Abstract
BACKGROUND
Children with cancer often face infertility as a long-term complication of their treatment. For boys, compromised testicular function is common after chemotherapy and currently there are no well-established options to prevent this damage. Platinum-based agents are used to treat a wide variety of childhood cancers. However, platinum agents are not currently included in the cyclophosphamide equivalent dose (CED), which is used clinically to assess the risks to fertility posed by combination chemotherapy in children with cancer.
OBJECTIVE AND RATIONALE
This was a systematic search of the literature designed to determine the evidence for effects of platinum-based cancer treatment on the prepubertal human testis in relation to subsequent testicular function and fertility.
SEARCH METHODS
PubMed and EMBASE were searched for articles published in English between 01 January 1966 and 05 April 2020 using search terms including ‘cancer treatment’, ‘chemotherapy’, ‘human’, ‘prepubertal’, ‘testis’, ‘germ cells’, ‘testosterone’ and related terms. Abstracts were screened and full-text articles were obtained for those that met the three major inclusion criteria (age ≤12 years at treatment, exposure to platinum-based chemotherapeutic and measure of reproductive function). Screening of bibliographies for full-text articles was used to identify additional studies.
OUTCOMES
Our initial search identified 1449 articles of which 20 (1.3%) studies (n = 13 759 males) met all inclusion criteria. A control group (healthy individuals or siblings) was included for 5/20 (25%) studies. A total of 10/20 (50%) studies provided sub-analysis of the relative gonadotoxicity of platinum-based agents.
The primary outcome measures were: pregnancies and fatherhood; semen analysis; and hormonal function. For pregnancies and fatherhood, three studies (n = 10 453 males) reported negative associations with platinum-agents, including the largest (n = 5640) controlled study (hazard ratio = 0.56, P = 0.0023), whilst two other studies (n = 1781) with platinum sub-analysis reported no association. For semen analysis (based on World Health Organization criteria), platinum-based chemotherapy was associated with azoospermia in one study (n = 129), whilst another (n = 44) found no association and the remainder did not perform platinum-based sub-analysis. For hormone analysis, conflicting results were obtained regarding potential associations between platinum-based agents and elevated FSH (a proxy for impaired spermatogenesis); however, the majority of these studies were based on low numbers of patients receiving platinum-based chemotherapy.
WIDER IMPLICATIONS
Overall, these results indicate that platinum-based chemotherapy should be included in clinical calculators, for example CED, used to determine gonadotoxicity for childhood cancer treatment. These findings have important implications for clinicians regarding counselling patients and their carer(s) on fertility risk, guiding requirements for fertility preservation strategies (e.g. testicular tissue cryopreservation) and modification of treatments to reduce or eliminate the risk of infertility in childhood cancer survivors.
Keywords: cancer treatment, chemotherapy, human, prepubertal, child, testis, fertility, cisplatin, carboplatin, platinum
Introduction
Childhood cancer rates have increased dramatically over recent decades and it is estimated that one in 530 young adults is a survivor of childhood cancer (Ward et al., 2014). The increasing incidence, coupled with remarkable improvements in cure rates (>80% 5-year survival), have resulted in an increase in young adults experiencing treatment-related morbidity (Anderson et al., 2015). Infertility is a well-recognized complication of cancer treatment, although the relative contribution of individual agents to fertility risk in childhood cancer survivors has not been fully elucidated. Amongst chemotherapeutics, alkylating agents (e.g. cyclophosphamide) are known to affect fertility in males and an objective measure of relative gonadotoxicity between chemotherapeutics, the cyclophosphamide equivalent dose (CED), has been established (Green et al., 2014b). This calculation can be used to estimate cumulative gonadotoxicity of multiple agents for a specific regimen.
Platinum-based chemotherapy (cisplatin or carboplatin) agents are used in treatment of a variety of childhood cancers including brain tumours, osteosarcoma, neuroblastoma, hepatoblastoma and germ cell tumours (GCTs). Whilst most of the commonly used chemotherapy drugs are included in the CED calculator, platinum-based chemotherapy is a notable exception. Therefore, the present study aimed to review the literature relating to effects of exposure to platinum-based chemotherapy during childhood on gonadal function and future fertility.
Methods
We performed a systematic search of the existing literature reporting the effects of platinum-based chemotherapy for childhood cancer on testicular development and function. We followed PRISMA guidelines for reporting systematic reviews (Moher et al., 2009). Given that the search was conducted to identify clinical and laboratory experimental data, the study was not registered with PROSPERO.
Information source
PubMed and EMBASE were searched (to 05 April 2020) to identify original clinical and experimental studies describing the testicular effects of platinum-based chemotherapy on prepubertal human testis. Additional studies were identified from a bibliographic screen of reference lists of included articles.
Inclusion criteria
The inclusion criteria for study selection were:
articles published between 01 January 1966 and 05 April 2020 and written in English;
clinical and experimental studies; and
outcomes relating to reproductive function including fatherhood, sperm counts, reproductive hormones (LH, FSH, testosterone, inhibin B and anti-Müllerian hormone, testicular volume and puberty).
Exclusion criteria
-
The exclusion criteria for the review were:
exposure to cancer treatment during (peri)puberty (≥12 years) and adulthood;
studies that did not include human data or experimental studies involving human tissue/cells; and
review publications.
Search and study selection
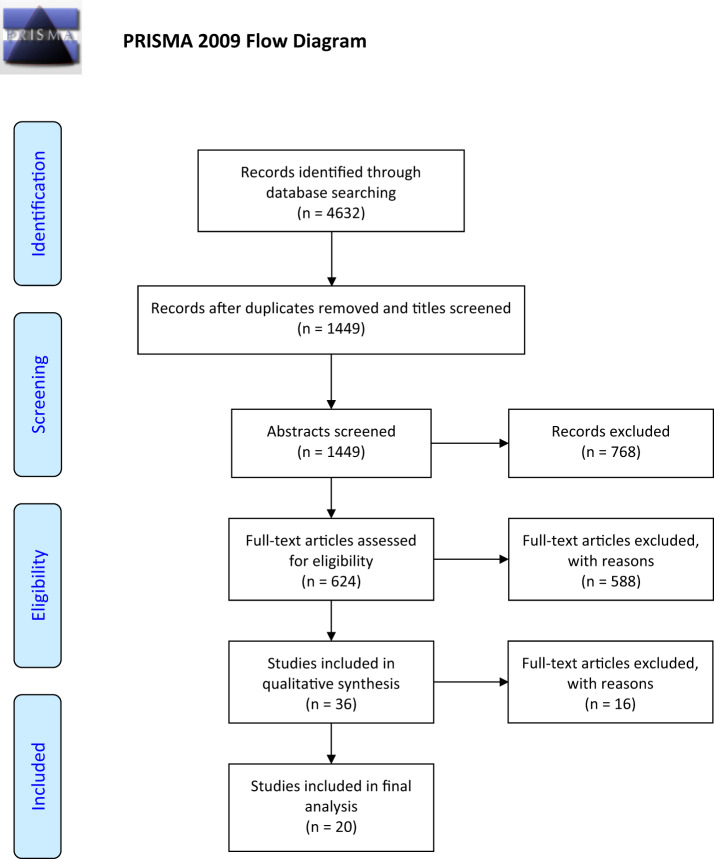
The search terms and strategy (Supplementary Table SI) used for the systematic review were adapted from a previous study (Skinner et al., 2017). After removing duplicates and screening by titles, we identified 1449 studies. R.T.M. and L.T.E. screened abstracts independently to determine eligibility. For abstracts that were not selected by both authors, these were discussed, and a joint decision made regarding inclusion for full-text screening. Full texts were obtained for 624 studies that met the criteria for inclusion. An initial screen of full texts identified 588 studies that were excluded for not meeting at least one aspect of the inclusion criteria (Supplementary Table SII). Of the 36 remaining papers that were subjected to a detailed assessment, a further 16 papers were excluded for a variety of reasons (Supplementary Table SIII). In total, 20 papers were included in the final analysis. A PRISMA flowchart for the search and study selection is presented in Fig. 1.
Figure 1.
PRISMA flow diagram for the selection of studies to assess the impacts of platinum-based chemotherapy on subsequent testicular function and fertility in boys with cancer. PRISMA, preferred reporting items for systematic reviews and meta-analysis.
Summary measures
Data were extracted in relation to four categories: pregnancy and fertility; semen analysis; hormones and testicular volume; and experimental studies (tissue and cells). Risk of bias within and between studies was assessed using the principles described for the assessment of risk of bias in non-randomized studies (Sterne et al., 2019). This included an assessment of risk of confounding and bias in selection, information and reporting for each study (Supplementary Table SIV). For each category, risk of bias was assessed as ‘low’, ‘moderate’, ‘serious’, ‘critical’ or ‘no information’. Given the heterogeneity of reported summary measures, all methods of reporting were included.
Results
Studies assessing testicular function and fertility after treatment with platinum agents
Overall, 20 studies reported testicular function and fertility following exposure to platinum-based agents (Table I). The risk of bias for each study is presented in Supplementary Table SIV. Overall risk of bias was lower in the studies that included a sub-analysis of platinum exposure, compared with cohort studies that did not include a sub-analysis. A detailed description of the data extracted from these studies is provided in Supplementary Table SV.
Table I.
Summary of 20 studies including use of platinum agents, prepubertal study participants and an outcome measure relating to fertility.
| Study | Study design | Level* | Control group | Fertility outcome |
|---|---|---|---|---|
| Flamant et al. (1984) | Multi-centre cohort | 3 | No | Pregnancy and fertility |
| Wallace et al. (1989) | Single-centre cohort | 3 | No | Gonadotrophins |
| Kiltie and Gattamaneni (1995) | Single-centre cohort | 3 | No | Gonadotrophins |
| Muller et al. (1996) | Single-centre cohort | 3 | Yesa | Semen analysis; testicular volume |
| Hale et al. (1999) | Single-centre cohort | 3 | No | Gonadotrophins |
| Relander et al. (2000) | Single-centre cohort | 3 | No | Semen analysis; gonadotrophins; testicular volume |
| Longhi et al. (2003) | Single-centre cohort | 3 | No | Semen analysis; gonadotrophins |
| Ridola et al. (2009) | Multi-centre cohort | 2 | No | Pregnancy and fertility; gonadotrophins |
| Romerius et al. (2011) | Single-centre cohort | 3 | No | Semen analysis |
| Tromp et al. (2011) | Single-centre cohort | 3 | No | Pregnancy and fertility; gonadotrophins |
| Odagiri et al. (2012) | Single-centre cohort | 3 | No | Gonadotrophins |
| Reinmuth et al. (2013) | Multi-centre cohort | 3 | No | Pregnancy and fertility |
| Green et al. (2014a) | Multi-centre cohort | 3 | No | Semen analysis |
| Green et al. (2014b) | Multi-centre cohort | 3 | No | Pregnancy and fertility |
| Wasilewski-Masker et al. (2014) | Multi-centre cohort | 3 | Yes | Pregnancy and fertility |
| Brignardello et al. (2016) | Single-centre cohort | 3 | No | Gonadotrophins |
| Chemaitilly et al. (2016) | Case report | 5 | No | Gonadotrophins; testicular volume |
| Chow et al. (2016) | Multi-centre cohort | 3 | Yesb | Pregnancy and fertility |
| Isaksson et al. (2018) | Multi-centre cohort | 2 | Yesa | Gonadotrophins |
| Utriainen et al. (2019) | Multi-centre cohort | 2 | Yesa | Pregnancy and fertility; gonadotrophins |
Level of Evidence (adapted from https://www.cebm.net/2016/05/ocebm-levels-of-evidence/).
1—Properly powered and conducted randomized clinical trial; systematic review with meta-analysis.
2—Well-designed controlled study without randomization; prospective comparative cohort study.
3—Case–control studies; retrospective cohort study.
4—Case series with or without intervention; cross-sectional study.
5—Opinion of respected authorities; case reports.
Healthy age-matched controls.
Healthy siblings.
Fertility and fatherhood
We identified eight papers which included pregnancy and fatherhood as outcome measures of fertility (Table II). Several papers reported data from a multi-centre cohort Childhood Cancer Survivor study (CCSS) (Robison et al., 2009). One questionnaire-based study including 1622 male cancer survivors aged 0–21 years at diagnosis reported that cisplatin exposure was not significantly (P > 0.20) associated with infertility, defined as difficulty in getting a female partner pregnant (>1 year) (Wasilewski-Masker et al., 2014).
Table II.
Summary of studies involving pregnancy and fertility outcome measures and reported to include platinum-including treatment regimens.
| Study | Diagnoses | Males (n) | Age at diagnosis (years) | Chemotherapy | Platinum analysis | Summary finding |
|---|---|---|---|---|---|---|
| Flamant et al. (1984) | Nonseminomatous GCT | 13 | 1–14 | Actinomycin D, Cyclophosphamide, Vincristine, Doxorubicin, Bleomycin, Cisplatin | No | One case of sterility attributed to whole abdominal irradiation. |
| Ridola et al. (2009) | Various | 159 | 9–19 | Ifosfamide versus Cyclophosphamide protocols | Yes | Platinum agents reported to have no impact on testicular function but data not shown. |
| Tromp et al. (2011) | Various | 565 | 0–17.8 | Various | Yes | 73 partner pregnancies (56 natural conception). Platinum sub-analysis for hormones only. |
| Reinmuth et al. (2013) | Various | 234 | 0–15 | Cyclophosphamide, Ifosfamide, Cis/Carboplatin, Etoposide | Yesc | Higher rate of infertility for platinum-based agents (carboplatin or cisplatin). |
| Green et al. (2014b) | Various | 4579 | 5–20 | Various | Yes | Lower rate of fatherhood after cisplatin compared to no cisplatin. Statistical data not shown. |
| Wasilewski-Masker et al. (2014) | Various | 1622 | 9a | Various | Yes | Platinum agents did not have independent association with fertility—preliminary modelling. |
| Chow et al. (2016) | Various | 5640 | ≤20 | Various | Yesc | Cisplatin associated with reduced pregnancy (≥488 mg/m2) and livebirth (≥355 mg/m2) rates. |
| Utriainen et al. (2019) | Neuroblastoma | 9 | 0.2–3.6b | Various, HSCT | Yes | d Cisplatin cumulative dose did not correlate with testicular size. |
Note that summary findings relate to patients receiving treatment when <12 years of age.
GCT, germ cell tumour; HSCT, haematopoietic stem cell transplant.
Mean age.
Interquartile range.
Multivariate and univariate regression analysis.
One patient fathered a child (patient received cisplatin).
Other studies from the CCSS have observed an impact on fertility with exposure to cisplatin. A study of 5640 male patients, of whom 455 were treated with cisplatin, reported that higher doses were significantly associated with a lower likelihood of siring pregnancies and livebirths (Chow et al., 2016). Multivariable models found that the upper tertile of cisplatin dose (≥488 mg/m2) was associated with a reduced likelihood of siring pregnancies (hazard ratio (HR) = 0.56, P = 0.0023), while lower doses of cisplatin (355–487 mg/m2) were associated with a reduced likelihood of siring livebirths (HR = 0.64, P = 0.071). Another study reported a lower percentage of fatherhood among patients who received cisplatin (10.9%) compared to patients who did not (14.9%), although no statistical data was provided (Green et al., 2014b).
Other studies have drawn conflicting conclusions on the impact of platinum agents on fertility. A study of 159 males ranging from 0 to 21 years at diagnosis using fatherhood and hormone measurements as outcome measures reported that addition of platinum agents to cyclophosphamide or ifosfamide did not increase the risk of gonadal dysfunction (statistical data not shown) (Ridola et al., 2009).
A study of 234 males aged 0–15 years reported an increased likelihood of infertility (unsuccessful at fathering a child or abnormal fertility test) among patients receiving cisplatin or carboplatin (Reinmuth et al., 2013). However, this increased risk was not statistically significant under both univariate and multivariate analysis, even at high doses (≥500 mg/m2 for cisplatin, ≥2000 mg/m2 for carboplatin). In a small study involving nine males with neuroblastoma ranging from 0.2 to 3.6 years of age at diagnosis, only one male patient was observed to have fathered a child (Utriainen et al., 2019). This patient had received cisplatin and etoposide as induction chemotherapy, followed by high-dose melphalan. However, participants had also received total body irradiation (TBI) and haematopoietic stem cell transplants (HSCTs), which are known to be sterilizing treatments (Freycon et al., 2019). A study of a cisplatin-including regimen for GCT in children also reported a single case of sterility, which was attributed to whole abdominal irradiation (Flamant et al., 1984). In a larger study of 565 males, aged 0–17.8 years at diagnosis, 73 of the participants sired pregnancies and 56 of these men conceived naturally (Tromp et al., 2011). A total of 120 conceptions were reported with 103 livebirths and 14 miscarriages. However, the study did not include an individual analysis of platinum agents with regards to pregnancy and fertility.
Semen analysis
Semen analysis represents a reliable indicator of fertility status in adult men. We identified five studies reporting the impact of platinum-based chemotherapy regimens for childhood cancer treatment on sperm count in adulthood (Table III). Methods and cut-offs for semen analysis varied between studies (Supplementary Table SV) and 3/5 studies reported the use of World Health Organization standards relevant to the period of study (Table III). All studies combined semen analysis with assessment of gonadotrophins, testicular volume or pubertal staging. A study involving 129 males diagnosed between the ages of 0–17 years reported azoospermia in 4/5 (80%) of those who had received what the authors considered a ‘sterilizing’ dose (>500 mg/m2) of cisplatin (without radiotherapy), compared with 0/8 (0%) of those who had received <500 mg/m2 of cisplatin (Romerius et al., 2011). Two studies included a control group of age-matched healthy participants (Muller et al., 1996; Utriainen et al., 2019). A study involving 33 males aged 0–19 years with various cancers showed azoospermia in 2/14 (14%) and oligozoospermia in 3/14 (21%) of long-term survivors compared with normospermia in 8/8 (100%) of healthy age-matched controls (Muller et al., 1996). Patients with azoospermia were significantly more likely to have been exposed to alkylating agents than those with normospermia. However, the contribution of cisplatin to the semen parameters was not reported (Muller et al., 1996). The second study involving a control group investigated nine male childhood cancer survivors who commenced treatment with alkylating-based chemotherapy, with or without cisplatin, between the ages of 0–3 years (Utriainen et al., 2019). Only one patient had a sperm count performed in adulthood. He had received cisplatin and had fathered a child, although interestingly he had a low total sperm count (0.8 × 106/ml) with oligo-asthenozoo-spermia (Utriainen et al., 2019). Specific correlation of semen analysis with platinum exposure was only reported in one study (Green et al., 2014a): this study involved 214 subjects treated for various cancers from the age of 0–19 years. Sub-analysis of those receiving cisplatin for neuroblastoma or osteosarcoma did not reveal a statistically significant effect of cisplatin on the risk of azoospermia (Green et al., 2014a). However, there were only 44 patients in this subgroup and these individuals had also received alkylating agents as part of their regimen, which may have affected the potential to detect a significant effect (Green et al., 2014a). A study involving 96 males, of whom 11 were prepubertal at diagnosis, reported one case of a prepubertal boy (>12 years old) in which semen analysis was performed in adulthood (Longhi et al., 2003). This individual was azoospermic and had received cisplatin treatment, in addition to ifosfamide, as part of his regimen. None of the patients who were ≤12 years old at diagnosis had semen analysis performed (Longhi et al., 2003).
Table III.
Summary of studies involving semen analysis in childhood cancer survivors exposed to treatment regimens that are reported to include platinum-based chemotherapy.
| Study | Diagnoses | Age at diagnosis (years) | Males (n) | Chemotherapy treatment | Platinum analysis | Summary finding |
|---|---|---|---|---|---|---|
| Muller et al. (1996) | Various | 7–17 | 33 | Various | No | Differences between azoospermic and normospermic survivors were not detected for non-alkylating agents. |
| Longhi et al. (2003) a | Osteosarcoma | 10–42 | 96 | Methotrexate, Cisplatin, Doxorubicin ± Ifosfamide ± Etoposide | No | None of the patients <12-years-old at diagnosis had a sperm count performed. |
| Romerius et al. (2011 a | Various | 0–17 | 129 | Alkylating agents/Cisplatin | No | Azoospermia in 4/5 (80%) versus 0/8 (0%) based on a threshold dose of >500 mg/m2 cisplatin. |
| Green et al. (2014a) a | Various | 0–19 | 214 | Various | Yesb | No increase in azoospermia in subgroup receiving cisplatin treatment for neuroblastoma/osteosarcoma. |
| Utriainen et al. (2019) | Neuroblastoma | 0–3 | 9 | Various | Yes | Sperm count performed in one patient who had received cisplatin—oligozoospermia although reported to have fathered a child. |
Note that summary findings relate to patients receiving treatment when <12 years of age.
Semen analysis reported to be based on World Health Organization criteria.
Sub-group analysis for cisplatin in osteosarcoma and neuroblastoma survivors.
Hormone analysis and puberty
Gonadotrophin measurements can be used to identify hypogonadism in adulthood. We identified 13 studies of gonadotrophins, testicular volume and puberty in childhood cancer survivors exposed to treatment regimens that are reported to include platinum-based chemotherapy (Table IV). A study including 33 pubertal males (aged 7–17 years at diagnosis) with a range of malignancies demonstrated a significant increase in basal FSH and LH when compared with healthy age-matched controls, indicating impaired spermatogenesis in the cancer survivor group. Of these, 29 males had received chemotherapy, which included alkylating agents for 24 patients (Muller et al., 1996). FSH levels correlated with testicular volumes and sperm counts (see above), providing further evidence to indicate impaired spermatogenesis. Despite the higher LH, suggesting a degree of Leydig cell failure, no differences in testosterone were identified between cancer survivors and controls (Muller et al., 1996). Whilst some patients had received cisplatin, the relationship between cumulative cisplatin dose and testicular function was not reported.
Table IV.
Summary of studies involving gonadotrophins, testicular volume and puberty in childhood cancer survivors exposed to treatment regimens that are reported to include platinum-based chemotherapy.
| Study | Diagnoses | Males (n) | Age at diagnosis (years) | Chemotherapy | Cranial irradiation | Platinum analysis | Summary finding |
|---|---|---|---|---|---|---|---|
| Wallace et al. (1989) | Various | 8 | 7–14 | Multiple regimens | No | No | d Gonadotrophins remained low at follow-up (≤3 years post-diagnosis) in keeping with retained prepubertal status. |
| Kiltie and Gattamaneni (1995) | IGCT | 18 | 3–15 | Multiple regimens | Yes (n = 18) | No | d Precocious puberty in two boys prior to treatment and normal puberty in another two. Specific impact of cisplatin not investigated. |
| Muller et al. (1996) | Various | 33 | 7–17 | Multiple regimens | Yes (n = 6) | No | Overall increase in basal FSH and LH compared with healthy controls. Specific impact of cisplatin not investigated. |
| Hale et al. (1999) | GCT | 26 | 0–16 | Multiple regimens | No | No | d Puberty normal in all patients. Specific impact of cisplatin not investigated. |
| Relander et al. (2000) | Various | 77 | 0–17 | Multiple regimens | Yes (n = 28) | No | Puberty and testosterone normal in 98% and 95%, respectively. Raised FSH in 14% of entire cohort. Only one patient received cisplatin. |
| Ridola et al. (2009) | Various | 159 | 0–20 | Cyclophosphamide versus Ifosfamide | No | Yes | Inclusion of platinum-based agents reported to have no effect on testicular function but data not shown. |
| Romerius et al. (2011) | Various | 129 | 0–17 | Multiple regimens | Yes (n = 61)a | No | Elevated FSH in 32.5% of the entire cohort. Specific impact of cisplatin not investigated. |
| Tromp et al. (2011) | Various | 565 | 0–17 | Multiple regimens | Yes (n = 135) | Yesc | Raised FSH in 33% and low testosterone in 12%. No association between cisplatin and FSH. |
| Odagiri et al. (2012) | IGCT | 14 | 8–19 | Carboplatin and Etoposide | Yes (n = 14) | No | No impairment of gonadotrophin secretion at 2–11 years post-diagnosis. |
| Brignardello et al. (2016) | Various | 199 | 0–18 | Various | Yes (n = 71) | Yesd | FSH or low Inhibin B in 35% (alkylators) versus 60% (alkylators + platinum agent). Compared with 12% (no alkylators or platinum agents). |
| Chemaitilly et al. (2016) | Glioma | 1 | 2 | Carboplatin, Vincristine, Temozolomide, Lomustine, Thioguanine, Procarbazine | No | No | d Case report aged 11 years. Tanner stage 4 with testicular volumes 4.5 ml indicating impaired spermatogenesis. |
| Isaksson et al. (2018) | Various | 121 | 5–15 | Multiple regimens | Yes (n = 12) | No | Raised FSH in 5.8% of childhood cancer survivors compared with 1% of healthy age-matched controls. |
| Utriainen et al. (2019) | Neuroblastoma | 9 | 0–3 | Multiple regimens | Yes (n = 4) | Yes | d Significantly raised FSH and reduced TV compared with healthy controls. No correlation between cumulative cisplatin dose and TV. |
IGCT, intracranial germ cell tumour; TV, testicular volume.
Includes all non-testicular irradiation.
Multivariate and univariate regression analysis.
Comparison of alkylators ± platinum agent.
Summary includes only patients receiving treatment <12 years of age.
Assessment of the hypothalamic–pituitary–gonadal (HPG) axis was conducted on a cohort of 14 young males (aged 8–19 years) with intracranial GCT (IGCT), of whom six were <12 years at diagnosis (Odagiri et al., 2012). All patients were treated with carboplatin and etoposide. However, patients also received cranial irradiation, which can impair pituitary gonadotrophin secretion. None of the six boys had impairment of gonadotrophin secretion by last assessment, which was 2–11 years after diagnosis (Odagiri et al., 2012). Whether this related only to hypogonadotrophic hypogonadism is not clear as no definition of what constituted abnormal gonadotrophin production was given.
Another study of patients with IGCT, including 18 boys aged 3–15 years, reported pubertal timing in four boys treated before the age of 12 years (Kiltie and Gattamaneni, 1995). Of these, two had precocious puberty prior to therapy, whilst two entered puberty at a normal age (11–12 years). Some patients in this study received platinum-based treatment; however, specific treatment regimens for individual patients were not described.
A case report of an 11-year-old boy treated with alkylating agents and carboplatin for a glioma at the age of 2 years reported puberty with evidence of impaired spermatogenesis (Chemaitilly et al., 2016). He was Tanner stage 4 with a testosterone of 8.9 nmol/l, although his testicular volumes were consistent with early puberty (4.5 ml). Subsequent follow-up into adulthood was not reported.
Normal puberty has been reported in 26 males (aged 0–18 years) treated for GCT (Hale et al., 1999). Treatment regimens were varied in terms of exposure to radiotherapy and/or platinum-based chemotherapy and the relative contribution of cisplatin cannot be concluded (Hale et al., 1999).
In a study involving 129 childhood cancer survivors (Romerius et al., 2011), gonadotrophins correlated with risk of azoospermia. Elevated FSH levels (>10.9 IU/l) were identified in 42 (32.5%) of patients of whom 22 were azoospermic giving a positive predictive value of 50% and a negative predictive value of 99%. Similar associations were seen between combined testicular volume ≤24 ml and azoospermia (Romerius et al., 2011). However, the study did not distinguish between impacts attributed to cisplatin and alkylating agents.
Gonadotrophins were evaluated in 159 boys (aged 4–20 years) treated for various malignancies (Ridola et al., 2009). Patients were grouped according to whether they received ifosfamide or cyclophosphamide as their sole alkylating agent in the treatment regimen and 42 had also received a platinum-based agent (cisplatin or carboplatin). Only two patients had a low testosterone within this cohort. Higher doses of cyclophosphamide (>12 g/m2) were associated with an increased risk of elevated FSH compared with lower doses and with all doses of ifosfamide. The inclusion of platinum-based agents to regimens was reported to have no impact on testicular function, although the data to support this conclusion was not shown (Ridola et al., 2009).
A study of 77 men who had been treated for a variety of malignancies included gonadotrophin and testosterone measurements in 66 (Relander et al., 2000). Testosterone was in the normal adult range for 65/66 and normal puberty was reported for 62/66 men. FSH was normal in 42/66 (63%) and in nine patients FSH was raised, indicating impaired spermatogenesis. Of these nine patients, seven had a sperm count performed and this was abnormal (five azoospermia and two oligozoospermia) in all cases. However, few patients in this study had received cisplatin. One patient with Ewing Sarcoma received cisplatin and cyclophosphamide and he was found to have testicular volumes <15 ml, whilst two patients with testicular GCT had received cisplatin but these patients were aged >14 years at diagnosis (Relander et al., 2000).
Long-term follow-up of gonadotrophin levels was conducted in a large cohort of 565 survivors of childhood cancer diagnosed between the ages 0–17 years and surviving for a minimum of 5 years (Tromp et al., 2011). The majority (90%) had received chemotherapy. FSH levels were obtained in 488 (86%) patients and were raised (>10 IU/l) in 121 (33%) cases. Raised LH and low testosterone, indicating primary Leydig cell failure, were reported in 2.9% and 12.4% of cases, respectively. The impact of platinum agents on FSH level was assessed using linear regression analysis and no significant association was identified either by univariate or multivariate analysis with odds ratios of 0.68 (0.35–1.32) and 2.29 (0.89–5.89), respectively. However, the number of patients receiving platinum-based chemotherapy represented a very small proportion of the cohort.
Cumulative exposure to cisplatin has been reported in relation to gonadotrophin data in a cohort of 20 patients (nine males) diagnosed with high-risk neuroblastoma at between 0 and 3 years of age (Utriainen et al., 2019). Cisplatin was part of their initial treatment and carboplatin was used for conditioning for HSCT, which included TBI in 10 (50%) cases. After a median follow-up time of 15 years, male survivors (n = 6) had significantly higher FSH (26.3 vs 3.6 IU, respectively; P < 0.001) and lower cumulative testicular volumes (8.5 vs 39 ml, respectively; P < 0.001) compared with healthy controls. Despite the high FSH, 3/5 patients in the non-TBI group had testicular volumes >15 ml, whereas all (4/4) patients receiving TBI had testicular volumes <10 ml. No correlation between cumulative cisplatin dose and testicular volume was identified, which may reflect the fact that these individuals also had HSCT and in many cases TBI. Of the nine patients, eight entered puberty spontaneously (three early), whilst one patient in the TBI group required pubertal induction. All four patients in the TBI group required testosterone replacement, whilst 0/5 in the no-TBI group required testosterone.
A study of 199 childhood cancer survivors aged <18 years at diagnosis (minimum follow-up of 5 years) had their HPG axis assessed (Brignardello et al., 2016). Impaired spermatogenesis, defined as FSH >10 IU/l or inhibin B <100 pg/ml, was identified in 52/130 (35%) patients treated with alkylating agents, compared to 14/23 (60%) patients treated with a combination of alkylating and platinum-based chemotherapy. For those treated with chemotherapy other than alkylating or platinum-based agents, impaired spermatogenesis only occurred in 2/17 (11.8%). Biochemical evidence for impaired spermatogenesis was confirmed by semen analysis in all patients (41/68) who provided a sample; however, the proportion that had received platinum-based chemotherapy was not described. In addition to impairment of spermatogenesis, Leydig cell failure (defined as raised LH and total testosterone <3 ng/dl) occurred in 13/147 (8.8%) of patients receiving alkylating chemotherapy, compared with 2/23 (8.7%) of those receiving alkylating agents in combination with platinum-based chemotherapy (Brignardello et al., 2016).
In a study of 14 male patients treated for CNS GCT, four patients were aged <12 years at diagnosis (Buckner et al., 1999). All four patients received chemotherapy, including etoposide and cisplatin, and localized radiotherapy. The study reported no post-treatment effects on growth and development; however, hormone levels were not reported.
Whilst not specifically reporting effects of cisplatin treatment, impaired spermatogenesis has also been shown in a cohort of 121 male childhood cancer survivors (Isaksson et al., 2018). Patients were diagnosed before the age of 18 years and were a minimum of 3 years post-treatment. Primary hypogonadism (definition includes FSH >10 IU/l) was reported in 7/121 (5.8%), compared to 1/122 (0.8%) of the control patients.
A study by Wallace et al. (1989) included eight male patients aged 7–12 years at diagnosis, who had received cisplatin treatment. Three were described as prepubertal at diagnosis. In the prepubertal patients, serum levels of gonadotrophins were low at follow-up, in keeping with their prepubertal status. However, no further follow-up data were available for these patients.
Experimental evidence for effects on testicular development and function
No laboratory studies were identified that investigated the effect of platinum-based chemotherapy on human testicular tissues or cells.
Discussion
The data on the impact of platinum-based chemotherapy in males on partner pregnancy rates provide conflicting results. Whilst one large study reported no effect of platinum-based chemotherapy on pregnancy rates (Wasilewski-Masker et al., 2014), the CCSS demonstrated a significant reduction in pregnancy rates based on a dose threshold of ∼500 mg/m2 (Green et al., 2014b) and another study involving the CCSS cohort reported similar findings with a lower threshold of ∼350 mg/m2 (Chow et al., 2016). A major limitation of the studies is the use of self-reporting of fertility using questionnaires. These may be prone to selection bias and variations in interpretation of the terms used to define infertility. In addition, it does not account for co-existing morbidities that may impact fertility e.g. obesity, or individual wish to father a child.
Semen analysis can provide an accurate objective measure of fertility potential in males and may be less prone to bias. However, obtaining a semen sample is challenging from a practical perspective and such studies are prone to participation bias. The majority of the studies that include sperm counts are limited by low numbers of patients providing a sample or by lack of sub-analysis to determine the contribution of platinum-based chemotherapy to azoospermia. The only study to specifically assess this did not find an association, although this may be confounded by the concomitant use of alkylating agents and low numbers of patients receiving platinum-based treatments (Green et al., 2014a).
Whilst the study of Isaksson et al. (2018) did not specifically report on cisplatin exposure in the childhood cancer survivor cohort, it did report a cohort of adult testicular cancer patients in whom there was a significantly increased risk of hypogonadism in those who received >4 cycles of cisplatin-based chemotherapy. This is in keeping with the studies reporting cisplatin-induced fertility impacts on men with testicular GCT (Lampe et al., 1997; Brydoy et al., 2010). The finding of persistent azoospermia in men after platinum-based chemotherapy suggests damage to the spermatogonial stem cell (SSC) population, which is also the likely target for damage by platinum-based chemotherapy during childhood, leading to impairment of fertility in adulthood. A reduced number of spermatogonia, including SSCs, has been reported in several recent studies involving histological examination of testicular biopsies in boys with cancer (Poganitsch-Korhonen et al., 2017; Stukenborg et al., 2018; Valli-Pulaski et al., 2019; Portela et al., 2020). However, these studies did not include data on the contribution of cisplatin to the germ cell loss.
Gonadotrophins provide an indirect assessment of testicular function and spermatogenesis. Raised gonadotrophins in the context of chemotherapy or radiotherapy indicate primary gonadal failure and an FSH threshold can be applied to identify azoospermia in adults with good sensitivity and specificity (Kelsey et al., 2017). However, the use of gonadotrophins in determining impairment to spermatogenesis and fertility in childhood cancer survivors is limited because these need to be measured post-puberty due to the quiescence of the HPG axis during prepuberty. The specific contribution of cisplatin was only assessed in 2/12 studies reporting gonadotrophin data, with conflicting results. One study showing no association was limited by the low numbers receiving cisplatin (Tromp et al., 2011). The other study did report an increased frequency of raised FSH in those receiving a combination of alkylators and cisplatin, compared to alkylators alone (Brignardello et al., 2016); however, the specific regimens and cumulative doses are not reported.
The major limitations of clinical studies involving childhood cancer survivors relate to the number of patients receiving specific regimens and the challenges of identifying the relative contribution of an individual agent to infertility in adulthood. Experimental studies can contribute to the understanding of the impact of platinum-based chemotherapy by exposing human testis tissues to single agent chemotherapy in human-relevant model systems. Such systems have recently been developed utilizing in vitro and xenograft approaches and can be applied to the study of exposure to pharmacological agents on germ and somatic cells of the testis (van den Driesche et al., 2015; Jorgensen et al., 2018). The in vitro approach has recently been used to expose prepubertal rodent testis tissue to a variety of chemotherapy drugs, including cisplatin (Smart et al., 2018; Allen et al., 2020). These studies report significant reductions in total germ cell and putative SSCs following exposure to concentrations of platinum-based chemotherapy that reflect serum levels in patients treated with these agents. To date, such experimental studies have not been replicated using human prepubertal testcicular tissues from patients who are having tissue stored for fertility preservation. In addition to conducting such studies, retrospective examination of archived testicular tissues that have already been exposed to platinum-based chemotherapy may be performed, similar to previous studies investigating the effects of exposure to alkylating agents (Poganitsch-Korhonen et al., 2017).
Overall, the results of this study indicate that platinum-based chemotherapy should be taken into consideration by clinicians when counselling patients and their carer(s) on the fertility risk associated with cancer treatment. Platinum-exposure should also be factored into discussions regarding fertility preservation options (e.g. testicular tissue cryopreservation) in prepubertal boys. However, it should be emphasized that identifying clear thresholds for testicular dysfunction following exposure to platinum-based chemotherapy in childhood requires further study. Children receiving treatment for osteosarcoma, hepatoblastoma and extracranial GCT may represent appropriate groups to determine the individual contribution of platinum agents, as these patients will often receive platinum-based chemotherapy without concomitant exposure to alkylating agents. These studies should include detailed clinical histories and drug exposures (including cumulative doses) for all patients. Regular follow-up of reproductive function, including pubertal assessment, hormone measurements and semen analysis, should also be performed.
Conclusion
Whilst many studies report the reproductive outcomes of childhood cancer survivors who have received platinum-based chemotherapy, relatively few have investigated the individual contribution of these agents to gonadal function, especially fertility. In addition, human-relevant experimental model systems in which to test the impact of specific chemotherapeutics on testicular development and function are lacking. Whilst this study indicates that platinum-based agents should be included in fertility risk assessments in prepubertal boys, it also highlights the importance of conducting large-scale prospective follow-up studies in these patients.
Supplementary Material
Acknowledgements
We thank Sheila Lane for her comments on the revised manuscript.
Authors’ roles
R.T.M. conceived and designed the study. R.T.M. and L.T.E. conducted the literature search and data collection. R.T.M. wrote the manuscript. W.H.B.W. and M.B. provided critical discussion of the data. All authors edited the manuscript and agreed upon the submitted manuscript.
Funding
R.T.M. is supported by UKRI Future Leaders Fellowship (MR/S017151/1). The MRC Centre for Reproductive Health is supported by MRC Centre Grant (MR/N022556/1).
Conflict of interest
The authors have no conflict of interest to declare.
Contributor Information
Lim Tian En, MRC Centre for Reproductive Health, The Queen’s Medical Research Institute, The University of Edinburgh, Edinburgh, UK.
Mark F H Brougham, Department of Paediatric Oncology, Royal Hospital for Sick Children, Edinburgh, UK.
William Hamish B Wallace, Department of Paediatric Oncology, Royal Hospital for Sick Children, Edinburgh, UK.
Rod T Mitchell, MRC Centre for Reproductive Health, The Queen’s Medical Research Institute, The University of Edinburgh, Edinburgh, UK; Department of Paediatric Endocrinology, Royal Hospital for Sick Children, Edinburgh, UK.
References
- Allen CM, Lopes F, Mitchell RT, Spears N. Comparative gonadotoxicity of the chemotherapy drugs cisplatin and carboplatin on prepubertal mouse gonads. Mol Hum Reprod 2020;26:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556–567. [DOI] [PubMed] [Google Scholar]
- Brignardello E, Felicetti F, Castiglione A, Nervo A, Biasin E, Ciccone G, Fagioli F, Corrias A. Gonadal status in long-term male survivors of childhood cancer. J Cancer Res Clin Oncol 2016;142:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydoy M, Fossa SD, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, Dahl O; Norwegian Urology Cancer Group III study group. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin-based chemotherapy. Eur Urol 2010;58:134–140. [DOI] [PubMed] [Google Scholar]
- Buckner JC, Peethambaram PP, Smithson WA, Groover RV, Schomberg PJ, Kimmel DW, Raffel C, O'Fallon JR, Neglia J, Shaw EG. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol 1999;17:933–940. [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Armstrong GT, Gajjar A, Hudson MM. Hypothalamic-pituitary axis dysfunction in survivors of childhood CNS tumors: importance of systematic follow-up and early endocrine consultation. J Clin Oncol 2016;34:4315–4319. [DOI] [PubMed] [Google Scholar]
- Chow EJ, Stratton KL,, Leisenring WM, Oeffinger KC, Sklar CA, Donaldson SS, Ginsberg JP, Kenney LB, Levine JM, Robison LL et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2016;17:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant F, Schwartz L, Delons E, Caillaud JM, Hartmann O, Lemerle J. Nonseminomatous malignant germ cell tumors in children. Multidrug therapy in Stages III and IV. Cancer 1984;54:1687–1691. [DOI] [PubMed] [Google Scholar]
- Freycon F, Casagranda L, Trombert-Paviot B. The impact of severe late-effects after 12 Gy fractionated total body irradiation and allogeneic stem cell transplantation for childhood leukemia (1988-2010). Pediatr Hematol Oncol 2019;36:86–102. [DOI] [PubMed] [Google Scholar]
- Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, Chemaitilly W, Pui CH, Klosky JL, Spunt SL et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014. a;15:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, Neglia JP, Sklar CA, Kaste SC, Hudson MM et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014. b;61:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale GA, Marina NM, Jones-Wallace D, Greenwald CA, Jenkins JJ, Rao BN, Luo X, Hudson MM. Late effects of treatment for germ cell tumors during childhood and adolescence. J Pediatr Hematol Oncol 1999;21:115–122. [DOI] [PubMed] [Google Scholar]
- Isaksson S, Bogefors K, Stahl O, Eberhard J, Giwercman YL, Leijonhufvud I, Link K, Ora I, Romerius P, Bobjer J et al. High risk of hypogonadism in young male cancer survivors. Clin Endocrinol (Oxf ) 2018;88:432–441. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Macdonald J, Nielsen JE, Kilcoyne KR, Perlman S, Lundvall L, Langhoff Thuesen L, Juul Hare K, Frederiksen H, Andersson AM et al. Nodal signaling regulates germ cell development and establishment of seminiferous cords in the human fetal testis. Cell Rep 2018;25:1924–1937.e4. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, Wallace WHB. Follicle Stimulating Hormone is an accurate predictor of azoospermia in childhood cancer survivors. PLoS One 2017;12:e0181377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiltie AE, Gattamaneni HR. Survival and quality of life of paediatric intracranial germ cell tumour patients treated at the Christie Hospital, 1972-1993. Med Pediatr Oncol 1995;25:450–456. [DOI] [PubMed] [Google Scholar]
- Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol 1997;15:239–245. [DOI] [PubMed] [Google Scholar]
- Longhi A, Macchiagodena M, Vitali G, Bacci G. Fertility in male patients treated with neoadjuvant chemotherapy for osteosarcoma. J Pediatr Hematol Oncol 2003;25:292–296. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HL, Klinkhammer-Schalke M, Seelbach-Gobel B, Hartmann AA, Kuhl J. Gonadal function of young adults after therapy of malignancies during childhood or adolescence. Eur J Pediatr 1996;155:763–769. [DOI] [PubMed] [Google Scholar]
- Odagiri K, Omura M, Hata M, Aida N, Niwa T, Ogino I, Kigasawa H, Ito S, Adachi M, Inoue T. Treatment outcomes, growth height, and neuroendocrine functions in patients with intracranial germ cell tumors treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys 2012;84:632–638. [DOI] [PubMed] [Google Scholar]
- Poganitsch-Korhonen M, Masliukaite I, Nurmio M, Lahteenmaki P, van Wely M, van Pelt AMM, Jahnukainen K, Stukenborg JB. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017;31:1460–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela JMD, Heckmann L, Wistuba J, Sansone A, Pelt A, Kliesch S, Schlatt S, Neuhaus N. Development and disease-dependent dynamics of spermatogonial subpopulations in human testicular tissues. J Clin Med 2020;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinmuth S, Hohmann C, Rendtorff R, Balcerek M, Holzhausen S, Muller A, Henze G, Keil T, Borgmann-Staudt A. Impact of chemotherapy and radiotherapy in childhood on fertility in adulthood: the FeCt-survey of childhood cancer survivors in Germany. J Cancer Res Clin Oncol 2013;139:2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relander T, Cavallin-Stahl E, Garwicz S, Olsson AM, Willen M. Gonadal and sexual function in men treated for childhood cancer. Med Pediatr Oncol 2000;35:52–63. [DOI] [PubMed] [Google Scholar]
- Ridola V, Fawaz O, Aubier F, Bergeron C, de Vathaire F, Pichon F, Orbach D, Gentet JC, Schmitt C, Dufour C et al. Testicular function of survivors of childhood cancer: a comparative study between ifosfamide- and cyclophosphamide-based regimens. Eur J Cancer 2009;45:814–818. [DOI] [PubMed] [Google Scholar]
- Robison LL, Armstrong GT, Boice JD, Chow EJ,, Davies SM, Donaldson SS, Green DM, Hammond S, Meadows AT, Mertens AC et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009;27:2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romerius P, Stahl O, Moell C, Relander T, Cavallin-Stahl E, Wiebe T, Giwercman YL, Giwercman A. High risk of azoospermia in men treated for childhood cancer. Int J Androl 2011;34:69–76. [DOI] [PubMed] [Google Scholar]
- Skinner R, Mulder RL, Kremer LC, Hudson MM,, Constine LS, Bardi E, Boekhout A, Borgmann-Staudt A, Brown MC, Cohn R et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol 2017;18:e75–e90. [DOI] [PubMed] [Google Scholar]
- Smart E, Lopes F, Rice S, Nagy B, Anderson RA, Mitchell RT, Spears N. Chemotherapy drugs cyclophosphamide, cisplatin and doxorubicin induce germ cell loss in an in vitro model of the prepubertal testis. Sci Rep 2018;8:1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Chapter 25: assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. 2019.
- Stukenborg JB, Alves-Lopes JP, Kurek M, Albalushi H, Reda A, Keros V, Tohonen V, Bjarnason R, Romerius P, Sundin M et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum Reprod 2018;33:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp K, Claessens JJ, Knijnenburg SL, van der Pal HJ, van Leeuwen FE, Caron HN, Beerendonk CC, Kremer LC. Reproductive status in adult male long-term survivors of childhood cancer. Hum Reprod 2011;26:1775–1783. [DOI] [PubMed] [Google Scholar]
- Utriainen P, Suominen A, Makitie O, Jahnukainen K. Gonadal failure is common in long-term survivors of childhood high-risk neuroblastoma treated with high-dose chemotherapy and autologous stem cell rescue. Front Endocrinol (Lausanne) 2019;10:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli-Pulaski H, Peters KA, Gassei K, Steimer SR, Sukhwani M, Hermann BP, Dwomor L, David S, Fayomi AP, Munyoki SK et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum Reprod 2019;34:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, Macdonald J, Anderson RA, Johnston ZC, Chetty T, Smith LB, McKinnell C, Dean A, Homer NZ, Jorgensen A et al. Prolonged exposure to acetaminophen reduces testosterone production by the human fetal testis in a xenograft model. Sci Transl Med 2015;7:288ra280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR, Price DA. Gonadal dysfunction due to cis-platinum. Med Pediatr Oncol 1989;17:409–413. [DOI] [PubMed] [Google Scholar]
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- Wasilewski-Masker K, Seidel KD, Leisenring W, Mertens AC, Shnorhavorian M, Ritenour CW, Stovall M, Green DM, Sklar CA, Armstrong GT et al. Male infertility in long-term survivors of pediatric cancer: a report from the childhood cancer survivor study. J Cancer Surviv 2014;8:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.