. The Fatigue Coalition. Semin. Hematol. 1997;34:4–12. [PubMed] [Google Scholar]
- 5.Penttinen H.M., Saarto T., Kellokumpu-Lehtinen P., Blomqvist C., Huovinen R., Kautiainen H., Jarvenpaa S., Nikander R., Idman I., Luoto R., et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psycho Oncol. 2011;20:1211–1220. doi: 10.1002/pon.1837. [DOI] [PubMed] [Google Scholar]
- 6.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual. Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan D.S., Vieira A.R., Aune D., Bandera E.V., Greenwood D.C., McTiernan A., Navarro Rosenblatt D., Thune I., Vieira R., Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Playdon M.C., Bracken M.B., Sanft T.B., Ligibel J.A., Harrigan M., Irwin M.L. Weight Gain After Breast Cancer Diagnosis and All-Cause Mortality: Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2015;107:djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell K.L., Winters-Stone K.M., Wiskemann J., May A.M., Schwartz A.L., Courneya K.S., Zucker D.S., Matthews C.E., Ligibel J.A., Gerber L.H., et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger A.M., Mooney K., Alvarez-Perez A., Breitbart W.S., Carpenter K.M., Cella D., Cleeland C., Dotan E., Eisenberger M.A., Escalante C.P., et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. JNCCN. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones L.W., Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7:1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- 12.Denlinger C.S., Ligibel J.A., Are M., Baker K.S., Demark-Wahnefried W., Dizon D., Friedman D.L., Goldman M., Jones L., King A., et al. Survivorship: Nutrition and weight management, Version 2.2014. Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2014;12:1396–1406. doi: 10.6004/jnccn.2014.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demark-Wahnefried W., Morey M.C., Sloane R., Snyder D.C., Miller P.E., Hartman T.J., Cohen H.J. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin P.J., Segal R.J., Vallis M., Ligibel J.A., Pond G.R., Robidoux A., Blackburn G.L., Findlay B., Gralow J.R., Mukherjee S., et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: The LISA trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 15.Rock C.L., Flatt S.W., Byers T.E., Colditz G.A., Demark-Wahnefried W., Ganz P.A., Wolin K.Y., Elias A., Krontiras H., Liu J., et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W., Case L.D., Blackwell K., Marcom P.K., Kraus W., Aziz N., Snyder D.C., Giguere J.K., Shaw E. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin. Breast Cancer. 2008;8:70–79. doi: 10.3816/CBC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 17.Djuric Z., Ellsworth J.S., Weldon A.L., Ren J., Richardson C.R., Resnicow K., Newman L.A., Hayes D.F., Sen A. A Diet and Exercise Intervention during Chemotherapy for Breast Cancer. Open Obes. J. 2011;3:87–97. doi: 10.2174/1876823701103010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carayol M., Ninot G., Senesse P., Bleuse J.P., Gourgou S., Sancho-Garnier H., Sari C., Romieu I., Romieu G., Jacot W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer. 2019;19:737. doi: 10.1186/s12885-019-5896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrier L., Foucaut A.M., Morelle M., Touillaud M., Kempf-Lepine A.S., Heinz D., Gomez F., Meyrand R., Baudinet C., Berthouze S., et al. Cost-effectiveness of an exercise and nutritional intervention versus usual nutritional care during adjuvant treatment for localized breast cancer: The PASAPAS randomized controlled trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2020;28:2829–2842. doi: 10.1007/s00520-019-05078-4. [DOI] [PubMed] [Google Scholar]
- 20.Carayol M., Romieu G., Bleuse J.P., Senesse P., Gourgou-Bourgade S., Sari C., Jacot W., Sancho-Garnier H., Janiszewski C., Launay S., et al. Adapted physical activity and diet (APAD) during adjuvant breast cancer therapy: Design and implementation of a prospective randomized controlled trial. Contemp. Clin. Trials. 2013;36:531–543. doi: 10.1016/j.cct.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Eisinger F., Viguier J., Touboul C., Coscas Y., Pivot X., Blay J.Y., Lhomel C., Morere J.F. Social stratification, risk factor prevalence and cancer screening attendance. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2015;24:S77–S81. doi: 10.1097/CEJ.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 22.Labbe E., Blanquet M., Gerbaud L., Poirier G., Sass C., Vendittelli F., Moulin J.J. A new reliable index to measure individual deprivation: The EPICES score. Eur. J. Public Health. 2015;25:604–609. doi: 10.1093/eurpub/cku231. [DOI] [PubMed] [Google Scholar]
- 23.Morere J.F., Eisinger F., Touboul C., Lhomel C., Couraud S., Viguier J. Decline in Cancer Screening in Vulnerable Populations? Results of the EDIFICE Surveys. Curr. Oncol. Rep. 2018;20:17. doi: 10.1007/s11912-017-0649-7. [DOI] [PubMed] [Google Scholar]
- 24.Roche H., Fumoleau P., Spielmann M., Canon J.L., Delozier T., Serin D., Symann M., Kerbrat P., Soulie P., Eichler F., et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 25.Clinton S.K., Giovannucci E.L., Hursting S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020;150:663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz K.H., Courneya K.S., Matthews C., Demark-Wahnefried W., Galvao D.A., Pinto B.M., Irwin M.L., Wolin K.Y., Segal R.J., Lucia A., et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 27.Courneya K.S., Mackey J.R., McKenzie D.C. Exercise for breast cancer survivors: Research evidence and clinical guidelines. Physician Sportsmed. 2002;30:33–42. doi: 10.3810/psm.2002.08.402. [DOI] [PubMed] [Google Scholar]
- 28.Wolin K.Y., Schwartz A.L., Matthews C.E., Courneya K.S., Schmitz K.H. Implementing the exercise guidelines for cancer survivors. J. Supportive Oncol. 2012;10:171–177. doi: 10.1016/j.suponc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roza A.M., Shizgal H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984;40:168–182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 30.Martin A., Touvier M., Volatier J.L. The basis for setting the upper range of adequate intake for regulation of macronutrient intakes, especially amino acids. J. Nutr. 2004;134:1625S–1629S. doi: 10.1093/jn/134.6.1625S. discussion 1630S–1632S, 1667S–1672S. [DOI] [PubMed] [Google Scholar]
- 31.Smets E.M., Garssen B., Cull A., de Haes J.C. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br. J. Cancer. 1996;73:241–245. doi: 10.1038/bjc.1996.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentile S., Delaroziere J.C., Favre F., Sambuc R., San Marco J.L. Validation of the French ‘multidimensional fatigue inventory’ (MFI 20) Eur. J. Cancer Care. 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 33.Razavi D., Delvaux N., Farvacques C., Robaye E. Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br. J. Psychiatry J. Ment. Sci. 1990;156:79–83. doi: 10.1192/bjp.156.1.79. [DOI] [PubMed] [Google Scholar]
- 34.Sprangers M.A., Cull A., Bjordal K., Groenvold M., Aaronson N.K. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: Guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1993;2:287–295. doi: 10.1007/BF00434800. [DOI] [PubMed] [Google Scholar]
- 35.Trinh O.T., Nguyen N.D., van der Ploeg H.P., Dibley M.J., Bauman A. Test-retest repeatability and relative validity of the Global Physical Activity Questionnaire in a developing country context. J. Phys. Act. Health. 2009;6:S46–S53. doi: 10.1123/jpah.6.s1.s46. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Casado A., Alejo L.B., Santos-Lozano A., Soria A., Ortega M.J., Pagola I., Fiuza-Luces C., Palomo I., Garatachea N., Cebolla H., et al. Validity of the Physical Activity Questionnaires IPAQ-SF and GPAQ for Cancer Survivors: Insights from a Spanish Cohort. Int. J. Sports Med. 2016;37:979–985. doi: 10.1055/s-0042-103967. [DOI] [PubMed] [Google Scholar]
- 37.Bull F.C., Maslin T.S., Armstrong T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health. 2009;6:790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 38.Biro G., Hulshof K.F., Ovesen L., Amorim Cruz J.A., Group E. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002;56:S25–S32. doi: 10.1038/sj.ejcn.1601426. [DOI] [PubMed] [Google Scholar]
- 39.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., de Haes J.C., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 40.Cornette T., Vincent F., Mandigout S., Antonini M.T., Leobon S., Labrunie A., Venat L., Lavau-Denes S., Tubiana-Mathieu N. Effects of home-based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016;52:223–232. [PubMed] [Google Scholar]
- 41.Courneya K.S., Segal R.J., Mackey J.R., Gelmon K., Reid R.D., Friedenreich C.M., Ladha A.B., Proulx C., Vallance J.K., Lane K., et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 42.Husebo A.M., Dyrstad S.M., Mjaaland I., Soreide J.A., Bru E. Effects of scheduled exercise on cancer-related fatigue in women with early breast cancer. Sci. World J. 2014;2014:271828. doi: 10.1155/2014/271828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutrie N., Campbell A.M., Whyte F., McConnachie A., Emslie C., Lee L., Kearney N., Walker A., Ritchie D. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. BMJ. 2007;334:517. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Travier N., Velthuis M.J., Steins Bisschop C.N., van den Buijs B., Monninkhof E.M., Backx F., Los M., Erdkamp F., Bloemendal H.J., Rodenhuis C., et al. Effects of an 18-week exercise programme started early during breast cancer treatment: A randomised controlled trial. BMC Med. 2015;13:121. doi: 10.1186/s12916-015-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Waart H., Stuiver M.M., van Harten W.H., Geleijn E., Kieffer J.M., Buffart L.M., de Maaker-Berkhof M., Boven E., Schrama J., Geenen M.M., et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 46.Van Vulpen J.K., Sweegers M.G., Peeters P.H.M., Courneya K.S., Newton R.U., Aaronson N.K., Jacobsen P.B., Galvao D.A., Chinapaw M.J., Steindorf K., et al. Moderators of Exercise Effects on Cancer-related Fatigue: A Meta-analysis of Individual Patient Data. Med. Sci. Sports Exerc. 2020;52:303–314. doi: 10.1249/MSS.0000000000002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furmaniak A.C., Menig M., Markes M.H. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016;9:CD005001. doi: 10.1002/14651858.CD005001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Courneya K.S., McKenzie D.C., Mackey J.R., Gelmon K., Friedenreich C.M., Yasui Y., Reid R.D., Cook D., Jespersen D., Proulx C., et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J. Natl. Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan P., Denieffe S., Murphy N.M., Harrison M. Exercise is more effective than health education in reducing fatigue in fatigued cancer survivors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2020;10 doi: 10.1007/s00520-020-05328-w. [DOI] [PubMed] [Google Scholar]
- 50.An K.Y., Morielli A.R., Kang D.W., Friedenreich C.M., McKenzie D.C., Gelmon K., Mackey J.R., Reid R.D., Courneya K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J. Cancer. 2020;146:150–160. doi: 10.1002/ijc.32493. [DOI] [PubMed] [Google Scholar]
- 51.Morey M.C., Snyder D.C., Sloane R., Cohen H.J., Peterson B., Hartman T.J., Miller P., Mitchell D.C., Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: A randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demark-Wahnefried W., Clipp E.C., Lipkus I.M., Lobach D., Snyder D.C., Sloane R., Peterson B., Macri J.M., Rock C.L., McBride C.M., et al. Main outcomes of the FRESH START trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 53.Demark-Wahnefried W., Clipp E.C., Morey M.C., Pieper C.F., Sloane R., Snyder D.C., Cohen H.J. Lifestyle intervention development study to improve physical function in older adults with cancer: Outcomes from Project LEAD. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams R.N., Mosher C.E., Blair C.K., Snyder D.C., Sloane R., Demark-Wahnefried W. Cancer survivors’ uptake and adherence in diet and exercise intervention trials: An integrative data analysis. Cancer. 2015;121:77–83. doi: 10.1002/cncr.28978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone C.R., Friedenreich C.M., O’Reilly R., Farris M.S., Vallerand J.R., Kang D.W., Courneya K.S. Predictors of Adherence to Different Volumes of Exercise in the Breast Cancer and Exercise Trial in Alberta. Ann. Behav. Med. Publ. Soc. Behav. Med. 2019;53:453–465. doi: 10.1093/abm/kay057. [DOI] [PubMed] [Google Scholar]
- 56.Buffart L.M., Sweegers M.G., May A.M., Chinapaw M.J., van Vulpen J.K., Newton R.U., Galvao D.A., Aaronson N.K., Stuiver M.M., Jacobsen P.B., et al. Targeting Exercise Interventions to Patients with Cancer in Need: An Individual Patient Data Meta-Analysis. J. Natl. Cancer Inst. 2018;110:1190–1200. doi: 10.1093/jnci/djy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.