Abstract
Mediterranean plant Helichrysum italicum represents a rich source of versatile bioactive compounds with potential benefits for human health. Despite extensive research on the plant’s active constituents, little attention has yet been paid to characterizing the relationship between its intra-specific genetic diversity and metabolite profile. The study aimed to determine metabolic profile of H. italicum ssp. italicum (HII) and ssp. tyrrhenicum (HIT) cultivated on the experimental plantation in Slovenia and to compare the chemical composition of extracts regarding the solvent extraction process. Extracts were prepared upon conventional extract preparation procedures: maceration with 50% methanol or ethanol and cold or hot water infusion and analyzed using High Performance Liquid Chromatography-Diode Array Detection-Electrospray Ionization-Quadrupole Time-of-Flight-Mass Spectrometry (HPLC-DAD-ESI-QTOF-MS). One hundred compounds were identified in the samples, among them several isomers and derivatives were reported for the first time, while caffeoylquinic acids and pyrones were the most abundant. Semi-quantitative comparison revealed that the extraction procedure had a greater impact on the chemical profile than genetic variability. All HIT extracts showed a higher total phenolic content compared to HII, while the antioxidant potential evaluated by 1,1-diphenyl-2-picrylhydrazil test was not proportionally higher. In addition, hot water extracts proved to be comparably active as alcoholic ones, confirming high commercial potential of Helichrysum italicum as herbal functional beverages.
Keywords: H. italicum, plant extracts, phenolic compounds, antioxidant activity, HPLC-DAD-ESI-QTOF-MS
1. Introduction
The plant Helichrysum italicum (Roth) G. Don is a perennial subshrub characteristic to the Mediterranean region. The genus Helichrysum Mill. (Asteraceae) is very complex, as it comprises cca. 500–600 species, which are geographically distributed also beyond the Mediterranean basin and thus diverse with respect to both phenotype and metabolite profile. Helichrysum italicum species itself differ in terms of morphological features, genetic variation and geographical distribution and are further divided into four subspecies (ssp.): italicum, microphyllum, siculum and tyrrhenicum. However, their correct taxonomic assignment and clear differentiation are sometimes difficult due to their phenotypic plasticity and existence of intermediates generated with spontaneous hybridization in areas where the subspecies overlap [1,2]. The plant naturally thrives in dry, sandy areas but is also extensively cultivated in several Mediterranean countries due to the high demand for its essential oil by the perfume and cosmetic industry [3]. Namely, its yellow fade-resistant inflorescences are a treasury of bioactive secondary metabolites that result from the plants adaptation to this challenging environment. Apart from volatile terpenes present in essential oils, H. italicum is also very rich in phenolic compounds, which are recognized as potential health promoting agents due to antioxidant properties they exert and their probable role in the prevention of various diseases associated with oxidative stress, such as cancer, cardiovascular and neurodegenerative diseases [4]. The health-beneficial potential of H. italicum has been reported in ethnopharmacological surveys and supported by numerous in vitro and in vivo experiments [5]. Despite the extensive research, little attention has been devoted to characterizing the relationship between its intra-specific genetic diversity and either growing environment or metabolite profile [6].
In addition to genetic and phenotypic differences, the type and concentration of herbal components and consequently their therapeutic effect is highly dependent on the extraction method used [5]. Biologically active compounds isolated from H. italicum ssp. italicum and microphyllum with regard to isolation procedure have already been summarized by Maksimović et al. [7]. Briefly, phenolic compounds previously reported in H. italicum comprise following chemical classes of structurally diverse substances: phenolic acids (hydroxybenzoic and hydroxycinnamic acids), hydroxycinnamic esters, coumarins, flavonoids (flavones, flavonols, flavanones, flavanols), flavonoid ethers, esters and glycosides, acetophenones as well as associates of those classes [7]. Since structures of the identified compounds have already been elucidated by spectroscopic methods, the chemical composition of extracts can be routinely investigated using chromatographic methods. Due to the complexity of the plant samples, a high mass resolution and accuracy, sensitivity as well as sophisticated data analysis software are needed to successfully perform phytochemical screening. A configuration, which is appropriate for phenolic compounds identification and fulfils above-mentioned requirements, is liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS). While there are numerous studies investigating essential oil composition of H. italicum (reviewed by Maksimovic et al. [7]), comprehensive chromatographic studies performed on crude solvent extracts are rather scarce. Even less investigated are water-based preparations, which are typically used in traditional medicine. An exception is the research by Pereira et al. [8] who investigated the chemical profile of infusions and decoctions of H. italicum ssp. picardii, growing in Portugal, in comparison to the commonly consumed tisanes of green tea and rooibos.
The aim of the study was to determine the metabolic profile of two different H. italicum subspecies (italicum and tyrrhenicum) cultivated on an experimental plantation in Slovenian Istria, as well as to compare the chemical composition of extracts in relation to the solvent extraction procedure. As the plants were grown and harvested under the same conditions, the environmental impact on differences in their metabolic profile was thus eliminated. The extracts were prepared upon conventional extract preparation procedures: maceration with 50 % methanol or ethanol and cold or hot water infusion [9]. To the best of our knowledge this is the first study comparing composition of crude hydroalcoholic and water extracts of two different H. italicum subspecies using High Performance Liquid Chromatography-Diode Array Detection-Electrospray Ionization-Quadrupole Time-of-Flight-Mass Spectrometry (HPLC-DAD-ESI-QTOF-MS). Additionally, antioxidant properties of extracts were evaluated using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) test and were correlated with the total phenolic content.
2. Results and Discussion
2.1. Qualitative Analysis
After interpretation of the fragmentation pattern from the collected mass spectra of the H. italicum ssp. italicum (HII) and tyrrhenicum (HIT) samples (methanol:water (1:1) extracts—MWEs, ethanol:water (1:1) extracts—EWEs, hot water extracts—HWEs and cold water extracts—CWEs), one hundred phenolic compounds were identified. Of these, five compounds could be identified unambiguously with a reference standard and seventy-five could be identified tentatively by matching with published MS/MS spectra. Nine compounds were only partially identified as a derivative of a known compound, according to characteristic fragments present in the spectra. For the remaining eleven compounds, without reference spectra available, identification is less confident, although, supported by accurate mass and isotope pattern match and additionally computed Molecular Structure Correlator (MSC) scores. Detailed MS/MS information of the identified compounds is available in Appendix A, Table A1. In Table A1, compounds are listed in order of elution from the column and numbered accordingly. The same numbering system is maintained throughout the text.
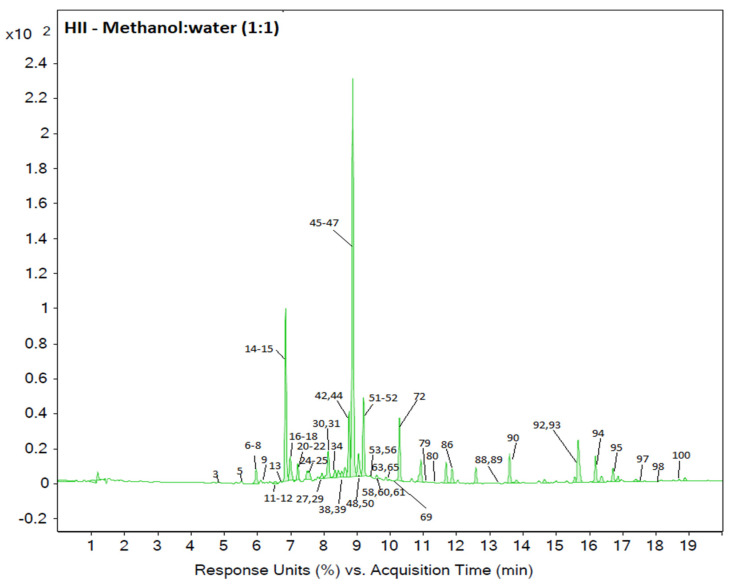
LC-ESI-QTOF-MS chromatograms (available in the Supplementary file, Figure S1) of H. italicum samples were relatively complex, containing peaks of several hydroxycinnamic and hydroxybenzoic acids, flavonoids, coumarins, pyrones as well as other chemical classes, such as isobenzofuranones, neolignans and acetophenones. The majority of the targeted compounds were detected better in ESI- mode, however, ESI+ spectra aided the identification of some compounds that lacked ESI- reference spectra or had more than one match. Figure 1 represents a DAD chromatogram of a HII MWE with compound numbers assigned to the peaks. The chromatogram was recorded at 280 nm, at which most of the phenolic compounds were detected.
Figure 1.
Representative DAD chromatogram (280 nm) of a H. italicum ssp. italicum (HII) sample with peak annotations representing identified compounds. Numbers correspond to each identified compound, listed in Appendix A, Table A1.
The applied method of analysis, along with the help of identification keys reported by other researchers, also enabled the distinguishing between isobaric compounds and identification of structural isomers. In the following sub-sections, the identification procedure of compounds is described in greater detail, with the emphasis on those more difficult to identify and compounds or isomers, which have not been previously reported in H. italicum.
2.1.1. Hydroxycinnamic Acids (Chlorogenic Acid Derivatives)
The class of chlorogenic acids (CGAs) represents a large group of compounds, among which the most characteristic are caffeoylquinic acids—conjugates of tetrahydroxy-cyclohexane carboxylic acid (quinic acid) and 3,4-dihydroxycinnamic acid (caffeic acid). Quinic acid can form even di- or tri-esters (e.g., di- or tri-caffeoylquinic acids; CQAs) or esters with several other trans-hydroxycinnamic acids, commonly ferulic and p-coumaric acids, which are then named feruloylquinic acid (FQA) and p-coumaroylquinic acid (pCoQA), respectively. Furthermore, ester mixes like caffeoyl-feruloylquinic acid (CFQA) can be formed as well, contributing to the even bigger complexity of this class [10,11].
Caffeic acid (21) was identified by comparing fragments with reference spectra. Four caffeic acid derivatives, three at m/z = 341 (9, 14, 16) and one at m/z = 567 (57) were observed. Compounds 9, 14 and 16, produced the same fragmentation ions corresponding to hexose moiety loss (162 Da) but with slightly different abundances and were identified as caffeic acid hexosides. Namely, the same [M−H]− at m/z = 341 is also generated by caffeoyl hexoses, where caffeic acid is connected with sugar moiety through ester bond instead of ether but in that case fragments characteristic for sugar moiety fragmentation are observed [12]. The fourth compound with fragment ion at m/z = 341 was semi-identified as a caffeic acid O-hexoside derivative (57). Glycosides eluted before its aglycones, which is in accordance with literature, in which several caffeic acid hexoside isomers have been identified before in tomato products by Vallverdú Queralt et al. [13]. Compound 57 and isomers of caffeic acid glycosides, have not been reported in H. italicum extracts before.
At least three positional isomers of monoacyl-CQAs are known to be present in H. italicum [14]. They all have identical molecular formula C16H18O9 with 354.0951 as the accurate theoretical mass. Pseudomolecular ion [M−H]− at m/z = 353 appeared at several retention times, indicating several CQA isomers. Compound 7 that eluted first was tentatively identified as a 3-O-CQA isomer (neochlorogenic acid), based on fragment ion abundances and literature data on retention times [13,15,16,17]. The most abundant was compound 15 (tR = 6.95 min) and was identified as 5-O-caffeoyl-quinic acid (5-CQA) (chlorogenic acid), which is also the most common CGA in nature [11]. Spectra matched well with those in the library but was also further confirmed with an authentic standard. Another peak matching chlorogenic acid, which was also observed in the standard solution, was present. The latter was less abundant but with a very similar fragmentation pattern, suggesting geometric isomerism. As cis-5-acyl isomers are reported to be appreciably more hydrophobic than their trans counterparts, the later eluting peak was tentatively identified as cis-5-O-CQA (25) [18]. Another compound (17) with [M−H]− at m/z = 353 was detected but presented quite different fragment abundances than features 7, 15 and 25 mentioned earlier. Fragmentation ions at m/z = 173 and m/z = 3 were highly abundant, which is characteristic for 4-O-CQA, as was ion at m/z = 191, which should not be so intense. Despite that we identified that peak as such, due to retention times that were in line with the literature [17,18]. It has been reported in the literature previously, that CGAs can readily transform to one another, especially during extraction procedures at elevated temperatures and in the presence of water. Chlorogenic acid (5-O-CQA) not only isomerizes to 3- and 4-O-CQA but also undergoes other transformations such as esterification and reactions with water (i.e., hydrolysis) [19]. However, it is also possible that geometrical isomerism was induced already in the plant by activators of plant defense and priming responses, considering a significant amount of the second peak [17]. To the best of our knowledge, this is the first time that geometrical isomer of chlorogenic acid is identified in H. italicum.
FQAs are a group of derivatives with pseudomolecular ion [M−H]− at m/z = 367. The same molecular mass fits also for caffeoylquinic acid methyl esters, among which 5-O-caffeoyl-4-methylquinic acid has been reported previously in H. italicum extract [20]. Compound 31 was identified as 5-O-FQA, as the base peak ion was at m/z = 191 and not 161 or 179, which are characteristic to methyl esters [15,21]. One feruloylquinic acid was also tentatively identified by Pereira et al. [8]. Ferulic acid (65) with [M−H]− at m/z = 193 was identified at later elution times which is in accordance with Pereira et al. [8]. Similar derivatives are CoQAs, which have a molecular ion at m/z = 337. As such was identified compound 26, whereas compound 34 produced a base fragment ion at m/z = 191 and was therefore tentatively identified as 5-O-CoQA [15]. Free coumaric acid (40) was also detected close after the compound 34. Similar retention times are also observed in the literature [13]. Compounds 19 and 24 were identified as coumaric acid hexosides (m/z = 325), with identifier ions at m/z = 163 and 119 indicating the presence of coumaric acid and typical loss of CO2 [M−H−44]− [13]. A coumaric acid hexoside has been reported in H. italicum previously by Pereira et al. [8].
As previously reported, both di-CQAs and CQA-glycosides produce an isobaric pseudomolecular ion at m/z = 515. Unlike the diCQA, the CQA glycosides produce distinctive ions at m/z = 341 ([caffeoyl glucoside-H]−) or/and 323 ([caffeoyl glucoside-H-H2O]−). A glycoside can be formed through an ether bond at either C-3 or C-4 on the aromatic caffeoyl ring. During MS fragmentation, these molecules give rise to ions at m/z = 341 which predominates in both cases, however, a peak at m/z = 323 is characteristic for glucosyl attachment at C-3 [17]. Compound 4 produced fragment ions only at m/z = 341 and was therefore tentatively identified as 3-O-(4′-O-caffeoyl glucosyl) quinic acid. For the compound 6, product ions at both m/z = 341 and 323 were observed but the latter was less abundant. The predominating fragment ion was at m/z = 191 but at higher collision energies, an ion at m/z = 161 was also observed. This feature was therefore tentatively identified as 5-O-(4′-O-caffeoyl glucosyl) quinic acid. Compound 12 was the most intense one, producing a base fragment at m/z = 323, which indicated the presence of an ether bond at the 3′ position. Based on the abundances of other fragments, its identity was predicted to be 5-O-(3′-O-caffeoyl glucosyl) quinic acid. Compound 12 has been reported before by de la Garza et al. [22], while isomers 4 and 6 are reported here for the first time. At later eluting times, di-CQAs are eluting and the isomers can be characterized based on identification keys and information published elsewhere [16,17,18,23]. Compounds 46, 49, 51 and 56 were tentatively identified as 3,4-diCQA, 3,5-diCQA, 1,5-diCQA and 4,5-diCQA, respectively. This identification is in accordance with the results for H. italicum methanolic extracts obtained by Gonçalves et al. [24] and by Zapesochnaya et al. [14] in terms of the number of isomers present and identified.
Structurally related but much less common, are esters formed by the reaction of quinic acid alkyl ester (quinate) with caffeic acid or of quinate with ferulic acid. They do appear in nature, although their isolation and identification are rather difficult. Methyl quinates might as well be the product of extraction with methanol and could frequently be found as artefacts in plant analysis [21]. Di-CQA methyl esters produce a pseudomolecular ion [M−H]− at m/z = 529 but so do FCQAs and FQA-glycosides. The parent ion at m/z = 529 was observed for compound 63, with a base fragment ion at m/z = 367, which is characteristic for deprotonated FQA and a fragment at m/z = 193 for ferulic acid. No ion at m/z = 337 ([feruloyl glucoside-H2O-H]−) was present, to indicate an ether linkage with hexose [10]. Also, FQA-glycosides have a similar polarity as other CQA glucosides and are therefore expected to elute earlier. Based on that information and the identification key from Clifford et al. [15], this feature was tentatively identified as 3-feruloyl-5-caffeoylquinic acid. Another compound (67) with [M−H]- at m/z = 529, produced a secondary product ion at m/z = 173 and was tentatively identified as 4-feruloyl-5-caffeoylquinic acid. Retention times were in accordance with Baeza et al. [25]. Pereira et al. [8] detected three features with a pseudomolecular ion at m/z = 529 but were tentatively identified as di-CQA methyl esters. 3,5-Di-CQA-methyl ester has been reported and unambiguously identified in H. italicum previously by Mari et al. [20] but with later retention times. In addition, the fragmentation profile of the 3,5-di-CQA-methyl ester isomer reported by Jaiswal and Kuhnert [21], did not match with the one observed by us.
TriCQAs produce a molecular ion at m/z = 677. For compound 36, the parent ion at m/z = 677 was detected along with its characteristic fragment ions at m/z = 515, 353 and 191. However, Clifford et al. [12] suggest that if a tentative tri-CQA isomer elutes before diCQA, it is too hydrophilic to be tri-CQA. Namely, under that molecular mass appear also compounds with either an additional caffeic acid residue (diCQA glycosides, C31H34O17) or an additional hexose residue (CQA diglycosides, C28H38O19). Discrimination between the two was possible based on the slight difference in monoisotopic mass and compound 36 was therefore identified as di-CQA glycoside. Compound 72 with [M−H]− at m/z = 677 and later retention time was identified as putative tri-CQA. Compounds with a pseudomolecular ion at m/z = 677 are reported in this study for the first time for H. italicum.
The unusual CQA derivatives were identified as well. Compound 52 was identified as tricaffeoylhexaric acid, based on the molecular ion at m/z = 695 and characteristic product ions at m/z = 533, 371 and 209. The proposed identification is in accordance with the literature, as this rare derivatives have been reported before for Asteraceae [26,27]. Similarly, compound 22, with a molecular ion at m/z = 533, was putatively identified as dicaffeoylhexaric acid. Compound 59 was identified as another CQA derivative with [M−H]− at m/z = 601 and with the main product ions at m/z = 395, 233 and 173. It has already been identified by Pereira et al. [8] as methoxyoxalyl dicaffeoylquinic acid. Compound 38, with a pseudomolecular ion at m/z = 747 and fragment ions with m/z = 585, 422 and 459, was detected by Pereira et al. [8] as well but remained unidentified. The fragment at m/z = 585 was produced after a glucose/caffeoyl moiety loss (162 Da) but our attempt in its identification was just as unsuccessful. Namely, the only relevant report with that molecular ion was for isobutyryl diCQA, published by Kłeczek et al. [27], which together with 162 Da, does produce the observed molecular ion. However, the molecular formula corresponding to either isobutyryl diCQA glycoside (C29H30O13) or isobutyryl triCQA (C34H30O15) does not match the observed most probable molecular formula, which was calculated as C34H36O19. The compound 38 was therefore semi-identified just as a caffeoyl derivative.
2.1.2. Hydroxybenzoic Acids and Their Glucosides
The first eluted compound (1) was identified as gallic acid glucoside, based on typical fragment ions corresponding to a hexoside group loss [M−H-162]− and for CO2 loss [M−H-162-44]−. Compound 3 was identified as 3,4-dihydroxybenzoic (protocatechuic) acid O-hexoside with a pseudomolecular ion [M−H]− at m/z = 315. It produced daughter ions at m/z = 153, corresponding to protocatechuic acid after the neutral loss of the hexoside group and at m/z = 109 produced after the neutral loss of a hexose moiety, followed by the neutral loss of CO2 (44 Da). This is a known phenolic compound [13,16] but has not yet been reported in H. italicum. However, its aglycone has been previously reported by Pereira et al. [8] and Gonçalves et al. [24] but the latter were unable to quantify it. Another compound (23) with a similar fragmentation pattern has been detected and its identification was predicted to be 2,4-dihydroxybenzoic acid. Compound 35, with parent ion at m/z = 197, was semi-identified as a dihydroxybenzoic acid derivative. Compound 18 was identified as vanillic acid hexoside, due to molecular ion at m/z = 329 and the most intense fragment ion at m/z = 167. Compounds 8, 27 and 29 with the same molecular ion at m/z = 329 and a somewhat improper fragmentation profiles were semi-identified as vanillic acid derivatives. Vanillic acid derivatives are common in plants but they have not yet been reported in H. italicum. Compounds 11 and 62 were identified based on their fragmentation profile and retention times as 4-hydroxybenzoic and 2-hydroxybenzoic acid (salicylic acid), respectively. Compound 13, with a pseudomolecular ion at m/z = 299 and fragment ions at m/z = 137 and 93, corresponding to a hexose moiety loss and additional neutral loss of CO2, was identified as hydroxybenzoic acid hexoside. Compound 10 (m/z = 331), with fragments characteristic for hydroxybenzoic acid, was semi-identified as a hydroxybenzoic acid derivative. Compounds 10 and 13 are reported for H. italicum here for the first time.
2.1.3. Flavonoids and Their Glycosides
The first eluting compound (30) of the flavonol class, presented a molecular ion at m/z = 609 and was tentatively identified as flavonoid dihexoside, due to the presence of a base peak at m/z = 285 [M−H-162-162]− and at m/z = 447 [M−H-162]−. The aglycone was identified as kaempferol, based on characteristic fragment ions at m/z = 225 and 227. Compound 54 was identified as kaempferol glycoside (m/z = 447). Both have already been reported in H. italicum before [22,28,29]. Compound 61, with a molecular ion at m/z = 489 and a fragment ion [M−H-204]− at m/z = 285 produced after acetylhexoside loss, was therefore identified as kaempferol acetylglycoside, which is reported here for the first time. Compound 75 was detected with [M−H]− at m/z = 593 or [M + H]+ at m/z = 595 and identified based on the fragmentation data reported previously [8] as tiliroside. Compounds 84 and 87 were identified as isokaempferides, presented by the matching fragment ions with those reported elsewhere [30]. Compound 81 was detected as kaempferol based on its characteristic fragment ions and compound 76, with the same molecular ion at m/z = 285, was identified as its isomer luteolin, whose identity was also confirmed by reference standard comparison.
Compounds 33, 41, 45 and 64 all presented a molecular ion at m/z = 463 and a fragment ion at m/z = 301 and were identified as quercetin O-hexosides, which is in accordance with Pereira et al. [8], who also observed four features at m/z = 463. Quercetin 3-O-galactoside and 3-O-glucoside have been reported previously for H. italicum [31], whereas additional isomers have not been identified yet. Separation of these two 3-O-isomers is difficult and as a result, inconsistent data on their identification is reported in the literature [8,16,31]. Compounds 43 and 50 were identified as quercetin malonylhexosides ([M−H]− at m/z = 549), due to fragment ions corresponding to the loss of acetylhexose (204 Da) after CO2 moiety loss (44 Da). Compounds 48 and 58 with a molecular ion at m/z = 477 produced fragments at m/z = 315 and 300, indicating a hexose moiety loss, followed by a methyl loss and were tentatively identified as isorhamnetin hexosides. Compound 58 produced slightly different fragments, which was in accordance with Pereira et al. [8]. Based on the information provided by Gu et al. [32], this derivative was probably isorhamnetin 3-O-glucoside. Compounds 77 and 83 were identified as isorhamnetin isomers. Similarly, compound 85 was identified as quercetin dimethyl ether, which has not been reported in H. italicum before. Compounds 68 and 70, with a molecular ion at m/z = 609, were identified as quercetin coumaroylglucoside, as by Pereira et al. [8]. This conclusion was based on the fragment ions [M−H-146]− at m/z = 463 and [M−H-162]− at m/z = 301, which indicates the substitution of coumaric acid and hexose. Compound 28 was identified as quercetin diglycoside, analogous to the kaempferol derivative (30), which has also not been reported before. Compound 73 was identified as quercetin by comparing the fragmentation pattern with literature [13,16].
Compounds 32, 39 and 47 were identified as myricetin derivatives. Compound 32 was identified as myricetin glucoside, which has already been reported for H. italicum [22], based on the fragment ion at m/z = 317, indicating a hexoside moiety loss and fragment ions characteristic for myricetin. Compound 39 with a parent ion at m/z = 565 and with the fragment ions corresponding to the loss of acetylhexose (204 Da) after a CO2 moiety loss (44 Da) was tentatively identified as myricetin malonylhexoside. This feature has been reported by Pereira et al. [8] but not identified. Similarly, compound 47 was identified as myricetin acetylglycoside but is reported here for the first time.
Compounds 90, 91 and 37 were identified as methyl derivatives of known flavonols, based on literature data on their fragmentation patterns [8,33]. The first was tentatively identified as gnaphaliin, the second as galangin methyl ether and the last as herbacetin methyl ether. All three compounds were reported in H. italicum before [34]. Compound 55 was tentatively identified as herbacetin, based on its fragmentation profile.
Flavanones were much less abundant as above described flavonols. Compound 60 was identified as eriodictyol O-hexoside based on the molecular ion at m/z = 449 and a product ion corresponding to the loss of a hexoside moiety [M−H-162]−. Compound 71 was identified as its aglycone. Eriodictyol, as well as its glycoside derivative have not been reported in H. italicum previously but are common in other plant derived products [13]. Compounds 78 and 80 were identified as naringenin and its isomer. The first presented a good literature fragment match, whereas for the second, different fragment ions were more abundant. Compound 89 was identified as pinocembrin and also confirmed with an authentic standard, whereas compound 82 was identified as its isomer, due to matching spectra with pinocembrin as well. Compound 74 produced a fragment ion at m/z = 255 and was semi-identified as a pinocembrin derivative. The pinocembrin isomer and pinocembrin derivative have not been reported before.
2.1.4. Coumarins
Compound 20 was identified based on the fragmentation profile as esculetin, which was also the most abundant coumarin present in the analyzed samples. It has also previously been identified in H. italicum extracts [35] but not in hydroalcoholic or water ones. The second detected coumarin was compound 42, which was identified as scopoletin based on MS/MS spectra and retention time comparison with an authentic standard. In many samples, fragmentation did not occur, probably due to low concentrations.
2.1.5. Arzanol and Other Pyrone Derivatives
Compound 93 was the most abundant compound of all the identified compounds. Its identity was confirmed as arzanol by comparison with a commercial reference standard. Arzanol is chemically characterized as prenylated heterodimeric phloroglucinyl α-pyrone. Compound 98 produced a very similar fragmentation profile to arzanol, so it was identified as an arzanol isomer. Compound 97, with [M−H]− at m/z = 567 and a fragment ion at m/z = 401, lead to its semi-identification as arzanol derivative. The arzanol isomer (98) and arzanol derivative (97) have not been reported before. Compound 96 appeared as a co-eluting peak and was tentatively identified as methylarzanol, based on the fragment ions comparison with Pereira et al. [8]. Compounds 92 and 99 were identified as heliarzanol 1 and 2, due to molecular ion at m/z = 445 and fragment ions similar to arzanol. However, no reference spectrum was available to confirm our findings. Compounds 95 and 100 were tentatively identified as italipyrone 1 and 2, also without the reference spectra to support it. The same counted for compound 86, whose identity was proposed as micropyrone. Despite the absence of reference spectra, the identification is likely to be correct as these compounds were isolated from H. italicum before. For compound 94 reference spectra were available and it was identified as helipyrone, as was already reported previously [8,36].
2.1.6. Other Phenolic Compounds
Compounds 44 and 53 were recognized as dihydrodehyrodiconiferyl glucoside derivatives, from the class of neolignans. The observed fragmentation pattern was plausible for the given formula. The observed fragments ions at m/z = 359 and 329 corresponded to the glucose moiety (162 Da) and sinapyl alcohol moiety loss (192 Da) followed by the loss of hydroxypropenyl moiety (46 Da). From the class of acetophenones, compound 88 was tentatively identified as 3-prenyl 4-hydroxyacetophenone, based on its fragmentation profile and reports from literature [8]. Compounds 66 and 69 were identified as 4-hydroxy-3-(2-hydroxy-3-isopentenyl) acetophenone 1 and 2, although reference spectra were not available. Isobenzofuranones and tremetones are the two groups of phenolic compounds that are readily extracted with methanol and have been previously reported in H. italicum [37]. However, they are rarely investigated in LC-MS studies and consequently reference spectral information is rather scarce. The reason for that lies in their lipophilicity, making them more suitable for GC-MS analysis [20]. Features 2 and 5 presented a molecular ion at m/z = 327 and product ions at m/z = 165 and 147, characteristic for a hexose moiety loss (162 Da) and additional water loss (18 Da). Based on the study by Lin et al. [38] and previous reports in H. italicum [37], they were identified as hydroxyphthalide glucosides. Compound 79 was identified as gnaphaliol glucopyranoside, a tremetone representative, based on fragment ions supporting the hexose moiety loss. No reference spectra were available.
2.2. Semi-Quantitative and Quantitative Analysis
Some differences in DAD profiles arising from genetic diversity of H. italicum as well as extraction solvent used can be observed immediately. To better estimate which has greater impact on the chemical composition, semi-quantitative comparison of different H. italicum extracts was made by comparing areas of EICs of individually identified compounds as well as summed areas of compounds belonging to the same chemical class. The results are graphically presented in Figure 2, whereas areas for each identified compound can be found in Supplementary File, Table S1.
Figure 2.
Heatmap representing the results of semi-quantitative analysis of putatively identified compounds in H. italicum extracts. Calculations were performed based on areas of extracted ion chromatograms (EICs), corrected for dilution factor during sample preparation. The abscissa is used to display the names of samples and the ordinate on the right is used to display the names of metabolites. The deeper the red color, the higher the content of the metabolites; the deeper the blue color, the lower the content of the metabolites. CQA—caffeoylquinic acids, HCA—hydroxycinnamic acids, HBA—hydroxybenzoic acids, TPC—total phenolic content (sum of all the identified and quantified compounds), der.—derivatives.
From the Figure 2 and the DAD chromatograms (Supplementary File, Figure S2), it can be seen that the EWEs and MWEs of the same species are the most alike, next to them are grouped the HWEs of both HII and HIT species, whereas CWEs are grouped completely separately. It is evident that the between-sample differences are primarily resulting from the extract preparation procedure, rather than the genetic differences of the subspecies. The differences in the composition of the analyzed extracts are understandable as the several extraction parameters, which can significantly influence the extraction yields of phenolic compounds, have been modified: extraction solvents (methanol, ethanol and water), temperature (room temperature and 100 °C), ultrasonic assistance and extraction time (5 days, 90 and 15 min). Preparation of extracts with water led to the loss of certain compound groups, such as di- and triCQAs, coumarins, quercetin and kaempferol derivatives in the case of cold water extraction with ultrasonic assistance, whereas other flavonols and flavanones were negatively affected in hot water extraction. To the contrary, some monoesters, diBAs, neolignans and caffeic acid derivatives, were more abundant in CWEs. On the other hand, some compound classes were basically unaffected by the extraction method used. Those were HCAs and pyrones, among which chlorogenic acid and arzanol were chosen for proper quantification.
The concentrations of two characteristic compounds in H. italicum extracts, obtained from calibration curves of the reference standards and the total phenolic content—TPC values are presented in Table 1. The TPC was calculated as a summation of all integrated DAD peaks and used for the comparison of the extracts’ strength.
Table 1.
Quantification of two most characteristic compounds (mg/g dried material) and the total phenolic content (TPC) of H. italicum extracts determined by DAD (280 nm).
| Sample | Chlorogenic Acid | Arzanol | TPC 1 |
|---|---|---|---|
| HII MWE | 4.6 ± 0.1 | 2.9 ± 0.1 | 31.4 ± 0.9 |
| HIT MWE | 5.7 ± 0.3 | 2.1 ± 0.2 | 84 ± 3 |
| HII EWE | 5.2 ± 0.2 | 6.72 ± 0.07 | 40.0 ± 0.7 |
| HIT EWE | 5.1 ± 0.1 | 3.50 ± 0.05 | 83.1 ± 0.5 |
| HII HWE | 4 ± 1 | <LOD | 19 ± 5 |
| HIT HWE | 5 ± 1 | <LOD | 43 ± 6 |
| HII CWE | <2.1 | <LOD | 40 ± 1 |
| HIT CWE | 3 ± 1 | <LOD | 153 ± 1 |
1 Results are expressed as chlorogenic acid equivalents (CAE) per dry mass. MWE—methanol:water extract EWE—ethanol:water extract, HWE—hot water extract, CWE—cold water extract, LOD—limit of detection.
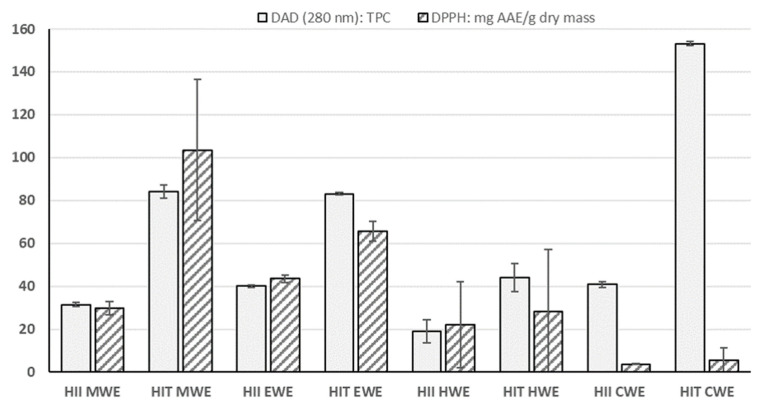
Chlorogenic acid was the most abundant in HIT MWE and HWE, whereas the lowest was in CWE. Arzanol, on the other hand, was the most abundant in the HII EWE, whereas in both types of water extracts it was below the detection limit. However, with LC-ESI-QTOF-MS, arzanol was detected in all samples. It is not uncommon, to miss some phenolic compounds with DAD due to its lesser sensitivity. Nevertheless, it can be seen that the cold-water extraction, even with the ultrasonic assistance, gives the lowest yields for both compounds and that the ethanol:water extraction gave better yields of arzanol for both subspecies, whereas for chlorogenic acid this was true only for HII. The results for the TPC ranged from 19 ± 5 to 153 ± 1 mg CAE/g dry mass and were contrary to what was expected. The sample with the highest values was CWE of HIT and the HWE of HII with the lowest. The reason for that is most probably due to the ultrasonic extraction assistance, which accelerates diffusion and enhances the mass transfer phenomena and enables good extraction yields even at lower processing temperatures [19]. On the other hand, with hot water extraction, the boiling water increases the extraction but can in turn cause the breakdown of heat-sensitive compounds. With ultrasonic extraction, heat is also a negative factor but if the extraction time is reasonably short and heat production minimized with addition of ice to the water bath, this problem can be avoided. Significant contribution to TPC in case of CWE must be attributed to some unidentified phenolic compounds. Substituting methanol for ethanol had little but significant overall effect on TPC in case of HII and no impact in case of HIT. Furthermore, the reduction of methanol to water ratio (from 3:1 to 1:1) decreased the TPC significantly (data not shown) but did not have proportionally negative impact on the bioactivity of the extracts. Namely, during the method optimization, higher methanol concentration was used for the extraction but later on proved to be of minor importance for the bioactivity, therefore it was not tested further. Based on the present results, we can conclude that HIT plant is richer in overall phenolic compounds than HII.
2.3. Antioxidant Activity
From the antioxidant test with DPPH radical, several values indicating the antioxidant potential of the extracts were determined—the EC50 value, indicating the concentration of the extract needed to inhibit 50% of the DPPH radical; maximal inhibition (inhmax), representing the highest value of DPPH inhibition; and the expression of the one tested concentration of the sample in mg of ascorbic acid equivalents per g of dried H. italicum material. The results are presented in Table 2.
Table 2.
Antioxidant potential of H. italicum samples determined by DPPH test and expressed as EC50 and ascorbic acid equivalents (AAE).
| Sample | EC50 [µg/mL] | Inhmax [%] | mg AAE/g Dry Mass |
|---|---|---|---|
| Ascorbic acid | 3.5 | 90.2 | / |
| HII MWE | 26 ± 1 a,b | 83 ± 3 | 30 ± 3 a |
| HIT MWE | 15 ± 1 | 82.75 ± 0.07 | 104 ± 33 c |
| HII EWE | 20 ± 1 | 83.0 ± 0.6 | 44 ± 2 a |
| HIT EWE | 19 ± 7 | 83 ± 2 | 66 ± 5 |
| HII HWE | 41 ± 4 a,d | 83.2 ± 0.9 d | 22 ± 20 d |
| HIT HWE | 29 ± 4 e | 82.8 ± 0.8 e | 28 ± 29 e |
| HII CWE | 268 ± 67 a | 75 ± 5 a | 3.8 ± 0.3 a |
| HIT CWE | 171 ± 63 | 86.6 ± 0.9 | 5.7 ± 0.9 |
MWE—methanol:water extract, EWE—ethanol:water extract, HWE—hot water extract and CWE—cold water extract. a Statistical significance (p < 0.01) between HII and HIT (MWE; EWE; CWE; HWE), b between HII MWE and HII EWE, c between HIT MWE and HIT EWE, d between HII HWE and HII CWE, e between HIT HWE and HIT CWE.
The lowest EC50 value was determined for the HIT MWE and was only 4-fold bigger as for ascorbic acid, followed by EWE and then by the HWE. CWEs had a 6-fold weaker potential than HWEs. A similar trend was observed for HII extracts. The maximum inhibition observed in the tested concentration range was around 82% for most samples. In the case of CWEs, it was 86 or 75% for HIT and HII extracts, respectively. Contrary to the highest TPC, HIT CWE did not have the best antioxidant potential, which suggests that bioactive compounds were deactivated during the extraction process or that mostly weakly active polyphenols were extracted. On the other hand, HWEs had a higher antioxidant potential as expected from the TPC, especially in the case of HII. Results were also expressed as mg AAE per g of dry mass for easier comparison of the antioxidant potential, where the higher values indicate higher antioxidant potential.
The relations between the antioxidant assay and TPC is shown in Figure 3. It can be seen that values correlated nicely for all the samples, except for CWEs. Pearson’s correlation coefficient of r = 0.89 (p < 0.001) was calculated when excluding CWEs. If the CWEs were included in the calculation, no significant relationships existed between TPC and antioxidant potential. This data further supports the findings, that cold water is not a good choice for extraction of the antioxidant active compounds, even with the assistance of ultrasound. Furthermore, the TPC and antioxidant activity were inversely proportioned for HIT (with the exception of methanol extract), whereas for HII antioxidant activity was higher compared to TPC values.
Figure 3.
Representation of DPPH results in comparison with TPC. MWE—methanol:water extract, EWE—ethanol:water extract, HWE—hot water extract and CWE—cold water extract.
3. Materials and Methods
3.1. Reagents and Chemicals
Reference standards of chlorogenic acid, scopoletin, pinocembrin, arzanol and luteolin were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and were of analytical or pharmaceutical primary standard grade. Mass spectrometer reference calibration mixtures and the tuning mix were purchased from Agilent (Agilent Technologies, Inc., Santa Clara, CA, USA). The mobile phase solvents methanol, water and formic acid were of LC-MS grade, whereas ethanol and methanol for extract preparation were of HPLC grade. All were purchased from Honeywell (Honeywell International Inc., Charlotte, NC, USA). Water used for cold and hot extraction was ultra-pure and obtained with the Purelab® Option-Q water purification system (Evoqua Water Technologies LLC, Pittsburgh, PA, USA). DPPH and ascorbic acid were purchased from Sigma-Aldrich as well.
3.2. Plant Collection
For research proposes, the Ex Situ experimental collection of Helichrysum italicum (Roth) G. Don of the University of Primorska was established in 2018 near Ankaran (45°34′19.3″ N 13°46′33.2″ E), Slovenia. Plant material for the collection was obtained with inventarization of some private, Slovenian home gardens where different H. italicum phenotypes are maintained for a decade. Plant material for the preparation of seedlings was acquired from morphological different mother plants grown in home gardens and was vegetative propagated in the nursery. Two-year seedlings were planted in rows at a distance of 0.7 m × 0.4 m according to the randomized block design in the experimental Ex Situ field in a well-drained soil with a sandy-loam texture. Both subspecies in the collection thrive under the uniform growing and ecological conditions of the sub Mediterranean climate. All plants in the collection were morphologically evaluated with the revised taxonomic identification key for H. italicum, which was recently developed by Herrando-Moraira et al. [1]. Based on the qualitative and quantitative morphological characters of vegetative parts (presence of axillary leaf fascicles, caulinar leaf length, leaf margin) and floral part (number of capitula per synflorescence), two different morphological variants were identified. One variant of H. italicum was identified as H. italicum ssp. italicum (HII) and another as H. italicum ssp. tyrrhenicum (HIT). Plants in the collection thrive under the uniform growing and ecological conditions of the sub Mediterranean climate. Aerial parts of two morphological variants were harvested at the stage of development of generative shoots before their full flowering period in June 2019. Sampling of each variant was performed on five randomly selected plants. Stems and leaves, along with flower-tops were cut into smaller pieces, frozen with liquid nitrogen and freeze-dried (Alpha 1–4 LSCplus; Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Dried plant material was then stored at −20 °C until use.
3.3. Extraction Procedures
Essentially, two conventional extraction procedures were used for the preparation of the extracts: maceration and infusion. In the preliminary experiments different solvent ratios were tested as reported in previously published studies on H. italicum [22,39]. Hydroalcoholic extracts were prepared by maceration of the dried and milled plant material (2.5 g) of each HII and HIT with 50 mL of methanol:water (1:1) and ethanol:water (1:1) solvent mixtures, which were chosen as the most appropriate in terms of extraction yields and desired future applications. After 5-day maceration at room temperature in the dark, extracts were filtered through Whatman No. 41 filter paper and concentrated by a rotary evaporator (Rotavapor® R-300; BÜCHI Labortechnik AG, Flawil, Switzerland). The dried residue was then dissolved in 5 mL of the original solvent mixture and kept at −20 °C until analysis. Cold water extracts were prepared by two successive macerations of 2.5 g of the plant material: first with 30 mL and the second with 20 mL of cold water, assisted with sonication (Elmasonic S 30 H, Elma Schmidbauer GmbH, Singen, Germany) in an ice bath for 60 min and 30 min, respectively. Ultrasonic assistance was used only in the case of cold water maceration, to improve poor extraction yields and shorten the time of maceration. Hot water extracts (infusions) were prepared just before analysis by immersing 1.25 g of milled plant material in hot water (50 mL) for 15 min and then filtered through filter paper. The drug to extract ratio used was the same as reported by Kazazic et al. [40]. Each extraction procedure was carried out in duplicates.
Samples for chemical analysis were diluted according to theirs estimated yields as follows: 1/50, 1/100 or 1/10 for hydroalcoholic, cold and hot water extracts, respectively, with the same solvent mixture as used for extraction. The diluted samples were passed through a 0.2 µm HPLC certified nylon membrane filter (Macherey-Nagel GmbH & Co KG, Düren, Germany) and kept in the amber HPLC vials (Agilent Technologies, Inc., Santa Clara, CA, USA) with PTFE/silicone septa caps at 4 °C until analysis.
3.4. HPLC-DAD-ESI-QTOF-MS Analysis
High performance liquid chromatography-mass spectrometry analysis of the reference standards and extracts samples was performed using an Agilent 1260 Infinity II HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a diode array detector (DAD, model G7115A) and coupled to an Agilent 6530 Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) MS system equipped with an Agilent Jet Stream dual electrospray ionization (ESI) source. The HPLC system included a binary pump (model G7112B), Agilent 1260 Autosampler (model G7129A) and a Poroshell 120, EC-C18, 2.1 × 150 mm, 2.7 µm column (693775-902, Agilent Technologies, Santa Clara, CA, USA). The following method for the HPLC-MS analysis rests on our previous studies of phenolic compounds investigation [41,42]. Separation was obtained with a linear gradient of (A) water + 0.1% formic acid (v/v) and (B) acetonitrile/methanol (50:50, v/v), starting at 3.0 % B and increased to 100.0% B in 15 min and held for 5 min (flow rate 0.30 mL/min, column temperature 50 °C, injection volume 1 µL). The separated compounds were first monitored using DAD at 280 nm and 330 nm and then MS scans were performed under the following conditions: gas temperature 250 °C, drying gas flow 8 L/min, nebulizer 35 psig, sheath gas temperature 375 °C, sheath gas flow 11 L/min, capillary voltage 1000 V and fragmentor voltage 150 V. The ion-source parameters were the same in both positive and negative ESI modes. Mass spectra were recorded as centroid data for m/z 100–1000 in MS mode and m/z 40–1000 in MS/MS mode, with an acquisition rate of 14.0 spectra/sec. The Automated MS/MS data-dependent acquisition was done for ions detected in the full scan above 2000 counts with a cycle time of 0.5 s, a quadrupole isolation width in narrow ~1.3 Da, using fixed collision energies of 10, 20 and 40 eV and a maximum of three selected precursor ions per cycle. The instrument was tuned in low mass range (1700 m/z) and in extended dynamic range (2 GHz) mode. In those conditions, the instrument is expected to provide experimental data with accuracy within ±3 ppm. The Agilent MassHunter Data Acquisition software was used to acquire data.
All the acquired data were first processed using MassHunter Qualitative Analysis Workflows (version B.08.00) and Qualitative Navigator (version B.08.00) software. The extracts were screened for the range of phenolic compounds previously reported in H. italicum and identified, based on the accurate mass of precursor ions with minimum 80 overall match scores and fragmentation profile obtained from METLIN Metabolite and Chemical Entity Database (The Scripps Research Institute, San Diego, CA, USA) or literature data, if available. Targets with no reference MS/MS data available, were evaluated just on MS level and processed further with in-silico fragment prediction software—Molecular Structure Correlator (MSC). For qualitative and semi-quantitative between-sample comparison, Agilent’s Mass Profinder (version B.08.00) was used for simultaneous targeted feature extraction.
Quantification was performed by an external calibration method using chromatograms measured with DAD at 280 nm. Standard solutions (10 µg/mL) were prepared from dimethyl sulfoxide or methanol stock solutions in LC-MS grade methanol. Chlorogenic acid and arzanol reference standards were used to construct the calibration graphs and to quantify the two most characteristic compounds, which were identified in H. italicum extracts. The calibration plots indicated good correlations between peak areas and commercial standard concentrations with regression coefficients higher than 0.996. The lowest calibration point included in the calibration curve was used to calculate the limit of quantifications (LOQs). The results are expressed as mg of a standard per g of the dried H. italicum sample. The total phenolic content of an extract was determined as a summation of areas for all integrated peaks with signal-to-noise ratio greater than 5:1 and expressed as mg of chlorogenic acid equivalents (CAE) per gram of dry mass of the plant material.
3.5. Antioxidant Assay
The antioxidant activity of H. italicum extracts was measured in terms of their radical-scavenging ability in the DPPH radical assay. The assay was performed as reported previously by Zegura et al. [43], with minor modifications. Briefly, reaction mixtures containing 7.8 to 2500 µg/mL of extracts and 0.1 mM DPPH in methanol were incubated at ambient temperature for 60 min in 96-well microtiter plates in the dark. The decrease in absorbance of the free radical DPPH was measured at 515 nm with a microplate reader Infinite F200 (Tecan Group Ltd., Zürich, Switzerland). Ascorbic acid was used as a positive control. The free radical scavenging activity was calculated as the percentage of DPPH radical that was scavenged, as follows:
| (1) |
Asample+DPPH: absorbance in the presence of H. italicum extracts or ascorbic acid,
Ablank+DPPH: absorbance of the control reaction (solvent without H. italicum extracts),
Asample: absorbance of the sample,
Ablank: absorbance of methanol.
EC50 values were determined graphically from the curves. Two independent experiments with at least three replicates each were performed. Results were also expressed as mg of ascorbic acid equivalents (AAE) per grams of dry plant material.
3.6. Statistical Analysis
The results were expressed as mean values ± standard deviation. One-way analysis of variance (ANOVA) and independent sample t-test were used to compare the differences in an antioxidant activity determined by DPPH test between two subspecies (HII vs. HIT) and between different extraction procedures. Levene’s test was performed to verify if there was homogeneity of variances. Pearson’s correlation analysis was performed to evaluate the relationship between total phenolic content (TPC) and antioxidant activity determined by DPPH test. All statistical outcomes with p values less than 0.05 (p < 0.05) were recognized as statistically significant. Statistical analyses were performed with the help of computer software—Statistical package for the social sciences (SPSS) version 23.0 (IBM Inc., Chicago, IL, USA). In addition, heatmap of hierarchical cluster analysis was conducted to present the results of semi-quantitative analysis of putatively identified compounds in H. italicum extracts. Calculations were performed based on areas of extracted ion chromatograms (EICs), corrected for dilution factor during sample preparation. Heatmap was conducted by the heatmap (Version 1.0.12) package of R software (Version 3.5.0).
4. Conclusions
The aim of this study was to examine the phytochemical profile of two Helichrysum italicum subspecies (HIT and HII) prepared with different extraction procedures and to investigate the differences between the extracts in terms of bioactive compounds resulting in possible distinction of antioxidant activity. In total, one hundred compounds were identified. Among them are several isomers and derivatives reported here for the first time (e.g., vanillic acid derivatives, di- and tricaffeoylhexaric acid, CoQA, FCQAs, CQA glucoside isomers, triCQA, eriodictyol and its derivatives). The most abundant compounds were caffeoylquinic acids and pyrones. This study is also noteworthy as it compares two subspecies of H. italicum grown under the same environmental conditions, between which no drastic differences in terms of qualitative composition were observed. Great similarities can also be drawn with the study of H. italicum ssp. picardii by Pereira et al. [13]. Although the morphological differences between the subspecies were obvious, a more accurate classification would only be possible with the help of DNA markers. Conversely, differences in the response to the extraction procedure and the parameters applied in the extraction process were evident. In case of HII, ethanol:water extracts gave better TPC yields than methanol:water, whereas the opposite was true for HIT. All HIT extracts had higher TPC content compared to HII, while the antioxidant potential was not proportionally higher. From these results, we can conclude that the antioxidant compounds in HII are either more potent but present in lesser amounts or that some non-phenolic substances contribute to the antioxidant activity. To better understand the mechanism of action and to confirm the potential use of these species in disease prevention or treatment, additional in vivo antioxidant assays are required. A key observation was that the hot water extracts proved to be comparably active as alcoholic ones, confirming the high commercial potential of Helichrysum italicum preparations as herbal functional beverages in the health-foods category. This study provided important information for selecting the best extract for further studies on the bioactivity of Helichrysum italicum.
Acknowledgments
The authors acknowledge the European Commission for funding InnoRenew CoE (grant agreement #739574), under the H2020 Widespread-Teaming program and Republic of Slovenia (investment funding of the Republic of Slovenia and the European Union’s European Regional Development Fund), the ARRS research project J4-1767. The authors would like to thank the InnoRenew CoE for providing the laboratory equipment for the sample preparation and the chemical analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/10/10/403/s1, Figure S1: Total ion chromatograms of the tested samples gathered in negative (A) and positive (B) ESI mode. Samples are numbered accordingly: (a) H. italicum ssp. italicum (HII) methanol:water extract in ratio 3:1 and (b) HII methanol:water extract in equal ratios, (c) H. italicum ssp. tyrrhenicum (HIT) methanol:water extract in equal ratios, (d) and (e) HII and HIT ethanol:water extracts in equal ratios, (f) and (g) HII and HIT hot water extracts, (h) and (i) cold water extracts., Figure S2: DAD chromatograms at 280 nm of the H. italicum samples overlaid accordingly: (A) and (B) representing differences in chemical profile between methanol and ethanol extracts of H. italicum ssp. italicum (HII) and H. italicum ssp. tyrrhenicum (HIT), respectively (C) and (D) representing differences in ethanol versus hot water extracts of the HII and HIT, respectively, (E) and (F) representing differences between HII versus HIT methanol and hot water extracts, respectively, Table S1: Results of the semi-quantitative analysis for the detected compounds in all tested samples. Values were calculated based on areas of extracted ion chromatograms (EICs) and corrected for dilution factor during sample preparation.
Appendix A
Table A1.
Comprehensive chromatographic and spectral data of the (tentatively) identified compounds in H. italicum extracts obtained with HPLC-DAD-ESI-QTOF-MS.
| No. | RT (Min) |
Compound Name | Compound Formula | Molar Mass | Diff. (ppm) |
m/z ESI− |
MS2 ESI− | m/z ESI+ | MS2 ESI+ | UVmax (nm) | Level of Identification 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.90 | Gallic acid glucoside | C13H16O10 | 332.0757 | −3.35 | 331.0682 | 331.1; 168.0; 125.0; 167.0; 149.9; 124.0; | NPR/MS2 | |||
| 2 | 4.25 | Hydroxyphthalide glucoside 1 | C14H16O9 | 328.079 | −0.24 0.92 |
327.0722 373.0773 |
165.1; 146.9; 103.0; 93.0; 77.0 | 329.0857 | 167.0; 121.0; 111.0; 149.0 | PR in [37]/MS | |
| 3 | 4.84 | Protocatechuic acid O−hexoside | C13H16O9 | 316.0789 | 1.83 | 315.0720 | 315.1; 152.0; 153.0; 108.0; 109.0 | 210 | NPR/MS2 | ||
| 4 | 5.49 | CQA−glucoside: 3−O−(4′−caffeoyl glucosyl) quinic acid | C22H28O14 | 516.1486 | −1.24 | 515.1413 | 515.1; 179.0; 341.1; 173.0; 353.1; 135.0 | NPR/MS2 | |||
| 5 | 5.55 | Hydroxyphthalide glucoside 2 | C14H16O9 | 328.079 | 1.17 | 327.0717 | 327.1; 165.0; 146.9; 121.0 | 207 | PR in [37]/MS | ||
| 6 | 5.95 | CQA−glucoside: 5−O−(4′caffeoyl glucosyl) quinic acid | C22H28O14 | 516.1469 | 1.87 | 515.1400 | 515.1; 191.1; 353.1; 341.1; 179.0; 323.1; 135.0; 161.0 | 517.1547 | 163.0; 325.1; 135.0 | 220; 324 | NPR/MS2 |
| 7 | 6.00 | Caffeoylquinic acid: 3−O−CQA (neochlorogenic acid) | C16H18O9 | 354.0947 | −0.11 | 353.0875 | 353.1; 191.1;179.0; 135.0; 146.9; 107.0 | 355.101 | 163.0; 145.0; 135.0; 117.0; 89.0 | PR in [14]/MS2 | |
| 8 | 6.08 | Vanillic acid derivative 1 | C14H18O9 | 330.0949 | 0.31 | 329.0876 | 329.1; 167.0; 123.0; 149.0; 102.9; 79.0 | 220; 204 280 |
NPR/SI | ||
| 9 | 6.18 | Caffeic acid hexoside 1 | C15H18O9 | 342.0956 | −1.76 | 341.0884 | 179.0; 341.1; 229.9; 135.0; 205.0; 133.0 | 202 | NPR/MS2 | ||
| 10 | 6.28 | Hydroxybenzoic acid derivative | C14H20O9 | 332.0543 | −3.49 | 331.0471 | 165.0; 123.0; 121.0; 93.0; 77.0; 137.0; 79.0 | NPR/SI | |||
| 11 | 6.47 | p−Hydroxybenzoic acid | C7H6O3 | 138.0313 | 0.19 | 137.0244 | 93.0; 137.0; 65.0; 41.0; 75.0; 49.0; 67.0 | 318; 226 | PR in [24]/MS2 | ||
| 12 | 6.58 | CQA−glucoside: 5−O−(3′−O−caffeoyl glucosyl) quinic acid | C22H28O14 | 516.1477 | −0.31 | 515.1404 | 515.1; 323.1; 191.1; 341.1; 161.0; 85.0 | 517.1546 | 163.0; 325.1; 517.1; 145.0; 85.0; 135.0 | PR in [22]/MS2 | |
| 13 | 6.75 | Hydroxybenzoic acid hexoside | C13H16O8 | 300.0843 | 0.54 | 299.0771 | 137.0; 299.1; 179.0; 59.0; 93.0; 71.0; 65.0 | 213 | NPR/MS2 | ||
| 14 | 6.82 | Caffeic acid hexoside 2 | C15H18O19 | 342.095 | −0.14 | 341.0874 | 341.1; 179.0; 135.0; 84.9; 73.9; 153.1 | 326; 297; 194; 218; 244 | NPR/MS2 | ||
| 15 | 6.88 | Caffeoylquinic acid: 5−O−CQA (chlorogenic acid) | C16H18O9 | 354.0953 | 0.49 | 353.0878 | 191.0; 353.1; 161.0; 85.0; 127.0; 87.0; 133.0; 83.0 | 355.1013 | 163.0; 135.0; 117.0; 135.0; 145.0 | PR in [14,22,24]/ STD |
|
| 16 | 7.01 | Caffeic acid hexoside 3 | C15H18O9 | 342.0951 | −0.01 | 387.0932 341.0894 |
179.0; 341.0; 135.0; 107.0 | 216; 259; 288; 330 | NPR/MS2 | ||
| 17 | 7.04 | Caffeoylquinic acid: 4−O−CQA (cryptochlorogenic acid) | C16H18O9 | 354.0951 | −0,07 | 353.0878 399.0944 |
191.1; 353.1; 173.0; 179.0; 161.0; 135.0; 93.0; 85.0 | 355.1011 | 163.0; 193.0; 145.0; 135.0; 117.0; 89.0; 193.0 | PR in [14]/MS2 | |
| 18 | 7.05 | Vanillic acid O−hexoside | C14H18O9 | 330.0951 | −0.16 | 329.0879 | 167.0; 329.1; 99.0; 230.7; 123.0; 125.0; 41.0 | NPR/MS2 | |||
| 19 | 7.08 | Coumaric acid hexoside 1 | C15H18O8 | 326.1009 | −2.2 | 325.0930 | 163.0; 325.1; 119.0; | 214 | PR in [8]/MS2 | ||
| 20 | 7.18 | Esculetin | C9H6O4 | 178.0266 | 0.2 | 177.0193 | 177.0; 133.0; 89.0; 41.0; 79.0; 53.0; | 326; 297; 194; 218; 240 | PR in [35]/MS2 | ||
| 21 | 7.24 | Caffeic acid | C9H8O4 | 180.0419 | 2.12 | 179.0346 | 135.0; 179.0; 164.0; 134.0; 45; 89.0 | PR in [22,35]/MS2 | |||
| 22 | 7.26 | Dicaffeoylhexaric acid | C23H34O14 | 534.1017 | −0.94 | 533.0939 | 371.1; 533.1; 209.0; 191.0; 85.0; 57.0; 147.0; 179.0 | NPR/MS2 | |||
| 23 | 7.45 | 2,4−Dihydroxybenzoic acid | C7H6O4 | 154.0259 | 4.21 | 153.0187 | 109.0; 153.0; 108.0; 65.0; 91.0 | 210; 245; 325; 295 | NPR/MS2 | ||
| 24 | 7.51 | Coumaric acid hexoside 2 | C15H18O8 | 326.0997 | 1.1 | 325.0925 | 265.1; 163.0; 205.0; 161.0; 145.0; 119.0; 117.0; 59.0 | 325;298; 210; 245 | PR in [8]/MS2 | ||
| 25 | 7.53 | Caffeoylquinic acid: cis−5−O−CQA (cis−chlorogenic acid) | C16H18O9 | 354.095 | 0.18 | 353.0878 | 191.1; 353.1; 85.0; 111.0; 127.0; 135.0 | 355.1015 | 163.0; 254.9; 145.0; 111.0; 117.0; 135.0; 89.0 | NPR/MS2 | |
| 26 | 7.60 | Coumaroylquinic acid | C16H18O8 | 338.1011 | −3.31 | 337.094 | 191.1; 337.1; 87.0; 69.0; 163.0; 93.0; 43.0; 67.0; | NPR/MS2 | |||
| 27 | 7.71 | Vanillic acid derivative 2 | C14H18O9 | 330.0955 | −0.53 | 329.088 | 167.0; 329.1; 108.0; 191.0 | 211; 204 | NPR/SI | ||
| 28 | 7.73 | Quercetin diglycoside | C27H30O17 | 626.15 | −1.41 | 625.1419 | 625.1; 593.1; 301.0; 463.1; 488.9; 300.0 | NPR/MS2 | |||
| 29 | 7.80 | Vanillic acid derivative 3 | C14H18O9 | 330.0955 | −1.89 | 329.0878 | 191.0; 167.0; 329.1; 123.0; 83.0; 81.0 | 216; 204 | NPR/SI | ||
| 30 | 7.97 | Kaempferol diglycoside | C27H30O16 | 610.1535 | −1.76 | 609.1472 | 609.2; 283.0; 49.4; 441.2; 285.0; 447.1; 328.0 | 200; 218; 180; 345 | NPR/MS2 | ||
| 31 | 7.97 | 5−O−Feruloylquinic acid | C17H20O9 | 368.1094 | −3.62 | 367.1037 | 191.1; 367.1; 173.0; 87.0; 93.0; 134.0; 85.0 | 369.1186 | 177.1; 369.1; 145.0; 117.0; 149.1 | PR in [8]/MS2 | |
| 32 | 8.0 | Myricetin glycoside | C21H20O13 | 480.0914 | −2.05 | 479.0842 | 479.1; 317.0; 316.0; 165.98; 139.0 | 481.0969 | 319.0; 481.1; 435.1; 169.0; 137.0 | PR in [22]/MS2 | |
| 33 | 8.15 | Quercetin O−hexoside 1 | C21H20O12 | 464.0959 | −0.24 | 463.0883 | 463.1; 300.0; 301.0; 137; 300.0 | PR in [20]/MS2 | |||
| 34 | 8.20 | 5−O−Cumaroylquinic acid | C16H18O8 | 338.1001 | −0.57 | 337.0931 | 191.1; 337.1; 145.0; 163.0, 81.0; 85.0; 93.0 | 320; 295; 195; 234 | NPR/MS2 | ||
| 35 | 8.3 | Dihydroxybenzoic acid derivative | C9H10O5 | 198.0532 | −2.67 | 197.455 | 197.0; 153.1; 138.0; 123.0; 109.0; 108.0; 137.0 | 320; 295; 195, 234 | NPR/SI | ||
| 36 | 8.37 | Dicaffeoylquinic acid glycoside | C31H34O17 | 678.1806 | −1.47 | 677.1734 | 677.2; 515.1; 353.1; 179.0; 191.1; 323.1 | NPR/MS2 | |||
| 37 | 8.40 | Herbacetin methyl ether | C16H12O17 | 316.0583 | 0.6 | 315.0508 | 315.0; 287.1; 255.0; 283.0; 227.0; 211.0; 183.0 | 216; 257; 291 | PR in [34]/MS2 | ||
| 38 | 8.42 | Caffeoyl derivative | C34H36O19 | 748.1856 | −1.71 | 747.1791 | 747.2; 585.1; 422.1; 459.1 | PR in [8]/SI | |||
| 39 | 8.49 | Myricetin malonylhexoside | C24H22O16 | 520.0861 | −1.39 | 565.0843 | 521.1; 317.0; 565.1; 178.9; 174.0; 161.0 | NPR/MS2 | |||
| 40 | 8.56 | Coumaric acid | C9H8O3 | 164.0472 | 1.06 | 163.0399 | 119.0; 163.0; 91.1; 93.0; 117.0; | 203; 228; 284; 340 | PR in [44,45]/MS2 | ||
| 41 | 8.59 | Quercetin O−hexoside 2 | C21H20O12 | 464.0955 | −3.28 | 463.097 | 463.1; 301.0; 300.0; 271 | 465.103 | 303.0; 465.1; 392.1; 229.0; 153.0; 285.0 | PR in [31]/MS2 | |
| 42 | 8.61 | Scopoletin | C10H8O4 | 192.0431 | −3.39 | 191.0359 | 176.0; 191.0; 148.0; 59; 104.0; 102.9; 120.0 | PR in [35]/STD | |||
| 43 | 8.64 | Quercetin malonylhexoside 1 | C24H22O15 | 550.0973 | −2.05 | 549.0897 | 505.1; 301.0; 445.1; 300.0 | NPR/MS2 | |||
| 44 | 8.66 | Dihydrodehyrodiconiferyl glycoside derivative 1 | C27H36O13 | 568.2157 | −0.21 | 567.2085 | 567.2; 359.1; 341.1; 521.2; 329.1; 44.9; | NPR/MS2 | |||
| 45 | 8.70 | Quercetin O−hexoside 3 | C21H20O12 | 464.0958 | −0.29 | 463.0882 | 463.1; 300.0; 301.0; 271.0; 255.0; 151.0 | 465.1027 | 303.0; 145.0; 85.0; 229.0; 97.0 | 196; 218: 244; 302; 328 | PR in [31]/MS2 |
| 46 | 8.79 | Dicaffeoyl quinic acid isomer: 3,4−diCQA |
C25H24O12 | 516.1264 | 0.76 | 515.1189 | 515.1; 353.1; 203.0; 173.0; 179.0; 191.1; 135.0 | 517.1339 | 163.0; 499.1; 135.0; 145.0; 89.0; 117.0 | PR in [14]/MS2 | |
| 47 | 8.83 | Myricetin acetylglycoside | C23H22O14 | 522.1012 | 0.28 | 521.0935 | 521.1; 503.1; 127; 367.1; 152.0; 108.0; 179.0 | NPR/MS2 | |||
| 48 | 8.87 | Isorhamnetin hexoside 1 | C22H22O12 | 478.1121 | −0.09 | 477.1039 | 477.1; 315.1; 314.0; 300.0; 299.0; 271.0; 201.0; 179.0 | 196; 218: 244; 302; 328 | PR in [20]/MS2 | ||
| 49 | 8.90 | Dicaffeoyl quinic acid isomer: 3,5−diCQA |
C25H24O12 | 516.1268 | 0.01 | 515.1193 | 353.1; 515.1; 191.1; 179.0; 135.0; 173.0; 161.0; | 517.1331 | 163.0; 499.1; 337.1; 145.0; 135.0; 117.0 | PR in [14]/MS2 | |
| 50 | 8.90 | Quercetin malonylhexoside 2 | C24H22O15 | 550.0963 | −0.58 | 549.0931 | 505.1; 300.4; 301.0; 287; 271.0 | 551.1028 | 303.0; 551.1; 127.0; 85.0; 109.0 | NPR/MS2 | |
| 51 | 9.07 | Dicaffeoyl quinic acid isomer: 1,5−diCQA |
C25H24O12 | 516.1271 | −0.14 | 515.1196 | 353.1; 515.1; 191.1; 179.0; 135.0 | 517.1332 | 163.0; 499.1; 319.1; 145.0; 89.0; 117.0 | 196; 218: 244; 302; 328 | PR in [14]/MS2 |
| 52 | 9.11 | Tricaffeoyl hexaric acid | C33H28O17 | 696.1331 | −0.14 | 695.1255 | 695.1; 533.1; 371.1; 209.0; 85.0; 191.0 | NPR/MS2 | |||
| 53 | 9.17 | Dihydrodehyrodiconiferyl glycoside derivative 2 | C27H36O13 | 568.2165 | −1.4 | 567.2091 | 521.2; 491.2; 503.2; 476.2; 250.1; 329.1; 341.1; 99.0; | 196; 218: 244; 302; 328 | NPR/MS | ||
| 54 | 9.20 | Kaempferol glycoside | C21H20O11 | 448.101 | −1.07 | 447.0936 | 447.1; 284.0; 285.0; 255.0; 227.0 | PR in [22]/MS2 | |||
| 55 | 9.21 | Herbacetin | C15H10O7 | 302.0428 | −0.94 | 301.0357 | 301.0; 268.0; 133.0; 211.0; 135.0; 159.0; 132.0 | 303.0496 | 303.0; 313.0; 163.0; 137.1; 135.0; 123.0; 169.0; 285.0 | NPR/MS2 | |
| 56 | 9.23 | Dicaffeoyl quinic acid isomer: 4,5−diCQA | C25H24O12 | 516.1268 | −0.11 | 515.1192 | 515.1; 353.1; 173.0; 179.0; 191.1; 135.0; 93.0 | 517.1333 | 163.0; 499.1; 145.0; 135.0; 117.0 | PR in [14]/MS2 | |
| 57 | 9.29 | Caffeic acid O−hexoside derivative | C27H36O13 | 568.2137 | 2.16 | 567.2071 | 179.1; 521.2; 341.1; 161.0; 326.1; 89.0; 71.0 | 207; 247, 305; 325 | NPR/SI | ||
| 58 | 9.33 | Isorhamnetin hexoside 2 | C22H22O12 | 478.1118 | −3.25 | 477.1051 | 477.1; 315.0; 314.0; 243.0; 271.0; 285.0; 299.0 | 479.1177 | 317.1; 479.1; 85.0; 302.0; 153.0; | PR in [20]/MS2 | |
| 59 | 9.35 | Methoxyoxalyl dicaffeoylquinic acid | C28H26O15 | 602.128 | −1.92 | 601.121 | 395.1; 439.1; 557.1; 353.1; 233.1; 191.0; 173.0; 179.0 | PR in [8]/MS2 | |||
| 60 | 9.44 | Eriodictyol O−hexoside | C21H22O11 | 450.1193 | −3.96 | 449.1107 | 449.1; 287.1; 135.0; 151.0; 123.0; 152.0 | NPR/MS2 | |||
| 61 | 9.47 | Kaempferol acetylglycoside | C23H22O12 | 490.1121 | −5.52 | 489.1065 | 489.1; 417.0; 285.0; 284.0; 255.0; 227.0 | NPR/MS2 | |||
| 62 | 9.59 | Salicylic acid | C7H6O3 | 138.0313 | 0.99 | 137.0244 | 93.0; 137.0; 65.0; 53.0 | 194; 224 | PR in [8]/MS2 | ||
| 63 | 9.61 | 3−Feruloyl−5−caffeoylquinic acid | C26H26O12 | 530.143 | −2.38 | 529.1364 | 367.1; 529.1; 193.0; 191.1; 134.0; 113 | 531.1493 | 177.1; 513.1; 531.2; 509.0; 163.0; 145.0; 117.0 | 295; 316; 250 | NPR/MS2 |
| 64 | 9.66 | Quercetin O−hexoside 4 | C21H20O12 | 464.096 | −1.07 | 463.0886 | 463.1; 301.0; 151.0; 178.9; 300.0; 121.0 | 465.1027 | 303.0; 465.1; 85.0; 97.0; 153.0; 61.0 | PR in [31]/MS2 | |
| 65 | 9.73 | Ferulic acid | C10H10O4 | 194.0579 | −0.21 | 193.0504 | 193.0; 161.0; 134.0; 132.0; 133.0; 104.0 | PR in [8,45]/MS2 | |||
| 66 | 9.84 | 4−hydroxy−3−(2−hydroxy−3− isopentenyl) acetophenone 1 |
C13H16O3 | 220.1095 | 1.94 | 219.1022 | 219.1; 201.1; 119.0; 218.9; 45; 189.1; 157.1; 41.0 | 196 | PR in [46]/MS | ||
| 67 | 9.90 | 4−Feruloyl−5−caffeoylquinic acid | C26H26O12 | 530.1433 | −3.1 | 529.1368 | 529.1;367.1; 353.1; 173.0; 193.1; 161.0 | NPR/MS2 | |||
| 68 | 9.98 | Quercetin coumaroylglucoside 1 | C30H26O14 | 610.1321 | −0.6 | 609.1254 | 609.1; 463.1; 300.0; 301.0; 255.0; 272.0; | 611.1393 | 147.0; 309.1; 303.0; | 208; 225; 265; 315; 360; 194; 292 | PR in [8]/MS2 |
| 69 | 10.04 | 4−hydroxy−3−(2−hydroxy−3− isopentenyl) acetophenone 2 |
C13H16O3 | 220.1095 | 1.79 | 219.1022 | 219.1; 201.1; 119.0; 218.9; 45; 143.0; 185.1; | PR in [46]/MS | |||
| 70 | 10.17 | Quercetin coumaroylglucoside 2 | C30H26O14 | 610.1319 | −1.91 | 609.1253 | 609.1; 463.1; 301.0; 300.0; 151.0; 255.0; | 204; 222; 295; 330 | PR in [8]/MS2 | ||
| 71 | 10.24 | Eriodictyol | C15H12O6 | 288.0641 | −1.76 | 287.0566 | 151.0; 287.1; 135.0; 65.0; 68.0; 41.0 | NPR/MS2 | |||
| 72 | 10.27 | Tricaffeoylquinic acid | C34H30O15 | 678.1579 | 0.89 | 677.1502 | 677.2; 515.1; 631.1; 515.1; 353.1; 179.0; 173.0; 335.1; 161.0. 191.1 | NPR/MS2 | |||
| 73 | 10.49 | Quercetin | C15H10O7 | 302.0431 | −0.59 | 301.0356 | 301.0; 179.0; 151.0; 121.0; 65.0; 63.0; 83.0; 107.0;93.0 | 303.049 | − | 192; 225 | PR in [8]/MS2 |
| 74 | 10.49 | Pinocembrin derivative | C27H32O15 | 596.1749 | 1.65 | 595.1678 | 549.2; 255.1; 279.1; 297.1 | NPR/SI | |||
| 75 | 10.50 | Tiliroside | C30H26O13 | 594.1389 | −2.71 | 593.131 | 593.1; 285.0; 284.0; 255.0; 145.0 | 595.1456 | 147.0; 309.1; 287.1; 165.1; 291.1; 91.1 | PR in [8,20]/MS2 | |
| 76 | 10.53 | Luteolin | C15H10O6 | 286.0483 | −0.46 | 285.0406 | 285.0; 151.0; 133.0; 107.0; 132.0; 63.0; 65.0; | PR in [22]/STD | |||
| 77 | 10.91 | Isorhamnetin 1 | C16H12O7 | 316.0591 | −2.45 | 315.0518 | 315.0;300.0; 271.0; 243.0; 255.0; 227.0 | 192; 225 | PR in [8,34]/MS2 | ||
| 78 | 11.06 | Naringenin | C15H12O5 | 272.0683 | 0.61 | 271.061 | 271.1; 151.0; 119.0; 65.0; 63.0; 41.0 | PR in [22,47]/MS2 | |||
| 79 | 11.12 | Gnaphaliol glucopyranoside | C19H24O9 | 396.1423 | −0.7 | 395.135 441.1419 |
233.1; 395.1; 190.9; 146.9; 189.1; 83.0; 41.0; 191.1 | 195; 335 | PR in [37]/MS | ||
| 80 | 11.34 | Naringenin isomer | C15H12O5 | 272.0683 | 0.61 | 271.061 | 271.1; 253.0; 151.0; 197.1; 63.0; 65.0; 83.0; 125.0; | 273.0751 | 227.1; 153.0; 255.1; 273.1; 199.1; | 209; 292 | PR in [8]/MS2 |
| 81 | 11.37 | Kaempferol | C15H10O6 | 286.0477 | 0.97 | 285.0405 | 285.0; 123.0; 185.1; 169.1; 159.0; 93.0; 155.1; 117.0 | PR in [8,20,22]/ MS2 |
|||
| 82 | 11.40 | Pinocembrin isomer | C15H12O4 | 256.0741 | −2.53 | 255.0669 | 255.1; 153.1; 213.0; 151.0; 171.0; 63.0; 65.0; 83.0 | NPR/MS2 | |||
| 83 | 11.63 | Isorhamnetin 2 | C16H12O7 | 316.0596 | −4.15 | 315.0525 | 315.0; 300.0; 60.7; 149.0; 271.0; 107.0; 83.0; 255.0 | PR in [8,34]/MS2 | |||
| 84 | 11.78 | Isokaempferide 1 | C16H12O6 | 300.0632 | 0.69 | 299.0559 | 299.1; 284.0; 256.0; 255.0; 239.0; 227.0; 132.0; 183.0 | 205; 290 | PR in [34]/MS2 | ||
| 85 | 11.85 | Quercetin dimethyl ether | C17H14O7 | 330.0749 | −2.53 | 329.0675 | 329.1; 314.0; 299.0; 271.0; 133.0; 215.0; | 331.0807 | 331.1; 316.1; 301.0; 273.0; 217.0; 121.0 | NPR/MS2 | |
| 86 | 11.90 | Micropyrone | C14H20O4 | 252.1345 | 0.01 | 251.1287 | 251.1; 207.1; 113.1; 151.1; 123.1; 85.1; 55.0 | PR in [36]/MS | |||
| 87 | 12.29 | Isokaempferide 2 | C16H12O6 | 300.0634 | −0.06 | 299.0567 | 299.1; 284.0; 256.0; 255.0; 239.0; 227.0; 132.0; 183.0 | 301.0707 | 301.1; 107.1; 269.2; 286.0; 285.0; 212.0 | PR in [34]/MS2 | |
| 88 | 13.16 | 3−Prenyl−4− hydroxyacetophenone |
C13H16O2 | 204.1147 | −1.45 | 203.108 | 203.1; 148.1; 71.0; 105.0 | 197; 228; 290 | PR in [46]/MS2 | ||
| 89 | 13.21 | Pinocembrin | C15H12O4 | 256.074 | −1.68 | 255.0667 | 255.1; 59.0; 151.0; 187.1; 213.0; 65.0; 83.0; 137.0 | PR in [34]/STD | |||
| 90 | 13.81 | Gnaphaliin | C17H14O6 | 314.0797 | −2.07 | 313.07 | 313.1; 298.0; 283.0; 227.0; 255.0; 199.0; 139.1; 183.0 | 315.086 | 315.1; 300.1; 285.0; 257.0; 138.9 | 210; 280 | PR in [8,34]/MS2 |
| 91 | 13.87 | Galangin methyl ether | C16H12O5 | 284.0689 | −1.44 | 283.0615 | 283.1; 268.0; 211.0; 239.0; 167.1; 195.0 | 285.075 | 285.3; 270.0; 269.0; 136.0; 241.0; 168.1 | PR in [34]/MS2 | |
| 92 | 15.53 | Heliarzanol 1 | C24H30O8 | 446.1941 | −0.8 | 445.1871 | 445.2; 279.1; 291.1; 235.1; 247.1; 205.1; 193.1; 191.1; | 210; 295 | PR in [44]/MS | ||
| 93 | 15.65 | Arzanol | C22H26O7 | 402.167 | −1.95 | 401.1606 | 401.2; 235.1; 247.1; 153.1; 191.1; 109.1; 205.1; 166.0; | 403.1743 | 249.1; 347.1; 237.1; 181.0; 403.2; 155.1; 193.0; 163.1; | PR in [8,36]/STD | |
| 94 | 16.21 | Helipyrone | C17H20O6 | 320.1258 | −1.09 | 319.1189 | 153.1; 109.1; 198.1; 41.0 | 321.1318 | 155.1; 321.1; 167.1; 139.1; 81.1; 57.0; 43.0 | 210; 230; 295 | PR in [8,36]/MS2 |
| 95 | 16.57 | Italipyrone 1 | C22H24O7 | 400.1531 | −0.66 | 399.1452 | 233.1; 399.1; 245.1; 153.1;109.1; 189.1 | 401.1601 | 401.2; 247.1; 235.1; 167.1; 187.0 | 19894 | PR in [36]/MS |
| 96 | 16.69 | 3−Methylarzanol | C23H28O7 | 416.1836 | −0.23 | 415.1769 | 415.2; 261.1; 249.1; 205.1; 180.0; 153.1; 109.1; 193.1 | 417.1909 | 263.1; 251.1; 361.1; 195.1; 155.1; 207.1; 177.1; 189.1 | 199; 225; 275 | PR in [8,48]/MS2 |
| 97 | 17.45 | Arzanol derivative | C31H36O10 | 568.2318 | −2.35 | 567.2249 | 567.2; 401.2; 413.2; 247.1; 235.1; 221.1; 191.1; 179.0 | 208; 295 | NPR/SI | ||
| 98 | 17.92 | Arzanol isomer | C22H26O7 | 402.167 | −1.95 | 401.1606 | 401.2;235.1; 153.1; 247.1; 109.1; 179.0; 166.0; 191.1 | 215; 295 | NPR/MS2 | ||
| 99 | 17.97 | Heliarzanol 2 | C24H30O8 | 446.1956 | −4.58 | 445.1888 | 445.1; 279.1; 291.1; 261.1; 109.1 | PR in [44]/MS | |||
| 100 | 18.73 | Italipyrone 2 | C22H24O7 | 400.1533 | −3.82 | 399.1455 | 233.1; 399.1; 245.1; 153.1; 109.1; 189.1 | 401.1595 | 401.2;235.1; 247.1; 193.0 | 292; 214 | PR in [36]/MS |
1—different confidence levels of identification: PR—previously reported compound and the most relevant reference, NPR—not previously reported (new compound) in H. italicum, STD—confirmed with standard comparison, MS2—confirmed with fragment pattern comparison (METLIN or literature), MS—confirmed only by exact mass match, isotopic pattern and further evaluated with in silico prediction tools (MSC score), SI—semi-identified. Underlined are fragments that were the most abundant at each collision energy.
Author Contributions
Conceptualization, D.B. and Z.J.P.; methodology, A.M.V. and K.K.; software, A.M.V., K.K.; validation, A.M.V. and K.K.; formal analysis, K.K., Z.J.P. and A.M.V.; investigation, K.K. and A.M.V.; resources, A.B.A., D.B., K.P., A.M.V. and K.K.; data curation, K.K., A.M.V. and Z.J.P.; writing—original draft preparation, K.K.; writing—review and editing, D.B., D.B.-M., K.P., A.M.V. and Z.J.P.; visualization, K.K. and Z.J.P.; supervision, D.B., Z.J.P. and D.B.-M.; project administration, A.M.V. and Z.J.P.; funding acquisition, D.B., Z.J.P. and D.B.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Slovenian Research Agency (research program P1-0386 and grant number 1000-18-1988 for junior researcher Katja Kramberger).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- 1.Herrando Moraira S., Blanco Moreno J.M., Sáez L., Galbany Casals M. Re-evaluation of Helichrysum italicum complex (Compositae: Gnaphalieae): A new species from Majorca (Balearic Islands) Collect. Bot. 2016;35:e009 [Google Scholar]
- 2.Galbany-Casals M., Sáez L., Benedí C. A taxonomic revision of Helichrysum sect. Stoechadina (Asteraceae, Gnaphalieae) Can. J. Bot. 2006;84:1203–1232. doi: 10.1139/b06-082. [DOI] [Google Scholar]
- 3.Ninčević T., Grdiša M., Šatović Z., Jug-Dujaković M. Helichrysum italicum (Roth) G. Don: Taxonomy, biological activity, biochemical and genetic diversity. Ind. Crop. Prod. 2019;138:111487. doi: 10.1016/j.indcrop.2019.111487. [DOI] [Google Scholar]
- 4.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 5.Antunes Viegas D., Palmeira-de-Oliveira A., Salgueiro L., Martinez-de-Oliveira J., Palmeira-de-Oliveira R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014;151:54–65. doi: 10.1016/j.jep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Melito S., Sias A., Petretto G.L., Chessa M., Pintore G., Porceddu A. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS ONE. 2013;8:e79043. doi: 10.1371/journal.pone.0079043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maksimovic S., Tadic V., Skala D., Zizovic I. Separation of phytochemicals from Helichrysum italicum: An analysis of different isolation techniques and biological activity of prepared extracts. Phytochemistry. 2017;138:9–28. doi: 10.1016/j.phytochem.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Pereira C.G., Barreira L., Bijttebier S., Pieters L., Neves V., Rodrigues M., Rivas R., Varela J., Custodio L. Chemical profiling of infusions and decoctions of Helichrysum italicum subsp. picardii by UHPLC-PDA-MS and in vitro biological activities comparatively with green tea (Camellia sinensis) and rooibos tisane (Aspalathus linearis) J. Pharm. Biomed. Anal. 2017;145:593–603. doi: 10.1016/j.jpba.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Kamil Hussain M., Saquib M., Faheem Khan M. Techniques for Extraction, Isolation, and Standardization of Bio-active Compounds from Medicinal Plants. In: Swamy M.K., Akhtar M.S., editors. Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices. Springer; Singapore: 2019. pp. 179–200. [Google Scholar]
- 10.Jaiswal R., Müller H., Müller A., Karar M.G.E., Kuhnert N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry. 2014;108:252–263. doi: 10.1016/j.phytochem.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Oestreich-Janzen S.H. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Elsevier; Amsterdam, The Netherlands: 2019. Chemistry of Coffee. [Google Scholar]
- 12.Clifford M.N., Wu W., Kirkpatrick J., Kuhnert N. Profiling the Chlorogenic Acids and Other Caffeic Acid Derivatives of Herbal Chrysanthemum by LC−MSn. J. Agric. Food Chem. 2007;55:929–936. doi: 10.1021/jf062314x. [DOI] [PubMed] [Google Scholar]
- 13.Vallverdú-Queralt A., Jáuregui O., Di Lecce G., Andrés-Lacueva C., Lamuela-Raventós R.M. Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC–ESI-QTOF) Food Chem. 2011;129:877–883. doi: 10.1016/j.foodchem.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Zapesochnaya G.G., Kurkin V.A., Kudryavtseva T.V., Karasartov B.S., Cholponbaev K.S., Tyukavkina N.A., Ruchkin V.E. Dicaffeolyquinic acids from Helichrysum italicum and Achillea cartilaginea. Chem. Nat. Compd. 1992;28:40–44. doi: 10.1007/BF00629790. [DOI] [Google Scholar]
- 15.Clifford M.N., Johnston K.L., Knight S., Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 16.Elsadig Karar M.G., Kuhnert N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen) J. Chem. Biol. 2016;1 doi: 10.4172/2572-0406.1000102. [DOI] [Google Scholar]
- 17.Ncube E.N., Mhlongo M.I., Piater L.A., Steenkamp P.A., Dubery I.A., Madala N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacumtissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014;8:66. doi: 10.1186/s13065-014-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clifford M.N., Kirkpatrick J., Kuhnert N., Roozendaal H., Salgado P.R. LC–MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 2008;106:379–385. doi: 10.1016/j.foodchem.2007.05.081. [DOI] [Google Scholar]
- 19.Gil M., Wianowska D. Chlorogenic acids—Their properties, occurrence and analysis. Ann. Univ. Mariae Curie Sklodowska Sect. AA Chem. 2017;72:61. doi: 10.17951/aa.2017.72.1.61. [DOI] [Google Scholar]
- 20.Mari A., Napolitano A., Masullo M., Pizza C., Piacente S. Identification and quantitative determination of the polar constituents in Helichrysum italicum flowers and derived food supplements. J. Pharm. Biomed. Anal. 2014;96:249–255. doi: 10.1016/j.jpba.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal R., Kuhnert N. How to identify and discriminate between the methyl quinates of chlorogenic acids by liquid chromatography–tandem mass spectrometry. J. Mass Spectrom. 2011;46:269–281. doi: 10.1002/jms.1889. [DOI] [PubMed] [Google Scholar]
- 22.de la Garza A.L., Etxeberria U., Lostao M.P., San Román B., Barrenetxe J., Martínez J.A., Milagro F.I. Helichrysum and Grapefruit Extracts Inhibit Carbohydrate Digestion and Absorption, Improving Postprandial Glucose Levels and Hyperinsulinemia in Rats. J. Agric. Food Chem. 2013;61:12012–12019. doi: 10.1021/jf4021569. [DOI] [PubMed] [Google Scholar]
- 23.Clifford M.N., Knight S., Kuhnert N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS(n) J. Agric. Food Chem. 2005;53:3821–3832. doi: 10.1021/jf050046h. [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves S., Moreira E., Grosso C., Andrade P.B., Valentão P., Romano A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017;54:219–227. doi: 10.1007/s13197-016-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeza G., Sarriá B., Bravo L., Mateos R. Exhaustive Qualitative LC-DAD-MSn Analysis of Arabica Green Coffee Beans: Cinnamoyl-glycosides and Cinnamoylshikimic Acids as New Polyphenols in Green Coffee. J. Agric. Food Chem. 2016;64:9663–9674. doi: 10.1021/acs.jafc.6b04022. [DOI] [PubMed] [Google Scholar]
- 26.Takenaka M., Yan X., Ono H., Yoshida M., Nagata T., Nakanishi T. Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius) J. Agric. Food Chem. 2003;51:793–796. doi: 10.1021/jf020735i. [DOI] [PubMed] [Google Scholar]
- 27.Kłeczek N., Michalak B., Malarz J., Kiss A.K., Stojakowska A. Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules. 2019;24:1614. doi: 10.3390/molecules24081614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J., Dunshea F.R., Suleria H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods. 2020;9:7. doi: 10.3390/foods9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z.-H., Guo H., Xu W.-B., Ge J., Li X., Alimu M., He D.-J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016;54:805–810. doi: 10.1093/chromsci/bmw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu B., Ji X., Zhao M., He F., Wang X., Wang Y., Liu P., Niu L. The influence of light quality on the accumulation of flavonoids in tobacco (Nicotiana tabacum L.) leaves. J. Photochem. Photobiol. B Biol. 2016;162:544–549. doi: 10.1016/j.jphotobiol.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Pietta P., Mauri P., Facino R.M., Carini M. Analysis of flavonoids by MECC with ultraviolet diode array detection. J. Pharm. Biomed. Anal. 1992;10:1041–1045. doi: 10.1016/0731-7085(91)80116-Q. [DOI] [PubMed] [Google Scholar]
- 32.Gu D., Yang Y., Abdulla R., Aisa H.A. Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012;26:83–100. doi: 10.1002/rcm.5289. [DOI] [PubMed] [Google Scholar]
- 33.Hussain F., Jahan N., Rahman K., Sultana B., Jamil S. Identification of Hypotensive Biofunctional Compounds of Coriandrum sativum and Evaluation of Their Angiotensin-Converting Enzyme (ACE) Inhibition Potential. [(accessed on 13 June 2020)]; doi: 10.1155/2018/4643736. Available online: https://www.hindawi.com/journals/omcl/2018/4643736/ [DOI] [PMC free article] [PubMed]
- 34.Wollenweber E., Christ M., Dunstan R.H., Roitman J.N., Stevens J.F. Exudate flavonoids in some Gnaphalieae and Inuleae (Asteraceae) Z. Nat. C J. Biosci. 2005;60:671–678. doi: 10.1515/znc-2005-9-1003. [DOI] [PubMed] [Google Scholar]
- 35.Karasartov B.S., Kurkin V.A., Zapesochnaya G.G. Coumarins and flavonoids of the flowers of Helichrysum italicum. Chem. Nat. Compd. 1992;28:504–505. doi: 10.1007/BF00630665. [DOI] [Google Scholar]
- 36.Appendino G., Ottino M., Marquez N., Bianchi F., Giana A., Ballero M., Sterner O., Fiebich B.L., Munoz E. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol alpha-Pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007;70:608–612. doi: 10.1021/np060581r. [DOI] [PubMed] [Google Scholar]
- 37.D’Abrosca B., Buommino E., D’Angelo G., Coretti L., Scognamiglio M., Severino V., Pacifico S., Donnarumma G., Fiorentino A. Spectroscopic identification and anti-biofilm properties of polar metabolites from the medicinal plant Helichrysum italicum against Pseudomonas aeruginosa. Bioorg. Med. Chem. 2013;21:7038–7046. doi: 10.1016/j.bmc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Lin H., Zhu H., Tan J., Wang H., Wang Z., Li P., Zhao C., Liu J. Comparative Analysis of Chemical Constituents of Moringa oleifera Leaves from China and India by Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry. Molecules. 2019;24:942. doi: 10.3390/molecules24050942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molnar M., Jerković I., Suknović D., Bilić Rajs B., Aladić K., Šubarić D., Jokić S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules. 2017;22:348. doi: 10.3390/molecules22030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazazic M. Department of Chemistry, Faculty of education, Džemal Bijedić University of Mostar, Bosnia and Herzegovina Antioxidant activity of water extracts of some medicinal plants from Herzegovina region. Int. J. Pure Appl. Biosci. 2016;4:85–90. doi: 10.18782/2320-7051.2251. [DOI] [Google Scholar]
- 41.Višnjevec A.M., Ota A., Skrt M., Butinar B., Možina S.S., Cimerman N.G., Nečemer M., Arbeiter A.B., Hladnik M., Krapac M., et al. Genetic, Biochemical, Nutritional and Antimicrobial Characteristics of Pomegranate (Punica granatum L.) Grown in Istria. Food Technol. Biotechnol. 2017;55:151–163. doi: 10.17113/ftb.55.02.17.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miklavčič Višnjevec A., Baker P., Charlton A., Preskett D., Peeters K., Tavzes Č., Kramberger K., Schwarzkopf M. Developing an olive biorefinery in Slovenia: Analysis of phenolic compounds found in olive mill effluents. (unpublished) [DOI] [PMC free article] [PubMed]
- 43.Zegura B., Dobnik D., Niderl M.H., Filipič M. Antioxidant and antigenotoxic effects of rosemary (Rosmarinus officinalis L.) extracts in Salmonella typhimurium TA98 and HepG2 cells. Environ. Toxicol. Pharmacol. 2011;32:296–305. doi: 10.1016/j.etap.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Taglialatela-Scafati O., Pollastro F., Chianese G., Minassi A., Gibbons S., Arunotayanun W., Mabebie B., Ballero M., Appendino G. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J. Nat. Prod. 2013;76:346–353. doi: 10.1021/np3007149. [DOI] [PubMed] [Google Scholar]
- 45.Zapesochnaya G.G., Dzyadevich T.V., Karasartov B.S. Phenolic compounds of Helichrysum italicum. Chem. Nat. Compd. 1990;26:342–343. doi: 10.1007/BF00597869. [DOI] [Google Scholar]
- 46.Sala A., Recio M.C., Giner R.M., Máñez S., Ríos J.L. New acetophenone glucosides isolated from extracts of Helichrysum italicum with antiinflammatory activity. J. Nat. Prod. 2001;64:1360–1362. doi: 10.1021/np010125x. [DOI] [PubMed] [Google Scholar]
- 47.Facino R.M., Carini M., Mariani M., Cipriani C. Anti-Erythematous and Photoprotective Activities in Guinea Pigs and in Man of Topically Applied Flavenoids from Helichysum italicum G. Don. Acta Ther. 1988;14:323–346. [Google Scholar]
- 48.Rosa A., Deiana M., Atzeri A., Corona G., Incani A., Melis M.P., Appendino G., Dessì M.A. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone–phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007;165:117–126. doi: 10.1016/j.cbi.2006.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.