Abstract
Simple Summary
Typhlocybinae currently is the second largest membracoid subfamily distributed worldwide and includes many important agricultural pests. The monophyly of Typhlocybinae has been supported by several recent analyses, but the relationships of its included tribes remain largely unexplored, particularly that of the phylogenetic relationship of Zyginellini and Typhlocybini. This study presented the annotated complete mitochondrial genome sequences of two zyginelline species, Limassolla sp. and Parazyginella tiani Gao, Huang and Zhang, 2012, and a comparative analysis of mitochondrial genomes within the Typhlocybini and Zyginellini. Typhlocybinae mitogenomes are highly conservative in overall organization, as have been found in some other Cicadellidae. The only unusual feature was found in the secondary structure of tRNAs, with the acceptor stem of trnR comprising only 5 or 6 bp in some species. This unusual feature was reported for the first time in Typhlocybinae. Phylogenetic analyses showed that the monophyly of tribe Zyginellini was not supported and should be treated as a synonym of Typhlocybini. Nevertheless, a broader analysis with a much larger sample of taxa is needed to confirm the present results.
Abstract
To explore the characteristics of mitogenomes and reveal phylogenetic relationships of the tribes of Zyginellini and Typhlocybini in Typhlocybinae, mitogenomes of two species of the Zyginellini, Parazyginella tiani and Limassolla sp., were sequenced. Mitogenomes of both species contain 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), two ribosomal RNA genes (rRNAs) and a large non-coding region (A + T-rich region). These characteristics are similar to other Membracoidea mitogenomes. All PCGs initiate with the standard start codon of ATN and terminate with the complete stop codon of TAA/G or with an incomplete T codon. All tRNAs have the typical clover-leaf structure, except trnS1 which has a reduced DHU arm and the acceptor stem of trnR is 5 or 6 bp in some species, an unusual feature here reported for the first time in Typhlocybinae. The A + T-rich region is highly variable in length and in numbers of tandem repeats present. Our analyses indicate that nad6 and atp6 exhibit higher evolutionary rates compared to other PCGs. Phylogenetic analyses by both maximum likelihood and Bayesian methods based on 13 protein-coding genes of 12 species of Typhlocybinae suggest that Zyginellini are paraphyletic with respect to Typhlocybini.
Keywords: mitochondrial genome, Typhlocybinae, Typhlocybini and Zyginellini, phylogenetic analysis
1. Introduction
Membracoidea is the largest hemipteroid superfamily, comprising nearly one-third of known hemipteran species, with approximately 24,000 valid species [1,2]. The leafhopper family Cicadellidae is among the 10 largest families of insects, and Typhlocybinae currently ranks as the second largest membracoid subfamily, with approximately 6000 described species [3,4,5]. The subfamily is distributed worldwide and includes many important agricultural pests such as the potato leafhopper (Empoasca fabae (Harris, 1841)), white apple leafhopper (Zonocyba pomaria (McAtee, 1926)), cotton leafhopper (Amrasca biguttula (Ishida, 1913)) and kaki leafhoppers (Limassolla hebeiensis Cai, Liang and Wang, 1992) [6,7,8].
The tribes of Typhlocybinae are most readily distinguished based on the wing venation. Although Oman et al. (1990) included ten valid tribes in Typhlocybinae [9], most recent eastern hemisphere workers recognize six tribes proposed by Dworakowska (1979): Alebrini, Dikraneurini, Empoascini, Erythroneurini, Typhlocybini and Zyginellini [10]. The monophyly of Typhlocybinae has been supported by several recent analyses [4,11,12,13], but the status and relationships of its included tribes remain largely unexplored, particularly that of the phylogenetic status of the tribe Zyginellini. Zyginellini and Typhlocybini were traditionally distinguished based on differences in hind wing venation [14] but more recently the former was considered to be a junior synonym of the latter [5]. The phylogenetic status and relationships of these two tribes have not yet been adequately tested by previous analyses of DNA sequence data and morphological characteristics [4,11,12]. Thus, additional data, such as complete mitogenomes, should be used to further investigate their phylogenetic relationships.
The insect mitogenome is typically a closed circular double-stranded DNA molecule, usually 15–18 kb in length and encoding 37 genes, including 13 protein-coding genes (PCG), 22 transfer RNA genes (tRNA), 2 ribosomal RNA genes (rRNA) and a control region (A + T-rich region) [15,16,17]. Because of maternal inheritance, absence of introns, high evolutionary rate, and rare recombination, the mitochondrial genome is considered ideal for phylogenetic and evolutionary analysis [18,19,20,21,22,23].
Currently, there are 14 complete or nearly complete mitogenomes of Typhlocybinae in GenBank [24,25,26,27,28,29,30,31,32,33,34,35]. To facilitate comparative studies and phylogenetic analyses, we sequenced the complete mitogenomes and provided functional annotations of two species in Zyginellini. Genomic structure, base composition, substitutional and evolutionary rates of six species of Typhlocybini and Zyginellini were comparatively analyzed. Combining mitogenome sequences of Typhlocybini and Zyginellini with previously available mitogenomes of other tribes of Typhlocybinae, i.e., Empoascini and Erythroneurini, we reconstructed phylogenetic relationships among major lineages of this subfamily to test the monophyly of Zyginellini and Typhlocybini.
2. Materials and Methods
2.1. Sample Preparation and DNA Extraction
Specimens of Limassolla sp. were collected at Hejiaping Village, Chongqing City, China and Parazyginella tiani Gao, Huang and Zhang, 2012 specimens were collected at Gutian Mountain, Quzhou City, Zhejiang Province, China. All specimens were preserved in 100% ethyl alcohol and stored at −20 °C in the Entomological Museum of the Northwest A&F University, Yangling, Shaanxi Province, China. Identification of adult specimens was based on the venation of the hind wing and male genitalia characteristics. Total DNA was extracted from muscle tissues of the thorax using the EasyPureR Genomic DNA Kit following the manufacturer’s instructions (TransGen, Beijing, China).
2.2. Sequence Analysis and Gene Annotation
Whole mitochondrial genome sequences of the 2 species, Limassolla sp. and Parazyginella tiani, were generated using an Illumina HiSeq platform with paired reads of 2 × 150 bp by the Biomarker Technologies Corporation (Beijing, China). First, the raw paired reads were quality-trimmed and assembled using Geneious 10.0.5 (Biomatters, Auckland, New Zealand) with default parameters [36] using the previously published mitochondrial genomes of L. lingchuanensis Chou and Zhang, 1985 and P. luodianensis Yuan and Song, 2019 as bait sequences for Limassolla sp. and P. tiani, respectively [25,26]. The genomes were annotated using Geneious 8.1.3 and L. lingchuanensis and P. luodianensis as references, respectively [25,26]. A total of 13 PCGs were identified as open reading frames based on the invertebrate mitochondrial genetic code; rRNA genes and control regions were identified by alignment with homologous genes from other Typhlocybinae species; tRNA genes were identified using the MITOS Web Server (http://mitos.bioinf.uni-leipzig.de/index.py) [37] and secondary structures were plotted with Adobe Illustrator CS5. Finally, a circular mitogenome map was drawn using CGView (http://stothard.afns.ualberta.ca/cgview_server/) [38]. Nucleotide composition, codon usage, composition skewness and relative synonymous codon usage (RSCU) were analyzed using PhyloSuite v 1.1.15 [39]. Tandem repeats in the A + T-rich region were predicted using the Tandem Repeats Finder program (http://tandem.bu.edu/trf/trf.html) [40]. The nucleotide diversity (Pi) and sliding window analysis (a sliding window of 200 bp and a step size of 20 bp) based on 13 aligned protein-coding genes (PCGs) were performed using DnaSP v 5.0. [41]. Kimura-2-parameter genetic distances were calculated using MEGA 7.0 [42]. Non-synonymous (dN) /synonymous (dS) mutation rate ratios among the 13 PCGs were calculated with DnaSP v 5.0. [41].
2.3. Phylogenetic Analysis
Mitochondrial genomes of 12 species of Typhlocybinae were selected, including 2 species from Empoascini, 4 species from Erythroneurini, 2 species from Typhlocybini and 4 species from Zyginellini. Two species belonging to different cicadellid subfamilies were chosen as outgroups: Scaphoideus varius Vilbaste, 1968 (Deltocephalinae: Scaphoideini) and Taharana fasciana Li, 1991 (Coelidiinae: Coelidiini) (Table 1) [43,44]. All previously available mitochondrial genomes in this study were acquired from GenBank. Statistical phylogenetic analysis of mitogenomes was conducted using PhyloSuite v 1.1.15 [39]. The nucleotide sequences of all 13 PCGs and 2 rRNA genes were used in our analyses. PCGs were aligned using the MAFFT v 7.313 plugin in PhyloSuite v 1.1.15 [39]. Nucleotide sequences were aligned using the G-INS-i (accurate) strategy and codon alignment mode; the two rRNAs were aligned with the Q-INS-i strategy [45]. Gaps and ambiguous sites were removed using Gblocks v 0.91b [46], then the results were concatenated in PhyloSuite v 1.1.15 [39]. PartitionFinder2 was used to select the optimal partition schemes and substitution models [47]; the results are shown in Supplementary Tables S2 and S3. We used the “greedy” algorithm with branch lengths estimated as “linked” and the Bayesian information criterion (BIC).
Table 1.
Summary of mitogenomic sequence information used in the present study.
| Subfamily | Tribe | Species | Accession Number | Reference |
|---|---|---|---|---|
| Coelidiinae | Coelidiini | Taharana fasciana | NC_036015 | [44] |
| Deltocephalinae | Scaphoideini | Scaphoideus varius | KY817245 | [43] |
| Typhlocybinae | Empoascini | Ghauriana sinensis | MN699874 | [28] |
| Empoasca onukii | NC_037210 | [32] | ||
| Erythroneurini | Mitjaevia protuberanta | NC_047465 | [30] | |
| Illinigina sp. | KY039129 | [27] | ||
| Empoascanara dwalata | MT350235 | [33] | ||
| Empoascanara sipra | MN604278 | [26] | ||
| Typhlocybini | Typhlocyba sp. | KY039138 | [27] | |
| Bolanusoides shaanxiensis | MN661136 | Unpublished | ||
| Zyginellini | Limassolla lingchuanensis | MN605256 | [24] | |
| Paraahimia luodianensis | NC_047464 | [25] | ||
| Limassolla sp. | MT683892 | This study | ||
| Parazyginella tiani | MT683891 | This study |
Alignments of individual genes were concatenated to generate 4 data sets: (1) the PCG12 matrix, including only the first and second codon positions of protein-coding genes; (2) the PCG12R matrix, including the first and second codon positions of protein-coding genes and the 2 rRNA genes; (3) the PCG123 matrix, including all 3 codon positions of protein-coding genes; (4) the PCG123R matrix, including all 3 codon positions of protein-coding genes and the 2 rRNA genes.
Phylogenetic trees were constructed using the IQ-TREE web server [48] and MrBayes 3.2.6 [49] based on maximum likelihood (ML) and Bayesian inference (BI), respectively. The ML tree was constructed with the ML + rapid bootstrap (BS) algorithm with 1000 replicates. For the Bayesian analysis, the default settings were used with 5 × 106 MCMC generations after reaching stationarity (average standard deviation of split frequencies <0.01), with estimated sample size >200, and potential scale reduction factor ≈1 [50].
3. Results and Discussion
3.1. Genome Organization and Base Composition
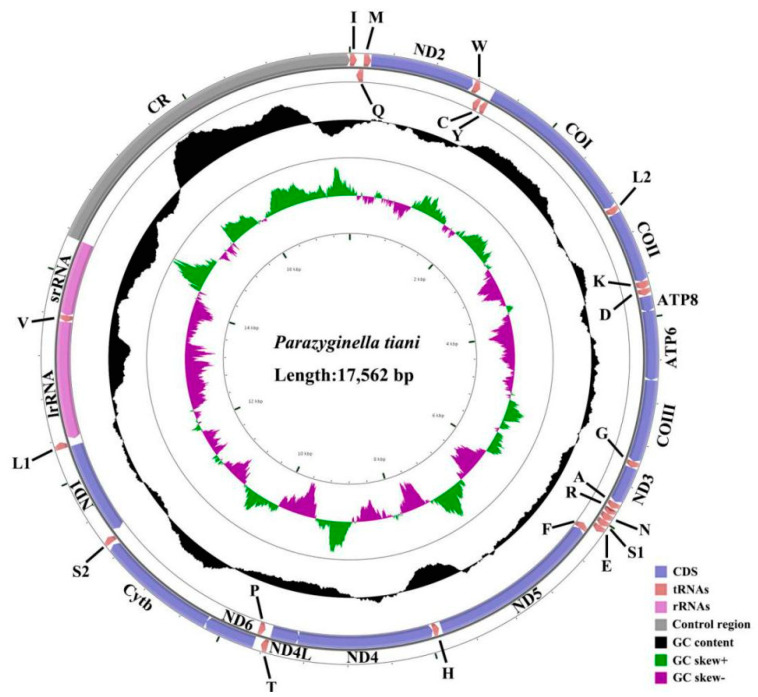
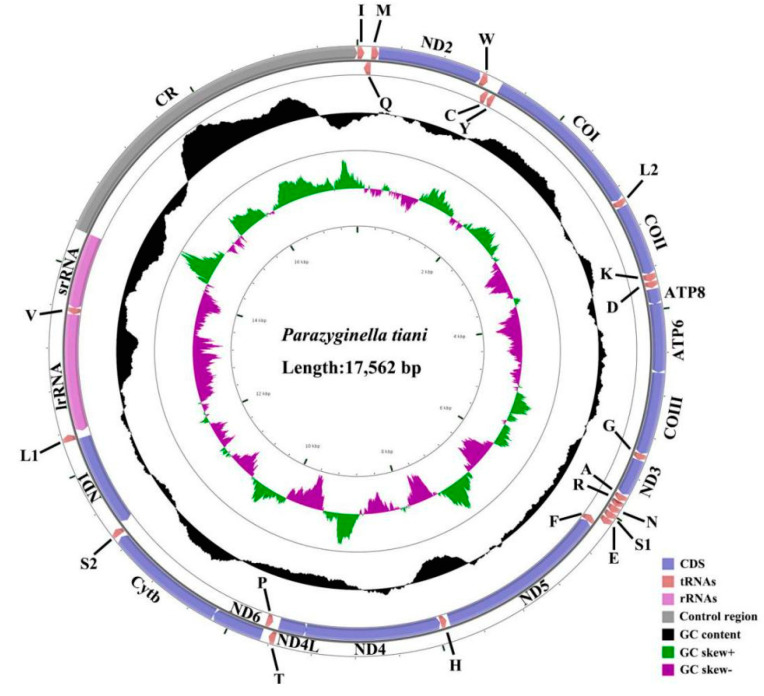
The complete mitogenome sequences of P. tiani and Limassolla sp. were 17,562 bp and 17,053 bp in length, respectively (Figure 1 and Figure 2). Known mitogenomes of Typhlocybinae range from 14,803 bp (Illinigina sp.) [27] to 16,497 bp (P. luodianensis) [25]. Length variation of Typhlocybinae mitogenomes is mainly due to variation in the size of the A + T-rich region. Each mitogenome includes the 37 standard mitochondrial genes; gene arrangement is the same as that of the hypothetical ancestral insect’s mitogenome in order and direction [51] (Figure 1 and Figure 2). Among the 37 mitochondrial genes, 23 genes (9 PCGs and 14 tRNAs) are transcribed from the majority strand (J-strand) and the remaining genes (four PCGs, eight tRNAs, and two rRNAs) are located on the minority strand (N-strand) (Table S1). The base composition of P. tiani is A = 42.1%, T = 34.3%, C = 13.2%, G = 10.4% and that of Limassolla sp. is A = 41.7%, T = 33.3%, C = 14.0%, G = 11.0%. The A/T nucleotide composition is 76.4% in P. tiani and 75.0% in Limassolla sp., thus exhibiting a strong A/T bias. The two species in this study have a positive AT skew and a negative GC as in the other four previously sequenced species of Typhlocybini and Zyginellini (Table 2 and Table 3).
Figure 1.
Organization of the complete mitogenome of P. tiani.
Figure 2.
Organization of the complete mitogenome of Limassolla sp.
Table 2.
Nucleotide composition and skewness of mitogenomes of P. tiani and Limassolla sp.
| Regions | Size (bp) | T(U) | C | A | G | AT (%) | GC (%) | AT Skew | GC Skew |
|---|---|---|---|---|---|---|---|---|---|
| Full genome | 17,562/17,053 | 34.3/33.3 | 13.2/14.0 | 42.1/41.7 | 10.4/11.0 | 76.4/75.0 | 23.6/25.0 | 0.103/0.112 | −0.117/−0.119 |
| PCGs | 10,938/10,926 | 42.9/41.5 | 12.4/12.9 | 33.3/33.3 | 11.4/12.3 | 76.2/74.8 | 23.8/25.2 | −0.126/−0.110 | −0.041/−0.025 |
| 1st codon position | 3646/3642 | 36.9/35.1 | 13.4/11.5 | 36.3/36.6 | 15.7/16.8 | 73.2/71.7 | 26.8/28.3 | −0.008/0.022 | 0.172/0.187 |
| 2nd codon position | 3646/3642 | 48.4/48.0 | 14.4/17.6 | 20.4/21.1 | 13.7/13.3 | 68.8/69.1 | 31.3/30.9 | −0.407/−0.389 | −0.123/−0.137 |
| 3rd codon position | 3646/3642 | 43.4/41.4 | 15.4/9.7 | 43.3/42.1 | 4.8/6.7 | 86.7/83.5 | 13.3/16.4 | −0.002/0.008 | −0.278/−0.182 |
| tRNAs | 1422/1432 | 36.6/40.2 | 16.4/8.4 | 40.6/39.7 | 12.2/11.8 | 77.2/79.9 | 22.7/20.2 | 0.052/0.006 | 0.077/0.170 |
| rRNAs | 1892/1976 | 47.1/46.1 | 17.4/8.5 | 35.6/32.4 | 10.8/13.0 | 82.7/78.5 | 17.4/21.5 | −0.139/−0.173 | 0.244/0.209 |
| A + T rich-region | 3230/2695 | 38.6/35.0 | 18.4/14.7 | 34.0/36.3 | 13.4/14.0 | 72.6/71.3 | 27.4/28.7 | −0.063/0.019 | −0.020/−0.026 |
Table 3.
Nucleotide composition of the Typhlocybini and Zyginellini mitochondrial genomes.
| Species | Whole Genome | AT Skew | GC Skew | PCGs | tRNAs | rRNAs | A + T-Rich Region | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (bp) | AT (%) | Size (bp) | AT (%) | Size (bp) | AT (%) | Size (bp) | AT (%) | Size (bp) | AT (%) | |||
| B. | 15,274 | 78.9 | 0.158 | −0.135 | 10,917 | 77.2 | 1469 | 79.7 | 1963 | 83.6 | 912 | 88.7 |
| T. | 15,223 | 77.1 | 0.138 | −0.138 | 10,950 | 75.2 | 1436 | 77.9 | 1866 | 82.3 | 945 | 85.9 |
| L1. | 15,716 | 78.8 | 0.096 | −0.098 | 10,932 | 76.3 | 1426 | 80.1 | 1911 | 83.1 | 1416 | 91.4 |
| P1. | 16,497 | 80.0 | 0.155 | −0.162 | 10,962 | 77.7 | 1427 | 79.0 | 1898 | 83.7 | 2193 | 88.4 |
| L2. | 17,053 | 75.0 | 0.112 | −0.119 | 10,926 | 74.8 | 1432 | 79.9 | 1976 | 78.5 | 2695 | 71.3 |
| P2. | 17,562 | 76.4 | 0.103 | −0.117 | 10,938 | 76.2 | 1422 | 77.2 | 1892 | 82.7 | 3230 | 72.6 |
B. shaanxiensis (B.); Typhlocyba sp. (T.); L. lingchuanensis (L1.); P. luodianensis (P1.); Limassolla sp. (L2.); P. tiani (P2.).
3.2. Protein-Coding Genes (PCGs)
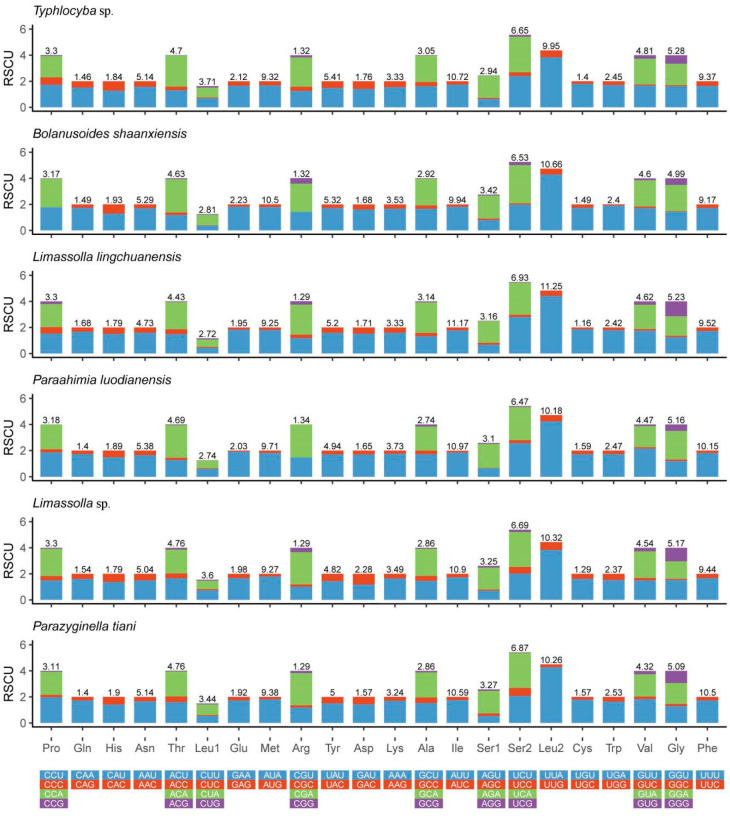
The 13 PCGs of P. tiani and Limassolla sp. comprise a total of 10,938 bp and 10,926 bp, respectively. The A + T content of the third codon positions (86.7%, 83.5%) is much higher than that of the first (73.2%, 71.7%) and the second (68.8%, 69.1%) positions. The two sequenced species show negative AT skew (−0.126, −0.110) and negative GC skew (−0.041, −0.025) in PCGs (Table 2). The majority of PCGs in mitogenomes have the typical start codon ATN and end with the TAA stop codon or its incomplete form T-. Incomplete stop codons are common in insects and believed to be completed by posttranscriptional polyadenylation [52]. Gene atp8 starts with TTG in Typhlocyba sp., Bolanusoides shaanxiensis Huang and Zhang, 2005, L. lingchuanensis and P. luodianensis [24,25,27]. Incomplete stop codon T- is present in cox2 and nad5 in Typhlocyba sp. [28]; B. shaanxiensis ends with T- in cox2, cox3, nad5 and nad4; L. lingchuanensis ends with T- in cox1, cox2 and nad4 [24]; P. luodianensis ends with T- in cox1, cox2, cox3 and nad5 [25]; Limassolla sp. ends with T- in cox1, cox2, cox3, nad4 and nad6 and P. tiani ends with T- in cox2, cox3, nad5 and nad1. Other genes end with a complete TAN codon (Table 4). Relative synonymous codon usage (RSCU) is summarized in Figure 3, indicating that the most frequently utilized amino acids are Leu, Ser, Ile, Phe and Met. Both newly sequenced species include all 62 available codons but the codons Pro (CCG) and Arg (CGC) are absent in B. shaanxiensis, Pro (CCG) and Arg (CGC/G) are absent in P. luodianensis. In the two new mitogenomes, as well as four other Typhlocybinae mitogenomes [24,25,28], the six most frequently used codons are all composed with A and T, which contribute to the high A + T bias of the entire mitogenomes.
Table 4.
Start and stop codons of the Typhlocybini and Zyginellini mitochondrial genomes. B. shaanxiensis (B.); Typhlocyba sp. (T.); L. lingchuanensis (L1.); P. luodianensis (P1.); Limassolla sp. (L2.); P. tiani (P2.).
| Gene | Start Codon/Stop Codon | |||||
|---|---|---|---|---|---|---|
| T. | B. | L1. | P1. | L2. | P2. | |
| nad2 | ATA/TAA | ATT/TAA | ATT/TAG | ATT/TAA | ATA/TAG | ATA/TAA |
| cox1 | ATG/TAA | ATG/TAA | ATG/T | ATG/T | ATG/T | ATG/TAA |
| cox2 | ATT/T | ATT/T | ATT/T | ATG/T | ATC/T | ATA/T |
| atp8 | TTG/TAA | TTG/TAA | TTG/TAA | TTG/TAA | ATC/TAA | ATA/TAA |
| atp6 | ATG/TAA | ATA/TAA | ATG/TAA | ATG/TAA | ATG/TAA | ATA/TAA |
| cox3 | ATG/TAA | ATG/T | ATG/TAA | ATG/T | ATG/T | ATG/T |
| nad3 | ATT/TAA | ATT/TAA | ATA/TAA | ATA/TAA | ATA/TAA | ATA/TAA |
| nad5 | ATG/T | TTG/T | ATA/TAA | ATG/T | ATT/TAA | ATT/T |
| nad4 | ATG/TAA | ATA/T | ATG/T | ATG/TAA | ATG/T | ATG/TAG |
| nad4L | ATG/TAA | ATG/TAA | ATG/TAA | ATG/TAA | ATG/TAA | ATG/TAA |
| nad6 | ATT/TAA | ATT/TAA | ATA/TAA | ATT/TAA | ATA/T | ATT/TAA |
| cytb | ATG/TAG | ATT/TAA | ATG/TAA | ATG/TAA | ATG/TAA | ATG/TAA |
| nad1 | ATT/TAA | ATT/TAA | ATT/TAA | ATT/TAA | ATT/TAA | ATA/T |
Figure 3.
Relative synonymous codon usage (RSCU) in the mitogenomes of 6 species.
3.3. Transfer and Ribosomal RNA Genes
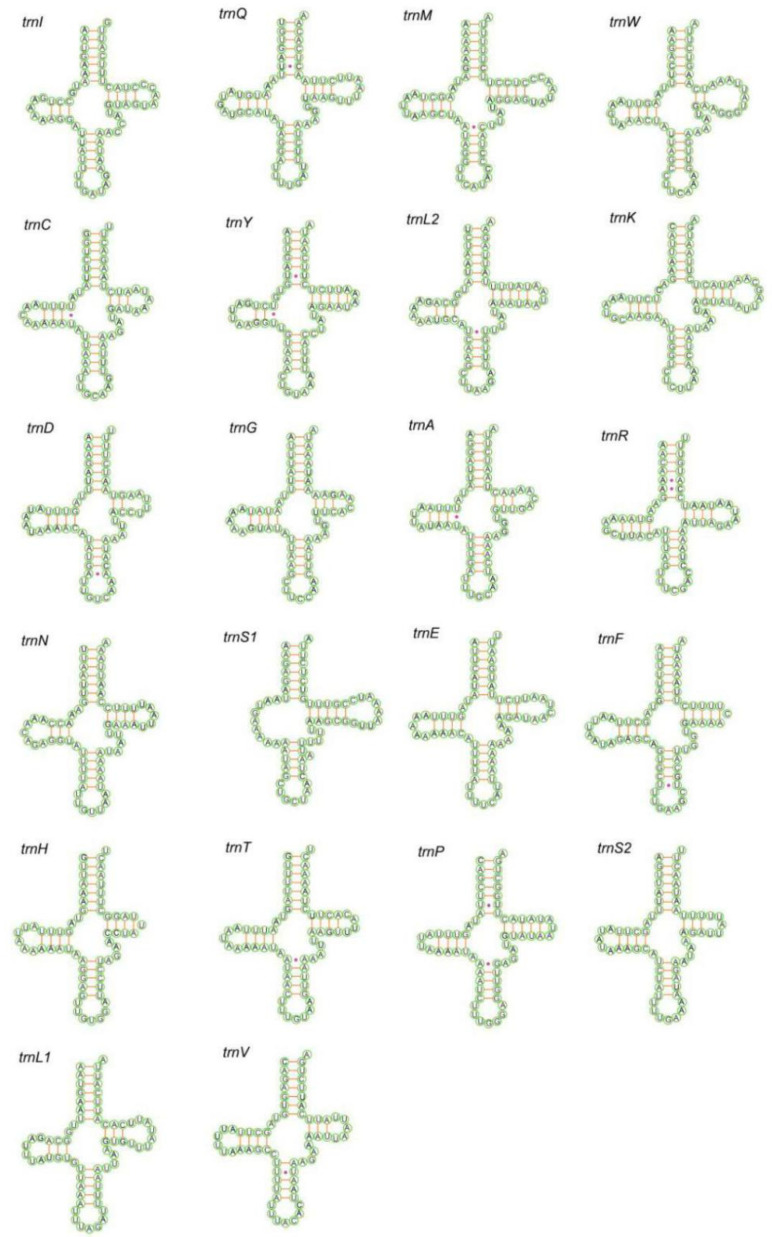
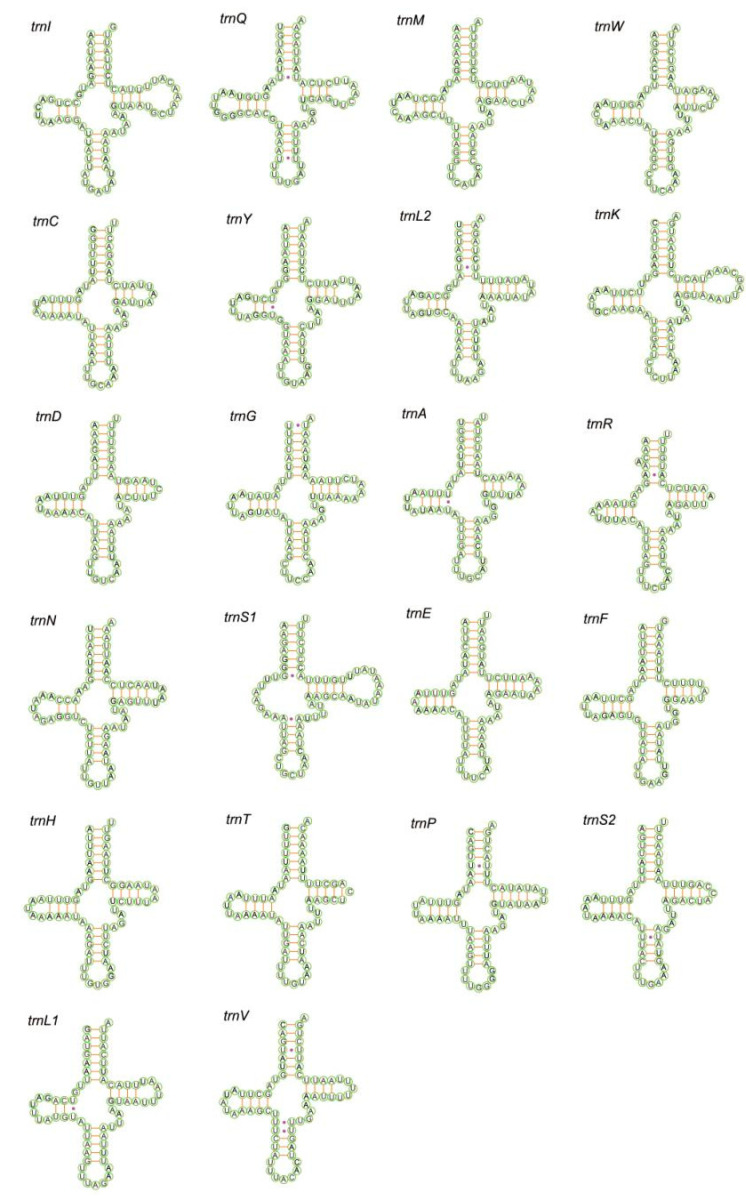
Each of the sequenced mitogenomes includes 22 tRNA genes; the total length of tRNA of P. tiani and Limassolla sp. is 1422 and 1432 bp, respectively; 14 tRNAs are encoded on the J-strand and the remainder are encoded on the N-strand; and the tRNAs of the two species have a positive AT and GC skew (Table 2). Secondary structures of all tRNAs are the typical cloverleaf secondary structure, except for that of trnS1, which lacks the dihydrouridine (DHU) arm forming a simple loop, as commonly found in other insect mitogenomes [16,53]. A cloverleaf secondary structure is conservative: 7 bp in the acceptor stem, 5 bp in the anticodon arm, with length of the DHU and TΨC arms variable. While the acceptor stem of trnR was 6 bp in Limassolla sp., L. lingchuanensis and Empoascanara sipra Dworakowska, 1980 [24,26], this stem is 5 bp in B. shaanxiens, Ghauriana sinensis Qin and Zhang, 2011, Illinigina sp. and Mitjaevia protuberanta Song, Li and Xiong, 2011 [27,28,30]. This result has not been previously reported in Typhlocybinae but such characteristics may be due to mitochondrial genome variation among different individuals [54]. Base pair mismatch is commonly found in the tRNA secondary structure of Cicadellidae [55]. In the arm structures of tRNAs of the two new mitogenomes, we recognized a total of six types of unmatched base pairs (G–U, U–U, A–A, G–A, U–C, A–C). The total number of unmatched base pairs found was 35 and 40 in P. tiani and Limassolla sp., respectively (Figure 4 and Figure 5). The large and small ribosomal RNA genes are located between trnL1 and trnV, and trnV and the A + T-rich region (Figure 1 and Figure 2). The lengths of rRNAs of P. tiani and Limassolla sp. are 1892 and 1976 bp and the AT content is 82.7% and 78.5%, respectively, with negative AT-skew and positive GC-skew (Table 2).
Figure 4.
Predicted secondary cloverleaf structure for the tRNAs of P. tiani.
Figure 5.
Predicted secondary cloverleaf structure for the tRNAs of Limassolla sp.
3.4. Gene Overlaps and Intergenic Spacers
There are 11 and 15 intergenic spacers in P.tiani and Limassolla sp. ranging from 1 to 28 bp in size. The longest (28 bp) intergenic spacer is found in Limassolla sp., between the nad5 and trnH genes. Gene overlaps range from 1 to 8 bp in the two mitogenomes, with the longest (8 bp) found between trnW and trnC genes in both species (Table S1). tRNA genes have more gene overlaps, which may reflect fewer evolutionary constraints on such genes [56].
3.5. A + T-Rich Region
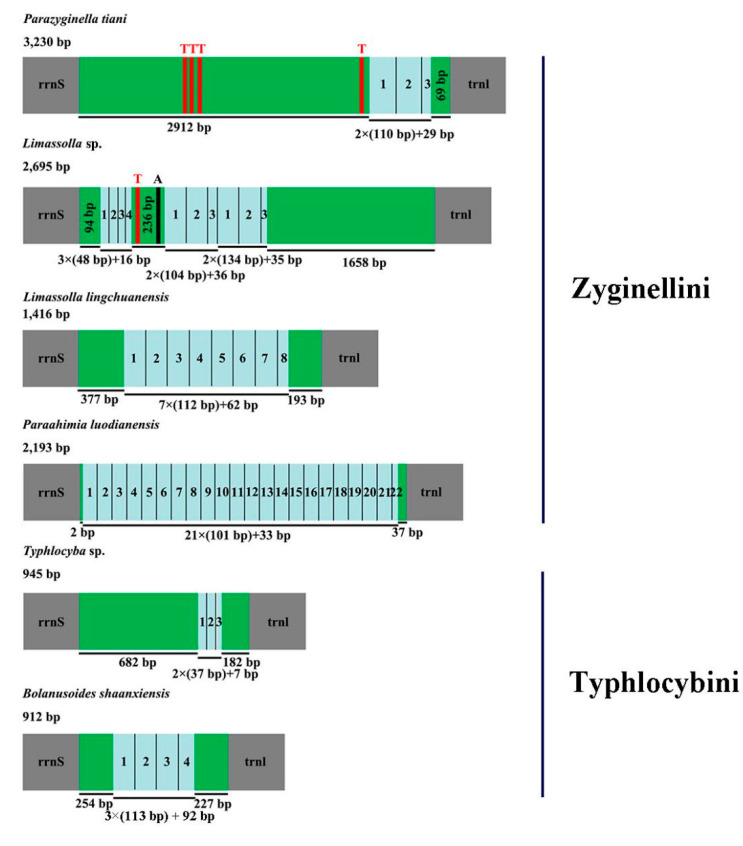
The A + T-rich region is the starting region of replication and the largest intergenic spacers are also believed to be involved in regulating transcription and replication of DNA in insects [57,58,59]. This region is located between rrnS and trnM in the two newly sequenced leafhoppers (Figure 1 and Figure 2). The length of this region ranges from 912 to 945 bp in Typhlocybini, and from 1416 to 3230 bp in Zyginellini (Table 3). One tandem repeat region is found in the control region of P. tiani, four Poly (T) are located in non-repeat regions. Three tandem repeat regions in the control region of Limassolla sp. include a Poly (T) and Poly (A) between the first and second tandem repeat region. The other four species have a single tandem repeat region. The length, nucleotide sequences and copy numbers of repeat units in the control region are highly variable among known Typhlocybinae mitochondrial genomes (Figure 6).
Figure 6.
Organization of the control regions in the mitochondrial genomes of Typhlocybini and Zyginellini. The boxes colored blue indicate the tandem repeats. Non-repeat regions are shown by green boxes. The red and black blocks represent the structures of poly (T) and poly (A), respectively.
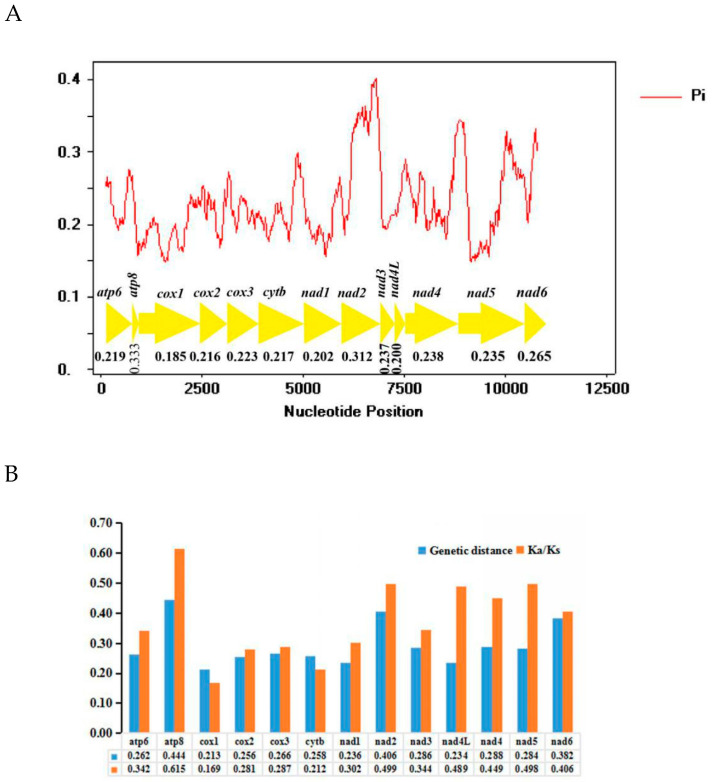
3.6. Nucleotide Diversity
Sliding window analysis was implemented to study the nucleotide diversity of 13 PCGs among six mitogenomes exhibited in Figure 7A. Nucleotide diversity values range from 0.185 (cox1) to 0.333 (atp8). Among the genes, atp8 (Pi = 0.333) has the highest variability, followed by nad2 (Pi = 0.312), nad6 (Pi = 0.265) and nad4 (Pi = 0.238). In contrast, cox1 (Pi = 0.185) and nad4L (Pi = 0.200) have comparatively low values and are the most conserved of the 13 PCGs. The nucleotide diversity is highly variable among the 13 PCGs. Pairwise genetic distances among these six mitogenomes produced results in Figure 7B. atp8 (0.444), nad2 (0.406) and nad6 (0.382) evolve comparatively faster, while cox1 (0.213), nad4L (0.234) and nad1 (0.236) evolve comparatively slowly (Figure 7B). Average non-synonymous (Ka) and synonymous (Ks) substitution rates can be used to estimate the evolutionary rate [60]. The pairwise Ka/Ks analyses showed that the average Ka/Ks ratios (ω) of 13 PCGs are from 0.169 to 0.615 (0 < ω < 1) (Figure 7B), indicating that these genes are under purifying selection [61]. The genes atp8 (0.615), nad2 (0.499) and nad5 (0.498) have comparatively high Ka/Ks ratios, while cox1 (0.169), cytb (0.212) and cox2 (0.281) have relatively low values (Figure 7B).
Figure 7.
(A) Sliding window analyses of 13 protein coding genes among 6 species’ mitogenomes. The red line shows the value of nucleotide diversity (Pi) in a sliding window analysis (a sliding window of 200 bp with the step size of 20 bp); the Pi value of each gene is shown under the gene name. (B) Genetic distances and the ratio of non-synonymous (Ka) to synonymous (Ks) substitution rates of 13 protein-coding genes among 6 species of Typhlocybini. The average value for each PCG is shown under the gene name.
Nucleotide diversity analyses are crucial for designing species-specific markers useful for insects belonging to groups difficult to distinguish by morphology alone [62,63]. The mitochondrial gene cox1 has long been used as a common barcode for identifying species and inferring the phylogenetic relationships in insects [64,65]. In Typhlocybinae, the cox1 gene exhibits a relatively slow evolutionary rate compared to other PCGs, while nad2 and nad6 genes evolve more quickly. Thus, nad2 and nad6 may be more suitable as barcode genes for species identification in Typhlocybinae.
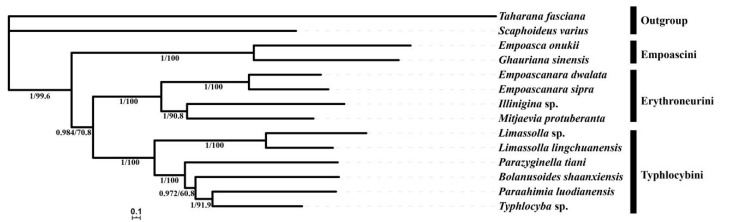
3.7. Phylogenetic Analyses
The best partitioning scheme and models for the different datasets as selected by PartitionFinder are listed in Tables S2 and S3. The phylogenetic topologies were highly consistent based on the four analyzed datasets (PCG123, PCG123R, PCG12 and PCG12R) with high nodal support values in BI and ML trees. The results (Figure 8) recovered Typhlocybinae as monophyletic. Empoascini, with Empoasca and Ghauriana sampled here, was resolved as monophyletic. Four included Erythroneurini, Empoascanara sipra, Empoascanara dwalata Dworakowska, 1971, Illinigina sp. and Mitjaevia protuberanta, forming a monophyletic clade sister to the clade comprising Typhlocybini and Zyginellini. All members of Typhlocybini and Zyginellini were grouped into a clade with high support (PP = 1, BS = 100), but the included Zyginellini formed a paraphyletic grade giving rise to Typhlocybini. Thus, the monophyly of tribe Zyginellini was not supported.
Figure 8.
Phylogenetic tree produced from ML and BI analysis based on 13 protein-coding genes. Numbers on branches are Bayesian posterior probabilities (PP) and bootstrap values (BS) (The Typhlocybini including former Zyginellini).
The tribe Typhlocybini was proposed by Distant [14] and is currently divided into two informal groups according to the number of cross veins on the hind wing: the Eupteryx complex with three cross veins and another one with two cross veins, including the Typhlocyba complex, Farynala complex, and Linnavuoriana complex [66]. The tribe Zyginellini was proposed by Dworakowska based on the presence of one cross vein on the hind wing and with vein CuA apparently directly connected to MP [67], but its status as a separate tribe has been questioned [68]. Balme (2007) and Dietrich (2013) treated it as a synonym of Typhlocybini based on analysis of morphological characteristics and histone H3 and 16S rDNA sequences [4,5]. Recent phylogenetic analyses of Cidadellidae and Membracoidea [12,13] also grouped members of Zyginellini with Typhlocybini but these analyses did not include a large enough taxon sample to test the monophyly of the tribes. In our study, members of the Typhlocyba complex of Typhlocybini are derived from within Zyginellini, consistent with treatment of the two tribes as synonyms. Nevertheless, a broader analysis with a much larger sample of taxa is needed to confirm the present results.
4. Conclusions
This study presents the annotated complete mitochondrial genome sequences of P. tinai and Limassolla sp. and a comparative analysis of mitochondrial genomes within the Typhlocybini and Zyginellini. Typhlocybinae mitogenomes are highly conservative in overall organization, exhibiting the hypothetical ancestral gene order for insects and lacking any gene rearrangements, as have been found in some other Cicadellidae. The only unusual feature was found in the secondary structure of tRNAs, with the acceptor stem of trnR comprising only 5 or 6 bp in some species. This unusual feature is here reported for the first time in Typhlocybinae. Phylogenetic analyses support treating Zyginellini as a synonym of Typhlocybini, as proposed by other recent authors. However, a much larger taxon with mitogenomes is still needed to reconstruct a better phylogeny tree to solve the tribal relationships within Typhlocybinae.
Acknowledgments
We are very grateful to Irena Dworakowska for her contribution to the knowledge of Chinese Typhlocybinae. Sincere thanks to students in the Entomological Museum of Northwest A&F University for their great help in obtaining specimens.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/10/684/s1, Table S1: Mitogenomic organization of P. tiani and Limassolla sp. Table S2: The best partitioning schemes and models for the Maximum likelihood (ML) method based on four datasets selected by PartitionFinder. Table S3: The best partitioning schemes and models for the Bayesian inference (BI) method based on four datasets selected by PartitionFinder.
Author Contributions
Conceptualization, X.Z. and M.H.; Funding acquisition, M.H.; Methodology, X.Z. and M.H.; Validation, C.H.D. and M.H.; Writing—original draft, X.Z.; Writing—review and editing, X.Z., C.H.D. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32070478, 31372233) and The Ministry of Science and Technology of the People’s Republic of China (2006FY120100, 2015FY210300).
Conflicts of Interest
The authors declare no conflicting interests.
References
- 1.McKamey S.H. Taxonomic catalogue of the Membracoidea (exclusive of leafhoppers); second supplement to Fascicle 1-Membracidae of the General Catalogue of the Hemiptera. Mem. Am. Entomol. Inst. 1998;60:1–377. [Google Scholar]
- 2.McKamey S.H. Leafhoppers of the World Database: Progress Report; Proceedings of the 11th International Auchenorrhyncha Congress; Potsdam/Berlin, Germany. 5–9 August 2002; Berlin, Germany: Museum für Naturkunde, Humboldt-Universität; 2002. p. 85. [Google Scholar]
- 3.Hamilton K.G.A. New world species of Chlorita, Notus, and Forcipata (Rhynchota: Homoptera: Cicadellidae: Typhlocybinae) with a new tribe Forcipatini. Can. Entomol. 1998;130:491–507. doi: 10.4039/Ent130491-4. [DOI] [Google Scholar]
- 4.Balme G.R. Ph.D. Thesis. North Carolina State University; Raleigh, NC, USA: 2007. Phylogeny and Systematics of the Leafhopper Subfamily Typhlocybinae (Insecta: Hemiptera: Typhlocybinae) pp. 1–149. [Google Scholar]
- 5.Dietrich C.H. South American leafhoppers of the tribe Typhlocybini (Hemiptera: Cicadellidae: Typhlocybinae) Zoologia. 2013;30:519–568. doi: 10.1590/S1984-46702013000500008. [DOI] [Google Scholar]
- 6.Cai P., Liang L.J., Wang F. A new species of Limassolla, pest of Diospyros Kaki (Homoptera: Cicadelloidea: Typhlocybidae) J. Anhui Agric. Univ. 1992;19:324–326. [Google Scholar]
- 7.Zhang Y.L. A Taxonomic Study of Chinese Cicadellidae (Homiptera) Tianze Eldonejo; Yangling, China: 1990. [Google Scholar]
- 8.Xu Y., Wang Y.R., Dietrich C.H., Fletcher M.J., Qin D.Z. Review of Chinese species of the leafhopper genus Amrasca Ghauri (Hemiptera, Cicadellidae, Typhlocybinae), with description of a new species, species checklist and notes on the identity of the Indian cotton leafhopper. Zootaxa. 2017;4353:360–370. doi: 10.11646/zootaxa.4353.2.7. [DOI] [PubMed] [Google Scholar]
- 9.Oman P.W., Knight W.J., Nielson M.W. Leafhoppers (Cicadellidae): A Bibliography, Generic Check-List and Index to the World Literature 1956–1985. C.A.B. International Institute of Entomology; London, UK: 1990. pp. 1–368. [Google Scholar]
- 10.Dworakowska I. The leafhopper tribe Zyginellini (Homoptera, Auchenorrhyncha, Cicadellidae, Typhlocybinae) Rev. Zool. Afr. 1979;93:299–331. [Google Scholar]
- 11.Dietrich C.H. Phylogeny of the leafhopper subfamily Evacanthinae with a review of Neotropical species and notes on related groups (Hemiptera: Membracoidea: Cicadellidae) Syst. Entomol. 2004;29:455–487. doi: 10.1111/j.0307-6970.2004.00250.x. [DOI] [Google Scholar]
- 12.Dietrich C.H., Allen J.M., Lemmon A.R., Lemmon E.M., Takiya D.M., Evangelista O., Walden K.K., Grady P.G., Johnson K.P., Wiegmann B. Anchored Hybrid Enrichment-Based Phylogenomics of Leafhoppers and Treehoppers (Hemiptera: Cicadomorpha: Membracoidea) Insect Syst. Divers. 2017;1:57–72. doi: 10.1093/isd/ixx003. [DOI] [Google Scholar]
- 13.Dietrich C.H., Rakitov R.A., Holmes J.L., Black W.C. Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera: Cicadomorpha) based on 28S rDNA Sequences. Mol. Phylogenet. Evol. 2001;18:293–305. doi: 10.1006/mpev.2000.0873. [DOI] [PubMed] [Google Scholar]
- 14.Distant W.L. The Fauna of British India including Ceylon and Burma. Volume 4. Secretary of State for India in Council; London, UK: 1908. Rhynchota—Homoptera; p. 501. [Google Scholar]
- 15.Boore J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu Rev. Entomol. 2014;59:95–117. doi: 10.1146/annurev-ento-011613-162007. [DOI] [PubMed] [Google Scholar]
- 17.Wolstenholme D.R. Geneticnovelties inmitochondrial genomes of multicellular animals. Curr. Opin. Genet. Dev. 1992;2:918–925. doi: 10.1016/S0959-437X(05)80116-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim M.J., Kang A.R., Jeong H.C., Kim K.G., Kim I. Reconstructing intraordinal relationships in Lepidoptera using mitochondrial genome data with the description of two newly sequenced Lycaenids, Spindasis takanonis and Protantigius superans (Lepidoptera: Lycaenidae) Mol. Phylogenet. Evol. 2011;61:436–445. doi: 10.1016/j.ympev.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Hebert P.D.N., Penton E.H., Burns J.M., Janzen D.H., Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. India Sect. A. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y.P., Song W., Shi S.L., Liu Y.Q., Pan M.H., Dai F.Y., Lu C., Xiang Z.H. Mitochondrial genome nucleotide substitution pattern between domesticated silkmoth, Bombyx mori, and its wild ancestors, Chinese Bombyx mandarina and Japanese Bombyx mandarina. Genet. Mol. Biol. 2010;33:186–189. doi: 10.1590/S1415-47572009005000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon C., Hadrys H. A comparative analysis of complete mitochondrial genomes among Hexapoda. Mol. Phylogenet. Evol. 2013;69:393–403. doi: 10.1016/j.ympev.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Simon C., Buckley T.R., Frati F., Stewart J.B., Beckenbach A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006;37:545–579. doi: 10.1146/annurev.ecolsys.37.091305.110018. [DOI] [Google Scholar]
- 23.Saccone C., Giorgi C.D., Gissi C., Pesole G., Reyes A. Evolutionary genomics in Metazoa: The mitochondrial DNA as a model system. Gene. 1999;238:195–209. doi: 10.1016/S0378-1119(99)00270-X. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X.W., Xiong K.N., Li C., Song Y.H. The complete mitochondrial genome of Limassolla lingchuanensis (Hemiptera: Cicadellidae: Typhlocybinae) Mitochondrial DNA Part B. 2020;5:229–230. doi: 10.1080/23802359.2019.1698354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y.H., Yuan X.W., Li C. The mitochondrial genome of Paraahimia luodianensis (Hemiptera: Cicadellidae: Typhlocybinae), a new genus and species from China. Mitochondrial DNA Part B. 2020;5:1351–1352. doi: 10.1080/23802359.2020.1735280. [DOI] [Google Scholar]
- 26.Tan C., Chen X.X., Li C., Song Y.H. The complete mitochondrial genome of Empoascanara sipra (Hemiptera: Cicadellidae: Typhlocybinae) with phylogenetic consideration. Mitochondrial DNA Part B. 2020;5:260–261. doi: 10.1080/23802359.2019.1698990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song N., Cai W., Li H. Deep-level phylogeny of Cicadomorpha inferred from mitochondrial genomes sequenced by NGS. Sci. Rep. 2017;7:10429. doi: 10.1038/s41598-017-11132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi R., Yu X.F., Yang M.F. Complete mitochondrial genome of Ghauriana sinensis (Hemiptera: Cicadellidae: Typhlocybinae) Mitochondrial DNA Part B. 2020;5:1367–1368. doi: 10.1080/23802359.2020.1735952. [DOI] [Google Scholar]
- 29.Zhou N.N., Wang M.X., Cui L., Chen X.X., Han B.Y. Complete mitochondrial genome of Empoasca vitis (Hemiptera: Cicadellidae) Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016;27:1052–1053. doi: 10.3109/19401736.2014.928863. [DOI] [PubMed] [Google Scholar]
- 30.Yuan X., Li C., Song Y.H. Characterization of the complete mitochondrial genome of Mitjaevia protuberanta (Hemiptera: Cicadellidae: Typhlocybinae) Mitochondrial DNA Part B. 2020;5:601–602. doi: 10.1080/23802359.2019.1710601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X., Chen Y., Chen C., Pu D.Q., Tang X.B., Zhang H., Lu D.H., Mao J.H. Characterization of the complete mitochondrial genome of Empoasca sp. (Cicadellidae: Hemiptera) Mitochondrial DNA Part B. 2019;4:1477–1478. doi: 10.1080/23802359.2019.1579066. [DOI] [Google Scholar]
- 32.Liu J.H., Sun C.Y., Long J., Guo J.J. Complete mitogenome of tea green leafhopper, Empoasca onukii (Hemiptera: Cicadellidae) from Anshun, Guizhou Province in China. Mitochondrial DNA Part B. 2017;2:808–809. doi: 10.1080/23802359.2017.1398616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X.X., Yuan Z.W., Li C., Song Y.H. Complete mitochondrial genome sequence of Empoascanara dwalata (Hemiptera: Cicadellidae: Typhlocybinae) Mitochondrial DNA Part B. 2020;5:2260–2261. doi: 10.1080/23802359.2020.1772141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X., Yuan Z.W., Li C., Song Y.H. Complete mitochondrial genome of Eupteryx (stacla) minusula (Hemiptera: Cicadellidae: Typhlocybinae) from China. Mitochondrial DNA Part B. 2020;5:2375–2376. doi: 10.1080/23802359.2020.1775146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J., Yuan X., Yuan Z., Song Y.H. The complete mitochondrial genome of Parathailocyba orla (Hemiptera: Cicadellidae: Typhlocybinae) Mitochondrial DNA Part B. 2020;5:1981–1982. doi: 10.1080/23802359.2020.1756952. [DOI] [Google Scholar]
- 36.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernt M., Donath A., Jühling F., Externbrink F., Florentz C., Fritzsch G., Pütz J., Middendorf M., Stadler P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013;69:313–319. doi: 10.1016/j.ympev.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Grant J.R., Stothard P. The CG View Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36:181–184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D., Gao F.L., Li W.X., Jakovlić I., Zou H., Zhang J., Wang G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. bioRxiv. 2018;20:489088. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
- 40.Benson G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du Y.M., Dai W., Dietrich C.H. Mitochondrial genomic variation and phylogenetic relationships of three groups in the genus Scaphoideus (Hemiptera: Cicadellidae: Deltocephalinae) Sci. Rep. 2017;7:16908. doi: 10.1038/s41598-017-17145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J.J., Li H., Dai R.H. Complete mitochondrial genome of Taharana fasciana (Insecta, Hemiptera: Cicadellidae) and comparison with other Cicadellidae insects. Genetica. 2017;145:593–602. doi: 10.1007/s10709-017-9984-8. [DOI] [PubMed] [Google Scholar]
- 45.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements inperformance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 47.Lanfear R., Frandsen P.B., Wright A.M., Senfeld T., Calcott B. Partition Finder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016;34:772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 50.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clary D.O., Wolstenholme D.R. The mitochondrial DNA molecule of Drosophila yakuba nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 1985;22:252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- 52.Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 53.Li H., Leavengood J.M., Chapman E.G., Burkhardt D., Song F., Jiang P., Liu J.P., Zhou X.G., Cai W.Z. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. Biol. Sci. 2017;284:20171223. doi: 10.1098/rspb.2017.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang K.J., Zhu W.C., Rong X., Ding X.L., Zhang Y.K., Liu J., Chen D.S., Du Y., Hong X.Y. The complete mitochondrial genomes of two rice planthoppers, Nilaparvata lugens and Laodelphax striatellus: Conserved genome rearrangement in Delphacidae and discovery of new characteristics of atp8 and tRNA genes. BMC Genom. 2013;14:417. doi: 10.1186/1471-2164-14-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo Z.L., Yuan M.L. Research progress of mitochondrial genomes of Hemiptera insects. Sci. Sin. Vitae. 2016;46:151–166. [Google Scholar]
- 56.Doublet V., Ubrig E., Alioua A., Bouchon D., Marcadé I., Maréchal-Drouard L. Large gene overlaps and tRNA processing in the compact mitochondrial genome of the crustacean Armadillidium vulgare. RNA Biol. 2015;12:1159–1168. doi: 10.1080/15476286.2015.1090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang D.X., Szymura J.M., Hewitt G.M. Evolution and structural conservation of the control region of insect mitochondrial DNA. J. Mol. Evol. 1995;40:382–391. doi: 10.1007/BF00164024. [DOI] [PubMed] [Google Scholar]
- 58.Huang W., Zheng J., He Y., Luo C. Tandem repeat modification during double-strand break repair induced by an engineered TAL effector nuclease in zebrafish genome. PLoS ONE. 2013;8:e84176. doi: 10.1371/journal.pone.0084176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levinson G., Gutman G.A. Slipped-strand mispairing: A major mechanism for DNA sequence evolution. Mol. Biol. Evol. 1987;4:203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- 60.Hurst L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002;18:486–487. doi: 10.1016/S0168-9525(02)02722-1. [DOI] [PubMed] [Google Scholar]
- 61.Mori S., Matsunami M.J.G. Signature of positive selection in mitochondrial DNA in Cetartiodactyla. Genes Genet. Syst. 2018;93:65–73. doi: 10.1266/ggs.17-00015. [DOI] [PubMed] [Google Scholar]
- 62.Jia W.Z., Yan H.B., Guo A.J., Zhu X.Q., Wang Y.C., Shi W.G., Chen H.T., Zhan F., Zhang S.H., Fu B.Q. Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: Additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genom. 2010;11:447. doi: 10.1186/1471-2164-11-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L.Y., Liu F.F., Chiba H., Yuan X.Q. The mitochondrial genomes of three skippers: Insights into the evolution of the family Hesperiidae (Lepidoptera) Genomics. 2019;112:422–441. doi: 10.1016/j.ygeno.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 64.Brabec J., Kostadinova A., Scholz T., Littlewood D.T.J. Complete mitochondrial genomes and nuclear ribosomal RNA operons of two species of Diplostomum (Platyhelminthes: Trematoda): A molecular resource for taxonomy and molecular epidemiology of important fish pathogens. Parasit. Vectors. 2015;8:336. doi: 10.1186/s13071-015-0949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demari-Silva B., Foster P.G., Oliveira-de T.M.P., Bergo E.S., Sanabani S.S., Pessôa R., Sallum M.A.M. Mitochondrial genomes and comparative analyses of Culex camposi, Culex coronator, Culex usquatus and Culex usquatissimus (Diptera: Culicidae), members of the Coronator group. BMC Genom. 2015;16:831. doi: 10.1186/s12864-015-1951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young D.A.J. A reclassification of western Hemisphere Typhlocybinae (Homoptera: Cicadellidae) Univ. Kans. Sci. Bull. 1952;35:3–217. doi: 10.5962/bhl.part.4327. [DOI] [Google Scholar]
- 67.Dworakowska I. On some Typhlocybinae from Vietnam (Homoptera: Cicadellidae) Folia Entomol. Hung. 1977;30:9–47. [Google Scholar]
- 68.Hamilton K.G.A. Classification, morphology and phylogeny of the family Cicadellidae (Rhynchota: Homoptera); Proceedings of the 1st International Workshop on Biotaxonomy, Classification and Biology of Leafhoppers and Planthoppers (Auchenorrhyncha) of Economic Importance; London, UK. 4–7 October 1983; pp. 15–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.