Abstract
Simple Summary
Familial clustering of cancer and identification of high- and low-risk cancer predisposition gene variants implicate that there are families that are at a high to moderate excess risk of cancer. We wanted to test genetically whether there are families protected from cancer. We whole-genome sequenced 51 elderly individuals without any personal or family history of cancer. We identified less high-risk loss-of-function variants in known and suggested cancer predisposition genes in these cancer-free individuals than in the general population. However, our results for low-risk variants were not conclusive. Our study suggests that random environmental causes of cancer are so dominant that a clear demarcation of cancer-free populations using genetic data may not be feasible. However, carrier identification of and counseling about prevalent high-risk cancer predisposition genes is useful.
Abstract
Familial clustering, twin concordance, and identification of high- and low-penetrance cancer predisposition variants support the idea that there are families that are at a high to moderate excess risk of cancer. To what extent there may be families that are protected from cancer is unknown. We wanted to test genetically whether cancer-free families share fewer breast, colorectal, and prostate cancer risk alleles than the population at large. We addressed this question by whole-genome sequencing (WGS) of 51 elderly cancer-free individuals whose numerous (ca. 1000) family members were found to be cancer-free (‘cancer-free families’, CFFs) based on face-to-face interviews. The average coverage of the 51 samples in the WGS was 42x. We compared cancer risk allele frequencies in cancer-free individuals with those in the general population available in public databases. The CFF members had fewer loss-of-function variants in suggested cancer predisposition genes compared to the ExAC data, and for high-risk cancer predisposition genes, no pathogenic variants were found in CFFs. For common low-penetrance breast, colorectal, and prostate cancer risk alleles, the results were not conclusive. The results suggest that, in line with twin and family studies, random environmental causes are so dominant that a clear demarcation of cancer-free populations using genetic data may not be feasible.
Keywords: predisposing genes, high-risk genes, polygenic risk, random environment
1. Introduction
Familial cancer (i.e., two or more first-degree relatives diagnosed with the same cancer) accounts for 25% of prostate cancer, 16% of breast cancer, and 15% of colorectal cancer [1]. For rarer cancers, the proportions go down to about 2%. These proportions are much lower than twin estimates on the heritability of various cancers [2,3]. This may imply, among various explanations, that population genetics is characterized by common genes and polygenes of low penetrance, which would rarely aggregate in families [1,4,5]. Germline genetics of cancer, as presently known, depends on the type of cancer. For common cancers, such as breast and colorectal cancers, mutations in high-risk predisposition genes BRCA1/2 and mismatch repair genes are rare, accounting for a small proportion of the particular cancers (depending on population, approximately 1%) [6,7,8]. A number of other high-risk genes are known, but mutations in these are even rarer [9]. In addition, numerous and ever-increasing numbers of low-risk gene variants have been described for these cancers [10,11]. For other common cancers, including prostate and lung cancers, high-penetrance genes are rarer but also for these cancers numerous low-risk variants have been identified [8,9]. Combined, the high and low-risk variants explain a small proportion of the known familial risk and even less about the heritability estimated on twins.
A three-generation analysis in the Swedish Family-Cancer Database found that 16% of cancers were diagnosed in the third generation individuals whose two older generations were cancer-free, yet the relative risk (RR) of 0.9 showed no dramatic protection [12]. Recently, a whole-genome sequencing (WGS) project among 2570 healthy elderly within the Medical Genome Reference Bank in Australia reported fewer disease-associated common and rare germline variants compared to both cancer cases and the gnomAD and UK biobank cohorts [13]. Here, we identified 51 elderly cancer-free index persons (born in the 1920s or 1930s) whose siblings and relatives in one or two older and younger generations were cancer-free. We used WGS to test genetically whether cancer-free families (CFFs) share fewer cancer risk alleles than the population at large. We estimated that the CFFs, from which an index individual was sequenced, covered a total of 1000 cancer-free individuals.
2. Results
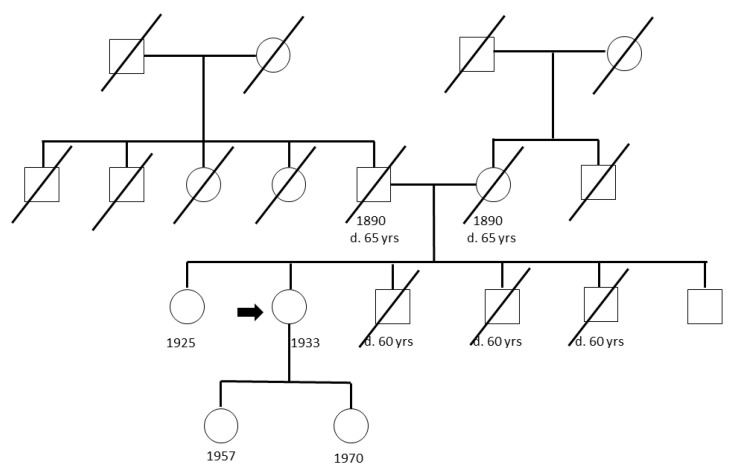
A pedigree of a CFF is shown in Figure 1 pointing out the 80-year-old index person with an arrow. In this, as in other families, the siblings as well as the individuals in the older generation(s) were either alive or had died due to reason other than cancer. The index case of each family was whole genome sequenced with an average coverage of 42x.
Figure 1.
Pedigree of one cancer-free family with the index case indicated by an arrow.
2.1. Low-Risk Variants
The analysis of the low-risk alleles included a total of 106 single-nucleotide polymorphisms (SNPs) for breast cancer, 81 SNPs for colorectal cancer, and 105 SNPs for prostate cancer identified in five large meta-analyses of whole-genome association studies (GWASs) [8,14,15,16]. The genotypes of these SNPs were determined from the WGS data of the CFFs based on the position of the SNP in the reference human genome (build GRCh37, assembly hs37d5). Table 1 compares the risk allele frequencies of the low-risk variants between the CFFs and the data from the gnomAD database. Only SNPs with nominally significant p-value < 0.05 in the analysis are shown. For breast cancer, risk allele frequencies for five SNPs were lower and for two SNPs higher than for the gnomAD data. The only variant for colorectal cancer was rarer in CFFs than in gnomAD and for prostate cancer risk allele frequencies for four SNPs were lower and for six SNPs higher in CFFs than in gnomAD.
Table 1.
Comparison of risk allele frequency between cancer-free families (CFFs) and gnomAD for breast, colorectal, and prostate cancers.
| Cancer | SNPID | Gene | Risk Allele | Frequency | OR | 95% CI | p 1 | ||
|---|---|---|---|---|---|---|---|---|---|
| GnomAD | CFF | ||||||||
| BC | rs10474352 | ARRDC3 | C | 0.83 | 0.74 | 0.56 | 0.36 | 0.87 | 0.0097 |
| rs16886181 | MAP3K1 | C | 0.17 | 0.08 | 0.41 | 0.20 | 0.85 | 0.0165 | |
| rs206966 | RP1-166H1.2 | T | 0.17 | 0.25 | 1.67 | 1.07 | 2.61 | 0.0248 | |
| rs2992756 | KLHDC7A | T | 0.49 | 0.37 | 0.62 | 0.41 | 0.93 | 0.0197 | |
| rs653465 | SLC4A7 | C | 0.47 | 0.57 | 1.48 | 1.00 | 2.20 | 0.0489 | |
| rs7072776 | DNAJC1 | A | 0.28 | 0.19 | 0.58 | 0.35 | 0.96 | 0.0348 | |
| rs889312 | MAP3K1 | C | 0.29 | 0.18 | 0.53 | 0.32 | 0.89 | 0.0154 | |
| CRC | rs17816465 | GREM1 | A | 0.20 | 0.10 | 0.43 | 0.23 | 0.84 | 0.0125 |
| PC | rs10460109 | TSHZ1 | T | 0.42 | 0.56 | 1.72 | 1.16 | 2.55 | 0.0067 |
| rs3850699 | TRIM8 | A | 0.68 | 0.56 | 0.6 | 0.41 | 0.89 | 0.0110 | |
| rs28607662 | TCF4 | C | 0.09 | 0.17 | 1.96 | 1.16 | 3.31 | 0.0118 | |
| rs2066827 | CDKN1B | T | 0.75 | 0.86 | 2.05 | 1.17 | 3.61 | 0.0125 | |
| rs2680708 | RNF43 | G | 0.60 | 0.48 | 0.62 | 0.42 | 0.91 | 0.0153 | |
| rs33984059 | RFX7 | A | 0.98 | 0.94 | 0.37 | 0.16 | 0.85 | 0.0193 | |
| rs12155172 | LINC01162 | A | 0.24 | 0.33 | 1.62 | 1.07 | 2.45 | 0.0216 | |
| rs6465657 | LMTK2 | C | 0.46 | 0.57 | 1.56 | 1.05 | 2.31 | 0.0265 | |
| rs12543663 | PCAT1 | C | 0.31 | 0.41 | 1.56 | 1.05 | 2.32 | 0.0270 | |
| rs9364554 | SLC22A3 | T | 0.27 | 0.19 | 0.60 | 0.37 | 1.00 | 0.0478 | |
1p-value for Bonferroni adjusted significance level: breast cancer (BC), 0.05/106 = 0.0005; colorectal cancer (CRC), 0.05/81 = 0.0006; prostate cancer (PC), 0.05/105 = 0.0005; OR: odds ratio; 95%CI: 95% confidence interval; SNPID, SNP identification number; p: p-value; bold values indicate statistical significance at p < 0.05.
The total number of risk alleles was calculated for each individual and their distribution is shown in Supplementary Figure S1. The aggregation of the low-risk alleles in CFF individuals were tested against the 1000 Genomes data for which individual genotype data were available (Table 2). Based on the total number of risk alleles, the individuals were divided in quartiles with approximately equal numbers of individuals in each quartile in the 1000 Genomes population. Compared to the 1000 Genomes population, the proportion of CFF individuals decreased with the increasing number of breast cancer risk alleles, for colorectal cancer there was no change, and for prostate cancer, the proportion of CFF individuals increased with the increasing number of risk alleles.
Table 2.
Combined effect of risk alleles related to breast, colorectal, and prostate cancers in cancer-free families (CFFs) and 1000 Genomes data.
| Cancer | No. Risk Alleles | 1000 Genomes No. | CFF No. | OR | 95%CI | p | |
|---|---|---|---|---|---|---|---|
| BC | ≤87 | 73 | 19 | 1.00 | - | - | |
| 88–91 | 72 | 12 | 0.64 | 0.29 | 1.42 | 0.27 | |
| 92–96 | 77 | 11 | 0.55 | 0.24 | 1.23 | 0.15 | |
| >96 | 72 | 9 | 0.48 | 0.20 | 1.13 | 0.09 | |
| p-trend = 0.07 | |||||||
| CRC | ≤71 | 75 | 13 | 1.00 | |||
| 72–76 | 90 | 13 | 0.83 | 0.36 | 1.91 | 0.67 | |
| 77–80 | 69 | 16 | 1.34 | 0.60 | 2.98 | 0.48 | |
| >80 | 60 | 9 | 0.87 | 0.35 | 2.16 | 0.76 | |
| p-trend = 0.88 | |||||||
| PC | ≤89 | 91 | 10 | 1.00 | |||
| 90–93 | 64 | 10 | 1.42 | 0.56 | 3.62 | 0.46 | |
| 94–97 | 86 | 10 | 1.06 | 0.42 | 2.67 | 0.90 | |
| >97 | 53 | 21 | 3.61 | 1.58 | 8.23 | 0.0023 | |
| p-trend = 0.0055 | |||||||
BC: breast cancer; CRC: colorectal cancer; PC: prostate cancer; OR: odds ratio; 95%CI: 95% confidence interval; p: p-value.
2.2. Suggested Cancer Predisposition Genes
Next, we calculated the probability of an individual in the CFFs and the ExAC population of carrying potentially pathogenic variants in suggested cancer predisposition genes obtained from two different sources [17,18] (Table 3). Pathogenicity was evaluated using the criteria of our in-house developed Familial Cancer Variant Prioritization Pipeline version 2 (FCVPPv2) [19]. We extracted all variants in these genes from the WGS data of the 51 CFF individuals and from the ExAC data. After filtering the variants according to the criteria of the FCVPPv2, 54 non-synonymous variants in 50 genes, and two loss-of-function variants in two genes were classified as potentially pathogenic in CFFs among the 723 genes reported by Wei et al. [18], while 23,419 non-synonymous variants in 367 genes and 3675 loss-of-function variants in 482 genes passed the filters in the ExAC population. Among the 114 cancer predisposition genes reported by Rahman [17], 18 non-synonymous variants in 14 genes and no loss-of-function variants were classified as potentially pathogenic in CFFs, while 5619 non-synonymous variants in 70 genes and 791 loss-of-function variants in 81 genes passed the filters in ExAC. The probability of carrying a non-synonymous variant in genes reported both by Wei et al. and Rahman was higher in CFFs than in ExAC, while the probability of a CFF individual to carry a loss-of-function variant was lower in genes of the Wei et al. list and no loss-of-function variants in genes of the Rahman list were detected.
Table 3.
Comparison of the probability of carrying potentially pathogenic non-synonymous and loss of function (LoF) variants within cancer predisposition genes (CPGs) in cancer-free families (pCFF) and in the ExAC population (pExAC). Pathogenicity was evaluated using the criteria of our in-house developed Familial Cancer Variant Prioritization Pipeline version 2 (FCVPPv2).
| Source of CPGs | CFF No. Variants |
P CFF | ExAC No. Variants |
P ExAC | OR | 95%CI | |
|---|---|---|---|---|---|---|---|
| Wei [18] non-synonymous | 54 | 67 % | 23419 | 63 % | 1.21 | 0.77 | 1.91 |
| Wei [18] LoF | 2 | 6 % | 3675 | 15 % | 0.35 | 0.00 | 0.53 |
| Rahman [17] non-synonymous | 18 | 31 % | 5619 | 22 % | 1.58 | 0.87 | 2.83 |
| Rahman [17] LoF | 0 | 0 % | 791 | 4 % | |||
LoF: loss-of-function, stop gain/loss, splice-site, and frameshift indel variants; P: probability; OR: odds ratio; 95%CI: 95% confidence interval.
2.3. High-Risk Breast, Colorectal, and Prostate Cancer Predisposition Genes
We searched the WGS data of the CFF individuals for missense and loss-of function variants within the known high-risk genes BRCA1 and BRCA2 for breast cancer, APC, MLH1, MSH2, MSH6, MUTYH, and PMS2 for colorectal cancer and HOXB13 for prostate cancer. In Table 4, we list the high-risk gene variants with MAF < 0.001 found in the CFF individuals and report the number of the missense and loss-of-function variants in ExAC and the probability of an ExAC individual to carry at least one pathogenic/likely pathogenic variant. For the CFF variants, the scaled PHRED-like Combined Annotation-Dependent Depletion CADD score, number of positive conservation (three tools) and deleteriousness (10 tools) predictions, and the ClinVar significance are shown. In the ExAC population, 1692 missense or loss-of-function variants were reported of which 98 were classified as pathogenic/likely pathogenic by ClinVar. In CFF, each of the listed 12 missense or loss-of-function variants occurred only once and none of them were classified as pathogenic. No variants were found for BRCA1 and HOXB13. ClinVar predicted all the CFF variants to be benign or likely benign, except that the MUTYH variant was reported to be likely pathogenic. Of note, MUTYH is a recessive cancer predisposition gene, and cancer might arise if a person inherited another mutated allele.
Table 4.
List of variants in known high-risk genes in breast, colorectal, and prostate cancers found in cancer-free families (CFFs) with annotations. For the ExAc population, probability of carrying a pathogenic/likely pathogenic variant is shown.
| Gene | Missense + LoF Variants in ExAC | Missense + LoF Variants in CFF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. | No. Pathogenic | p ExAC 1 | SNP ID | Chr | Position | Ref/Alt | Prevalence ExAC NFE |
CADD | Positive Conservation Scores | Positive Prediction Tools | ClinVar Significance | |
| BRCA2 | 691 | 40 | 0.112% | rs397507270 | 13 | 32907128 | A/G | 1.51 × 10−5 | 0.11 | 0 | 1 | Likely benign/US |
| rs56087561 | 13 | 32913562 | A/C | 3.65 × 10−4 | 24.1 | 2 | 5 | Benign | ||||
| rs80358768 | 13 | 32913947 | C/T | 3.45 × 10−4 | 0.2 | 0 | 1 | Benign | ||||
| APC | 481 | 2 | 0.003% | rs748940586 | 5 | 112178309 | A/C | 1.51 × 10−5 | 22.7 | 3 | 8 | US |
| No dbSNP | 5 | 112178460 | GTAT/G | . | 21.8 | . | . | - | ||||
| MLH1 | 167 | 3 | 0.008% | rs41294980 | 3 | 37067306 | G/A | 1.18 × 10−3 | 7.3 | 1 | 0/4 2 | Benign |
| rs63751225 | 3 | 37090075 | T/C | 1.80 × 10−4 | 22.1 | 3 | 4 | US | ||||
| MSH2 | 246 | 4 | 0.006% | rs116117580 | 2 | 47739533 | G/A | 1.99 × 10−2 | 0.003 | 0 | 1 | Not provided |
| MSH6 | 359 | 8 | 0.017% | rs752887988 | 2 | 48010377 | C/T | 0 | 33 | 3 | 7 | - |
| rs267608075 | 2 | 48028282 | A/T | 1.83 × 10−4 | 13.0 | 3 | 5 | Benign/US | ||||
| MUTYH | 174 | 12 | 0.079% | rs36053993 | 1 | 45797228 | C/T | 3.96 × 10−3 | 29.4 | 3 | 3/4 2 | Likely Pathogenic/Pathogenic |
| PMS2 3 | 172 | 12 | 0.021% | No dbSNP | 7 | 6043400 | T/C | . | 24.9 | 3 | 6 | - |
| BRCA1 | 344 | 17 | 0.071% | Not found | - | - | - | - | - | - | - | - |
| HOXB13 3 | 62 | 0 | Not found | - | - | - | - | - | - | - | - | |
LoF: loss-of-function, stop gain/loss, splice-site, and frameshift indel variants; No: number; NFE: Non-Finnish European; US: uncertain significance; Conservational Scores: Genomic Evolutionary Rate Profiling (GERP), PhastCons, and Phylogenetic P-value (PhyloP); inclusion cutoff ≥ 2/3; Prediction Tools: Sorting Intolerant from Tolerant (SIFT), Polymorphism Phenotyping version-2 (PolyPhen-2) HDIV (HumDiv), PolyPhen-v2 HVAR (HumVar), Log ratio test (LRT), MutationTaster, Mutation Assessor, Functional Analysis Through Hidden Markov Models (FATHMM), MetaSVM, MetaLR, Protein Variation Effect Analyzer (PROVEAN); inclusion cutoff ≥ 6/10; 1 probability of carrying pathogenic/likely pathogenic non-synonymous and loss of function (LoF) variants in the ExAC population; 2 data from 4 prediction tools available; 3 the high-risk status of PMS2 and HOXB13 is under discussion.
3. Discussion
In Poland, some 25% of all deaths are due to cancer, which is close to the average in Europe as reported by the World Health Organization (WHO) (http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer/data-and-statistics). All persons with a cancer diagnosis do not die of cancer, and we can assume that 35% of Poles have a cancer in their lifetime. This would imply that among fully aged families of 10 persons, less than 1% would be cancer-free. Thus, such rare lucky families may exist by chance. However, although twin data suggest that cancer is largely a random environmental disease, family studies show that familial cancer is largely genetic, except for lung and cervical cancer with a large environmental component [2,3,20]. Therefore, the investigated 51 CFFs can be expected to show a reduced genetic predisposition to cancer.
The strongest evidence for lower predisposition to cancer in CFFs was that these individuals carried a lower frequency of loss-of-function alleles in suggested cancer predisposition genes but not of missense variants, as shown in Table 3. A relatively poor discrimination of missense variants for cancer risk has been reported earlier [4]. In the same vein, analysis of variants in high-risk cancer predisposition genes showed that the CFF population had 12 missense but no loss-of-function variants and none of these were classified as pathogenic by ClinVar, whereas in ExAC 98 of the 1692 identified variants were classified as pathogenic/likely pathogenic. The lack of loss-of-function variants in CFF was probably not surprising because only 51 individuals were tested. The 12 missense variants were benign as judged by the ClinVar significance, with one exception, MUTYH, which is a recessive cancer predisposition gene. Interestingly even though the ClinVar score indicated benign phenotype, the CADD scores were high (>20) for many of the variants.
The testing of low-risk variants did not give conclusive results. The frequencies of risk alleles in CFFs varied inconsistently around the frequencies in the gnomAD database (Table 1). Similarly, when CFF and the 1000 Genomes individuals were compared by the number of risk alleles, the proportion of CFF individuals decreased with the increasing number of breast cancer risk alleles, while an opposite trend was observed in prostate cancer. Data from GWASs on many cancers show that even collectively low-risk alleles explain a small proportion of the empirical familial risk [8,21]. It is known that usually low-risk alleles are moderately enriched in familial compared to sporadic cases, but even opposite results have been reported [22,23]. Improvement of risk prediction by adding a polygenetic risk score to prediction models that include the family history indicate only partial overlapping of these factors [24,25].
Overall, our results are concordant with the recent study on 2570 healthy elderly within the Medical Genome Reference Bank in Australia [13]. In that study, the participants did not have any personal history of cancer, cardiovascular disease, or dementia, while our study participants did not have any personal or family history of cancer in one or two older and younger generations that included around 1000 cancer-free individuals. A study of 51 individuals may not be impressive if one fails to recognize that all the index cases were over 70 years old and that these represent families each with an average of 20 elderly relatives none of whom were diagnosed with cancer. Unfortunately, the age of death data were not complete, although most of the deceased were known to have reached an age of late adulthood. Both studies reported fewer pathogenic/likely pathogenic variants in high-risk cancer predisposition genes, while we also showed that loss-of-function variants within suggested cancer predisposition genes were depleted in CFFs compared to the ExAC data. On the other hand, the Australian study showed depletion of common cancer risk alleles among the elderly population, which was not obvious in our study with 51 sequenced individuals.
It would also be interesting to search for genetic variants protecting against cancer, however, that would require large, well-characterized elderly population without any personal or family history of cancer. Even identification of cancer risk alleles is a challenging task, as shown by the GWASs on common cancers of breast, colorectum, and prostate in which over 100,000 individuals were genotyped [8,14,15,16].
Sample size was a limitation of the study even though the 51 sequenced individuals represented 1000 other individuals without known cancers. Unreported cancers may be another weakness of the study because information on cancer in relatives was based on anecdotal data. However, the family history data were collected by face-to-face interviews of individuals who had reported no cancer family history in questionnaires within a large population screening conducted earlier; thus, the data are likely to be more reliable than postal or telephone interviews. If the index persons were 80 years in 2010 their grandparents were 80 years at around 1950. Even though cancer was a known disease at that time, the incidence rates were earlier lower and thus the probability of being cancer-free was higher. Yet even currently well-functioning national cancer registries may miss up to 10% cancers, characterized by elderly patients and cancers, which may be diagnosed with debilitating comorbidities such as lung cancer [26]. Nevertheless, the overall cancer incidence in Poland is at a low European level, except for colorectal cancer, which is relatively common as shown in the Cancer Statistics-Specific Cancers by the European Union with data extracted in August 2020 (https://ec.europa.eu/eurostat/statistics-explained/pdfscache/39738.pdf). Another minor weakness is the likely genotypic stratification between the Polish population and the referent European populations. Overall, the European population is genetically very homogenous, although a more detailed analysis of population genetic structure using autosomal, Y-chromosome, and mitochondrial markers have shown closest Polish resemblance to the Eastern neighbors Russians, Belarusians, and Ukrainians, followed by Czechs, Slovaks, and Baltic populations [27,28,29,30]. To diminish bias related to population stratification and to exclude cancer patients from the analyses, we included only the non-Finnish European non-TCGA data from the ExAC and the gnomAD in our study. This may, however, have caused bias on our analyses, as the samples from CFFs and the ExAC and the gnomAD populations were sequenced on different platforms and the quality control was done separately. To avoid this bias, we used the quality filtering protocol, as suggested [31].
In conclusion, no striking genetic differences between the CFF and the unselected reference populations were detected. However, loss-of-function variants appeared to be at a lower frequency in CFF members, and for high-risk cancer genes, no loss-of-function variants were found in CFFs. The results appear to be consistent with the earlier finding from the Swedish Family-Cancer Database that the overall cancer risk is not markedly depressed (RR 0.9) if two previous generations are cancer-free because of random environmental and polygenic causes. They further agree with the notions suggesting that carrier identification of and counseling about prevalent high-risk cancer predisposition genes is useful, but the prospects of defining genetic basis for cancer protection may not be promising [32].
4. Materials and Methods
4.1. Study Populations
The CFF group contained 51 individuals recruited by the Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland. Family histories were collected through face-to-face detailed interviews. An average interview took 20–30 min. In West-Pomeranian region of Poland, population screening was performed mainly in years 2000–2001, in which questionnaires about cancer family history were collected from about 1.25 million (~70%) of inhabitants. Persons with negative cancer family history were invited to outpatient clinics and asked to agree for recruitment to control group. In such a way, the group of about 1000 adult individuals was established. Persons selected for the present study were part of this control group. They all were over 70 years old at the time of recruitment.
Different reference groups were used to perform distinct statistical analyses; these included data from 64,603 (56,885 exome and 7718 genome individuals), 33,370, and 294 non-Finnish European (NFE) individuals extracted from the Genome Aggregation Database (gnomAD) (https://gnomad.broadinstitute.org/), the Exome Aggregation Consortium (ExAC) [33], and the 1000 Genomes database (https://www.internationalgenome.org/1000-genomes-browsers), respectively.
4.2. Ethics Statement
The ethical approval for this study design was obtained from the Bioethics Committee of the Pomeranian Medical Academy in Szczecin No: BN-001/174/05. Sample collection was performed following the guidelines proposed by this Committee. A written informed consent was signed by each participant in accordance with the Helsinki declaration.
4.3. Whole-Genome Sequencing
Whole-genome sequencing (WGS) of the cancer-free persons considered in the present study was performed in the Illumina X10 platform using DNA extracted from the blood samples. WGS was carried out as paired-end sequencing with a read length of 150 bp. Sequences were mapped to the reference human genome (build GRCh37, assembly hs37d5) using BWA mem (version 0.7.15) and duplicates were removed using Sambamba (version 0.1.19). Variants were called by using Platypus (version 0.8.1) and annotated using ANNOVAR [34], dbSNP [35], 1000 Genomes phase III [36], dbNSFP v3.0 [37], and ExAC [33], respectively. Variant filtering was carried out by considering a minimum of 5 reads coverage and a QUAL score higher than 20. To check for family relatedness, a pairwise comparison of variants among the cohort was performed. CFF, gnomAD, and ExAC data were filtered separately based on the criteria described in [31] and bases with a minimum of 10 reads coverage in at least 90% of samples were included in the analysis.
4.4. Low-Risk Variants
Five large recently published meta-analyses were used to collect single nucleotide polymorphisms (SNPs) predicted by genome-wide association studies (GWASs) to be associated with the risk of breast [8,15], colorectal [8,14], and prostate cancers [16] at the genome-wide significance level. SNPs with any of the following criteria were filtered out: (1) unspecified risk allele, (2) unspecified minor allele frequency (MAF) or MAF between 0.45 and 0.55, (3) effect size as odds ratio (OR) of the risk allele below 1.04, (4) only estrogen receptor (ER) status/histology-specific associations, (5) absence in the 1000 Genomes data, and (6) from two or more SNPs with pairwise linkage equilibrium (r2) higher than 0.8, only one was included. After filtering, 106, 81, and 105 SNPs for breast, colorectal, and prostate cancers, respectively, were used for further analyses. Logistic regression was performed to compare risk allele frequencies of the selected SNPs between CFFs and gnomAD data (used as the reference population). To account for the high number of tests, the significance level was adjusted using Bonferroni correction. In order to calculate a polygenic risk score, the logistic regression model was used to compare the number of risk alleles between CFFs and 294 non-Finnish European individuals from 1000 Genomes for which individual genotype data were available. The trend test was performed after dividing the individuals into quartiles based on the total number of risk alleles in individuals in 1000 Genomes and considering the groups as continuous variables.
4.5. Suggested Cancer Predisposition Genes
A comprehensive list of cancer predisposition genes was extracted from [17,18]. All missense and loss-of-function variants listed for each of these genes were downloaded from the ExAC data. Variants were filtered using the criteria of our in-house developed Familial Cancer Variant Prioritization Pipeline version 2 (FCVPPv2) [19]. MAF of 0.1% was used with respect to 1000 Genomes phase III, non-Finnish European non-TCGA ExAC data, and local datasets.
To select the top 10% of potentially deleterious variants in the human genome a scaled PHRED-like Combined Annotation-Dependent Depletion (CADD) score greater than 10 was applied [38]. Assuming that variants in genes intolerant to variation are likely to be deleterious, a screening for intolerance was performed; three different intolerance scores based on NHLBI-ESP6500 [39], ExAC datasets [33], and a local dataset with allele frequency data were considered. Additionally, the Z-score, developed by the ExAC consortium for missense and synonymous variants, was utilized [33].
To assess the evolutionary conservation of the variant position, three tools were used: Genomic Evolutionary Rate Profiling (GERP >2.0) [40], PhastCons (>0.3) [41], and Phylogenetic p-value (PhyloP ≥ 3.0) [42] with an inclusion of variants predicted to be located at a conserved genomic position by at least two tools.
To evaluate the deleteriousness of the coding variants, prediction tools Sorting Intolerant from Tolerant (SIFT) [43], Polymorphism Phenotyping version-2 (PolyPhen-2) HDIV (HumDiv) [44], PolyPhen-v2 HVAR (HumVar) [44], Log ratio test (LRT) [45], MutationTaster [46], Mutation Assessor [47], Functional Analysis Through Hidden Markov Models (FATHMM) [48], MetaSVM [37], MetaLR [37], and Protein Variation Effect Analyzer (PROVEAN) [49] were used. Variants predicted to be deleterious by more than 50% of these tools were included in the further analyses.
To evaluate the probability that one individual from the CFFs (PCFF) and the ExAC (PExAC) population, respectively, carries at least one potentially pathogenic variant, we used the method described by Castera et al. [50]. PExAC and PCFF were calculated using the following formula: 1- the probability of one individual not carrying any pathogenic variants. Therefore, (1) in which (2) represented the probability that one ExAC individual from non-Finish European population carried the ith variant among the k potentially pathogenic variants identified. OR was estimated by computing (3) and bias-corrected and accelerated (BCa) bootstrapping was performed to calculate 95% confidence interval (95%CI) of OR with 10,000 resampling [51].
| (1) |
| (2) |
| (3) |
4.6. Variants in High-Risk Genes of Breast, Colorectal, and Prostate Cancer
We searched the WGS data of CFFs for missense and loss-of function variants within the known high-risk genes BRCA1 and BRCA2 for breast cancer, APC, MLH1, MSH2, MSH6, MUTYH, and PMS2 for colorectal cancer and HOXB13 for prostate cancer. The pathogenicity was evaluated using the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/). We also screened the ExAC non-Finnish European data for missense and loss-of-function variants with MAF <0.001 that passed the ExAC QC filters. The probability that one individual of the ExAC carries at least one pathogenic/likely pathogenic variant reported in the ClinVar database was evaluated as described above.
All the statistical analyses were done using SAS version 9.4 and R version 3.5 (SAS Institute Inc., Cary, NC, USA).
5. Conclusions
Our whole-genome germline sequencing effort on 51 elderly cancer-free individuals whose numerous (ca. 1000) family members were found to be cancer-free implicated that the cancer-free family members had no pathogenic variants in high-risk breast, colorectal, and prostate cancer predisposition genes. They also had fewer loss-of-function variants in suggested cancer predisposition genes compared to the ExAC data. For common low-penetrance breast, colorectal, and prostate cancer risk alleles, the results were not conclusive. The results suggest that, in line with twin and family studies, random environmental causes are so dominant that a clear demarcation of cancer-free populations using genetic data may not be feasible.
Acknowledgments
The authors thank the Genomics and Proteomics Core Facility (GPCF) and the Omics IT and Data Management Core Facility (ODCF) of the German Cancer Research Center (DKFZ) for excellent technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/10/2770/s1, Figure S1: Distribution of the number of GWAS-identified risk alleles in the cancer free families (CFFs) and the 1000 Genomes population for the (a) breast cancer, (b) colorectal cancer, and (c) prostate cancer risk loci.
Author Contributions
Conceptualization, O.R.B., K.H. and A.F.; data curation, G.Z., C.C. and N.P.; formal analysis, G.Z., C.C. and S.C.; funding acquisition, K.H.; investigation, G.Z., C.C., N.P. and S.C.; methodology, G.Z., C.C., N.P., S.C. and M.S.; project administration, K.H. and A.F.; resources, N.P., M.S., D.D., J.L. and L.H.; software, G.Z., C.C., N.P., S.C. and M.S.; supervision, K.H. and A.F.; validation, G.Z.,C.C.,N.P., S.C. and M.S.; visualization, G.Z., C.C. and A.F.; writing—original draft, K.H. and A.F.; writing—review and editing, G.Z., C.C., O.R.B., N.P..S.C., M.S., R.S., A.H., D.D., J.L., K.H. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the European Union’s Horizon 2020 research and innovation programme, No 856620. A.H. was supported by Jane and Aatos Erkko Foundation, HUCH Research Funds (VTR), Sigrid Juselius Foundation, Finnish Cancer Organizations, University of Helsinki, Novo Nordisk Foundation, Päivikki and Sakari Sohlberg Foundation, The Finnish Society of Sciences and Letters. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
A.H. is shareholder in Targovax ASA, A.H. is employee and shareholder in TILT Biotherapeutics Ltd, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Other authors declare no conflict of interest.
References
- 1.Frank C., Sundquist J., Yu H., Hemminki A., Hemminki K. Concordant and discordant familial cancer: Familial risks, proportions and population impact. Int. J. Cancer. 2017;140:1510–1516. doi: 10.1002/ijc.30583. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein P., Holm N.V., Verkasalo P.K., Iliadou A., Kaprio J., Koskenvuo M., Pukkala E., Skytthe A., Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from sweden, denmark, and finland. N. Engl. J. Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 3.Mucci L.A., Hjelmborg J.B., Harris J.R., Czene K., Havelick D.J., Scheike T., Graff R.E., Holst K., Moller S., Unger R.H., et al. Familial risk and heritability of cancer among twins in nordic countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artomov M., Joseph V., Tiao G., Thomas T., Schrader K., Klein R.J., Kiezun A., Gupta N., Margolin L., Stratigos A.J., et al. Case-control analysis identifies shared properties of rare germline variation in cancer predisposing genes. Eur. J. Hum. Genet. 2019;27:824–828. doi: 10.1038/s41431-019-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson J.N., Wheeler W.A., Yeager M., Panagiotou O., Wang Z., Berndt S.I., Lan Q., Abnet C.C., Amundadottir L.T., Figueroa J.D., et al. Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J. Natl. Cancer Inst. 2015;107:e279. doi: 10.1093/jnci/djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chubb D., Broderick P., Frampton M., Kinnersley B., Sherborne A., Penegar S., Lloyd A., Ma Y.P., Dobbins S.E., Houlston R.S. Genetic diagnosis of high-penetrance susceptibility for colorectal cancer (crc) is achievable for a high proportion of familial crc by exome sequencing. J. Clin. Oncol. 2015;33:426–432. doi: 10.1200/JCO.2014.56.5689. [DOI] [PubMed] [Google Scholar]
- 7.Palomaki G.E. Is it time for brca1/2 mutation screening in the general adult population?: Impact of population characteristics. Genet. Med. 2015;17:24–26. doi: 10.1038/gim.2014.167. [DOI] [PubMed] [Google Scholar]
- 8.Sud A., Kinnersley B., Houlston R.S. Genome-wide association studies of cancer: Current insights and future perspectives. Nat. Rev. Cancer. 2017;17:692–704. doi: 10.1038/nrc.2017.82. [DOI] [PubMed] [Google Scholar]
- 9.Huang K.L., Mashl R.J., Wu Y., Ritter D.I., Wang J., Oh C., Paczkowska M., Reynolds S., Wyczalkowski M.A., Oak N., et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173:355–370. doi: 10.1016/j.cell.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michailidou K., Lindstrom S., Dennis J., Beesley J., Hui S., Kar S., Lemacon A., Soucy P., Glubb D., Rostamianfar A., et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmit S.L., Edlund C.K., Schumacher F.R., Gong J., Harrison T.A., Huyghe J.R., Qu C., Melas M., Van Den Berg D.J., Wang H., et al. Novel common genetic susceptibility loci for colorectal cancer. J. Natl. Cancer Inst. 2019;111:146–157. doi: 10.1093/jnci/djy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H., Frank C., Sundquist J., Hemminki A., Hemminki K. Common cancers share familial susceptibility: Implications for cancer genetics and counselling. J. Med. Genet. 2017;54:248–253. doi: 10.1136/jmedgenet-2016-103932. [DOI] [PubMed] [Google Scholar]
- 13.Pinese M., Lacaze P., Rath E.M., Stone A., Brion M.J., Ameur A., Nagpal S., Puttick C., Husson S., Degrave D., et al. The medical genome reference bank contains whole genome and phenotype data of 2570 healthy elderly. Nat. Commun. 2020;11:e435. doi: 10.1038/s41467-019-14079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huyghe J.R., Bien S.A., Harrison T.A., Kang H.M., Chen S., Schmit S.L., Conti D.V., Qu C., Jeon J., Edlund C.K., et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat. Genet. 2019;51:76–87. doi: 10.1038/s41588-018-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilyquist J., Ruddy K.J., Vachon C.M., Couch F.J. Common genetic variation and breast cancer risk-past, present, and future. Cancer. Epidemiol. Biomark. Prev. 2018;27:380–394. doi: 10.1158/1055-9965.EPI-17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumacher F.R., Al Olama A.A., Berndt S.I., Benlloch S., Ahmed M., Saunders E.J., Dadaev T., Leongamornlert D., Anokian E., Cieza-Borrella C., et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 2018;50:928–936. doi: 10.1038/s41588-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman N. Realizing the promise of cancer predisposition genes. Nature. 2014;505:302–308. doi: 10.1038/nature12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei R., Yao Y., Yang W., Zheng C.H., Zhao M., Xia J. Dbcpg: A web resource for cancer predisposition genes. Oncotarget. 2016;7:37803–37811. doi: 10.18632/oncotarget.9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A., Bandapalli O.R., Paramasivam N., Giangiobbe S., Diquigiovanni C., Bonora E., Eils R., Schlesner M., Hemminki K., Forsti A. Familial cancer variant prioritization pipeline version 2 (fcvppv2) applied to a papillary thyroid cancer family. Sci. Rep. 2018;8:11635. doi: 10.1038/s41598-018-29952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czene K., Lichtenstein P., Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the swedish family-cancer database. Int. J. Cancer. 2002;99:260–266. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell J.S., Li N., Weinhold N., Forsti A., Ali M., van Duin M., Thorleifsson G., Johnson D.C., Chen B., Halvarsson B.M., et al. Genome-wide association study identifies multiple susceptibility loci for multiple myeloma. Nat. Commun. 2016;7:12050. doi: 10.1038/ncomms12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremers R.G., Galesloot T.E., Aben K.K., van Oort I.M., Vasen H.F., Vermeulen S.H., Kiemeney L.A. Known susceptibility snps for sporadic prostate cancer show a similar association with "hereditary" prostate cancer. Prostate. 2015;75:474–483. doi: 10.1002/pros.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archambault A.N., Su Y.R., Jeon J., Thomas M., Lin Y., Conti D.V., Win A.K., Sakoda L.C., Lansdorp-Vogelaar I., Peterse E.F., et al. Cumulative burden of colorectal cancer-associated genetic variants is more strongly associated with early-onset vs late-onset cancer. Gastroenterology. 2019;158:1274–1286. doi: 10.1053/j.gastro.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cust A.E., Drummond M., Kanetsky P.A., Mann G.J., Schmid H., Hopper J.L., Aitken J.F., Armstrong B.K., Giles G.G., Holland E., et al. Assessing the incremental contribution of common genomic variants to melanoma risk prediction in two population-based studies. J. Invest. Derm. 2018;138:2617–2624. doi: 10.1016/j.jid.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigl K., Thomsen H., Balavarca Y., Hellwege J.N., Shrubsole M.J., Brenner H. Genetic risk score is associated with prevalence of advanced neoplasms in a colorectal cancer screening population. Gastroenterology. 2018;155:88–98. doi: 10.1053/j.gastro.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji J., Sundquist K., Sundquist J., Hemminki K. Comparability of cancer identification among death registry, cancer registry and hospital discharge registry. Int. J. Cancer. 2012;131:2085–2093. doi: 10.1002/ijc.27462. [DOI] [PubMed] [Google Scholar]
- 27.Andersen M.M., Eriksen P.S., Morling N. Cluster analysis of european y-chromosomal str haplotypes using the discrete laplace method. Forensic Sci. Int. Genet. 2014;11:182–194. doi: 10.1016/j.fsigen.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Heath S.C., Gut I.G., Brennan P., McKay J.D., Bencko V., Fabianova E., Foretova L., Georges M., Janout V., Kabesch M., et al. Investigation of the fine structure of european populations with applications to disease association studies. Eur. J. Hum. Genet. 2008;16:1413–1429. doi: 10.1038/ejhg.2008.210. [DOI] [PubMed] [Google Scholar]
- 29.Mielnik-Sikorska M., Daca P., Malyarchuk B., Derenko M., Skonieczna K., Perkova M., Dobosz T., Grzybowski T. The history of slavs inferred from complete mitochondrial genome sequences. PLoS ONE. 2013;8:e54360. doi: 10.1371/journal.pone.0054360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelis M., Esko T., Magi R., Zimprich F., Zimprich A., Toncheva D., Karachanak S., Piskackova T., Balascak I., Peltonen L., et al. Genetic structure of europeans: A view from the north-east. PLoS ONE. 2009;4:e5472. doi: 10.1371/journal.pone.0005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo M.H., Plummer L., Chan Y.M., Hirschhorn J.N., Lippincott M.F. Burden testing of rare variants identified through exome sequencing via publicly available control data. Am. J. Hum. Genet. 2018;103:522–534. doi: 10.1016/j.ajhg.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbull C., Sud A., Houlston R.S. Cancer genetics, precision prevention and a call to action. Nat. Genet. 2018;50:1212–1218. doi: 10.1038/s41588-018-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K., Li M., Hakonarson H. Annovar: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic. Acids. Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smigielski E.M., Sirotkin K., Ward M., Sherry S.T. Dbsnp: A database of single nucleotide polymorphisms. Nucleic. Acids. Res. 2000;28:352–355. doi: 10.1093/nar/28.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genomes Project C., Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Wu C., Li C., Boerwinkle E. Dbnsfp v3.0: A one-stop database of functional predictions and annotations for human nonsynonymous and splice-site snvs. Hum. Mutat. 2016;37:235–241. doi: 10.1002/humu.22932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovski S., Wang Q., Heinzen E.L., Allen A.S., Goldstein D.B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/annotation/32c8d343-9e1d-46c6-bfd4-b0cd3fb7a97e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper G.M., Stone E.A., Asimenos G., Program N.C.S., Green E.D., Batzoglou S., Sidow A. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 2005;15:901–913. doi: 10.1101/gr.3577405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siepel A., Bejerano G., Pedersen J.S., Hinrichs A.S., Hou M., Rosenbloom K., Clawson H., Spieth J., Hillier L.W., Richards S., et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollard K.S., Hubisz M.J., Rosenbloom K.R., Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the sift algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 44.Adzhubei I., Jordan D.M., Sunyaev S.R. Predicting functional effect of human missense mutations using polyphen-2. Curr. Protoc. Hum. Genet. 2013;7:e20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chun S., Fay J.C. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz J.M., Rodelsperger C., Schuelke M., Seelow D. Mutationtaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 47.Reva B., Antipin Y., Sander C. Predicting the functional impact of protein mutations: Application to cancer genomics. Nucleic. Acids. Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shihab H.A., Gough J., Cooper D.N., Stenson P.D., Barker G.L., Edwards K.J., Day I.N., Gaunt T.R. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden markov models. Hum. Mutat. 2013;34:57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi Y., Sims G.E., Murphy S., Miller J.R., Chan A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castera L., Harter V., Muller E., Krieger S., Goardon N., Ricou A., Rousselin A., Paimparay G., Legros A., Bruet O., et al. Landscape of pathogenic variations in a panel of 34 genes and cancer risk estimation from 5131 hboc families. Genet. Med. 2018;20:1677–1686. doi: 10.1038/s41436-018-0005-9. [DOI] [PubMed] [Google Scholar]
- 51.Haukoos J.S., Lewis R.J. Advanced statistics: Bootstrapping confidence intervals for statistics with "difficult" distributions. Acad. Emerg. Med. 2005;12:360–365. doi: 10.1197/j.aem.2004.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.