Abstract
BACKGROUND
Information regarding the possible influence of immunosuppressive drugs on male sexual function and reproductive outcomes is scarce. Men diagnosed with immune-mediated diseases and a wish to become a father represent an important neglected population since they lack vital information to make balanced decisions about their treatment.
OBJECTIVE AND RATIONALE
The aim of this research was to systematically review the literature for the influence of paternal immunosuppressive drug use on many aspects of male sexual health, such as sexual function, fertility, pregnancy outcomes and offspring health outcomes.
SEARCH METHODS
A systematic literature search was performed in the bibliographic databases: Embase (via Elsevier embase.com), MEDLINE ALL via Ovid, Cochrane Central Register of Trials (via Wiley) and Web of Science Core Collection. Additionally, Google Scholar and the Clinical trial registries of Europe and the USA were searched. The databases were searched from inception until 31 August 2019. The searches combined keywords regarding male sexual function and fertility, pregnancy outcomes and offspring health with a list of immunosuppressive drugs. Studies were included if they were published in English and if they included original data on male human exposure to immunosuppressive drugs. A meta-analysis was not possible to perform due to the heterogeneity of the data.
OUTCOMES
A total of 5867 references were identified, amongst which we identified 161 articles fulfilling the eligibility criteria. Amongst these articles, 50 included pregnancy and offspring outcomes and 130 included sexual health outcomes. Except for large Scandinavian cohorts, most of the identified articles included a small number of participants. While a clear negative effect on sperm quality was evident for sulfasalazine and cyclophosphamide, a dubious effect was identified for colchicine, methotrexate and sirolimus. In three articles, exposure to tumour necrosis factor-α inhibitors in patients diagnosed with ankylosing spondylitis resulted in improved sperm quality. The information regarding pregnancy and offspring outcomes was scant but no large negative effect associated with paternal immunosuppressive drug exposure was reported.
WIDER IMPLICATIONS
Evidence regarding the safety of immunosuppressive drugs in men with a wish to become a father is inconclusive. The lack of standardisation on how to evaluate and report male sexual function, fertility and reproduction as study outcomes in men exposed to immunosuppressive drugs is an important contributor to this result. Future research on this topic is needed and should be preferably done using standardised methods.
Keywords: immunosuppressive agents, semen analysis, male infertility, sexual health, sexual dysfunction, hypogonadism, teratogenicity, pregnancy, paternal exposure, gonadal steroid hormones
Introduction
Men with immune-mediated diseases (IMDs) and a wish to become a father represent an important neglected population. The question on how they should be treated to improve (or at least not impair) their chances of achieving a successful pregnancy and a healthy offspring remains a challenge for physicians and researchers all around the world.
Based on data from Denmark, the Netherlands and Norway, it is estimated that 5.6–7.6% of fathers could be exposed to non-steroidal anti-inflammatory drugs (NSAIDs) or anti-rheumatic drugs during the pre-conceptional period (3 or 6 months before pregnancy) (Schirm et al., 2004; Crijns et al., 2012; Engeland et al., 2013). Many factors are contributing to a substantial number of men with a wish to become a father being exposed to immunosuppressive drugs; some IMDs can affect men at a young age (i.e. juvenile idiopathic arthritis), then the prevalence of other IMDs increases during the peak of the male reproductive lifespan (i.e. rheumatoid arthritis (RA) or inflammatory bowel disease (IBD)), and furthermore in many parts of the world, men are becoming fathers at an older age (Khandwala et al., 2017).
It is known that immunosuppressive drugs can affect male sexual health and reproduction via multiple mechanisms: by altering reproductive hormone secretion and/or action, by disrupting spermatogenesis or sperm motility and by causing sexual dysfunction (Sasaki et al., 2011).
Furthermore, many of the available immunosuppressive drugs, such as methotrexate or sulfasalazine, were approved by the Food and Drug Administration (FDA) and/or the European Medicines Agency (EMA) before it was required to perform mandatory evaluations of male reproductive toxicity (FDA, 2015; EMA, 2017)
On the contrary, to get approval, new drugs are facing more strict protocols. Testicular toxicity is first evaluated in animal studies. When evidence suggests adverse events on the male reproductive system, complex trials in humans should follow. Importantly, in animal studies, the FDA considers histopathological evaluation to be an appropriate endpoint. In the case of human studies, semen analyses at baseline, at one spermatogenic cycle after exposure and at 13 weeks after drug discontinuation) become the most important marker of fertility. For further reassurance of testicular safety, the FDA recommends conducting randomised, double-blind, placebo-controlled, parallel-arm trials including ∼200 men in a 1:1 ratio (drug:placebo) (FDA, 2015).
For men of reproductive age, the decision on which immunosuppressive drug to prescribe is not straightforward. Information regarding the possible effects on male sexual health and reproduction is still lacking for most of the commonly used immunosuppressive drugs.
The objective of our study is to provide this information in the form of a ‘state of the art’ systematic review. Our goal is to review the available information about the influence of paternal immunosuppressive drug exposure on many aspects of male sexual and reproductive health, such as sexual function, reproductive hormones, fertility, pregnancy and offspring outcomes.
Methods
Protocol and registration
The protocol for this systematic review was registered with the International Prospective Register of Systematic Reviews (Registration no. CRD42018096898, https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=96898) and undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses protocols (PRISMA-P) guidelines (Moher et al., 2015).
Eligibility criteria
The literature search was limited to the English language and human subjects. Case–control studies, cohort studies, cross-sectional studies, case reports and case series were included. In vitro studies using human material were also included. Conference abstracts published after April 2016 were included. Publications without original data, such as reviews, were excluded. Publications concerning the use of immunosuppressive drugs for the treatment of any form of cancer were excluded.
The outcome data should include at least one of the following outcomes: sexual function, reproductive hormones, fertility, pregnancy outcomes or offspring outcomes. For pregnancy outcomes, publications were included if paternal exposure of immunosuppressive drugs took place in the 6 months before or around the time of conception, and in case of studies reporting sexual function or fertility parameters (i.e. semen analysis, sexual dysfunction and testosterone levels), publications were included if male exposure of immunosuppressive drugs was taken into consideration. For both categories, no restrictions were made regarding the comparison groups.
Information sources and search terms
A search strategy was developed by an experienced medical librarian (W.B.) using a structured methodology (Bramer et al., 2018a,b). The searches combined keywords regarding male sexual function and fertility, pregnancy outcomes and offspring health with a list of immunosuppressive drugs collected by experts in the fields of Rheumatology, Gastroenterology, Dermatology and Nephrology. Our full electronic search strategy is provided in Supplementary Table S1.
Subsequently, a systematic literature search was performed in the bibliographic databases: Embase (via Elsevier embase.com), MEDLINE ALL (via Ovid), Cochrane Central Register of Trials (via Wiley) and Web of Science Core Collection. Additionally, Google Scholar and the Clinical trial registries of Europe and the USA were searched. We also included references from the primary search publications, in case these were missed in our search and when relevant data were missing, we contacted authors for further information. These databases were searched from inception until 31 August 2019.
Study selection and data extraction
All articles were imported into EndNote X9. After removal of duplicates with the method described by Bramer et al. (2016), two reviewers (L.F.P.-G. and B.t.W.) independently screened titles, abstracts and full-text of the records for eligibility. Disagreements were resolved by consensus with the help of a third reviewer (R.J.E.M.D.). Two reviewers (L.F.P.-G. and B.t.W.) extracted relevant information for each studied outcome from the included articles.
Risk of bias in individual studies
The methodological quality of the studies was assessed with the Newcastle–Ottawa Scale (NOS), developed for case–control and cohort studies (Wells et al., 2013). In the case of cross-sectional studies, an adapted scale was used (Modesti et al., 2016). Using these methods, points were awarded to each publication, related to the selection of the study group, the comparability of the study groups and the ascertainment of the outcomes. The score ranges from 0 to 9, with scores >5 representing good-quality studies. The results are presented in Tables I and II. Case reports were not graded. Quality assessment was done by L.F.P-G. for the sexual function, reproductive hormones and fertility data, and by B.t.W. and S.V. for pregnancy and child outcome data.
Table I.
Summary of study characteristics and main findings for sperm quality, sexual function and reproductive hormones outcomes.
| Reference Country | Number of cases and controls (with mean age in years) | Disease | Key findings | Effect on fertility | Effect on reproductive hormones | Effect on sexual function | NOS quality assessment |
|---|---|---|---|---|---|---|---|
| Study type | |||||||
| Aminosalicylic acid and similar agents | |||||||
|
|
IBD | All sperm samples had abnormalities, mainly in motility. Sperm quality improved after stopping SSZ or switching to 5-ASA. | − | − | NR |
|
|
| |||||||
|
|
IBD | Oligospermia was detected in 72% of samples. After switching to 5-ASA, all samples showed improvement in sperm counts. | − | * | NR |
|
|
| |||||||
|
IBD | Oligospermia was detected on 40% of samples. After switching to mesalazine, samples showed improvement in sperm counts. | − | NR | NR |
|
|
|
| |||||||
|
IBD | Mean number of sperm count and of normal morphology was significantly lower. In five patients who stopped SSZ, improvement in sperm quality was observed. | − | * | NR |
|
|
|
| |||||||
|
|
Healthy participants | Prostaglandin levels in seminal plasma decreased by 36% secondary to SSZ exposure. | − | NR | NR |
|
|
| |||||||
|
IBD | SSZ exposure was associated with significant decrease in sperm counts, motility and increase in abnormal sperm morphology. | − | * | NR |
|
|
|
| |||||||
|
|
IBD | Sperm motility was reduced in all cases and serum testosterone levels were significantly lower in exposed cases. | − | − | NR |
|
|
| |||||||
|
IBD | Sperm head size was significantly larger in cases than in controls. | − | NR | NR |
|
|
|
IBD | Lower progressive motility in SSZ-exposed group. | − | NR | NR |
|
|
|
| |||||||
|
|
IBD | Case report: reversible infertility after stopping SSZ, patient on high dose GCs. | − | * | NR |
|
|
| |||||||
|
IBD | Exposed samples showed reduced sperm motility and density and altered morphology. After withdrawal, sperm density and motility improved significantly but not sperm morphology. | − | * | NR |
|
|
|
| |||||||
|
IBD |
|
− | NR | NR |
|
|
|
| |||||||
|
|
IBD | Head, midpiece and tail abnormalities were detected in spermatozoa of SSZ-exposed patients. | − | NR | NR |
|
|
| |||||||
|
IBD |
|
− | NR | NR |
|
|
|
| |||||||
|
Healthy participants | Sperm motility decreased 15% after exposure to SSZ. | − | NR | NR |
|
|
|
| |||||||
|
|
IBD | Case report: SSZ-exposed patient who was diagnosed with infertility and achieved a successful pregnancy after switching therapy from SSZ to 5-ASA. | − | NR | NR |
|
|
|
IBD | 26.23% of SSZ-exposed patients developed oligospermia. This is the first article to comment on the possible effect by disease activity. | − | NR | NR |
|
|
| |||||||
|
IBD | Case report: oligospermia associated with exposure to SSZ. | − | NR | NR |
|
|
|
| |||||||
|
IBD | Case report: pregnancy achieved after stopping SSZ therapy. | − | NR | NR |
|
|
|
| |||||||
|
IBD | Case report: SSZ-induced infertility case confirmed by sperm penetration assay (sperm analysis was normal). | −* | NR | NR |
|
|
|
| |||||||
|
IBD | 86% of SSZ-exposed patients had abnormal semen analysis (72% had oligospermia). | − | NR | NR |
|
|
|
| |||||||
|
|
IBD | Case report: reversible oligospermia in two cases exposed to SSZ. Both cases achieved pregnancies after drug withdrawal. | −* | NR | NR |
|
|
| |||||||
| Antimalarials | |||||||
|
|
Healthy participants | No differences in sperm quality parameters and reproductive hormones were found between exposed and non-exposed after exposure of chloroquine 1 g/day for 2 days and then 500 mg/day for 1 day. | * | * | NR |
|
|
| |||||||
| NR | Healthy participants | Chloroquine had a dual in vitro effect, enhancing rapid motility at low concentrations but inhibiting it at higher concentrations. At 250 µg/ml chloroquine, all spermatozoa were static. |
|
NR | NR |
|
|
|
|
Healthy participants | Chloroquine is present in seminal plasma even after long time of no exposure. | NR | NR | NR |
|
|
| |||||||
|
|
Healthy participants | Chloroquine crosses the BTB, probably by passive diffusion. | NR | NR | NR |
|
|
| |||||||
| Calcineurin inhibitors (CsP, ciclosporine; EVE, everolimus; SIR, sirolimus; TAC, tacrolimus) | |||||||
|
NR |
|
In vitro study showing that ciclosporine exerts deleterious effects on sperm, which become immotile and nonviable. | − | NR | NR |
|
|
| |||||||
|
|
With the exemption of a low semen volume, ciclosporine A at 3 mg/kg/day did not result in other sperm quality or hormonal abnormalities. | * | * | NR |
|
|
|
| |||||||
|
|
Pretreatment (pre-transplant) testosterone levels were below normal in 80%. After 12 months of treatment with CsP and other immunosuppressive drugs, testosterone levels significantly increased in all 10 cases. | NR | + | NR |
|
|
|
| |||||||
|
|
|
Sperm concentration was inversely correlated to the CsP whole blood levels. | − | * | + |
|
|
| |||||||
|
|
|
Testosterone levels increased from baseline in EVE and EVE-CsP groups. | NR | + | NR |
|
|
| |||||||
|
|
|
No statistical differences in baseline levels of serum FSH, LH, testosterone and PRL between CsP- and TAC-treated patients. All results were in normal ranges. | NR | * | NR |
|
|
| |||||||
|
|
|
Serum levels of reproductive hormones were normal in CsP exposed cases. | NR | * | NR |
|
| 1 (40) |
|
Case report: patient was infertile while on Sirolimus he developed oligospermia with normal hormone levels after switching to tacrolimus he was able to conceive. | −* | * | NR |
|
|
|
| |||||||
|
|
Case series: infertile patients with oligospermia, after discontinuing SRL, all patients had increased sperm counts and were able to conceive. | −* | NR | + |
|
|
|
| |||||||
|
|
|
Sirolimus-exposed patients had lower sperm counts and motility. The fathered pregnancy rate was significantly lower in exposed patients than in non-exposed. | − | NR | NR |
|
|
| |||||||
|
|
|
Recovery of spermatogenesis after cessation of sirolimus. | −* | − | NR |
|
|
| |||||||
|
1 (26) |
|
Benign Leydig cell tumour in a patient exposed to sirolimus lead to testicular biopsy that showed testicular atrophy and signs of impaired spermatogenesis. | −* | − | NR |
|
|
| |||||||
|
1 (36) |
|
Case report: low sperm count and motility with abnormal morphology associated with sirolimus exposure. These changes were reversed after switching therapy to tacrolimus.* | −* | NR | NR | NA |
|
| |||||||
|
|
|
Patients exposed to sirolimus had significantly lower serum testosterone levels and higher FHS/LH levels than control group. | NR | − | NR |
|
|
| |||||||
|
|
Patients exposed to sirolimus had significantly lower serum testosterone levels and higher FHS/LH levels than control group. | NR | − | NR |
|
|
|
|
|
Sirolimus daily dose and testosterone concentrations were significantly inversely correlated (r = –0.383). | NR | − | NR |
|
|
| |||||||
|
|
Significantly reduced levels of circulating testosterone amongst patients receiving sirolimus alone compared to those treated with calcineurin inhibitors alone were identified. | NR | − | NR |
|
|
|
| |||||||
| Colchicine | |||||||
|
|
Gout | Cytogenic analysis of sperm (FISH) revealed no damage secondary to colchicine use. | * | NR | NR |
|
|
| |||||||
|
Retinal vasculitis | Case report: reversible azoospermia. | − | NR | NR |
|
|
|
| |||||||
|
|
Behçet syndrome | The longer the use of colchicine, the more serious the adverse events on sperm count | − | + | NR |
|
|
| |||||||
|
|
Healthy participants | In vitro study, high concentrations of colchicine may affect in vitro motility of sperms, probably by its direct effect on the microtubules. | − | NR | NR |
|
|
| |||||||
|
|
FMF | After being advised to stop treatment with colchicine prior to attempt conception, sperm analysis was within normal limits in all six patients. | * | NR | NR |
|
|
| |||||||
|
Healthy participants | Colchicine caused no significant changes in sperm quality or reproductive hormones levels after 3 or 6 months of treatment. | * | * | NR |
|
|
|
| |||||||
|
|
Gout | Case report: azoospermia believed to be associated with colchicine use. Colchicine was stopped and after 3 months, sperm count improved and wife became pregnant. | − | NR | NR |
|
|
|
FMF | Mean colchicine dose at the time of sperm analysis was higher in patients with low sperm motility than that with normal sperm motility. | − | NR | NR |
|
|
| |||||||
| Cyclophosphamide | |||||||
|
|
SLE | The median serum inhibin B was lower in patients treated with CYC compared with those without this therapy. | − | − | NR |
|
|
| |||||||
|
|
SLE |
|
− | − | NR |
|
|
| |||||||
|
|
Bone marrow transplantation | 10% of patients who received CYC showed azoospermia, and recovery of spermatogenesis was observed in 60% of patients. | − | NR | NR |
|
|
| |||||||
|
|
Nephrotic syndrome | Significant inverse correlation between sperm density and CYC dosage and duration of treatment. | − | NR | NR |
|
|
| |||||||
|
|
Nephrotic syndrome | Altered spermatogenesis was found in 41.6% of adult patients treated with CYC during childhood (1.8–5.5 mg/kg/day for 12 weeks). No significant inverse correlation of total dose of the drug with sperm density. | − | − | NR |
|
|
| |||||||
|
|
Nephrotic syndrome | A significant inverse correlation was evident between sperm density and CYC dosage. Recovery of sperm count after prolonged interval after treatment is possible. | − | − | * |
|
|
|
Nephrotic syndrome | Histologic oligospermic changes were observed in three patients treated with high doses (10.6–16.2 g during 125–432 days). | − | NR | NR |
|
|
| |||||||
|
|
Behcet syndrome |
|
− | − | NR |
|
|
| |||||||
|
Nephrotic syndrome | Lower ejaculate volumes and sperm densities and higher percentage of immotile and abnormal forms in CYC exposed group. | − | − | NR |
|
|
|
| |||||||
|
Nephrotic syndrome | All patients showed abnormalities: oligospermia (1), azoospermia (1) and aplasia of germinal epithelium (1). | − | NR | NR |
|
|
|
| |||||||
|
|
Nephrotic syndrome | Sperm quality abnormalities found in 63%. An increase in the total dosage and in duration of the treatment was associated with a higher incidence of testicular dysfunction. | − | − | NR |
|
|
| |||||||
|
Nephrotic syndrome | Low doses (2–4 mg/kg/day) did not influence pituitary gonadal function (confirmed by biopsy). | − | − | NR |
|
|
|
| |||||||
|
Nephrotic syndrome | Serum testosterone levels were normal in CYC-treated patients | NR | * | NR |
|
|
|
| |||||||
|
Nephrotic syndrome | Sperm quality was uniformly decreased in CYC-treated patients and high FSH levels were common. | − | − | NR |
|
|
|
| |||||||
|
Nephrotic syndrome | All eight biopsy specimens had evidence of testicular atrophy, and it was profound in 6. | − | NR | NR |
|
|
|
Nephrotic syndrome | Biopsies confirmed absent spermatogenesis in azoospermic patients and FSH elevation correlated with degree of testicular damage. | − | − | NR |
|
|
|
| |||||||
|
|
Nephrotic syndrome | First case report that reported azoospermia associated with CYC exposure. | − | NR | NR |
|
|
| |||||||
|
|
Nephrotic syndrome | All 15 patients received CYC and became azoospermic or oligospermic. Five patients received testosterone (100 mg intramuscularly every 15 days during CYC therapy). After CYC treatment, normal sperm analysis was reported in all five patients who received testosterone (vs 1/10) | − | NR | NR |
|
|
| |||||||
|
|
NR | Testicular biopsy was performed on five patients who were receiving CYC and no spermatogenesis was found. | − | NR | NR |
|
|
| |||||||
| Methotrexate | |||||||
|
Psoriasis | Case report: reversible oligospermia secondary to MTX. | − | NR | NR |
|
|
|
| |||||||
|
Psoriasis | Sperm count was reduced to 63–97% at 2 weeks after a single IV injection of MTX. | − | NR | NR |
|
|
|
| |||||||
|
|
Psoriasis |
|
* | NR | NR |
|
|
|
Psoriasis | Sperm abnormalities found in 40% of MTX-treated patients but sperm quality was better than in patients treated with glucocorticoids. | + | NR | NR |
|
|
| |||||||
|
IBD | In all MTX-treated patients, basic semen analyses were within normal limits |
|
NR | NR |
|
|
|
| |||||||
|
1 (50) | Psoriasis | Case report: gynaecomastia and oligospermia secondary to MTX | − | NR | NR |
|
|
| |||||||
| NSAIDs | |||||||
|
|
|
Experiment: exposure to ibuprofen in adult testis explants caused a state of compensated hypogonadism. | NR | − | NR |
|
|
| |||||||
|
|
In vitro study: salicylate significantly decreases sperm motility | − | NR | NR |
|
|
|
| |||||||
|
|
|
Treatment with naproxen significantly reduces the concentration of all PGs present in human seminal fluid. | NR | NR | NR |
|
|
| |||||||
|
NA |
|
In vitro study: production of testosterone by Leydig cells was altered by exposure to all these drugs | NR | − | NR |
|
|
| |||||||
|
|
|
Exposure to indomethacin led to lower PGs levels in seminal plasma but unchanged sperm quality parameters and levels of reproductive hormones. | * | * | NR |
|
| Retinoids | |||||||
|
|
|
After 3 months of treatment at doses of 20 mg/day and 30 mg/day, sperm quality did not differ between cases and controls. | * | * | NR |
|
|
| |||||||
|
|
|
After 3 months of treatment at doses of 20 mg or 40 mg/day alitretinoin and 4-oxo-alitretinoin were detected in 11 of 12 semen samples.Concentrations detected are unlikely associated with teratogenicity. | NR | NR | NR |
|
|
| |||||||
|
|
Case report: 39-year old diagnosed with psoriasis reported erectile dysfunction after starting treatment with acitretin (25 mg/day). After 2 weeks of drug withdrawal, patient reported normalisation of sexual activity. | NR | NR | − |
|
|
|
| |||||||
|
|
|
After 3 months of treatment at doses of 25–50 mg/day, sperm quality did not differ between cases and controls | * | * | NR |
|
|
| |||||||
|
|
|
After 6 months of treatment at doses of 120 mg/day, all the sperm quality parameters changed positively and reproductive hormone levels did not differ. | + | * | NR |
|
|
| |||||||
|
|
|
After 4 months of treatment at doses of 1 mg/kg/day, sperm motility increased significantly and the other sperm quality parameters did not differ. | + | NR | NR |
|
|
| |||||||
|
|
Case report of ejaculatory failure associated with isotretinoin (1 mg/kg/day). | NR | NR | − |
|
|
|
|
Independent drug safety website (RxISK.org) data: isotretinoin commonly associated with SD. | NR | NR | − |
|
|
|
| |||||||
| Systemic glucocorticoids | |||||||
|
RA | Case series: biopsies performed after exposure to 75 mg of cortisone, and no negative effect was observed. | * | NR | NR |
|
|
|
| |||||||
|
RA | Compared to healthy controls, RA patients taking prednisone had significantly lower testosterone levels and slightly elevated levels of FSH and LH. | NR | − | NR |
|
|
|
| |||||||
| Thiopurines (AZA, Azathioprine) | |||||||
|
|
|
Semen analyses of 23 patients with IBD showed no negative association between AZA therapy and sperm quality. | * | NR | NR |
|
|
| |||||||
|
|
80% of patients had oligospermia. | − | NR | NR |
|
|
|
| |||||||
|
|
No correlation between poor spermatogenesis and AZA was reported. | * | * | NR |
|
|
|
| |||||||
|
|
|
Sperm motility was decreased in patients, DFI was similar. |
|
* | NR |
|
|
| |||||||
| TNF-α inhibitors (INF, infliximab; ETN, etanercept; CZP, certolizumab pegol; ADA, adalimumab; GOL, golimumab) | |||||||
|
|
|
Compared with baseline, no significant differences in mean total sperm number, sperm concentration, total and progressive motility nor other semen parameters were noticed during follow-up. | * | NR | NR |
|
|
|
|
*In vitro study: TNF-α had a detrimental effect on sperm function and in vitro etanercept counteracted this toxic action of TNF-α. | + | NR | NR |
|
|
| |||||||
|
|
|
Improvement in semen parameters after 12 months of TNF-α inhibitor treatment was reported. | + | * | NR |
|
|
| |||||||
|
|
|
Exposure of 20 patients to three different types of anti-TNFs did not have a negative impact on sperm quality after 3–6 months and in six cases after 12 months of treatment. | * | NR | NR |
|
|
| |||||||
|
|
|
Sperm abnormalities were comparable in patients and controls after 6 months of TNF-α inhibitor therapy. | * | * | NR |
|
|
| |||||||
|
|
|
|
+ | * | NR |
|
|
| |||||||
|
|
Sperm motility, or the percentage of sperm that show flagellar motion, was below normal in study patients after INF treatment. | − | NR | NR |
|
|
|
| |||||||
|
|
CZP treatment was found to have no effect on the semen quality variables assessed vs placebo | * | NR | NR |
|
|
|
| |||||||
|
|
|
|
|
NR | NR |
|
|
| |||||||
|
|
|
Case series reporting asthenoazoospermia in two out of three patients using infliximab. | −* | NR | NR |
|
|
| |||||||
|
|
|
Case report: oligoasthenozoospermia and decreased motility reversed after stopping drug. | −* | NR | NR |
|
|
| |||||||
|
1 (50) |
|
Case report: low sperm count, concentration increased after stopping IFX. | −* | NR | NR |
|
|
| |||||||
|
|
|
Normospermia before and after TNF-α therapy initiation. | * | NR | NR |
|
|
| |||||||
| 1 (58) |
|
Case report: priapism associated with adalimumab. | NR | NR | − |
|
|
|
| |||||||
|
|
|
Anti-TNF-α-treated patients showed significant improvements in four out of the five IIEF domains. | NR | NR | + |
|
| Verdolizumab | |||||||
|
|
IBD |
|
* | * | NR |
|
H, high; L, low; NA, not applicable; NR, not reported; *, no differences reported; +, positive effect; −, negative effect; −*, reversible negative effect upon withdrawal; CC, case–control study; Ch, cohort study; CR, case report; CS, cross-sectional study; RCT, randomised controlled trial.
Table II.
Summary of study characteristics and main findings for pregnancy and child outcomes.
| Data source Country Author Year of publication | Type of study Study period Number of cases Number of controls Unit cases | Exposure period | Inclusion Cases Controls | Pregnancy outcome Live births (LB) Spontaneous abortions (SA) ETOP* (ET) Stillbirths (SB) Pending/LTFU* (PL) Neonatal death (ND) Other (OT) n (%) | Gestational age (GA in weeks, mean ± SD) Preterm birth (PB, n (%)) | Birth weight (BW in gram, mean ± SD) Low birth weight (LBW, n (%)) Small for gestational age (SGA, n (%)) | Birth defects (BD, n (%)) | Quality assessment |
|---|---|---|---|---|---|---|---|---|
| Calcineurin inhibitors (CsP, ciclosporine; SIR, sirolimus; TAC, tacrolimus) | ||||||||
|
|
3 months prior to conception | Ciclosporine | LB 1 | NS | NR |
|
L |
|
| ||||||||
|
|
Long term |
|
LB 3 | NR |
|
|
L |
|
| ||||||||
|
|
Long term | Ciclosporine | LB 167 |
|
|
|
L |
|
| ||||||||
|
|
Long term |
|
LB 2 |
|
|
|
L |
|
| ||||||||
|
|
Long term | Sirolimus |
|
NR | NR |
|
L |
|
| ||||||||
|
|
3 months prior to conception and during the first trimester | Ciclosporine/no immunosuppressants | NA |
|
|
|
H |
| Colchicine | ||||||||
|
| ||||||||
|
|
3 months prior to conception | Colchicine | LB 3 | NR | NR | NR | L |
|
| ||||||||
|
|
3 months prior to conception | Colchicine |
|
NR | NR | NR | L |
|
| ||||||||
|
|
3 months prior to conception | Colchicine |
|
NR | NR | NR | L |
|
| ||||||||
| Cyclophosphamide | ||||||||
|
|
Long term | Cyclophosphamide | LB 1 | NR | NR |
|
L |
|
| ||||||||
| Interleukin inhibitors | ||||||||
|
|
Long term | Tocilizumab |
|
NR | NR |
|
L |
|
|
Long term |
|
|
|
|
|
L |
|
| ||||||||
|
|
At the time of conception | Secukinumab |
|
|
NR |
|
L |
|
| ||||||||
| Methotrexate | ||||||||
|
|
|
LB 1 |
|
|
|
NA | |
|
| ||||||||
|
|
|
LB 1 | NR |
|
|
NA | |
|
| ||||||||
|
|
At the time of conception | LB 1 | NR |
|
|
NA | |
|
| ||||||||
|
|
3 months prior to conception |
|
|
|
|
L | |
|
|
At the time of conception |
|
NR | NR |
|
L | |
|
| ||||||||
|
|
3 months prior to conception | SA 0 |
|
|
|
L | |
|
| ||||||||
|
|
3 months prior to conception |
|
|
|
|
L | |
|
| ||||||||
|
|
3 months prior to conception | Methotrexate/no methotrexate | NA |
|
|
|
H |
|
|
3 months prior to conception and during the first trimester | Methotrexate/no methotrexate |
|
|
|
NA | L |
|
| ||||||||
| Mycophenolate acid products | ||||||||
|
|
Long term | MPA/no MPA |
|
|
|
|
H |
|
| ||||||||
|
|
Long term | MPA/no MPA |
|
|
|
|
H |
|
| ||||||||
|
|
3 months prior to conception and during the first trimester | Mycophenolate mofetil/no immunosuppressants | NA |
|
|
|
H |
|
| ||||||||
|
|
Long term | MPA/no MPA |
|
|
|
L | |
| Other selective immunosuppressants | ||||||||
|
| ||||||||
|
|
At the time of conception | Leflunomide | LB 1 |
|
|
|
NA |
|
| ||||||||
|
|
At the time of conception | Abatacept |
|
NR | NR |
|
L |
|
| ||||||||
|
|
At the time of conception | Tofacitinib |
|
NR | NR |
|
L |
|
| ||||||||
| Retinoids | ||||||||
|
|
Long term | Etretinate | LB 3 | NR | NR |
|
L |
|
| ||||||||
|
|
3 months prior to conception | Isotretinoin/NR | NR |
|
NR |
|
L |
|
|
3 months prior to conception and during first trimester | Acitretin/NR |
|
NR | NR | BD | L |
|
| ||||||||
| Systemic corticosteroids | ||||||||
|
|
Long term | Prednisone |
|
NR | NR |
|
L |
|
| ||||||||
|
|
Long term | Prednisolone | LB 11 |
|
|
|
L |
|
| ||||||||
|
|
Long term | Prednisone | LB 167 |
|
|
|
L |
|
| ||||||||
|
|
3 months prior to conception | Prednisolone |
|
|
|
|
L |
|
|
3 months prior to conception |
|
NA |
|
|
|
H |
|
| ||||||||
| Thiopurines (AZA, azathioprine; 6MP, 6-mercaptopurine) | ||||||||
|
|
Long term | Azathioprine |
|
NR | NR |
|
L |
|
| ||||||||
|
|
Long term | Azathioprine or 6-mercaptopurine | LB 1 | NR | NR |
|
NA |
|
| ||||||||
|
|
Long term | Azathioprine |
|
|
|
|
L |
|
|
Long term | Azathioprine | LB 167 |
|
|
|
L |
|
| ||||||||
|
|
3 months prior to conception and during the first trimester | 6-Mercaptopurine/never taken 6MP or only after conception | SA 2 (15)/2 (2.2) | NR | NR |
|
L |
|
| ||||||||
|
|
3 months prior to conception | 37 Azathioprine or 9-mercaptopurine/no exposure to thiopurines in 3 months prior to conception |
|
|
|
|
H |
|
| ||||||||
|
|
At the time of conception | 6-Mercaptopurine/pregnancies prior to treatment 6 MP |
|
|
|
|
L |
|
| ||||||||
|
|
101 until conception or longer, others not specified | 108 Azathioprine, seven 6-mercaptopurine/pregnancies not exposed to teratogens and no paternal reported immunosuppressive drugs or otherwise risky treatment |
|
|
|
|
L |
|
|
3 months prior to conception | At least one filled prescription of AZA or 6MP within 3 months before the date of conception/no filled prescription of AZA or 6MP within 3 months before the date of conception | NA |
|
|
|
H |
|
| ||||||||
| TNF-α inhibitors (INF, infliximab; ETN, etanercept; CZP, certolizumab pegol; ADA, adalimumab; GOL, golalinumab) | ||||||||
|
|
At the time of conception | Infliximab | LB 1 | NR |
|
|
NA |
|
| ||||||||
|
|
Long term | Infliximab |
|
|
|
|
|
|
| ||||||||
|
|
At the time of conception | TNF-α inhibitor |
|
|
|
|
L |
|
|
NR | Etanercept | LB 3 | NR | NR |
|
L |
|
| ||||||||
|
|
At the time of conception | Infliximab |
|
|
NR |
|
L |
|
| ||||||||
|
|
At the time of conception | Certolizumab Pegol |
|
NS | NS | NS | L |
|
| ||||||||
|
|
3 months prior to conception |
|
NA |
|
|
|
H |
|
| ||||||||
|
|
Long term | TNF-α I/general population data |
|
|
|
|
L |
H, high; L, low; NA, not applicable; NR, not reported; PBR, population-based registry; presc., prescriptions; TC, transplantation centre; TIS, Teratology Information Service.
Regarding pregnancy and child outcomes data, the following ‘rules’ were applied. Ascertainment exposure/outcome was graded ‘1’ (structured questionnaire equals structured interview). The question ‘outcome not present at the start’ was always graded ‘0’. Follow-up length was considered long enough for congenital anomalies if at least 1-year follow-up was reported. For long-term outcomes, the follow-up needed to last until children were 18 years of age. In cases where publications included maternal and paternal outcomes, the score was based only on the paternal outcomes.
Synthesis of results
Sexual health outcomes were classified into two categories: (i) sexual function, reproductive hormones and fertility (e.g. sexual dysfunction, testosterone and sperm quality) and (ii) pregnancy and offspring outcomes (e.g. live births, spontaneous abortions, premature birth, low birth weight and congenital anomalies).
Additional analysis
A meta-analysis was not possible to perform due to the heterogenicity of the data.
Results
Study selection and characteristics
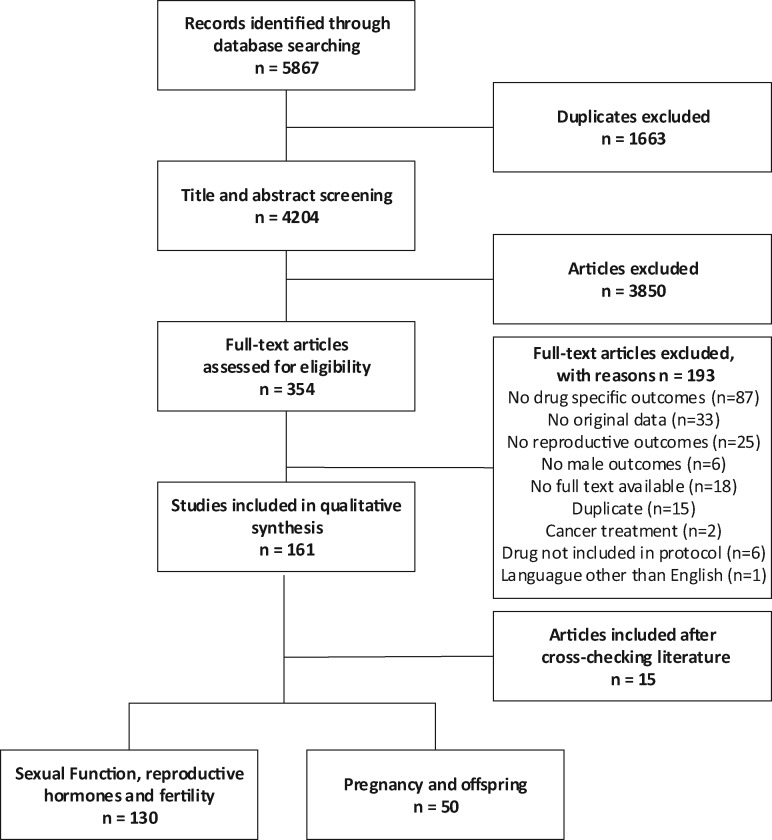
A total of 5867 references were identified (2366 from Embase, 2023 from Medline, 1315 from Web of Science and 163 from Cochrane central) and imported into EndNote X9. After removing 1663 duplicates, 4204 articles were eligible for title and abstract screening. During this phase, 3850 articles were excluded and 354 articles were eligible for full-text reading. Then 193 articles were excluded after full-text reading (see flowchart in Fig. 1). Additionally 15 articles that fulfilled the inclusion criteria and that were not identified by our search strategy or that were missed during the screening titles and abstracts procedure were identified by cross-checking relevant literature. In total, 176 articles fulfilled the inclusion criteria.
Figure 1.
Flow diagram for study selection.
Description of participants
A brief description of participants’ characteristics is provided throughout the text and/or in the tables.
Description of interventions
In general, sexual function and fertility outcomes were evaluated in a few studies before and after exposure to immunosuppressive drugs. In cross-sectional studies, disease activity and co-medication used during the study were not uniformly reported.
The publications regarding pregnancy and child outcomes were observational; no standardised interventions were studied.
Risk of bias within studies
Regarding sexual function, reproductive hormones and male fertility, the overall quality of the included studies was low to moderate and the number of exposed cases was low for all the drugs included in this systematic review. Regarding pregnancy outcomes, case series and small cohorts were of low quality (<5) in general. High scores (≥5) were given to the population-based registries from Denmark and Norway and transplantation registries.
Outcomes
In the upcoming text, we provide a summary of the main outcomes from the included studies. More in-depth information regarding the findings and study quality per study is presented in Tables I and II and in the Supplementary tables. Table I contains information regarding fertility, reproductive hormones and sexual function outcomes. Table II contains information about pregnancy outcomes, gestational age, birth weight and birth defects. Supplementary Table S2 contains more study specifications. Reported specification of the birth defects is presented in Supplementary Table S3. Other maternal and child outcomes are reported in Supplementary Table S4.
Paternal exposure was included in this systematic review if paternal exposure occurred 6 months before conception or around the time of conception. Some of the included studies also presented results for exposure at any time before conception. Comparison between ‘exposure 3 months prior to conception’ and ‘exposure at any time before conception’ was possible in publications of the Danish registry data (Larsen et al., 2016; Egeberg et al., 2017; Nørgaard and Andersen, 2019). See Supplementary Table S5 for outcomes after exposures any time before conception. No major changes were found in the risk estimates, only small changes were seen with a very low number of cases.
Aminosalicylic acid and similar agents
Sexual function, reproductive hormones and fertility.
There were 22 studies with data on a total of 329 exposed men to sulfasalazine identified. Sperm analysis abnormalities were reported in 40–100% of those patients exposed to sulfasalazine (doses ranged from 2 to 4 mg/day). The most common sperm abnormality reported was asthenozoospermia (decreased motility) followed by decreased sperm counts and abnormal morphology. Data extracted from case reports and small case series showed that oligospermia and asthenozoospermia were severe enough to cause male infertility. In all studies where follow-up samples were available, sperm quality improved after sulfasalazine was withdrawn for 3 months. The majority of these studies were published between 1979 and 1987 and included patients diagnosed with IBD (Levi et al., 1979; Toth, 1979; Traub et al., 1979; Birnie et al., 1981; Heineman et al., 1981; Toovey et al., 1981; Freeman et al., 1982; Hudson et al., 1982; Tobias et al., 1982; Cann and Holdsworth, 1984; Cosentino et al., 1984; Freixa et al., 1984; McIntyre and Lennard-Jones, 1984; O’Morain et al., 1984; Ragni et al., 1984; Shaffer et al., 1984; Iglesias-cortit et al., 1985; Riley et al., 1987; Chatzinoff et al., 1988; Zelissen et al., 1988; Di Paolo et al., 2001; Ganatra et al., 2018). Importantly, most of these studies are case reports and case series.
Pregnancy and child outcomes.
No studies were identified.
Antimalarials (chloroquine and hydroxychloroquine)
Sexual function, reproductive hormones and fertility.
Four studies that included data from 37 healthy men were identified. One study reported sperm quality parameters and three studies evaluated the ability of chloroquine to cross the blood-testis barrier (Ette et al., 1988; Adeeko and Dada, 1994; Hargreaves et al., 1998; Ejebe et al., 2008). As it is the case for other human tissues and fluids, chloroquine can be found on seminal plasma even after long-term withdrawal. One in vitro study reported that high concentrations of chloroquine in seminal plasma inhibited sperm motility. No studies reporting these outcomes were identified for hydroxychloroquine.
Pregnancy and child outcomes.
No studies were identified.
Calcineurin inhibitors (ciclosporine, sirolimus and tacrolimus)
Sexual function, reproductive hormones and fertility.
Fifteen studies including a total of 263 cases and 229 controls were identified. All of these cases were receiving sirolimus or ciclosporine for organ transplantation (mainly kidney transplantation). In all 11 studies included, sperm quality abnormalities and reproductive hormonal alterations (low testosterone and high FSH/LH levels) were reported after sirolimus exposure (Misro et al., 1999; Bererhi et al., 2003; Fritsche et al., 2004; Kaczmarek et al., 2004; Lee et al., 2005; Tondolo et al., 2005; Deutsch et al., 2007; Skrzypek and Krause, 2007; Zuber et al., 2008; Boobes et al., 2010; Sajad Hussain et al., 2015). In addition, reversible infertility associated with sirolimus was reported in three studies. One prospective study reported that testosterone levels increased from baseline levels (pre-transplant) in an undefined number of patients using everolimus (Kramer et al., 2005). Despite the lack of reproductive safety information for tacrolimus in humans, in these studies, patients were switched from sirolimus to tacrolimus and their sperm quality improved.
Nine post-kidney transplant patients exposed to ciclosporine provided semen samples and no relevant sperm quality abnormalities were reported. From this group of patients, partners of three out of four patients were able to conceive while being exposed to ciclosporine (Haberman et al., 1991; Misro et al., 1999). A prospective study that included pre- and post-kidney transplantation data of 10 men, reported that hypogonadism was present before initiating treatment with ciclosporine. After 12 months of ciclosporine exposure, levels of testosterone exceeded pre-transplant levels (Samojlik et al., 1992). Sexual hormone levels were normal and comparable amongst 21 ciclosporine- and 16 tacrolimus-exposed renal transplant male patients (Kantarci et al., 2004). Similar results were reported by others (Peces et al., 1994). Sperm concentration was inversely correlated to the ciclosporine whole blood levels in one study (Eid et al., 1996).
Pregnancy and child outcomes.
Six studies were aimed at determining the impact of the use of ciclosporine, tacrolimus or sirolimus on pregnancy and child-related outcomes. Transplant recipients used these medications often in combinations with other drugs.
Three case reports/case series and two transplantation registries found no abnormal outcomes (Moskovitz et al., 1988; Holmgren et al., 2004; Xu et al., 2009; Ecevit et al., 2012; Moritz et al., 2017; Schopf, 2017). A population-based registry found a higher risk of birth defects although this was not statistically significant (Egeberg et al., 2017). Seven of the 67 children were diagnosed with a congenital anomaly (CA) after paternal use of ciclosporine. No details of the CAs were provided.
Colchicine
Sexual function, reproductive hormones and fertility.
Eight studies including a total of 166 cases were identified. Most of these studies were published before 2000. Colchicine exposure (1–2 mg/day) was associated with low sperm counts and motility in five studies (Merlin, 1972; Ben-Chetrit et al., 1993; Sarica et al., 1995; Kirchin et al., 1999; Kaya Aksoy et al., 2019). Abnormal sperm analysis was reported in 40–58% of patients exposed to colchicine (Sarica et al., 1995; Kaya Aksoy et al., 2019). One study reported normal cytogenic sperm analysis in two patients diagnosed with gout and exposed to colchicine (Kastrop et al., 1999), one study reported no significant sperm analysis abnormalities in patients previously exposed to colchicine (Levy and Eliakim, 1977) and finally, one study reported no significant sperm abnormalities in healthy volunteers exposed to colchicine (Bremner and Paulsen, 1976). A possible adverse effect on sperm quality associated with disease activity was discussed in the most recent study by Kaya Aksoy et al. (2019).
Pregnancy and child outcomes.
Three older studies from an Israeli hospital followed patients with familial Mediterranean fever (FMF) treated with colchicine (Levy and Eliakim, 1977; Ehrenfeld et al., 1986; Ben-Chetrit et al., 2004). Only one study reported specific data on colchicine and spontaneous abortions (no increased risk) (Ben-Chetrit et al., 2004); the other studies did not report specific outcomes for colchicine-treated patients.
Cyclophosphamide
Sexual function, reproductive hormones and fertility.
There were 20 studies identified, and most of them included patients who were exposed to cyclophosphamide (CYC) to treat nephrotic syndromes associated with glomerulonephritis (73%). Most of these studies reported fertility outcomes from young adults who were exposed to CYC during their childhood. Unfortunately, the mean age of these participants was not reported in many studies. From these studies, a clear negative effect on sperm quality and reproductive hormones, mainly causing low sperm counts and high FSH levels, from CYC is evident (Fairley et al., 1972; Feng et al., 1972; Kumar et al., 1972; Penso et al., 1974; Pennisi et al., 1975; Etteldorf et al., 1976; Kirkland et al., 1976; Hsu et al., 1979; Marina and Barcelo, 1979; Fukutani et al., 1981; Trompeter et al., 1981; Ogata et al., 1982; Watson et al., 1985; Perrone et al., 1989; Bogdanovic et al., 1990; Masala et al., 1997; Anserini et al., 2002; Soares et al., 2007; Suehiro et al., 2008). Reversibility (improvement in sperm counts after CYC withdrawal) with a possible dose-dependent effect was a repetitive finding in some studies. Because of substantial methodological problems (selection bias, loss of follow-up and no baseline samples), reversibility and a dose-dependent effect cannot be interpreted as conclusive evidence.
Pregnancy and child outcomes.
In 1983, a case report was published noting that a child was born with an absent hand after paternal exposure to cyclophosphamide and dexamethasone (Balci and Sarikayalar, 1983).
Interleukin inhibitors
Sexual function, reproductive hormones and fertility.
No studies were identified.
Pregnancy and child outcomes.
Three case series from different sources focused mainly on maternal exposures and briefly mentioned paternal exposures (Weber-Schoendorfer and Schaefer, 2016; Youngstein et al., 2017; Warren et al., 2018). The paternal exposures included two pregnancies where the male partners were on tocilizumab (one healthy liveborn and one spontaneous abortion), and 54 pregnancies on where the male partners were on secukinumab (Weber-Schoendorfer and Schaefer, 2016). Outcomes were not available for 20 pregnancies (7 were pending and 13 were lost to follow-up), while known outcomes were 29 liveborn with one malformation, four spontaneous abortions and one elective termination (Warren et al., 2018).
Youngstein et al. reported six children with paternal exposure of anakinra and five children with paternal exposure of canakinumab; no malformations were reported (Youngstein et al., 2017).
Methotrexate
Sexual function, reproductive hormones and fertility.
Six studies reporting fertility outcomes in patients exposed to methotrexate (MTX) were identified. These studies included a total of 47 cases (40 men diagnosed with psoriasis and seven with IMD) and 1912 controls (all controls come from one study (Ley et al., 2018)). In patients exposed to MTX, sperm concentration decreased in three studies (Van Scott and Reinertson, 1959; Sussman and Leonard, 1980; Pandhi et al., 2006), no differences were reported in two studies (one study reported five normal testicular biopsies after MTX exposure) (El-Beheiry et al., 1979; Ley et al., 2018) and the sperm quality of one group of patients diagnosed with psoriasis and exposed to MTX was better than patients treated with high dose glucocorticoids (Grunnet et al., 1977).
Pregnancy and child outcomes.
Three case reports from before 2000 reported only healthy liveborn children (Perry, 1983; Griggs and Schwartz, 2006; Lamboglia et al., 2009). More recent case series and cohort studies (169 pregnancies and 193 liveborn children) found no increased risk of birth defects associated with paternal MTX exposure (Beghin et al., 2011; Engeland et al., 2013; Weber-schoendorfer et al., 2014; Winter et al., 2017; Drenches et al., 2018). This also applies to the rate of spontaneous abortions, preterm birth and small for gestational age (SGA; Weber-schoendorfer et al., 2014; Winter et al., 2017; Andersen et al., 2018).
Friedman et al. used the Danish registries to look at long-term outcomes and no negative impact of paternal preconception use of MTX was reported (Friedman et al., 2017).
Mycophenolate acid products
Sexual function, reproductive hormones and fertility.
No studies were identified.
Pregnancy and child outcomes.
Four data sources have published data with 295 pregnancies and 189 children included: three registries, two population-based and one pregnancy transplantation with medical records from one hospital in Spain (Jones et al., 2013; Åsberg et al., 2017; Egeberg et al., 2017; Midtvedt et al., 2017; Moritz et al., 2017; Lopez-Lopez et al., 2018). No major differences were found compared to transplantation patients not taking mycophenolate acid products (MPAs). In these studies, MPA was often used in combination with calcineurin inhibitors and corticosteroids.
Non-steroidal anti-inflammatory drugs (NSAIDs)
Sexual function, reproductive hormones and fertility.
For NSAIDs as a group, no studies were identified in our population of interest. Nonetheless, six studies that included healthy participants were identified. Exposure to salicylate decreased sperm motility, and for naproxen one study concluded that sperm quality abnormalities were similar between pre- and post- exposed samples (Bendvold et al., 1985; Knuth et al., 1989; Poratsoldin and Soldin, 1992). A study from Kristensen et al. concluded that ibuprofen exposure results in a state of compensated hypogonadism (Kristensen et al., 2018). One in vitro study using adult human testis explants demonstrated that exposure to indomethacin and aspirin altered the production of testosterone by Leydig cells (Albert et al., 2013).
Pregnancy and child outcomes.
No studies were identified.
Retinoids (acitretin, etretinate and isotretinoin)
Sexual function, reproductive hormones and fertility.
Eight studies that included a total of 203 cases and 20 controls were identified (Torok et al., 1987; Parsch et al., 1990; Coleman and MacDonald, 1994; Rossi and Pellegrino, 2009; Schmitt-Hoffmann et al., 2011; Çinar et al., 2016; Liu et al., 2017; Healy et al., 2018). Low concentrations of retinoids that are unlikely associated with a teratogenic risk can be found in seminal plasma of exposed patients (Schmitt-Hoffmann et al., 2011). No negative effect on sperm quality was reported in four studies (130 exposed men). Sexual dysfunction in the form of ejaculatory failure and erectile dysfunction associated with acitretin has been reported (Healy et al., 2018).
Pregnancy and child outcomes.
Three studies report paternal retinoid use and pregnancy-related outcomes. Two included only liveborn children. A long-term general follow-up study after etretinate use, with 18 male patients, found three healthy children (Katugampola and Finlay, 2006). Population-based registries from Norway found a higher risk (OR (95% CI) 1.8 (0.81–3.8) for preterm birth (live birth after at least 22 and prior to 37 weeks of gestation) based on 80 isotretinoin cases (Engeland et al., 2013). Based on population-based registries, a study from Denmark found no risk for spontaneous abortion after acitretin use (Nørgaard and Andersen, 2019).
Systemic corticosteroids
Sexual function, reproductive hormones and fertility.
Two studies were identified. In a study from 1956, seven patients diagnosed with RA were treated with 75 mg of cortisone over periods ranging from 23 to 334 days. Pre- and post-treatment testicular biopsies were performed for six patients, in which no significant changes were reported (McDonald and Heckel, 1956). In a small study that included 36 men with long standing active RA, the use of prednisone at doses ranging from 5 to 10 mg/day was associated with significantly lower testosterone levels and lower levels of FSH and LH compared to men with long standing RA but without prednisone treatment (Martens et al., 1994). Because of the scope of our systematic review, studies that reported the effect of corticosteroids on the male reproductive health of healthy controls were excluded. A review on this topic can be found elsewhere (Drobnis and Nangia, 2017).
Pregnancy and child outcomes.
A high number of cases were reported in the population-based studies of Norway and Denmark. Data from Norway refer to prednisolone (Engeland et al., 2013). No drug-specific information about systemic corticosteroids in the Danish data was available and both registries have no information about the used dose or indication. Larsen et al. only found a higher risk of birth defects in the Danish registries, although it was not statistically significant, after one or two redeemed prescriptions (Larsen et al., 2018). Smaller numbers are reported in transplantation patients (Penn et al., 1971; McGeown and Nevin, 1978; Xu et al., 2011).
Thiopurines
Sexual function, reproductive hormones and fertility.
Four studies that included a total of 75 cases and 40 controls exposed to azathioprine were included (Baumgarten et al., 1977; Farthing and Dawson, 1983; Dejaco et al., 2001; Grosen et al., 2019c). Sperm quality, sperm DNA fragmentation index (DFI) and the male endocrine reproductive axis appear not to be negatively affected by azathioprine exposure.
Pregnancy and child outcomes.
Azathioprine is the most frequently reported thiopurine followed by 6-mercaptopurine. The first hospital case series were reported in the 1970s (Penn et al., 1971; McGeown and Nevin, 1978). In the early 2000s, other case series followed and the largest study up to now was based on the Danish registries from 2017. No differentiation between the two drugs was made (Rajapakse et al., 2000; Francella et al., 2003; Xu et al., 2009, 2011; Teruel et al., 2010; Hoeltzenbein et al., 2012; Nørgård et al., 2017). In total 192 males, 211 pregnancies and 669 children were included in these studies. Overall, no increased risks were detected.
Friedman et al. used the Danish registries to look at long-term outcomes and no negative impact of paternal preconception use of azathioprine or 6-mercaptopurine was reported (Friedman et al., 2017).
Tumour necrosis factor-α inhibitors
Sexual function, reproductive hormones and fertility.
We identified 15 studies that evaluated the effect of tumour necrosis factor (TNF)-α inhibitors on male sexual health. Thirteen studies reported fertility or sperm quality outcomes, one study reported sexual function as an outcome (Oh et al., 2009) and priapism secondary to the use of adalimumab was reported in one case report (Kreitenberg et al., 2015). In total, outcomes of interest were reported in 156 men diagnosed with ankylosing spondylitis (AS), psoriasis, RA and IBD exposed to TNF-α inhibitors and in 225 men who participated in these studies as healthy controls.
Regarding sperm quality before and after TNF-α inhibitor use, one small randomised control trial (RCT) that included data of 20 men concluded that certolizumab pegol had no adverse event on sperm quality compared to placebo (Perrier d’Hauterive et al., 2012). In studies where a comparison between baseline samples before TNF-α inhibitor exposure and follow-up samples was available, no differences on sperm quality were reported in five studies (Almeida et al., 2013; Micu et al., 2014, 2019; Heppt et al., 2017; Grosen et al., 2019a), while in three studies sperm quality improved after exposure (Villiger et al., 2010; Ramonda et al., 2014; Pascarelli et al., 2017) and in one study sperm quality worsened after exposure (Mahadevan et al., 2005). A possible positive effect on sperm quality using TNF-α inhibitors could be the result of decreasing disease activity in patients with AS. These findings should not be extrapolated to other diseases until more research is available.
One study showed that exposure to TNF-α inhibitors in a group of men diagnosed with AS resulted in improvement of sexual function scores (Oh et al., 2009).
Pregnancy and child outcomes.
Eight small studies and one large population-based cohort were identified (Lamboglia et al., 2009; Paschou et al., 2009; Saougou et al., 2013; Clowse et al., 2015; Larsen et al., 2016; Hoxha et al., 2017; Uyaroglu et al., 2017; Lichtenstein et al., 2018; Micu et al., 2019). In total, 61 males, 121 pregnancies and 372 children were included. Overall, no increased risk was found. Larsen found a higher risk for SGA infants based on 16 cases, although this was not statistically significant.
Verdolizumab
Sexual function, reproductive hormones and fertility.
One study from Denmark that included data on 15 male patients diagnosed with IBD with a mean age of 33 years and 40 healthy controls with a mean age of 23 years was identified. After exposure to verdolizumab, the sperm DFI was similar amongst the two groups (Grosen et al., 2019b).
Pregnancy and child outcomes.
No studies were identified.
Other selective immunosuppressants
Pregnancy and child outcomes.
All publications contained mostly maternal exposure cases and only briefly mention paternal exposures. The results for the paternal exposure were one case report on leflunomide reporting a healthy child (De Santis et al., 2005) and two case series from the industry on abatacept (10 pregnancies) and tofacitinib (84 pregnancies) revealing no safety concerns (Kumar et al., 2015; Mahadevan et al., 2018).
Immunosuppressive drugs without available information
For many immunosuppressive drugs, no studies were identified. In Table III, the most relevant immunosuppressive drugs where no available data were available are presented.
Table III.
Immunosuppressive drugs included in the search strategy without studies included in the final data analysis.
| Sexual function, reproductive hormones and fertility | Pregnancy outcomes | ||
|---|---|---|---|
| Anakinra | JAK inhibitors | Apremilast | JAK inhibitors |
| Apremilast | Leflunomide | Belimumab | NSAIDs |
| Belimumab | Rituximab | Canakinumab | Rituximab |
| Canakinumab | Ruxolitinib | COX 2 inhibitors | Ruxolitinib |
| Human immunoglobulin | Secukinumab | Everolimus | Sulfasalazine |
| Hydroxychloroquine | Tacrolimus | Human immunoglobulin | Tioguanine |
| Ixekizumab | Tocilizumab | Ixekizumab | Tocilizumab |
Treatment of antisperm antibodies
Antisperm antibodies are considered as an important cause of male infertility and often are associated with autoimmunity. Although not included in the original scope of our systematic review, we identified a considerable number of studies regarding the treatment of antisperm antibodies (mainly associated with male infertility) using glucocorticoids. These studies reported mixed results and overall the risks associated with glucocorticoid therapy outweighed the benefits. In-depth information can be found elsewhere (Drobnis and Nangia, 2017).
Discussion
Summary of evidence
Sexual function, reproductive hormones and fertility.
Regarding sexual function, reproductive hormones and fertility, most of the available information focuses on the effect of immunosuppressive drugs on male fertility (specifically on sperm quality). Less information was available for sexual function or reproductive hormones.
Based on the available information on the effect of immunosuppressive drugs on male sexual function, reproductive hormones and fertility, the following classification is provided.
No negative effect was observed for acitretin, azathioprine, ciclosporine, isotretinoin, TNF-α inhibitors and verdolizumab.
Negative effects were reported for cyclophosphamide, sirolimus and sulfasalazine.
Unclear effects were noted for chloroquine, colchicine, methotrexate, NSAIDs and systemic glucocorticoids.
Worth mentioning is that TNF-α plays an important role in spermatogenesis and testicular homeostasis; one of the main findings for this group of drugs is that disease activity itself might play a role in baseline sperm quality characteristics and on the subsequent effect that TNF-α inhibitors have on sperm quality. At least for patients diagnosed with AS, TNF-α inhibitors appeared to have a positive effect on sperm quality. As it is the case for most of the drugs included in this systematic review, further research is needed.
Disease activity was taken into consideration in the study design of a few studies. By doing this, authors showed that disease activity can also induce sperm abnormalities (Villiger et al., 2010; Ganatra et al., 2018; Kaya Aksoy et al., 2019). Considering that IMDs have different inflammatory phenotypes, the effect of disease activity could be an important confounder in future studies on the impact of medications on sperm quality.
Pregnancy and child outcomes.
Regarding pregnancy and child outcomes, we found no clear evidence to support restriction in the prescription of these drugs. Although the number of patients was low in case reports and series, and in small cohorts, in some cases, detailed information is available. In contrast, in population-based registries, predominantly from Denmark, larger numbers of patients have been reported. In these populations, odds ratios or hazard ratios can be calculated but they lack important detailed information about the dose, indication and co-medication.
Findings
Sexual function, reproductive hormones and fertility.
The effects of many immunosuppressive drugs on sexual function, reproductive hormones and fertility have not been properly evaluated. Many factors can contribute to this situation, for example, sperm samples are needed to evaluate sperm quality and this may lead to many logistic problems. In addition, there is a general misconception that male contributions to pregnancy are not important, which can contribute to a lack of interest by researchers and clinicians.
Furthermore, the effect of immunosuppressive drugs on sexual function, reproductive hormones and fertility cannot be studied separately. Multiple factors are interconnected in this process and should be considered in clinical practice and in future research.
Pregnancy and child outcomes.
The possible influence of paternal exposure before conception on pregnancy and child outcomes is also a neglected topic. In the last years, the number of publications has been increasing. In most cases, these studies include maternal and paternal exposures with little attention to the outcomes secondary to paternal exposure. Most of the time, no in-depth details of the paternal cases were available.
Strengths and limitations
The strengths of this study are based on the design and conduction of the systematic review. It followed strict pre-specified and reproducible methods. A comprehensive search strategy was developed to summarise the available information on many aspects of sexual health and reproduction. We did not restrict the search to a specific disease or drug (group) but tried to compile information about all important drugs (groups) used for several IMDs. Systematic reviews can also demonstrate where knowledge is lacking, and we consider that this is another major strength of this review. Major areas of opportunities for future research regarding this topic were identified.
Unfortunately, several limitations should be addressed. Most of the studies included small numbers of patients and controls. In addition, studies about sexual function, reproductive hormones and fertility in men with IMDs suffer from an inconsistent methodological quality; disease activity was not evaluated as a potential confounder in many studies; relevant comorbidities that also have a direct effect on these outcomes were not reported in all studies. Results might only apply to the specific populations studied.
Importantly, our findings should be interpreted with caution since a significant proportion of our included studies are case reports and small case series that tend to overestimate the outcomes of interest.
Regarding the pregnancy and child outcomes, the level of detail and specific information that is available in the publications needs to improve.
In this review, no animal studies were included. Animal studies show effects on reproductive outcomes for drugs such as methotrexate and thiopurines (Grosen et al., 2017; Simsek et al., 2018). Outcomes of animal studies are not always predictive for humans. Based on these outcomes and in addition to the lack of well-documented human paternal exposures in large studies, a restrictive wording is placed in the summary of product characteristics by the regulatory agencies.
Research recommendations
For many immunosuppressive drugs that are prescribed to millions of men with IMD, such as methotrexate or hydroxychloroquine, the possibility of re-evaluating their reproductive toxicity is of major importance and should be discussed. Semen analysis is still considered to be the most valid method to evaluate testicular toxicity in humans. Ideally, RCTs and case–control well-designed prospective cohort studies should be designed to reach conclusive evidence.
While experimental studies are not ethical for assessing reproductive toxicity, observational studies such as those utilising case–control or cohort designs may be and should be considered. As a rule of thumb, if no increased incidence of malformations is observed within at least 1000 prospectively collected pregnancies, a conclusion might be reached that the drug of interest is not responsible for a 2-fold or more increase of the overall incidence of malformations (EMA, 2008).
Several factors such as the number of exposed patients, the incidence of the outcome in the unexposed control group, the minimum relative risk to be detected and the ratio of unexposed control to exposed study subjects can affect the power of a cohort study and should be considered during the early stages of a study design (Strom, 2020).
Focusing only on the prevalence of exposure and in order to put this into perspective, for frequently used drugs (>10% of the population), such as paracetamol, smaller sample sizes are needed to detect an increased risk of congenital malformations. For such drugs, the required sample size ranges from ∼8140 participants in a population cohort study to 614 participants in a case–control study. This range increases drastically for less frequently used drugs such as rituximab (<1% of the population), where ∼814 000 participants are needed in population cohort studies to 51 116 participants in case–control studies.
Based on these recommendations and following the example of the large Scandinavian cohorts here presented, collecting prospective data on current paternal exposures should be strongly recommended and considered for future research on this topic. In the meantime, health care professionals should think about these potential adverse events and intervene appropriately (see Table IV for research recommendations for standardisation).
Table IV.
Research recommendations to conduct future research on these topics.
| Sexual function |
|
| Sperm quality |
|
| Reproductive hormones |
|
| Pregnancy and offspring outcomes |
|
Recently, it has been shown that disease activity can also impair fertility in men with rheumatic diseases (Villiger et al., 2010; Ganatra et al., 2018; Kaya Aksoy et al., 2019; Perez-Garcia et al., 2020). In consequence, it is very important to start focusing on developing well-designed epidemiological studies where study design and data analysis consider how diseases and drugs together can affect sexual health and reproduction.
To improve the overall quality of research on this topic, a call to action initiative that gets scientists from many different fields involved in the topic is needed. This could result in an organised plan to study and report future research.
Furthermore, access to information is more important than ever since effective communication has become an essential part of the treatment shared decision process between health care professionals and patients. Therefore, discussing the possible effect(s) of immunosuppressive drugs on male sexual health and reproduction should be considered for every man, irrespective of whether they have a wish to become a father or not.
Conclusion
There is little scientific evidence regarding the potential adverse events on male sexual function, reproductive hormones and fertility of many of the commonly used immunosuppressive drugs. Most of the included studies are heterogeneous and cannot be generalised to a wider population. With a lack of conclusive evidence, it is expected that clinicians and patients are confronted with difficult treatment decisions.
The results of this systematic review did not reveal major safety issues concerning paternal exposure to immunosuppressive drugs. However, we have to keep in mind that the numbers are low and an increased risk cannot be excluded. Well-designed and fully powered observational cohort studies with longitudinal data should be conducted to properly label these drugs. In cases where the number of patients included in a study is considered to be too low to reach adequate power, researchers should use standardised methods to measure outcomes of interest, ensure the quality of the collected variables and report their findings according to the STROBE statement (Vandenbroucke et al., 2007; von Elm et al., 2008). This will provide the scientific community with valuable information and allow it to perform meta-analyses in the future.
In cases of men with a wish to become a father, the sometimes very restrictive wording in the summary of product characteristics might not be necessary. In the meantime, in case the patient wishes to become a father, clinicians must discuss the pros and cons of stopping or changing drug treatment. The potential negative effects of the disease on reproductive outcomes and potential flares need to be weight against theoretical concerns of the drug effects.
Supplementary Material
Acknowledgements
The authors acknowledge the infrastructure and support of the Erasmus MC Medical Library. They also gratefully acknowledge H.T.W. Smeele for his support in improving the quality of the figures and tables of this manuscript. And lastly, they sincerely acknowledge the authors of several articles cited in this manuscript, who kindly replied to their e-mails and provided them with relevant unpublished data that helped them improve the overall quality of their manuscript.
Funding
This research was funded by three organisations: The Netherlands Organization for Health Research and Development (ZonMw), The Dutch Arthritis Association (ReumaNederland, previously known as Reumafonds) and Consejo Nacional de Ciencia y Tecnologia (CONACYT). These organisations were not involved in any part of the project, nor in preparing the protocol, nor conducting, analysing, interpreting or publishing the research.
Conflict of interest
R.J.E.M.D. received an unrestricted research grant from UCB Pharma BV. Otherwise there are no conflicts of interest.
Contributor Information
L F Perez-Garcia, Department of Rheumatology, Erasmus MC, University Medical Center, 3000 CA Rotterdam, The Netherlands.
R J E M Dolhain, Department of Rheumatology, Erasmus MC, University Medical Center, 3000 CA Rotterdam, The Netherlands.
S Vorstenbosch, Netherlands Pharmacovigilance Centre Lareb, 5237 MH ’s-Hertogenbosch, The Netherlands.
W Bramer, Medical Library, Erasmus MC, University Medical Center, 3000 CA Rotterdam, The Netherlands.
E van Puijenbroek, Netherlands Pharmacovigilance Centre Lareb, 5237 MH ’s-Hertogenbosch, The Netherlands; Groningen Research Institute of Pharmacy, PharmacoTherapy, Epidemiology and Economics, University of Groningen, 9712 CP Groningen, The Netherlands.
J M W Hazes, Department of Rheumatology, Erasmus MC, University Medical Center, 3000 CA Rotterdam, The Netherlands.
B te Winkel, Netherlands Pharmacovigilance Centre Lareb, 5237 MH ’s-Hertogenbosch, The Netherlands.
Authors’ roles
L.F.P-G., B.t.W., R.J.E.M.D. and W.B. contributed to the conception and study design. L.F.P-G. and B.t.W. analysed the data and contributed to the interpretation of the data. L.F.P-G. and B.t.W. wrote the first version of the manuscript and R.J.E.M.D., J.M.W.H., S.V., E.v.P. and W.B. revised it critically. All the authors read and approved the final manuscript.
References
- Adeeko AO, Dada OA. Chloroquine excretion in semen following antimalarial-drug administration. Andrologia 1994;26:165–166. [DOI] [PubMed] [Google Scholar]
- Albert O, Desdoits-Lethimonier C, Lesné L, Legrand A, Guillé F, Bensalah K, Dejucq-Rainsford N, Jégou B. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod 2013;28:1890–1898. [DOI] [PubMed] [Google Scholar]
- Almeida BP, Saad CGS, Souza FHC, Moraes JCB, Nukumizu LA, Viana VST, Bonfá E, Silva CA. Testicular Sertoli cell function in ankylosing spondylitis. Clin Rheumatol 2013;32:1075–1079. [DOI] [PubMed] [Google Scholar]
- Andersen J, Askaa B, Broedbaek K. Paternal exposure to methotrexate and the risk of miscarriage-a register based nationwide cohort study. Pharmacoepidemiol Drug Saf 2018;27:231. [Google Scholar]
- Andersen J Askaa B Jensen T, Horwitz H, Vermehren C, Broedbaek K.. P06 paternal exposure to methotrexate and the risk of miscarriage – a register based nationwide cohort study. Arch Dis Child 2019;104:e19–e20. [Google Scholar]
- Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, Bacigalupo A. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant 2002;30:447–451. [DOI] [PubMed] [Google Scholar]
- Åsberg A, Reisæter AV, Bergan S, Vikse BE, Midtvedt K. Exposure to mycophenolate and fatherhood-is there a risk? Transplant Int 2017;30:152–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci S, Sarikayalar F. Absence of a hand (acheiria) in a child whose father was treated with cyclophosphamide for Behcet’s disease. Turk J Pediatr 1983;25:55–58. [PubMed] [Google Scholar]
- Baumgarten SR, Lindsay GK, Wise GJ. Fertility problems in the renal transplant patient. J Urol 1977;118:991–993. [DOI] [PubMed] [Google Scholar]
- Beghin D, Cournot MP, Vauzelle C, Elefant E. Paternal exposure to methotrexate and pregnancy outcomes. J Rheumatol 2011;38:628–632. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit A, Ben-Chetrit E, Nitzan R, Ron M. Colchicine inhibits spermatozoal motility in vitro. Int J Fertil 1993;38:301–304. [PubMed] [Google Scholar]
- Ben-Chetrit E, Berkun Y, Ben-Chetrit E, Ben-Chetrit A. The outcome of pregnancy in the wives of men with familial Mediterranean fever treated with colchicine. Semin Arthritis Rheum 2004;34:549–552. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Z Ackerman Z. WAGR syndrome in a baby–the result of 6-MP treatment in afather affected by Crohn's disease? Am J Gastroenterol 2001;96:251. [DOI] [PubMed] [Google Scholar]
- Bendvold E, Gottlieb C, Svanborg K. The effect of naproxen on the concentration of prostaglandins in human seminal fluid. Fertil Steril 1985;43:922–926. [DOI] [PubMed] [Google Scholar]
- Bererhi L, Flamant M, Martinez F, Karras A, Thervet E, Legendre C. Rapamycin-induced oligospermia. Transplantation 2003;76:885–886. [DOI] [PubMed] [Google Scholar]
- Birnie GG, McLeod TI, Watkinson G. Incidence of sulphasalazine-induced male infertility. Gut 1981;22:452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic R, Banicevic M, Cvoric A. Testicular function following cyclophosphamide treatment for childhood nephrotic syndrome: long-term follow-up study. Pediatr Nephrol 1990;4:451–454. [DOI] [PubMed] [Google Scholar]
- Boobes Y, Bernieh B, Saadi H, Hakim MRA, Abouchacra S. Gonadal dysfunction and infertility in kidney transplant patients receiving sirolimus. Int Urol Nephrol 2010;42:493–498. [DOI] [PubMed] [Google Scholar]
- Bramer WM, de Jonge GB, Rethlefsen ML, Mast F, Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc 2018. a;106:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer WM, Rethlefsen ML, Mast F, Kleijnen J. Evaluation of a new method for librarian-mediated literature searches for systematic reviews. Res Synth Methods 2018. b;9:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner WJ, Paulsen CA. Colchicine and testicular function in man. New Engl J Med 1976;294:1384–1385. [DOI] [PubMed] [Google Scholar]
- Cann PA, Holdsworth CD. Reversal of male infertility on changing treatment from sulphasalazine to 5-aminosalicylic acid. Lancet 1984;1:1119. [DOI] [PubMed] [Google Scholar]
- Chatzinoff M, Guarino JM, Corson SL, Batzer FR, Friedman LS. Sulfasalazine-induced abnormal sperm penetration assay reversed on changing to 5-aminosalicylic acid enemas. Dig Dis Sci 1988;33:108–110. [DOI] [PubMed] [Google Scholar]
- Çinar L, Kartal D, Ergin C, Aksoy H, Karadag MA, Aydin T, Cinar E, Borlu M. The effect of systemic isotretinoin on male fertility. Cutaneous Ocul Toxicol 2016;35:296–299. [DOI] [PubMed] [Google Scholar]
- Clowse ME. Feldman SR. Isaacs JD, Kimball AB, Strand V, Warren RB, Xibillé D, Chen Y, Frazier D, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016;39:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowse MEB, Wolf DC, Förger F, Cush JJ, Golembesky A, Shaughnessy L, De Cuyper D, Mahadevan U. Pregnancy outcomes in subjects exposed to certolizumab pegol. J Rheumatol 2015;42:2270–2278. [DOI] [PubMed] [Google Scholar]
- Coleman R, MacDonald D. Effects of isotretinoin on male reproductive system. Lancet 1994;344:198. [DOI] [PubMed] [Google Scholar]
- Cosentino MJ, Chey WY, Takihara H, Cockett ATK. The effects of sulfasalazine on human male fertility potential and seminal prostaglandins. J Urol 1984;132:682–686. [DOI] [PubMed] [Google Scholar]
- Crijns I, Bos J, Knol M, Straus S, de Jong-van den Berg L. Paternal drug use: before and during pregnancy. Expert Opin Drug Saf 2012;11:513–518. [DOI] [PubMed] [Google Scholar]
- De Santis M, Straface G, Cavaliere A, Carducci B, Caruso A. Paternal and maternal exposure to leflunomide: pregnancy and neonatal outcome. Ann Rheum Dis 2005;64:1096–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejaco C, Mittermaier C, Reinisch W, Gasche C, Waldhoer T, Strohmer H, Moser G. Azathioprine treatment and male fertility in inflammatory bowel disease. Gastroenterology 2001;121:1048–1053. [DOI] [PubMed] [Google Scholar]
- Deutsch MA, Kaczmarek I, Huber S, Schmauss D, Beiras-Fernandez A, Schmoeckel M, Ochsenkuehn R, Meiser B, Mueller-Hoecker J, Bruno Reichart B. Sirolimus-associated infertility: case report and literature review of possible mechanisms. Am J Transplant 2007;7:2414–2421. [DOI] [PubMed] [Google Scholar]
- Di Paolo MC, Paoluzi OA, Pica R, Iacopini F, Crispino P, Rivera M, Spera G, Paoluzi P. Sulphasalazine and 5-aminosalicylic acid in long-term treatment of ulcerative colitis: report on tolerance and side-effects. Dig Liver Dis 2001;33:563–569. [DOI] [PubMed] [Google Scholar]
- Drenches P, Klotsche J, Niewerth M, Horneff G, Minden K. Pregnancy outcomes in partners of DMARD exposed men with juvenile idiopathic arthritis-an observational study. Arthritis Rheum 2018;70:1580–1581. [Google Scholar]
- Drobnis EZ, Nangia AK. Immunosuppressants and male reproduction. Adv Exp Med Biol 2017;1034:179–210. [DOI] [PubMed] [Google Scholar]
- Ecevit C, Ünal F, Baran M, Aydoǧdu S. Parenthood in pediatric liver transplant patients. Pediatr Transplant 2012;16:346–349. [DOI] [PubMed] [Google Scholar]
- Eck LK, Jensen TB Mastrogiannis D, Torp-Pedersen A, Askaa B, Nielsen TK, Poulsen HE, Jimenez-Solem E, Andersen JT. Risk of adverse pregnancy outcome after paternal exposure to methotrexate within 90 days before pregnancy. Obstet Gynecol 2017;129:707-714. [DOI] [PubMed] [Google Scholar]
- Egeberg A, Gislason GH, Nast A. Birth outcomes in children fathered by men treated with immunosuppressant drugs before conception—a Danish population-based cohort study. J Invest Dermatol 2017;137:1790–1792. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld M, Levy M, Margalioth EJ, Eliakim M. The effects of long-term colchicine therapy on male fertility in patients with familial Mediterranean fever. Andrologia 1986;18:420–426. [DOI] [PubMed] [Google Scholar]
- Eid MM,, Abdel-Hamid IA, Sobh MA, el-Saied MA. Assessment of sperm motion characteristics in infertile renal transplant recipients using computerized analysis. Int J Androl 1996;19:338–344. [DOI] [PubMed] [Google Scholar]
- Ejebe DE, Ojieh AE, Ovuakporaye SI, Odion-Obomhense HK, Adegor EC, Amadi CN, Nwadito C, Emudainohwo JOT, Ozoko TC. Effects of anti-malarial alkaloids on the sperm properties and blood levels of reproductive hormones of adult men. Afr J Biotechnol 2008;7:3395–3400. [Google Scholar]
- El-Beheiry A, El-Mansy E, Kamel N, Salama N. Methotrexate and fertility in men. Arch Androl 1979;3:177–179. [DOI] [PubMed] [Google Scholar]
- EMA. Guideline on Risk Assessment of Medicinal Products on Human Reproduction and Lactation: From Data to Labelling European Medicines Agency, 2008, 1–18. https://www.ema.europa.eu/en/risk-assessment-medicinal-products-human-reproduction-lactation-data-labelling.
- EMA. ICH S5 (R3) Guideline on Reproductive Toxicology: Detection of Toxicity to Reproduction for Medicinal Products including Toxicity to Male Fertility European Medicines Agency, 2017. https://www.ema.europa.eu/en/ich-s5-r3-guideline-reproductive-toxicology-detection-toxicity-reproduction-human-pharmaceuticals.
- Engeland A, Bjørge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, Furu K. Effects of preconceptional paternal drug exposure on birth outcomes: cohort study of 340000 pregnancies using Norwegian population-based databases. Br J Clin Pharmacol 2013;75:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ette EI, Ogonor JI, Essien EE. Passage of chloroquine into semen. Br J Clin Pharmacol 1988;26:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etteldorf JN, West CD, Pitcock JA, Williams DL. Gonadal function, testicular histology, and meiosis following cyclophosphamide therapy in patients with nephrotic syndrome. J Pediatr 1976;88:206–212. [DOI] [PubMed] [Google Scholar]
- Fairley KF, Barrie JU, Johnson W. Sterility and testicular atrophy related to cyclophosphamide therapy. Lancet 1972;1:568–569. [DOI] [PubMed] [Google Scholar]
- Farthing MJG, Dawson AM. Impaired semen quality in Crohn’s disease - drugs, ill health, or undernutrition? Scand J Gastroenterol 1983;18:57–60. [DOI] [PubMed] [Google Scholar]
- FDA. Testicular Toxicity: Evaluation during Drug Development Guidance for Industry, Draft Guidance FDA: Center for Drug Evaluation and Research, 2015. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/testicular-toxicity-evaluation-during-drug-development.
- Feng PH, George CR, Evans RA, Murkin GE, Spicer E, Thomas BS, Hellmann K, Hassib H, Hassib F. Cyclophosphamide and infertility. Lancet 1972;1:840–842. [DOI] [PubMed] [Google Scholar]
- Francella A, Dyan A, Bodian C, Rubin P, Chapman M, Present DH. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: a retrospective cohort study. Gastroenterology 2003;124:9–17. [DOI] [PubMed] [Google Scholar]
- Freeman JG, Reece VAC, Venables CW. Sulphasalazine and spermatogenesis. Digestion 1982;23:68–71. [DOI] [PubMed] [Google Scholar]
- Freixa R, Rosello Catafau J, Gelpi E. Comparative study of antiinflammatory drugs and sulphasalazine in relation to prostaglandin E and 19 hydroxylated prostaglandin E levels and human male fertility. Prostaglandins Leukot Med 1984;16:359–369. [DOI] [PubMed] [Google Scholar]
- Friedman S, Larsen MD, Magnussen B, Jølving LR, de Silva P, Nørgård BM. Paternal use of azathioprine/6-mercaptopurine or methotrexate within 3 months before conception and long-term health outcomes in the offspring—a nationwide cohort study. Reprod Toxicol 2017;73:96–200. [DOI] [PubMed] [Google Scholar]
- Fritsche L, Budde K, Dragun D, Einecke G, Diekmann F, Neumayer HH. Testosterone concentrations and sirolimus in male renal transplant patients. Am J Transplant 2004;4:130–131. [DOI] [PubMed] [Google Scholar]
- Fukutani K, Ishida H, Shinohara M. Suppression of spermatogenesis in patients with Behcet’s disease treated with cyclophosphamide and colchicine. Fertil Steril 1981;36:76–80. [DOI] [PubMed] [Google Scholar]
- Ganatra A, Shaikh A, Chandak S, Kalke S, Bhojani N, Bhojani K. Effect of sulfasalazine on fertility of spondyloarthropathy patients. Indian J Rheumatol 2018;13:S239. [Google Scholar]
- Griggs LR, Schwartz DA. Successful paternity of a healthy child while taking methotrexate for Crohn’s disease. Am J Gastroenterol 2006;101:2893–2894. [DOI] [PubMed] [Google Scholar]
- Grosen A, Bungum M, Christensen LA, Cordelli E, Larsen OH, Leter G, Julsgaard M, Vestergaard T, Villani P, Hvas CL et al. Semen quality and sperm DNA integrity in patients with severe active inflammatory bowel disease and effects of tumour necrosis factor-alpha inhibitors. J Crohns Colitis 2019. a:13;564–571. [DOI] [PubMed] [Google Scholar]
- Grosen A, Bungum M, Hvas CL, Julsgaard M, Cordelli E, Kelsen J. Vedolizumab does not impair sperm DNA integrity in men with inflammatory bowel disease. Gastroenterology 2019. b:156;2342–2344. [DOI] [PubMed] [Google Scholar]
- Grosen A, Kelsen J, Hvas CL, Bellaguarda E, Hanauer SB. The influence of methotrexate treatment on male fertility and pregnancy outcome after paternal exposure. Inflamm Bowel Dis 2017;23:561–569. [DOI] [PubMed] [Google Scholar]
- Grosen A, Nersting J, Bungum M, Christensen LA, Schmiegelow K, Spanò M, Julsgaard M, Cordelli E, Leter G, Larsen PB et al. Sperm DNA integrity is unaffected by thiopurine treatment in men with inflammatory bowel disease. J Crohn’s Colitis 2019. c;13;3–11. [DOI] [PubMed] [Google Scholar]
- Grunnet E, Nyfors A, Hansen KB. Studies of human semen in topical corticosteroid-treated and in methotrexate-treated psoriatics. Dermatologica 1977;154:78–84. [DOI] [PubMed] [Google Scholar]
- Haberman J, Karwa G, Greenstein SM, Soberman R, Glicklich D, Tellis V, Melman A. Male fertility in cyclosporine-treated renal transplant patients. J Urol 1991;145:294–296. [DOI] [PubMed] [Google Scholar]
- Hargreaves CA, Rogers S, Hills F, Rahman F, Howell RJS, Homa ST. Effects of co-trimoxazole, erythromycin, amoxycillin, tetracycline and chloroquine on sperm function in vitro. Hum Reprod 1998;13:1878–1886. [DOI] [PubMed] [Google Scholar]
- Healy D, Le Noury J, Mangin D. Enduring sexual dysfunction after treatment with antidepressants, 5α-reductase inhibitors and isotretinoin: 300 cases. Int J Risk Saf Med 2018;29:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman MJ, Dony JMJ, Rolland R. Salicylazosulfapyridine and male infertility. Eur J Obstet Gynecol Reprod Biol 1981;12:297–303. [DOI] [PubMed] [Google Scholar]
- Heppt F, Colsman A, Maronna A, Uslu U, Heppt MV, Kiesewetter F, Sticherling M. Influence of TNF-alpha inhibitors and fumaric acid esters on male fertility in psoriasis patients. J Eur Acad Dermatol Venereol 2017;31:1860–1866. [DOI] [PubMed] [Google Scholar]
- Hoeltzenbein M, Weber-Schoendorfer C, Borisch C, Allignol A, Meister R, Schaefer C. Pregnancy outcome after paternal exposure to azathioprine/6-mercaptopurine. Reprod Toxicol 2012;34:364–369. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Lundgren HE, Suhr OB. Successful pregnancies and fatherhood in familial amyloidotic polyneuropathy (FAP Val30Met) patients with liver transplantation. Amyloid 2004;11:125–129. [DOI] [PubMed] [Google Scholar]
- Hoxha A, Calligaro A, Di Poi E, Peccatori S, Favaro M, Del Ross T, Ramonda R, Grava C, Raffeiner B, Ravagni P et al. Pregnancy and foetal outcomes following anti-tumor necrosis factor alpha therapy: a prospective multicentre study. Jt Bone Spine 2017;84:169–173. [DOI] [PubMed] [Google Scholar]
- Hsu AC, Folami AO, Bain J, Rance CP. Gonadal function in males treated with cyclophosphamide for nephrotic syndrome. Fertil Steril 1979;31:173–177. [DOI] [PubMed] [Google Scholar]
- Hudson E, Dore C, Sowter C. Sperm size in patients with inflammatory bowel disease on sulfasalazine therapy. Fertil Steril 1982;38:77–84. [DOI] [PubMed] [Google Scholar]
- Iglesias-cortit JL, Paz JL, Ballesca JL, Valles A, Iglesias-guiu J, Freixa R, Rosello J, Gelpi E, Puig-parellada P. Effects of sulphasalazine, lysine acetylsalicylate and flurbiprofen on human spermatozoa. Adv Contracept Deliv Syst 1985;92–96. [PubMed] [Google Scholar]
- Jones A, Clary MJ, McDermott E, Coscia LA, Constantinescu S, Moritz MJ, Armenti VT. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant 2013;23:153–157. [DOI] [PubMed] [Google Scholar]
- Kaczmarek I, Groetzner J, Adamidis I, Landwehr P, Mueller M, Vogeser M, Gerstorfer M, Uberfuhr P, Meiser B, Reichart B. Sirolimus impairs gonadal function in heart transplant recipients. Am J Transplant 2004;4:1084–1088. [DOI] [PubMed] [Google Scholar]
- Kantarci G, Sahin S, Uras AR, Ergin H. Effects of different calcineurin inhibitors on sex hormone levels in transplanted male patients. Transplant Proc 2004;36:178–179. [DOI] [PubMed] [Google Scholar]
- Kastrop P, Kimmel I, Bancsi L, Weima S, Giltay J. The effect of colchicine treatment on spermatozoa: a cytogenetic approach. J Assist Reprod Genet 1999;16:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katugampola RP, Finlay AY. Oral retinoid therapy for disorders of keratinization: single-centre retrospective 25 years’ experience on 23 patients. Br J Dermatol 2006;154:267–276. [DOI] [PubMed] [Google Scholar]
- Kaya Aksoy G, Koyun M, Usta MF, Çomak E, Akman S. Semen analysis in adolescents with familial Mediterranean fever. J Pediatr Urol 2019;15:342.e1–342.e7. [DOI] [PubMed] [Google Scholar]
- Khandwala YS, Zhang CA, Lu Y, Eisenberg ML. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod 2017;32:2110–2116. [DOI] [PubMed] [Google Scholar]
- Kirchin VS, Southgate HJ, Beard RC. Colchicine: an unusual cause of reversible azoospermia. BJU Int 1999;83:156. [DOI] [PubMed] [Google Scholar]
- Kirkland RT, Bongiovanni AM, Cornfeld D. Gonadotropin responses to luteinizing release factor in boys treated with cyclophosphamide for nephrotic syndrome. J Pediatr 1976;89:941–944. [DOI] [PubMed] [Google Scholar]
- Knuth UA, Kuhne J, Crosby J, Bals-Pratsch M, Kelly RW, Nieschlag E. Indomethacin and oxaprozin lower seminal prostaglandin levels but do not influence sperm motion characteristics and serum hormones of young healthy men in a placebo-controlled double-blind trial. J Androl 1989;10:108–119. [DOI] [PubMed] [Google Scholar]
- Kramer BK, Neumayer HH, Stahl R, Pietrzyk M, Kruger B, Pfalzer B, Bourbigot B, Campbell S, Whelchel J, Eris J et al. Graft function, cardiovascular risk factors, and sex hormones in renal transplant recipients on an immunosuppressive regimen of everolimus, reduced dose of cyclosporine, and basiliximab. Transplant Proc 2005;37:1601–1604. [DOI] [PubMed] [Google Scholar]
- Kreitenberg AJ, Ortiz EC, Arkfeld DG. Priapism after tumor necrosis factor alpha inhibitor use. Clin Rheumatol 2015;34:801–802. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Desdoits-Lethimonier C, Mackey AL, Dalgaard MD, De Masi F, Munkbøl CH, Styrishave B, Antignac JP, Le Bizec B, Platel C et al. Ibuprofen alters human testicular physiology to produce a state of compensated hypogonadism. Proc Natl Acad Sci U S A 2018;115:E715–E724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Ray L, Vemuri S, Simon T. Pregnancy outcomes following exposure to abatacept during pregnancy. Intern Med J 2015; 45:31. [DOI] [PubMed] [Google Scholar]
- Kumar R, Biggart JD, McEvoy J, McGeown MG. Cyclophosphamide and reproductive function. Lancet 1972;1:1212–1214. [DOI] [PubMed] [Google Scholar]
- Lamboglia F, D’Incà R, Oliva L, Bertomoro P, Sturniolo GC. Patient with severe Crohn’s disease became a father while on methotrexate and infliximab therapy. Inflamm Bowel Dis 2009;15:648–649. [DOI] [PubMed] [Google Scholar]
- Larsen MD, Friedman S, Magnussen B, Nørgård BM. Birth outcomes in children fathered by men treated with anti-TNF-alpha agents before conception. Am J Gastroenterol 2016;111:1608–1613. [DOI] [PubMed] [Google Scholar]
- Larsen MD, Friedman S, Magnussen B, Norgard BM. Birth outcome of children fathered by men treated with systemic corticosteroids during the conception period - a cohort study based on nationwide data. Basic Clin Pharmacol Toxicol 2018;122:133–138. [DOI] [PubMed] [Google Scholar]
- Lee S, Coco M, Greenstein SM, Schechner RS, Tellis VA, Glicklich DG. The effect of sirolimus on sex hormone levels of male renal transplant recipients. Clin Transplant 2005;19:162–167. [DOI] [PubMed] [Google Scholar]
- Levi AJ, Fischer AM, Hughes L, Hendry WF. Male infertility due to sulphasalazine. Lancet 1979;2:276–278. [DOI] [PubMed] [Google Scholar]
- Levy M, Eliakim M. Long-term colchicine prophylaxis in familial Mediterranean fever. Br Med J 1977;2:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley D, Jones J, Parrish J, Salih S, Caldera F, Tirado E, Leader B, Saha S. Methotrexate reduces DNA integrity in sperm from men with inflammatory bowel disease. Gastroenterology 2018;154:2064–2067. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Mahadevan U, Salzberg BA, Langholff W, Morgan GJ, Safdi M, Nissinen R, Taillard F, Sandborn WJ et al. Pregnancy outcomes reported during the 13-year TREAT registry: a descriptive report. Am J Gastroenterol 2018;113:1678–1688. [DOI] [PubMed] [Google Scholar]
- Liu H, Li J, Yu L. Effects of acitretin on semen quality and reproductive hormone levels in patients with psoriasis vulgaris. Dermatol Sin 2017;35:55–58. [Google Scholar]
- Lopez-Lopez I, Rodelo-Haad C, Agüera ML, Cabello-Jabalquinto R, Esquivias-Motta E, Navarro MD, Aljama P, Rodriguez-Benot A. Administration of mycophenolic acid is not associated with malformations in descendants from kidney transplanted males. PLoS One 2018;13:e0202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan U, Dubinsky MC, Su C, Lawendy N, Jones TV, Marren A, Zhang H, Graham D, Clowse MEB, Feldman SR et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis 2018;24:2494–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan U, Terdiman JP, Aron J, Jacobsohn S, Turek P. Infliximab and semen quality in men with inflammatory bowel disease. Inflamm Bowel Dis 2005;11:395–399. [DOI] [PubMed] [Google Scholar]
- Marina S, Barcelo P. Permanent sterility after immunosuppressive therapy. Int J Androl 1979;2:6–13. [Google Scholar]
- Martens HF, Sheets PK, Tenover JS, Dugowson CE, Bremner WJ, Starkebaum G. Decreased testosterone levels in men with rheumatoid arthritis: effect of low dose prednisone therapy. J Rheumatol 1994;21:1427–1431. [PubMed] [Google Scholar]
- Masala A, Faedda R, Alagna S, Satta A, Chiarelli G, Rovasio PP, Ivaldi R, Taras MS, Lai E, Bartoli E. Use of testosterone to prevent cyclophosphamide-induced azoospermia. Ann Intern Med 1997;126:292–295. [DOI] [PubMed] [Google Scholar]
- McDonald JH, Heckel NJ. The effect of cortisone on the spermatogenic function of the human testes. J Urol 1956;75:527–529. [DOI] [PubMed] [Google Scholar]
- McGeown MG, Nevin NC. Cytogenetic analysis on children born of parents treated with immunosuppressive drugs. Proc Eur Dial Transplant Assoc 1978;15:384–390. [PubMed] [Google Scholar]
- McIntyre PB, Lennard-Jones JE. Reversal with balsalazide of infertility caused by sulphsalazine. Br Med J (Clin Res Ed) 1984;288:1652–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin HE. Azoospermia caused by colchicine–a case report. Fertil Steril 1972;23:180–181. [DOI] [PubMed] [Google Scholar]
- Micu MC, Micu R, Surd S, Gîrlovanu M, Bolboacǎ SD, Ostensen M. TNF-α inhibitors do not impair sperm quality in males with ankylosing spondylitis after short-term or long-term treatment. Rheumatology 2014;53:1250–1255. [DOI] [PubMed] [Google Scholar]
- Micu MC, Ostensen M, Bojinca V, Serban O, Mihai M, Suta C, Ramazan A, Enache L, Bobirca A, Patcas S et al. Pregnancy outcomes in couples with males exposed to longterm anti-tumor necrosis factor-α inhibitor therapies: a prospective study. J Rheumatol 2019;46:1084–1088. [DOI] [PubMed] [Google Scholar]
- Midtvedt K, Bergan S, Reisæter AV, Vikse BE, Åsberg A. Exposure to mycophenolate and fatherhood. Transplantation 2017;101:e214–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misro MM, Chaki SP, Srinivas M, Chaube SK. Effect of cyclosporine on human sperm motility in vitro. Arch Androl 1999;43:215–220. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Reboldi G, Cappuccio FP,, Agyemang C, Remuzzi G, Rapi S, Perruolo E, Parati G; ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One 2016;11:e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna GL Malesci D Buono R Valentini G. Asthenoazoospermia in patients receivinganti-tumour necrosis factor α agents. Ann Rheum Dis 2005;64:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Coscia L, Armenti D, Constantinescu S. Transplant pregnancy registry international: fathered pregnancy outcomes with exposure to mycophenolic acid products. Transplant Int 2017;30:135. [Google Scholar]
- Moskovitz B, Lin R, Nassar S, Levin DR. Effect of diclofenac sodium (Voltaren) on spermatogenesis of infertile oligospermic patients. Eur Urol 1988;14:395–397. [DOI] [PubMed] [Google Scholar]
- Nørgård BM, Magnussen B, Larsen MD, Friedman S. Reassuring results on birth outcomes in children fathered by men treated with azathioprine/6-mercaptopurine within 3 months before conception: a nationwide cohort study. Gut 2017;66:1761–1766. [DOI] [PubMed] [Google Scholar]
- Nørgaard M, Andersen JT. Paternal acitretin exposure and pregnancy risks. Arch Dis Child 2019;104:e6. [Google Scholar]
- Ogata H, Shibata T, Hirai Y. Effect of cyclophosphamide on the reproductive function. (Study of testicular histology in male patients with nephrotic syndrome). Nephron 1982;32:294. [PubMed] [Google Scholar]
- Oh JS, Heo HM, Kim YG, Lee SG, Lee CK, Yoo B. The effect of anti-tumor necrosis factor agents on sexual dysfunction in male patients with ankylosing spondylitis: a pilot study. Int J Impot Res 2009;21:372–375. [DOI] [PubMed] [Google Scholar]
- O’Morain C, Smethurst P, Dore CJ, Levi AJ. Reversible male infertility due to sulphasalazine: studies in man and rat. Gut 1984;25:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandhi D, Gupta R, Singal A. Gynaecomastia with oligospermia: an unusual complication of low-dose methotrexate for pustular psoriasis. Clin Exp Dermatol 2006;31:138–140. [DOI] [PubMed] [Google Scholar]
- Parsch EM, Ruzicka T, Przybilla B, Schill WB. Andrological investigation in men treated with acitretin (Ro 10-1670). Andrologia 1990;22:479–482. [DOI] [PubMed] [Google Scholar]
- Pascarelli NA, Fioravanti A, Moretti E, Guidelli GM, Mazzi L, Collodel G. The effects in vitro of TNF-α and its antagonist ‘etanercept’ on ejaculated human sperm. Reprod Fertil Dev 2017;29:1169–1177. [DOI] [PubMed] [Google Scholar]
- Paschou S, Voulgari PV, Vrabie IG, Saougou IG, Drosos AA. Fertility and reproduction in male patients with ankylosing spondylitis treated with infliximab. J Rheumatol 2009;36:351–354. [DOI] [PubMed] [Google Scholar]
- Peces R, Delatorre M, Urra JM. Pituitary testicular function in cyclosporine-treated renal-transplant patients. Nephrol Dial Transplant 1994;9:1453–1455. [PubMed] [Google Scholar]
- Penn I, Makowski E, Droegemueller W, Halgrimson CG, Starzl TE. Parenthood in renal homograft recipients. JAMA 1971;216:1755–1761. [PMC free article] [PubMed] [Google Scholar]
- Pennisi AJ, Grushkin CM, Lieberman E. Gonadal function in children with nephrosis treated with cyclophosphamide. Am J Dis Child 1975;129:315–318. [DOI] [PubMed] [Google Scholar]
- Penso J, Lippe B, Ehrlich R, Smith FG. Testicular function in prepubertal and pubertal male patients treated with cyclophosphamide for nephrotic syndrome. J Pediatr 1974;84:831–836. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia LF, Te Winkel B, Carrizales JP, Bramer W, Vorstenbosch S, van Puijenbroek E, Hazes JMW, Dolhain R. Sexual function and reproduction can be impaired in men with rheumatic diseases: a systematic review. Semin Arthritis Rheum 2020;50:557–573. [DOI] [PubMed] [Google Scholar]
- Perrier d’Hauterive S, Kesseler S, Ruggeri P, Timmermans M, Gaspard O, Kumke T, Parker G. Certolizumab PEGOL did not result in a decrease in semen quality in healthy volunteers: results from a phase 1 study. Ann Rheum Dis 2012; 71:365–366. [Google Scholar]
- Perrone L, Sinisi AA, Del Gado R, Del Gaizo D, Bellastella A, Faggiano M. Late effects of cyclophosphamide on testicular function in prepubertal boys and adults. J Pediatr Endocrinol 1989;3:105–108. [Google Scholar]
- Perry WH. Methotrexate and teratogenesis. Arch Dermatol 1983;119:874–875. [PubMed] [Google Scholar]
- Poratsoldin O, Soldin SJ. Preliminary studies on the in vitro and in vivo effect of salicylate on sperm motility. Ther Drug Monit 1992;14:366–370. [DOI] [PubMed] [Google Scholar]
- Ragni G, Bianchi Porro G, Ruspa M. Abnormal semen quality and low serum testosterone in men with inflammatory bowel disease treated for a long time with sulfasalazine. Andrologia 1984;16:162–167. [DOI] [PubMed] [Google Scholar]
- Rajapakse RO, Korelitz BI, Zlatanic J, Baiocco PJ, Gleim GW. Outcome of pregnancies when fathers are treated with 6-mercaptopurine for inflammatory bowel disease. Am J Gastroenterol 2000;95:684–688. [DOI] [PubMed] [Google Scholar]
- Ramonda R, Foresta C, Ortolan A, Bertoldo A, Oliviero F, Lorenzin M, Pizzol D, Punzi L, Garolla A. Influence of tumor necrosis factor α inhibitors on testicular function and semen in spondyloarthritis patients. Fertil Steril 2014;101:359–365. [DOI] [PubMed] [Google Scholar]
- Riley SA, Lecarpentier J, Mani V. Sulphasalazine induced seminal abnormalities in ulcerative colitis: results of mesalazine substitution. Gut 1987;28:1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Pellegrino M. Acitretin-associated erectile dysfunction: a case report. Cases J 2009; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad Hussain S, Farhat S, Wani I. Oligospermia secondary to sirolimus. Indian J Transplant 2015;9:119–121. [Google Scholar]
- Samojlik E, Kirschner MA, Ribot S, Szmal E. Changes in the hypothalamic-pituitary-gonadal axis in men after cadaver kidney transplantation and cyclosporine therapy. J Androl 1992;13:332–336. [PubMed] [Google Scholar]
- Saougou I, Markatseli TE, Papagoras C, Kaltsonoudis E, Voulgari PV, Drosos AA. Fertility in male patients with seronegative spondyloarthropathies treated with infliximab. Jt Bone Spine 2013;80:34–37. [DOI] [PubMed] [Google Scholar]
- Sarica K, Suzer O, Gurler A, Baltact S, Ozdiler E, Dincel C. Urological evaluation of Behcet patients and the effect of colchicine on fertility. Eur Urol 1995;27:39–42. [DOI] [PubMed] [Google Scholar]
- Sasaki JC, Chapin RE, Hall DG, Breslin W, Moffit J, Saldutti L, Enright B, Seger M, Jarvi K, Hixon M et al. Incidence and nature of testicular toxicity findings in pharmaceutical development. Birth Defects Res B Dev Reprod Toxicol 2011;92:511–525. [DOI] [PubMed] [Google Scholar]
- Schirm E, Pedersen L, Tobi H, Nielsen GL, Sorensen HT, de Jong-van den Berg LTW. Drug use among fathers around time of conception: two register based surveys from Denmark and the Netherlands. Pharmacoepidemiol Drug Saf 2004;13:609–613. [DOI] [PubMed] [Google Scholar]
- Schmitt-Hoffmann AH, Roos B, Sauer J, Brown T, Weidekamm E, Meyer I, Schleimer M, Maares J. Low levels of alitretinoin in seminal fluids after repeated oral doses in healthy men. Clin Exp Dermatol 2011;36:12–17. [DOI] [PubMed] [Google Scholar]
- Schopf R. Cyclosporine treatment for psoriasis followed by fathering of a healthy child in a previously infertile male. J Am Acad Dermatol 2017;76:AB71. [Google Scholar]
- Shaffer JL, Kershaw A, Berrisford MH. Sulphasalazine-induced infertility reversed on transfer to 5-aminosalicylic acid. Lancet 1984;1:1240. [DOI] [PubMed] [Google Scholar]
- Simsek M, Lambalk CB, Wilschut JA, Mulder CJJ, De Boer NKH. The associations of thiopurines with male fertility and paternally exposed offspring: a systematic review and meta-analysis. Hum Reprod Update 2018;24:192–206. [DOI] [PubMed] [Google Scholar]
- Skrzypek J, Krause W. Azoospermia in a renal transplant recipient during sirolimus (rapamycin) treatment. Andrologia 2007;39:198–199. [DOI] [PubMed] [Google Scholar]
- Soares PMF, Borba EF, Bonfa E, Hallak J, Corrêa AL, Silva CAA. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum 2007;56:2352–2361. [DOI] [PubMed] [Google Scholar]
- Strom BL. Sample size considerations for pharmacoepidemiologic studies In: Pharmacoepidemiology, 6th edn Hoboken, NJ: Wiley-Blackwell, 2020; 60–70. [Google Scholar]
- Suehiro RM, Borba EF, Bonfa E, Okay TS, Cocuzza M, Soares PMF, Silva CAA. Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology (UK) 2008;47:1692–1697. [DOI] [PubMed] [Google Scholar]
- Sussman A, Leonard JM. Psoriasis, methotrexate, and oligospermia. Arch Dermatol 1980;116:215–217. [PubMed] [Google Scholar]
- Teruel C, Román ALS, Bermejo F, Taxonera C, Pérez-Calle JL, Gisbert JP, Martín-Arranz M, Ponferrada A, Van Domselaar M, Algaba A et al. Outcomes of pregnancies fathered by inflammatory bowel disease patients exposed to thiopurines. Am J Gastroenterol 2010;105:2003–2008. [DOI] [PubMed] [Google Scholar]
- Tobias R, Coetzee T, Sapire KE, Marks IN. Male infertility due to sulphasalazine. Postgrad Med J 1982;58:102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondolo V, Citterio F, Panocchia N, Nanni G, Favi E, Brescia A, Castagneto M. Gonadal function and immunosuppressive therapy after renal transplantation. Transplant Proc 2005;37:1915–1917. [DOI] [PubMed] [Google Scholar]
- Toovey S, Hudson E, Hendry WF, Levi AJ. Sulphasalazine and male infertility: reversibility and possible mechanism. Gut 1981;22:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok L, Kadar L, Kasa M. Spermatological investigations in patients treated with etretinate and isotretinoin. Andrologia 1987;19:629–633. [DOI] [PubMed] [Google Scholar]
- Toth A. Reversible toxic effect of salicylazosulfapyridine on semen quality. Fertil Steril 1979;31:538–540. [DOI] [PubMed] [Google Scholar]
- Traub AI, Thompson W, Carville J. Male infertility due to sulphasalazine. Lancet 1979;2:639–640. [DOI] [PubMed] [Google Scholar]
- Trompeter RS, Evans PR, Barratt TM. Gonadal function in boys with steroid-responsive nephrotic syndrome treated with cyclophosphamide for short periods. Lancet 1981;1:1177–1179. [DOI] [PubMed] [Google Scholar]
- Uyaroglu OA, Seyhoglu E, Erden A, Kilic L, Armagan B, Sari A, Karadag O,, Akdogan A, Bilgen SA, Kiraz S et al. Pregnancy outcomes in male patients using anti-tumor necrosis factor alpha patients with inflammatory arthritis; Hur-BIO real life experiences. Arthritis Rheum 2017;69. [Google Scholar]
- Van Scott EJ, Reinertson RP. Morphologic and physiologic effects of chemotherapeutic agents in psoriasis. J Invest Dermatol 1959;33:357–369. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ,, Poole C, Schlesselman JJ, Egger M, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger PM, Caliezi G, Cottin V, Förger F, Senn A, Østensen M. Effects of TNF antagonists on sperm characteristics in patients with spondyloarthritis. Ann Rheum Dis 2010;69:1842–1844. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- Warren R, Reich K, Langley R, Strober B, Gladman D, Deodhar A, Bachhuber T, Bao W, Altemeyer E, Hussain S et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Acta Derm Venereol 2018;98:11. [DOI] [PubMed] [Google Scholar]
- Watson AR, Rance CP, Bain J. Long term effects of cyclophosphamide on testicular function. Br Med J 1985;291:1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-schoendorfer C, Hoeltzenbein M, Wacker E, Meister R, Schaefer C. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose methotrexate: an observational cohort study. Rheumatology 2014;53:757–763. [DOI] [PubMed] [Google Scholar]
- Weber-Schoendorfer C, Schaefer C. Pregnancy outcome after tocilizumab therapy in early pregnancy-case series from the German Embryotox Pharmacovigilance Center. Reprod Toxicol 2016;60:29–32. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses The University of Ottawa, 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Wildi LM, Haraoui B. Reversible male infertility under treatment with an anti-TNFα agent: a case report. Ann Rheum Dis 2012;71:473–474. [DOI] [PubMed] [Google Scholar]
- Winter RW, Larsen MD, Magnussen B, Friedman S, Kammerlander H, Nøgård BM. Birth outcomes after preconception paternal exposure to methotrexate: a nationwide cohort study. Reprod Toxicol 2017;74:219–223. [DOI] [PubMed] [Google Scholar]
- Xu L, Han S, Liu Y, Wang H, Yang Y, Qiu F, Peng W, Tang L, Fu J, Zhu XF et al. The influence of immunosuppressants on the fertility of males who undergo renal transplantation and on the immune function of their offspring. Transplant Immunol 2009;22:28–31. [DOI] [PubMed] [Google Scholar]
- Xu LG, Yang YR, Wang HW, Qiu F, Peng WL, Xu HM, Han S, Liu Y, Tang LG, Fu J. Characteristics of male fertility after renal transplantation. Andrologia 2011;43:203–207. [DOI] [PubMed] [Google Scholar]
- Youngstein T, Hoffmann P, Gül A, Lane T, Williams R, Rowczenio DM, Ozdogan H, Ugurlu S, Ryan J, Harty L et al. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 2017;56:2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis S, Rimar D. Slobodin G. Boulman N. Rozenbaum M. Rosner I . Effect of infliximab on male fertility: Comment on the article “Fertility male patients with seronegative spondyloarthropathies treated with infliximab” by Saougou et al., Joint Bone Spine 2013; 80,34-37. Joint Bone Spine 2014;81:102–103. [DOI] [PubMed] [Google Scholar]
- Zelissen PMJ, Van Hattum J, Poen H, Scholten P, Gerritse R, Te Velde ER. Influence of salazosulphapyridine and 5-aminosalicylic acid on seminal qualities and male sex hormones. Scand J Gastroenterol 1988;23:1100–1104. [DOI] [PubMed] [Google Scholar]
- Zuber J, Anglicheau D, Elie C, Bererhi L, Timsit MO, Mamzer-Bruneel MF, Ciroldi M, Martinez F, Snanoudj R, Hiesse C et al. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant 2008;8:1471–1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.