Abstract
The nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway is an important cell signaling mechanism in maintaining redox homeostasis in humans. The role of dietary flavonoids in activating Nrf2/ARE in relation to cancer chemoprevention or cancer promotion is not well established. Here we summarize the dual effects of flavonoids in cancer chemoprevention and cancer promotion with respect to the regulation of the Nrf2/ARE pathway, while underlying the possible cellular mechanisms. Luteolin, apigenin, quercetin, myricetin, rutin, naringenin, epicatechin, and genistein activate the Nrf2/ARE pathway in both normal and cancer cells. The hormetic effect of flavonoids has been observed due to their antioxidant or prooxidant activity, depending on the concentrations. Reported in vitro and in vivo investigations suggest that the activation of the Nrf2/ARE pathway by either endogenous or exogenous stimuli under normal physiological conditions contributes to redox homeostasis, which may provide a mechanism for cancer chemoprevention. However, some flavonoids, such as luteolin, apigenin, myricetin, quercetin, naringenin, epicatechin, genistein, and daidzein, at low concentrations (1.5 to 20 µM) facilitate cancer cell growth and proliferation in vitro. Paradoxically, some flavonoids, including luteolin, apigenin, and chrysin, inhibit the Nrf2/ARE pathway in vitro. Therefore, even though flavonoids play a major role in cancer chemoprevention, due to their possible inducement of cancer cell growth, the effects of dietary flavonoids on cancer pathophysiology in patients or appropriate experimental animal models should be investigated systematically.
Keywords: cancer chemoprevention, cancer promotion, polyphenols, oxidative homeostasis, Keap1/Nrf2
1. Introduction
1.1. Oxidative Stress and Antioxidant Defense System in Relation to Cancer
Several biological reactions that occur in the human body generate reactive oxygen species (ROS) [1]. Excessive generation of ROS can lead to oxidative stress that can potentially cause over 40 non-communicable diseases, such as certain cancers, diabetes mellitus, neurodegenerative diseases, and accelerated aging [2]. In addition to the primary endogenous ROS generation by many mechanisms, including the mitochondrial electron transport chain [1], exogenous stimuli, such as air pollutants, cigarette smoke, ionization radiation, xenobiotics, atmospheric pressure plasmas, and hypoxia, could induce ROS generation [3,4,5,6]. Non-communicable diseases and rapid aging induced by oxidative stress are mainly due to unrecoverable damages that occur to biological macromolecules, such as nucleic acids, proteins, and membranes [3,7]. For instance, DNA can be damaged in the forms of single-strand or double-strand DNA breakage, and stable modifications in the nitrogen bases of the pentose-phosphate backbone of DNA due to the ROS-induced oxidative stress [8,9]. If these damages are not repaired, they could lead to epigenetic alterations in proto-oncogenes and tumor suppressor genes, somatic gene mutations, and genomic instability, which could initiate carcinogenesis [8,10,11,12]. Therefore, it is essential to maintain the ROS at the non-deleterious basal level in cells, also called redox homeostasis, to prevent oxidative stress-induced DNA damage and the initiation of carcinogenesis, leading to neoplastic diseases [3,13].
The cellular antioxidant defense system is the primary mechanism to protect biological macromolecules from oxidative stress [3]. The enzymatic and non-enzymatic antioxidants of the antioxidant defense system are capable of neutralizing ROS, such as superoxide anion radical, hydrogen peroxide, and hydroxyl radical, and the secondary reactive species such as peroxyl and alkoxyl radicals, generated by their further oxidation [3,14,15]. In a cellular environment, superoxide anion radical is generated mainly due to the activities of lipoxygenase, nicotine adenine dinucleotide phosphate (NADPH) oxidase, cyclooxygenase, cytochrome P450, and xanthine oxidase [16,17]. This free radical is converted to hydrogen peroxide by superoxide dismutase (SOD) [18]. Hydrogen peroxide can also be produced by NADPH oxidase [19], xanthine oxidase [20] and amino acid oxidase enzymes [21], or as a result of oxygen consumption in the metabolic reactions that happen in peroxisome [22]. Hydrogen peroxides are further converted into water and oxygen by catalase (CAT) and glutathione peroxidase (GPx). GPx needs secondary enzymes such as glutathione reductase (GR) and co-factors, reduced glutathione and NADPH, to catalyze the conversion of hydrogen peroxide into water [18,23]. If hydrogen peroxides are not neutralized by CAT and GPx enzymes, hydrogen peroxides can react with superoxide radical, or undergo Fenton reactions or Haber–Weiss reactions in the presence of metal ions, such as copper and ferrous, to generate hydroxyl radical [24,25]. Hydroxyl radicals cause severe oxidative damage to DNA, and the inefficient or mis-repair of DNA can promote DNA mutations and genomic instability that can initiate carcinogenesis [3,13]. Therefore, it is essential to eliminate ROS to prevent oxidative stress-induced DNA damage. As such, pathways that regulate the expression of proteins related to the antioxidant defense system and other cytoprotective genes are vital in managing oxidative stress-induced DNA damage, and thus play a role in the prevention of cancer by limiting genomic instability [14,26].
In addition to oxidative stress-mediated cancer initiation, ROS play several roles in cancer therapy [27]. The ROS production in a cancer cell is higher than in a normal cell due to its hypermetabolism [28]. However, in cancer cells, oxidative balance is achieved due to their marked antioxidant capacity, facilitated by the activation of the antioxidant defense system and mediated by pathways such as Nrf2/ARE [27]. In anticancer therapy, oxidative stress-mediated damages to cancer cells are achieved by the induction of accelerated ROS generation and the inhibition of antioxidant defense systems, mainly through exogenous stimuli such as chemotherapeutic drugs, i.e., cisplatin, doxorubicin, and 2-methoxyestradiol [27,29,30]. ROS-mediated anticancer therapies are based on the generation of excessive ROS over the cytotoxic limit, which disrupts the redox homeostasis that kills cancer cells [31].
1.2. Mechanisms of Activation of the Antioxidant Defense System and Other Cytoprotective Genes
Activation of the antioxidant defense system and other cytoprotective genes, such as phase 2 detoxification enzymes, is mainly due to the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway in a cellular environment upon oxidative stress [32,33]. Briefly, the activation of this pathway is initiated by a transcription factor, Nrf2, which binds to the promoter region of the ARE, leading to the transcription of genes of antioxidant defense enzymes and phase 2 detoxifying enzymes. Thereby, these proteins restore the redox homeostasis by managing oxidative stress [34,35,36,37,38].
For the initiation of this pathway, Nrf2, a basic leucine-zipper transcription factor, needs to be activated. Under normal cellular physiological conditions, Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1), which is an endogenous inhibitor of Nrf2, bound to actin fibers [39,40,41]. Interactions between Keap1 and Nrf2 via its motifs (Neh2 ETGE and DLG) lead to the activation of the Nrf2 ubiquitination process, which is mediated by the Cullin 3 (Cul3)-based E3 ligase complex [42]. The degradation of Nrf2 is rapidly undertaken in the 26S proteasome, leading to low levels of Nrf2 in the cytoplasm. This avoids the stabilization, phosphorylation and nuclear translocation of Nrf2, resulting in 15–40 min of Nrf2 half-life time depending on the type of cell [40,43,44,45].
However, oxidative stress or the presence of electrophilic compounds induce the activation of the Nrf2 pathway through canonical mechanisms. Herein, the cysteine residues (Cys151, Cys257, Cys273, Cys288, and Cys297) in Keap1 undergo conformational changes upon oxidation or alkylation, and dissociate Nrf2 from Keap1 [46,47,48]. In addition, the non-canonical activation of Nrf2 by the influence of proteins such as p62, p21, dipeptidyl peptidase III (DPP3), Wilms tumor gene on X chromosome (WTX), breast cancer gene 1 (BRCA1), and partner and localizer of BRCA2 (PALB2) also leads to the cytoplasmic stabilization of Nrf2 [48]. These proteins disrupt the direct interactions of Keap1 with Nrf2, by binding to either Keap1 or Nrf2. Collectively, the canonical and non-canonical activation of Nrf2 results in a reduction of Nrf2 ubiquitination and, consequently, its degradation [48]. The detached Nrf2 can be negatively regulated by several other proteins, such as glycogen synthase kinase 3 beta (GSK-3β). Herein, GSK-3β-mediated phosphorylation of specific serine residues, such as Ser335 and Ser338 (numbers in mouse sequence), in the Neh6 domain of Nrf2 creates a degradation domain, which can be recognized by the ubiquitin ligase adapter E3 ubiquitin–protein ligase. Thereafter, the proteasomal degradation of Nrf2 is facilitated via the Cul3-based E3 ligase complex [41]. However, the phosphorylation of serine 558 residue is located in the canonical nuclear export signal of the Nrf2 protein by 5′ adenosine monophosphate (AMP)-activated protein kinase (AMPK), which leads to the improved stability of the Nrf2 protein facilitating the nuclear translocation [49,50]. Once Nrf2 is translocated into the nucleus, it begins heterodimerization with another transcription factor called musculoaponeurotic fibrosarcoma (sMaf) [44,45]. The resulting complex binds to ARE and initiates the transcription of downstream genes belonging to the antioxidant defense system and phase 2 detoxifying enzymes. Once these proteins are expressed, functions such as the oxidizing of xenobiotics or drugs, the conjugation of oxidized metabolites, and the transportation of final metabolites out of the intracellular environment will ensure cytoprotection by restoring redox homeostasis [33]. Therefore, the identification of endogenous and exogenous molecules that can activate the Nrf2/ARE pathway offers potential protection against oxidative stress-mediated diseases.
Among many other phytochemicals, flavonoids have shown the potential to activate the Nrf2/ARE pathway in the absence of oxidative inducers [38,51]. However, the activation of the Nrf2/ARE pathway has not always been beneficial because the same flavonoids may promote the growth of cancer cells as well [52]. In normal cells, chemoprevention can be achieved by expressing Nrf2/ARE-driven cytoprotective genes [34,35,37,53,54,55]. However, a study conducted by Harris and colleagues in 2015 showed that the biosynthesis of cytoprotective molecules glutathione (GSH) and thioredoxin synergistically facilitates cancer initiation and progression in genetically engineered mice of mammary tumor initiation [56]. In cancer cells, the constitutive activation of the Nrf2/ARE pathway promotes cell growth [57], cell survival [58], continuous proliferation, and the renewal of stem cells of several types of cancers, as well as resistance to chemotherapies [33]. Some cancer chemotherapeutics are designed to inhibit the Nrf2/ARE pathway in cancer cells [59]. Therefore, certain flavonoids may have the potential for use in cancer treatment [60,61,62,63]. Thus, the remainder of this review focuses on understanding the dual role of flavonoids in cancer chemoprevention and the effects on cancer cell growth or cancer promotion with respect to the regulation of the Nrf2/ARE pathway.
1.3. Hormetic Effects of Dietary Flavonoids
The hormetic behavior of phytochemicals should be taken into consideration when determining the safe and effective concentrations for cancer prevention or cancer treatment. Hormesis is the bi-phasic concentration/dose–response, often depicted as the U-shaped dose–response curve of some dietary antioxidants, drugs, and toxins [64]. Accordingly, at low concentrations/doses, biologically active molecules such as flavonoids exert beneficial effects, such as stimulation for either adaptation or protection from a stress factor [64,65,66,67]. At high concentrations/doses, they may exert either detrimental/inhibitory or toxic effects on the cells or tissue microenvironment [64,66,67,68]. As such, hormetic compounds act as antioxidants at low doses and prooxidants at high doses [13,40,69,70,71]. For example, apigenin and luteolin at low concentrations (6.25 µM) exert stimulatory effects on the Nrf2/ARE pathway in human hepatocellular carcinoma HepG2 cells, significantly increasing the mRNA and protein expression of Nrf2 and heme oxygenase 1 (HO-1) with the activation of phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) and ERK1/2 signaling [65]. However, at high concentrations (50 and 100 µM), apigenin reduces the mRNA and protein levels of Nrf2, CAT activity, and intracellular glutathione levels in HepG2 cells [68]. Similarly, at low concentrations (10 µM), luteolin increases the GSH protein expression in human epithelial colorectal adenocarcinoma Caco-2 cells, and higher concentrations (above 15 µM) of luteolin decrease GSH expression, showing the hormetic effects [72]. Therefore, when considering flavonoids in cancer treatment and developing experiments to evaluate the effectiveness, their hormetic effect should be taken into consideration.
2. Role of the Nrf2/ARE Pathway in Cancer Chemoprevention
Activation of the Nrf2/ARE pathway in normal cells has been shown to have cancer chemopreventive effects on non-malignant cells under normal physiological conditions [33,40,73]. This can be mainly achieved by controlling redox homeostasis [74], which leads to genomic stability and cell survival that is facilitated by the activities of antioxidant defense enzymes (SOD, catalase, GPx, GSH synthase, glutathione S-transferase, thioredoxin, and GSH reductase) and phase 2 and 3 detoxifying enzymes (HO-1 and NAD(P)H quinone dehydrogenase 1 (NQO1), aldo-keto reductase, multidrug resistance-associated proteins, P-glycoprotein, organic anion-transporting polypeptide, ATP-binding cassette, heat shock proteins, glycation defense enzymes, and ferritin) [12,33,37,53,54]. These expressed proteins avoid oxidative stress-induced DNA damage by either reducing the exposure of DNA to carcinogens (exogenous or endogenous), inhibiting the activation of pro-carcinogens, or increasing the rate of detoxification of carcinogens [26,75,76]. Therefore, the inactivation of the Nrf2/ARE pathway could increase oxidative stress by generating ROS, create mutagenesis, and initiate carcinogenesis and tumor formation in normal cells [26,75,76]. For example, a decreased expression of the Nrf2 gene increases the risk of lung cancer among smokers [77]. Furthermore, decreased levels of phase 2 enzymes, such as HO-1 and Nrf2 proteins, in Nrf2-knockout animal models, such as female C57BL/6 mice, increases susceptibility towards 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis [78]. Therefore, many investigations propose the activation of the Nrf2/ARE signaling pathway as a potential cell signaling pathway in cancer chemoprevention [35].
2.1. Activators of Nrf2/ARE in Non-Cancer Experimental Models
Investigations into the activation of the Nrf2/ARE pathway have shown that some vitamins and a diverse range of dietary phytochemicals, including flavonoids, sulforaphanes, alkaloids and polyphenols, activate the pathway by different mechanisms in non-cancer experimental models [40] (Table 1). Further, several endogenous signaling molecules, such as protein kinase-like endoplasmic reticulum-resident kinase (PERK), c-Jun n-terminal kinase (JNK), extracellular signal-regulated protein kinase (ERK) and p38, are under normal conditions known to give similar results [79,80]. The activation of the Nrf2/ARE pathway mainly occurs through the disruption of Keap1 and Nrf2 interactions (either through canonical or non-canonical mechanisms) [48], Nrf2 phosphorylation [47], and the prevention of Nrf2 ubiquitination [81]. In addition, some of the activators facilitate Nrf2 nuclear translocation and the transcription of cytoprotective genes associated with ARE [81].
Table 1.
Activators of the Nrf2/ARE pathway in non-cancer experimental models: phytochemicals and other signal molecules.
| Group | Compound | Effective Concentration | Experimental Model | Mode of Action | Reference |
|---|---|---|---|---|---|
| Phytochemicals–Polyphenols | |||||
| Phenolic acid | Ellagic acid | 25–50 µM | Human keratinocyte HaCaT cells | ↑ Nrf2 nuclear translocation. ↑ SOD enzyme activity. |
[141] |
| Chlorogenic acid | 100 µM | Human retinal pigment epithelial ARPE-19 cells | ↑ mRNA expression of Nrf2 and SOD. | [142] | |
| 500 mg/kg of body weight orally | Sprague-Dawley rats | ↑ mRNA expression of Nrf2. ↑ SOD and GSH activities. |
[143] | ||
| Proanthocyanidin | Procyanidin C1 | 5–10 µM | Mouse hippocampal neuronal HT22 cells | ↑ Nrf2 nuclear translocation. ↑ HO-1 protein. |
[144] |
| Lignans | Sesamin | 100 mg/kg body weight intraperitoneally | C57BL/6 mice | ↑ SOD and CAT activities. ↑ GSH and Nrf2 protein. |
[145] |
| 10 µM | Primary chondrocytes | ↑ Nrf2 and HO-1 proteins. | [146] | ||
| Coumarins | Fraxin | 50 mg/kg of body weight up to 5 days orally | Sprague-Dawley rats | ↑ cellular GSH levels. | [147] |
| Stilbene derivative | Resveratrol | 5 µM | Primary human coronary artery endothelial cells | ↑ mRNA expression of NQO1. | [148] |
| 500 mg in a tablet/day up to 30 days in the morning fasting | Phase 3 clinical trial on chronic subclinical inflammation and redox status | ↑ electrophilic modification of Keap1-Cys-151 | [47,85] | ||
| 215 mg in a tablet/day up to 52 weeks | Phase 2 clinical trials on Alzheimer’s disease | ↑ electrophilic modification of Keap1-Cys-151 | [47,149] | ||
| Curcumminoid | Curcumin | 5 µM | Human extravillous trophoblast HTR8/Sveo cells | ↑ CAT and GSH activities. | [150] |
| 400 mg/kg body weight/day orally up to 21 days | White Pekin ducklings | ↑ CAT, SOD, and GPx activities. | [151] | ||
| 800 mg/day in two capsules up to 7 days | Phase 3 clinical on diabetic nephropathy | ↑ electrophilic modifications of Keap1-Cys-151 | [47,82] | ||
| 15 µM | Human retinal pigment epithelial ARPE-19 cells | ↑ Nrf2 protein. ↑ HO-1 activity. |
[152] | ||
| 15–30 µM | Porcine renal epithelial proximal tubule LLC PK1 cells | ↑ ARE binding activity. ↑ Nrf2 protein. ↑ HO-1 activity. |
[153] | ||
| 10 µM | Rat kidney epithelial NRK-52E cells | ↑ ARE binding activity. | [153] | ||
| 200 mg/kg body weight twice a week for 6 weeks | Kunming (KM) mice | ↑ Nrf2 nuclear translocation. ↑ HO-1 and NQO-1 proteins. |
[154] | ||
| Phytochemicals–Polyphenols–Flavonoids | |||||
| Flavone | Luteolin | 0.1 mg/kg body weight/day for 7 days at two time points orally. | ICR mice | ↑ Nrf2 nuclear translocation. ↑ HO-1 and NQO-1 proteins. |
[51] |
| 10 mg/kg body weight intracerebrally injected | Sprague-Dawley rats | ↑ Nrf2 nuclear translocation. ↑ HO-1 and NQO-1 proteins. |
[131] | ||
| 5–10 µM | Rat myoblast H9c2 cells | ↑ Nrf2 protein. ↑ mRNA expression of SOD, NQO-1 and HO-1. |
[93] | ||
| 5 µM | Mouse testis sertoli TM4 cells | ↑ Nrf2 nuclear translocation. | [121] | ||
| 3,5-di-O-Methyl Gossypetin | 10–25 µg/mL | Human keratinocyte HaCaT cells | ↑ Nrf2 nuclear translocation. ↑ GSH, SOD and HO-1 proteins. |
[55] | |
| Baicalein | 160 mg/kg/day for 8 weeks orally | T2DM Kunming mice | ↑ Nrf2 nuclear translocation. ↑ SOD, CAT, GSH proteins. |
[120] | |
| 50 µM | Human liver L-02 cells | ↑ p62 protein. ↑ Nrf2 dissociation from Keap1. ↑ Nrf2 phosphorylation via phosphorylation of ERK1/2 and protein kinase C. |
[117] | ||
| 20 µM | Human liver HL-7702 cells | ↑ Nrf2 nuclear translocation. ↑ SOD, CAT, GSH protein. |
[120] | ||
| Baicalin | 50 mg/kg body weight twice after 2 and 12 h of subarachnoid hemorrhage intraperitoneally. | Sprague-Dawley rats | ↑ SOD, GSH, NQO-1, and Nrf2 proteins. ↑ mRNA expression of HO-1. |
[94] | |
| 75 µM | Rat myoblast H9C2 cells | ↑ Nrf2 and HO-1 proteins. | [97] | ||
| 450 mg/kg body weight/day up to 7 days orally. | Chicken | ↑ Nrf2 and HO-1 proteins. ↑ mRNA expression of Nrf2 and HO-1. |
[116] | ||
| 50 µM | Human liver L-02 cells | ↑ p62 protein. ↑ Nrf2 dissociation from Keap1. ↑ Nrf2 phosphorylation via phosphorylation of ERK1/2 and protein kinase C. |
[117] | ||
| Chrysin | 10–25 µM | Rat hepatocytes | ↑ Nrf2 nuclear translocation via ERK2 signaling. ↑cellular GSH protein. ↑ ARE binding ability. |
[124] | |
| Apigenin | 400 µM | Human retinal pigment epithelial ARPE-19 cells | ↑ mRNA expression of Nrf2. ↑ Nrf2 protein. ↑ Nrf2 nuclear translocation. ↑SOD, CAT, and GPx activities. |
[95] | |
| 200 µM | Human renal tubular epithelial HK-2 cells | ↑ mRNA expression of Nrf2 and HO-1. ↑ Nrf2 protein. |
[100] | ||
| Flavonol | Myricetin | 100 mg/kg/day for 6 weeks orally | Kungming mice | ↑ Nrf2 nuclear translocation. | [132] |
| Quercetin | 30 µM | Human keratinocyte HaCaT and BJ foreskin fibroblast cells | ↑ Nrf2 protein. | [102] | |
| 200 mg/kg body weight/day for 20 days orally | Broiler chicken | ↑ Nrf2 protein. ↑ Nrf2 nuclear translocation. ↑ SOD and CAT proteins. |
[111] | ||
| 100 µM | Intestinal epithelial IEC-6 cells | ↑ Nrf2 nuclear translocation. | [155] | ||
| 10 µM | Human umbilical endothelial cells | ↑ Nrf2 protein. | [108] | ||
| Rutin | 44 µM | Human keratinocyte HaCaT cells | ↑ mRNA expression of HO-1 and NQO-1. | [140] | |
| Flavanone | Naringenin | 80 µM | Sprague-Dawley rat neuron cells | ↑ Nrf2, HO-1, and NQO-1 proteins. | [103] |
| 70 mg/kg body weight/day up to 4 days orally | C57BL/6 mice | ↑ Nrf2 protein. | [112] | ||
| Hesperidin | 50 mg/kg body weight for 28 days orally | Sprague-Dawley rat | ↑ Nrf2 and HO-1 proteins. ↑ mRNA expression of HO-1. |
[104] | |
| Flavan-3-ol | Epicatechin | 10–100 µM | Primary astrocytes from WT and Nrf2 deficient KO mice | ↑ Nrf2 nuclear translocation. | [122] |
| Epigallocatechin-3-gallate (EGCG) |
40 mg/kg body weight/day for 3 days intraperitoneally or a single dose of 50 mg/kg body weight intraperitoneally | Sprague-Dawley rat | ↑ Nrf2 protein. | [105,156] | |
| Isoflavones | Genistein | 1 mg/kg body weight intraperitoneally | Sprague-Dawley rat | ↑ Nrf2 nuclear translocation. ↑ HO-1 protein. |
[138] |
| Anthocyanin | Cyanidin-3-O-glucoside (C3G) |
20–40 µM | Human umbilical vein epithelial cells | ↑ Nrf2 nuclear translocation. ↑mRNA expression of NQO-1 and HO-1. |
[128] |
| Chalcone | Butein | 20 µM | Human dental pulp cells | ↑ Nrf2 nuclear translocation. | [130] |
| Other Phytochemicals | |||||
| Sulfur-containing | Sulforaphane | 5 µM | Mouse skin JB6 P+ cells | ↑ Nrf2 nuclear translocation. ↑ HO-1 and NQO-1 proteins. |
[157] |
| Diallyl sulfide | 15 µM | Human embryonic lung MRC-5 cells | Dissociates Nrf2 from Keap1 through phosphorylated ERK and p38 interactions. ↑ Nrf2 nuclear translocation. |
[89] | |
| 150 mg/kg body weight/day intraperitoneally for 6 days | Wistar rats | ↑ Nrf2 protein. ↑SOD, CAT, GPx, GR, GST, and quinone reductase activities. |
[158] | ||
| Alkaloids | Berberine | 200 mg/kg body weight/day orally for 16 weeks | Wistar rats | ↑ mRNA expression of Nrf2. | [159] |
| Vitamins | |||||
| Fat-soluble vitamins | Vitamin D | 40 000 U/kg/week of body weight intratracheally for 8 weeks | C57BL/6 mice | ↑ mRNA expression Aldo-keto reductase family 1 member C1 (AKR1C1) and GCLM. | [160] |
| Vitamin E | 100 mg/kg body weight/day intraperitoneally for 6 days | Balb/c mice | ↑ Nrf2 and HO-1 protein levels. | [161] | |
| Vitamin A | 100,000 U/kg body weight/day subcutaneously for 14 days | Wistar rats | ↑ Nrf2 nuclear translocation. ↑ HO-1 and NQO 1 proteins. |
[162] | |
| Water-soluble vitamins | Vitamin C | 27–65 mg/kg body feed twice a day for 8 weeks | Juvenile Sillago sihama | ↑ mRNA expression of Nrf2, CAT, SOD, GPx, GR, and GST in intestine and liver cells. | [163] |
| Vitamin B2 | 30 mg/kg body weight/day intra-gastrically | APP/PS1 double transgenic mice | ↑ SOD, CAT, GSH, and GPx activities. ↑ Nrf2 expression. ↓ Keap1 expression. |
[164,165] | |
| Endogenous Signaling Molecules | |||||
| Protein kinases | PI3K | N/A | Human retinal pigment epithelial RPE-19 cells | ↑ Nrf2 nuclear translocation. | [92] |
| JNK 1 & 2 | N/A | Human embryonic kidney HEK 293T cells | Phosphorylates Nrf2 at S212, S408, S558, S577, and T559. | [80] | |
| p38 | N/A | Human embryonic kidney HEK 293T cells | Phosphorylates Nrf2 at Ser212, Ser408, Ser558, Ser577, and Thre559. | [80] | |
| AMPK | N/A | Human embryonic kidney HEK 293T cells | Phosphorylates Nrf2 at the Ser558 residue. ↑ Nrf2 nuclear translocation. |
[49] | |
| ERK2 | N/A | Human embryonic kidney HEK 293T cells | Phosphorylates Nrf2 at Ser212, Ser408, Ser558, Ser577, and Thre559. | [80] | |
| Casein kinase 2 | N/A | Human embryonic kidney HEK 293T cells | ↑ Nrf2 phosphorylation. ↑ Nrf2 nuclear translocation. |
[90] | |
| PKC | N/A | New Zealand white rabbits | ↑ Nrf2 nuclear translocation. | [91] | |
| PERK | N/A | Mouse embryonic fibroblasts | Dissociates Nrf2 from Keap1 by phosphorylation of Nrf2. | [79] | |
| Autophagy-substrate proteins | Sequestosome 1 (p62) | N/A | Human aortic smooth muscle cells | Competes with Nrf2 to bind with Keap1. | [88] |
| Synthetic compounds | |||||
| Synthetic triterpenoids | Bradoxolone-methyl (CDDO-Me) | 25–50 mg/day for 52 weeks | Phase 2 clinical on diabetic nephropathy | ↑ electrophilic modification of Keap1-Cys-151. | [47,84] |
| Synthetic triterpenoids | RTA-408 (omaveloxolone) | 1% ophthalmic suspension for twice a day for 14 days | Phase 2 clinical trial on inflammation and pain following ocular surgery | ↑ electrophilic modification of Keap1-Cys-151. | [47,83] |
| Synthetic lignans | LGM2605 (Secoisolariciresinol diglucoside) | 50 µM | Murine peritoneal macrophages derived from C57BL/6J mice | ↑ mRNA expression of GST and redoxin reductase 1. | [166] |
Abbreviations—HaCaT: human skin keratinocytes; ARPE-19: human retinal pigment epithelial cell line; HT22: mouse hippocampal neuronal cell line; LLC PK1: porcine renal epithelial proximal tubule cell line; NRK-52E: rat kidney epithelial cell line; IEC-6: intestinal epithelial cell line; HK-2: human renal tubular epithelial cell line; HL-7702/ L-02: human liver cell line; MRC5: human embryonic lung cell line; HTR8/Sveo: extravillous trophoblast cell line; HEK 293T: human embryonic kidney 293T cell line; HT22: mouse neuronal cell line; H9C2: rat myoblast cell line: TM4: mouse Sertoli cell line; T2DM mice: type 2 diabetes mellitus mice; BJ: human foreskin fibroblast cell line; WT: wild type; KO: knock-out; JB6 P+: mouse skin cells; APP/PSI: ARTE1; GCLM: glutamate-cysteine ligase modifier; GSH: glutathione; CAT: catalase; SOD; superoxide dismutase; GPx: glutathione peroxidase; ARE: antioxidant response element; ERK: extracellular signal-regulated protein kinase; GSK-3β: glycogen synthase kinase 3; Akt: protein kinase B; GR: glutathione reductase; NQO-1: NAD(P)H quinone dehydrogenase 1; HO-1: heme oxygenase 1; GST- glutathione S-transferase; Ser212: serine residue 212; Ser408: serine residue; Ser558: serine residue 558; Ser577: serine residue 577; Thre559: threonine residue 559; PI3K: phosphorylation of phosphatidylinositol 3-kinase; JNK: N-terminal kinase; AMPK: 5′ adenosine monophosphate-activated protein kinase; PKC: protein kinase C; PERK: protein kinase-like endoplasmic reticulum-resident kinase; p62: sequestosome 1; Keap1: Kelch-like ECH-associated protein 1; Nrf2: Nuclear factor erythroid 2 p45 (NF-E2)-related factor; ↑ increase; ↓ decrease.
Resveratrol, a stilbene derivative, and RTA-408 (omaveloxolone), a synthetic terpenoid, activate the Nrf2 pathway through canonical mechanisms [47,82,83,84,85]. Canonical activators that obstruct the interaction of the Keap1/Nrf2 system possess electrophilic properties and react with the cysteine residues (i.e., Cys151, Cys257, Cys273, Cys288, and Cys297) of Keap1 via either oxidation or alkylation in order to dissociate Nrf2 from Keap1 [35,46,47,53]. For example, in a phase 3 clinical trial on chronic subclinical inflammation and redox status, 500 mg of resveratrol in one tablet/day up to 30 days (Table 1) has shown to be disruptive to the Nrf2–Keap1 interactions via conformational changes. These changes occurred due to electrophilic modifications in the Keap1-Cys151 thiol group [47,85]. The Cul3-based E3 ligase complex binds and interacts with Keap1 to facilitate Nrf2 polyubiquitination, which promotes Nrf2 degradation at the 26S proteasome. Therefore, post-translational modifications in Cys151 lead to the dissociation of the Cul3-based E3 ligase complex from Keap1 and Nrf2 stabilization [43,44,45,47,82]. Thereby, it prevents Nrf2 proteasomal degradation by ubiquitination, and facilitates ARE-mediated gene expression [81,86,87]. Similar results were observed with RTA-408 in a clinical trial on inflammation and pain due to ocular surgery (1% ophthalmic suspension of RTA-408 twice a day for 14 days) [47,83].
Sequestosome-1, an endogenous signaling molecule, activates the Nrf2 pathway via non-canonical mechanisms by blocking Nrf2 binding to Keap1 [88] (Table 1). Sequestosome 1, also called p62, not only competes with Nrf2 to bind to Keap1 and block the formation of Nrf2–Keap1 complex, but also promotes the autophagic degradation of Keap1 [48,88]. For example, Nrf2 silencing downregulates p62 expression while upregulating Keap1 expression at the mRNA and protein levels in vascular smooth muscle cells. Conversely, p62 silencing dramatically upregulates Keap1 and downregulates Nrf2 at the mRNA and protein levels, suggesting p62 may be effective in downregulating Keap1 protein via autophageal degradation [88].
Most of the endogenous activators of the Nrf2/ARE pathway act by stimulating the phosphorylation of Nrf2, which leads to the detachment of Nrf2 from Keap1 [53]. For example, the PERK-mediated direct phosphorylation of Nrf2 in mouse embryonic fibroblasts results in the dissociation of Nrf2 from Keap1 (Table 1) [79]. Similarly, JNK 1 and 2, ERK2 and p38 phosphorylate Nrf2 at the serine (Ser212, Ser400, Ser558, Ser577) and threonine (Thre559) residues in human embryonic kidney HEK 293T cells [80]. It is also suggested that the above-mentioned endogenous activators of the Nrf2/ARE pathway can be activated by phytochemicals such as diallyl sulfide. Diallyl sulfide phosphorylates ERK and p38 in human embryonic lung MRC-5 cells, and facilitates the dissociation of Nrf2 from Keap1 and nuclear translocation [89].
Most of the endogenous activators of Nrf2 are protein kinases, which seem to facilitate the nuclear translocation of phosphorylated Nrf2 [49,90,91]. Nrf2 phosphorylation mediated by AMPK, casein kinase 2, PERK, ERK and p38 facilitates Nrf2 nuclear translocation [90]. PI3K signaling is also involved in the activation of the Nrf2/ARE pathway through its downstream regulator Akt, which facilitates Nrf2 nuclear translocation and the following ARE gene transactivation [92]. Therefore, the influence of endogenous signaling molecules, phytochemicals and synthetic chemicals upon activation of Nrf2/ARE at different stages of the pathway will be vital in exerting chemopreventive effects upon the activation of the Nrf2/ARE pathway [40,47,79,80].
2.2. Flavonoids: Nrf2/ARE Activation in Non-Cancer Experimental Models
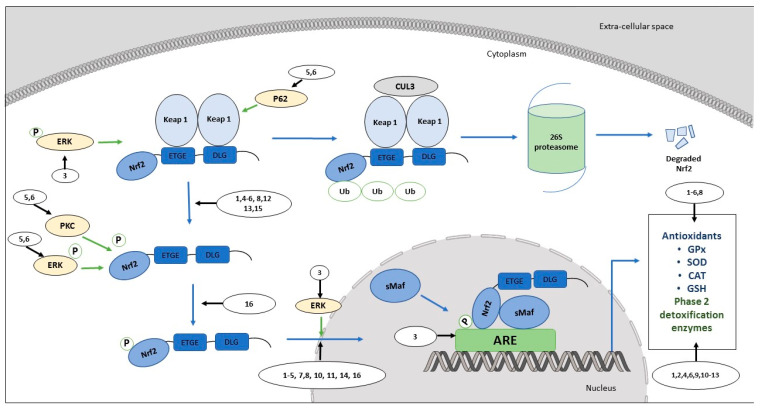
Flavonoids are among the most noticeable dietary phytochemicals that activate the Nrf2/ARE pathway under normal physiological and induced conditions (Table 1) (Figure 1). The sub-class flavones (luteolin, baicalin and apigenin) are more prominent in upregulating Nrf2 protein expression in both in vitro and pre-clinical studies [93,94,95]. For example, luteolin upregulates Nrf2 protein expression in relieving high glucose-induced cell injury in rat myoblast H9C2 cells at rat-physiological concentrations [93,96]. Baicalin also increases Nrf2 protein expression against hypoxia-induced apoptosis in rat myoblast H9C2 cells, although the required concentration is higher than rat-physiological concentrations [97,98]. Furthermore, the intraperitoneal administration of baicalin demonstrates a similar effect in male Sprague-Dawley rats after inducing subarachnoid hemorrhage by endovascular perforation [94,99]. A study conducted by Xu and colleagues showed that apigenin increases the Nrf2 protein level as a response to tert-butyl hydroperoxide (t-BHP)-induced oxidative cell injury in human retinal pigment epithelial ARPE-19 cells, at much higher concentrations compared to other flavones [95]. Similarly, apigenin upregulated Nrf2 expression at transcriptional and translational levels, at much higher concentrations, against hydrogen peroxide-induced oxidative stress and cell injury in human renal tubular epithelial HK-2 cells [100]. However, the tested concentrations in both ARPE-19 and HK-2 cell lines were much higher than the bioavailable apigenin levels in human plasma [95,100,101].
Figure 1.
Role of dietary flavonoids in the regulation of the Nrf2/ARE pathway in normal cells.
Further, the upregulation of Nrf2 protein was observed in flavonols (quercetin), flavanones (naringenin, hesperidin), and flavan-3-ols (epigallocatechin-3-gallate) [102,103,104,105]. Naringenin, a flavanone found in citrus fruits, upregulates the Nrf2 protein in hypoxia-induced neuron cells derived from neonatal Sprague-Dawley rats [103]. However, this was at comparatively higher concentrations that may not be achievable in pre-clinical studies, considering the limited bioavailability of naringenin in rats [103,106,107]. Quercetin upregulates Nrf2 protein expression in different human cell lines, such as human skin keratinocytes HaCaT, BJ foreskin fibroblast, and human umbilical vein endothelial cells (HUVECs), but at concentrations higher than physiologically relevant concentrations considering the low bioavailability of quercetin in the human diet [102,108,109,110]. Furthermore, the oral administration of quercetin upregulates Nrf2 protein levels against lipopolysaccharide (LPS)-induced intestinal oxidative stress in broiler chicken [111]. Furthermore, pre-clinical studies on the oral administration of naringenin show the upregulation of the Nrf2 protein in male C57BL/6 mice with 6-hydroxydopamine (6-OHDA)-induced neurotoxicity at a concentration which is not toxic [112,113]. Furthermore, both hesperidin (oral) and epigallocatechin-3-gallate (intraperitoneal) have shown that similar upregulations can be achievable at non-toxic concentrations in male Sprague-Dawley rats with methotrexate (MTX)-induced hepatotoxicity and testicular ischemia-induced oxidative stress, respectively [104,105,114,115]. In contrast, mRNA levels of Nrf2 were upregulated only in flavones such as baicalin (chicken with Mycoplasma gallisepticum infection-induced oxidative stress) and apigenin (human retinal pigment epithelial ARPE-19 cells with t-BHP-induced oxidative cell injury) in pre-clinical and in vitro models [95,116]. However, the molecular mechanisms of the flavonoid-mediated increase of cellular Nrf2 mRNA and protein levels remain unclear.
The activation of Nrf2 is necessary for the progression of the pathway either by canonical or non-canonical mechanisms [46,47,48]. Baicalin and its aglycon baicalein activate the Nrf2/ARE pathway in acetaminophen-induced L-02 (synonym: HL-7702) human liver cells through non-canonical activation of Nrf2 via p62 [117]. Baicalin and baicalein increase the expression of the p62 protein in L-02 cells, which competes with Nrf2 for binding to the Nrf2 binding site in the Keap1-kelch domain [117]. The inductive effects of baicalin and baicalein in L-02 cells further continues as baicalin, and baicalein itself, compete with Nrf2 to bind to the Nrf2 binding site [117]. Hydroxyl groups at C5 and C6 of the A-ring of these two flavones might be useful in anchoring them to the Nrf2 binding site in the Keap1-kelch domain through hydrogen bond formation [118]. Furthermore, baicalin and baicalein phosphorylate ERK1/2 and protein kinase C (PKC). Thereby, phosphorylated ERK1/2 and PKC phosphorylate Nrf2. Therefore, these flavones increase Nrf2 stabilization by preventing Nrf2 ubiquitination [117]. However, the inductive effects of effective baicalin and baicalein concentrations in L-02 cells are higher than the physiological (intraperitoneally administered) concentrations of these two flavones in human primates and monkeys [117,119,120].
The nuclear translocation of phosphorylated Nrf2 is a necessity in order to proceed with ARE-driven gene transcription. [49,50]. Many subclasses of flavonoids, including flavones (luteolin, baicalein, chrysin, and apigenin), flavonols (myricetin and quercetin), flavanones (eriodyctiol), flavan-3-ols (epicatechin), isoflavones (genistein), anthocyanidins (cyanidin-3-O-glucoside; C3G) and chalcones (butein), promote Nrf2 nuclear translocation (Table 1). Luteolin and epicatechin upregulate the Nrf2 nuclear translocation in mice cells; mouse testis Sertoli TM4 (triptolide-induced apoptosis) and hemoglobin toxicity induce the primary astrocytes of mice, respectively [121,122]. Similarly, chrysin and quercetin upregulate Nrf2 nuclear translocation in rat hepatocytes (t-BHP-induced oxidative stress) and rat intestinal epithelial IEC-6 cells [123,124]. Furthermore, in rat hepatocytes, the chrysin-mediated upregulation of the phosphorylated ERK1 increases Nrf2 nuclear translocation [124]. Therefore, ERK1-mediated influences may be due to the improved stability of Nrf2 upon Nrf2 phosphorylation, which prevents Nrf2 ubiquitination and degradation [117]. However, the tested concentrations of luteolin (mouse testis Sertoli TM4 cells), epicatechin (primary astrocytes from mice), quercetin (rat hepatocytes) and chrysin (IEC-6 cells) in the above murine cells are higher than the achievable physiological concentrations in murine models upon oral administration [51,125,126,127]. Baicalein upregulates the Nrf2 nuclear translocation against high glucose-induced oxidative stress in L-02 liver cells [119,120]. Furthermore, C3G upregulates Nrf2 nuclear translocation in HUVECs challenged with tumor necrosis factor-α [128]. However, the tested concentrations of C3G on HUVECs are much higher than the serum levels that can be achieved in humans upon oral uptake [129]. Apigenin also facilitates nuclear translocation in human retinal epithelial ARPE-19 cells at concentrations higher than those physiologically available in humans [95,101]. Similarly, butein upregulates Nrf2 nuclear translocation against hydrogen peroxide-induced oxidative stress in human dental pulp cells [55,130]. Further, due to the lack of availability of clinical data on the bioavailability of butein, the physiological relevance of tested concentrations of butein is mostly unknown.
Furthermore, promising results on the upregulation of Nrf2 nuclear translocation were observed in several pre-clinical studies (ICR mice, Sprague-Dawley rats, Kunming mice, and broiler chicken) [51,111,120,131,132]. Luteolin, baicalein, myricetin, quercetin and genistein demonstrate their ability in upregulating Nrf2 nuclear translocation [51,111,120,131,132]. Luteolin increases Nrf2 nuclear translocation in male Sprague-Dawley rats with intracerebral hemorrhage-induced secondary brain damage (intraperitoneal administration) and male ICR mice (oral administration) at concentrations which are not toxic [51,131,133,134,135]. Similarly, the oral administration of baicalein facilitates Nrf2 nuclear translocation in male type 2 diabetes mellitus (T2DM) Kunming mice with high glucose-induced oxidative stress, at a concentration much lower than the maximum tolerable levels for mice [120,136]. Further, myricetin (oral administration) was effective in upregulating Nrf2 nuclear translocation against cuprizone-induced demyelination in male Kunming mice [132]. The concentrations of myricetin tested on Kunming mice are much lower than the sub-lethal concentrations of myricetin for mice [132,137]. Furthermore, both quercetin (oral administration) and genistein (intraperitoneal administration) upregulate Nrf2 nuclear translocation in broiler chickens (LPS-induced intestinal oxidative stress) and male Sprague-Dawley rats (cerebral ischemia-induced oxidative stress) [111,138]. Further, the tested concentrations of genistein on Sprague-Dawley rats were much lower than concentrations that show toxic effects in mice [139].
The downstream activation of the Nrf2/ARE pathway upon Nrf2 nuclear translocation by flavonoids has been demonstrated. The overexpression of antioxidant defense genes (GSH, SOD, GPx and CAT) and phase 2 detoxifying genes (HO-1 and NQO-1) was observed due to flavones (luteolin, apigenin, baicalin, baicalein, chrysin), flavanones (naringenin, and hesperidin), flavonols (quercetin, rutin), anthocyanins (C3G) and isoflavones (genistein) (Table 1). Baicalein upregulates the expression of downstream target genes, SOD, CAT, and GSH against high glucose-induced oxidative stress in L-02 liver cells at physiologically higher concentrations [119,120].
Chrysin upregulates cellular GSH proteins (antioxidant defense gene) in relieving the t-BHP-induced oxidative stress of rat hepatocytes [124]. Baicalin and naringenin upregulate phase 2 detoxifying enzymes at the protein level against hypoxia-induced oxidative stress or apoptosis in H9C2 (HO-1) and Sprague-Dawley neuronal cells (HO-1 and NQO-1), respectively [97,103]. Although the concentrations tested were higher than the physiologically relevant range, rutin and C3G upregulate the expressions of HO-1 and NQO-1 in the mRNA levels of HaCaT and HUVECs, respectively [110,128,129,140]. Further, apigenin enhances the activity of SOD, CAT, and GPx in human retinal epithelial ARPE-19 cells, and the expression of HO-1 in the mRNA levels of human renal tubular epithelial HK-2 cells, but at much higher concentrations than can be achieved physiologically in humans [95,100,101].
In preclinical studies, the oral administration of baicalein and quercetin upregulates proteins related to antioxidant defense genes against relieving oxidative stress in high glucose-induced male T2DM Kunming mice (SOD, CAT, and GSH) and LPS-induced broiler chicken (SOD and CAT), respectively [111,120]. In contrast, the upregulation of both antioxidant defense (SOD and GSH) and phase 2 detoxifying genes (NQO-1 and mRNA HO-1) was observed upon intraperitoneal administration of baicalin in male Sprague-Dawley rats after inducing subarachnoid hemorrhage [94]. Luteolin upregulates phase 2 detoxifying enzymes (HO-1 and NQO-1) in male Sprague-Dawley rats with intracerebral hemorrhage-induced secondary brain damage (intraperitoneal administration) and male ICR mice (oral administration) [51,131]. In contrast, the upregulation of HO-1 was observed at the protein level against cerebral ischemia-induced oxidative stress in Sprague-Dawley rats upon intraperitoneal administration of genistein [138]. Furthermore, the oral administration of baicalin and hesperidin upregulated HO-1 at both the protein and mRNA levels against Mycoplasma gallisepticum infection-induced oxidative stress in chicken and MTX-induced hepatotoxicity in male Sprague-Dawley rats, respectively [104,116]. More importantly, the above upregulations of either or both antioxidant and phase 2 detoxifying enzymes by luteolin (ICR mice and Sprague-Dawley rats), baicalein (T2DM Kunming mice), baicalin (Sprague-Dawley rats), hesperidin (Sprague-Dawley rats) and genistein (Sprague-Dawley rats) were observed in concentrations lower than toxic or lethal in in vivo studies [99,114,133,134,136,139]. Based on the reported literature, further investigations should be carried out so as to better understand the molecular mechanisms of the effects of flavonoids in facilitating the activation, stabilization and nuclear translocation of Nrf2, and ARE-driven gene expression.
In normal cells, flavonoids have been shown to activate the Nrf2/ARE pathway in maintaining redox homeostasis. Under normal physiological conditions, Keap1 protein inhibits the activation of the Nrf2 protein by its interactions with the Nrf2 protein and ubiquitination-associated Nrf2 degradation. Upon oxidative stress caused by ROS, the oxidation of cysteine residues of Keap1 makes the Nrf2 dissociate from the Keap1 protein, followed by the stabilization of Nrf2 via phosphorylation. Phosphorylated Nrf2 translocates into the nucleus and binds to ARE along with the sMaf transcription factor. ARE-driven downstream antioxidant defenses and phase 2 detoxifying proteins will be expressed, leading to the restoration of normal physiological conditions via the detoxification of xenobiotics, drug transportation, and the neutralization of reactive species avoiding DNA damage and subsequent carcinogenesis. Dietary flavonoids activate the Nrf2/ARE pathway by influencing the pathway at different stages, and thus have potential effects on cancer chemoprevention.
1: Luteolin; 2: 3,5-di-O-Methyl Gossypetin; 3: Chrysin; 4: Apigenin; 5: Baicalein; 6: Baicalin; 7: Myricetin; 8: Quercetin; 9: Rutin; 10: Genistein; 11: C3G; 12: Naringenin; 13: Hesperidin; 14: Epicatechin; 15: EGCG; 16: Butein.
Keap1: Kelch-like ECH-associated protein 1; Nrf2: Nuclear factor erythroid 2 p45 (NF-E2)-related factor; sMaf: Small musculoaponeurotic fibrosarcoma protein; ARE: Antioxidant response element; GSH: glutathione; SOD: superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase.
(The figure was adapted from Wu et al., 2019 [33])
3. Promotion of Cancer Cell Proliferation by Activation of Nrf2/ARE: Nrf2-Associated Cell Signaling and Mechanisms
The constitutive activation of Nrf2 promotes the development of different types of cancers as well as the resistance of cells to anti-cancer drugs [167]. The cellular mechanisms that over-activate the Nrf2/ARE pathway include disruption of interactions between Nrf2 and Keap1, the reduction of Keap1 protein expression, and the increase in Nrf2 protein expression [33]. The interactions between Nrf2 and Keap1 are inhibited by somatic mutations acquired in the Nrf2, CUL3 and/or Keap1 genes in cancer cells [168,169,170]. Furthermore, the Nrf2 protein can acquire mutations during protein translation by skipping exons of the Nrf2-coding mRNA strand [171]. The resultant Nrf2 or/and Keap1 mutants disrupt Nrf2 binding to Keap1 [33,169,170,171]. Similarly, the generated Keap1 and/or CUL3 mutants in cancer cells prevent CUL3–Keap1–Nrf2 complex formation, blocking Nrf2 ubiquitination [33,168,170]. Further, Nrf2 ubiquitination and the binding affinity of Nrf2 to Keap1 is reduced in cancer cells by the competition of endogenous signaling molecules, such as p62, partner and localizer of BRCA2 (PALB2), and dipeptidyl-peptidase 3 (DPP3), with Nrf2 to bind to Keap1 [172,173,174,175,176]. Furthermore, the succination of cysteine molecules in Keap1 facilitates the dissociation of Nrf2 from Keap1 [177]. The reduction of Keap1 protein levels in cancer cells is mostly due to the epigenetic alteration of Keap1 through the hypermethylation of the CpG islands in the Keap1 promoter region [178], which thereby releases Nrf2 from the inhibitory regulation of Keap1 [178]. Further, the transcription of the Nrf2 protein is increased by either epigenetic changes in Nrf2, mutations on specific tumor suppressor genes (PTEN: Phosphatase and tensin homolog), or oncogenes (Myc, K-Ras, and B-Raf) [33,179,180]. However, the mechanism of the overactivation of Nrf2/ARE could be different from one cancer type to another [181]. As a result of the overactivation of the Nrf2/ARE pathway, cancer cells continue to grow and proliferate continuously, evading cellular apoptotic signals and promoting the self-renewal capacity of cancer stem cells [33].
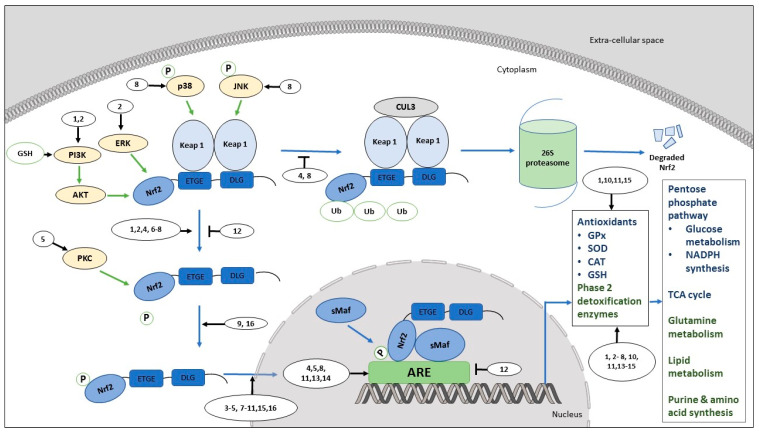
The promotion of cancer cell proliferation by over activation of the Nrf2/ARE pathway is associated with the stimulation of several metabolic pathways, such as glutathione synthesis [180,182], fatty acid and lipid biosynthesis [180,182,183,184,185], the pentose phosphate pathway, and the tricarboxylic acid cycle [186] (Figure 2). In both normal and cancer cells, reduced-glutathione (GSH) maintains redox homeostasis to facilitate cell proliferation [180,187,188,189]. The overactivation of Nrf2 expresses genes, such as malic enzyme 1, isocitrate dehydrogenase-1, 6-phosphogluconate dehydrogenase, and glucose-6-phosphate dehydrogenase, which may be involved in the generation of NADPH [190]. NADPH is an essential co-factor that is required for glutathione synthesis [180,182]. Furthermore, the metabolic reprogramming of cell proliferation is a result of the Nrf2 overactivation-mediated expression of several metabolic enzymes (i.e., transketolase, phosphogluconate dehydrogenase, glucose-6-phosphate dehydrogenase, malic enzyme 1, isocitrate dehydrogenase) in cancer cells. These enzymes facilitate the metabolism of glucose in the pentose phosphate pathway, as well as glutamine and the synthesis of purine and amino acids [180]. In addition, Nrf2 upregulates the expression of genes (i.e., prostaglandin reductase-1 in rat hepatocarcinogenesis) that facilitate the fatty acids and lipids metabolism [180,182,183,184,185]. Furthermore, the reduction of the proliferation capacity of Nrf2-dependent and siRNA-mediated adenocarcinomic human alveolar basal epithelial A549 cancer cells with reduced Nrf2 levels suggests that the activation of Nrf2 is necessity to promote A549 cell proliferation [180]. Furthermore, Nrf2 appears to induce the direct carbon influx to the pentose phosphate pathway and the tricarboxylic acid cycle by regulating microRNA miR-1 and miR-206 [186]. Furthermore, direct carbon influx is associated with accelerated metabolic reactions in the pentose phosphate pathway and the tricarboxylic acid cycle, which facilitates cancer cell growth and proliferation [33,186].
Figure 2.
Role of dietary flavonoids in the Nrf2/ARE pathway in cancer cells.
Nrf2 is found to be a regulator of the cell cycle and PI3K/AKT signaling, and in ensuring healthy mitochondrial functions and lifespans in facilitating cancer cell proliferation [191]. Reddy and colleagues [192] showed that Nrf2 deficiency arrests the cell cycle progression at the G2/M phase of primary cortical neuron cultures, avoiding further proliferation. Furthermore, the authors suggest that these anti-proliferative effects are related to diminished glutathione levels [192]. Reduced antioxidant activity leads to an inactivation of the PI3K/AKT pathway, which is restored upon glutathione supplementation, confirming the role of antioxidant defense mechanisms in the anti-proliferative effect of the hepatocytes of Nrf2-deficient C57B/SV129 mice [192,193]. Furthermore, the knockdown of Nrf2 in A549 lung carcinoma cells facilitates the cell cycle arrest at the G1 phase, with the reduction of the phosphorylated retinoblastoma tumor suppressor protein [194]. Nrf2 has a role in cancer proliferation in pancreatic cancer as well [195]. Nrf2 modulates mRNA translation in murine pancreatic organoids by preventing the oxidation of mRNA translational regulatory proteins (i.e., pyruvate kinase PKM, elongation factor 2, 40S ribosomal protein S2, 40S ribosomal protein S4, and valine-tRNA ligase) [195]. Further, the deficient state of Nrf2 (shNrf2) in SUIT-2 pancreatic cancer cells and murine pancreatic organoids has also been shown to impair epidermal growth factor signaling and translational regulatory proteins, which causes impaired mRNA translation, leading to defects in cell proliferation [195]. In addition, activation of the Nrf2/ARE pathway facilitates the proliferation of both malignant and non-malignant cells by its effects on the function and lifespan of mitochondria, which promote healthy aging [191]. The smooth functioning of mitochondria is assured through increasing substrate availability for mitochondria through the activation of Nrf2, which increases mitochondrial membrane potential, ATP levels, rate of respiration, and the efficacy of oxidative phosphorylation. Furthermore, mitochondrial life spans are increased by inducing biogenesis, maintenance, and the removal of the damaged mitochondria while maintaining homeostasis [196,197].
In summary, the activation of the Nrf2/ARE pathway leads to the proliferation of cancer cells by regulating cell cycle proteins, PI3K/AKT signaling, the regulation of cellular metabolism, and maintaining mitochondrial health [191]. Therefore, the downregulation of the Nrf2/ARE pathway by inhibiting Nrf2 activation in cancer cells would be ideal for downregulating cancer cell proliferation and progression [194,198].
3.1. Flavonoids: Nrf2/ARE Activation in Cancer Cells
Flavonoids promote cancer in preclinical conditions while exerting positive effects on cancer cell survival, growth, and proliferation in vitro, by activating the Nrf2/ARE pathway through several different mechanisms (Table 2) (Figure 2). Luteolin activates the Nrf2/ARE pathway in multiple human and murine cancer cell lines at several different stages. Studies show not only that luteolin increases the activation of the Nrf2/ARE pathway; it also induces the upregulation of downstream Nrf2/ARE-targeted molecules. Luteolin increases the mRNA and protein levels of Nrf2 in human hepatocellular carcinoma HepG2 cells [65,199]. The inductive effects of luteolin on HepG2 continue further downstream, as evidenced by an increased expression of HO-1 at transcriptional and translational levels [65]. The treatment of another human colorectal cancer cell line (Caco-2) with luteolin increases the nuclear translocation of Nrf2 and increases the GSH level at a downstream level [72]. However, the tested concentrations of luteolin in HepG2 and Caco-2 cells are higher than the bioavailable luteolin from a human diet [199]. Pandurangan and co-workers in 2014 reported a similar effect of luteolin on Nrf2 in a rodent model (azoxymethane-induced colorectal cancer) of BALB/c male mice, with the further activation of GST as well [200]. Furthermore, the tested concentration that activates Nrf2/ARE in BALB/c mice is much lower than concentrations that are toxic to mice upon intraperitoneal administration [201]. Apigenin increases the mRNA and protein expression of Nrf2 in HepG2 human hepatocellular carcinoma cells with oxidative stress [65]. The inductive effects of apigenin on HepG2 continue further downstream, as evidenced by an increased expression of HO-1 at the transcriptional and translational levels [65]. Furthermore, the apigenin-mediated upregulation of Nrf2/ARE in HepG2 is associated with the activation of PI3K /Akt and ERK1/2 signaling [65]. However, the tested concentrations of apigenin in HepG2 cells are higher than the reported physiological concentrations of apigenin in humans [65,101,202].
Table 2.
Flavonoids: Nrf2/ARE regulation in cancer cells.
| Sub-Group | Compound | Effective Concentration/Concentration Range | Model | Mode of Action | Reference |
|---|---|---|---|---|---|
| Flavone | Luteolin | 30 µM | Human colorectal carcinoma HCT116 cells | ↑ mRNA expression of Nrf2. ↑ Nrf2 protein. |
[211] |
| 10–15 µM | Human epithelial colorectal adenocarcinoma Caco-2 cells | ↑ Nrf2 nuclear translocation. ↑ GSH levels. |
[72] | ||
| 1.2 mg/kg body weight/day intraperitoneal injection once a week for 3 weeks | Colorectal cancer in Balb/C mice | ↑ Nrf2 and GST proteins. | [200] | ||
| 1.5–6.25 µM | Human hepatocellular carcinoma HepG2 cells | ↑ mRNA and protein expression of Nrf2 and HO-1 via PI3K/Akt. | [65] | ||
| 1–10 µM | Human epithelial colorectal adenocarcinoma Caco-2 cells | ↓ mRNA expression of Nrf2, HO-1, NQO-1, aldo-keto reductases1C1 and C2 (AKR1C), glutamate-cysteine ligase catalytic subunit (GCLC), and multidrug resistance-associated protein (MRP) 2. | [61] | ||
| 5–10 µM | Human alveolar basal epithelial adenocarcinoma A549 cells | ↓ mRNA expression of HO-1, NQO-1, AKR1C, GCLC, and MRP2. ↓ Nrf2 protein. |
[61] | ||
| 1–10 µM | Human breast cancer MCF7 cells | ↓ mRNA expression of Nrf2, HO-1, NQO-1, AKR1C, GCLC, and MRP2. | [61] | ||
| 25 µM | Opisthorchiasis-associated cholangiocarcinoma KKU-100 cells | ↓ Nrf2, gamma-glutamylcysteine ligase and HO-1 proteins | [60] | ||
| Chrysin | 10–20 µM | Hepatocellular carcinoma BEL-7402/ADM cells | ↓ mRNA and protein expression of Nrf2, HO-1, MRP5, and aldo-keto reductase family 1 member B10 (AKR1B10) | [63] | |
| 20 µM | Human breast cancer MCF7 cells | ↓ mRNA expression of Nrf2, MRP1, NQO-1, and HO-1. | [209] | ||
| Apigenin | 1.56–6.25 µM | Human hepatocellular carcinoma HepG2 cells | ↑ mRNA expression of Nrf2. ↑ Nrf2 and HO-1 proteins. ↑ mRNA expression of Nrf2 and HO-1. Activates PI3K/Akt and ERK1/2 signaling. |
[65] | |
| 10–20 µM | Hepatocellular carcinoma BEL-7402/ADM cells | ↓ mRNA expression of Nrf2. ↓ mRNA and protein expression of HO-1, MRP5, and AKR1B10. |
[62] | ||
| 100 µM | Human hepatocellular carcinoma HepG2 cells | ↓ mRNA expression of GSH and GPx. ↓ GSH and GPx activity. |
[68] | ||
| Tangeretin | 20 µM | Human hepatocellular carcinoma HepG2 cells | ↑ Nrf2 nuclear translocation ↑mRNA and protein expression of HO-1 and NQO-1. |
[212] | |
| Flavonol | Myricetin | 10–40 µM | Human hepatocellular carcinoma HepG2 cells | Activates Nrf2 by modifying Keap1 protein. ↓ Nrf2 ubiquitination. ↑ Nrf2 nuclear translocation. ↑ ARE binding ability. ↑ Nrf2 protein levels but not Keap1. ↑ protein expression of HO-1. |
[203] |
| Quercetin | 10 µM | Human hepatocellular carcinoma HepG2 cells | ↑ Nrf2 nuclear translocation. ↑ARE binding activity of Nrf2. |
[204] | |
| 10 µM | Human neuroblastoma SH-SY5Y cells | ↑ Nrf2 nuclear translocation. ↑ Nrf2 phosphorylation via PKC activation. ↑ protein and mRNA expression of glyoxalase-1. ↑ glyoxalase-1 activity |
[123] | ||
| Rutin | 44 µM | Human epithelial colorectal adenocarcinoma Caco-2 cells | ↑ mRNA expression of Nrf2, HO-1 and NQO-1 without changing Keap1 mRNA levels. | [140] | |
| Flavanone | Hesperetin | 40 µM | Murine macrophage Raw 264.7 cells | ↑ Nrf2 nuclear translocation. ↑ degradation of Keap1. ↑ protein expression of HO-1 |
[205] |
| Neohesperidin dihydrochalcone |
30 µM | Human hepatocellular carcinoma HepG2 cells | ↑ Keap1 modifications ↑ Nrf2 nuclear translocation. ↑ Nrf2 ARE binding ability. ↓ Nrf2 ubiquitination. ↑ phosphorylated JNK and p38 dependent protein expression of HO-1 and NQO-1. |
[207] | |
| Naringenin | 20–80 µM | Human neuroblastoma SH-SY5Y cells | ↑ Nrf2 nuclear translocation. ↑ GSH protein ↑ protein expression of HO-1 |
[112] | |
| Flavan-3-ol | Epicatechin | 10 µM | Human hepatocellular carcinoma HepG2 cells | ↑ Nrf2 phosphorylation. ↑ Nrf2 nuclear translocation. |
[213] |
| Morin | 5–10 µM | Rat insulinoma INS-1E cells | ↑ Nrf2 phosphorylation. ↑ Nrf2 nuclear translocation. |
[214] | |
| Isoflavones | Daidzein | 5 µM | Murine hepatoma Hepa-1c1c7 cells | ↑ mRNA expression of quinone reductase. ↑ quinone reductase activity. ↑ ARE binding ability |
[208] |
| Genistein | 5 µM | Murine hepatoma Hepa-1c1c7 cells | ↑ mRNA expression of quinone reductase. ↑ quinone reductase activity. ↑ ARE binding ability. |
[208] | |
| Chalcone | Phloretamide | 20 µM | Human hepatocellular carcinoma HepG2 cells | ↑ Nrf2 nuclear translocation. ↑mRNA expression of GST and NQO-1. |
[215] |
Abbreviations—HCT116: human colorectal carcinoma cell line; Caco-2: human epithelial colorectal adenocarcinoma cell line; KKU-100: opisthorchiasis-associated cholangiocarcinoma cell line; A549: human alveolar basal epithelial adenocarcinoma cell line; MCF7: human breast cancer cell line; BEL-7402/ADM: hepatocellular carcinoma cell line; HepG2: human hepatocellular carcinoma cell line; SH-SY5Y: human neuroblastoma cells; Raw 264.7: murine macrophage cell line; INS-1E: rat insulinoma cell line; Hepa-1c1c7: murine hepatoma cell line; GST: glutathione S-transferase; NQO-1: NAD(P)H quinone dehydrogenase 1; HO-1: heme oxygenase 1; AKR1C: aldo-keto reductases1 C1 and C2; GCLC: glutamate-cysteine ligase catalytic subunit; MRP: multidrug resistance-associated protein; AKR1B10: aldo-keto reductase family 1 member B10; PKC: protein kinase C; ERK: extracellular signal-regulated protein kinase; PI3K/Akt: phosphorylation of phosphatidylinositol 3-kinase/protein kinase B; JNK: N terminal kinase; Keap1: Kelch-like ECH-associated protein 1; Nrf2: Nuclear factor erythroid 2 p45 (NF-E2)-related factor; ↑ increase; ↓ decrease.
Myricetin activates Nrf2 in HepG2 human hepatocellular carcinoma cells through canonical activation, via modifying the Keap1 protein [203]. These modifications lead to unchanged levels of Keap1 but inhibit Nrf2 ubiquitination through interfering with the CUL3–Keap1–Nrf2 complex, which activates Nrf2 [203]. Furthermore, myricetin upregulates Nrf2 nuclear translocation following Nrf2 activation in HepG2 [203]. The inductive effects of myricetin on HepG2 continue further downstream, as evidenced by an increased expression of HO-1 at the translational level [203]. However, the bioavailability of tested concentrations of myricetin through human dietary intake is mostly unknown.
Quercetin activates the Nrf2/ARE pathway in chronic high glucose-induced human neuroblastoma cells (SH-SY5Y), with oxidative stress, through sustained phosphorylation in Nrf2 by PKC [123]. The inductive effect of quercetin continues further downstream on SH-SY5Y cells via the upregulation of the nuclear localization of Nrf2 and the expression of phase 2 detoxifying enzyme glyoxalase 1 at transcriptional and translational levels, in addition to increasing its enzymatic activity [123]. Furthermore, quercetin shows similar upregulations of Nrf2 nuclear translocation in t-BHP-induced HepG2 cells with oxidative stress [204]. However, the tested concentrations of quercetin in SH-SY5Y and HepG2 cells are higher than bioavailable quercetin from a human diet [110].
Hesperetin, the aglycone of hesperidin, activates the Nrf2/ARE pathway in LPS-induced murine macrophage Raw 264.7 cells [205]. Hesperetin increases the degradation of the Keap1 protein and Nrf2 nuclear translocation in Raw 264.7 [205]. The inductive effects of hesperetin continue further downstream on Raw 264.7 cells, as evidenced by an increased expression of HO-1 at translational levels [205]. Furthermore, the tested concentrations of hesperetin in Raw 264.7 cells are much higher than the physiologically bioavailable hesperetin in human subjects derived from the diet [206]. Neohesperidin dihydrochalcone reduces Nrf2 ubiquitination through the canonical activation of Nrf2 by modifying the Keap1 protein in CCl4-induced HepG2 cells with oxidative stress [207]. Furthermore, modifications observed in Keap1 may have interfered with the CUL3–Keap1–Nrf2 complex, which reduces Nrf2 ubiquitination [203,207]. Further, the inductive effects of neohesperidin dihydrochalcone on HepG2 continue further downstream upon Nrf2 activation, as evidenced by Nrf2 nuclear localization and the increased expression of HO-1 and NQO-1 at the translational level [207]. The expression of downstream target proteins (HO-1 and NQO-1) in HepG2 cells depends on the increased levels of phosphorylated p38 and JNK signaling molecules [207]. However, the bioavailability of neohesperidin dihydrochalcone from a human diet is mostly unknown, due to a lack of studies on the bioavailability of neohesperidin in clinical studies.
Genistein and daidzein, two major isoflavones of soybean, upregulate quinone reductase at the transcriptional level and its activity in Hepa-1c1c7 murine hepatoma cells at human physiological concentrations [208]. Furthermore, several other flavonoids belong to flavan-3-ol, and chalcones show their potential in activating the Nrf2/ARE pathway in different cancer models (Table 2).
Overall, most of the flavonoids tested have exhibited their potential in activating the Nrf2/ARE pathway in different cancer models, either under induced or non-induced conditions. However, the exact molecular mechanism of the activation of the Nrf2/ARE pathway by these flavonoids in cancer cells is yet to be explored.
3.2. Flavonoids: Nrf2/ARE Inhibition in Cancer Cells
Identification of specific flavonoids that inhibit the Nrf2/ARE pathway in cancer cells would be interesting for exploring their applications in cancer treatment. Interestingly, three flavones, luteolin, apigenin and chrysin, were found to be effective in inhibiting the Nrf2/ARE pathway in different cancer cell lines [60,61,62,63,68,209].
Luteolin inhibits the Nrf2-ARE pathway and downregulates ARE-driven enzymes such as gamma-glutamylcysteine ligase and HO-1 in opisthorchiasis-associated cholangiocarcinoma KKU-100 cells [60]. Luteolin induced these effects, leading to an elevation of superoxide radical levels followed by the mitochondrial depolarization-mediated apoptosis of KKU-100 cells [60]. Another study shows that luteolin reduces the mRNA levels of phase 2 drug detoxifying enzymes, such as HO-1, NQO-1, aldo-keto reductases1 C1 and C2 (AKR1C), glutamate-cysteine ligase catalytic subunit (GCLC), and multidrug resistance-associated protein (MRP) 2 in human cancer cells, A549, siGFP-C5 (a stable lung cancer cell line developed from A549 cells by transfecting pRS-GFP), Caco-2 cells, and human breast cancer MCF7 cells [61]. Interestingly, luteolin sensitizes siGFP-C5 to oxaliplatin, bleomycin and doxorubicin by reducing the IC50 values of these chemotherapeutic drugs that are used to treat lung cancer. However, the tested concentrations of luteolin in KKU-100, A549, siGFP-C5, Caco-2 and MCF7 are higher than the physiologically available luteolin in humans through the diet [199].
Apigenin inhibits the Nrf2/ARE pathway in doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM and HepG2 cancer cells [62,68]. Apigenin inhibits Nrf2 at the mRNA level, and phase 2 detoxifying enzymes at the protein and mRNA levels in BEL-7402/ADM cells [62]. Furthermore, apigenin sensitizes doxorubicin-resistant BEL-7402/ADM cells to doxorubicin by reducing the IC50 value of doxorubicin [62,63]. Furthermore, these observations are associated with the improved cellular uptake of doxorubicin with the downregulation of MRP5 gene expression and the downregulation of the PI3K/Akt pathway by reducing phosphorylated Akt, leading to reduced Nrf2 nuclear translocation [62]. Furthermore, the intraperitoneal administration of apigenin significantly reduces the growth of BEL-7402 tumors transplanted into male BALB/c mice without inducing hepatotoxicity [62]. Interestingly, the co-administration of apigenin and doxorubicin in BALB/c male mice shows a greater reduction of BEL-7402 tumor size than the apigenin or doxorubicin treatment alone [62]. Apigenin facilitates the inhibition of cell growth and the ROS-mediated apoptosis of HepG2 cells [68]. Further, apigenin reduces the mRNA levels of antioxidant defense genes (GSH and GPx) in HepG2, as well as the activity of GSH and GPx [68].
The inhibitory effect of chrysin on the Nrf2/ARE pathway in several cancer cells has been shown. Chrysin reduces the mRNA expression of Nrf2, MRP1, NQO-1, and HO-1 in breast cancer MCF7 cells [209]. Chrysin sensitizes MCF7 cells to doxorubicin, and the co-treatment with chrysin and doxorubicin increases the apoptotic MCF7 cell population when compared to chrysin or doxorubicin treatment alone [209]. Similar to apigenin, chrysin downregulates the expression of Nrf2 and phase 2 detoxifying enzymes in doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells [63]. Further, the chrysin-mediated downregulation of the Nrf2/ARE pathway is associated with the inhibition of PI3K/Akt and ERK signaling, and the increased uptake of doxorubicin upon inhibition of MRP5 by BEL-7402/ADM cells [63]. Furthermore, chrysin sensitizes BEL-7402/ADM cells to doxorubicin by reducing the IC50 of doxorubicin [63]. However, the reported effective concentrations of chrysin in MCF7 and BEL-7402/ADM cell lines may not be achievable through a human diet or supplementation, due to the low bioavailability of oral administration [210].
In cancer cells, flavonoids can activate the Nrf2/ARE pathway, subsequently leading to the restoration of redox homeostasis via the detoxification of xenobiotics, drug transportation, and the neutralization of reactive species. This thereby facilitates cancer cell survival and cancer promotion. The expression of several ARE-driven genes facilitates cancer cell proliferation by facilitating metabolic reactions such as glutamine synthesis [180,182], NADPH and glucose metabolism in the pentose phosphate pathway [180,182,183,184,185] and the tricarboxylic acid cycle, in addition to fatty acid and lipid metabolism [180,182,183,184,185], and purine and amino acids synthesis [180,182,183,184,185]. Further, the activation of the Nrf2/ARE pathway was achieved, with influential crosstalk facilitated by several flavonoids in between other signaling molecules and the Nrf2/ARE pathway. Therefore, it will be beneficial if the Nrf2/ARE pathway can be inhibited by certain flavonoids in cancer cells [40].
1: Luteolin; 2: Apigenin; 3: Tangeretin; 4: Myricetin; 5: Quercetin; 6: Rutin; 7: Hesperetin; 8: Neohesperidin dihydrochalcone; 9: Epicatechin; 10: Naringenin; 11: Phloretomide; 12: Chrysin; 13: Daidzein; 14: Genistein; 15: Naringenin; 16: Morin.
Keap1: Kelch-like ECH-associated protein 1; Nrf2: Nuclear factor erythroid 2 p45 (NF-E2)-related factor; sMaf: Small musculoaponeurotic fibrosarcoma protein; ARE: Antioxidant response element; GSH: Glutathione; SOD: superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase, FA: fatty acid; AA: Amino acid; NADPH: nicotine adenine dinucleotide phosphate; TCA: tricarboxylic acid.
(The figure was adapted from Wu et al., 2019 [33])
4. Flavonoids and Nrf2/ARE: A Friend or Foe
The role of flavonoids in the activation of the Nrf2/ARE pathway has led to controversial debates over their dual roles in cancer prevention (in normal cells) and cancer promotion (in cancer cells) [33]. Certain flavonoids have the potential to promote or inhibit cancer/cancer cell survival, growth, and proliferation by regulating the Nrf2/ARE pathway differently [65,93].
As previously described, luteolin exhibits dual roles in cancer cell growth in vitro and cancer promotion in vivo, in addition to cancer prevention in vitro and in vivo. Luteolin activates Nrf2/ARE in cancer models, such as human colorectal carcinoma HCT116, Caco-2, and HepG2 [65,72,200]. In comparison, luteolin activates the Nrf2/ARE pathway in H9C2 and mouse testis Sertoli TM4 cells to protect cells from oxidative stress-mediated apoptosis [93,121]. Luteolin reduces LPS-induced severe acute pancreatitis in ICR mice [193]. Furthermore, luteolin upregulates the Nrf2/ARE pathway in ICR mice under normal physiological conditions, and Sprague-Dawley rats with intracerebral hemorrhage [51,131]. Further, luteolin mediates the upregulation of Nrf2 at both the mRNA and protein levels in both non-cancer (H9C2) and cancer models (HCT116, HepG2 cells, and azoxymethane-induced colorectal cancer induced BALB/c mice) [65,72,93,200]. Furthermore, luteolin facilitates Nrf2 nuclear translocation in non-cancer (TM4 cells, liver cells of ICR mice, and basal ganglia cells of Sprague-Dawley rats) and cancer (HepG2 and Caco-2 cells) models [51,65,72,121,131]. Furthermore, luteolin is effective in expressing the HO-1 protein in ICR mice, Sprague-Dawley rats with intracerebral hemorrhage, and human HepG2 cancer cells [51,65,131].
Interestingly, the upregulation of Nrf2 at both the mRNA and protein levels in HCT116 cells is associated with cancer prevention through the reducing/reversing of epigenetic modifications (CpG methylation) in the Nrf2 gene promoter, and blocking HCT116 cell proliferation and transformation [211]. Furthermore, further exposure to azoxymethane in azoxymethane-induced colorectal cancer BALB/c mice shows that the upregulation of GST in the liver and colon by luteolin detoxifies azoxymethane to avoid the further initiation of normal cells to colorectal cancer cells [200]. However, cancer-initiated cells may survive with the upregulation of the Nrf2/ARE pathway and lead to cancer progression, even though exposure to carcinogens is dealt with, as the activation of Nrf2/ARE can lead to cancer cell survival and proliferation [33,200]. Further, low concentrations of luteolin significantly increase cell viability in HepG2 cancer cells, which may have the potential to facilitate cancer progression [65]. In contrast, luteolin exhibits inhibitory effects on KKU-100, siGFP-C5, MCF7, Caco-2, and A549 cells by downregulating the Nrf2/ARE pathway [61]. However, luteolin exhibits hormetic effects in MCF7, Caco-2, siGFP-C5, and A549 cells in a dose-dependent manner. At low concentrations, luteolin increases the expression of HO-1, NQO-1, AKR1C, GCLC, and MRP2 genes (protein and mRNA levels) in MCF7, Caco-2, siGFP-C5, and A549 cells, and high concentrations of luteolin significantly reduce the gene expression. Furthermore, the luteolin-induced downregulation of the expression of ARE-driven enzymes increases the sensitivity of siGFP-C5 cells, which is transfected with pRS-GFP (expresses siRNA against GFP mRNA; siNrf2-C27), to chemopreventive drugs such as oxaliplatin, bleomycin, and doxorubicin [61]. Furthermore, a study carried out by Yang and colleagues (2014) showed that low doses of luteolin encourage the upregulation of cellular GSH, and comparatively higher concentrations of luteolin encourage the reduction of GSH content in Caco-2 cell line [72]. Thus, luteolin may have the potential to exert anti-cancer effects via its hormetic effects.
Apigenin (flavone) also showed dual roles in vitro with respect to cancer prevention and cancer cell survival, growth, and proliferation in different non-cancer (ARPE-19) and cancer (HepG2) cell lines, respectively [65,95,100]. The expression of Nrf2 at both the mRNA and protein levels is commonly seen in ARPE-19and HepG2 cells [65,95]. In ARPE-19 cells, apigenin alleviates t-BHP-induced oxidative stress with the increased activities of antioxidant defense enzymes (SOD, CAT, and GPx), and reduces cell apoptosis by restoring normal physiological concentrations [95]. Further, at the tested low-concentrations, apigenin alone significantly improved cell viability in HepG2 cancer cells [65]. Thus, along with the Nrf2/ARE activation, cell growth and cell proliferation have been increased in HepG2 cells at the tested concentrations (1.5-6.25 µM) [65]. Contrarily, the hormetic effect of apigenin is evident, as higher concentrations (100 µM) inhibit cancer cell survival in HepG2 cancer cells via the excessive generation of ROS-mediated apoptosis [68]. Furthermore, the possible use of low doses of apigenin in treating hepatocellular carcinoma has been validated in both BEL-7402/ADM cells (10–20 µM) and BEL-7402 transplanted BALB/c mice (50 mg/kg), with the inhibition of cancer cells and tumors in the animal model, respectively. Furthermore, apigenin sensitizes hepatocellular carcinoma cells (BEL-7402/ADM) to chemotherapeutic drugs such as doxorubicin, indicating the promising synergistic effects of the co-treatment of apigenin and chemotherapeutic drugs [62].
Chrysin activates the Nrf2/ARE pathway in rat hepatocytes to attenuate t-BHP-induced oxidative stress, and restores redox homeostasis [124]. Furthermore, the inhibitory effects of apigenin were observed in both cancer (BEL-7402/ADM and MCF7) and non-cancer (HUVEC) cells [124]. At the same time, chrysin sensitizes MCF7 and BEL-7402/ADM cancer cells to doxorubicin, indicating the potential use of chrysin along with chemotherapy to treat breast cancer and hepatocellular carcinoma after further validation in pre-clinical and clinical studies [63,209]. However, chrysin acts as an inhibitor of Nrf2 in HUVECs, as it downregulates the protective effects of hydrogen. Molecular hydrogen protects HUVECs from anti-senescence by activating the Nrf2/ARE pathway [216]. Hydrogen treatment upregulates the phosphorylated Nrf2 and phase 2 detoxifying enzymes, such as HO-1 and NQO-1, at protein levels [216]. However, chrysin treatments inhibit the antioxidant and anti-senescence activities of hydrogen by downregulating HO-1 and NQO-1 proteins and increasing DNA damage, as shown by the significantly increased 8-hydroxydeoxyguanosine level in HUVEC cells [216]. Thus, chrysin exhibits its potential to inhibit the Nrf2/ARE pathway in HUVECs under normal physiological conditions, while activating it in non-cancer (rat hepatocytes) cells with induced conditions.
Baicalin and its aglycone baicalein activate the Nrf2/ARE pathway in non-cancer experimental models [94,117,120]. Baicalin activates the Nrf2/ARE pathway in both human liver L-02 and murine H9C2 cells, and in Sprague-Dawley rats. Further, baicalin upregulates Nrf2 at the translational level in H9C2 and the brain tissues of Sprague-Dawley cells [94,97]. Furthermore, baicalin protects H9C2 cells from hypoxia-aroused apoptosis, and reduces early brain injury in Sprague-Dawley rats after subarachnoid hemorrhage [97]. Further, the inductive effects of both baicalein and baicalin show that human liver L-02 cells can be protected from acetaminophen-induced oxidative stress and liver cell injury [117]. In another study, Dong and colleagues (2020) showed that baicalein upregulates Nrf2 nuclear translocation in high glucose-induced human liver L-02 (synonym: HL-7702) cells, and the liver cells of T2DM Kunming mice, and reduces oxidative stress in both the L-02 and the liver cells of T2DM Kunming mice, in addition to the reduction of L-02 cell apoptosis [120].
Quercetin, a common flavonol aglycone, exhibits the activation of the Nrf2/ARE pathway in both cancer (HepG2 and SH-SY5Y) and non-cancer cells (HaCaT and BJ foreskin fibroblasts, IEC-6 and HUVECs), and in animal models of broiler chicken [102,108,111,123,155,204]. Quercetin exerts inductive effects on Nrf2 nuclear translocation in HepG2, SH-SY5Y, broiler chicken, and IEC-6 cells. Further, the upregulation of the Nrf2 protein is common among non-cancer cells (HUVECs, HaCaT, and BJ foreskin fibroblasts) and in broiler chickens. The quercetin-mediated induction of metallothionein and the activation of the Nrf2/ARE pathway in HepG2 protects from t-BHP-induced oxidative stress [204]. Furthermore, the expression of Nrf2 downstream protein metallothionein is associated with protecting liver cells from acute heavy metal toxicity through binding heavy metals intracellularly [204,217,218]. Therefore, the hepatoprotection exerted by the quercetin-mediated induction of metallothionein in HepG2 cells may be partly due to the binding of metallothionein with metals present in the liver (i.e., Fe) that have the potential to participate in Fenton reaction and Haber–Weiss reactions, to generate further ROS [204,219]. Furthermore, the quercetin-mediated activation of the Nrf2/ARE pathway protects SH-SY5Y cancer cells from chronic high glucose-induced oxidative cell injury, and exerts neuroprotection in addition to improving cell viability [123]. Therefore, the protective effects of quercetin with the induction of the Nrf2/ARE pathway may facilitate cancer cell survival and proliferation in HepG2 and SH-SY5Y cells. In contrast, quercetin activates the Nrf2/ARE pathway in HaCaT and BJ foreskin fibroblasts under normal physiological conditions, without any oxidative inducers [102]. Furthermore, quercetin activates Nrf2/ARE with the presence of oxidative stress inducers in hydrogen peroxide-induced IEC-6 and HUVECs cells, and in LPS-induced broiler chickens, and restores normal physiological conditions [108,111,155].
Myricetin and rutin are another two flavanols that activate the Nrf2/ARE pathway in both cancer and non-cancer experimental models [132,140,203]. Myricetin treated HepG2, and normal Kunming mice show similar mechanisms of Nrf2/ARE activation in terms of the upregulation of Nrf2 nuclear translocation. Furthermore, myricetin shows that it can activate the Nrf2/ARE pathway in HepG2 cells and Kunming mice without any oxidative inducers [132,203]. Furthermore, rutin activates the Nrf2/ARE pathway, leading to a subsequent upregulation of HO-1 and NQO-1 at the transcriptional level in human keratinocyte HaCaT cells and colorectal cancer cells (Caco-2), in an oxidative inducer-independent manner [140]. Further, rutin did not increase ROS production in HaCaT and Caco-2 cells, which suggests that rutin alone acts as an activator of the Nrf2/ARE pathway without increasing oxidative stress [140].
Naringenin activates the Nrf2/ARE pathway in 6-OHDA-induced SH-SY5Y cancer cells and male C57BL/6 mice, attenuating oxidative stress [112]. Furthermore, naringenin protects SH-SY5Y cancer cells against 6-OHDA-induced oxidative stress-mediated apoptosis [112]. Further, the inductive effects of naringenin continue as it activates the Nrf2/ARE pathway in normal Sprague-Dawley neuron cells in vitro in the absence of oxidative inducers, and reduces oxidative stress-mediated mitochondrial dysfunctions [103]. Furthermore, the upregulation of HO-1 at the translational level was observed in Sprague-Dawley rat neuron cells and 6-OHDA-induced SH-SY5Y cells [103,112]. Furthermore, low concentrations of naringenin elevate ROS production in Sprague-Dawley neurons and, therefore, the activation of the Nrf2/ARE pathway in Sprague-Dawley neurons upon naringenin treatment is partly attributed to ROS production [103].
Epicatechin, a flavan-3-ol, facilitates the Nrf2/ARE pathway by upregulating Nrf2 nuclear translocation in primary mouse astrocytes of wild type (WT) and Nrf2 knock-out (KO) C57BL/6 mice, and in HepG2 cells [213]. Furthermore, epicatechin protects primary mouse astrocytes from hemoglobin-induced oxidative stress, and improves HepG2 cell survival and cell proliferation by activating the Nrf2/ARE pathway [122]. Even though genistein activates the Nrf2/ARE pathway against cerebral ischemia-induced oxidative stress, in Sprague-Dawley rats and normal murine hepatoma Hepa-1c1c cells without oxidative inducers similar mechanisms of activation were not observed [138,208].
Overall, flavones (i.e., luteolin, apigenin, chrysin), flavonols (i.e., myricetin, quercetin, and rutin), flavan-3-ols (i.e., epicatechin), flavanones (i.e., naringenin), and isoflavones (i.e., genistein) exhibit dual roles in the activation of the Nrf2/ARE pathway in both cancer and normal cells/experimental animals. Therefore, flavonoids can be considered as a double-edged sword, or a friend and a foe, as the same compounds have the potential to act as a chemopreventive agent as well as a compound that may have the potential to facilitate cancer cell survival, growth and proliferation. Most of the studies related to flavonoids are reported in the in vitro settings, and there are no clinical data to comment on the dual action of flavonoids in the activation/inhibition of the Nrf2/ARE pathway in cancer and normal cells.
5. Conclusions and Future Directions
The effect of flavonoids on the regulation of the Nrf2/ARE pathway in non-cancer (Figure 1) and cancer (Figure 2) is mostly reported using in vitro experimental models. Only a few in vivo studies have been conducted to date. Therefore, the influence of the dietary intake of flavonoids on the Nrf2/ARE pathway associated with physiological benefits is mostly unknown. Based on the reported in vitro and in vivo investigations, it is suggested that if efficacious concentrations of flavonoids can be reached in targeted tissues, flavonoids could contribute to the management of oxidative homeostasis, and thus reduce the risk of cancer. Further, the observed cytotoxicity and anti-proliferative activity in cancer cells of luteolin, apigenin and chrysin should be further validated using in vivo experiments. When considering the bioavailability of flavonoids, most of the effective concentrations of flavonoids that are reported in cell-based assays might not be physiologically relevant [220]. The physiological concentrations that can be achieved through the human diet/oral administration are much lower than most of the tested concentrations in normal (5-400 µM) and cancer (1.5–80 µM) cell lines [221]. For example, the maximum peak plasma concentration of chrysin in healthy human volunteers, after consumption of two 200 mg capsules containing chrysin in the morning following overnight fasting, is 0.01–0.06 nM (3–16 ng/mL) [210]. Therefore, the tested high concentrations of chrysin will never be realized in vivo or in clinical studies in order to have benefits upon oral administration. Further, the peak plasma concentration of chrysin metabolite, chrysin sulfate, is 30 times higher than the administered chrysin concentration in healthy human volunteers (two capsules of 200 mg chrysin via oral administration), despite this being in low concentrations [210]. During phase 2 metabolism, flavonoids undergo sulfation, glucuronidation and methylation in the enterocytes of the small intestine and the liver, resulting in different metabolites. Thus, phase 2 metabolism alters the bioavailability and bioefficacy of the parent compound/flavonoid [222,223]. Hence it is ideal for testing the effectiveness of dietary flavonoids within the physiologically relevant concentration range, in addition to evaluating the effectiveness of metabolites of dietary flavonoids at physiologically relevant concentrations in both in vitro and in vivo experiments. Further, flavonoids such as chrysin have been shown exert positive effects on cancer cell inhibition at comparatively low concentrations, through synergistic effects influenced by one or more other phytochemicals and in low concentrations, compared to acting alone. Therefore, the study of the combinatorial effects of multiple flavonoids/flavonoids and phytochemicals in in vitro, pre-clinical and clinical conditions can be encouraged, which may have the ability to exert chemopreventive effects at lower concentrations [224,225]. Furthermore, due to the low oral bioavailability of luteolin, apigenin and chrysin, the special emphasis should be given to alternative modes of administration, such as intraperitoneal administration, concerning the potential use of these compounds in cancer treatment, but at safer doses [101,199,210].
A cohort study conducted by Bondonno and colleagues, lasting 23 years and using 56,048 cancer-free participants, has shown that the moderate habitual flavonoid intake from a Danish diet (about 500 mg flavonoids/day) is inversely associated with cancer-related mortality [226]. This association is much stronger among participants who consume tobacco and alcohol [226], suggesting that the benefits of dietary flavonoids are noticeable among high-risk groups of cancer. Similar results were observed in a cohort study conducted using 34,708 postmenopausal women who were former or current smokers. The dietary intake of foods rich in flavanones and proanthocyanins reduced the risk of lung cancer incidence, while the intake of foods rich in isoflavones reduced the risk of overall cancer incidence [227]. Considering the digestive tract cancers, a meta-analysis of 23 studies has shown that an increased dietary intake of flavonoids reduces the risk of gastric cancer in European populations [228]. Furthermore, the risk of colorectal cancer among humans is inversely associated with the increased intake of foods rich in flavonols, flavan-3-ols, anthocyanidins and proanthocyanidins [229]. A meta-analysis of 12 epidemiological studies, including five cohort and seven case-control studies, suggests that the risk of colon cancer can be reduced by a high intake of dietary flavonols, whereas the risk of rectal cancer can be reduced by a high intake of flavones [230]. Furthermore, a population-based case-control study by Gates and colleagues shows that the dietary intake of apigenin reduces the risk of ovarian cancer [231]. In contrast, in a health study of 38,408 women aged ≥45 years, Wang and colleagues did not find a major role of five individual flavonols (quercetin, kaempferol and myricetin) and flavones (apigenin and luteolin), or selected flavonoid-rich foods, in cancer prevention [232]. In another study, total flavonoid, quercetin, myricetin and kaempferol intake was not inversely associated with colorectal cancer risk among 71,976 women and 35,425 men involved in the health profession in the US [233]. The above observed lack of correlation could be due to the low intake of flavonoids in the studied populations. Overall, the reported studies suggest that the dietary intake of flavonoids has the potential to reduce the risk of several cancers in most of the studied populations. However, since there are limited or no promising in vivo and clinical data related to the concern that flavonoids may promote already existing cancer growth through the activation of the Nrf2/ARE pathway, further investigations are warranted.
Furthermore, most of the in vitro studies on the activation of the Nrf2/ARE pathway by different flavonoids have been carried out for limited types of cancer cell lines, i.e., HepG2 and Caco-2 cells. The reported studies reveal that the activation or inhibition of the Nrf2/ARE pathway in cancer or normal cells is dependent on flavonoid structure. Therefore, in the future, it is essential to study the structure of the flavonoid vs. its function using different cell lines with dose–response in mind. Furthermore, in silico molecular docking can be used for predicting the influence of the flavonoid structure’s interaction with various proteins associated with the mechanisms of activation of the Nrf2/ARE pathway. These preliminary studies should proceed for a better understanding of the roles of flavonoids in cancer prevention and treatment, before going towards clinical studies. Single flavonoids have been used extensively in the reported molecular mechanistic studies. This is appropriate in fundamental studies as well as in flavonoid-inspired drug discovery. However, in food systems, flavonoids do not present as individual compounds, but exist as a mixture of compounds along with other types of phytochemicals in a food matrix. These complex compound mixtures present in food systems may interact in unpredictable ways with the patients. Therefore, it is also important to assess well-characterized and standardized natural phytochemical mixtures, crude extracts of flavonoids or flavonoid-rich food in light of their effects on the Nrf2/ARE pathway-related management of certain cancers. As reviewed above, some dietary flavonoids could interfere with conventional anticancer therapies by upregulating Nrf2/ARE, which could lead to cytoprotective mechanisms in malignant cells. Recently, Milkovic and colleagues reviewed the controversy around the pharmacological modulation of Nrf2 in relation to cancer therapy, and emphasized the need for a further investigation into inhibitors of Nrf2, which could be a proto-oncogene of cancer patients [234].
In conclusion, the Nrf2/ARE pathway plays a dual role in cancer management via cytoprotection in both normal and cancer cells. Therefore, dietary flavonoids have the potential to exert either cancer-preventive or cancer-promotive influences, depending on the stage of carcinogenesis. Since certain flavonoids can activate the Nrf2/ARE pathway in cancer cells, flavonoid-rich food or supplements given to cancer patients may promote the disease, or interfere with radiotherapy and many forms of chemotherapeutics. Therefore, properly designed clinical studies are required to validate this presumption.
Abbreviations
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| Cul3 | Cullin 3 |
| ERK | Extracellular signal-regulated protein kinase |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GSK-3β | Glycogen synthase kinase 3 beta |
| HO-1 | Heme oxygenase 1 |
| HUVECs | Human umbilical vein epithelial cells |
| JNK | n- terminal kinase |
| Keap1 | Kelch-like ECH associated protein 1 |
| NADPH | Nicotine adenine dinucleotide phosphate |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2/ARE | Nuclear factor erythroid 2-related factor 2/Antioxidant response element |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| T2DM | Type 2 diabetes mellitus |
| t-BHP | tert-butyl hydroperoxide |
| p62 | Sequestosome 1 |
| PERK | Protein kinase-like endoplasmic reticulum-resident kinase |
| PI3K | Phosphatidylinositol 3-kinase |
| PKC | Protein kinase C |
| 6-OHDA | 6-hydroxydopamine |
Author Contributions
Conceptualization: H.P.V.R.; writing—original draft preparation: T.L.S.; writing—review and editing: G.D., Z.X., and H.P.V.R.; visualization: T.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Discovery Grant of the Natural Sciences and Engineering Research Council of Canada (RGPIN2016-05369; H.P.V.R.) and the Cancer Research Training Program of the Beatrice Hunter Cancer Research Institute with funds provided by CIBC (BHCRI-CRTP2020; T.L.S.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hancock J.T., Desikan R., Neill S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001;29:345–350. doi: 10.1042/bst0290345. [DOI] [PubMed] [Google Scholar]
- 2.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 5.Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra S., Nguyen L.N., Akter M., Park G., Choi E.H., Kaushik N.K. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers. 2019;11:1030. doi: 10.3390/cancers11071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apak R., Gorinstein S., Böhm V., Schaich K.M., Özyürek M., Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report) Pure Appl. Chem. 2013;85:957–998. doi: 10.1351/PAC-REP-12-07-15. [DOI] [Google Scholar]
- 8.Lodish H., Berk A., Zipursky S.L., Matsudaira P., Baltimore D., Darnell J. Molecular Cell Biology. 4th ed. W.H. Freeman and Company; New York, NY, USA: 2000. DNA damage and repair and their role in carcinogenesis. [Google Scholar]
- 9.Sipowicz M.A., Amin S., Desai D., Kasprzak K.S., Anderson L.M. Oxidative DNA damage in tissues of pregnant female mice and fetuses caused by the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Cancer Lett. 1997;117:87–91. doi: 10.1016/S0304-3835(97)00208-5. [DOI] [PubMed] [Google Scholar]
- 10.Wajed S.A., Laird P.W., DeMeester T.R. DNA methylation: An alternative pathway to cancer. Ann. Surg. 2001;234:10–20. doi: 10.1097/00000658-200107000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broustas C.G., Lieberman H.B. DNA damage response genes and the development of cancer metastasis. Radiat. Res. 2014;181:111–130. doi: 10.1667/RR13515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George V.C., Dellaire G., Rupasinghe H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017;45:1–14. doi: 10.1016/j.jnutbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Trachootham D., Lu W., Ogasawara M.A., Valle N.R.-D., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 15.Kalam S., Singh R., Mani A., Patel J., Khan F.N., Pandey A. Antioxidants: Elixir of life. Int. Multidiscip. Res. J. 2012;2:18–34. [Google Scholar]
- 16.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/S0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 17.Vignais P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteaga O., Álvarez A., Revuelta M., Santaolalla F., Urtasun A., Hilario E. Role of antioxidants in neonatal hypoxic-ischemic brain injury: New therapeutic approaches. Int. J. Mol. Sci. 2017;18:265. doi: 10.3390/ijms18020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirokmány G., Geiszt M. The relationship of NADPH oxidases and heme peroxidases: Fallin’ in and out. Front. Immunol. 2019;10:394. doi: 10.3389/fimmu.2019.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley E.E., Khoo N.K.H., Hundley N.J., Malik U.Z., Freeman B.A., Tarpey M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Yang Q., Sun M., Teng M., Niu L. Hydrogen peroxide produced by two amino acid oxidases mediates antibacterial actions. J. Microbiol. 2004;42:336–339. [PubMed] [Google Scholar]
- 22.Elsner M., Gehrmann W., Lenzen S. Peroxisome-generated hydrogen Peroxide as Important Mediator of Lipotoxicity in Insulin-Producing Cells. Diabetes. 2011;60:200–208. doi: 10.2337/db09-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Hinkhouse M.M., Sun W., Weydert C.J., Ritchie J.M., Oberley L.W., Cullen J.J. Redox regulation of pancreatic cancer cell growth: Role of glutathione peroxidase in the suppression of the malignant phenotype. Hum. Gene Ther. 2004;15:239–250. doi: 10.1089/104303404322886093. [DOI] [PubMed] [Google Scholar]
- 24.Lyngsie G., Krumina L., Tunlid A., Persson P. Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-29075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehrer J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 26.Jeeva J.S., Sunitha J., Ananthalakshmi R., Rajkumari S., Ramesh M., Krishnan R. Enzymatic antioxidants and its role in oral diseases. J. Pharm. Bioallied. Sci. 2015;7:S331–S333. doi: 10.4103/0975-7406.163438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.J., Kim H.S., Seo Y.R. Understanding of ROS-inducing strategy in anticancer therapy. Oxid. Med. Cell. Longev. 2019;2019:e5381692. doi: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storz P. KRas, ROS and the initiation of pancreatic cancer. Small GTPases. 2017;8:38–42. doi: 10.1080/21541248.2016.1192714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Yi J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008;7:1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 31.Raza M.H., Siraj S., Arshad A., Waheed U., Aldakheel F., Alduraywish S., Arshad M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017;143:1789–1809. doi: 10.1007/s00432-017-2464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsuki A., Yamamoto M. Cis-element architecture of Nrf2–sMaf heterodimer binding sites and its relation to diseases. Arch. Pharm. Res. 2020;43:275–285. doi: 10.1007/s12272-019-01193-2. [DOI] [PubMed] [Google Scholar]
- 33.Wu S., Lu H., Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krajka-Kuźniak V., Paluszczak J., Baer-Dubowska W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017;69:393–402. doi: 10.1016/j.pharep.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Tsushima M., Liu J., Hirao W., Yamazaki H., Tomita H., Itoh K. Emerging evidence for crosstalk between Nrf2 and mitochondria in physiological homeostasis and in heart disease. Arch. Pharm. Res. 2019;43:21–43. doi: 10.1007/s12272-019-01188-z. [DOI] [PubMed] [Google Scholar]
- 37.Furfaro A.L., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U.M., Pronzato M.A., Nitti M. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid. Med. Cell. Longev. 2016;2016:1958174. doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefanson A.L., Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients. 2014;6:3777–3801. doi: 10.3390/nu6093777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Liu H., Davies K.J.A., Sioutas C., Finch C.E., Morgan T.E., Forman H.J. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic. Biol. Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernando W., Rupasinghe H.P.V., Hoskin D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019;452:168–177. doi: 10.1016/j.canlet.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Tong K.I., Katoh Y., Kusunoki H., Itoh K., Tanaka T., Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi A., Kang M.-I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuadrado A., Manda G., Hassan A., Alcaraz M.J., Barbas C., Daiber A., Ghezzi P., León R., López M.G., Oliva B., et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Joo M.S., Kim W.D., Lee K.Y., Kim J.H., Koo J.H., Kim S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at Serine 550. Mol. Cell. Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmermann K., Baldinger J., Mayerhofer B., Atanasov A.G., Dirsch V.M., Heiss E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis–A role for the unfolded protein response. Free Radic. Biol. Med. 2015;88:417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitakaze T., Makiyama A., Yamashita Y., Ashida H. Low dose of luteolin activates Nrf2 in the liver of mice at start of the active phase but not that of the inactive phase. PLoS ONE. 2020;15:e0231403. doi: 10.1371/journal.pone.0231403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y., Li W., Su Z., Kong A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abed D.A., Goldstein M., Albanyan H., Jin H., Hu L. Discovery of direct inhibitors of Keap1–Nrf2 protein–protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B. 2015;5:285–299. doi: 10.1016/j.apsb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung K.-A., Kwak M.-K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobeh M., Petruk G., Osman S., El Raey M.A., Imbimbo P., Monti D.M., Wink M. Isolation of myricitrin and 3,5-di-O-methyl gossypetin from Syzygium samarangense and Evaluation of their involvement in protecting keratinocytes against oxidative stress via activation of the Nrf-2 pathway. Molecules. 2019;24:1839. doi: 10.3390/molecules24091839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris I.S., Treloar A.E., Inoue S., Sasaki M., Gorrini C., Lee K.C., Yung K.Y., Brenner D., Knobbe-Thomsen C.B., Cox M.A., et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H.-S., Du G.-Y., Zhang Z.-G., Zhou Z., Sun H.-L., Yu X.-Y., Shi Y.-T., Xiong D.-N., Li H., Huang Y.-H. NRF2 facilitates breast cancer cell growth via HIF1ɑ-mediated metabolic reprogramming. Int. J. Biochem. Cell Biol. 2018;95:85–92. doi: 10.1016/j.biocel.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Lu K., Alcivar A.L., Ma J., Foo T.K., Zywea S., Mahdi A., Huo Y., Kensler T.W., Gatza M.L., Xia B. NRF2 induction supporting breast cancer cell survival is enabled by oxidative stress–induced DPP3–KEAP1 interaction. Cancer Res. 2017;77:2881–2892. doi: 10.1158/0008-5472.CAN-16-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panieri E., Saso L. Potential applications of NRF2 inhibitors in cancer therapy. Oxid. Med. Cell. Longev. 2019;2019:8592348. doi: 10.1155/2019/8592348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kittiratphatthana N., Kukongviriyapan V., Prawan A., Senggunprai L. Luteolin induces cholangiocarcinoma cell apoptosis through the mitochondrial-dependent pathway mediated by reactive oxygen species. J. Pharm. Pharmacol. 2016;68:1184–1192. doi: 10.1111/jphp.12586. [DOI] [PubMed] [Google Scholar]
- 61.Tang X., Wang H., Fan L., Wu X., Xin A., Ren H., Wang X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Gao A.-M., Ke Z.-P., Wang J.-N., Yang J.-Y., Chen S.-Y., Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 63.Gao A.-M., Ke Z.-P., Shi F., Sun G.-C., Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Calabrese V., Cornelius C., Trovato A., Cavallaro M., Mancuso C., Di Rienzo L., Condorelli D., De Lorenzo A., Calabrese E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. Des. 2010;16:877–883. doi: 10.2174/138161210790883615. [DOI] [PubMed] [Google Scholar]
- 65.Paredes-Gonzalez X., Fuentes F., Jeffery S., Saw C.L.-L., Shu L., Su Z.-Y., Kong A.-N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015;36:440–451. doi: 10.1002/bdd.1956. [DOI] [PubMed] [Google Scholar]
- 66.Rattan S.I.S. Hormesis in aging. Ageing Res. Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Calabrese E.J. Astrocytes: Adaptive responses to low doses of neurotoxins. Crit. Rev. Toxicol. 2008;38:463–471. doi: 10.1080/10408440802004023. [DOI] [PubMed] [Google Scholar]
- 68.Valdameri G., Trombetta-Lima M., Worfel P.R., Pires A.R.A., Martinez G.R., Noleto G.R., Cadena S.M.S.C., Sogayar M.C., Winnischofer S.M.B., Rocha M.E.M. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem. Biol. Interact. 2011;193:180–189. doi: 10.1016/j.cbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res. Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martins L.A.M., Coelho B.P., Behr G., Pettenuzzo L.F., Souza I.C.C., Moreira J.C.F., Borojevic R., Gottfried C., Guma F.C.R. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biochem. Biophys. 2014;68:247–257. doi: 10.1007/s12013-013-9703-8. [DOI] [PubMed] [Google Scholar]
- 71.Franco R., Navarro G., Martínez-Pinilla E. Hormetic and mitochondria-related mechanisms of antioxidant action of phytochemicals. Antioxidants. 2019;8:373. doi: 10.3390/antiox8090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y., Cai X., Yang J., Sun X., Hu C., Yan Z., Xu X., Lu W., Wang X., Cao P. Chemoprevention of dietary digitoflavone on colitis-associated colon tumorigenesis through inducing Nrf2 signaling pathway and inhibition of inflammation. Mol. Cancer. 2014;13:48. doi: 10.1186/1476-4598-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwak M.-K., Kensler T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basak P., Sadhukhan P., Sarkar P., Sil P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017;4:306–318. doi: 10.1016/j.toxrep.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 76.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki T., Shibata T., Takaya K., Shiraishi K., Kohno T., Kunitoh H., Tsuta K., Furuta K., Goto K., Hosoda F., et al. Regulatory nexus of synthesis and degradation deciphers cellular Nrf2 expression levels. Mol. Cell. Biol. 2013;33:2402–2412. doi: 10.1128/MCB.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu C., Huang M.-T., Shen G., Yuan X., Lin W., Khor T.O., Conney A.H., Kong A.-N.T. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2–related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 79.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Z., Huang Z., Zhang D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giudice A., Arra C., Turco M.C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- 82.Curcumin on NFE2L2 Gene Expression, Antioxidant Capacity and Renal Function According to rs35652124 in Diabetic Nephropathy. [(accessed on 8 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03262363.
- 83.RTA 408 Ophthalmic Suspension for the Treatment of Ocular Inflammation and Pain Following Ocular Surgery. [(accessed on 8 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02065375.
- 84.A 52-Week, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Phase IIb Trial to Determine the Effects of Bardoxolone Methyl (RTA 402) on eGFR in Patients with Type 2 Diabetes and Chronic Kidney Disease with an eGFR of 20–45 mL/Min/1.73m2. [(accessed on 8 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT00811889.
- 85.Anti-Inflammatory and Antioxidant Effects of Resveratrol on Healthy Adults. [(accessed on 8 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT01492114.
- 86.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang D.D., Lo S.-C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei R., Enaka M., Muragaki Y. Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-46824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho C.-Y., Cheng Y.-T., Chau C.-F., Yen G.-C. Effect of diallyl sulfide on in vitro and in vivo Nrf2-mediated pulmonic antioxidant enzyme expression via activation ERK/p38 signaling pathway. J. Agric. Food Chem. 2012;60:100–107. doi: 10.1021/jf203800d. [DOI] [PubMed] [Google Scholar]
- 90.Apopa P.L., He X., Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J. Biochem. Mol. Toxicol. 2008;22:63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Xiao Z., Yao J., Zhao G., Fa X., Niu J. Participation of protein kinase C in the activation of Nrf2 signaling by ischemic preconditioning in the isolated rabbit heart. Mol. Cell. Biochem. 2013;372:169–179. doi: 10.1007/s11010-012-1458-9. [DOI] [PubMed] [Google Scholar]
- 92.Wang L., Chen Y., Sternberg P., Cai J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest. Ophthalmol. Vis. Sci. 2008;49:1671–1678. doi: 10.1167/iovs.07-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li L., Luo W., Qian Y., Zhu W., Qian J., Li J., Jin Y., Xu X., Liang G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. 2019;59:152774. doi: 10.1016/j.phymed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H., Tu X., Song S., Liang R., Shi S. Baicalin reduces early brain injury after subarachnoid hemorrhage in rats. Chin. J. Integr. Med. 2020;26:510–518. doi: 10.1007/s11655-020-3183-7. [DOI] [PubMed] [Google Scholar]
- 95.Xu X., Li M., Chen W., Yu H., Yang Y., Hang L. Apigenin attenuates oxidative injury in ARPE-19 cells thorough activation of Nrf2 pathway. Oxid. Med. Cell. Longev. 2016;2016:4378461. doi: 10.1155/2016/4378461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarawek S., Derendorf H., Butterweck V. Pharmacokinetics of luteolin and metabolites in rats. Nat. Prod. Commun. 2008;3:2029–2036. doi: 10.1177/1934578X0800301218. [DOI] [Google Scholar]
- 97.Yu H., Chen B., Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif. Cells Nanomed. Biotechnol. 2019;47:3657–3663. doi: 10.1080/21691401.2019.1657879. [DOI] [PubMed] [Google Scholar]
- 98.Zhao L., Wei Y., Huang Y., He B., Zhou Y., Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: In vitro and in vivo evaluation. Int. J. Nanomed. 2013;8:3769–3779. doi: 10.2147/IJN.S51578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang H.-M., But P.P.-H. Pharmacology and applications of Chinese materia medica. World Sci. 1986;1:304. [Google Scholar]
- 100.Zhang J., Zhao X., Zhu H., Wang J., Ma J., Gu M. Apigenin protects against renal tubular epithelial cell injury and oxidative stress by high glucose via regulation of NF-E2-related factor 2 (Nrf2) pathway. Med. Sci. Monit. 2019;25:5280–5288. doi: 10.12659/MSM.915038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meyer H., Bolarinwa A., Wolfram G., Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann. Nutr. Metab. 2006;50:167–172. doi: 10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- 102.Schadich E., Hlaváč J., Volná T., Varanasi L., Hajdúch M., Džubák P. Effects of ginger phenylpropanoids and quercetin on Nrf2-ARE pathway in human BJ fibroblasts and HaCaT keratinocytes. BioMed Res. Int. 2016;2016:2173275. doi: 10.1155/2016/2173275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang K., Chen Z., Huang L., Meng B., Zhou X., Wen X., Ren D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med. 2017;40:1582–1590. doi: 10.3892/ijmm.2017.3134. [DOI] [PubMed] [Google Scholar]
- 104.Abdelaziz R.M., Abdelazem A.Z., Hashem K.S., Attia Y.A. Protective effects of hesperidin against MTX-induced hepatotoxicity in male albino rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393:1405–1417. doi: 10.1007/s00210-020-01843-z. [DOI] [PubMed] [Google Scholar]
- 105.Al-Maghrebi M., Alnajem A.S., Esmaeil A. Epigallocatechin-3-gallate modulates germ cell apoptosis through the SAFE/Nrf2 signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:663–671. doi: 10.1007/s00210-019-01776-2. [DOI] [PubMed] [Google Scholar]
- 106.Li S.-Q., Dong S., Su Z.-H., Zhang H.-W., Peng J.-B., Yu C.-Y., Zou Z.-M. Comparative pharmacokinetics of naringin in rat after oral administration of Chaihu-Shu-Gan-San aqueous extract and naringin alone. Metabolites. 2013;3:867–880. doi: 10.3390/metabo3040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salehi B., Fokou P.V.T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019;12:11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tian R., Yang Z., Lu N., Peng Y.-Y. Quercetin, but not rutin, attenuated hydrogen peroxide-induced cell damage via heme oxygenase-1 induction in endothelial cells. Arch. Biochem. Biophys. 2019;676:108157. doi: 10.1016/j.abb.2019.108157. [DOI] [PubMed] [Google Scholar]
- 109.Egert S., Wolffram S., Bosy-Westphal A., Boesch-Saadatmandi C., Wagner A.E., Frank J., Rimbach G., Mueller M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 110.Erlund I., Kosonen T., Alfthan G., Mäenpää J., Perttunen K., Kenraali J., Parantainen J., Aro A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000;56:545–553. doi: 10.1007/s002280000197. [DOI] [PubMed] [Google Scholar]
- 111.Sun L., Xu G., Dong Y., Li M., Yang L., Lu W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules. 2020;25:1053. doi: 10.3390/molecules25051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lou H., Jing X., Wei X., Shi H., Ren D., Zhang X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology. 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 113.Ortiz-Andrade R.R., Sánchez-Salgado J.C., Navarrete-Vázquez G., Webster S.P., Binnie M., García-Jiménez S., León-Rivera I., Cigarroa-Vázquez P., Villalobos-Molina R., Estrada-Soto S. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes. Metab. 2008;10:1097–1104. doi: 10.1111/j.1463-1326.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 114.Li Y., Kandhare A.D., Mukherjee A.A., Bodhankar S.L. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2019;105:77–85. doi: 10.1016/j.yrtph.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Chen J.-H., Tipoe G.L., Liong E.C., So H.S.H., Leung K.-M., Tom W.-M., Fung P.C.W., Nanji A.A. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am. J. Clin. Nutr. 2004;80:742–751. doi: 10.1093/ajcn/80.3.742. [DOI] [PubMed] [Google Scholar]
- 116.Ishfaq M., Chen C., Bao J., Zhang W., Wu Z., Wang J., Liu Y., Tian E., Hamid S., Li R., et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019;98:6296–6310. doi: 10.3382/ps/pez406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shi L., Hao Z., Zhang S., Wei M., Lu B., Wang Z., Ji L. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem. Pharmacol. 2018;150:9–23. doi: 10.1016/j.bcp.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 118.Pang C., Zheng Z., Shi L., Sheng Y., Wei H., Wang Z., Ji L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Rad. Biol. Med. 2016;91:236–246. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 119.Tian S., He G., Song J., Wang S., Xin W., Zhang D., Du G. Pharmacokinetic study of baicalein after oral administration in monkeys. Fitoterapia. 2012;83:532–540. doi: 10.1016/j.fitote.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Dong Y., Xing Y., Sun J., Sun W., Xu Y., Quan C. Baicalein alleviates liver oxidative stress and apoptosis induced by high-level glucose through the activation of the PERK/Nrf2 signaling pathway. Molecules. 2020;25:599. doi: 10.3390/molecules25030599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma B., Zhang J., Zhu Z., Zhao A., Zhou Y., Ying H., Zhang Q. Luteolin ameliorates testis injury and blood–testis barrier disruption through the Nrf2 signaling pathway and by upregulating Cx43. Mol. Nutr. Food Res. 2019;63:1800843. doi: 10.1002/mnfr.201800843. [DOI] [PubMed] [Google Scholar]
- 122.Lan X., Han X., Li Q., Wang J. (−)-Epicatechin, a natural flavonoid compound, protects astrocytes against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways. Mol. Neurobiol. 2017;54:7898–7907. doi: 10.1007/s12035-016-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y.-W., Liu X.-L., Kong L., Zhang M.-Y., Chen Y.-J., Zhu X., Hao Y.-C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019;109:2145–2154. doi: 10.1016/j.biopha.2018.11.066. [DOI] [PubMed] [Google Scholar]
- 124.Huang C.-S., Lii C.-K., Lin A.-H., Yeh Y.-W., Yao H.-T., Li C.-C., Wang T.-S., Chen H.-W. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 2013;87:167–178. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- 125.Noh K., Oh D.G., Nepal M.R., Jeong K.S., Choi Y., Kang M.J., Kang W., Jeong H.G., Jeong T.C. Pharmacokinetic interaction of chrysin with caffeine in rats. Biomol. Ther. 2016;24:446–452. doi: 10.4062/biomolther.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manach C., Morand C., Demigné C., Texier O., Régérat F., Rémésy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409:12–16. doi: 10.1016/S0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 127.Ishii S., Kitazawa H., Mori T., Kirino A., Nakamura S., Osaki N., Shimotoyodome A., Tamai I. Identification of the catechin uptake transporter responsible for intestinal absorption of epigallocatechin gallate in mice. Sci. Rep. 2019;9:11014. doi: 10.1038/s41598-019-47214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Speciale A., Anwar S., Canali R., Chirafisi J., Saija A., Virgili F., Cimino F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013;57:1979–1987. doi: 10.1002/mnfr.201300102. [DOI] [PubMed] [Google Scholar]
- 129.Czank C., Cassidy A., Zhang Q., Morrison D.J., Preston T., Kroon P.A., Botting N.P., Kay C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 130.Lee D.-S., Li B., Kim K.-S., Jeong G.-S., Kim E.-C., Kim Y.-C. Butein protects human dental pulp cells from hydrogen peroxide-induced oxidative toxicity via Nrf2 pathway-dependent heme oxygenase-1 expressions. Toxicol. Vitr. 2013;27:874–881. doi: 10.1016/j.tiv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 131.Tan X., Yang Y., Xu J., Zhang P., Deng R., Mao Y., He J., Chen Y., Zhang Y., Ding J., et al. Luteolin exerts neuroprotection via modulation of the p62/Keap1/Nrf2 pathway in intracerebral hemorrhage. Front. Pharmacol. 2020;10:1551. doi: 10.3389/fphar.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Q., Li Z., Wu S., Li X., Sang Y., Li J., Niu Y., Ding H. Myricetin alleviates cuprizone-induced behavioral dysfunction and demyelination in mice by Nrf2 pathway. Food Funct. 2016;7:4332–4342. doi: 10.1039/C6FO00825A. [DOI] [PubMed] [Google Scholar]
- 133.Yang D., Tan X., Lv Z., Liu B., Baiyun R., Lu J., Zhang Z. Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci. Rep. 2016;6:37157. doi: 10.1038/srep37157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang H., Tan X., Yang D., Lu J., Liu B., Baiyun R., Zhang Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget. 2017;8:40982–40993. doi: 10.18632/oncotarget.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dai L.M., Cheng H., Li W.P., Liu S.Q., Chen M.Z., Xu S.Y. The influence of luteolin on experimental inflammatory models in rats. Acta Anhui Med. Univ. 1985;20:1–3. [Google Scholar]
- 136.Chen L., Dou J., Su Z., Zhou H., Wang H., Zhou W., Guo Q., Zhou C. Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antivir. Res. 2011;91:314–320. doi: 10.1016/j.antiviral.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 137.Yang Y., Choi J.K., Jung C.H., Koh H.J., Heo P., Shin J.Y., Kim S., Park W.-S., Shin H.-J., Kweon D.-H. SNARE-wedging polyphenols as small molecular botox. Planta Med. 2012;78:233–236. doi: 10.1055/s-0031-1280385. [DOI] [PubMed] [Google Scholar]
- 138.Wang R., Tu J., Zhang Q., Zhang X., Zhu Y., Ma W., Cheng C., Brann D.W., Yang F. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus. 2013;23:634–647. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- 139.Singh P., Sharma S., Rath S.K. Genistein induces deleterious effects during its acute exposure in Swiss mice. BioMed Res. Int. 2014;2014:e619617. doi: 10.1155/2014/619617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elle R.E., Rahmani S., Lauret C., Morena M., Bidel L.P.R., Boulahtouf A., Balaguer P., Cristol J.-P., Durand J.-O., Charnay C., et al. Functionalized mesoporous silica nanoparticle with antioxidants as a new carrier that generates lower oxidative stress impact on cells. Mol. Pharm. 2016;13:2647–2660. doi: 10.1021/acs.molpharmaceut.6b00190. [DOI] [PubMed] [Google Scholar]
- 141.Hseu Y.-C., Chou C.-W., Kumar K.J.S., Fu K.-T., Wang H.-M., Hsu L.-S., Kuo Y.-S., Wu C.-R., Chen S.-C., Yang H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012;50:1245–1255. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 142.Tate P.S., Marazita M.C., Marquioni-Ramella M.D., Suburo A.M. Ilex paraguariensis extracts and its polyphenols prevent oxidative damage and senescence of human retinal pigment epithelium cells. J. Funct. Foods. 2020;67:103833. doi: 10.1016/j.jff.2020.103833. [DOI] [Google Scholar]
- 143.Liu D., Wang H., Zhang Y., Zhang Z. Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Des. Dev. Ther. 2020;14:51–60. doi: 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Song J.H., Lee H.-J., Kang K.S. Procyanidin C1 activates the Nrf2/HO-1 signaling pathway to prevent glutamate-induced apoptotic HT22 cell death. Int. J. Mol. Sci. 2019;20:142. doi: 10.3390/ijms20010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rousta A.-M., Mirahmadi S.-M.-S., Shahmohammadi A., Nourabadi D., Khajevand-Khazaei M.-R., Baluchnejadmojarad T., Roghani M. Protective effect of sesamin in lipopolysaccharide-induced mouse model of acute kidney injury via attenuation of oxidative stress, inflammation, and apoptosis. Immunopharmacol. Immunotoxicol. 2018;40:423–429. doi: 10.1080/08923973.2018.1523926. [DOI] [PubMed] [Google Scholar]
- 146.Kong P., Chen G., Jiang A., Wang Y., Song C., Zhuang J., Xi C., Wang G., Ji Y., Yan J. Sesamin inhibits IL-1β-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget. 2016;7:83720–83726. doi: 10.18632/oncotarget.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang B.Y., Jung Y.S., Yoon C.-S., Oh J.S., Hong J.H., Kim Y.-C., Kim S.Y. Fraxin prevents chemically induced hepatotoxicity by reducing oxidative stress. Molecules. 2017;22:587. doi: 10.3390/molecules22040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ungvari Z., Bagi Z., Feher A., Recchia F.A., Sonntag W.E., Pearson K., de Cabo R., Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pilot Study of the Effects of Resveratrol Supplement in Mild-To-Moderate Alzheimer’s Disease. [(accessed on 8 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT00743743.
- 150.Qi L., Jiang J., Zhang J., Zhang L., Wang T. Curcumin protects human trophoblast HTR8/SVneo cells from H2O2-induced oxidative stress by activating Nrf2 signaling pathway. Antioxidants. 2020;9:121. doi: 10.3390/antiox9020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhai S.S., Ruan D., Zhu Y.W., Li M.C., Ye H., Wang W.C., Yang L. Protective effect of curcumin on ochratoxin A–induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult. Sci. 2020;99:1124–1134. doi: 10.1016/j.psj.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bucolo C., Drago F., Maisto R., Romano G.L., D’Agata V., Maugeri G., Giunta S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J. Cell. Physiol. 2019;234:17295–17304. doi: 10.1002/jcp.28347. [DOI] [PubMed] [Google Scholar]
- 153.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao S., Duan X., Wang X., Dong D., Liu D., Li X., Sun G., Li B. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem. Toxicol. 2013;59:739–747. doi: 10.1016/j.fct.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 155.Barraza-Garza G., Pérez-León J.A., Castillo-Michel H., de la Rosa L.A., Martinez-Martinez A., Cotte M., Alvarez-Parrilla E. Antioxidant effect of phenolic compounds (PC) at different concentrations in IEC-6 cells: A spectroscopic analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;227:117570. doi: 10.1016/j.saa.2019.117570. [DOI] [PubMed] [Google Scholar]
- 156.Han J., Wang M., Jing X., Shi H., Ren M., Lou H. (−)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem. Res. 2014;39:1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 157.Su Z.-Y., Zhang C., Lee J.H., Shu L., Wu T.-Y., Khor T.O., Conney A.H., Lu Y.-P., Kong A.-N.T. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prev. Res. 2014;7:319–329. doi: 10.1158/1940-6207.CAPR-13-0313-T. [DOI] [PubMed] [Google Scholar]
- 158.Kalayarasan S., Prabhu P.N., Sriram N., Manikandan R., Arumugam M., Sudhandiran G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharmacol. 2009;606:162–171. doi: 10.1016/j.ejphar.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 159.Yuan X., Wang J., Tang X., Li Y., Xia P., Gao X. Berberine ameliorates non alcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015;13:24. doi: 10.1186/s12967-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tao S., Zhang H., Xue L., Jiang X., Wang H., Li B., Tian H., Zhang Z. Vitamin D protects against particles-caused lung injury through induction of autophagy in an Nrf2-dependent manner. Environ. Toxicol. 2019;34:594–609. doi: 10.1002/tox.22726. [DOI] [PubMed] [Google Scholar]
- 161.Duan L., Li J., Ma P., Yang X., Xu S. Vitamin E antagonizes ozone-induced asthma exacerbation in Balb/c mice through the Nrf2 pathway. Food Chem. Toxicol. 2017;107:47–56. doi: 10.1016/j.fct.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 162.Wang G., Xiu P., Li F., Xin C., Li K. Vitamin A Supplementation alleviates extrahepatic cholestasis liver injury through Nrf2 activation. Oxid. Med. Cell. Longev. 2014;2014:273692. doi: 10.1155/2014/273692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang Q., Zhang S., Du T., Yang Q., Chi S., Liu H., Yang Y., Dong X., Tan B. Modulation of growth, immunity and antioxidant-related gene expressions in the liver and intestine of juvenile Sillago sihama by dietary vitamin C. Aquac. Nutr. 2020;26:338–350. doi: 10.1111/anu.12996. [DOI] [Google Scholar]
- 164.Zhao R., Wang H., Qiao C., Zhao K. Vitamin B2 blocks development of Alzheimer’s disease in APP/PS1 transgenic mice via anti-oxidative mechanism. Trop. J. Pharm. Res. 2018;17:1049–1054. doi: 10.4314/tjpr.v17i6.10. [DOI] [Google Scholar]
- 165.Said H.M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem. J. 2011;437:357–372. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pietrofesa R.A., Chatterjee S., Park K., Arguiri E., Albelda S.M., Christofidou-Solomidou M. Synthetic lignan secoisolariciresinol diglucoside (LGM2605) reduces asbestos-induced cytotoxicity in an Nrf2-dependent and -independent manner. Antioxidants. 2018;7:38. doi: 10.3390/antiox7030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Solis L.M., Behrens C., Dong W., Suraokar M., Ozburn N.C., Moran C.A., Corvalan A.H., Biswal S., Swisher S.G., Bekele B.N., et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin. Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yoo N.J., Kim H.R., Kim Y.R., An C.H., Lee S.H. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60:943–952. doi: 10.1111/j.1365-2559.2012.04178.x. [DOI] [PubMed] [Google Scholar]
- 169.Kim Y.R., Oh J.E., Kim M.S., Kang M.R., Park S.W., Han J.Y., Eom H.S., Yoo N.J., Lee S.H. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J. Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 170.Ooi A., Dykema K., Ansari A., Petillo D., Snider J., Kahnoski R., Anema J., Craig D., Carpten J., Teh B.-T., et al. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res. 2013;73:2044–2051. doi: 10.1158/0008-5472.CAN-12-3227. [DOI] [PubMed] [Google Scholar]
- 171.Goldstein L.D., Lee J., Gnad F., Klijn C., Schaub A., Reeder J., Daemen A., Bakalarski C.E., Holcomb T., Shames D.S., et al. Recurrent loss of NFE2L2 exon 2 is a mechanism for Nrf2 pathway activation in human cancers. Cell Rep. 2016;16:2605–2617. doi: 10.1016/j.celrep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 172.Chen W., Sun Z., Wang X.-J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21Cip1/WAF1 upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma J., Cai H., Wu T., Sobhian B., Huo Y., Alcivar A., Mehta M., Cheung K.L., Ganesan S., Kong A.-N.T., et al. PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol. Cell. Biol. 2012;32:1506–1517. doi: 10.1128/MCB.06271-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 175.Hast B.E., Goldfarb D., Mulvaney K.M., Hast M.A., Siesser P.F., Yan F., Hayes D.N., Major M.B. Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Res. 2013;73:2199–2210. doi: 10.1158/0008-5472.CAN-12-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Orthwein A., Noordermeer S.M., Wilson M.D., Landry S., Enchev R.I., Sherker A., Munro M., Pinder J., Salsman J., Dellaire G., et al. A mechanism for the suppression of homologous recombination in G1 cells. Nature. 2015;528:422–426. doi: 10.1038/nature16142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177.Adam J., Hatipoglu E., O’Flaherty L., Ternette N., Sahgal N., Lockstone H., Baban D., Nye E., Stamp G.W., Wolhuter K., et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: Roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hanada N., Takahata T., Zhou Q., Ye X., Sun R., Itoh J., Ishiguro A., Kijima H., Mimura J., Itoh K., et al. Methylation of the KEAP1 gene promoter region in human colorectal cancer. BMC Cancer. 2012;12:66. doi: 10.1186/1471-2407-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 181.Taguchi K., Yamamoto M. The KEAP1–NRF2 system in cancer. Front. Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. J. Soc. Toxicol. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kitteringham N.R., Abdullah A., Walsh J., Randle L., Jenkins R.E., Sison R., Goldring C.E.P., Powell H., Sanderson C., Williams S., et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J. Proteom. 2010;73:1612–1631. doi: 10.1016/j.jprot.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee J.-M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 185.Sánchez-Rodríguez R., Torres-Mena J.E., Quintanar-Jurado V., Chagoya-Hazas V., Rojas del Castillo E., del Pozo Yauner L., Villa-Treviño S., Pérez-Carreón J.I. Ptgr1 expression is regulated by NRF2 in rat hepatocarcinogenesis and promotes cell proliferation and resistance to oxidative stress. Free Radic. Biol. Med. 2017;102:87–99. doi: 10.1016/j.freeradbiomed.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 186.Singh A., Happel C., Manna S.K., Acquaah-Mensah G., Carrerero J., Kumar S., Nasipuri P., Krausz K.W., Wakabayashi N., Dewi R., et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013;123:2921–2934. doi: 10.1172/JCI66353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reddy N.M., Kleeberger S.R., Cho H.-Y., Yamamoto M., Kensler T.W., Biswal S., Reddy S.P. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am. J. Respir. Cell. Mol. Biol. 2007;37:3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Reddy N.M., Kleeberger S.R., Yamamoto M., Kensler T.W., Scollick C., Biswal S., Reddy S.P. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genom. 2007;32:74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 189.Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H., et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 190.Kim S.K., Yang J.W., Kim M.R., Roh S.H., Kim H.G., Lee K.Y., Jeong H.G., Kang K.W. Increased expression of Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant breast cancer cells. Free Radic. Biol. Med. 2008;45:537–546. doi: 10.1016/j.freeradbiomed.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 191.Murakami S., Motohashi H. Roles of Nrf2 in cell proliferation and differentiation. Free Radic. Biol. Med. 2015;88:168–178. doi: 10.1016/j.freeradbiomed.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 192.Reddy N.M., Kleeberger S.R., Bream J.H., Fallon P.G., Kensler T.W., Yamamoto M., Reddy S.P. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene. 2008;27:5821–5832. doi: 10.1038/onc.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beyer T.A., Xu W., Teupser D., auf dem Keller U., Bugnon P., Hildt E., Thiery J., Kan Y.W., Werner S. Impaired liver regeneration in Nrf2 knockout mice: Role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Homma S., Ishii Y., Morishima Y., Yamadori T., Matsuno Y., Haraguchi N., Kikuchi N., Satoh H., Sakamoto T., Hizawa N., et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 2009;15:3423–3432. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 195.Chio I.I.C., Jafarnejad S.M., Ponz-Sarvise M., Park Y., Rivera K., Palm W., Wilson J., Sangar V., Hao Y., Öhlund D., et al. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell. 2016;166:963–976. doi: 10.1016/j.cell.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Holmström K.M., Baird L., Zhang Y., Hargreaves I., Chalasani A., Land J.M., Stanyer L., Yamamoto M., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Itoh K., Ye P., Matsumiya T., Tanji K., Ozaki T. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J. Clin. Biochem. Nutr. 2015;56:91–97. doi: 10.3164/jcbn.14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kitamura H., Motohashi H. NRF2 addiction in cancer cells. Cancer Sci. 2018;109:900–911. doi: 10.1111/cas.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cao J., Zhang Y., Chen W., Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br. J. Nutr. 2010;103:249–255. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- 200.Pandurangan A.K., Sadagopan S.K.A., Dharmalingam P., Ganapasam S. Luteolin, a bioflavonoid inhibits Azoxymethane-induced colorectal cancer through activation of Nrf2 signaling. Toxicol. Mech. Methods. 2014;24:13–20. doi: 10.3109/15376516.2013.843111. [DOI] [PubMed] [Google Scholar]
- 201.Xiong J., Wang K., Yuan C., Xing R., Ni J., Hu G., Chen F., Wang X. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017;39:113–125. doi: 10.3892/ijmm.2016.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ding S., Zhang Z., Song J., Cheng X., Jiang J., Jia X. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014;9:2327–2333. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Qin S., Chen J., Tanigawa S., Hou D.-X. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Mol. Nutr. Food Res. 2013;57:435–546. doi: 10.1002/mnfr.201200563. [DOI] [PubMed] [Google Scholar]
- 204.Weng C.-J., Chen M.-J., Yeh C.-T., Yen G.-C. Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. New Biotechnol. 2011;28:767–777. doi: 10.1016/j.nbt.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 205.Ren H., Hao J., Liu T., Zhang D., Lv H., Song E., Zhu C. Hesperetin suppresses inflammatory responses in lipopolysaccharide-induced RAW 264.7 cells via the inhibition of NF-κB and activation of Nrf2/HO-1 pathways. Inflammation. 2016;39:964–973. doi: 10.1007/s10753-016-0311-9. [DOI] [PubMed] [Google Scholar]
- 206.Takumi H., Nakamura H., Simizu T., Harada R., Kometani T., Nadamoto T., Mukai R., Murota K., Kawai Y., Terao J. Bioavailability of orally administered water-dispersible hesperetin and its effect on peripheral vasodilatation in human subjects: Implication of endothelial functions of plasma conjugated metabolites. Food Funct. 2012;3:389–398. doi: 10.1039/c2fo10224b. [DOI] [PubMed] [Google Scholar]
- 207.Su C., Xia X., Shi Q., Song X., Fu J., Xiao C., Chen H., Lu B., Sun Z., Wu S., et al. Neohesperidin dihydrochalcone versus CCl₄-induced hepatic injury through different mechanisms: The implication of free radical scavenging and Nrf2 activation. J. Agric. Food Chem. 2015;63:5468–5475. doi: 10.1021/acs.jafc.5b01750. [DOI] [PubMed] [Google Scholar]
- 208.Froyen E.B., Steinberg F.M. Soy isoflavones increase quinone reductase in hepa-1c1c7 cells via estrogen receptor beta and nuclear factor erythroid 2-related factor 2 binding to the antioxidant response element. J. Nutr. Biochem. 2011;22:843–848. doi: 10.1016/j.jnutbio.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 209.Sabzichi M., Mohammadian J., Bazzaz R., Pirouzpanah M.B., Shaaker M., Hamishehkar H., Chavoshi H., Salehi R., Samadi N. Chrysin loaded nanostructured lipid carriers (NLCs) triggers apoptosis in MCF-7 cancer cells by inhibiting the Nrf2 pathway. Process Biochem. 2017;60:84–91. doi: 10.1016/j.procbio.2017.05.024. [DOI] [Google Scholar]
- 210.Walle T., Otake Y., Brubaker J.A., Walle U.K., Halushka P.V. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001;51:143–146. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zuo Q., Wu R., Xiao X., Yang C., Yang Y., Wang C., Lin L., Kong A.-N. The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell Biochem. 2018;119:9573–9582. doi: 10.1002/jcb.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liang F., Fang Y., Cao W., Zhang Z., Pan S., Xu X. Attenuation of tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in HepG2 Cells by tangeretin: Relevance of the Nrf2–ARE and MAPK signaling pathways. J. Agric. Food Chem. 2018;66:6317–6325. doi: 10.1021/acs.jafc.8b01875. [DOI] [PubMed] [Google Scholar]
- 213.Granado-Serrano A.B., Martín M.A., Haegeman G., Goya L., Bravo L., Ramos S. Epicatechin induces NF-κB, activator protein-1 (AP-1) and nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2) via phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) and extracellular regulated kinase (ERK) signalling in HepG2 cells. Br. J. Nutr. 2010;103:168–179. doi: 10.1017/S0007114509991747. [DOI] [PubMed] [Google Scholar]
- 214.Vanitha P., Senthilkumar S., Dornadula S., Anandhakumar S., Rajaguru P., Ramkumar K.M. Morin activates the Nrf2-ARE pathway and reduces oxidative stress-induced DNA damage in pancreatic beta cells. Eur. J. Pharmacol. 2017;801:9–18. doi: 10.1016/j.ejphar.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 215.Krajka-Kuźniak V., Paluszczak J., Celewicz L., Barciszewski J., Baer-Dubowska W. Phloretamide, an apple phenolic compound, activates the Nrf2/ARE pathway in human hepatocytes. Food Chem. Toxicol. Int. J. 2013;51:202–209. doi: 10.1016/j.fct.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 216.Hara F., Tatebe J., Watanabe I., Yamazaki J., Ikeda T., Morita T. Molecular hydrogen alleviates cellular senescence in endothelial cells. Circ. J. 2016;80:2037–2046. doi: 10.1253/circj.CJ-16-0227. [DOI] [PubMed] [Google Scholar]
- 217.Juárez-Rebollar D., Rios C., Nava-Ruíz C., Méndez-Armenta M. Metallothionein in brain disorders. Oxid. Med. Cell. Longev. 2017;2017:5828056. doi: 10.1155/2017/5828056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gu J., Cheng Y., Wu H., Kong L., Wang S., Xu Z., Zhang Z., Tan Y., Keller B.B., Zhou H., et al. Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66:529–542. doi: 10.2337/db15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Singh N., Haldar S., Tripathi A.K., Horback K., Wong J., Sharma D., Beserra A., Suda S., Anbalagan C., Dev S., et al. Brain iron homeostasis: From molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid. Redox Signal. 2013;20:1324–1363. doi: 10.1089/ars.2012.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thilakarathna S.H., Rupasinghe H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 222.Mullen W., Edwards C.A., Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006;96:107–116. doi: 10.1079/BJN20061809. [DOI] [PubMed] [Google Scholar]
- 223.Rupasinghe H.P.V., Ronalds C.M., Rathgeber B., Robinson R.A. Absorption and tissue distribution of dietary quercetin and quercetin glycosides of apple skin in broiler chickens. J. Sci. Food Agric. 2010;90:1172–1178. doi: 10.1002/jsfa.3944. [DOI] [PubMed] [Google Scholar]
- 224.Javan N., Ansari M.H.K., Dadashpour M., Khojastehfard M., Bastami M., Rahmati-Yamchi M., Zarghami N. Synergistic antiproliferative effects of co-nanoencapsulated curcumin and chrysin on MDA-MB-231 breast cancer cells through upregulating miR-132 and miR-502c. Nutr. Cancer. 2019;71:1201–1213. doi: 10.1080/01635581.2019.1599968. [DOI] [PubMed] [Google Scholar]
- 225.Lotfi-Attari J., Pilehvar-Soltanahmadi Y., Dadashpour M., Alipour S., Farajzadeh R., Javidfar S., Zarghami N. Co-Delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells. Nutr. Cancer. 2017;69:1290–1299. doi: 10.1080/01635581.2017.1367932. [DOI] [PubMed] [Google Scholar]
- 226.Bondonno N.P., Dalgaard F., Kyrø C., Murray K., Bondonno C.P., Lewis J.R., Croft K.D., Gislason G., Scalbert A., Cassidy A., et al. Flavonoid intake is associated with lower mortality in the Danish diet cancer and health cohort. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-11622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cutler G.J., Nettleton J.A., Ross J.A., Harnack L.J., Jacobs D.R., Scrafford C.G., Barraj L.M., Mink P.J., Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. Int. J. Cancer. 2008;123:664–671. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bo Y., Sun J., Wang M., Ding J., Lu Q., Yuan L. Dietary flavonoid intake and the risk of digestive tract cancers: A systematic review and meta-analysis. Sci. Rep. 2016;6:24836. doi: 10.1038/srep24836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Woo H.D., Kim J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013;19:1011–1019. doi: 10.3748/wjg.v19.i7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chang H., Lei L., Zhou Y., Ye F., Zhao G. Dietary flavonoids and the risk of colorectal cancer: An updated meta-analysis of epidemiological studies. Nutrients. 2018;10:950. doi: 10.3390/nu10070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gates M.A., Vitonis A.F., Tworoger S.S., Rosner B., Titus-Ernstoff L., Hankinson S.E., Cramer D.W. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int. J. Cancer. 2009;124:1918–1925. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang L., Lee I.M., Zhang S.M., Blumberg J.B., Buring J.E., Sesso H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009;89:905–912. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin J., Zhang S.M., Wu K., Willett W.C., Fuchs C.S., Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am. J. Epidemiol. 2006;164:644–651. doi: 10.1093/aje/kwj296. [DOI] [PubMed] [Google Scholar]
- 234.Milkovic L., Zarkovic N., Saso L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol. 2017;12:727–732. doi: 10.1016/j.redox.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]