Abstract
The volatile secondary metabolite, isoprene, is released by trees to the atmosphere in enormous quantities, where it has important effects on air quality and climate. Oil palm trees, one of the highest isoprene emitters, are increasingly dominating agroforestry over large areas of Asia, with associated uncertainties over their effects on climate. Microbes capable of using isoprene as a source of carbon for growth have been identified in soils and in the tree phyllosphere, and most are members of the Actinobacteria. Here, we used DNA stable isotope probing to identify the isoprene-degrading bacteria associated with oil palm leaves and inhabiting the surrounding soil. Among the most abundant isoprene degraders of the leaf-associated community were members of the Sphingomonadales, although no representatives of this order were previously known to degrade isoprene. Informed by these data, we obtained representatives of the most abundant isoprene degraders in enrichments, including Sphingopyxis strain OPL5 (Sphingomonadales), able to grow on isoprene as the sole source of carbon and energy. Sequencing of the genome of strain OPL5, as well as a novel Gordonia strain, confirmed their pathways of isoprene degradation and broadened our knowledge of the genetic and taxonomic diversity of this important bacterial trait.
Keywords: isoprene degradation, targeted isolation, DNA-SIP, isoA, isoprene monooxygenase, Sphingopyxis
1. Introduction
Isoprene (2-methyl-1,3-butadiene) is a climate-active gas, with global emissions estimated at around 400–600 Tg per year, making it the most abundant biological volatile organic compound (BVOC) produced in the biosphere [1,2]. Unlike the potent greenhouse gas methane, which is produced in similar amounts, isoprene is a highly reactive compound that has multiple effects on climate and air quality [3]. In polluted urban regions where there are high concentrations of NOx, isoprene causes an increase in tropospheric ozone, whereas in pristine environments isoprene reacts with ozone, resulting in its depletion, and also with hydroxyl radicals which thus indirectly increases the residence time of methane in the atmosphere, leading to global warming [4]. Oxidation products of isoprene in the atmosphere form secondary aerosols that can increase cloud albedo and thus potentially result in global cooling [5]. Thus, isoprene is a climate-active BVOC with the potential to influence climate in several ways. Isoprene is produced by some bacteria, archaea, algae, fungi, protists, and animals, but the vast majority (>90%) of isoprene is from trees [6]. Not all trees produce isoprene, and the exact reasons why some trees can lose 1–2% of their photosynthetically fixed carbon as isoprene are not fully understood [7,8]. Isoprene has been shown to protect plants from heat and oxidative stress and has been implicated as a signaling molecule and regulator of expression of plant genes (reviewed in [9]). Isoprene-emitting trees include poplar and willow, which are often cultivated as biofuel crops, and oil palm, which is one of the highest isoprene-emitting trees [10,11]. Oil palm is a major crop plant grown in vast amounts particularly in southeast Asia, and is the source of around a third of the world’s vegetable oil [12]. Expansion of growth of such crop plants that produce isoprene has raised concerns about their long-term effects on air quality [13].
It has been known for a number of years that soils can be a sink for isoprene [14,15] and this has stimulated research on the microbiology of isoprene metabolism. The biological consumption of isoprene by bacteria and their role in the isoprene cycle has been reviewed in detail recently ([16,17,18]). In brief, a number of Gram-positive bacteria, mainly Actinobacteria of the genera Rhodococcus [19,20], Gordonia, Mycobacterium [21], and Nocardioides [22] have been isolated and characterized. The best studied is Rhodococcus strain AD45, isolated from freshwater sediment, which has become a model bacterium for the study of isoprene metabolism [23,24]. There are fewer cultivated Gram-negative bacteria that have been reported to degrade isoprene [25,26]. The most well-characterized of these is Variovorax strain WS11, originally isolated from soil [25,26,27]. All isoprene degraders characterized to date contain a multi-subunit isoprene monooxygenase, encoded by the genes isoABCDEF, which catalyzes the oxidation of isoprene to epoxyisoprene, and the gene cluster isoGHIJ encoding a glutathione transferase and enzymes catalyzing further steps in the isoprene oxidation pathway [23]. The isoprene monooxygenase (IsoMO) can be distinguished from related soluble di-iron monooxygenases (SDIMOs, [28]) that are involved in metabolism of alkenes, as well as alkanes and aromatic compounds (reviewed in [18]). In Variovorax strain WS11 and Rhodococcus strain AD45, iso genes are carried on megaplasmids [24,26]. New understanding of the physiology, biochemistry, and molecular biology of isoprene metabolism has provided the tools for designing functional gene primers and probes based on isoA, encoding a key component of the IsoMO [19,29]. Surveys with samples from the terrestrial environment, mainly soils and the phyllosphere have revealed the presence of isoprene-degrading bacteria, especially in environments containing trees that produce high concentrations of isoprene [19,25,27,29,30]. DNA-stable isotope probing (DNA-SIP) experiments have also revealed the diversity of active isoprene degraders present in these environments, which are often Actinobacteria, especially Rhodococcus species, but also include other Gram-negative isoprene degraders [19,25,27]. Oil palms may provide “hotspots” of isoprene production where isoprene degraders are likely to thrive, so we examined an oil palm tree in the Palm House at Kew Gardens, London. The aim of this study was to investigate the diversity of active isoprene-degrading bacteria in the phyllosphere and soil around an oil palm tree using DNA-SIP experiments. Here we report on these experiments, together with the isolation, characterization, and genome sequence of a new isoprene-degrading Sphingopyxis species.
2. Materials and Methods
2.1. Sampling, Enrichment Assays, and DNA-SIP Experiments
Samples were collected (November 2016 and February 2017) from a 5-meter-high oil palm tree (germinated in 2002 and located in the Palm House, Kew Gardens, London, UK) and transported back to the laboratory for processing. During the first visit, leaflets and leaf swabs (three leaflets or approx. 34 g of leaves) were collected by cutting off a compound leaf from the oil palm tree. Swabs were collected on site according to Hedin et al. 2010 [31]. Leaves were also sampled for microbes by pressing them onto the surface of fresh, sterile Ewers minimal medium [32] (hereafter referred to as Ewers minimal medium) agar plates for preliminary enrichment and isolation of isoprene-degrading bacteria. In the laboratory, cells were detached from leaflets and leaf swabs by washing into Ewers minimal medium as previously described [27,31] and the resulting medium containing epiphytic cells was enriched with 20 ppmv of isoprene according to Crombie et al. 2018 [27] (Figure S1). Plates, inoculated directly by pressing leaves onto minimal medium agar plates, were incubated in sealed jars at 30 °C in the presence of approximately 1% (v/v) isoprene and checked for growth every day. During the second sampling, leaflets from the same oil palm tree and soil surrounding the base of the tree (at a depth of 0–5 cm) were taken for DNA-SIP experiments. DNA-SIP incubations were set up in triplicate using either 4 g soil in 40 mL of sterile water (as detailed in Larke-Mejía et al. 2019 [25]) or leaf washings with 40 mL of minimal medium (as described in [27]) in 2 liter sealed glass bottles supplied with 25–50 ppmv of either 12C- or 13C-labelled isoprene, as reported in [19], in order to promote enrichment of diverse isoprene degraders as seen in [25]. Isoprene consumption (Figure S2) in both sets of DNA-SIP experiments was closely monitored using gas chromatography as described previously [27]. 13C incorporation into biomass over time was then estimated (Table S1). Isoprene was replenished frequently to restore the concentration of isoprene to between 25 and 50 ppmv throughout the DNA-SIP incubations [25]. Aliquots (approx. 10 mL) of the DNA-SIP incubations were harvested before enrichment (T0) and after incorporation of approximately 25, 50, and 75 µmol g−1 of 13C (Table S1). Aliquots were stored at −20 °C until DNA extraction with FastSpin DNA soil kit (MP Biomedicals, Santa Ana, CA, US) following the manufacturer’s instructions.
DNA (soil: 5 µg, leaves: up to 1 µg) was separated into 13C-labelled (“heavy”) DNA and 12C-unlabelled (“light”) DNA fractions by isopycnic ultracentrifugation as described previously [19]. DNA concentration and density of each fraction was determined with a QubitTM dsDNA HS Assay kit (Thermo Fisher Scientific, UK) and an AR200 digital refractometer (Reichert, USA), respectively. Denaturing gradient gel electrophoresis of 16S rRNA gene amplicons (not shown) and fractionation plots of DNA abundance against fraction density (Figure S3) for each time-point were used to determine which fractions contained “heavy” and “light” DNA to be used for further analysis. Comparing fractionation plots from different time-points in both experiments also confirmed the progressive incorporation of the 13C-label during the enrichment, observed as the migration of DNA to “heavy” and “light” regions of ultracentrifuge tubes in 13C incubations compared to only a “light” region for 12C incubations [27], and development of a larger “heavy” peak as the isoprene enrichment increased.
2.2. Identification of Active Isoprene-Degrading Bacteria from Oil Palm Soils and Leaves
The datasets generated for this study can be found in the GeneBank repository (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA272922). Bacterial community profiles from unenriched (T0) and 13C-enriched DNA at each time-point were analyzed by partial 16S rRNA gene sequencing (performed with 341f and 785r primers, [33]) utilizing an Illumina MiSeq platform and carried out at MR DNA (Molecular Research LP) (Shallowater, TX, USA). 16S rRNA gene sequences (SRA accession number SRR12533424) from these PCR amplicons were processed as described by Larke-Mejía et al. (SRA accession number SRR12533424) [25].
2.3. Enrichment and Isolation of Isoprene-Degrading Bacteria from Oil Palm Samples
At the end of DNA-SIP experiments, 10 mL samples were transferred to 120-mL glass vials containing 10 mL of fresh Ewers minimal medium [32] pH 6.5, supplemented with 1 ml per liter vitamins v10 solution (DSMZ). Vials were then sealed with butyl rubber stoppers and isoprene was injected to maintain a concentration of 25–50 ppmv. After several subcultures, isoprene-degrading bacteria were selected and purified as previously described [25]. For subsequent characterization, isoprene-degrading strains were grown in Ewers minimal medium containing 1% (v/v) isoprene. DNA from cultures was extracted using the FastSpin DNA soil kit (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions, and used as a template for PCR amplification of 16S rRNA genes [34] and isoA genes (using primers isoA14f and isoA1019r, [29]). The 16S rRNA gene was used to identify isoprene-degrading isolates of interest by comparing with 16S rRNA gene sequences from characterized isoprene-degrading bacteria, and also by comparing with 16S rRNA genes from the most abundant OTUs during the DNA-SIP experiments (targeted isolation). 16S rRNA gene sequence alignments and maximum likelihood trees of 16S rRNA genes were constructed as described previously [25].
2.4. Genome Sequencing and Analysis
The genomes of the new isoprene-degrading bacteria Sphingopyxis strain OPL5 (GenBank accession CP060725.1) and Gordonia strain OPL2 (NCBI Reference Sequence NZ_RKME00000000.1) were sequenced and assembled by MicrobesNG (University of Birmingham, Birmingham, UK) using Oxford Nanopore and Ilumina HiSeq 2500 platforms, respectively. After growth on isoprene and confirmation of purity by microscopy, Sphingopyxis strain OPL5 and Gordonia strain OPL2 were grown at 30 °C for 7 days on minimal media plates supplemented with approximately 1% (v/v) isoprene supplied to the headspace in a sealed jar. The biomass was collected from plates and deposited to bead tubes supplied by MicrobesNG (University of Birmingham, UK). Ilumina genome sequencing was conducted by MicrobesNG as follows: “Three beads were washed with extraction buffer containing lysozyme and RNase A and incubated for 25 min at 37 °C. Proteinase K (0.05 µg/mL) and RnaseA (0.1 µg/mL) were added and incubated for 5 min at 65 °C. Genomic DNA was purified using an equal volume of SPRI beads and resuspended in EB buffer. DNA was quantified in triplicate with the Quantit dsDNA HS assay in an Eppendorff AF2200 plate reader. Genomic DNA libraries were prepared using Nextera XT Library Prep Kit (Illumina, San Diego, CA, USA) following the manufacturer’s protocol with the following modifications: two nanograms of DNA instead of one were used as input, and PCR elongation time was increased to 1 min from 30 s. Pooled libraries were quantified using the Kapa Biosystems Library Quantification Kit for Illumina on a Roche light cycler 96 qPCR machine. Libraries were sequenced on the Illumina HiSeq instrument using a 250 bp paired end protocol. Reads were adapter trimmed using Trimmomatic version 0.30 (26) with a sliding window quality cutoff of Q15. De novo assembly was performed on samples using SPAdes version 3.7 [35].” For the enhanced genome sequencing, libraries using Oxford Nanopore were prepared as follows: “Broth cultures of each isolate were pelleted and the pellet was resuspended in the cryoperservative of a Microbank™ (Pro-Lab Diagnostics UK, United Kingdom) tube and stored in the tube. Approximately 2× 109 cells were used for high molecular weight DNA extraction using Nanobind CCB Big DNA Kit (Circulomics, Baltimore, MD, USA). DNA was quantified with the Qubit dsDNA HS assay in a Qubit 3.0 ((Invitrogen) Eppendorf UK Ltd., Stevenage, UK). Long read genomic DNA libraries were prepared with an Oxford Nanopore SQK-RBK004 kit and/or SQK-LSK109 kit with Native Barcoding EXP-NBD104/114 (ONT, Oxford, UK) using 400–500 ng of HMW DNA. Twelve to twenty-four barcoded samples were pooled together into a single sequencing library and loaded in a FLO-MIN106 (R.9.4 or R.9.4.1) flow cell in a GridION (ONT, UK). The contigs were annotated using Prokka 1.11. [36]”
2.5. Genome Analysis
Genome characteristics were assessed with the use of the Rapid Annotation using Subsystem Technology (RAST server) version 2.0 at https://rast.nmpdr.org/ [37] and the MicroScope Microbial Genome Annotation and Analysis Platform version 3.13.5 at https://mage.genoscope.cns.fr/microscope [38]. These two platforms were used to determine the general characteristics of the genomes of Sphingopyxis strain OPL5 and Gordonia strain OPL2 and to search for the genes of interest. Amino acid sequences that were likely candidates for enzymes involved in the isoprene degradation pathway were compared to a personally curated database of such proteins with the use of BLASTp https://blast.ncbi.nlm.nih.gov/Blast.cgi [39]. The Microbial Genome Atlas (MiGA) http://microbial-genomes.org [40] was used to determine taxonomic affiliation, novelty and gene diversity with the use of the NCBI prokaryotic genome database. Gene Graphics [41] was used to visualize the isoprene metabolic genes for Figure 3.
2.6. Isoprene Oxidation Assays
Sphingopyxis strain OPL5 was grown in a 4 L working volume fermenter (Electrolab, Tewkesbury, UK) with Ewers minimal medium and isoprene as the sole carbon and energy source. Optimal growth conditions for Sphingopyxis strain OPL5 were used (30 °C, 160 rpm, pH 6.5, 1 mL per liter vitamins v10 solution, and air flow 2.4 L/min). Isoprene was supplied by bubbling air (1 mL min−1) through a small volume of liquid isoprene contained in a 30-mL vial, held on ice (as described in [26]). Cells were harvested three times, at an optical density (540 nm) of 1.0, 2.5, and 3.1, respectively (Figure S7) by centrifuging at 7000× g for 20 min and resuspending in 50 mM HEPES, pH 6.5, before snap-freezing and storing at −80 °C. Frozen cells retained full isoprene oxidation activity over several months. Isoprene oxidation rates were calculated using a Clark-type oxygen electrode [42]. Frozen cells were thawed and resuspended to an OD540 of 2.0 in 3 mL of 0.5 M phosphate buffer (pH 6.5) and equilibrated to 30 °C for 2–3 min with stirring. Substrate-induced rates of oxygen depletion were calculated for concentrations of isoprene from 3 μM to 30 μM (Figure S8) as detailed in [26].
3. Results and Discussion
3.1. Testing Methods to Recover Leaf Epiphytes
In preparation for DNA-SIP incubations, an initial experiment was carried out to determine the better method to recover epiphytes from the leaves. Epiphytes were recovered either by washing cells from leaves or by removing with swabs using the methods described by Hedin et al. 2010 [31]. Cells were then incubated with 25 ppmv isoprene and the isoprene consumption rate with cells retrieved by oil palm leaf (OPL) washings and swabbing (OPL swabs) was monitored (Figure S1) and a lag in initiation of isoprene consumption was observed with both incubations. After isoprene consumption commenced (approx. 110 h) the rate of isoprene consumption with bacterial cells retrieved by leaf-washing was marginally faster than with leaf swabs, and so the leaf-washing method was used subsequently.
3.2. Active Isoprene-Degrading Bacteria Associated with an Oil Palm Tree
In order to determine the diversity of isoprene degraders associated with an oil palm tree, two separate DNA-SIP experiments were performed in triplicate using: (a) Cells washed from the surface of oil palm leaves (hereafter termed leaf-washings), to recover epiphytic cells present on leaves; and (b) soil from the vicinity of this tree. 13C-labelled isoprene (with 12C-isoprene controls) was added to 25 ppmv and the consumption of isoprene for each microcosm was monitored over approximately 6–9 days. Over the time-course of the DNA-SIP experiments, between approximately 25 and 86 µmol 13C was incorporated per gram of soil or per gram of washed leaf which is within the range of 13C incorporation required for a successful DNA-SIP experiment, as recommended by Neufeld et al. [43]. DNA-SIP incubations with soil began consuming isoprene after approximately 2 days of incubation, while incubations with leaf washings consumed isoprene after a lag of approximately 4 days. Once isoprene was depleted, it was added again to 25 ppmv over the course of the DNA-SIP experiments, and the repeated supply of isoprene after its depletion led to rapid degradation (Figure S2). The total amount of isoprene consumed by the incubations was used as a proxy for the amount of 13C-label incorporation into biomass and this allowed the selection of harvesting times for samples from DNA-SIP microcosms for DNA extraction at three time points corresponding to 25, 50, and 75 µmol of 13C-label incorporated per gram of starting material (142, 166, and 190 h for soils and approximately 220, 280, and 310 h for leaf washings, Figure S2 and Table S1). DNA-SIP enrichments were harvested at these times based on the assumption that approximately half of the carbon, added to enrichments as 13C-isoprene, was incorporated into biomass, and estimates of the amount of 13C-label incorporated are summarized in Table S1. This yielded eight samples (each in triplicate) for soil and leaf washing incubations, at time points T1, T2, and T3, with T0 being a sample retained at the start of DNA-SIP incubations.
DNA was extracted from samples and then fractionated to separate heavy 13C-DNA from light 12C-DNA. Comparison of 13C-DNA and 12C-DNA profiles (controls) across 13C-isoprene and 12C-isoprene SIP enrichments confirmed the incorporation of 13C-label into microbial biomass over the course of the incubations with both soil samples and leaf-washings (Figure S3). The fractions representing 12C-DNA (light) and 13C-DNA (heavy) samples obtained from soil and leaf washings, taken at T1, T2, and T3, are also indicated in Figure S3. The change in the ratio of heavy DNA to light DNA throughout the DNA-SIP experiments was different between soils and leaves because soils contained higher biomass at the beginning of the enrichment, shown as a larger 12C-unlabeled peak.
3.3. Bacterial Community Composition in SIP Enrichments
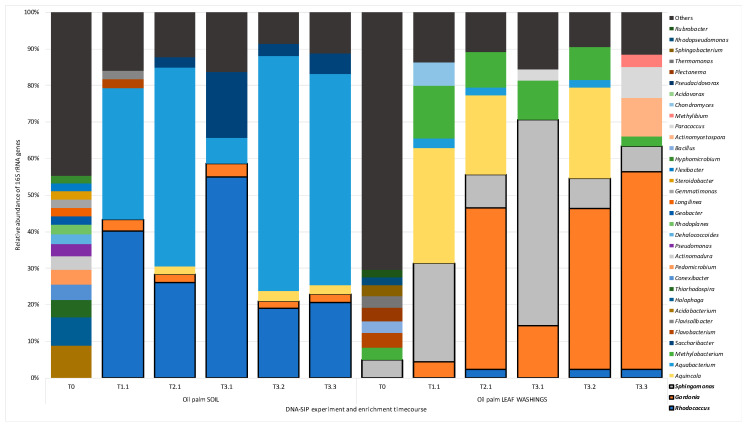
Changes in the bacterial communities in DNA-SIP enrichments were followed by comparing the labelled 16S rRNA gene profiles in samples that were incubated with 13C-isoprene at T1, T2, and T3, with the T0 community. Figure S4 and Figure 1 depict the relative abundance of 16S rRNA genes present in these samples, at the order and genus level respectively, and the changes in bacterial community composition over the time-course of the DNA-SIP experiments. For clarity, in Figure S4 and Figure 1, we followed the enrichment of replicate 1 throughout enrichment (T1 to T3, shown as T1.1, T2.1 and T3.1) and the T3 samples are depicted as T3.1, T3.2, and T3.3 to show the variation in relative abundance of 16S rRNA genes across replicates at the end of the enrichment experiments. 16S rRNA gene analysis of non-enriched soil (T0) and non-enriched leaves (T0) revealed the diversity of organisms from six main orders (Figure S4): the Rhizobiales (13.4% in soil and 13.9% in leaves), the Actinomycetales (9.3% and 13.3%, respectively), Bacillales (2.8% and 8.7%, respectively), Sphingomonadales (1.5% and 7.7%, respectively), Sphingobacteriales (2.2% and 7.6%, respectively), and Burkholderiales (2.9% and 4.6%, respectively). The order Acidobacteriales was also abundant only in soils (8.9%). After enrichment with 13C-labelled isoprene, 13C labelled heavy DNA samples from both soils and leaves were dominated by Actinomycetales (21–60% and 7.6–69.3%, respectively) and Burkholderiales (8.9–68.6% and 3.7–38.6%, respectively). Of significant interest was the substantial enrichment of 16S rRNA genes from the Sphingomonadales, at 7–57% relative abundance in 13C-DNA samples from DNA-SIP experiments with oil palm leaf-washings (Figure S4).
Figure 1.
Relative abundance of bacterial genera during DNA-stable isotope probing (DNA-SIP) enrichments. Figure 1 shows the relative abundance of bacterial 16S rRNA genes (at the genus level), obtained by PCR of enriched and un-enriched DNA extracted from oil palm soil samples and leaf washings. Results include un-enriched (un-fractionated) samples and heavy DNA fractions (Figure S3) for three time points T1, T2 for one replicate (shown as T1.1, T2.1) and T3 for all three replicates (shown as T3.1, T3.2, and T3.3; refer to Table S1 for the time course). Only 16S rRNA gene sequences with a relative abundance of greater than 2% or over are shown. 16S rRNA gene sequences with a relative abundance of less than 2% are grouped together as “others.” Sequences from the genera (Rhodococcus, Gordonia, and Sphingomonas), highlighted with a black border, were identified as putative isoprene-degrading bacteria according to 13C-labelling.
3.4. 13C—Labelling of Putative Isoprene Degraders in Leaf Washings
Analysis at the genus level (Figure 1) revealed the presence in leaf washing incubations of labelled members of the genus Gordonia in leaf washing incubations, (4.4% at T1 up to 54.2% at T3.2) some of which are known to grow on isoprene [21]. Sequences affiliated with Aquincola, a member of the Comamonadaceae family (along with the isoprene degraders Variovorax strain WS11 and Ramlibacter strain WS9 [25,26,27]) were also present in 13C-DNA. Approximately 22–31% relative abundance of Aquincola-like sequences in T1 and T2 samples and one T3 sample (T3.1) from leaf washing SIP incubations, but were less abundant in two of the T3 samples (T3.2 and T3.3) suggesting that this might have been an as-yet unidentified isoprene-degrader that was outcompeted by other isoprene degraders over time. An example of an important member of this genus, found to degrade important alkenes, is Aquincola tertiaricarbonis L108. This strain is an example of a member of this genus able to degrade aerobic fuel oxygenate, tert-alkyl ethers, and alcohols by expression of cytochrome P450, EthABCD monooxygenase, and possibly the tertiary alcohol monooxygenase MdpJ which produces and then degrades the hemiterpene 2-methyl-3-buten-2-ol [44,45].
Of significance was the presence of relatively high numbers of 16S rRNA gene sequences from Sphingomonas in oil palm leaf washings (6.9–56.3% relative abundance), especially dominating 13C-DNA samples from T3.1. Thus far there were no known isoprene-degrading Sphingomonas strains and so this became a target for isolation (see later). Also of note was the enrichment of members of the genus Methylobacterium in DNA-SIP experiments with oil palm leaf washings (Figure 1) with the relative abundance of 16S rRNA genes in 13C-DNA increasing from 3.4% at T0 to 9.6% at T2 and to approximately 2.7–10.6% relative abundance in T3 samples. This was interesting because Methylobacterium is well-known as an inhabitant of the phyllosphere since it grows on methanol produced from the degradation of pectin [46] and it has also been reported to be an isoprene-degrader [47]. Despite our efforts, we were not subsequently able to isolate an isoprene-degrading Methylobacterium but recently McGenity and colleagues ([48] and manuscript in preparation) have reported the isolation of isoprene-degrading Methylobacterium from poplar and willow leaves.
3.5. 13C—Labelling of Putative Isoprene Degraders in Soil
In 13C-DNA samples retrieved from DNA-SIP enrichments using soil from the vicinity of the oil palm tree, 16S rRNA genes from the genera Rhodococcus (19–55% relative abundance) and Aquabacterium (from the Comamonadaceae family, 7–64% relative abundance) dominated the 13C-isoprene enrichments across all time points (Figure 1). Rhodococcus strain AD45 is a well-characterized isoprene degrader that has become a model for the study of isoprene metabolism [24]. Rhodococcus has also been observed in high abundance in other DNA-SIP experiments with estuarine samples [21], willow soils [19,25], poplar leaves [27] and so it was perhaps not surprising to see members of this genus enriched in oil palm soil samples. Analysis of 13C-DNA samples from SIP enrichments with soil also revealed the enrichment of Saccharibacter from the Rhodospirillales order (present at a relative 16S rRNA gene abundance of around 3–18%) which was also interesting because there are also no reports of isoprene-degrading isolates from this genus. Labelled Gordonia 16S rRNA gene sequences were also noted from soil for all enriched time-points (present at around 2–4% relative abundance) which was not unexpected as isoprene-degrading Gordonia have previously been reported [21].
3.6. Targeted Isolation of Isoprene Degraders from Oil Palm
For the targeted isolation of isoprene degraders identified by the 16S rRNA gene profiling of bacterial communities in DNA extracted from SIP experiments (Figure 1), the DNA-SIP microcosms were continuously enriched with 25 ppmv of isoprene and samples were sub-cultured into Ewers minimal medium supplied with 25 ppmv isoprene. Subsequent serial dilution, plating of enrichments, and isoA PCR were used to confirm the presence of the isoA gene of Rhodococcus sp. strain OPL1, Gordonia strain OPL2, and Sphingopyxis strain OPL5 from oil palm leaf enrichments. From the strains isolated, the latter two were of most interest because members of these families were found to be enriched during DNA-SIP experiments with leaf washings from oil palm (Figure 1). Most importantly, there is only one other isolated isoprene-degrading representative from the Gordonia genus (Gordonia i37, [21]) and no Sphingopyxis have been reported previously to grow on isoprene. Unfortunately, Aquabacterium, Saccharibacter, and Methylobacterium from which 16S rRNA gene sequences of which were enriched in 13C-DNA from the SIP experiments described above, were not isolated. All three isolates that were obtained subsequently grew well (OD540 > 1.0) on Ewers minimal medium containing up to 1% (v/v) isoprene as their sole carbon and energy source. Since Rhodococcus species growing on isoprene have previously been described in detail [19,20,24], Rhodococcus strain OPL1, which has 100% 16S rRNA gene sequence identity to Rhodococcus strain MAK1 from a coal tar-contaminated aquifer located in South Glens Falls, New York [49], was not characterized further.
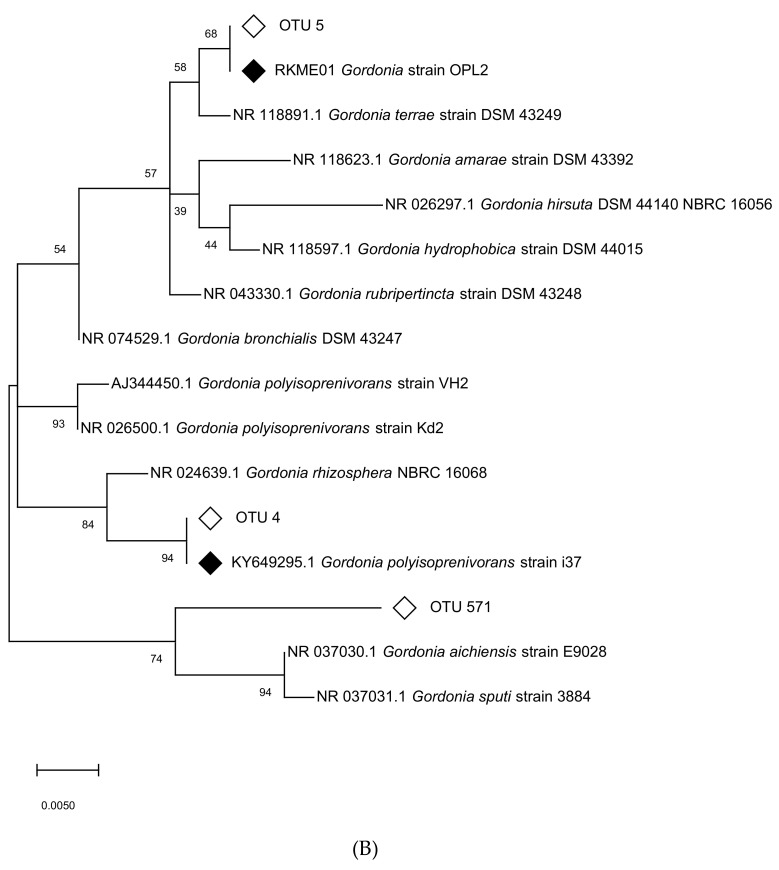
The pink-pigmented oil palm leaf isolate Gordonia strain OPL2 (growth rate 0.12 h−1) was the second isoprene degrader isolated from this genus and the first isoprene degrader from the Gordonia genus isolated from the phyllosphere of an isoprene-producing tree. It had 97.53% sequence identity with the 16S rRNA gene of Gordonia polyisoprenivorans strain i37, an isoprene degrader isolated from an estuary [21]. The closest relative of strain OPL2 in terms of 16S rRNA gene was Gordonia strain 647 W.R.1a.05, at 99.92% sequence identity, isolated from the venom duct of the cone snail, Conus circumcises [50] and Gordonia terrae strain DSM 43249, with 99.55% sequence identity (Figure S5).
Sphingopyxis strain OPL5, a member of the Sphingomonadaceae in the Alphaproteobacteria, was isolated from isoprene enrichments arising from the oil palm leaf-washing DNA-SIP experiments. This yellow-pigmented isolate had high 16S rRNA gene sequence identity with the characterized Sphingopyxis panaciterrae strain Gsoil124 (99.48% 16S rRNA gene sequence), which was isolated from a soil sample taken from a ginseng field in Pocheon Province, South Korea [51] (Figure S6). In a survey of isoA gene diversity, Carrión et al. (2018) [29] reported the presence of Sphingopyxis-like isoA from the same oil palm leaves described here (60% relative abundance of Sphingopyxis-like isoA sequences), and from freshwater sediment (>40%) and poplar leaves. Gordonia-like isoA sequences were found in poplar leaf samples, in oil palm leaves from this study (40% of sequences affiliated with Gordonia) and Malaysian oil palm leaves (over 98%) [29]. In a similar DNA-SIP experiment, enriching leaves and soil from a Malaysian oil palm tree, Carrión et al. showed the presence of Gordonia and members of the Sphingomonadaceae family enriched in the soil and phyllosphere communities [30].
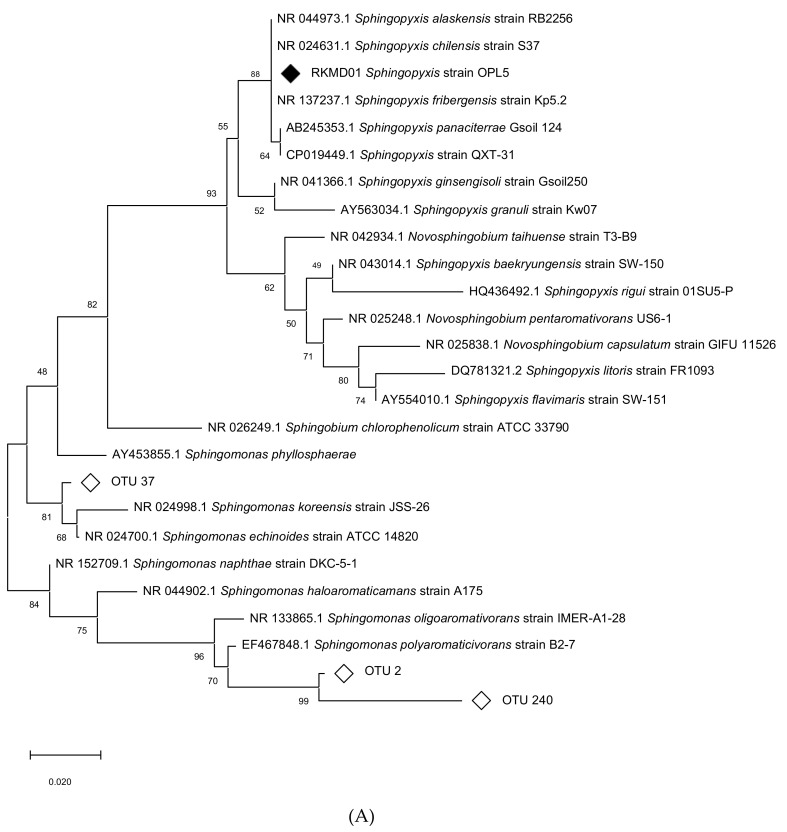
In order to assess the relative abundance and importance of these isolates during the enrichments, two phylogenetic trees were constructed, placing the abundant OTUs obtained from oil palm leaf SIP in context with the isolates obtained here together with representatives from the Sphingomonadaceae family (Figure 2A) and representatives of the Gordonia genus (Figure 2B). The most abundant members of the Sphingomonadaceae were Sphingomonas-like OTU 240 and OTU 2 which cluster closely with Sphingomonas polyaromaticivorans B2-7 and Sphingomonas oligoaromativorans strain IMER-A1-28, and OTU 37 (18.7%, 7.5%, and 1.2% relative abundance at T1, respectively). The most abundant Gordonia-like sequences were, OTU 4 (2.5% at T1, 36.4% at T2, and 39% at T3), identical to estuarine isolate Gordonia polyisoprenivorans strain i37 [21], OTU 5 (0.6% at T1, 2.9% at T2, and 1.8% at T3), identical to our isolate Gordonia OPL2, and OTU 571 (up to 3.5% abundant at T2), distinct from known isoprene-degrading isolates (Figure 2B). According to the DNA-SIP amplicon sequencing data, Gordonia strain OPL2 was the second most abundant isoprene-degrader from the Gordonia genus in the enrichments. Although Sphingopyxis strain OPL5 was not among the abundant isoprene degraders during DNA-SIP enrichments with oil palm leaf washings (Figure 1), targeted isolation, which was informed by these SIP experiments, enabled the isolation of Sphingopyxis strain OPL5 as a representative isoprene-degrader of the Sphingomonadaceae, as well as Gordonia strain OPL2.
Figure 2.
Partial 16S rRNA gene phylogenetic trees showing the oil palm leaf isolates, the most abundant closely related OTUs during DNA-SIP enrichments and related representatives from the databases: Sphingopyxis strain OPL5 (A) and Gordonia strain OPL2 (B).Trees were constructed using the Maximum Likelihood method using the partial 16S rRNA gene (V3 to V4 variable regions) amplified using 341f and 785r primers [1]. Following removal of gaps and missing data, there were 418 bp (A) and 409 bp (B) in the alignment. Bootstrap values (1000 replications) are shown. Strains isolated in this study are indicated with black diamonds and the most abundant closely related OTUs with empty diamonds. Relative abundance (%) of the most abundant OTUs before (T0) and throughout the DNA-SIP experiments (T1, T2, and T3) for soils and leaf washings were as follows: A) Sphingomonadaceae-like OTU 240 (in soils < 0.6% and on leaves 1.4% at T0, 18.7% at T1), OTU 2 (in soils <0.4% and leaves 7.5% at T1) and OTU 37 (in soils <0.1% and leaves 1.2% at T1). B) Gordonia-like: OTU 4 (in soils 1.5% at T1 and up to 39.0% at T3), OTU 571 (soils <0.2% and leaves up to 3.8% at T2) and OTU 5 (soils up to 1.1% at T1 and leaves 2.9% at T2). The scale bar shows nucleotide substitutions per site.
3.7. Genome Sequencing and Characterization of Isoprene Gene Clusters
The genomes of strains OPL2 and OPL5 were sequenced to compare the newly isolated Gordonia strain OPL2 with Gordonia strain i37 [21] and to provide deeper insights into the mechanisms of isoprene metabolism in Sphingopyxis strain OPL5, the first strain of this genus found to grow on isoprene. The Gordonia strain OPL2 genome was 5.8 Mbp and had a GC content of 67.3% GC which is very similar to that of Gordonia i37 at 6.2 Mbp and a GC content of 66.8% (Table 1).
Table 1.
General characteristics of the genomes of Gordonia strain OPL2 and Sphingopyxis strain OPL5.
| Gordonia OPL2 | Sphingopyxis OPL5 | |
|---|---|---|
| NCBI Tax ID | 2486274 | 2486273 |
| Length (bp) | 5,759,526 | 4,676,975 |
| GC (%) | 67.3 | 65.89 |
| Contigs | 132 | 1 |
| N50 | 80,039 | 4,676,975 |
| CDS (total) | 5313 | 4403 |
| Genes (coding) | 5200 | 4392 |
| Genes (RNA) | 55 | 51 |
| rRNAs (5S, 16S, 23S) | 3, 3, 1 | 1, 1, 1 |
| tRNAs | 45 | 55 |
| Pseudogenes (total) | 113 | 69 |
| Coding Density (%) | 91.2 | 91.9 |
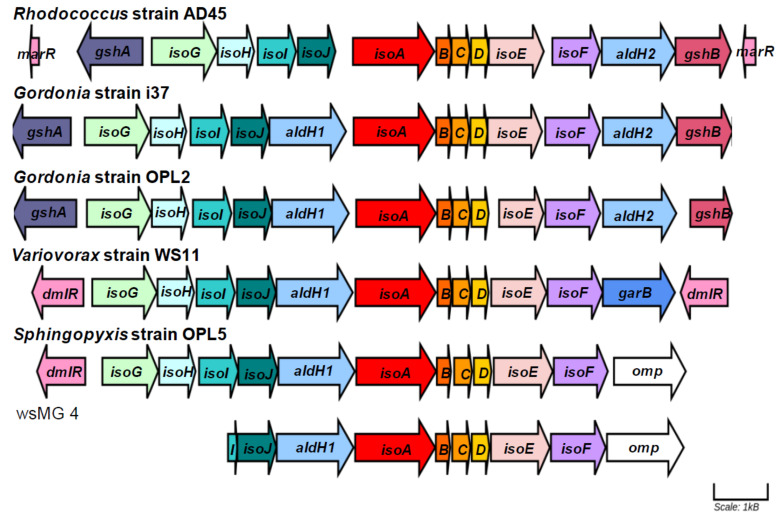
The complete genome of Sphingopyxis strain OPL5 comprised of one contig of 4.67 Mbp and had a GC content of 65.89% (Table 1). The genome size and GC content are comparable with those of other Sphingopyxis species, the genomes of which vary considerably within the broad range of 3.0–6.0 Mbp [52]. Specifically, the genome of the closest sequenced relative of strain OPL5, Sphingopyxis QXT-31 (see Figure 2), was 4.3 Mbp and had a GC content of 66.5%. Focusing on genes that are known to be involved in isoprene metabolism [24], the organization of iso genes from Gordonia sp. strain OPL2 and Sphingopyxis strain OPL5 were compared with those of the most well-characterized iso gene cluster of Rhodococcus strain AD45 [24], the estuarine isoprene-degrader Gordonia i37 [21] and the most well-characterized Gram-negative isoprene degrader Variovorax strain WS11 [25,26] (Figure 3). Figure 3 and Table S2 also include the comparison of the iso gene cluster of strain OPL5 with a partial iso cluster from a Sphingopyxis-like MAG (metagenome assembled genome, wsMG4), recovered from a DNA-SIP experiment performed with willow soil [25]. The recovery of this MAG suggests the presence of other isoprene-degrading bacteria from the Sphingomonadales order in the willow soil environment.
Figure 3.
Isoprene metabolic gene clusters. Isoprene metabolic gene clusters in isoprene-degrading isolates (including Sphingopyxis strain OPL5 and Gordonia strain OPL2 isolated in this study), and a representative contig with the iso gene cluster obtained from metagenome co-assembly of 13C-DNA from isoprene-enriched willow soil (wsMG4). The isoA gene that codes for the alpha-subunit of the monooxygenase is shown in red. The % identity of iso gene-encoded polypeptides to the corresponding Iso polypeptides of the well-characterized Rhodococcus strain AD45 are shown in Table S2. omp: outer membrane protein transfer.
The cluster shows similarities and differences in the organization of the 10 main iso genes in isoprene degraders. The first difference observed is that all iso genes of the Gram-positive model isoprene degrader Rhodococcus strain AD45 are clustered together (isoGHIJABCDEF), while other isoprene clusters have a aldH1 gene, coding for an aldehyde dehydrogenase, between isoJ and isoA (Figure 3); Rhodococcus strain AD45 has the gene aldH1 upstream isoG. When comparing the protein sequence similarity, all iso gene products from Gram-positive isoprene degraders have closer percentage similarity between each other, and the same is observed when iso gene products from Gram-negative bacteria are compared. For example, IsoA is 86–87% similar between both Gordonia isolates and Rhodococcus strain AD45, while the number decreases to 72–73% when comparing the same IsoA sequence from Rhodococcus AD45 with IsoA from the Gram-negative isoprene-degraders Variovorax strain WS11 and Sphingopyxis strain OPL5.
3.8. Sphingopyxis Strain OPL5 Growth and Affinity for Isoprene
Sphingopyxis strain OPL5 grew best at 30 °C, with shaking at 160 rpm, at a pH between 6.0 and 7.0, and while supplementing Ewers medium with 1 mL per liter v10 vitamins solution. Sphingopyxis strain OPL5 grown with the previous conditions and supplemented with 1% v/v isoprene had a specific growth rate of 0.14 h−1 and doubling time of approximately 5.0 h. Interestingly, the growth rate was very similar between isoprene concentrations of 1–10% (v/v), with no inhibition at higher concentrations of isoprene. A morphological change to elongated rods at the highest isoprene concentration was also noted. These optimized conditions were then used to grow Sphingopyxis strain OPL5 in a fed-batch bioreactor with Ewers minimal medium and isoprene as the sole carbon and energy source, resulting in the growth rate varying with a doubling time of around 3.6 to 8.4 hrs (Figure S7).
In order to estimate substrate-induced oxidation rates, using concentrations of isoprene between 3 μM and 30 μM, Sphingopyxis strain OPL5 cells were used in oxygen-electrode experiments. Km and Vmax values were calculated using Lineweaver-Burk, Eadie-Hoffstee, and Hanes plots, yielding values of 2.6 μM and 10.6 nmol/min/mg; 2.2 μM and 10.1 nmol/min/mg; 2.8 μM and 10.7 nmol/min/mg dry weight of cells, respectively (Figure S8). The rates of oxidation of isoprene by Sphingopyxis strain OPL5 were approximately three times lower than Variovorax strain WS11 (31.2 nmol/min/mg [26]) and seven times lower than Rhodococcus strain AD45 (reported oxidation rates of 76 nmol min−1 mg−1 [53]).
4. Conclusions
DNA-SIP enrichment experiments with soil and leaves from an oil palm tree in the UK provided insights into the diversity and abundance of active isoprene-degrading bacteria that are present on and around this high isoprene-emitting species of tree. Informed by 16S rRNA gene amplicon sequencing, we carried out targeted isolation of new isoprene degraders in DNA-SIP enrichments. The isolation of three isoprene-degrading isolates from the phyllosphere of this oil palm tree, from taxonomic groups identified as active by DNA-SIP experiments, highlights the importance of using cultivation-independent methods to inform cultivation-dependent strategies. The genomes of Gordonia strain OPL2 and Sphingopyxis strain OPL5 have increased the known diversity of isoprene degraders and their iso genes from different environments. Isolation and characterization of Sphingopyxis strain OPL5 has also been important in enabling the identification of Sphingopyxis-like sequences recovered from a wide range of environments in previous cultivation-independent studies and provides further insights into the broader diversity of isoprene-degrading Proteobacteria from the Sphingomonadales family.
Acknowledgments
We thank David Cooke and Silke Roch from the Palm House at Kew Gardens, London, for their help in accessing and collecting soil and leaf samples from oil palm trees.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/10/1557/s1. Figure S1: Isoprene consumption in microcosms from oil palm leaf (OPL) washings and swabs. Figure S2: Isoprene consumption in microcosms used in DNA-SIP experiments with oil palm soil (A) and leaf washings (B). Figure S3: Separation 12C and 13C DNA of oil palm soil (A) and oil palm leaf washings (B) throughout the DNA-SIP experiments. Figure S4: Relative abundance of bacterial orders during DNA-SIP enrichments. Figure S5: 16S rRNA gene phylogenetic tree of representative members of the Gordonia genus and the oil palm leaf isolate Gordonia strain OPL2. Figure S6: 16S rRNA gene phylogenetic tree of representative members of the Sphingomonadaceae family and the oil palm leaf isolate Sphingopyxis sp. OPL5. Figure S7: Growth of Sphingopyxis sp. OPL5 in a 4-liter fermenter with isoprene. Figure S8: Rate of oxygen uptake (V0) as a function of isoprene concentration [S0]. Table S1: Incorporation of 13C-labelled carbon during DNA-SIP experiments with oil palm soil and leaf washings. Table S2: Identity of Iso polypeptides encoded by iso gene clusters when compared to the corresponding Iso polypeptides from Rhodococcus sp. strain AD45.
Author Contributions
N.L.L.-M., A.T.C., T.J.M., and J.C.M. planned the experiments. N.L.L.-M. (with O.C. in February 2017) carried out the DNA-SIP enrichments. N.L.L.-M. carried out all other experiments. N.L.L.-M., A.T.C., and J.C.M. analyzed the results. N.L.L.-M., J.C.M., and A.T.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work on this project was funded through the European Research Council (ERC) Advanced Grant (IsoMet 694578) to J.C.M., Natural Environment Research Council (NERC) grants to J.C.M. (NE/J009725/1), and T.J.M. (NE/J009555/1) and Earth and Life Systems Alliance (ELSA) at the University of East Anglia. Colombian Government Scholarship No. 646, Colciencias/Newton Fund (2014 was awarded to N.L.M. We acknowledge the award of a Leverhulme Early Career Fellowship (ECF-2016-626) to A.T.C.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Arneth A., Monson R.K., Schurgers G., Niinemets Ü., Palmer P.I. Why are estimates of global terrestrial isoprene emissions so similar (and why is this not so for monoterpenes)? Atmos. Chem. Phys. 2008;8:4605–4620. doi: 10.5194/acp-8-4605-2008. [DOI] [Google Scholar]
- 2.Guenther A.B., Jiang X., Heald C.L., Sakulyanontvittaya T., Duhl T., Emmons L.K., Wang X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012;5:1471–1492. doi: 10.5194/gmd-5-1471-2012. [DOI] [Google Scholar]
- 3.Guenther A. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995;100:8873–8892. doi: 10.1029/94JD02950. [DOI] [Google Scholar]
- 4.Atkinson R., Arey J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003;103:4605–4638. doi: 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- 5.Carlton A.G., Wiedinmyer C., Kroll J.H. A review of secondary organic aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009;9:4987–5005. doi: 10.5194/acp-9-4987-2009. [DOI] [Google Scholar]
- 6.Guenther A., Karl T., Harley P., Wiedinmyer C., Palmer P.I., Geron C. Estimates of global terrestrial isoprene emissions using MEGAN. Atmos. Chem. Phys. Discuss. 2006;6:107–173. doi: 10.5194/acpd-6-107-2006. [DOI] [Google Scholar]
- 7.Loreto F., Sharkey T.D. On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta. 1993;189:420–424. doi: 10.1007/BF00194440. [DOI] [PubMed] [Google Scholar]
- 8.Lantz A.T., Allman J., Weraduwage S.M., Sharkey T.D. Isoprene: New insights into the control of emission and mediation of stress tolerance by gene expression. Plant Cell Environ. 2019;42:2808–2826. doi: 10.1111/pce.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharkey T.D., Monson R.K. Isoprene research – 60 years later, the biology is still enigmatic. Plant Cell Environ. 2017;40:1671–1678. doi: 10.1111/pce.12930. [DOI] [PubMed] [Google Scholar]
- 10.Klinger L.F., Greenburg J., Guenther A., Tyndall G., Zimmerman P., M’Bangui M., Moutsamboté J.-M., Kenfack D. Patterns in volatile organic compound emissions along a savanna-rainforest gradient in central Africa. J. Geophys. Res. Atmos. 1998;103:1443–1454. doi: 10.1029/97JD02928. [DOI] [Google Scholar]
- 11.Hewitt C.N.N., Street R.A. A qualitative assessment of the emission of non-methane hydrocarbon compounds from the biosphere to the atmosphere in the U.K.: Present knowledge and uncertainties. Atmos. Environ. Part A Gen. Top. 1992;26:3069–3077. doi: 10.1016/0960-1686(92)90463-U. [DOI] [Google Scholar]
- 12.Cheng Y., Yu L., Xu Y., Lu H., Cracknell A.P., Kanniah K., Gong P. Mapping oil palm plantation expansion in Malaysia over the past decade (2007–2016) using ALOS-1/2 PALSAR-1/2 data. Int. J. Remote Sens. 2019;40:7389–7408. doi: 10.1080/01431161.2019.1580824. [DOI] [Google Scholar]
- 13.Hewitt C.N., MacKenzie A.R., Di Carlo P., Di Marco C.F., Dorsey J.R., Evans M., Fowler D., Gallagher M.W., Hopkins J.R., Jones C.E., et al. Nitrogen management is essential to prevent tropical oil palm plantations from causing ground-level ozone pollution. Proc. Natl. Acad. Sci. USA. 2009;106:18447–18451. doi: 10.1073/pnas.0907541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Ginkel C.G., De Jong E., Tilanus J.W.R., De Bont J.A.M. Microbial oxidation of isoprene, a biogenic foliage volatile and of 1,3-butadiene, an anthropogenic gas. FEMS Microbiol. Lett. 1987;45:275–279. doi: 10.1016/0378-1097(87)90004-8. [DOI] [Google Scholar]
- 15.Fall R., Copley S.D. Bacterial sources and sinks of isoprene, a reactive atmospheric hydrocarbon. Environ. Microbiol. 2000;2:123–130. doi: 10.1046/j.1462-2920.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 16.Murrell J.C., McGenity T.J., Crombie A.T. Microbial metabolism of isoprene: A much-neglected climate-active gas. Microbiology. 2020:1–25. doi: 10.1099/mic.0.000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrión O., McGenity T.J., Murrell J.C. Molecular ecology of isoprene-degrading bacteria. Microorganisms. 2020;8:967. doi: 10.3390/microorganisms8070967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGenity T.J., Crombie A.T., Murrell J.C. Microbial cycling of isoprene, the most abundantly produced biological volatile organic compound on Earth. ISME J. 2018;12:931–941. doi: 10.1038/s41396-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Khawand M., Crombie A.T., Johnston A., Vavlline D.V., McAuliffe J.C., Latone J.A., Primak Y.A., Lee S.K., Whited G.M., McGenity T.J., et al. Isolation of isoprene degrading bacteria from soils, development of isoA gene probes and identification of the active isoprene-degrading soil community using DNA-stable isotope probing. Environ. Microbiol. 2016;18:2743–2753. doi: 10.1111/1462-2920.13345. [DOI] [PubMed] [Google Scholar]
- 20.Crombie A.T., Emery H., McGenity T.J., Murrell J.C. Draft genome sequences of three terrestrial isoprene-degrading Rhodococcus strains. Genome Announc. 2017;5:e01256-17. doi: 10.1128/genomeA.01256-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston A., Crombie A.T., El Khawand M., Sims L., Whited G.M., McGenity T.J., Murrell J.C. Identification and characterisation of isoprene-degrading bacteria in an estuarine environment. Environ. Microbiol. 2017;19:3526–3537. doi: 10.1111/1462-2920.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson L., Larke-Mejía N.L., Murrell J.C. Complete genome of isoprene degrading Nocardioides sp. WS12. Microorganisms. 2020;8:889. doi: 10.3390/microorganisms8060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hylckama Vlieg J.E.T.T., Leemhuis H., Lutje Spelberg J.H., Janssen D.B., Jeffrey H., Spelberg L., Janssen D.B., Ad S., Vlieg J.E.T.V.H., Leemhuis H., et al. Characterization of the gene cluster involved in isoprene metabolism in Rhodococcus sp. strain AD45. J. Bacteriol. 2000;182:1956–1963. doi: 10.1128/JB.182.7.1956-1963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crombie A.T., Khawand M.E., Rhodius V.A., Fengler K.A., Miller M.C., Whited G.M., McGenity T.J., Murrell J.C. Regulation of plasmid-encoded isoprene metabolism in Rhodococcus, a representative of an important link in the global isoprene cycle. Environ. Microbiol. 2015;17:3314–3329. doi: 10.1111/1462-2920.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larke-Mejía N.L., Crombie A.T., Pratscher J., McGenity T.J., Murrell J.C. Novel isoprene-degrading Proteobacteria from soil and leaves identified by cultivation and metagenomics analysis of stable isotope probing experiments. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson R.A., Larke-Mejía N.L., Crombie A.T., Ul Haque M.F., Murrell J.C. Isoprene oxidation by the Gram-negative model bacterium Variovorax sp. WS11. Microorganisms. 2020;8:349. doi: 10.3390/microorganisms8030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crombie A.T., Larke-Mejia N.L., Emery H., Dawson R., Pratscher J., Murphy G.P., McGenity T.J., Murrell J.C. Poplar phyllosphere harbors disparate isoprene-degrading bacteria. Proc. Natl. Acad. Sci. USA. 2018;115:13081–13086. doi: 10.1073/pnas.1812668115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman N.V., Bui N.B., Holmes A.J. Soluble di-iron monooxygenase gene diversity in soils, sediments and ethene enrichments. Environ. Microbiol. 2006;8:1228–1239. doi: 10.1111/j.1462-2920.2006.01015.x. [DOI] [PubMed] [Google Scholar]
- 29.Carrión O., Larke-Mejía N.L., Gibson L., Farhan Ul Haque M., Ramiro-García J., McGenity T.J., Murrell J.C. Gene probing reveals the widespread distribution, diversity and abundance of isoprene-degrading bacteria in the environment. Microbiome. 2018;6:219. doi: 10.1186/s40168-018-0607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrión O., Gibson L., Elias D.M.O., McNamara N.P., van Alen T.A., Op den Camp H.J.M., Supramaniam C.V., McGenity T.J., Murrell J.C. Diversity of isoprene-degrading bacteria in phyllosphere and soil communities from a high isoprene-emitting environment: a Malaysian oil palm plantation. Microbiome. 2020;8:81. doi: 10.1186/s40168-020-00860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedin G., Rynbäck J., Loré B. New technique to take samples from environmental surfaces using flocked nylon swabs. J. Hosp. Infect. 2010;75:314–317. doi: 10.1016/j.jhin.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Ewers J., Freier-Schröder D., Knackmuss H.J. Selection of trichloroethene (TCE) degrading bacteria that resist inactivation by TCE. Arch. Microbiol. 1990;154:410–413. doi: 10.1007/BF00276540. [DOI] [PubMed] [Google Scholar]
- 33.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:1–11. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc.; New York, NY, USA: 1991. [Google Scholar]
- 35.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 37.Aziz R.K., Bartels D., Best A., DeJongh M., Disz T., Edwards R.A., Formsma K., Gerdes S., Glass E.M., Kubal M., et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008;9:1–15. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallenet D., Engelen S., Mornico D., Cruveiller S., Fleury L., Lajus A., Rouy Z., Roche D., Salvignol G., Scarpelli C., et al. MicroScope: a platform for microbial genome annotation and comparative genomics. Database (Oxford). 2009;2009:bap021. doi: 10.1093/database/bap021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altschul S. Basic Local Alignment Search Tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-R L.M., Gunturu S., Harvey W.T., Rosselló-Mora R., Tiedje J.M., Cole J.R., Konstantinidis K.T. The Microbial Genomes Atlas (MiGA) webserver: Taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res. 2018;46:W282–W288. doi: 10.1093/nar/gky467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durinck S., Bullard J., Spellman P.T., Dudoit S. GenomeGraphs: Integrated genomic data visualization with R. BMC Bioinform. 2009;10:2. doi: 10.1186/1471-2105-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark L.C., Wolf R., Granger D., Taylor Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- 43.Neufeld J.D., Vohra J., Dumont M.G., Lueders T., Manefield M., Friedrich M.W., Murrell C.J. DNA stable-isotope probing. Nat. Protoc. 2007;2:860–866. doi: 10.1038/nprot.2007.109. [DOI] [PubMed] [Google Scholar]
- 44.Schäfer F., Muzica L., Schuster J., Treuter N., Rosell M., Harms H., Müller R.H., Rohwerder T. Alkene formation from tertiary alkyl ether and alcohol degradation by Aquincola tertiaricarbonis L108 and Methylibium spp. Am. Soc. Microbiol. 2011;77:5981–5987. doi: 10.1128/AEM.00093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster J., Schäfer F., Hübler N., Brandt A., Rosell M., Härtig C., Harms H., Müller R.H., Rohwerder T. Bacterial degradation of tert-amyl alcohol proceeds via hemiterpene 2-methyl-3-buten-2-ol by employing the tertiary alcohol desaturase function of the Rieske nonheme mononuclear iron oxygenase MdpJ. J. Bacteriol. 2012;194:972–981. doi: 10.1128/JB.06384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sy A., Timmers A.C.J., Knief C., Vorholt J.A. Methylotrophic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Appl. Environ. Microbiol. 2005;71:7245–7252. doi: 10.1128/AEM.71.11.7245-7252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srivastva N., Vishwakarma P., Bhardwaj Y., Singh A., Manjunath K., Dubey S.K. Kinetic and molecular analyses reveal isoprene degradation potential of Methylobacterium sp. Bioresour. Technol. 2017;242:87–91. doi: 10.1016/j.biortech.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Murphy G.P. Ph.D Thesis. University of Essex; Colchester, UK: 2016. Isoprene degradation in the terrestrial environment. [Google Scholar]
- 49.Posman K.M., DeRito C.M., Madsen E.L. Benzene degradation by a Variovorax species within a coal tar-contaminated groundwater microbial community. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.02658-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Z., Marett L., Hughen R.W., Flores M., Forteza I., Ammon M.A., Concepcion G.P., Espino S., Olivera B.M., Rosenberg G., et al. Neuroactive diol and acyloin metabolites from cone snail-associated bacteria. Bioorganic Med. Chem. Lett. 2013;23:4867–4869. doi: 10.1016/j.bmcl.2013.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H.W., Ten I.L., Jung H.M., Liu Q.M., Im W.T., Lee S.T. Sphingopyxis panaciterrae sp. nov., isolated from soil of ginseng field. J. Microbiol. Biotechnol. 2008;18:1011–1015. [PubMed] [Google Scholar]
- 52.Verma H., Dhingra G.G., Sharma M., Gupta V., Negi R.K., Singh Y., Lal R. Comparative genomics of Sphingopyxis spp. unravelled functional attributes. Genomics. 2020;112:1956–1969. doi: 10.1016/j.ygeno.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Van Hylckama Vlieg J.E., Kingma J., van den Wijngaard A.J., Janssen D.B. A glutathione S-transferase with activity towards cis-1, 2-dichloroepoxyethane is involved in isoprene utilization by Rhodococcus sp. strain AD45. Appl. Environ. Microbiol. 1998;64:2800–2805. doi: 10.1128/AEM.64.8.2800-2805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.