Abstract
Bacterial biofilm contributes to antibiotic resistance. Developing antibiofilm agents, more favored from natural origin, is a potential method for treatment of highly virulent multidrug resistant (MDR) bacterial strains; The potential of Pimenta dioica and Pimenta racemosa essential oils (E.Os) antibacterial and antibiofilm activities in relation to their chemical composition, in addition to their ability to treat Acinetobacter baumannii wound infection in mice model were investigated; P. dioica leaf E.O at 0.05 µg·mL−1 efficiently inhibited and eradicated biofilm formed by A. baumannii by 85% and 34%, respectively. Both P. diocia and P. racemosa leaf E.Os showed a bactericidal action against A. baumanii within 6h at 2.08 µg·mL−1. In addition, a significant reduction of A. baumannii microbial load in mice wound infection model was found. Furthermore, gas chromatography mass spectrometry analysis revealed qualitative and quantitative differences among P. racemosa and P. dioica leaf and berry E.Os. Monoterpene hydrocarbons, oxygenated monoterpenes, and phenolics were the major detected classes. β-Myrcene, limonene, 1,8-cineole, and eugenol were the most abundant volatiles. While, sesquiterpenes were found as minor components in Pimenta berries E.O; Our finding suggests the potential antimicrobial activity of Pimenta leaf E.O against MDR A. baumannii wound infections and their underlying mechanism and to be further tested clinically as treatment for MDR A. baumannii infections.
Keywords: Acinetobacter baumannii; MDR; biofilm; antimicrobial; Pimenta; Myrtaceae; wound infection; eugenol; 1,8-cineole; GC/MS
1. Introduction
Wound healing is a complex biological process in the human body, achieved through programmed phases. Potential factors, including infection, can interfere with these phases and impair healing. There is increasing evidence that bacteria can delay healing processes by living within biofilm communities in which the bacteria are protected from the host immune system and to further develop antibiotic drug resistance. Referring to the previous findings, wound infection is detrimental to wound healing and there is a great demand for the development of more effective treatment for infected and poorly healing wounds [1,2,3].
Acinetobacter baumannii is one of the most important opportunistic nosocomial pathogens. This pathogen was recorded, with high incidence, among immune-compromised patients as the causative agent of wound infections [2]. Based on its pathogenicity, including biofilm formation and antimicrobial resistance, A. baumannii is regarded as a multidrug-resistant pathogen (MDR) that usually complicates wound infection prognosis [4], the phenomenon that warrants further investigation of both nosocomial and community-acquired infections [5]. The World Health Organization recognizes antimicrobial resistance as one of the three most important issues facing human health [6]. The need to develop new spectrum antibiotics to tackle multidrug resistance is still ongoing. Plant-based antimicrobials present a potential source of medication that is successful in treating infectious diseases while minimizing many of the side effects that are frequently associated with synthetic antimicrobials [7,8].
Pimenta genus is an important myrtaceous taxa that encompasses 15 species, mostly found in the Americas Caribbean area, and commonly used for several medicinal purposes [9,10]. Pimenta dioica and Pimenta racemosa are the most recognized in that genus to exhibit potential pharmacological effects owing to its rich essential oil composition [11]. Traditionally, different Pimenta plant parts have been utilized for the alleviation of common cold, viral infections, bronchitis, dental and muscle aches, rheumatic pains, and arthritis. Furthermore, its berry oil is reported to exhibit antimicrobial, antiseptic, anesthetic, and analgesic properties [12]. Essential oils of Pimenta are ethno-pharmacologically relevant in traditional management of microbial infections [5,13]. Pimenta spp. antimicrobial and antiviral activities have been previously reported against several microbes including Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and antifungal activity against Candida albicans, Aspergillus niger, and Abisidia corymbifera. P. racemosa is used in folk medicine for the treatment of influenza, pneumonia, fever rheumatism, toothache, and abdominal pains [14,15,16].
Studies on P. dioica leaf essential oil showed that eugenol, methyl eugenol, β-caryophyllene, and myrcene were the most abundant constituents, while limonene, 1,8-cineole, terpinolene, β-caryophyllene, β-selinene, and methyl eugenol were the major constituents in other studies [13,17,18].
In the preset study, antimicrobial and antibiofilm activities of Pimenta essential oils (E.Os) grown in Egypt were evaluated against A. baumannii using in vitro and in vivo models to assess their role as antimicrobial agents in wound infections. To the best of our knowledge, this is the first study reporting on the in vitro and in vivo antimicrobial efficacy of Pimenta oils against A. baumannii pathogen.
2. Results and Discussion
A major goal of this study was to assess the potential antimicrobial effect for Pimenta essential oils (E.Os) represented by its different organ or species type. The minimum inhibitory concentration (MIC) values of the E.Os obtained from Pimenta racemosa and Pimenta dioica leaves and berries against 15 bacterial isolates were in the range of 0.51–5.2 µg·mL−1 (Table 1). Among the tested plant parts, leaves’ E.Os demonstrated the strongest antimicrobial activities in terms of smaller MIC values and were thus chosen for further in vivo wound infection. Eugenol exhibited the strongest antimicrobial activity with the lowest MIC values (ranging from 0.1 to 5.2 µg·mL−1 as compared to all tested E.Os). While P. dioica berry oil displayed the weakest antimicrobial activity (average MIC value 3.38 µg·mL−1) compared to other E.Os. Besides, MIC values of β-myrcene, a monoterpene hydrocarbon was higher than 4 µg.mL−1 against all tested strains.
Table 1.
The minimum inhibitory concentration values (MIC), in µg·mL−1, of tested Pimenta essential oils, eugenol, and cefepime antibiotic tested against the clinical isolates of Acinetobacter baumanii (AB), the results are represented as the mean of three replicates ± standard deviation.
| Isolate | P. dioica Leaf a | P. racemosa Leaf a | P. dioica Berry a | P. racemosa Berry a | Eugenol a | Cefepime b |
|---|---|---|---|---|---|---|
| AB-1 | 0.69 ± 0.2 | 0.52 ± 0.0 | 0.69 ± 0.2 | 0.69 ± 0.2 | 0.10 ± 0.0 | 10 ± 0.0 |
| AB-2 | 0.86 ± 0.2 | 4.84 ± 0.5 | 5.18 ± 0.0 | 0.69 ± 0.2 | 0.20 ± 0.0 | 5.0 ± 0.0 |
| AB-3 | 5.18 ± 0.0 | 1.03 ± 0.0 | 1.03 ± 0.0 | 1.03 ± 0.0 | 0.53 ± 0.0 | 0.1 ± 0.0 |
| AB-5 | 0.86 ± 0.2 | 0.86 ± 0.2 | 0.86 ± 0.2 | 0.86 ± 0.2 | 0.21 ± 0.0 | 5.0± 0.0 |
| AB-6 | 0.69 ± 0.2 | 0.52 ± 0.0 | 0.69 ± 0.2 | 0.69 ± 0.2 | 0.53 ± 0.0 | 0.6 ± 0.0 |
| AB-8 | 0.52 ± 0.0 | 0.52 ± 0.0 | 5.18 ± 0.0 | 0.69 ± 0.2 | 0.21 ± 0.0 | 6.7 ± 2.8 |
| AB-9 | 0.52 ± 0.0 | 0.52 ± 0.0 | 4.49 ± 1.1 | 1.03 ± 0.0 | 0.53±0.0 | 5.0 ± 0.0 |
| AB-10 | 5.18 ± 0.0 | 0.69 ± 0.2 | 5.18 ± 0.0 | 0.69 ± 0.2 | 0.53 ± 0.0 | 0.1 ± 0.0 |
| AB-13 | 1.03 ± 0.0 | 0.52 ± 0.0 | 0.52 ± 0.0 | 1.03 ± 0.0 | 0.45 ± 0.0 | 1.3 ± 0.0 |
| AB-15 | 1.03 ± 0.0 | 1.03 ± 0.0 | 5.18 ± 0.0 | 1.03 ± 0.0 | 5.3 ± 0.0 | 5.0 ± 0.0 |
| AB-11Q | 0.86 ± 0.2 | 1.03 ± 0.0 | 5.18 ± 0.0 | 1.03 ± 0.0 | 0.53 ± 0.0 | 0.1 ± 0.0 |
| AB-14T | 1.03 ± 0.0 | 4.66 ± 0.8 | 5.18 ± 0.0 | 5.18 ± 0.0 | 0.78 ± 0.2 | 0.1 ± 0.0 |
| AB-16Q | 1.03 ± 0.0 | 1.03 ± 0.0 | 5.18 ± 0.0 | 5.87 ± 1.1 | 0.88 ± 0.2 | 0.3 ± 0.0 |
| AB-7T | 4.49 ± 1.1 | 5.18 ± 0.0 | 5.18 ± 0.0 | 5.18 ± 0.0 | 0.53 ± 0.0 | 1.0 ± 0.30 |
| ATCC 19606 | 0.69 ± 0.2 | 1.03 ± 0.0 | 0.86 ± 0.2 | 1.03 ± 0.0 | 0.47 ± 0.0 | 0.1 ± 0.0 |
| Average MIC values (µg·mL−1) | 1.65 | 1.60 | 3.38 | 1.78 | 0.79 | 2.70 |
(a,b) A statistically significant difference exists between the antimicrobial activity of cefepime and each of the tested E.Os and eugenol (Kruskal-Wallis test-Dunn’s multiple comparisons test, p-value < 0.0001).
Although, the order of activity according to the average MIC values was as follows:
Eugenol > P. dioica leaf/P. racemosa leaf > P. racemosa berry > cefepime > P. dioica berry.
No statistically significant difference was observed among the activities of the four tested E.Os, eugenol, while a statistically significant difference existed between cefepime and each of the tested E.Os and eugenol (Kruskal–Wallis test, Dunn’s multiple comparisons test, p-value < 0.0001).
Semiquantitative determination of biofilm formation by the examined five A. baumannii strains revealed that AB-R strain is a strong biofilm forming (BF), and both AB-8 and AB-16 are moderate BF while AB-11 and AB-13 strains are negative BF. Therefore, the effect of E.Os on biofilm formation and eradication was tested on three strains; AB-R, AB-8, and AB-16.
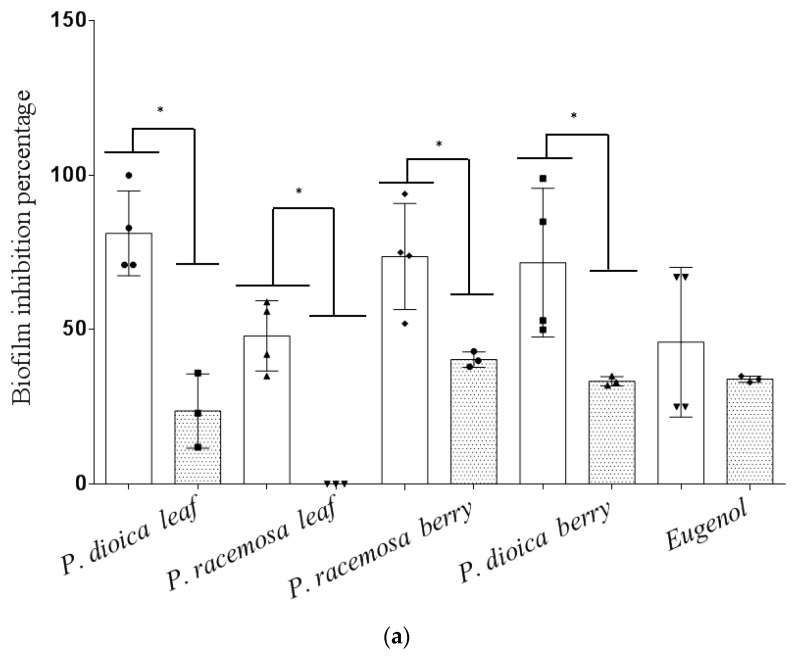
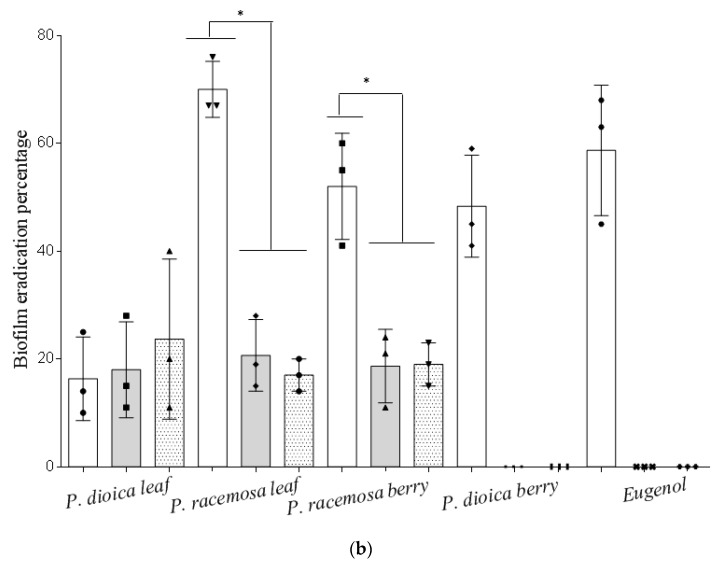
Unexpectedly, no inhibition activity was detected against the strong biofilm formed by the standard A. baumanii strain (AB-R). However, all Pimenta oils, as well as eugenol, could inhibit biofilm formation by AB-8 and AB-16, at 0.1× MIC (0.05 and 0.10 µg·mL−1, respectively), except for P. racemosa leaf E.O, which could inhibit biofilm formed by AB-8 only at 0.05 µg·mL−1 (0.1× MIC). In detail, P. dioica leaf oil was found superior as antibiofilm relative to eugenol and other tested oils (Figure 1a). Comparing leaf to berry oil, Pimenta berry oils showed stronger effect as antibiofilm than that of P. racemosa leaf oil. The observed antibiofilm activity of Pimenta oils was higher than that of standard eugenol, though with no significant difference (one-way ANOVA, followed by Tukey’s multiple comparisons p-value < 0.0001).
Figure 1.
(a) Biofilm inhibition percentages of Pimenta essential oils/eugenol at a dose of 0.1× MIC against MDR AB-8 (white) and AB-16 (light shade) isolates. (b) Biofilm eradication percentages of the tested E.Os/eugenol at a dose of 0.1× MIC against MDR AB-8 (white), AB-R (gray), and AB-16 (light shade) isolates. The symbols in each column (●, ■, ▲, ◆, ▼, ×, +) represent each data point of the triplicate experiments performed for each E.O and eugenol against the tested bacterial isolates. Data represent the means of biofilm inhibition/eradication percentages ± SD, n = 3 (one-way ANOVA, followed by Tukey’s multiple comparisons, p-value < 0.0001). (*) Means statistically significant difference exists between columns.
The same concentrations of all tested E.Os and eugenol, which inhibited biofilm formation, could also eradicate biofilm formed by AB-8, while only P. dioica leaf only, P. racemosa leaf and berry E.Os could eradicate biofilm formed by AB-R and AB-16 strains. Eradication of AB-8 strain biofilm by the E.Os and eugenol was more statistically significant than the eradication effect on biofilm formed by other strains (AB-R and AB-16), except for P. dioica leaf E.O (one-way ANOVA followed by Tukey’s multiple comparisons p-value < 0.0001) (Figure 1b). The reported biofilm eradication activity of Pimenta E.Os herein is in agreement with Vázquez-Sánchez et al. [19] who demonstrated the eradication of 24h-old biofilm of L. monocytogenes by Pimenta pseudochariophyllus E.O at doses of 2.75% and 3% within 30 min of application. In another study, the same E.O could also eradicate 24h-old Staphylococcus aureus biofilm at doses of 1.75% and 3%. Eugenol and 1,8-cineol were among the main reported components of this E.O [20].
In this study, eugenol could eradicate the biofilm formed by AB-8 strain only and did not affect AB-R or AB-16 preformed biofilms. In a previous study performed by Yadav et al. [21], it was reported that eugenol (at 0.5× MIC) could eradicate MRSA and MSSA preformed biofilms, these authors reported that within 6 h, eugenol could reduce biomass and cell viability of the preformed biofilms by two mechanisms, the first is by lysis of bacterial cells within biofilms, and the second mechanism is via disruption of the cell-to-cell connections leading to biofilm organization disassembly.
The antimicrobial and antibiofilm activity of Pimenta spp. E.Os presented herein, is in agreement with recent studies reporting the potential of myrtaceous plants as efficient inhibitors of bacterial quorum sensing and to possess pronounced antimicrobial and antibiofilm activities [18,22]. A study performed by Vasavi et al. [23] revealed a strong antiquorum sensing activity for the ethyl acetate fraction of ethanolic extract of P. dioica, this fraction could inhibit AHL-mediated violacein pigment production in Chromobacterium violaceum, ATCC12472, at dose-dependent manner, the authors related this effect to polyphenolic compounds like the E.O components of the tested extract.
The minimum bactericidal concentration (MBC) values of Pimenta oils were estimated at different dose levels, where all of the tested oils exhibited bactericidal effect after 24 h incubation. P. dioica leaf oil exhibited the most pronounced bactericidal action at 1 µg·mL−1 (2× MIC) against the tested AB-8 isolate (Table 2). Both P. racemosa leaf and berry E.Os exhibited the same bactericidal activity at 2.08 and 2.76 µg·mL−1, respectively. The bactericidal activity of eugenol was inferior to that of P. dioica leaf oil. In addition, P. dioica berries E.O at 41.4 µg·mL−1 (8× MIC) results in two-log cycle colony count reduction as compared to the control untreated bacteria. While, as a consequence, higher concentration, than 41.4 µg·mL−1, of this E.O was required to achieve a complete bactericidal activity which was not tested.
Table 2.
The determined MBC values of Pimenta spp. leaf and berry essential oils tested against A. baumanii isolate-8 (AB-8) and their MIC values equivalents.
| Essential Oils/Standard Compound | MBC Value | MIC Values Equivalents |
|---|---|---|
| P. dioica leaf | 1.0 µg·mL−1 | 2× MIC |
| P. racemosa leaf | 2.0 µg·mL−1 | 4× MIC |
| P. racemosa berry | 2.76 µg·mL−1 | 4× MIC |
| P. dioica berry * | More than 41.4 µg·mL−1 | More than 8× MIC |
| Eugenol | 1.2 µg·mL−1 | 6× MIC |
* Concentration equivalent to 8× MIC caused only 2-log cycle reduction of the bacterial load.
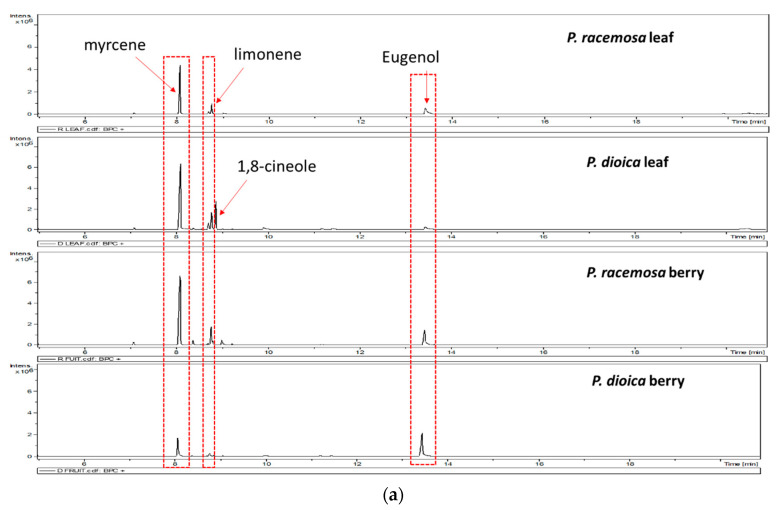
GC/MS analysis was employed to account for volatiles’ compositions in tested Pimenta E.Os and mediating for the difference observed in the antimicrobial effects, results are depicted in Table 3. It was revealed that monoterpene hydrocarbons prevail in both P. racemosa and P. dioica leaf oils, constituting up to 64.4% predominance, with β-myrcene as the major identified component accounting for 39.6% and 44.1%, respectively (Figure 2a,b, Table 3).
Table 3.
Relative percentile levels of volatiles identified in Pimenta berry and leaf essential oils using GC/MS.
| RI * Calculated. | RI Reported | Name | Class | Essential Oil | |||
|---|---|---|---|---|---|---|---|
| P. racemosa Leaf | P. dioica Leaf | P. racemosa Berry | P. dioica Berry | ||||
| 1186 | 1201 | Decanal | aldehyde/ketone | 0.0 | 0.03 | 0.0 | 0.0 |
| Total aldehyde/ketone | 0.0 | 0.03 | 0.0 | 0.0 | |||
| 906 | 932 | α-Pinene | 1.3 | 1.0 | 1.5 | 0.2 | |
| 965.2 | 988 | β-Myrcene | 39.6 | 44.1 | 42.3 | 13.9 | |
| 978.8 | 1003 | p-Mentha-1(7),8-diene | 0.9 | 0.2 | 2.9 | 0.0 | |
| 980.9 | 1005 | α-Phellandrene | 1.0 | 0.6 | 0.6 | 1.0 | |
| 991.2 | 1014 | α-Terpinene | 0.6 | 0.4 | 0.9 | 0.0 | |
| 993 | 1014 | 4-carene | 0.0 | 2.1 | 0.0 | 0.4 | |
| 1001 | 1020 | p-Cymene | 2.3 | 2.1 | 0.9 | 0.4 | |
| 1004.2 | 1024 | Limonene | 15.5 | 11.7 | 14.3 | 4.6 | |
| 1019 | 1032 | β-cis-Ocimene | 2.8 | 0.6 | 4.6 | 1.1 | |
| 1033 | 1054 | γ-Terpinene | 0.2 | 0.4 | 0.7 | 0.4 | |
| 1063 | 1085 | p-Mentha-2,4(8)-diene | 0.1 | 0.0 | 0.4 | 0.3 | |
| Total Monoterpene hydrocarbons | 64.4 | 63.3 | 69.1 | 22.2 | |||
| 1009.5 | 1026 | 1,8-cineol | Oxygenated monoterpene | 0.0 | 18.8 | 0.0 | 1.5 |
| 1080 | 1095 | β-Linalool | 2.1 | 5.3 | 1.6 | 3.6 | |
| 1163 | 1174 | Terpinen-4-ol | 0.8 | 1.5 | 1.0 | 1.4 | |
| 1181.5 | 1186 | α-Terpineol | 0.3 | 2.5 | 0.0 | 2.2 | |
| 1250 | 1247 | Chavicol | 1.5 | 0.0 | 0.2 | 1.5 | |
| 1250.1 | 1264 | Geranial | 0.0 | 0.0 | 0.0 | 0.6 | |
| Total oxygenated Monoterpene | 4.7 | 28.1 | 2.8 | 10.8 | |||
| 1334.5 | 1356 | Eugenol | Phenols | 31.0 | 8.6 | 27.7 | 65.6 |
| 1350 | 1345 | α-Cubebene | Sesquiterpene hydrocarbon | 0.0 | 0.0 | 0.0 | 0.1 |
| 1349 | 1374 | α-Copaene | 0.0 | 0.0 | 0.3 | 0.0 | |
| 1396 | 1417 | β-Caryophyllene | 0.0 | 0.0 | 0.0 | 0.3 | |
| 1430 | 1452 | α-Humulene | 0.0 | 0.0 | 0.0 | 0.2 | |
| 1444.8 | 1484 | Germacrene D | 0.0 | 0.0 | 0.0 | 0.2 | |
| 1478 | 1522 | δ-Cadinene | 0.0 | 0.0 | 0.0 | 0.6 | |
| Total sesquiterpenes | 0.0 | 0.0 | 0.3 | 1.4 | |||
| Total | 100 | 100 | 100 | 100 | |||
RI *, retention index on DB-5-MS column relative to n-alkanes C8-C20.
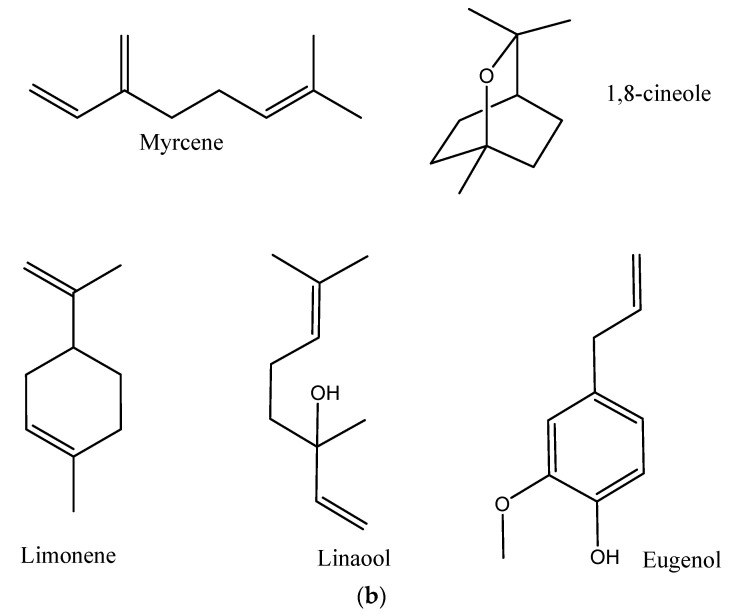
Figure 2.
(a) Representative GC/MS chromatograms of P. racemosa and P. dioica leaf and berry essential oils. (b) Structures of the major volatile constituents detected in P. racemosa and P. dioica essential oils.
Next, limonene was identified as a second major monoterpene hydrocarbon in P. racemosa leaf and berry at 14–15%, and to less extent in P. dioica berry oil (4.6%). In contrast, 1,8-cineole recorded the highest levels in P. dioica leaf oil (18.8%) compared to its berries, this was previously reported in the Cuban oil of P. dioica leaf (14.69%) [24]. 1,8-Cineole, among reported volatiles, is likely to mediate for the observed antimicrobial effects, in agreement with previously reported its potent antimicrobial effect against Streptococcus mutans [25]. In a recent study, Merghni et al. [26] recorded high MIC values displayed by 1,8-cineole against MRSA strains (≥29 mm) and suggestive for its positive effect against MDR bacteria. The antibiofilm activity of P.dioica leaf oil could be correlated to its enrichment in 1,8-cineole (18.80%). A study performed by Santos and Rao (2001) [27] reported the ability of 1,8-cineole to alleviate gastric mucosa injury and damage induced by absolute ethanol in rats. Stojkovic et al. [28] investigated the inhibitory effect of 1,8-cineole on apple rot disease caused by Aspergillus niger at concentrations of 3% and 6% of 1,8-cineole applied for 3 days, 100% inhibition of apple rot disease was observed. Merghni et al. [26] also reported 1,8-cineole biofilm inhibition effect against S. aureus ATCC 6538. Our results suggest possible synergistic antibiofilm activities of volatile oil constituents in Pimenta spp.
Eugenol dominated P. dioica berries essential oil composition (65.6%). Eugenol was found as a second predominant constituent in P. racemosa leaf and berry E.Os at comparable level of 27–31%. In the present study, it was observed that eugenol could inhibit A. baumannii biofilm formation at 0.02 µg·mL−1 which is in agreement with a study conducted by Kim et al. [29]. Where, more than 75% inhibition of E. coli O157:H7 biofilm was observed by eugenol, P. racemose, and P. dioica berries oils at slightly higher levels (0.052 µg·mL−1), though a different organism was tested. Noteworthy, eugenol is well-characterized as an antimicrobial agent against oral pathogens, infective diseases, and foodborne infections [30,31].
Further, sesquiterpene hydrocarbons were only detected in Pimenta berries oils at low levels, reaching 0.3% and 1.4% in P. racemosa and P. dioica berries, respectively. Major sesquiterpenes included δ-Cadinene in P. dioica berry oil followed by β-caryophyllene, α-humulene, germacrene D, and α-cubebene.
Although cefepime is considered to be the drug of choice for treatment of A. baumanii infections [32], its MIC values varied between 0.1 and 10 µg·mL−1 which can be attributed to the variable susceptibilities of the MDR isolates used herein.
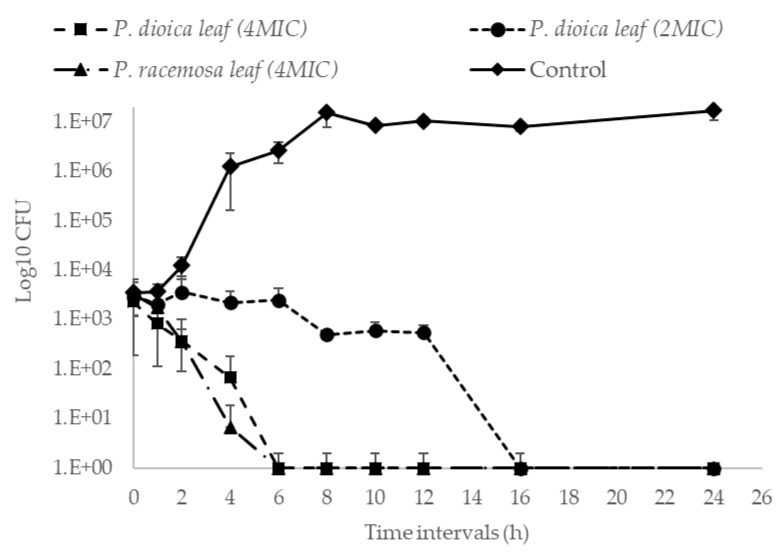
Accordingly, killing kinetics of AB-8 strain by P. dioica and P. racemosa leaf oils were further investigated. Within 6h of incubation, both oils could kill AB-8 strain at 2 µg·mL−1 (4× MIC) (Figure 3), while longer time (16h) was needed for P. dioica leaf oil to kill the bacterium at 1 µg·mL−1 (2× MIC). In contrast, the E.O of P. racemosa leaf, at 1 µg·mL−1, could not kill the bacteria nor achieve any level of bacterial colony count reduction.
Figure 3.
The killing kinetics of Pimenta dioica and Pimenta racemosa leaf essential oils tested against AB-8 isolate. (■) P. dioica leaf E.O. tested at concentration of 2.0 µg·mL−1 (4× MIC), (●) P. dioica leaf E.O. tested at concentration of 1.0 µg·mL−1 (2× MIC), (▲) P. racemosa leaf E.O. tested at concentration of 2.0 µg·mL−1 (4× MIC). Data is represented by means of the number of recovered colonies counted at each time point ± SD, n = 3.
The observed bactericidal action of eugenol is attributed to several mechanisms, involving alteration of bacterial membrane permeability causing leakage of ions leading to cell death [33], and in correlation to its phenolic nature which facilitates its binding to target proteins on the bacterial cell [34]. On the other hand, the monoterpene hydrocarbons viz., β-myrcene, α-pinene, and limonene are generally known to exert less antibacterial effects in comparison to oxygenated monoterpenes and phenolics [35] and might act to synergize the main action of cineole and eugenol in Pimenta oil, thus accounting for the superior antibiofilm effect of the oil compared to eugenol standard tested alone (Figure 1). The lipophilic nature of terpene hydrocarbons greatly affects bacterial membrane permeability and might improve uptake of E.O components into bacterial cells enhancing its penetration into the bacterial biofilms and thus exerting its antimicrobial and antibiofilm action and further potentiating the effect of other concomitant antibiotics [36,37].
According to the obtained MIC, MBC values, and antibiofilm activities, both P. dioica and P. racemosa leaf oils were selected to test their in vivo effectiveness as antimicrobial agents in a mouse model of wound infection as they showed the lowest MIC and MBC values together with strong antibiofilm activity (Table 1, Figure 1 and Figure 3).
Acinetobacter is regarded as one of the pathogens involved in nosocomial infections and outbreaks. In a previous epidemiological study, skin colonization with Acinetobacter spp. was found at a higher rate in hospitalized patients than in healthy people [38], thus a wound infection animal model was tested.
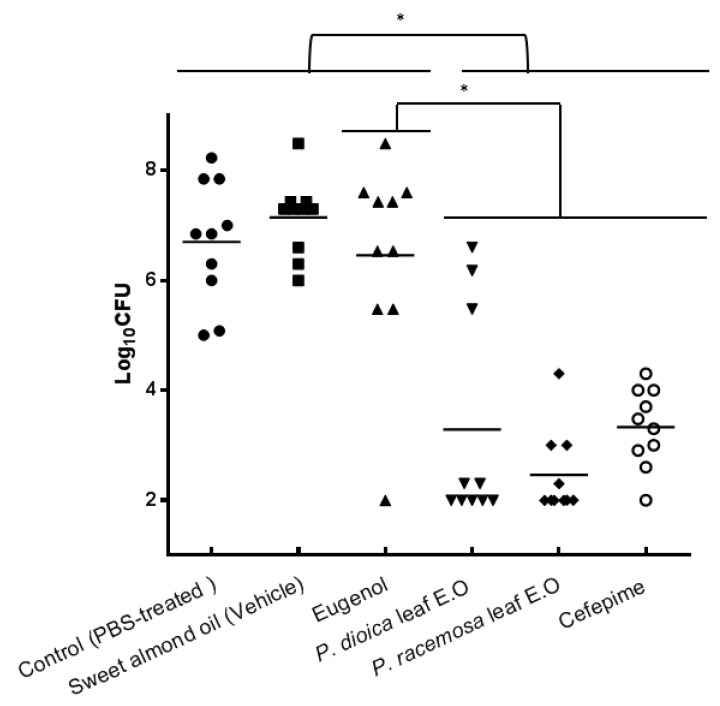
Interestingly, the microbial load of infected wounds in the mice groups treated with each of the E.Os and cefepime demonstrated a statistically significant reduction compared to other groups including the group treated by eugenol (one-way ANOVA followed by Tukey’s multiple comparisons test, p-value = 0.0047). This is suggestive that the E.O is more active than standard eugenol due to possible synergistic action of volatile oil constituents. Three log cycle reduction was observed in the total bacterial count of groups treated with E.Os compared to infected PBS-treated control group (Figure 4). P. dioica leaf oil did not show a significantly higher activity than that of P. racemosa on reducing bacterial count recovered from both treatments. No significant differences were recorded among the infected untreated group and groups treated with either almond oil vehicle or eugenol indicating that eugenol was less active in this in vivo assay. Nevertheless, Kim et al. [29] showed that eugenol could prolong the lifespan of a nematode worm infected with EHEC. Yadav et al. [21] reported eugenol, at sub-MIC, to be effective in reducing S. aureus colonization by 88% in a rat middle ear infection model.
Figure 4.
The antimicrobial effect of Pimenta dioica and Pimenta racemosa leaf essential oils and eugenol at 5.2 µg·mL−1 (10× MIC) concentration and cefepime at 25 µg·g−1 of mouse weight, tested against AB-8 isolate in a skin wound infection mouse model. Data is represented by the mean colony counts recovered from each of the six infected groups ± SD, six days post-infection. (●) data points of control infected PBS-treated group, (■) data points of sweet almond-treated group (negative (vehicle) control group), (▲) data points of eugenol-treated group, (▼) data points of P. dioica leaf E.O-treated group, (♦) data points of P. racemosa leaf E.O-treated group, (○) data points of cefepime-treated group (positive drug control group). n = 10 mice per group. (One-way ANOVA followed by Tukey’s multiple comparisons test, p-value = 0.0047). (*) Means statistically significant difference exists between groups.
The antimicrobial activity of P. dioica leaf oil could be attributed to its 1,8-cineole enriched content in addition to eugenol [39]. Cefepime showed a better reduction of microbial load than that observed due to P. racemosa leaf E.O and a comparable effect to P. dioica leaf E.O but these differences were statistically nonsignificant (one-way ANOVA followed by Tukey’s multiple comparisons test, p-value = 0.0047).
Another study performed by Karumathil et al. [40] revealed the in vitro ability of eugenol to reduce A. baumannii adhesion and invasion to human keratinocytes and also to inhibit biofilm formation in an in vitro collagen matrix wound model.
Nevertheless, few studies have investigated the in vivo antimicrobial effects of plant-derived E.Os against A. baumannii infections and likely to exert a stronger effect being composed of complex volatiles compared to a single chemical component. This study is the first to report the in vivo antimicrobial effect of P. dioica and P. racemosa E.Os in an A. baumannii wound infection model. In the same context, Tsai et al. [41] screened 30 Chinese herbs for antimicrobial effect against extensively drug-resistant A. baumannii isolates. Where, only Scutellaria barbata aqueous extract showed the highest activity in vitro and is likely to be mediated by polar type compounds in contrast to lipophilic nature of E.O reported herein and suggesting that different chemically based metabolites could evoke antimicrobial action against A. baumannii.
3. Materials and Methods
3.1. Plant Material
Leaves and berries of Pimenta dioica (L.) Merr. and Pimenta racemosa (Mill.) J.W. Moore were collected from Zohria Botanical Garden in May 2017. They were identified by Mrs. Therese Labib, Consultant of Plant Taxonomy at the Ministry of Agriculture and Orman Botanical Garden, Giza, Egypt. A voucher specimen of the plant material was deposited at Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Egypt.
3.2. Preparation of Essential Oil
Leaf and berry of Pimenta species under investigation were hydro-distilled separately in a Clevenger-type apparatus for 4h, according to the procedure described in the Egyptian pharmacopeia (2005) [42]. The obtained oils were dehydrated by filtration through anhydrous sodium sulfate and kept in a refrigerator for subsequent GC/MS analysis and antimicrobial assays.
3.3. GC/MS Analysis of the Volatile Oil Composition
E.Os. prepared from P. dioica and P. racemosa leaf and berry were subjected to GC/MS analysis. The injection volume was 1 µL. The instrument was controlled by the Shimadzu Class-5000 Version 2.2 software containing a NIST62 (National Institute of Standards and Technology) MS library. Volatile components were separated on a DB5-MS column (30 m length, 0.25 mm inner diameter, and 0.25 µm film thickness (J&W Scientific, Santa Clara, CA, USA). Injections were made in the split mode for 30 s, and the gas chromatograph was operated under the following conditions: injector 220 °C and column oven 40 °C for 3 min, then programmed at a rate of 12 °C/min to 180 °C, kept at 180 °C for 5 min, and finally ramped at a rate of 40 °C/min to 220 °C and kept for 2 min, He carrier gas at 1 mL/min. The transfer line and ion-source temperatures were adjusted at 230 and 180 °C, respectively. The HP quadrupole mass spectrometer was operated in the electron ionization mode at 70 eV. The scan range was set at 40–500 m/z.
The percentages of different components in each oil sample were determined by computerized peak area measurements relative to each other. Volatile components were identified using the procedure described in Farag and Wessjohann [43]. The peaks were first deconvoluted using AMDIS software (www.amdis.net) and identified by its retention indices (RI) relative to n-alkanes (C6–C20), mass spectrum matching to NIST, WILEY library database. Results are depicted in Table 2.
3.4. Bacterial Isolates and Culture Conditions
Fourteen MDR clinical isolates of Acinetobacter baumanii, in addition to one reference strain (ATCC 19606), were used in this study. They were isolated from patients in Kasr El-Ainy hospital, Cairo, Egypt [44]. The strains were grown aerobically on Luria–Bertani (L.B) agar/broth (Oxoid, UK) with shaking at 180 rpm at 37 °C for 24 h.
3.5. Determination of the Minimum Inhibitory Concentration (MIC) by Agar Microdilution Technique
MIC was determined according to the method described by Golus et al. [45]. Briefly, freshly prepared two-fold serial dilutions of the test essential oil (E.O.)/standard volatiles (eugenol and myrcene) were prepared in dimethyl sulfoxide (DMSO). In total, 1 µL of each E.O dilution was dispensed into the U-shaped bottom 96-well microplates. Aliquots of 100 µL of the molten L.B agar medium (at ≈60 °C) were mixed with E.O. in each well before solidification. Then, 2 µL of a freshly prepared bacterial suspension (in sterile normal saline (107 CFU·mL−1)) was inoculated onto the surface of the solidified mixture (L.B agar medium + E.O/standard compound).
The lowest concentration showing no visible bacterial growth after incubation at 30 °C for 24 h were recorded. Negative and positive controls (L.B agar medium + DMSO and L.B agar medium + DMSO + bacteria, respectively) and antibiotic control (cefepime) were included. The test was performed in triplicate.
3.6. Effect of Essential Oils on Biofilm Formation and Eradication
Five A. baumannii strains, namely AB-8, AB-R, AB-11, AB-13, and AB-16, were semiquantitatively examined for their biofilm forming ability in a 96-well flat bottom microtiter plate according to a formula reported by Naves et al. [46]:
where:
BF, biofilm formation
AB, stained bacteria cells attached to the wells
CW, stained control wells
Bacterial isolate is considered a strong, moderate, weak, or negative biofilm forming if BF is ≥0.300, 0.200–0.299, 0.100–0.199, and <0.100, respectively [46].
Then, strong or moderate biofilm forming strains were selected to perform biofilm inhibition/eradication assays.
3.6.1. Biofilm Inhibition Assay
The assay was performed according to Hussein et al. [47]. To estimate the ability of E.O/standard to inhibit biofilm formation by bacteria, an overnight culture of the corresponding isolate in L.B broth, adjusted at OD600 of 1, was diluted 1:100 in fresh LB broth. Then, 200 µL of each culture were dispensed in the wells of 96-well flat-shaped bottom microplates. A total of 1 µL of sub-MIC concentrations of each E.O./standard compound (0.5× MIC, 0.25× MIC, and 0.1× MIC) was mixed with LB broth in the wells and then incubated overnight at 37 °C. Growth was monitored by recording the optical density at 600 nm using microplate reader (BioTek Synergy 2, Winooski, VTUSA). Plates were then washed gently twice with phosphate-buffered saline to remove planktonic cells without disturbing the biofilm. Plates were left to completely dry in a laminar air flow cabinet. For biofilm visualization, 200 µL of 0.5% crystal violet solution were added to each well and left still for 30 min. Plates were then washed twice with distilled water to remove excess stain and left to dry completely. To extract the color of crystal violet, 150 µL of 99% ethanol were added per well and plates were left for 15 min with gentle shaking. The extracted color was measured colorimetrically at 570 nm in order to estimate the extent of biofilm formation. Negative and positive controls (broth only and broth inoculated with bacteria, respectively) were included. The test was performed in triplicate.
3.6.2. Biofilm Eradication Assay
Test strains were allowed to form mature biofilm for 24 h in the wells of 96-well flat-shaped bottom microplates under the same conditioned mentioned in biofilm inhibition assay without any E.O or inhibitory compound. After that, the spent media containing the planktonic cells were discarded and fresh L.B broth with different concentrations of the test E.O./standard compound (0.5× MIC, 0.25× MIC, and 0.1× MIC) were added to the mature biofilm in the wells. The plates were further incubated for 24 h at 37 °C, then they were washed and stained for biofilm visualization according to the steps mentioned in biofilm inhibition assay. Negative and positive controls (broth only and broth inoculated with bacteria, respectively) were included. The test was performed in triplicate.
3.7. Determination of the Minimum Bactericidal Concentration (MBC) by Broth Microdilution Technique
Amounts of 100 µL of L.B broth were dispensed into the U-shaped bottom 96-well microplates, concentrations equivalent to 1× MIC, 2× MIC, 4× MIC, 6× MIC, and 8× MIC of each E.O/standard compound, against A. baumannii strain-8 (AB-8), were tested. In total, 2 µL of a freshly prepared bacterial suspension (107 CFU·mL−1) was inoculated into the mixture, plates were then incubated at 37 °C for 24 h. Viable colony count on L.B agar medium was performed to determine the MBC at which no colonies appear. Negative and positive controls were included (L.B broth + DMSO and L.B broth + DMSO + bacteria, respectively). The test was performed in triplicate.
3.8. Kill Kinetics Assay
Time–kill kinetics of P. dioica and P. racemosa leaf E.O/standard compound against AB-8 strain was performed according to the method of [48]. The killing kinetics of the E.O were assayed at the bactericidal concentrations. First, 2 µL of 107 CFU·mL−1 suspension of AB-8 strain was incubated with MBCs of E.Os in L.B broth for up to 24 h. Then, samples of each test were withdrawn at time intervals of 0, 1, 2, 4, 6, 8, 10, 12, 16, and 24 h, diluted and subjected to viable colony count on L.B agar medium. Plates were then incubated at 37 °C for 24 h and then the visible colonies were counted. Positive and negative controls of DMSO + AB-8 in L.B broth and DMSO in L.B broth, respectively, were included. The assay was performed in triplicate.
3.9. In Vivo Wound Infection Animal Model
3.9.1. Ethical Statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the Faculty of Pharmacy, Cairo University, Cairo, Egypt (Approval no. MI-2364).
3.9.2. Experimental Design and Induction of Infection
The animal model was performed according to Wang et al. [49] as follows: 60 adult 6–8 weeks old male mice weighing 25–35 g were obtained from the Modern Veterinary Office for Laboratory Animals, Giza, Egypt. Animals were kept in cages under well-defined and standardized conditions (humidity and temperature-controlled room; 12-h light and 12-h dark cycle). The mice were initially examined to exclude any sign of skin inflammation and were fed with a standard dry food and water on demand.
Back hair was clipped from the cervical to mid-lumbar dorsum, and the skin was rinsed with ethanol. The skin on the shaved back of the mice was lifted with forceps and a 1.0 × 1.0 cm full thickness excisional wound was made by removing full thickness skin with a scissors. Infection was induced by adding aliquots of 10 µL freshly prepared suspension of isolate AB-8 in LB broth containing 107–108 CFU·mL−1 into the wound and allowed to be absorbed (the experiment was performed twice, once using 107 and then using 108 CFU·mL−1. Each time, the used inoculum was determined by viable colony count at time zero). P. dioica and P. racemosa leaf E.O/eugenol were prepared as 10× MIC which is equivalent to 5× MBC (5.2 µg·mL−1) in sweet almond oil as the vehicle. Cefepime was used as a positive drug control at a dose of 25 µg·g−1 of mice weight [31].
Twenty-four hours post-infection, animals were divided into six groups, 10 mice each, and treatment proceeded for 6 days.
Each group received 25 µL of respective treatment:
Group 1: P. dioica leaf E.O. dissolved in sweet almond oil (5.2 µg·mL−1).
Group 2: P. racemosa leaf E.O. dissolved in sweet almond oil (5.2 µg·mL−1).
Group 3: Eugenol dissolved in sweet almond oil (2 µg·mL−1)
Group 4: Cefepime solution (25 µg per gram of mouse weight).
Group 5: Vehicle (sweet almond oil).
Group 6: Phosphate-buffered saline.
Animals were then sacrificed and the wounded skin was removed for viable count technique (counts recovered from groups were compared), in brief, the wounded skins were cut into small pieces using sterile scalpels and then homogenized with PBS. Then, 20 μL aliquots of each suspension were 10-fold serially diluted using PBS, then, 10 μL of each dilution was spotted onto the surface of L.B agar medium, incubated at 37 °C for 24 h and then, the recovered colonies were counted and groups compared.
3.10. Statistical Analysis
One-way ANOVA, followed by Tukey’s multiple comparisons and Kruskal–Wallis test, Dunn’s multiple comparisons test (p-value < 0.05) were performed using GraphPad Prism 6.01 (GraphPad Software, Inc., San Diego, CA, USA).
4. Conclusions
Chemical characterization of Pimenta racemosa and Pimenta dioica leaf and berry E.Os revealed the abundance of oxygenated monoterpenes and phenolics’ which are likely to contribute to the oil antimicrobial effect. The strong antimicrobial and antibiofilm activities of both P. dioica and P. racemosa leaf oils pose them for future incorporation in treatment of MDR A. baumanii infections. Our future perspective will focus on designing and characterization of a suitable pharmaceutical nano-formulation containing the Pimenta E.Os, to be further up-scaled to be incorporated in antimicrobial and antibiofilm adjuvant therapy after sufficient clinical trials.
Acknowledgments
The authors would like to acknowledge Mona Tawfik Al-Kashef and Omneya Helmy, Microbiology and Immunology Faculty of Pharmacy, Cairo University, Egypt, who generously donated the clinical bacterial isolates used in this study.
Author Contributions
Conceptualization, M.A.F., F.R.S. and M.M.I.; methodology, M.A.F., M.M.I., F.R.S., R.S. and S.R.A.; software, M.A.F., F.R.S. and M.M.I.; validation, F.R.S., M.M.I. and R.S.; formal analysis, M.M.I. and F.R.S.; investigation, F.R.S., M.M.I. and R.S.; resources, M.A.F.; data curation, M.A.F., F.R.S, M.M.I. and R.S., writing—original draft preparation, F.R.S., M.M.I., R.S. and S.R.A.; writing—review and editing M.A.F., F.R.S., M.M.I., R.S. and S.R.A.; visualization, M.A.F., F.R.S., M.M.I. and R.S.; supervision, M.A.F.; project administration, M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, this work was supported by Faculty of Pharmacy, Cairo University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Guo S.A., DiPietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mihu M.R., Sandkovsky U., Han G., Friedman J.M., Nosanchuk J.D., Martinez L.R. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 2010;1:62–67. doi: 10.4161/viru.1.2.10038. [DOI] [PubMed] [Google Scholar]
- 3.Edwards R., Harding K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004;17:91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Howard A., O’Donoghue M., Feeney A., Sleator R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassetti M., Ginocchio F., Mikulska M. New treatment options against gram-negative organisms. Crit. Care. 2011;15:501–515. doi: 10.1186/cc9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekh J., Jadeja D., Chanda S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 2006;29:203–210. [Google Scholar]
- 9.Morton J.F. Atlas of Medicinal Plants of Middle America: Bahamas to Yucatan. Charles C. Thomas; Springfield, IL, USA: 1981. [Google Scholar]
- 10.Chaverri C., Cicció J.F. Leaf and fruit essential oil compositions of Pimenta guatemalensis (Myrtaceae) from Costa Rica. Rev. De Biol. Trop. 2015;63:303–311. doi: 10.15517/rbt.v63i1.14580. [DOI] [PubMed] [Google Scholar]
- 11.Contreras-Moreno B.Z. Chemical Composition of Essential Oil of Genus Pimenta (Myrtaceae) In: El-Shemy H., editor. Potential of Essential Oils. IntechOpen Limited; London, UK: 2018. p. 21. [DOI] [Google Scholar]
- 12.Seidemann J. World Spice Plants: Economic Usage, Botany, Taxonomy. Springer Science & Business Media; Berlin, Germany: 2005. [Google Scholar]
- 13.Oussalah M., Caillet S., Saucier L., Lacroix M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006;73:236–244. doi: 10.1016/j.meatsci.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Tucker A.O., Maciarello M.J., Adams R.P., Landrum L.R., Zanoni T.A. Volatile leaf oils of Caribbean Myrtaceae. I. Three varieties of Pimenta racemosa (Miller) J. Moore of the Dominican Republic and the commercial bay oil. J. Essent. Oil Res. 1991;3:323–329. doi: 10.1080/10412905.1991.9697952. [DOI] [Google Scholar]
- 15.Ayedoun A.M., Adeoti B.S., Setondji J., Menut C., Lamaty G., Bessiére J.-M. Aromatic plants from Tropical West Africa. IV. Chemical composition of leaf oil of Pimenta racemosa (Miller) JW Moore var. racemosa from Benin. J. Essent. Oil Res. 1996;8:207–209. doi: 10.1080/10412905.1996.9700597. [DOI] [Google Scholar]
- 16.Alitonou G.A., Noudogbessi J.-P., Sessou P., Tonouhewa A., Avlessi F., Menut C., Sohounhloue D. Chemical composition and biological activities of essential oils of Pimenta racemosa (Mill.) JW Moore. from Benin. Int. J. Biosci. 2012;2:1–12. [Google Scholar]
- 17.Tucker A.O., Maciarello M.J., Landrum L.R. Volatile leaf oils of Caribbean Myrtaceae. II. Pimenta dioica (L.) Merr. of Jamaica. J. Essent. Oil Res. 1991;3:195–196. doi: 10.1080/10412905.1991.9700504. [DOI] [Google Scholar]
- 18.Prasad M.A., Zolnik C.P., Molina J.J. Leveraging phytochemicals: The plant phylogeny predicts sources of novel antibacterial compounds. Future Sci. OA. 2019;5:FSO407. doi: 10.2144/fsoa-2018-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vázquez-Sánchez D., Galvão J.A., Ambrosio C.M., Gloria E.M., Oetterer M. Single and binary applications of essential oils effectively control Listeria monocytogenes biofilms. Ind. Crop. Prod. 2018;121:452–460. doi: 10.1016/j.indcrop.2018.05.045. [DOI] [Google Scholar]
- 20.Vázquez-Sánchez D., Galvão J.A., Mazine M.R., Gloria E.M., Oetterer M. Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int. J. Food Microbiol. 2018;286:128–138. doi: 10.1016/j.ijfoodmicro.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Yadav M.K., Chae S.-W., Im G.J., Chung J.-W., Song J.-J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE. 2015;10:e0119564. doi: 10.1371/journal.pone.0119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shehabeldine A.M., Ashour R.M., Okba M.M., Saber F.R. Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020;254:112669. doi: 10.1016/j.jep.2020.112669. [DOI] [PubMed] [Google Scholar]
- 23.Vasavi H.S., Arun A.B., Rekha P.D. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pac. J. Trop. Biomed. 2013;3:954–959. doi: 10.1016/S2221-1691(13)60185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández Díaz L., Rodríguez Jorge M., García D., Pino Alea J. Actividad antidermatofítica in vitro de aceites esenciales. Rev. Cuba. Plantas Med. 2003;8 [Google Scholar]
- 25.Goldbeck J.C., do Nascimento J.E., Jacob R.G., Fiorentini Â.M., da Silva W.P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crop. Prod. 2014;60:304–309. doi: 10.1016/j.indcrop.2014.05.030. [DOI] [Google Scholar]
- 26.Merghni A., Noumi E., Hadded O., Dridi N., Panwar H., Ceylan O., Mastouri M., Snoussi M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1, 8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018;118:74–80. doi: 10.1016/j.micpath.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Santos F., Rao V. 1, 8-cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Digest. Dis. Sci. 2001;46:331–337. doi: 10.1023/A:1005604932760. [DOI] [PubMed] [Google Scholar]
- 28.Stojković D., Soković M., Glamočlija J., Džamić A., Ćirić A., Ristić M., Grubišić D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011;128:1017–1022. [Google Scholar]
- 29.Kim Y.-G., Lee J.-H., Gwon G., Kim S.-I., Park J.G., Lee J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157: H7. Sci. Rep. 2016;6:36377. doi: 10.1038/srep36377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhara L., Tripathi A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur. J. Integr. Med. 2013;5:527–536. doi: 10.1016/j.eujim.2013.08.005. [DOI] [Google Scholar]
- 31.Marchese A., Barbieri R., Coppo E., Orhan I.E., Daglia M., Nabavi S.F., Izadi M., Abdollahi M., Nabavi S.M., Ajami M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017;43:668–689. doi: 10.1080/1040841X.2017.1295225. [DOI] [PubMed] [Google Scholar]
- 32.Cunha B.A. Optimal therapy for multidrug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 2010;16:170–171. doi: 10.3201/eid1601.090922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devi K.P., Sakthivel R., Nisha S.A., Suganthy N., Pandian S.K. Eugenol alters the integrity of cell membrane and acts against the nosocomial pathogen Proteus mirabilis. Arch. Pharmacal Res. 2013;36:282–292. doi: 10.1007/s12272-013-0028-3. [DOI] [PubMed] [Google Scholar]
- 34.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiolology. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Trombetta D., Castelli F., Sarpietro M.G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q., Niu H., Zhang W., Mu H., Sun C., Duan J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiolol. 2015;60:421–430. doi: 10.1111/lam.12401. [DOI] [PubMed] [Google Scholar]
- 37.Freitas P.R., de Araújo A.C.J., dos Santos Barbosa C.R., Muniz D.F., da Silva A.C.A., Rocha J.E., de Morais Oliveira-Tintino C.D., Ribeiro-Filho J., da Silva L.E., Confortin C. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Ind. Crop. Prod. 2020;145:112106. doi: 10.1016/j.indcrop.2020.112106. [DOI] [Google Scholar]
- 38.Seifert H., Dijkshoorn L., Gerner-Smidt P., Pelzer N., Tjernberg I., Vaneechoutte M. Distribution of Acinetobacter species on human skin: Comparison of phenotypic and genotypic identification methods. J. Clin. Microbiol. 1997;35:2819–2825. doi: 10.1128/JCM.35.11.2819-2825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aldoghaim F.S., Flematti G.R., Hammer K.A. Antimicrobial activity of several cineole-rich Western Australian Eucalyptus essential oils. Microorganisms. 2018;6:122. doi: 10.3390/microorganisms6040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karumathil D.P., Surendran-Nair M., Venkitanarayanan K. Efficacy of Trans-cinnamaldehyde and Eugenol in Reducing Acinetobacter baumannii Adhesion to and Invasion of Human Keratinocytes and Controlling Wound Infection In Vitro. Phytother. Res. 2016;30:2053–2059. doi: 10.1002/ptr.5713. [DOI] [PubMed] [Google Scholar]
- 41.Tsai C.-C., Lin C.-S., Hsu C.-R., Chang C.-M., Chang I.-W., Lin L.-W., Hung C.-H., Wang J.-L. Using the Chinese herb Scutellaria barbata against extensively drug-resistant Acinetobacter baumannii infections: In vitro and in vivo studies. Bmc Complementary Altern. Med. 2018;18:96. doi: 10.1186/s12906-018-2151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egyptian Pharmacopeia . Central Administration of Pharmaceutical Affairs (CAPA) 4th ed. Ministry of Health and Population; Cairo, Egypt: 2005. pp. 31–33. [Google Scholar]
- 43.Farag M.A., Wessjohann L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012;77:C1179–C1184. doi: 10.1111/j.1750-3841.2012.02927.x. [DOI] [PubMed] [Google Scholar]
- 44.Kashif M.T., Yassin A.S., Hosny A.E.-D.M. Detection of AmpC beta-lactamases using sodium salicylate. J. Microbiol. Methods. 2012;91:354–357. doi: 10.1016/j.mimet.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Golus J., Sawicki R., Widelski J., Ginalska G. The agar microdilution method–a new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016;121:1291–1299. doi: 10.1111/jam.13253. [DOI] [PubMed] [Google Scholar]
- 46.Naves P., Del Prado G., Huelves L., Gracia M., Ruiz V., Blanco J., Rodríguez-Cerrato V., Ponte M., Soriano F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J. Appl. Microbiol. 2008;105:585–590. doi: 10.1111/j.1365-2672.2008.03791.x. [DOI] [PubMed] [Google Scholar]
- 47.Hussein S.H., Samir R., Aziz R.K., Toama M.A. Two putative MmpL homologs contribute to antimicrobial resistance and nephropathy of enterohemorrhagic E. coli O157: H7. Gut Pathog. 2019;11:15. doi: 10.1186/s13099-019-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stratton C., Weeks L., Aldridge K. Comparison of kill-kinetic studies with agar and broth microdilution methods for determination of antimicrobial activity of selected agents against members of the Bacteroides fragilis group. J. Clin. Microbiol. 1987;25:645–649. doi: 10.1128/JCM.25.4.645-649.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X., Ghasri P., Amir M., Hwang B., Hou Y., Khilili M., Lin A., Keene D., Uitto J., Woodley D.T. Topical application of recombinant type VII collagen incorporates into the dermal–epidermal junction and promotes wound closure. Mol. Ther. 2013;21:1335–1344. doi: 10.1038/mt.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]