Abstract
Studying the complex molecular mechanisms involved in traumatic brain injury (TBI) is crucial for developing new therapies for TBI. Current treatments for TBI are primarily focused on patient stabilization and symptom mitigation. However, the field lacks defined therapies to prevent cell death, oxidative stress, and inflammatory cascades which lead to chronic pathology. Little can be done to treat the mechanical damage that occurs during the primary insult of a TBI; however, secondary injury mechanisms, such as inflammation, blood-brain barrier (BBB) breakdown, edema formation, excitotoxicity, oxidative stress, and cell death, can be targeted by therapeutic interventions. Elucidating the many mechanisms underlying secondary injury and studying targets of neuroprotective therapeutic agents is critical for developing new treatments. Therefore, we present a review on the molecular events following TBI from inflammation to programmed cell death and discuss current research and the latest therapeutic strategies to help understand TBI-mediated secondary injury.
Keywords: neurotrauma, neuroinflammation, excitotoxicity, oxidative stress, apoptosis, edema, brain injury, therapeutic strategies
1. Introduction
Traumatic brain injury (TBI), a leading cause of death and disability, is an international public health concern. An estimated 53–69 million individuals worldwide sustain a TBI annually [1], and up to 2 percent of the population lives with neurological disabilities caused by a TBI [2,3]. TBI occurs when an external mechanical force causes a disruption in normal brain functioning. While commonly discussed as a single clinical entity, TBI embodies a complex and heterogeneous pathology (Figure 1 and Figure 2). As such, comprehensive knowledge of the cellular and molecular events post-TBI remains a long-standing goal of preclinical research, with the hope that this knowledge will spur the expansion of novel therapeutics.
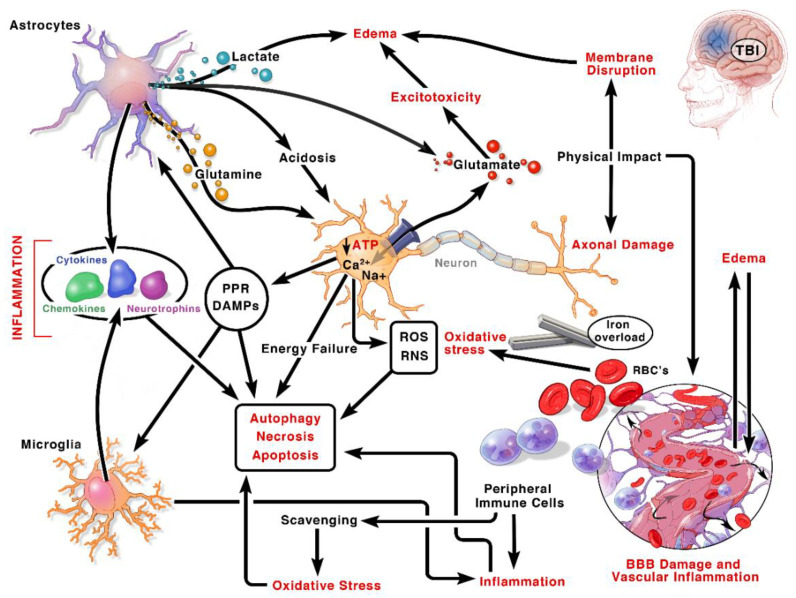
Figure 1.
Pathophysiology of TBI. A schematic flow chart of the pathological changes after TBI that lead to acute and chronic neurovascular damage and immune activation. Immediately after the insult neurovascular damage occurs, and large amounts of DAMPs are released causing gliosis and peripheral immune cell infiltration. The initial function of these immune cells is to contain the injury and remove debris and dead cells. However, unregulated immune cells cause enhanced inflammation and injury progression. Furthermore, energy failure, oxidative stress, prolonged inflammation, and excitotoxicity lead to progressive injury with white matter damage and chronic behavioral deficits. Abbreviations: DAMP: Damage associated molecular patterns; PRR: Pattern recognition receptors; ROS: Reactive oxygen species; RNS: Reactive nitrogen species; RBC: Red blood cells; Na+: Sodium ion; Ca2+: Calcium ion; ATP: Adenosine triphosphate; TBI: Traumatic brain injury.
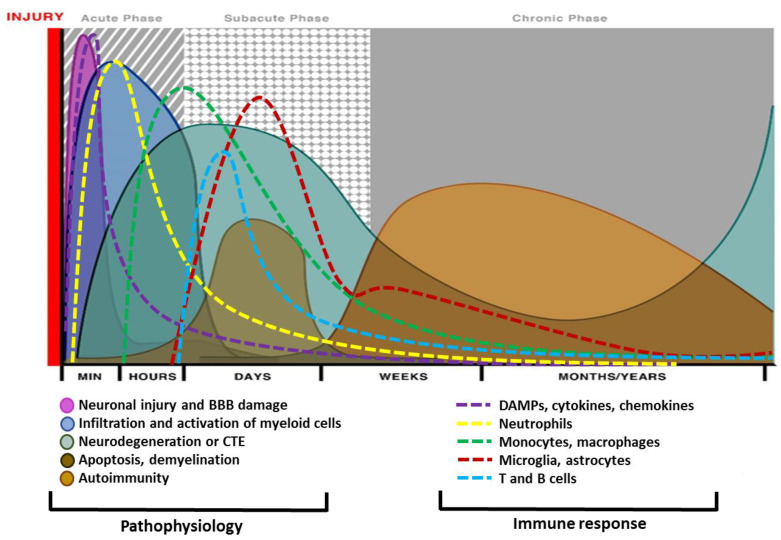
Figure 2.
Different phases of traumatic brain injury (TBI) pathophysiology and relative immune response. Mechanical insult leads to acute neuronal injury and blood-brain barrier (BBB) damage, which initiates gliosis and glial injury minutes after TBI and continues for days after injury. Necrotic and apoptotic cell death start immediately after the insult and peak within h to days. Axonal shearing is another event that leads to demyelination and white matter injury. Neurodegeneration, traumatic encephalopathy, and axonal injury may sustain for years after a single TBI. Acute insult and neurovascular damage lead to myeloid accumulation and recruitment of T-cells that last for years and may cause chronic neurodegeneration and neuropathology. Immune cells respond to trauma in a timely manner and a differential pattern of activations has been observed by various studies. An impact to the head leads to cellular damage and results in the rapid release of damage-associated molecular patterns (DAMPs). DAMPs stimulate local cells to release inflammatory mediators such as cytokines and chemokines. These mediators recruit myeloid cells specifically neutrophils as first responders, which phagocytize debris and damaged cells promoting the containment of the injury site. As neutrophil numbers begin to decline, infiltrated monocytes and glia get activated and accumulate around the site of injury to perform further phagocytic or repair functions. Depending on the severity of the brain injury, myeloid cells can recruit T and B cells. T and B cells appear at the sites of brain pathology at later time points in the response (3–7 days post-injury) and may persist for weeks to months. Other abbreviation is as CTE: Chronic traumatic encephalopathy.
TBI is categorized according to pathophysiology, etiology, and severity, as assessed by neuroimaging and physiological responses. The Glasgow Coma Scale (GCS) is most commonly utilized to define the severity of brain injury in clinical settings, where patients are assessed following initial resuscitation and within 48 h post-injury [4]. A GCS score of 13–15 is classified as mild injury, a score of 9–12 is classified as moderate injury, and a score of <9 is classified as severe injury. Another assessment tool similar to the GCS is the Full Outline of Unresponsiveness (FOUR) score, which can be used in intubated patients and includes an assessment of brainstem function [5].
The pathogenesis of TBI may be divided into two injury-mechanisms: primary and secondary injury. Primary injury entails the direct brain damage that occurs immediately after the impact. The initial injury mechanisms could give rise to extraparenchymal hemorrhages (epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intraventricular hemorrhage); focal contusions and intraparenchymal hemorrhages; traumatic axonal (focal or diffuse) injury (TAI) due to shearing of WM tracts; and cerebral edema (Figure 1 and Figure 2). Secondary injury mechanisms are also initiated at the moment of the traumatic incident but are believed to continue for many years through a series of cellular, physiological and molecular processes impacting all kinds of cells in the brain. Blood-brain barrier (BBB)-disturbance, excitotoxicity, mitochondrial dysfunction, oxidative stress, inflammation, and cell loss are the principal identified mechanisms orchestrating secondary injury mechanisms [6]. Thus, a pathophysiological and anatomical based classification system for TBI that links the precise pathological mechanisms with the appropriate therapeutic interventions would enhance the translation of therapies from bench to bedside [7]. Therefore, this review provides a synopsis of the main mechanisms of secondary brain damage, along with targeted current and potential neuroprotective therapeutic interventions in preclinical and clinical settings. In the following sections, we will present the historical context for targeting a number of secondary injury pathways. We will discuss the rationale, preclinical evidence, and where appropriate, the translational data in humans. This will provide a segue to why we discuss all these topics, show where we have failed, and perhaps gives a clue why some targets were unsuccessful. This can guide both experimental studies and clinical trial design as we seek efficacy treatments.
2. Excitotoxicity
Excitotoxicity is a pathological process where accumulation of excitatory neurotransmitters, usually glutamate and over-activation of their receptors (NMDAR), causes BBB damage, loss of neuronal membrane integrity, edema, and cell loss after TBI [8]. Studies involving the administration of membrane-resealing polymers following controlled cortical impact (CCI) reported reduced BBB permeability and cerebral damage, and improved functional recovery [9,10] but failed to rescue degenerating cells [11]. This may suggest that resealing of these membranes prevents further alterations of the membrane but did not rescue degenerating cells that were already damaged by the initial episode of TBI-induced excitotoxicity. Similarly, persistent elevated glutamate in cerebral tissue and CSF link with TBI severity in patients [12,13]. Although NMDAR antagonism mitigated TBI-induced neurological damage in rodents [14], global NMDAR antagonists showed side effects and were associated with poor therapeutic windows [15]. Thus, revelation of the mechanisms linking glutamate excitotoxicity, NMDAR activation, and consequent neurological damage, may offer a roadmap to improve neurological outcomes without any adverse effect.
2.1. Glutamate
Glutamate, the principal excitatory neurotransmitter, is essential for normal brain function; however, metabolic perturbations occurring immediately after neurotrauma result in loss of ATP production and subsequent failure of neuronal Na+-K+ ATPases. These changes disrupt the homeostatic balance of the electrochemical gradient, causing intracellular sodium accumulation and neuronal depolarization to exacerbate release of synaptic glutamate. In addition, mechanical stretching of neuronal membranes induces micropore formation to aggravate intracellular sodium influx. This ionic shift exacerbates the opening of voltage-gated calcium channels and neuronal depolarization to further the excessive synaptic release of glutamate. Microdialysis studies have shown that increased levels of extracellular glutamate post-TBI correlated with the severity of injury, while elevated glutamate levels in CSF and brain tissues correlated with worse outcomes after clinical TBI [12,16,17,18,19].
2.2. Glutamate Receptors
Glutamate receptors are present on membranes of neurons and glial cells both at synaptic and extra-synaptic regions. There are two types of glutamate receptors: (GluR)-ionotropic (iGluR) and metabotropic (mGluR). When glutamate binds to iGluRs (ligand-gated nonselective cation channels), it activates the ion channels, and when it binds to mGluRs (G protein-coupled receptors), it either upregulates or downregulates signal transduction pathways. iGluRs are in turn categorized into four subtypes, including N-methyl-D-aspartate receptors (NMDAR), kainate receptors (KAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), and delta receptors. Glutamate increases intracellular calcium primarily through activation of postsynaptic ionotropic receptors, such as NMDARs [20,21]; however, KARs and calcium-permeable AMPARs also may add to elevated intracellular calcium. Excessive inflow of calcium activates phospholipases, endonucleases, and proteases (calpains), which then precipitate neuronal cell loss via a process deemed excitotoxicity. In addition, GluRs, which are particularly sensitive to mechanical injury, may also contribute to delayed depolarization and injury [22].
2.2.1. Synaptic and Extrasynaptic NMDARs
Based on their location, NMDARs can exert opposing effects. Although stimulation of synaptic NMDAR upregulates brain-derived neurotrophic factor (BDNF) and cAMP response element-binding protein (CREB) activity to promote neuronal survival, extrasynaptic NMDAR activation leads to excitotoxicity and neuronal cell death. This occurs via promotion of a CREB shut-off pathway and inhibition of BDNF gene expression. Specific targeting of NMDARs based on their location could pave the way towards more effective neuroprotective therapies [23,24,25].
2.2.2. NMDAR Subunits
A more comprehensive analysis of NMDARs shows that they can be made up of seven variable subunits, including a GluNR1 subunit, four GluNR2 subunits (GluN2A, GluN2B, GluN2C, or GluN2D), and two GluNR3 subunits (GluNR3A and GluNR3B). NMDARs are heterotetramers with a strictly regulated subunit composition [26]. Further, extra-synaptic and synaptic NMDARs have different subunit compositions which could be a reason for their opposing cellular effects. The majority of extrasynaptic NMDARs are composed of NR1/NR2B subunits, while synaptic receptors also contain NR2A subunits. Moreover, NMDARs at immature sites markedly contain NR1/NR2B subunits, while NMDAR composition is more diverse at mature sites and switches from NR2B to NR2A on synaptic maturation, termed as NR2B to NR2A developmental switch [27]. Recently, it has been reported that higher expression of the NMDAR subunit NR2A protected neuronal connectivity in the injured brain, while activation of NR2B-containing NMDARs contributed towards loss of connectivity, suggesting a potential role for NMDARs in the restructuring of the neuronal network post-TBI [28].
2.2.3. Therapeutic Strategies Targeting NMDARs
Many therapeutic interventions have been designed based on targeting NMDARs, including NMDAR antagonists, NMDAR subunit inhibitors, and partial agonists of glycine/NMDAR.
NMDAR Antagonists
Administration of NMDAR antagonists (e.g., MK-801) improved outcomes after experimental TBI [29,30,31,32]; yet clinical trials exploring the efficacy of NMDAR antagonists were halted due to poor efficacy, major side effects, poor drug efficacy, restricted therapeutic windows, and interference with normal synaptic transmission. These disappointing results, which suggest limited utility of broad-spectrum NMDAR antagonists after acute brain injury, illustrate the translational challenges involved in limiting the detrimental effects of glutamate and suggest the need for alternative approaches [15,33,34,35].
MK-801: Rats treated with MK-801 prior to injury demonstrated enhanced cognition and axo-dendritic integrity [30], and co-application of MK-801 with other NMDAR antagonists had additive neuroprotective effects [31]. Treatment with MK-801 prior to injury, significantly attenuated neurological deficits; however, MK-801 had minimal influence on neurologic scores when was administered post-injury [32].
Memantine: Intraperitoneal treatment of memantine (10 and 20 mg/kg), a non-competitive NMDAR antagonist, immediately after TBI inhibits neuronal death in rats [36]. Memantine (1–10 μM), when applied to rat hippocampal neurons in vitro, inhibited extrasynaptic NMDAR-induced currents while largely sparing synaptic activity [37]. In a randomized controlled trial of moderate TBI patients, enteral administration of memantine (30 mg twice daily for 7 days) post-injury resulted in substantial improvement in GCS scores at 3 days and significant reduction in neuronal damage at 7 days, as evident from reduced serum levels of neuron-specific enolase (NSE) [38].
Ketamine: In a moderate TBI rat model, administration of ketamine, a non-competitive NMDAR antagonist, at a sub-anesthetic dose (10 mg/kg daily) for 7 days resulted in protection of neuronal dendrites and spines, attenuation of post-traumatic neuroinflammation, and thus, improved neurobehavioral outcomes [39]. Similarly, a significant association between ketamine treatment and reduced spreading depolarization events have been reported in TBI, SAH, and hemispheric stroke patients. Spreading depolarizations are linked with neuronal damage and poor outcome [40].
Magnesium: Magnesium can bind to NMDARs, blocking the passage of ions through the channel, and can therefore be utilized to augment neuroprotection. A study in rats reported that magnesium deficiency worsened TBI outcomes while magnesium administration improved neurological outcomes and mortality after TBI [41]. A Cochrane systematic review including three randomized controlled trials (RCTs) published in 2008, found no beneficial role for magnesium treatment in acute TBI patients in terms of improving neurological outcomes or mortality and therefore did not support its use [42]. Another methodical review of the use of magnesium sulfate in acute TBI management, including eight RCTs published in 2015, also found no significant improvement in mortality but did report a nonsignificant improvement in GOS and GCS scores with magnesium therapy [43].
NMDAR Subunit Inhibitors
Ifenprodil: Ifenprodil, a selective inhibitor of NR2B subunit, can differentially suppress extrasynaptic NMDARs, and therefore could attenuate cell death cascades [44]. Similarly, Ifenprodil treatment mitigated brain edema and reduced injury volume in a CCI model of rats [45]. In an in vitro model of TBI, Ifenprodil reduced NMDA-activated currents but failed to limit fluid shear stress-induced Ca2+ influx in primary rat astrocytes [46].
Ro25-6981: TBI modifies NMDAR expression and functioning. For example, moderate TBI in rats caused rapid recruitment of NR2B to membrane rafts successively inducing autophagy. Ro25-6981, a selective inhibitor of NR2B, markedly mitigated autophagy [47]. Activation of NR2B-containing NMDARs may be further involved in the insertion of calcium-permeable AMPARs (CP-AMPARs) in an in vitro model of TBI, further worsening the intracellular calcium overload [48]. Stretch injury of cultured cortical neurons resulted in enhanced extrasynaptic current transmission facilitated by NR2B-units of NMDARs, with no marked variations in synaptic transmission through NMDAR [49]. In addition, either Ro25-6981 or memantine treatment barred this injury-induced increase in CP-AMPAR activity [49]. Further, administration of Ro25-6981 (6 mg/kg, i.p.), attenuated edema post-TBI in mice and limited the NMDA-induced excitotoxicity and release of HMGB1 from injured cortical neurons in vitro [14].
Traxoprodil (CP-101,606): Intravenous (IV) infusion of traxoprodil in patients, another NR2B antagonist, for up to 72 h post-injury was reported to be safe and well-tolerated in all cases of TBI [50,51]. Although in an RCT where severe TBI patients were given a 72-h infusion of traxoprodil within 8 h post-injury, improvements in the dichotomized Glasgow Outcome Scale (dGOS) at 6 months and mortality rate were noticed; however, this improvement was not significant [52].
Temporal alteration of NMDARs and the partial agonist D-cycloserine (DCS): TBI in rodent models, leads to dynamic changes in NMDARs with early hyperactivation followed by weeks of functional loss. Subacute administration (24 to 72 h post-TBI) of DCS, a partial agonist of glycine/NMDARs, upregulated BDNF expression, restored impaired hippocampal long-term potentiation, and enhanced recovery of neurobehavioral and cognitive functions in mice [53]. The complex role of NMDARs in TBI pathophysiology adds to the translational challenges faced by therapeutic interventions targeting this mechanism.
2.3. Postsynaptic Density Protein 95 (PSD-95) and PSD-95 Inhibitors
PSD-95 is a membrane-associated guanylate kinase (MAGUK) that interacts with NMDARs, AMPARs, and potassium channels and plays a role in synaptic plasticity [54]. PSD-95 combines the NR2B subunit of NMDARs with neuronal nitric oxide synthase (nNOS), to form a complex NMDAR/PSD-95/nNOS, and adds to neurotoxicity [55]. Interestingly, inhibition of nNOS by disturbing NMDAR-PSD-95 interactions prevented nitration of protein and cell death in vitro [56]. ZL006, an inhibitor of nNOS-PSD95 interaction, prevented neuronal apoptosis and improved sensorimotor and cognitive outcomes in rodents [57]. Similarly, disruption of NMDAR-PSD-95 interaction by a synthetic peptide (now known as NA-1) blocked excitotoxicity in cultured neurons, limited ischemic cerebral damage, and improved neurological function in rats without altering calcium influx or synaptic activity [58]. The strategy appears to be very promising as inhibition of this interaction between PSD-95 and NMDAR-mediated neurotoxic signaling pathways has demonstrated reduced infarct size and improved outcomes after stroke in macaques [59]. Further, NMDAR-PSD-95-nNOS complex inhibitor, known as NA-1, has been shown to treat ruptured cerebral aneurism, reduce ischemic brain damage, and improve neurological scores, and has become the first stroke therapy to demonstrate efficacy in humans (NCT00728182) after initial results in primates [60,61,62]. In addition, two phase III clinical trials (NCT02315443; NCT02930018) have been completed in acute stroke and awaiting publication. Interestingly, TBI activated endoplasmic reticulum-associated PKR-like ER kinase (PERK) which phosphorylates CAMP response element-binding protein (CREB) and PSD-95, resulting in reduced brain-derived neurotropic factor (BDNF) and PSD-95 in the injured cortex. However, either PERK inhibitor GSK2656157 or overexpression of kinase-dead mutant of PERK (PERK-K618A) in primary neurons rescued loss of dendrites and memory in mice [63]. Further, a rodent model of CCI showed loss of PSD-95 with loss of neuronal NeuN in contused cortex and directly correlated with behavioral abnormalities [64]. UCCB01-147 [also known as Tat-NPEG4(IETDV)(2), (Tat-N-dimer)], a dimeric PSD-95 inhibitor, was observed to be neuroprotective in an experimental stroke model [65] but failed to demonstrate those beneficial effects in experimental TBI [66]. Therefore, it can be argued that PSD-95 alone or with other effector proteins may provide a potential clinical therapeutic target to improve memory and learning deficits but must be translated carefully for better results post-TBI.
2.4. mGluR2 Receptors and Gap Junctions
Recently, it was reported that mGluR2 receptors in a fluid percussion TBI model once activated, upregulated gap junction protein expression, suggesting a possible role for mGluR2 and gap junctions in secondary brain injury [67]. Gap junctions play critical roles in neuronal differentiation and circuit formation in the developing CNS and allow for the passage of ions, small molecules, and secondary messengers (Ca, IP3, cAMP, etc.) in the adult brain [68,69]. However, upregulated gap junctions in insulted brain may enhance the secretion of pro-apoptotic factors and secondary messengers such as Ca2+ to add to cell death. Therefore, mefloquine, a gap-junction blocker may be a valuable therapeutic tool in mitigating TBI-induced excitotoxicity.
2.5. Glutamate Transporters
The solute carrier 1 (SLC1) family of neurotransmitter transporters includes a five-member family of excitatory amino acid transporters (EAAT) that mediate the rapid uptake of synaptic glutamate via a process coupled to ion gradients [70]. Amongst the EAAT, EAAT1 [Glutamate Aspartate Transporter (GLAST)] (human/rodent homolog) and EAAT2 [Glutamate Transporter 1 (GLT-1)] are essential for glutamate clearance related to neurotransmission, whereas EAAT3, EAAT4, and EAAT5 exhibit less prominent parts in regulating neuronal excitability [70]. In particular, EAAT2 is expressed in glia and mediates 95 percent of glutamate uptake in CNS [71]. Notably, when ionic gradients are lost, sodium-glutamate transporters can reverse the transport direction to secrete a high amount of glutamate [72]. Given that excessive glutamate is associated with excitotoxicity, targeted enhancement of EAAT2 may circumvent the issues associated with administration of NMDAR antagonists. Postmortem analysis of human brains obtained after TBI showed lower expression of the glial glutamate transporter EAAT2, which might have impaired reuptake of extracellular glutamate and thus, have led to excitotoxicity [73,74]. Similarly, lowered EAAT2 expression inversely correlated with CSF glutamate levels up to 7 days post-injury in CCI model of rodents [75,76].
MS-153 (GLT-1 activator): Functionally, administration of GLT-1 antisense oligodeoxynucleotides exacerbated hippocampal injury and increased mortality after TBI [76], whereas acute administration of (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153), a GLT-1 activator, decreased neurodegeneration and attenuated calpain activation in cortical and hippocampal tissue for up to two weeks after fluid percussion injury. While these latter findings warrant further exploration of GLT-1 activators, MS-153 upregulated GLT-1 in the naïve brain but not after brain injury in rats, suggesting that mechanisms independent from GLT-1 may mediate the observed beneficial effects [77]. Future studies incorporating more selective pharmacological activators and genetic overexpression approaches will provide clarity regarding the translational potential of targeting GLT-1 after TBI.
2.6. Blood Glutamate Scavengers
Glutamate transporters located on brain capillary endothelial cells facilitate brain-to-blood efflux of glutamate and play a role in glutamate homeostasis. When glutamate concentrations are kept low in blood, this results in a larger concentration gradient of glutamate and enhances its brain-to-blood efflux [78,79]. Glutamate levels in the blood might be reduced via two enzymes: glutamate-oxaloacetate transaminase (GOT or AST) (l-glutamate + oxaloacetate ⇌ α-ketoglutarate + l-aspartate) and glutamate-pyruvate transaminase (GPT or ALT) (l-glutamate + pyruvate ⇌ α-ketoglutarate + l-alanine). The two serum enzymes (SGOT and SGPT) and co-substrates (oxaloacetate and pyruvate) may potentially act as glutamate scavengers. In agreement, treatment with co-substrate, oxaloacetate, and pyruvate resulted in a reduction of glutamate in blood and enhanced neuronal survival and neurological outcomes in experimental TBI studies [80,81]. In addition, recombinant GOT1 has also shown promising results in TBI [82], and ischemic stroke [83,84]. Of note, riboflavin (Vitamin B2) was found to be a potent scavenger of blood glutamate, resulting in reduced infarct size after ischemia [85]. Similarly, Hoane and colleagues found that riboflavin significantly reduced sensorimotor and cognitive impairment, reduced edema, and astrogliosis after TBI [86] Furthermore, a double-blind, randomized phase IIb clinical trial in stroke patients also demonstrated riboflavin significantly scavenged glutamate in patient blood [85]. Taken together, these studies suggest that therapies utilizing blood glutamate scavenging may be promising therapeutic avenues for reducing glutamate-induced excitotoxicity.
2.7. GABAergic Excitotoxicity
Although glutamate is a major player in excitotoxicity, elevated concentrations of other neurotransmitters have also been detected in the extracellular space of injured brains. These other neurotransmitters, such as GABA, may also aid in excitotoxicity in both specific and distant cell populations. A microdialysis study in experimental open-skull weight drop TBI, showed elevated GABA in the cortical extracellular space [87]. Similarly, hippocampal cell loss has been reported in multiple experimental models of TBI and may correlate with neurocognitive deficits that occur in TBI patients [88,89]. Immature neurons in the adult hippocampal sub-granular zone express voltage-gated channels similar to their embryonic equivalents and may be depolarized by GABAergic activity via chloride gradient reversal [90,91]. Since selective necrotic cell loss among immature adult-born neurons has been reported after CCI injury; therefore, the properties of these immature neurons may be relevant to pathophysiology in TBI [92,93,94]. However, the mechanism behind this particular cellular selectivity for necrosis among immature cells is still known. GABAergic excitotoxicity also contributes to neuronal loss as exposure of isoflurane to the immature pyramidal cells in culture increased intracellular calcium and led to cellular death [95], suggesting that intracellular calcium overload may add to immature neuronal death. These studies, coupled with the increased extracellular GABA concentrations as observed following TBI [87], may implicate an unexplored role for GABAergic excitotoxicity post-TBI. Understanding the mechanisms of GABAergic excitotoxicity in the post-traumatic brain may be valuable therapeutically, as several GABA antagonists, approved by the U.S. Food and Drug Administration (FDA), are available. In terms of treating more canonical glutamatergic excitotoxicity, it may be argued that antagonists to GluR or inhibitors that block the release of glutamate inhibitors (e.g., lamotrigine) exert beneficial effects not only through inhibition of either GluR or glutamate, but also by minimizing the neuronal metabolism. However, given the contradictory findings of clinical trials utilizing magnesium sulfate as an NMDAR antagonist in acute stroke patients [96,97], a more comprehensive knowledge of excitotoxicity following TBI, including non-canonical mechanisms (such as GABAergic excitotoxicity), is critical for developing effective therapeutic strategies. Further, excitotoxicity and as a result, influx of excessive calcium into cells lead to oxidative stress, mitochondrial dysfunction, activation of Nox family member, and oxidation of cellular biomolecules such as lipids, proteins, and DNA. Furthermore, excessive amount of intracellular calcium activates several proteases and phospholipases, and thus, mediates degradation of cellular proteins and lipids, and enhances ROS production, and contributes significantly to secondary injury post-TBI [98,99].
3. Oxidative Stress
TBI results in cerebral circulation dysfunction, microvascular impairment, and moderate hypoxia [100,101]. Although primary traumatic injury occurs as a result of the physical impact, tissue injury is amplified by secondary injury mechanisms. Oxidative damage is unambiguously one of the most confirmed “secondary injury” pathways observed in TBI. The brain is very sensitive to free radical-mediated damage because of the presence of abundant polyunsaturated lipids and a high rate of endogenous oxidative metabolism. Therefore, a balance between oxidant production and antioxidant machinery is essential for normal functioning of the brain.
3.1. Oxidant-Antioxidant Balance
The reperfusion of blood circulation after trauma ensures the survival of neurons but also elevates the generation of free radicals and reactive oxygen species (ROS) [102,103,104]. The generated free radicals, such as hydrogen peroxide, superoxides, nitric oxide (NO), etc., also cause excitotoxicity and impair the metabolic activity of cells. Further, superoxide radicals generated due to catalytic activity after TBI react with NO to form another potent oxidant peroxynitrite, which impairs cerebrovascular function [105,106]. The ROS possesses an unpaired electron and thus, readily binds to different macromolecules such as protein, nucleic acid, or lipid to cause damage. The endogenous antioxidant system comprises of glutathione (GSH), and various enzymes (i.e., glutathione reductase (GR), glutathione-S-transferase (GST), glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), and uric acid) neutralizes these ROS, preventing the oxidation of macromolecules. In a normal brain, oxidants and antioxidants exist in equilibrium; however, the unwarranted production of ROS following brain injury overburdens the efficiency of the endogenous antioxidants and shifts the equilibrium towards oxidants by depleting endogenous antioxidants. This disrupted balance increases membranous lipid peroxidation, oxidation of proteins, DNA break, and inhibition of the mitochondrial respiration, which ultimately throws cells into apoptosis or necrosis [107]. Overall, increased oxidants and reduced activity of antioxidant defense systems may contribute toward the pathogenesis post-TBI.
3.2. Superoxide Radicals and Superoxide Scavengers
Kontos and colleagues demonstrated an acute increase in brain microvascular superoxide radical (O2•−) contents as a result of compromised autoregulatory function after fluid percussion injury [108,109]. Within an injured brain, several possible sources of O2•− may be operating from the very first minute of impact to a few h post-injury, including the arachidonic acid-prostaglandin cascade, oxidation of leaked hemoglobin and biogenic amines (e.g., norepinephrine, dopamine, 5-hydroxytryptamine), xanthine oxidase activity, and mitochondrial leakage. At later time points, activated microglia and infiltrating neutrophils and macrophages also provide additional sources of O2•− Superoxide O2•− is less reactive to biological substrates than hydrogen peroxide (H2O2). Once formed, O2•− undergoes dismutation to form H2O2 in a reaction that is catalyzed by SOD: O2•− + O2− + 2H+ → H2O2 + O2 [110]. The H2O2 formed is altered to water by peroxidases, such as Gpx and peroxiredoxin, or is dismuted to water and oxygen by CAT. Both CAT and GPx enzymes are abundant in the brain, though the latter has a sevenfold greater activity [111]. In the absence of the antioxidant system, as in TBI, O2•− actually exists in equilibrium with hydroperoxyl radicals (HO2•): O2•− + H+ → HO2•, which is a considerably more powerful oxidizing or reducing agent [112]. O2•−/HO2• cause the pH to fall into acidic ranges (i.e., tissue acidosis), fueling an equilibrium shift in favor of HO2•, which is much more reactive than O2•−, particularly toward lipids.
SOD and polyethylene glycol (PEG)-conjugated SOD (PEG-SOD): In humans, the three forms of SOD are SOD1 (Cu/Zn-SOD), SOD2 (Mn-SOD), and SOD3 (Cu/Zn-SOD), which are respectively sited in the cytoplasm, mitochondria, and outside the cell. In cats, the administration of SOD reverses the microvascular dysfunction post-TBI [113]. Transgenic mice overexpressing human Cu/Zn SOD activity have reduced acute injuries, prevented brain edema, and inhibited BBB permeability following TBI [114,115]. Studies using both Cu/Zn-SOD and Mn-SOD transgenic and knockout mice have further solidified the protective role of these enzymes against head trauma [115,116,117,118,119]. In a randomized controlled phase-II trial with more metabolically stable PEG-SOD (2000–10,000 U/kg intravenously administered 4 h post-TBI), the higher doses (5000 and 10,000 U/kg) decreased the duration when ICP > 20 mm of Hg. There was a statistically significant improvement in patient outcomes measured using the Glasgow Outcome Scale (GOS) at 3 and 6 months post-injury between patients who received placebo and those who received 10,000 U/kg PEG-SOD [120]. However, a subsequent larger phase-III randomized trial with higher doses of PEG-SOD (10,000 or 20,000 U/kg within 8 h post-TBI failed to show significant improvement in neurological outcomes or patient survival [121]. Nevertheless, it is imperative to shed light on a 4-h difference in the time of administration of PEG-SOD post-injury between these two trials.
OPC-14117: The superoxide radical scavenger “OPC-14117” reduced cortical damage and improved neuronal survival and cognitive functions following CCI in rats [122]. A controlled randomized trial assessing the safety of OPC-14117 (240 mg per day) in HIV-associated cognitive impairment found it to be tolerable and resulted in improvement of clinical global impression scale scores and nonsignificant enhancement of cognitive test scores [123]. In light of the neuroprotective effects of OPC-14117 in this preclinical TBI study and the safety of use in humans, the potential benefits of OPC-14117 treatment after TBI are worth investigating.
3.3. Iron, Hydroxyl Radicals, and Iron Chelators
The abundance of iron in the CNS makes it vulnerable to oxidative insult [124]. Under normal circumstances, iron is maintained in a non-catalytic state by plasma transferrin and intracellular ferritin [110]. However, in the event of tissue acidosis, when pH falls below 6, both proteins readily release their iron into the traumatized brain parenchyma. Further, hemorrhage occurs as a result of mechanical impact provides an obvious pool of iron released from hemoglobin via interaction with H2O2 or lipid hydroperoxides (LOOH) at acidic pH [125,126]. Once released into the brain parenchyma, iron actively catalyzes Fenton’s reaction to generate ROS [110]. Free iron or iron compounds participate in production of ROS in two ways: First, autoxidation of Fe2+ produces O2•− [110]: Fe2+ + O2 → Fe3+ + O2•− and/or secondly, oxidation of Fe2+ by H2O2 gives hydroxyl radicals (•OH) via Fenton’s reaction: Fe2+ + H2O2 → Fe3+ + •OH + OH−.
Deferoxamine and dextran-conjugated deferoxamine: Experimental TBI studies of deferoxamine (iron chelator) treatment in rodents have shown neuroprotective effects [127,128]. However, IV infusion of deferoxamine may cause profound hypotension, but binding of deferoxamine to dextran may alleviate this effect as low dose dextran-conjugated deferoxamine following TBI improved neurological outcomes as compared to the deferoxamine group alone [129].
N,N’-Di(2-hydroxybenzyl)ethylenediamine-N,N’-diacetic acid monohydrochloride (HBED): HBED is an iron chelator, can cross the BBB, and has a relatively longer half-life compared to that of deferoxamine [130]. HBED treatment resulted in reduced cortical damage and restored neurological functions in mice post-TBI [131].
3.4. Nitric oxide Synthase (NOS) and NOS Inhibitors
NOS catalyzes l-arginine to give NO and citrulline at the expense of NADPH. The three NOS isoforms [neuronal (nNOS or NOS1), inducible (iNOS or NOS2), and endothelial (eNOS or NOS3)] are acutely upregulated in rats following TBI, with levels peaking at 6 to 12 h post-injury and then declining. eNOS is expressed solely in endothelial cells, nNOS predominantly in neurons but also in polymorphonuclear cells, and iNOS in immune cells such as myeloid cells [132]. Subsequent to injury, an upsurge of NO occurs, possibly because of the hyperactivity of iNOS, and the inhibition of iNOS could be neuroprotective [133]. Clinically, NO levels can be assessed indirectly through CSF measurement of the end products of NO metabolism, and a peak concentration is found at 1–3 days following TBI [134,135,136].
nNOS, NG-nitro l-arginine methyl ester (l-NAME), and 7-nitro indazole (7-NI): Both l-NAME (nonselective NOS inhibitor) and 7-NI (nNOS inhibitor) were reported to exert neuroprotection only when administered within an hour of injury in mice, indicating a narrow therapeutic window post-TBI. L-arginine, the physiological precursor of NO, when co-administered with NOS inhibitors reverses their protective effects [137]. Pretreatment with l-NAME or 7-NI reduced NOS activity after FPI in rat, while 7-NI also improved neurobehavioral outcomes post-TBI [138]. However, another FPI model of TBI study in rats found no beneficial role of l-NAME administration post-TBI with regards to mortality, and pretreatment leads to prolonged hypertensive episodes and increased mortality [139].
eNOS and l-arginine: TBI-induced upregulation of eNOS plays a beneficial role by maintaining cerebral blood flow (CBF) post-head injury. Administration of l-arginine post-TBI in rats activated eNOS, improved CBF, and attenuated neurological deficits without altering cerebral perfusion pressure (CPP) [140]. Similarly, eNOS-deficient mice subjected to CCI have shown lower CBF at the cortical injury site compared to wild-type mice, and l-arginine did not improve the CBF nor the contusion volume in eNOS-deficient mice [141]. In humans, microdialysates from severe TBI patients showed elevated levels of NO metabolites in the first 24 h post-injury followed by a gradual decline over 5 days. There was also a significant direct relationship between the concentration of NO metabolites and regional CBF [142]. In severe TBI patients, l-arginine administration at 48 h post-injury had a better response in improving internal carotid artery flow volume than at 12 h following brain injury [143]. The above findings further emphasize the importance of determining the effective therapeutic window for drug administration following brain injury. In humans, the NOS3 (eNOS) gene, located at 7q35–36, has several allelic variations and patients having the −786C allele showed lower CBF values than other severe TBI patients. Thus, genetic makeup may be a potential contributing factor to the variability in TBI patient outcomes [144].
Statins: Statins are HMG-CoA reductase inhibitors with proven neuroprotective activity in experimental TBI studies through targeting of multiple secondary injuries [103]. More precisely, statins upregulate eNOS, have a palliative effect on cerebral autoregulation (CA), and improve stroke outcomes [145].
iNOS and iNOS inhibitors (Aminoguanidine (AG), L-NIL and 1400W): iNOS is an inducible type of NOS which is stimulated by injury-induced stimuli, such as inflammatory modulators, ROS, etc. [146,147,148,149]. In rats exposed to FPI, intraperitoneal injection of 100 mg/kg aminoguanidine (AG) twice daily for 3 days reduced the total cortical necrotic neuron counts but not the contusion volume [150]. Blast induced-TBI in rats showed that those receiving AG either prophylactically or after the injury performed better on neurobehavioral tests and had reduced cortical neuron degeneration compared with those receiving saline injection [151]. In an FPI model of brain trauma in rats at 6 h after injury, the following 3 iNOS inhibitors were given at 6 h post-injury: aminoguanidine (AG; 100 mg/kg intraperitoneally), l-N-iminoethyl-lysine (l-NIL; 20 mg/kg intraperitoneally), or N-[3-(aminomethyl)benzyl]acetamide (1400W; 20 mg/kg subcutaneously). All three improved neurofunctional outcomes, but AG also reduced brain edema [152].
Ronopterin (also termed 4-amino-tetrahydrobiopterin or VAS203): In a phase IIa RCT with moderate to severe TBI subjects (NO Synthase inhibition in traumatic brain injury, NOSTRA), patients were given various IV infusion doses (15–30 mg/kg) of ronopterin, a NOS inhibitor. Ronopterin treatment showed no marked alteration in ICP, CPP, or brain metabolism. Other than a transitory acute kidney injury in half of the patients receiving the highest dose, no other toxic side effects were reported. Additionally, ronopterin had a neuroprotective role shown by significant improvement of extended Glasgow Outcome Scores (eGOS) at 6 months [153]. These positive results lead NOSTRA trial into phase III which is current and whose study protocol has been published [154].
3.5. Peroxynitrite and Peroxynitrite Scavengers
Peroxynitrite is formed by the reaction of superoxide and NO radicals, which contributes to impaired cerebral vascular reactivity after TBI [105,106]. Peroxynitrite interacts with DNA, proteins, and lipids via oxidizing or radical-mediated mechanisms. In addition, it reacts with tyrosine residues of proteins to yield nitrotyrosine and impairs activity [155].
Penicillamine and penicillamine methyl ester: The thiol-containing compound penicillamine and the more brain-permeable penicillamine methyl ester are sulfhydryl-based scavengers of peroxynitrite. Both of these compounds have improved early neurological recovery in TBI mice. Although penicillamine remains mainly within the cerebral microvasculature, it showed greater neurological recovery than highly penetrable penicillamine methyl ester, highlighting the significance of early scavenging of intravascular peroxynitrite [156].
Tempol and α-phenyl-N-tert-butyl-nitrone (PBN): The peroxynitrite radical scavengers tempol [157,158,159] and PBN [160] have demonstrated neuroprotective activity in experimental TBI and could be good candidates to minimize nitrosative stress.
3.6. Lipid Peroxidation (LP) and LP Inhibitors
Free radical-mediated LP is an extensively studied mechanism of oxidative injury in TBI [161]. The brain cell membrane is abundant in polyunsaturated fatty acids e.g., arachidonic acid (AA), which is extremely susceptible to •OH-induced peroxidation. LP starts when a radical species, such as •OH, extracts hydrogen from an allyl group (AA + R• → AA• + RH), converting this allylic carbon into an “alkyl” radical (AA•). During the propagation stage, the resulting alkyl radical binds with a molecule of oxygen to form a lipid peroxyl radical (AA-OO•; AA• + O2 → AA-OO•). The peroxyl radical then reacts with a neighboring AA within the membrane and gains its electron to generate a lipid hydroperoxide (AA-OOH) and a resultant alkyl radical (AA•; AA-OO• + AA → AA-OOH + AA•). This cycle of generation of alkyl radicals is continuous and compromises cellular and sub-cellular membranous integrity. Finally, in the termination step of the LP, lipid radials react with another radical, giving rise to highly reactive, potentially neurotoxic aldehydes known as carbonyls. The neurotoxic aldehydes, 4-hydroxynonenal (4-HNE), and 2-propenal (acrolein) bind to basic amino acids (arginine, histidine, and lysine) and sulfhydryl-containing cysteine residues in cellular proteins, and alter their conformation and function.
Tirilazad (lazaroid, 21-aminosteroid, a LP inhibitor): Previously, tirilazad has been shown to enhance neurological recovery and survival in experimental TBI [162,163,164]. In a multicenter phase III study, moderate-severely injured patients, treated with tirilazard mesylate (10 mg/kg intravenous), starting within 4 h post-injury and repeated for every 6 h up to 5 days, did not show significant GOS on recovery/survival at 6 months. However, a post hoc analysis of data discovered that tirilazad lowered mortality rates in male TBI patients with accompanying traumatic subarachnoid hemorrhage (tSAH) [165].
U83836E: U83836E is a very effective second-generation lazaroid with a unique structure giving it the ability to scavenge lipid peroxyl and to inhibit LP. Further, U83836E also showed the ability to preserve mitochondrial respiratory function in rodents post-TBI [159].
LP carbonyl (4-HNE or acrolein) scavengers
Hydralazine: Despite being shown to have a good ability to scavenge LP carbonyls, hydralazine is a powerful vasodilator exacerbating hypotension in TBI and therefore, is not recommended post-TBI [166,167].
Phenelzine: Phenelzine, a monoamine oxidase inhibitor (MAO-I), is also a good scavenger of LP carbonyls because of the presence of its hydrazine functional group. Phenelzine inhibited oxidative damage and mitigated mitochondrial dysfunction in isolated rat brain mitochondria when subjected to exogenous acrolein or 4-HNE. Further, rats subjected to CCI and given a single dose of 10 mg/kg phenelzine subcutaneously 15 min post-TBI, protected cortical tissue from injury at 2 weeks [168]. Additionally, repeated doses of phenelzine (an initial dose of 10 mg/kg subcutaneous 15 min post-TBI followed by a repeated dose of 5 mg/kg subcutaneous every 12 h up to 60 h post-TBI) protected cortical tissue loss and attenuated mitochondrial impairment in CCI rats [169].
β-Phenylethylidenehydrazine (PEH): PEH is an active metabolite of phenelzine (β-phenylethylhydrazine). Both phenelzine and PEH possess a hydrazine functional group, and therefore, react with LP carbonyls and other LP aldehydes to form hydrazones. Because the compounds have different impacts on MAO inhibition, use of PEH may avoid the drug interactions seen with the use of phenelzine and certain sympathomimetics (tyramine) [170].
3.7. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-Antioxidant Response Element (ARE) Pathway
Kelch-like ECH-associated protein 1 (Keap1)-Nrf2-ARE signaling regulates endogenous antioxidant defense system and thus, plays an important role in protecting cells from intrinsic and extrinsic oxidants and electrophiles. Nrf2 heterodimerizes with other transcription factors and binds to ARE leading to expression of ARE-regulated genes that enhance cell survival. Kelch ECH associating protein 1 (Keap1) is a cytosolic repressor of Nrf2, which enhances its proteasomal degradation by binding with it [171]. Following TBI, Nrf2-knockout mice exhibited exacerbated brain injury with increased expression of inflammatory cytokines tumor necrotic factor-α (TNF-α), and interleukins (IL-1β and IL-6), and decreased activity of antioxidant enzymes NADPH: quinone oxidoreductase-1 (NQO-1) and glutathione S-transferase alpha-1 (GST-alpha1) [172].
Nrf2 activators: Sulforaphane (SFN), an Nrf2 activator, resulted in upregulation of the antioxidant enzymes heme oxygenase 1 (HO-1) and NQO-1 and lead to significant reduction in neurological dysfunction, injury volume, and neuronal death in rodent CCI models [173]. Tetra-butylhydroquinone (tBHQ), another Nrf2 activator, reduced nuclear factor kappa B (NF-κB) activation and inflammatory cytokines (TNF-α, IL-1β, IL-6) and attenuated cortical injury and brain edema in closed head-injured mice [174]. In another closed-head mouse model, tBHQ activated Nrf2 and attenuated NADPH oxidase (NOX2) in order to reduce cerebral edema and neurologic deficits [175]. Carnosic acid (CA) activates Nrf2 and in turn upregulates cytoprotective (ARE) genes and inhibits pro-inflammatory genes (through suppression of NF-κB). Early (15 min) and delayed (8 h) administration of CA after CCI in mice, resulted in restored mitochondrial respiration, and reduced neuronal cytoskeletal breakdown [167,176]. In mice subjected to repetitive mild TBI, CA administration improved cognitive and motor functions [177].
Melatonin and N-acetylserotonin (NAS): Melatonin (N-acetyl 5-methoxytryptamine) is a neurohormone with multiple physiological functions and has demonstrated to have anti-inflammatory, antioxidant, and antiapoptotic properties [178,179,180]. NAS, a precursor of melatonin, is a melatonin receptor 1C (MT3) agonist that is also shown to exhibit neuroprotection against TBI in preclinical studies. It exhibits antioxidant properties by directly scavenging oxidants and indirectly acting through antioxidant enzymes [181]. Melatonin might act through Nrf2-ARE signaling, as melatonin treatment in rodents upregulated antioxidant enzymes (HO-1 and NQO-1) downstream to Nrf2, while knockout of Nrf2 partially reversed its neuroprotective effects [182]. Moreover, a double-blinded randomized placebo-control clinical trial is investigating the sublingual melatonin in the treatment of post-concussion syndrome following mild pediatric TBI [183].
N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA): Administration of both NAC and its more BBB-permeable form, NACA, reduced cortical damage in rodents after experimental TBI [184]. NACA treatment, following TBI in rats, activated the Nrf2-ARE pathway, attenuated oxidative stress, and inhibited neuronal degeneration [185]. A systematic review including twenty animal studies and three human trials concluded that although there is sufficient preclinical evidence for neuroprotective effects of NAC/NACA after TBI, well-designed clinical studies are lacking [186].
3.8. Endothelial Targeted Antioxidant Enzyme Therapy
Following TBI, the damaged endothelium is a key site for oxidative stress, and the damaged endothelial cells upregulate the expression of cell adhesion molecules, such as Intercellular Adhesion Molecule 1 (ICAM-1). A novel approach is the use of targeted endothelial nanomedicine/antibodies. For example, anti-ICAM-1/CAT is an anti-ICAM-1 antibody conjugated to the antioxidant enzyme CAT. In an experimental model of TBI, anti-ICAM-1/CAT treatment reduced oxidative stress at the BBB and attenuated neuropathological outcomes [187].
4. Inflammation
Post-traumatic cerebral inflammation starts within minutes of injury and is characterized by upregulation and secretion of mediators (such as DAMPs, cytokines, and chemokines), infiltration of neutrophils and other myeloid cells, and subsequent glial activation and leukocyte recruitment (Figure 2) [188]. BBB impairment during the acute post-traumatic period allows for the entry of circulating neutrophils, monocytes, and lymphocytes to the injury site and directly influences neuronal survival and death [188,189,190]. The accumulated peripheral and resident immune cells in the brain parenchyma release inflammatory mediators, including but not limited to DAMPs, cytokine, chemokines, ROS, prostaglandins, and complement factors [20], which further potentiates inflammation in the injured brain by recruiting more immune cells to the injury site [191]. However, over time, subsequent production of anti-inflammatory mediators and endogenous protectants suppress both humoral and cellular immune activation (Figure 2). In addition to the infiltrating peripheral blood cells, the resident microglia are activated. These activated microglia help in clearing cell debris and promote tissue remodeling. However, activated microglia also release various neurotoxic substances, such as ROS, RNS, and excitatory neurotransmitters, such as glutamate, that may exacerbate neuronal death [192]. In addition, proliferation and migration of reactive astrocytes, and the development of a glial scar after brain trauma impair axonal regrowth. Overall, complex astrogliosis and the trafficking of immune cells to the injury site can promote tissue repair and neurogenesis via the release of neurotrophic factors, [29] or can exacerbate tissue damage through increased inflammatory mechanisms as well (Figure 2).
4.1. Inflammatory Mediators
Within minutes to hours after the injury, damaged cells release many intracellular components, such as heat shock proteins (HSP 60 and 70), nucleic acids, and high mobility group protein B1 (HMGB1) into circulation and the extracellular space [14,193,194]. These released intracellular components act as damage-associated molecular patterns (DAMPs) and activate pattern recognition receptors (PRR) for downstream cell signaling [194,195,196]. In response, astrocytes, microglia, and neurons at the injury site begin secreting cytokines and chemokines [191,197]. In addition to their contribution in immune processes and roles in homeostasis, cytokines also function as messengers of intracellular communication [198], while the chemotactic cytokines, or chemokines, regulate leukocyte activation and migration [199]. These inflammatory signals activate microglia and astrocytes, recruit peripheral immune cells, and increase migration to the site of injury. Once inside the brain, peripheral immune cells secrete large amounts of inflammatory mediators, which add to further tissue damage and remodeling [200,201,202,203]. Many studies have reported upregulated expression of IL-1β, TNF-α, IL-6, CCL2, CCL3, CXCL1, CXCL2, CXCL8/IL-8, CXCL10, CCR2, CCR5, CXCR4, and CX3CR1 within 6 h of TBI [204,205,206]. Similar to animal models of TBI, the levels of many cytokines and chemokines peak at 4 h post-injury in patients [207,208]. Collectively, the previous reports suggest that early upregulation of inflammatory mediators is a robust response to injury that also adds to the subsequent secondary injury and chronic neuropathological processes.
4.2. Cellular (Innate and Adaptive) Responses
The inflammatory mediators released post-TBI not only alter the residential CNS cells, but also recruit peripheral cells into the brain. These immune cells polarize toward pro-or anti-inflammatory phenotypes due to surrounding signals from the tissue microenvironment [194,205,209,210]. In cortical impact TBI models, neutrophils are among the first cell types to respond to injury. Upregulated adhesion molecules on vascular endothelium mediate neutrophil entry into the traumatized brain during the early h of the first day post-injury [195,211,212]. Despite the essential role of neutrophil recruitment during peripheral infections and damage, they release huge amounts of ROS and RNS in traumatized brain causing oxidative cellular damage. The presence of neutrophils in the injured brain becomes greatly reduced by 3–5 days post-TBI, and mononuclear leukocytes begin to predominate [195,211,213]. These infiltrated cells are mostly CD45hiCCR2+Ly6C+ monocytes, with a small number of dendritic cells (DCs), T lymphocytes, and natural killer (NK) cells. DCs and T cells infiltrate the brain in lower numbers in a similar fashion as monocytes, and perform specific functions depending on the subpopulations of cells present. DCs are categorized into two- T-cell stimulating conventional dendritic cells (cDCs), and interferon-α secreting plasmacytoid (pDCs) [213,214,215]. Further, T cells are categorized into four sub-types- T helper (TH), memory T, cytotoxic T (TC), and NK cells, each serve distinct functions. Besides infiltrating peripheral cells, residential microglia and astrocytes simultaneously get activated during early immune response. Activated microglia, along with infiltrating macrophages, phagocytose cellular debris, secrete inflammatory mediators, and add to the local inflammation [211,216,217,218].
Meningeal lymphatic vessels are specialized to facilitate drainage of immune cells and macromolecules into the deep cervical lymph nodes and act as sites for immune surveillance of the CNS [219,220]. Notably, following TBI, activated macrophages may drain into cervical lymph nodes and mediate long-term adaptive response through MHC Class II-dependent antigen presentation [213,221,222]. In agreement, T-lymphocytes get activated and recruited within deep cervical lymph nodes by antigen-presenting cells rather than at the site of CNS injury [223]. Interestingly, HLA-DR, an MHC Class II antigen expressed on macrophages, initiated adaptive immune responses by binding myelin basic protein (MBP) [224,225]. Thus, myelin-loaded macrophages may initiate white matter injury (WMI) post-TBI in a similar way to the autoimmune-mediated demyelination in multiple sclerosis [226]. The fact that pharmacological inhibition of MHC Class II reduced neurodegeneration after TBI [227], therefore, activated macrophages perhaps be a connected link between TBI and chronic adaptive immune responses.
T-lymphocytes do not routinely cross the BBB [228,229] but are functionally diverse subsets that mediate the specific adaptive responses against a presented antigen. In particular, TH cells stimulate antibody production and release cytokines that potentiate activation of macrophages and TC cells. Thus, infiltrating macrophages and TH cells cause neurodegeneration as evident in traumatized brain tissue from animals and TBI patients [227,230,231]. Naïve TH cells differentiate into three TH subtypes, such as TH1, TH2, and TH17 on the basis of clues obtained from secreted cytokines by activated macrophages [209,232,233,234].
Further, TH17 cells promoted microglial polarization after experimental autoimmune encephalomyelitis (EAE), while, in vitro, myelin-specific T-lymphocytes induced a pro-inflammatory phenotype in microglia via IL-17 [235,236]. Furthermore, inhibition of TH17 influx into the brain protected WM and prevented chronic neurological deficits post-neonatal hypoxia-ischemic injury [237]. In agreement, myelin-reactive TH17 cells were found to induce demyelination and to compromise remyelination in animal models of WMI and in multiple sclerosis patients [238,239,240,241]. Moreover, curcumin, an anti-inflammatory compound in the curry spice turmeric, improved TBI outcomes [242] and attenuated activation of TH17 in ovalbumin-sensitized mice and in acute graft versus host disease [243,244]. Curcumin further mitigated RORγT-mediated TH17 differentiation, decreased MBP-reactive T cells, and attenuated IL-17 secretion by activated TH17 cells in EAE [245]. Therefore, therapeutic strategies aiming at TH17 production/activity and/or MHC-II inhibition may provide potential possibilities to improve chronic functional outcomes following TBI.
By 10–14 days post-injury, most of the circulating immune cells are largely absent from the injury site. However, F4/80+ macrophages and glial fibrillary acidic protein (GFAP)+ astrocytes have been detected at distant sites far from the primary damage. In addition, injured thalamic neurons and WMI have been seen many months after the initial impact and thus indicate an effect of chronic diffuse injury [217,246,247]. More detailed literature on the neuroimmunology of TBI and various components of the post-TBI immune response can be obtained from the recent reviews by McKee and Lukens and Jassam et al. [248,249].
Contrary to previous dogma, cerebral inflammation is now considered to have both injurious and beneficial roles in TBI resolution. The traumatized brain can benefit from inflammation if regulated; otherwise, excessive and chronic inflammatory cascades can take over and contribute to numerous neuropathologies [250]. Therefore, many therapies targeting either specific immune cells or inflammation are gaining interests scientifically and clinically.
4.3. Therapies Targeting Inflammation in TBI
Glucocorticoids: Glucocorticoids have broad anti-inflammatory actions. A randomized placebo-controlled trial (Corticosteroid Randomisation After Significant Head injury, CRASH) investigated the effects of an IV corticosteroid (methylprednisolone) infusion within 8 h of TBI in adult patients with a GCS score of 14 or less. They reported higher mortality rates at 2 and 24 weeks in the glucocorticoid-treated patients [251,252].
TNF-α inhibitors: In neuroinflammation, TNF-α induces microglial and astrocytic activation, and influences BBB permeability, glutamatergic transmission, and synaptic plasticity [253]. Treatment with intraperitoneal etanercept, a TNF blocker (repeated every 12 h up to 3 days starting immediately following injury) lowered neuronal and glial apoptosis, attenuated microglial and astrocytic activation, reduced the cerebral damage, and improved cognition and motor ability [254]. Additionally, systemic entanercept adminstration was able to penetrate the contused brain tissue reducing the brain contents of TNF-α, and also stimulated newly formed neuronogenesis [255,256]. Clinically, perispinal administration of etanercept (PSE) has shown early promising results in several studies investigating its effects in neurological recovery and chronic pain post-brain injury [257,258,259,260]. An observational study investigating the role of PSE in chronic management of TBI and stroke patients reported significant improvements of chronic neurological dysfunctions. This beneficial effect was observed irrespective of the duration of ailment with improvements noted in patients treated more than a decade after stroke and TBI [261]. The wide therapeutic window of etanercept makes it a valuable therapeutic tool in the management of patients after TBI. 3,6′-dithiothalidomide, a TNF-α synthesis inhibitor, has also shown neuroprotective effects in experimental mild TBI (mTBI) studies when given up to 12 h after injury [262,263]. However, mice lacking TNF-α showed increased post-traumatic mortality without altering the sequele of TBI pathophysiology, and number of infiltrating cells, suggesting a protective role after TBI [264] and could be modulated wisely to extract better outcomes.
IL-1 inhibitors: IL-1 receptor antagonist (IL-1ra) (Anakinra, recombinant human IL-1ra, rhIL-1ra): Both IL-1α and IL-1β bind to IL-1 receptor type 1 (IL-1r1) and initiate signaling. IL-1ra is an endogenous antagonist of the IL-1r1 and blocks receptor activation by IL-1 [265]. IL-1ra overexpressing transgenic mice showed improved neurological functions with a delayed secretion of pro-inflammatory cytokines in a closed-head injury (CHI) model [266]. A review examined previous experimental studies using anakinra in TBI, elucidated that anakinra has a narrow early therapeutic window with less neuroprotective effects when given at 2 h compared to 5 min or 15 min post-injury [267]. In a randomized controlled phase II trial, 100 mg anakinra was administered subcutaneously once daily for 5 days to severe TBI patients and was observed to be safe, penetrated into plasma and brain extracellular fluid, and modified the neuroinflammatory response. However, only twenty patients were recruited, and so the therapeutic effect of anakinra could not be concluded in the study. Furthermore, considering the evidence from experimental studies regarding the narrow acute therapeutic window for administration of anakinra, it would have been advisable to administer the medication at an earlier time point which could be a limitation of the study due to ethical committee requirements [268].
IL-1β neutralizing antibody (anti-IL-1β antibody, IgG2a/k): Intraventricular infusion of anti-IL-1β antibody (starting 5 min post-injury up to 14 days) reduced cortical microglial activation, minimized neutrophil and T cell cortical infiltration, diminished lesion volume, and improved cognitive function [269]. Further, intraperitoneal anti-IL-1β antibody at 30 min and 7 days post-CCI brain injury in mice reduced the ipsilateral hemispheric edema [270]. It has been reported that TBI leads to activation of NOX2 and subsequent NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. The activation of NLRP3 inflammasome may lead to recruitment of IL-1 and caspase-1 following TBI [271,272]. Clinically, expressions of IL-18, IL-1β, caspase-1, and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) were found to be consistent with poor outcomes after TBI [273], and targeting inflammasome through inhibiting ASC could show a promising result in curbing inflammation in mice [274].
IL-1α inhibitors: IL-1α is another important early mediator of inflammation following acute TBI and its inhibition may be neuroprotective [275]. An experimental research study has investigated the selective and combined inhibition of both IL-1 subtypes in TBI. They investigated the effect of inhibiting IL-1α (IL-1α-deficient mice), IL-1β (IL-1β-deficient mice), and IL-1r1 [IL-1r1-deficient mice and anakinra (IL-1ra)] in cortical FPI. IL-1r1 blockade caused a greater reduction in diffuse cytokine expression compared to individual ablation of IL-1α or IL-1β. Both genetic (IL-1r1-deficient mice) and pharmacological (anakinra) blockade of IL-1r1 protected mice from cognitive dysfunction, which was not seen with selective ablation of both IL-1 subtypes [276]. Therefore, broad targeting of IL-1r1 may be a more effective neuroprotective approach.
IL-6 inhibitors: In experimental mild TBI models, IL-6 and keratinocyte-derived chemokine (KC) were elevated 90 min post-injury, and IL-6 levels correlated with injury levels. This makes serum IL-6 a possible biomarker of TBI severity [277]. In a severe injury, serum IL-6 levels were used as a valid prognostic marker of ICP elevation only in TBI patients but could not be used in patients having both polytrauma and TBI [278]. In one study, mice were subjected to mild TBI, treated with rat monoclonal anti-IL-6 antibodies 10 min after injury, and then immediately exposed to hypoxia for 30 min. Anti-IL-6 treatment reduced brain inflammation and neuronal injury. Additionally, anti-IL-6 antibody administration abrogated the motor incoordination prompted by mTBI and hypoxia [279].
Clinically, there are many medications available for IL-6 inhibition, some of them are monoclonal anti-IL-6 antibodies (siltuximab) or anti-IL6-receptor antibodies (tocilizumab, sarilumab). Thus, IL-6 inhibition has a protective effect following TBI and may be the focus of future extensive TBI studies. Given that IL-1 inhibition improved cognitive but not motor functions and that IL-6 inhibition improved motor coordination, collective inhibition of both IL-1 and IL-6 could be a promising therapeutic avenue for further research.
HMGB1 inhibitors
TLR4 inhibitor: HMGB1 is a DAMP and binds to PRRs such as TLR2, TLR4, or receptors for advanced glycation end products (RAGE). As neuronal HMGB1 leads TLR4-mediated secretion of IL-6 from activated microglial/macrophages, either pharmacological inhibition of TLR4 by VGX-1027 or genetic mutation limited post-traumatic edema and inflammation in mice post-TBI [14].
Glycyrrhizin (Gly): Gly is a natural triterpenoid, which possesses antiviral and anti-inflammatory activities and suppresses HMGB1 activities via binding with its HMG box [280]. Previously, glycyrrhizic acid (600 mg/kg, i.p.) was reported to reduce ipsilateral brain edema 24 h post-TBI when administered 15 min prior to injury [14]. Similarly, IV administration of Gly 30 min post-injury downregulates HMGB1-(TLR4/RAGE)-NF-κB inflammatory pathway, resulting in attenuation of brain edema and improvement of motor function in TBI rats [281].
HMGB1 A-box: HMGB1 is comprised of an acidic C-terminus and the DNA-binding domains A-box (contains binding sites) and B-box (pro-inflammatory domain). Therefore, recombinant HMGB1 A-box fragment may compete with HMGB1 for binding to corresponding receptor, and thus, may be exert neuroprotection. In a CCI model of TBI in mice, IV administration of HMGB1 A-box daily for 3 days lowered pro-inflammatory cytokine levels, attenuated BBB breakdown, reduced cerebral edema, and enhanced neurobehavioral outcomes [282].
Other anti-inflammatory interventions
Natural anti-inflammatory compounds [curcumin and epigallocatechin-3-gallate (EGCG)]: Curcumin, the active compound in the spice turmeric, decreased glial activation, reduced cerebral edema, and improved neurological functions after CCI injury in mice, possibly by suppressing IL-1β, inhibiting NFκB, and downregulating AQP4 [242]. Natural compounds such as curcumin, which have broad anti-inflammatory effects, may prove to be effective treatments for brain injury since they likely target multiple inflammatory pathways to prevent cell death. For example, EGCG, an antioxidant in green tea, reduced cerebral edema and microglial activation following TBI by lowering expression of AQP4 and GFAP. EGCG further decreased oxidative stress by inhibiting NADPH oxidase activation and increasing SOD activity [283].
Cannabinoids: A fast-growing field of therapeutic intervention in different brain disorders is to modulate the endocannabinoid (eCB) system to harness favorable outcomes [205,284]. Cannabis-based research has come a long way from its introduction in 1838 by William O’Shaughnessy [285] for the treatment of migraines [286,287] and neuropathic pain [285,288,289]. The current cannabis preparations available clinically are Cesamet (nabilone), Marinol (dronabinol: Δ9-tetrahydrocannabinol [Δ9-THC]), and Sativex also branded as Nabiximols (Δ9-THC with cannabidiol) [290]. Sativex has been approved in thirty countries for multiple sclerosis-associated spasticity and central neuropathic pain [291], and for opioid-resistant cancer pain as well [292]. The eCB system is an endogenous system that gets activated by natural cannabinoids or cannabinoid-mimicking substances. The eCB system consists of two main cannabinoid receptors (CB1 and CB2), their endogenous ligands 2-arachidonyl glycerol [(2-AG) and N-arachidonoylethanolamine (anandamide)], and ligand-synthesizing and metabolizing enzymes regulating the secreted ligands [293,294]. The eCB system is essential for cellular homeostasis and physiology, and may have an important contribution in repair processes either after injury or during disease [294,295,296,297,298]. However, various inhibitors to AEA-metabolizing enzyme fatty acid amide hydrolase (FAAH) did not advance to phase III clinical trials as neurological therapeutics [299]. Additionally, an IV cannabinoid analog, dexanabinol (HU-211), has failed to show protective effect after head trauma in phase III clinical trials [300], but the scientific quest continues in the hope for other therapeutic preparations for the treatment of stroke and other brain pathologies [301,302,303]. Treatment with exogenous 2-AG attenuated inflammation, cerebrovascular injury, and subsequent neurological deficits after TBI [304]. Recently, we reported that selective activation of CB2R helped to reduce inflammatory macrophages and thus, protected CBF and behavioral function [205]. Synthetic CB2R agonists such as HU-910 and HU-914 showed enhanced recovery in rodents after closed-head injury [304]. Thus, eCB possesses potential targets for the modulation in diverse TBI pathologies. Exogenous compounds, such as the plant-derived phytocannabinoids or synthetic cannabinoids are being highly incorporated in basic research in TBI therapy. However, full characterization of the eCB system in the settings of TBI and other brain injuries is not fully revealed, but efforts are being made to understand its important part in brain homeostasis.
Remote Ischemic Conditioning (RIC): Most studies involving TBI therapies focus on various drugs that can target secondary injury mechanisms; however, a novel treatment known as RIC involves a non-pharmacological approach [305]. This treatment involves applying a blood pressure-type cuff on the arm, which tightens and loosens, subjecting the limb to short cycles of alternate ischemia and subsequent reperfusion to protect distant organs such as the brain, from any injury [306]. Previously, RIC has been stated to improve CBF [307], possibly through the secretion of humoral factors, such as endothelial NOS, NO, and/or nitrites [308], and anti-inflammatory factors that activate protective pathways against ischemia. These mechanisms may also activate mitochondrial ATP-sensitive potassium channels [309] that can restore the mitochondrial membrane potential and can suppress apoptosis following ischemia-reperfusion [310]. Recently, we reported that RIC improved hematoma resolution in a murine model of intracerebral hemorrhage (ICH) via modulation of AMP-activated protein kinase (AMPK) in macrophages [210]. RIC is gaining popularity, and more and more research has started focusing on this method in an effort to enhance endogenous protection [311,312,313,314,315]. A recent clinical trial on RIC following severe TBI found that RIC significantly decreased levels of blood biomarkers after TBI [313]. However, this clinical trial was relatively small and only looked at biomarkers in patient blood at 0, 6, and 24 h after RIC. Further clinical trials on RIC as a treatment for TBI are desired to evaluate its effectiveness, particularly in regard to patient outcomes, cognition, and standards of living. RIC appears to be a promising and non-invasive treatment for numerous conditions and can be easily combined with other treatments. For example, RIC combined with minocycline was proved to be a safe and low-cost intervention in a mouse thromboembolic stroke model [307]. Moreover, exposing rodents to intermittent and sub-acute RIC upregulated endogenous protection mechanisms limiting secondary injury following TBI [311,312]. Non-invasive approaches, such as RIC, will be better tools to provide neuroprotection after TBI but will still require further testing in clinical settings.
5. Programmed Cell Death (PCD)
PCD is another major cause of neuronal cell loss that can continue for days following TBI and is associated with poor prognosis [316]. Important PCD processes include cell cycle activation-dependent cell death, cell death mediated by caspases and pro-apoptotic members of Bcl-2 family, PARP/AIF-dependent death, and calpain/cathepsis-dependent death [317,318]. Understanding these many mechanisms of PCD is essential for developing a therapeutic intervention since multiple mechanisms of cell death are often simultaneously and excessively activated in response to injury. Blocking any one individual cell death mechanism may not be beneficial as other mechanisms can still compensate and lead to cell loss.
5.1. Cell Cycle Activation-Dependent Neuronal Cell Death
In several neurodegenerative disorders, markers of cell cycle reentry can be detected long before actual neuronal death, suggesting that mature neurons may re-enter the cell cycle and that these cell cycle events (CCE) may be upstream of neuronal cell death pathways [319]. TBI activates neuronal apoptosis, upregulating cell cycle markers (e.g., Cyclin D1, CDK4, E2F5, c-myc, and PCNA), while downregulating various endogenous cell cycle inhibitors [320,321,322]. Pharmacological inhibitors of cyclin-dependent kinases (CDKs), which regulate the cell cycle, have been stated to attenuate neuronal cell death and significantly improve outcomes after TBI in rodent models [321,323].
5.2. Caspase-Dependent Cell Death
Morphological cell changes, such as nuclear fragmentation, chromatic condensation, and membrane budding following TBI, occur via caspase-dependent cleavage of specific apoptotic substrates. Caspase-3 activation can follow either the extrinsic pathway, involving TNF and FAS receptors, or the intrinsic pathway, involving mitochondrial outer membrane permeabilization (MOMP). MOMP induces the release of cytochrome c (cyt c), an inner mitochondrial membranous protein, into the cytosol. There, cyt c binds with apoptosis-inducing factor (Apaf-1) to form an ATP-dependent complex, which in turn activates caspase-9 and caspase-3 [324].
Caspase-3 is an important effector caspase, which plays an important role in injury-induced neuronal loss after TBI [324]. Many studies have reported the link between activation of caspases and neuronal apoptosis in both clinical and pre-clinical TBI [325,326,327,328]. Treatment with various caspase inhibitors improves outcomes after experimental TBI, is effective therapeutically, and has a broad safety window [327,329]. Caspase-12 can be activated by endoplasmic reticulum (ER) stress, triggering apoptosis and resulting in neuronal cell death [328,330]. Bcl-2 and Bcl-xL are anti-apoptotic proteins that either inhibit MOMP directly or inhibit pro-apoptotic proteins to regulate MOMP and caspases. Pro-apoptotic proteins, consist of three subtypes; the first subtype consists of BAX and BAK, which can directly permeabilize mitochondrial membrane; the second subtype includes BID and BIM, which activate the first subtype; and the third type includes BAD, PUMA, and NOXA which can inactivate Bcl-2 and Bcl-xL [318]. The balance between the activities of pro- and anti-apoptotic members of Bcl-2 family is a major determinant of apoptosis after TBI. Increased Bcl-2 and Bcl-xL expression leads to survival of cells [331] while upregulation of BAX, BAD, or BIM promotes cell demise in the post-TBI brain [316].
5.3. Caspase-Independent Cell Death Pathways
Following MOMP, mitochondrial inner membrane proteins, such as cyt c, Smac/DIABLO, AIF, and endonuclease G (endoG), may be released into the cytosol and modulate cell death. Calpain I, a Ca2+-dependent cysteine protease, is activated following TBI [332,333,334]. Activated calpain I cleaves the death-promoting Bcl-2 family members BID [335,336] and BAX [337], which then translocate to mitochondrial membranes. This results in the release of truncated apoptosis-inducing factor (tAIF) [335], cyt c [338], and endoG [336] in the case of BID or cyt c in the case of BAX [337,338]. These released proteins cause damage to nucleic acids and potentiate the release of inner membrane proteins [339,340,341]. While endoG cleaves internucleosomal (180 base pair) DNA [342], tAIF causes DNA cleavage on a large scale via interaction with phosphorylated histone H2AX (γH2AX) and cyclophilin A after translocation into nuclei [343,344]. Further, activated calpain I cleaves the Na+-Ca2+ exchanger, which leads to accumulation of intracellular Ca2+ [345]. Cyt c has also been shown to translocate to the nucleus and is linked with cytosolic translocation of acetylated histone H2A in irradiated HeLa cells [346,347,348].
Most studies have confirmed that AIF mediates cell death, independent of caspase, Apaf-1, or cyt c [349,350]. PAR polymerase-1 (PARP-1), Cyclophilin A, and HSP-70 are key regulators of AIF and are responsible for translocation of AIF from mitochondria into the nucleus, which is partly mediated by activation of PARP-1 [351,352]. PARP-1 activation causes depletion of cytosolic NAD+ and subsequent mitochondrial dysfunction, which mediates the release of AIF from mitochondria [353,354]. The end-product of PARP-1 activation, poly (ADP-ribose) (PAR) polymer, also cause direct or calpain-mediated mitochondrial impairment and MOMP [355]. PARP-1 is activated in response to DNA damage and forms PAR polymers to repair DNA nicks. However, when DNA loss is extensive, PAR starts building up in the nucleus, and eventually translocates to mitochondria, and causes release of AIF [356,357,358]. Further, nuclear PAR glycohydrolase (PARG) hydrolyzes excessive PAR into ADP-ribose. These ADP-ribose translocate into the plasma membrane to stimulate melastatin-like transient receptor potential 2 (TRPM-2) channels to cause excessive Ca2+ influx into neurons [359]. In the main region of injury following cerebral ischemia, where bioenergetics conditions are compromised, cellular death takes place via AIF- and PARP-1-mediated processes rather than caspase-mediated cell death [349]. In fact, in PARP-1-dependent cell death, mitochondria release AIF and cyt c; however, caspases do not become activated because of depleted ATP [353,355].
5.4. Therapies Targeting Cell Death Pathways
HSP70: HSP70 is an interesting molecule that is extremely important in neuronal cell survival [360,361]. This mechanism of neuroprotection includes binding of HSP70 to Apaf-1 and AIF, thereby blocking the creation of apoptosome complexes, and subsequent activation of caspase-3 [362,363] and attenuating nuclear translocation of AIF [361,364,365]. In addition, deletion of HSP70 or HSP110 caused increased cell death with upregulated expression of ROS-induced P53-target genes, such as pig1, pig8, and pig12, while HSP70/110 boosting drug celastrol improved behavioral outcomes and protected brain cells from secondary injury following TBI [366]. It must be noted that stress-induced cellular death likely involves multiple pathways [318,367,368]. A key determinant in cell death dynamics in TBI is likely the cellular bioenergetics of the brain. When bioenergetic processes are preserved, caspase-dependent cell death mechanisms predominate, while under deficient bioenergetic conditions, when caspase is not activated, AIF may facilitate cell death. As AIF and caspases act through parallel pathways in apoptosis, targeting both pathways would have potentially additive therapeutic effects [369].
CDK inhibitors: Selective CDK inhibitors inhibit the cell cycle that leads to glial activation and neuronal apoptosis in TBI [370]. These CDK inhibitors are toxic when given chronically, as is done in cancer treatments; however, short-term treatments, such as could be the case for acute TBI, would pose less of an issue. In addition, roscovitine and CR-8 show strong neuroprotective effects when administered as a single dose at a clinically relevant delayed time point [370]. Another TBI study found that CDK inhibitors decreased neuronal death, lesion volume, astroglial scarring, microglial activation, and improved motor and cognition functions [321].
Minocycline: Minocycline is a second-generation tetracycline and has been reported to inhibit microglial activation and subsequent excitotoxicity in TBI [371,372,373,374]. Minocycline also inhibited caspase-dependent and independent mitochondrial cell death pathways by preventing release of cyt c in a chronic neurodegeneration model [375]. However, one study found only transient neuroprotection and no change in apoptosis [189]. Minocycline is already FDA approved as an antibiotic, has a long half-life, can readily cross the BBB, and is well tolerated in high doses [376]. In addition, clinical trials of minocycline treatment in acute spinal cord injury found improvements in neurological outcomes and no significant adverse effects [377]. Therefore, animal studies and clinical trials warrant further investigation of minocycline as a possible therapeutic intervention in TBI.
Progesterone: Progesterone treatment leads to reduced edema, neuroinflammation, neuronal excitotoxicity, and apoptosis after TBI in both animal studies and initial clinical trials [378]. Progesterone modulated AQP4 expression on astrocytes and decreased cerebral edema in rats after TBI [379]. Progesterone may also reduce LP and oxidative stress by upregulating antioxidant enzymes, such as SOD [380], and ROS scavengers, such as mitochondrial glutathione [381]. Progesterone also attenuated neuronal excitoxicity by inhibiting voltage-gated calcium channels [382]. Studies have also shown that progesterone may inhibit activity of cyt c and caspase-3, and upregulate anti-apoptotic Bcl-2 proteins [382]. Despite the widespread success in experimental TBI [378], stage III clinical trials found no significant improvements in progesterone-treated TBI patients [383]. Similar to progesterone studies, many seemingly promising TBI treatments also failed in later clinical trials [384]. For example, erythropoietin restored mitochondrial function in TBI, and thereby reduced oxidative stress and inflammation [385] but failed to show significant improvements in TBI patients in clinical trials [386]. These failures may be attributed to many different factors including mechanistic and physiological differences in animal systems, heterogeneity of the injuries in patients, and/or issues with dosage and durations of treatment [387]. For example, no drug optimization studies were done prior to the phase III progsterone trials even though pre-clinical trials found many parameters that were critical for treatment effectiveness [384]. Studies have stated that growth hormone replacement therapy, in patients with post-traumatic hypopituitarism, partially reversed cognitive impairment, and improved processing speed and memory after TBI [388,389]. Incidence of endocrine insufficiency/failure after TBI is quite high but its cognitive symptoms are often mistaken for signs of residual injury [390]. These hormone deficiencies may be easily treated with hormone replacement therapy, and clinical symptoms respond well to treatment. Greater awareness of hypopituitarism and adrenal and endocrine failure following TBI is desirable in order to better manage the chronic effects of TBI.
microRNAs (miRNAs): Widespread research on therapies utilizing miRNAs, small non-coding RNA molecules that regulate gene expression, is performed in many different disease research fields [391]. miRNAs have demonstrated great therapeutic potential; however, research on these therapies is still in its infancy and the part of miRNAs in secondary injury in TBI remains largely unexplored [392]. Recently, Sabirzhanov et al. found that upregulation of miR-711 in TBI coincided with downregulation of the pro-survival protein Akt and subsequent activation of apoptotic PUMA and BIM, and cytosolic translocation of cytochrome c and AIF [392]. Inhibitor of miR-711 decreased apoptosis, restored Akt, and attenuated long-term neurological deficits after TBI. Another miRNA, miR-21 repressed apoptosis and supported angiogenesis by increasing Bcl-2 expression, inhibiting BAX and caspase-3, and activating PTEN-Akt signaling [393,394]. Going forward, miRNA therapies may be a promising future direction for the development of novel interventions in TBI by enabling direct targeting and inhibition of cell loss and concurrent targeting of multiple effectors.
6. Conclusions
In conclusion, a comprehensive understanding of TBI pathophysiology will allow for the development of effective drugs or drug combinations that target multiple secondary injury mechanisms and can be administered during optimal therapeutic windows. This approach could augment the bench to bedside translation of neuroprotective treatments. A few noteworthy treatment strategies have made it to the clinical trial stage for the treatment of brain injury, including tranexamic acid, CDP-choline, methylphenidate, NA-1, CBD, and non-invasive RIC. However, there is still a lack of effective therapeutics for TBI, with treatment mainly consisting of emergency surgeries, maintaining ICP, and rehabilitation therapies. The heterogeneous nature and complex pathophysiology of TBI necessitates a combination of therapies to ameliorate secondary injury and post-traumatic deficits. The fact that neurons and other supporting cells, such as astrocytes, microglia, oligodendrocytes, and the brain vasculature can all undergo degeneration quickly after trauma in the injured brain, further complicates its management. Astrogliosis and neuroinflammation are key secondary injury events that contribute to neurological deficits and even to chronic neurodegeneration after TBI. Moreover, death of non-neuronal cells can compromise recovery and hinder neurotransmission. Future therapeutic strategies should be focused on secondary injury, aiming to minimize detrimental events such as neuroinflammation and create optimal conditions for regeneration and repair post-injury. Additionally, the classification of patients according to GCS score alone may not be an effective method for patient inclusion in clinical trials. Advanced analysis of brain imaging has emerged as a vital tool for identifying progression of disease and efficacy of treatment clinically. Therefore, utilization of novel imaging methods and biomarkers, in addition to GCS scores, may be more accurate criteria for patient recruitment in clinical trials of neuroprotective medications.
Acknowledgments
The authors thank Colby Zahn for illustration provided in this review.
Author Contributions
A.J., M.B., M.A., K.M.D., and K.V. drafted the manuscript. R.V.G., M.W., S.M., P.A., J.R.V., and F.L.V. provided intellectual input. All authors have read and agreed to the published version of the manuscript.
Funding
Authors’ research is supported by grants from the National Institutes of Neurological Diseases and Stroke (NS114560 to KV, NS065172, NS097825 to KMD and NS110378 to BB/KMD), National Institutes of Child Health and Development (HD094606 to KV), AURI (MCGFD08343 to KV), and American Heart Association (GRNT33700286 to KMD).
Conflicts of Interest
Authors declare no financial or competing conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Dewan M.C., Rattani A., Gupta S., Baticulon R.E., Hung Y.C., Punchak M., Agrawal A., Adeleye A.O., Shrime M.G., Rubiano A.M., et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018 doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson P.J., O’Connell M.T., Rothwell N.J., Hopkins S.J., Nortje J., Carpenter K.L., Timofeev I., Al-Rawi P.G., Menon D.K., Pickard J.D. Inflammation in human brain injury: Intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J. Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 3.Langlois J.A., Rutland-Brown W., Thomas K.E. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. [(accessed on 1 September 2020)]; Available online: http://www.ncdsv.org/images/CDC_TBIintheUSEDVisitsHospitalizationsAndDeaths_2006.pdf.
- 4.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (Lond. Engl.) 1974;2:81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 5.Wijdicks E.F., Bamlet W.R., Maramattom B.V., Manno E.M., McClelland R.L. Validation of a new coma scale: The FOUR score. Ann. Neurol. 2005;58:585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 6.Loane D.J., Faden A.I. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., Manley G.T., Workshop Scientific T., Advisory Panel M. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ankarcrona M., Dypbukt J.M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S.A., Nicotera P. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 9.Whalen M.J., Dalkara T., You Z., Qiu J., Bermpohl D., Mehta N., Suter B., Bhide P.G., Lo E.H., Ericsson M., et al. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008;28:490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbye L.H., Keles E., Tao L., Zhang J., Chung J., Larvie M., Koppula R., Lo E.H., Whalen M.J. Kollidon VA64, a membrane-resealing agent, reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2012;32:515–524. doi: 10.1038/jcbfm.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller B.F., Keles E., Tien L., Zhang J., Kaplan D., Lo E.H., Whalen M.J. The pharmacokinetics and pharmacodynamics of Kollidon VA64 dissociate its protective effects from membrane resealing after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2014;34:1347–1353. doi: 10.1038/jcbfm.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faden A.I., Demediuk P., Panter S.S., Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 13.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird M.D., Shields J.S., Sukumari-Ramesh S., Kimbler D.E., Fessler R.D., Shakir B., Youssef P., Yanasak N., Vender J.R., Dhandapani K.M. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikonomidou C., Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/S1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 16.Palmer A.M., Marion D.W., Botscheller M.L., Bowen D.M., DeKosky S.T. Increased transmitter amino acid concentration in human ventricular CSF after brain trauma. Neuroreport. 1994;6:153–156. doi: 10.1097/00001756-199412300-00039. [DOI] [PubMed] [Google Scholar]
- 17.Hong Z., Xinding Z., Tianlin Z., Liren C. Excitatory Amino Acids in Cerebrospinal Fluid of Patients with Acute Head Injuries. Clin. Chem. 2001;47:1458. doi: 10.1093/clinchem/47.8.1458. [DOI] [PubMed] [Google Scholar]
- 18.Baker A.J., Moulton R.J., MacMillan V.H., Shedden P.M. Excitatory amino acids in cerebrospinal fluid following traumatic brain injury in humans. J. Neurosurg. 1993;79:369–372. doi: 10.3171/jns.1993.79.3.0369. [DOI] [PubMed] [Google Scholar]
- 19.Chamoun R., Suki D., Gopinath S.P., Goodman J.C., Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010;113:564–570. doi: 10.3171/2009.12.JNS09689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 21.Greve M.W., Zink B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. N.Y. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 22.Tavalin S.J., Ellis E.F., Satin L.S. Mechanical perturbation of cultured cortical neurons reveals a stretch-induced delayed depolarization. J. Neurophysiol. 1995;74:2767–2773. doi: 10.1152/jn.1995.74.6.2767. [DOI] [PubMed] [Google Scholar]
- 23.Parsons M.P., Raymond L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82:279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Hardingham G.E., Fukunaga Y., Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 25.Hardingham G.E., Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanz-Clemente A., Nicoll R.A., Roche K.W. Diversity in NMDA receptor composition: Many regulators, many consequences. Neuroscientist. 2013;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tovar K.R., Westbrook G.L. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel T.P., Ventre S.C., Geddes-Klein D., Singh P.K., Meaney D.F. Single-neuron NMDA receptor phenotype influences neuronal rewiring and reintegration following traumatic injury. J. Neurosci. 2014;34:4200–4213. doi: 10.1523/JNEUROSCI.4172-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bush T.G., Puvanachandra N., Horner C.H., Polito A., Ostenfeld T., Svendsen C.N., Mucke L., Johnson M.H., Sofroniew M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/S0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 30.Phillips L.L., Lyeth B.G., Hamm R.J., Reeves T.M., Povlishock J.T. Glutamate antagonism during secondary deafferentation enhances cognition and axo-dendritic integrity after traumatic brain injury. Hippocampus. 1998;8:390–401. doi: 10.1002/(SICI)1098-1063(1998)8:4<390::AID-HIPO7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Faden A.I., O’Leary D.M., Fan L., Bao W., Mullins P.G., Movsesyan V.A. Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improvesoOutcome after brain trauma. Exp. Neurol. 2001;167:435–444. doi: 10.1006/exnr.2000.7577. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh T.K., Vink R., Soares H., Hayes R., Simon R. Effects of the N-methyl-D-aspartate receptor blocker MK-801 on neurologic function after experimental brain injury. J. Neurotrauma. 1989;6:247–259. doi: 10.1089/neu.1989.6.247. [DOI] [PubMed] [Google Scholar]
- 33.Muir K.W. Glutamate-based therapeutic approaches: Clinical trials with NMDA antagonists. Curr. Opin. Pharm. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Roesler R., Quevedo J., Schroder N. Is it time to conclude that NMDA antagonists have failed? Lancet Neurol. 2003;2:13. doi: 10.1016/S1474-4422(03)00260-6. [DOI] [PubMed] [Google Scholar]
- 35.Hoyte L., Barber P.A., Buchan A.M., Hill M.D. The rise and fall of NMDA antagonists for ischemic stroke. Curr. Mol. Med. 2004;4:131–136. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- 36.Rao V.L., Dogan A., Todd K.G., Bowen K.K., Dempsey R.J. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- 37.Xia P., Chen H.S., Zhang D., Lipton S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokhtari M., Nayeb-Aghaei H., Kouchek M., Miri M.M., Goharani R., Amoozandeh A., Akhavan Salamat S., Sistanizad M. Effect of Memantine on Serum Levels of Neuron-Specific Enolase and on the Glasgow Coma Scale in Patients With Moderate Traumatic Brain Injury. J. Clin. Pharm. 2018;58:42–47. doi: 10.1002/jcph.980. [DOI] [PubMed] [Google Scholar]
- 39.Wang C.Q., Ye Y., Chen F., Han W.C., Sun J.M., Lu X., Guo R., Cao K., Zheng M.J., Liao L.C. Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience. 2017;343:30–38. doi: 10.1016/j.neuroscience.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Hertle D.N., Dreier J.P., Woitzik J., Hartings J.A., Bullock R., Okonkwo D.O., Shutter L.A., Vidgeon S., Strong A.J., Kowoll C., et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain. 2012;135:2390–2398. doi: 10.1093/brain/aws152. [DOI] [PubMed] [Google Scholar]
- 41.McIntosh T.K., Faden A.I., Yamakami I., Vink R. Magnesium deficiency exacerbates and pretreatment improves outcome following traumatic brain injury in rats: 31P magnetic resonance spectroscopy and behavioral studies. J. Neurotrauma. 1988;5:17–31. doi: 10.1089/neu.1988.5.17. [DOI] [PubMed] [Google Scholar]
- 42.Arango M.F., Bainbridge D. Magnesium for acute traumatic brain injury. Cochrane Database Syst. Rev. 2008 doi: 10.1002/14651858.CD005400.pub3. [DOI] [PubMed] [Google Scholar]
- 43.Li W., Bai Y.A., Li Y.J., Liu K.G., Wang M.D., Xu G.Z., Shang H.L., Li Y.F. Magnesium sulfate for acute traumatic brain injury. J. Craniofac. Surg. 2015;26:393–398. doi: 10.1097/SCS.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 44.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: Selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharm. 1993;44:851–859. [PubMed] [Google Scholar]
- 45.Dempsey R.J., Başkaya M.K., Doğan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47:399–404; discussion 404–406. doi: 10.1097/00006123-200008000-00024. [DOI] [PubMed] [Google Scholar]
- 46.Maneshi M.M., Maki B., Gnanasambandam R., Belin S., Popescu G.K., Sachs F., Hua S.Z. Mechanical stress activates NMDA receptors in the absence of agonists. Sci. Rep. 2017;7:39610. doi: 10.1038/srep39610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bigford G.E., Alonso O.F., Dietrich D., Keane R.W. A novel protein complex in membrane rafts linking the NR2B glutamate receptor and autophagy is disrupted following traumatic brain injury. J. Neurotrauma. 2009;26:703–720. doi: 10.1089/neu.2008.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spaethling J., Le L., Meaney D.F. NMDA receptor mediated phosphorylation of GluR1 subunits contributes to the appearance of calcium-permeable AMPA receptors after mechanical stretch injury. Neurobiol. Dis. 2012;46:646–654. doi: 10.1016/j.nbd.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrario C.R., Ndukwe B.O., Ren J., Satin L.S., Goforth P.B. Stretch injury selectively enhances extrasynaptic, GluN2B-containing NMDA receptor function in cortical neurons. J. Neurophysiol. 2013;110:131–140. doi: 10.1152/jn.01011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merchant R.E., Bullock M.R., Carmack C.A., Shah A.K., Wilner K.D., Ko G., Williams S.A. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann. N. Y. Acad. Sci. 1999;890:42–50. doi: 10.1111/j.1749-6632.1999.tb07979.x. [DOI] [PubMed] [Google Scholar]
- 51.Bullock M.R., Merchant R.E., Carmack C.A., Doppenberg E., Shah A.K., Wilner K.D., Ko G., Williams S.A. An open-label study of CP-101,606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage. Ann. N. Y. Acad. Sci. 1999;890:51–58. doi: 10.1111/j.1749-6632.1999.tb07980.x. [DOI] [PubMed] [Google Scholar]
- 52.Yurkewicz L., Weaver J., Bullock M.R., Marshall L.F. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J. Neurotrauma. 2005;22:1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- 53.Shohami E., Biegon A. Novel approach to the role of NMDA receptors in traumatic brain injury. CNS Neurol. Disord. Drug Targets. 2014;13:567–573. doi: 10.2174/18715273113126660196. [DOI] [PubMed] [Google Scholar]
- 54.Funke L., Dakoji S., Bredt D.S. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev. Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 55.Christopherson K.S., Hillier B.J., Lim W.A., Bredt D.S. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 56.Arundine M., Aarts M., Lau A., Tymianski M. Vulnerability of Central Neurons to Secondary Insults after In Vitro Mechanical Stretch. J. Neurosci. 2004;24:8106–8123. doi: 10.1523/JNEUROSCI.1362-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu W., Liu N.K., Wu X., Wang Y., Xia Y., Sun Y., Lai Y., Li R., Shekhar A., Xu X.M. Disrupting nNOS-PSD95 Interaction Improves Neurological and Cognitive Recoveries after Traumatic Brain Injury. Cereb. Cortex. 2020;30:3859–3871. doi: 10.1093/cercor/bhaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aarts M., Liu Y., Liu L., Besshoh S., Arundine M., Gurd J.W., Wang Y.-T., Salter M.W., Tymianski M. Treatment of Ischemic Brain Damage by Perturbing NMDA Receptor—PSD-95 Protein Interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 59.Cook D.J., Teves L., Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 60.Hill M.D., Martin R.H., Mikulis D., Wong J.H., Silver F.L., Terbrugge K.G., Milot G., Clark W.M., Macdonald R.L., Kelly M.E., et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11:942–950. doi: 10.1016/S1474-4422(12)70225-9. [DOI] [PubMed] [Google Scholar]
- 61.Ballarin B., Tymianski M. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharm. Sin. 2018;39:661–668. doi: 10.1038/aps.2018.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q.J., Tymianski M. Targeting NMDA receptors in stroke: New hope in neuroprotection. Mol. Brain. 2018;11:15. doi: 10.1186/s13041-018-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sen T., Gupta R., Kaiser H., Sen N. Activation of PERK Elicits Memory Impairment through Inactivation of CREB and Downregulation of PSD95 After Traumatic Brain Injury. J. Neurosci. 2017;37:5900–5911. doi: 10.1523/JNEUROSCI.2343-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wakade C., Sukumari-Ramesh S., Laird M.D., Dhandapani K.M., Vender J.R. Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J. Neurosurg. 2010;113:1195–1201. doi: 10.3171/2010.3.JNS091212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bach A., Clausen B.H., Moller M., Vestergaard B., Chi C.N., Round A., Sorensen P.L., Nissen K.B., Kastrup J.S., Gajhede M., et al. A high-affinity, dimeric inhibitor of PSD-95 bivalently interacts with PDZ1-2 and protects against ischemic brain damage. Proc. Natl. Acad. Sci. USA. 2012;109:3317–3322. doi: 10.1073/pnas.1113761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommer J.B., Bach A., Mala H., Stromgaard K., Mogensen J., Pickering D.S. In vitro and in vivo effects of a novel dimeric inhibitor of PSD-95 on excitotoxicity and functional recovery after experimental traumatic brain injury. Eur. J. Neurosci. 2017;45:238–248. doi: 10.1111/ejn.13483. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Song J.H., Denisova J.V., Park W.M., Fontes J.D., Belousov A.B. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J. Neurosci. 2012;32:713–725. doi: 10.1523/JNEUROSCI.3872-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartfield E.M., Rinaldi F., Glover C.P., Wong L.F., Caldwell M.A., Uney J.B. Connexin 36 expression regulates neuronal differentiation from neural progenitor cells. Plos ONE. 2011;6:e14746. doi: 10.1371/journal.pone.0014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Todd K.L., Kristan W.B., Jr., French K.A. Gap junction expression is required for normal chemical synapse formation. J. Neurosci. 2010;30:15277–15285. doi: 10.1523/JNEUROSCI.2331-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amara S.G., Fontana A.C. Excitatory amino acid transporters: Keeping up with glutamate. Neurochem. Int. 2002;41:313–318. doi: 10.1016/S0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 71.Suchak S.K., Baloyianni N.V., Perkinton M.S., Williams R.J., Meldrum B.S., Rattray M. The ‘glial’ glutamate transporter, EAAT2 (Glt-1) accounts for high affinity glutamate uptake into adult rodent nerve endings. J. Neurochem. 2003;84:522–532. doi: 10.1046/j.1471-4159.2003.01553.x. [DOI] [PubMed] [Google Scholar]
- 72.Li S., Stys P.K. Na(+)-K(+)-ATPase inhibition and depolarization induce glutamate release via reverse Na(+)-dependent transport in spinal cord white matter. Neuroscience. 2001;107:675–683. doi: 10.1016/S0306-4522(01)00385-2. [DOI] [PubMed] [Google Scholar]
- 73.Beschorner R., Dietz K., Schauer N., Mittelbronn M., Schluesener H.J., Trautmann K., Meyermann R., Simon P. Expression of EAAT1 reflects a possible neuroprotective function of reactive astrocytes and activated microglia following human traumatic brain injury. Histol. Histopathol. 2007;22:515–526. doi: 10.14670/HH-22.515. [DOI] [PubMed] [Google Scholar]
- 74.van Landeghem F.K., Weiss T., Oehmichen M., von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J. Neurotrauma. 2006;23:1518–1528. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- 75.van Landeghem F.K., Stover J.F., Bechmann I., Bruck W., Unterberg A., Buhrer C., von Deimling A. Early expression of glutamate transporter proteins in ramified microglia after controlled cortical impact injury in the rat. Glia. 2001;35:167–179. doi: 10.1002/glia.1082. [DOI] [PubMed] [Google Scholar]
- 76.Rao V.L., Dogan A., Bowen K.K., Todd K.G., Dempsey R.J. Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur. J. Neurosci. 2001;13:119–128. [PubMed] [Google Scholar]
- 77.Fontana A.C., Fox D.P., Zoubroulis A., Mortensen O.V., Raghupathi R. Neuroprotective Effects of the Glutamate Transporter Activator (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153) following Traumatic Brain Injury in the Adult Rat. J. Neurotrauma. 2016;33:1073–1083. doi: 10.1089/neu.2015.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gottlieb M., Wang Y., Teichberg V.I. Blood-mediated scavenging of cerebrospinal fluid glutamate. J. Neurochem. 2003;87:119–126. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- 79.Helms H.C.C., Nielsen C.U., Waagepetersen H.S., Brodin B. Glutamate Transporters in the Blood-Brain Barrier. Adv. Neurobiol. 2017;16:297–314. doi: 10.1007/978-3-319-55769-4_15. [DOI] [PubMed] [Google Scholar]
- 80.Zlotnik A., Gruenbaum S.E., Artru A.A., Rozet I., Dubilet M., Tkachov S., Brotfain E., Klin Y., Shapira Y., Teichberg V.I. The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: Evidence from the use of maleate. J. Neurosurg. Anesth. 2009;21:235–241. doi: 10.1097/ANA.0b013e3181a2bf0b. [DOI] [PubMed] [Google Scholar]
- 81.Zlotnik A., Sinelnikov I., Gruenbaum B.F., Gruenbaum S.E., Dubilet M., Dubilet E., Leibowitz A., Ohayon S., Regev A., Boyko M., et al. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology. 2012;116:73–83. doi: 10.1097/ALN.0b013e31823d7731. [DOI] [PubMed] [Google Scholar]
- 82.Boyko M., Gruenbaum S.E., Gruenbaum B.F., Shapira Y., Zlotnik A. Brain to blood glutamate scavenging as a novel therapeutic modality: A review. J. Neural. Transm. (Vienna) 2014;121:971–979. doi: 10.1007/s00702-014-1181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campos F., Sobrino T., Ramos-Cabrer P., Argibay B., Agulla J., Pérez-Mato M., Rodríguez-González R., Brea D., Castillo J. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: An experimental study. J. Cereb. Blood Flow Metab. 2011;31:1378–1386. doi: 10.1038/jcbfm.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pérez-Mato M., Ramos-Cabrer P., Sobrino T., Blanco M., Ruban A., Mirelman D., Menendez P., Castillo J., Campos F. Human recombinant glutamate oxaloacetate transaminase 1 (GOT1) supplemented with oxaloacetate induces a protective effect after cerebral ischemia. Cell Death Dis. 2014;5:e992. doi: 10.1038/cddis.2013.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Da Silva-Candal A., Pérez-Díaz A., Santamaría M., Correa-Paz C., Rodríguez-Yáñez M., Ardá A., Pérez-Mato M., Iglesias-Rey R., Brea J., Azuaje J., et al. Clinical validation of blood/brain glutamate grabbing in acute ischemic stroke. Ann. Neurol. 2018;84:260–273. doi: 10.1002/ana.25286. [DOI] [PubMed] [Google Scholar]
- 86.Hoane M.R., Wolyniak J.G., Akstulewicz S.L. Administration of riboflavin improves behavioral outcome and reduces edema formation and glial fibrillary acidic protein expression after traumatic brain injury. J. Neurotrauma. 2005;22:1112–1122. doi: 10.1089/neu.2005.22.1112. [DOI] [PubMed] [Google Scholar]
- 87.Nilsson P., Hillered L., Ponten U., Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 88.Anderson K.J., Miller K.M., Fugaccia I., Scheff S.W. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 89.Sato M., Chang E., Igarashi T., Noble L.J. Neuronal injury and loss after traumatic brain injury: Time course and regional variability. Brain Res. 2001;917:45–54. doi: 10.1016/s0006-8993(01)02905-5. [DOI] [PubMed] [Google Scholar]
- 90.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Reviews. Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mongiat L.A., Schinder A.F. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur. J. Neurosci. 2011;33:1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- 92.Gao X., Deng-Bryant Y., Cho W., Carrico K.M., Hall E.D., Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao X., Chen J. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J. Neurotrauma. 2009;26:1325–1335. doi: 10.1089/neu.2008.0744. [DOI] [PubMed] [Google Scholar]
- 94.Zhou H., Chen L., Gao X., Luo B., Chen J. Moderate traumatic brain injury triggers rapid necrotic death of immature neurons in the hippocampus. J. Neuropathol. Exp. Neurol. 2012;71:348–359. doi: 10.1097/NEN.0b013e31824ea078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Y.L., Xiang Q., Shi Q.Y., Li S.Y., Tan L., Wang J.T., Jin X.G., Luo A.L. GABAergic excitotoxicity injury of the immature hippocampal pyramidal neurons’ exposure to isoflurane. Anesth. Analg. 2011;113:1152–1160. doi: 10.1213/ANE.0b013e318230b3fd. [DOI] [PubMed] [Google Scholar]
- 96.Afshari D., Moradian N., Rezaei M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin. Neurol. Neurosurg. 2013;115:400–404. doi: 10.1016/j.clineuro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Saver J.L., Starkman S., Eckstein M., Stratton S.J., Pratt F.D., Hamilton S., Conwit R., Liebeskind D.S., Sung G., Kramer I., et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 2015;372:528–536. doi: 10.1056/NEJMoa1408827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khatri N., Thakur M., Pareek V., Kumar S., Sharma S., Datusalia A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets. 2018;17:689–695. doi: 10.2174/1871527317666180627120501. [DOI] [PubMed] [Google Scholar]
- 99.Angeloni C., Prata C., Vieceli Dalla Sega F., Piperno R., Hrelia S. Traumatic Brain Injury and NADPH Oxidase: A Deep Relationship. Oxidative Med. Cell. Longev. 2015;2015:370312. doi: 10.1155/2015/370312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toth P., Szarka N., Farkas E., Ezer E., Czeiter E., Amrein K., Ungvari Z.I., Hartings J.A., Buki A., Koller A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanism and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016 doi: 10.1152/ajpheart.00267.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veenith T.V., Carter E.L., Geeraerts T., Grossac J., Newcombe V.F., Outtrim J., Gee G.S., Lupson V., Smith R., Aigbirhio F.I., et al. Pathophysiologic Mechanisms of Cerebral Ischemia and Diffusion Hypoxia in Traumatic Brain Injury. JAMA Neurol. 2016;73:542–550. doi: 10.1001/jamaneurol.2016.0091. [DOI] [PubMed] [Google Scholar]
- 102.Ansari M.A., Roberts K.N., Scheff S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cornelius C., Crupi R., Calabrese V., Graziano A., Milone P., Pennisi G., Radak Z., Calabrese E.J., Cuzzocrea S. Traumatic brain injury: Oxidative stress and neuroprotection. Antioxid. Redox Signal. 2013;19:836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 104.Readnower R.D., Chavko M., Adeeb S., Conroy M.D., Pauly J.R., McCarron R.M., Sullivan P.G. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010;88:3530–3539. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DeWitt D.S., Prough D.S. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J. Neurotrauma. 2009;26:877–887. doi: 10.1089/neu.2007.0439. [DOI] [PubMed] [Google Scholar]
- 106.Vuceljic M., Zunic G., Romic P., Jevtic M. Relation between both oxidative and metabolic-osmotic cell damages and initial injury severity in bombing casualties. Vojnosanit. Pregl. 2006;63:545–551. doi: 10.2298/VSP0606545V. [DOI] [PubMed] [Google Scholar]
- 107.Tran L.V. Understanding the pathophysiology of traumatic brain injury and the mechanisms of action of neuroprotective interventions. J. Trauma Nurs. 2014;21:30–35. doi: 10.1097/JTN.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 108.Povlishock J.T., Kontos H.A. Continuing axonal and vascular change following experimental brain trauma. Cent. Nerv. Syst. Trauma. 1985;2:285–298. doi: 10.1089/cns.1985.2.285. [DOI] [PubMed] [Google Scholar]
- 109.Kontos H.A., Wei E.P. Superoxide production in experimental brain injury. J. Neurosurg. 1986;64:803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- 110.Halliwell B., Gutteridge J. Free Radicals in Biology and Medicine. Oxford University Press; Oxfard, UK: 2007. [Google Scholar]
- 111.Marklund S.L., Westman N.G., Lundgren E., Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982;42:1955–1961. [PubMed] [Google Scholar]
- 112.Smith S.L., Andrus P.K., Zhang J.R., Hall E.D. Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J. Neurotrauma. 1994;11:393–404. doi: 10.1089/neu.1994.11.393. [DOI] [PubMed] [Google Scholar]
- 113.Kontos H.A., Povlishock J.T. Oxygen radicals in brain injury. Cent. Nerv. Syst. Trauma. 1986;3:257–263. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- 114.Chan P.H., Epstein C.J., Li Y., Huang T.T., Carlson E., Kinouchi H., Yang G., Kamii H., Mikawa S., Kondo T., et al. Transgenic mice and knockout mutants in the study of oxidative stress in brain injury. J. Neurotrauma. 1995;12:815–824. doi: 10.1089/neu.1995.12.815. [DOI] [PubMed] [Google Scholar]
- 115.Mikawa S., Kinouchi H., Kamii H., Gobbel G.T., Chen S.F., Carlson E., Epstein C.J., Chan P.H. Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J. Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- 116.Lewen A., Fujimura M., Sugawara T., Matz P., Copin J.C., Chan P.H. Oxidative stress-dependent release of mitochondrial cytochrome c after traumatic brain injury. J. Cereb. Blood Flow Metab. 2001;21:914–920. doi: 10.1097/00004647-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 117.Lewen A., Sugawara T., Gasche Y., Fujimura M., Chan P.H. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol. Dis. 2001;8:380–390. doi: 10.1006/nbdi.2001.0396. [DOI] [PubMed] [Google Scholar]
- 118.Xiong Y., Shie F.S., Zhang J., Lee C.P., Ho Y.S. Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J. Neurochem. 2005;95:732–744. doi: 10.1111/j.1471-4159.2005.03412.x. [DOI] [PubMed] [Google Scholar]
- 119.Pineda J.A., Aono M., Sheng H., Lynch J., Wellons J.C., Laskowitz D.T., Pearlstein R.D., Bowler R., Crapo J., Warner D.S. Extracellular superoxide dismutase overexpression improves behavioral outcome from closed head injury in the mouse. J. Neurotrauma. 2001;18:625–634. doi: 10.1089/089771501750291864. [DOI] [PubMed] [Google Scholar]
- 120.Muizelaar J.P., Marmarou A., Young H.F., Choi S.C., Wolf A., Schneider R.L., Kontos H.A. Improving the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated superoxide dismutase: A phase II trial. J. Neurosurg. 1993;78:375–382. doi: 10.3171/jns.1993.78.3.0375. [DOI] [PubMed] [Google Scholar]
- 121.Muizelaar J.P., Kupiec J.W., Rapp L.A. PEG-SOD after head injury. J. Neurosurg. 1995;83:942. doi: 10.3171/jns.1995.83.5.0942. [DOI] [PubMed] [Google Scholar]
- 122.Aoyama N., Katayama Y., Kawamata T., Maeda T., Mori T., Yamamoto T., Kikuchi T., Uwahodo Y. Effects of antioxidant, OPC-14117, on secondary cellular damage and behavioral deficits following cortical contusion in the rat. Brain Res. 2002;934:117–124. doi: 10.1016/S0006-8993(02)02366-1. [DOI] [PubMed] [Google Scholar]
- 123.The Dana Consortium Safety and tolerability of the antioxidant OPC-14117 in HIV-associated cognitive impairment. The Dana Consortium on the Therapy of HIV Dementia and Related Cognitive Disorders. Neurology. 1997;49:142–146. doi: 10.1212/WNL.49.1.142. [DOI] [PubMed] [Google Scholar]
- 124.Zaleska M.M., Floyd R.A. Regional lipid peroxidation in rat brain in vitro: Possible role of endogenous iron. Neurochem. Res. 1985;10:397–410. doi: 10.1007/BF00964608. [DOI] [PubMed] [Google Scholar]
- 125.Sadrzadeh S.M., Graf E., Panter S.S., Hallaway P.E., Eaton J.W. Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 1984;259:14354–14356. [PubMed] [Google Scholar]
- 126.Sadrzadeh S.M., Eaton J.W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J. Clin. Investig. 1988;82:1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Long D.A., Ghosh K., Moore A.N., Dixon C.E., Dash P.K. Deferoxamine improves spatial memory performance following experimental brain injury in rats. Brain Res. 1996;717:109–117. doi: 10.1016/0006-8993(95)01500-0. [DOI] [PubMed] [Google Scholar]
- 128.Zhang L., Hu R., Li M., Li F., Meng H., Zhu G., Lin J., Feng H. Deferoxamine attenuates iron-induced long-term neurotoxicity in rats with traumatic brain injury. Neurol. Sci. 2013;34:639–645. doi: 10.1007/s10072-012-1090-1. [DOI] [PubMed] [Google Scholar]
- 129.Panter S.S., Braughler J.M., Hall E.D. Dextran-coupled deferoxamine improves outcome in a murine model of head injury. J. Neurotrauma. 1992;9:47–53. doi: 10.1089/neu.1992.9.47. [DOI] [PubMed] [Google Scholar]
- 130.Daglas M., Adlard P.A. The Involvement of Iron in Traumatic Brain Injury and Neurodegenerative Disease. Front. Neurosci. 2018;12:981. doi: 10.3389/fnins.2018.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khalaf S., Ahmad A.S., Chamara K., Dore S. Unique Properties Associated with the Brain Penetrant Iron Chelator HBED Reveal Remarkable Beneficial Effects after Brain Trauma. J. Neurotrauma. 2018 doi: 10.1089/neu.2017.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gahm C., Holmin S., Mathiesen T. Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery. 2000;46:169–177. doi: 10.1093/neurosurgery/46.1.169. [DOI] [PubMed] [Google Scholar]
- 133.Cherian L., Hlatky R., Robertson C.S. Nitric oxide in traumatic brain injury. Brain Pathol. (Zur. Switz.) 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toczylowska B., Chalimoniuk M., Wodowska M., Mayzner-Zawadzk E. Changes in concentration of cerebrospinal fluid components in patients with traumatic brain injury. Brain Res. 2006;1104:183–189. doi: 10.1016/j.brainres.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 135.Clark R.S., Kochanek P.M., Obrist W.D., Wong H.R., Billiar T.R., Wisniewski S.R., Marion D.W. Cerebrospinal fluid and plasma nitrite and nitrate concentrations after head injury in humans. Crit. Care Med. 1996;24:1243–1251. doi: 10.1097/00003246-199607000-00030. [DOI] [PubMed] [Google Scholar]
- 136.Uzan M., Tanriover N., Bozkus H., Gumustas K., Guzel O., Kuday C. Nitric oxide (NO) metabolism in the cerebrospinal fluid of patients with severe head injury. Inflammation as a possible cause of elevated no metabolites. Surg. Neurol. 2001;56:350–356. doi: 10.1016/S0090-3019(01)00633-4. [DOI] [PubMed] [Google Scholar]
- 137.Mesenge C., Verrecchia C., Allix M., Boulu R.R., Plotkine M. Reduction of the neurological deficit in mice with traumatic brain injury by nitric oxide synthase inhibitors. J. Neurotrauma. 1996;13:11–16. doi: 10.1089/neu.1996.13.11. [DOI] [PubMed] [Google Scholar]
- 138.Wada K., Chatzipanteli K., Busto R., Dietrich W.D. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain injury in the rat. J. Neurotrauma. 1999;16:203–212. doi: 10.1089/neu.1999.16.203. [DOI] [PubMed] [Google Scholar]
- 139.Lu Y.C., Liu S., Gong Q.Z., Hamm R.J., Lyeth B.G. Inhibition of nitric oxide synthase potentiates hypertension and increases mortality in traumatically brain-injured rats. Mol. Chem. Neuropathol. 1997;30:125–137. doi: 10.1007/BF02815154. [DOI] [PubMed] [Google Scholar]
- 140.Cherian L., Chacko G., Goodman J.C., Robertson C.S. Cerebral hemodynamic effects of phenylephrine and L-arginine after cortical impact injury. Crit. Care Med. 1999;27:2512–2517. doi: 10.1097/00003246-199911000-00031. [DOI] [PubMed] [Google Scholar]
- 141.Hlatky R., Lui H., Cherian L., Goodman J.C., O’Brien W.E., Contant C.F., Robertson C.S. The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J. Neurotrauma. 2003;20:995–1006. doi: 10.1089/089771503770195849. [DOI] [PubMed] [Google Scholar]
- 142.Hlatky R., Goodman J.C., Valadka A.B., Robertson C.S. Role of nitric oxide in cerebral blood flow abnormalities after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23:582–588. doi: 10.1097/01.WCB.0000059586.71206.F3. [DOI] [PubMed] [Google Scholar]
- 143.Rangel-Castilla L., Ahmed O., Goodman J.C., Gopinath S., Valadka A., Robertson C. L-arginine reactivity in cerebral vessels after severe traumatic brain injury. Neurol. Res. 2010;32:1033–1040. doi: 10.1179/016164110X12767786356598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Robertson C.S., Gopinath S.P., Valadka A.B., Van M., Swank P.R., Goodman J.C. Variants of the endothelial nitric oxide gene and cerebral blood flow after severe traumatic brain injury. J. Neurotrauma. 2011;28:727–737. doi: 10.1089/neu.2010.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giannopoulos S., Katsanos A.H., Tsivgoulis G., Marshall R.S. Statins and cerebral hemodynamics. J. Cereb. Blood Flow Metab. 2012;32:1973–1976. doi: 10.1038/jcbfm.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cho H., Yun C.W., Park W.K., Kong J.Y., Kim K.S., Park Y., Lee S., Kim B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004;49:37–43. doi: 10.1016/S1043-6618(03)00248-2. [DOI] [PubMed] [Google Scholar]
- 147.Khan A., Vaibhav K., Javed H., Tabassum R., Ahmed M.E., Khan M.M., Khan M.B., Shrivastava P., Islam F., Siddiqui M.S., et al. 1,8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: Relevance to Alzheimer’s disease. Neurochem. Res. 2014;39:344–352. doi: 10.1007/s11064-013-1231-9. [DOI] [PubMed] [Google Scholar]
- 148.Tabassum R., Vaibhav K., Shrivastava P., Khan A., Ahmed M.E., Ashafaq M., Khan M.B., Islam F., Safhi M.M., Islam F. Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-kappaB in middle cerebral artery occlusion rats. Eur. J. Pharmacol. 2015;747:190–199. doi: 10.1016/j.ejphar.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 149.Vaibhav K., Shrivastava P., Javed H., Khan A., Ahmed M.E., Tabassum R., Khan M.M., Khuwaja G., Islam F., Siddiqui M.S., et al. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-kappaB in middle cerebral artery occlusion rat model. Mol. Cell. Biochem. 2012;367:73–84. doi: 10.1007/s11010-012-1321-z. [DOI] [PubMed] [Google Scholar]
- 150.Wada K., Chatzipanteli K., Kraydieh S., Busto R., Dietrich W.D. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998;43:1427–1436. doi: 10.1097/00006123-199812000-00096. [DOI] [PubMed] [Google Scholar]
- 151.Moochhala S.M., Md S., Lu J., Teng C.H., Greengrass C. Neuroprotective role of aminoguanidine in behavioral changes after blast injury. J. Trauma. 2004;56:393–403. doi: 10.1097/01.TA.0000066181.50879.7A. [DOI] [PubMed] [Google Scholar]
- 152.Louin G., Marchand-Verrecchia C., Palmier B., Plotkine M., Jafarian-Tehrani M. Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury. Neuropharmacology. 2006;50:182–190. doi: 10.1016/j.neuropharm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 153.Stover J.F., Belli A., Boret H., Bulters D., Sahuquillo J., Schmutzhard E., Zavala E., Ungerstedt U., Schinzel R., Tegtmeier F., et al. Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: A placebo-controlled randomized Phase IIa trial (NOSTRA) J. Neurotrauma. 2014;31:1599–1606. doi: 10.1089/neu.2014.3344. [DOI] [PubMed] [Google Scholar]
- 154.Tegtmeier F., Schinzel R., Beer R., Bulters D., LeFrant J.Y., Sahuquillo J., Unterberg A., Andrews P., Belli A., Ibanez J., et al. Efficacy of Ronopterin (VAS203) in Patients with Moderate and Severe Traumatic Brain Injury (NOSTRA phase III trial): Study protocol of a confirmatory, placebo-controlled, randomised, double blind, multi-centre study. Trials. 2020;21:80. doi: 10.1186/s13063-019-3965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hall E.D., Kupina N.C., Althaus J.S. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann. N. Y. Acad. Sci. 1999;890:462–468. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- 157.Zhang R., Shohami E., Beit-Yannai E., Bass R., Trembovler V., Samuni A. Mechanism of brain protection by nitroxide radicals in experimental model of closed-head injury. Free Radic. Biol. Med. 1998;24:332–340. doi: 10.1016/S0891-5849(97)00267-0. [DOI] [PubMed] [Google Scholar]
- 158.Bonini M.G., Mason R.P., Augusto O. The Mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem. Res. Toxicol. 2002;15:506–511. doi: 10.1021/tx015571z. [DOI] [PubMed] [Google Scholar]
- 159.Hall E.D., Vaishnav R.A., Mustafa A.G. Antioxidant therapies for traumatic brain injury. Neurother. J. Am. Soc. Exp. Neurother. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marklund N., Clausen F., Lewen A., Hovda D.A., Olsson Y., Hillered L. alpha-Phenyl-tert-N-butyl nitrone (PBN) improves functional and morphological outcome after cortical contusion injury in the rat. Acta Neurochir. 2001;143:73–81. doi: 10.1007/s007010170141. [DOI] [PubMed] [Google Scholar]
- 161.Gutteridge J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995;41:1819–1828. doi: 10.1093/clinchem/41.12.1819. [DOI] [PubMed] [Google Scholar]
- 162.Hall E.D., Yonkers P.A., McCall J.M., Braughler J.M. Effects of the 21-aminosteroid U74006F on experimental head injury in mice. J. Neurosurg. 1988;68:456–461. doi: 10.3171/jns.1988.68.3.0456. [DOI] [PubMed] [Google Scholar]
- 163.McIntosh T.K., Thomas M., Smith D., Banbury M. The novel 21-aminosteroid U74006F attenuates cerebral edema and improves survival after brain injury in the rat. J. Neurotrauma. 1992;9:33–46. doi: 10.1089/neu.1992.9.33. [DOI] [PubMed] [Google Scholar]
- 164.Dimlich R.V., Tornheim P.A., Kindel R.M., Hall E.D., Braughler J.M., McCall J.M. Effects of a 21-aminosteroid (U-74006F) on cerebral metabolites and edema after severe experimental head trauma. Adv. Neurol. 1990;52:365–375. [PubMed] [Google Scholar]
- 165.Marshall L.F., Maas A.I., Marshall S.B., Bricolo A., Fearnside M., Iannotti F., Klauber M.R., Lagarrigue J., Lobato R., Persson L., et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J. Neurosurg. 1998;89:519–525. doi: 10.3171/jns.1998.89.4.0519. [DOI] [PubMed] [Google Scholar]
- 166.Galvani S., Coatrieux C., Elbaz M., Grazide M.H., Thiers J.C., Parini A., Uchida K., Kamar N., Rostaing L., Baltas M., et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic. Biol. Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 167.Hall E.D., Wang J.A., Miller D.M., Cebak J.E., Hill R.L. Newer pharmacological approaches for antioxidant neuroprotection in traumatic brain injury. Neuropharmacology. 2019;145:247–258. doi: 10.1016/j.neuropharm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 168.Singh I.N., Gilmer L.K., Miller D.M., Cebak J.E., Wang J.A., Hall E.D. Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J. Cereb. Blood Flow Metab. 2013;33:593–599. doi: 10.1038/jcbfm.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cebak J.E., Singh I.N., Hill R.L., Wang J.A., Hall E.D. Phenelzine Protects Brain Mitochondrial Function In Vitro and In Vivo following Traumatic Brain Injury by Scavenging the Reactive Carbonyls 4-Hydroxynonenal and Acrolein Leading to Cortical Histological Neuroprotection. J. Neurotrauma. 2017;34:1302–1317. doi: 10.1089/neu.2016.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Baker G., Matveychuk D., MacKenzie E.M., Holt A., Wang Y., Kar S. Attenuation of the effects of oxidative stress by the MAO-inhibiting antidepressant and carbonyl scavenger phenelzine. Chem. Biol. Interact. 2019;304:139–147. doi: 10.1016/j.cbi.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 171.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharm. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 172.Jin W., Wang H., Yan W., Zhu L., Hu Z., Ding Y., Tang K. Role of Nrf2 in protection against traumatic brain injury in mice. J. Neurotrauma. 2009;26:131–139. doi: 10.1089/neu.2008.0655. [DOI] [PubMed] [Google Scholar]
- 173.Hong Y., Yan W., Chen S., Sun C.R., Zhang J.M. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharm. Sin. 2010;31:1421–1430. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jin W., Kong J., Wang H., Wu J., Lu T., Jiang J., Ni H., Liang W. Protective effect of tert-butylhydroquinone on cerebral inflammatory response following traumatic brain injury in mice. Injury. 2011;42:714–718. doi: 10.1016/j.injury.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 175.Lu X.Y., Wang H.D., Xu J.G., Ding K., Li T. Pretreatment with tert-butylhydroquinone attenuates cerebral oxidative stress in mice after traumatic brain injury. J. Surg. Res. 2014;188:206–212. doi: 10.1016/j.jss.2013.11.1106. [DOI] [PubMed] [Google Scholar]
- 176.Miller D.M., Singh I.N., Wang J.A., Hall E.D. Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 2015;264:103–110. doi: 10.1016/j.expneurol.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maynard M.E., Underwood E.L., Redell J.B., Zhao J., Kobori N., Hood K.N., Moore A.N., Dash P.K. Carnosic Acid Improves Outcome after Repetitive Mild Traumatic Brain Injury. J. Neurotrauma. 2019;36:2147–2152. doi: 10.1089/neu.2018.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin C., Chao H., Li Z., Xu X., Liu Y., Hou L., Liu N., Ji J. Melatonin attenuates traumatic brain injury-induced inflammation: A possible role for mitophagy. J. Pineal Res. 2016;61:177–186. doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- 179.Ding K., Xu J., Wang H., Zhang L., Wu Y., Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem. Int. 2015;91:46–54. doi: 10.1016/j.neuint.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 180.Wu H., Shao A., Zhao M., Chen S., Yu J., Zhou J., Liang F., Shi L., Dixon B.J., Wang Z., et al. Melatonin attenuates neuronal apoptosis through up-regulation of K(+) -Cl(−) cotransporter KCC2 expression following traumatic brain injury in rats. J. Pineal Res. 2016;61:241–250. doi: 10.1111/jpi.12344. [DOI] [PubMed] [Google Scholar]
- 181.Luo C., Yang Q., Liu Y., Zhou S., Jiang J., Reiter R.J., Bhattacharya P., Cui Y., Yang H., Ma H., et al. The multiple protective roles and molecular mechanisms of melatonin and its precursor N-acetylserotonin in targeting brain injury and liver damage and in maintaining bone health. Free Radic. Biol. Med. 2019;130:215–233. doi: 10.1016/j.freeradbiomed.2018.10.402. [DOI] [PubMed] [Google Scholar]
- 182.Ding K., Wang H., Xu J., Li T., Zhang L., Ding Y., Zhu L., He J., Zhou M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014;73:1–11. doi: 10.1016/j.freeradbiomed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 183.Barlow K.M., Brooks B.L., MacMaster F.P., Kirton A., Seeger T., Esser M., Crawford S., Nettel-Aguirre A., Zemek R., Angelo M., et al. A double-blind, placebo-controlled intervention trial of 3 and 10 mg sublingual melatonin for post-concussion syndrome in youths (PLAYGAME): Study protocol for a randomized controlled trial. Trials. 2014;15:271. doi: 10.1186/1745-6215-15-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pandya J.D., Readnower R.D., Patel S.P., Yonutas H.M., Pauly J.R., Goldstein G.A., Rabchevsky A.G., Sullivan P.G. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 2014;257:106–113. doi: 10.1016/j.expneurol.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou Y., Wang H.D., Zhou X.M., Fang J., Zhu L., Ding K. N-acetylcysteine amide provides neuroprotection via Nrf2-ARE pathway in a mouse model of traumatic brain injury. Drug Des. Devel. 2018;12:4117–4127. doi: 10.2147/DDDT.S179227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bhatti J., Nascimento B., Akhtar U., Rhind S.G., Tien H., Nathens A., da Luz L.T. Systematic Review of Human and Animal Studies Examining the Efficacy and Safety of N-Acetylcysteine (NAC) and N-Acetylcysteine Amide (NACA) in Traumatic Brain Injury: Impact on Neurofunctional Outcome and Biomarkers of Oxidative Stress and Inflammation. Front. Neurol. 2017;8:744. doi: 10.3389/fneur.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lutton E.M., Farney S.K., Andrews A.M., Shuvaev V.V., Chuang G.Y., Muzykantov V.R., Ramirez S.H. Endothelial Targeted Strategies to Combat Oxidative Stress: Improving Outcomes in Traumatic Brain Injury. Front. Neurol. 2019;10:582. doi: 10.3389/fneur.2019.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Morganti-Kossmann M.C., Rancan M., Otto V.I., Stahel P.F., Kossmann T. Role of cerebral inflammation after traumatic brain injury: A revisited concept. Shock (Augustaga.) 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 189.Bye N., Habgood M.D., Callaway J.K., Malakooti N., Potter A., Kossmann T., Morganti-Kossmann M.C. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 2007;204:220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 190.Kubes P., Ward P.A. Leukocyte recruitment and the acute inflammatory response. Brain Pathol. (Zur. Switz.) 2000;10:127–135. doi: 10.1111/j.1750-3639.2000.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lucas S.M., Rothwell N.J., Gibson R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147(Suppl. 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kreutzberg G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 193.King M.D., Laird M.D., Ramesh S.S., Youssef P., Shakir B., Vender J.R., Alleyne C.H., Dhandapani K.M. Elucidating novel mechanisms of brain injury following subarachnoid hemorrhage: An emerging role for neuroproteomics. Neurosurg. Focus. 2010;28:E10. doi: 10.3171/2009.10.FOCUS09223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Braun M., Vaibhav K., Saad N.M., Fatima S., Vender J.R., Baban B., Hoda M.N., Dhandapani K.M. White matter damage after traumatic brain injury: A role for damage associated molecular patterns. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2017;1863:2614–2626. doi: 10.1016/j.bbadis.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rhodes J. Peripheral immune cells in the pathology of traumatic brain injury? Curr. Opin. Crit. Care. 2011;17:122–130. doi: 10.1097/MCC.0b013e3283447948. [DOI] [PubMed] [Google Scholar]
- 196.Kigerl K.A., de Rivero Vaccari J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rothwell N.J. Annual review prize lecture cytokines - killers in the brain? J. Physiol. 1999;514(Pt 1):3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang C.X., Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog. Neurobiol. 2002;67:161–172. doi: 10.1016/S0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 199.Lu W., Gersting J.A., Maheshwari A., Christensen R.D., Calhoun D.A. Developmental expression of chemokine receptor genes in the human fetus. Early Hum. Dev. 2005;81:489–496. doi: 10.1016/j.earlhumdev.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 200.Dalgard C.L., Cole J.T., Kean W.S., Lucky J.J., Sukumar G., McMullen D.C., Pollard H.B., Watson W.D. The cytokine temporal profile in rat cortex after controlled cortical impact. Front. Mol. Neurosci. 2012;5:6. doi: 10.3389/fnmol.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shein S.L., Shellington D.K., Exo J.L., Jackson T.C., Wisniewski S.R., Jackson E.K., Vagni V.A., Bayir H., Clark R.S., Dixon C.E., et al. Hemorrhagic shock shifts the serum cytokine profile from pro- to anti-inflammatory after experimental traumatic brain injury in mice. J. Neurotrauma. 2014;31:1386–1395. doi: 10.1089/neu.2013.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Redell J.B., Moore A.N., Grill R.J., Johnson D., Zhao J., Liu Y., Dash P.K. Analysis of functional pathways altered after mild traumatic brain injury. J. Neurotrauma. 2013;30:752–764. doi: 10.1089/neu.2012.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.White T.E., Ford G.D., Surles-Zeigler M.C., Gates A.S., Laplaca M.C., Ford B.D. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genom. 2013;14:282. doi: 10.1186/1471-2164-14-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vaibhav K., Braun M., Alverson K., Khodadadi H., Kutiyanawalla A., Ward A., Banerjee C., Sparks T., Malik A., Rashid M.H., et al. Neutrophil extracellular traps exacerbate neurological deficts after traumatic brain injury. Sci. Adv. 2020 doi: 10.1126/sciadv.aax8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Braun M., Khan Z.T., Khan M.B., Kumar M., Ward A., Achyut B.R., Arbab A.S., Hess D.C., Hoda M.N., Baban B., et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018;68:224–237. doi: 10.1016/j.bbi.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tweedie D., Karnati H.K., Mullins R., Pick C.G., Hoffer B.J., Goetzl E.J., Kapogiannis D., Greig N.H. Time-dependent cytokine and chemokine changes in mouse cerebral cortex following a mild traumatic brain injury. ELife. 2020;9 doi: 10.7554/eLife.55827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Helmy A., Carpenter K.L., Menon D.K., Pickard J.D., Hutchinson P.J. The cytokine response to human traumatic brain injury: Temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Helmy A., Antoniades C.A., Guilfoyle M.R., Carpenter K.L., Hutchinson P.J. Principal component analysis of the cytokine and chemokine response to human traumatic brain injury. Plos ONE. 2012;7:e39677. doi: 10.1371/journal.pone.0039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Braun M., Vaibhav K., Saad N., Fatima S., Brann D.W., Vender J.R., Wang L.P., Hoda M.N., Baban B., Dhandapani K.M. Activation of Myeloid TLR4 Mediates T Lymphocyte Polarization after Traumatic Brain Injury. J. Immunol. 2017;198:3615–3626. doi: 10.4049/jimmunol.1601948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vaibhav K., Braun M., Khan M.B., Fatima S., Saad N., Shankar A., Khan Z.T., Harris R.B.S., Yang Q., Huo Y., et al. Remote ischemic post-conditioning promotes hematoma resolution via AMPK-dependent immune regulation. J. Exp. Med. 2018 doi: 10.1084/jem.20171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Soares H.D., Hicks R.R., Smith D., McIntosh T.K. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Carlos T.M., Clark R.S., Franicola-Higgins D., Schiding J.K., Kochanek P.M. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 213.Holmin S., Mathiesen T., Shetye J., Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir. 1995;132:110–119. doi: 10.1007/bf01404857. [DOI] [PubMed] [Google Scholar]
- 214.Hausmann R., Kaiser A., Lang C., Bohnert M., Betz P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int. J. Leg. Med. 1999;112:227–232. doi: 10.1007/s004140050241. [DOI] [PubMed] [Google Scholar]
- 215.Hsieh C.L., Kim C.C., Ryba B.E., Niemi E.C., Bando J.K., Locksley R.M., Liu J., Nakamura M.C., Seaman W.E. Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kelley B.J., Lifshitz J., Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exp. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- 217.Cao T., Thomas T.C., Ziebell J.M., Pauly J.R., Lifshitz J. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience. 2012;225:65–75. doi: 10.1016/j.neuroscience.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Semple B.D., Kossmann T., Morganti-Kossmann M.C. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Holmin S., Schalling M., Hojeberg B., Nordqvist A.C., Skeftruna A.K., Mathiesen T. Delayed cytokine expression in rat brain following experimental contusion. J. Neurosurg. 1997;86:493–504. doi: 10.3171/jns.1997.86.3.0493. [DOI] [PubMed] [Google Scholar]
- 222.Oehmichen M., Jakob S., Mann S., Saternus K.S., Pedal I., Meissner C. Macrophage subsets in mechanical brain injury (MBI)--a contribution to timing of MBI based on immunohistochemical methods: A pilot study. Leg. Med. 2009;11:118–124. doi: 10.1016/j.legalmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 223.Walsh J.T., Zheng J., Smirnov I., Lorenz U., Tung K., Kipnis J. Regulatory T Cells in Central Nervous System Injury: A Double-Edged Sword. J. Immunol. 2014;193:5013–5022. doi: 10.4049/jimmunol.1302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pizzolla A., Gelderman K.A., Hultqvist M., Vestberg M., Gustafsson K., Mattsson R., Holmdahl R. CD68-expressing cells can prime T cells and initiate autoimmune arthritis in the absence of reactive oxygen species. Eur. J. Immunol. 2011;41:403–412. doi: 10.1002/eji.201040598. [DOI] [PubMed] [Google Scholar]
- 225.Vergelli M., Pinet V., Vogt A.B., Kalbus M., Malnati M., Riccio P., Long E.O., Martin R. HLA-DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. Eur. J. Immunol. 1997;27:941–951. doi: 10.1002/eji.1830270421. [DOI] [PubMed] [Google Scholar]
- 226.Berger T., Rubner P., Schautzer F., Egg R., Ulmer H., Mayringer I., Dilitz E., Deisenhammer F., Reindl M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 2003;349:139–145. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 227.Tobin R.P., Mukherjee S., Kain J.M., Rogers S.K., Henderson S.K., Motal H.L., Newell Rogers M.K., Shapiro L.A. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol. Commun. 2014;2:143. doi: 10.1186/s40478-014-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mosley R.L., Hutter-Saunders J.A., Stone D.K., Gendelman H.E. Inflammation and Adaptive Immunity in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hickey W.F., Hsu B.L., Kimura H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 230.Holmin S., Söderlund J., Biberfeld P., Mathiesen T. Intracerebral Inflammation after Human Brain Contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. [DOI] [PubMed] [Google Scholar]
- 231.Hua R., Mao S.S., Zhang Y.M., Chen F.X., Zhou Z.H., Liu J.Q. Effects of pituitary adenylate cyclase activating polypeptide on CD4(+)/CD8(+) T cell levels after traumatic brain injury in a rat model. World J. Emerg. Med. 2012;3:294–298. doi: 10.5847/wjem.j.issn.1920-8642.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gutcher I., Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kabelitz D., Medzhitov R. Innate immunity-cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr. Opin. Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 234.Fu H., Wang A., Mauro C., Marelli-Berg F. T lymphocyte trafficking: Molecules and mechanisms. Front. Biosci. 2013;18:422–440. doi: 10.2741/4111. [DOI] [PubMed] [Google Scholar]
- 235.Murphy A.C., Lalor S.J., Lynch M.A., Mills K.H. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 236.Rostami A., Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013;333:76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang D., Sun Y.-Y., Bhaumik S.K., Li Y., Baumann J.M., Lin X., Zhang Y., Lin S.-H., Dunn R.S., Liu C.-Y., et al. Blocking Lymphocyte Trafficking with FTY720 Prevents Inflammation-Sensitized Hypoxic–Ischemic Brain Injury in Newborns. J. Neurosci. 2014;34:16467–16481. doi: 10.1523/JNEUROSCI.2582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Baxi E.G., DeBruin J., Tosi D.M., Grishkan I.V., Smith M.D., Kirby L.A., Strasburger H.J., Fairchild A.N., Calabresi P.A., Gocke A.R. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J. Neurosci. 2015;35:8626–8639. doi: 10.1523/JNEUROSCI.3817-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cao Y., Goods B.A., Raddassi K., Nepom G.T., Kwok W.W., Love J.C., Hafler D.A. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7:287ra274. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Becher B., Segal B.M. T(H)17 cytokines in autoimmune neuro-inflammation. Curr. Opin. Immunol. 2011;23:707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Grifka-Walk H.M., Lalor S.J., Segal B.M. Highly polarized Th17 cells induce EAE via a T-bet independent mechanism. Eur. J. Immunol. 2013;43:2824–2831. doi: 10.1002/eji.201343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Laird M.D., Sukumari-Ramesh S., Swift A.E., Meiler S.E., Vender J.R., Dhandapani K.M. Curcumin attenuates cerebral edema following traumatic brain injury in mice: A possible role for aquaporin-4? J. Neurochem. 2010;113:637–648. doi: 10.1111/j.1471-4159.2010.06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ma C., Ma Z., Fu Q., Ma S. Curcumin attenuates allergic airway inflammation by regulation of CD4+CD25+ regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized mice. Fitoterapia. 2013;87:57–64. doi: 10.1016/j.fitote.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 244.Park M.J., Moon S.J., Lee S.H., Yang E.J., Min J.K., Cho S.G., Yang C.W., Park S.H., Kim H.Y., Cho M.L. Curcumin attenuates acute graft-versus-host disease severity via in vivo regulations on Th1, Th17 and regulatory T cells. Plos ONE. 2013;8:e67171. doi: 10.1371/journal.pone.0067171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xie L., Li X.K., Funeshima-Fuji N., Kimura H., Matsumoto Y., Isaka Y., Takahara S. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int. Immunopharmacol. 2009;9:575–581. doi: 10.1016/j.intimp.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 246.Hernandez-Ontiveros D.G., Tajiri N., Acosta S., Giunta B., Tan J., Borlongan C.V. Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., Faden A.I. Progressive Neurodegeneration after Experimental Brain Trauma: Association with Chronic Microglial Activation. J. Neuropathol. Exp. Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.McKee C.A., Lukens J.R. Emerging Roles for the Immune System in Traumatic Brain Injury. Front. Immunol. 2016;7:556. doi: 10.3389/fimmu.2016.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jassam Y.N., Izzy S., Whalen M., McGavern D.B., El Khoury J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Correale J., Villa A. The neuroprotective role of inflammation in nervous system injuries. J. Neurol. 2004;251:1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- 251.Roberts I., Yates D., Sandercock P., Farrell B., Wasserberg J., Lomas G., Cottingham R., Svoboda P., Brayley N., Mazairac G., et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet (Lond. Engl.) 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 252.Edwards P., Arango M., Balica L., Cottingham R., El-Sayed H., Farrell B., Fernandes J., Gogichaisvili T., Golden N., Hartzenberg B., et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet (Lond. Engl.) 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 253.Tuttolomondo A., Pecoraro R., Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: A review of the evidence to date. Drug Des. Dev. 2014;8:2221–2238. doi: 10.2147/DDDT.S67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chio C.C., Lin J.W., Chang M.W., Wang C.C., Kuo J.R., Yang C.Z., Chang C.P. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J. Neurochem. 2010;115:921–929. doi: 10.1111/j.1471-4159.2010.06969.x. [DOI] [PubMed] [Google Scholar]
- 255.Cheong C.U., Chang C.P., Chao C.M., Cheng B.C., Yang C.Z., Chio C.C. Etanercept attenuates traumatic brain injury in rats by reducing brain TNF- alpha contents and by stimulating newly formed neurogenesis. Mediat. Inflamm. 2013;2013:620837. doi: 10.1155/2013/620837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chio C.C., Chang C.H., Wang C.C., Cheong C.U., Chao C.M., Cheng B.C., Yang C.Z., Chang C.P. Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci. 2013;14:33. doi: 10.1186/1471-2202-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tobinick E., Rodriguez-Romanacce H., Levine A., Ignatowski T.A., Spengler R.N. Immediate neurological recovery following perispinal etanercept years after brain injury. Clin. Drug Investig. 2014;34:361–366. doi: 10.1007/s40261-014-0186-1. [DOI] [PubMed] [Google Scholar]
- 258.Tobinick E. Immediate Resolution of Hemispatial Neglect and Central Post-Stroke Pain After Perispinal Etanercept: Case Report. Clin. Drug Investig. 2020;40:93–97. doi: 10.1007/s40261-019-00864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ralph S.J., Weissenberger A., Bonev V., King L.D., Bonham M.D., Ferguson S., Smith A.D., Goodman-Jones A.A., Espinet A.J. Phase I/II parallel double-blind randomized controlled clinical trial of perispinal etanercept for chronic stroke: Improved mobility and pain alleviation. Expert Opin. Investig. Drugs. 2020;29:311–326. doi: 10.1080/13543784.2020.1709822. [DOI] [PubMed] [Google Scholar]
- 260.Ignatowski T.A., Spengler R.N., Dhandapani K.M., Folkersma H., Butterworth R.F., Tobinick E. Perispinal etanercept for post-stroke neurological and cognitive dysfunction: Scientific rationale and current evidence. CNS Drugs. 2014;28:679–697. doi: 10.1007/s40263-014-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tobinick E., Kim N.M., Reyzin G., Rodriguez-Romanacce H., DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: An observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs. 2012;26:1051–1070. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- 262.Baratz R., Tweedie D., Rubovitch V., Luo W., Yoon J.S., Hoffer B.J., Greig N.H., Pick C.G. Tumor necrosis factor-alpha synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J. Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Baratz R., Tweedie D., Wang J.Y., Rubovitch V., Luo W., Hoffer B.J., Greig N.H., Pick C.G. Transiently lowering tumor necrosis factor-alpha synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice. J. Neuroinflamm. 2015;12:45. doi: 10.1186/s12974-015-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Stahel P.F., Shohami E., Younis F.M., Kariya K., Otto V.I., Lenzlinger P.M., Grosjean M.B., Eugster H.P., Trentz O., Kossmann T., et al. Experimental closed head injury: Analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 265.Thome J.G., Reeder E.L., Collins S.M., Gopalan P., Robson M.J. Contributions of Interleukin-1 Receptor Signaling in Traumatic Brain Injury. Front. Behav. Neurosci. 2019;13:287. doi: 10.3389/fnbeh.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tehranian R., Andell-Jonsson S., Beni S.M., Yatsiv I., Shohami E., Bartfai T., Lundkvist J., Iverfeldt K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J. Neurotrauma. 2002;19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- 267.Bergold P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016;275 Pt. 3:367–380. doi: 10.1016/j.expneurol.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Helmy A., Guilfoyle M.R., Carpenter K.L., Pickard J.D., Menon D.K., Hutchinson P.J. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: A phase II randomized control trial. J. Cereb. Blood Flow Metab. 2014;34:845–851. doi: 10.1038/jcbfm.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Clausen F., Hanell A., Bjork M., Hillered L., Mir A.K., Gram H., Marklund N. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2009;30:385–396. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- 270.Clausen F., Hanell A., Israelsson C., Hedin J., Ebendal T., Mir A.K., Gram H., Marklund N. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2011;34:110–123. doi: 10.1111/j.1460-9568.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- 271.Ma M.W., Wang J., Dhandapani K.M., Brann D.W. NADPH Oxidase 2 Regulates NLRP3 Inflammasome Activation in the Brain after Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2017;2017:6057609. doi: 10.1155/2017/6057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ismael S., Ahmed H.A., Adris T., Parveen K., Thakor P., Ishrat T. The NLRP3 inflammasome: A potential therapeutic target for traumatic brain injury. Neural Regen. Res. 2020;16:49–57. doi: 10.4103/1673-5374.286951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kerr N., Lee S.W., Perez-Barcena J., Crespi C., Ibañez J., Bullock M.R., Dietrich W.D., Keane R.W., de Rivero Vaccari J.P. Inflammasome proteins as biomarkers of traumatic brain injury. Plos ONE. 2018;13:e0210128. doi: 10.1371/journal.pone.0210128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Desu H.L., Plastini M., Illiano P., Bramlett H.M., Dietrich W.D., de Rivero Vaccari J.P., Brambilla R., Keane R.W. IC100: A novel anti-ASC monoclonal antibody improves functional outcomes in an animal model of multiple sclerosis. J. Neuroinflamm. 2020;17:143. doi: 10.1186/s12974-020-01826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Brough D., Denes A. Interleukin-1alpha and brain inflammation. IUBMB Life. 2015;67:323–330. doi: 10.1002/iub.1377. [DOI] [PubMed] [Google Scholar]
- 276.Newell E.A., Todd B.P., Mahoney J., Pieper A.A., Ferguson P.J., Bassuk A.G. Combined Blockade of Interleukin-1alpha and -1beta Signaling Protects Mice from Cognitive Dysfunction after Traumatic Brain Injury. ENeuro. 2018;5 doi: 10.1523/ENEURO.0385-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yang S.H., Gustafson J., Gangidine M., Stepien D., Schuster R., Pritts T.A., Goodman M.D., Remick D.G., Lentsch A.B. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J. Surg. Res. 2013;184:981–988. doi: 10.1016/j.jss.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hergenroeder G.W., Moore A.N., McCoy J.P., Jr., Samsel L., Ward N.H., 3rd, Clifton G.L., Dash P.K. Serum IL-6: A candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J. Neuroinflamm. 2010;7:19. doi: 10.1186/1742-2094-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yang S.H., Gangidine M., Pritts T.A., Goodman M.D., Lentsch A.B. Interleukin 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock (Augustaga.) 2013;40:471–475. doi: 10.1097/SHK.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mollica L., De Marchis F., Spitaleri A., Dallacosta C., Pennacchini D., Zamai M., Agresti A., Trisciuoglio L., Musco G., Bianchi M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 281.Gu X.J., Xu J., Ma B.Y., Chen G., Gu P.Y., Wei D., Hu W.X. Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin. J. Traumatol. 2014;17:1–7. [PubMed] [Google Scholar]
- 282.Yang L., Wang F., Yang L., Yuan Y., Chen Y., Zhang G., Fan Z. HMGB1 a-Box Reverses Brain Edema and Deterioration of Neurological Function in a Traumatic Brain Injury Mouse Model. Cell. Physiol. Biochem. 2018;46:2532–2542. doi: 10.1159/000489659. [DOI] [PubMed] [Google Scholar]
- 283.Zhang B., Wang B., Cao S., Wang Y. Epigallocatechin-3-Gallate (EGCG) Attenuates Traumatic Brain Injury by Inhibition of Edema Formation and Oxidative Stress. Korean J. Physiol. Pharmacol. 2015;19:491–497. doi: 10.4196/kjpp.2015.19.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Reddy V., Grogan D., Ahluwalia M., Salles É.L., Ahluwalia P., Khodadadi H., Alverson K., Nguyen A., Raju S.P., Gaur P., et al. Targeting the endocannabinoid system: A predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies. EPMA J. 2020 doi: 10.1007/s13167-020-00203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Russo E.B. History of cannabis as medicine: Nineteenth century irish physicians and correlations of their observations to modern research. In: Chanda S., Lata H., Elsohly M.., editors. Cannabis Sativa L.: Botany and Biotechnology. Springer International Publishing; Cham, Switzerland: 2017. pp. 63–78. [Google Scholar]
- 286.Russo E.B. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016;1:154–165. doi: 10.1089/can.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Rhyne D.N., Anderson S.L., Gedde M., Borgelt L.M. Effects of Medical Marijuana on Migraine Headache Frequency in an Adult Population. Pharmacotherapy. 2016;36:505–510. doi: 10.1002/phar.1673. [DOI] [PubMed] [Google Scholar]
- 288.Russo E.B., Hohmann A.G. Role of cannabinoids in pain management. In: Deer T., Gordin V., editors. Comprehensive Treatment of Chronic Pain by Medical, Interventional and Behavioral Approaches. Springer; New York, NY, USA: 2013. pp. 181–197. [Google Scholar]
- 289.Serpell M., Ratcliffe S., Hovorka J., Schofield M., Taylor L., Lauder H., Ehler E. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur. J. Pain (Lond. Engl.) 2014;18:999–1012. doi: 10.1002/j.1532-2149.2013.00445.x. [DOI] [PubMed] [Google Scholar]
- 290.Chen D.J., Gao M., Gao F.F., Su Q.X., Wu J. Brain cannabinoid receptor 2: Expression, function and modulation. Acta Pharm. Sin. 2017;38:312–316. doi: 10.1038/aps.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rog D.J., Nurmikko T.J., Friede T., Young C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 292.Johnson J.R., Burnell-Nugent M., Lossignol D., Ganae-Motan E.D., Potts R., Fallon M.T. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manag. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 293.Benyo Z., Ruisanchez E., Leszl-Ishiguro M., Sandor P., Pacher P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H785–H801. doi: 10.1152/ajpheart.00571.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Schurman L.D., Lichtman A.H. Endocannabinoids: A Promising Impact for Traumatic Brain Injury. Front. Pharmacol. 2017;8:69. doi: 10.3389/fphar.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Paloczi J., Varga Z.V., Hasko G., Pacher P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2018;29:75–108. doi: 10.1089/ars.2017.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Fernandez-Ruiz J., Moro M.A., Martinez-Orgado J. Cannabinoids in Neurodegenerative Disorders and Stroke/Brain Trauma: From Preclinical Models to Clinical Applications. Neurother. J. Am. Soc. Exp. Neurother. 2015;12:793–806. doi: 10.1007/s13311-015-0381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Habib A., Chokr D., Wan J., Hegde P., Mabire M., Siebert M., Ribeiro-Parenti L., Le Gall M., Letteron P., Pilard N., et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut. 2018 doi: 10.1136/gutjnl-2018-316137. [DOI] [PubMed] [Google Scholar]
- 298.Kho D.T., Glass M., Graham E.S. Is the Cannabinoid CB2 Receptor a Major Regulator of the Neuroinflammatory Axis of the Neurovascular Unit in Humans? Adv. Pharmacol. (San Diegocalif.) 2017;80:367–396. doi: 10.1016/bs.apha.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 299.Nozaki C., Markert A., Zimmer A. Inhibition of FAAH reduces nitroglycerin-induced migraine-like pain and trigeminal neuronal hyperactivity in mice. Eur. Neuropsychopharmacol. 2015;25:1388–1396. doi: 10.1016/j.euroneuro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 300.Maas A.I., Murray G., Henney H., 3rd, Kassem N., Legrand V., Mangelus M., Muizelaar J.P., Stocchetti N., Knoller N. Efficacy and safety of dexanabinol in severe traumatic brain injury: Results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5:38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 301.Latorre J.G., Schmidt E.B. Cannabis, Cannabinoids, and Cerebral Metabolism: Potential Applications in Stroke and Disorders of the Central Nervous System. Curr. Cardiol. Rep. 2015;17:627. doi: 10.1007/s11886-015-0627-3. [DOI] [PubMed] [Google Scholar]
- 302.Russo E.B. Synthetic and natural cannabinoids: The cardiovascular risk. Br. J. Cardiol. 2015;22:7–9. [Google Scholar]
- 303.Pacher P., Steffens S., Hasko G., Schindler T.H., Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018;15:151–166. doi: 10.1038/nrcardio.2017.130. [DOI] [PubMed] [Google Scholar]
- 304.Magid L., Heymann S., Elgali M., Avram L., Cohen Y., Liraz-Zaltsman S., Mechoulam R., Shohami E. Role of CB2 Receptor in the Recovery of Mice after Traumatic Brain Injury. J. Neurotrauma. 2019;36:1836–1846. doi: 10.1089/neu.2018.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hess D.C., Blauenfeldt R.A., Andersen G., Hougaard K.D., Hoda M.N., Ding Y., Ji X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015;11:698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- 306.Saxena P., Newman M.A., Shehatha J.S., Redington A.N., Konstantinov I.E. Remote ischemic conditioning: Evolution of the concept, mechanisms, and clinical application. J. Card. Surg. 2010;25:127–134. doi: 10.1111/j.1540-8191.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 307.Hoda M.N., Fagan S.C., Khan M.B., Vaibhav K., Chaudhary A., Wang P., Dhandapani K.M., Waller J.L., Hess D.C. A 2 × 2 factorial design for the combination therapy of minocycline and remote ischemic perconditioning: Efficacy in a preclinical trial in murine thromboembolic stroke model. Exp. Transl. Stroke Med. 2014;6:10. doi: 10.1186/2040-7378-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Hess D.C., Hoda M.N., Khan M.B. Humoral Mediators of Remote Ischemic Conditioning: Important Role of eNOS/NO/Nitrite. Acta Neurochir. Suppl. 2016;121:45–48. doi: 10.1007/978-3-319-18497-5_8. [DOI] [PubMed] [Google Scholar]
- 309.Loukogeorgakis S.P., Williams R., Panagiotidou A.T., Kolvekar S.K., Donald A., Cole T.J., Yellon D.M., Deanfield J.E., MacAllister R.J. Transient Limb Ischemia Induces Remote Preconditioning and Remote Postconditioning in Humans by a KATP Channel–Dependent Mechanism. Circulation. 2007;116:1386–1395. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 310.Xu M., Wang Y., Ayub A., Ashraf M. Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1295–H1303. doi: 10.1152/ajpheart.2001.281.3.H1295. [DOI] [PubMed] [Google Scholar]
- 311.Vaibhav K., Baban B., Khan M.B., Liu J.Y., Huo Y., Hess D.C., Dhandapani K.M., Hoda M.N. Remote ischemic preconditioning protects from traumatic brain injury (TBI) J. Neurotrauma. 2014;31:A87. [Google Scholar]
- 312.Vaibhav K., Baban B., Wang P., Khan M.B., Pandya C., Ahmed H., Chaudhary A., Ergul A., Heger I., Hess D.C., et al. Remote Ischemic Conditioning (RIC) Attenuates Post-TBI Ischemic Injury and Improves Behavioral Outcomes. Stroke A J. Cereb. Circ. 2015;46:ATP92. [Google Scholar]
- 313.Joseph B., Pandit V., Zangbar B., Kulvatunyou N., Khalil M., Tang A., O’Keeffe T., Gries L., Vercruysse G., Friese R.S., et al. Secondary brain injury in trauma patients: The effects of remote ischemic conditioning. J. Trauma Acute Care Surg. 2015;78:698–703; discussion 703–705. doi: 10.1097/TA.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 314.Pandit V., Khan M., Zakaria E.R., Largent-Milnes T.M., Hamidi M., O’Keeffe T., Vanderah T.W., Joseph B. Continuous remote ischemic conditioning attenuates cognitive and motor deficits from moderate traumatic brain injury. J. Trauma Acute Care Surg. 2018;85:48–53. doi: 10.1097/TA.0000000000001835. [DOI] [PubMed] [Google Scholar]
- 315.Sandweiss A.J., Azim A., Ibraheem K., Largent-Milnes T.M., Rhee P., Vanderah T.W., Joseph B. Remote ischemic conditioning preserves cognition and motor coordination in a mouse model of traumatic brain injury. J. Trauma Acute Care Surg. 2017;83:1074–1081. doi: 10.1097/TA.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 316.Minambres E., Ballesteros M.A., Mayorga M., Marin M.J., Munoz P., Figols J., Lopez-Hoyos M. Cerebral apoptosis in severe traumatic brain injury patients: An in vitro, in vivo, and postmortem study. J. Neurotrauma. 2008;25:581–591. doi: 10.1089/neu.2007.0398. [DOI] [PubMed] [Google Scholar]
- 317.Bredesen D.E. Key note lecture: Toward a mechanistic taxonomy for cell death programs. Stroke A J. Cereb. Circ. 2007;38:652–660. doi: 10.1161/01.STR.0000257802.82826.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bredesen D.E. Programmed cell death mechanisms in neurological disease. Curr. Mol. Med. 2008;8:173–186. doi: 10.2174/156652408784221315. [DOI] [PubMed] [Google Scholar]
- 319.Stoica B.A., Byrnes K.R., Faden A.I. Cell cycle activation and CNS injury. Neurotox. Res. 2009;16:221–237. doi: 10.1007/s12640-009-9050-0. [DOI] [PubMed] [Google Scholar]
- 320.Di Giovanni S., Movsesyan V., Ahmed F., Cernak I., Schinelli S., Stoica B., Faden A.I. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Cernak I., Stoica B., Byrnes K.R., Di Giovanni S., Faden A.I. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle (Georget. Tex.) 2005;4:1286–1293. doi: 10.4161/cc.4.9.1996. [DOI] [PubMed] [Google Scholar]
- 322.Stoica B., Byrnes K., Faden A.I. Multifunctional Drug Treatment in Neurotrauma. Neurother. J. Am. Soc. Exp. Neurother. 2009;6:14–27. doi: 10.1016/j.nurt.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hilton G.D., Stoica B.A., Byrnes K.R., Faden A.I. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 2008;28:1845–1859. doi: 10.1038/jcbfm.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yakovlev A.G., Faden A.I. Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol. 2001;24:131–144. doi: 10.1385/mn:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- 325.Wan J., Wang J., Cheng H., Yu Y., Xing G., Oiu Z., Qian X., He F. Proteomic analysis of apoptosis initiation induced by all-trans retinoic acid in human acute promyelocytic leukemia cells. Electrophoresis. 2001;22:3026–3037. doi: 10.1002/1522-2683(200108)22:14<3026::AID-ELPS3026>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 326.Zhang X., Alber S., Watkins S.C., Kochanek P.M., Marion D.W., Graham S.H., Clark R.S. Proteolysis consistent with activation of caspase-7 after severe traumatic brain injury in humans. J. Neurotrauma. 2006;23:1583–1590. doi: 10.1089/neu.2006.23.1583. [DOI] [PubMed] [Google Scholar]
- 327.Yakovlev A.G., Knoblach S.M., Fan L., Fox G.B., Goodnight R., Faden A.I. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 1997;17:7415–7424. doi: 10.1523/JNEUROSCI.17-19-07415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Larner S.F., Hayes R.L., McKinsey D.M., Pike B.R., Wang K.K. Increased expression and processing of caspase-12 after traumatic brain injury in rats. J. Neurochem. 2004;88:78–90. doi: 10.1046/j.1471-4159.2003.02141.x. [DOI] [PubMed] [Google Scholar]
- 329.Knoblach S.M., Nikolaeva M., Huang X., Fan L., Krajewski S., Reed J.C., Faden A.I. Multiple caspases are activated after traumatic brain injury: Evidence for involvement in functional outcome. J. Neurotrauma. 2002;19:1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- 330.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 331.Nathoo N., Narotam P.K., Agrawal D.K., Connolly C.A., van Dellen J.R., Barnett G.H., Chetty R. Influence of apoptosis on neurological outcome following traumatic cerebral contusion. J. Neurosurg. 2004;101:233–240. doi: 10.3171/jns.2004.101.2.0233. [DOI] [PubMed] [Google Scholar]
- 332.Brophy G.M., Pineda J.A., Papa L., Lewis S.B., Valadka A.B., Hannay H.J., Heaton S.C., Demery J.A., Liu M.C., Tepas J.J., 3rd, et al. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma. 2009;26:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.McGinn M.J., Kelley B.J., Akinyi L., Oli M.W., Liu M.C., Hayes R.L., Wang K.K., Povlishock J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol. Exp. Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mondello S., Robicsek S.A., Gabrielli A., Brophy G.M., Papa L., Tepas J., Robertson C., Buki A., Scharf D., Jixiang M., et al. alphaII-spectrin breakdown products (SBDPs): Diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Polster B.M., Basanez G., Etxebarria A., Hardwick J.M., Nicholls D.G. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J. Biol. Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- 336.Takano J., Tomioka M., Tsubuki S., Higuchi M., Iwata N., Itohara S., Maki M., Saido T.C. Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: Evidence from calpastatin mutant mice. J. Biol. Chem. 2005;280:16175–16184. doi: 10.1074/jbc.M414552200. [DOI] [PubMed] [Google Scholar]
- 337.Gao G., Dou Q.P. N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J. Cell. Biochem. 2000;80:53–72. doi: 10.1002/1097-4644(20010101)80:1<53::AID-JCB60>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 338.Mandic A., Viktorsson K., Strandberg L., Heiden T., Hansson J., Linder S., Shoshan M.C. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: Two separate pathways in cisplatin-induced apoptosis. Mol. Cell. Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.van Loo G., Saelens X., van Gurp M., MacFarlane M., Martin S.J., Vandenabeele P. The role of mitochondrial factors in apoptosis: A Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 340.Daugas E., Nochy D., Ravagnan L., Loeffler M., Susin S.A., Zamzami N., Kroemer G. Apoptosis-inducing factor (AIF): A ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/S0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- 341.Li L.Y., Luo X., Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 342.Ishihara Y., Shimamoto N. Involvement of endonuclease G in nucleosomal DNA fragmentation under sustained endogenous oxidative stress. J. Biol. Chem. 2006;281:6726–6733. doi: 10.1074/jbc.M510382200. [DOI] [PubMed] [Google Scholar]
- 343.Artus C., Boujrad H., Bouharrour A., Brunelle M.N., Hoos S., Yuste V.J., Lenormand P., Rousselle J.C., Namane A., England P., et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. Embo J. 2010;29:1585–1599. doi: 10.1038/emboj.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zhang X., Chen J., Graham S.H., Du L., Kochanek P.M., Draviam R., Guo F., Nathaniel P.D., Szabo C., Watkins S.C., et al. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem. 2002;82:181–191. doi: 10.1046/j.1471-4159.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- 345.Bano D., Munarriz E., Chen H.L., Ziviani E., Lippi G., Young K.W., Nicotera P. The plasma membrane Na+/Ca2+ exchanger is cleaved by distinct protease families in neuronal cell death. Ann. N. Y. Acad. Sci. 2007;1099:451–455. doi: 10.1196/annals.1387.006. [DOI] [PubMed] [Google Scholar]
- 346.Nur E.K.A., Gross S.R., Pan Z., Balklava Z., Ma J., Liu L.F. Nuclear translocation of cytochrome c during apoptosis. J. Biol. Chem. 2004;279:24911–24914. doi: 10.1074/jbc.C400051200. [DOI] [PubMed] [Google Scholar]
- 347.Zhao S., Aviles E.R., Jr., Fujikawa D.G. Nuclear translocation of mitochondrial cytochrome c, lysosomal cathepsins B and D, and three other death-promoting proteins within the first 60 min of generalized seizures. J. Neurosci. Res. 2010;88:1727–1737. doi: 10.1002/jnr.22338. [DOI] [PubMed] [Google Scholar]
- 348.Fujikawa D.G., Shinmei S.S., Cai B. Kainic acid-induced seizures produce necrotic, not apoptotic, neurons with internucleosomal DNA cleavage: Implications for programmed cell death mechanisms. Neuroscience. 2000;98:41–53. doi: 10.1016/S0306-4522(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 349.Cregan S.P., Dawson V.L., Slack R.S. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 350.Cande C., Vahsen N., Garrido C., Kroemer G. Apoptosis-inducing factor (AIF): Caspase-independent after all. Cell Death Differ. 2004;11:591–595. doi: 10.1038/sj.cdd.4401400. [DOI] [PubMed] [Google Scholar]
- 351.Hong S.J., Dawson T.M., Dawson V.L. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 352.Whalen M.J., Clark R.S., Dixon C.E., Robichaud P., Marion D.W., Vagni V., Graham S.H., Virag L., Hasko G., Stachlewitz R., et al. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 1999;19:835–842. doi: 10.1097/00004647-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 353.Alano C.C., Ying W., Swanson R.A. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 354.Ying W., Alano C.C., Garnier P., Swanson R.A. NAD+ as a metabolic link between DNA damage and cell death. J. Neurosci. Res. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- 355.Moubarak R.S., Yuste V.J., Artus C., Bouharrour A., Greer P.A., Menissier-de Murcia J., Susin S.A. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell. Biol. 2007;27:4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Andrabi S.A., Kim N.S., Yu S.W., Wang H., Koh D.W., Sasaki M., Klaus J.A., Otsuka T., Zhang Z., Koehler R.C., et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yu S.W., Wang H., Poitras M.F., Coombs C., Bowers W.J., Federoff H.J., Poirier G.G., Dawson T.M., Dawson V.L. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 358.Yu S.W., Andrabi S.A., Wang H., Kim N.S., Poirier G.G., Dawson T.M., Dawson V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Blenn C., Wyrsch P., Bader J., Bollhalder M., Althaus F.R. Poly(ADP-ribose)glycohydrolase is an upstream regulator of Ca2+ fluxes in oxidative cell death. Cell. Mol. Life Sci. Cmls. 2011;68:1455–1466. doi: 10.1007/s00018-010-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yasuda H., Shichinohe H., Kuroda S., Ishikawa T., Iwasaki Y. Neuroprotective effect of a heat shock protein inducer, geranylgeranylacetone in permanent focal cerebral ischemia. Brain Res. 2005;1032:176–182. doi: 10.1016/j.brainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 361.Lee S.H., Kwon H.M., Kim Y.J., Lee K.M., Kim M., Yoon B.W. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke A J. Cereb. Circ. 2004;35:2195–2199. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- 362.Beere H.M., Wolf B.B., Cain K., Mosser D.D., Mahboubi A., Kuwana T., Tailor P., Morimoto R.I., Cohen G.M., Green D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 363.Parcellier A., Gurbuxani S., Schmitt E., Solary E., Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem. Biophys. Res. Commun. 2003;304:505–512. doi: 10.1016/S0006-291X(03)00623-5. [DOI] [PubMed] [Google Scholar]
- 364.Gurbuxani S., Schmitt E., Cande C., Parcellier A., Hammann A., Daugas E., Kouranti I., Spahr C., Pance A., Kroemer G., et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 2003;22:6669–6678. doi: 10.1038/sj.onc.1206794. [DOI] [PubMed] [Google Scholar]
- 365.Matsumori Y., Hong S.M., Aoyama K., Fan Y., Kayama T., Sheldon R.A., Vexler Z.S., Ferriero D.M., Weinstein P.R., Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J. Cereb. Blood Flow Metab. 2005;25:899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- 366.Eroglu B., Kimbler D.E., Pang J., Choi J., Moskophidis D., Yanasak N., Dhandapani K.M., Mivechi N.F. Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J. Neurochem. 2014;130:626–641. doi: 10.1111/jnc.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Proskuryakov S.Y., Konoplyannikov A.G., Gabai V.L. Necrosis: A specific form of programmed cell death? Exp. Cell Res. 2003;283:1–16. doi: 10.1016/S0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 368.Volbracht C., Leist M., Kolb S.A., Nicotera P. Apoptosis in caspase-inhibited neurons. Mol. Med. (Camb. Mass.) 2001;7:36–48. doi: 10.1007/BF03401837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Zhu C., Wang X., Huang Z., Qiu L., Xu F., Vahsen N., Nilsson M., Eriksson P.S., Hagberg H., Culmsee C., et al. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–784. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 370.Kabadi S.V., Faden A.I. Selective CDK inhibitors: Promising candidates for future clinical traumatic brain injury trials. Neural Regen. Res. 2014;9:1578–1580. doi: 10.4103/1673-5374.141779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tikka T., Fiebich B.L., Goldsteins G., Keinanen R., Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Siopi E., Llufriu-Daben G., Fanucchi F., Plotkine M., Marchand-Leroux C., Jafarian-Tehrani M. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: The effect of minocycline. Neurosci. Lett. 2012;511:110–115. doi: 10.1016/j.neulet.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 373.Homsi S., Piaggio T., Croci N., Noble F., Plotkine M., Marchand-Leroux C., Jafarian-Tehrani M. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: A twelve-week follow-up study. J. Neurotrauma. 2010;27:911–921. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- 374.Kovesdi E., Kamnaksh A., Wingo D., Ahmed F., Grunberg N., Long J., Kasper C., Agoston D. Acute Minocycline Treatment Mitigates the Symptoms of Mild Blast-Induced Traumatic Brain Injury. Front. Neurol. 2012;3 doi: 10.3389/fneur.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wang X., Zhu S., Drozda M., Zhang W., Stavrovskaya I.G., Cattaneo E., Ferrante R.J., Kristal B.S., Friedlander R.M. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kim H.S., Suh Y.H. Minocycline and neurodegenerative diseases. Behav. Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 377.Casha S., Zygun D., McGowan M.D., Bains I., Yong V.W., John Hurlbert R. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 378.Wei J., Xiao G.-M. The neuroprotective effects of progesterone on traumatic brain injury: Current status and future prospects. Acta Pharm. Sin. 2013;34:1485–1490. doi: 10.1038/aps.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Guo Q., Sayeed I., Baronne L.M., Hoffman S.W., Guennoun R., Stein D.G. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp. Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 380.Moorthy K., Sharma D., Basir S.F., Baquer N.Z. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp. Gerontol. 2005;40:295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 381.Robertson C.L., Saraswati M. Progesterone protects mitochondrial function in a rat model of pediatric traumatic brain injury. J. Bioenerg. Biomembr. 2015;47:43–51. doi: 10.1007/s10863-014-9585-5. [DOI] [PubMed] [Google Scholar]
- 382.Djebaili M., Hoffman S.W., Stein D.G. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 383.Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., Stocchetti N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014;371:2467–2476. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 384.Stein D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29:1259–1272. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Xiong Y., Chopp M., Lee C.P. Erythropoietin improves brain mitochondrial function in rats after traumatic brain injury. Neurol. Res. 2009;31:496–502. doi: 10.1179/174313208X353703. [DOI] [PubMed] [Google Scholar]
- 386.Nichol A., French C., Little L., Haddad S., Presneill J., Arabi Y., Bailey M., Cooper D.J., Duranteau J., Huet O., et al. Erythropoietin in traumatic brain injury (EPO-TBI): A double-blind randomised controlled trial. Lancet (Lond. Engl.) 2015;386:2499–2506. doi: 10.1016/S0140-6736(15)00386-4. [DOI] [PubMed] [Google Scholar]
- 387.Maas A.I.R., Menon D.K., Lingsma H.F., Pineda J.A., Sandel M.E., Manley G.T. Re-Orientation of Clinical Research in Traumatic Brain Injury: Report of an International Workshop on Comparative Effectiveness Research. J. Neurotrauma. 2012;29:32–46. doi: 10.1089/neu.2010.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.High W.M., Jr., Briones-Galang M., Clark J.A., Gilkison C., Mossberg K.A., Zgaljardic D.J., Masel B.E., Urban R.J. Effect of growth hormone replacement therapy on cognition after traumatic brain injury. J. Neurotrauma. 2010;27:1565–1575. doi: 10.1089/neu.2009.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Moreau O.K., Cortet-Rudelli C., Yollin E., Merlen E., Daveluy W., Rousseaux M. Growth hormone replacement therapy in patients with traumatic brain injury. J. Neurotrauma. 2013;30:998–1006. doi: 10.1089/neu.2012.2705. [DOI] [PubMed] [Google Scholar]
- 390.Powner D.J., Boccalandro C., Alp M.S., Vollmer D.G. Endocrine failure after traumatic brain injury in adults. Neurocritical Care. 2006;5:61–70. doi: 10.1385/NCC:5:1:61. [DOI] [PubMed] [Google Scholar]
- 391.Christopher A.F., Kaur R.P., Kaur G., Kaur A., Gupta V., Bansal P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016;7:68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sabirzhanov B., Stoica B.A., Zhao Z., Loane D.J., Wu J., Dorsey S.G., Faden A.I. miR-711 upregulation induces neuronal cell death after traumatic brain injury. Cell Death Differ. 2016;23:654–668. doi: 10.1038/cdd.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Ge X.T., Lei P., Wang H.C., Zhang A.L., Han Z.L., Chen X., Li S.H., Jiang R.C., Kang C.S., Zhang J.N. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci. Rep. 2014;4:6718. doi: 10.1038/srep06718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Han Z., Chen F., Ge X., Tan J., Lei P., Zhang J. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014;1582:12–20. doi: 10.1016/j.brainres.2014.07.045. [DOI] [PubMed] [Google Scholar]