Abstract
Inherited retinal diseases (IRDs), which are among the most common genetic diseases in humans, define a clinically and genetically heterogeneous group of disorders. Over 80 forms of syndromic IRDs have been described. Approximately 200 genes are associated with these syndromes. The majority of syndromic IRDs are recessively inherited and rare. Many, although not all, syndromic IRDs can be classified into one of two major disease groups: inborn errors of metabolism and ciliopathies. Besides the retina, the systems and organs most commonly involved in syndromic IRDs are the central nervous system, ophthalmic extra-retinal tissues, ear, skeleton, kidney and the cardiovascular system. Due to the high degree of phenotypic variability and phenotypic overlap found in syndromic IRDs, correct diagnosis based on phenotypic features alone may be challenging and sometimes misleading. Therefore, genetic testing has become the benchmark for the diagnosis and management of patients with these conditions, as it complements the clinical findings and facilitates an accurate clinical diagnosis and treatment.
Keywords: retina, inherited retinal diseases, syndrome
1. Introduction
The retina is a multi-layered sensory tissue that lines the back of the eye. Its main function is the transduction of light energy into an electrical potential change, via a process known as phototransduction. The light-sensitive elements of the retina are the photoreceptor cells. The retina contains two types of photoreceptors, rods and cones. Rods (approximately 120 million in the human eye) are in charge of night vision, while cones (6 to 7 million in the human eye) are in charge of visual acuity and color vision. The highest cone concentration is found in the central region of the retina, known as the macula. Photoreceptors are highly compartmentalized cells, with the nucleus and other cellular organs located in the inner segment (IS), while the entire phototransduction machinery is included in the outer segment (OS).
Inherited retinal diseases (IRDs), which are among the most common genetic diseases in humans, define a clinically heterogeneous group of disorders, which cause visual loss due to improper development, dysfunction or premature death of the retinal photoreceptors [1]. IRDs are distinguished by several factors, including the type and location of affected cells and the timing of disease onset. The most common form of IRD is retinitis pigmentosa (RP) (also known as rod–cone dystrophy) [2]. Other IRD forms include cone/cone–rod dystrophy (CD/CRD) [3]; Leber congenital amaurosis (LCA) [4]; macular dystrophy (MD); and achromatopsia (rod monochromatism) [5], among others.
IRD is also one of the most genetically heterogeneous groups of disorders in humans, with over 260 genes identified to date (RetNet at https://sph.uth.edu/retnet/). It can be inherited as autosomal recessive (AR), autosomal dominant (AD) or X-linked (XL). Mitochondrial and digenic modes of inheritance have also been described. While in most cases of IRD the disease is limited to the eye (non-syndromic), over 80 forms of syndromic IRD have been described. Approximately 200 genes are associated with these syndromes (Table 1). In some cases of syndromic IRD, the retinal disease may be the presenting symptom and other systemic findings evolve during childhood, puberty or later on in life. In other cases, the first identifiable symptom of the syndrome is non-ocular and the retinal phenotype is revealed only later in life.
Table 1.
Syndromic inherited retinal diseases (IRDs).
| Syndrome (MIM/Reference) |
Gene | Inheritance * | Main Ocular Phenotypes # | Main Extra-Ocular Phenotypes ¶ |
|---|---|---|---|---|
| Abetalipoproteinemia; ABL (#200100) | MTTP | AR | RP | Fat malabsorption, neurodegeneration, acanthocytosis |
| Aicardi Syndrome; AIC (#304050) | Xp22 abnormalities | XLD | Chorioretinopathy, OA, microphthalmia, optic nerve coloboma, cataract | Callosal agenesis, PGR, microcephaly, ID, skeletal anomalies, neoplasia |
| Alagille Syndrome 1; ALGS1 (#118450) | JAG1 | AD | Iris stromal hypoplasia, posterior embryotoxon, microcornea, anomalous optic disc, peripapillary retinal depigmentation, chorioretinopathy | Liver disease, skeletal and renal involvement, characteristic facial features, ID, FTT |
| Alport Syndrome 1; ATS1 (#3010150) |
COL4A5 | XLD | Fleck retinopathy, cataract, myopia, corneal abnormalities | HL, renal disease |
| Alstrom Syndrome; ALMS (#203800) | ALMS1 | AR | CRD, MD, cataract | DD, SS, obesity, HL, cardiac, skeletal, hepatic, renal and endocrine involvement |
| Alpha-Methylacyl-CoA Racemase Deficiency; AMACRD (#614307) | AMACR | AR | RP | Neurodegeneration |
| Autoimmune Polyendocrine Syndrome, Type I, with or without Reversible Metaphyseal Dysplasia; APS1 (#240300) | AIRE | AD, AR | RP, keratopathy, keratoconjunctivitis | Multiple autoantibodies, anemia, hepatic, gastrointestinal, dental, skin, hair and endocrine involvement, hypogonadism |
| Bardet–Biedl Syndrome; BBS (#209900, #615981, #600151, #615982, #615983, #605231, #615984, #615985, #615986, #615987, #615988, #615989, #615990, #615991, 615992, #615993, #615994, #615995, #615996, #617119, #617406) [7] |
BBS1, BBS2, ARL6, BBS4, BBS5, MKKS, BBS7, TTC8, PTHB1, BBS10, TRIM32, BBS12, MKS1, CEP290, WDPCP, SDCCAG8, LZTFL1, BBIP1, IFT27, IFT74, C8ORF37, CEP164 | AR | RP, strabismus, cataract | ID, SS, obesity, hypogonadism, renal disease, polydactyly |
| Cerebellar Atrophy with Pigmentary Retinopathy [8] | MSTO1 | AR | RD | Cerebellar atrophy, ID, PGR |
| Congenital Disorder of Glycosylation; CDG (#212065, #617082, #613861, #608799, #300896) |
PMM2, NUS1, DHDDS, DPM1, SLC35A2 | AR | RP | FTT, microcephaly, ID, neurodegeneration, cardiac, hepatic, gastrointestinal, renal and hematological involvement |
| Congenital Disorder of Glycosylation with Defective Fucosylation 2; CDGF2 (#618324) | FCSK | AR | MD, OA, strabismus | FTT, ID, hypotonia, neurodegeneration, gastrointestinal anomalies |
| Cranioectodermal Dysplasia 4; CED4 (#614378) | WDR19 | AR | RP | Skeletal anomalies, SS, respiratory, hepatic and renal involvement |
| Ceroid Lipofuscinosis, Neuronal; CLN (#256730, #204500, #204200, #256731, #601780, #610951, #600143, #610127, #614706) |
PPT1, TPP1, CLN3, CLN5, CLN6, MFSD8, CLN8, CTSD, GRN | AR | RP, CRD, OA | Microcephaly, ID, neurodegeneration |
| Cohen Syndrome; COH1 (#216550) | VPS13B | AR | RD, OA, strabismus, high myopia | ID, DD, microcephaly, SS, obesity, skeletal, cardiac, hematological and endocrine involvement |
| Coenzyme Q10 Deficiency, Primary, 1; COQ10D1 (#607426) | COQ2 | AR | RP | ID, cerebellar atrophy, HL, cardiac, hepatic, renal and muscular involvement |
| Combined Oxidative Phosphorylation Deficiency 29; COXPD29 (#616811) | TXN2 | AR | RD, OA | Microcephaly, hypotonia, DD, ID, neurodegeneration |
| Charcot–Marie–Tooth Disease, X-linked recessive, 5; CMTX5 (#311070) | PRPS1 | XLR | RP, OA | Peripheral neuropathy, HL |
| Cone–Rod Dystrophy and Hearing Loss 1; CRDHL1 (#617236) | CEP78 | AR | CRD | HL |
| Cockayne Syndrome; CS (#216400, #133540) | ERCC8, ERCC6 | AR | RD, OA, cataract, strabismus | IUGR, PGR, microcephaly, ID, neurodegeneration, HL, renal, skeletal and skin involvement |
| Cystinosis, Nephropathic; CTNS (#219800, #219900) |
CTNS | AR | RD, corneal crystals | Renal disease, neurodegeneration, skeletal and endocrine anomalies |
| Danon Disease (#300257) | LAMP2 | XLD | RD | Cardiac disease, myopathy, ID |
| Diabetes and Deafness, Maternally Inherited; MIDD (#520000) | MTTL1, MTTE, MTTK, mitochondrial DNA rearrangements | Mi | RD, MD, ophthalmoplegia | HL, cardiac and neurological anomalies, diabetes mellitus |
| Dyskeratosis Congenita, Autosomal Dominant 3; DKCA3 (#613990) | TINF2 | AD | RD, blockage of lacrimal ducts | IUGR, SS, microcephaly, ID, HL, respiratory, skin, skeletal and hematological involvement, neoplasia |
| Hypobetalipoproteinemia, Familial, 1; FHBL1 (#615558) | APOB | AR | RP | Fat malabsorption, neurodegeneration, acanthocytosis |
| Hypobetalipoproteinemia, Acanthocytosis, Retinitis Pigmentosa and Pallidal Degeneration; HARP (#607236) | PANK2 | AR | RP | Fat malabsorption, neurodegeneration, acanthocytosis |
| Hypotrichosis, Congenital, with Juvenile Macular Dystrophy; HJMD (#601553) | CDH3 | AR | MD | Hypotrichosis |
| Hermansky–Pudlak Syndrome; HPS (#614072, #614073, #614077) |
HPS3, HPS4, BLOC1S3 | AR | Hypopigmentation of retina and choroid, foveal hypoplasia, nystagmus, iris transillumination | Skin and hair hypopigmentation, bleeding diathesis |
| Hyper-IgD Syndrome; HIDS (#260920) | MVK | AR | RP | Hematological anomalies, gastrointestinal and skeletal involvement, periodic fever |
| Hyperoxaluria, Primary, Type I; HP1 (#259900) | AGXT | AR | RD, OA | Renal disease, dental, cardiovascular and skin involvement, peripheral neuropathy |
| Intellectual Developmental Disorder and Retinitis Pigmentosa; IDDRP (#618195) | SCAPER | AR | RP, MD, cataract | ID, skeletal abnormalities, male sterility |
| Jalili Syndrome (#217080) | CNNM4 | AR | CRD | Amelogenesis imperfecta |
| Joubert Syndrome; JBTS (#213300, #608091, #608629, #610188, #610688, #611560, #612291, #612285, #614464, #614465, #614844, #614970, #615636, #615665, #616781, #617121, #617562, #617622, #618161, #300804) |
INPP5E, TMEM216, AHI1, CEP290, TMEM67, RPGRIP1L, ARL13B, CC2D2A, CEP41, TMEM138, ZNF423, TMEM231, CSPP1, PDE6D, CEP104, MKS1, TMEM107, ARMC9, ARL3 | AR | RD, chorioretinal coloboma, optic nerve coloboma, microphthalmia, oculomotor apraxia, esotropia, ptosis | Brain structural anomalies, FTT, macrocephaly, ID, neurodegeneration, genitourinary, hepatic, respiratory and skeletal involvement |
| OFD1 | XLR | |||
| Kearns–Sayre Syndrome; KSS (#530000) | Mitochondrial DNA deletions | Mi | RD, ophthalmoplegia | SS, microcephaly, neurodegeneration, cardiac, renal and endocrine involvement |
| Laurence–Moon Syndrome; LNMS (#245800) | PNPLA6 | AR | Chorioretinal degeneration | ID, neurodegeneration, genitourinary abnormalities |
| Leber Congenital Amaurosis with Early-Onset Deafness; LCAEOD (#617879) | TUBB4B | AD | LCA | HL |
| Lipodystrophy, familial partial, type7; FPLD7 (#606721) | CAV1 | AD | RD, cataract | Lack of facial fat, orthostatic hypotension, neurological and skin involvement |
| Methylmalonic Aciduria and Homocystinuria, cblC type; MAHCC (#277400) | MMACHC | AR | RP, CRD | FTT, microcephaly, ID, neurodegeneration, renal and hematological involvement |
| Mevalonic Aciduria; MEVA (#610377) | MVK | AR | RP, OA, cataract | FTT, DD, neurodegeneration, spleen, hepatic, skeletal, skin and hematological involvement |
| Microcephaly and Chorioretinopathy, autosomal recessive; MCCRP (#251270, #616171, #616335) | TUBGCP6, PLK4, TUBGCP4 | AR | Chorioretinopathy, OA, microphthalmia, microcornea, cataract | IUGR, microcephaly, brain structural anomalies, DD, ID, neurodegeneration, SS |
| Microcephaly with or without Chorioretinopathy, Lymphedema or Mental Retardation; MCLMR (#152950) | KIF11 | AD | Chorioretinopathy, myopia, hypermetropia, corneal opacity, microcornea, microphthalmia, cataract | Microcephaly, ID, neurodegeneration, lymphedema |
| Microphthalmia, Syndromic 5; MCOPS5 (#610125) | OTX2 | AD | RD, microphthalmia, anophthalmia, optic nerve hypoplasia or agenesis, microcornea, cataract | Brain structural anomalies, hypotonia, pituitary dysfunction, DD, SS, cleft palate, abnormal genitalia, joint laxity |
| Mitochondrial Complex II Deficiency (#252011) | SDHA, SDHD, SDHAF1 | AR | RD, OA, ptosis, ophthalmoplegia | SS, cardiac, skeletal, muscular and neurological involvement |
| Mitochondrial Complex IV Deficiency (#220110) | APOPT1, COA3, COX6A2, COX6B1, COX8A, COX10, COX14, COX20, PET100, TACO1 | AR | RD, OA, ptosis | FTT, brain structural anomalies, ID, HL, cardiac, respiratory, hepatic, renal and muscular involvement |
| Mucolipidosis III alpha/beta; MLIII A/B (#252600) | GNPTAB | AR | RD, corneal clouding | Neurodegeneration, ID, SS, coarse facies, skeletal, cardiac and skin involvement |
| Mucolipidosis IV; ML4 (#252650) | MCOLN1 | AR | RD, OA, corneal disease, strabismus | Microcephaly, ID, neurodegeneration |
| Mucopolysaccharidosis; MPS (#309900, #252930, #607014, #253000, #253010) |
IDS | XLR | RP, ptosis, corneal clouding | Neurodegeneration, ID, SS, coarse facies, HL, skeletal, cardiac, respiratory, hepatic, gastrointestinal and skin involvement |
| HGSNAT, IDUA, GALN5, GLB1 | AR | |||
| Nephronophthisis 15; NPHP15 (#614845) |
CEP164 | AR | LCA | Renal disease |
| Neurodegeneration with Brain Iron Accumulation 1; NBIA1 (#234200) | PANK2 | AR | RD, OA, eyelid apraxia | Neurodegeneration, gastrointestinal, skeletal, skin and muscular involvement |
| Neuropathy, Ataxia and Retinitis Pigmentosa; NARP (#551500) | MTATP6 | Mi | RP | Neurodegeneration, ataxia |
| Norrie Disease; ND (#310600) | NDP | XLR | Retinal dysgenesis, retinal dysplasia, OA, microphthalmia, vitreous atrophy, corneal opacities, iris atrophy, cataract | HL, ID, neurodegeneration |
| Oculoauricular Syndrome; OCACS (#612109) | HMX1 | AR | RP, microphthalmia, microcornea, cataract, microphakia, sclerocornea, increased intraocular pressure | External ear abnormalities |
| Orofaciodigital Syndrome XVI; OFD16 (#617563) | TMEM107 | AR | RD, oculomotor apraxia, ptosis | Facial anomalies, breathing abnormalities, polydactyly, hypotonia, ID, neurological anomalies |
| Oliver–McFarlane Syndrome; OMCS (#275400) | PNPLA6 | AR | Chorioretinopathy, OA | SS, ID, neurodegeneration, obesity, male external genitalia abnormalities, endocrine anomalies |
| Peroxisomal Acyl-CoA Oxidase Deficiency (#264470) | ACOX1 | AR | RD, OA, strabismus | Neurodegeneration, ID, HL, liver disease |
| Peroxisome Biogenesis Disorder; PBD (#214100, #614866, #601539, #234580, #614879, #266510) | PEX1, PEX2, PEX5, PEX6, PEX7, PEX12 | AR | RD, OA, corneal clouding, cataract | FTT, neurodegeneration, ID, HL, dental, cardiac, hepatic, genitourinary and skeletal involvement |
| Posterior Column Ataxia with Retinitis Pigmentosa; AXPC1 (#609033) | FLVCR1 | AR | RP, OA | Posterior column ataxia, neurodegeneration, gastrointestinal and skeletal involvement |
| Polyneuropathy, Hearing Loss, Ataxia, Retinitis Pigmentosa and Cataract; PHARC (#612674) | ABHD12 | AR | RP, OA, cataract | Ataxia, neurodegeneration, HL |
| Pseudoxanthoma Elasticum; PXE (#264800) | ABCC6 | AR | RD, MD, choroidal neovascularization | Skin lesions, cardiovascular disease, gastrointestinal and genitourinary involvement |
| Refsum Disease, classic (#266500) | PHYH | AR | RP | Neurodegeneration, ataxia, HL, anosmia, cardiac, skeletal and skin involvement |
| Retinal Dystrophy, Iris Coloboma and Comedogenic Acne Syndrome; RDCCAS (#615147) | RPB4 | AR | RD, coloboma of the iris, displacement of the pupil, microcornea, cataract | Comedogenic acne |
| Retinal Dystrophy and Iris Coloboma with or without Cataract; RDICC (#616722) | MIR204 | AD | RD, coloboma of the iris, congenital cataract | |
| Retinal Dystrophy, Juvenile Cataracts and Short Stature Syndrome; RDJCSS (#616108) | RDH11 | AR | RD, juvenile cataracts | SS, DD, ID, dental anomalies |
| Retinal Dystrophy and Obesity; RDOB (#616188) | TUB | AR | RD | Obesity |
| Revesz Syndrome (#268130) | TINF2 | AD | RD | IUGR, brain structural anomalies, neurodegeneration, ID, aplastic anemia, skin, hair and nail abnormalities |
| Retinitis Pigmentosa–Deafness Syndrome (#500004) | MTTS2 | Mi | RP | HL |
| Retinitis Pigmentosa and Erythrocytic Microcytosis; RPEM (#616959) | TRNT1 | AR | RP | Erythrocytic microcytosis and additional hematologic abnormalities |
| Retinitis Pigmentosa, Hypopituitarism, Nephronophtisis and mild Skeletal Dysplasia; RHYNS (#602152) | TMEM67 | AR | RP | Hypopituitarism, renal disease, skeletal anomalies, HL |
| Retinitis Pigmentosa 82 with or without Situs Inversus; RP82 (#615434) | ARL2BP | AR | RP | Situs inversus, male infertility |
| Retinitis Pigmentosa with or without Skeletal Anomalies; RPSKA (#250410) | CWC27 | AR | RP | SS, skeletal anomalies, ID |
| Retinitis Pigmentosa, X-linked and Sinorespiratory Infections, with or without Deafness (#300455) | RPGR | XL | RP | Recurrent respiratory infections, HL |
| Senior–Løken Syndrome; SLSN (#266900, #606996, #609254, #610189, #613615, #616307, #616629) |
NPHP1, NPHP4, IQCB1, CEP290, SDCCAG8, WDR19, TRAF3IP1 | AR | RP, LCA | Renal disease |
| Short Stature, Hearing Loss, Retinitis Pigmentosa and Distinctive Facies; SHRF (#617763) | EXOSC2 | AR | RP, corneal dystrophy, glaucoma, strabismus | SS, facial anomalies, HL, neurodegeneration, DD, ID |
| Sideroblastic Anemia with B-cell Immunodeficiency, Periodic Fevers and Developmental Delay; SIFD (#616084) | TRNT1 | AR | RP | Sideroblastic anemia, immunodeficiency, growth retardation, DD, periodic fever, HL, neurological, cardiac and renal involvement |
| Spondylometaphyseal Dysplasia with Cone–Rod Dystrophy; SMDCRD (#608940) | PCYT1A | AR | CRD | Skeletal anomalies, PGR |
| Spondylometaphyseal Dysplasia, Axial; SMDAX (#602271) | CFAP410 | AR | RP, CRD, OA | Skeletal anomalies, respiratory disease, reduced sperm motility |
| Short-Rib Thoracic Dysplasia 9 with or without Polydactyly; SRTD9 (#266920) | IFT140 | AR | RP | Skeletal anomalies, renal disease, ID |
| Thiamine-Responsive Megaloblastic Anemia Syndrome; TRMA (#249270) | SLC19A2 | AR | OA, RD | Megaloblastic anemia, diabetes mellitus, HL |
| Usher Syndrome; USH (#276900, #276904, #601067, #602083, #606943, #614869, #276901, #605472, #611383, #276902, #614504) |
MYO7A, USH1C, CDH23, PCDH15, USH1G, CIB2, USH2A, ADGRV1, WHRN, CLRN1, HARS1 | AR | RP | HL, vestibular dysfunction |
| Wolfram Syndrome 1, WFS1 (#222300) | WFS1 | AR | OA, RD | Diabetes mellitus, diabetes insipidus, HL, neurodegeneration, genitourinary and neurologic involvement |
| White–Sutton Syndrome, WHSUS (#616364) | POGZ | AD | RP, OA, cortical blindness | DD, characteristic facial features, hypotonia, HL, joint laxity, gastrointestinal anomalies |
| Xeroderma Pigmentosum, group B; XPB (#610651) | ERCC3 | AR | RD, OA, micropathalmia | Neoplasia, skin anomalies, SS, microcephaly, HL, ID, brain structural anomalies, neurodegeneration |
* AD, autosomal dominant; AR, autosomal recessive; Mi, mitochondrial; XL, X-linked; XLD, X-linked dominant; XLR, X-linked recessive. # CRD, cone–rod dystrophy; LCA, Leber congenital amaurosis; MD, macular dystrophy; OA, optic atrophy; RD, retinal dystrophy; RP, retinitis pigmentosa. ¶ DD, developmental delay; FTT, failure to thrive; HL, hearing loss; ID, intellectual disability; IUGR, intrauterine growth restriction; PGR, postnatal growth retardation; SS, short stature.
The topic of systemic diseases associated with IRDs has been reviewed before, including the description of some of these syndromes [6]. In the current review, we provide a comprehensive summary of the vast majority of syndromic IRD forms reported to date, for which the underlying gene/s have been identified (as listed in OMIM-Online Mendelian Inheritance in Man, https://www.ncbi.nlm.nih.gov/omim, and reported in the literature). We discuss different aspects, including the marked genetic heterogeneity of some of these syndromes, phenotypic overlap and diagnostic approaches.
2. Syndromic IRD Types
The majority of syndromic IRDs are recessively inherited and rare. Many, although not all, syndromic IRDs can be classified into one of two major disease groups: inborn errors of metabolism (IEM) and ciliopathies.
IEMs are genetic disorders leading to failure of carbohydrate metabolism, protein metabolism, fatty acid oxidation or glycogen storage. Many IEMs present with neurologic symptoms [9]. The retina develops from an embryonic forebrain pouch and is considered an extension of the brain. Therefore, neurodegeneration resulting from IEMs often involves retinal degeneration (RD) as well. Major forms of syndromic IRD that belong to the IEM group include congenital disorders of glycosylation (CDG) [10], neuronal ceroid lipofuscinoses (CLNs) [11], mucopolysaccharidoses (MPSs) [12], peroxisomal diseases [13] and more (Table 1).
Ciliopathies are a group of genetic diseases caused by mutations in genes associated with the structure and function of primary cilia. Primary cilia function as signaling hubs that sense environmental cues and are pivotal for organ development and function, and for tissue homeostasis. By their nature, cilia defects are usually pleiotropic, affecting more than one system [14]. Photoreceptor OSs are highly modified primary sensory cilia. The proximal end of the OS is linked to the cell body (i.e., the IS) via a connecting cilium which is structurally homologous to the transition zone of primary cilia [15]. Consequently, retinal pathogenesis is a common finding in ciliopathies. Other organs which are commonly affected in ciliopathies are the central nervous system (CNS), kidney, liver, skeleton and inner ear. Major forms of syndromic IRD that belong to the ciliopathy group include Bardet–Biedl Syndrome (BBS) [16], Joubert Syndrome (JBTS) [17], Usher Syndrome (USH) [18], Senior–Løken Syndrome (SLN) [19] and Alstrom Syndrome (ALMS) [20] (Table 1).
3. Genetic Heterogeneity in Syndromic IRDs
Over 80 forms of syndromic IRD have been described (Table 1). Most of these syndromes are caused by a single gene. However, 14 of 81 (17%) of the syndromes listed in Table 1 are genetically heterogeneous, and some of them are associated with multiple causative genes. The most genetically heterogeneous forms of syndromic IRD are three recessively inherited ciliopathies: BBS, JBTS and USH. The protein products of the genes associated with each one of these ciliopathies tend to form multi-protein complexes in the retina and in additional tissues, thus explaining the similar phenotypes caused by mutations in each of these genes.
BBS (prevalence of about 1/125,000) is characterized by a combination of RP, postaxial polydactyly (and other skeletal abnormalities), hypogonadism, renal disease, intellectual disability (ID) and truncal obesity [16]. Twenty-one causative genes have been reported to date (OMIM) (Table 1). Their protein products are involved in lipid homeostasis, intraflagellar transport, establishing planar cell polarity, regulation of intracellular trafficking and centrosomal functions. Eight of these genes encode for subunits of a protein complex, the BBSome, which is integral in ciliary as well as intracellular trafficking [21]. In the retina, the BBSome is required for photoreceptor OS formation and maintenance [22], as well as for retinal synaptogenesis [23].
JBTS (prevalence of 1/55,000–1/200,000) is characterized by a peculiar midbrain–hindbrain malformation, known as the molar tooth sign. The neurological presentation of JBTS includes hypotonia that evolves into ataxia, developmental delay, abnormal eye movements and neonatal breathing abnormalities. This picture is often associated with variable multiorgan involvement, mainly of the retina, kidney and liver [17]. RD has been reported in 38% of patients [24]. To date, 36 causative genes have been identified, all encoding for proteins expressed in the primary cilium or its apparatus (OMIM). Mutations in 20 of these genes (listed in Table 1) have been specifically associated with RD and additional ocular abnormalities (such as nystagmus and oculomotor apraxia). Ocular abnormalities have also been reported in patients with mutations in most other JBTS genes. However, since RD was not specifically reported in these patients, these genes are not listed in Table 1. Given the marked phenotypic heterogeneity found in JBTS patients, it is very likely that a retinal phenotype will be associated with these genes in the future, as additional patients are discovered.
USH (prevalence of 1–4/25,000) is characterized by the combination of RP and sensorineural hearing loss (HL). Based on the severity and progression of HL, age at onset of RP and the presence or absence of vestibular impairment, the majority of USH cases can be classified into one of three clinical subtypes (USH1-3). Eleven USH genes have been identified to date (OMIM) (Table 1). Their protein products are associated with a wide range of functions, including actin-binding molecular motors, cell adhesion, scaffolding and cellular trafficking. USH proteins form complexes and function cooperatively in neurosensory cells of both the retina and the inner ear (reviewed in [18,25]).
4. Phenotypic Overlap in Syndromic IRDs
When referring to syndromic IRD, phenotypic overlap is a common phenomenon, which can be divided into three groups, as detailed below:
4.1. Phenotypic Overlap between Different IRD Syndromes
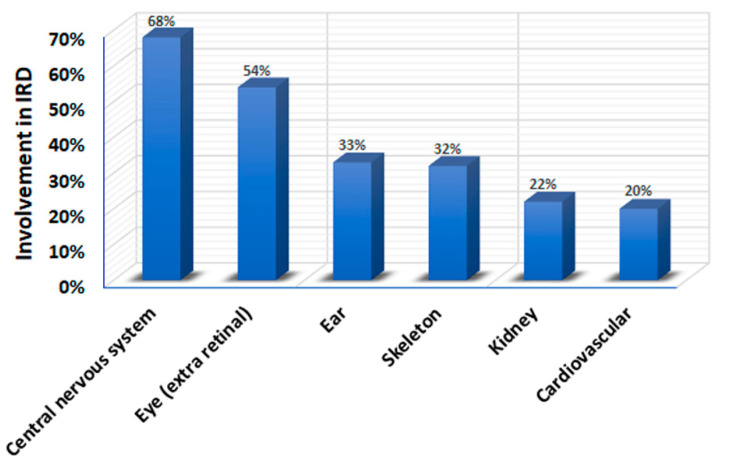
Many types of syndromic IRD have a multi-systemic nature. Certain organs are commonly involved in syndromic IRDs. Specifically, CNS involvement (usually manifested as ID) is found in 68% of IRD syndromes (Table 1 and Figure 1), and over 80 genes are associated with the combination of IRD and ID [26] (Table 1). In addition to ID, the most common findings in syndromic IRD are extra-retinal eye abnormalities and ear, skeletal, renal and cardiovascular involvement (Figure 1). Most of these syndromes are phenotypically heterogeneous, with many patients exhibiting only some of the phenotypic features. These factors lead to a marked phenotypic overlap between different syndromes, and to a diagnostic challenge. For example, the combination of RD, ID, renal disease and skeletal abnormalities is found in numerous forms of syndromic IRD, including BBS, JBTS and ALMS, among others (Table 1). The combination of retinal abnormalities and HL as prominent symptoms is found in USH, as well as in CRD and HL 1 syndrome [27], Leber congenital amaurosis with early-onset deafness syndrome [28], Norrie disease [29], peroxisome biogenesis disorders, Refsum disease [30] and more (Table 1). These overlaps may often lead to diagnostic mistakes [27,31,32].
Figure 1.
Systems and organs most commonly involved in syndromic IRDs.
4.2. Syndromic Versus Non-Syndromic IRD Caused by the Same Genes
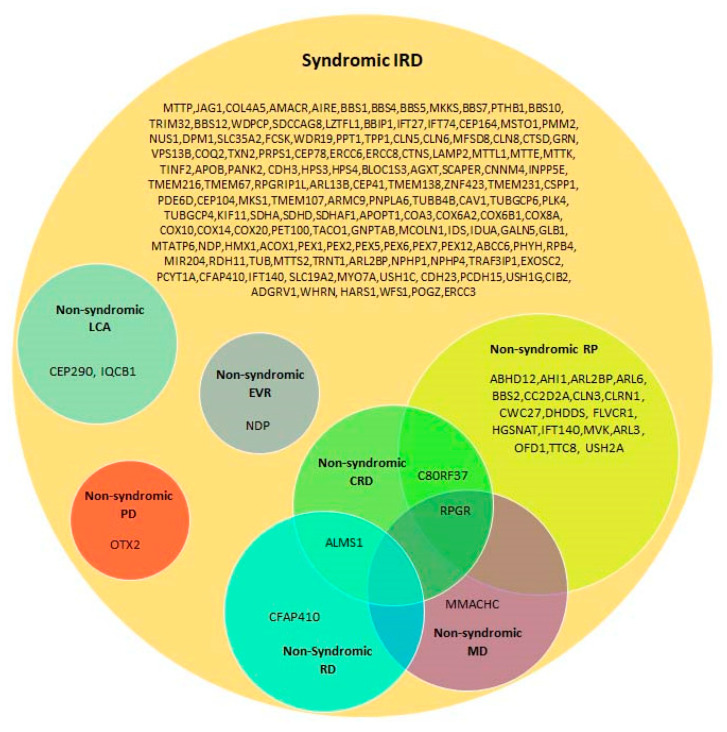
Twenty-eight of the genes listed in Table 1 can cause both syndromic and non-syndromic IRD (Table 2 and Figure 2). In general, milder hypomorphic mutations in these genes are associated with non-syndromic IRD, while null mutations lead to the involvement of additional tissues. In addition, the involvement of additional genetic and environmental factors in the determination of the final phenotypic outcome cannot be excluded. A prominent example is the USH2A gene. Mutations in this gene are the most common cause of USH, and specifically of USH2 (RP with congenital, mild to moderate sensorineural HL) [33]. Moreover, USH2A variants are also one of the commonest causes of AR non-syndromic RP worldwide [34,35,36]. It appears that the specific combination of USH2A variants determines whether one has USH2 or non-syndromic RP [37,38,39]. In addition, RD is more severe in patients with USH2A-related USH2 than in patients with USH2A-related non-syndromic RP. However, the reason is not completely understood [38].
Table 2.
Genes underlying both syndromic and non-syndromic IRDs.
| Gene | Syndromic IRD | Non-Syndromic IRD (MIM) | Reference |
|---|---|---|---|
| ABHD12 | PHARC | arRP | [40] |
| AHI1 | JBTS3 | arRP | [41] |
| ALMS1 | ALMS | arCRD, arEORD | [42] |
| ARL2BP | RP with situs inversus | arRP (#615434) | [43,44] |
| ARL3 | JBTS35 | adRP (#618173) | [45] |
| ARL6 | BBS3 | arRP (#613575) | [46] |
| BBS2 | BBS2 | arRP (#616562) | [47] |
| C8ORF37 | BBS21 | arCRD, arRP (#614500) | [48] |
| CC2D2A | JBTS9, MKS6 | arRP | [49] |
| CEP290 | BBS14, JBTS5, MKS4, SLSN6 | arLCA (#611755) | [50] |
| CFAP410 | SMDAX | arRD with or without macular staphyloma (#617547) | [51,52] |
| CLN3 | CLN3 | arRP | [53] |
| CLRN1 | USH3A | arRP (#614180) | [54] |
| CWC27 | RPSKA | arRP (#250410) | [55] |
| DHDDS | CDG1BB | arRP (#613861) | [56,57] |
| FLVCR1 | PCARP | arRP | [58] |
| HGSNAT | MPS3C | arRP (#616544) | [59] |
| IFT140 | SRTD9 with/without polydactyly | arRP (#617781) | [60] |
| IQCB1 | SLSN5 | arLCA | [61] |
| MFSD8 | CLN7 | arMD (#616170), arRD | [62] |
| MMACHC | MAHCC | arMD | [63] |
| MVK | HIDS, MEVA | arRP | [64] |
| NDP | ND | XL EVR (#305390) | [65] |
| OFD1 | JBTS10 | XL RP (#300424) | [66] |
| OTX2 | RD with pituitary dysfunction | adPD (#610125) | [67] |
| RPGR | RP, sinorespiratory infections and deafness | XL CRD (#304020), XL MD (#300834), XL RP (#300029) | [68] |
| TTC8 | BBS8 | arRP (#613464) | [69] |
| USH2A | USH2A | arRP (#613809) | [70] |
ALMS: Alstrom syndrome; ar: autosomal recessive; ad: autosomal dominant; BBS: Bardet–Biedl syndrome; CDG: congenital disorder of glycosylation; CLN: ceroid lipofuscinosis neuronal; CRD: cone–rod dystrophy; EORD: early-onset retinal degeneration; EVR: exudative vitreoretinopathy; HIDS: hyper-IgD syndrome; JBTS: Joubert syndrome; LCA: Leber congenital amaurosis; MAHCC: methylmalonic aciduria and homocystinuria, cblC type; MD: macular dystrophy; MEVA: mevalonic aciduria; MKS: Meckel syndrome; MPS: mucopolysaccharidosis; ND: Norrie disease; PCARP: posterior column ataxia with retinitis pigmentosa; PD: pattern dystrophy; PHARC: polyneuropathy, hearing loss, ataxia, retinitis pigmentosa and cataract; RD: retinal dystrophy; RP: retinitis pigmentosa; RPSKA: retinitis pigmentosa with skeletal anomalies; SLSN: Senior–Løken syndrome; SMDAX: spondylometaphyseal dysplasia axial; SRTD: short-rib thoracic dysplasia; USH: Usher syndrome; XL: X-linked.
Figure 2.
A Venn diagram showing the involvement of syndromic IRD genes in non-syndromic IRD phenotypes. CRD: cone–rod dystrophy; EVR: exudative vitreoretinopathy; LCA: Leber congenital amaurosis; MD: macular dystrophy; PD: pattern dystrophy; RD: retinal dystrophy; RP: retinitis pigmentosa.
4.3. Co-Existence of Non-Syndromic IRD and Additional Non-Ocular Diseases
IRD is one of the most genetically heterogeneous groups of disorders in humans, and most cases of IRD are non-syndromic. Non-syndromic IRD may coincide with other genetic (and non-genetic) rare conditions, leading to a clinical suspicion or diagnosis of a syndrome. For example, co-occurrence of non-syndromic RP and non-syndromic HL in a family may appear as USH [71].
5. Diagnostic Challenges
Due to the high degree of phenotypic variability and phenotypic overlap found in syndromic IRD, as described above, correct diagnosis based on phenotypic features alone may be challenging and sometimes misleading. Therefore, genetic testing has become the benchmark for the diagnosis and management of patients with these conditions, as it complements the clinical findings and facilitates an accurate clinical diagnosis. Establishing a correct diagnosis is important for both the patients and their family members, for multiple reasons: it enables the understanding of the natural history course, and the prediction of disease prognosis; it aids in tailoring correct follow-up and treatment, including potential gene-targeted therapies [72]; it leads to a reduction in disease prevalence, by genetic screening and counseling in high-risk populations; it allows the patients to pursue prenatal counseling and reproductive planning; and it enables identification of novel disease genes and mechanisms.
The existence of common founder mutations in certain populations allows for quick and efficient mutation screening in affected individuals, based on the relevant phenotype and ethnic background. This is performed by PCR-based DNA amplification and Sanger sequencing, or by specifically designed assays. Some examples are common USH3A-, USH1F-, ML4- and BBS2-causative mutations found in the Ashkenazi Jewish population [73,74,75,76]; and USH3A- and MKS1-causative mutations found in the Finnish population [77,78]. Nevertheless, for most syndromic IRD patients worldwide, this strategy is not effective.
Currently, the most efficient approach for genetic diagnosis in monogenic diseases, including IRDs, is next-generation sequencing (NGS). NGS technologies facilitate the screening of the entire genome (whole genome sequencing, WGS); of all protein-coding regions (whole exome sequencing, WES); or of protein-coding regions of pre-determined panels of genes (targeted NGS, T-NGS) [79,80]. Since protein-coding regions comprise only 1–2% of the entire genome while harboring over 85% of variants causing Mendelian disorders, WES is still considered as the method of choice for genetic analysis, in both clinical and research settings. However, worldwide diagnostic yields of IRD patients by WES only range between 60% and 70% [36,81,82]. The missing mutations can be divided into four groups: (1) mutations located within exons, but missed due to technical issues, e.g., lack of coverage; (2) mutations located within covered exons, but missed due to limitations in data analysis and interpretation; (3) non-coding variants that may affect gene expression, mRNA stability, splicing and more; and (4) structural variants, such as large deletions, duplications and inversions, which are missed by WES. The latter two may be identified by WGS [34].
6. Summary and Conclusions
Over 80 forms of syndromic IRDs have been described, and approximately 200 causative genes identified. Due to the high degree of phenotypic variability and phenotypic overlap found in syndromic IRD, correct diagnosis based on phenotypic features alone is insufficient, and genetic testing has become the benchmark for the diagnosis and management of patients with these conditions. For most patients, molecular diagnosis should be based on NGS technologies. Currently, WES is the most popular approach for genetic analysis in patients with monogenic diseases, including IRDs. However, the continuous progress in both technical and bioinformatic aspects, as well as the reduction of costs, is already leading to a shift towards WGS as the method of choice.
Author Contributions
Conceptualization, T.B.-Y.; methodology, T.B.-Y.; validation, Y.T. and T.B.-Y.; formal analysis, Y.T.; investigation, Y.T. and T.B.-Y.; resources, T.B.-Y.; data curation, Y.T. and T.B.-Y.; writing—original draft preparation, T.B.-Y.; writing—review and editing, T.B.-Y.; supervision, T.B.-Y.; funding acquisition, T.B.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Israel Science Foundation (grant number 525/19).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Duncan J.L., Pierce E.A., Laster A.M., Daiger S.P., Birch D.G., Ash J.D., Iannaccone A., Flannery J.G., Sahel J.A., Zack D.J., et al. Inherited Retinal Degenerations: Current Landscape and Knowledge Gaps. Transl. Vis. Sci. Technol. 2018;7:6. doi: 10.1167/tvst.7.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verbakel S.K., van Huet R.A.C., Boon C.J.F., den Hollander A.I., Collin R.W.J., Klaver C.C.W., Hoyng C.B., Roepman R., Klevering B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Thiadens A.A., Phan T.M., Zekveld-Vroon R.C., Leroy B.P., van den Born L.I., Hoyng C.B., Klaver C.C., Roosing S., Pott J.W., van Schooneveld M.J., et al. Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology. 2012;119:819–826. doi: 10.1016/j.ophtha.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Kumaran N., Moore A.T., Weleber R.G., Michaelides M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017;101:1147–1154. doi: 10.1136/bjophthalmol-2016-309975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang S.H., Sharma T. Rod Monochromatism (Achromatopsia) Adv. Exp. Med. Biol. 2018;1085:119–123. doi: 10.1007/978-3-319-95046-4_24. [DOI] [PubMed] [Google Scholar]
- 6.Werdich X.Q., Place E.M., Pierce E.A. Systemic diseases associated with retinal dystrophies. Semin. Ophthalmol. 2014;29:319–328. doi: 10.3109/08820538.2014.959202. [DOI] [PubMed] [Google Scholar]
- 7.Shamseldin H.E., Shaheen R., Ewida N., Bubshait D.K., Alkuraya H., Almardawi E., Howaidi A., Sabr Y., Abdalla E.M., Alfaifi A.Y., et al. The morbid genome of ciliopathies: An update. Genet. Med. 2020;22:1051–1060. doi: 10.1038/s41436-020-0761-1. [DOI] [PubMed] [Google Scholar]
- 8.Iwama K., Takaori T., Fukushima A., Tohyama J., Ishiyama A., Ohba C., Mitsuhashi S., Miyatake S., Takata A., Miyake N., et al. Novel recessive mutations in MSTO1 cause cerebellar atrophy with pigmentary retinopathy. J. Hum. Genet. 2018;63:263–270. doi: 10.1038/s10038-017-0405-8. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira C.R., van Karnebeek C.D.M. Inborn errors of metabolism. Handb. Clin. Neurol. 2019;162:449–481. doi: 10.1016/B978-0-444-64029-1.00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeze H.H., Schachter H., Kinoshita T. Genetic Disorders of Glycosylation. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2017. Chapter 45. [Google Scholar]
- 11.Nita D.A., Mole S.E., Minassian B.A. Neuronal ceroid lipofuscinoses. Epileptic Disord. 2016;18:73–88. doi: 10.1684/epd.2016.0844. [DOI] [PubMed] [Google Scholar]
- 12.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl. 5):v4–v12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 13.Imanaka T. Biogenesis and Function of Peroxisomes in Human Disease with a Focus on the ABC Transporter. Biol. Pharm. Bull. 2019;42:649–665. doi: 10.1248/bpb.b18-00723. [DOI] [PubMed] [Google Scholar]
- 14.Sreekumar V., Norris D.P. Cilia and development. Curr. Opin. Genet. Dev. 2019;56:15–21. doi: 10.1016/j.gde.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 15.May-Simera H., Nagel-Wolfrum K., Wolfrum U. Cilia—The sensory antennae in the eye. Prog. Retin. Eye Res. 2017;60:144–180. doi: 10.1016/j.preteyeres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Tsang S.H., Aycinena A.R.P., Sharma T. Ciliopathy: Bardet-Biedl Syndrome. Adv. Exp. Med. Biol. 2018;1085:171–174. doi: 10.1007/978-3-319-95046-4_33. [DOI] [PubMed] [Google Scholar]
- 17.Valente E.M., Dallapiccola B., Bertini E. Joubert syndrome and related disorders. Handb. Clin. Neurol. 2013;113:1879–1888. doi: 10.1016/B978-0-444-59565-2.00058-7. [DOI] [PubMed] [Google Scholar]
- 18.Geleoc G.G.S., El-Amraoui A. Disease mechanisms and gene therapy for Usher syndrome. Hear. Res. 2020;394:107932. doi: 10.1016/j.heares.2020.107932. [DOI] [PubMed] [Google Scholar]
- 19.Tsang S.H., Aycinena A.R.P., Sharma T. Ciliopathy: Senior-Loken Syndrome. Adv. Exp. Med. Biol. 2018;1085:175–178. doi: 10.1007/978-3-319-95046-4_34. [DOI] [PubMed] [Google Scholar]
- 20.Tsang S.H., Aycinena A.R.P., Sharma T. Ciliopathy: Alstrom Syndrome. Adv. Exp. Med. Biol. 2018;1085:179–180. doi: 10.1007/978-3-319-95046-4_35. [DOI] [PubMed] [Google Scholar]
- 21.Petriman N.A., Lorentzen E. Moving proteins along in the cilium. Elife. 2020;9:e55254. doi: 10.7554/eLife.55254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu Y., Garrison J.E., Kim G., Schmitz A.R., Searby C.C., Zhang Q., Datta P., Nishimura D.Y., Seo S., Sheffield V.C. BBSome function is required for both the morphogenesis and maintenance of the photoreceptor outer segment. PLoS Genet. 2017;13:e1007057. doi: 10.1371/journal.pgen.1007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu Y., Garrison J.E., Seo S., Sheffield V.C. The absence of BBSome function decreases synaptogenesis and causes ectopic synapse formation in the retina. Sci. Rep. 2020;10:8321. doi: 10.1038/s41598-020-65233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S.F., Kowal T.J., Ning K., Koo E.B., Wu A.Y., Mahajan V.B., Sun Y. Review of Ocular Manifestations of Joubert Syndrome. Genes. 2018;9:605. doi: 10.3390/genes9120605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Amraoui A., Petit C. The retinal phenotype of Usher syndrome: Pathophysiological insights from animal models. Comptes Rendus Biol. 2014;337:167–177. doi: 10.1016/j.crvi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Yang X.R., Benson M.D., MacDonald I.M., Innes A.M. A diagnostic approach to syndromic retinal dystrophies with intellectual disability. Am. J. Med. Genet. C Semin. Med. Genet. 2020;184:538–570. doi: 10.1002/ajmg.c.31834. [DOI] [PubMed] [Google Scholar]
- 27.Namburi P., Ratnapriya R., Khateb S., Lazar C.H., Kinarty Y., Obolensky A., Erdinest I., Marks-Ohana D., Pras E., Ben-Yosef T., et al. Bi-allelic Truncating Mutations in CEP78, Encoding Centrosomal Protein 78, Cause Cone-Rod Degeneration with Sensorineural Hearing Loss. Am. J. Hum. Genet. 2016;99:777–784. doi: 10.1016/j.ajhg.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luscan R., Mechaussier S., Paul A., Tian G., Gerard X., Defoort-Dellhemmes S., Loundon N., Audo I., Bonnin S., LeGargasson J.F., et al. Mutations in TUBB4B Cause a Distinctive Sensorineural Disease. Am. J. Hum. Genet. 2017;101:1006–1012. doi: 10.1016/j.ajhg.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims K.B. NDP-Related Retinopathies. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington; Seattle, WA, USA: 2014. [Google Scholar]
- 30.Tsang S.H., Sharma T. Inborn Errors of Metabolism: Refsum Disease. Adv. Exp. Med. Biol. 2018;1085:191–192. doi: 10.1007/978-3-319-95046-4_39. [DOI] [PubMed] [Google Scholar]
- 31.Raas-Rothschild A., Wanders R.J., Mooijer P.A., Gootjes J., Waterham H.R., Gutman A., Suzuki Y., Shimozawa N., Kondo N., Eshel G., et al. A PEX6-defective peroxisomal biogenesis disorder with severe phenotype in an infant, versus mild phenotype resembling Usher syndrome in the affected parents. Am. J. Hum. Genet. 2002;70:1062–1068. doi: 10.1086/339766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith C.E., Poulter J.A., Levin A.V., Capasso J.E., Price S., Ben-Yosef T., Sharony R., Newman W.G., Shore R.C., Brookes S.J., et al. Spectrum of PEX1 and PEX6 variants in Heimler syndrome. Eur. J. Hum. Genet. 2016;24:1565–1571. doi: 10.1038/ejhg.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Quesne Stabej P., Saihan Z., Rangesh N., Steele-Stallard H.B., Ambrose J., Coffey A., Emmerson J., Haralambous E., Hughes Y., Steel K.P., et al. Comprehensive sequence analysis of nine Usher syndrome genes in the UK National Collaborative Usher Study. J. Med. Genet. 2012;49:27–36. doi: 10.1136/jmedgenet-2011-100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carss K.J., Arno G., Erwood M., Stephens J., Sanchis-Juan A., Hull S., Megy K., Grozeva D., Dewhurst E., Malka S., et al. Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. Am. J. Hum. Genet. 2017;100:75–90. doi: 10.1016/j.ajhg.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dockery A., Stephenson K., Keegan D., Wynne N., Silvestri G., Humphries P., Kenna P.F., Carrigan M., Farrar G.J. Target 5000: Target Capture Sequencing for Inherited Retinal Degenerations. Genes. 2017;8:304. doi: 10.3390/genes8110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharon D., Ben-Yosef T., Goldenberg-Cohen N., Pras E., Gradstein L., Soudry S., Mezer E., Zur D., Abbasi A.H., Zeitz C., et al. A nationwide genetic analysis of inherited retinal diseases in Israel as assessed by the Israeli inherited retinal disease consortium (IIRDC) Hum. Mutat. 2019;41:140–149. doi: 10.1002/humu.23903. [DOI] [PubMed] [Google Scholar]
- 37.Lenassi E., Vincent A., Li Z., Saihan Z., Coffey A.J., Steele-Stallard H.B., Moore A.T., Steel K.P., Luxon L.M., Heon E., et al. A detailed clinical and molecular survey of subjects with nonsyndromic USH2A retinopathy reveals an allelic hierarchy of disease-causing variants. Eur. J. Hum. Genet. 2015;23:1318–1327. doi: 10.1038/ejhg.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierrache L.H., Hartel B.P., van Wijk E., Meester-Smoor M.A., Cremers F.P., de Baere E., de Zaeytijd J., van Schooneveld M.J., Cremers C.W., Dagnelie G., et al. Visual Prognosis in USH2A-Associated Retinitis Pigmentosa Is Worse for Patients with Usher Syndrome Type IIa Than for Those with Nonsyndromic Retinitis Pigmentosa. Ophthalmology. 2016;123:1151–1160. doi: 10.1016/j.ophtha.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Sengillo J.D., Cabral T., Schuerch K., Duong J., Lee W., Boudreault K., Xu Y., Justus S., Sparrow J.R., Mahajan V.B., et al. Electroretinography Reveals Difference in Cone Function between Syndromic and Nonsyndromic USH2A Patients. Sci. Rep. 2017;7:11170. doi: 10.1038/s41598-017-11679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiguchi K.M., Avila-Fernandez A., van Huet R.A., Corton M., Perez-Carro R., Martin-Garrido E., Lopez-Molina M.I., Blanco-Kelly F., Hoefsloot L.H., van Zelst-Stams W.A., et al. Exome sequencing extends the phenotypic spectrum for ABHD12 mutations: From syndromic to nonsyndromic retinal degeneration. Ophthalmology. 2014;121:1620–1627. doi: 10.1016/j.ophtha.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen T.T., Hull S., Roepman R., van den Born L.I., Oud M.M., de Vrieze E., Hetterschijt L., Letteboer S.J.F., van Beersum S.E.C., Blokland E.A., et al. Missense mutations in the WD40 domain of AHI1 cause non-syndromic retinitis pigmentosa. J. Med. Genet. 2017;54:624–632. doi: 10.1136/jmedgenet-2016-104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aldrees A., Abdelkader E., Al-Habboubi H., Alrwebah H., Rahbeeni Z., Schatz P. Non-syndromic retinal dystrophy associated with homozygous mutations in the ALMS1 gene. Ophthalmic Genet. 2019;40:77–79. doi: 10.1080/13816810.2018.1551495. [DOI] [PubMed] [Google Scholar]
- 43.Audo I., El Shamieh S., Mejecase C., Michiels C., Demontant V., Antonio A., Condroyer C., Boyard F., Letexier M., Saraiva J.P., et al. ARL2BP mutations account for 0.1% of autosomal recessive rod-cone dystrophies with the report of a novel splice variant. Clin. Genet. 2017;92:109–111. doi: 10.1111/cge.12909. [DOI] [PubMed] [Google Scholar]
- 44.Davidson A.E., Schwarz N., Zelinger L., Stern-Schneider G., Shoemark A., Spitzbarth B., Gross M., Laxer U., Sosna J., Sergouniotis P.I., et al. Mutations in ARL2BP, encoding ADP-ribosylation-factor-like 2 binding protein, cause autosomal-recessive retinitis pigmentosa. Am. J. Hum. Genet. 2013;93:321–329. doi: 10.1016/j.ajhg.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holtan J.P., Teigen K., Aukrust I., Bragadottir R., Houge G. Dominant ARL3-related retinitis pigmentosa. Ophthalmic Genet. 2019;40:124–128. doi: 10.1080/13816810.2019.1586965. [DOI] [PubMed] [Google Scholar]
- 46.Aldahmesh M.A., Safieh L.A., Alkuraya H., Al-Rajhi A., Shamseldin H., Hashem M., Alzahrani F., Khan A.O., Alqahtani F., Rahbeeni Z., et al. Molecular characterization of retinitis pigmentosa in Saudi Arabia. Mol. Vis. 2009;15:2464–2469. [PMC free article] [PubMed] [Google Scholar]
- 47.Shevach E., Ali M., Mizrahi-Meissonnier L., McKibbin M., El-Asrag M., Watson C.M., Inglehearn C.F., Ben-Yosef T., Blumenfeld A., Jalas C., et al. Association Between Missense Mutations in the BBS2 Gene and Nonsyndromic Retinitis Pigmentosa. JAMA Ophthalmol. 2015;133:312–318. doi: 10.1001/jamaophthalmol.2014.5251. [DOI] [PubMed] [Google Scholar]
- 48.Khan A.O., Decker E., Bachmann N., Bolz H.J., Bergmann C. C8orf37 is mutated in Bardet-Biedl syndrome and constitutes a locus allelic to non-syndromic retinal dystrophies. Ophthalmic Genet. 2016;37:290–293. doi: 10.3109/13816810.2015.1066830. [DOI] [PubMed] [Google Scholar]
- 49.Mejecase C., Hummel A., Mohand-Said S., Andrieu C., El Shamieh S., Antonio A., Condroyer C., Boyard F., Foussard M., Blanchard S., et al. Whole exome sequencing resolves complex phenotype and identifies CC2D2A mutations underlying non-syndromic rod-cone dystrophy. Clin. Genet. 2019;95:329–333. doi: 10.1111/cge.13453. [DOI] [PubMed] [Google Scholar]
- 50.den Hollander A.I., Koenekoop R.K., Yzer S., Lopez I., Arends M.L., Voesenek K.E., Zonneveld M.N., Strom T.M., Meitinger T., Brunner H.G., et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan A.O., Eisenberger T., Nagel-Wolfrum K., Wolfrum U., Bolz H.J. C21orf2 is mutated in recessive early-onset retinal dystrophy with macular staphyloma and encodes a protein that localises to the photoreceptor primary cilium. Br. J. Ophthalmol. 2015;99:1725–1731. doi: 10.1136/bjophthalmol-2015-307277. [DOI] [PubMed] [Google Scholar]
- 52.Suga A., Mizota A., Kato M., Kuniyoshi K., Yoshitake K., Sultan W., Yamazaki M., Shimomura Y., Ikeo K., Tsunoda K., et al. Identification of Novel Mutations in the LRR-Cap Domain of C21orf2 in Japanese Patients With Retinitis Pigmentosa and Cone-Rod Dystrophy. Investig. Ophthalmol. Vis. Sci. 2016;57:4255–4263. doi: 10.1167/iovs.16-19450. [DOI] [PubMed] [Google Scholar]
- 53.Ku C.A., Hull S., Arno G., Vincent A., Carss K., Kayton R., Weeks D., Anderson G.W., Geraets R., Parker C., et al. Detailed Clinical Phenotype and Molecular Genetic Findings in CLN3-Associated Isolated Retinal Degeneration. JAMA Ophthalmol. 2017;135:749–760. doi: 10.1001/jamaophthalmol.2017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan M.I., Kersten F.F., Azam M., Collin R.W., Hussain A., Shah S.T., Keunen J.E., Kremer H., Cremers F.P., Qamar R., et al. CLRN1 mutations cause nonsyndromic retinitis pigmentosa. Ophthalmology. 2011;118:1444–1448. doi: 10.1016/j.ophtha.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 55.Xu M., Xie Y.A., Abouzeid H., Gordon C.T., Fiorentino A., Sun Z., Lehman A., Osman I.S., Dharmat R., Riveiro-Alvarez R., et al. Mutations in the Spliceosome Component CWC27 Cause Retinal Degeneration with or without Additional Developmental Anomalies. Am. J. Hum. Genet. 2017;100:592–604. doi: 10.1016/j.ajhg.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lam B.L., Zuchner S.L., Dallman J., Wen R., Alfonso E.C., Vance J.M., Pericak-Vance M.A. Mutation K42E in dehydrodolichol diphosphate synthase (DHDDS) causes recessive retinitis pigmentosa. Adv. Exp. Med. Biol. 2014;801:165–170. doi: 10.1007/978-1-4614-3209-8_21. [DOI] [PubMed] [Google Scholar]
- 57.Zelinger L., Banin E., Obolensky A., Mizrahi-Meissonnier L., Beryozkin A., Bandah-Rozenfeld D., Frenkel S., Ben-Yosef T., Merin S., Schwartz S.B., et al. A missense mutation in DHDDS, encoding dehydrodolichyl diphosphate synthase, is associated with autosomal-recessive retinitis pigmentosa in Ashkenazi Jews. Am. J. Hum. Genet. 2011;88:207–215. doi: 10.1016/j.ajhg.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuehlewein L., Schols L., Llavona P., Grimm A., Biskup S., Zrenner E., Kohl S. Phenotypic spectrum of autosomal recessive retinitis pigmentosa without posterior column ataxia caused by mutations in the FLVCR1 gene. Graefes Arch. Clin. Exp. Ophthalmol. 2019;257:629–638. doi: 10.1007/s00417-018-04233-7. [DOI] [PubMed] [Google Scholar]
- 59.Haer-Wigman L., Newman H., Leibu R., Bax N.M., Baris H.N., Rizel L., Banin E., Massarweh A., Roosing S., Lefeber D.J., et al. Non-syndromic retinitis pigmentosa due to mutations in the mucopolysaccharidosis type IIIC gene, heparan-alpha-glucosaminide N-acetyltransferase (HGSNAT) Hum. Mol. Genet. 2015;24:3742–3751. doi: 10.1093/hmg/ddv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu M., Yang L., Wang F., Li H., Wang X., Wang W., Ge Z., Wang K., Zhao L., Li H., et al. Mutations in human IFT140 cause non-syndromic retinal degeneration. Hum. Genet. 2015;134:1069–1078. doi: 10.1007/s00439-015-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone E.M., Cideciyan A.V., Aleman T.S., Scheetz T.E., Sumaroka A., Ehlinger M.A., Schwartz S.B., Fishman G.A., Traboulsi E.I., Lam B.L., et al. Variations in NPHP5 in patients with nonsyndromic leber congenital amaurosis and Senior-Loken syndrome. Arch. Ophthalmol. 2011;129:81–87. doi: 10.1001/archophthalmol.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan K.N., El-Asrag M.E., Ku C.A., Holder G.E., McKibbin M., Arno G., Poulter J.A., Carss K., Bommireddy T., Bagheri S., et al. Specific Alleles of CLN7/MFSD8, a Protein That Localizes to Photoreceptor Synaptic Terminals, Cause a Spectrum of Nonsyndromic Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2017;58:2906–2914. doi: 10.1167/iovs.16-20608. [DOI] [PubMed] [Google Scholar]
- 63.Collison F.T., Xie Y.A., Gambin T., Jhangiani S., Muzny D., Gibbs R., Lupski J.R., Fishman G.A., Allikmets R. Whole Exome Sequencing Identifies an Adult-Onset Case of Methylmalonic Aciduria and Homocystinuria Type C (cblC) with Non-Syndromic Bull’s Eye Maculopathy. Ophthalmic Genet. 2015;36:270–275. doi: 10.3109/13816810.2015.1010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siemiatkowska A.M., van den Born L.I., van Hagen P.M., Stoffels M., Neveling K., Henkes A., Kipping-Geertsema M., Hoefsloot L.H., Hoyng C.B., Simon A., et al. Mutations in the mevalonate kinase (MVK) gene cause nonsyndromic retinitis pigmentosa. Ophthalmology. 2013;120:2697–2705. doi: 10.1016/j.ophtha.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z.Y., Battinelli E.M., Fielder A., Bundey S., Sims K., Breakefield X.O., Craig I.W. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat. Genet. 1993;5:180–183. doi: 10.1038/ng1093-180. [DOI] [PubMed] [Google Scholar]
- 66.Webb T.R., Parfitt D.A., Gardner J.C., Martinez A., Bevilacqua D., Davidson A.E., Zito I., Thiselton D.L., Ressa J.H., Apergi M., et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23) Hum. Mol. Genet. 2012;21:3647–3654. doi: 10.1093/hmg/dds194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vincent A., Forster N., Maynes J.T., Paton T.A., Billingsley G., Roslin N.M., Ali A., Sutherland J., Wright T., Westall C.A., et al. OTX2 mutations cause autosomal dominant pattern dystrophy of the retinal pigment epithelium. J. Med. Genet. 2014;51:797–805. doi: 10.1136/jmedgenet-2014-102620. [DOI] [PubMed] [Google Scholar]
- 68.Tee J.J., Smith A.J., Hardcastle A.J., Michaelides M. RPGR-associated retinopathy: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2016;100:1022–1027. doi: 10.1136/bjophthalmol-2015-307698. [DOI] [PubMed] [Google Scholar]
- 69.Riazuddin S.A., Iqbal M., Wang Y., Masuda T., Chen Y., Bowne S., Sullivan L.S., Waseem N.H., Bhattacharya S., Daiger S.P., et al. A splice-site mutation in a retina-specific exon of BBS8 causes nonsyndromic retinitis pigmentosa. Am. J. Hum. Genet. 2010;86:805–812. doi: 10.1016/j.ajhg.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rivolta C., Sweklo E.A., Berson E.L., Dryja T.P. Missense mutation in the USH2A gene: Association with recessive retinitis pigmentosa without hearing loss. Am. J. Hum. Genet. 2000;66:1975–1978. doi: 10.1086/302926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ehrenberg M., Weiss S., Orenstein N., Goldenberg-Cohen N., Ben-Yosef T. The co-occurrence of rare non-ocular phenotypes in patients with inherited retinal degenerations. Mol. Vis. 2019;25:691–702. [PMC free article] [PubMed] [Google Scholar]
- 72.Ku C.A., Pennesi M.E. The new landscape of retinal gene therapy. Am. J. Med. Genet. C Semin. Med. Genet. 2020;184:846–859. doi: 10.1002/ajmg.c.31842. [DOI] [PubMed] [Google Scholar]
- 73.Bach G., Webb M.B., Bargal R., Zeigler M., Ekstein J. The frequency of mucolipidosis type IV in the Ashkenazi Jewish population and the identification of 3 novel MCOLN1 mutations. Hum. Mutat. 2005;26:591. doi: 10.1002/humu.9385. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Yosef T., Ness S.L., Madeo A.C., Bar-Lev A., Wolfman J.H., Ahmed Z.M., Desnick R.J., Willner J.P., Avraham K.B., Ostrer H., et al. A mutation of PCDH15 among Ashkenazi Jews with the type 1 Usher syndrome. N. Engl. J. Med. 2003;348:1664–1670. doi: 10.1056/NEJMoa021502. [DOI] [PubMed] [Google Scholar]
- 75.Fedick A., Jalas C., Abeliovich D., Krakinovsky Y., Ekstein J., Ekstein A., Treff N.R. Carrier frequency of two BBS2 mutations in the Ashkenazi population. Clin. Genet. 2014;85:578–582. doi: 10.1111/cge.12231. [DOI] [PubMed] [Google Scholar]
- 76.Ness S.L., Ben-Yosef T., Bar-Lev A., Madeo A.C., Brewer C.C., Avraham K.B., Kornreich R., Desnick R.J., Willner J.P., Friedman T.B., et al. Genetic homogeneity and phenotypic variability among Ashkenazi Jews with Usher syndrome type III. J. Med. Genet. 2003;40:767–772. doi: 10.1136/jmg.40.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joensuu T., Hamalainen R., Yuan B., Johnson C., Tegelberg S., Gasparini P., Zelante L., Pirvola U., Pakarinen L., Lehesjoki A.E., et al. Mutations in a novel gene with transmembrane domains underlie Usher syndrome type 3. Am. J. Hum. Genet. 2001;69:673–684. doi: 10.1086/323610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kyttala M., Tallila J., Salonen R., Kopra O., Kohlschmidt N., Paavola-Sakki P., Peltonen L., Kestila M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat. Genet. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 79.Branham K., Schlegel D., Fahim A.T., Jayasundera K.T. Genetic testing for inherited retinal degenerations: Triumphs and tribulations. Am. J. Med. Genet. C Semin. Med. Genet. 2020;184:571–577. doi: 10.1002/ajmg.c.31835. [DOI] [PubMed] [Google Scholar]
- 80.Mansfield B.C., Yerxa B.R., Branham K.H. Implementation of a registry and open access genetic testing program for inherited retinal diseases within a non-profit foundation. Am. J. Med. Genet. C Semin. Med. Genet. 2020;184:838–845. doi: 10.1002/ajmg.c.31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone E.M., Andorf J.L., Whitmore S.S., DeLuca A.P., Giacalone J.C., Streb L.M., Braun T.A., Mullins R.F., Scheetz T.E., Sheffield V.C., et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology. 2017;124:1314–1331. doi: 10.1016/j.ophtha.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haer-Wigman L., van Zelst-Stams W.A., Pfundt R., van den Born L.I., Klaver C.C., Verheij J.B., Hoyng C.B., Breuning M.H., Boon C.J., Kievit A.J., et al. Diagnostic exome sequencing in 266 Dutch patients with visual impairment. Eur. J. Hum. Genet. 2017;25:591–599. doi: 10.1038/ejhg.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]