Abstract
Simple Summary
There is currently no consensus on a widely accepted algorithm for imaging Merkel cell carcinoma (MCC) patients. Baseline, tomographic imaging is not generally recommended in early-stage disease, but its value in locally advanced and/or distant metastatic MCC has been well established. In this context, the hybrid imaging modality positron emission tomography/computed tomography (PET/CT) is increasingly applied in the workup of metastatic or unresectable MCC, providing essential information for staging, restaging, and treatment monitoring of the disease. Although the role of PET/CT in the management of loco-regional MCC is still limited and less well-defined, current evidence suggests its important contribution also in cases of localized MCC. Herein, we provide a structured literature review summarizing the most important studies on the role of PET or PET/CT with different radiopharmaceuticals in the clinical care of MCC.
Abstract
Merkel cell carcinoma (MCC) is a rare neuroendocrine skin malignancy usually arising as a nonspecific nodule on sun-exposed areas of the head and neck. Given the poor prognosis of this aggressive tumor, assessment of disease burden in pre- and post-treatment care may ensure an optimal management with significant implications for patient surveillance and prognosis. Although imaging has established its role in locally advanced or distant metastatic MCC, a standard imaging algorithm is yet to be determined and respective recommendations are mainly based on melanoma. Positron emission tomography/computed tomography (PET/CT) is increasingly evolving as a valuable imaging tool in metastatic or unresectable MCC, mostly utilizing the glucose analogue 18F-fluorodeoxyglucose (18F-FDG) as a radiotracer. Despite being inferior in detecting the disease in its early stages compared to the “gold standard” of sentinel lymph node biopsy, recent evidence suggests an important role for 18F-FDG PET/CT in the routine workup of localized MCC. Moreover, 68Ga-labeled somatostatin analogues have been employed as PET tracers in the field of MCC with promising, yet comparable to 18F-FDG, results. This article provides a structured literature review of the most important studies investigating the role of PET or PET/CT in the clinical practice of MCC.
Keywords: Merkel cell carcinoma (MCC), positron emission tomography/computed tomography (PET/CT), 18F-fluorodeoxyglucose (18F-FDG), 68Ga-labeled somatostatin analogues
1. Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine skin malignancy usually arising as a red/bluish or flesh-colored nodule on sun-exposed areas of the head or neck in elderly, fair-skinned, and/or immunocompromised patients [1,2]. Due to its aggressive nature and nonspecific clinical features, diagnostic delays correlated with high rates of regional (26%) or distant (8%) metastatic spread at presentation are common [1]. Following resection of the primary lesion, recurrence will occur in nearly 30% of cases [3]. The five-year overall survival (OS) is reported to be as high as 51%, 35%, and 14% for local, nodal, and distant disease, respectively [1,4]. Based on the poor prognosis of MCC, assessment of disease burden in pre- and post-treatment care may thus ensure an optimal management adding diagnostic value in staging/restaging and providing therapeutic guidance.
For the management of MCC, the National Comprehensive Cancer Network (NCCN) and the European consensus-based interdisciplinary guidelines propose specific treatment algorithms based on disease extent at presentation [5]. Initial management of loco-regional MCC typically involves complete surgical excision of the tumor lesion (wide, local excision with resection margins of 1–2 cm or Mohs micrographic surgery) followed by adjuvant radiotherapy of the primary site. In the metastatic setting, a case-by-case multidisciplinary approach is recommended. While mono- or poly-chemotherapy alone or in combination with radiotherapy has commonly been used to treat advanced forms, innovative therapies, mostly utilizing immune checkpoint inhibitors of the programmed death-ligand 1 (PD-L1; avelumab) and programmed cell death-1 (PD-1; pembrolizumab, nivolumab) axis, should be considered, if indicated. Moreover, targeted molecular therapies, i.e., somatostatin (SST) analogues, tyrosine kinase inhibitors, and mammalian target of rapamycin inhibitors, are currently in development [6,7,8].
Among imaging modalities, brain magnetic resonance imaging (MRI), neck/chest/abdomen/pelvis computed tomography (CT), and/or whole-body positron emission tomography/computed tomography (PET/CT) appear to be essential for evaluating MCC, especially in advanced stages. However, a standard imaging protocol remains to be established [1,2,9]. Given the much lower incidence compared to melanoma, current MCC imaging guidance reflects the melanoma guidelines [5]. In line with melanoma, whole-body baseline imaging is not generally recommended in early-stage MCC but is of value in locally advanced or distant metastatic disease [5]. However, emerging evidence suggests that baseline imaging enables detection of clinically occult metastatic spread in 12.5% of “localized” MCC cases [10].
In this setting, PET/CT imaging, combining the functional information of PET with the anatomic details of CT, offers superior diagnostic capabilities in several malignancies including MCC [11,12,13]. Particularly, PET/CT using the radiotracer 18F-fluorodeoxyglucose (18F-FDG) is evolving as a powerful imaging tool in the management of MCC, providing high levels of sensitivity and specificity in documenting the disease burden [13]. Herein, we provide a structured literature review summarizing the most important studies on the role of PET or PET/CT in the clinical practice of MCC.
2. Search Strategy and Study Selection
The PubMed/MEDLINE and Scopus databases were searched (last updated in August 2020) for studies investigating the performance of PET and/or PET/CT in MCC patients. The search algorithm was based on the following keywords: “Merkel cell carcinoma”, “MCC”, “Positron Emission Tomography”, “Positron Emission Tomography/Computed Tomography”, “PET”, “PET/CT”, “PET-CT” AND imaging, as well as on combinations of these terms. Additional relevant references were also isolated from citations in the reviewed articles. We focused only on non-preclinical data from English-language medical literature. Of the 22 original articles selected, the full-text versions were retrieved and discussed in this review (Table 1).
Table 1.
Summary of the published studies (including >1 patient) on positron emission tomography (PET) in Merkel cell carcinoma (in chronological order).
| Author (Year) | Study Design | No. of Patients (Mean Age; % Male) | PET Radiotracers | Main Findings |
|---|---|---|---|---|
| Scanga et al. (2004) [14] | Retrospective | 2 (n.r.) | 18F-FDG | 1 TP patient, 1 TN patient |
| Yao et al. (2005) [15] | Report of 2 cases | 2 (68 years; 100%) | 18F-FDG | Pre-treatment PET scans revealed metastatic disease not detected in CT. Post-treatment PET imaging predicted response to therapy. |
| Talbot et al. (2005) [16] | Case series | 3 (63 years; 67%) |
18F-FDOPA 18F-FDG |
2 TP cases with both 18F-FDOPA and 18F-FDG. No uptake of 18F-FDOPA, and unclear findings with 18F-FDG in the TN patient. Lower image contrast with 18F-FDOPA compared to 18F-FDG. |
| Belhocine et al. (2006) [17] | Retrospective | 11 (64 years; 36%) | 18F-FDG | Sensitivity 92% (11 TP, 1 FN), specificity 100% (3 TN, 0 FP). Contributive PET findings in 10/11 MCC cases. |
| Iagaru et al. (2006) [18] | Retrospective case series | 6 (69 years; 67%) | 18F-FDG | 9 TP, 7 TN, 1 FP, and 1 FN lesions. |
| Concannon et al. (2009) [19] | Retrospective | 18 (74 years; 67%) | 18F-FDG | PET/CT altered staging in 33% and management in 43% of cases. Sensitivity 94%. |
| Peloschek et al. (2010) [20] | Retrospective | 16 (75 years; 69%) |
18F-FDG (16 pts.) 18F-FDOPA (5 pts) |
18F-FDG: Sensitivity 85.7%, specificity 96.2% 18F-FDOPA: Negative findings in all cases (19 TN, 2 FN). |
| Maury et al. (2011) [21] | Retrospective | 15 (68 years; 60%) | 18F-FDG | PET/CT had significant impact on staging and management in 46% of cases vs. clinical examination alone. Sensitivity, specificity, PPV, and NPV were 89%, 100%, 100%, and 93%, respectively. |
| Lu et al. (2012) [22] | Retrospective | 9 (70 years; 78%) | 18F-FDG | 18F-FDG PET/CT detected more lesions and staged patients more accurately than 111In-Pentetreotide scintigraphy. 6 TP, and 4 TN scans. |
| Colgan et al. (2012) [23] | Retrospective | 33 (70 years; 72%) * | 18F-FDG | Sensitivity 83%, specificity 95%, PPV 91%, NPV 91% in detecting nodal basin disease. |
| Hawryluk et al. (2012) [24] | Retrospective | 97 (70 years; 58%) | 18F-FDG | PET/CT detected regional nodal disease in 14% of patients, and upstaged 16% of more advanced cases. |
| Schmidt et al. (2012) [25] | Report of 2 cases | 2 (72 years; 50%) | 68Ga-DOTATATE | Disease extent was determined in both cases leading to management changes with inclusion of DOTATATE-peptide receptor radiotherapy in the therapeutic regimen. |
| Siva et al. (2013) [26] | Retrospective | 102 (77 years; n.r.) | 18F-FDG | PET-based staging had a significant impact on management in 37% of cases. High- and medium-impact scans were recorded for 22% and 15% of patients, respectively. PET staging results differed from conventional staging results in 22% of patients. In stratification by PET-defined stage, the 5-year OS was 67% in stage I/II patients and 31% in stage III cases (log-rank p < 0.001). On multivariate analysis, PET staging was significantly associated with OS (p < 0.001). |
| Ibrahim et al. (2013) [27] | Retrospective | 20 (58 years; 45%) | 18F-FDG | Changes in tumor status and management occurred in 20% and 15% of cases, respectively, as a direct result of PET/CT. |
| George et al. (2014) [28] | Retrospective | 23 (74 years #; 57%) | 18F-FDG | Sensitivity 97%, specificity 89%, PPV 94%, NPV 94%, with 2 FP and 1 FN results. Lesions neglected clinically or by conventional imaging were revealed in 44% of PET/CTs at initial presentation and during follow-up, with, respectively, 50% and 41% of scans identifying new lesions. At initial presentation, PET/CT altered tumor staging in 39% of cases. Management was modified by PET/CT in one-third of cases (33% at initial presentation; 32% during follow-up; 36% during evaluation of chemotherapy response). |
| Buder et al. (2014) [29] | Retrospective | 24 (68 years; 67%) |
68Ga-DOTATOC 68Ga-DOTATATE |
Sensitivity 73% for nodal, 100% for bone, and 67% for soft-tissue metastases. Up-staging and management changes in 17% and 13% of cases, respectively, as a result of PET. |
| Byrne et al. (2015) [30] | Retrospective | 62 (n.r.) | 18F-FDG | The impact of PET on disease restaging was high in 45% and medium in 11% of cases, respectively. Patients who achieved no CMR had a 15% 1-year OS, while those with CMR had an 88% 2-year and a 68% 5-year OS. Both CMR achievement and nodal disease were significantly prognostic of the OS. |
| Sollini et al. (2015) [31] | Retrospective | 23 (70 years; 78%) |
68Ga-DOTATOC 68Ga-DOTANOC 68Ga-DOTATATE |
11 TP, 8 TN, 3 FP, and 1 FN cases. Sensitivity 92%, specificity 73%, and diagnostic accuracy 83%. Impact on management in 30% of cases. |
| Ben-Haim et al. (2016) [32] | Retrospective | 46 (68 years; 61%) | 18F-FDG | PET/CT altered disease stage in 26% resulting in management changes in 15% of cases. |
| Liu et al. (2017) [33] | Retrospective | 16 (69 years; 75%) | 18F-FDG | In stage I-II MCC, PET/CT was less sensitive (6% positive results) vs. SLNB (63% positive results) in detecting occult nodal metastasis. |
| Poulsen et al. (2017) [34] | Prospective | 58 (68 years; 78%) | 18F-FDG | Sensitivity 95%, specificity 88%, PPV 95%, NPV 88%. Pre-treatment PET impacted treatment decisions in 27.6% of cases, leading to upstaging in 25.9% of them. |
| Taralli et al. (2018) [35] | Retrospective | 15 (70 years; 80%) |
18F-FDG 68Ga-DOTATOC 68Ga-DOTANOC 68Ga-DOTATATE |
On patient-based analysis, 18F-FDG and 68Ga-somatostatin analogs showed both 100% sensitivity, and 85.7% and 71.4% specificity, respectively, without significant difference. On lesion-based analysis, 18F-FDG detected 89% and 68Ga-somatostatin analogs 92% of the lesions, without significant difference. |
| Singh et al. (2020) [10] | Retrospective | 352 (nr.; nr.) | 18F-FDG | PET/CT upstaged 16.8% of patients. Higher sensitivity vs. CT. Baseline imaging led to upstaging also in patients with clinically uninvolved regional nodes. |
TP, true positive; TN, true negative; FP, false positive; FN, false negative; PPV, positive predictive value; NPV, negative predictive value; OS, overall survival; SLNB, sentinel lymph node biopsy; MCC, Merkel cell carcinoma; CMR, complete metabolic response; n.r., not reported. * The numbers refer to the whole study population of 99 patients. # Median age (mean age not provided).
3. 18F-FDG PET/CT in MCC
18F-FDG, a glucose analogue radiolabeled with fluorine-18 (18F), is the major workhorse in PET imaging. The rationale for using this radiotracer in nuclear oncology is based on the increased glucose uptake encountered in tumor cells. 18F-FDG is actively transported and phosphorylated into cancer cells but, unlike glucose, it cannot undergo further metabolism and remains intracellularly trapped, thus enabling PET/CT to detect areas of disease activity and spread by illustrating functional changes between normal and malignant tissue [36,37].
Currently, 18F-FDG PET/CT has emerged at the forefront for various oncologic applications, including melanoma, lymphoma, lung, head/neck, and colorectal cancer [38,39,40,41,42]. Apart from providing both functional and anatomical information in a single session, this modality also enables quantification of tumor radiotracer uptake via the standardized uptake value (SUV), an index reflecting the intensity of tracer activity in the visualized part of interest [43]. This allows an objective characterization of PET/CT scans beyond the ‘standard’ visual evaluation of 18F-FDG uptake with significant implications on patient follow-up and prognosis [44,45,46,47].
MCC tumors are typically highly metabolic, showing intense 18F-FDG uptake at PET [13,48]. Particularly for primary lesions, increased SUV max values (4.0–6.5) have been reported [48]. Thus, 18F-FDG appears to be an efficient PET tracer in this setting. In the following section, the most important studies as well as significant milestones regarding the use of 18F-FDG PET and PET/CT in MCC will be presented.
3.1. Initial Studies
The first reported use of 18F-FDG PET in the field of MCC involved a female patient with recurrent disease in 1998. Since baseline scans revealing multiple 18F-FDG-avid lesions were suggestive of metastases, isolated limb chemotherapy (melphalan plus tumor necrosis factor) was introduced. Post-treatment PET images showed complete metabolic response (CMR) of the lesions, demonstrating for the first time the potential utility of 18F-FDG PET for MCC staging/restaging, and monitoring therapy response [49]. Since then, an era of PET emerged, in which several case articles supported the favorable performance of 18F-FDG PET in the clinical care of MCC [14,15,50,51,52,53,54].
In 2006, Belhocine and colleagues were the first to retrospectively study the diagnostic accuracy of 18F-FDG PET or PET/CT in a case series of 11 MCC patients, comparing the findings with histological or clinical and radiological (CT, MRI, and bone scan) follow-up data. 18F-FDG PET was proven contributive in 10/11 cases, while it revealed second unexpected neoplasms in 4/11 patients. Overall, sensitivity and specificity of the modality in detecting MCC and other malignancies were 92% (11 true positive (TP), 1 false negative (FN)) and 100% (3 true negative (TN), 0 false positive (FP)), respectively [17]. Similar results were obtained in the same year in a case series of six MCC patients, where PET/CT (12 examinations) was TP in nine, TN in seven, FP in one, and FN in one lesion [18]. These early, promising data paved the way for clinical research on larger MCC patient cohorts, which will be discussed below.
3.2. Regional Lymph Node Evaluation
Since the sentinel lymph node (SLN) status is a major predictor of overall and disease-free survival, SLN biopsy (SLNB) should be regarded as essential or standard of care for MCC patients, comprising the most reliable staging tool for identifying subclinical nodal disease [5,55].
A number of studies have compared the information obtained by 18F-FDG PET or PET/CT with the “gold standard” of pathological/biopsy nodal evaluation, reporting quite different levels of sensitivity [23,24,33]. In the retrospective study by Colgan et al. [23], the findings of different imaging approaches (CT, MRI, PET, and PET/CT) were compared to conventional histology after SLNB and/or elective lymph node dissection (LND). Regarding detection of nodal spread, CT, MRI, and 18F-FDG PET or PET/CT exhibited a sensitivity of 47%, 0%, and 83%, and a specificity of 97%, 86%, and 95%, respectively. However, in the study by Hawryluk et al. [24], PET/CT was reported to be less capable of detecting loco-regional disease (3/21 scans; 14%); among PET/CT “negative” results (18/21 scans; 86%), 13 (72%) cases of micrometastatic disease were revealed only by means of immunohistochemistry. Another retrospective analysis of 16 stage I-II MCC patients recorded a concordance between PET/CT and histopathological findings in one of 10 patients with histologically positive nodes [33]. As similar findings have been reported for other imaging modalities [56], the NCCN panel does not recommend routine baseline imaging for clinically node-negative patients with localized MCC [5].
However, Singh et al. [10], in a recent large-scale study (n = 584), supported that baseline cross-sectional imaging is frequently positive, detecting occult metastatic disease in a non-negligible number of MCC cases. In this cohort, imaging upstaged 13.2% (65/492) of patients without clinically evident regional spread (8.9% in regional nodes, 4.3% in distant sites), markedly affecting management and prognosis. These findings are of high clinical importance and suggest that the current melanoma-derived imaging recommendations may be of questionable value in MCC management, underlying the potential role of imaging in routine screening of MCC patients with clinically uninvolved regional nodes.
3.3. Distant Metastasis Staging/Impact on Management
Compared to loco-regional MCC, the role of 18F-FDG PET/CT in assessing the burden of metastatic disease appears to be essential and, certainly, more well-defined. Indeed, several studies have sought to evaluate the impact of 18F-FDG PET/CT on stratification and management of MCC patients at virtually any stage of the disease with a reported sensitivity and specificity ranging between 86–100% and 89–100%, respectively. Notably, almost all studies have demonstrated that PET/CT, as part of the initial diagnostic workup, resulted in restaging, guiding therapeutic plans in 6–46% of cases [19,20,21,24,26,27,28,30,32,34] (Table 1).
In a first attempt to evaluate the effect of different morphological and functional imaging approaches on tumor staging and post-treatment evaluation, Peloschek et al. [20] screened 16 MCC patients with sonography, CT, MRI, and PET, comparing the findings with a combined standard of reference (histopathology, clinical, and/or radiological follow-up). Overall, 18F-FDG PET had a sensitivity of 85.7% and specificity of 96.2%, while the combined sensitivity and specificity for morphological imaging methods was 95.5% and 89.1%, respectively. Moreover, in regions where PET and conventional imaging correlated to the standard of reference, 18F-FDG PET showed 85% sensitivity and 95% specificity, while conventional imaging yielded 95% and 90%, respectively. No significant differences between the methods tested were observed.
In a retrospective chart review comprising 18 MCC cases, most staged as II/III disease, Concannon et al. [19] reported that 18F-FDG PET/CT resulted in restaging and influenced management plans in seven (33%) and nine (43%) cases, respectively. The modality showed a 94% sensitivity for histologically proven disease (or 100% of all lesions >5 mm). Accordingly, in the series by Maury et al. [21] (n = 15), 18F-FDG PET/CT performed at initial staging and/or during follow-up led to significant changes in disease status and management in 46% of cases compared with clinical examination alone. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were, respectively, 89%, 100%, 100%, and 93% for both CT and PET/CT.
In the study by Hawryluk et al. [24], PET/CT conducted as part of the baseline workup upstaged 16% of patients, mainly in the more advanced stages. Regarding surveillance, their study confirmed the increased efficacy of the modality in detecting sites of metastatic disease missed on CT, especially in bone/bone marrow.
In line with previous reports, changes in tumor status as a result of PET/CT (39 scans) occurred in 20% (4/20) of cases, altering treatment decision making in 15% (3/20) [27]. Similarly, Ben-Haim et al. [32], in a cohort of 46 MCC patients, also reported changes in disease stage and management in 26% and 15% of cases, respectively. In another study of 23 MCC cases explored with 18F-FDG PET/CT (66 scans) at initial diagnosis or during subsequent monitoring, the modality exhibited 97% sensitivity, 89% specificity, 94% PPV, and 94% NPV. At initial presentation, PET/CT was able to restage tumor status in 39% of patients, modifying therapeutic plans in 33% of cases [28].
Siva et al. [26] retrospectively reviewed the clinical impact of PET imaging on the staging and management of 102 MCC patients. PET staging results had an impact on management in 37% of patients (p < 0.003) and differed from conventional staging in 22% of cases. In stratification by PET-defined stage, the five-year OS was 67% in stage I/II patients but only 31% in stage III patients (log-rank p < 0.001). On multivariate analysis, PET stage was significantly associated with OS (p < 0.001). This MCC cohort was partly followed up and evaluated a few years later. After definitive treatment of 62 patients, the impact of follow-up 18F-FDG PET on disease restaging, including identifying patients suitable for salvage treatment, was high in 45%, medium in 11%, and low in 43% of cases. With regard to prognosis, the status of post-treatment PET was reported to be highly prognostic of the OS, as patients who achieved a CMR assessed via PET had a two- and five- year OS of 88% and 68%, respectively, compared to 15% one-year OS in cases with residual activity [30].
In 2017, the first prospective phase II study, involving 58 MCC patients with IIA-IIIB-stage disease, evaluated the role of 18F-FDG PET in the management of MCC (Trans Tasman Radiation Oncology Group TROG 09.03 trial). Pre-treatment scans showed a sensitivity of 94.7%, a specificity of 88.2%, a PPV of 94.7%, and a NPV of 88.2%. Initial PET screening also provided treatment guidance in 27.6% of cases; upstaging occurred in 25.9% with no instances of downstaging. Contrary to Byrne et al. [30], no prognostic impact related to post-treatment PET was detected [34].
In the largest-to-date cohort of 352 MCC patients explored with PET/CT, Singh et al. [10] recently investigated the clinical utility of baseline cross-sectional imaging (CT, PET/CT, or MRI) focusing on patients presenting with primary cutaneous MCC and no evident distant metastatic spread. As mentioned above, imaging upstaged one in eight cases with no clinically evident regional spread, while 10.8% (10/92) of clinically node-positive patients were upstaged to distant metastatic disease. Of note, in this cohort PET/CT was more precise in accurate disease staging than CT, upstaging 16.8% of 352 cases compared to 6.9% of 231 cases who underwent CT alone (p = 0.0006) [10]. Table 2 provides a summary of the published studies involving a direct comparison between 18F-FDG PET or 18F-FDG PET/CT and CT.
Table 2.
Summary of the published studies (including >1 patient) presenting a direct comparison between 18F-fluorodeoxyglucose (18F-FDG) PET or 18F-FDG PET/CT and CT in Merkel cell carcinoma (in chronological order).
| Author (Year) | No. of Patients Undergoing PET/No. of Patients Undergoing CT | Main Findings |
|---|---|---|
| Yao et al. (2005) [15] | 2/2 | Pre-treatment PET detected metastatic disease in both patients not appreciated in CT. |
| Peloschek et al. (2010) [20] | 16/16 | PET: Sensitivity 85.7%, specificity 96.2% Morphological imaging (CT, MRI, US): Combined sensitivity 95.5%, combined specificity 89.1%. |
| Maury et al. (2011) [21] | 15/15 | PET: Sensitivity 89%, specificity 100%, PPV 100%, NPV 93%. CT: Sensitivity 89%, specificity 100%, PPV 100%, NPV 93%. |
| Colgan et al. (2012) [23] | 33/69 | PET and PET/CT: Sensitivity 83%, specificity 95%, PPV 91%, NPV 91% in detecting nodal basin disease. CT: Sensitivity 47%, specificity 97%, PPV 94%, NPV 68% in detecting nodal basin disease. PET was significantly more sensitive and equally specific in comparison with CT. |
| Hawryluk et al. (2012) [24] | 97/97 | Bone/bone marrow metastases in 10 cases were revealed only on PET with no CT correlate. |
| George et al. (2014) [28] | 23/n.r. | All lesions identified by CT were also detected by PET. Lesions not detected clinically or by conventional imaging (not further specified) were found in 44% of PET/CTs performed at initial presentation and subsequent monitoring with, respectively, 50% and 41% of scans identifying new lesions. |
| Poulsen et al. (2017) [34] | 58/58 | PET led to upstaging in 15 (25.9%) of patients, with no cases of downstaging. Upstaging was due to detection of distant metastases (4 cases) or regional nodes (6 cases) that were not reported on CT. |
| Singh et al. (2020) [10] | 352/231 | PET/CT upstaged patients (16.8% of 352) significantly more often than CT alone (6.9% of 231). |
US, ultrasound; PPV, positive predictive value; NPV, negative predictive value; n.r., not reported.
In the era of immunotherapy, the advent of immune checkpoint inhibitors has profoundly enriched the treatment landscape of several malignancies, including melanoma, offering crucial survival benefits. Although there are no randomized comparative trials demonstrating the superiority of immune checkpoint blockade over conventional chemotherapy in metastatic MCC, preliminary results are rapidly becoming promising for PD-L1/PD-1 inhibitors [57,58]; avelumab, nivolumab, and pembrolizumab are currently recommended as first-line, systemic treatment options for advanced MCC [5].
In this evolving field, treatment response evaluation is now at the forefront of cancer management, given the different mode of action between immunotherapy and conventional chemotherapy [59,60,61]. Although 18F-FDG PET/CT has yielded favorable results for response assessment in melanoma immunotherapy [62,63], there is still little evidence to unravel its full potential for evaluating immunotherapy response in the field of MCC, mainly due to the rarity of this tumor. However, considering the melanoma-derived data as well as published MCC case reports/series and summarizing the experience from our institution, the modality is emerging as an attractive tool for monitoring therapeutic outcomes, enabling the evaluation of tumor burden at different time points over the course of treatment [64,65,66,67,68] (Figure 1, Figure 2 and Figure 3).
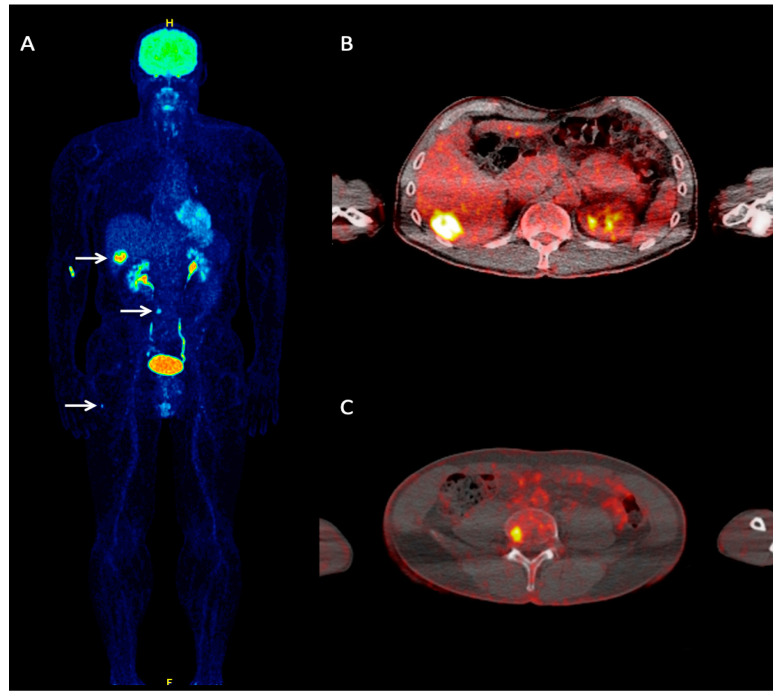
Figure 1.
A 60-year-old Merkel cell carcinoma (MCC) patient referred to our department for staging purposes due to clinical suspicion of hepatic metastases. Whole-body 18F-FDG PET maximum intensity projection (MIP) (A) demonstrated foci of increased tracer uptake in the liver, lumbar spine, and right femur, corresponding to metastases (arrows). Transaxial, fused 18F-FDG PET/CT at the hepatic level (B) shows a focal site of increased tracer uptake in liver segment VI, corresponding to a hepatic metastasis. Transaxial, fused 18F-FDG PET/CT of the lower abdomen (C) shows pathologic tracer accumulation in the fourth lumbar vertebrae, representing an osseous metastasis.
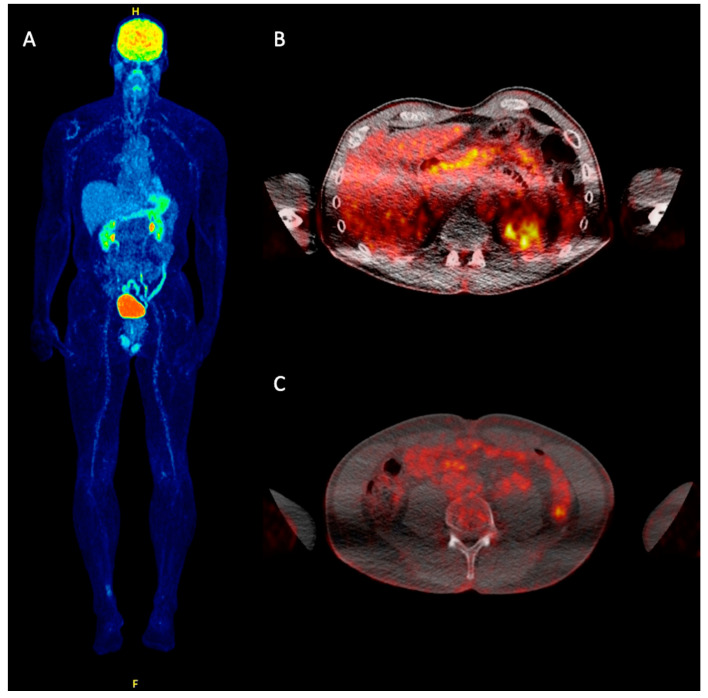
Figure 2.
Post-treatment follow-up 18F-FDG PET/CT of the same patient as in Figure 1 after one year. Following anti-programmed death-ligand 1 (PD-L1) immunotherapy (avelumab), both whole-body MIP (A) and transaxial, fused 18F-FDG PET/CT images (B,C) demonstrated complete metabolic remission (CMR) of the previously observed metastases as response to treatment.
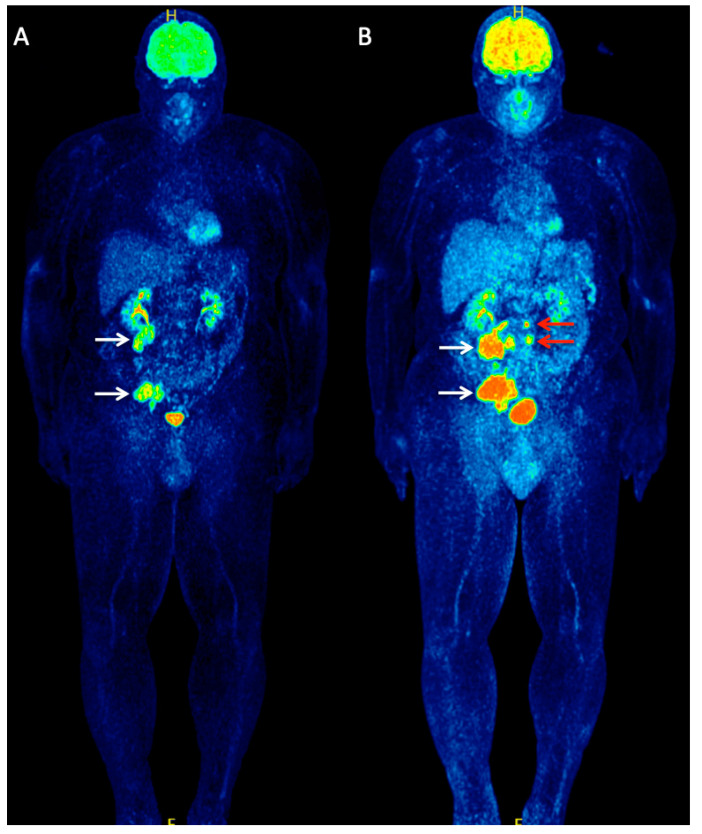
Figure 3.
A 56-year-old patient with metastatic MCC of unknown primary referred to our department for staging purposes before initiation of immunotherapy. Whole-body 18F-FDG PET (MIP) (A) demonstrated two large hypermetabolic lesions in the abdomen and pelvis, corresponding to lymph node metastases (white arrows). The patient received treatment with the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, ipilimumab, considered at the time of scanning as a potentially beneficial immunotherapeutic agent for MCC. After receiving two cycles of ipilimumab, the patient underwent an interim follow-up 18F-FDG PET/CT for early treatment response evaluation. Whole-body 18F-FDG PET (MIP) (B) revealed a clear disease progression with an increase in size and metabolism of the previously observed metastases (white arrows) but also detected newly appeared metastatic retroperitoneal lymph nodes (red arrows).
3.4. Limitations of 18F-FDG PET/CT
Disadvantages of 18F-FDG PET/CT include its limited availability mainly because of its high cost compared to conventional imaging modalities. Since 18F-FDG uptake is not specific for cancer, both false positive (i.e., inflammation, post-surgical areas, recent chemotherapy, fractures) and false negative (i.e., hyperglycemia, recent high-dose steroid therapy) results may be encountered. Moreover, the application of PET/CT in specific body areas, such as the brain, heart, and kidneys, may occasionally be suboptimal due to increased physiologic radiotracer uptake in these organs [43].
4. Non-18F-FDG PET Tracers in MCC
This section intends to address the most important non-18F-FDG PET tracers that have been utilized as potential imaging biomarkers in the field of MCC.
4.1. Somatostatin (SST) Analogues’ Imaging
Imaging with radiolabeled SST analogues has been well established in the management of neuroendocrine tumors (NETs) [69]. For high-grade NETs, like MCC, SST receptor (SSTR) expression can also be utilized for visualization of disease burden and potentially theranostics [48]. In this setting, 111In-pentetreotide scintigraphy (OctreoScan) has been used in the workup of MCC, with reported results ranging from initially promising to reserved or even disappointing [70,71,72]. In the largest cohort study, 85% of MCC patients (n = 39) had at least some degree of 111In-pentetreotide uptake in SSTR scintigraphy, with the majority (75%) showing low to medium tracer uptake. However, the SSTR expression status, assessed by scintigraphy, did not significantly correlate with clinical outcomes of SSTR-targeted therapy [73].
Although extensive comparative studies of OctreoScan and 18F-FDG PET have not yet been carried out, a limited number of case reports and case series studies have all demonstrated the superiority of PET, which can partly be due to its improved image resolution and sensitivity over scintigraphy [16,22,50]. In particular, regarding disease staging, Lu et al. confirmed the enhanced performance of 18F-FDG PET/CT compared to scintigraphy in a series of nine MCC cases, upstaging 56% of patients and altering clinical decisions in all cases. Interestingly, no lesions identified by OctreoScan were missed on PET/CT [22].
The introduction of 68Ga-labelled dodecane tetraacetic acid (DOTA)-peptides with high affinity for SSTR in PET imaging, such as 68Ga-DOTA-d-Phe1-Tyr3–Octreotide (DOTATOC), 68Ga-DOTA-Tyr3-Octreotate (DOTATATE), and 68Ga-DOTA-Nal3-Octreotide (DOTANOC), has optimized NETs’ detection and characterization, providing higher diagnostic efficiency and accuracy over scintigraphic approaches [74,75,76].
Concerning MCC, 68Ga-labeled SST analogues’ PET and PET/CT have shown highly promising results. Salavati and coauthors in 2012 were the first to perform PET/CT examinations using 68Ga-DOTATOC and 18F-FDG in a patient with stage IV MCC, demonstrating similar performance of both tracers in detecting metastatic disease. Based on the intense uptake of SST analogues by metastatic lesions, the authors took a step further and administered adjuvant peptide receptor radionuclide therapy (PRRNT) with 177Lu-DOTATATE in combination with doxorubicin chemotherapy, implementing the first documented ‘theranostic’ strategy in MCC management. However, follow-up PET/CT scans showed mixed patterns of response; despite a significant decline in tumor size and SSTR expression at specific sites, progressive disease with new skin and nodal lesions was also detected [77].
A similar approach was subsequently adopted by Schmidt et al. [25] in two MCC cases with extensive lymph node involvement. Following confirmation of SSTR expression in metastatic lesions using 68Ga-DOTATATE PET/CT, both patients were introduced to combination therapy with PRRNT (90Y-DOTATATE or 177Lu-DOTATATE) and capecitabine followed by external beam radiotherapy in one case. Despite a temporary partial response in both patients, however, fatal outcomes could not be prevented [25].
Further case reports have supported the usefulness of 68Ga-labelled tracers for MCC detection and management in the context of theranostics [78,79,80]. In 2014, Buder et al. [29] retrospectively studied the largest MCC cohort (n = 24) using SSTR-PET with 68Ga-DOTATOC and 68Ga-DOTATATE radiotracers in comparison to CT. Nodal, bone, and soft-tissue metastases were revealed by SSTR-PET with a sensitivity of 73%, 100%, and 67%, respectively. Based on PET findings, four (17%) patients were upstaged and management was modified in three (13%) cases.
One year later, another retrospective analysis comprising 23 MCC patients evaluated the role of 68Ga-DOTA-peptides PET/CT. Overall, the modality showed a sensitivity, specificity, and diagnostic accuracy of 92%, 73%, and 83%, respectively. Higher diagnostic accuracy was obtained for staging compared to restaging (88% vs. 73%; p = 0.7) and for 68Ga-DOTANOC compared to 68Ga-DOTATOC or 68Ga-DOTATATE (100% vs. 71% and 75%, respectively; p = 0.56). Disease management was influenced by PET/CT findings in almost 30% (7/23) of cases [31].
Further, Taralli et al. [35] compared the impact of 18F-FDG and 68Ga-labeled SST-analogues’ PET/CT on staging, restaging, or treatment response evaluation in a series of 15 MCC patients. Using histology or clinical/radiological follow-up as the reference standard, both approaches showed good and comparable diagnostic performance; 18F-FDG and 68Ga-labeled SST-analogues’ PET/CT had both a sensitivity of 100% and a specificity of 85.7% and 71.4%, respectively, with no significant differences. The authors thus concluded that 68Ga-SST analogue PET/CT cannot replace but rather supplement 18F-FDG PET/CT, according to clinical indication.
4.2. 18F-Fluorodihydroxyphenylalanine (18F-DOPA)
6-Fluoro-(18F)-L-3,4-dihydroxyphenylalanine (18F-DOPA) is a neutral amino acid analogue employed as a PET tracer. When injected intravenously, this molecule can cross the blood–brain barrier to reach the dopaminergic neurons where it is used as a precursor of the neurotransmitter dopamine. In the field of nuclear oncology, the main clinical application of imaging with 18F-DOPA is for the management of NETs and brain tumors [81,82]. On the basis of the knowledge that the amino acid DOPA is a precursor of melanin, 18F-FDOPA PET has also been experimentally applied in melanoma patients in combination with 18F-FDG. However, its sensitivity as well as the tracer uptake in melanoma lesions was lower in comparison to 18F-FDG [83].
Given that NET cells are able to uptake, decarboxylate, and store biogenic amines [16], 18F-DOPA PET has been used in few case series of MCC patients. In their 2006 retrospective case study (n = 3), Talbot et al. [16] were the first to evaluate and compare the performance of 18F-DOPA PET, 18F-FDG PET, and SSTR scintigraphy. Despite 18F-FDOPA uptake by MCC lesions, 18F-FDOPA PET added no further information than 18F-FDG PET in the two TP patients, while it provided inferior contrast of images. Moreover, in one case suspected of recurrence at scintigraphy and with inconclusive 18F-FDG PET, the 18F-FDOPA PET result was proven TN [16].
A few years later, Peloschek et al. [20], in a retrospective chart review, compared imaging findings obtained via 18F-DOPA PET with a standard of reference (histopathology or clinical/radiological follow-up) in five MCC cases. FDOPA results were negative in all anatomical sites (19 TN, two FN), correlating to the standard of reference in 21/144 regions. These findings further suggested the limited clinical utility of 18F-FDOPA PET in MCC diagnostics.
Given the limited, but discouraging, data on the use of 18F-DOPA PET in MCC, as well as the practical and logistical issues regarding the complicated labeling process of the tracer, the potential role of this modality in the management of MCC seems to be rather poor.
5. Conclusions
In summary, there is currently no consensus on a widely accepted imaging algorithm for MCC, and respective recommendations are still based mainly on melanoma. While baseline imaging is not generally encouraged in early-stage MCC, its value in locally advanced and/or distant metastatic disease has been well established. In this context, PET/CT, mostly utilizing 18F-FDG, is increasingly applied in the workup of metastatic or unresectable MCC, providing essential information for initial staging, therapy response evaluation, and monitoring of recurrent disease. Although the accuracy of the modality in detecting the disease in its early (I/II) stages appears to be inferior compared to SLNB, current evidence suggests an important contribution also in cases of localized MCC. In addition, 68Ga-labeled SST analogues have also been used as PET tracers with promising results, given that SSTR expression may be utilized as a potential target for visualizing MCC, according to clinical indication. Despite the limited experience, 18F-DOPA PET imaging seems to be less valuable in the field of MCC diagnostics.
Author Contributions
C.S. and P.S. wrote the manuscript. C.S. and P.S. conceived the original idea, N.D., J.C.H., and A.D.-S. provided review and editing support. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Xue Y., Thakuria M. Merkel Cell Carcinoma Review. Hematol. Clin. N. Am. 2019;33:39–52. doi: 10.1016/j.hoc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Coggshall K., Tello T.L., North J.P., Yu S.S. Merkel cell carcinoma: An update and review: Pathogenesis, diagnosis, and staging. J. Am. Acad. Dermatol. 2018;78:433–442. doi: 10.1016/j.jaad.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Garkaby J., Israel O., Epelbaum R. Metabolic assessment of Merkel cell cancer-The role of FDG-PET/CT in rare tumors. J. Nucl. Med. 2013;54:107. [Google Scholar]
- 4.Harms K.L., Healy M.A., Nghiem P., Sober A.J., Johnson T.M., Bichakjian C.K., Wong S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016;23:3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) Merkel Cell Carcinoma Version1. 2 October 2019. [(accessed on 25 August 2020)];2020 Available online: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf.
- 6.Lebbé C., Becker J.C., Grob J.-J., Malvehy J., Del Marmol V., Pehamberger H., Peris K., Saiag P., Middleton M., Bastholt L., et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer. 2015;51:2396–2403. doi: 10.1016/j.ejca.2015.06.131. [DOI] [PubMed] [Google Scholar]
- 7.Cornejo C., Miller C.J. Merkel Cell Carcinoma: Updates on Staging and Management. Dermatol. Clin. 2019;37:269–277. doi: 10.1016/j.det.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Tello T.L., Coggshall K., Yom S.S., Yu S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018;78:445–454. doi: 10.1016/j.jaad.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Grandhaye M., Teixeira P.G., Henrot P., Morel O., Sirveaux F., Verhaeghe J.-L., Blum A. Focus on Merkel cell carcinoma: Diagnosis and staging. Skelet. Radiol. 2015;44:777–786. doi: 10.1007/s00256-015-2104-x. [DOI] [PubMed] [Google Scholar]
- 10.Singh N., Alexander N.A., Lachance K., Lewis C.W., McEvoy A., Akaike G., Byrd D., Behnia S., Bhatia S., Paulson K.G., et al. Clinical Benefit of Baseline Imaging in Merkel Cell Carcinoma: Analysis of 584 Patients. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer A. A systematic review of PET and PET/CT in oncology: A way to personalize cancer treatment in a cost-effective manner? BMC Health Serv. Res. 2010;10:283. doi: 10.1186/1472-6963-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen H., Holdgaard P.C., Madsen P.H., Knudsen L.M., Gad D., Gravergaard A.E., Rohde M., Godballe C., Engelmann B.E., Bech K., et al. FDG PET/CT in cancer: Comparison of actual use with literature-based recommendations. Eur. J. Nucl. Med. Mol. Imaging. 2015;43:695–706. doi: 10.1007/s00259-015-3217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treglia G., Kakhki V.R.D., Giovanella L., Sadeghi R. Diagnostic Performance of Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography in Patients with Merkel Cell Carcinoma: A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2013;14:437–447. doi: 10.1007/s40257-013-0040-x. [DOI] [PubMed] [Google Scholar]
- 14.Scanga D.R., Martin W.H., Delbeke D. Value of FDG PET Imaging in the Management of Patients With Thyroid, Neuroendocrine, and Neural Crest Tumors. Clin. Nucl. Med. 2004;29:86–90. doi: 10.1097/01.rlu.0000109329.34975.9f. [DOI] [PubMed] [Google Scholar]
- 15.Yao M., Smith R.B., Hoffman H.T., Funk G.F., Graham M.M., Buatti J.M. Merkel Cell Carcinoma: Two case reports focusing on the role of fluorodeoxyglucose positron emission tomography imaging in staging and surveillance. Am. J. Clin. Oncol. 2005;28:205–210. doi: 10.1097/01.coc.0000144850.68142.68. [DOI] [PubMed] [Google Scholar]
- 16.Talbot J.-N., Kerrou K., Missoum F., Grahek D., Aide N., Lumbroso J., Montravers F. 6-[F-18]Fluoro-l-DOPA Positron Emission Tomography in the Imaging of Merkel Cell Carcinoma: Preliminary Report of Three Cases with 2-Deoxy-2-[F-18]Fluoro-d-Glucose Positron Emission Tomography or Pentetreotide-(111In) SPECT Data. Mol. Imaging Biol. 2005;7:257–261. doi: 10.1007/s11307-005-0006-3. [DOI] [PubMed] [Google Scholar]
- 17.Belhocine T.-Z., Pierard G., Frühling J., Letesson G., Bolle S., Hustinx R., Dargent J.-L., Flamen P., Rigo P. Clinical added-value of 18FDG PET in neuroendocrine-merkel cell carcinoma. Oncol. Rep. 2006;16:347–352. doi: 10.3892/or.16.2.347. [DOI] [PubMed] [Google Scholar]
- 18.Iagaru A., Quon A., McDougall I.R., Gambhir S.S. Merkel Cell Carcinoma: Is there a Role for 2-Deoxy-2-[F-18]fluoro-d-glucose-Positron Emission Tomography/Computed Tomography? Mol. Imaging Biol. 2006;8:212–217. doi: 10.1007/s11307-006-0047-2. [DOI] [PubMed] [Google Scholar]
- 19.Concannon R., Larcos G.S., Veness M. The impact of 18F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: Results from Westmead Hospital, Sydney, Australia. J. Am. Acad. Dermatol. 2010;62:76–84. doi: 10.1016/j.jaad.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Peloschek P., Novotny C., Mueller-Mang C., Weber M., Sailer J., Dawid M., Czerny C., Dudczak R., Kletter K., Becherer A. Diagnostic imaging in Merkel cell carcinoma: Lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur. J. Radiol. 2010;73:317–323. doi: 10.1016/j.ejrad.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Maury G., Dereure O., Du-Thanh A., Mariano-Goulart D., Guillot B. Interest of (18)F-FDG PET-CT scanning for staging and management of merkel cell carcinoma: A retrospective study of 15 patients. J. Eur. Acad. Dermatol. Venereol. 2011;25:1420–1427. doi: 10.1111/j.1468-3083.2011.03994.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y., Fleming S.E., Fields R.C., Coit D.G., Carrasquillo J.A. Comparison of 18F-FDG PET/CT and 111In Pentetreotide Scan for Detection of Merkel Cell Carcinoma. Clin. Nucl. Med. 2012;37:759–762. doi: 10.1097/RLU.0b013e31825ae8e7. [DOI] [PubMed] [Google Scholar]
- 23.Colgan M.B., Tarantola T.I., Weaver A.L., Wiseman G.A., Roenigk R.K., Brewer J.D., Otley C.C. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J. Am. Acad. Dermatol. 2012;67:1250–1256. doi: 10.1016/j.jaad.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Hawryluk E.B., O’Regan K.N., Sheehy N., Guo Y., Dorosario A., Sakellis C.G., Jacene H.A., Wang L.C. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: A study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J. Am. Acad. Dermatol. 2013;68:592–599. doi: 10.1016/j.jaad.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M., Uhrhan K., Markiefka B., Hasselbring L., Schlaak M., Cremer B., Kunze S., Baum R.P., Dietlein M. 68Ga-DotaTATE PET-CT followed by Peptide Receptor Radiotherapy in combination with capecitabine in two patients with Merkel Cell Carcinoma. Int. J. Clin. Exp. Med. 2012;5:363–366. [PMC free article] [PubMed] [Google Scholar]
- 26.Siva S., Byrne K., Seel M., Bressel M., Jacobs D., Callahan J., Laing J., MacManus M.P., Hicks R.J. 18F-FDG PET Provides High-Impact and Powerful Prognostic Stratification in the Staging of Merkel Cell Carcinoma: A 15-Year Institutional Experience. J. Nucl. Med. 2013;54:1223–1229. doi: 10.2967/jnumed.112.116814. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim S.F., Ahronowitz I., McCalmont T.H., Pampaloni M.H., Ryan J.L., Yu S.S. 18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography Imaging in the Management of Merkel Cell Carcinoma: A Single-Institution Retrospective Study. Dermatol. Surg. 2013;39:1323–1333. doi: 10.1111/dsu.12246. [DOI] [PubMed] [Google Scholar]
- 28.George A., Girault S., Testard A., Delva R., Soulié P., Couturier O.-F., Morel O. The impact of 18F-FDG-PET/CT on Merkel cell carcinoma management. Nucl. Med. Commun. 2014;35:282–290. doi: 10.1097/MNM.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 29.Buder K., Lapa C., Kreissl M.C., Schirbel A., Herrmann K., Schnack A., Bröcker E.-B., Goebeler M., Buck A.K., Becker J.C. Somatostatin receptor expression in Merkel cell carcinoma as target for molecular imaging. BMC Cancer. 2014;14:268. doi: 10.1186/1471-2407-14-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne K., Siva S., Chait L., Callahan J., Bressel M., Seel M., MacManus M.P., Hicks R.J. 15-year Experience of 18F-FDG PET Imaging in Response Assessment and Re-staging after Definitive Treatment of Merkel Cell Carcinoma. J. Nucl. Med. 2015;56:1328–1333. doi: 10.2967/jnumed.115.158261. [DOI] [PubMed] [Google Scholar]
- 31.Sollini M., Taralli S., Milella M., Erba P., Rubagotti S., Fraternali A., Roncali M., Moscarella E., Perotti G., Rufini V., et al. Somatostatin receptor positron emission tomography/computed tomography imaging in Merkel cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2015;30:1507–1511. doi: 10.1111/jdv.13405. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Haim S., Garkaby J., Primashvili N., Goshen E., Shapira R., Davidson T., Israel O., Epelbaum R. Metabolic assessment of Merkel cell carcinoma. Nucl. Med. Commun. 2016;37:865–873. doi: 10.1097/MNM.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Larcos G., Howle J., Veness M. Lack of clinical impact of 18F-fluorodeoxyglucose positron emission tomography with simultaneous computed tomography for stage I and II Merkel cell carcinoma with concurrent sentinel lymph node biopsy staging: A single institutional experience from Westme. Australas. J. Dermatol. 2015;58:99–105. doi: 10.1111/ajd.12400. [DOI] [PubMed] [Google Scholar]
- 34.Poulsen M., Macfarlane D., Veness M., Estall V., Hruby G., Kumar M., Pullar A., Tripcony L., Rischin D. Prospective analysis of the utility of 18-FDG PET in Merkel cell carcinoma of the skin: A Trans Tasman Radiation Oncology Group Study, TROG 09:03. J. Med. Imaging Radiat. Oncol. 2018;62:412–419. doi: 10.1111/1754-9485.12705. [DOI] [PubMed] [Google Scholar]
- 35.Taralli S., Sollini M., Milella M., Perotti G., Filice A., Menga M., Versari A., Rufini V. 18F-FDG and 68Ga-somatostatin analogs PET/CT in patients with Merkel cell carcinoma: A comparison study. EJNMMI Res. 2018;8:64. doi: 10.1186/s13550-018-0423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith T.A.D. FDG uptake, tumour characteristics and response to therapy. Nucl. Med. Commun. 1998;19:97–106. doi: 10.1097/00006231-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kapoor V., McCook B.M., Torok F.S. An Introduction to PET-CT Imaging1. Radiographics. 2004;24:523–543. doi: 10.1148/rg.242025724. [DOI] [PubMed] [Google Scholar]
- 38.Fischer B.M., Lassen U., Mortensen J., Larsen S., Loft A., Bertelsen A., Ravn J., Clementsen P., Høgholm A., Larsen K., et al. Preoperative Staging of Lung Cancer with Combined PET–CT. New Engl. J. Med. 2009;361:32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 39.Hutchings M., Loft A., Hansen M., Pedersen L.M., Buhl T., Jurlander J., Buus S., Keiding S., D’Amore F., Boesen A.-M., et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 40.Schröer-Günther M.A., Wolff R.F., Westwood M., Scheibler F., Schürmann C., Baumert B.G., Sauerland S., Kleijnen J. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: A systematic review. Syst. Rev. 2012;1:62. doi: 10.1186/2046-4053-1-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.W., Roh J.L., Kim J.S., Lee J.H., Cho K.J., Choi S.H., Nam S.I., Kim S.Y. (18)FFDG PET/CT surveillance at 3–6 and 12 months for detection of recurrence and second primary cancer in patients with head and neck squamous cell carcinoma. Br. J. Cancer. 2013;109:2973–2979. doi: 10.1038/bjc.2013.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel S., McCall M., Ohinmaa A., Bigam D., Dryden D.M. Positron Emission Tomography/Computed Tomographic Scans Compared to Computed Tomographic Scans for Detecting Colorectal Liver Metastases. Ann. Surg. 2011;253:666–671. doi: 10.1097/SLA.0b013e31821110c9. [DOI] [PubMed] [Google Scholar]
- 43.Ziessman H.A., O’Malley J.P., Thrall J.H. Oncology: Positron Emission Tomography. In: Ziessman H.A., O’Malley J.P., Thrall J.H., Fahey F.H., editors. Nuclear Medicine: The Requisites. 4th ed. Elsevier Mosby; Philadelphia, PA, USA: 2014. [Google Scholar]
- 44.Pandit-Taskar N., Gonen M., Krug L., Larson S.M. Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:78–84. doi: 10.1007/s00259-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 45.Borst G.R., Belderbos J.S., Boellaard R., Comans E.F., De Jaeger K., Lammertsma A.A., Lebesque J.V. Standardised FDG uptake: A prognostic factor for inoperable non-small cell lung cancer. Eur. J. Cancer. 2005;41:1533–1541. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Kidd E.A., Siegel B.A., Dehdashti F., Grigsby P.W. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–1744. doi: 10.1002/cncr.22974. [DOI] [PubMed] [Google Scholar]
- 47.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akaike G., Akaike T., Fadl S.A., Lachance K., Nghiem P., Behnia S. Imaging of Merkel Cell Carcinoma: What Imaging Experts Should Know. Radiographics. 2019;39:2069–2084. doi: 10.1148/rg.2019190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lampreave J.L., Bénard F., Alavi A., Jimenez-Hoyuela J., Fraker D. PET evaluation of therapeutic limb perfusion in Merkel’s cell carcinoma. J. Nucl. Med. 1998;39:2087–2090. [PubMed] [Google Scholar]
- 50.Wong C. F-18 FDG Accumulation in an Octreotide Negative Merkel Cell Tumor. Clin. Positron Imaging. 2000;3:71–73. doi: 10.1016/S1095-0397(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen B.D. Positron Emission Tomographic Imaging of Merkel Cell Carcinoma. Clin. Nucl. Med. 2002;27:922–923. doi: 10.1097/00003072-200212000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Lin O., Thomas A., Singh A., Greenspan B. Complementary Role of Positron Emission Tomography in Merkel Cell Carcinoma. South Med. J. 2004;97:1110–1112. doi: 10.1097/01.SMJ.0000140856.66693.17. [DOI] [PubMed] [Google Scholar]
- 53.Golan H., Volkov O., Linchinsky O., Melloul M. FDG-PET imaging in Merkel cell carcinoma—Value of head-to-toe scan. Nucl. Med. Rev. 2005;8:135–136. [PubMed] [Google Scholar]
- 54.Greene G.S., Mezheritskiy I., Biko D.M. PET/CT imaging of metastatic Merkel cell carcinoma to the adrenal glands. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.03.2010.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemos B.D., Storer B.E., Iyer J.G., Phillips J.L., Bichakjian C.K., Fang L.C., Johnson T.M., Liegeois-Kwon N.J., Otley C.C., Paulson K.G., et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J. Am. Acad. Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S.G., Wang L.C., Fernández-Peñas P., Gellenthin M., Lee S.J., Nghiem P. Sentinel Lymph Node Biopsy for Evaluation and Treatment of Patients With Merkel Cell Carcinoma. Arch. Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 57.Nghiem P., Bhatia S., Lipson E.J., Kudchadkar R.R., Miller N.J., Annamalai L., Berry S., Chartash E.K., Daud A., Fling S.P., et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topalian S.L., Bhatia S., Hollebecque A., Awada A., De Boer J.P., Kudchadkar R.R., Gonçalves A., Delord J.-P., Martens U.M., Picazo J.M.L., et al. Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC); Proceedings of the Clinical Trials; American Association for Cancer Research (AACR); Washington, DC, USA. 1–5 April 2017; p. CT074. [Google Scholar]
- 59.Gilardi L., Grana C.M., Paganelli G. Evaluation of response to immunotherapy: New challenges and opportunities for PET imaging. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:2090–2092. doi: 10.1007/s00259-014-2848-x. [DOI] [PubMed] [Google Scholar]
- 60.Tirumani S.H., Ramaiya N.H., Keraliya A., Bailey N.D., Ott P.A., Hodi F.S., Nishino M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015;3:1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amin A., Lawson D.H., Salama A.K.S., Koon H.B., Guthrie T., Thomas S.S., O’Day S.J., Shaheen M., Zhang B., Francis S., et al. Phase II study of vemurafenib followed by ipilimumab in patients with previously untreated BRAF-mutated metastatic melanoma. J. Immunother. Cancer. 2016;4:44. doi: 10.1186/s40425-016-0148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimitrakopoulou-Strauss A. Monitoring of patients with metastatic melanoma treated with immune checkpoint inhibitors using PET–CT. Cancer Immunol. Immunother. 2018;68:813–822. doi: 10.1007/s00262-018-2229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sachpekidis C., Dimitrakopoulou-Strauss A. Atlas of Response to Immunotherapy. Springer Science and Business Media LLC; Cham, Switzerland: 2019. Melanoma: 18F-FDG PET/CT for Response Assessment of Melanoma Following Immunotherapy; pp. 55–65. [Google Scholar]
- 64.Winkler J., Dimitrakopoulou-Strauss A., Sachpekidis C., Enk A., Hassel J.C. Ipilimumab has efficacy in metastatic Merkel Cell Carcinoma: A case series of five patients. J. Eur. Acad. Dermatol. Venereol. 2017;31 doi: 10.1111/jdv.14193. [DOI] [PubMed] [Google Scholar]
- 65.Eshghi N., Lundeen T.F., MacKinnon L., Avery R., Kuo P.H. 18F-FDG PET/CT for Monitoring Response of Merkel Cell Carcinoma to the Novel Programmed Cell Death Ligand 1 Inhibitor Avelumab. Clin. Nucl. Med. 2018;43:e142–e144. doi: 10.1097/RLU.0000000000002051. [DOI] [PubMed] [Google Scholar]
- 66.Vellani C., D’Ambrosio D., Licata L., Vacchieri I., Bernardo A., Trifirò G. Monitoring response of advanced Merkel cell carcinoma to Avelumab with 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2018;46:1197–1198. doi: 10.1007/s00259-018-4230-x. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen M.H., Leong S.P., Abendroth R., Kashani-Sabet M., Kim K.B. Complete clinical response to intralesional talimogene laherparepvec injection in a patient with recurrent, regionally advanced Merkel cell carcinoma. JAAD Case Rep. 2019;5:849–851. doi: 10.1016/j.jdcr.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giroulet F., Tabotta F., Pomoni A., Prior J. Primary parotid Merkel cell carcinoma: A first imagery and treatment response assessment by 18F-FDG PET. BMJ Case Rep. 2019;12:e226511. doi: 10.1136/bcr-2018-226511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maecke H.R., Hofmann M., Haberkorn U. Ga-68-labeled peptides in tumor imaging. J. Nucl. Med. 2005;46(Suppl. 1):172S–178S. [PubMed] [Google Scholar]
- 70.Kwekkeboom D.J., Hoff A.M., Lamberts S.W., Oei H.Y., Krenning E.P. Somatostatin analogue scintigraphy. A simple and sensitive method for the in vivo visualization of Merkel cell tumors and their metastases. Arch. Dermatol. 1992;128:818–821. doi: 10.1001/archderm.1992.01680160102014. [DOI] [PubMed] [Google Scholar]
- 71.Guitera-Rovel P., Lumbroso J., Gautier-Gougis M.S., Spatz A., Mercier S., Margulis A., Mamelle G., Kolb F., Lartigau E., Avril M.F. Indium-III octreotide scintigraphy of Merkel cell carcinomas and their metastases. Ann. Oncol. 2001;12:807–811. doi: 10.1023/A:1011142410535. [DOI] [PubMed] [Google Scholar]
- 72.Durani B., Klein A., Henze M., Haberkorn U., Hartschuh W. Somatostatin analogue scintigraphy in Merkel cell tumours. Br. J. Dermatol. 2003;148:1135–1140. doi: 10.1046/j.1365-2133.2003.05338.x. [DOI] [PubMed] [Google Scholar]
- 73.Akaike T., Qazi J., Anderson A.J., Behnia F., Shinohara M., Akaike G., Hippe D.S., Thomas H., Takagishi S., Lachance K., et al. High somatostatin receptor expression and efficacy of somatostatin analogues in patients with metastatic Merkel cell carcinoma. Br. J. Dermatol. 2020;10 doi: 10.1111/bjd.19150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofmann M., Maecke H., Börner A., Weckesser E., Schöffski P., Oei M., Schumacher J., Henze M., Heppeler A., Meyer G., et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: Preliminary data. Eur. J. Nucl. Med. Mol. Imaging. 2001;28:1751–1757. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 75.Gabriel M., Decristoforo C., Kendler D., Dobrozemsky G., Heute D., Uprimny C., Kovacs P., Von Guggenberg E., Bale R., Virgolini I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 76.Buchmann I., Henze M., Engelbrecht S., Eisenhut M., Runz A., Schäfer M., Schilling T., Haufe S., Herrmann T., Haberkorn U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 77.Salavati A., Prasad V., Schneider C.-P., Herbst R., Baum R.P. Peptide receptor radionuclide therapy of Merkel cell carcinoma using 177lutetium-labeled somatostatin analogs in combination with radiosensitizing chemotherapy: A potential novel treatment based on molecular pathology. Ann. Nucl. Med. 2012;26:365–369. doi: 10.1007/s12149-012-0578-3. [DOI] [PubMed] [Google Scholar]
- 78.Schneider C., Schlaak M., Bludau M., Markiefka B., Schmidt M.C. 68Ga-DOTATATE-PET/CT Positive Metastatic Lymph Node in a 69-Year-Old Woman With Merkel Cell Carcinoma. Clin. Nucl. Med. 2012;37:1108–1111. doi: 10.1097/RLU.0b013e318266d3b3. [DOI] [PubMed] [Google Scholar]
- 79.Epstude M., Tornquist K., Riklin C., Di Lenardo F., Winterhalder R., Hug U., Strobel K. Comparison of 18F-FDG PET/CT and 68Ga-DOTATATE PET/CT Imaging in Metastasized Merkel Cell Carcinoma. Clin. Nucl. Med. 2013;38:283–284. doi: 10.1097/RLU.0b013e318281658e. [DOI] [PubMed] [Google Scholar]
- 80.Basu S., Ranade R. Metastatic Merkel Cell Carcinoma responding favourably to targeted therapy with 177Lu-DOTATATE: Will PRRT evolve as an important treatment approach in Receptor positive cases? J. Nucl. Med. Technol. 2015;44:85–87. doi: 10.2967/jnmt.115.163527. [DOI] [PubMed] [Google Scholar]
- 81.Balogova S., Talbot J.-N., Nataf V., Michaud L., Huchet V., Kerrou K., Montravers F. 18F-Fluorodihydroxyphenylalanine vs. other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:943–966. doi: 10.1007/s00259-013-2342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giammarile F., Castellucci P., Dierckx R., Lobato E.E., Farsad M., Hustinx R., Jalilian A., Pellet O., Rossi S., Paez D. Non-FDG PET/CT in Diagnostic Oncology: A pictorial review. Eur. J. Hybrid. Imaging. 2019;3:1–46. doi: 10.1186/s41824-019-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dimitrakopoulou-Strauss A., Strauss L.G., Burger C. Quantitative PET studies in pretreated melanoma patients: A comparison of 6-[18F]fluoro-L-dopa with 18F-FDG and (15)O-water using compartment and noncompartment analysis. J. Nucl. Med. 2001;42:248–256. [PubMed] [Google Scholar]