Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is rapidly becoming a global health problem. Cardiovascular diseases (CVD) are the most common cause of mortality in NAFLD patients. NAFLD and CVD share several common risk factors including obesity, insulin resistance, and type 2 diabetes (T2D). Atherogenic dyslipidemia, characterized by plasma hypertriglyceridemia, increased small dense low-density lipoprotein (LDL) particles, and decreased high-density lipoprotein cholesterol (HDL-C) levels, is often observed in NAFLD patients.
Scope of review
In this review, we highlight recent epidemiological studies evaluating the link between NAFLD and CVD risk. We further focus on recent mechanistic insights into the links between NAFLD and altered lipoprotein metabolism. We also discuss current therapeutic strategies for NAFLD and their potential impact on NAFLD-associated CVD risk.
Major conclusions
Alterations in hepatic lipid and lipoprotein metabolism are major contributing factors to the increased CVD risk in NAFLD patients, and many promising NASH therapies in development also improve dyslipidemia in clinical trials.
Keywords: Dyslipidemia, NAFLD, Cardiovascular disease, Metabolic syndrome
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) prevalence is estimated at nearly 25% worldwide due to its close association with metabolic disorders such as obesity and type 2 diabetes (T2D) [1] and will soon become the most common indication for liver transplantation in the US and Europe. NAFLD encompasses a wide spectrum of liver pathologies ranging from isolated hepatic steatosis (non-alcoholic fatty liver, NAFL) to non-alcoholic steatohepatitis (NASH), which combines steatosis with hepatocyte ballooning and inflammation and favors development of fibrosis [2]. NAFLD can be divided into three stages with increasing severity: hepatic steatosis, NASH without fibrosis, and NASH with fibrosis. Disease progression from steatosis to NASH and fibrosis is heterogeneous and occurs over years or even decades. Consequently, the mechanisms involved in the evolution of NAFLD remain poorly understood. However, numerous lines of evidence point to alterations in hepatic and extra-hepatic lipid metabolism as central drivers [3]. For example, mutations in several genes involved in the control of lipid metabolism (for example, patatin-like phospholipase domain containing 3, PNPLA3 [4], transmembrane 6 superfamily member 2, TM6SF2 [5], farnesyl-diphosphate farnesyltransferase 1, FDFT1 [6], and membrane bound O-acyltransferase domain containing 7, MBOAT7 [7]) are associated with increased risk of NAFL or NASH.
Importantly, NASH also increases the risk of extra-hepatic complications, especially cardiovascular diseases (CVD), which are among the most common causes of death in NASH patients [8]. Indeed, the alterations in hepatic lipid metabolism that lead to NAFLD also drive the development of atherogenic dyslipidemia, especially elevated plasma triglycerides (TG) and remnant lipoprotein cholesterol levels, and small dense LDL particles that infiltrate the arterial wall and promote the development of atherosclerotic plaques. Altered glucose metabolism and insulin resistance, also hallmarks of NAFLD, can further exacerbate CVD risk in these patients. In this review, we summarize and analyze current data on the links between NAFLD and CVD with a specific focus on the mechanisms of dyslipidemia and their effect on outcomes for patients with NASH.
2. Epidemiology
Although NAFLD shares many common risk factors with CVD (that is, obesity, insulin resistance, T2D, and atherogenic dyslipidemia), there is emerging evidence that NAFL and NASH directly impact CVD risk. This section highlights recent studies assessing the relative contributions of insulin resistance, dyslipidemia, and inflammation to NAFLD-associated CVD.
2.1. NAFLD drives CVD risk independent of obesity, insulin resistance, T2D, and atherogenic dyslipidemia
Several recent studies have investigated the risk of fatal and non-fatal cardiovascular (CV) events in cohorts of NAFLD patients, and there is general agreement that all stages of NAFLD (isolated steatosis and NASH, among others) can increase the risk of CV events such as myocardial infarction, stroke, revascularization, or death. For example, compared to individuals without NAFLD, patients with fatty liver already show an elevated risk of CV events independent of metabolic syndrome risk factors, and this risk further increases when fibrosis is present [9,10]. Surprisingly, even in patients with body mass index (BMI) < 25 kg/m2, ultrasound defined-NAFLD is associated with a higher incidence of CV events, indicating that NAFLD may act independent of obesity [11]. Importantly, these observations have been partly confirmed in recent meta-analyses. A first meta-analysis of 16 studies showed an increased risk of non-fatal CV events in NAFLD patients compared to those without NAFLD [12]. However, the risk of CVD mortality primarily increased in patients with severe NAFLD, as defined by the concomitant presence of imaging-diagnosed steatosis and elevated gamma-glutamyl transpeptidase (GGT), high NAFLD fibrosis scores (NFS), high hepatic fluorodeoxyglucose (FDG) uptake, and/or high histological fibrosis stage. A second smaller meta-analysis [13] generally confirmed these findings, despite methodological weaknesses such as inclusion of low numbers of NASH patients (discussed in detail in Liu et al. [14]). Surprisingly, a third meta-analysis found increased liver-related but not CVD mortality in patients with NAFLD [14].
One major limitation of these studies lies in the diagnostic criteria of NASH. The previously described meta-analyses combined studies using a variety of non-invasive diagnostic measures and relatively few patients with histological evaluation of NASH (the current gold standard). Further long-term assessment of larger numbers of histologically diagnosed patients is essential to improve our understanding of the causes of mortality in NASH. NASH diagnosis may result in more aggressive management, potentially leading to reduced CVD-related events and mortality [14].
In conclusion, these studies suggest that NAFLD increases the risk of CV events independent of other major CVD risk factors (such as dyslipidemia, hypertension, obesity, T2D, and insulin resistance) and suggest that other factors link the liver to arterial wall pathologies. CV events such as myocardial infarction or stroke are the result of dysfunction of the vascular system leading to atherosclerosis. This raises the question of whether NAFLD increases CVD risk by favoring the development of atherosclerosis.
2.2. Association of NAFLD and atherosclerosis
Atherosclerosis, characterized by the development of neo-intimal plaques in large arteries, drives CV events such as myocardial infarction and stroke. Atherosclerotic plaques develop progressively over several decades, during which the plaque composition changes through distinct processes such as lipid-deposition, inflammation, fibrosis and calcification (reviewed in detail by Libby et al. [15]). Evaluation of subclinical atherosclerosis commonly relies on non-invasive detection of plaque features such as lipid deposition, calcification, and inflammatory cells. Common methods include measuring the coronary artery calcification (CAC) score via computed tomography (CT) and estimating vascular inflammation by FDG position emission tomography (PET). Other methods evaluate plaque size by measuring carotid intima-media thickness (CIMT) or arterial stiffness via brachial-ankle pulse wave velocity (ba-PWV). Some invasive procedures also exist (for example, intravascular ultrasonography, optical coherence tomography, and invasive angiography), although these are generally not used for the initial investigation of atherosclerosis [15]. All these methods have been validated as measuring markers of CV risk.
In line with epidemiological evidence of increased risk of CVD events, several studies demonstrated increased subclinical atherosclerosis in NAFLD patients by measuring calcification and aortic stiffness. Cross-sectional studies have shown that ultrasound or magnetic resonance spectroscopy (MRS)-diagnosed NAFLD patients display higher CAC scores than those without NAFLD [16,17], even among patients with BMI < 25 kg/m2 [16]. This was further confirmed in a meta-analysis including 12 studies [18]. Interestingly, the annual rate of CAC progression was significantly higher in participants with ultrasound defined-NAFLD compared to those without NAFLD at baseline and was further increased in subjects with elevated NFS independent of obesity, hypertension, dyslipidemia, and diabetes [19]. CIMT is also increased in patients with NAFLD (diagnosed by ultrasound or CT) independent of dyslipidemia and hypertension [17,20]. Interestingly, a prospective study showed that increased CIMT and ba-PWV at baseline were associated with a higher risk of developing NAFLD (assessed by ultrasound) and ba-PWV was associated with a higher likelihood of fibrosis as assessed by the NFS, fibrosis-4 (FIB4), and aspartate transaminase (AST) to platelet ratio index (APRI) scores [21]. This suggests that vascular dysfunction could also drive NAFLD development. However, more studies with bona fide histological assessment of NASH and fibrosis are needed to further explore this hypothesis. Interestingly, some studies failed to find an association between ultrasound-defined NAFLD and subclinical atherosclerosis by measuring ba-PWV [20], carotid to femoral (cf-) PWV [22], or CAC-defined calcification (higher CAC cutoff >10) [23] after adjustment for other risk factors.
While most studies focused on coronary artery plaque calcification, an investigation by CT of several locations (carotid artery, coronary artery, thoracic aorta, iliac artery, renal artery, celiac trunk, and superior mesenteric artery) found a positive association of NAFLD (diagnosed by CT) with calcification in the thoracic aorta and celiac trunk but not in the coronary artery [24]. Moreover, this study demonstrated that NAFLD patients are more susceptible to developing multi-arterial calcification. Surprisingly, two studies indicated that NAFLD is associated with non-calcified plaques measured by cardiac CT rather than with calcified plaques, the former possibly being more vulnerable to rupture and subsequent CV events [23,25]. In line, hepatic steatosis (diagnosed by CT or ultrasound) is associated with increased plaque FDG-PET/CT, an indicator of plaque vulnerability [26,27].
In conclusion, the majority of studies indicate that NAFLD increases the risk of atherosclerosis and seems to favor the development of unstable plaques, adding to traditional CV risk factors such as obesity, diabetes, hypertension, and dyslipidemia. However, adjustments for systemic inflammation (for example, by plasma C-reactive protein (CRP) or interleukin 6 (IL-6) levels) have not always been carefully performed. Considering the role of inflammation as a driver of atherosclerosis, future studies should focus on the contribution of inflammation vs other factors in defining the NAFLD-associated increase in CVD risk. Still, genetic evidence points to NAFLD-associated dyslipidemia as an important contributor to elevated CVD risk. Intriguingly, certain genetic polymorphisms favoring the development of NAFLD unexpectedly decrease CVD risk (discussed as follows).
2.3. Genetic variation dissociates NAFLD and CVD incidence
A number of genetic studies have identified variants associated with an increased risk of NAFLD development. Among the most frequent are PNPLA3 rs738409 I148M, which affects remodeling of fatty acid chains in liver TG [28], TM6SF2 rs58542926 E167K, which reduces hepatic VLDL secretion [5], and MBOAT7 rs641738 [7], an enzyme involved in phospholipid acyl-chain remodeling, with respective minor (risk) allele frequencies of 24%, 7%, and 43% (based on CARDIoGRAMplusC4D consortium data [29]). While these variants were identified based on MRS-screened hepatic steatosis, the impact of the PNPLA3 and TM6SF2 variants has been confirmed in histologically diagnosed NAFLD [30,31]. However, although steatosis in NAFLD is thought to be the result of increased lipogenesis and increased flux of adipose-derived FA [32,33], there are currently no data linking these variants to these pathways.
Given the impact of these variants on hepatic lipid metabolism, their modulatory role in CVD risk has also been investigated. The PNPLA3 [[34], [35], [36]] and TM6SF2 [34,[36], [37], [38]] NAFLD risk variants are associated with a lower-risk lipoprotein profile characterized by lower plasma TG and/or LDL-C levels. Interestingly, the effect size of the TM6SF2 E167K variant on plasma TG levels is comparable to that of a lipoprotein lipase (LPL) activating variant [34]. This translates into a modest protection of E167K carriers (∼5–15% depending on the study) against CVD development [34,[39], [40], [41]]. The effect on CVD risk is less clear for PNPLA3 I148M carriers. Association studies have shown either no effect [29,[42], [43], [44]] or modest protection [34] from coronary artery disease (CAD) when comparing I148M carriers to non-carriers. Two studies that specifically matched for NAFLD presence (by histology or ultrasound) found no protection from CAD in I148M carriers [43,45].
Unexpectedly, genetic polymorphisms associated with NAFLD tend to decrease CVD risk, indicating that high levels of liver fat, by any mechanism, are not sufficient to increase CVD risk. Nevertheless, these findings indicate a role for NAFLD-driven dyslipidemia as an important mediator of elevated CVD risk.
3. Dyslipidemia: linking hepatic lipid metabolism and the heart
There are likely several mechanisms behind the close association between CVD and NAFLD. Insulin resistance is an important driver of NAFLD and especially NASH. It affects several processes, such as dyslipidemia, hyperglycemia, activation of oxidative stress and inflammation, endothelial dysfunction, and ectopic lipid accumulation, together creating a pro-atherogenic environment favoring CVD development (reviewed in detail Ormazabal et al. [46]). Additionally, hepatic and circulating immune cell populations are highly correlated in patients with NASH [47] and display alterations comparable to those observed in CVD, suggesting a common immune-inflammatory landscape (reviewed in detail by Gehrke et al. [48]).
Atherogenic dyslipidemia, characterized by plasma hypertriglyceridemia, increased small dense LDL particles, and decreased HDL-C levels, is variably present in patients with NAFLD [[49], [50], [51], [52]]. The liver plays a central role in lipoprotein metabolism as it participates in the production and/or clearance of all classes of lipoprotein particles. In addition to its role in the metabolism of lipoprotein particles, the liver is also a major site of metabolism for their substituent triglycerides and especially cholesterol. There is consequently an intricate link between hepatic metabolic dysfunction in NAFLD and altered lipoprotein metabolism and composition (Figure 1). Although low HDL-C is a consistent hallmark of NASH in humans, the underlying mechanism driving this effect (and the resultant change in CVD risk) remains an area of active research [53]. In this section, we focus on recent mechanistic insights into the links between NAFLD and altered VLDL and LDL metabolism.
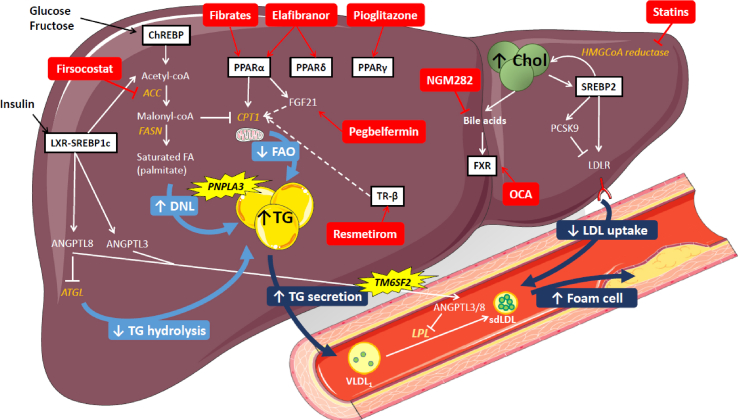
Figure 1.
A summary of hepatic lipid metabolism pathways altered in NAFLD and driving dyslipidemia. Increased hepatic TG in NAFLD is a result of several processes. Elevated plasma insulin and glucose levels respectively activate the LXR and ChREBP pathways, which increase de novo lipogenesis (DNL). Through the action of ACC, DNL increases the concentration of malonyl-coA, leading to inhibition of CPT1 and consequently reducing fatty acid oxidation (FAO) and mitochondrial function. In parallel, LXR increases the expression of ANGPTL8 and 3, two inhibitors of LPL. Moreover, ANGPTL8 contributes to increase hepatic TG by decreasing intracellular TG hydrolysis via inhibiting ATGL. Increased hepatic TG content leads to higher TG secretion and, as a consequence, increased plasma TG levels. Increased intracellular cholesterol in the liver inhibits the SREBP2 pathway. SREBP2 increases LDLR and PCSK9 mRNA expression. These changes combined with additional post-transcriptional regulation of PCSK9 lead to reduced membrane-bound LDLR, which leads to decreased LDL uptake by the liver. The ensemble of these changes contribute to increased large VLDL1 and the formation of small dense LDL, which favors foam cell formation and ultimately atherosclerosis. A number of potential NASH therapies directly target metabolic pathways of lipid metabolism. For example, firsocostat inhibits ACC, reducing DNL and hepatic TG accumulation. Statins inhibit HMGCoA reductase, the rate-limiting enzyme in cholesterol biosynthesis. Other strategies focus on activating different nuclear receptors that more broadly control lipid and glucose metabolism. These include nuclear receptors from the PPAR family, FXR and TR-β. Among those treatments, fibrates are PPARα agonists, pegbelfermin is a FGF21 analog (a PPARα target gene), thiazolidinediones (pioglitazone and rosiglitazone) are PPARγ agonists, elafibranor is a dual-PPARα/δ agonist, lanifibranor is a pan-PPAR agonist, obeticholic acid (OCA) is an FXR agonist, NGM282 is an FGF19 analog (FXR target gene in the intestine), and resmetirom is a TR-β agonist.
3.1. Hepatic TG metabolism and hypertriglyceridemia
3.1.1. Hepatic de novo lipogenesis drives liver injury in NAFLD
De novo lipogenesis (DNL) is considered an important driver of NAFLD despite relatively low absolute levels of DNL in humans compared to rodents. Hyperinsulinemia in metabolic syndrome leads to excessive DNL via activation of the liver X receptor alpha-sterol regulatory element-binding protein 1c (LXRα-SREBP1c) cascade [54]. In addition, nutrient signaling through mechanistic targeting of rapamycin complex 1 (mTORC1) by amino acids and carbohydrate response element-binding protein (ChREBP) by glucose and fructose can further stimulate this pathway. In physiological states, mTORC1-mediated signaling can compensate for the loss of insulin action [55]. Conversely, when challenged with a high-fructose diet, receptor-level ablation of hepatic insulin signaling protects from hepatic steatosis in murine models. Importantly, stable isotope studies have indicated that hepatic DNL is elevated in non-diabetic patients with high intrahepatic TG (measured by MRS) [32,33,56] and is closely associated with altered glucose homeostasis in these patients [56]. These results suggest that hyperinsulinemia (even without overt T2D) likely drives steatosis development in the context of NAFLD.
Whether enhanced DNL is truly detrimental and drives progression to NASH and fibrosis remains a point of debate. During lipogenesis, acetyl-CoA carboxylase (ACC) catalyzes the production of malonyl-CoA, which is subsequently elongated by fatty acid synthase (FASN) into long-chain fatty acids such as palmitate (C16:0). In addition to serving as a substrate for lipid synthesis, malonyl-coA reduces fatty acid oxidation by inhibiting carnitine palmitoyltransferase I (CPT1) [57]. DNL is also closely linked with cellular saturated fatty acid levels [58] and ceramide synthesis [54], both of which can lead to hepatocyte apoptosis and metabolic dysfunction. Conversely, mice lacking hepatic ChREBP, a glucose-induced activator of glycolytic and lipogenic gene expression, showed more pronounced liver damage when challenged with a high-fat high-fructose diet [59]. Moreover, hepatic overexpression of PHD finger protein 2 (Phf2), a histone demethylase that mediates some of ChREBP's actions on gene transcription, induced marked steatosis in chow-fed mice. However, Phf2-overexpressing mice were protected from steatosis-associated endoplasmic reticulum-stress induced inflammation and hepatic metabolic dysfunction likely due to a fatty acid profile enriched in monounsaturated fatty acid [60]. When challenged with a high-fat high-sucrose diet, Phf2 overexpression protected against hepatic inflammation and fibrosis. Thus, ChREBP-Phf2-coordinated regulation of lipogenesis as well as fatty acid elongation/desaturation plays an important role in maintaining the balance of saturated to unsaturated fatty acid.
Similar findings resulted from human trials with ACC inhibitors. A proof-of-concept study in obese patients with elevated intrahepatic TG as assed by non-invasive imaging showed that ACC1 and ACC2 inhibition potently and rapidly decreased liver TG content (−36% in 4 weeks) [61]. However, this was associated with elevated plasma TG due to compensatory activation of mitochondrial glycerol-3-phosphate acyltransferase 1 (GPAT1), which is induced by intracellular polyunsaturated fatty acid deficiency [61]. In contrast, elevated DNL observed in patients with high hepatic TG content (∼18% measured by MRS) was also associated with increased hepatic VLDL-TG secretion [33]. Thus, increased DNL in NAFLD favors liver fat accumulation by the combination of increased TG synthesis and decreased fatty acid catabolism but also drives VLDL-TG production, leading to dyslipidemia.
3.1.2. VLDL-TG secretion correlates positively with intrahepatic TG content
VLDL are liver-derived, TG-carrying particles that contribute the majority (by mass) of fasting plasma TG levels. In humans, each VLDL particle contains 1 molecule of full-length apolipoprotein (Apo) B (ApoB100), whereas intestinally produced chylomicrons contain the truncated ApoB isoform (ApoB48). Ultracentrifugation-isolated VLDL separates in two subclasses (VLDL1 and VLDL2) differing in size and metabolic function. VLDL1 are larger with higher TG content, while VLDL2 are smaller and denser. Importantly, VLDL1 are reportedly more pro-atherogenic, since they give rise to small dense LDL [62]. Moreover, increased VLDL1 but not VLDL2 particle numbers are observed in overweight diabetic patients, identifying VLDL1 production as a driver of dyslipidemia [62].
NAFLD patients often display hypertriglyceridemia [[49], [50], [51], [52]] and elevated remnant lipoprotein particle concentrations, which are indicative of delayed intravascular TG metabolism [63]. Both features increase cardiovascular risk. Indeed, the risk of major adverse CV events (MACE) inversely correlates with remnant cholesterol levels, with a 0.8 mmol/L reduction in remnant cholesterol levels lowering the risk of MACE by 20% [64]. Intrahepatic TG content directly correlates with hepatic VLDL production in obese non-diabetic patients with normal liver fat content [65,66]. Surprisingly, this direct correlation was lost in patients with intrahepatic TG content higher than 10% (as assessed by MRS) [65,66]. These results suggest that hepatic VLDL-TG secretion can only compensate for increased liver fat content to a certain extent. Beyond this limit of 10%, it is possible that the correct balance of TG and phospholipids, which coat the VLDL particle (discussed to follow), can no longer be maintained, and VLDL production lags behind further increases in hepatic TG content. In parallel, insulin resistance in more severe steatosis and NASH leads to reduced plasma VLDL clearance (discussed to follow) that can further exacerbate plasma hypertriglyceridemia. Together, these factors may explain the co-existence of hepatic steatosis and hypertriglyceridemia in NAFLD patients (Figure 2). Importantly, the molar ratio of VLDL-TG (particle TG content) to VLDL-ApoB100 (the number of particles) is elevated in non-diabetic obese individuals with high intrahepatic TG (measured by MRS), suggesting that the secreted VLDL particles are larger and resemble the pro-atherogenic VLDL1 [65]. Since other studies indicated that plasma ApoB concentration is a stronger predictor of CVD risk than LDL-C or plasma TG concentrations [67], further investigation is required to dissect the contribution of ApoB particle concentration vs particle content (TG or cholesterol) in determining CVD risk. It is important to bear in mind that the majority of the aforementioned studies focused on differences in intrahepatic TG and not necessarily NASH per se. Moreover, the relative impact of insulin resistance vs elevated intrahepatic TG on hepatic VLDL-TG secretion remains an open question. In insulin-sensitive individuals, insulin inhibits hepatic VLDL production and favors LPL activity, decreasing plasma VLDL [46]. However, in NAFLD patients (defined by MRS) hyperinsulinemia increases VLDL-TG secretion [68], indicative of a loss of insulin-dependent inhibition of VLDL production.
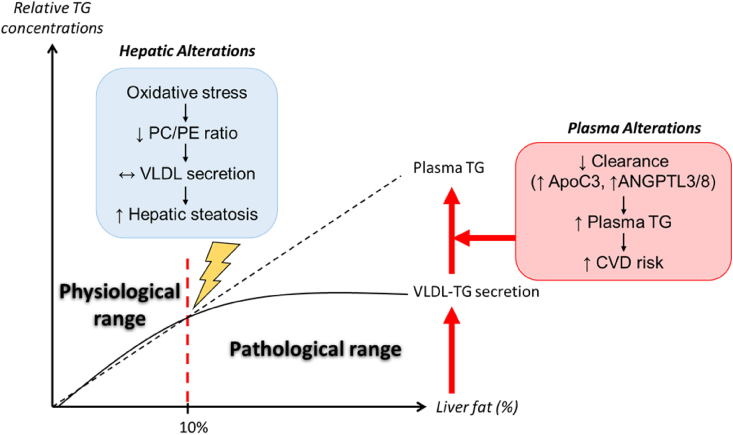
Figure 2.
The dynamic balance between hepatic VLDL-TG secretion and plasma clearance determines the association between NAFLD and plasma triglycerides. When intrahepatic triglycerides reach of a ∼10% level, oxidative stress increases, leading to decreased PC availability, preventing further increases in hepatic VLDL-TG secretion, thereby favoring hepatic steatosis. In parallel, circulating inhibitors of lipoprotein lipase such as ApoC3 and ANGPTL3/8 increase due to the presence of insulin resistance and exacerbate plasma hypertriglyceridemia by reducing intravascular TG hydrolysis. The dynamic balance between these factors explains the positive correlation between plasma hypertriglyceridemia and hepatic steatosis.
Alterations in phospholipid homeostasis may also contribute to altered VLDL regulation in NAFLD. Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are the major phospholipid components that form the surface monolayer of VLDL (and other) particles. Several studies have shown that PC is crucial for VLDL assembly and secretion [69]. Lowering PE levels can also reduce VLDL secretion as ∼30% of hepatic PC production comes from the conversion of PE to PC by phosphatidylethanolamine N-methyltransferase (PEMT) [70]. Moreover, a decreased PC/PE ratio [71] and lower levels of PEMT [72] were observed in patients with NASH. Likewise, diets that reduce PC availability, such as methionine- and choline-deficient diets, reduce hepatic VLDL-TG secretion and are commonly used to induce features of NAFLD in mice. Studies in mice indicate that in addition to steatosis, reduced PC availability may also contribute to hepatic injury in NASH. For example, high-fat diet (HFD)-fed PEMT-deficient mice develop more severe hepatic inflammation and fibrosis, likely due to cholestasis [73]. Similarly, patients with histological NASH and methionine- and choline-deficient-fed mice [74] displayed increased hepatic glutaminase 1 (GLS1), which is associated with reduced PC synthesis. Inhibition of GLS1 in the methionine and choline-deficient diet mouse model reduced oxidative stress by limiting anaplerotic reactions for the tricarboxylic acid (TCA) cycle. By reducing oxidative stress, serine was used preferentially in the 1 carbon cycle to generate methyl donors for use in the conversion of PE to PC and VLDL-TG output was consequently restored [74]. This study provides evidence that oxidative stress induced by hepatic lipid overload could indirectly impact the PC/PE balance, impacting hepatic VLDL-TG output (Figure 2). Together, these results highlight important links between phospholipid metabolism, amino acid metabolism, and oxidative stress in the development of NAFLD-associated dyslipidemia.
3.1.3. Reduced plasma VLDL clearance results in the accumulation of remnant particles in NAFLD
While hepatic VLDL output is increased in NAFLD, triglyceride-rich lipoprotein (TRL, that is, VLDL and chylomicron remnants) clearance is reduced, further exacerbating hypertriglyceridemia in these patients. Circulating TRLs are metabolized by LPL to deliver fatty acids to peripheral tissues. Importantly, several exchangeable apolipoproteins modulate LPL activity, including ApoC2 and ApoA5, which stimulate LPL activity, and ApoC1, ApoC3, and ApoE, which inhibit LPL activity. Additionally, angiopoietin-like proteins ANGPTL3 and ANGPTL4 also inhibit LPL individually, particularly when complexed with ANGPTL8 [75].
Elevated non-fasting plasma TG and remnant cholesterol levels (defined as total cholesterol, LDL-C, and HDL-C) due to genetic ApoA5 polymorphisms causally increased the risk of myocardial infarction (independent of hypertension and T2D) [76]. Moreover, loss of function mutations in ApoC3 [77,78] and ANGPTL4 [79] decreased plasma TG and CVD risk. In line, decreased plasma levels of ANGPTL8 [80] and ANGPTL3 [81] are both associated with decreased CVD risk (defined by the presence of atherosclerotic plaques or myocardial infarction). Conversely, decreased plasma ANGPTL4, which is transcriptionally activated by peroxisome proliferator-activated receptor (PPAR) α, is also associated with carotid artery stenosis (vessel wall thickness measured by magnetic resonance imaging, MRI) [82], suggesting increased CVD risk. In ApoE∗3-Leiden mice, ANGPTL4 overexpression decreased foam cell formation and protected against atherosclerosis formation via lipid-independent anti-inflammatory effects [82].
ApoC3 variants clearly impact plasma TG levels but they do not seem to have a consistent effect on hepatic steatosis [[83], [84], [85], [86], [87]]. In line, overexpression of ApoC3 in HFD-fed mice did not change steatosis despite markedly elevating plasma triglycerides due to impaired TG clearance [88]. Thus, ApoC3 more likely plays a role in NAFLD-associated dyslipidemia. Mechanistically, ApoC3 is transcriptionally activated by glucose [89] and repressed by PPARα, itself reduced in patients with NASH [90]. Since ApoC3-mediated clearance explains ∼75% of plasma TG variations in obese non-diabetic patients [91], these findings support the idea that NAFLD favors increased ApoC3 transcription, suppressing TRL clearance.
Several studies have observed elevated ANGPTL protein levels in NAFLD patients. Plasma ANGPTL3 levels are higher in biopsy-proven NASH patients [92,93], and plasma [94,95] and hepatic [96] ANGPTL8 are elevated in patients with fatty liver independent of insulin resistance [94]. In addition to inhibiting LPL activity, ANGPTL8 seems to favors TG accumulation in hepatocytes. In vitro, ANGPTL8 knockdown decreased TG accumulation in hepatocytes after fatty acid treatment [96], whereas incubation of hepatocytes with recombinant ANGPTL8 increased TG accumulation likely due to decreased activity of adipose triglyceride lipase (ATGL) [97]. Overall, several regulators of LPL activity are perturbed in patients with NAFLD, which may contribute to increasing the risk of CVD.
After TG lipolysis, TG-poor VLDL become remnant particles enriched in cholesterol [98]. Ultrasound-diagnosed NAFLD patients displayed higher fasting remnant cholesterol concentrations, indicating impaired clearance of remnant particles. Moreover, NAFLD patients with above-median concentrations of remnant cholesterol display higher CV event rates than NAFLD patients with below-median concentrations of remnant particles [99]. Indeed, similar to LDL, remnant particles can cross the arterial wall [100]. NAFLD patients also display elevated levels of small dense LDL particles, which are more atherogenic than large buoyant LDL [51,101]. Moreover, NAFL patients (defined by ultrasound or biopsy) display higher activity of cholesteryl ester transfer protein (CETP), an enzyme that exchanges cholesterol between HDL and ApoB particles, thereby favoring the formation of small dense LDL [102,103]. Together, these changes lead to increased abundance and altered metabolism of TRL, which are major contributors to CVD risk in NAFLD.
3.2. Intracellular cholesterol metabolism and elevated plasma LDL-C
3.2.1. Lipid-lowering therapy
Statins have been investigated as a potential therapy for NASH due to their actions in correcting total plasma and LDL-C levels and subsequent CVD risk reduction. Initial studies using indirect measures of NAFLD showed promising results, including decreased alanine transaminase (ALT) and AST levels or reduced steatosis on ultrasound [104]. However, randomized trials evaluating the impact of statin therapy on biopsy-proven NASH are lacking [105]. A post hoc analysis of a randomized, placebo-controlled trial assessing pioglitazone in NASH revealed that patients on statin therapy either at enrollment or starting therapy during the trial displayed lower plasma ALT and liver fat content on MRS [106]. However, histological analysis did not show statistically significant improvements in steatosis, inflammation, ballooning, or fibrosis [106]. Very surprisingly, the use of lipid-lowering agents (statins or other lipid-lowering agents) was reported not to reduce CV mortality in NAFLD patients [107]. However, the groups in this study were not well matched for other co-morbidities increasing CVD risk (T2D or a history of CVD), which could mask the potential beneficial effect of lipid-lowering therapy on CVD risk. A transcriptomic analysis of liver biopsies from obese patients revealed that statin treatment is associated with increased expression of genes involved in DNL [108]. Altogether, while statins are clearly safe drugs to use in NAFLD patients, whether they also improve NAFLD is doubtful.
3.2.2. Receptor-mediated LDL clearance
Unlike mice, humans metabolize the majority of their VLDL particles to LDL. Elevated concentrations of LDL-C, an undisputed CVD risk factor, are often but not always observed in NAFLD patients [50,52]. Several cell surface receptors facilitate the removal of LDL and remnant lipoprotein particles from the circulation by the liver, the best characterized being the LDL receptor (LDLR) and the closely related LDLR related-protein 1 (LRP1). However, LDLR is negatively regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9), a protease whose best-known function is to bind LDLR at the plasma membrane. Upon binding, PCSK9 promotes intracellular degradation of LDLR by blocking its recycling in the endosomal compartment. This leads to reduced LDLR presence at the plasma membrane and less LDL uptake as a consequence. Regulation of LDLR activity by PCSK9 has been shown to impact CVD. Several studies showed dysregulated levels of these proteins in NAFLD. Plasma levels of PCSK9 positively correlated with histological hepatic steatosis [109,110], whereas low levels of hepatic LRP1 expression are associated with poor prognosis in hepatocellular carcinoma, an outcome of NAFLD [111].
Recent preclinical studies suggest that PCSK9 and LRP1 could modulate NAFLD pathogenesis. PSCK9 deficiency exacerbated the development of hepatic steatosis in HFD-fed mice [112]. Moreover, hepatic steatosis decreased cell surface LDLR expression and increased plasma ApoB and total cholesterol in a PCSK9-dependent manner [110]. Indeed, increased intracellular lipid induces endoplasmic reticulum stress, which can activate sterol regulatory element-binding protein 2 (SREBP2), a transcriptional regulator of PCSK9 expression [110]. These results demonstrate the dual role of PCSK9. By decreasing cholesterol uptake through LDLR inhibition, PCSK9 protects the liver from excess intrahepatic cholesterol accumulation. However, this leads to increased plasma LDL-C levels, increasing CVD risk.
High-fat high-cholesterol diet-fed liver-specific LRP1-deficient mice showed accelerated NAFLD development characterized by increased steatosis, inflammation, and fibrosis independent of any changes in plasma lipids [113]. Worsening of NAFLD was attributed to a marked increase in hepatic cholesterol in liver-specific LRP1-deficient mice associated with increased cell death and stellate cell activation [113]. The lack of effect on plasma lipoproteins in this model was surprising but is not consistent across all murine models. For example, hepatocyte LRP1 deficiency increased atherosclerosis severity in ApoE/LDLR double-knockout mice despite decreased plasma cholesterol levels [114]. Collectively, these data suggest that dysregulation of receptors mediating hepatic LDL-C uptake induces liver injury by increasing intrahepatic cholesterol. Moreover, insulin signaling strongly induces LRP1 translocation to the membrane, highlighting a further mechanism by which insulin resistance could contribute to remnant accumulation observed in NAFLD patients [115].
Dysregulation of intracellular cholesterol metabolism is also associated with NASH severity in humans, and excess dietary cholesterol drives hepatic inflammation in murine models of NASH [116]. The mechanisms underlying these findings are likely complex. Intracellular cholesterol directly regulates gene transcription by suppressing SREBP2 activity, or indirectly via activating LXRs and farnesoid X receptor (FXR) by cholesterol-derived metabolites (oxysterols and bile acids, respectively). Moreover, the transcriptional regulator tafazzin (TAZ) has been identified as another cholesterol-sensitive pathway implicated in NASH progression [117]. Excess intracellular cholesterol stabilizes TAZ, which in turn activates the expression of pro-fibrotic genes in hepatocytes. In line, in vitro studies showed that increased intracellular cholesterol as a result of LDL loading provoked mitochondrial injury and induced several signs of lipotoxicity such as apoptosis, necrosis, and oxidative stress [118]. These studies highlight an important role of alterations in intracellular cholesterol homeostasis as a driver of NASH.
4. Therapeutic strategies and their potential impact on CVD risk in NAFLD
4.1. Lifestyle interventions and bariatric surgery to treat NAFLD: impact on CVD risk factors
4.1.1. Lifestyle interventions
Lifestyle interventions such as diet modification and increased physical activity are cornerstones for the prevention and treatment of NAFLD. Several dietary intervention strategies have been tested: modification of diet composition (for example, Mediterranean, low-carbohydrate, low-fat, and ketogenic diets) or feeding behavior (time-restricted feeding or intermittent fasting) with the Mediterranean diet showing the most promising results for reducing hepatic steatosis, dyslipidemia, and other metabolic comorbidities (reviewed by Saeed et al.) [119]. A recent study of overweight and obese patients showed that consumption of a ketogenic diet reduced intrahepatic fat content (measured by MRS) and markedly increased hepatic mitochondrial activity [120]. This was paralleled by a 25% reduction in plasma TG levels and improved insulin sensitivity despite minimal (∼3%) weight loss. Similarly, in NAFLD patients, 2 weeks of a low-carbohydrate diet also strongly decreased liver fat (assessed by MRS) associated with decreased hepatic DNL, increased mitochondrial β-oxidation, ∼50% reduced VLDL-TG, improved insulin sensitivity, and decreased plasma IL-6 and tumor necrosis factor α (TNF-α) levels despite only modest weight loss (∼2%) [121].
Increasing physical activity has also shown promise for treating NAFLD and associated comorbidities. High-intensity interval training improved several metabolic parameters including liver fat content (measured by MRI), BMI, plasma lipids, and insulin resistance in obese NAFLD patients (diagnosed by ultrasound or CT) with T2D [122]. Similarly, supervised exercise training, including a combination of aerobic and resistance training, effectively decreased body weight, hepatic steatosis, and adipose depot size (measured by MRI) compared to standard lifestyle advice [123]. Moreover, these changes were accompanied by improvements in several CVD risk parameters, including decreased plasma LDL-C, increased clearance of large TG-rich VLDL1 particles, improved insulin sensitivity, and reduced arterial stiffness [123].
Overall, these studies indicate that short-term reductions in liver fat and improvements in CVD risk factors can be achieved independent of weight loss. However, more significant weight loss (>7%) is needed to achieve resolution of biopsy-proven NASH [124,125], and fibrosis regression was observed only after ≥ 10% weight loss [124]. Future studies should address whether dietary interventions such as ketogenic or Mediterranean diets lead to durable reductions in NASH and CVD events with or without significant weight loss.
4.1.2. Bariatric surgery
Bariatric surgery is highly effective for treating hepatic steatosis and NASH as an alternative to diet and exercise, especially in the context of severe and morbid obesity. Bariatric surgery refers to several types of surgical interventions that mechanically restrict food intake, including gastric bypass (most commonly Roux-en-Y gastric bypass, RYGB), gastric banding, and sleeve gastrectomy. Gastric bypass and gastric banding have been shown to significantly improve histological features of NASH one [126] and five [127] years after the intervention and progressively decrease histological fibrosis in NASH patients [128]. Gastric bypass was the most efficient procedure to improve NASH [126], correlating with the degree of weight loss. Both studies also found durable improvements in dyslipidemia, insulin resistance, and markers of systemic inflammation, indicating that CVD risk may also be reduced. Histological improvements in NASH (steatosis, inflammation, and fibrosis) after RYGB or gastric banding have been confirmed in several smaller studies with median follow up varying from 6 months [129,130] to 40 months [131].
Prior to studies on NASH, bariatric surgery was found to rapidly and markedly improve several CVD risk factors, especially insulin resistance and dyslipidemia (reviewed by Tailleux et al.) [132]. A 12-year follow up study demonstrated that RYGB is associated with persistent weight loss, T2D remission, increased HDL-C, and decreased LDL-C and plasma TG compared to obese patients not undergoing surgical weight loss intervention [133]. Importantly, bariatric surgery also reduced CVD events and mortality [134] among T2D and obese patients. Therefore, it is highly likely that bariatric surgery will also reduce CV events in severely and morbidly obese patients with NASH, although this remains to be directly proven. Moreover, despite these benefits, bariatric surgery is not without risks [135] and is unlikely to become a universal treatment for metabolic diseases, including NAFLD.
4.2. Potential impact of pharmacological interventions targeting NASH on CVD risk
There are currently no approved pharmacological therapies for NASH. However, several clinical phase 2 and 3 trials assessing metabolically oriented strategies are ongoing (Figure 1). Many of these therapies are nuclear receptor agonists or hormone analogs that mainly stimulate metabolic pathways to reduce hepatic fat accumulation and decrease liver injury. Interestingly, the majority of these compounds also showed atheroprotection in preclinical studies. In this chapter, we highlight the current advancement of selected clinical trials for NASH and report specifically on associated effects on dyslipidemia and CVD risk reduction when data are available.
4.2.1. Bile acid metabolism modulators
4.2.1.1. FXR agonists
FXR is a nuclear receptor activated by bile acids that is highly expressed in the enterohepatic system. In addition to suppressing bile acid synthesis, FXR activation protects against lipid accumulation in the liver by decreasing hepatic lipogenesis, favoring fatty acid oxidation as well as decreasing inflammation by repressing nuclear factor kappa B (NF-κB) signaling [136].
Obeticholic acid (OCA) is a semi-synthetic steroidal FXR agonist. Phase 2 (FLINT) and phase 3 (REGENERATE) trial data indicate that OCA treatment improves fibrosis without worsening NASH (ClinicalTrials.gov identifier: NCT02548351). However, complete NASH resolution was not achieved. Unfortunately, these benefits are associated with an increase in LDL-C by approximately 20 mg/dL [137,138], increasing the CVD risk score category from low or medium to high risk in 7.6% of patients [139]. Detailed lipoprotein profile analysis in the FLINT trial patients revealed that OCA treatment increased both large buoyant (less atherogenic) and small dense (more atherogenic) LDL particles. Moreover, despite similar total VLDL levels, OCA-treated patients displayed a shift from large VLDL1 toward less atherogenic small VLDL2 particles [140]. Importantly, the OCA-induced LDL-C increase was reversed by concomitant treatment with statins [138,141]. This finding suggests that the OCA-associated increase in LDL-C likely results from FXR-mediated reduction of cytochrome P450 family 7 subfamily A member 1 (CYP7A1) expression in hepatocytes, leading to decreased conversion of intrahepatic cholesterol to bile acids. Increased intrahepatic cholesterol represses the statin-sensitive SREBP2 pathway, reducing LDLR transcription and decreasing plasma LDL clearance [136].
Several other synthetic FXR agonists with potential selective modulator actions are currently being evaluated in phase 2 clinical trials enrolling patients with NASH. One such compound, cilofexor (ClinicalTrials.gov identifier: NCT02854605), an intestinally targeted, non-steroidal FXR agonist, decreases hepatic steatosis (assessed by MRI) without increasing LDL-C [142]. Further studies should address whether these novel FXR agonists also impact CVD risk.
Agonism of the FXR pathways has the potential to treat fibrotic NASH but the current class of compounds likely needs refinement to improve the metabolic and CV risk profile. Indeed, FXR activation favors reverse cholesterol transport, reduces VLDL secretion, and decreases lipoprotein (Lp) (a) levels but also increases plasma LDL-C by decreasing bile acid synthesis (which leads to decreased LDLR expression) and increasing CETP expression [136].
4.2.1.2. Fibroblast growth factor 19 analogs
The non-tumorigenic fibroblast growth factor (FGF) 19 analog NGM282 is also under evaluation for NASH treatment (ClinicalTrials.gov identifier: NCT02443116). FGF19, a hormone produced by the distal intestine and FXR target gene, participates in a feedback loop to reduce bile acid synthesis. FGF19 also exerts metabolic actions that may be beneficial against NAFLD such as lowering liver TG and reducing plasma TG levels [143]. Interestingly, circulating FGF19 is decreased in NASH patients, providing further therapeutic rationale [144].
In two 12-week trials, NGM282 improved all of the histological parameters of NASH and fibrosis and reduced ALT and AST [145,146]. However, as with OCA, NGM282 treatment increased plasma LDL-C [145] mostly as large, buoyant LDL particles [147]. Plasma TG levels decreased [145] as a result of fewer large VLDL particles [147]. The net effect of these changes on CVD risk remains unclear. Preclinical studies of ApoE-deficient mice demonstrated a clear atheroprotective action of NGM282 [148]. In humans, FGF19 levels correlated negatively with CAD (defined by coronary angiography) independent of BMI, hypertension, dyslipidemia, and diabetes [149]. As with OCA, increased plasma LDL-C was blunted by statin treatment [146,147], suggesting that the action mechanism of NGM282 is similar to OCA. Larger phase 3 trials are ongoing.
4.2.1.3. Future directions for treatments targeting the bile acid pathways
Volixibat (an intestinal ASBT inhibitor that prevents reabsorption of bile acids) decreased plasma total cholesterol and LDL-C but did not improve NASH in an interim analysis from a phase 2 trial (ClinicalTrials.gov identifier: NCT02787304) [150]. Bile acid sequestrants, such as colesevelam and cholestyramine, improve glucose metabolism in T2D and lower CV risk (WHO trial) without improving liver histology (ClinicalTrials.gov identifier: NCT01066364) [151]. Therefore, intra-vascular cholesterol and lipid metabolism appear to be regulated in a dissociated manner from liver histological NAFLD.
4.2.2. PPAR agonists
Several clinical trials are currently assessing agonists of different members of the PPAR family to treat NASH. This family is composed of three isoforms, PPARα, PPARβ (or PPARδ), and PPARγ, which have distinct and partially overlapping functions in the control of lipid and glucose metabolism (for a detailed review, see Dubois et al. [152]). Each PPAR isoform can be specifically activated by appropriately designed synthetic agonists. For example, fibrates (PPARα agonists) and thiazolidinediones (TZDs, PPARγ agonists) are used to treat hyperlipidemia and diabetes, respectively.
4.2.2.1. Single PPAR agonists
Since hepatic PPARα expression is downregulated in NASH and restored PPARα activity is observed upon NASH resolution [90], activation of this pathway appears to be a reasonable approach to treat NASH. Fibrates (for example, fenofibrate, gemfibrozil, and pemafibrate, among others) mainly target PPARα and thereby improve atherogenic dyslipidemia observed in obesity, T2D, and NAFLD. Pre-statin era and post hoc analysis of the FIELD and ACCORD studies indicated a reduction in CV risk, especially in high TG/low HDL-C subpopulations [153]. Several small studies have found poor efficacy for older fibrates (fenofibrate, gemfibrozil, and clofibrate) on histological improvements for NASH despite correction of dyslipidemia [154]. Pemafibrate, a new PPARα agonist, is currently being evaluated for CV risk in the PROMINENT trial (ClinicalTrials.gov identifier: NCT03071692). Studies of NASH assessed by non-invasive methods (MRI for steatosis, FIB4, NFS, and enhanced liver fibrosis [ELF] tests) are underway.
Thiazolidinediones (TZD) such as pioglitazone and rosiglitazone are PPARγ agonists used in T2D patients to increase peripheral insulin sensitivity. Because of the strong interaction between diabetes and NAFLD, several trials tested their ability to improve NASH. A meta-analysis of 8 randomized clinical trials evaluating pioglitazone and rosiglitazone therapy on biopsy-proven NASH revealed that pioglitazone, but not rosiglitazone, promotes NASH resolution and reduces liver fibrosis [155]. While rosiglitazone treatment reduced steatosis and plasma ALT, it did not improve lobular inflammation, ballooning, or fibrosis [156,157]. Conversely, pioglitazone improved all histological features of NASH and fibrosis, including improving dyslipidemia [[158], [159], [160]]. Pioglitazone is also superior to rosiglitazone in terms of CVD risk reduction in T2D patients. Rosiglitazone increases plasma LDL-C and HDL-C levels [161], whereas pioglitazone improves dyslipidemia by increasing plasma HDL-C levels and decreasing plasma TG levels without raising LDL-C [162] or increasing large, buoyant LDL [161,163]. While long-term rosiglitazone treatment of T2D patients may slightly increase the risk of myocardial infarction [164], pioglitazone somewhat decreases the risk of MACE (although the risk of heart failure increases with all glitazones) [165,166]. In biopsy-proven NASH patients, rosiglitazone treatment did not modify plasma TG or HDL-C levels but increased LDL-C [156]. Conversely, pioglitazone treatment resulted in similar plasma lipid improvements in biopsy-proven NASH patients as observed in subjects with T2D [159,167]. In the PIVENS trial, pioglitazone reduced plasma TG levels and VLDL particle size, while plasma HDL-C and LDL particle size increased, especially in patients with NASH resolution [168,169], further underscoring the close association between NASH and atherogenic dyslipidemia. These different effects of rosiglitazone and pioglitazone may be due to the fact that pioglitazone also activates PPARα [170,171]. Thus, whereas selective PPARγ activators may reduce steatosis by lowering adipose-liver free fatty acid flux as a result of enhanced insulin sensitivity, pioglitazone's additional beneficial effects on inflammation and fibrosis may be a result of its modest activation of PPARα.
Seladelpar is a PPARδ agonist analyzed in a phase 2 clinical trial (ClinicalTrials.gov identifier: NCT03551522). Importantly, the trial was prematurely stopped after interim analysis due to the presence of abnormal hepatic inflammation during interim analysis, reportedly unrelated to seladelpar treatment, despite improvements in liver function biomarkers. Another trial in patients with mixed dyslipidemia showed that seladelpar decreased plasma LDL-C and TG and increased HDL-C levels. Improvements in insulin sensitivity and plasma levels of alkaline phosphatase and GGT, markers of liver injury, were also observed in seladelpar-treated patients [172].
4.2.2.2. Dual and pan-PPAR agonists
Different dual- and triple-PPAR agonists that leverage the complementary actions of each PPAR isoform are also being evaluated for NASH. Elafibranor is a dual-PPARα/δ agonist that in a phase 2b study demonstrated improvements in histological ballooning and lobular inflammation without worsening of fibrosis, benefits that were most apparent in patients with more severe NASH at baseline (ClinicalTrials.gov identifier: NCT01694849) [173]. Improvements in insulin sensitivity and decreased systemic inflammation markers (measured by CRP, fibrinogen, and haptoglobin) were also observed in elafibranor-treated patients. Decreases in plasma TG and LDL-C were observed with concomitant increases in HDL-C, even among patients treated with statins. However, recently reported interim phase 3 results did not show statistically significant differences in resolving NASH between elafibranor and placebo. Long-term evaluation of CVD risk reduction by elafibranor in NASH patients has not yet been demonstrated.
Saroglitazar is a dual-PPARα/γ agonist currently in phase 3 clinical trials in the US and Europe (ClinicalTrials.gov identifier: NCT03061721) already approved for treating NASH in India. Lanifibranor is a triple-PPARα/γ/δ agonist that was recently reported to reach primary and secondary endpoints by significantly decreasing the Steatosis Activity Fibrosis Score (SAF), inducing NASH resolution and lowering fibrosis in the NATIVE phase 2b trial (ClinicalTrials.gov identifier: NCT03008070). Preclinical results indicated that saroglitazar decreases plasma TG levels, reduces hepatic steatosis, ballooning, and inflammation in the choline-deficient, l-amino acid-defined, high-fat diet NASH mouse model, and protects against carbon tetrachloride (CCl4)-induced fibrosis [174], whereas lanifibranor treatment decreases CCl4-induced hepatic fibrosis, plasma glucose, and TG levels in db/db mice [175].
4.2.2.3. FGF21 analog
FGF21, a hepatokine and PPARα target gene, displays many favorable metabolic actions, including enhancing fatty acid oxidation, reducing lipogenesis, and improving dyslipidemia and insulin sensitivity [176]. Accordingly, a recombinant analog of human FGF21, pegbelfermin (ClinicalTrials.gov identifier: NCT02413372), reduced hepatic fat (assessed by MRI) and plasma transaminases and improved dyslipidemia (reduced plasma TG and LDL-C and increased HDL-C) in a phase 2 study of biopsy-diagnosed NASH patients (no post-treatment biopsy was performed) [177]. In ApoE-deficient mice, FGF21 protects against atherosclerosis progression acting on several pathways by reducing plasma cholesterol levels and cholesterol biosynthesis in the liver and reducing apoptosis in the aortic roots [178,179].
4.2.3. Thyroid hormone receptor β agonists
Thyroid hormones (TH) regulate several pathways of hepatic lipid metabolism mainly through TH receptor β (TR-β), the major isoform in the liver. In NAFLD, TR-β activation induces fatty acid uptake and oxidation and enhances mitochondrial biogenesis and activity. TR-β agonism also favors LDLR-mediated endocytosis and reverse cholesterol transport [180]. At least two TR-β selective agonists are under investigation for NASH, resmetirom (ClinicalTrials.gov identifier: NCT02912260) and VK2809 (ClinicalTrials.gov identifier: NCT04173065), employing distinct pharmacological mechanisms for liver selectivity. Resmetirom treatment decreased liver fat content (assessed by MRI) and NAS on biopsy in patients with biopsy-proven NASH with fibrosis (stages 1–3) [181]. Moreover, resmetirom treatment improved dyslipidemia by decreasing plasma levels of TG, ApoB, Lp(a), ApoCIII, and LDL-C, especially reducing the proportion of small LDL and large VLDL [181]. Thus, TR-β activation reduces both liver fat content and plasma lipid CVD risk factors, which may result in improved CV function.
4.2.4. Other treatments in clinical trials
The glucagon-like peptide-1 (GLP1) analog liraglutide used for T2D treatment showed promise for NASH treatment with improvements in body weight and glycemic control in 9 biopsy-proven NASH patients (ClinicalTrials.gov identifier: NCT01237119) [182], prompting confirmation in larger studies with histological endpoints. Interestingly, the GLP1 receptor (GLP1-R) agonists liraglutide [183] and semaglutide [184] both reduce CVD risk in T2D patients. Considering the lack of GLP1-R expression in hepatocytes, it is unclear how GLP1 analogs improve NAFLD (either through non-parenchymal actions on the liver or by enhancing systemic insulin sensitivity and weight loss). In Western diet-fed ApoE or LDLR-deficient mice, both liraglutide and semaglutide decrease atherosclerosis through anti-inflammatory effects independent of cholesterol lowering and weight loss [185]. Moreover, GLP1 may prevent macrophage foam cell formation [186]. In high-fat diet-fed ApoE∗3-Leiden CETP-transgenic mice, GLP1-R agonist treatment decreased hepatic steatosis and plasma TG by respectively decreasing DNL and VLDL production [187].
Several drugs in development target the increase in DNL observed in NASH patients [32]. Recent phase 2 studies with the ACC1/ACC2 inhibitor firsocostat showed reduced hepatic steatosis but increased plasma VLDL particles and TG levels (ClinicalTrials.gov identifier: NCT02856555) [188]. Thus, despite improving hepatic steatosis, firsocostat may increase CVD risk by increasing the amount of atherogenic particles.
In conclusion, several therapeutic strategies for NAFLD with evidence or potential for CVD protection are emerging. However, a detailed assessment of CV outcomes (MACE and subclinical atherosclerosis) is lacking, and in several short-term studies, changes in plasma lipid profiles were unreported. More importantly, the efficacy of these therapies on NASH remains to be demonstrated. Nevertheless, it remains a major challenge to identify NASH patients at the highest risk of CVD to appropriately target them for management.
5. Conclusion
NAFLD is closely associated with CVD. While epidemiological studies do not consistently show increased CVD mortality in NAFLD patients, there is clear evidence for increased (non-fatal) MACE. Alterations in lipid and lipoprotein metabolism are major contributing factors linking NAFLD to CVD. Moreover, many promising NASH therapies in development also improve dyslipidemia in clinical trials. Given the current lack of approved pharmacological therapies for NASH, a clear understanding of the underlying factors that drive elevated CVD risk in NAFLD will be critical for the effective care and management of this growing patient population.
Funding sources
This study was supported by the French National Research Agency (ANR-10-LABX-46 and ANR-16-RHUS-0006). B.S. is a recipient of an ERC Advanced Grant (694717). A.D. was supported by a doctoral fellowship from the University of Lille as well as support from the Association Française pour l’Étude du Foie (AFEF) and Fondation pour la Recherche Médicale.
Contributor Information
Joel T. Haas, Email: joel.haas@pasteur-lille.fr.
Bart Staels, Email: bart.staels@pasteur-lille.fr.
Conflict of interest
B.S. is a consultant for Genfit S.A. All other authors have nothing to declare.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Haas J.T., Francque S., Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annual Review of Physiology. 2016;78:181–205. doi: 10.1146/annurev-physiol-021115-105331. [DOI] [PubMed] [Google Scholar]
- 3.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 4.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjærg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani N., Guo X., Loomba R., Goodarzi M.O., Haritunians T., Kwon S. Genome-wide association study identifies variants associated with histologic features of nonalcoholic Fatty liver disease. Gastroenterology. 2010;139(5):1567–1576. doi: 10.1053/j.gastro.2010.07.057. 1576.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230. doi: 10.1053/j.gastro.2016.01.032. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A., Scorletti E., Mosca A., Alisi A., Byrne C.D., Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism: Clinical and Experimental. 2020 doi: 10.1016/j.metabol.2020.154170. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Baratta F., Pastori D., Angelico F., Balla A., Paganini A.M., Cocomello N. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2019;18(10):2324–2331. doi: 10.1016/j.cgh.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Sinn D.H., Kang D., Chang Y., Ryu S., Cho S.J., Paik S.W. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: a cohort study. Journal of Gastroenterology and Hepatology. 2020;35(5):833–839. doi: 10.1111/jgh.14856. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitaka H., Hamaguchi M., Kojima T., Fukuda T., Ohbora A., Fukui M. Nonoverweight nonalcoholic fatty liver disease and incident cardiovascular disease: a post hoc analysis of a cohort study. Medicine. 2017;96(18) doi: 10.1097/MD.0000000000006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. Journal of Hepatology. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Wu S., Wu F., Ding Y., Hou J., Bi J., Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Scientific Reports. 2016;6:33386. doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Zhong G.-C., Tan H.-Y., Hao F.-B., Hu J.-J. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Scientific Reports. 2019;9(1):11124. doi: 10.1038/s41598-019-47687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S. Atherosclerosis. Nature Reviews. Disease Primers. 2019;5(1):56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y., Ryu S., Sung K.-C., Cho Y.K., Sung E., Kim H.-N. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut. 2019;68(9):1667–1675. doi: 10.1136/gutjnl-2018-317666. [DOI] [PubMed] [Google Scholar]
- 17.Oni E., Budoff M.J., Zeb I., Li D., Veledar E., Polak J.F. Nonalcoholic fatty liver disease is associated with arterial distensibility and carotid intima-media thickness: (from the multi-ethnic study of atherosclerosis) The American Journal of Cardiology. 2019;124(4):534–538. doi: 10.1016/j.amjcard.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Kapuria D., Takyar V.K., Etzion O., Surana P., O'Keefe J.H., Koh C. Association of hepatic steatosis with subclinical atherosclerosis: systematic review and meta-analysis. Hepatology Communications. 2018;2(8):873–883. doi: 10.1002/hep4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinn D.H., Kang D., Chang Y., Ryu S., Gu S., Kim H. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66(2):323–329. doi: 10.1136/gutjnl-2016-311854. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J., Zhou Y., Zhang K., Qi Y., An S., Wang S. Association between nonalcoholic fatty liver disease and subclinical atherosclerosis: a cross-sectional study on population over 40 years old. BMC Cardiovascular Disorders. 2018;18(1):147. doi: 10.1186/s12872-018-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin Z., Zhu Y., Wang S., Liu S., Xu M., Wang T. Associations of subclinical atherosclerosis with nonalcoholic fatty liver disease and fibrosis assessed by non-invasive score. Liver International: Official Journal of the International Association for the Study of the Liver. 2020;40(4):806–814. doi: 10.1111/liv.14322. [DOI] [PubMed] [Google Scholar]
- 22.Harada P.H., Bensenõr I.J.M., Drager L.F., Goulart A.C., Mill J.G., Lotufo P.A. Non-alcoholic fatty liver disease presence and severity are associated with aortic stiffness beyond abdominal obesity: the ELSA-Brasil. Atherosclerosis. 2019;284:59–65. doi: 10.1016/j.atherosclerosis.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.B., Park G.-M., Lee J.-Y., Lee B.U., Park J.H., Kim B.G. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. Journal of Hepatology. 2018;68(5):1018–1024. doi: 10.1016/j.jhep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Koo B.K., Allison M.A., Criqui M.H., Denenberg J.O., Wright C.M. The association between liver fat and systemic calcified atherosclerosis. Journal of Vascular Surgery. 2020;71(1):204–211. doi: 10.1016/j.jvs.2019.03.044. e4. [DOI] [PubMed] [Google Scholar]
- 25.Park H.E., Lee H., Choi S.-Y., Kwak M.-S., Yang J.I., Yim J.Y. Clinical significance of hepatic steatosis according to coronary plaque morphology: assessment using controlled attenuation parameter. Journal of Gastroenterology. 2019;54(3):271–280. doi: 10.1007/s00535-018-1516-5. [DOI] [PubMed] [Google Scholar]
- 26.Moon S.H., Noh T.S., Cho Y.S., Hong S.P., Hyun S.H., Choi J.Y. Association between nonalcoholic fatty liver disease and carotid artery inflammation evaluated by 18F-fluorodeoxyglucose positron emission tomography. Angiology. 2015;66(5):472–480. doi: 10.1177/0003319714537872. [DOI] [PubMed] [Google Scholar]
- 27.Lee H.J., Lee C.H., Kim S., Hwang S.Y., Hong H.C., Choi H.Y. Association between vascular inflammation and non-alcoholic fatty liver disease: analysis by 18F-fluorodeoxyglucose positron emission tomography. Metabolism: Clinical and Experimental. 2017;67:72–79. doi: 10.1016/j.metabol.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Luukkonen P.K., Nick A., Hölttä-Vuori M., Thiele C., Isokuortti E., Lallukka-Brück S. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4(16) doi: 10.1172/jci.insight.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwers M.C.G.J., Simons N., Stehouwer C.D.A., Koek G.H., Schaper N.C., Isaacs A. Relationship between nonalcoholic fatty liver disease susceptibility genes and coronary artery disease. Hepatology Communications. 2019;3(4):587–596. doi: 10.1002/hep4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R., Kim L.J., Palmer C.D. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genetics. 2011;7(3) doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anstee Q.M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically-characterised cohort. Journal of Hepatology. 2020;73(3):505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D.J., Peloso G.M., Yu H., Butterworth A.S., Wang X., Mahajan A. Exome-wide association study of plasma lipids in >300,000 individuals. Nature Genetics. 2017;49(12):1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rüschenbaum S., Schwarzkopf K., Friedrich-Rust M., Seeger F., Schoelzel F., Martinez Y. Patatin-like phospholipase domain containing 3 variants differentially impact metabolic traits in individuals at high risk for cardiovascular events. Hepatology Communications. 2018;2(7):798–806. doi: 10.1002/hep4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Llauradó G., Orešič M., Hyötyläinen T., Orho-Melander M., Yki-Järvinen H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. Journal of Hepatology. 2015;62(3):657–663. doi: 10.1016/j.jhep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Sliz E., Sebert S., Würtz P., Kangas A.J., Soininen P., Lehtimäki T. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Human Molecular Genetics. 2018;27(12):2214–2223. doi: 10.1093/hmg/ddy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirola C.J., Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology. 2015;62(6):1742–1756. doi: 10.1002/hep.28142. [DOI] [PubMed] [Google Scholar]
- 39.Simons N., Isaacs A., Koek G.H., Kuč S., Schaper N.C., Brouwers M.C.G.J. PNPLA3, TM6SF2, and MBOAT7 genotypes and coronary artery disease. Gastroenterology. 2017;152(4):912–913. doi: 10.1053/j.gastro.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Holmen O.L., Zhang H., Fan Y., Hovelson D.H., Schmidt E.M., Zhou W. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nature Genetics. 2014;46(4):345–351. doi: 10.1038/ng.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dongiovanni P., Petta S., Maglio C., Fracanzani A.L., Pipitone R., Mozzi E. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 42.Lauridsen B.K., Stender S., Kristensen T.S., Kofoed K.F., Køber L., Nordestgaard B.G. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: mendelian randomization and meta-analysis of 279 013 individuals. European Heart Journal. 2018;39(5):385–393. doi: 10.1093/eurheartj/ehx662. [DOI] [PubMed] [Google Scholar]
- 43.Käräjämäki A.J., Hukkanen J., Kauma H., Kesäniemi Y.A., Ukkola O. Metabolic syndrome but not genetic polymorphisms known to induce NAFLD predicts increased total mortality in subjects with NAFLD (OPERA study) Scandinavian Journal of Clinical and Laboratory Investigation. 2020;80(2):106–113. doi: 10.1080/00365513.2019.1700428. [DOI] [PubMed] [Google Scholar]
- 44.Unalp-Arida A., Ruhl C.E. Patatin-like phospholipase domain-containing protein 3 I148M and liver fat and fibrosis scores predict liver disease mortality in the U.S. Population. Hepatology. 2020;71(3):820–834. doi: 10.1002/hep.31032. [DOI] [PubMed] [Google Scholar]
- 45.Grimaudo S., Pipitone R.M., Pennisi G., Celsa C., Cammà C., Di Marco V. Association between PNPLA3 rs738409 C>G variant and liver-related outcomes in patients with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2020;18(4):935–944. doi: 10.1016/j.cgh.2019.08.011. e3. [DOI] [PubMed] [Google Scholar]
- 46.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovascular Diabetology. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas J.T., Vonghia L., Mogilenko D.A., Verrijken A., Molendi-Coste O., Fleury S. Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nature Metabolism. 2019;1(6):604–614. doi: 10.1038/s42255-019-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gehrke N., Schattenberg J.M. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158(7):1929–1947. doi: 10.1053/j.gastro.2020.02.020. e6. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z., Qin H., Qiu S., Chen G., Chen Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: a retrospective cohort research. Lipids in Health and Disease. 2019;18(1):162. doi: 10.1186/s12944-019-1104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M.H., Sung J., Gwak G.-Y. The associations between apolipoprotein B, A1, and the B/A1 ratio and nonalcoholic fatty liver disease in both normal-weight and overweight Korean population. Journal of Clinical Lipidology. 2016;10(2):289–298. doi: 10.1016/j.jacl.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Bril F., Sninsky J.J., Baca A.M., Superko H.R., Portillo Sanchez P., Biernacki D. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. Journal of Clinical Endocrinology & Metabolism. 2016;101(2):644–652. doi: 10.1210/jc.2015-3111. [DOI] [PubMed] [Google Scholar]
- 52.Peng K., Mo Z., Tian G. Serum lipid abnormalities and nonalcoholic fatty liver disease in adult males. The American Journal of the Medical Sciences. 2017;353(3):236–241. doi: 10.1016/j.amjms.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Ben-Aicha S., Badimon L., Vilahur G. Advances in HDL: much more than lipid transporters. International Journal of Molecular Sciences. 2020;21(3) doi: 10.3390/ijms21030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hodson L., Gunn P.J. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Nature Reviews Endocrinology. 2019;15(12):689–700. doi: 10.1038/s41574-019-0256-9. [DOI] [PubMed] [Google Scholar]
- 55.Haas J.T., Miao J., Chanda D., Wang Y., Zhao E., Haas M.E. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metabolism. 2012;15(6):873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGarry J.D., Leatherman G.F., Foster D.W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. Journal of Biological Chemistry. 1978;253(12):4128–4136. [PubMed] [Google Scholar]
- 58.Roumans K.H.M., Lindeboom L., Veeraiah P., Remie C.M.E., Phielix E., Havekes B. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nature Communications. 2020;11(1):1891. doi: 10.1038/s41467-020-15684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang D., Tong X., VanDommelen K., Gupta N., Stamper K., Brady G.F. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. Journal of Clinical Investigation. 2017;127(7):2855–2867. doi: 10.1172/JCI89934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bricambert J., Alves-Guerra M.-C., Esteves P., Prip-Buus C., Bertrand-Michel J., Guillou H. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nature Communications. 2018;9(1):2092. doi: 10.1038/s41467-018-04361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim C.-W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metabolism. 2017;26(2):394–406. doi: 10.1016/j.cmet.2017.07.009. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adiels M., Olofsson S.-O., Taskinen M.-R., Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(7):1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 63.Nordestgaard B.G., Nicholls S.J., Langsted A., Ray K.K., Tybjærg-Hansen A. Advances in lipid-lowering therapy through gene-silencing technologies. Nature Reviews Cardiology. 2018;15(5):261–272. doi: 10.1038/nrcardio.2018.3. [DOI] [PubMed] [Google Scholar]
- 64.Langsted A., Madsen C.M., Nordestgaard B.G. Contribution of remnant cholesterol to cardiovascular risk. Journal of Internal Medicine. 2020;288(1):116–127. doi: 10.1111/joim.13059. [DOI] [PubMed] [Google Scholar]
- 65.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mittendorfer B., Yoshino M., Patterson B.W., Klein S. VLDL triglyceride kinetics in lean, overweight, and obese men and women. Journal of Clinical Endocrinology & Metabolism. 2016;101(11):4151–4160. doi: 10.1210/jc.2016-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richardson T.G., Sanderson E., Palmer T.M., Ala-Korpela M., Ference B.A., Davey Smith G. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Medicine. 2020;17(3) doi: 10.1371/journal.pmed.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poulsen M.K., Nellemann B., Stødkilde-Jørgensen H., Pedersen S.B., Grønbæk H., Nielsen S. Impaired insulin suppression of VLDL-triglyceride kinetics in nonalcoholic fatty liver disease. Journal of Clinical Endocrinology & Metabolism. 2016;101(4):1637–1646. doi: 10.1210/jc.2015-3476. [DOI] [PubMed] [Google Scholar]
- 69.van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2017;1859(9 Pt B):1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 70.DeLong C.J., Shen Y.J., Thomas M.J., Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. Journal of Biological Chemistry. 1999;274(42):29683–29688. doi: 10.1074/jbc.274.42.29683. [DOI] [PubMed] [Google Scholar]
- 71.Li Z., Agellon L.B., Allen T.M., Umeda M., Jewell L., Mason A. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metabolism. 2006;3(5):321–331. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Nakatsuka A., Matsuyama M., Yamaguchi S., Katayama A., Eguchi J., Murakami K. Insufficiency of phosphatidylethanolamine N-methyltransferase is risk for lean non-alcoholic steatohepatitis. Scientific Reports. 2016;6(21721) doi: 10.1038/srep21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan S., Kuipers F., Havinga R., Ando H., Vance D.E., Jacobs R.L. Impaired hepatic phosphatidylcholine synthesis leads to cholestasis in mice challenged with a high-fat diet. Hepatology Communications. 2019;3(2):262–276. doi: 10.1002/hep4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simon J., Nuñez-García M., Fernández-Tussy P., Barbier-Torres L., Fernández-Ramos D., Gómez-Santos B. Targeting hepatic glutaminase 1 ameliorates non-alcoholic steatohepatitis by restoring very-low-density lipoprotein triglyceride assembly. Cell Metabolism. 2020;31(3):605–622. doi: 10.1016/j.cmet.2020.01.013. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y.Q., Pottanat T.G., Siegel R.W., Ehsani M., Qian Y.-W., Zhen E.Y. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. Journal of Lipid Research. 2020;61(8):1203–1220. doi: 10.1194/jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jørgensen A.B., Frikke-Schmidt R., West A.S., Grande P., Nordestgaard B.G., Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. European Heart Journal. 2013;34(24):1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 77.Jørgensen A.B., Frikke-Schmidt R., Nordestgaard B.G., Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. New England Journal of Medicine. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 78.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Crosby J., Peloso G.M., Auer P.L., Crosslin D.R., Stitziel N.O. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. New England Journal of Medicine. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dewey F.E., Gusarova V., O'Dushlaine C., Gottesman O., Trejos J., Hunt C. Inactivating Variants in ANGPTL4 and risk of coronary artery disease. New England Journal of Medicine. 2016;374(12):1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niki H., Kishimoto Y., Saita E., Ohmori R., Kondo K., Momiyama Y. Plasma betatrophin levels and carotid atherosclerosis. Disease Markers. 2019;2019(4214650) doi: 10.1155/2019/4214650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stitziel N.O., Khera A.V., Wang X., Bierhals A.J., Vourakis A.C., Sperry A.E. ANGPTL3 Deficiency and protection against coronary artery disease. Journal of the American College of Cardiology. 2017;69(16):2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Georgiadi A., Wang Y., Stienstra R., Tjeerdema N., Janssen A., Stalenhoef A. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(7):1529–1537. doi: 10.1161/ATVBAHA.113.301698. [DOI] [PubMed] [Google Scholar]
- 83.Zhang R.-N., Zheng R.-D., Mi Y.-Q., Zhou D., Shen F., Chen G.-Y. APOC3 rs2070666 is associated with the hepatic steatosis independently of PNPLA3 rs738409 in Chinese han patients with nonalcoholic fatty liver diseases. Digestive Diseases and Sciences. 2016;61(8):2284–2293. doi: 10.1007/s10620-016-4120-7. [DOI] [PubMed] [Google Scholar]
- 84.Petersen K.F., Dufour S., Hariri A., Nelson-Williams C., Foo J.N., Zhang X.-M. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. New England Journal of Medicine. 2010;362(12):1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verrijken A., Beckers S., Francque S., Hilden H., Caron S., Zegers D. A gene variant of PNPLA3, but not of APOC3, is associated with histological parameters of NAFLD in an obese population. Obesity. 2013;21(10):2138–2145. doi: 10.1002/oby.20366. [DOI] [PubMed] [Google Scholar]
- 86.Hyysalo J., Stojkovic I., Kotronen A., Hakkarainen A., Sevastianova K., Makkonen J. Genetic variation in PNPLA3 but not APOC3 influences liver fat in non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2012;27(5):951–956. doi: 10.1111/j.1440-1746.2011.07045.x. [DOI] [PubMed] [Google Scholar]
- 87.Niu T.-H., Jiang M., Xin Y.-N., Jiang X.-J., Lin Z.-H., Xuan S.-Y. Lack of association between apolipoprotein C3 gene polymorphisms and risk of nonalcoholic fatty liver disease in a Chinese Han population. World Journal of Gastroenterology. 2014;20(13):3655–3662. doi: 10.3748/wjg.v20.i13.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng X., Yamauchi J., Lee S., Zhang T., Gong Z., Muzumdar R. APOC3 protein is not a predisposing factor for fat-induced nonalcoholic fatty liver disease in mice. Journal of Biological Chemistry. 2017;292(9):3692–3705. doi: 10.1074/jbc.M116.765917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caron S., Verrijken A., Mertens I., Samanez C.H., Mautino G., Haas J.T. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(3):513–519. doi: 10.1161/ATVBAHA.110.220723. [DOI] [PubMed] [Google Scholar]
- 90.Francque S., Verrijken A., Caron S., Prawitt J., Paumelle R., Derudas B. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. Journal of Hepatology. 2015;63(1):164–173. doi: 10.1016/j.jhep.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 91.Borén J., Watts G.F., Adiels M., Söderlund S., Chan D.C., Hakkarainen A. Kinetic and related determinants of plasma triglyceride concentration in abdominal obesity: multicenter tracer kinetic study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(10):2218–2224. doi: 10.1161/ATVBAHA.115.305614. [DOI] [PubMed] [Google Scholar]
- 92.Yilmaz Y., Ulukaya E., Atug O., Dolar E. Serum concentrations of human angiopoietin-like protein 3 in patients with nonalcoholic fatty liver disease: association with insulin resistance. European Journal of Gastroenterology and Hepatology. 2009;21(11):1247–1251. doi: 10.1097/MEG.0b013e32832b77ae. [DOI] [PubMed] [Google Scholar]
- 93.Barchetta I., Cimini F.A., Chiappetta C., Bertoccini L., Ceccarelli V., Capoccia D. Relationship between hepatic and systemic angiopoietin-like 3, hepatic Vitamin D receptor expression and NAFLD in obesity. Liver International: Official Journal of the International Association for the Study of the Liver. 2020;40(9):2139–2147. doi: 10.1111/liv.14554. [DOI] [PubMed] [Google Scholar]
- 94.Lee Y.-H., Lee S.-G., Lee C.J., Kim S.H., Song Y.-M., Yoon M.R. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Scientific Reports. 2016;6:24013. doi: 10.1038/srep24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hong B.S., Liu J., Zheng J., Ke W., Huang Z., Wan X. Angiopoietin-like protein 8/betatrophin correlates with hepatocellular lipid content independent of insulin resistance in non-alcoholic fatty liver disease patients. Journal of Diabetes Investigation. 2018;9(4):952–958. doi: 10.1111/jdi.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.García-Monzón C., Petrov P.D., Rey E., Marañón P., Del Pozo-Maroto E., Guzmán C. Angiopoietin-like protein 8 is a novel vitamin D receptor target gene involved in nonalcoholic fatty liver pathogenesis. American Journal Of Pathology. 2018;188(12):2800–2810. doi: 10.1016/j.ajpath.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y., Li S., Donelan W., Xie C., Wang H., Wu Q. Angiopoietin-like protein 8 (betatrophin) is a stress-response protein that down-regulates expression of adipocyte triglyceride lipase. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2016;1861(2):130–137. doi: 10.1016/j.bbalip.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 98.He P.-P., Jiang T., OuYang X.-P., Liang Y.-Q., Zou J.-Q., Wang Y. Lipoprotein lipase: biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clinica Chimica Acta. 2018;480:126–137. doi: 10.1016/j.cca.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Pastori D., Baratta F., Novo M., Cocomello N., Violi F., Angelico F. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non-alcoholic fatty liver disease. Journal of Clinical Medicine. 2018;7(11):378. doi: 10.3390/jcm7110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nordestgaard B.G., Wootton R., Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(4):534–542. doi: 10.1161/01.atv.15.4.534. [DOI] [PubMed] [Google Scholar]
- 101.Ivanova E.A., Myasoedova V.A., Melnichenko A.A., Grechko A.V., Orekhov A.N. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxidative Medicine and Cellular Longevity. 2017;2017:1273042. doi: 10.1155/2017/1273042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCullough A., Previs S.F., Dasarathy J., Lee K., Osme A., Kim C. HDL flux is higher in patients with nonalcoholic fatty liver disease. American Journal of Physiology. Endocrinology and Metabolism. 2019;317(5):E852–E862. doi: 10.1152/ajpendo.00193.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lucero D., Zago V., López G.I., Graffigna M., López G.H., Fainboim H. Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome? Clinica Chimica Acta. International Journal of Clinical Chemistry. 2011;412(7–8):587–592. doi: 10.1016/j.cca.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Tziomalos K., Athyros V.G., Paschos P., Karagiannis A. Nonalcoholic fatty liver disease and statins. Metabolism. 2015;64(10):1215–1223. doi: 10.1016/j.metabol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Barb D., Cusi K. Reply to “statins and non-alcoholic steatohepatitis. Metabolism. 2017;66:e3–e5. doi: 10.1016/j.metabol.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 106.Bril F., Portillo Sanchez P., Lomonaco R., Orsak B., Hecht J., Tio F. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: post hoc analysis of a randomized trial. Journal of Clinical Endocrinology & Metabolism. 2017;102(8):2950–2961. doi: 10.1210/jc.2017-00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shahab O., Biswas R., Paik J., Bush H., Golabi P., Younossi Z.M. Among patients with NAFLD, treatment of dyslipidemia does not reduce cardiovascular mortality. Hepatology Communications. 2018;2(10):1227–1234. doi: 10.1002/hep4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Margerie D., Lefebvre P., Raverdy V., Schwahn U., Ruetten H., Larsen P. Hepatic transcriptomic signatures of statin treatment are associated with impaired glucose homeostasis in severely obese patients. BMC Medical Genomics. 2019;12(1):80. doi: 10.1186/s12920-019-0536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruscica M., Ferri N., Macchi C., Meroni M., Lanti C., Ricci C. Liver fat accumulation is associated with circulating PCSK9. Annals of Medicine. 2016;48(5):384–391. doi: 10.1080/07853890.2016.1188328. [DOI] [PubMed] [Google Scholar]
- 110.Lebeau P.F., Byun J.H., Platko K., MacDonald M.E., Poon S.V., Faiyaz M. Diet-induced hepatic steatosis abrogates cell-surface LDLR by inducing de novo PCSK9 expression in mice. Journal of Biological Chemistry. 2019;294(23):9037–9047. doi: 10.1074/jbc.RA119.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang X.-Y., Shi G.-M., Devbhandari R.P., Ke A.-W., Wang Y., Wang X.-Y. Low level of low-density lipoprotein receptor-related protein 1 predicts an unfavorable prognosis of hepatocellular carcinoma after curative resection. PloS One. 2012;7(3) doi: 10.1371/journal.pone.0032775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lebeau P.F., Byun J.H., Platko K., Al-Hashimi A.A., Lhoták Š., MacDonald M.E. Pcsk9 knockout exacerbates diet-induced non-alcoholic steatohepatitis, fibrosis and liver injury in mice. JHEP Reports: Innovation in Hepatology. 2019;1(6):418–429. doi: 10.1016/j.jhepr.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamlin A.N., Chinnarasu S., Ding Y., Xian X., Herz J., Jaeschke A. Low-density lipoprotein receptor-related protein-1 dysfunction synergizes with dietary cholesterol to accelerate steatohepatitis progression. Journal of Biological Chemistry. 2018;293(25):9674–9684. doi: 10.1074/jbc.RA118.001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Espirito Santo S.M.S., Pires N.M.M., Boesten L.S.M., Gerritsen G., Bovenschen N., van Dijk K.W. Hepatic low-density lipoprotein receptor-related protein deficiency in mice increases atherosclerosis independent of plasma cholesterol. Blood. 2004;103(10):3777–3782. doi: 10.1182/blood-2003-11-4051. [DOI] [PubMed] [Google Scholar]
- 115.Laatsch A., Merkel M., Talmud P.J., Grewal T., Beisiegel U., Heeren J. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2009;204(1):105–111. doi: 10.1016/j.atherosclerosis.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 116.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends in Endocrinology and Metabolism: Trends in Endocrinology and Metabolism. 2016;27(2):84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 117.Wang X., Cai B., Yang X., Sonubi O.O., Zheng Z., Ramakrishnan R. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metabolism. 2020;31(5):969–986. doi: 10.1016/j.cmet.2020.03.010. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gan L.T., Van Rooyen D.M., Koina M.E., McCuskey R.S., Teoh N.C., Farrell G.C. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. Journal of Hepatology. 2014;61(6):1376–1384. doi: 10.1016/j.jhep.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 119.Saeed N., Nadeau B., Shannon C., Tincopa M. Evaluation of dietary approaches for the treatment of non-alcoholic fatty liver disease: a systematic review. Nutrients. 2019;11(12):3064. doi: 10.3390/nu11123064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luukkonen P.K., Dufour S., Lyu K., Zhang X.-M., Hakkarainen A., Lehtimäki T.E. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(13):7347–7354. doi: 10.1073/pnas.1922344117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mardinoglu A., Wu H., Bjornson E., Zhang C., Hakkarainen A., Räsänen S.M. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metabolism. 2018;27(3):559–571. doi: 10.1016/j.cmet.2018.01.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abdelbasset W.K., Tantawy S.A., Kamel D.M., Alqahtani B.A., Soliman G.S. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine. 2019;98(12) doi: 10.1097/MD.0000000000014918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shojaee-Moradie F., Cuthbertson D.J., Barrett M., Jackson N.C., Herring R., Thomas E.L. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. Journal of Clinical Endocrinology & Metabolism. 2016;101(11):4219–4228. doi: 10.1210/jc.2016-2353. [DOI] [PubMed] [Google Scholar]
- 124.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. doi: 10.1053/j.gastro.2015.04.005. e5; quiz e14-15. [DOI] [PubMed] [Google Scholar]
- 125.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lassailly G., Caiazzo R., Buob D., Pigeyre M., Verkindt H., Labreuche J. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–388. doi: 10.1053/j.gastro.2015.04.014. quiz e15-16. [DOI] [PubMed] [Google Scholar]
- 127.Caiazzo R., Lassailly G., Leteurtre E., Baud G., Verkindt H., Raverdy V. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Annals of Surgery. 2014;260(5):893–899. doi: 10.1097/SLA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 128.Lassailly G., Caiazzo R., Ntandja-Wandji L.-C., Gnemmi V., Baud G., Verkindt H. Gastroenterology; 2020. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 129.Manco M., Mosca A., De Peppo F., Caccamo R., Cutrera R., Giordano U. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. The Journal of Pediatrics. 2017;180:31–37.e2. doi: 10.1016/j.jpeds.2016.08.101. [DOI] [PubMed] [Google Scholar]
- 130.Praveen Raj P., Gomes R.M., Kumar S., Senthilnathan P., Karthikeyan P., Shankar A. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: “NASHOST” prospective observational trial. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2015;11(6):1315–1322. doi: 10.1016/j.soard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Schneck A.-S., Anty R., Patouraux S., Bonnafous S., Rousseau D., Lebeaupin C. Roux-en Y gastric bypass results in long-term remission of hepatocyte apoptosis and hepatic histological features of non-alcoholic steatohepatitis. Frontiers in Physiology. 2016;7(344) doi: 10.3389/fphys.2016.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tailleux A., Rouskas K., Pattou F., Staels B. Bariatric surgery, lipoprotein metabolism and cardiovascular risk. Current Opinion in Lipidology. 2015;26(4):317–324. doi: 10.1097/MOL.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 133.Adams T.D., Davidson L.E., Litwin S.E., Kim J., Kolotkin R.L., Nanjee M.N. Weight and metabolic outcomes 12 Years after gastric bypass. New England Journal of Medicine. 2017;377(12):1143–1155. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aminian A., Zajichek A., Arterburn D.E., Wolski K.E., Brethauer S.A., Schauer P.R. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. Journal of the American Medical Association. 2019;322(13):1271–1282. doi: 10.1001/jama.2019.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thereaux J., Lesuffleur T., Czernichow S., Basdevant A., Msika S., Nocca D. Long-term adverse events after sleeve gastrectomy or gastric bypass: a 7-year nationwide, observational, population-based, cohort study. The Lancet. Diabetes & Endocrinology. 2019;7(10):786–795. doi: 10.1016/S2213-8587(19)30191-3. [DOI] [PubMed] [Google Scholar]
- 136.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679–1694. doi: 10.1053/j.gastro.2017.01.055. e3. [DOI] [PubMed] [Google Scholar]
- 137.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet (London, England) 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (London, England) 2019;394(10215):2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 139.Labenz C., Prochaska J.H., Huber Y., Nagel M., Straub B.K., Wild P. Cardiovascular risk categories in patients with nonalcoholic fatty liver disease and the role of low-density lipoprotein cholesterol. Hepatology Communications. 2019;3(11):1472–1481. doi: 10.1002/hep4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siddiqui M.S., Van Natta M.L., Connelly M.A., Vuppalanchi R., Neuschwander-Tetri B.A., Tonascia J. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. Journal of Hepatology. 2020;72(1):25–33. doi: 10.1016/j.jhep.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pockros P.J., Fuchs M., Freilich B., Schiff E., Kohli A., Lawitz E.J. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver International: Official Journal of the International Association for the Study of the Liver. 2019;39(11):2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 142.Patel K., Harrison S.A., Elkashab M., Trotter J.F., Herring R., Rojter S. Cilofexor, a nonsteroidal FXR agonist, in non-cirrhotic patients with nonalcoholic steatohepatitis: a phase 2 randomized controlled trial. Hepatology. 2020;72(1):58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 143.Somm E., Jornayvaz F.R. Fibroblast growth factor 15/19: from basic functions to therapeutic perspectives. Endocrine Reviews. 2018;39(6):960–989. doi: 10.1210/er.2018-00134. [DOI] [PubMed] [Google Scholar]
- 144.Eren F., Kurt R., Ermis F., Atug O., Imeryuz N., Yilmaz Y. Preliminary evidence of a reduced serum level of fibroblast growth factor 19 in patients with biopsy-proven nonalcoholic fatty liver disease. Clinical Biochemistry. 2012;45(9):655–658. doi: 10.1016/j.clinbiochem.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 145.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (London, England) 2018;391(10126):1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 146.Harrison S.A., Rossi S.J., Paredes A.H., Trotter J.F., Bashir M.R., Guy C.D. NGM282 improves liver fibrosis and histology in 12 Weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2019;71(4):1198–1212. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rinella M.E., Trotter J.F., Abdelmalek M.F., Paredes A.H., Connelly M.A., Jaros M.J. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. Journal of Hepatology. 2019;70(4):735–744. doi: 10.1016/j.jhep.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 148.Zhou M., Learned R.M., Rossi S.J., Tian H., DePaoli A.M., Ling L. Therapeutic FGF19 promotes HDL biogenesis and transhepatic cholesterol efflux to prevent atherosclerosis. Journal of Lipid Research. 2019;60(3):550–565. doi: 10.1194/jlr.M089961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hao Y., Zhou J., Zhou M., Ma X., Lu Z., Gao M. Serum levels of fibroblast growth factor 19 are inversely associated with coronary artery disease in Chinese individuals. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0072345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Newsome P.N., Palmer M., Freilich B., Sheikh M.Y., Sheikh A., Sarles H. Volixibat in adults with non-alcoholic steatohepatitis: 24-week interim analysis from a randomized, phase II study. Journal of Hepatology. 2020;73(2):231–240. doi: 10.1016/j.jhep.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 151.Le T.-A., Chen J., Changchien C., Peterson M.R., Kono Y., Patton H. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56(3):922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dubois V., Eeckhoute J., Lefebvre P., Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. Journal of Clinical Investigation. 2017;127(4):1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Staels B., Maes M., Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nature Clinical Practice. Cardiovascular Medicine. 2008;5(9):542–553. doi: 10.1038/ncpcardio1278. [DOI] [PubMed] [Google Scholar]
- 154.Liss K.H.H., Finck B.N. PPARs and nonalcoholic fatty liver disease. Biochimie. 2017;136:65–74. doi: 10.1016/j.biochi.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Musso G., Cassader M., Paschetta E., Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Internal Medicine. 2017;177(5):633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology. 2008;135(1):100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 157.Ratziu V., Charlotte F., Bernhardt C., Giral P., Halbron M., Lenaour G. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51(2):445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 158.Musso G., Cassader M., Paschetta E., Gambino R. Pioglitazone for advanced fibrosis in nonalcoholic steatohepatitis: new evidence, new challenges. Hepatology. 2017;65(3):1058–1061. doi: 10.1002/hep.28960. [DOI] [PubMed] [Google Scholar]
- 159.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Annals of Internal Medicine. 2016;165(5):305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 160.Bril F., Kalavalapalli S., Clark V.C., Lomonaco R., Soldevila-Pico C., Liu I.-C. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2018;16(4):558–566. doi: 10.1016/j.cgh.2017.12.001. e2. [DOI] [PubMed] [Google Scholar]
- 161.Goldberg R.B., Kendall D.M., Deeg M.A., Buse J.B., Zagar A.J., Pinaire J.A. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28(7):1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 162.Filipova E., Uzunova K., Kalinov K., Vekov T. Effects of pioglitazone therapy on blood parameters, weight and BMI: a meta-analysis. Diabetology & Metabolic Syndrome. 2017;9:90. doi: 10.1186/s13098-017-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dormandy J.A., Charbonnel B., Eckland D.J.A., Erdmann E., Massi-Benedetti M., Moules I.K. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial. Lancet (London, England) 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 164.Wallach J.D., Wang K., Zhang A.D., Cheng D., Grossetta Nardini H.K., Lin H. Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ. 2020;368:l7078. doi: 10.1136/bmj.l7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Jong M., van der Worp H.B., van der Graaf Y., Visseren F.L.J., Westerink J. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovascular Diabetology. 2017;16(1):134. doi: 10.1186/s12933-017-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kernan W.N., Viscoli C.M., Furie K.L., Young L.H., Inzucchi S.E., Gorman M. Pioglitazone after ischemic stroke or transient ischemic attack. New England Journal of Medicine. 2016;374(14):1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. New England Journal of Medicine. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corey K.E., Wilson L.A., Altinbas A., Yates K.P., Kleiner D.E., Chung R.T. Relationship between resolution of non-alcoholic steatohepatitis and changes in lipoprotein sub-fractions: a post-hoc analysis of the PIVENS trial. Alimentary Pharmacology & Therapeutics. 2019;49(9):1205–1213. doi: 10.1111/apt.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Corey K.E., Vuppalanchi R., Wilson L.A., Cummings O.W., Chalasani N. NASH resolution is associated with improvements in HDL and triglyceride levels but not improvement in LDL or non-HDL-C levels. Alimentary Pharmacology & Therapeutics. 2015;41(3):301–309. doi: 10.1111/apt.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Orasanu G., Ziouzenkova O., Devchand P.R., Nehra V., Hamdy O., Horton E.S. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. Journal of the American College of Cardiology. 2008;52(10):869–881. doi: 10.1016/j.jacc.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sakamoto J., Kimura H., Moriyama S., Odaka H., Momose Y., Sugiyama Y. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochemical and Biophysical Research Communications. 2000;278(3):704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 172.Bays H.E., Schwartz S., Littlejohn T., Kerzner B., Krauss R.M., Karpf D.B. MBX-8025, a novel peroxisome proliferator receptor-delta agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. Journal of Clinical Endocrinology & Metabolism. 2011;96(9):2889–2897. doi: 10.1210/jc.2011-1061. [DOI] [PubMed] [Google Scholar]
- 173.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147–1159. doi: 10.1053/j.gastro.2016.01.038. e5. [DOI] [PubMed] [Google Scholar]
- 174.Jain M.R., Giri S.R., Bhoi B., Trivedi C., Rath A., Rathod R. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver International: Official Journal of the International Association for the Study of the Liver. 2018;38(6):1084–1094. doi: 10.1111/liv.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Boubia B., Poupardin O., Barth M., Binet J., Peralba P., Mounier L. Design, synthesis, and evaluation of a novel series of indole sulfonamide peroxisome proliferator activated receptor (PPAR) α/γ/δ triple activators: discovery of lanifibranor, a new antifibrotic clinical candidate. Journal of Medicinal Chemistry. 2018;61(6):2246–2265. doi: 10.1021/acs.jmedchem.7b01285. [DOI] [PubMed] [Google Scholar]
- 176.Staiger H., Keuper M., Berti L., Hrabě de Angelis M., Häring H.-U. Fibroblast growth factor 21—metabolic role in mice and men. Endocrine Reviews. 2017;38(5):468–488. doi: 10.1210/er.2017-00016. [DOI] [PubMed] [Google Scholar]
- 177.Sanyal A., Charles E.D., Neuschwander-Tetri B.A., Loomba R., Harrison S.A., Abdelmalek M.F. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet (London, England) 2019;392(10165):2705–2717. doi: 10.1016/S0140-6736(18)31785-9. [DOI] [PubMed] [Google Scholar]
- 178.Lin Z., Pan X., Wu F., Ye D., Zhang Y., Wang Y. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131(21):1861–1871. doi: 10.1161/CIRCULATIONAHA.115.015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yan X., Gou Z., Li Y., Wang Y., Zhu J., Xu G. Fibroblast growth factor 21 inhibits atherosclerosis in apoE-/- mice by ameliorating Fas-mediated apoptosis. Lipids in Health and Disease. 2018;17(1):203. doi: 10.1186/s12944-018-0846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ritter M.J., Amano I., Hollenberg A.N. Thyroid hormone signaling and the liver. Hepatology. 2020;72(2):742–752. doi: 10.1002/hep.31296. [DOI] [PubMed] [Google Scholar]
- 181.Harrison S.A., Bashir M.R., Guy C.D., Zhou R., Moylan C.A., Frias J.P. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (London, England) 2019;394(10213):2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 182.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet (London, England) 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 183.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F.E., Nauck M.A. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jódar E., Leiter L.A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 185.Rakipovski G., Rolin B., Nøhr J., Klewe I., Frederiksen K.S., Augustin R. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC. Basic to Translational Science. 2018;3(6):844–857. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nagashima M., Watanabe T., Terasaki M., Tomoyasu M., Nohtomi K., Kim-Kaneyama J. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54(10):2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Parlevliet E.T., Wang Y., Geerling J.J., Schröder-Van der Elst J.P., Picha K., O'Neil K. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE∗3-Leiden mice. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0049152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463–1473. doi: 10.1053/j.gastro.2018.07.027. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]