Abstract
Background and Objectives: Syzygium gratum (SG) is a local vegetable and widely consumed in Thailand. Previously, a strong antioxidative effect of SG extract has been reported. The effects of SG extract on hypertension have remained unknown. The effect of SG aqueous extract on blood pressure and vascular changes were examined in L-NAME-induced hypertensive rats (LHR), and its potential active constituents were also explored. Materials and Methods: Male Sprague Dawley rats were allocated to control, L-NAME (40 mg/kg/day), L-NAME + SG (100, 300, 500 mg/kg/day), or captopril (5 mg/kg/day) groups. The components of SG extract were analyzed. Results: The analysis of aqueous SG extract was carried out using HPLC-Mass spectroscopy, and phenolic compounds could be identified as predominant components which might be responsible for its antihypertensive effects observed in the LHR model (p < 0.05). Additionally, SG extract also improved vascular responses to acetylcholine and decreased vascular remodeling in LHR (p < 0.05). Enhancements of eNOS expression and plasma nitric oxide metabolite levels, and attenuation of angiotensin converting enzyme (ACE) activity and plasma angiotensin II levels were observed in the LHR group treated with SG. Moreover, SG exhibited strong antioxidant activities by reducing vascular superoxide generation and systemic malondialdehyde in LHRs. Captopril suppressed high blood pressure and alleviated vascular changes and ACE activity in LHRs, similar to those of the SG extract (p < 0.05). Conclusion: Our results suggest that the SG extract exhibited antihypertensive effects, which is relevant to alleviation of vascular dysfunction and vascular remodeling of LHRs. These effects might be mediated by phenolic compounds to inhibit ACE activity and scavenge reactive oxygen species in LHR.
Keywords: Syzygium gratum, hypertensive rats, L-NAME, vascular dysfunction and hypertrophy, renin angiotensin system
1. Introduction
In general, nitric oxide (NO) is synthesized from vascular endothelium to mediate vascular smooth muscle relaxation and vascular protective effects [1]. NO deficiency induced by N-nitro-L-arginine-methyl ester (L-NAME), an L-arginine analogue, can enhance systemic vasoconstriction and arterial hypertension in rats [2]. Furthermore, other cardiovascular complications, such as cardiac dysfunction, heart failure, vascular dysfunction, and hypertrophy in long-term treatment of L-NAME in animal models have been noted [3,4]. Impairment of endothelium-dependent vasodilation in rats induced by L-NAME linked with reductions of eNOS protein expression and plasma nitrate/nitrite concentrations have been shown [5]. Moreover, aortic hypertrophy, including increases in vascular wall thickness, cross-sectional area, and wall-to-lumen ratio occurred after chronic L-NAME administration was shown [6,7]. Renin angiotensin system (RAS) activation characterized by increases in angiotensin converting enzyme activity and systemic angiotensin II (Ang II) concentration has also been observed in hypertensive rats with NO depletion [3,8]. This RAS activation is a consequence of renal vasoconstriction caused by L-NAME [9]. It has been known that Ang II is a potent vasoconstrictor and mediates cardiovascular hypertrophy [10]. Furthermore, Ang II might be a major contributor of oxidative stress in L-NAME hypertension [11].
Previous studies demonstrated that oxidative stress plays an important role in the pathology of hypertension [12,13,14]. In L-NAME hypertensive rats, oxidative stress has been reported to increase lipid and protein peroxidation, plasma malondialdehyde (MDA) levels, and protein carbonyl content [15]. Furthermore, vascular superoxide production is high in L-NAME-treated rats [16]. It is well-established that increases in reactive oxygen species can quench systemic NO bioavailability [17] and subsequently impair vascular function. Therefore, various substances with antioxidant properties could be considered to cause the enhancement of NO bioavailability and the suppression of hypertension.
Syzygium gratum (SG) (S. gratum, synonym: Eugenia grata Wight) of theMyrtaceae family has been known as a local vegetable in South-East Asia. The nutraceutical merit of its leaves has been reported to contain a high content of protein, beta-carotene, and iron. The biological effects of SG extract has been demonstrated in both in vivo and in vitro studies. Kukongviriyapan and coworkers found that SG extract had high free radical scavenging and antioxidant activities and alleviated phenylhydrazine-induced vascular alterations in rats [18]. The SG aqueous extract had relatively high antioxidant activity assayed by ferric-reducing antioxidant power and total phenolic assay and increased the cytoprotective enzyme, heme oxygenase (HO-1) activity, as well as the expression of HO-1 mRNA in C57BL/6J mice [19]. SG extract was speculated to have potential anti-cancer activity, due to its cytotoxic effect on cancer cells in vitro [20]. A recent study showed that phenolic compounds in SG methanolic extract might mediate cytotoxic activity in MCF-7 breast adenocarcinoma and MDA-MB-231 breast cancer cell lines [21]. This current study was designed to investigate the effect of SG aqueous extract on blood pressure, vascular dysfunction, and hypertrophy in hypertensive rats induced by a NOS inhibitor.
2. Materials and Methods
2.1. Animals and Experimental Protocol
The present study was approved by the Animal Ethics Committee of Khon Kaen University, Khon Kaen, Thailand (ACUC-KKU-29/60), and followed the guidelines for the care and use of experimental animals. Experiments were performed with male Sprague-Dawley rats weighing 200–250 g (Nomura Siam International Co., Ltd., Bangkok, Thailand), housed under controlled environmental conditions (25 ± 2 °C, 12 h light/dark cycle) with free access to food and water. Before starting the experiment, all rats were acclimatized for seven days and then were randomly allocated into six experimental groups (n = 8/each) including control, L-NAME, L-NAME+SG extract (100, 300, or 500 mg/kg/day), and L-NAME + 5 mg/kg/day captopril. For five weeks of the study, the control rats were given tap water, while the hypertensive rats were given L-NAME (Sigma-Aldrich, St. Louis, MO, USA, 40 mg/kg/day) in their drinking water to induce hypertension. Various doses of SG extract, vehicle, or captopril were orally administrated every day for the last two weeks of the study period.
2.2. Preparation of SG Extract and Chemicals Analysis of the Extract
The fresh SG leaves were cultivated in local agricultural fields of the Khon Kaen province, Thailand, and the leaves were identified as SG by the same method as previously described by Kukongviriyapan et al., in 2007 [18]. After that, the leaves were weighed, chopped, and boiled in distilled water for 30 min. Thereafter, the extract was dried using a spray-drying machine, which yielded 16.7% SG [19]. Biochemical components of SG extract were analyzed using reversed phase ultra-high-performance liquid chromatography coupled with quadruple time-of-flight mass spectrometry (RP-UHPLC-QTOF-MS).
2.3. Measurement of Systolic Blood Pressure
To monitor changes in blood pressure induced by L-NAME, systolic blood pressures (SBP) and heart rates (HR) were determined in all rats using tail-cuff methods (IITC/Life Science Instrument model 229 and model 179 amplifier (Woodland Hills, CA, USA)) throughout the five weeks of the experimental period.
2.4. Vascular Function Study
At the end of the experiment, the rats were anesthetized and killed by exsanguination. The thoracic aortas were removed and placed in Kreb’s solution. After cleaning off connective tissue, the aortas were cut into rings for tension measurement. To examine the vascular response to various vasoactive agents, the rings were pre-contracted with phenylephrine (10 µM) before acetylcholine (ACh, 0.01 µM–3 µM) or sodium nitroprusside (SNP, 0.01 µM–3 µM) were cumulatively applied with various concentrations in the baths. The results are expressed as percent relaxation from the phenylephrine-induced contraction.
2.5. Measurement of Oxidative Stress Markers and Plasma Nitrate/Nitrite
Superoxide (O2•−) generated in the carotid artery was assessed using the lucigenin-enhanced chemiluminescence method following the previously published method [16]. Plasma NOx levels were determined using an enzymatic conversion method reported by Verdon et al., 1995 [22] with some modification [16] to evaluate plasma NOx levels. Firstly, the plasma protein was filtrated using nanosep (Pall Life sciences, Portsmouth, UK) and the filtrate (80 µL) was collected and mixed with NADPH, G-6-P, G-6-PD and nitrate reductase, and then incubated at 30 °C for 30 min. The reaction mixture was mixed with a Griess solution (100 µL), and kept at room temperature for 15 min. Then, the absorbance at 540 nm was measured using a microplate reader (Tecan GmbH., Groding Australia). The serial dilution of NaNO2 stock solution was carried out to prepare standard solutions reported previously [16].
2.6. Morphometric Measurement
The thoracic aortas at the third to sixth vertebral levels were separated and fixed with 4% paraformaldehyde. The tissues were processed and embedded in paraffin and cut before staining with hematoxylin and eosin. Images were viewed under a DS-2Mv light microscope (Nikon, Tokyo, Japan). Morphometric evaluations and vascular smooth muscle cell numbers (VSMC) were analyzed and counted under Image J morphometric software (National Institutes of Health, Bethesda, MD, USA).
2.7. Measurement of Angiotensin Converting Enzyme (ACE) Activity and Serum Angiotensin II (Ang II) Concentration
Serum ACE activity was determined using a fluorescence assay, as previously described [23], with minor modifications. The reaction mixtures containing the serum mixed with hippuryl-L-histidyl-L-leucine (HHL) in assay buffer were incubated at 37 °C for 30 min. After that, NaOH was added to stop the reaction and the product of the reaction was fluorogenically labeled with o-phthaldialdehyde (OPA). The fluorescence was read at 355 nm excitation; 535 nm emission using a fluorescent plate reader. The activity of ACE was reported as mU/mL. In addition, an Ang II Enzyme immunoassay (EIA) kit (St. Louis, MO, USA) was used to determine the level of serum Ang II.
2.8. eNOS Protein Expression Assay
Thoracic aortas were collected to determine the expression of eNOS protein in the tissues following a previous method [16]. The expression of β-actin was also determined as an internal standard.
2.9. Statistical Analysis
Results were expressed as the mean ± the standard error (SEM) and the statistical analysis was carried out using GraphPad prism 8.3 software (San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by a post-hoc Turkey’s test was performed to test the differences between groups. p < 0.05 was considered to be statistically significant.
3. Results
3.1. Biochemical Components of SG Extract
Components of aqueous SG extract were analyzed using reverse phase ultra-high-performance liquid chromatography coupled with quadruple time-of-flight mass spectrometry (RP-UHPLC-QTOF-MS). A total of 2048 signals were detected in the positive electrospray ionization mode and 374 signals in the negative electrospray ionization mode. Background signals were removed by use of the coefficient of variation of less than 30%, and metabolite identification was then performed. The main compounds in the aqueous extract of SG are listed in Table 1.
Table 1.
Major components of aqueous Syzygium gratum (SG) extract analyzed using RP-UHPLC-QTOF-MS.
| Retention Time | [M+H]+ (m/z) |
Identified Compound | Formula | Class of Phytochemical |
|---|---|---|---|---|
| 10.78 | 274.27 | C16 Sphinganine | C16H35NO2 | Phospholipid |
| 12.52 | 271.10 | Pinostrobin | C16H14O4 | Flavonoids |
| 11.7 | 257.08 | Pinocembrin | C15H12O4 | Flavanone |
| 8.52 | 419.13 | Neoliquiritin | C21H22O9 | Flavonoids |
| 6.65 | 459.09 | Epigallocatechin-3-Gallate | C22H18O11 | Flavonoids |
| 8.51 | 257.08 | Aloe emodin anthrone | C15H12O4 | Anthraquinone |
| 5.63 | 205.10 | d-Tryptophan | C11H12N2O2 | Amino acid |
| 5.91 | 307.08 | Epigallocatechin | C15H14O7 | Flavonoids |
| 7 | 481.10 | Myricetin 3-glucoside | C21H20O13 | Flavonoid-3-o-glycosides |
| 1.35 | 268.10 | Adenosine | C10H13N5O4 | Purine nucleoside base |
| 7.17 | 617.11 | Quercetin 4-6-galloylglucoside | C28H24O16 | Flavonol |
| 7.59 | 319.04 | Myricetin | C15H10O8 | Flavonol |
| 7.37 | 465.10 | Myricitrin | C21H20O12 | Flavonoid glycoside |
| 10.18 | 297.24 | Dimorphecolic acid | C18H32O3 | Endogenous fatty acid |
| 7.91 | 449.11 | Quercitrin | C21H20O11 | Flavonol |
| 8.42 | 273.08 | Naringenin | C15H12O5 | Flavanone |
| 6.64 | 291.09 | Epicatechin | C15H14O6 | Flavonoids |
| 14.75 | 277.18 | Buddledin A | C17H24O3 | Terpenoids |
| 13.32 | 239.13 | 2,2,4,4-Tetramethyl-6-1-oxopropyl-1,3,5-cyclohexanetrione | C13H18O4 | Lipid |
| 7.43 | 443.10 | Catechin 7-O-gallate | C22H18O10 | Flavonoid-7-o-glycosides |
| 7.69 | 265.14 | Abscisic Acid | C15H20O4 | Isoprenoid plant hormone |
| 14.88 | 473.36 | Maslinic Acid | C30H48O4 | Triterpenoids |
| 10.36 | 295.23 | 12,13-Epoxy-9,15-octadecadienoic acid | C18H30O3 | Long-chain fatty acids |
| 8.1 | 229.09 | cis-Resveratrol | C14H12O3 | Polyphenol |
| 14.37 | 478.29 | Lysophosphatidylethanolamine (18:2) | C23H44NO7P | Lipid |
| 5.82 | 655.19 | Rhamnazin 3-sophoroside | C29H34O17 | Flavonoid glycoside |
| 5.19 | 627.15 | 6-Hydroxyluteolin 7-gentiobioside | C27H30O17 | Flavonoid |
| 11.09 | 281.17 | Brefeldin A | C16H24O4 | Lactone |
3.2. SG Extract Alleviated Systolic Blood Pressure and Heart Rates in LHR
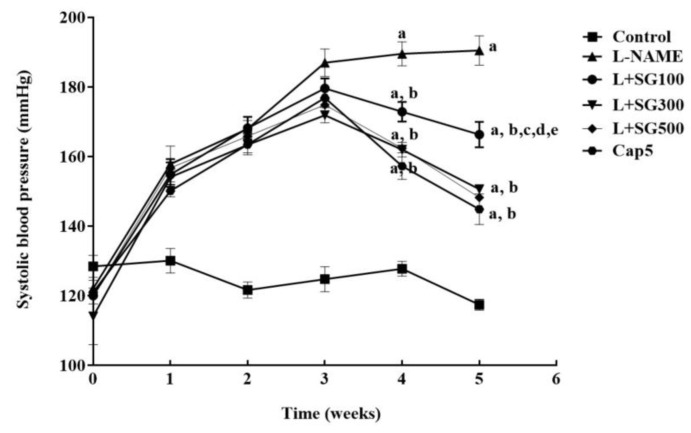
Adding L-NAME in drinking water for five weeks gradually caused the elevation of systolic blood pressure in LHRs compared to control rats (190.49 ± 4.2 vs. 117.42 ± 1.58 mmHg, p < 0.05). Administration of SG extract markedly reduced systolic blood pressure at doses of 100 (166.33 ± 3.68 mmHg), 300 (150.6 ± 0.76 mmHg), and 500 mg/kg/day (148.21 ± 1.11 mmHg) or captopril (144.83 ± 4.38 mmHg) (p < 0.05, Figure 1). In addition, LHR caused a rise from 425.19 ± 4.51 to 549.94 ± 12.38 beats/min (p < 0.05) at week five. SG extract at 100 (481.72 ± 24.76 beats/min), 300 (455.86 ± 1012 beats/min), and 500 mg/kg/day (471.73 ± 13.64 beats/min) and captopril treatment reduced HR in LHR compared to untreated rats at week five (474.75 ± 19.56 beats/min, p < 0.05).
Figure 1.
Changes of systolic blood pressure during five weeks of experiments in all groups of rats. Results are represented as mean ± SEM (n = 6–8). a < 0.05 vs. control group, b < 0.05 vs. L-NAME group, c < 0.05 vs. L + 100 group, d < 0.05 vs. L + 300 group, e < 0.05 vs. L + Cap5 group.
3.3. SG Extract Improved Vascular Responses in Aortic Rings
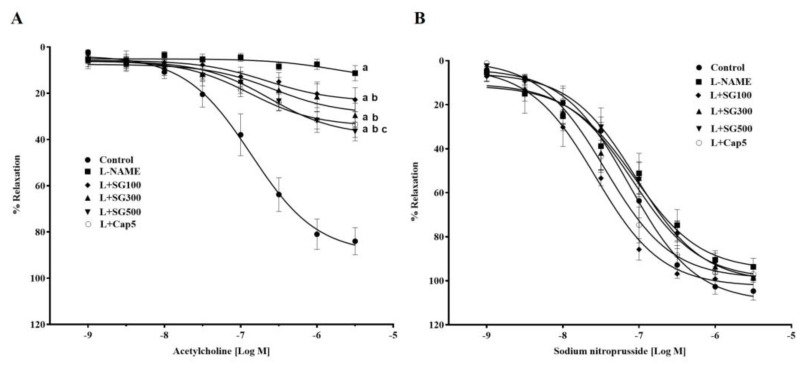
The vasorelaxation response of aortic rings to ACh (0.01–3 mM) was significantly attenuated in LHRs compared to control rats (3µM, 11.26 ± 5.81 vs. 84.01 ± 3.22%) (p < 0.05). The response to ACh was improved with SG 100 (29.47% ± 5.15%), 300 (34.78 ± 3.78%) and 500 mg/kg/day (40.16 ± 5.70%) or captopril supplementation (33.53 ± 5.53%) compared to LHRs p < 0.05) (Figure 2A). The vasorelaxation responses to SNP were almost similar in all groups (Figure 2B).
Figure 2.
Effect of SG extract on vascular responses to exogenous acetylcholine (A), and sodium nitroprusside (B) in the thoracic aortas of all groups. Results are shown as mean ± SEM (n = 6–8/group), a < 0.05 vs. control, b < 0.05 vs. L-NAME, c < 0.05 vs. L + SG100.
3.4. SG Extract Enhanced Expression eNOS Protein and Plasma Nitrate/Nitrite Level
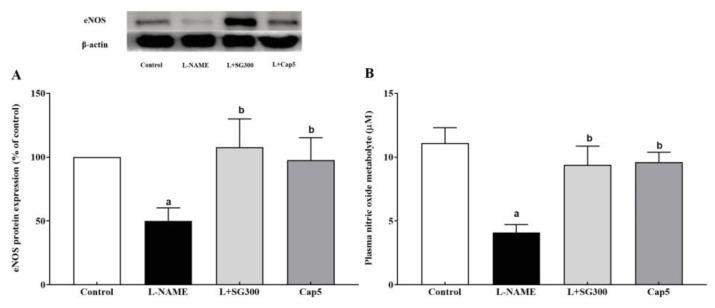
A significant decrease of eNOS protein expression was detected in aortas from LHRs compared to those of control rats (p < 0.05). Treated with SG extract at a dose of 300 mg/kg/day or captopril at a dose of 5 mg/kg/day markedly raised expression of the eNOS protein compared to LHRs (p < 0.05) (Figure 3A). In addition, a low level of plasma NOx was found in LHRs compared to the control rats (4.09 ± 0.63 vs. 11.10 ± 1.20 µM). This reduction was improved by SG extract and captopril supplementation (9.40 ± 1.50 and 9.61 ± 0.76 µM, Figure 3B).
Figure 3.
Effect of SG extract on plasma NOx (A) and eNOS protein expression (B) in the thoracic aorta. Data are expressed as mean ± SEM, (n = 6–8/group), a < 0.05 vs. control group, b < 0.05 vs. L-NAME group.
3.5. SG Extract Suppressed Vascular Remodeling of Thoracic Aorta
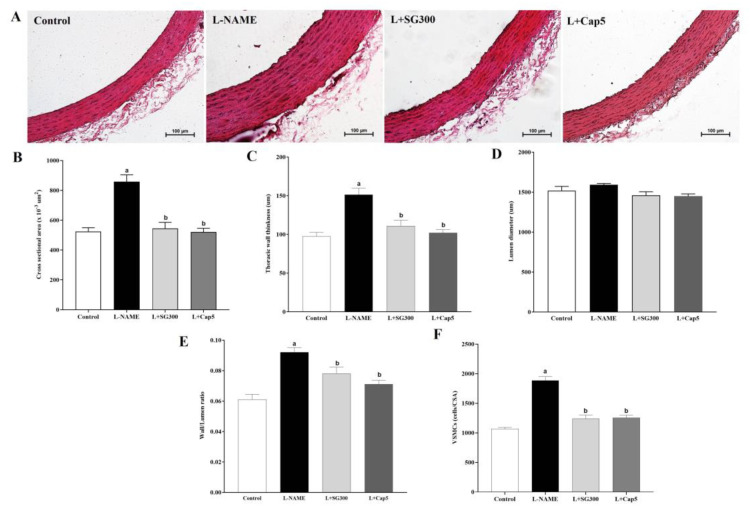
Significant increases in the indices of vascular remodeling, such as the cross-sectional area (CSA), wall thickness, lumen diameter, wall thickness/lumen ratio, and VSMC were observed in thoracic aortas obtained from LHRs (Figure 4A–F, p < 0.05). Interestingly, SG extract or captopril treatment alleviated thickenings of aortic walls, CSA, and VSMC compared with LHRs (p < 0.05).
Figure 4.
Morphological changes in aortic hypertrophy in all groups of rats. Illustrative images of thoracic aortas stained by hematoxylin and eosin, original magnification = X 20 (A) and values of cross-sectional areas (B), thoracic wall thicknesses (C), Luminal diameter (D), wall/lumen ratios (E) and vascular smooth muscle cell numbers (F) (n = 6). Data are mean ± SEM, p < 0.05, a < 0.05 vs. control group, b < 0.05 vs. L-NAME group.
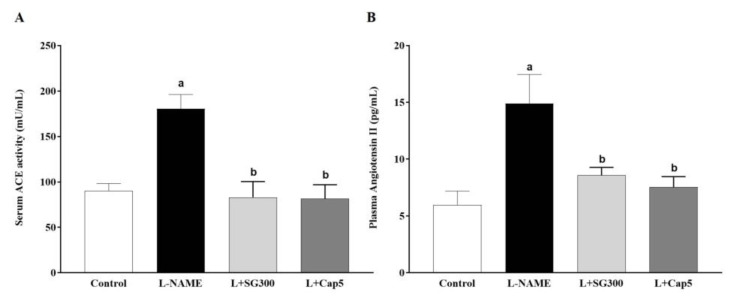
3.6. SG Extract Reduced Plasma Ang II Level and Serum ACE Activity
A high level of serum ACE activity was observed in LHRs compared to those of control rats (180.62 ± 15.45 vs. 90.12 ± 8.15 mU/mL, p < 0.01). Treatment with SG extract or captopril prevented the L-NAME-induced increase in serum ACE activity (82.96 ± 17.43 mU/mL, 81.42 ± 15.46 mU/mL, Figure 5A). Moreover, plasma Ang II was markedly increased in LHRs compared to the control rats (14.89 ± 2.56 vs. 5.99 ± 1.20 pg/mL, p < 0.01). SG extract (300 mg/kg/day, 8.59 ± 0.69 pg/mL) and captopril (7.54 ± 0.92 pg/mL) significantly decreased concentrations of plasma Ang II compared to LHRs (Figure 5B).
Figure 5.
Serum angiotensin converting enzyme (ACE) activity (A) and plasma angiotensin II levels (B) in all rats (n = 6–8). Data are mean ± SEM, p < 0.05. a < 0.05 vs. control rats, b < 0.05 vs. L-NAME rats.
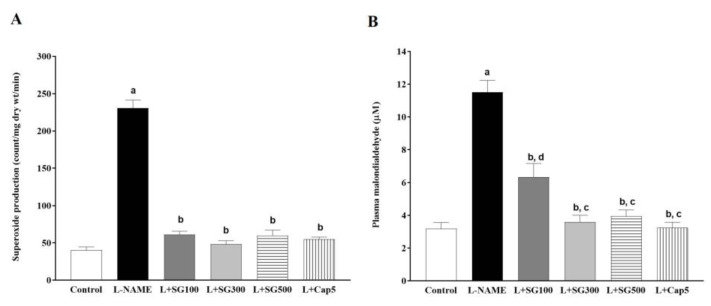
3.7. Effect of SG Extract on Markers of Oxidative Stress
Vascular O2•− generation was high in LHRs compared to control rats (230.59 ± 10.78 vs. 40.25 ± 4.49 count/mg dry wt/min, p < 0.05). SG extract in BW/day (100, 61.29 ± 4.42, 300, 48.49 ± 4.35, 500 mg/kg/day, 59.85 ± 7 count/mg dry wt/min), and captopril at 5 mg/kg/day (54.79 ± 3.00 count/mg dry wt/min) significantly decreased vascular O2•− generation compared to LHRs, p < 0.05) (Figure 6A). Subsequently, plasma MDA was raised in LHRs compared with the control rats (11.50 ± 0.76 vs. 3.18 ± 0.38 µM, p < 0.05). Interestingly, SG extract or captopril restored the L-NAME-induced high levels of plasma MDA compared to LHRs (6.33 ± 0.82, 3.59 ± 0.41, 3.96 ± 0.36 and 3.24 ± 0.32 µM, p < 0.05, Figure 6B).
Figure 6.
Effect of SG extract on vascular superoxide generation (A), and levels of plasma malondialdehyde (MDA) (B) in rats. Data are expressed as mean ± SEM, (n = 6–8/group), p < 0.05, a < 0.05 vs. control group, b < 0.05 vs. L-NAME group, c < 0.05 vs. L + SG100.
4. Discussion
This study suggests that the major components of SG extract might be phenolic compounds. SG extract caused the reduction of blood pressure in a dose-dependent manner in the hypertensive rat model produced by chronic L-NAME administration. Endothelial dysfunction characterized by a decrease in vascular response to Ach and aortic hypertrophy were observed in aortic rings prepared from hypertensive rats. These vascular alterations were attenuated by SG extract in LHRs. SG extract also increased eNOS protein expression and plasma NOx in LHRs. RAS activation, including increases in serum ACE activity and plasma Ang II in LHRs, was suppressed by the SG extract. SG also exhibited an antioxidant property by reducing vascular superoxide generation and lipid peroxidation products, plasma MDA in LHRs. Captopril was used as a positive control, and it exerted antihypertensive effects and restored aortic dysfunction and hypertrophy relevant to reducing RAS activation and oxidative stress in LHRs.
Several biochemical compounds were identified in the SG extract, and most of them were phenolic compounds. Phenolic compounds have been known as secondary metabolites isolated from most food plants [24]. There are several subgroups of phenolic compounds characterized by their chemical structures, such as phenolic acids, flavonoids, tannins, coumarins, lignans, quinones, stilbens, and curcuminoids [25,26]. In this study, flavonoid bioactive substances listed in Table 1 were shown to be abundant in SG extract. Flavonoids, a large class of polyphenols, have contributed to health maintenance, since they exert antioxidant, inflammatory, anti-hypertensive, and anti-cancer activities [27,28]. Thus, the biological effects of SG extract on L-NAME-induced high blood pressure in rats will be discussed further.
LHR developed high blood pressure and increased HR that were associated with impairment of vascular responses to ACh and aortic hypertrophy. Blood pressure is influenced by two main factors—cardiac output, and vascular resistance. In LHR, reduction of NO generation, resulting in alterations of vascular endothelial function and an increase in peripheral vascular resistance have been established [29,30]. Sympathetic overactivity induced by chronic inhibition of NO synthesis by L-NAME has been suggested to raise heart rate in this animal model [31,32]. Aortic hypertrophy is caused by the vascular adaptive response to long-term hemodynamic changes, especially high blood pressure [33]. Furthermore, lacking NO has been proposed to promote vascular structural changes in hypertension [34]. Activation of RAS, such as high levels of serum Ang II, renin, ACE activity, and AT1R expression in LHR has been observed, and has contributed to hypertension and vascular remodeling in this animal model [35,36]. The current study findings showed that endothelial dysfunction and aortic hypertrophy were the consequence of decreased eNOS expression and plasma NOx, as well as increased serum ACE activity and ang II in LHR. Furthermore, enhancement of superoxide generation from eNOS uncoupling induced by L-NAME was revealed, and this ROS subsequently quenched NO to become ONOO-, a powerful oxidizing agent.
SG extract decreased systolic blood pressure and HR associated with improvement of vascular alterations in LHR. It restored expression of protein, eNOS, and NO metabolites in LHRs. Additionally, SG extract also suppressed the elevation of ACE activity and serum Ang II, as well as oxidative stress markers in LHRs. With the suppression of RAS activation, Ang II levels observed in this study might have been due to the ACE inhibitory activity of the SG extract. The ACE inhibitor property of SG extract is likely to be mediated by its main components, phenolic compounds, as mentioned above. There is a study to demonstrate the inhibitory effect of phenolic compounds on ACE activity suggesting that the carboxylate and hydroxyl groups can bind to the zinc ion at the active site of ACE and stop the enzyme reaction [37]. The bioactive molecules in the SG extract that were involved in the antioxidant effects might have been phenolic compounds, especially flavonoids, such as pinostrobin, pinocembrin, epigallocatechin-3-Gallate, neoliquiritin, and epigallocatechin. It has been known that flavonoids have the ability to reduce free radical formation and to scavenge free radicals [38]. The current results were accompanied by a previous study demonstrating that SG extract has potent antioxidant capacity using the FRAP and DPPH assays [19]. This antioxidant property of SG extract can prevent the reaction of O2•− and NO and raise systemic NO levels to reduce vascular resistance and blood pressure. Captopril produced an antihypertensive effect via alleviation of vascular dysfunction and remodeling in LHRs. Additionally, captopril also reduced oxidative stress and RAS activation in LHRs. Therefore, it is possible that the SG extract had an antihypertensive effect, probably through the same mechanism as captopril.
5. Conclusions
SG extract mainly contained phenolic compounds. SG extract had anti-hypertensive and vascular effects that were allied with restorations of eNOS protein expression and plasma nitrate/nitrite, RAS activation, and oxidative status in LHR.
Acknowledgments
We would like to acknowledge James A. Will, for editing the MS via Publication Clinic KKU, Thailand.
Author Contributions
P.P. performed the experiments and the data analysis, and wrote the manuscript; S.M., C.S., J.P. and S.B. performed the experiments and P.M. designed and supervised the study and checked the final manuscript. All authors have read and agreed to the final version of this manuscript.
Funding
This research was supported by Invitation Research Fund [IN60317], Faculty of Medicine, Khon Kaen University, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Loscalzo J., Jin R.C. Vascular nitric oxide: Formation and function. J. Blood Med. 2010;1:147–162. doi: 10.2147/JBM.S7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribeiro M.O., Antunes E., De Nucci G., Lovisolo S.M., Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20:298–303. doi: 10.1161/01.HYP.20.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Wunpathe C., Maneesai P., Rattanakanokchai S., Bunbupha S., Kukongviriyapan U., Tong-Un T., Pakdeechote P. Tangeretin mitigates l-NAME-induced ventricular dysfunction and remodeling through the AT1R/pERK1/2/pJNK signaling pathway in rats. Food Funct. 2020;11:1322–1333. doi: 10.1039/C9FO02365H. [DOI] [PubMed] [Google Scholar]
- 4.Gao Y., Wang Z., Zhang Y., Liu Y., Wang S., Sun W., Guo J., Yu C., Wang Y., Kong W., et al. Naringenin inhibits N(G)-nitro-L-arginine methyl ester-induced hypertensive left ventricular hypertrophy by decreasing angiotensin-converting enzyme 1 expression. Exp. Ther. Med. 2018;16:867–873. doi: 10.3892/etm.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkban T., Boonprom P., Bunbupha S., Welbat J.U., Kukongviriyapan U., Kukongviriyapan V., Pakdeechote P., Prachaney P. Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of eNOS and p47phox Expression in Rats. Nutrition. 2015;7:5265–5280. doi: 10.3390/nu7075222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira L.M., Bezerra D.G., Mandarim-de-Lacerda C.A. Aortic wall remodeling in rats with nitric oxide deficiency treated by enalapril or verapamil. Pathol. Res. Pr. 2004;200:211–217. doi: 10.1016/j.prp.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Potue P., Wunpathe C., Maneesai P., Kukongviriyapan U., Prachaney P., Pakdeechote P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019;10:1880–1892. doi: 10.1039/C8FO02408A. [DOI] [PubMed] [Google Scholar]
- 8.Usui M., Egashira K., Kitamoto S., Koyanagi M., Katoh M., Kataoka C., Shimokawa H., Takeshita A. Pathogenic role of oxidative stress in vascular angiotensin-converting enzyme activation in long-term blockade of nitric oxide synthesis in rats. Hypertension. 1999;34:546–551. doi: 10.1161/01.HYP.34.4.546. [DOI] [PubMed] [Google Scholar]
- 9.Zanchi A., Schaad N.C., Osterheld M.C., Grouzmann E., Nussberger J., Brunner H.R., Waeber B. Effects of chronic NO synthase inhibition in rats on renin-angiotensin system and sympathetic nervous system. Am. J. Physiol. Circ. Physiol. 1995;268:H2267–H2273. doi: 10.1152/ajpheart.1995.268.6.H2267. [DOI] [PubMed] [Google Scholar]
- 10.Fyhrquist F., Metsärinne K., Tikkanen I. Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J. Hum. Hypertens. 1995;9(Suppl. 5):19–24. [PubMed] [Google Scholar]
- 11.Rincon J., Correia D., Arcaya J., Finol E., Fernández A., Perez M., Yaguas K., Talavera E., Chávez M., Summer R., et al. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015;124:81–90. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touyz R.M. Reactive Oxygen Species, Vascular Oxidative Stress, and Redox Signaling in Hypertension. Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigo R., Gonzalez J., Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011;34:431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri N.D., Wang X.Q., Oveisi F., Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.HYP.36.1.142. [DOI] [PubMed] [Google Scholar]
- 15.Jan-On G., Sangartit W., Pakdeechote P., Kukongviriyapan V., Sattayasai J., Senaphan K., Kukongviriyapan U. Virgin rice bran oil alleviates hypertension through the upregulation of eNOS and reduction of oxidative stress and inflammation in L-NAME–induced hypertensive rats. Nutrition. 2020;69:110575. doi: 10.1016/j.nut.2019.110575. [DOI] [PubMed] [Google Scholar]
- 16.Bunbupha S., Pakdeechote P., Kukongviriyapan U., Prachaney P., Kukongviriyapan V. Asiatic Acid Reduces Blood Pressure by Enhancing Nitric Oxide Bioavailability with Modulation of eNOS and p47phoxExpression inl-NAME-induced Hypertensive Rats. Phytotherapy Res. 2014;28:1506–1512. doi: 10.1002/ptr.5156. [DOI] [PubMed] [Google Scholar]
- 17.Guzik T.J., West N.E., Pillai R., Taggart D.P., Channon K.M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.HYP.0000018041.48432.B5. [DOI] [PubMed] [Google Scholar]
- 18.Kukongviriyapan U., Luangaram S., Leekhaosoong K., Kukongviriyapan V., Preeprame S. Antioxidant and vascular protective activities of Cratoxylum formosum, Syzygium gratum and Limnophila aromatica. Biol. Pharm. Bull. 2007;30:661–666. doi: 10.1248/bpb.30.661. [DOI] [PubMed] [Google Scholar]
- 19.Senggunprai L., Kukongviriyapan V., Prawan A., Kukongviriyapan U. Consumption of Syzygium gratum Promotes the Antioxidant Defense System in Mice. Plant Foods Hum. Nutr. 2010;65:403–409. doi: 10.1007/s11130-010-0200-6. [DOI] [PubMed] [Google Scholar]
- 20.Stewart P., Boonsiri P., Puthong S., Rojpibulstit P. Antioxidant activity and ultrastructural changes in gastric cancer cell lines induced by Northeastern Thai edible folk plant extracts. BMC Complement. Altern. Med. 2013;13:60. doi: 10.1186/1472-6882-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocchetti G., Lucini L., Ahmed S.R., Saber F.R. In vitro cytotoxic activity of six Syzygium leaf extracts as related to their phenolic profiles: An untargeted UHPLC-QTOF-MS approach. Food Res. Int. 2019;126:108715. doi: 10.1016/j.foodres.2019.108715. [DOI] [PubMed] [Google Scholar]
- 22.Verdon C., Burton B., Prior R. Sample Pretreatment with Nitrate Reductase and Glucose-6-Phosphate Dehydrogenase Quantitatively Reduces Nitrate While Avoiding Interference by NADP+ When the Griess Reaction Is Used to Assay for Nitrite. Anal. Biochem. 1995;224:502–508. doi: 10.1006/abio.1995.1079. [DOI] [PubMed] [Google Scholar]
- 23.Friedland J., Silverstein E. A Sensitive Fluorimetric Assay for Serum Angiotensin-con venrting Enzyme. Am. J. Clin. Pathol. 1976;66:416–424. doi: 10.1093/ajcp/66.2.416. [DOI] [PubMed] [Google Scholar]
- 24.Ho C.-T. Phenolic Compounds in Food and Their Effects on Health I. ACS Publications; Wahington, DC, USA: 1992. Phenolic compounds in food: An overview. [Google Scholar]
- 25.Cai Y.-Z., Sun M., Xing J., Luo Q., Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Anantharaju P.G., Gowda P.C., Vimalambike M.G., Madhunapantula S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016;15:99. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos M.C., Toson N.S., Pimentel M.C., Bordignon S.A., Mendez A.S., Henriques A.T. Polyphenols composition from leaves of Cuphea spp. and inhibitor potential, in vitro, of angiotensin I-converting enzyme (ACE) J. Ethnopharmacol. 2020;255:112781. doi: 10.1016/j.jep.2020.112781. [DOI] [PubMed] [Google Scholar]
- 28.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicine. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner S.M., Compton A.M., Kemp P.A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-l-arginine methyl ester in conscious, Long Evans rats. Br. J. Pharmacol. 1990;101:625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakdeechote P., Prachaney P., Berkban W., Kukongviriyapan U., Kukongviriyapan V., Khrisanapant W., Phirawatthakul Y. Vascular and Antioxidant Effects of an Aqueous Mentha cordifolia Extract in Experimental NG-Nitro-L-arginine Methyl Ester-Induced Hypertension. Z. Nat. C. 2014;69:35–45. doi: 10.5560/znc.2012-0212. [DOI] [PubMed] [Google Scholar]
- 31.Cunha R.S., Cabral A.M., Vasquez E.C. Evidence that the Autonomic Nervous System Plays a Major Role in the L-NAME—Induced Hypertension in Conscious Rats. Am. J. Hypertens. 1993;6:806–809. doi: 10.1093/ajh/6.9.806. [DOI] [PubMed] [Google Scholar]
- 32.Souza H.C.D., Ballejo G., Salgado M.C.O., Da Silva V.J.D., Salgado H.C. Cardiac sympathetic overactivity and decreased baroreflex sensitivity in L-NAME hypertensive rats. Am. J. Physiol. Circ. Physiol. 2001;280:H844–H850. doi: 10.1152/ajpheart.2001.280.2.H844. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi K., Naiki T. Adaptation and remodeling of vascular wall; biomechanical response to hypertension. J. Mech. Behav. Biomed. Mater. 2009;2:3–19. doi: 10.1016/j.jmbbm.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Rudic R.D., Shesely E.G., Maeda N., Smithies O., Segal S.S., Sessa W.C. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Investig. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maneesai P., Prasarttong P., Bunbupha S., Kukongviriyapan U., Kukongviriyapan V., Tangsucharit P., Prachaney P., Pakdeechote P. Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in l-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1R Expression. Nutrition. 2016;8:122. doi: 10.3390/nu8030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okazaki H., Minamino T., Tsukamoto O., Kim J., Okada K.-I., Myoishi M., Wakeno M., Takashima S., Mochizuki N., Kitakaze M. Angiotensin II Type 1 Receptor Blocker Prevents Atrial Structural Remodeling in Rats with Hypertension Induced by Chronic Nitric Oxide Inhibition. Hypertens. Res. 2006;29:277–284. doi: 10.1291/hypres.29.277. [DOI] [PubMed] [Google Scholar]
- 37.Al Shukor N., Van Camp J., Gonzales G.B., Staljanssens D., Struijs K., Zotti M.J., Raes K., Smagghe G. Angiotensin-Converting Enzyme Inhibitory Effects by Plant Phenolic Compounds: A Study of Structure Activity Relationships. J. Agric. Food Chem. 2013;61:11832–11839. doi: 10.1021/jf404641v. [DOI] [PubMed] [Google Scholar]
- 38.Pietta P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]