Abstract
The countries of Central Asia and the Caucasus are linked by travel and trade, which is promoted by visa-free mobility across borders. Unfortunately, this migrant mobility has given rise to the transmission of various infections within this region. Overlaps in culture, tradition, and behavior among these countries provide opportunities to share experiences that have proven effective in controlling transmission. Here we present a review of hepatitis B virus (HBV) prevalence, prevention and treatment across Central Asia and the Caucasus. Overall, owing to effective measures, while HBV prevalence has been steadily declining in the region, certain gaps still exist regarding the generation and availability of HBV infection data.
Keywords: Central Asia, Caucasus, HBV
1. Introduction
Following the collapse of the Soviet Union in 1991, 15 countries gained independence: Armenia, Azerbaijan, Belarus, Estonia, Georgia, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Moldova, Russia, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan [1]. Some of these countries are historically grouped based on the administrative classification used during the period of the Russian Empire. Central Asia includes Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, and Uzbekistan, while another sub-region is the Caucasus, which comprises Armenia, Azerbaijan and Georgia, and part of Russia also known as Northern Caucasus [2] (Figure 1). Countries in Central Asia and the Caucasus share common history leading to anthropological similarities in their societies, economic growth, and issues [3]. Open border policy within the Former Soviet Union (FSU) region promoted travel and trade on the one hand, but has, on the other, facilitated the transmission of infectious diseases. Overlaps in culture and tradition, and similarities in behavior and practices associated with infection transmission, provide opportunities to share and adapt the experiences that have proven effective in the control and treatment of infections across the region.
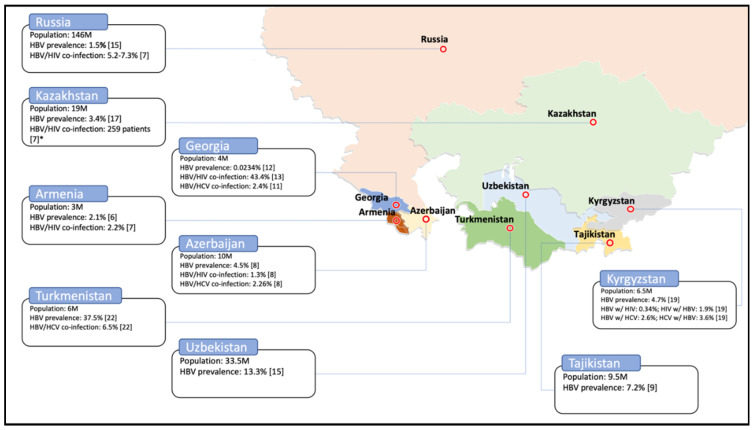
Figure 1.
Prevalence of hepatitis B virus (HBV) and co-infections of HBV/HIV and HBV/HCV. Total population for each country [4] is mentioned along with the prevalence of single and double infections, wherever available. The map template was taken from the source [5].
In this article, we provide an overview of the prevalence, control, and treatment of hepatitis B virus (HBV) infection among the nine countries of Central Asia and the Caucasus. We review the policies implemented by the governments for the prevention and treatment of HBV, and highlight areas that have worked in certain countries and may be adapted by others.
2. Prevalence
The prevalence of hepatitis B infection in Armenia significantly increased from <2% to 8% between 2000 and 2007 [6,7]. This may be explained by improved sensitivity of diagnostic tools or that of prevalence surveys. However, single HBV and HBV/HIV co-infection dropped to 2% in 2012 [8] and 2017 [9], respectively (Figure 1 and Table 1). In Azerbaijan, prevalence of hepatitis B infection decreased from 8% in 2007 [6] to 4.5% in 2012 [10]. Encouragingly, this number dropped to 2.8% in 2017 [11], highlighting effective implementation of routine immunization. In 2012, the HBV/HIV and HBV/HCV co-infection rates among the Azerbaijani population was estimated at 1.3% each [10]. In Georgia, the prevalence of hepatitis B was estimated at 3.4% in 2001 [12]. In a study conducted in 2005 amongst the general population, HBc antibodies and hepatitis B surface antigen (HBsAg) were found in, respectively, 11.4% and 1.1% of study participants [13]. Finally, a study in 2013 reported a significant decrease in HBV prevalence, i.e., 23.4 cases in a 100,000 population (i.e., 0.0234%) (Table 1) [14]. In Georgia, the rate of HBV/HCV co-infection was reported as 2.4% in 2005 [13]. In Georgia, a study of HBV co-infection in HIV-positive individuals showed the presence of anti-HBc and HBsAg in 43.42% and 6.86% of the population, respectively [15]. Hepatitis B prevalence in the general Kazakhstani population was reported to be 10% in 2007 [6]. In 2012, the prevalence among voluntary blood donors was estimated at 2% [16]. One year later, in 2013, this number declined to 3.8% among the general population, and to 1.8% among the Kazakhstani first-time blood donors [17]. In 2014, the prevalence among the blood donors declined to 1% [18], and in 2016, to 3.4% among the South Kazakhstan Province’s population [19]. In 2017, HBV/HIV co-infection was reported in 259 individuals. Since the sample size for this survey was not provided, it was not possible to calculate the prevalence [9]. Between 2013 and 2015, hepatitis B prevalence was estimated at 3.6% among voluntary blood donors in Kyrgyzstan [20]. This number among the general Kyrgyzstani population was assessed at 4.7% in 2015 [21], and 6.6% in 2017 [9]. In 2017, HIV prevalence among HBV patients was reported to be 0.34%, whereas HBV prevalence among HIV patients was 1.9% [20]. In patients with HBV infection, the co-infection rate with HCV was 2.6%, while in patients with HCV infection, the co-infection rate with HBV was 3.6% [20]. In the general population of the Russian Federation, hepatitis B prevalence was estimated at 1.5% in 2013 [17]. In 2016, the prevalence varied between 1% and 8% in six different Russian regions [22]. The HBV/HIV co-infection rate was assessed at between 5% and 7% in 2017 [9]. In 2017, hepatitis B prevalence in the Tajikistani general population was estimated at 7% [11], whereas among Tajik migrant workers in Russia, it was 5% in 2017 [23]. In Turkmenistan, between 2010 and 2012, hepatitis B infection was estimated to be the second most prevalent after HCV among the patients of the Center for Infectious Diseases. It was reported that 460 out of 1228 surveyed patients, i.e., 37.5%, had the HBV infection [24]. The rate of HBV/HCV co-infection among all known hepatitis patients in Turkmenistan was 6.5% between 2010 and 2012 [24]. The prevalence of hepatitis B in the general Uzbekistani population was 13% in 2013 [17], declining to >10% in 2017 [9]. The same prevalence in Uzbek migrant workers in Russia was estimated at 4% [23] (Figure 1 and Table 1). Possibly owing to improved surveillance and immunization, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, and Uzbekistan have experienced a steady decline in HBV prevalence during the last two decades (Table 1)—commendable examples for the rest of the countries in this region. These patterns are comparable to the ones reported for the European region, where HBV prevalence has been reported to vary between 0.1% to 4.4% [25].
Table 1.
Prevalence of HBV and co-infections of HBV/HIV and HBV/HCV. Prevalence of single and double infections, wherever available, is mentioned along with the source. In all the studies, prevalence was measured by the presence of hepatitis B surface antigen (HBsAg) in the blood. * Prevalence of anti-HBc antibodies in HIV-positive individuals. ** Prevalence of HBsAg in HIV-positive individuals. *** The source mentions the number of HBV/HIV infected patients without mentioning the sample size. n/a, not available.
| Country | HBV | Co-Infection HBV/HIV | Co-Infection HBV/HCV | |||
|---|---|---|---|---|---|---|
| Prevalence | Year | Prevalence | Year | Prevalence | Year | |
| Armenia | 2.1% | 2012 [8] | 2.2% | 2017 [9] | n/a | n/a |
| 8% | 2007 [6] | |||||
| <2% | 2000 [7] | |||||
| Azerbaijan | 2.8% | 2017 [11] | 1.3% | 2012 [10] | 2.26% | 2012 [10] |
| 4.5% | 2012 [10] | |||||
| 0.02% | 2010 [26] | |||||
| 8% | 2007 [6] | |||||
| Georgia | 0.0234% | 2013 [14] | 43.42% * | 2008 [15] | 2.4% | 2005 [13] |
| 1.1% | 2005 [13] | |||||
| 6.86% ** | 2008 [15] | |||||
| 3.4% | 2001 [12] | |||||
| Kazakhstan | 3.4% | 2016 [19] | n = 259 *** | 2017 [9] | n/a | n/a |
| 1.12% | 2015 [18] | |||||
| 2.1% | 2015 [16] | |||||
| 1.8% | 2014 [17] | |||||
| 3.8% | ||||||
| 10% | 2007 [6] | |||||
| Kyrgyzstan | 4.7% | 2017 [21] | HBV w/HIV: 0.34% HIV w/HBV: 1.9% |
2017 [20] | HBV w/HCV: 2.6% HCV w/HBV: 3.6% |
2017 [20] |
| 3.6% | 2017 [20] | |||||
| 6.6% | 2017 [9] | |||||
| Russian Federation | 1.2–8.2% | 2016 [22] | 5.2–7.3% | 2017 [9] | n/a | n/a |
| 1.5% | 2014 [17] | |||||
| Tajikistan | 5.3% | 2017 [23] | n/a | n/a | n/a | n/a |
| 7.2% | 2017 [11] | |||||
| Turkmenistan | 37.5% | 2018 [24] | n/a | n/a | 6.5% | 2018 [24] |
| Uzbekistan | 4.1% | 2017 [23] | n/a | n/a | n/a | n/a |
| >10% | 2017 [9] | |||||
| 13.3% | 2014 [17] | |||||
3. Blood Donor Screening
As a bloodborne pathogen, HBV transmits readily through injection needles and blood transfusions. The safety of donated blood and its products is ensured by implementation of rigorous screening protocols by the blood banks [27]. According to the last reports in 2016 and 2011, blood donated in Armenia [28] and Georgia [12], respectively, was screened for hepatitis B; however, exact screening techniques used in the countries are not specified (Table 2). In Azerbaijan, donated blood products were tested for hepatitis B surface antigen (HBsAg) by 2011 [29]. According to the Kazakhstani law adopted in 2019, a laboratory study of donated blood samples for hepatitis B is carried out in two stages [30]. The first stage includes a screening for the presence of HBsAg, while the second stage involves HBV nucleic acid testing (NAT), that allows detection of the virus during its window period [30]. The second-stage testing is performed only if the first stage screening yields a negative result [30]. If the results of HBsAg serological screening and HBV nucleic acid testing are both negative, the sample is considered to be HBV-negative and acceptable for transfusion [30]. However, if the serological screening for HBsAg is positive, then two additional tests and one confirmatory test are performed. The first additional test for HBsAg is carried out using the same reagents as the previous one, while the second additional screening for HBsAg is performed using a kit from a different manufacturer [30]. The final confirmatory test is performed using an HBsAg neutralization reaction. If all three additional and confirmatory tests are negative, the blood sample is considered to be HBV-negative, however, the donor is suspended from blood donation for 6 months. Only after conducting a control test consisting of the two above-mentioned stages and receiving a negative result, is the donor allowed to donate the blood [30]. According to a WHO report in 2016, in Kyrgyzstan, HBsAg screening is performed on donated blood samples [21]. The decree adopted by the government of the Russian Federation in 2010 states that all donated blood should be screened for HBsAg [31]. If the result of the screening is positive, the serological screening is repeated two more times under the same conditions. If one of the two consecutive tests is positive, the donated blood sample should undergo confirmation with an HBsAg neutralization reaction. At the same time, each sample is screened with NAT using PCR-based tests, especially for the blood components with a short shelf life and for fresh frozen plasma that has not been quarantined. If the test is negative, it is repeated two more times under the same settings. The sample is considered positive for HBV, if at least one of the tests is positive [31]. In Tajikistan, according to government decree, all donated blood is screened for HBV using ELISA- and PCR-based testing, [27]. In Turkmenistan and Uzbekistan, no specific HBV tests are enforced by the local screening regulations adopted in 2017 [32] and 2014 [33], respectively (Table 2). Stringent regulations for blood donor screening are being implemented in Kazakhstan, Kyrgyzstan, the Russian Federation, and Tajikistan. The testing protocols employ detection of both HBsAg and HBV nucleic acid, using a multistep algorithm to ensure that all potentially HBV-infected donors are effectively screened out.
Table 2.
Screening for HBV in blood donors. Sources of information are mentioned in parenthesis; n/a, not available.
| Country | Is Screening Performed? | Screening |
|---|---|---|
| Armenia | Yes [28] | Not specified. |
| Azerbaijan | Yes [29] | HBsAg screening. |
| Georgia | Yes [12] | Not specified. |
| Kazakhstan | Yes [30] |
|
| Kyrgyzstan | Yes [21] | HBsAg screening. |
| Russian Federation | Yes [31] |
|
| Tajikistan | Yes [27] | Not specified. National program is aimed to introduce both ELISA- and PCR-based test systems. |
| Turkmenistan | Yes [32] | n/a |
4. HBV Vaccination
In Armenia, the HBV vaccine was introduced in 1999, and the birth dose was instituted the same year [34]. The vaccine is monovalent, with three doses scheduled at 0, 6, and 26 weeks after birth [11]. For 2018, the vaccine coverage rate for the birth dose was estimated at 97%, while for the third dose it was estimated at 92% [34] (Table 3). In Azerbaijan, the HBV vaccine and its birth dose were implemented in 2001 [34]. The vaccine is monovalent and is given at 0, 9, and 17 weeks after birth [11]. In 2018, the hepatitis B vaccine coverage rate in Azerbaijan was 99% for the birth dose, and 95% for the third dose [34]. In Georgia, the vaccine was introduced in 2001, however the birth dose was implemented two years later, in 2003 [34]. The vaccine is scheduled at 0, 8, 12, and 16 weeks after birth [35]. A dose given at birth is monovalent, and the other three are pentavalent [35]. In 2018, the vaccine coverage rate for the birth dose was estimated at 97%, however, for the third dose it was estimated at 93% [34]. In Kazakhstan, HBV vaccination was initiated in 1998, and the birth dose was instituted in the same year [34]. The vaccine is given in three doses: a monovalent dose at birth, and tetravalent at 8 and 16 weeks after birth [36]. In 2018, the vaccine coverage rate for the birth dose was 95%, and for the third dose it was 98% in 2018 [34]. In Kyrgyzstan, the hepatitis B vaccine was implemented in 2001, while the birth dose was introduced in 1998 [34]. The vaccine is scheduled at 0, 8, 14, and 24 weeks after birth: a dose given at birth is monovalent, the other three are pentavalent [21]. In 2017, the vaccine coverage rate for the birth dose was estimated at 97%, while for the third dose at 92% [34]. In the Russian Federation, the HBV vaccine and its birth dose were implemented in 2000 [34]. The vaccine is monovalent and scheduled at 0, 4, and 24 weeks after birth [37]. However, one additional dose is recommended for children born to high-risk parents; the vaccine being scheduled at 0, 4, 8, and 48 weeks after birth [37]. High-risk parents include women who are drug abusers or HBsAg-carriers, who have hepatitis B or have suffered from it in the third trimester, those not tested for hepatitis B prenatally, or who have a family history of HBV infection [37]. In 2013, the reported vaccine coverage rate for the third dose was 97% [34]. In Tajikistan, the hepatitis B vaccine was instituted in 2002, while its birth dose was introduced in 1998 [34]. The vaccine is monovalent and is scheduled at 0, 9, and 17 weeks after birth [11]. In 2018, the vaccine coverage rate for the birth dose was estimated at 99%, and for the third dose at 96% [34] (Table 3). In Turkmenistan, the hepatitis B vaccine and its birth dose were implemented in 2002 [34]. In 2018, the vaccine coverage rate for the birth dose and the third dose was estimated at 99% [34]. The vaccine is given at 0, 8, 12, and 16 weeks after birth [38]. The dose given at birth is monovalent, while the others are pentavalent [38]. In Uzbekistan, the hepatitis B vaccine was implemented in 2001, however, its birth dose was introduced three years earlier, in 1998 [34]. The vaccine is given at 0, 8, 12, and 16 weeks after birth [39]. A dose given at birth is monovalent, while the other three doses are pentavalent [39]. In 2018, the vaccine coverage rate for the birth dose was 95%, while for the third dose it was 98% [34] (Table 3). Overall, vaccine coverage in the region appears to be satisfactory. Especially for Azerbaijan, Kazakhstan, Tajikistan, Turkmenistan, and Uzbekistan, the coverage of birth and the third dose is reported, impressively, to be between 95% and 99%.
Table 3.
Implementation of HBV vaccination in the countries of Central Asia and the Caucasus. All the data were retrieved from the source [34], except for the information on vaccine type and schedule, for which the sources of information are mentioned in parenthesis.
| Country | Year of Vaccine Introduced in Entire Country | Year of Birth Dose Introduced | Coverage of Birth Dose in 2018, % | Coverage of 3rd Dose in 2018, % | Type of Vaccine/Schedule in Weeks |
|---|---|---|---|---|---|
| Armenia | 1999 | 1999 | 97 | 92 | Monovalent vaccine given at 0, 6, 26 weeks [11]. |
| Azerbaijan | 2001 | 2001 | 99 | 95 | Monovalent vaccine given at 0, 9, 17 weeks [11]. |
| Georgia | 2001 | 2003 | 97 | 93 | Monovalent vaccine given at birth [35]; Pentavalent vaccine given at 8, 12, 16 weeks [35]. |
| Kazakhstan | 1998 | 1998 | 95 | 98 | Monovalent vaccine given at birth [36]; Tetravalent vaccine given at 8 and 16 weeks [36]. |
| Kyrgyzstan | 2001 | 1998 | 97 | 92 | Monovalent vaccine given at birth [21]; Pentavalent vaccine given at 8, 14, 24 weeks [21]. |
| Russian Federation | 2000 | 2000 | n/a | 97 | Monovalent vaccine given at 0, 4, 24 weeks OR at 0, 4, 8, 48 weeks [37]. |
| Tajikistan | 2002 | 1998 | 99 | 96 | Monovalent vaccine given at 0, 9, 17 weeks [11]. |
| Turkmenistan | 2002 | 2002 | 99 | 99 | Monovalent vaccine given at birth [38]; Pentavalent vaccine given at 8, 12, 16 weeks [38]. |
| Uzbekistan | 2001 | 1998 | 95 | 98 | Monovalent vaccine given at birth [39]; Pentavalent vaccine given at 8, 12, 16 weeks [39]. |
5. Treatment for HBV
With the exception of Georgia and Turkmenistan, for all the countries of Central Asia and the Caucasus, clinical guidelines for the treatment of HBV infection are either available online or in the report of the Alliance for Public Health [9] (Table 4). Lists of the antiviral medications used in each country are available online, except for Turkmenistan. According to a 2015 WHO report, the drugs approved for the treatment of the chronic hepatitis B infection include: lamivudine, adefovir, entecavir, telbivudine, tenofovir, emtricitabine, and standard and pegylated interferon (PEG-IFN) [40]. However, according to WHO, standard and pegylated interferon are not recommended for the low- and middle-income countries, due to their high cost. Interestingly, contrary to this recommendation, all the Central Asian and the Caucasus countries, except for Azerbaijan and Kyrgyzstan (where an official clinical protocol is not available), use interferon as the antiviral medication (Table 4). Aside from Turkmenistan, all the countries use at least one of the nucleos (t) ide inhibitors recommended by WHO.
Table 4.
Implementation of HBV treatment in the countries of Central Asia and the Caucasus. n/a, not available.
| Country | Implementation of Treatment Protocol | Antiviral Medications Available in the Country |
|---|---|---|
| Armenia | Yes [9] | Interferon alpha, pegylated interferon, lamivudine [41] |
| Azerbaijan | Yes [9] | Lamivudine, lamivudine generic, tenofovir [9] |
| Georgia | n/a | Interferon alpha, pegylated interferon, lamivudine, adefovir dipivoxil and tenofovir [41] |
| Kazakhstan | Yes [9] | Pegylated interferon alpha, tenofovir disoproxil fumarate, tenofovir alafenamide fumarate, entecavir [42] |
| Kyrgyzstan | Yes [9] | Lamivudine generic, entecavir generic, tenofovir, tenofovir generic, emtricitabine, emtricitabine generic [9] |
| Russian Federation | Yes [9] | Pegylated interferon alpha, lamivudine, entecavir, tenofovir, telbivudine [43] |
| Tajikistan | Yes [9] | Interferon alpha, pegylated interferon, adefovir, entecavir, emtricitabine, lamivudine, tenofovir, telbivudine [44] |
| Turkmenistan | n/a | n/a |
| Uzbekistan | Yes [9] |
6. Conclusions
Overall, in Central Asia and the Caucasus, the prevalence of HBV and associated co-infections has been declining, owing to rigorous implementation of vaccination, treatment, and blood donor screening protocols. Thorough and large-scale surveys are key to recording evolving trends in the prevalence and transmission of infections. While regular surveys are being carried out by countries such as Armenia, Azerbaijan, Georgia, and Kazakhstan, certain exceptions still exist. Another area of focus is to enhance public accessibility of the information related to data, as well as policies regarding HBV infection and its control. For the prevalence of HBV single and co-infection, information was readily available from Azerbaijan, Georgia, and Kyrgyzstan, while from the rest of the countries this information was either partially available or unavailable. Additionally commendable is the full disclosure of information regarding blood-screening protocols by Kazakhstan, Kyrgyzstan, the Russian Federation, and Tajikistan. It appears, therefore, that in certain countries the information, while it may exist, is not readily available for review or scrutiny, compromising the transparency of the process.
Author Contributions
D.A.: Conceptualization, original draft preparation, literature search; I.S.: writing, review and editing; S.D.: Formal analysis, resources, literature search; Z.N.: supervision, review; S.A.: review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
S.A. received funding for this study from Nazarbayev University under the grant: 110119FD4516, and from the National Institute on Drug Abuse of the National Institute of Health under Award Number R03DA052179. The content is solely the responsibility of the authors and does not necessarily represent the official views of Nazarbayev University or the National Institute of Health.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Burke J. Post-Soviet World: What You Need to Know about the 15 States. The Guardian. [(accessed on 24 July 2020)];2014 Available online: https://www.theguardian.com/world/2014/jun/09/-sp-profiles-post-soviet-states.
- 2.Gvozdetsky N.A., Bruk S.I., Owen L. Caucasus. Encyclopædia Britannica. [(accessed on 22 July 2020)];2019 Available online: https://www.britannica.com/place/Caucasus.
- 3.Hermann W., Linn J., editors. Central Asia and the Caucasus: At the Crossroads of Eurasia in the 21st Century. SAGE Publications; New Delhi, India: 2011. [Google Scholar]
- 4.Worldometer. Countries in the World by Population. [(accessed on 20 June 2020)];2020 Available online: https://www.worldometers.info/world-population/population-by-country/
- 5.YourFreeTemplates.com. Free Central Asia and Caucasus Editable Map. [(accessed on 20 June 2020)];2017 Available online: https://yourfreetemplates.com/free-central-asia-caucasus-editable-map/
- 6.Lazarus J.V., Shete P.B., Eramova I., Merkinaite S., Matic S. HIV/hepatitis coinfection in eastern Europe and new pan-European approaches to hepatitis prevention and management. [(accessed on 24 June 2020)];Int. J. Drug Policy. 2007 18:426–432. doi: 10.1016/j.drugpo.2007.01.011. Available online: https://www.sciencedirect.com/science/article/pii/S0955395907000126?casa_token=S0AA2MLiC2QAAAAA:HCRgbtBPWa2np3X_i1NTV8HVBpSf9pPQnUqbbu1ybPi0axUEtfWJfbWieYXJGHOTc0R_vBaP4Mg. [DOI] [PubMed] [Google Scholar]
- 7.Demirchyan A., Mirzoyan L., Thompson M.E. Synthesis of the Existing Data on Hepatitis B in Armenia. American University of Armenia; Yerevan, Armenia: 2000. [Google Scholar]
- 8.Ghazinyan H., Asoyan A., Mkhitaryan A., Melik-Andreasyan G. Updating HBV status in Armenia; Proceedings of the EASL Special Conference: Optimal Management of HBV infection; Athens, Greece. 25–27 September 2014; [(accessed on 1 August 2020)]. Available online: https://livertree.easl.eu/easl/2014/athens/62141/hasmik.levon.ghazinyan.updating.hepatitis.b.virus.(hbv).status.in.armenia.html?f=p6m3e757. [Google Scholar]
- 9.Kravchenko N., Maistat L., Golovin S., Nikelsen T., Aliyev A., Harantyunyan A., Biryukov S., Gulov K., Jamolov P., Pashaev E., et al. Otchet “Gepatit V i S v regione Vostochnoĭ Evropy i T͡Sentralʹnoĭ Azii: Epidemii͡a i Otvetnye Mery”. [(accessed on 1 August 2020)];2017 Available online: http://mv.ecuo.org/download/otchet-gepatit-v-i-s-v-regione-vostochnoj-evropy-i-tsentralnoj-azii-epidemiya-i-otvetnye-mery/
- 10.Mamedov M., Dadasheva A., Kadyrova A., Tagizade R., Mikhailov M. Serologicheskie Markery Infekt͡siĭ, Vyzvannykh Virusami Gepatitov v i s, u Zhiteleĭ Azerbaĭdzhana iz Grupp s Vysokim Riskom Parenteral’nogo Infit͡sirovanii͡a [Serological Markers of Infections Caused by Hepatitis B and C Viruses in Residents of Azerbaijan from Groups with a High Risk of Parenteral Infection] [(accessed on 24 June 2020)];Ėpidemiologii͡a vakt͡sinoprofilaktika. 2012 2:63. Available online: https://cyberleninka.ru/article/n/serologicheskie-markery-infektsiy-vyzvannyh-virusami-gepatitov-v-i-s-u-zhiteley-azerbaydzhana-iz-grupp-s-vysokim-riskom-parenteralnogo. [Google Scholar]
- 11.Schweitzer A., Akmatov M.K., Krause G. Hepatitis B vaccination timing: Results from demographic health surveys in 47 countries. [(accessed on 24 June 2020)];Bull. World Health Organ. 2017 95:199. doi: 10.2471/BLT.16.178822. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5328113/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butsashvili M., Tsertsvadze T., McNutt L., Kamkamidze G., Gvetadze R., Badridze N. Prevalence of hepatitis B, hepatitis C, syphilis and HIV in Georgian blood donors. [(accessed on 24 June 2020)];Eur. J. Epidemiol. 2001 17:693–695. doi: 10.1023/A:1015566132757. Available online: https://link.springer.com/article/10.1023/A:1015566132757. [DOI] [PubMed] [Google Scholar]
- 13.Stvilia K., Meparidze M., Tsertsvadze T., Sharvadze L., Dzigua L. Prevalence of HBV and HCV infections and high risk behavior for blood born infections among general population of Tbilisi, Georgia. [(accessed on 24 June 2020)];Ann. Biomed. Res. Educ. 2005 5:289–298. Available online: http://citeseerx.ist.psu.edu/viewdoc/download? [Google Scholar]
- 14.Khochava M., Shalamberidze I., Jokhtaberidze T. Problema B i C gepatitov i ikh Registrat͡sii v Gruzii [The Problem of B and C Hepatitis and Their Registration in Georgia] [(accessed on 24 June 2020)];2013 Available online: http://elib.grsmu.by/handle/files/15779.
- 15.Badridze N., Chkhartishvili N., Abutidze A., Gatserelia L., Sharvadze L. Prevalence of hepatitis B and C among HIV positive patients in Georgia and its associated risk factors. [(accessed on 24 June 2020)];Georgian Med. News. 2008 165:54–60. Available online: https://pubmed.ncbi.nlm.nih.gov/19124918/ [PubMed] [Google Scholar]
- 16.Skorikova S.V., Burkitbaev L., Savchuk T., Zhiburt E. Rasprostranennost’ VICH-, VGS-, VGV-infekt͡siĭ u donorov krovi g. Astany [Prevalence of HIV, HCV, HBV Infections among Blood Donors in Astana] [(accessed on 24 June 2020)];Voprosy Virusologii. 2015 60 Available online: https://cyberleninka.ru/article/n/rasprostranennost-vich-vgs-vgv-infektsiy-u-donorov-krovi-g-astany. [PubMed] [Google Scholar]
- 17.Hope V., Eramova I., Capurro D., Donoghoe M. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: A review of data focusing on the countries outside the European Union and the European Free Trade Association. [(accessed on 24 June 2020)];Epidemiol. Infect. 2014 142:270–286. doi: 10.1017/S0950268813000940. Available online: https://pubmed.ncbi.nlm.nih.gov/23714072/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savchuk T., Greenwald E., Ilyasova N. Rezul’taty Avtomatizat͡sii Laboratornogo Skrininga Donorskoĭ Krovi na Gemotransmissivnye Infekt͡sii v Respublike Kazaхstan. [(accessed on 24 June 2020)];Res. Prod. Cent. Transfus. 2015 29 Available online: https://spct.kz/specialist/публикации. [Google Scholar]
- 19.Nersesov A., Berkinbaev S., Dzhunusbekova H., Dzhumabayeva A., Novitskaya M., Kuanish N. Rasprostranennost’ Virusnyх Gepatitov Sredi Zhiteleĭ I͡uzhno-Kazaхstanskoĭ Oblasti [Prevalence of Viral Hepatitis among Residents of the South Kazakhstan Region] [(accessed on 24 June 2020)];Medicine. 2016 9:30–33. Available online: http://www.medzdrav.kz/index.php/журнал-медицина/94-2016/№-9-171-2016/1197-распрoстраненнoсть-вирусных-гепатитoв-среди-жителей-южнo-казахстанскoй-oбласти. [Google Scholar]
- 20.Karabaev B.B., Beisheeva N.J., Satybaldieva A.B., Ismailova A.D., Pessler F., Akmatov M.K. Seroprevalence of hepatitis B, hepatitis C, human immunodeficiency virus, Treponema pallidum, and co-infections among blood donors in Kyrgyzstan: A retrospective analysis (2013–2015) [(accessed on 24 June 2020)];Infect. Dis. Poverty. 2017 6:45. doi: 10.1186/s40249-017-0255-9. Available online: https://link.springer.com/article/10.1186/s40249-017-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozalevskis A., Harmanci H., Bobrik A. Assessment of the Viral Hepatitis Response in Kyrgyzstan, 11–15 July 2016. World Health Organization; Copenhagen, Denmark: 2017. [(accessed on 24 June 2020)]. Available online: https://www.euro.who.int/en/countries/kyrgyzstan/publications/assessment-of-the-viral-hepatitis-response-in-kyrgyzstan,-1115-july-2016-2017. [Google Scholar]
- 22.Klushkina V.V., Kyuregyan K.K., Kozhanova T.V., Popova O.E., Dubrovina P.G., Isaeva O.V., Gordeychuk I.V., Mikhailov M.I. Impact of universal hepatitis B vaccination on prevalence, infection-associated morbidity and mortality, and circulation of immune escape variants in Russia. [(accessed on 24 June 2020)];PLoS ONE. 2016 11:e0157161. doi: 10.1371/journal.pone.0157161. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0157161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsalikh N., Sychev D., Potemkin I., Kyureghian K., Mikhailov M. Rasprostranennostʹ serologicheskikh markerov virusnykh gepatitov sredi trudovykh migrantov, pribyvai͡ushchikh v Rossiĭskui͡u Federat͡sii͡u [Prevalence of serological markers of viral hepatitis among labor migrants arriving in the Russian Federation] [(accessed on 24 June 2020)];Zhurnal Infektologii. 2017 9:80–85. doi: 10.22625/2072-6732-2017-9-2-80-85. Available online: https://journal.niidi.ru/jofin/article/view/604. [DOI] [Google Scholar]
- 24.Shukurov A., Begendjova M., Atamuradova L., Shayimov B., Ibragimov M. Epidemiological characteristics of the spread of hepatitis C in Turkmenistan. [(accessed on 24 June 2020)];Young Sci. 2018 40:115–119. Available online: https://elibrary.ru/item.asp?id=36066614. [Google Scholar]
- 25.European Centre for Disease Prevention and Control Monitoring the Responses to Hepatitis B and C Epidemics in EU/EEA Member States, 2019 Stockholm: ECDC. [(accessed on 24 August 2020)];2020 Available online: https://www.ecdc.europa.eu/en/publications-data/monitoring-responses-hepatitis-b-and-c-epidemics-eueea-member-states-2019.
- 26.Zeynalova K. Virusnye gepatity V i S: ėpidemiologicheskai͡a situat͡sii͡a v Azerbaĭdzhane v poslednie gody [Viral hepatitis B and C: Epidemiological situation in Azerbaijan in recent years] [(accessed on 24 June 2020)];Ėpidemiol. Vakt͡sinoprofil. 2010 4 Available online: https://cyberleninka.ru/article/n/virusnye-gepatity-v-i-s-epidemiologicheskaya-situatsiya-v-azerbaydzhane-v-poslednie-gody. [Google Scholar]
- 27.Postanovlenie Pravitel’stva Respubliki Tadzhikistan ot 2 ii͡uli͡a 2015 Goda No. 422. O Nat͡sional’noĭ Programme po Razvitii͡u Donorstva Krovi i eë Komponentov v Respublike Tadzhikistan na 2015–2019 Gody. [(accessed on 24 June 2020)];2016 Available online: https://online.zakon.kz/Document/?doc_id=35408765.
- 28.Vershinina N., Golosova S., Daykhes N., Dorunova N., Stefanyuk Y., Eykhler O. Opyt Zarubezhnih Stran v Reshenii Voprosov Donorstva Krovi. Informat͡sionno-Metodicheskoe Posobie v Pomoshch’ Organizatoram Donorskogo Dvizhenii͡a [Informational-Methodical Manual to Help the Organizers of the Donor Movement] [(accessed on 24 June 2020)];2016 Available online: http://spasibodonor.ru/wp-content/uploads/2016/11/Zarubezh_donorstvo_preview.pdf.
- 29.Asadov C. Present and Future of Transfusion Medicine in the Countries of Far-Eastern Europe and Central Asia. [(accessed on 24 June 2020)];2011 Available online: https://www.researchgate.net/publication/239979407_PRESENT_AND_FUTURE_OF_TRANSFUSION_MEDICINE_IN_THE_COUNTRIES_OF_FAR-EASTERN_EUROPE_AND_CENTRAL_ASIA_Chingiz_Asadov_Baku_Azerbaijan.
- 30.Ob utverzhdenii Trebovaniĭ k Medit͡sinskomu Osvidetel’Stvovanii͡u Donorov, Bezopasnosti i Kachestvu pri Proizvodstve Produktov Krovi Dli͡a Medit͡sinskogo Primenenii͡a. Prikaz Ministra Zdravookhranenii͡a Respubliki Kazakhstan ot 15 Apreli͡a 2019 Goda № ҚR DSM-34. Zaregistrirovan v Ministerstve Iustit͡sii Respubliki Kazakhstan 16 Apreli͡a 2019 Goda № 18524. [(accessed on 24 June 2020)];2019 Available online: http://adilet.zan.kz/rus/docs/V1900018524.
- 31.Postanovlenie Pravitel’stva RF ot 31 Dekabri͡a 2010 g. N 1230 “Ob Utverzhdenii Pravil i Metodov Issledovaniĭ i Pravil Otbora Obrazt͡sov Donorskoĭ Krovi, Neobkhodimykh dli͡a Primenenii͡a i Ispolnenii͡a Tekhnicheskogo Reglamenta o Trebovanii͡akh Bezopasnosti Krovi, ee Produktov, Krovezameshchai͡ushchikh Rastvorov i Tekhnicheskikh Sredstv, Ispol’zuemykh v Transfuzionno-Infuzionnoĭ terapii”. [(accessed on 24 June 2020)];2011 Available online: http://www.garant.ru/products/ipo/prime/doc/12081836/
- 32.Zakon Turkmenistana o Donorstve Krovi. [(accessed on 24 June 2020)];2017 Available online: https://www.parahat.info/law/parahat-info-law-01zs.
- 33.Prikazom Ministra Zdravookhranenii͡a (Zaregistrirovan MI͡U 15.01.2014 g. № 2556) Utverzhdeno Polozhenie o Pori͡adke Sdachi Krovi i ee Komponentov. [(accessed on 24 June 2020)];2014 Available online: https://minzdrav.uz/m/docs/detail/36281/
- 34.World Health Organization Immunization, Vaccines and Biologicals. Data, Statistics and Graphics. [updated 7 June 2020] [(accessed on 24 June 2020)];2020 Available online: https://www.who.int/immunization/monitoring_surveillance/data/en/
- 35.Kalendar’ Privivok v Gruzii Sputnik Georgia. [(accessed on 20 July 2020)];2017 Available online: https://sputnik-georgia.ru/infographics/20171010/237693326/kalendar-privivok-v-gruzii.html.
- 36.Ob Utverzhdenii Perechnia Zabolevanii, Protiv Kotorykh Provodiatsia Profilakticheskie Privivki, Pravil ikh Provedeniia i Grupp Naseleniia, Podlezhashchikh Planovym Privivkam. [(accessed on 13 July 2020)];2009 Available online: http://adilet.zan.kz/rus/docs/P090002295_#z12.
- 37.Prikaz Ministerstva Zdravookhranenii͡a RF ot 21 Marta 2014 g. N 125n “Ob Utverzhdenii Nat͡sional’nogo Kalendari͡a Profilakticheskikh Privivok i Kalendari͡a Profilakticheskikh Privivok po Epidemicheskim Pokazanii͡am” (s Izmenenii͡ami i Dopolnenii͡ami) [(accessed on 13 July 2020)];2014 Available online: https://base.garant.ru/70647158/
- 38.Yagudina T. Turkmenistan Rasshiri͡aet Kalendar’ Privivok, Soglasno Rekomendat͡sii͡am VOZ. [(accessed on 19 July 2020)];2020 Available online: https://arzuw.news/turkmenistan-rasshirjaet-kalendar-privivok-soglasno-rekomendacijam-voz.
- 39.Saydaliev S., Tursunova Y., Khalilova G., Mullaeva L., Mirzabaev D., Kim L. Sanitarnye Pravila, Normy, Gigienicheskie Normativy, Immunoprofilaktika, Infekt͡sionnykh Zabolevaniĭ v Respublike Uzbekistan. [(accessed on 19 July 2020)];2015 Available online: https://www.minzdrav.uz/documentation/detail.php?ID=45175.
- 40.World Health Organization . Guidelines for the Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection: Mar-15. World Health Organization; Geneva, Switzerland: 2015. [PubMed] [Google Scholar]
- 41.World Health Organization Global policy report on the prevention and control of viral hepatitis in WHO Member States. [(accessed on 19 July 2020)];2013 Available online: https://www.who.int/hepatitis/publications/global_report/en/
- 42.Republican Center for Healthcare Development of the Ministry of Health of the Republic of Kazakhstan Chronic Hepatitis B in Adults. 2019 [Updated 19 November 2019] [(accessed on 19 July 2020)]; Available online: https://diseases.medelement.com/disease/хрoнический-гепатит-в-у-взрoслых-2019/16388.
- 43.Ivashkin V., Yushchuk N., Mayevskaya M., Znojko O., Dudin K., Karetkina G., Klimova S.L., Maksimov Y.V., Martynov I.V., Maev H.S., et al. Mezhdunarodnai͡a Koalit͡sii͡a po Gotovnosti k Lechenii͡u Vostochnai͡a Evropa i T͡Sentral’nai͡a Azii͡a. Klinicheskiĭ Protokol Respubliki Tadzhikistan “Gepatit V i VICH-infekt͡sii͡a: Taktika Vedenii͡a Pat͡sientov s Koinfekt͡sieĭ”. [(accessed on 19 July 2020)];2011 Available online: https://itpcru.org/2015/09/16/13495/
- 44.Ministerstvo Zdravookhranenii͡a Respubliki Uzbekistan. Klinicheskoe Rukovodstvo po Diagnostike, Lechenii͡u i Profilaktike Khronicheskikh Gepatitov u Vzroslykh v Pervichnom Zvene Zdravookhranenii͡a. [(accessed on 19 July 2020)];2013 Available online: https://www.minzdrav.uz/documentation/detail.php?ID=41092. (In Russian)