Abstract
BACKGROUND
Eosinophilic gastritis and duodenitis are characterized by gastrointestinal mucosal eosinophilia, chronic symptoms, impaired quality of life, and a lack of adequate treatments. Mast-cell activity may contribute to the pathogenesis of the conditions. AK002 (lirentelimab) is an anti–Siglec-8 antibody that depletes eosinophils and inhibits mast cells and that has shown potential in animal models as a treatment for eosinophilic gastritis and duodenitis.
METHODS
In this phase 2 trial, we randomly assigned adults who had symptomatic eosinophilic gastritis, eosinophilic duodenitis, or both conditions in a 1:1:1 ratio to receive four monthly infusions of low-dose AK002, high-dose AK002, or placebo. The primary end point was the change in gastrointestinal eosinophil count from baseline to 2 weeks after the final dose; to maximize statistical power, we evaluated this end point in the placebo group as compared with the combined AK002 group. Secondary end points were treatment response (>30% reduction in total symptom score and >75% reduction in gastrointestinal eosinophil count) and the change in total symptom score.
RESULTS
Of the 65 patients who underwent randomization, 43 were assigned to receive AK002 and 22 were assigned to receive placebo. The mean percentage change in gastrointestinal eosinophil count was −86% in the combined AK002 group, as compared with 9% in the placebo group (least-squares mean difference, −98 percentage points; 95% confidence interval [CI], −121 to −76; P<0.001). Treatment response occurred in 63% of the patients who received AK002 and in 5% of the patients who received placebo (difference, 58 percentage points; 95% CI, 36 to 74; P<0.001). The mean change in total symptom score was −48% with AK002 and −22% with placebo (least-squares mean difference, −26 percentage points; 95% CI, −44 to −9; P = 0.004). Adverse events associated with AK002 were similar to those with placebo, with the exception of higher percentages of patients having mild-to-moderate infusion-related reactions with AK002 (60% in the combined AK002 group and 23% in the placebo group).
CONCLUSIONS
In patients with eosinophilic gastritis or duodenitis, AK002 reduced gastrointestinal eosinophils and symptoms. Infusion-related reactions were more common with AK002 than with placebo. (Funded by Allakos; ENIGMA ClinicalTrials.gov number, NCT03496571.)
Eosinophilic gastrointestinal diseases are chronic inflammatory conditions characterized by gastrointestinal symptoms and eosinophilic infiltration of the gastrointestinal mucosa.1–3 Emerging evidence suggests a role for mast cells in the pathogenesis and symptomatic burden of these conditions.4–11 Whereas eosinophilic esophagitis is relatively well characterized, less is known about eosinophilic gastrointestinal diseases that are distal to the esophagus. Eosinophilic gastritis and eosinophilic duodenitis are defined by eosinophilia of the stomach and duodenum, respectively, and sometimes include concomitant esophageal involvement.1,12–16 Although a U.S. population-based study of eosinophilic gastritis and duodenitis indicated a diagnosed prevalence of approximately 15 cases per 100,000 population,17 other findings have suggested that this may be an underestimate.18–20 The clinical presentation of eosinophilic gastritis and duodenitis commonly consists of abdominal pain, bloating, early satiety, abdominal cramping, diarrhea, nausea or vomiting, and, in more severe cases, hypoproteinemia.1–3,12–14 Patients with eosinophilic gastritis or duodenitis have an impaired quality of life and barriers to care, including delayed diagnosis, misdiagnosis, and a lack of treatment options.21,22 There are no approved therapies targeting these conditions, and current regimens, such as glucocorticoids and elimination diets, have limitations.1–3,13,14
Sialic acid–binding immunoglobulin-like lectin 8 (Siglec-8) is an inhibitory receptor that is selectively expressed on mature eosinophils and mast cells, with low expression on basophils.5,23–26 AK002 (recently given the nonproprietary name lirentelimab) is a first-in-class, humanized, non-fucosylated IgG1 anti–Siglec-8 monoclonal antibody that depletes eosinophils through natural killer cell–mediated antibody-dependent cellular cytotoxicity (in the blood) and apoptosis (in tissues).5,24 AK002 and other anti–Siglec-8 antibodies have been shown to inhibit mast-cell activation, thereby reducing degranulation, secretion of inflammatory mediators, and recruitment of additional mast cells, eosinophils, and other immune cells to tissue.5,24 Open-label clinical studies of AK002 have indicated activity in various allergic diseases, such as chronic urticaria (ClinicalTrials.gov number, NCT03436797) and severe allergic conjunctivitis (NCT03379311).27,28
We hypothesized that because it depletes eosinophils and inhibits mast cells, AK002 could potentially be used to treat eosinophilic gastrointestinal diseases. Proof of concept was shown in a murine model of eosinophilic gastritis and duodenitis, in which an anti–Siglec-8 monoclonal antibody reduced allergen-induced gastric and duodenal eosinophilia and inhibited mast-cell activation.5 The aim of our trial (ENIGMA) was to assess the efficacy and safety of AK002 in adult patients with eosinophilic gastritis, eosinophilic duodenitis, or both conditions.
METHODS
TRIAL DESIGN AND OVERSIGHT
In this multicenter, randomized, double-blind, placebo-controlled, phase 2 trial involving adults with eosinophilic gastritis, eosinophilic duodenitis, or both conditions, eligible patients were randomly assigned in a 1:1:1 ratio to receive four monthly intravenous infusions of low-dose AK002 (0.3, 1, 1, and 1 mg per kilogram of body weight), high-dose AK002 (0.3, 1, 3, and 3 mg per kilogram), or placebo (volumetric equivalent) on days 1, 29, 57, and 85 (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Patients who completed treatment through day 113 had the option to continue in a separate open-label extension study. Patients who declined participation in the open-label extension had follow-up visits on days 113 and 141. For details of randomization and blinding, see the Supplemental Methods section in the Supplementary Appendix.
The trial was initiated at 22 sites across the United States (17 of the 22 sites enrolled patients) and was performed in accordance with the Declaration of Helsinki and in compliance with Good Clinical Practice Guidelines, the institutional review boards of the 22 trial sites, and applicable laws. All patients provided written informed consent before trial entry. An independent data and safety monitoring committee provided additional oversight. The trial was designed by Allakos (the commercial sponsor) in collaboration with an advisory board of investigators (see the Supplementary Appendix). The investigators collected the data, and the commercial sponsor analyzed the data. The academic authors had access to the data. The commercial sponsor and the members of the steering committee contributed to the trial design, data analysis and interpretation, and critical review of the manuscript. The first draft of the manuscript was prepared by a medical writer (paid by the commercial sponsor), with direction and content driven by the first author. The manuscript was reviewed and approved by all the authors. The authors vouch for the completeness and accuracy of the data and for fidelity of the trial to the protocol, available at NEJM.org. All the investigators had confidentiality agreements with the commercial sponsor.
PATIENTS
Adults who were 18 to 80 years of age and had eosinophilic gastritis, eosinophilic duodenitis, or both conditions were eligible for inclusion if they reported moderate-to-severe symptoms, defined as a mean daily symptom score of 3 or higher (scores range from 0 to 10, with 0 indicating no symptoms and 10 indicating the worst possible symptoms) over a period of 7 days for abdominal pain, diarrhea, or nausea on a patient-reported outcome questionnaire that was completed for at least 2 weeks (see Appendix 1 in the protocol); a minimum of four questionnaires had to be completed each qualifying week during screening. Patients who met the symptomatic criteria underwent esophagogastroduodenoscopy with biopsy and histopathologic evaluation and were required to have gastrointestinal eosinophilia, defined as at least 30 eosinophils per high-power field29 in five high-power fields in the stomach (for eosinophilic gastritis), at least 30 eosinophils per high-power field in three high-power fields in the duodenum (for eosinophilic duodenitis), or both, without any other cause of gastrointestinal eosinophilia (see the Supplementary Appendix). (Eosinophilic duodenitis is referred to as “eosinophilic gastroenteritis” in the protocol and statistical analysis plan.) Esophageal biopsy specimens were obtained for histopathological evaluation from patients with a history of eosinophilic esophagitis (a previous documented clinical diagnosis) or if esophageal involvement was suspected on upper endoscopy.
Key exclusion criteria were celiac disease, active Helicobacter pylori infection, inflammatory bowel disease, helminthic parasitic infection in the preceding 6 months, or the use of immunosuppressive or immunomodulatory drugs other than glucocorticoids. (Prednisone at a dose of ≤10 mg daily [or equivalent] was allowed if it was already being taken and the regimen remained unchanged during screening and throughout the trial; single-use glucocorticoid treatment to prevent or manage infusion-related reactions was allowed at the investigators’ discretion.) Pre-existing dietary restrictions and permitted pre-trial medications were allowed if they were maintained throughout the trial (see the protocol).
END POINTS
The primary end point was the percentage change in mean peak eosinophil count in gastric or duodenal mucosa from baseline to the end of treatment, defined as day 99 (2 weeks after the final dose). If eosinophilia was present in both gastric and duodenal tissue at baseline, the end point was assessed in the compartment with the highest baseline eosinophil count. The first secondary end point was treatment response (defined as both a >30% reduction in total symptom score [scores range from 0 to 80, with higher values indicating greater symptom severity] and a >75% reduction in gastrointestinal eosinophil count), and the second secondary end point was the percentage change in total symptom score from baseline to end of treatment, defined as week 13 or 14 (mean of daily scores in the 14-day window between the last dose and the end-of-treatment biopsy). The total symptom score was calculated from daily patient-reported outcome questionnaire entries made by patients into the electronic data capture system. The questionnaire assessed eight individual symptoms (each on a scale of 0 to 10, combining to form the total symptom score): abdominal pain, nausea, vomiting, early satiety, loss of appetite, abdominal cramping, bloating, and diarrhea. The questionnaire was developed in accordance with methods described in the Food and Drug Administration (FDA) Guidance on Patient-Reported Outcome Measures30; before its use in this trial, evidence of psychometric reliability and validity was obtained in a 30-day observational longitudinal study. All other prespecified end points are summarized in Table S1.
SAFETY
All adverse events were monitored, assigned a severity grade, and assessed for clinical significance and relationship to AK002 or placebo by a principal investigator. Additional safety evaluations included clinical laboratory tests, physical examinations, and assessment of vital signs.
STATISTICAL ANALYSIS
The intention-to-treat population included all patients who underwent randomization. The primary and secondary efficacy end points were assessed in the intention-to-treat population. Patients who were excluded from the per-protocol population (for withdrawal from the trial, protocol violations, or missing outcome data) were considered to have had treatment failure in intention-to-treat analyses; for these patients, we used baseline values for the post-treatment tissue eosinophil count and total symptom scores and used a percentage change of 0 for these end points. Prespecified analyses of all efficacy end points, as defined in the statistical analysis plan, were conducted in the per-protocol population. The analysis of safety included the population of all patients who underwent randomization, which was identical to the intention-to-treat population. Both the per-protocol and the safety populations were predefined in the statistical analysis plan before database lock, which occurred on July 10, 2019.
The primary efficacy end point was analyzed by analysis of covariance (ANCOVA), with treatment as factor and baseline and randomization stratum as covariates. The first secondary end point was analyzed with Fisher’s exact test. The second secondary end point was analyzed by ANCOVA, with treatment as factor and baseline total symptom score as covariate.
Statistical comparisons between the combined, high-dose, and low-dose AK002 groups and the placebo group with respect to the primary and secondary end points were performed with the use of a prespecified hierarchical testing procedure to control the familywise type I error rate. The confidence interval for the difference in the percentage of patients with a treatment response was based on the inverse of the exact tests with the use of the score statistic.31 Additional details of multiplicity adjustment and hierarchical testing schema, the approach to missing data and repeated measures, and other statistical methods are described in the statistical analysis plan and the Supplementary Appendix.
Sample size was determined by assuming a mean reduction in gastrointestinal eosinophil count of 30% in either AK002 group and 10% in the placebo group, with a standard deviation of 20% for the reduction from baseline in the overall trial population. With 20 patients in each group and a 5% significance level, the trial had an estimated 87% power for the analysis of the primary end point. (The sample size was planned on the basis of a comparison between the placebo group and each AK002 dose group; to enhance statistical power, a decision was made before database lock for the primary analyses to compare the placebo group with the combined AK002 group.)
RESULTS
PATIENT CHARACTERISTICS
We enrolled patients who had active eosinophilic gastritis or eosinophilic duodenitis (or both conditions) that was not adequately controlled with pharmacologic therapy or dietary modification (Table 1 and Table S2). In total, 65 patients received at least one dose of AK002 or placebo (22 in the low-dose AK002 group, 21 in the high-dose AK002 group, and 22 in the placebo group) (Fig. S2). The baseline characteristics of the patients were generally similar in all the groups, although the percentage of patients who were male was numerically higher and the median age was younger in the placebo group, and the baseline peripheral blood eosinophil count was numerically higher in the combined AK002 group (Table 1). In total, 10 patients (15%) had a diagnosis of gastritis, 25 patients (38%) had a diagnosis of duodenitis, and 30 patients (46%) had concurrent gastritis and duodenitis. At baseline, the mean (±SD) number of gastrointestinal eosinophils per high-power field was 84±52, and the total symptom score was 32±14.
Table 1.
Patient Characteristics at Baseline (Intention-to-Treat and Safety Population).*
| Characteristic | AK002 | Placebo (N = 22) | Overall (N = 65) | ||
|---|---|---|---|---|---|
| Low Dose (N = 22) | High Dose (N = 21) | Combined (N = 43) | |||
| Median age (range) — yr | 39 (18–74) | 41 (20–67) | 40 (18–74) | 36 (18–67) | 40 (18–74) |
| Female sex — no. (%) | 17 (77) | 13 (62) | 30 (70) | 10 (45) | 40 (62) |
| Race or ethnic group — no. (%)† | |||||
| White | 21 (95) | 17 (81) | 38 (88) | 22 (100) | 60 (92) |
| Asian | 0 | 1 (5) | 1 (2) | 0 | 1 (2) |
| Black | 1 (5) | 2 (10) | 3 (7) | 0 | 3 (5) |
| American Indian or Alaska Native | 0 | 1 (5) | 1 (2) | 0 | 1 (2) |
| Median weight (range) — kg | 78 (54–127) | 82 (42–121) | 80 (42–127) | 78 (46–141) | 80 (42–141) |
| Median body-mass index (range)‡ | 26 (19–50) | 27 (16–43) | 27 (16–50) | 24 (19–53) | 27 (16–53) |
| Primary diagnosis — no. (%) | |||||
| Eosinophilic gastritis | 4 (18) | 1 (5) | 5 (12) | 5 (23) | 10 (15) |
| Eosinophilic duodenitis | 6 (27) | 10 (48) | 16 (37) | 9 (41) | 25 (38) |
| Eosinophilic gastritis and duodenitis | 12 (55) | 10 (48) | 22 (51) | 8 (36) | 30 (46) |
| History of eosinophilic gastritis or duodenitis — no. (%)§ | 20 (91) | 15 (71) | 35 (81) | 17 (77) | 52 (80) |
| Median time since diagnosis of eosinophilic gastritis or duodenitis (range) — yr | 6 (0–18) | 4 (1–16) | 5 (0–18) | 4 (0–16) | 4 (0–18) |
| History of eosinophilic esophagitis — no. (%)§ | 15 (68) | 8 (38) | 23 (53) | 12 (55) | 35 (54) |
| Baseline use of low-dose systemic glucocorticoid — no. (%)¶ | 2 (9) | 3 (14) | 5 (12) | 2 (9) | 7 (11) |
| Baseline use of proton-pump inhibitor — no. (%) | 14 (64) | 10 (48) | 24 (56) | 8 (36) | 32 (49) |
| Baseline use of physician-prescribed diet regimen — no. (%)‖ | 3 (14) | 4 (19) | 7 (16) | 4 (18) | 11 (17) |
| Total serum IgE concentration — kU/liter | 716±1624 | 309±395 | 517±1198 | 635±1225 | 556±1198 |
| Blood absolute eosinophil count/mm3 | 991±1369 | 497±847 | 750±1158 | 562±537 | 686±991 |
| Gastrointestinal eosinophil count — eosinophils/high-power field** | 101±66 | 76±40 | 89±55 | 74±46 | 84±52 |
| Gastrointestinal mast-cell count — cells/high-power field** | 74±25 | 58±26 | 66±27 | 58±19 | 64±25 |
| Total symptom score†† | 34±13 | 33±14 | 33±13 | 29±14 | 32±14 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the patient.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
A history of the condition was considered to be present if a documented clinical diagnosis had been made by the patient’s physician and was noted in the patient’s clinical chart history.
Prednisone at a dose no higher than 10 mg daily (or equivalent) was permitted if it was a preexisting regimen and was taken throughout the trial.
Patients were required to maintain their baseline diet regimen throughout the study.
The included count was for the site (gastric or duodenal) with the highest number of cells per high-power field at baseline.
The total symptom score was determined with a patient-reported outcome questionnaire (see Appendix 1 in the protocol). The questionnaire assessed eight symptoms on a daily basis: abdominal pain, nausea, vomiting, early satiety, loss of appetite, abdominal cramping, bloating, and diarrhea. Individual symptom scores ranged from 0 to 10, allowing for a maximum daily total symptom score of 80, with higher scores indicating greater severity.
Four patients (9%) who had been receiving AK002 and 2 (9%) who had been receiving placebo were excluded from the per-protocol population and were considered to have had treatment failures in the intention-to-treat analyses (see Supplemental Methods): 2 patients who received one dose of AK002 and were withdrawn from the trial because they had hypereosinophilic syndrome and did not receive the two-dose minimum for inclusion in the efficacy analyses; 2 patients in the placebo group and 1 patient in the AK002 group who began to receive or changed the dose of daily systemic glucocorticoid treatment, which could have confounded interpretation of the safety and efficacy data; and 1 patient in the AK002 group with missing outcome data, which precluded calculation of end points (Fig. S2). The baseline characteristics of the remaining 59 patients (19 patients in the low-dose AK002 group, 20 in the high-dose AK002 group, and 20 in the placebo group) were similar in the three groups (Table S3). The frequency of single-use systemic glucocorticoid treatment (i.e., with prednisone or methylprednisolone) for prophylaxis or treatment of infusion-related reactions was balanced among the groups (7 patients in the low-dose AK002 group, 4 in the high-dose AK002 group, and 7 in the placebo group).
EFFICACY
Change in Gastrointestinal Eosinophil Count
Patients in all three AK002 groups (combined, high-dose, and low-dose) had a significantly greater mean percentage reduction in gastrointestinal eosinophil count from baseline to the end of treatment than patients in the placebo group (Table 2). In the intention-to-treat analysis, the mean change from baseline in the gastrointestinal eosinophil count among patients receiving AK002 was −86%, as compared with 9% in the placebo group. This corresponded to a least-squares mean difference as compared with placebo of −98 percentage points (95% confidence interval [CI], −121 to −76; P<0.001). In the prespecified per-protocol analysis, the change from baseline in the gastrointestinal eosinophil count was −95% in the AK002 group, as compared with 10% in the placebo group (least-squares mean difference, −105 percentage points; 95% CI, −128 to −83; P<0.001) (Table 2 and Fig. S3). Patients in all three AK002 groups had a greater absolute change in gastrointestinal eosinophil count from baseline to end of treatment than patients in the placebo group (Table S4).
Table 2.
Primary and Secondary Efficacy End Points.*
| End Point | Intention-to-Treat Analysis† | Per-Protocol Analysis‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Low-Dose AK002 (N = 22) | High-Dose AK002 (N = 21) | Combined AK002 (N = 43) | Placebo (N = 22) | Low-Dose AK002 (N = 19) | High-Dose AK002 (N = 20) | Combined AK002 (N = 39) | Placebo (N = 20) | |
| Primary end point | ||||||||
| Mean change in gastrointestinal eosinophil count (95% CI) — % | −79 (−95 to −59) | −92 (−100 to −81) | −86 (−94 to −71) | 9 (−15 to 31) | −92 (−100 to −70) | −97 (−100 to −89) | −95 (−100 to −83) | 10 (−14 to 40) |
| Least-squares mean difference from placebo | −95 (−122 to −68) | −102 (−128 to −76) | −98 (−121 to −76) | — | −103 (−130 to −77) | −107 (−133 to −81) | −105 (−128 to −83) | — |
| P value | <0.001 | <0.001 | <0.001 | — | <0.001 | <0.001 | <0.001 | — |
| Secondary end points | ||||||||
| Treatment response§ | ||||||||
| No. of patients | 13 | 14 | 27 | 1 | 13 | 14 | 27 | 1 |
| Percentage of patients (95% CI) | 59 (36 to 79) | 67 (43 to 85) | 63 (47 to 77) | 5 (0 to 23) | 68 (43 to 87) | 70 (46 to 88) | 69 (52 to 83) | 5 (0 to 25) |
| Difference from placebo | 55 (27 to 76) | 62 (34 to 82) | 58 (36 to 74) | — | 63 (33 to 84) | 65 (36 to 85) | 64 (41 to 80) | — |
| P value¶ | <0.001 | <0.001 | <0.001 | — | <0.001 | <0.001 | <0.001 | — |
| Mean percentage change in total symptom score | −42 (−56 to −28) | −55 (−70 to −40) | −48 (−58 to −39) | −22 (−37 to −7) | −49 (−63 to −35) | −58 (−72 to −43) | −53 (−63 to −44) | −24 (−41 to −8) |
| Least-squares mean difference from placebo | −20 (−40 to 0) | −33 (−53 to −13) | −26 (−44 to −9) | — | −26 (−46 to −5) | −35 (−55 to −14) | −30 (−48 to −12) | — |
| P value | 0.05 | 0.002 | 0.004 | — | 0.02 | 0.001 | 0.001 | — |
End points are shown in the order used in the prespecified hierarchical testing procedure. CI denotes confidence interval.
The intention-to-treat analysis included all patients who underwent randomization, with patients who had major protocol violations or missing data treated as having treatment failure. For patients with treatment failure, the baseline values were imputed to provide outcomes of zero percentage change.
The prespecified analyses of all efficacy end points, as defined in the statistical analysis plan, were performed with the per-protocol population, which excluded patients who had major protocol deviations that could confound the efficacy interpretation.
A treatment response was defined as both a reduction of more than 30% in the total symptom score and a reduction of more than 75% in the gastrointestinal eosinophil count.
P value was calculated with Fisher’s exact test.
In an additional prespecified per-protocol analysis, a histologic response (defined as <30 gastrointestinal eosinophils per high-power field at the end of treatment) was observed in 37 patients (95%; 95% CI, 83 to 99) who received AK002 and in 3 (15%; 95% CI, 3 to 38) who received placebo. In a post hoc analysis, the percentage of AK002-treated patients who had a gastrointestinal eosinophil count at the end of treatment that was no higher than 6 eosinophils per high-power field was 95% (95% CI, 83 to 99), and the percentage with 0 or 1 eosinophils per high-power field was 85% (95% CI, 70 to 94), as compared with 0% (95% CI, 0 to 17) for both of these end points in the placebo group.
In a prespecified analysis involving 23 patients (14 in the combined AK002 group and 9 in the placebo group) who had concomitant esophageal eosinophilia (≥15 eosinophils per high-power field) at screening, esophageal histologic remission (≤6 eosinophils per high-power field) was found in 13 of 14 patients (93%; 95% CI, 66 to 100) who received AK002 and in 1 of 9 (11%; 95% CI, 0 to 48) who received placebo (Table S5). In prespecified analyses, AK002 treatment also resulted in rapid reductions in blood absolute eosinophil count (Fig. S4) and decreases in gastrointestinal mast-cell counts, whereas these measures increased from baseline in the placebo group (Table S6).
Treatment Response and Symptomatic Improvement
Significantly more patients in the three AK002 groups than in the placebo group had a treatment response, in both the intention-to-treat and the per-protocol populations (Table 2). In addition, the mean total symptom score showed a significantly greater decrease in the combined and high-dose AK002 groups than in the placebo group (Table 2). Low-dose AK002 resulted in a significantly greater mean decrease in the total symptom score than did placebo in the per-protocol analysis, but this difference was not significant in the intention-to-treat analysis (Table 2). Patients in all three AK002 groups had a greater absolute change in total symptom score from baseline to end of treatment than patients in the placebo group (Table S4).
A prespecified per-protocol analysis of changes from baseline in the weekly total symptom score showed symptomatic improvements at each time point assessed, from 1 week after the first dose to the end of the treatment period (Fig. 1). Furthermore, patients who received AK002 had reductions in the eight assessed individual symptom scores from baseline to the end of the treatment period (Fig. S5). In a post hoc analysis of the percentage of patients with a decrease in mean total symptom score of at least 50% from baseline to the end of treatment, we identified 25 patients (64%; 95% CI, 47 to 79) in the AK002 group who had a response, as compared with 3 patients (15%; 95% CI, 3 to 38) in the placebo group (Fig. S6). Among patients who reported dysphagia symptoms, the mean percentage decrease in symptom severity from baseline to week 14 was greater among patients who received AK002 than among those who received placebo (Table S7).
Figure 1. Change in Total Symptom Score.
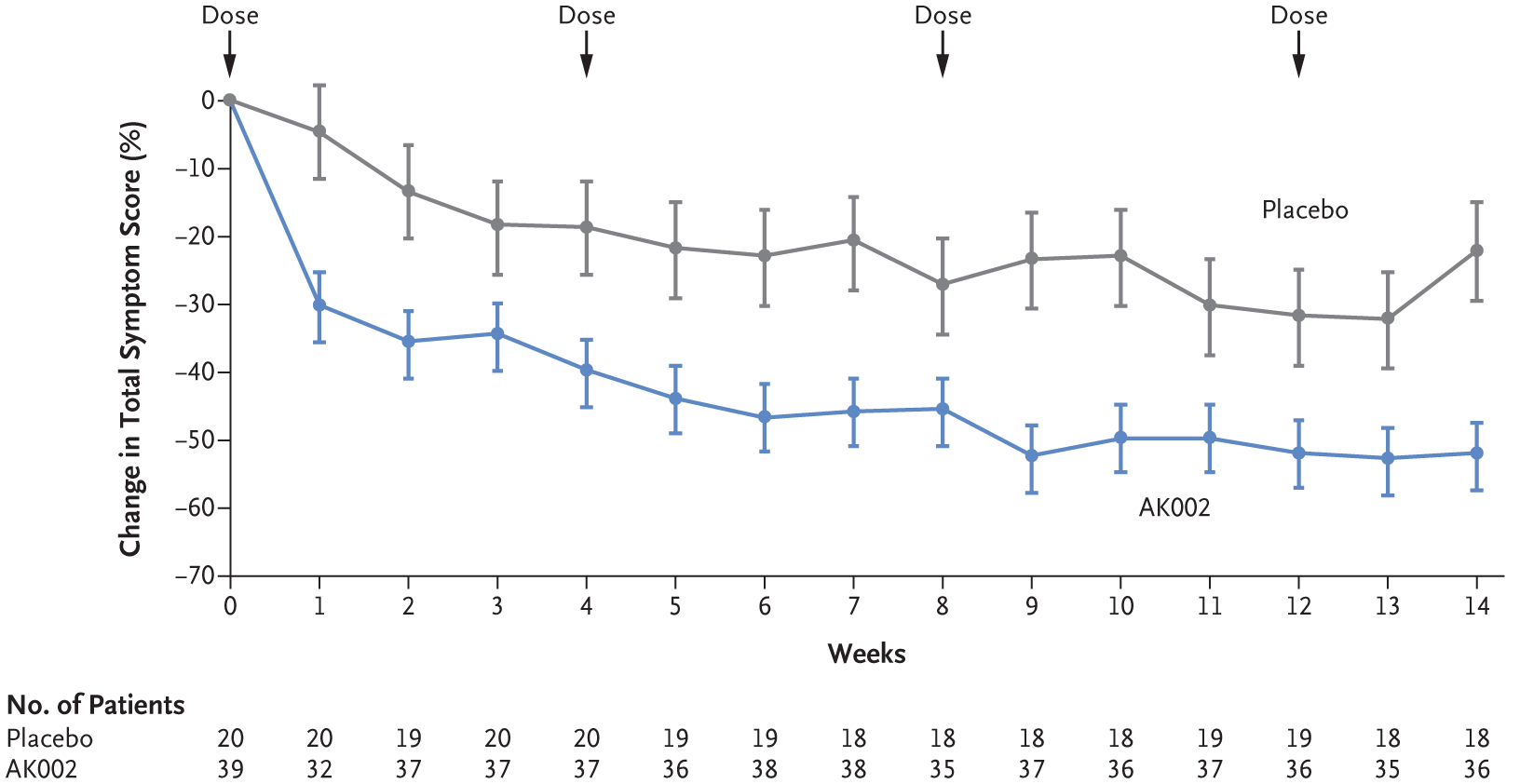
Shown is the least-squares mean percentage change from baseline in total symptom score over time. The total symptom score was based on a daily patient-reported outcome questionnaire. The questionnaire assessed eight individual symptoms (each on a scale of 0 to 10, combining to form the total symptom score): abdominal pain, nausea, vomiting, early satiety, loss of appetite, abdominal cramping, bloating, and diarrhea. The total symptom score ranges from 0 to 80, with higher scores indicating greater symptom severity. I bars indicate the standard error.
Other Exploratory End Points
The results with respect to the primary and secondary efficacy end points were not affected by the occurrence of infusion-related reactions, single-use glucocorticoid treatment for the prevention or management of reactions (Tables S8 and S9), or ongoing low-dose maintenance treatment with glucocorticoids. Patients who received AK002 had better outcomes with respect to changes from baseline in every component of the 36-Item Short Form Survey quality-of-life questionnaire than did patients who received placebo (Fig. S7). In the open-label extension study, patients who received 52 weeks of AK002 treatment had sustained tissue eosinophil depletion, as well as a further decrease in the total symptom score (mean change, −68%; 95% CI, −78 to −58) (see Open-Label Extension Interim Results in the Supplementary Appendix).
SAFETY
The number of patients who had at least one adverse event during treatment was 39 (91%) in the combined AK002 group and 18 (82%) in the placebo group (Table 3 and Table S10). The most common adverse event during treatment was an infusion-related reaction, which occurred in 26 patients (60%) who received AK002, as compared with 5 (23%) who received placebo. Most reactions (93%) were mild to moderate and consisted of flushing, a feeling of warmth, headache, nausea, or dizziness. Other adverse events during treatment occurred in similar percentages of patients in the two groups.
Table 3.
Adverse Events (Safety Population).
| Adverse Event | AK002 (N = 43) | Placebo (N = 22) |
|---|---|---|
| no. of patients (%) | ||
| Any serious event | 4 (9)* | 3 (14)† |
| Any event during treatment that occurred in ≥5% of patients in either group | 39 (91) | 18 (82) |
| Infusion-related reaction‡ | 26 (60) | 5 (23) |
| Headache | 4 (9) | 2 (9) |
| Upper respiratory tract infection | 4 (9) | 2 (9) |
| Urinary tract infection | 4 (9) | 1 (5) |
| Nausea | 3 (7) | 3 (14) |
| Fatigue | 3 (7) | 2 (9) |
| Diarrhea | 2 (5) | 2 (9) |
| Nasopharyngitis | 2 (5) | 2 (9) |
| Abdominal pain | 1 (2) | 2 (9) |
| Dehydration | 1 (2) | 2 (9) |
| Viral gastroenteritis | 1 (2) | 2 (9) |
| Pyrexia | 1 (2) | 2 (9) |
| Sinusitis | 1 (2) | 2 (9) |
| Cough | 0 | 2 (9) |
| Influenza | 0 | 2 (9) |
| Increase in white-cell count | 0 | 2 (9) |
The serious adverse events that occurred in the combined AK002 group were moderate hypoxia and moderate chest pain (classified as a serious adverse event because the patient was hospitalized) in 1 patient, abdominal pain in 1 patient, dehydration in 1 patient, and anemia and a grade 4 infusion-related reaction in 1 patient. The only serious adverse event that was deemed to be related to AK002 was the infusion-related reaction.
The serious adverse events that occurred in the placebo group were mild dehydration in 1 patient (classified as a serious adverse event because the patient was hospitalized), severe anemia in 1 patient, and a change in mental status in 1 patient.
Infusion-related reactions included flushing, a feeling of warmth, headache, nausea, or dizziness. Infusion-related reactions predominantly occurred on the first or second infusions, in which patients in both AK002 groups received identical doses, and the number of infusion-related reactions was numerically higher in the low-dose group (Table S10).
Serious adverse events occurred in 4 patients (9%) who received AK002 and in 3 (14%) who received placebo. The only serious adverse event that was deemed to be related to AK002 or placebo was a grade 4 infusion-related reaction in the high-dose AK002 group that led to discontinuation of participation in the trial. Transient lymphopenia was detected after infusion in 30 of 35 patients (86%) who received AK002 and 8 of 17 (47%) who received placebo; none had any clinical consequence. The incidence of antidrug antibodies during treatment was balanced among the groups, occurring in 2 patients (10%) in the high-dose AK002 group, 2 patients (9%) in the low-dose AK002 group, and 2 patients (9%) in the placebo group. Antidrug antibodies had no effect on safety, efficacy, or histopathological measures. In an ongoing open-label extension study, the most common adverse event was mild-to-moderate infusion-related reactions, although none were observed in patients who received a single dose of prednisone on the day before the infusion (see the Supplementary Appendix).
DISCUSSION
In this trial, AK002 was more effective than placebo with respect to all the prespecified primary and secondary end points. Patients who received low-dose or high-dose AK002 had significant reductions in tissue eosinophil counts, accompanied by substantial reductions in symptoms, whether assessed in the predefined per-protocol analysis or in an intention-to-treat analysis. AK002 treatment led to eosinophil depletion in gastric, duodenal, and esophageal tissues. Symptomatic improvement was observed within a week of dosing and was maintained throughout the trial. AK002 also resulted in alleviation of dysphagia in patients with a history of concomitant eosinophilic esophagitis. The most common adverse event related to AK002 that occurred during treatment was mild-to-moderate infusion-related reactions; these reactions occurred mostly on first infusion and were hypothesized to be triggered by rapid depletion of blood eosinophils through antibody-dependent cellular cytotoxicity. Studies of other infused monoclonal antibodies that deplete cells through antibody-dependent cellular cytotoxicity have also shown infusion-related reactions.32,33 Reactions were less frequent or did not occur with continued AK002 treatment, a finding that may have reflected lower blood eosinophil levels after AK002 treatment. Other adverse events occurred in similar percentages of patients in the AK002 group and the placebo group. Long-term treatment with AK002 in the ongoing open-label extension study resulted in further symptomatic improvements and sustained depletion of tissue and blood eosinophils. In the open-label extension study, infusion-related reactions were not seen in patients who received a single dose of oral prednisone the day before the first infusion.
There are no FDA-approved therapies for eosinophilic gastritis or duodenitis, and limited published studies are available for comparison. The current standard of care consists of topical and systemic glucocorticoids, diet modification, or both. Systemic and topical glucocorticoids have been reported to reduce symptoms, but their toxicity limits long-term use.1,2,13,15,34,35 Data from studies of dietary therapy for eosinophilic gastritis and duodenitis are difficult to interpret because of their small sample sizes and inconsistent results, but efficacy is not universal, and adherence is difficult. Off-label use of biologics (e.g., targeting IgE, the interleukin-5 pathway, or integrins) has been shown to reduce tissue eosinophilia, but reductions in symptoms have not been shown consistently.1,36,37 In our trial, 20% of the patients who underwent randomization had no previous diagnosis of eosinophilic gastritis or duodenitis. The majority (10 of 13) of these chronically symptomatic patients came from nonacademic, community sites. This suggests that these conditions may not be as rare as previously described and adds urgency to the need for a safe, effective treatment.
AK002 depletes eosinophils and inhibits mast-cell activity; consequently, the combined activity could explain the histologic and clinical improvements observed in this trial. Although eosinophils have long been recognized as being pathogenic in eosinophilic gastrointestinal diseases, mast cells also may contribute to disease pathogenesis.5,24,38 Evidence supporting a key role for mast cells includes the presence of activated mast cells at elevated levels in biopsy specimens from patients with eosinophilic gastritis, duodenitis, or esophagitis4–11 and the high incidence of allergic coexisting conditions, such as rhinitis, asthma, and atopic dermatitis, among patients with eosinophilic gastrointestinal disease.1–3,12–14,17 The effect of AK002 on both eosinophils and mast cells makes it a potential therapeutic candidate for eosinophilic gastritis, duodenitis, and esophagitis and for a variety of allergic and inflammatory conditions.
One limitation of this trial was the small sample size. Therefore, there were numeric differences in baseline characteristics between the AK002 and placebo groups (i.e., sex, primary diagnosis, and blood eosinophil count). However, post hoc subgroup analyses did not indicate that any of these differences in baseline characteristics favored a response to AK002, and current literature does not suggest that sex or primary disease location influences outcomes. Larger studies are necessary to assess the safety and efficacy of AK002. An additional limitation was the potential confounding factor of single-use glucocorticoid treatment to manage infusion-related reactions. Post hoc analyses showed similar improvements with respect to all efficacy end points regardless of whether infusion-related reactions occurred or glucocorticoids were used. The fact that we did not perform esophageal biopsies in all patients was also a limitation, since we may not have captured possible concomitant esophageal eosinophilia in patients who did not have a previous diagnosis of eosinophilic esophagitis. Although long-term suppression of eosinophils could, in theory, increase the risk of helminth infection, this has not been seen in studies of AK002 to date, nor has this been seen or reported in the clinical development or postmarketing use of other eosinophil-depleting agents. Longer-term treatment with AK002 in larger number of patients is necessary to assess this safety concern.
In this phase 2 trial, AK002 treatment reduced gastrointestinal eosinophil burden and led to reductions in symptoms in adult patients with eosinophilic gastritis, eosinophilic duodenitis, or both conditions, as compared with placebo. A phase 3 trial of AK002 in patients with eosinophilic gastritis or duodenitis (NCT04322604) and a phase 2–3 trial involving patients with eosinophilic esophagitis (NCT04322708) are currently under way.
Supplementary Material
Acknowledgments
Supported by Allakos. The work of Drs. Khoury and Klion is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Dr. Murray’s work is supported in part by a grant (UL1 TR002377) from the National Center for Advancing Translational Sciences.
We thank the patients who participated in the trial; Cory Mekelburg, B.S., and Lauren Gehman, Ph.D., employees of Allakos, for data analysis and assistance in the preparation of the submitted manuscript; staff members of Etera Solutions for providing analysis support, funded by Allakos; and Jocelyn Hybiske, Ph.D., for writing and editing assistance (funded by Allakos).
Footnotes
References
- 1.Gonsalves N Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol 2019; 57: 272–85. [DOI] [PubMed] [Google Scholar]
- 2.Egan M, Furuta GT. Eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis. Ann Allergy Asthma Immunol 2018; 121: 162–7. [DOI] [PubMed] [Google Scholar]
- 3.Fleischer DM, Atkins D. Evaluation of the patient with suspected eosinophilic gastrointestinal disease. Immunol Allergy Clin North Am 2009; 29: 53–63. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell JM, Collins MH, Stucke EM, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol 2014; 134: 1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youngblood BA, Brock EC, Leung J, et al. AK002, a humanized sialic acid-binding immunoglobulin-like lectin-8 antibody that induces antibody-dependent cell-mediated cytotoxicity against human eosinophils and inhibits mast cell-mediated anaphylaxis in mice. Int Arch Allergy Immunol 2019; 180: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu Blatman KS, Gonsalves N, Hirano I, Bryce PJ. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J Allergy Clin Immunol 2011; 127(5): 1307.e3–1308.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abonia JP, Blanchard C, Butz BB, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol 2010; 126: 140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton SM, Kagalwalla AF, Arva NC, et al. Mast cell infiltration is associated with persistent symptoms and endoscopic abnormalities despite resolution of eosinophilia in pediatric eosinophilic esophagitis. Am J Gastroenterol 2020; 115: 224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2013; 304: G1087–G1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon ES, Chen X, Miller CR, et al. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. Am J Gastroenterol 2011; 106: 264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed CC, Genta RM, Youngblood BA, Wechsler JB, Dellon ES. Mast cell and eosinophil counts in gastric and duodenal biopsies from patients with and without eosinophilic gastroenteritis. Clin Gastroenterol Hepatol 2020. August 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesek RD, Reed CC, Muir AB, et al. Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol 2019; 114: 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis 2015; 47: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko HM, Morotti RA, Yershov O, Chehade M. Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol 2014; 109: 1277–85. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990; 31: 54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JY, Choung RS, Lee RM, et al. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastroenterol Hepatol 2010; 8: 669–75. [DOI] [PubMed] [Google Scholar]
- 17.Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr 2016; 62: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhmoud T, Hanson JA, Parasher G. Eosinophilic gastroenteritis: an underdiagnosed condition. Dig Dis Sci 2016; 61: 2585–92. [DOI] [PubMed] [Google Scholar]
- 19.Chehade M, Gehman LT, Singh B, Rasmussen HS. The tortuous path to diagnosis (Dx) of eosinophilic gastritis and eosinophilic gastroenteritis (EG/EGE) in the United States: a real-world, population-based study. Allergy 2019; 74(S106): 375 abstract. [Google Scholar]
- 20.Licari A, Votto M, Scudeller L, et al. Epidemiology of nonesophageal eosinophilic gastrointestinal diseases in symptomatic patients: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2020; 8(6): 1994.e2–2003.e2. [DOI] [PubMed] [Google Scholar]
- 21.Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol 2018; 42: 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedell A, Taft T, Craven MR, Guadagnoli L, Hirano I, Gonsalves N. Impact on health-related quality of life in adults with eosinophilic gastritis and gastroenteritis: a qualitative assessment. Dig Dis Sci 2018; 63: 1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther 2012; 135: 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youngblood BA, Brock EC, Leung J, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 2019; 4(19): e126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legrand F, Cao Y, Wechsler JB, et al. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: receptor expression and targeting using chimeric antibodies. J Allergy Clin Immunol 2019; 143(6): 2227.e10–2237.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikly KK, Bochner BS, Freeman SD, et al. Identification of SAF-2, a novel Siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol 2000; 105: 1093–100. [DOI] [PubMed] [Google Scholar]
- 27.Altrichter S, Staubach P, Pasha M, et al. Clinical activity of AK002, an anti-Siglec-8 antibody, in multiple forms of uncontrolled chronic urticaria. Ann Allergy Asthma Immunol 2019; 123: Suppl: S27–S28. abstract. [Google Scholar]
- 28.Levine T, Tauber J, Nguyen Q, et al. Clinical activity of AK002, an anti-Siglec-8 antibody, in severe allergic conjunctivitis and comorbid atopic diseases. Ann Allergy Asthma Immunol 2019; 123: Suppl: S17 abstract. [Google Scholar]
- 29.Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am 2014; 43: 257–68. [DOI] [PubMed] [Google Scholar]
- 30.Patient-reported outcome measures: use in medical product development to support labeling claims: guidance for industry. Silver Spring, MD: Food and Drug Administration, December 2009. [Google Scholar]
- 31.Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999; 55: 1202–9. [DOI] [PubMed] [Google Scholar]
- 32.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 2010; 125(6): 1237.e2–1244.e2. [DOI] [PubMed] [Google Scholar]
- 33.Levin AS, Otani IM, Lax T, Hochberg E, Banerji A. Reactions to rituximab in an outpatient infusion center: a 5-year review. J Allergy Clin Immunol Pract 2017; 5(1): 107.e1–113.e1. [DOI] [PubMed] [Google Scholar]
- 34.Siewert E, Lammert F, Koppitz P, Schmidt T, Matern S. Eosinophilic gastroenteritis with severe protein-losing enteropathy: successful treatment with budesonide. Dig Liver Dis 2006; 38: 55–9. [DOI] [PubMed] [Google Scholar]
- 35.Tan AC, Kruimel JW, Naber TH. Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur J Gastroenterol Hepatol 2001; 13: 425–7. [DOI] [PubMed] [Google Scholar]
- 36.Foroughi S, Foster B, Kim N, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol 2007; 120: 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prussin C, James SP, Huber MM, Klion AD, Metcalfe DD. Pilot study of anti-IL-5 in eosinophilic gastroenteritis. J Allergy Clin Immunol 2003; 111: Suppl 1: S275 abstract. [Google Scholar]
- 38.Ramsay DB, Stephen S, Borum M, Voltaggio L, Doman DB. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y) 2010; 6: 772–7. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


