Abstract
The use of controlled mixed inocula of Saccharomyces cerevisiae and non-Saccharomyces yeasts is a common practice in winemaking, with Torulaspora delbrueckii, Lachancea thermotolerans and Metschnikowia pulcherrima being the most commonly used non-Saccharomyces species. Although S. cerevisiae is usually the dominant yeast at the end of mixed fermentations, some non-Saccharomyces species are also able to reach the late stages; such species may not grow in culture media, which is a status known as viable but non-culturable (VBNC). Thus, an accurate methodology to properly monitor viable yeast population dynamics during alcoholic fermentation is required to understand microbial interactions and the contribution of each species to the final product. Quantitative PCR (qPCR) has been found to be a good and sensitive method for determining the identity of the cell population, but it cannot distinguish the DNA from living and dead cells, which can overestimate the final population results. To address this shortcoming, viability dyes can be used to avoid the amplification and, therefore, the quantification of DNA from non-viable cells. In this study, we validated the use of PMAxx dye (an optimized version of propidium monoazide (PMA) dye) coupled with qPCR (PMAxx-qPCR), as a tool to monitor the viable population dynamics of the most common yeast species used in wine mixed fermentations (S. cerevisiae, T. delbrueckii, L. thermotolerans and M. pulcherrima), comparing the results with non-dyed qPCR and colony counting on differential medium. Our results showed that the PMAxx-qPCR assay used in this study is a reliable, specific and fast method for quantifying these four yeast species during the alcoholic fermentation process, being able to distinguish between living and dead yeast populations. Moreover, the entry into VBNC status was observed for the first time in L. thermotolerans and S. cerevisiae during alcoholic fermentation. Further studies are needed to unravel which compounds trigger this VBNC state during alcoholic fermentation in these species, which would help to better understand yeast interactions.
Keywords: non-Saccharomyces, Saccharomyces cerevisiae, wine yeast, viable but non culturable, viability qPCR
1. Introduction
Alcoholic fermentation of grape must is mainly driven by Saccharomyces cerevisiae, which quickly dominates the fermentation of grape must in wine production. However, in the last years, there has been an increasing interest in studying other fermentative yeasts, which are generally referred to as non-Saccharomyces yeasts, due to the ability of some of them to improve the complexity of wines by increasing the concentration of aromatic molecules, such as terpenoids, esters, higher alcohols or other molecules of interest, such as glycerol [1,2,3,4,5,6]. In addition, another advantage is the potential of some species to reduce the alcohol content of wines, a feature increasingly sought after in this industry [7,8,9]. In contrast, some of these non-Saccharomyces yeasts are known to spoil wines and cause stuck fermentations [10,11]. However, several studies have shown that when selected non-Saccharomyces yeasts are used as starters together with S. cerevisiae, they are able to participate in the fermentations until late stages, and produce wines with the previously mentioned characteristics [3,12]. Among them, it is important to highlight those species that are already available as commercial strains, Metschnikowia pulcherrima, Torulaspora delbrueckii and Lachancea thermotolerans. All of them showed the ability to reduce ethanol content, mainly M. pulcherrima [9,13,14,15,16,17]. In addition, M. pulcherrima has been described as a yeast that makes wines fruitier and fresher, positively modulating the wine aroma profile [13,18]. L. thermotolerans increases wine natural acidity due to increased lactic acid production [13,19,20] and T. delbrueckii has a positive influence on the overall impression of the obtained wines, improving the aroma quality and the varietal character [19,21,22].
Therefore, although it has been proved that the use of some of these yeasts together with S. cerevisiae as starters produces wines with improved qualities, their interactions at the microbiological level are still not completely understood. Very early studies of alcoholic fermentation confirmed that S. cerevisiae quickly dominates the fermentation process, resulting in the disappearance of the non-Saccharomyces yeasts, which was initially attributed to different ethanol and SO2 sensitivities of the latter [23]. However, different studies during the last 20 years have demonstrated the ability of many non-Saccharomyces yeasts to survive until the end of the fermentation, with a broad array of interactions among different yeasts [2,3,12]. Indeed, the yeast interactions are highly dependent on the species and strains used [24,25]. From the beginning of the fermentation, there is competition for nutrients (mainly for nitrogen) and oxygen, which can influence the behavior of these yeasts and, consequently, the final wine. During alcoholic fermentation, some studies have demonstrated that the secretion of antimicrobial peptides by S. cerevisiae is responsible for the premature death of non-Saccharomyces yeasts [26,27,28]. Furthermore, direct physical contact between non-Saccharomyces and S. cerevisiae yeasts can also cause different responses in the cells. In fact, several studies have shown early growth arrest of different non-Saccharomyces yeasts in co-fermentations with S. cerevisiae due to a cell–cell contact mechanism [29,30,31,32].
The detection and quantification of the yeasts involved in wine production have been studied for many years and with different methodologies. The most widely used method is based on the culturability on different solid media. The use of differential media, such as Wallerstein Laboratory Nutrient (WLN) medium, allows the differentiation of some non-Saccharomyces yeasts, but not all can be differentiated with this method, as not all the species present different colony morphologies [33]. Indeed, culture-dependent methods are time-consuming and unable to detect viable but non-culturable (VNBC) microorganisms, and even some slow-growing microorganisms may also be undetected. Thus, to achieve an accurate study of population dynamics, it is important to use culture-independent techniques, which will allow the identification and quantification of the different yeasts present during alcoholic fermentation regardless of their culturability. Several molecular tools have been developed, with quantitative polymerase chain reaction (qPCR) being one of the most applied methods [34,35,36,37]. Several studies have demonstrated that qPCR is much more specific than other molecular tools in the detection of different yeast and bacteria during fermentation [38,39]. In addition, qPCR has been shown to be sensitive enough to detect even underrepresented strains among not only yeasts [35,40,41], but also other types of wine microorganisms, such as bacteria [42,43].
As mentioned before, interactions among yeasts during alcoholic fermentation can lead to cell death or growth arrest. If this occurs, the qPCR results will not be accurate enough since the test quantifies the total DNA, not differentiating between dead and living yeasts. To solve this problem, some studies have applied reverse-transcription PCR (RT-PCR), a technique that amplifies the genetic material only from viable cells [34,44,45]. However, as RNA handling is more demanding and prone to degradation by contamination with RNA-degrading enzymes, this tool requires trained personnel and may result in problems of reproducibility. A simpler procedure is the use of viability-qPCR, which includes a pretreatment of the sample with DNA-binding dyes, such as ethidium monoazide (EMA) or propidium monoazide (PMA). These dyes enter cells with damaged membranes and bind to DNA in a covalent manner after photoactivation, preventing its amplification by subsequent PCR. This means that only DNA from viable cells (with membrane integrity) will be susceptible to being amplified by qPCR [38,46,47,48,49]. Several studies performed in bacteria and parasites showed that PMA is an excellent choice to determine the viability of cells due to its high specificity and sensitivity in different food matrices, such as oysters, meat and wastewater [50,51,52]. Indeed, PMA seemed to be more selective for dead cells than EMA [53,54]. A new and improved version of PMA, PMAxx, has been shown to be better at discriminating between living and dead bacterial cells or viruses [55,56,57]. However, only a few studies have been conducted using viability-qPCR to determine yeast population dynamics through alcoholic fermentation [38,44,45,58].
Our aim in this study was to analyze the population dynamics of mixed alcoholic fermentation without the use of SO2 in order to eliminate competitive advantages for S. cerevisiae, and to determine the VBNC population. For that reason, we simultaneously inoculated a synthetic must with S. cerevisiae and three non-Saccharomyces yeasts commonly used in mixed wine industrial fermentations (T. delbrueckii, L. thermotolerans and M. pulcherrima), and analyzed yeast dynamics and viability for each species by PMAxx-qPCR and compared the results with those from non-dyed qPCR and colony counting.
2. Materials and Methods
2.1. Strains and Culture Conditions
Four yeast species were used in this study: S. cerevisiae QA23 (Lallemand Inc., Montreal, QC, Canada) (Sc), T. delbrueckii Biodiva (Lallemand Inc., Canada) (Td), L. thermotolerans 1 (provided by Agrovin S.A., Alcázar de San Juan, Spain) (Lt) and M. pulcherrima CECT 13131 (Mp) isolated from grape must from the Priorat region (URV collection) [25]. Strains were plated on YPD solid medium (2% (w/v) glucose, 2% (w/v) yeast extract, 1% (w/v) peptone and 1.7% (w/v) agar) and on Wallerstein Laboratory Nutrient (WLN) agar (Becton, Dickinson and Company, Le Point de Claix, France) from frozen stocks, and one colony was used to develop the inoculum. Before starting fermentations, routine species confirmation of the different strains was performed by restriction analysis (PCR-RFLP) of 5.8 S-ITS rDNA [59].
2.2. Fermentation Procedure
Single colonies of each strain were grown individually in YPD liquid medium for 24 h at 28 °C. Cells were counted in a Neubauer chamber and 5 × 105 cells/mL of each strain were simultaneously inoculated in 230 mL of a synthetic must [60], contained in 250 mL bottles. Thus, the total inoculated population was 2 × 106 cells/mL. Fermentations were performed in triplicate and incubation was at 22 °C with stirring (120 rpm).
Fermentations were monitored by measuring must density (electronic densitometer, Densito 30PX Portable Density Meter; Mettler Toledo, Spain) and optical density at 600 nm (Ultrospec 2100 Pro; Biotech Ltd., Cambridge, England). Fermentations were considered to be finished when residual sugars were determined to be less than 5 g/L (Miura One Rev; I.S.E. Srl, Italy).
2.3. Population Dynamics by Plating
The population dynamics were monitored by plating samples at different points of fermentation on two solid media. Briefly, samples were serially diluted in sterile water and the number of colony-forming units per milliliter (CFU/mL) was determined by plating 100 μL of the corresponding dilutions on YPD for total yeast population and on WLN for the morphological differentiation and counting of the four species present in the sample.
2.4. Population Dynamics by qPCR
2.4.1. Living/Dead Cell Amplification: Heat Shock and PMAxx Treatment
To better differentiate between living and dead yeast cells and to ensure that the qPCR only amplifies living cells, the PMAxxTM viability dye (Biotium Inc., Fremont, CA, USA) was used. First, we validated the effectiveness of this dye using living cells and heat-treated cells (as dead cells).
One yeast colony was inoculated in YPD liquid medium and incubated overnight at 28 °C. Then, two aliquots of 1 mL sample (106 cells/mL) were centrifuged at 6000 rpm for 5 min and the pellet was resuspended in 1 mL of sterile distilled water. One of the aliquots was subjected to heat shock (95 °C for 10 min) and both were stained with PMAxx according to the manufacturer’s protocol, with the modification of the cross-link time. Briefly, the PMAxx dye (25 µM) was added to the sample, followed by an incubation time of 10 min in the dark. Then, based on previous studies [38], the sample was exposed twice to light for 30 s and to ice for 60 s in between light exposures. Cells, not treated with PMAxx or light exposure, were used as controls to evaluate the effect of the dyes. The pellet was recovered by centrifugation at 13,500 rpm for 2 min and DNA was extracted.
The effectiveness of heat shock treatment was confirmed by the absence of growth of the heat-treated cells on YPD solid medium compared with the growth observed for non-treated cells. The procedure was repeated at least twice with each species.
2.4.2. PMAxx Treatment of Samples from Fermentation
Two 500 µL aliquots of must were centrifuged at 7800 rpm for 2 min and the pellets were washed with sterile distilled water. One aliquot was resuspended in 500 µL of sterile distilled water and then was treated with the PMAxx dye as previously described. The other aliquot was not treated (untreated cells). Then, DNA from both aliquots was extracted as described below.
2.4.3. DNA Extraction and qPCR Analysis
DNA was extracted using the DNeasy Plant Mini Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Primers used to amplify non-Saccharomyces species were published by García et al. [36], S. cerevisiae primers were published by Hierro et al. [61] and the total yeast population was quantified using primers described by Hierro et al. [34] (Table A1). All qPCR amplifications were carried out in triplicate with a final volume of 20 µL using TB GreenTM Premix Ex TaqTM II (Takara Bio Inc., Kusatsu, Japan) according to the manufacturer’s instructions in a QuantStudioTM 5 real-time PCR instrument (Applied Biosystems by Thermo Fisher Scientific, Waltham, MA, USA). The amplification process included an initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturing at 95 °C for 5 s and annealing at 60 °C for 30 s. Cycle threshold (Ct) was determined using the Standard Curve application (Applied Biosystems by Thermo Fisher Scientific, Waltham, MA, USA). Primer specificity was checked by performing the same qPCR with DNA of all species involved in the study. Milli-Q water (Millipore, Molsheim, France) was used as a negative control of amplification (NTC).
For each species, standard curves, with and without PMAxx dye treatment, were created by plotting the average Ct values of a tenfold serial dilution of DNA from 107 to 10 cells/mL against the log of cells/mL; and each dilution was assayed in triplicate. Standard curves were constructed using the Standard Curve application.
2.4.4. Limit of Detection and Quantification by PMAxx-qPCR
The limit of detection (LoD) and quantification (LoQ) by qPCR of living cells treated with PMAxx were calculated in pure and mixed cultures. For the latter, two different types of combinations were prepared for each yeast species. In living/dead mixed cultures, tenfold serial dilutions of living cell DNA (from 104 to 10 cells/mL) were mixed with DNA extracted from 106 cells/mL of dead cells from the same species. Additionally, in living/living mixed cultures, the same serial dilutions of DNA from living cells were mixed with DNA extracted from 107 cells/mL of living cells from another yeast species (Sc was mixed with Td; and Td, Lt and Mp were mixed with Sc). According to the Clinical and Laboratory Standards Institute (CLSI), the LoD is the lowest amount of analyte in a sample that can be detected with probability, although perhaps not quantified as an exact value (Ct < 40). The LoQ is the lowest amount of measurand in a sample that can be quantitatively determined with acceptable precision and stated, acceptable accuracy, under stated experimental conditions (Ct < 30).
2.5. Statistical Analysis
All analyses were performed using GraphPad Prism® version 6 (GraphPad Software, San Diego, CA, USA). Results are expressed as the mean ± SD. Student’s t-tests were applied to analyze differences between living and dead cells and the influence of PMAxx treatment (p < 0.05).
3. Results
3.1. Optimization of PMAxx-qPCR for Yeast Viability Determination
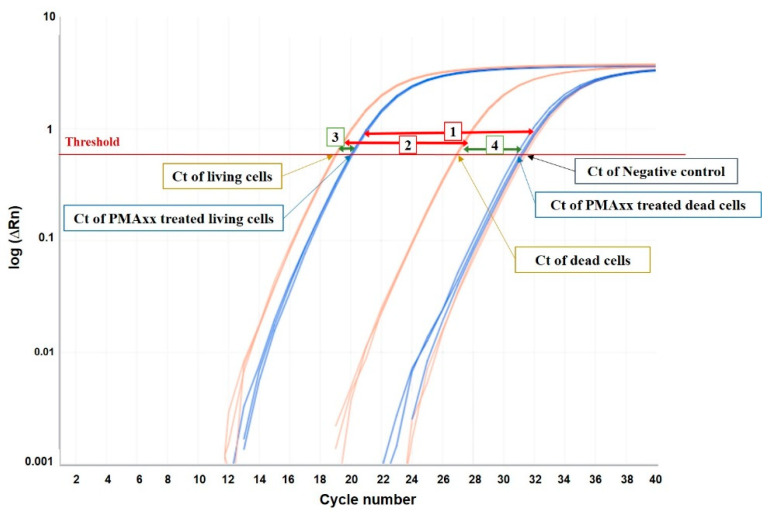
PMAxx is an optimized version of PMA, a fluorescent photoaffinity dye that binds covalently to DNA after photoactivation, inhibiting its amplification by PCR. As PMAxx can only enter cells with compromised or damaged membranes, but not intact cells, predominantly, DNA from living cells will be detected by qPCR. In our study, dead cells treated with PMAxx showed the maximum possible Ct reduction (amplifying from 4.834 to 10.4 cycles later than untreated dead cells, depending on the yeast; Figure 1, Table 1a), as the Ct of these samples clustered with the negative controls (NTC) (Figure 1). Dead cells without PMA treatment amplified much earlier, but it was still later than the Ct of living cells. In contrast, living cells treated with PMAxx showed a small Ct reduction compared with untreated cells, being also dependent on the yeast (from 0.5525 to 2.171 cycles; Figure 1, Table 1a). Therefore, the impact of the treatment with the PMAxx dye on the DNA of living cells, although subtle, has to be considered in its quantification. Hence, when the means of Ct values from living and dead cells were compared (Figure 1, Table 1b), the PMAxx treatment resulted in a Ct reduction from 11.33 to 15.42 cycles (the maximum possible reduction), while the reduction in untreated cells showed significantly lower values (between 7.39 and 8.81 cycles).
Figure 1.
Schematic representation of the effect of PMAxx treatment in living and dead cells. Red arrows represent the cycle threshold (Ct) reduction obtained by subtracting the mean Ct values of living cells from those of dead cells, (1) with or (2) without PMAxx treatment. Green arrows represent the Ct reduction obtained by subtracting the mean Ct values obtained from PMAxx-qPCR of (3) living or (4) dead cells from the mean Ct values obtained from non-dyed qPCR.
Table 1.
Effect of PMAxx treatment in 106 living or dead cells/mL of Saccharomyces cerevisiae (Sc), Torulaspora delbrueckii (Td), Lachancea thermotolerans (Lt) and Metschnikowia pulcherrima (Mp). (a) Ct reduction was obtained by subtracting the mean Ct values obtained from PMAxx treatment of living or dead cells from the mean Ct values obtained from untreated cells. (b) Ct reduction was obtained by subtracting the mean Ct values of dead cells from those of living cells, with or without PMAxx treatment. The results are expressed as the mean ± SD. The significance level for the unpaired t-test was p < 0.05.
| (a) ΔCt (PMAxx Treated–Untreated Cells) |
(b) ΔCt (Dead–Living Cells) |
|||||
|---|---|---|---|---|---|---|
| Living Cells | Dead Cells | p Value | PMAxx | non-PMAxx | p Value | |
| S. cerevisiae | 2.003 ± 0.428 | 10.4 ± 1.558 | 0.001 | 15.42 ± 0.888 | 7.76 ± 1.447 | 0.018 |
| T. delbrueckii | 1.631 ± 1.040 | 5.566 ± 1.498 | 0.029 | 11.33 ± 1.736 | 7.392 ± 1.099 | 0.020 |
| L. thermotolerans | 2.171 ± 0.395 | 8.22 ± 0.310 | 0.010 | 13.46 ± 1.708 | 8.814 ± 0.3917 | 0.003 |
| M. pulcherrima | 0.5525 ± 0.665 | 4.834 ± 0.984 | 0.041 | 11.83 ± 1.170 | 7.548 ± 0.4837 | 0.036 |
Since the treatment with PMAxx affected the quantification of both living and dead cells, two standard curves were performed for each species to accurately quantify both treated and untreated samples—with and without PMAxx treatment of living cells. The correlation coefficients, slopes and efficiencies of the amplification of standard curves are shown in Table 2. Standard curves obtained with the different species had similar slopes, with efficiencies ranging between 97.1% and 108.7%, and with an R2 close or equal to 1 (Table 2). Indeed, we obtained better efficiencies (closer to 100%) with DNA from PMAxx-treated cells. The LoQ (Table 2) for all the species treated with PMAxx was 103 cells/mL, which was linear over five orders of magnitude, except for Mp, which was 104 cells/mL. Untreated cells of Sc and Td showed a lower LoQ (102 cells/mL), while for Lt and Mp, no differences in LoQ were observed between PMAxx-treated and untreated cells. The LoD was one log lower in all cases (Table 2).
Table 2.
Slopes, Y-intersections, correlation coefficients (R2), efficiencies (%), standard errors, limits of quantification (LoQs) and limits of detection (LoDs) of standard curves obtained from serially diluted DNA of S. cerevisiae, T. delbrueckii, L. thermotolerans, M. pulcherrima and total yeasts, with or without the PMAxx dye. Efficiency (E) was calculated using the formula E = (10−1/slope)-1. ND, not determined.
| qPCR | |||||||
|---|---|---|---|---|---|---|---|
| Slope | Y-Intersection | R 2 | Efficiency (%) | Error | LoQ | LoD | |
| S. cerevisiae | −3.275 | 37.526 | 0.999 | 102 | 0.041 | 102 | 10 |
| T. delbrueckii | −3.149 | 37.174 | 1 | 107.77 | 0.027 | 102 | 10 |
| L. thermotolerans | −3.226 | 38.466 | 1 | 104.17 | 0.02 | 103 | 102 |
| M. pulcherrima | −3.389 | 44.651 | 0.996 | 97.287 | 0.071 | 104 | 103 |
| Total yeast | −3.539 | 40.168 | 0.995 | 91.657 | 0.085 | ND | ND |
| PMAxx-qPCR | |||||||
| Slope | Y-Intersection | R2 | Efficiency (%) | Error | LoQ | LoD | |
| S. cerevisiae | −3.353 | 38.009 | 0.999 | 98.719 | 0.011 | 103 | 102 |
| T. delbrueckii | −3.542 | 38.1 | 0.998 | 91.552 | 0.011 | 103 | 102 |
| L. thermotolerans | −3.24 | 39.945 | 0.998 | 103.5 | 0.015 | 103 | 102 |
| M. pulcherrima | −3.34 | 41.802 | 0.999 | 99.214 | 0.023 | 104 | 103 |
| Total yeast | −3.129 | 37.672 | 0.994 | 108.73 | 0.074 | ND | ND |
To determine if the presence of dead cells from the same species or living cells from other species modified the sensitivity of our detection and quantification, the LoD and LoQ of living cells treated with PMAxx were also calculated in mixed cultures. In all the species, both limits remained identical to those obtained in pure cultures (Table A2).
3.2. Monitoring Yeast Population Dynamics of Mixed Fermentation and Differentiation of VBNC Yeasts
Sc, Td, Lt and Mp were simultaneously inoculated in the synthetic must in the same proportion (1:1:1:1) to test if PMAxx-qPCR could be used to monitor the viable population dynamics of each species during mixed fermentation. The results of PMAxx-qPCR were compared with those from non-dyed qPCR and with a culture-dependent technique, using YPD and WLN differential media.
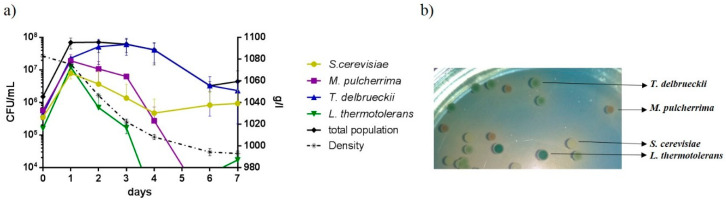
Mixed fermentations were completed in 7 days (Figure 2a). First, viability was analyzed using classical microbiological methods after plating the samples on YPD and WLN media. The four yeast species used in this study showed different colony morphology on WLN medium, allowing the counting of each strain separately on the same plate (Figure 2b). During fermentation and based on colony growth on WLN medium, at day 1, all species were able to grow and reached the same population (approximately 107 CFU/mL), but since day 2, each species followed different dynamics. Td was the species with the highest number of CFU/mL counted on WLN plates, in all sampling points, with its maximum growth at day 3 (6.17 × 107 CFU/mL). Sc colonies were detected throughout fermentation, but, surprisingly, Sc was not the main strain at the end of fermentation. In contrast, Mp and Lt colonies were not detected at all sampling points. Hence, Mp suffered a drastic fall in plate counts starting at day 3, so that the last colonies were observed at day 4. Lt was the species with the fastest decrease in plate viability, as no colony was observed after day 3; however, surprisingly, at day 7, some colonies were recovered in two out of three replicates. The absence of Lt colonies at days 4 and 6 showed that colony counting is not a suitable method for the detection of minority strains. The sample dilution necessary to have a proper number of colonies in a plate (30–300 CFU/mL) rules out the possibility of detecting low-abundance strains.
Figure 2.
(a) Density (g/L) and viable population (CFU/mL) dynamics during fermentation. The total population was determined by plating on YPD medium and the viable population for each strain was determined by plating on WLN medium. The results are expressed as the mean ± SD of three fermentations. (b) Colony morphologies of the four species on WLN plates.
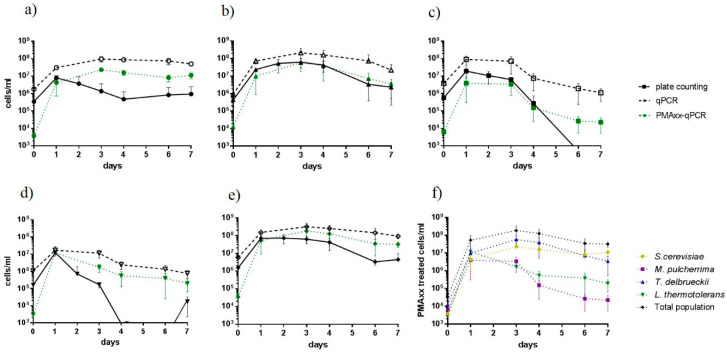
Plating results were then compared with qPCR analysis. For qPCR, samples of days 0 (1 h after inoculation), 1, 3, 4, 6 and 7, treated and untreated with PMAxx, were analyzed (Figure 3). In a general overview, for all yeast species, the results obtained with PMAxx-qPCR were closer to cell count, whereas non-dyed qPCR results showed population overestimation (Figure 3). Focusing on PMAxx-qPCR results, we observed that the populations estimated for Td and Mp species, as well as for total yeasts, were similar to those obtained by colony counting (except for the days with undetected Mp colonies on WLN medium). However, for Sc and Lt species, the culturable populations were lower than those determined by PMAxx-qPCR from day 3 to the end of fermentation. This difference was higher in Lt, reaching more than a 3-log unit difference at days 4 and 6. Therefore, if we observed the fermentation dynamics analyzed by PMAxx-qPCR (Figure 3f), expected results were obtained, with Sc being the main species detected at the end of the fermentation. Td presented a population similar to Sc, and both species (the ones with higher fermentative capacity) represented more than 98% of the yeast population detected at the end of fermentation. Interestingly, 1 h after inoculation, all yeasts showed a low viable cell number by PMAxx-qPCR, reaching up to two logarithm units less than colonies counted on plates.
Figure 3.
Yeast population analysis based on colony counting performed with WLN plates (black lines) and qPCR with or without PMAxx treatment (green dotted lines or black dashed lines, respectively) of (a) S. cerevisiae, (b) T. delbrueckii, (c) M. pulcherrima, (d), L. thermotolerans and (e) and total yeast population during fermentation. (f) Growth dynamics of the different yeast species based on PMAxx-qPCR results.
4. Discussion
The study of microbial population in alcoholic fermentation has been traditionally performed by the classical microbiological colony-counting technique [4,23]. However, in the last 30 years, new methodologies have been applied because more accurate and specific techniques are needed to satisfy the demands of the winemaking industry. In our study, we aimed to validate PMAxx-qPCR as a tool to monitor the population dynamics throughout alcoholic fermentation of the most common yeast species (S. cerevisiae, T. delbrueckii, L. thermotolerans and M. pulcherrima) currently used in the wine industry, in comparison to non-dyed qPCR and classical microbiological methods.
The non-Saccharomyces species used in this study are all commercially available as active dry yeast starters and are used in mixed or sequential fermentations with S. cerevisiae due to the positive characteristics given to the final wine [14,16,18,19,62]. Thus, having a reliable methodology to analyze the population dynamics when using those species in mixed fermentations will be very useful to understand the survival of the different microorganisms and the imprint of the different species in the final product.
When analyzing the population dynamics of spontaneous or mixed fermentations, the classical solid medium used to study the cell population presents important drawbacks to properly distinguish and count all the species present [35,36], and further molecular analyses are needed to identify the different yeasts, usually by culture-dependent molecular techniques (RFLP-PCR of rDNA, rDNA sequencing, etc.) [59]. Some differential media such as WLN can overcome some of these problems since it is possible to distinguish and count different species based on colony morphologies [33]. However, not all species can be easily differentiated because some of them present similar aspects; additionally, colony morphology is strongly dependent on yeast physiological status. In our study, as mentioned above, yeast species were first selected based on their enological potential, but also because it was possible to differentiate the four strains on WLN medium, which allowed us to monitor them by colony counting. The WLN medium enabled us to determine the presence of the four yeast species until mid-fermentation (days 3 and 4). When the populations of some species (Mp and Lt) began to decrease, only few or no colonies could be detected at the chosen dilution. As all yeast species were detected in the same plate, the dilution rate had to be chosen depending on the total population, which allowed only the dominant species to be detected (species with populations 2 log units lower than the main species cannot be detected). Similar results have been observed in different studies [36,41,63], where the detection of minor strains could only be achieved after using qPCR. For these reasons, we consider that colony counting is not an accurate technique to follow the population dynamics when several yeasts are involved in fermentation, and at different cell populations, as they occur in natural habitats. Indeed, WLN medium can be used to detect and count species with similar log populations, but it cannot detect differences higher than 1 or 2 log units.
Opposite to colony counting, the use of qPCR allowed the detection of the four yeast species throughout the fermentation. In agreement with previous studies, the cell population numbers obtained by an independent-culture technique, such as qPCR, were higher than the results obtained by plating [36,38,39,61,63], and the difference between methodologies was higher as the fermentation progressed. This fact could be due to the inability of qPCR to differentiate DNA from living and dead cells. Therefore, as fermentation progresses, the number of dead cells increases, and, if the cell death rate is greater than the DNA degradation rate, the difference between colony counting and qPCR also increases.
To achieve an exact quantification of viable cells, samples were treated with PMAxx, so that only DNA from cells with an intact membrane, impermeable to this dye, can be amplified. Several studies have proven that this kind of dye, known as a viability dye, is useful to assume that the amplified population can be considered as viable cells [38,46,49,53,55]. In the case of studies of yeast viability performed by qPCR, only EMA and/or PMA have been used as viable dyes [38,44,45,58]; PMAxx has not yet been tested to determine its usefulness in yeast. The significant Ct reduction obtained between treated and untreated dead cells allowed us to validate the effectiveness of the treatment in all yeast species under study. Likewise, the Ct reduction between living and dead cells treated with PMAxx was significantly higher than that with untreated cells. However, a subtle Ct reduction was also obtained when living cells were treated with PMAxx. This fact exposed the need to calculate different standard curves depending on whether they are treated or not. According to results obtained by other authors [38,44], the quantification of a sample of treated living cells was not altered by the presence of DNA from dead cells of the same species (106). In this study, the interference with DNA from different living cells (107) was also tested, and again, linearity of standard curves was not influenced. In addition, LoD and LoQ were calculated in the presence of both exogenous DNA molecules and these limits were not changed in any condition.
The application of PMAxx-qPCR allowed us to obtain a more accurate determination of the population dynamics. At all sampling points analyzed, except for time 0, the quantification obtained by PMAxx-qPCR was closer to the results of colony counting, while non-dyed qPCR overestimated the population in all cases. As we have previously noted, this may be due to the ability of PMAxx to bind the DNA from dead cells, impairing its amplification, so that qPCR will mainly amplify and detect the DNA from viable cells [38,45,50]. Thus, at the sampling points where no species were detected by colony counting, PMAxx-qPCR results will be more reliable than qPCR ones since they are more accurate for viable cell counting. Similar conclusions were drawn by Vendrame et al. [45], but using PMA as a viability dye to assess wines spontaneously and artificially contaminated with Brettanomyces bruxellensis, obtaining better results by PMA-qPCR than by colony counting, qPCR or even RT-qPCR.
Nevertheless, some discordant points were detected. One of them was the low number of viable cells obtained by PMAxx-qPCR after one hour of must inoculation compared with colony counting or non-dyed qPCR. Capusoni et al. [64] showed how the permeability of the cell membrane of S. cerevisiae changes due to cultivation in a hyperosmotic medium (high NaCl concentration); this change allows the entry of several DNA-binding dyes into cells. Grape must is a hostile and stressful medium for yeast cells [65,66,67]. When yeast cells come into contact with grape juice, a sudden change in the expression of stress genes takes place, and GPD1, an osmotic stress gene, is activated within the first hour after inoculating grape juice from a stationary preculture [68]. In this way, this stress condition can also generate a change in the permeability of the cell membrane and therefore, allow the entry of PMAxx into viable cells, explaining the lower quantification after qPCR at this early time point.
However, the most important aspect revealed by the use of this viable dye was the existence of S. cerevisiae and L. thermotolerans in a VBNC state during fermentation. This behavior has been widely described by several authors in different bacteria and is considered as a survival strategy that permits resilience to unfavorable environmental conditions [50,69,70]. These cells retain an intact membrane, undamaged DNA and metabolic activity, but they are not culturable using laboratory media [71]. Some studies have also evidenced this phenomenon in wine yeasts [24,72] and explored the different stress conditions that could lead to the VBNC state in non-Saccharomyces yeasts. Thermosonication in B. bruxellensis [73] and hypoxic growth conditions in Cryptococcus neoformans [74] have triggered this state. Moreover, Wang et al. [24] showed that cell-free S. cerevisiae supernatant produced the loss of culturability of Hanseniaspora uvarum, M. pulcherrima and T. delbrueckii, suggesting that the presence of some antimicrobial metabolites could induce the VBNC state. More specifically, Branco et al. [26] showed how antimicrobial peptides produced by S. cerevisiae caused the loss of culturability in H. uvarum. Regarding S. cerevisiae, for the first time, our study evidenced the presence of the VBNC state in mixed fermentation. This VBNC population was found in a high proportion (more than 85%) from the middle to the end of fermentation, reaching 93.74 ± 10.07%. Previous studies have demonstrated that S. cerevisiae is able to enter into a VBNC state under SO2 and heat shock stress [75,76]. In addition, Petitgonnet et al. [77] demonstrated that cell-to-cell contact between S. cerevisiae and L. thermotolerans modifies yeast metabolism and the exometabolome, and cell contact can also influence S. cerevisiae viability. In fact, common winemaking procedures include the use of SO2 to prevent the growth of unwanted microorganisms, among them, non-Saccharomyces yeasts. Industrial wine S. cerevisiae yeasts are known to present resistance to SO2, and this is one of the mechanisms of inducing VBNC status in non-Saccharomyces yeasts [23] and inducing the predominance of S. cerevisiae during the winemaking process. For this reason, in the present work, SO2 was not used to prevent the bias that would be introduced by its addition. Furthermore, the use of SO2 in the wine industry has been challenged due to side effects in human health; thus, our conditions are also useful in this new SO2-free scenario.
5. Conclusions
In summary, the use of the viable dye PMAxx coupled with qPCR is a reliable, specific and fast method for monitoring population dynamics in mixed fermentations. In addition to detecting minority yeasts until the end of fermentation, the presence of a VBNC state in S. cerevisiae was revealed for the first time during mixed alcoholic fermentation. Further research is still required to understand how the interactions between S. cerevisiae and non-Saccharomyces yeasts impact their physiological and metabolic status and which conditions produced during fermentation are causing this state.
Acknowledgments
The authors would like to thank Braulio Esteve-Zarzoso for support on the qPCR design.
Appendix A
Table A1.
Primer sequences used for quantitative PCR analysis.
| Target Species | Primer Name | Sequence 5′-3′ | Reference |
|---|---|---|---|
| S. cerevisiae | CESP-F | ATCGAATTTTTGAACGCACATTG | Hierro et al. [61] |
| SCER-R | CGCAGAGAAACCTCTCTTTGGA | ||
| T. delbrueckii | Tods L2 | CAAAGTCATCCAAGCCAGC | García et al. [36] |
| Tods R2 | TTCTCAAACAATCATGTTTGGTAG | ||
| L. thermotolerans | LTH2-F | CGCTCCTTGTGGGTGGGGAT | García et al. [36] |
| LTH2-R | CTGGGCTATAACGCTTCTCC | ||
| M. pulcherrima | MP2-F | AGACACTTAACTGGGCCAGC | García et al. [36] |
| MP2-R | GGGGTGGTGTGGAAGTAAGG | ||
| Total yeast | YEAST-F | GAGTCGAGTTGTTTGGGAATGC | Hierro et al. [34] |
| YEAST-R | TCTCTTTCCAAAGTTCTTTTCATCTTT |
Table A2.
Limit of Quantification (LoQ) and Limit of Detection (LoD) of S. cerevisiae (Sc), T. delbrueckii (Td), L. thermotolerans (Lt) and M. pulcherrima (Mp) obtained by PMAxx-qPCR. DNA from living cells of each species (Sc, Td, Lt or Mp) was mixed with DNA from dead cells of the same species (living/dead mixed cultures), or with DNA from living cells of another yeast species (living/living mixed cultures, Sc/Td, Td/Sc, Lt/Sc or Mp/Sc). Cycle threshold was calculated by triplicate and standard deviation was calculated (Ct ± SD).
| Living/Dead Mixed Cultures | |||
|---|---|---|---|
| Living Yeast (evaluated concentrations) | Dead Yeast | LoQ (Ct ± SD) | LoD (Ct ± SD) |
| Sc (104 to 10 CFU/mL) |
Sc (106 CFU/mL) | 103
(27.907 ± 0.124) |
102 (30.841 ± 0.468) |
| Td (104 to 10 CFU/mL) |
Td (106 CFU/mL) | 103 (28.670 ± 0.139) |
102 (30.817 ± 0.273) |
| Lt (104 to 10 CFU/mL) |
Lt (106 CFU/mL) | 103 (29.594 ± 0.180) |
102 (32.255 ± 0.483) |
| Mp (104 to 10 CFU/mL) |
Mp (106 CFU/mL) | 104 (28.570 ± 0.190) |
103 (32.030 ± 0.940) |
| Living/Living Mixed Cultures | |||
| Living Yeast (evaluated concentrations) | Living Yeast | LoQ (Ct ± SD) | LoD (Ct ± SD) |
| Sc (104 to 10 CFU/mL) |
Td (107 CFUs/mL) | 103 (28.057 ± 0.089) |
102 (30.992 ± 0.207) |
| Td (104 to 10 CFU/mL) |
Sc (107 CFUs/mL) | 103 (29.922 ± 0.387) |
102 (32.121 ± 0.185) |
| Lt (104 to 10 CFU/mL) |
Sc (107 CFUs/mL) | 103 (29.692 ± 0.280) |
102 (31.995 ± 0.759) |
| Mp (104 to 10 CFU/mL) |
Sc (107 CFUs/mL) | 104 (29.597 ± 0.495) |
103 (31.173 ± 1.799) |
Author Contributions
Conceptualization, Y.N., M.-J.T., G.B. and A.M.; methodology, Y.N. and M.-J.T.; validation, Y.N., M.-J.T. and G.B.; formal analysis, Y.N.; investigation, Y.N.; resources, M.-J.T., G.B. and A.M.; data curation, Y.N.; writing-original draft preparation, Y.N.; writing-review and editing, M.-J.T., G.B. and A.M.; visualization, Y.N.; supervision, M.-J.T., G.B. and A.M.; project administration, G.B. and A.M.; funding acquisition, G.B. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Innovation and Universities, Spain (Project CoolWine, PCI2018-092962), under the call ERA-NET ERA COBIOTECH. Yurena Navarro is a postdoctoral fellow of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu P.T., Lu L., Duan C.Q., Yan G.L. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT Food Sci. Technol. 2016;71:356–363. doi: 10.1016/j.lwt.2016.04.031. [DOI] [Google Scholar]
- 2.Jolly N.P., Augustyn O.P.H., Pretorius I.S. The effect of Non-Saccharomyces yeasts on fermentation and wine quality. S. Afr. J. Enol. Vitic. 2003;24:55–62. doi: 10.21548/24-2-2638. [DOI] [Google Scholar]
- 3.Comitini F., Gobbi M., Domizio P., Romani C., Lencioni L., Mannazzu I., Ciani M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Fleet G.H. Wine yeasts for the future. FEMS Yeast Res. 2008;8:979–995. doi: 10.1111/j.1567-1364.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 5.Padilla B., Gil J.V., Manzanares P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016;7:411. doi: 10.3389/fmicb.2016.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemente-Jimenez J.M., Mingorance-Cazorla L., Martínez-Rodríguez S., Las Heras-Vázquez F.J., Rodríguez-Vico F. Influence of sequential yeast mixtures on wine fermentation. Int. J. Food Microbiol. 2005;98:301–308. doi: 10.1016/j.ijfoodmicro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Varela J., Varela C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019;56:88–96. doi: 10.1016/j.copbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Ciani M., Morales P., Comitini F., Tronchoni J., Canonico L., Curiel J.A., Oro L., Rodrigues A.J., Gonzalez R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016;7:642. doi: 10.3389/fmicb.2016.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quirós M., Rojas V., Gonzalez R., Morales P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014;181:85–91. doi: 10.1016/j.ijfoodmicro.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Fleet G.H., Lafon-Lafourcade S., Ribereau-Gayon P. Evolution of yeasts and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 1984;48:1034–1038. doi: 10.1128/AEM.48.5.1034-1038.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loureiro V., Malfeito-Ferreira M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003;86:23–50. doi: 10.1016/S0168-1605(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 12.Ciani M., Comitini F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015;1:1–6. doi: 10.1016/j.cofs.2014.07.001. [DOI] [Google Scholar]
- 13.Binati R.L., Lemos Junior W.J.F., Luzzini G., Slaghenaufi D., Ugliano M., Torriani S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020;318:108470. doi: 10.1016/j.ijfoodmicro.2019.108470. [DOI] [PubMed] [Google Scholar]
- 14.Canonico L., Comitini F., Ciani M. Metschnikowia pulcherrima selected strain for ethanol reduction in wine: Influence of cell immobilization and aeration condition. Foods. 2019;8:378. doi: 10.3390/foods8090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras A., Hidalgo C., Schmidt S., Henschke P.A., Curtin C., Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Puškaš V.S., Miljić U.D., Djuran J.J., Vučurović V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Sci. Nutr. 2020;8:1489–1498. doi: 10.1002/fsn3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X., Navarro Y., Mas A., Torija M.J., Beltran G. A rapid method for sSelecting Non-Saccharomyces strains with a lLow ethanol yield. Microorganism. 2020;8:658. doi: 10.3390/microorganisms8050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz J., Belda I., Beisert B., Navascués E., Marquina D., Calderón F., Rauhut D., Santos A., Benito S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018;102:8501–8509. doi: 10.1007/s00253-018-9255-3. [DOI] [PubMed] [Google Scholar]
- 19.Benito S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018;102:6775–6790. doi: 10.1007/s00253-018-9117-z. [DOI] [PubMed] [Google Scholar]
- 20.Gobbi M., Comitini F., Domizio P., Romani C., Lencioni L., Mannazzu I., Ciani M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013;33:271–281. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Bely M., Stoeckle P., Masneuf-Pomarède I., Dubourdieu D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008;122:312–320. doi: 10.1016/j.ijfoodmicro.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 22.González-Royo E., Pascual O., Kontoudakis N., Esteruelas M., Esteve-Zarzoso B., Mas A., Canals J.M., Zamora F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015;240:999–1012. doi: 10.1007/s00217-014-2404-8. [DOI] [Google Scholar]
- 23.Ribéreau-Gayon P., Dubourdieu D., Donèche B., Lonvaud A. Handbook of Enology: Volume 1, The Microbiology of Wine and Vinifications. John Wiley & Sons Ltd.; West Sussex, UK: 2006. [Google Scholar]
- 24.Wang C., Mas A., Esteve-Zarzoso B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016;7:502. doi: 10.3389/fmicb.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla B., García-Fernández D., González B., Izidoro I., Esteve-Zarzoso B., Beltran G., Mas A. Yeast biodiversity from DOQ priorat uninoculated fermentations. Front. Microbiol. 2016;7:930. doi: 10.3389/fmicb.2016.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branco P., Viana T., Albergaria H., Arneborg N. Antimicrobial peptides (AMPs) produced by Saccharomyces cerevisiae induce alterations in the intracellular pH, membrane permeability and culturability of Hanseniaspora guilliermondii cells. Int. J. Food Microbiol. 2015;205:112–118. doi: 10.1016/j.ijfoodmicro.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Albergaria H., Arneborg N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016;100:2035–2046. doi: 10.1007/s00253-015-7255-0. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Nevado F., Albergaria H., Hogg T., Girio F. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006;108:336–345. doi: 10.1016/j.ijfoodmicro.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Nissen P., Nielsen D., Arneborg N. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell—cell contact-mediated mechanism. Yeast. 2003;20:331–341. doi: 10.1002/yea.965. [DOI] [PubMed] [Google Scholar]
- 30.Wang C., Mas A., Esteve-Zarzoso B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2015;206:67–74. doi: 10.1016/j.ijfoodmicro.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Englezos V., Rantsiou K., Giacosa S., Río Segade S., Rolle L., Cocolin L. Cell-to-cell contact mechanism modulates Starmerella bacillaris death in mixed culture fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2019;289:106–114. doi: 10.1016/j.ijfoodmicro.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Renault P.E., Albertin W., Bely M. An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 2013;97:4105–4119. doi: 10.1007/s00253-012-4660-5. [DOI] [PubMed] [Google Scholar]
- 33.Pallmann C.L., Brown J.A., Olineka T.L., Cocolin L., Mills D.A., Bisson L.F. Use of WL medium to profile native flora fermentations. Am. J. Enol. Vitic. 2001;52:198–203. [Google Scholar]
- 34.Hierro N., Esteve-Zarzoso B., González Á., Mas A., Guillamón J.M. Real-time quantitative PCR (QPCR) and reverse transcription-QPCR for detection and enumeration of total yeasts in wine. Appl. Environ. Microbiol. 2006;72:7148–7155. doi: 10.1128/AEM.00388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Díaz C., Molina A.M., Nähring J., Fischer R. Characterization and dynamic behavior of wild yeast during spontaneous wine fermentation in steel tanks and amphorae. Biomed Res. Int. 2013;2013:540465. doi: 10.1155/2013/540465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García M., Esteve-Zarzoso B., Crespo J., Cabellos J.M., Arroyo T. Yeast monitoring of wine mixed or sequential fermentations made by native strains from D.O. “Vinos de Madrid” using real-time quantitative PCR. Front. Microbiol. 2017;8:2520. doi: 10.3389/fmicb.2017.02520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Glawe D.A., Weller D.M., Okubara P.A. Real-time PCR assays for the quantification of native yeast DNA in grape berry and fermentation extracts. J. Microbiol. Methods. 2020;168:105794. doi: 10.1016/j.mimet.2019.105794. [DOI] [PubMed] [Google Scholar]
- 38.Andorrà I., Esteve-Zarzoso B., Guillamón J.M., Mas A. Determination of viable wine yeast using DNA binding dyes and quantitative PCR. Int. J. Food Microbiol. 2010;144:257–262. doi: 10.1016/j.ijfoodmicro.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Maturano Y.P., Mestre M.V., Combina M., Toro M.E., Vazquez F., Esteve-Zarzoso B. Culture-dependent and independent techniques to monitor yeast species during cold soak carried out at different temperatures in winemaking. Int. J. Food Microbiol. 2016;237:142–149. doi: 10.1016/j.ijfoodmicro.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Andorrà I., Landi S., Mas A., Guillamón J.M., Esteve-Zarzoso B. Effect of oenological practices on microbial populations using culture-independent techniques. Food Microbiol. 2008;25:849–856. doi: 10.1016/j.fm.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Zott K., Claisse O., Lucas P., Coulon J., Lonvaud-Funel A., Masneuf-Pomarede I. Characterization of the yeast ecosystem in grape must and wine using real-time PCR. Food Microbiol. 2010;27:559–567. doi: 10.1016/j.fm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Vendrame M., Iacumin L., Manzano M., Comi G. Use of propidium monoazide for the enumeration of viable Oenococcus oeni in must and wine by quantitative PCR. Food Microbiol. 2013;42:196–204. doi: 10.1016/j.fm.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Torija M.J., Mateo E., Guillamón J.M., Mas A. Identification and quantification of acetic acid bacteria in wine and vinegar by TaqMan-MGB probes. Food Microbiol. 2010;27:257–265. doi: 10.1016/j.fm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Wang C., Esteve-Zarzoso B., Cocolin L., Mas A., Rantsiou K. Viable and culturable populations of Saccharomyces cerevisiae, Hanseniaspora uvarum and Starmerella bacillaris (synonym Candida zemplinina) during Barbera must fermentation. Food Res. Int. 2015;78:195–200. doi: 10.1016/j.foodres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Vendrame M., Manzano M., Comi G., Bertrand J., Iacumin L. Use of propidium monoazide for the enumeration of viable Brettanomyces bruxellensis in wine and beer by quantitative PCR. Food Microbiol. 2014;42:196–204. doi: 10.1016/j.fm.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Kim S.Y., Ko G. Using propidium monoazide to distinguish between viable and nonviable bacteria, MS2 and murine norovirus. Lett. Appl. Microbiol. 2012;55:182–188. doi: 10.1111/j.1472-765X.2012.03276.x. [DOI] [PubMed] [Google Scholar]
- 47.Nogva H.K., Dromtorp S.M., Nissen H., Rudi K. Ethidium monoazide for DNA-based differentiation of viable and dead bacteria by 5′-nuclease PCR. Biotechniques. 2003;34:804–813. doi: 10.2144/03344rr02. [DOI] [PubMed] [Google Scholar]
- 48.Nocker A., Camper A.K. Selective removal of DNA from dead cells of mixed bacterial communities by use of ethidium monoazide. Appl. Environ. Microbiol. 2006;72:1997–2004. doi: 10.1128/AEM.72.3.1997-2004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudi K., Naterstad K., Drømtorp S.M., Holo H. Detection of viable and dead Listeria monocytogenes on gouda-like cheeses by real-time PCR. Lett. Appl. Microbiol. 2005;40:301–306. doi: 10.1111/j.1472-765X.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 50.Miotto M., Barretta C., Ossai S.O., da Silva H.S., Kist A., Vieira C.R.W., Parveen S. Optimization of a propidium monoazide-qPCR method for Escherichia coli quantification in raw seafood. Int. J. Food Microbiol. 2020;318:108467. doi: 10.1016/j.ijfoodmicro.2019.108467. [DOI] [PubMed] [Google Scholar]
- 51.Ravindran V.B., Shahsavari E., Soni S.K., Ball A.S. Viability determination of Ascaris ova in raw wastewater: A comparative evaluation of culture-based, BacLight Live/Dead staining and PMA-qPCR methods. Water Sci. Technol. 2019;80:817–826. doi: 10.2166/wst.2019.286. [DOI] [PubMed] [Google Scholar]
- 52.Dorn-In S., Gareis M., Schwaiger K. Differentiation of live and dead Mycobacterium tuberculosis complex in meat samples using PMA qPCR. Food Microbiol. 2019;84:103275. doi: 10.1016/j.fm.2019.103275. [DOI] [PubMed] [Google Scholar]
- 53.Pan Y., Breidt F. Enumeration of viable Listeria monocytogenes cells by real-time PCR with propidium monoazide and ethidium monoazide in the presence of dead cells. Appl. Environ. Microbiol. 2007;73:8028–8031. doi: 10.1128/AEM.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nocker A., Cheung C.Y., Camper A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods. 2006;67:310–320. doi: 10.1016/j.mimet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Sicard A., Merfa M.V., Voeltz M., Zeilinger A.R., De La Fuente L., Almeida R.P.P. Discriminating between viable and membrane-damaged cells of the plant pathogen Xylella fastidiosa. PLoS ONE. 2019;14:e0221119. doi: 10.1371/journal.pone.0221119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Randazzo W., López-Gálvez F., Allende A., Aznar R., Sánchez G. Evaluation of viability PCR performance for assessing norovirus infectivity in fresh-cut vegetables and irrigation water. Int. J. Food Microbiol. 2016;229:1–6. doi: 10.1016/j.ijfoodmicro.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Han S., Jiang N., Lv Q., Kan Y., Hao J., Li J., Luo L. Detection of Clavibacter michiganensis subsp. michiganensis in viable but nonculturable state from tomato seed using improved qPCR. PLoS ONE. 2018;13:e0196525. doi: 10.1371/journal.pone.0196525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi H., Xu W., Trinh Q., Luo Y., Liang Z., Li Y., Huang K. Establishment of a viable cell detection system for microorganisms in wine based on ethidium monoazide and quantitative PCR. Food Control. 2012;27:81–86. doi: 10.1016/j.foodcont.2012.02.035. [DOI] [Google Scholar]
- 59.Esteve-Zarzoso B., Belloch C., Uruburu F., Querol A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999;49:329–337. doi: 10.1099/00207713-49-1-329. [DOI] [PubMed] [Google Scholar]
- 60.Beltran G., Novo M., Rozès N., Mas A., Guillamón J.M. Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Res. 2004;4:625–632. doi: 10.1016/j.femsyr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Hierro N., Esteve-Zarzoso B., Mas A., Guillamón J.M. Monitoring of Saccharomyces and Hanseniaspora populations during alcoholic fermentation by real-time quantitative PCR. FEMS Yeast Res. 2007;7:1340–1349. doi: 10.1111/j.1567-1364.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 62.Contreras A., Curtin C., Varela C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015;99:1885–1895. doi: 10.1007/s00253-014-6193-6. [DOI] [PubMed] [Google Scholar]
- 63.Padilla B., Zulian L., Ferreres À., Pastor R., Esteve-Zarzoso B., Beltran G., Mas A. Sequential inoculation of native non-Saccharomyces and Saccharomyces cerevisiae strains for wine making. Front. Microbiol. 2017;8:1293. doi: 10.3389/fmicb.2017.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Capusoni C., Arioli S., Donzella S., Guidi B., Serra I., Compagno C. Hyper-osmotic stress elicits membrane depolarization and decreased permeability in halotolerant marine Debaryomyces hansenii strains and in Saccharomyces cerevisiae. Front. Microbiol. 2019;10:64. doi: 10.3389/fmicb.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira J., Du Toit M., Du Toit W.J. The effects of copper and high sugar concentrations on growth, fermentation efficiency and volatile acidity production of different commercial wine yeast strains. Aust. J. Grape Wine Res. 2006;12:50–56. doi: 10.1111/j.1755-0238.2006.tb00043.x. [DOI] [Google Scholar]
- 66.Larue F., Lafon-Lafourcade S., Ribereau-Gayon P. The various functions of steroids on the yeast metabolism in grape must during fermentation: The notion of survival factor. Ann. Microbiol. 1979;130:231–243. [PubMed] [Google Scholar]
- 67.Attfield P.V. Stress tolerance: The key to effective strains of industrial baker’s yeast. Nat. Biotechnol. 1997;15:1351–1357. doi: 10.1038/nbt1297-1351. [DOI] [PubMed] [Google Scholar]
- 68.Pérez-Torrado R., Carrasco P., Aranda A., Gimeno-Alcañiz J., Pérez-Ortín J.E., Matallana E., Del Olmo M. Study of the first hours of microvinification by the use of osmotic stress-response genes as probes. Syst. Appl. Microbiol. 2002;25:153–161. doi: 10.1078/0723-2020-00087. [DOI] [PubMed] [Google Scholar]
- 69.Colwell R.R. Global climate and infectious disease: The cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 70.Overney A., Jacques-André-Coquin J., Ng P., Carpentier B., Guillier L., Firmesse O. Impact of environmental factors on the culturability and viability of Listeria monocytogenes under conditions encountered in food processing plants. Int. J. Food Microbiol. 2017;244:74–81. doi: 10.1016/j.ijfoodmicro.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Ayrapetyan M., Oliver J.D. The viable but non-culturable state and its relevance in food safety. Curr. Opin. Food Sci. 2016;8:127–133. doi: 10.1016/j.cofs.2016.04.010. [DOI] [Google Scholar]
- 72.Millet V., Lonvaud-Funel A. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 2000;30:136–141. doi: 10.1046/j.1472-765x.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- 73.Križanović S., Tomašević M., Režek Jambrak A., Ćurko N., Gracin L., Lukić K., Kovačević Ganić K. Effect of thermosonication and physicochemical properties of wine on culturability, viability, and metabolic activity of Brettanomyces bruxellensis yeast in red wines. J. Agric. Food Chem. 2019;68:3302–3311. doi: 10.1021/acs.jafc.9b03661. [DOI] [PubMed] [Google Scholar]
- 74.Hommel B., Sturny-Leclère A., Volant S., Veluppillai N., Duchateau M., Yu C.H., Hourdel V., Varet H., Matondo M., Perfect J.R., et al. Cryptococcus neoformans resists to drastic conditions by switching to viable but non-culturable cell phenotype. PLoS Pathog. 2019;15:e1007945. doi: 10.1371/journal.ppat.1007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salma M., Rousseaux S., Sequeira-Le Grand A., Divol B., Alexandre H. Characterization of the viable but nonculturable (VBNC) state in Saccharomyces cerevisiae. PLoS ONE. 2013;8:e77600. doi: 10.1371/journal.pone.0077600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munna M.S., Humayun S., Noor R. Influence of heat shock and osmotic stresses on the growth and viability of Saccharomyces cerevisiae SUBSC01. BMC Res. Notes. 2015;8:369. doi: 10.1186/s13104-015-1355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petitgonnet C., Klein G.L., Roullier-Gall C., Schmitt-Kopplin P., Quintanilla-Casas B., Vichi S., Julien-David D., Alexandre H. Influence of cell-cell contact between L. thermotolerans and S. cerevisiae on yeast interactions and the exo-metabolome. Food Microbiol. 2019;83:122–133. doi: 10.1016/j.fm.2019.05.005. [DOI] [PubMed] [Google Scholar]