Abstract
Lynch syndrome (LS), also known as hereditary nonpolyposis colorectal cancer (HNPCC), is a disorder caused by an autosomal dominant heterozygous germline mutation in one of the DNA mismatch repair (MMR) genes. Individuals with LS are at an increased risk of developing colorectal and extracolonic cancers, such as endometrial, small bowel, or ovarian. In this review, the mutations involved with LS and their diagnostic methods are described and compared, as are their current uses in clinical decision making. Nowadays, LS diagnosis is based on a review of family medical history, and when necessary, microsatellite instability (MSI) or/and immunohistochemistry (IHC) analyses should be performed. In the case of a lack of MMR protein expression (dMMR) or MSI-H (MSI-High) detection in tumor tissue, molecular genetic testing can be undertaken. More and more genetic testing for LS is based mainly on next-generation sequencing (NGS) and multiplex ligation-dependent probe amplification (MLPA), which provide better and quicker information about the molecular profile of patients as well as individuals at risk. Testing based on these two methods should be the standard and commonly used. The identification of individuals with mutations provides opportunities for the detection of cancer at an early stage as well as the introduction of proper, more effective treatment, which will result in increased patient survival and reduced costs of medical care.
Keywords: Lynch syndrome, hereditary cancer, colorectal cancer, MMR, diagnostics, IHC, MSI, NGS, MLPA
1. Introduction
Colorectal cancer (CRC) is currently one of the most commonly diagnosed cancers, taking third place in men and fourth in women. Only in 2018, more than 1.8 million new cases and almost 900 thousand deaths were recorded worldwide. Approximately 70–80% of them are sporadic cancers, while genetic factors are responsible for the remaining 20–30% of known cases [1,2]. In people with genetic load, familial cancer syndromes, such as Lynch syndrome (LS) or familial adenomatous polyposis, are most commonly the culprits of cancer development. LS, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is associated with 3–4% of hereditary cancers, while familial adenomatous polyposis-approximately 1% [3].
LS is characterized by a predisposition to a spectrum of cancers, mainly colorectal and endometrial cancer. It is associated with autosomal heterozygous germline mutations in either one of the DNA mismatch repair system (MMR) genes-MLH1, MSH2, MSH6, PMS2 or with the epithelial cell adhesion molecule (EPCAM) gene [4,5]. Mutations in MLH1 and MSH2 are the most common ones and represent approximately 80–90% of all cases, while the other 10–20% applies to mutations in MSH6 and PMS2 genes. Mutations in EPCAM are rare and constitute about 3% of cases [5].
In cells, in which a malfunction of the MMR system is observed, mutations usually occur in short tandem repeats (STRs), and the said mutations are referred to as microsatellite instability (MSI) [6,7]. Carriers of germline mutations in MMR genes display an 80% risk of developing cancer by the age of 70, and the average age of onset in LS is 45, compared with an average of 60 in sporadic CRC. LS is usually divided into two types—I and II [8].
1.1. LS I-Clinical Presentation
Colorectal cancer is the main type of cancer observed in LS patients. Individuals with LS are also at increased risk of developing synchronous (primary tumors diagnosed within six months of each other) and metachronous (primary tumors occurring >6 months apart) cancers. The risk of CRC development is significantly increased in patients with mutations in MSH2 and MLH1 genes compared with ones with MSH6 and PMS2 mutations. CRCs in LS are predominantly right-sided mucinous tumors [9,10,11], usually occurring at a young age [10] and evolving from pre-existing adenomas, which more likely and more rapidly progress to cancer in people with LS than in people with sporadic adenomas (2–3 vs. 8–10 years) [7]. Histologically, these cancers are poorly differentiated, which makes the identification difficult [10,12].
1.2. LS II-Clinical Presentation
Individuals with LS are at an increased risk of developing not only CRC but also extracolonic cancers, like endometrial, ovarian, stomach, small intestine, urinary tract, pancreatic, brain, and that of the cutaneous sebaceous glands also known as Muir–Torre syndrome. Endometrial cancer (EC) is the most common extracolonic cancer and occurs with similar frequency to CRC in women [11,12]. However, the risk of developing EC in patients with LS by the age of 70 ranges from 14% to 71% and is dependent on a mutation in a particular gene-approximately 14–54% for patients with MLH1/MSH2 mutations, 17–71% for patients with a mutation in MSH6 and 15% in for instances of PMS2 [13]. Interestingly, in women with an MSH6 mutation, EC will more likely develop than CRC [14]. Moreover, EC associated with Lynch syndrome is usually located in the low uterine segment and it is mostly observed in individuals with an MSH2 mutation [15,16,17]. For comparison, the risk of developing ovarian cancer is much lower (4–20%) [13], and is also associated mostly with a mutation in the MSH2 gene [14].
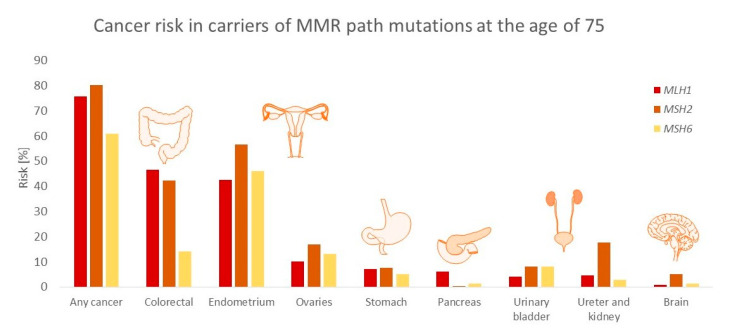
The risk of developing small bowel cancer ranges from 0.4% to 12%, and the average age of onset is 46–49 vs. 50–70 in the overall population [13,18]. The tumor is usually located in the duodenum and jejunum, less frequently in the ileum, and is mostly observed in individuals with a mutation in MLH1 [19]. The risk, depending on the cancer type and MMR mutation, is presented in Figure 1.
Figure 1.
Cancer risk in carriers depending on the mutated mismatch repair (MMR) path gene (MLH1/MSH/MSH6) at the age of 75. The risk of endometrial and ovarian cancers was calculated in females, all others-in both sexes [16].
2. Function and Mutations in Genes Responsible for DNA Repair and Involved in LS
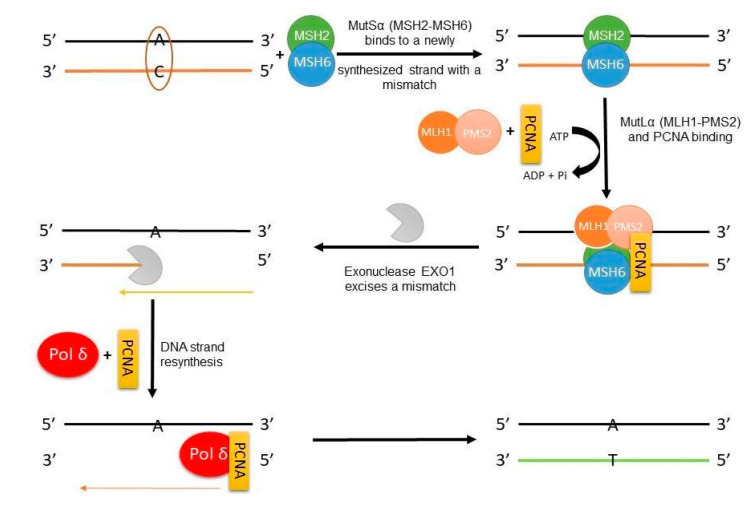
The DNA mismatch repair system enhances genome stability by recognizing and repairing polymerase errors. In LS, germline variants are mutated in the genes that encode MMR proteins. Mutations in MMR proteins result in microsatellite instability, which is observed in some cancers, including those associated with LS. Originally, the MMR system was identified and characterized in Escherichia coli, and it requires MutS, MutL, and MutH proteins. In humans, the mismatch repair system consists of six MMR proteins, MLH1, PMS2, MSH2, MSH6, MSH3, and PMS1, which are E. coli protein homologs [20,21]. The mechanism of their role in DNA repair is presented in Figure 2.
Figure 2.
The MMR mechanism in a eukaryotic cell on the example of a single-base mismatch. PCNA—proliferating cell nuclear antigen; Pol δ—polymerase delta.
The mechanism of mismatch base repair in eukaryotic cells is initiated by a MutSα (MSH2-MSH6) heterodimer, which recognizes mismatched single bases or small insertions/deletions (ID) (1–2 nucleotides long), or by the MutSβ (MSH2-MSH3) heterodimer, which recognizes larger insertions/deletions, up to 16 nucleotides. Both of the mentioned heterodimers are ATPases, and this activity is crucial for proper initiation and recognition [20,21]. Through ATP-dependent activation, MutS undergoes a conformational change into the sliding clamp, which moves along the DNA [22], and this state allows further recruitment and activation of the MutLα complex (MLH1-PMS2). PMS2, included in MutLα, is an endonuclease dependent on the PCNA (proliferating cell nuclear antigen) and RFC (replication factor C) (PCNA activates MutLα endonuclease activity). The complex MutLα is responsible for the mismatched base’s incision and, thus, has an essential role in MMR. Interestingly, the endonuclease activity of MutLα is essentially required in 3′ nick directed excision and is not obligatorily needed in 5′ nick mismatch excision [20,21,22,23,24].
In 3′ nick directed excision, the MLH1-PMS2, with the help of PCNA, initiates the incision of the strand in 3′ heteroduplex, resulting in a strand break usually 5′ from the mismatch, which is later used as a starting point for the EXO1 (exonuclease 1) 5′→3′ excision of the intermittent strand with the included mismatch, so in the next step, polymerase δ, after binding with PCNA, can re-synthesize the correct DNA strand, Figure 2 [20,21,22,23,24]. When a gap is located 5′ to the mismatch, the interaction between MutS, EXO1, and replication protein A (RPA) is needed. The MutS activates the EXO1, and RPA stimulates the whole process. Nevertheless, MutLα might play a role in this excision as well, although a non-compulsory one. It is suspected that the MLH1-PMS2 complex takes part in the termination of the 5′ excision right after cutting the mismatch [20,21,22,23,24,25].
However, besides the EXO1-dependent MMR, there is also the possibility of an EXO1-independent pathway, where repeated strand breaks by MutLα might be required. That would further lead to the production of 3′ ends or the excision of a newly synthesized strand near the mismatch, then directly to polymerase δ binding and later to displacement strand resynthesis [24,26]. Aside from the role played by MutLα in DNA MMR, it also participates in cell damage signaling, the control of the cell cycle checkpoint, and directing the cell into the apoptosis pathway [20,21]
Mutations in MMR genes, MLH1, PMS2, MSH2, MSH6, are associated with LS [27,28]. A loss of MMR system functions results in the accumulation of mismatches in microsatellite sequences, which is referred to as MSI [29]. MSI is observed in over 90% of colon tumors in LS patients and only 10–15% in patients with sporadic CRC [13]. In the second case, the presence of microsatellite instability is due to the hypermethylation of the MLH1 promoter and not due to a mutation in the germline [13,30].
Mutations of MSH2 and MLH1 are the most frequent due to their obligatory functions in the MMR system. The most commonly observed types of mutations in these genes are nonsense mutations, in which the substitution of a single base leads to the appearance of a stop codon and results in the reduction of a polypeptide chain (the protein is usually shortened and nonfunctional). Additionally, missense mutations can also be observed [31]. A significant part of the mutations in the MMR genes is unique, characteristic for the family. However, many mutations are already known and are quite commonly observed in LS patients [32].
According to the InSiGHT database, there are over 3000 different variants of MMR genes that predispose LS: MLH1 mutations constitute 40% of the variants, MSH2-30%, MSH6-20% and PMS2-10%. The type of mutations mostly observed in these genes are point mutations, and quite often-significant rearrangement, deletions or insertions, are as well. According to Knudson’s two-hit hypothesis, both copies of the MMR gene have to be inactivated for the tumor’s phenotype manifestation [33]. In an LS-associated cancer, the first hit mutation is usually an inherited point mutation or a massive rearrangement, and the second-a loss of the wild-type allele or gene conversion. Recently, it was observed that constitutional epimutations could serve as the first hit mutation and promoter methylations as the second [34].
In LS, the most common constitutional epimutation is the hypermethylation of the MLH1 promoter in one of two alleles, which leads to silenced gene expression in most somatic tissues and the hypermethylation of MSH2 caused by EPCAM gene mutation [34]. The LS families, in which no mutation in MMR genes’ sequence has ever been previously observed, need to be diagnosed for epigenetic mutations [34].
The epithelial cell adhesion molecule (EPCAM/CD326) is a 39–42 kDa transmembrane glycoprotein almost exclusively expressed in epithelial tissue and epithelial-derived cancers and functions not only in cellular adhesion but also signaling, migration, proliferation and differentiation [35]. Due to a high and stable expression of EPCAM in primary cancers, adenocarcinomas, metastases and malignant effusions and cancer stem cells, including circulating cancer stem cells, it could be used as a biomarker [35]. The expression of EPCAM can be regulated by epigenetic mechanisms, for example, DNA promoter hypomethylation, as well as elements of signaling pathways, for example, the WNT signaling pathway [36]. Proteolytic changes in the transmembrane EPCAM protein leads to the release of extra- and intracellular domains. The cytoplasmic form creates a transcriptional complex with WNT signaling and influences genes connected with cell proliferation and stemness maintenance. The second component, extracellular form, functions as a ligand, which stimulates, for instance, PI3K/AKT/mTOR pathways and supports cancer cell growth [36].
Mutation in EPCAM genes can cause two different, unrelated diseases such as LS or congenital tufting enteropathy (CTE), which depends on the type and nature of changes [37]. The EPCAM gene is located 15 kb upstream from the MSH2 gene and deletions of the 3′ end of the EPCAM gene, including its polyadenylation signal, first cause changes and eventually the inactivation of the promoter of MSH2, while maintaining the expression of EPCAM [38]. It should be noted that these changes are not to be observed in the promoter or start site of MSH2, but only in the polyadenylation signal of the EPCAM gene [39]. It was found that twenty-five 3′ deletions of EPCAM at the sequence level are implicated in causing LS. Most of these deletions are in exons 8 and 9 and are mediated by a recombination between imperfectly homologous Alu repeats [39]. These mutations could cause the loss of the intracellular element of EPCAM without changes in the transmembrane, as well as extracellular domains. However, it is unclear if these truncated EPCAM proteins are created because EPCAM-MSH2 fusions are indicated [37].
Rumilla et al. checked the frequency of deletions of EPCAM (TACSTD1) in MSH2-associated LS cases. They indicated that 20% to 25% of cases possess deletions that were suspected of having a mutation in MSH2, but in which a germline mutation was not detected [40]. In the case of CRC connected with EPCAM deletions, the observed risk of disease is comparable to that of MSH2 mutation. Contrary to this, the risk for endometrial cancer is lower compared to MSH2 mutation carriers. It should, however, be noted that this depends on the size and location of the EPCAM deletion [38]. In CTE, 19 different EPCAM mutations were indicated and they were grouped to chromosomal deletions, non-coding/splicing, frameshift/truncation and missense type. The frameshift mutation c.499dupC is the most important from a clinical point of view [37]. The detailed characteristics of MSH2, MLH1, MSH6, and EPCAM is presented in Table 1.
Table 1.
Characteristics of MSH2, MLH1, MSH6, and EPCAM.
| Gene | Localization on Chromosome | Protein | Type of Mutations Leading to LS | Ref. |
|---|---|---|---|---|
| MSH2 (human mutS homolog 2), 16 exons | 2p21–p16.3 | DNA binding domain, 2 domains interacting with MSH6/MSH3 and MutL homolog | large deletions (whole exons) approx. 30%, secondary epimutation due to the loss of 3′ end of the EPCAM gene | [32,41,42,43,44] |
| MLH1 (human mutL homolog 1), 19 exons | 3p22.2 | 3 domains (ATPase, MutS homologs, PMS2/MLH3/PMS1 interaction domain) | mainly missense, nonsense mutations, splicing aberrations and large rearrangements or constitutional epimutation resulting in hypermethylation of MLH1 promoter | [32,34,45,46] |
| MSH6 (human mutS homolog 6), 10 exons | 2p16.3 | an ATPase domain, a conservative sequence and adenine-repeats consisting motif | mostly missense or nonsense | [29,47] |
| EPCAM (epithelial cell adhesion molecule), 9 exons | 2p21.2 | intracellular domain (EpICD) regulating the expression of other genes responsible for growth, proliferation, migration, and differentiation of cell | deletion of the 3′ end resulting in the loss of termination sequence and production of EPCAM-MSH2 hybrid transcript, and eventually resulting in hypermethylation of MSH2 promoter | [29,41,44,48,49] |
3. Diagnostics of LS
The diagnostics, treatment, and care of patients with LS should differ from methods used in patients with sporadic colorectal cancer, due to genetic and clinical differences of these disease types. Efficient and cost-effective diagnostics of people with suspected LS is crucial to implement an appropriate prevention program or treatment regimen [50].
Diagnostics begin with the identification of families at high risk for cancer through family history and other distinguishing features, such as early age of onset or metachronous disease. The Amsterdam criteria and the Revised Bethesda Guidelines are used in traditional testing for initial identification, Table 2. When a patient fulfills all three of the Amsterdam criteria or at least one criterion of the Revised Bethesda Guidelines, then microsatellite instability testing or/and immunohistochemistry (IHC) testing should be performed [13,51]. However, a universal strategy is becoming more commonly used, and it involves screening all individuals with newly diagnosed CRC with a tumor testing (MSI/IHC) [13,52]. In a patient with an MSI-H (MSI-High) tumor and/or lack of expression of one of MMR proteins, further genetic testing for the identification of a specific mutation or epimutation causing LS should be performed. Such families should receive comprehensive care focused on early diagnosis, which would enable earlier treatment and, at the same time, increase the survival rate among high-risk patients [13,51].
Table 2.
| Amsterdam Criteria I: |
| At least three relatives with histologically verified colorectal cancer and one of which is a first-degree relative of the other two *, At least two successive generations affected, At least one of the relatives with colorectal cancer diagnosed at < 50 years of age. |
| Amsterdam Criteria II: |
| At least three relatives with histologically verified HNPCC-associated cancer (colorectal cancer, endometrial, stomach, ovary, ureter/renal pelvis, brain, small bowel, hepatobiliary tract and skin (sebaceous tumors) and one of which is a first-degree relative of the other two *, At least two successive generations affected, At least one of the HNPCC-associated cancers should be diagnosed at < 50 years of age. |
| Revised Bethesda Guidelines: |
| CRC diagnosed at < 50 years of age, Presence of synchronous or metachronous CRC or other LS-associated tumors ** regardless of age, Colorectal cancer with MSI-H histology diagnosed in a patient < 60 years of age, Colorectal cancer or LS-associated * tumor diagnosed under the age of 50 years in at least one first-degree relative, Colorectal cancer or LS-associated tumor ** diagnosed at any age in two first- or second-degree relatives |
* Familial adenomatous polyposis should be excluded. ** LS-associated tumors include a tumor of the colorectum, endometrium, ovary, pancreas, stomach, renal pelvis, ureter, brain, biliary tract, small bowel, sebaceous glands, and keratoacanthomas.
3.1. Clinical Diagnostics
Clinical diagnostics of LS are mainly based on the Amsterdam criteria I and II, and the Revised Bethesda Guidelines, Table 2. The application of the Amsterdam criteria I involves recognition of patients at a higher risk of developing LS-associated colorectal cancer based on patients’ family history and clinical evaluation [7,13]. However, the Amsterdam criteria I was revised due to the subsequent discoveries of extracolonic cancers also being associated with LS. From now on, the Amsterdam criteria II also included the occurrence of LS-associated extracolonic cancer [13,53].
Nevertheless, several studies reveal the low sensitivity (22%) and specificity (98%) of the Amsterdam criteria. For this reason, new criteria for the identification of LS patients were introduced, namely the Revised Bethesda Guidelines. In patients meeting at least one of the guidelines, screening for mutation carriers in MLH1, MSH2, MSH6, or PMS2 genes is performed by way of immunohistochemistry and/or microsatellite instability testing [13]. It is also recommended to perform both screening tests also in patients’ first degree relatives [55]. Unfortunately, both the Amsterdam criteria and the Revised Bethesda Guidelines are not sensitive enough to detect all patients with LS. The patients’ pedigree is not always reliable or available. Furthermore, not every patient with LS fulfills all of the Amsterdam criteria [7,56]. Adar et al. noted that the Amsterdam II criteria and the Revised Bethesda Guidelines would have missed nearly 62.5% and 50% of the LS cases in the study, respectively, and also recommend future development of a universal screening program for CRCs as well as ECs, which would increase the identification of LS [57].
Additionally, clinical prediction models are also in use, such as MMRpredict, MMRpro, Prediction of Mismatch Repair Gene Mutations in MLH1, MSH2, and MSH6 (PREMM 1, 2, 6) [13,58] or PREdiction Model for gene Mutations (PREMM5), which may be considered as PREMM1,2,6 replacement [59]. Their function is to determine the risk of carrying mutations in MMR genes in individuals with suspected LS. The aforementioned prediction models are useful when the suspected individual is unaffected, or the performance of MSI testing is impossible [60]. Thus, they can also be used as one of the criteria allowing further MSI/IHC testing when the calculated risk is ≥5% [13,60] or ≥2.5% in PRIMM5 [59]. The used clinical criteria, function, sensitivity, and specificity of the models are presented in Table 3.
Table 3.
| Prediction Model | Analyzed Criteria | Models’ Function | Sensitivity [%] | Specificity [%] |
|---|---|---|---|---|
| MMRpredict | Sex, age of CRC diagnosis, tumor location, synchronous or metachronous CRCs, EC in first-degree relatives and age of diagnosis | Calculating risk of carrying characteristics for Lynch syndrome mutations | 69 | 90 |
| MMRpro | Personal and family history of CRC and EC, age of diagnosis, if available—results of molecular testing for MMR genes | Calculating the risk of carrying germline mutations in any of the MLH1/MSH2/MSH6 genes and risk of developing LS-associated cancer | 89 | 85 |
| PREMM 1, 2, 6 | Sex, personal and family history of LS-associated cancers | Calculating the risk of carrying mutations in MLH1/MSH2/MSH6 in the individual with suspected LS | 90 | 67 |
| PREMM5 | Sex, age at genetic testing, personal and family cancer history | Calculating the risk of carrying mutations in MLH1/MSH2/MSH6/PMS2/EPCAM in the individual with suspected LS | 89.4 | 49 |
3.2. Immunohistochemistry and Microsatellite Instability Testing
Before performing costly molecular testing in individuals with suspected LS, it is recommended to undertake screening tests first, which may suggest the probability of MMR gene mutation. MSI and IHC are used to identify patients at high risk of LS for further genetic testing [7]. The screening tests are based on tumor testing performed on formalin-fixed tissue from surgical specimens [61].
Immunohistochemistry can directly indicate a lack of a particular MMR protein expression, saving time and costs of testing other MMR genes. IHC uses primary monoclonal antibodies directed against specific proteins, in diagnostics for LS, against MSH2, MLH1, MSH6, and PMS2 proteins. The sensitivity and specificity of immunohistochemistry are approximately 83% and 89%, respectively [7,13,61,62].
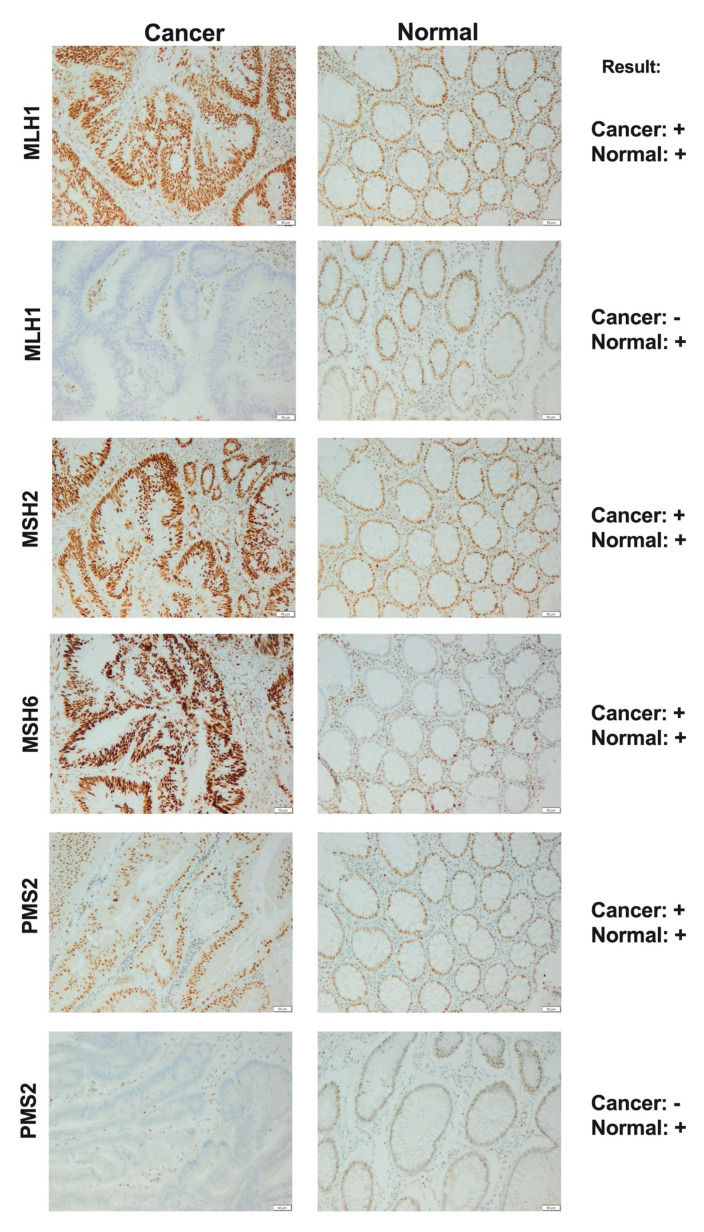
MSH2 and MLH1 are obligatory proteins in the MMR system and they dimerize with secondary proteins-MSH6 and PMS2, respectively. When the mandatory protein is defective, the whole heterodimer becomes unstable, and the secondary protein becomes degraded. On the other hand, when the secondary protein is altered, the heterodimer can remain stable since obligatory proteins can also form heterodimers with other secondary proteins (MSH3, MLH3, PMS1) [7,61]. Shia et al. [61] showed that in IHC testing, a 2-antibody panel (MSH6 and PMS2) might be as predictive as a widely used 4-antibody panel (MSH2, MLH1, MSH6, PMS2), Figure 3. The antibody against MSH6 can detect a loss of protein expression both in MSH6 and MSH2, and the antibody against PMS2-in both PMS2 and MLH. The proposed approach may increase cost-effectiveness in an already widely used screening method. However, the 2-antibody panel should be used only as a first-line screening method and if any abnormality is detected, the secondary IHC with the use of additional antibodies should be undertaken.
Figure 3.
Immunohistochemistry staining of MLH1, MSH2, MSH6 and PMS2 proteins in colon cancer and normal tissue as the example of a diagnostic panel. The scale bar represents 50 μm; “+”—positive staining; “−”—negative staining.
When it comes to the interpretation of IHC results, a loss of expression of the MMR protein is confirmed based on the complete absence of staining in the tumor tissue. However, in some cases, a heterogeneity of staining can be observed. This includes both areas with weak and thus uncertain staining as well as areas with both strong and no staining. Such heterogeneity can create false positive or false negative results [63,64,65]. The possible IHC results and their interpretations are presented in Table 4.
Table 4.
| IHC Results | Interpretation |
|---|---|
| Retained MMR proteins expression | (a) When MSI-H tumor—germline mutation in MMR/EPCAM genes but possibly maintained protein expression (b) when MSI−L/MSS-sporadic cancer |
| Heterogeneity of MMR protein expression | If heterogeneity is observed despite proper performance of IHC, it might be reasonable to consider further molecular testing. |
| Loss of MSH2 protein expression | Germline MSH2 mutation |
| Loss of MSH6 protein expression | Germline MSH6 mutation, rarely MSH2 |
| Loss of MSH2 and MSH6 protein expression | Germline MSH2/EPCAM mutation, rarely MSH6 |
| Loss of MLH1 and PMS2 protein expression | Sporadic cancer or germline MLH1 mutation—recommendation: further BRAF/MLH1 methylation testing |
| Loss of MLH1 protein expression | Germline MLH1 mutation |
| Loss of PMS2 protein expression | Germline PMS2 mutation, rarely MLH1 |
Microsatellite instability testing is also a screening test performed on patients who fulfill the Amsterdam criteria I or II or the Revised Bethesda Guidelines, or due to the universal screening strategy-all new patients affected by CRC [13,52,66].
MSI can be used for the identification of tumors caused by a defective MMR system. Microsatellites are the DNA stretches especially susceptible to acquiring errors when the MMR system is faulty. The identification process is based on analyzing the difference in the length of microsatellite repeats in tumor tissue compared to the healthy non-neoplastic tissue surrounding the tumor. Generally, panels of 10 markers [67] and commercially available kits [68,69] are in use, as well as a panel proposed by Bethesda [7,70,71].
Before DNA amplification, the microdissection of examined tissue must be performed. Microsatellite instability is considered to be present when, in DNA samples from tumor tissue, an additional amplicon of a different size is observed, compared to DNA samples from healthy tissue from the same patient. The additional amplicon indicates insertion/deletion in the examined microsatellite sequence and its length is changed. When ≥30% of the markers are unstable, the tumor is considered MSI-H, when less than 30% of the markers are unstable, the tumor is considered MSI-L (MSI-Low). When no additional amplicons are observed, the tumor is considered microsatellite stable (MSS). Nevertheless, it was also noted that microsatellite instability could be stated if at least one mononucleotide marker is unstable, which can reduce the number of markers in the panel and makes all diagnostic processes more cost-effective [7,62,72].
Alternatively, microsatellite testing can also be carried out using next-generation sequencing (NGS). No healthy tissue is needed as a benchmark in this method, which makes it possible to perform MSI testing even in individuals from whom obtaining healthy tissue is problematic or impossible. MSI-NGS can easily be included in panels already used in molecular diagnostics, which would reduce both the number of tests and overall costs [73].
Sometimes, in tumors in patients with LS-associated cancer, all four MMR proteins are present, but nevertheless microsatellite instabilities are observed. This phenomenon can be explained by the type of mutation in the altered gene. In this case, the expression of a stable protein with a regular epitope, although unfunctional, is observed [7,72].
When MSI-H and lack of expression of both MLH1 and PMS2 are observed, tumor BRAF V600E mutation and/or MLH1 promoter hypermethylation testing is recommended to distinguish sporadic colorectal cancer from CRC caused by an MMR defective system [13,52,62,66].
BRAF mutation analysis is performed using immunohistochemistry and the VE1 antibody, the PCR reaction, or exon 15 sequencing. BRAF testing is widely used in LS diagnostics since the V600E mutation does not occur in LS patients but it is observed in half of the patients with sporadic CRC [74,75]. When BRAF mutation occurs, no further diagnostics for LS is necessary (LS excluded). If there is no BRAF mutation in a tumor, performing further genetic tests for MLH1 and PMS2 germline mutations is recommended [76]. The sensitivity and specificity of BRAF V600E IHC are 69 and 99%, respectively [77].
Besides BRAF mutation analysis, MLH1 promoter methylation also can be performed if MLH1 and PMS2 proteins are absent. If the hypermethylation and no BRAF mutation occur in the tumor tissue, it is recommended to analyze MLH1 promoter methylation results in the healthy tissue to determine epimutation. Epigenetic mutations that cause LS are rare but may be present in both tumor and healthy tissue [13].
Microsatellite instability in tumor tissue is evidence of MMR germline mutation. However, through MSI testing, a specific altered gene cannot be determined. It is also impossible to distinguish between LS-associated and sporadic cancer. On the other hand, immunohistochemistry allows to identify the specific, mutated gene in the examined individual but does not differentiate a somatic and germline mutation. Therefore, using both methods guarantees extensive and reliable screening diagnostics for patients with LS [55,78]. However, if only one of the tumor tests can be performed, the choice between MSI and IHC should depend on the preferences of the physician, staff opinion, or the available technologies [79]. The MSI and IHC diagnostics are significant also because of the different responses to treatment of dMMR (MMR-deficient)/MSI tumors. In patients with dMMR/MSI tumors, distant and local lymph node metastases are rarer and fewer advanced stage tumors are observed [5,72].
3.3. Molecular Testing
Further molecular diagnostics should be performed in patients and their families in which MSI-H and/or lack of expression of at least one MMR protein were observed. The molecular diagnostics should be complex because none of the methods can detect all types of possible mutations in MMR/EPCAM genes. When the family mutation is known, the appropriate diagnostic method should be used, depending on the type of mutation. If a mutation is unknown, methods that enable gene scanning should be used. Gene scanning methods for unknown mutations and their mechanisms are presented in Table 5. Currently, next-generation sequencing (NGS) is the most commonly used [55,59]. However, the Sanger sequencing method is still considered to be the gold standard [77]. DNA sequencing allows us to detect and identify both point mutations and small deletions/insertions [7].
Table 5.
Gene scanning methods for unknown mutations and their mechanisms.
| Method | The Mechanism/Application | Ref. |
|---|---|---|
| Single-Strand Conformation Polymorphism (SSCP) |
|
[80,81] |
| Denaturing Gradient Gel Electrophoresis (DGGE) |
|
[80,81] |
| Denaturing High-Pressure Liquid Chromatography (DHPLC) |
|
[82,83] |
| Conformation-sensitive Gel Electrophoresis (CSGE) |
|
[84] |
| High-Resolution Melting (HRM) |
|
[85] |
Comprehensive analysis of MMR genes can be problematic due to their large size. Therefore, high throughput and massive parallel methods are required, such as next-generation sequencing (NGS) technology [85].
With the NGS method, it is possible to sequence the whole genome (WGS, whole-genome sequencing), whole exome (WES, whole-exome sequencing), or to perform targeted gene sequencing. The main advantage of NGS is the ability to detect single nucleotide variations (SNVs) or small insertions/deletions in several genes simultaneously [7,86,87]. Useful features include short time of analysis, detection of meager input of nucleic acids [86], and sensitivity and specificity reaching 99.9% for both parameters [77].
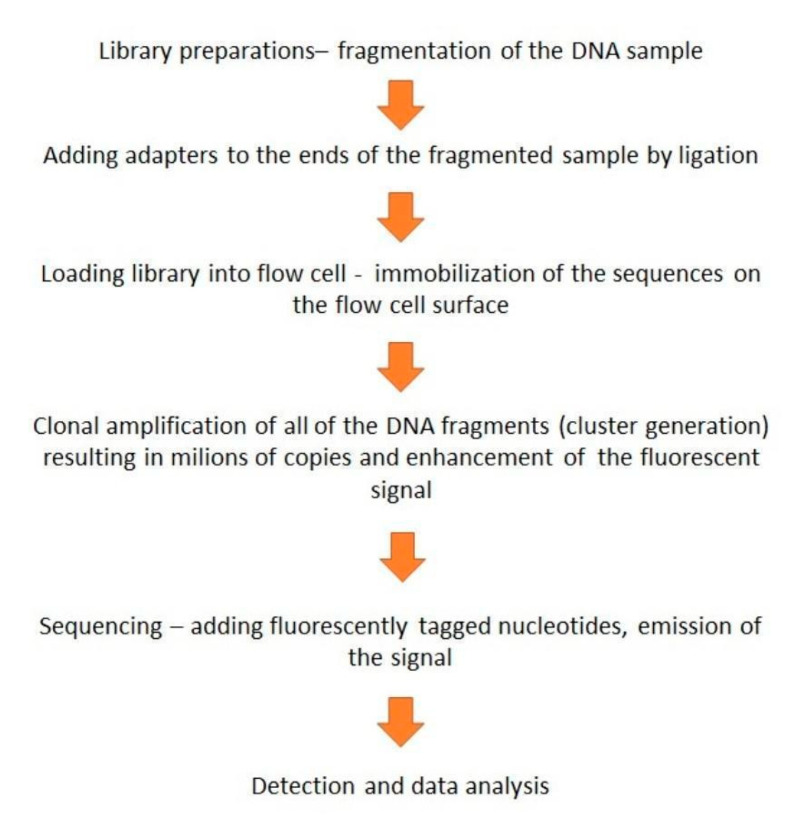
Targeted gene panels save time, reduce costs, and enable the exclusive analysis of areas of interest. Also, the sequencing is performed at a high depth, up to 500–1000× while in WGS the level of coverage is at 30–50×. The most commonly used type of NGS is sequencing by synthesis, the method developed by Illumina [88]. The process of sequencing is presented in Figure 4.
Figure 4.
Schematically presented stages of next-generation sequencing (NGS) sequencing by synthesis based on Illumina’s method [88,89].
In LS diagnostics, commercial targeted gene panels, including exons and exon-intron regions of MMR/EPCAM genes, are available (e.g., HNPCC MASTR Plus, Agilent) [86]. However, it is also possible to design panels depending on needs. To simultaneously sequence several genes, a multiplex PCR is performed. Multiple libraries are pooled together and sequenced in the same run by adding discriminatory barcodes to each library [88]. Sanger sequencing as the gold standard is often performed to verify NGS results [77].
When no point mutation is detected, it is recommended to use methods that allow for the identification of more extensive rearrangements, deletions, and insertions, such as Southern blot, Array Comparative Genomic Hybridization (aCGH microarrays) or Multiplex Ligation-dependent Probe Amplification (MLPA) [7,55,62].
MLPA is the most commonly used method in LS diagnostics and makes possible the detection of significant structural mutations, such as genomic deletions, duplications or rearrangements of one or more exons [7], which represent from 5% to 20% of all of the MMR genes mutations [50]. In MLPA, instead of DNA sequencing, the probes are amplified. In the end, the peak pattern analysis indicates which of the sequences shows incorrect copy numbers. The method consists of 5–6 steps and was described in detail for the first time by Schouten et al. [90].
The results are presented as a ratio of 1.0 when both copies of the gene are noted in an examined sample, which means that each probe detected the same amount of the gene copies in both the tested and reference samples (no detected aberrations). A ratio of 0.5 indicates heterozygous deletion and 1.5 indicates heterozygous duplication. For LS, there are already designed sets of probes, which include MLH1 and MSH2 or all MMR and EPCAM genes [91,92]. It is also possible to study the level of methylation of genes using MS-MLPA (Methylation-Specific MLPA). This variant of the MLPA method can be used both for methylation profiling and copy number quantification. The performance of MS-MLPA is similar to a standard MLPA, except for the fact that the MS-MLPA generates two samples-one for copy number detection and one for methylation profiling, and the last one undergoes digestion with the HhaI enzyme, directly after ligation of the probes. Hybrids of probes and unmethylated DNA sequences are digested and do not generate a signal during capillary electrophoresis, unlike hybrids of probes and methylated samples [92,93]. With MS-MLPA, it is possible to detect hypermethylation of MLH1 and MSH2 genes. A summary of the advantages and limitations of molecular applications is presented in Table 6.
Table 6.
Comparison of selected diagnostics methods.
| Method | Advantages, Applications | Disadvantages, Limitations |
|---|---|---|
| Next-generation sequencing (NGS) | sensitivity and specificity >99% [77], massively parallel sequencing in several genes simultaneously, a relatively short time of analysis, detection of low input of DNA samples [86,88], detection of SNVs and small insertions/deletions [94,95] |
advanced bioinformatics systems and large data storage potential [96,97], filtering and data interpretation (various variants can be found when a large number or whole genes are sequenced) [94,97], issues with detecting structural rearrangements or copy number variations (CNVs) [95] |
| Multiplex Ligation-dependent Probe Amplification (MLPA) |
wide diagnostic applications—copy numbers, point mutations detection, methylation profiling, also detected simultaneously, washing unbounded probes are not necessary, a simple and cost-effective method, easy analysis of the results [91,92] |
does not detect balanced mutations, like balanced translocations or inversions (detects only ones which affect the probe binding sequence), probes can be designed only for known mutations—impossible to detect an unknown mutation, the heterozygous deletions analysis is reliable when tumor cells constitute 20–30% of the sample, heterozygous duplication—about 40% [91,92], does not provide precise deletion/insertion characteristics‘ [98] |
| High-Resolution Melting (HRM) |
simple after proper optimization, fast, high-throughput, software supporting optimization available, relatively simple and not-expensive equipment needed [98,99] |
detected variants not characterized, further characterization with another method, e.g., sequencing needed [98,99] |
| Sanger sequencing | the gold standard, mainly for detecting point mutations, high quality reads [98] | not cost-effective when a large number of samples and long sequences are analyzed, technically demanding method [98] |
| Single-Strand Conformation Polymorphism (SSCP) |
detection of point mutations, deletions, and insertions, detection of unknown variants, simple and quite fast method [81] |
low sensitivity and repeatability, amplicons not longer than 200–300 bp, detected variants not characterized, further characterization with another method, e.g., sequencing needed [80] |
| Conformation-sensitive Gel Electrophoresis (CSGE) |
detection of single-nucleotide mutations, small insertions, and deletions, relatively high sensitivity and specificity, cost-effective [84] |
detected aberrations need to be sequenced time-consuming method [84] |
| Denaturing Gradient Gel Electrophoresis (DGGE) |
detection of unknown variants [80], relatively cheap, reliable heteroduplexes detection [98] |
technically demanding, results must be characterized by another method, e.g., sequencing [80,98], GC-rich regions can be difficult to optimize and analyze [98,100] |
| Denaturing High-Pressure Liquid Chromatography (DHPLC) |
sensitivity nearly 100% [82], a wide spectrum of applications: mutations and SNP detection, gene mapping, gene expression and methylation analysis [82,101], does not require modified primers or specific reagents [101], relatively cheap [81] |
does not detect copy number aberrations [92], detected variants need to be characterized by sequencing, when more than one melting domain in tested amplicon-analysis of several temperatures required [82], chemical waste generation, not a high-throughput method [85] |
| Southern blot | detection of large insertions/deletions [82] | not always small deletions are detected [82] time-consuming [98] |
4. The Care and Treatment of Patients with LS and Their Families
The risk of developing cancer in individuals with LS before the age of 70 is approximately 80%. Therefore, it is crucial to identify patients with MMR/EPCAM mutations as soon as possible. This allows for the commencement of observation and care programs for affected individuals and their families. Early cancer detection will result in increased treatment efficiency and patients’ survival rates [13,52,102]. It is recommended for LS patients to attend genetic counseling before and after genetic testing to clarify any clinical, ethical, financial, or social issues that may arise during the diagnostics process. Also, educating the patient about the disease, the cancer risk, discussing the test results, and presenting further diagnostics or treatment options are also crucial factors. Patients diagnosed with LS should also be undergoing some form of psychological care [13,102].
Individuals with LS or high risk patients are usually recommended to perform a colonoscopy every 1–2 years starting at 20–25 years old if they carry mutations in MSH2 or MLH1 genes [13,52,66]. However, some studies indicate that colonoscopy surveillance once every two years might be more cost-effective than the annual approach [103,104]. In the case of an individual carrying a mutation in MSH6 or PMS2, some sources advise considering the later inception of colonoscopy surveillance due to lower rates of CRC in these individuals [13,52,66,105]. In short, there are no formal or compelling guidelines for prophylactic colorectal surgery in LS [106].
Patients at high risk of LS should also be screened for extracolonic cancers. Several approaches are proposed for EC and ovarian cancer screening, such as pelvic examinations and endometrial sampling, starting at the age of 30–35 years every year, a transvaginal ultrasound or determination of CA-125 concentration in serum. Nevertheless, there is still a need for more evidence pointing to these screening methods’ influence on the mortality or cancer rate [13,52]. Prophylactic hysterectomy and salpingo-oophorectomy should also be considered for women with LS who have given birth or are in their 40s [13,52,56]. For gastric cancer screening, it is recommended to undertake esophagogastroduodenoscopy (EGD) with gastric biopsy at age 30–35 years and H. pylori testing at least [13,52,107]. Currently, the surveillance guidelines for the small intestine, urinary tract, pancreas, or prostate cancer in LS patients are still lacking or are limited [13,52].
Numerous studies suggest the role of aspirin in preventing cancer, but the evidence is not strong enough to make a recommendation for its regular use. Despite that, it might be suggested to consume 600 mg of aspirin daily for a minimum of two years to reduce LS-associated cancer risk [13,52]. It was also noted that dMMR tumors might respond differently to treatment, for example, 5-fluorouracil treatment. This type of chemotherapy is less effective, whereas irinotecan treatment is characterized by an increased response [5]. Furthermore, MMR-deficient ECs seem to be great candidates for immune checkpoint inhibitor therapies due to significantly increased PD-L1 (programmed cell death ligand) expression both in tumor and immune stromal, compared to carcinomas with sporadic MLH1 hypermethylation [52,108,109]. Hence treatment with anti-PD-1 antibodies, such as pembrolizumab, has great potential, mainly in the treatment of ECs. Pembrolizumab was approved by the Food and Drug Administration (FDA) for the treatment of patients with unresectable/metastatic, MSI-H, or dMMR tumors [108,110,111]. EPCAM was also mentioned as it is correlated with worse survival, and metastasis is the target of therapy. The trifunctional anti-EpCAM with anti-CD3 antibody catumaxomab, under the trade name Removab, is used for intraperitoneal treatment for EPCAM-positive malignant ascites [112]. It should be noted that, additionally, other anti-EPCAM antibodies were investigated with good prognosis for their clinical implication in many cancers [113].
With different responses of MSI-H or dMMR tumors to several treatment approaches, optimizing a personalized treatment strategy for patients with LS-associated cancer might prove to be more promising and may result in decreased mortality. This highlights the need for the detection of individuals with LS.
5. Conclusions and Future Perspectives
The identification of individuals with Lynch syndrome has evolved in the past and continues to rapidly improve. The development of molecular testing allowed for the replacement of time-consuming and demanding screening methods, such as SSCP, DGGE, or DHPLC, with NGS technology. NGS makes it possible to sequence whole gene panels for many patients simultaneously, making the diagnostics process quick, accurate, and reliable. Also, MSI assay can be performed using the next-generation sequencing method and can easily be included in panels already used in molecular diagnostics, which would reduce the costs even further. Although NGS still has its limitations, there is no doubt that this method has revolutionized the whole diagnostics system.
On the other hand, there is still a need for more accurate clinical criteria that would include all LS families. There are still unidentified LS families that were missed in the current approaches. Diagnostics should also be more focused on the identification of unaffected LS individuals rather than individuals with a newly developed tumor. This would lead to early detections and more frequent observations of people with MMR mutation and the introduction of a screening program, which in case of cancer development, will result in quick and more efficient treatment and thus increase the patient survival rate.
Acknowledgments
This work was supported by Poznan University of Medical Sciences, Chair of Medical Biotechnology, Department of Cancer Immunology. Language correction was done by Adam Slowinski from Universal Translations (www.biurotlumaczen.co).
Author Contributions
J.S., T.K.—investigation writing, original draft writing, review and editing; A.T., N.B.-L., M.K., K.G., A.P.—investigation writing, review and editing; V.F., E.B.-R.—investigation; K.L., A.M.—supervision. J.S. and T.K. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuipers E.J., Grady W.M., Lieberman D., Seufferlein T., Sung J.J., Boelens P.G., Van De Velde C.J.H., Watanabe T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller M.F., Ibrahim A.E.K., Arends M.J. Molecular pathological classification of colorectal cancer. Virchows Archiv für Pathologische Anatomie und Physiologie und für klinische Medizin. 2016;469:125–134. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch H.T., Snyder C.L., Shaw T.G., Heinen C.D., Hitchins M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer. 2015;15:181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal R., Sheahan K., O’Connell P.R., Hanly A.M., Martin S.T., Winter D.C. Lynch Syndrome: An Updated Review. Genes. 2014;5:497–507. doi: 10.3390/genes5030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwari A., Roy H.K., Lynch H. Lynch syndrome in the 21st century: Clinical perspectives. Qjm Int. J. Med. 2015;109:151–158. doi: 10.1093/qjmed/hcv137. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami H., Zaanan A., Sinicrope F.A. Microsatellite instability testing and its role in the management of colorectal cancer. Curr. Treat. Options Oncol. 2015;16:30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snowsill T., Coelho H., Huxley N., Jones-Hughes T., Briscoe S., Frayling I.M., Hyde C. Molecular testing for Lynch syndrome in people with colorectal cancer: Systematic reviews and economic evaluation. Heal. Technol. Assess. 2017;21:1–238. doi: 10.3310/hta21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hereditary Nonpolyposis Colorectal Cancer: Practice Essentials, Background, Pathophysiology. [(accessed on 5 May 2020)];2020 Available online: https://emedicine.medscape.com/article/188613-overview.
- 9.Haraldsdottir S., Hampel H., Wu C., Weng D.Y., Shields P.G., Frankel W.L., Pan X., De La Chapelle A., Goldberg R.M., Bekaii-Saab T. Patients with colorectal cancer associated with Lynch syndrome and MLH1 promoter hypermethylation have similar prognoses. Genet. Med. 2016;18:863–868. doi: 10.1038/gim.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liccardo R., De Rosa M., Izzo P., Duraturo F. Novel Implications in Molecular Diagnosis of Lynch Syndrome. Gastroenterol. Res. Pr. 2017;2017:1–12. doi: 10.1155/2017/2595098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinke V., Engel C., Büttner R., Schackert H.K., Schmiegel W.H., Propping P. Hereditary Nonpolyposis Colorectal Cancer (HNPCC)/Lynch Syndrome. Dtsch. Aerzteblatt Online. 2013;110:32–38. doi: 10.3238/arztebl.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastrinos F., Stoffel E.M. History, genetics, and strategies for cancer prevention in Lynch syndrome. Clin. Gastroenterol. Hepatol. 2013;12:715–727. doi: 10.1016/j.cgh.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giardiello F.M., Allen J.I., Axilbund J.E., Boland C.R., Burke C.A., Burt R.W., Church J., Dominitz J.A., Johnson D.A., Kaltenbach T., et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2014;147:502–526. doi: 10.1053/j.gastro.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Wang Y., Li J., Cragun J., Hatch K.D., Chambers S.K., Zheng W. Lynch syndrome related endometrial cancer: Clinical significance beyond the endometrium. J. Hematol. Oncol. 2013;6:22. doi: 10.1186/1756-8722-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonadona V., Bonaiti B., Olschwang S., Grandjouan S., Huiart L., Longy M., Guimbaud R., Buecher B., Bignon Y.-J., Caron O., et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 16.Møller P., Seppälä T., Bernstein I., Holinski-Feder E., Sala P., Evans G.D., Lindblom A., Macrae F., Blanco I., Sijmons R.H., et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut. 2017;67:1306–1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westin S.N., Lacour R.A., Urbauer D.L., Luthra R., Bodurka D.C., Lu K.H., Broaddus R.R. Carcinoma of the Lower Uterine Segment: A Newly Described Association With Lynch Syndrome. J. Clin. Oncol. 2008;26:5965–5971. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowotwory jelita cienkiego (C17)|KRN. [(accessed on 2 June 2019)]; Available online: http://onkologia.org.pl/nowotwory-jelita-cienkiego-c17/?fbclid=IwAR1dPUXhvZHN_HDMKSlgtQbKBf323zYw-fnTG2u4CQHmJxZzhompLuv4A9c.
- 19.Bansidhar B.J. Extracolonic Manifestations of Lynch Syndrome. Clin. Colon Rectal Surg. 2012;25:103–110. doi: 10.1055/s-0032-1313781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarné A. The Functions of MutL in Mismatch Repair. Prog. Mol. Biol. Transl. Sci. 2012;110:41–70. doi: 10.1016/b978-0-12-387665-2.00003-1. [DOI] [PubMed] [Google Scholar]
- 21.Li G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2007;18:85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 22.Groothuizen F.S., Winkler I., Cristovão M., Fish A., Winterwerp H.H., Reumer A., Marx A.D., Hermans N., Nicholls R.A., Murshudov G.N., et al. MutS/MutL crystal structure reveals that the MutS sliding clamp loads MutL onto DNA. eLife. 2015;4:e06744. doi: 10.7554/eLife.06744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukui K. DNA Mismatch Repair in Eukaryotes and Bacteria. J. Nucleic Acids. 2010;2010:1–16. doi: 10.4061/2010/260512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadyrova L.Y., A Kadyrov F. Endonuclease activities of MutLα and its homologs in DNA mismatch repair. DNA Repair. 2016;38:42–49. doi: 10.1016/j.dnarep.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishel R. Mismatch Repair. J. Biol. Chem. 2015;290:26395–26403. doi: 10.1074/jbc.R115.660142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goellner E.M., Putnam C.D., Kolodner R.D. Exonuclease 1-dependent and independent mismatch repair. DNA Repair. 2015;32:24–32. doi: 10.1016/j.dnarep.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-López J.V., Fishel R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Fam. Cancer. 2013;12:159–168. doi: 10.1007/s10689-013-9635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sijmons R.H., Hofstra R.M. Review: Clinical aspects of hereditary DNA Mismatch repair gene mutations. DNA Repair. 2016;38:155–162. doi: 10.1016/j.dnarep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Mahalingam M. MSH6, Past and Present and Muir–Torre Syndrome—Connecting the Dots. Am. J. Dermatopathol. 2017;39:239–249. doi: 10.1097/DAD.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 30.Boland C.R., Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterol. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genetyka, krótkie wykłady, Fletcher, Hickey, Winter-Pobierz pdf z Docer.pl, Docer.pl. [(accessed on 4 June 2019)]; Available online: https://docer.pl/doc/n11180s.
- 32.Peltomaki P. Update on Lynch syndrome genomics. Fam. Cancer. 2016;15:385–393. doi: 10.1007/s10689-016-9882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knudson A.G. Mutation and Cancer: Statistical Study of Retinoblastoma. Proc. Natl. Acad. Sci. USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peltomaki P. Epigenetic mechanisms in the pathogenesis of Lynch syndrome. Clin. Genet. 2014;85:403–412. doi: 10.1111/cge.12349. [DOI] [PubMed] [Google Scholar]
- 35.Patriarca C., Macchi R.M., Marschner A.K., Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012;38:68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Mohtar M.A., Syafruddin S.E., Nasir S.N., Low T.Y., Low T.Y. Revisiting the Roles of Pro-Metastatic EpCAM in Cancer. Biomolecules. 2020;10:255. doi: 10.3390/biom10020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pathak S.J., Mueller J.L., Okamoto K., Das B., Hertecant J., Greenhalgh L., Cole T., Pinsk V., Yerushalmi B., Gürkan Ödül E., et al. EPCAM mutation update: Variants associated with congenital tufting enteropathy and Lynch syndrome. Hum. Mutat. 2018;40:142–161. doi: 10.1002/humu.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ligtenberg M.J.L., Kuiper R.P., Van Kessel A.G., Hoogerbrugge N. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam. Cancer. 2012;12:169–174. doi: 10.1007/s10689-012-9591-x. [DOI] [PubMed] [Google Scholar]
- 39.Lynch H.T., Riegert-Johnson D.L., Snyder C., Lynch J.F., Hagenkord J., Boland C.R., Rhees J., Thibodeau S.N., Boardman L.A., Davies J., et al. Lynch Syndrome-Associated Extracolonic Tumors Are Rare in Two Extended Families With the Same EPCAM Deletion. Am. J. Gastroenterol. 2011;106:1829–1836. doi: 10.1038/ajg.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rumilla K., Schowalter K.V., Lindor N.M., Thomas B.C., Mensink K.A., Gallinger S., Holter S., Newcomb P.A., Potter J.D., Jenkins M.A., et al. Frequency of Deletions of EPCAM (TACSTD1) in MSH2-Associated Lynch Syndrome Cases. J. Mol. Diagn. 2011;13:93–99. doi: 10.1016/j.jmoldx.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domingo E., Schwartz S.J. MSH2 (human mutS homolog 2) Atlas Genet. Cytogenet. Oncol. Haematol. 2011 doi: 10.4267/2042/38240. [DOI] [Google Scholar]
- 42.Niessen R.C., Hofstra R.M., Westers H., Ligtenberg M.J.L., Kooi K., Jager P.O.J., De Groote M.L., Dijkhuizen T., Olderode-Berends M.J.W., Hollema H., et al. Germline hypermethylation ofMLH1andEPCAMdeletions are a frequent cause of Lynch syndrome. Genes Chromosom. Cancer. 2009;48:737–744. doi: 10.1002/gcc.20678. [DOI] [PubMed] [Google Scholar]
- 43.G. H. Reference, “MSH2 gene,” Genetics Home Reference. [(accessed on 23 February 2019)]; Available online: https://ghr.nlm.nih.gov/gene/MSH2.
- 44.Schnell U., Cirulli V., Giepmans B.N. EpCAM: Structure and function in health and disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2013;1828:1989–2001. doi: 10.1016/j.bbamem.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Domingo E., Schwartz S.J. MLH1 (human mutL homolog 1) Atlas Genet. Cytogenet. Oncol. Haematol. 2011 doi: 10.4267/2042/38179. [DOI] [Google Scholar]
- 46.Morak M., Koehler U., Schackert H.K., Steinke V., Royer-Pokora B., Schulmann K., Kloor M., Höchter W., Weingart J., Keiling C., et al. Biallelic MLH1 SNP cDNA expression or constitutional promoter methylation can hide genomic rearrangements causing Lynch syndrome. J. Med. Genet. 2011;48:513–519. doi: 10.1136/jmedgenet-2011-100050. [DOI] [PubMed] [Google Scholar]
- 47.MSH6 (mutS homolog 6; E. Coli) [(accessed on 17 March 2019)]; Available online: http://atlasgeneticsoncology.org//Genes/MSH6ID344ch2p16.html.
- 48.EPCAM (Tumor-Associated Calcium Signal Transducer 1) [(accessed on 17 March 2019)]; Available online: http://atlasgeneticsoncology.org//Genes/TACSTD1ID42459ch2p21.html.
- 49.EPCAM gene [(accessed on 26 March 2019)];Genetics Home Reference. Available online: https://ghr.nlm.nih.gov/gene/EPCAM.
- 50.Pérez-Cabornero L., Velasco E.A., Infante M., Sanz D., Lastra E., Hernández L., Miner C., Durán M. A new strategy to screen MMR genes in Lynch Syndrome: HA-CAE, MLPA and RT-PCR. Eur. J. Cancer. 2009;45:1485–1493. doi: 10.1016/j.ejca.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Maradiegue A., Jasperson K., Edwards Q.T., Lowstuter K., Weitzel J. Scoping the family history: Assessment of Lynch syndrome (hereditary nonpolyposis colorectal cancer) in primary care settings—A primer for nurse practitioners. J. Am. Acad. Nurse Pr. 2008;20:76–84. doi: 10.1111/j.1745-7599.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 52.Yurgelun M.B., Hampel H. Recent Advances in Lynch Syndrome: Diagnosis, Treatment, and Cancer Prevention. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:101–109. doi: 10.1200/EDBK_208341. [DOI] [PubMed] [Google Scholar]
- 53.Vasen H.F.A., Watson P., Mecklin J., Lynch H.T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/S0016-5085(99)70510-X. [DOI] [PubMed] [Google Scholar]
- 54.Lynch H.T., Boland C.R., Gong G., Shaw T.G., Lynch P.M., Fodde R., Lynch J.F., De La Chapelle A. Phenotypic and genotypic heterogeneity in the Lynch syndrome: Diagnostic, surveillance and management implications. Eur. J. Hum. Genet. 2006;14:390–402. doi: 10.1038/sj.ejhg.5201584. [DOI] [PubMed] [Google Scholar]
- 55.Nallamilli B.R.R., Hegde M. Genetic Testing for Hereditary Nonpolyposis Colorectal Cancer (HNPCC) Curr. Protoc. Hum. Genet. 2017;94:10.12.1–10.12.23. doi: 10.1002/cphg.40. [DOI] [PubMed] [Google Scholar]
- 56.Wang A., McCracken J., Li Y., Xu L. The practice of universal screening for Lynch syndrome in newly diagnosed endometrial carcinoma. Health Sci. Rep. 2018;1:e43. doi: 10.1002/hsr2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adar T., Rodgers L., Shannon K.M., Yoshida M., Ma T., Mattia A., Lauwers G.Y., Iafrate A.J., Hartford N.M., Oliva E., et al. Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer. 2018;124:3145–3153. doi: 10.1002/cncr.31534. [DOI] [PubMed] [Google Scholar]
- 58.Kastrinos F., Ojha R.P., Leenen C., Alvero C., Mercado R.C., Balmana J., Valenzuela I., Balaguer F., Green R., Lindor N.M., et al. Comparison of Prediction Models for Lynch Syndrome Among Individuals With Colorectal Cancer. J. Natl. Cancer Inst. 2015;108 doi: 10.1093/jnci/djv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kastrinos F., Uno H., Ukaegbu C., Alvero C., McFarland A., Yurgelun M.B., Kulke M.H., Schrag D., Meyerhardt J.A., Fuchs C.S., et al. Development and Validation of the PREMM5 Model for Comprehensive Risk Assessment of Lynch Syndrome. J. Clin. Oncol. 2017;35:2165–2172. doi: 10.1200/JCO.2016.69.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luba D.G., DiSario J.A., Rock C., Saraiya D., Moyes K., Brown K., Rushton K., Ogara M.M., Raphael M., Zimmerman D., et al. Community Practice Implementation of a Self-administered Version of PREMM1,2,6 to Assess Risk for Lynch Syndrome. Clin. Gastroenterol. Hepatol. 2017;16:49–58. doi: 10.1016/j.cgh.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shia J., Tang L.H., Vakiani E., Guillem J.G., Stadler Z.K., Soslow R.A., Katabi N., Weiser M.R., Paty P.B., Temple L.K., et al. Immunohistochemistry as First-line Screening for Detecting Colorectal Cancer Patients at Risk for Hereditary Nonpolyposis Colorectal Cancer Syndrome. Am. J. Surg. Pathol. 2009;33:1639–1645. doi: 10.1097/PAS.0b013e3181b15aa2. [DOI] [PubMed] [Google Scholar]
- 62.Hegde M., A Working Group of the American College of Medical Genetics and Genomics (ACMG) Laboratory Quality Assurance Committee. Ferber M., Mao R., Samowitz W., Ganguly A. ACMG technical standards and guidelines for genetic testing for inherited colorectal cancer (Lynch syndrome, familial adenomatous polyposis, and MYH-associated polyposis) Genet. Med. 2013;16:101–116. doi: 10.1038/gim.2013.166. [DOI] [PubMed] [Google Scholar]
- 63.McCarthy A.J., Capo-Chichi J.-M., Spence T., Grenier S., Stockley T., Kamel-Reid S., Serra S., Sabatini P., Chetty R. Heterogenous loss of mismatch repair (MMR) protein expression: A challenge for immunohistochemical interpretation and microsatellite instability (MSI) evaluation. J. Pathol. Clin. Res. 2018;5:115–129. doi: 10.1002/cjp2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joost P., Veurink N., Holck S., Klarskov L., Bojesen A., Harbo M., Baldetorp B., Rambech E., Nilbert M. Heterogenous mismatch-repair status in colorectal cancer. Diagn. Pathol. 2014;9:126. doi: 10.1186/1746-1596-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W., Pearlman R., Hampel H., Pritchard C.C., Markow M., Arnold C., Knight D., Frankel W.L. MSH6 immunohistochemical heterogeneity in colorectal cancer: Comparative sequencing from different tumor areas. Hum. Pathol. 2020;96:104–111. doi: 10.1016/j.humpath.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syngal S., Brand R.E., Church J.M., Giardiello F.M., Hampel H.L., Burt R.W. American College of Gastroenterology ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am. J. Gastroenterol. 2015;110:223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poynter J.N., Siegmund K.D., Weisenberger D.J., Long T.I., Thibodeau S.N., Lindor N.M., Young J., Jenkins M.A., Hopper J.L., Baron J.A., et al. Molecular Characterization of MSI-H Colorectal Cancer by MLHI Promoter Methylation, Immunohistochemistry, and Mismatch Repair Germline Mutation Screening. Cancer Epidemiol. Biomark. Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suraweera N., Duval A., Reperant M., Vaury C., Furlan D., Leroy K., Seruca R., Iacopetta B., Hamelin R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123:1804–1811. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L. Immunohistochemistry versus Microsatellite Instability Testing for Screening Colorectal Cancer Patients at Risk for Hereditary Nonpolyposis Colorectal Cancer Syndrome. J. Mol. Diagn. 2008;10:301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Losso G.M., Moraes R.D.S., Gentili A.C., Messias-Reason I.T. Microsatellite instability--MSI markers (BAT26, BAT25, D2S123, D5S346, D17S250) in rectal cancer. ABCD Arquivos Brasileiros Cirurgia Digestiva (São Paulo) 2013;25:240–244. doi: 10.1590/S0102-67202012000400006. [DOI] [PubMed] [Google Scholar]
- 71.Umar A., Boland C.R., Terdiman J.P., Syngal S., De La Chapelle A., Rüschoff J., Fishel R., Lindor N.M., Burgart L.J., Hamelin R., et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibson J., Lacy J., Matloff E., Robert M. Microsatellite Instability Testing in Colorectal Carcinoma: A Practical Guide. Clin. Gastroenterol. Hepatol. 2014;12:171–176.e1. doi: 10.1016/j.cgh.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 73.VanderWalde A., Spetzler D., Xiao N., Gatalica Z., Marshall J.L. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7:746–756. doi: 10.1002/cam4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capper D., Voigt A.Y., Bozukova G., Ahadova A., Kickingereder P., Von Deimling A., Doeberitz M.V.K., Kloor M. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int. J. Cancer. 2013;133:1624–1630. doi: 10.1002/ijc.28183. [DOI] [PubMed] [Google Scholar]
- 75.Roth R.M., Hampel H., Arnold C.A., Yearsley M.M., Marsh W.L., Frankel W.L. A Modified Lynch Syndrome Screening Algorithm in Colon Cancer. Am. J. Clin. Pathol. 2015;143:336–343. doi: 10.1309/AJCP4D7RXOBHLKGJ. [DOI] [PubMed] [Google Scholar]
- 76.Jin M., Hampel H., Zhou X., Schunemann L., Yearsley M., Frankel W.L. BRAF V600E Mutation Analysis Simplifies the Testing Algorithm for Lynch Syndrome. Am. J. Clin. Pathol. 2013;140:177–183. doi: 10.1309/AJCPB9FOVH1HGKFR. [DOI] [PubMed] [Google Scholar]
- 77.Castellanos E., Gel B., Rosas I., Tornero E., Santín S., Pluvinet R., Velasco J., Sumoy L., Del Valle J., Perucho M., et al. A comprehensive custom panel design for routine hereditary cancer testing: Preserving control, improving diagnostics and revealing a complex variation landscape. Sci. Rep. 2017;7:39348. doi: 10.1038/srep39348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Svrcek M., Lascols O., Cohen R., Collura A., Jonchère V., Fléjou J.-F., Buhard O., Duval A. MSI/MMR-deficient tumor diagnosis: Which standard for screening and for diagnosis? Diagnostic modalities for the colon and other sites: Differences between tumors. Bull. Cancer. 2019;106:119–128. doi: 10.1016/j.bulcan.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 79.Di Marco M., D’Andrea E., Panić N., Baccolini V., Migliara G., Marzuillo C., De Vito C., Pastorino R., Boccia S., Villari P. Which Lynch syndrome screening programs could be implemented in the “real world”? A systematic review of economic evaluations. Genet. Med. 2018;20:1131–1144. doi: 10.1038/gim.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Konstantinos K.V., Panagiotis P., Antonios V.T., Agelos P., Argiris N.V. PCR–SSCP: A Method for the Molecular Analysis of Genetic Diseases. Mol. Biotechnol. 2007;38:155–163. doi: 10.1007/s12033-007-9006-7. [DOI] [PubMed] [Google Scholar]
- 81.Matyjasik J., Masojć B., Kurzawski G. Molecular analyzes of DNA and RNA in detecting hereditary predisposition to cancer. Postępy Nauk Medycznych, Wydawnictwo Borgis Sp. zoo. 2008:431–440. [Google Scholar]
- 82.Marsh D.J., Howell V.M. The Use of Denaturing High Performance Liquid Chromatography (DHPLC) for Mutation Scanning of Hereditary Cancer Genes. Adv. Struct. Saf. Stud. 2010;653:133–145. doi: 10.1007/978-1-60761-759-4_8. [DOI] [PubMed] [Google Scholar]
- 83.Tysarowski1 A., Fabisiewicz1 A., Kolasa I., Kupryjańczyk J., Ścieglińska D., Rusin M., Krawczyk Z., Woźniak A., Morzuch L., Limon J., et al. Validation of selected molecular methods for the mutations determinationin codons 12 and 13 of K-RAS gene in five Polish oncological research centers. Onkol. Prak. Klin. 2008;6:232–244. [Google Scholar]
- 84.Ganguly A. An update on conformation sensitive gel electrophoresis. Hum. Mutat. 2002;19:334–342. doi: 10.1002/humu.10059. [DOI] [PubMed] [Google Scholar]
- 85.Obul J., Itoga S., Abliz M., Sato K., Ishige T., Utsuno E., Matsushita K., Matsubara H., Nomura F. High-Resolution Melting Analyses for Gene Scanning of APC, MLH1, MSH2, and MSH6 Associated with Hereditary Colorectal Cancer. Genet. Test. Mol. Biomark. 2012;16:406–411. doi: 10.1089/gtmb.2011.0166. [DOI] [PubMed] [Google Scholar]
- 86.Kašubová I., Holubekova V., Janíková K., Váňová B., Sňahničanová Z., Kalman M., Plank L., Lasabova Z. Next Generation Sequencing in Molecular Diagnosis of Lynch Syndrome – a Pilot Study Using New Stratification Criteria. Acta Med. (Hradec Kralove, Czech Republic) 2018;61:98–102. doi: 10.14712/18059694.2018.125. [DOI] [PubMed] [Google Scholar]
- 87.Talseth-Palmer B.A., Bauer D.C., Sjursen W., Evans T.J., McPhillips M., Proietto A., Otton G., Spigelman A.D., Scott R.J. Targeted next-generation sequencing of 22 mismatch repair genes identifies Lynch syndrome families. Cancer Med. 2016;5:929–941. doi: 10.1002/cam4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Next-Generation Sequencing (NGS) | Explore the Technology. [(accessed on 19 April 2019)]; Available online: https://www.illumina.com/science/technology/next-generation-sequencing.html.
- 89.Bronner I.F., Quail M.A., Turner D.J., Swerdlow H. Improved Protocols for Illumina Sequencing. Curr. Protoc. Hum. Genet. 2014;18:18.2.1–18.2.42. doi: 10.1002/0471142905.hg1802s80. [DOI] [PubMed] [Google Scholar]
- 90.Schouten J.P., McElgunn C.J., Waaijer R., Zwijnenburg D., Diepvens F., Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hömig-Hölzel C., Savola S. Multiplex Ligation-dependent Probe Amplification (MLPA) in Tumor Diagnostics and Prognostics. Diagn. Mol. Pathol. 2012;21:189–206. doi: 10.1097/PDM.0b013e3182595516. [DOI] [PubMed] [Google Scholar]
- 92.MRC-Holland-Technology-MLPA-MLPA Technique. [(accessed on 23 April 2019)]; Available online: http://www.mrc-holland.com/WebForms/WebFormMain.aspx?Tag=_hS-AvFINWhkPMYt9ZIZdCx7-VkDGgJqQ1uzZmJTgWTQ.
- 93.Nygren A.O.H., Ameziane N., Duarte H.M.B., Vijzelaar R.N.C.P., Waisfisz Q., Hess C.J., Schouten J.P., Errami A. Methylation-Specific MLPA (MS-MLPA): Simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 2005;33:e128. doi: 10.1093/nar/gni127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandez-Marmiesse A., Gouveia S., Couce M.-L. NGS Technologies as a Turning Point in Rare Disease Research, Diagnosis and Treatment. Curr. Med. Chem. 2018;25:404–432. doi: 10.2174/0929867324666170718101946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yohe S., Thyagarajan B. Review of Clinical Next-Generation Sequencing. Arch. Pathol. Lab. Med. 2017;141:1544–1557. doi: 10.5858/arpa.2016-0501-RA. [DOI] [PubMed] [Google Scholar]
- 96.Ari S., Arikan M. Next-Generation Sequencing: Advantages, Disadvantages, and Future. Plant Omics: Trends Appl. 2016:109–135. [Google Scholar]
- 97.Strengths and Limitations of Next-Generation Sequencing. [(accessed on 3 May 2020)]; Available online: https://www.healio.com/hematology-oncology/learn-genomics/whole-genome-sequencing/strengths-and-limitations-of-next-generation-sequencing.
- 98.Traeger-Synodinos J., Harteveld C.L., Old J.M., Petrou M., Galanello R., Giordano P., Angastioniotis M., De La Salle B., Henderson S., May A., et al. EMQN Best Practice Guidelines for molecular and haematology methods for carrier identification and prenatal diagnosis of the haemoglobinopathies. Eur. J. Hum. Genet. 2014;23:426–437. doi: 10.1038/ejhg.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wittwer C.T. High-resolution DNA melting analysis: Advancements and limitations. Hum. Mutat. 2009;30:857–859. doi: 10.1002/humu.20951. [DOI] [PubMed] [Google Scholar]
- 100.A Knapp L. Denaturing gradient gel electrophoresis and its use in the detection of major histocompatibility complex polymorphism. Tissue Antigens. 2005;65:211–219. doi: 10.1111/j.1399-0039.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 101.Berginc G., Glavač D. Rapid and Accurate Approach for Screening of Microsatellite Unstable Tumours Using Quasimonomorphic Mononucleotide Repeats and Denaturating High Performance Liquid Chromatography (DHPLC) Dis. Mark. 2009;26:19–26. doi: 10.1155/2009/901532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jagadeesh D., Syngal S. Genetic testing for hereditary nonpolyposis colorectal cancer. Curr. Opin. Gastroenterol. 2003;19:57–63. doi: 10.1097/00001574-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Engel C., Vasen H.F., Seppälä T., Aretz S., Bigirwamungu-Bargeman M., De Boer S.Y., Bucksch K., Büttner R., Holinski-Feder E., Holzapfel S., et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries With Different Lynch Syndrome Surveillance Policies. Gastroenterology. 2018;155:1400–1409.e2. doi: 10.1053/j.gastro.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 104.Peterse E.F.P., Naber S.K., Daly C., Pollett A., Paszat L.F., Spaander M.C., Aronson M., Gryfe R., Rabeneck L., Lansdorp-Vogelaar I., et al. Cost-effectiveness of Active Identification and Subsequent Colonoscopy Surveillance of Lynch Syndrome Cases. Clin. Gastroenterol. Hepatol. 2019 doi: 10.1016/j.cgh.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryan N.A.J., Morris J., Green K., Lalloo F., Woodward E.R., Hill J., Crosbie E.J., Evans G.D. Association of Mismatch Repair Mutation With Age at Cancer Onset in Lynch Syndrome. JAMA Oncol. 2017;3:1702–1706. doi: 10.1001/jamaoncol.2017.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menahem B., Alves A., Regimbeau J., Sabbagh C. Lynch Syndrome: Current management In 2019. J. Visc. Surg. 2019;156:507–514. doi: 10.1016/j.jviscsurg.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 107.Biller L.H., Syngal S., Yurgelun M.B. Recent advances in Lynch syndrome. Fam. Cancer. 2019;18:211–219. doi: 10.1007/s10689-018-00117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hacking S., Jin C., Komforti M., Liang S., Nasim M. MMR deficient undifferentiated/dedifferentiated endometrial carcinomas showing significant programmed death ligand-1 expression (sp 142) with potential therapeutic implications. Pathol. Res. Pr. 2019;215:152552. doi: 10.1016/j.prp.2019.152552. [DOI] [PubMed] [Google Scholar]
- 109.Sloan E.A., Ring K.L., Willis B.C., Modesitt S.C., Mills A.M. PD-L1 Expression in Mismatch Repair-deficient Endometrial Carcinomas, Including Lynch Syndrome-associated and MLH1 Promoter Hypermethylated Tumors. Am. J. Surg. Pathol. 2017;41:326–333. doi: 10.1097/PAS.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 110.Ferriss J.S., Williams-Brown M.Y. Immunotherapy: Checkpoint Inhibitors in Lynch-Associated Gynecologic Cancers. Curr. Treat. Options Oncol. 2019;20:75. doi: 10.1007/s11864-019-0676-8. [DOI] [PubMed] [Google Scholar]
- 111.Lemery S., Keegan P., Pazdur R. First FDA Approval Agnostic of Cancer Site — When a Biomarker Defines the Indication. N. Engl. J. Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 112.Heiss M.M., Murawa P., Koralewski P., Kutarska E., Kolesnik O.O., Ivanchenko V.V., Dudnichenko A.S., Aleknaviciene B., Razbadauskas A., Gore M., et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int. J. Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eyvazi S., Farajnia S., Dastmalchi S., Kanipour F., Zarredar H., Bandehpour M., Bandehpour M. Antibody Based EpCAM Targeted Therapy of Cancer, Review and Update. Curr. Cancer Drug Targets. 2018;18:857–868. doi: 10.2174/1568009618666180102102311. [DOI] [PubMed] [Google Scholar]