Abstract
Simple Summary
Organometallics, such as copper compounds, are cancer chemotherapeutics used alone or in combination with other drugs. One small group of copper complexes exerts an effective inhibitory action on topoisomerases, which participate in the regulation of DNA topology. Copper complexes of topoisomerase inhibitors work by different molecular mechanisms that have repercussions on the cell cycle checkpoints and death effectors. The expansion of this family of highly active anticancer drugs and their use in combination with other emerging cancer therapies opens new avenues for the treatment of cancers.
Abstract
Organometallics, such as copper compounds, are cancer chemotherapeutics used alone or in combination with other drugs. One small group of copper complexes exerts an effective inhibitory action on topoisomerases, which participate in the regulation of DNA topology. Copper complexes inhibitors of topoisomerases 1 and 2 work by different molecular mechanisms, analyzed herein. They allow genesis of DNA breaks after the formation of a ternary complex, or act in a catalytic mode, often display DNA intercalative properties and ROS production, and sometimes display dual effects. These amplified actions have repercussions on the cell cycle checkpoints and death effectors. Copper complexes of topoisomerase inhibitors are analyzed in a broader synthetic view and in the context of cancer cell mutations. Finally, new emerging treatment aspects are depicted to encourage the expansion of this family of highly active anticancer drugs and to expend their use in clinical trials and future cancer therapy.
Keywords: copper complexes, topoisomerase inhibitor, DNA damage response, cell cycle, cell death, chemotherapy
1. Introduction
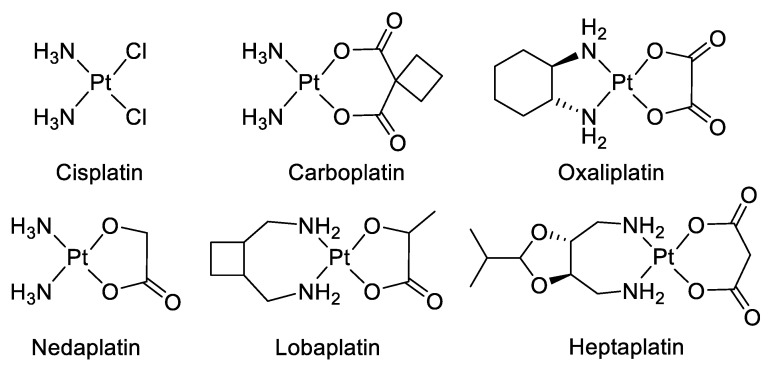
Chemotherapy is a systemic treatment proposed to patients suffering from cancer. It is often a complementary approach to surgery or radiotherapy. The discovery of platinum’s inhibitory effect on tumor cell growth in the 1960s [1] was a milestone for anticancer drug application in medicine [2]. Platinum (II) sets at the center of the squared planar structure of cisplatin and is coordinated with two chlorides and two ammonia molecules in a cis configuration. Cisplatin and its derivative drugs (carboplatin of second generation and oxaliplatin of third generation) are used worldwide in clinical applications and several other platinum analogs (lobaplatin, nedaplatin, and heptaplatin) are approved in several countries (Figure 1) [3,4]. However, serious side effects including toxicities on the kidney, heart, ear, and liver, decrease in immunity, hemorrhage, and gastrointestinal disorders limit the use of platinum derivatives [5,6,7]. The appearance of drug resistances, issuing from acquired or intrinsic multiple genetic and epigenetic changes, has also limited the clinical use of platinum-derived drugs [8]. Platinum-based treatment efficiency is challenged by cross-resistance and multiple changes including a decreased accumulation of the drug, a reduction in DNA–drug adducts, a modification in cell survival gene expression, an alteration of DNA damage repair mechanisms, modifications of transporters, protein trafficking, and altered cell metabolism [9,10,11,12,13,14].
Figure 1.
Platinum (II) complexes.
To circumvent drug resistance, a possible approach consists of designing and developing new therapeutic metal-based anticancer drugs [15,16,17,18,19,20,21]. Several transition metals from the d-block of the periodic table (groups 3 to 12) and particularly essential trace metals [15,22,23], such as copper [24,25,26,27,28,29], are useful for the implementation of metal-based complexes in anticancer therapies. Copper plays central roles in various cellular processes being an essential micronutrient and an important cofactor for several metalloenzymes involved in mitochondrial metabolism (cytochrome c oxidase), or cellular radical detoxification against reactive oxygen species (ROS) (superoxide dismutase) [30]. Copper is essential for angiogenesis, proliferation, and migration of endothelial cells [31,32,33]. Elevated copper favors tumor growth and metastasis. It is detected in several brain [34], breast [35], colon, prostate [36], and lung [37] tumors and serves as an indicator of the course of the disease [38]. The differences in tumor cells’ responses to copper compared to normal cells laid the foundation of copper complexes’ (CuC) evolution as anticancer agents. Numerous developed CuC contain different sets of N, S, or O ligands and demonstrate high cytotoxicity and efficient antitumor activity [25]. Different mechanisms are involved in copper drugs’ anticancer effect. They act as chelators, and interact with and sequester endogenous copper, reducing its availability for tumor growth and angiogenesis [39]. On the contrary, ionophores trigger intracellular copper accumulation, cytotoxicity, and activate apoptosis inhibitor factor (XIAP) [24,40,41,42,43,44,45,46]. Other CuC are proteasome inhibitors [47,48]. Several CuC are actually on clinical trials: a number of copper/disulfiram-based drug combinations for therapy and as diagnostic tools (metastatic breast cancer and germ cell tumor), several casiopeínas compounds and elesclomol (leukemia), and thiosemicarbazone-based copper complexes labeled with a radioactive isotope for positron emission tomography imaging of hypoxia (in head and neck cancers) [49].
The cisplatin DNA-targeting principle of action also conditioned the development of anticancer copper-based drugs [4,23,50]. Antitumor activities of copper-based drugs are based on the interactive properties of both copper and the ligand. Copper toxicity results from its redox capacities (Cu(I) and Cu(II) redox states’ interconversion in oxidation–reduction cycles), the property to displace other ions from the enzyme binding sites, a high DNA binding affinity, and the ability to promote DNA breaks [28,51]. In most cases, copper modifies the backbone of the complexed ligand and grants better DNA affinity, specificity, and stability [52]. Copper derivatives can interact with DNA without the formation of covalent adducts. The noncovalent interactions with DNA include binding along with the major or the minor DNA grooves, intercalation, or electrostatic binding. Some copper-based drugs generate reactive oxygen species (ROS) that overwhelm cellular antioxidant defenses to produce oxidative damages in the cytoplasm, mitochondria, and DNA [53]. An important class of CuC, actually on focus for chemotherapy, inhibits topoisomerases (Top) 1 and 2, resulting in severe DNA damages, cell cycle arrest, and death [40,54,55,56,57]. Chemotherapeutics that target Top as poisons convert a transient DNA-enzyme complex into lethal DNA breaks [58,59,60,61,62]. However, topoisomerase inhibitors’ activity and their multifaceted binding modes to DNA, the effects, and the modulations they produce on the control of cancer cell division necessitate better understanding to optimize their efficiency.
This review focuses on CuC targeting human Top1 and Top2, the molecular mechanism of induced DNA damages, cell cycle arrest, programmed cell death responses, and emerging research strategies.
2. Copper Complexes as Topoisomerases Inhibitors
DNA topoisomerases have been molecular targets for anticancer agents since their discovery in 1971 [63]. Topoisomerases regulate DNA winding and play essential functions in DNA replication and transcription [59,64]. Topoisomerase 1 (Top1) creates transient single-DNA nicks, while topoisomerases 2 (Top2α and Top2β) produce transient double-stranded DNA breaks. Both nuclear Top1 and Top2 are important targets for cancer chemotherapy, and Top inhibitors are used in therapeutic protocols [65,66,67]. Top inhibitors are classified into two groups: poisons and catalytic inhibitors. Top poisons (or interfacial poisons) stabilize the reversible cleavage complex formed between Top and DNA and form a ternary complex. Top2 catalytic inhibitors can prevent DNA strands cleavage through inhibition of the ATPase activity (novobiocin, merbarone), by impeding ATP hydrolysis to block Top dissociation from the DNA (ICRF-193), or by DNA intercalation at the Top fixation site (aclarubicinet) see [68]. In all cases, inhibitors convert the indispensable nuclear Top enzyme into a killing tool.
Top inhibitors’ activity increases upon complexation with copper ion. Top1, Top2, or Top1/2 inhibitors synthesized in the form of copper complexes (CuC) are mostly mononuclear Cu(II) complexes associated with a variety of ligands (Table 1). Different strategies are currently proposed to design and develop Top inhibitory agents based on ligands’ properties [69]. If both Top1 and Top2 inhibitors CuC primarily target DNA by a direct interaction through intercalation or cleavage, their antiproliferative activity is reinforced by ROS production and other molecular targets (Table 1) [25,52].
Table 1.
Copper complexes inhibitors of topoisomerases: targeted top isoforms, cancer cell lines responses, and molecular mechanisms are summarized. * Tests were realized in vitro with human Top1 or Top2α/β unless specified. IC50: half-maximal inhibitory concentration. EC50: half-maximal effective concentration. GI50: half-average of growth inhibition.
| Ligand Class of Cu-C | Compound Number | Targeted Top(s) | Inhibition of DNA Relaxation Total (µM) (minimal (µM)) | Inhibition Mecanism | Cancer Cell Lines | IC50 (µM) | Cell Cycle Arrest | Cell Death Type | Other Specificity | Reference Number |
|---|---|---|---|---|---|---|---|---|---|---|
| Oxindolimine | 1 | Top1 | 50 (25) |
Fixation in the DNA Top1 binding site |
Neuroblastoma SH-SY5Y Promonocytic U937 |
G2/M arrest | Apoptosis | ROS induction | [70,71,72,73] | |
| Hydrazone with triphenylphosphonium | 2 | Top1 | 40 | DNA Binding | Lung A549 | 4.2 ± 0.8 | [74] | |||
| Enzyme complex formation | Prostatic PC-3 | 3.2 ± 0.2 | ||||||||
| Plumbagin | 3 | Top1 | 1.56 | DNA intercalation | Breast MCF-7 | 3.2 ± 1.1 | [75] | |||
| Colon HCT116 | 5.9 ± 1.4 | |||||||||
| Hepatoma BEL7404 | 12.9 ± 3.6 | |||||||||
| Hepatoma HepG2 | 9.0 ± 0.7 | |||||||||
| Kidney 786-O | 2.5 ± 0.9 | |||||||||
| Lung NCI-H460 | 2.0 ± 1.2 | |||||||||
| Nasopharyngeal cancer CNE2 | 11.8 ± 5.9 | |||||||||
| Phenanthroline with amino acids |
4 | Top1 | 50 | DNA intercalation | Nasopharyngeal cancer HK1 | 2.2–5.2 | Apoptosis | [76] | ||
| (10) | ||||||||||
| Pyrophosphate | 5 | Top1 | 500 | DNA interaction | Ovarian A2780/AD | 0.64 ± 0.12 | [77] | |||
| Heterobimetallic Cu(II)-Sn2(IV) phenanthroline |
6 | Top1 | 20 | DNA intercalation | Breast Zr-75–1 | [78] | ||||
| cleavage | Cervix SiHa | |||||||||
| Colon HCT15, SW620 | <10 (GI50) | |||||||||
| Kidney 786-O, A498 | ||||||||||
| Lung Hop-62, A569 | ||||||||||
| Pancreatic MIA PaCa-2 | ||||||||||
| Neuroblastoma SH-SY5Y | 2–8 | Apoptosis | [79] | |||||||
| Analogs | [80] | |||||||||
| Tridentate chiral Schiff base | 7, 8 | Top1 | 25 | DNA binding | Hepatoma HuH7 | 25 | ROS | [81,82] | ||
| (15) | major groove | Hepatoma HepG2 | 6.2 ± 10 | Cytokine TGFb | ||||||
| mRNA upregulation | ||||||||||
| Salicylidene | 9 | Top1 | (E. coli) * | DNA binding | Prostatic PC-3 | 7.3 ± 0.2 | antimetastasis | [83] | ||
| DNA cleavage | Breast MCF7 | 51.1 ± 1.6 | [84] | |||||||
| Colon HT29 | 16.6 ± 0.6 | |||||||||
| Hepatoma HepG2 | 2.3 ± 0.1 | |||||||||
| Lung A549 | 16.8 ± 1.0 | |||||||||
| Ovary A2780 | 14.6 ± 0.2 | |||||||||
| Prostatic LNCaP | 25.4 ± 0.8 | |||||||||
| Chalcone-derived Thiosemicarbazone |
10 | Top1 | 3 | DNA binding | Breast MCF-7 | 0.16 ± 0.06 | [85] | |||
| (0.75) | DNA cleavage | Leukemia THP-1 | 0.20 ± 0.06 | |||||||
| Religation inhibition | ||||||||||
| Pyridyl-substituted tetrazolopyrimidie | 11 | Top1 | (Molecular docking) * |
DNA binding | Cervix HeLa | 0.565 ± 0.01 | Apoptosis | CDK receptor | [86] | |
| groove mode | Colon HCT-15 | 0.358 | binding | |||||||
| Lung A549 | 0.733 | |||||||||
| Tetrazolopyrimidine Diimine |
Top1 | 102 ± 1.1 | DNA binding | Cervical HeLa | 0.620 ± 0.0013 | Apoptosis | vEGF receptor | [87] | ||
| groove mode | Colon HCT-15 | 0.540 ± 0.00015 | binding | |||||||
| Lung A549 | 0.120 ± 0.002 | |||||||||
| Piperazine | 12 | Top1 | 12.5 | DNA binding | SOD mimic | [88] | ||||
| (5) | minor groove | |||||||||
| Elesclomol | 13 | Top1 | 50 | Poison | Erythroleukemic K562 | 0.0075 | Apoptosis | Copper chelator | [89] | |
| Necrosis | Not a substrat for | |||||||||
| Oxidative stress | ABC transporters | |||||||||
| Cu(SBCM)2 | 14 | Top1 | * (Molecular | DNA intercalation | Breast MCF7 | 27 | G2/M arrest | Apoptosis | p53 increase | [90] |
| docking) | DNA binding | Breast MDA-MB-231 | 18.7 ± 3.1 | No ROS | [91] | |||||
| TSC and TSC CuC | [92,93,94,95,96,97] | |||||||||
| Pyridine-TSC | 15 | Top2a | 50 | Breast MDA-MB-231 | 1.01 | [98] | ||||
| (10) | Breast MCF7 | 0.0558 | ||||||||
| 50 | ATP hydrolysis inhibition | [99] | ||||||||
| Top2β | (5) | ATP hydrolysis inhibition | [100] | |||||||
| Piperazine-TSC | 16 | Top2a | 0.9 ± 0.7 | Potentially catalytic | Breast MCF7 | 4.7 ± 0.3 | [101,102] | |||
| Breast SK-BR-3 | 1.3 ± 0.3 | [99] | ||||||||
| Thiazole-TSC | 17 | Top2a | 4 | Breast MDA-MB-231 | 1.41 (EC50) | [103] | ||||
| (2) | Breast MCF7 | 0.13 (EC50) | ||||||||
| 17–18 | Top2a | 25 | ATP hydrolysis inhibition | Breast | [104,105] | |||||
| (10) | + Poison | HCC 70, HCC 1395, | 1 to 20 | |||||||
| HCC 1500, and HCC 1806 | ||||||||||
| Colon | 0.83 to 41.2 | |||||||||
| Caco-2, HCT-116 and HT-29 | ||||||||||
| L- and D-Proline-TSC | 19 | Top2a | 300 | Ovarian carcinoma CH1 | 113 ± 16 | [106] | ||||
| Quinoline-TSC | 20 | Top2a | 0.48 | Potentially catalytic | Lymphoma U937 | 0.48-16.2 | [107] | |||
| Naphthoquinone-TSC | 21 | Top2α | 1 mM | Breast MCF7 | 3.98 ± 1.01 | No apoptosis | [108] | |||
| Bis-TSC | 22 | Top2a | 100 | Poison | Breast MDA-MB-231 | 1.45 ± 0.07 | G2/M arrest | Apoptosis | DNA synthesis | [109] |
| (5) | Colon HCT116 | 1.23 ± 0.27 | inhibition | |||||||
| Keratinocyte HaCaT | 0.65 ± 0.07 | No ROS | ||||||||
| Colon HCT116 | Delayed mice xenograft | |||||||||
| Carbohydrazone | 23 | Top2α | 250 | DNA binding | Breast MCF7 | 9.916 | Apoptosis | [110] | ||
| (25) | major groove | Breast MDA-MB-231 | 7.557 | |||||||
| Breast HCC 1937 | 3.278 | |||||||||
| Breast MX1 | 4.534 | |||||||||
| Breast MDA-MB-436 | 5.249 | |||||||||
| Breast MX-1 | Reducted mice xenograft (83%) | |||||||||
| Chromone | 24 | Top2a | 25 | DNA binding | Breast MCF7 | 18.6 (GI 50) | [111] | |||
| (15) | major groove | Breast Zr-75-1 | 25.2 (GI 50) | |||||||
| Colon HT29 | >80 (GI 50) | |||||||||
| Cervix SiHa | 34.6 (GI 50) | |||||||||
| Kidney A498 | 73.3 (GI 50) | |||||||||
| Lung A549 | 31.7 (GI 50) | |||||||||
| Ovary A2780 | 17.4 (GI 50) | |||||||||
| Quinolinone Shiff Base | 25 | Top2α | 9 | No intercalation | Hepatic HepG2 | 17.9 ± 3.8 | DNA synthesis | [112] | ||
| inhibition | ||||||||||
| Slight substrate | ||||||||||
| for ABC transporter | ||||||||||
| Bis-pyrazolyl Carboxylate | 26 | Dual Top1/Top2 |
(Molecular docking) * |
ATP entry (potentially) | Hepatic HepG2 | 3.3 ± 0.02 | Apoptosis | DNA replication | [113] | |
| DNA religation inhibition (potentially) | ROS |
2.1. CuC Top1 Inhibitors
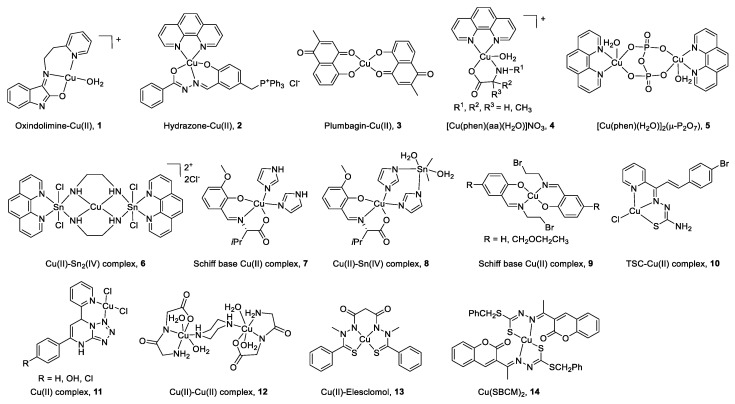
All the structures of CuC Top1 inhibitors are reported in Figure 2 and the main characteristics in Table 1. Oxindolimine-Cu(II) Top1 inhibitors such as 1 are planar copper compounds [70] that do not permit enzyme-DNA complex formation [71,72,73]. Besides, they produce ROS [70]. Cu(II) derivative complexes of the hydrazone ligand with triphenylphosphonium moiety 2 can bind DNA and the Top enzyme [74]. Plumbagin-Cu(II) 3 selectively intercalates into DNA [75]. The latter compound [75] and the phenanthroline-Cu(II) complexes modulated by amino acids 4 [76] can induce cancer cell apoptosis via mitochondrial signaling. Copper pyrophosphate-bridged binuclear complex 5 interacts with DNA, and based on the redox chemistry of copper, induces significant oxidative stress in cancer cell lines [77].
Figure 2.
Structure of Cu(II) complexes as Top1 inhibitors.
In the heterobimetallic Cu(II)-Sn2(IV) (copper/tin) complex 6, the planar phenanthroline heterocyclic ring approaches the Top−DNA complex Cu(II)-Sn2(IV) toward the DNA cleavage site and forms a stable complex with Top1 [78,79]. Other Cu(II)-Sn2(IV) analogs induce apoptosis [80]. Chiral monometallic or heterobimetallic complexes 7 and 8 with tridentate chiral Schiff base–ONO-ligand are DNA groove binders and produce ROS [81,82].
Salicylidene-Cu(II) derivative 9 of 2-[2-bromoethyliminomethyl] phenol [83,84] is a bifunctional drug that inhibits both cancer cell growth and metastasis.
Chalcone-derived thiosemicarbazone (TSC) Cu(II) complex 10 prevents the DNA cleavage step of the Top1 catalytic cycle and DNA relegation [85].
Tetrazolo[1,5-a]pyrimidine-based Cu(II) complexes 11 have a square planar geometry, and despite their high capability to inhibit Top1, interact with CDK for 11 [86] and VEGF receptors for an analog of 11 [87]. Binuclear Cu(II) dipeptide piperazine-bridged complex 12 recognizes specific sequences in the DNA, oxidatively cleaves DNA, and displays superoxide dismutase (SOD) activity [88].
Derived from elesclomol (in clinical trials: phase 3 against melanoma and randomized phases 2 and 3 for the treatment of a variety of other cancers), the elesclomol-Cu(II) complex 13 inhibits Top1 and induces apoptosis in cancer cells [89].
As recently studied, Cu(II)(SBCM)2 14 derived from S-benzyldithiocarbazate and 3-acetylcoumarin intercalates into DNA, induces ROS production, and has an antiproliferative activity in breast cancer lines [90,91].
2.2. CuC Top2α Inhibitors
Due to its cell cycle phase dependence and its high expression in proliferating cells, the Top2α isoform is primarily targeted by copper complexes (CuC), whereas Top2β remains unchanged during the course of the cell cycle [66]. Another reason to limit the clinical application of Top2β inhibitors is the strong unwanted side effects produced (secondary leukemia, myelodysplastic syndrome (MDS), and cardiac toxicity [92,93]).
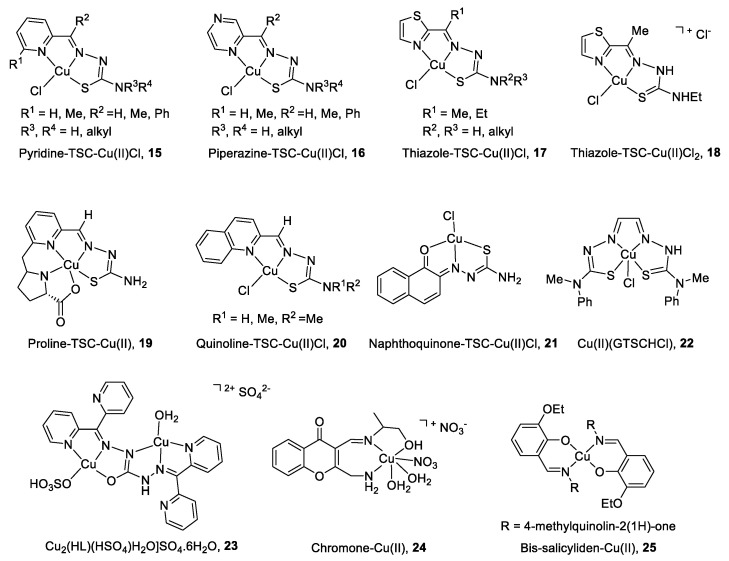
The main characteristics and structures of CuC Top2 inhibitors are reported in Figure 3 and Table 1. Several α-(N)-heterocyclic thiosemicarbazone (TSC) CuC [94,95] present a greater inhibitory effect on Top2α than corresponding TSC ligands alone [96,97] due to a square planar structure around the Cu(II) ion. A specific subset of pyridine-TSC CuC 15 inhibits Top2α [98] acting as ATP hydrolysis inhibitors in a non-competitive mode [94,99,100]. Another pyridine-TSC CuC inhibits Top2β [100]. Molecular modeling supports the binding of the complexes near but outside the ATP binding pocket in communication with the DNA cleavage/ligation site of Top2. Piperazine-TSCs based CuC 16 inhibit Top2α [101,102] by a strong interaction with the ATP-binding pocket residues [99] without ROS production [102]. Thiazole-TSC CuC 17 and 18 are Top2α catalytic inhibitors [103,104] or poisons [105]. The highly water-soluble proline-TSC CuC series 19 inhibit Top2α and cell proliferation [106]. Quinoline-TSC CuC 20 interact with the DNA phosphate group preventing relegation. The presence of two methyl groups on the terminal nitrogen is responsible for high activity and confers a cationic nature responsible for easier passive access into the cell [107].
Figure 3.
Structure of Cu(II) complexes as Top2 inhibitors.
Non-heterocycle naphthoquinone-TSC CuC 21 [108] and bis-TSC CuC 22 [109] are Top2α inhibitors acting as poisons [109]; they induce apoptosis in various human cancer cell lines and delay colorectal growth of carcinoma xenografts in mice [109]. Carbohydrazone CuC 23 [110] is a Top2α inhibitor that binds DNA, induces apoptosis, and reduces mice xenograft (83% after a treatment of 2 mg/kg). Chiral chromone Cu(II)/Zn(II) 24 [111] revealed catalytic inhibition of Top2α with DNA binding in the major groove. Quinolinone CuC 25 [112] inhibit Top2α and DNA synthesis without DNA intercalation and are only minimized PGP (P-glycoprotein efflux transporter) substrates.
2.3. CuC Dual Top1/Top2α Inhibitors
Heteroleptic Cu(I) complexes of the bis-pyrazolyl carboxylate ligand with auxiliary phosphine 26 (Figure 4) may inhibit Top1 by blocking the relegation step and inhibit Top2α by preventing ATP hydrolysis, as proposed by molecular docking analysis. They also perturb DNA replication, generate ROS, and induce apoptosis [113].
Figure 4.
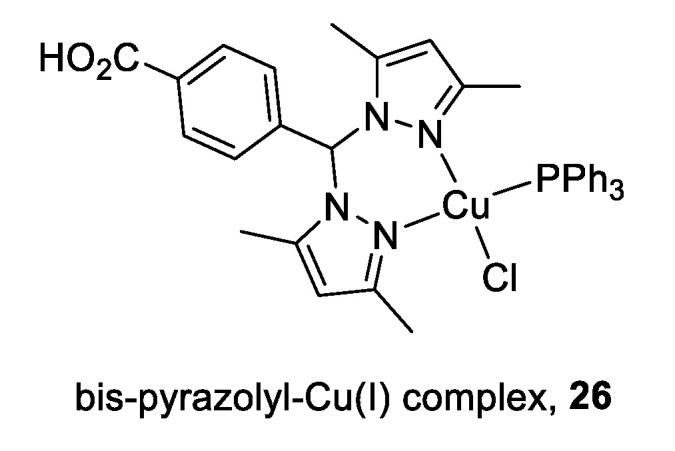
Structure of Cu(I) complex as a Top1/2α dual inhibitor.
3. Cell Cycle Regulation by Copper Complexes and Top Inhibitors
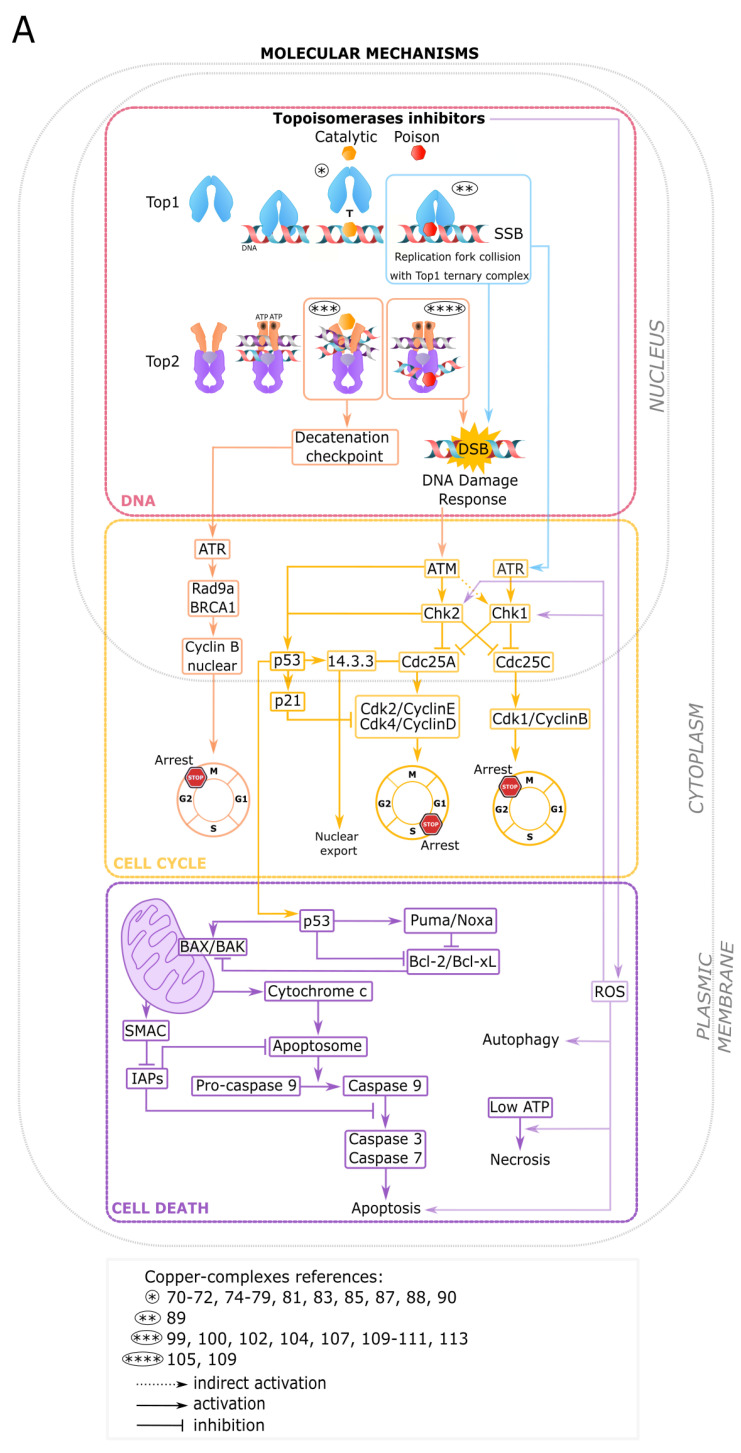
CuC inhibitors targeting Top1 [72,90] or Top2 [109] as DNA-damaging drugs or poisons arrest cancer cells in G2/M (Table 1). This common G2/M arrest involves the activation of two different cell cycle pathways: the DNA damage response (DDR) and the decatenation checkpoint.
Both Top1 and Top2 CuC inhibitors produce DNA damages. Top2 poisons prevent DNA relegation and stabilize an enzyme–DNA complex with the double-stranded cleaved DNA [114]. Top1 poisons induce single-stranded DNA breaks and associated signaling cascades. The collision between the Top1 cleavage complexes and the DNA replication forks ends up generating double-strand breaks [115] (Figure 5A). Top1- and Top2-induced DNA breaks trigger a DDR executed by ATM-, ATR-, and DNA-PK-related kinases, and an arrest of the cell cycle machinery [116,117,118]. ATM- and ATR-dependent phosphorylations of p53, Chk1, and Chk2 regulate the G1/S, S, or G2/M cell cycle checkpoints. Chk1 and Chk2 inhibit Cdc25 phosphatases (A,B,C) required for Cdks activation. Phosphorylated and ubiquitinated Cdc25A (Ser123) is degraded, leading to the absence of activation of the Cdk2/Cyclin E and the Cdk4/cyclin D complexes and followed by an arrest in G1/S. Phosphorylated Cdc25C (Ser216) binds to 14-3-3, prevents Cdk1/Cyclin B (MPF) activation, and induces a G2/M arrest (Figure 5A). Cdc25B inactivation also results in a G2 arrest [119,120]. The DNA damage-induced cell cycle arrest in G1 is dependent on p53 phosphorylation by ATM (Ser15) and Chk2 (Ser20) but arrest in S and G2 phases is p53-independent [121,122,123,124]. Phosphorylated p53 dissociates from MDM2 and activates the transcription of Cdk inhibitor p21WAF1 [125,126]. In several CuC (Top1 DNA binding CuC inhibitors [72,82,88] and a dual Top1/2 inhibitor with heteroleptic CuC [113]), Cu(II) exhibits a high redox potential and reinforces DDR activation by ROS production. ROS are also involved in a G2/M arrest through the decrease in Cdc25C [127] and Cdc25A levels [128], the activation of Chk1 [129] and Chk2 [130], and genomic instability through induced-DNA damages [131] (Figure 5A).
Figure 5.
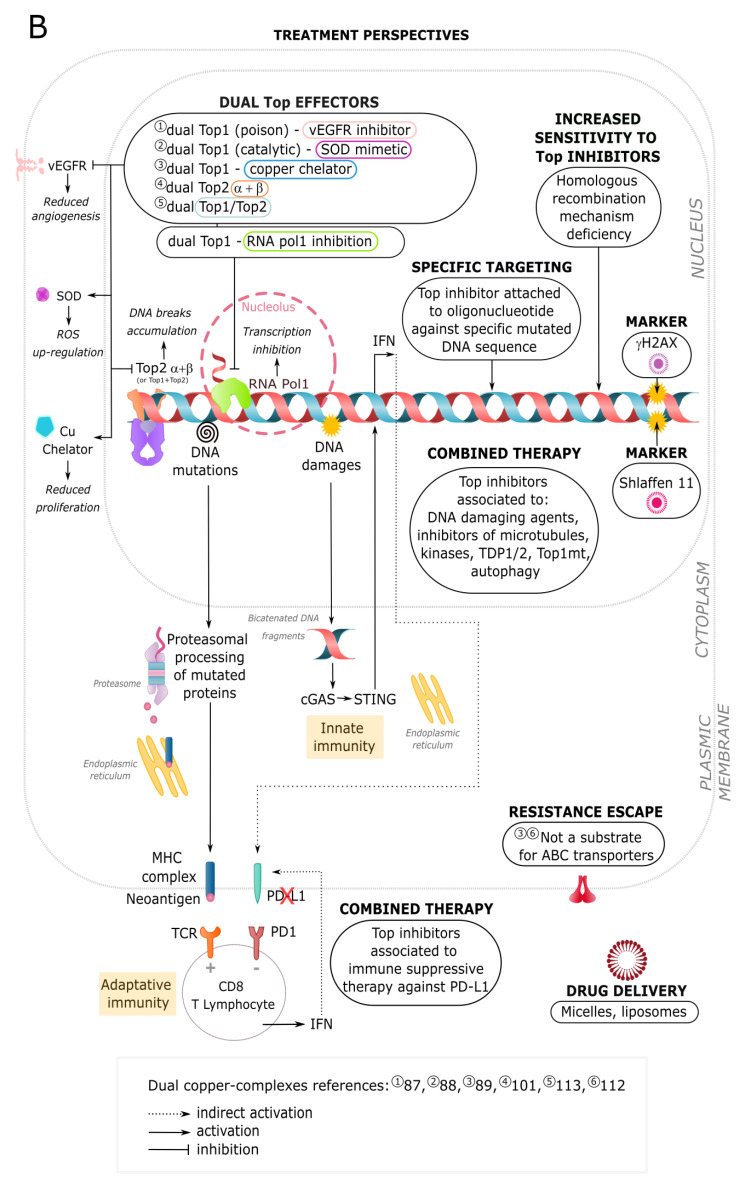
Molecular mechanisms and treatment perspectives for copper complexes (CuC) drugs. (A) Molecular checkpoints and networks involved in DNA damage (red), cell cycle regulation (yellow), and death response (violet) triggered by topoisomerase inhibitors (poison and catalytic), including CuC of topoisomerase inhibitors. (B) Treatment perspectives alone or in association with other chemotherapeutics (see text for more details).
By contrast to poisons, Top2 catalytic inhibitors do not form cleavable complexes. They function by enzymatic activity deprivation and cell cycle arrest in G2 through a decatenation checkpoint distinct from the DNA damage checkpoint. To delay the mitotic entry, an insufficient decatenation engages molecular components from the DDR and the spindle assembly checkpoint (SAC) (Rad9a, ATR, and BRCA1), SUMOylation and phosphorylation of Top2, the p38 and the MAPK pathways, and several decatenation checkpoint effectors but not p53 [66,132,133,134,135,136] (Figure 5A).
Cell cycle checkpoint effectors arrest DNA-damaged cells and induce their death providing that cell cycle regulatory networks are effective. Cell cycle checkpoint effectors integrity influences responses to Top2 inhibitors [137]. Besides, cancer disease is associated with multiple overexpression and mutations [138] in Cdc25 [139,140] and p53 [141,142], to a loss of Cdk inhibitors expression and/or overexpression of cell cycle-regulated protein [143,144], Top deregulation, and multidrug resistance [145,146,147]. Moreover, cell cycle variation of Top2α is regulated by post-translational modifications that represent potential targets. These alterations include ubiquitination by Cdk-1 [148], sumoylation [149], phosphorylation by polo-like kinase 1, Cdc7 [150], protein kinase C, Ca/calmodulin-dependent kinase II, and casein kinase [151], and the association with 14-3-3 [152]. Rewiring cellular pathways leading to cell death is a challenge that requires targeting specific molecular checkpoint effectors [153]. For example, a mutated p53 pathway arrests the cell cycle but avoids DDR-induced cell death [154]. Some anticancer therapeutic strategies (e.g., Chk1/2 pathways targeting drugs associated with DNA-damaging drugs) can force cancer cells to bypass S and G2/M arrest, enter mitosis with damaged DNA, and finally undergo a mitotic catastrophe and death [155]. ATR inhibition is another strategy to overcome the resistance of BRCA-deficient cancers [156].
4. Programmed Cell Death Engaged by Copper Complexes and Top Inhibitors
Multiple stress factors ranging from various cell damages, ATP levels, and specific pathways (e.g., caspases) determine the type of cell death [157]. Most Top1 CuC inhibitors that interact with DNA [70,76,79,86,87,90], Top1 poison [89], Top2α CuC poison [109], or dual Top1/Top2 inhibitor [113] trigger apoptotic programmed cell death. Genetic damages and oxidative stress activate an intrinsic mitochondrial response [158]. Pro-apoptotic members of the Bcl-2 family (Bid, Noxa, Puma, BAX, BAK) neutralize the anti-apoptotic members (Bcl-2, Bcl-xL, and Mcl-1), disrupt the mitochondrial outer membrane, and allow cytoplasmic cytochrome-c release. The binding of cytochrome c to the apoptotic protease activating factor-1 (Apaf-1), ATP, and the pro-caspase-9 create the apoptosome protein complex. Pro-caspase 9 is cleaved into its active caspase-9 form, which in turn cleaves pro-caspase-3 into caspase-3 effector, and the downstream executor caspase-7. SMAC (second mitochondria-derived activator of caspases), and Omi/HtrA2 (high-temperature requirement protein A2) are simultaneously released from mitochondria and deactivate the IAPs factors (inhibitors of apoptosis proteins). p53, activated by the DNA damage, contributes to apoptosis through the translation of several pro-apoptotic members of the Bcl-2 family (Bid, Puma) that inhibit the pro-survival action of Bcl-2 on BAX (Figure 5A). Most cancer cells evade apoptosis through caspase inhibition, upregulation of Bcl-2 (in more than 50% of all types of cancers), and loss of BAX/BAK and become resistant to anticancer drugs [159].
A Top1 DNA-damaging CuC inhibitor induces necrotic cell death. To facilitate cell destruction, necrosis is activated by ROS or ATP metabolic stresses in crosstalk with apoptosis [160]. When the intracellular energy/ATP level is low, the apoptotic cell death is converted into necrosis [161] (Figure 5A). However, necrosis releases pro-inflammatory and tumor-promoting cytokine HMGB1 [162] into the extracellular space reported to stimulate inflammation and angiogenesis, and promote tumor progression [163].
Apoptosis and necrosis often co-exist with another cell death with controversial pro-death and pro-survival functions: autophagy [164]. Up to the current study, no CuC Top inhibitors are involved in autophagic or necroptotic programmed cell death (Table 1). However, some CuC trigger stress-mediated protective autophagy in response to ROS that impedes apoptosis and creates survival of malignant cells [165]. Moreover, topoisomerase inhibition-induced autophagy is associated with cancer resistance [166].
5. Future Strategies for Copper Complexes as Top Inhibitors in Cancer Cell Treatments
The development of new effective anticancer drugs is a major research area against the continuing increase in cancers worldwide. Top inhibitors used in chemotherapy are limited in number [61,167,168]. Top1 inhibitors’ camptothecin derivatives used are irinotecan (colorectal [169], pancreatic (in combination) [170], and small cell lung cancers (in clinical trials and in combination) [171,172]), and topotecan (ovarian [173,174], cervical [175], and small cell lung cancers [176]). Top2 anticancer drugs commonly used are from the anthracycline group such as doxorubicin (acute leukemia [177], lymphomas [178], sarcomas [179,180], and solid tumors [181]), epirubicin (breast cancer [182]), valrubicin (bladder cancer [183]), and idarubicin (acute myeloid leukemia [184]), from the anthracenedione classes: mitoxantron and pixantron (lymphoma, [185,186,187]), and from the epipodopodophyllotoxins group such as etoposide (testicular [188] and small cell lung cancers [189]) and teniposide (brain [190] and small cell lung [191] cancers, acute lymphocytic leukemia [192]). Only a few numbers of Top1 inhibitors are in clinical trials including the promising indenoisoquinoline derivatives LMP400 (Indotecan), LMP776 (Indimitecan) (phase I), and LMP744 examined in a phase I study on lymphoma in dogs [193]. In addition to better stability, and milder side effects, they can escape ABC transporter efflux and the drug resistance mechanism, as Elesclomol-CuC Top complexes 13 [89] or Quinolinone-CuC 25 [112]. Perspectives to use CuC of Top inhibitors in clinical trials are summarized in Figure 5B. Development and optimization in CuC of Top inhibitors imply structure modifications that must encompass several specific strategies [194], such as scaffold hopping [195], pharmacophore hybridization [196], bioisosteric replacement [197], and conformational restrictions. Generally, a rigidification of the ligand heterocycle structure with a copper metal [78] provides a planar configuration that facilitates DNA intercalation and Top-DNA ternary complex formation compared to the molecular backbone alone.
Top inhibitors in clinical use and particularly Top poison display unwanted drawbacks, such as cumulative cardiotoxicity in long-term protocols, secondary malignancies, and drug resistance [198]. A therapeutic option would be to use preferentially catalytic Top agents that disturb the catalytic cycle without the formation of a ternary complex. CuC Top catalytic inhibitors, listed in Table 1, exhibit high antitumor effects on cancer cell lines and for some compounds on tumor growth in animal models, compared to their respective ligands (see Table 1). They constitute a reservoir of anticancer drugs. For example, TSC-based CuC Top2 inhibitors (Figure 3) [98,102,103,105,107] have demonstrated strong inhibition of tumor growth compared to TSC derivatives currently used in cancer chemotherapies [199].
Considering that cancer is a multigenetic and multifactorial disease that recruits numerous molecular effectors, monotherapies (based on Top inhibitors) do not provide the optimal curative effects. Combination therapy with a few numbers of therapeutics against two or more biotargets is the base of promising treatments such as the association of a Top 2 inhibitor (vosaroxin) with a DNA methyltransferase inhibitor (decitabine) in AML [200,201]. Inhibitors of Top1 and Top2, currently developed, also exert their effect against other cancer-related targets [202]. Dual Top inhibitors, e.g., Top 1/2 [203], Top2/microtubule [204], or Top2/histone deacetylase [205], may exert improved efficacy. Besides, Top1 inhibitors are nonspecific RNA polymerase inhibitors. An RNA Pol1-mediated ribosomal RNA gene increase is involved in cancer progression, through the control of cellular checkpoints and chromatin structure and is, therefore, an interesting co-target [206]. CuC dual Top inhibitors display a high antiproliferative activity. Particularly, some CuC and non-CuC are dual inhibitors of Top1 and superoxide dismutase agonist [88,207,208] or Cdk receptor, like VEGF inhibitors, involved in cancer cells proliferation [86,87,209,210] (Figure 5B). Another strategy to improve therapies is the association of a CuC with a TDP1/2 (tyrosyl-DNA-phosphodiesterase 1/2) inhibitor. TDP1/2 are enzymes responsible for the reparation of DNA breaks induced by topoisomerase poisons [57,211,212]. TDP1/2 inhibitors are capable of improving cancer cells’ sensitivity to these poisons [213].
Autophagy, an essential mechanism for cell integrity and survival, is stimulated in cancer cells under several chemotherapeutic drugs and acts as an unwanted protective system towards tumor cells. Association of specific autophagic inhibitors with Cu-C treatment (disulfiram) in non-small cell lung cancer [214] has proven to be a novel efficient strategy to enhance apoptosis in cancer therapy.
Immunogenic cell death is an important mechanism used in chemotherapy. Association of CuC with immune checkpoint therapies is certainly a new avenue in cancer treatment. CuC and non-CuC Top inhibitors induce DNA damages and are linked to adaptive and innate immunities [215]. Top poisons promote immunogenicity in various ways [216]. Top1 poison camptothecin enhances the adaptive immune response [217]. Top inhibitors also increase chromosomal instability and mutations accumulated by cancer cells [59,218]. Consequently, due to their high number of mutations, tumors display more neoantigens presented at their surface by the major histocompatibility complex class I (MHCI) and recruit lymphocytes T harboring TCR (T cell receptor) and CD8 co-receptor (adaptive immunity). This response is counterbalanced by the overexpression of immune checkpoint modulators, such as the immune-suppressive ligand PD-L1 (programmed death-ligand 1) targeted in immune therapies [219] (Figure 5B). DNA-damaging agents such as Top2 poison anthracycline also interfere with the innate immune response. They enhance the malignant formation of cytosolic bicatenated DNA fragments that activate the cyclic GMP-AMP synthase-stimulator of the interferon (IFN) gene pathway (cGAS-STING) and initiate innate anti-cancer immunity. cGAS-STING agonist serves as a sensitizer in immunotherapies [220]. Top1-DNA covalent cleavage complex enables cGAS-mediated cytoplasmic chromatin recognition and immune checkpoint response [221] (Figure 5B). Top2 inhibitors teniposide and doxorubicin potentiate the therapeutic immune checkpoint blockade therapies based on anti-PD-1 (programmed cell death 1) in multiple types of mouse tumor models [222,223]. Besides, ROS produced by Top inhibitors alter the molecular pattern recognized as immunogenic structures and enhance apoptosis [224] (Figure 5B).
As DDR gene mutations exist in a large range of tumor types, the determination of tumor-specific mutations is another accurate strategy to generate chemotypes with beneficial efficacies superior to adverse effects [225,226]. In each tumor, the signaling components of the DDR exhibit numerous defects that result in a unique mutational signature [227]. Cancer cells with defects in their homologous recombination mechanism are more sensitive to Top2 inhibitory therapies that generate DNA double-strand breaks [228]. Moreover, the prediction of anticancer treatments determined by the clinical stage and the pathological features of the tumor does not always ascertain a cancer death response. Cellular biomarkers that may predict sensitivity or resistance to therapy based on DNA damage induced by Top inhibitors would be useful. Insights into the Top2 regulatory mechanisms have identified genetic markers to allow the prediction of an overcome treatment with a Top inhibitor. γ-H2AX is a DNA-damaged marker, recruited on DNA breaks after Top poison action, currently evaluated [229]. Schlaffen is also a promising marker for an accurate response to Top1 and Top2 inhibitors, especially for colon and ovarian adenocarcinomas [56,230] (Figure 5B).
Recently, cancer cells were targeted specifically by a Top2 inhibitor, etoposide, attached to a single-stranded oligonucleotide with a complementary sequence to a DNA cleavage hotspot corresponding to a translocated region only present in promyelocytic leukemia cells [231].
Finally, to overcome toxicity to normal cells, Top drugs could be attached to vehicles. Top2 inhibitors delivery has been optimized using liposomes [232], micelles [233], or functionalized nanoparticles [234] (Figure 5B).
Topoisomerases are present in mitochondria where they participate in mitochondrial DNA replication and transcription. Mitochondrial Top1 isoform (Top1mt) is involved in the metabolism of cancer cells providing energy to tumors surrounded by a nutrient-low microenvironment. Exposures to a Top1 inhibitor (lamellarin D) or Top2 inhibitors (doxorubicin or fluoroquinolones) exert mitochondrial toxicity [235]. However, the loss of Top1mt in liver cancers correlates with increased survival of hepatocellular carcinoma patients, showing that co-targeting Top1mt in addition to nuclear topoisomerases is another option for anticancer therapies [236].
6. Conclusions
In a multifactorial disease such as cancer, Top inhibitors are efficient anticancer compounds used in monotherapy or polypharmacological strategies. They certainly have to target closely related modulators of the cellular checkpoints’ networks. CuC Top inhibitors are particularly adapted to fulfill this role. A perspective in anticancer strategy is to increase and to enlarge this family of highly active anticancer drugs.
Acknowledgments
The authors are thankful to the Research Federation FRABio (FR 3688, FRABio, Biochimie Structurale et Fonctionnelle des Assemblages Biomoléculaires) and to E. Germain (Inserm U1003) for reading the manuscript.
Author Contributions
Writing—original draft preparation: C.M., K.C., A.M. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
C.M. is a recipient from a doctoral fellowship from the French ministry. The scientific supports were provided by the “Centre National de la Recherche Scientifique ”, “Université de Lille ”, and the “Ligue Contre le Cancer”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rosenberg B., VanCamp L., Trosko J.E., Mansour V.H. Platinum compounds: A new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 2.Alderden R.A., Hall M.D., Hambley T.W. The discovery and development of cisplatin. J. Chem. Educ. 2006;83:728–734. doi: 10.1021/ed083p728. [DOI] [Google Scholar]
- 3.Dilruba S., Kalayda G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharm. 2016;77:1103–1124. doi: 10.1007/s00280-016-2976-z. [DOI] [PubMed] [Google Scholar]
- 4.Bergamo A., Dyson P.J., Sava G. The mechanism of tumour cell death by metal-based anticancer drugs is not only a matter of DNA interactions. Coord. Chem. Rev. 2018;360:17–33. doi: 10.1016/j.ccr.2018.01.009. [DOI] [Google Scholar]
- 5.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharm. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manohar S., Leung N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 7.Herradón E., González C., Uranga J.A., Abalo R., Martín M.I., López-Miranda V. characterization of cardiovascular alterations induced by different chronic cisplatin treatments. Front. Pharm. 2017;8:196–211. doi: 10.3389/fphar.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen D.W., Pouliot L.M., Hall M.D., Gottesman M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharm. Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S.H., Chang J.Y. New insights into mechanisms of cisplatin resistance: From tumor cell to microenvironment. Int. J. Mol. Sci. 2019;20:4136. doi: 10.3390/ijms20174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obrist F., Michels J., Durand S., Chery A., Pol J., Levesque S., Joseph A., Astesana V., Pietrocola F., Wu G.S., et al. Metabolic vulnerability of cisplatin-resistant cancers. EMBO J. 2018;37:e98597. doi: 10.15252/embj.201798597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amable L. Cisplatin resistance and opportunities for precision medicine. Pharm. Res. 2016;106:27–36. doi: 10.1016/j.phrs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 13.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S. Drug resistance in cancer: An overview. Cancers. 2014;6:1769. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinho N., Santos T., Florindo H.F., Silva L.C. Cisplatin-membrane interactions and their influence on platinum complexes activity and toxicity. Front. Physiol. 2019;9:1898–1913. doi: 10.3389/fphys.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng L., Gupta P., Chen Y., Wang E., Ji L., Chao H., Chen Z.-S. The development of anticancer ruthenium(ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017;46:5771–5804. doi: 10.1039/C7CS00195A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P., Sadler P.J. Advances in the design of organometallic anticancer complexes. J. Org. Chem. 2017;839:5–14. doi: 10.1016/j.jorganchem.2017.03.038. [DOI] [Google Scholar]
- 17.Jaouen G., Vessières A., Top S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015;44:8802–8817. doi: 10.1039/C5CS00486A. [DOI] [PubMed] [Google Scholar]
- 18.Gianferrara T., Bratsos I., Alessio E. A categorization of metal anticancer compounds based on their mode of action. Dalton Trans. 2009;37:7588–7598. doi: 10.1039/b905798f. [DOI] [PubMed] [Google Scholar]
- 19.Hartinger C.G., Dyson P.J. Bioorganometallic chemistry from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009;38:391–401. doi: 10.1039/B707077M. [DOI] [PubMed] [Google Scholar]
- 20.Wambang N., Schifano-Faux N., Martoriati A., Henry N., Baldeyrou B., Bal-Mahieu C., Bousquet T., Pellegrini S., Meignan S., Cailliau K., et al. Synthesis, structure, and antiproliferative activity of ruthenium(ii) arene complexes of indenoisoquinoline derivatives. Organometallics. 2016;35:2868–2872. doi: 10.1021/acs.organomet.6b00440. [DOI] [Google Scholar]
- 21.Wambang N., Schifano-Faux N., Aillerie A., Baldeyrou B., Jacquet C., Bal-Mahieu C., Bousquet T., Pellegrini S., Ndifon T.P., Meignan S., et al. Synthesis and biological activity of ferrocenyl indeno[1,2-c]isoquinolines as topoisomerase II inhibitors. Bioorg. Med. Chem. 2016;24:651–660. doi: 10.1016/j.bmc.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Komeda S., Casini A. Next-generation anticancer metallodrug. Curr. Top. Med. Chem. 2012;12:219–235. doi: 10.2174/156802612799078964. [DOI] [PubMed] [Google Scholar]
- 23.Mejía C., Ortega-Rosales S., Ruiz-Azuara L. Mechanism of action of anticancer metallodrugs. In: Rai M., Ingle A., Medici S., editors. Biomedical Applications of Metals. Volume 10. Springer; Berlin/Heidelberg, Germany: 2018. pp. 213–234. [Google Scholar]
- 24.Denoyer D., Clatworthy S.A.S., Cater M.A. Copper complexes in cancer therapy. Met. Ions Life Sci. 2018;18:469–506. doi: 10.1515/9783110470734-022. [DOI] [PubMed] [Google Scholar]
- 25.Santini C., Pellei M., Gandin V., Porchia M., Tisato F., Marzano C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014;114:815–862. doi: 10.1021/cr400135x. [DOI] [PubMed] [Google Scholar]
- 26.Jungwirth U., Kowol C.R., Keppler B.K., Hartinger C.G., Berger W., Heffeter P. Anticancer activity of metal complexes: Involvement of redox processes. Antioxid. Redox Signal. 2011;15:1085–1127. doi: 10.1089/ars.2010.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tardito S., Marchiò L. Copper compounds in anticancer strategies. Curr. Med. Chem. 2009;16:1325–1348. doi: 10.2174/092986709787846532. [DOI] [PubMed] [Google Scholar]
- 28.Marzano C., Pellei M., Tisato F., Santini C. Copper complexes as anticancer agents. Anticancer Agents Med. Chem. 2009;9:185–211. doi: 10.2174/187152009787313837. [DOI] [PubMed] [Google Scholar]
- 29.Kellett A., Molphy Z., McKee V., Slator C. Recent advances in anticancer copper compounds. In: Vessieres I.A., Meier-Menches S.M., Casini A., editors. Metal-Based Anticancer Agents. Volume 14. Royal Society of Chemistry, RSC Metallobiology; London, UK: 2019. pp. 91–119. [Google Scholar]
- 30.Hordyjewska A., Popiołek L., Kocot J. The many “faces” of copper in medicine and treatment. Biometals. 2014;27:611–621. doi: 10.1007/s10534-014-9736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urso E., Maffia M. Behind the link between copper and angiogenesis: Established mechanisms and an overview on the role of vascular copper transport systems. J. Vasc. Res. 2015;52:172–196. doi: 10.1159/000438485. [DOI] [PubMed] [Google Scholar]
- 32.Lowndes S.A., Harris A.L. The role of copper in tumour angiogenesis. J. Mammary Gland. Biol. Neoplasia. 2005;10:299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 33.Hu G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998;69:326–335. doi: 10.1002/(SICI)1097-4644(19980601)69:3<326::AID-JCB10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida D., Ikeda Y., Nakazawa S. Quantitative analysis of copper, zinc and copper/zinc ratio in selected human brain tumors. J. Neurooncol. 1993;16:109–115. doi: 10.1007/BF01324697. [DOI] [PubMed] [Google Scholar]
- 35.Geraki K., Farquharson M.J., Bradley D.A. Concentrations of Fe, Cu and Zn in breast tissue: A synchrotron XRF study. Phys. Med. Biol. 2002;47:2327–2339. doi: 10.1088/0031-9155/47/13/310. [DOI] [PubMed] [Google Scholar]
- 36.Nayak S.B., Bhat V.R., Upadhyay D., Udupa S.L. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian J. Physiol. Pharm. 2003;47:108–110. [PubMed] [Google Scholar]
- 37.Díez M., Arroyo M., Cerdàn F.J., Muñoz M., Martin M.A., Balibrea J.L. Serum and tissue trace metal levels in lung cancer. Oncology. 1989;46:230–234. doi: 10.1159/000226722. [DOI] [PubMed] [Google Scholar]
- 38.Kaiafa G.D., Saouli Z., Diamantidis M.D., Kontoninas Z., Voulgaridou V., Raptaki M., Arampatzi S., Chatzidimitriou M., Perifanis V. Copper levels in patients with hematological malignancies. Eur. J. Intern. Med. 2012;23:738–741. doi: 10.1016/j.ejim.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Baldari S., Di Rocco G., Toietta G. Current biomedical use of copper chelation therapy. Int. J. Mol. Sci. 2020;21:1069. doi: 10.3390/ijms21031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denoyer D., Masaldan S., La Fontaine S., Cater M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics. 2015;7:1459–1476. doi: 10.1039/C5MT00149H. [DOI] [PubMed] [Google Scholar]
- 41.Cater M.A., Pearson H.B., Wolyniec K., Klaver P., Bilandzic M., Paterson B.M., Bush A.I., Humbert P.O., La Fontaine S., Donnelly P.S., et al. Increasing intracellular bioavailable copper selectively targets prostate cancer cells. ACS Chem. Biol. 2013;8:1621–1631. doi: 10.1021/cb400198p. [DOI] [PubMed] [Google Scholar]
- 42.Cater M.A., Haupt Y. Clioquinol induces cytoplasmic clearance of the X-linked inhibitor of apoptosis protein (XIAP): Therapeutic indication for prostate cancer. Biochem. J. 2011;436:481–491. doi: 10.1042/BJ20110123. [DOI] [PubMed] [Google Scholar]
- 43.Cheriyan V.T., Wang Y., Muthu M., Jamal S., Chen D., Yang H., Polin L.A., Tarca A.L., Pass H.I., Dou Q.P., et al. Disulfiram suppresses growth of the malignant pleural mesothelioma cells in part by inducing apoptosis. PLoS ONE. 2014;9:e93711. doi: 10.1371/journal.pone.0093711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan L., Shen H., Zhao G., Yang R., Cai X., Zhang L., Jin C., Huang Y. Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2014;446:1010–1016. doi: 10.1016/j.bbrc.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 45.Jivan R., Damelin L.H., Birkhead M., Rousseau A.L., Veale R.B., Mavri-Damelin D. Disulfiram/copper-disulfiram damages multiple protein degradation and turnover pathways and cytotoxicity is enhanced by metformin in oesophageal squamous cell carcinoma cell lines. J. Cell. Biochem. 2015;116:2334–2343. doi: 10.1002/jcb.25184. [DOI] [PubMed] [Google Scholar]
- 46.Safi R., Nelson E.R., Chitneni S.K., Franz K.J., George D.J., Zalutsky M.R., McDonnell D.P. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014;74:5819–5831. doi: 10.1158/0008-5472.CAN-13-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F., Jiao P., Qi M., Frezza M., Dou Q.P., Yan B. Turning tumor-promoting copper into an anti-cancer weapon via high-throughput chemistry. Curr. Med. Chem. 2010;17:2685–2698. doi: 10.2174/092986710791859315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z., Wang H., Yan M., Wang H., Zhang C. Novel copper complexes as potential proteasome inhibitors for cancer treatment. Mol. Med. Rep. 2017;15:3–11. doi: 10.3892/mmr.2016.6022. [DOI] [PubMed] [Google Scholar]
- 49.Krasnovskaya O., Naumov A., Guk D., Gorelkin P., Erofeev A., Beloglazkina E., Majouga A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020;21:3965. doi: 10.3390/ijms21113965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brissos R.F., Caubet A., Gamez P. Possible DNA-interacting pathways for metal-based compounds exemplified with copper coordination compounds. Eur. J. Inorg. Chem. 2015;16:2633–2645. doi: 10.1002/ejic.201500175. [DOI] [Google Scholar]
- 51.Kagawa T.F., Geierstanger B.H., Wang A.H.J., Ho P.S. Covalent modification of guanine bases in double-stranded DNA. J. Biol. Chem. 1991;266:20175–20184. doi: 10.2210/pdb1d39/pdb. [DOI] [PubMed] [Google Scholar]
- 52.Ceramella J., Mariconda A., Iacopetta D., Saturnino C., Barbarossa A., Caruso A., Rosano C., Sinicropi M.S., Longo P. From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg. Med. Chem. Lett. 2020;30:126905–126916. doi: 10.1016/j.bmcl.2019.126905. [DOI] [PubMed] [Google Scholar]
- 53.Shobha Devi C., Thulasiram B., Aerva R.R., Nagababu P. Recent advances in copper intercalators as anticancer agents. J. Fluoresc. 2018;28:1195–1205. doi: 10.1007/s10895-018-2283-7. [DOI] [PubMed] [Google Scholar]
- 54.Liang X., Wu Q., Luan S., Yin Z., He C., Yin L., Zou Y., Yuan Z., Li L., Song X., et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur. J. Med. Chem. 2019;171:129–168. doi: 10.1016/j.ejmech.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 55.Cuya S.M., Bjornsti M.A., van Waardenburg R.C.A.M. DNA topoisomerase-targeting chemotherapeutics: What’s new? Cancer Chemother. Pharmacol. 2017;80:1–14. doi: 10.1007/s00280-017-3334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas A., Pommier Y. Targeting topoisomerase I in the era of precision medicine. Clin. Cancer Res. 2019;25:6581–6589. doi: 10.1158/1078-0432.CCR-19-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pommier Y. Drugging topoisomerases: Lessons and challenges. ACS Chem. Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjornsti M.A., Kaufmann S.H. Topoisomerases and cancer chemotherapy: Recent advances and unanswered questions. F1000 Res. 2019;8 doi: 10.12688/f1000research.20201.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pommier Y., Sun Y., Huang S.N., Nitiss J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell. Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pommier Y., Leo E., Zhang H., Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hevener K., Verstak T.A., Lutat K.E., Riggsbee D.L., Mooney J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B. 2018;8:844–861. doi: 10.1016/j.apsb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y., Her C. Inhibition of Topoisomerase (DNA) I (TOP1): DNA damage repair and anticancer therapy. Biomolecules. 2015;5:1652. doi: 10.3390/biom5031652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J.C. Interaction between DNA and an Escherichia coli protein omega. J. Mol. Biol. 1971;55:523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- 64.Madabhushi R. The roles of DNA topoisomerase IIβ in transcription. Int. J. Mol. Sci. 2018;19:1917. doi: 10.3390/ijms19071917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakasai R., Iwabuchi K. The distinctive cellular responses to DNA strand breaks caused by a DNA topoisomerase I poison in conjunction with DNA replication and RNA transcription. Genes Genet. Syst. 2016;90:187–194. doi: 10.1266/ggs.15-00023. [DOI] [PubMed] [Google Scholar]
- 66.Lee J.H., Berger J.M. Cell cycle-dependent control and roles of DNA topoisomerase II. Genes. 2019;10:859. doi: 10.3390/genes10110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M., Liu Y. Topoisomerase I in human disease pathogenesis and treatments. Genom. Proteom. Bioinform. 2016;14:166–171. doi: 10.1016/j.gpb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen A.K., Escargueil A.E., Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharm. Ther. 2003;99:167–181. doi: 10.1016/S0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 69.Hu W., Huang X.S., Wu J.F., Yang L., Zheng Y.T., Shen Y.M., Li Z.Y., Li X. Discovery of novel topoisomerase II inhibitors by medicinal chemistry approaches. J. Med. Chem. 2018;61:8947–8980. doi: 10.1021/acs.jmedchem.7b01202. [DOI] [PubMed] [Google Scholar]
- 70.Castelli S., Goncalves M.B., Katkar P., Stuchi G.C., Couto R.A.A., Petrilli H.M., da Costa Ferreira A.M. Comparative studies of oxindolimine-metal complexes as inhibitors of human DNA topoisomerase IB. J. Inorg. Biochem. 2018;186:85–94. doi: 10.1016/j.jinorgbio.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Katkar P., Coletta A., Castelli S., Sabino G.L., Alves Couto R.A., da Costa Ferreira A.M., Desideri A. Effect of oxindolimine copper(ii) and zinc(ii) complexes on human topoisomerase I activity. Metallomics. 2014;6:117–125. doi: 10.1039/C3MT00099K. [DOI] [PubMed] [Google Scholar]
- 72.Cerchiaro G., Aquilano K., Filomeni G., Rotilio G., Ciriolo M.R., Ferreira A.M. Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J. Inorg. Biochem. 2005;99:1433–1440. doi: 10.1016/j.jinorgbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Filomeni G., Cerchiaro G., Da Costa Ferreira A.M., De Martino A., Pedersen J.Z., Rotilio G., Ciriolo M.R. Pro-apoptotic activity of novel Isatin-Schiff base copper(II) complexes depends on oxidative stress induction and organelle-selective damage. J. Biol. Chem. 2007;282:12010–12021. doi: 10.1074/jbc.M610927200. [DOI] [PubMed] [Google Scholar]
- 74.Chew S.T., Lo K.M., Lee S.K., Heng M.P., Teoh W.Y., Sim K.S., Tan K.W. Copper complexes with phosphonium containing hydrazone ligand: Topoisomerase inhibition and cytotoxicity study. Eur. J. Med. Chem. 2014;76:397–407. doi: 10.1016/j.ejmech.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 75.Chen Z.F., Tan M.X., Liu L.M., Liu Y.C., Wang H.S., Yang B., Peng Y., Liu H.G., Liang H., Orvig C. Cytotoxicity of the traditional chinese medicine (tcm) plumbagin in its copper chemistry. Dalton Trans. 2009;48:10824–10833. doi: 10.1039/b910133k. [DOI] [PubMed] [Google Scholar]
- 76.Seng H.L., Wang W.S., Kong S.M., Alan Ong H.K., Win Y.F., Raja Abd Rahman R.N.Z., Chikira M., Leong W.K., Ahmad M., Khoo A.S.B., et al. Biological and cytoselective anticancer properties of copper(II)-polypyridyl complexes modulated by auxiliary methylated glycine ligand. BioMetals. 2012;25:1061–1081. doi: 10.1007/s10534-012-9572-4. [DOI] [PubMed] [Google Scholar]
- 77.Ikotun O.F., Higbee E.M., Ouellette W., Doyle R.P. Pyrophosphate-bridged complexes with picomolar toxicity. J. Inorg. Biochem. 2009;103:1254–1264. doi: 10.1016/j.jinorgbio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Tabassum S., Afzal M., Arjmand F. Synthesis of heterobimetallic complexes: In vitro DNA binding, cleavage and antimicrobial studies. J. Photochem. Photobiol. B Biol. 2012;114:108–118. doi: 10.1016/j.jphotobiol.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 79.Chauhan M., Banerjee K., Arjmand F. DNA binding studies of novel copper(ii) complexes containing l-tryptophan as chiral auxiliary: in vitro antitumor activity of cu−sn2 complex in human neuroblastoma cells. Inorg. Chem. 2007;46:3072–3082. doi: 10.1021/ic061753a. [DOI] [PubMed] [Google Scholar]
- 80.Afzal M., Al-Lohedan H.A., Usman M., Tabassum S. Carbohydrate-based heteronuclear complexes as topoisomerase Iα inhibitor: Approach toward anticancer chemotherapeutics. J. Biomol. Struct. Dyn. 2019;37:1494–1510. doi: 10.1080/07391102.2018.1459321. [DOI] [PubMed] [Google Scholar]
- 81.Tabassum S., Ahmad A., Khan R.A., Hussain Z., Srivastav S., Srikrishna S., Arjmand F. Chiral heterobimetallic complexes targeting human DNA-topoisomerase Iα. Dalton Trans. 2013;42:16749–16761. doi: 10.1039/c3dt51209f. [DOI] [PubMed] [Google Scholar]
- 82.Tabassum S., Asim A., Khan R.A., Arjmand F., Rajakumar D., Balaji P., Akbarsha A.M. A multifunctional molecular entity CuII–SnIV heterobimetallic complex as a potential cancer chemotherapeutic agent: DNA binding/cleavage, SOD mimetic, topoisomerase Ia inhibitory and in vitrocytotoxic activities. RSC Adv. 2015;5:47439–47450. doi: 10.1039/C5RA07333B. [DOI] [Google Scholar]
- 83.Lee S.K., Tan K.W., Ng S.W. Zinc, copper and nickel derivatives of 2-[2-bromoethyliminomethyl]phenol as topoisomerase inhibitors exhibiting anti-proliferative and antimetastatic properties. RSC Adv. 2014;4:60280–60292. doi: 10.1039/C4RA09256B. [DOI] [Google Scholar]
- 84.Lee S.K., Tan K.W., Ng S.W. Topoisomerase I inhibition and DNA cleavage by zinc, copper, and nickel derivatives of 2-[2-bromoethyliminomethyl]-4-[ethoxymethyl]phenol complexes exhibiting anti-proliferation and anti-metastasis activity. J. Inorg. Biochem. 2016;159:14–21. doi: 10.1016/j.jinorgbio.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Vutey V., Castelli S., D’Annessa I., Sâmia L.B., Souza-Fagundes E.M., Beraldo H., Desideri A. Human topoisomerase IB is a target of a thiosemicarbazone copper(II) complex. Arch. Biochem. Biophys. 2016;606:34–40. doi: 10.1016/j.abb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Haleel A.K., Mahendiran D., Rafi U.M., Veena V., Shobana S., Rahiman A.K. Tetrazolo[1,5-a]pyrimidine-based metal(II) complexes as therapeutic agents: DNA interaction, targeting topoisomerase I and cyclin-dependent kinase studies. Inorg. Nano Met. Chem. 2019;48:569–582. doi: 10.1080/24701556.2019.1571514. [DOI] [Google Scholar]
- 87.Haleel A.K., Mahendiran D., Veena V., Sakthivel N., Rahiman A.K. Antioxidant, DNA interaction, VEGFR2 kinase, topoisomerase I and in vitro cytotoxic activities of heteroleptic copper(II) complexes of tetrazolo[1,5-a]pyrimidines and diimines. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;68:366–382. doi: 10.1016/j.msec.2016.05.120. [DOI] [PubMed] [Google Scholar]
- 88.Tabassum S., Al-Asbahy W.M., Afzal M., Arjmand F., Bagchi V. Molecular drug design, synthesis and structure elucidation of a new specific target peptide based metallo drug for cancer chemotherapy as topoisomerase I inhibitor. Dalton Trans. 2012;41:4955–4964. doi: 10.1039/c2dt12044e. [DOI] [PubMed] [Google Scholar]
- 89.Hasinoff B.B., Wu X., Yadav A.A., Patel D., Zhang H., Wang D.S., Chen Z.S., Yalowich J.C. Cellular mechanisms of the cytotoxicity of the anticancer drug elesclomol and its complex with Cu(II) Biochem. Pharm. 2015;93:266–276. doi: 10.1016/j.bcp.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 90.Foo J.B., Ng L.S., Lim J.H., Tan P.X., Lor Y.Z., Loo J.S., Low M.L., Chan L.C., Beh C.Y., Leong S.W., et al. Induction of cell cycle arrest and apoptosis by copper complex Cu(SBCM)2 towards oestrogen-receptor positive MCF-7 breast cancer cells. RSC Adv. 2019;9:18359–18370. doi: 10.1039/C9RA03130H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foo J.B., Low M.L.L., Lim J.H., Lor Y.Z., Abidin R.Z., Dam V.E., Rahman N.A., Beh C.Y., Chan L.C., How C.W., et al. Copper complex derived fromS-benzyldithiocarbazate and 3-acetylcoumarin induced apoptosis in breast cancer cell. Biometals. 2018;4:505–515. doi: 10.1007/s10534-018-0096-4. [DOI] [PubMed] [Google Scholar]
- 92.Yeh E.T.H., Ewer M., Moslehi J., Dlugosz-Danecka M., Banchs J., Chang H.M., Minotti G. Mechanisms and clinical course of cardiovascular toxicity of cancer treatment I. Oncology. Semin. Oncol. 2019;46:397–402. doi: 10.1053/j.seminoncol.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Pendleton M., Lindsey R.H., Jr., Felix C.A., Grimwade D., Osheroff N. Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 2014;1310:98–110. doi: 10.1111/nyas.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.West D.X., Thientanavanich I., Liberta A.E. Copper(II) complexes of 6-methyl-2-acetylpyridine N(4)-substituted thiosemicarbazones. Trans. Met. Chem. 1995;20:303–308. doi: 10.1007/BF00143498. [DOI] [Google Scholar]
- 95.Miller M.C., Bastow K.F., Stineman C.N., Vance J.R., Song S.C., West D.X., Hall I.H. The Cytotoxicity of 2-Formyl and 2-Acetyl-(6-picolyl)-4 N-Substituted Thiosemicarbazones and Their Copper(II) Complexes. Arch. Pharm. Pharm. Med. Chem. 1998;331:121–127. doi: 10.1002/(SICI)1521-4184(199804)331:4<121::AID-ARDP121>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 96.Khan T., Rahmad R., Joshi S., Khan A.R. Anticancer potential of metal thiosemicarbazone complexes: A review. Chem. Sin. 2015;6:1–11. [Google Scholar]
- 97.Huang H., Chen Q., Xin K., Meng L., Lin L., Wang X., Zhu C., Wang Y., Chen Z., Li M., et al. A series of α-heterocyclic carboxaldehyde thiosemicarbazones inhibit topoisomerase IIα catalytic activity. J. Med. Chem. 2010;53:3048–3064. doi: 10.1021/jm9014394. [DOI] [PubMed] [Google Scholar]
- 98.Conner J.D., Medawala W., Stephens M.T., Morris W.H., Deweese J.E., Kent P.L., Rice J.J., Jiang X., Lisic E.C. Cu(II) benzoylpyridine thiosemicarbazone complexes: Inhibition of human topoisomerase IIα and activity against breast cancer cells. Open J. Inorg. Chem. 2016;6:146–154. doi: 10.4236/ojic.2016.62010. [DOI] [Google Scholar]
- 99.Wilson J.T., Jiang X., McGill B.C., Lisic E.C., Deweese J.E. Examination of the impact of copper(ii) α-(n)-heterocyclic thiosemicarbazone complexes on dna topoisomerase IIα. Chem. Res. Toxicol. 2016;29:649–658. doi: 10.1021/acs.chemrestox.5b00471. [DOI] [PubMed] [Google Scholar]
- 100.Keck J.M., Conner J.D., Wilson J.T., Jiang X., Lisic E.C., Deweese J.E. Clarifying the mechanism of copper(II) α-(N)-heterocyclic thiosemicarbazone complexes on DNA topoisomerase IIα and IIβ. Chem. Res. Toxicol. 2019;32:2135–2143. doi: 10.1021/acs.chemrestox.9b00311. [DOI] [PubMed] [Google Scholar]
- 101.Miller M.C., Stineman C.N., Vance J.R., West D.X. Multiple Mechanisms for Cytotoxicity Induced by Copper(II) Complexes of 2-Acetylpyrazine-N-substituted Thiosemicarbazones. Appl. Organometal. Chem. 1999;13:9–19. doi: 10.1002/(SICI)1099-0739(199901)13:1<9::AID-AOC818>3.0.CO;2-#. [DOI] [Google Scholar]
- 102.Zeglis B.M., Divilov V., Lewis J.S. Role of metalation in the topoisomerase IIα inhibition and antiproliferation activity of a series of α-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes. J. Med. Chem. 2011;54:2391–2398. doi: 10.1021/jm101532u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lisic E.C., Rand V.G., Ngo L., Kent P., Rice J., Gerlach D., Papish E.T., Jiang X. Cu(II) propionyl-thiazole thiosemicarbazone complexes: Crystal structure, inhibition of human topoisomerase IIα, and activity against breast cancer cells. Open J. Med. Chem. 2018;8:30–46. doi: 10.4236/ojmc.2018.82004. [DOI] [Google Scholar]
- 104.Morris W.H., Ngo L., Wilson J.T., Medawala W., Brown A.R., Conner J.D., Fabunmi F., Cashman D.J., Lisic E., Yu T., et al. Structural and metal ion effects on human topoisomerase IIα inhibition by α-(N)-heterocyclic thiosemicarbazones. Chem. Res. Toxicol. 2019;32:90–99. doi: 10.1021/acs.chemrestox.8b00204. [DOI] [PubMed] [Google Scholar]
- 105.Sandhaus S., Taylor R., Edwards T., Huddleston A., Wooten Y., Venkatraman R., Weber R.T., González-Sarrías A., Martin P.M., Cagle P., et al. A novel copper(II) complex identified as a potent drug against colorectal and breast cancer cells and as a poison inhibitor for human topoisomerase IIα. Inorg. Chem. Commun. 2016;64:45–49. doi: 10.1016/j.inoche.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bacher F., Enyedy É., Nagy N.V., Rockenbauer A., Bognár G.M., Trondl R., Novak M.S., Klapproth E., Kiss T., Arion V.B. Copper(II) complexes with highly water-soluble L- and D-proline-thiosemicarbazone conjugates as potential inhibitors of Topoisomerase IIα. Inorg. Chem. 2013;52:8895–8908. doi: 10.1021/ic401079w. [DOI] [PubMed] [Google Scholar]
- 107.Bisceglie F., Musiari A., Pinelli S., Alinovi R., Menozzi I., Polverini E., Tarasconi P., Tavone M., Pelosi G. Quinoline-2-carboxaldehyde thiosemicarbazones and their Cu(II) and Ni(II) complexes as topoisomerase IIa inhibitors. J. Inorg. Biochem. 2015;152:10–19. doi: 10.1016/j.jinorgbio.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 108.Chen J., Huang Y.W., Liu G., Afrasiabi Z., Sinn E., Padhye S., Ma Y. The cytotoxicity and mechanisms of 1,2-naphthoquinone thiosemicarbazone and its metal derivatives against MCF-7 human breast cancer cells. Toxicol. Appl. Pharm. 2004;197:40–48. doi: 10.1016/j.taap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Palanimuthu D., Shinde S.V., Somasundaram K., Samuelson A.G. In vitro and in vivo anticancer activity of copper bis(thiosemicarbazone) complexes. J. Med. Chem. 2013;56:722–734. doi: 10.1021/jm300938r. [DOI] [PubMed] [Google Scholar]
- 110.Nair R.S., Potti M.E., Thankappan R., Chandrika S.K., Kurup M.R., Srinivas P. Molecular trail for the anticancer behavior of a novel copper carbohydrazone complex in BRCA1 mutated breast cancer. Mol. Carcinog. 2017;56:1501–1514. doi: 10.1002/mc.22610. [DOI] [PubMed] [Google Scholar]
- 111.Arjmand F., Jamsheera A., Afzal M., Tabassum S. Enantiomeric specificity of biologically significant Cu(II) and Zn(II) chromone complexes towards DNA. Chirality. 2012;24:977–986. doi: 10.1002/chir.22081. [DOI] [PubMed] [Google Scholar]
- 112.Duff B., Thangella V.R., Creaven B.S., Walsh M., Egan D.A. Anti-cancer activity and mutagenic potential of novel copper(II) quinolinone Schiff base complexes in hepatocarcinoma cells. Eur. J. Pharm. 2012;689:45–55. doi: 10.1016/j.ejphar.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 113.Khan R.A., Usman M., Dhivya R., Balaji P., Alsalme A., AlLohedan H., Arjmand F., AlFarhan K., Akbarsha M.A., Marchetti F., et al. Heteroleptic copper(I) complexes of “scorpionate” bis-pyrazolyl carboxylate ligand with auxiliary phosphine as potential anticancer agents: An insight into cytotoxic mode. Sci. Rep. 2017;7:45229–45246. doi: 10.1038/srep45229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ross W., Rowe T., Glisson B., Yalowich J., Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984;44:5857–5860. [PubMed] [Google Scholar]
- 115.Furuta T., Takemura H., Liao Z.Y., Aune G.J., Redon C., Sedelnikova O.A., Pilch D.R., Rogakou E.P., Celeste A., Chen H.T., et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 116.Bonner W.M., Redon C.E., Dickey J.S., Nakamura A.J., Sedelnikova O.A., Solier S., Pommier Y. Gamma H2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sordet O., Redon E.C., Guirouilh-Barbat J., Smith S., Solier S., Douarre C., Conti C., Nakamura J.A., Das B.B., Nicolas E., et al. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 2009;10:887–893. doi: 10.1038/embor.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pilié P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hakem R. DNA-damage repair; the good, the bad and the ugly. EMBO J. 2008;27:589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Donzelli M., Draetta F.G. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 2003;4:671–677. doi: 10.1038/sj.embor.embor887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Banin S., Moyal L., Shieh S., Taya Y., Anderson C.W., Chessa L., Smorodinsky N.I., Prives C., Reiss Y., Shiloh Y., et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;28:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 122.Canman C.E., Lim D.S., Cimprich K.A., Taya Y., Tamai K., Sakaguchi K., Appella E., Kastan M.B., Siliciano J.D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 123.Hirao A., Kong Y.Y., Matsuoka S., Wakeham A., Ruland J., Yoshida H., Liu D., Elledge S.J., Mak T.W. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 124.Shieh S.Y., Ahn J., Tamai K., Taya Y., Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 125.Xiong Y., Hannon G.J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 126.Hengstschläger M., Braun K., Soucek T., Miloloza A., Hengstschläger-Ottnad E. Cyclin-dependent kinases at the G1-S transition of the mammalian cell cycle. Mutat. Res. 1999;436:1–9. doi: 10.1016/S1383-5742(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 127.Xiao D., Herman-Antosiewicz A., Antosiewicz J., Xiao H., Brisson M., Lazo J.S., Singh S.V. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc 25 C. Oncogene. 2005;24:6256–6268. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- 128.Okoh V.O., Garba N.A., Penney R.B., Das J., Deoraj A., Singh K.P., Sarkar S., Felty Q., Yoo C., Jackson R.M., et al. Redox signalling to nuclear regulatory proteins by reactive oxygen species contributes to oestrogen-induced growth of breast cancer cells. Br. J. Cancer. 2015;112:1687–1702. doi: 10.1038/bjc.2014.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Macip S., Kosoy A., Lee S.W., O’Connell M.J., Aaronson S.A. Oxidative stress induces a prolonged but reversible arrest in p53-null cancer cells, involving a Chk1-dependent G2 checkpoint. Oncogene. 2006;25:6037–6047. doi: 10.1038/sj.onc.1209629. [DOI] [PubMed] [Google Scholar]
- 130.He L., Nan M.H., Oh H.C., Kim Y.H., Jang J.H., Erikson R.L., Ahn J.S., Kim B.Y. Asperlin induces G2/M arrest through ROS generation and ATM pathway in human cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2011;409:489–493. doi: 10.1016/j.bbrc.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 131.Tubbs A., Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168:644–656. doi: 10.1016/j.cell.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deiss K., Lockwood N., Howell M., Segeren H.A., Saunders R.E., Chakravarty P., Soliman T.N., Martini S., Rocha N., Semple R., et al. A genome-wide RNAi screen identifies the SMC5/6 complex as a non-redundant regulator of a Topo2a-dependent G2 arrest. Nucleic Acids Res. 2019;47:2906–2921. doi: 10.1093/nar/gky1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bower J.J., Zhou Y., Zhou T., Simpson D.A., Arlander S.J., Paules R.S., Cordeiro-Stone M., Kaufmann W.K. Revised genetic requirements for the decatenation G2 checkpoint: The role of ATM. Cell Cycle. 2010;9:1617–1628. doi: 10.4161/cc.9.8.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bower J.J., Karaca G.F., Zhou Y., Simpson D.A., Cordeiro-Stone M., Kaufmann W.K. Topoisomerase IIalpha maintains genomic stability through decatenation G(2) checkpoint signaling. Oncogene. 2010;29:4787–4799. doi: 10.1038/onc.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoshida C., Hishiyama K., Miyazaki K., Watanabe M., Kanbe M., Yamada Y., Matsuzaki K., Miyashita K., Kitanaka S., Miyata S. Analysis of inhibition of topoisomerase IIalpha and cancer cell proliferation by ingenolEZ. Cancer Sci. 2010;101:374–378. doi: 10.1111/j.1349-7006.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dykhuizen E.C., Hargreaves D.C., Miller E.L., Cui K., Korshunov A., Kool M., Pfister S., Cho Y.J., Zhao K., Crabtree G.R. BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature. 2013;497:624–627. doi: 10.1038/nature12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.D Arcy N., Gabrielli B. Topoisomerase II inhibitors and poisons, and the influence of cell cycle checkpoints. Curr. Med. Chem. 2017;24:1504–1519. doi: 10.2174/0929867323666161205122613. [DOI] [PubMed] [Google Scholar]
- 138.Hjaltelin J.X., Izarzugaza J., Jensen L.J., Russo F., Westergaard D., Brunak S. Identification of hyper-rewired genomic stress non-oncogene addiction genes across 15 cancer types. NPJ Syst. Biol. Appl. 2019;5:27–37. doi: 10.1038/s41540-019-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Al-Matouq J., Holmes T.R., Hansen L.A. CDC25B and CDC25C overexpression in nonmelanoma skin cancer suppresses cell death. Mol. Carcinog. 2019;58:1691–1700. doi: 10.1002/mc.23075. [DOI] [PubMed] [Google Scholar]
- 140.Butz H., Németh K., Czenke D., Likó I., Czirják S., Zivkovic V., Baghy K., Korbonits M., Kovalszky I., Igaz P., et al. Systematic investigation of expression of G2/M transition genes reveals CDC25 alteration in nonfunctioning pituitary adenomas. Pathol. Oncol. Res. 2017;23:633–641. doi: 10.1007/s12253-016-0163-5. [DOI] [PubMed] [Google Scholar]
- 141.Mantovani F., Collavin L., Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayman L., Chaudhry W.R., Revin V.V., Zhelev N., Bourdon J.C. What is the potential of p53 isoforms as a predictive biomarker in the treatment of cancer? Expert Rev. Mol. Diagn. 2019;19:149–159. doi: 10.1080/14737159.2019.1563484. [DOI] [PubMed] [Google Scholar]
- 143.Lin Z.P., Zhu Y.L., Ratner E.S. Targeting cyclin-dependent kinases for treatment of gynecologic cancers. Front. Oncol. 2018;8:303–314. doi: 10.3389/fonc.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roskoski R., Jr. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharm. Res. 2019;139:471–488. doi: 10.1016/j.phrs.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 145.Kachalaki S., Ebrahimi M., Mohamed Khosroshahi L., Mohammadinejad S., Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: A brief overview. Eur. J. Pharm. Sci. 2016;89:20–30. doi: 10.1016/j.ejps.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 146.Gongora C., Vezzio-Vie N., Tuduri S., Denis V., Causse A., Auzanneau C., Collod-Beroud G., Coquelle A., Pasero P., Pourquier P., et al. New Topoisomerase I mutations are associated with resistance to camptothecin. Mol. Cancer. 2011;10:64–77. doi: 10.1186/1476-4598-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsurutani J., Nitta T., Hirashima T., Komiya T., Uejima H., Tada H., Syunichi N., Tohda A., Fukuoka M., Nakagawa K. Point mutations in the topoisomerase I gene in patients with non-small cell lung cancer treated with irinotecan. Lung Cancer. 2002;35:299–304. doi: 10.1016/S0169-5002(01)00425-1. [DOI] [PubMed] [Google Scholar]
- 148.Bassermann F., Eichner R., Pagano M. The ubiquitin proteasome system–implications for cell cycle control and the targeted treatment of cancer. Biochim. Biophys. Acta. 2014;1843:150–162. doi: 10.1016/j.bbamcr.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee K.C., Swan R.L., Sondka Z., Padget K., Cowell I.G., Austin C.A. Effect of TDP2 on the Level of TOP2-DNA Complexes and SUMOylated TOP2-DNA Complexes. Int. J. Mol. Sci. 2018;19:2056. doi: 10.3390/ijms19072056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gardner L., Malik R., Shimizu Y., Mullins N., ElShamy W.M. Geminin overexpression prevents the completion of topoisomerase IIα chromosome decatenation, leading to aneuploidy in human mammary epithelial cells. Breast Cancer Res. 2011;13:R53. doi: 10.1186/bcr2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rozav A.G., Chikamori K., Kozuki T., Grabowski D.R., Bukowski R.M., Willard B., Kinter M., Andersen A.H., Ganapathi R., Ganapathi M.K. Casein kinase I delta phosphorylates topoisomerase II at serine-1106 and modulates DNA cleavage activity. Nucleic Acids Res. 2009;37:382–392. doi: 10.1093/nar/gkn934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kurz E.U., Leader K.B., Kroll D.J., Clark M., Gieseler F. Modulation of human DNA topoisomerase II function by interaction with 14–3-3”. J. Biol. Chem. 2000;275:13948–13954. doi: 10.1074/jbc.275.18.13948. [DOI] [PubMed] [Google Scholar]
- 153.Visconti R., Della Monica R., Grieco D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016;35:153–161. doi: 10.1186/s13046-016-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Allday M.J., Inman G.J., Crawford D.H., Farrell P.J. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 1995;14:4994–5005. doi: 10.1002/j.1460-2075.1995.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vitale I., Galluzzi L., Castedo M., Kroemer G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 156.Yazinski S.A., Comaills V., Buisson R., Genois M.M., Nguyen H.D., Ho C.K., Todorova Kwan T., Morris R., Lauffer S., Nussenzweig A., et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev. 2017;31:318–332. doi: 10.1101/gad.290957.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Su Z., Yang Z., Xu Y., Chen Y., Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer. 2015;14:48–62. doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pfeffer C.M., Singh A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee S.Y., Ju M.K., Jeon H.M., Jeong E.K., Lee Y.J., Kim C.H., Park H.G., Han S.I., Kang H.S. Regulation of tumor progression by programmed necrosis. Oxid. Med. Cell. Longev. 2018;2018:3537471–3537499. doi: 10.1155/2018/3537471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eguchi Y., Shimizu S., Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 162.Vakkila J., Lotze M.T. Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 163.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., Huang J., Yu Y., Fan X.G., Yan Z., et al. MGB1 in health and disease. Mol. Asp. Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomes L.R., Menck C.F.M., Leandro G.S. Autophagy roles in the modulation of DNA repair pathways. Int. J. Mol. Sci. 2017;18:2351. doi: 10.3390/ijms18112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang Y., Li C., Fu Y., Liu Y., Zhang Y., Zhang Y., Zhou P., Yuan Y., Zhou S., Li S., et al. Redox cycling of a copper complex with benzaldehyde nitrogen mustard-2-pyridine carboxylic acid hydrazone contributes to its enhanced antitumor activity, but no change in the mechanism of action occurs after chelation. Oncol. Rep. 2016;3:1636–1644. doi: 10.3892/or.2015.4530. [DOI] [PubMed] [Google Scholar]
- 166.Chen C., Lu L., Yan S., Yi H., Yao H., Wu D., He G., Tao X., Deng X. Autophagy and doxorubicin resistance in cancer. Anticancer Drugs. 2018;29:1–9. doi: 10.1097/CAD.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 167.Delgado J.L., Hsieh C.M., Chan N.L., Hiasa H. Topoisomerases as anticancer targets. Biochem. J. 2018;475:373–398. doi: 10.1042/BCJ20160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dehshahri A., Ashrafizadeh M., Ghasemipour Afshar E., Pardakhty A., Mandegary A., Mohammadinejad R., Sethi G. Topoisomerase inhibitors: Pharmacology and emerging nanoscale delivery systems. Pharm. Res. 2020;151:104551–104563. doi: 10.1016/j.phrs.2019.104551. [DOI] [PubMed] [Google Scholar]
- 169.Fujita K., Kubota Y., Ishida H., Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015;21:12234–12248. doi: 10.3748/wjg.v21.i43.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Woo W., Carey E.T., Choi M. Spotlight on liposomal irinotecan for metastatic pancreatic cancer: Patient selection and perspectives. Onco Targets Ther. 2019;12:1455–1463. doi: 10.2147/OTT.S167590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kondo R., Watanabe S., Shoji S., Ichikawa K., Abe T., Baba J., Tanaka J., Tsukada H., Terada M., Sato K., et al. A Phase II Study of Irinotecan for Patients with Previously Treated Small-Cell Lung Cancer. Oncology. 2018;94:223–232. doi: 10.1159/000486622. [DOI] [PubMed] [Google Scholar]
- 172.Xu F., Ren X., Chen Y., Li Q., Li R., Chen Y., Xia S. Irinotecan-platinum combination therapy for previously untreated extensive-stage small cell lung cancer patients: A meta-analysis. BMC Cancer. 2018;18:808–820. doi: 10.1186/s12885-018-4715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lihua P., Chen X.Y., Wu T.X. Topotecan for ovarian cancer. Cochrane Databse Syst. Rev. 2008;2008:CD005589. doi: 10.1002/14651858.CD005589.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pignata S., Pisano C., Di Napoli M., Cecere S.C., Tambaro R., Attademo L. Treatment of recurrent epithelial ovarian cancer. Cancer. 2019;24:4609–4615. doi: 10.1002/cncr.32500. [DOI] [PubMed] [Google Scholar]
- 175.Rosen V.M., Guerra I., McCormack M., Nogueira-Rodrigues A., Sasse A., Munk V.C., Shang A. Systematic review and network meta-analysis of bevacizumab plus first-line topotecan-paclitaxel or cisplatin-paclitaxel versus non-bevacizumab-containing therapies in persistent, recurrent, or metastatic cervical cancer. Int. J. Gyn. Cancer. 2017;27:1237–1246. doi: 10.1097/IGC.0000000000001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qin A., Kalemkerian G.P. Treatment options for relapsed small-cell lung cancer: What progress have we made? J. Oncol. Pract. 2018;14:369–370. doi: 10.1200/JOP.18.00278. [DOI] [PubMed] [Google Scholar]
- 177.Armenian S., Bhatia S. Predicting and preventing anthracycline-related cardiotoxicity. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:3–12. doi: 10.1200/EDBK_100015. [DOI] [PubMed] [Google Scholar]
- 178.Vu K., Ai W. Update on the treatment of anaplastic large cell lymphoma. Curr. Hematol. Malig. Rep. 2018;13:135–141. doi: 10.1007/s11899-018-0436-z. [DOI] [PubMed] [Google Scholar]
- 179.Liu W., Jiang Q., Zhou Y. Advances of systemic treatment for adult soft-tissue sarcoma. Chin. Clin. Oncol. 2018;7:42–55. doi: 10.21037/cco.2018.08.02. [DOI] [PubMed] [Google Scholar]
- 180.D’Ambrosio L., Touati N., Blay J.Y., Grignani G., Flippot R., Czarnecka A.M., Piperno-Neumann S., Martin-Broto J., Sanfilippo R., Katz D., et al. European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: A propensity score matching analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer. 2020;126:2637–2647. doi: 10.1002/cncr.32795. [DOI] [PubMed] [Google Scholar]
- 181.Carvalho C., Santos R.X., Cardoso S., Correia S., Oliveira P.J., Santos M.S., Moreira P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 182.Banke A., Fosbøl E.L., Møller J.E., Gislason G.H., Andersen M., Bernsdorf M., Jensen M.B., Schou M., Ejlertsen B. Long-term effect of epirubicin on incidence of heart failure in women with breast cancer: Insight from a randomized clinical trial. Eur. J. Heart Fail. 2018;20:1447–1453. doi: 10.1002/ejhf.1168. [DOI] [PubMed] [Google Scholar]
- 183.Werntz R.P., Adamic B., Steinberg G.D. Emerging therapies in the management of high-risk non-muscle invasive bladder cancer (HRNMIBC) World J. Urol. 2019;37:2031–2040. doi: 10.1007/s00345-018-2592-0. [DOI] [PubMed] [Google Scholar]
- 184.Ravandi F., Assi R., Daver N., Benton C.B., Kadia T., Thompson P.A., Borthakur G., Alvarado Y., Jabbour E.J., Konopleva M., et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019;6:e480–e488. doi: 10.1016/S2352-3026(19)30114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Evison B.J., Sleebs B.E., Watson K.G., Phillips D.R., Cutts S.M. Mitoxantrone, More than Just another topoisomerase II poison. Med. Res. Rev. 2016;36:248–299. doi: 10.1002/med.21364. [DOI] [PubMed] [Google Scholar]
- 186.Barrenetxea Lekue C., Grasso Cicala S., Leppä S., Stauffer Larsen T., Herráez Rodríguez S., Alonso Caballero C., Jørgensen J.M., Toldbod H., Leal Martínez I., D’Amore F. Pixantrone beyond monotherapy: A review. Ann. Hematol. 2019;98:2025–2033. doi: 10.1007/s00277-019-03749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Minotti G., Han H., Cattan V., Egorov A., Bertoni F. Pixantrone: Novel mode of action and clinical readouts. Expert Rev. Hematol. 2018;11:587–596. doi: 10.1080/17474086.2018.1476848. [DOI] [PubMed] [Google Scholar]
- 188.Alsdorf W., Seidel C., Bokemeyer C., Oing C. Current pharmacotherapy for testicular germ cell cancer. Expert Opin. Pharm. 2019;20:837–850. doi: 10.1080/14656566.2019.1583745. [DOI] [PubMed] [Google Scholar]
- 189.Bernhardt E.B., Jalal S.I. Small Cell Lung Cancer. Cancer Treat. Res. 2016;170:301–322. doi: 10.1007/978-3-319-40389-2_14. [DOI] [PubMed] [Google Scholar]
- 190.Reveiz L., Rueda J.R., Cardona A.F. Chemotherapy for brain metastases from small cell lung cancer. Cochrane Databse Syst. Rev. 2012;6:CD007464. doi: 10.1002/14651858.CD007464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li J., Chen W., Zhang P., Li N. Topoisomerase II trapping agent teniposide induces apoptosis and G2/M or S phase arrest of oral squamous cell carcinoma. World J. Surg. Oncol. 2006;4:41–47. doi: 10.1186/1477-7819-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Joyce M.J., Pollock B.H., Devidas M., Buchanan G.R., Camitta B. Chemotherapy for initial induction failures in childhood acute lymphoblastic leukemia: A Children’s Oncology Group Study (POG 8764) J. Pediatr. Hematol. Oncol. 2013;35:32–35. doi: 10.1097/MPH.0b013e318279afdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pommier Y., Cushman M., Doroshow J.H. Novel clinical indenoisoquinoline topoisomerase I inhibitors: A twist around the camptothecins. Oncotarget. 2018;9:37286–37288. doi: 10.18632/oncotarget.26466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bailly C. Contemporary challenges in the design of topoisomerase II inhibitors for cancer chemotherapy. Chem. Rev. 2012;112:3611–3640. doi: 10.1021/cr200325f. [DOI] [PubMed] [Google Scholar]
- 195.Lovrics A., Pape V.F.S., Szisz D., Kalászi A., Heffeter P., Magyar C., Szakács G. Identifying new topoisomerase II poison scaffolds by combining publicly available toxicity data and 2D/3D-based virtual screening. J. Cheminform. 2019;11:67–81. doi: 10.1186/s13321-019-0390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ortega J.A., Riccardi L., Minniti E., Borgogno M., Arencibia J.M., Greco M.L., Minarini A., Sissi C., De Vivo M. Pharmacophore hybridization to discover novel topoisomerase II poisons with promising antiproliferative activity. J. Med. Chem. 2018;61:1375–1379. doi: 10.1021/acs.jmedchem.7b01388. [DOI] [PubMed] [Google Scholar]
- 197.Beck D.E., Abdelmalak M., Lv W., Reddy P.V., Tender G.S., O’Neill E., Agama K., Marchand C., Pommier Y., Cushman M. Discovery of potent indenoisoquinoline topoisomerase I poisons lacking the 3-nitro toxicophore. J. Med. Chem. 2015;58:3997–4015. doi: 10.1021/acs.jmedchem.5b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nitiss J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu T., Karlsen M., Karlberg A.M., Redalen K.R. Hypoxia imaging and theranostic potential of [64Cu][Cu(ATSM)] and ionic Cu(II) salts: A review of current evidence and discussion of the retention mechanisms. Ejnmmi Res. 2020;10:33–47. doi: 10.1186/s13550-020-00621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kantarjian H. Acute myeloid leukemia-Major progress over four decades and glimpses into the future. Am. J. Hematol. 2016;91:131–145. doi: 10.1002/ajh.24246. [DOI] [PubMed] [Google Scholar]
- 201.Bornhäuser M. Vosaroxin in acute myeloid leukaemia. Lancet Oncol. 2015;16:1000–1001. doi: 10.1016/S1470-2045(15)00165-5. [DOI] [PubMed] [Google Scholar]
- 202.Skok Ž., Zidar N., Kikelj D., Ilaš J. Dual inhibitors of human DNA topoisomerase II and other cancer-related targets. J. Med. Chem. 2020;63:884–904. doi: 10.1021/acs.jmedchem.9b00726. [DOI] [PubMed] [Google Scholar]
- 203.Kim S.O., Sakchaisri K., Thimmegowda N.R., Soung N.K., Jang J.H., Kim Y.S., Lee K.S., Kwon Y.T., Asami Y., Ahn J.S., et al. STK295900, a dual inhibitor of topoisomerase 1 and 2, induces G(2) arrest in the absence of DNA damage. PLoS ONE. 2013;8:e53908. doi: 10.1371/journal.pone.0053908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yi J.M., Zhang X.F., Huan X.J., Song S.S., Wang W., Tian Q.T., Sun Y.M., Chen Y., Ding J., Wang Y.Q., et al. Dual targeting of microtubule and topoisomerase II by α-carboline derivative YCH337 for tumor proliferation and growth inhibition. Oncotarget. 2015;6:8960–8973. doi: 10.18632/oncotarget.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Seo Y.H. Dual inhibitors against topoisomerases and histone deacetylases. J. Cancer Prev. 2015;20:85–91. doi: 10.15430/JCP.2015.20.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ferreira R., Schneekloth J.S., Jr., Panov K.I., Hannan K.M., Hannan R.D. Targeting the RNA polymerase I transcription for cancer therapy comes of age. Cells. 2020;9:266. doi: 10.3390/cells9020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li X., Chen Y., Zhao J., Shi J., Wang M., Qiu S., Hu Y., Xu Y., Cui Y., Liu C., et al. The specific inhibition of SOD1 selectively promotes apoptosis of cancer cells via regulation of the ROS signaling network. Oxid. Med. Cell Longev. 2019;2019:9706792–9706814. doi: 10.1155/2019/9706792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huang P., Feng L., Oldham E.A., Keating M.J., Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 209.Ceci C., Atzori M.G., Lacal P.M., Graziani G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: Experimental evidence in different metastatic cancer models. Int. J. Mol. Sci. 2020;21:1388. doi: 10.3390/ijms21041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Roskoski R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharm. Res. 2020;152:104609–104628. doi: 10.1016/j.phrs.2019.104609. [DOI] [PubMed] [Google Scholar]
- 211.Yang S.W., Burgin A.B., Jr., Huizenga B.N., Robertson C.A., Yao K.C., Nash H.A. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc. Natl. Acad. Sci. USA. 1996;93:11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cortes Ledesma F., El Khamisy S.F., Zuma M.C., Osborn K., Caldecott K.W. A human 5’-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 213.Zakharenko A., Dyrkheeva N., Lavrik O. Dual DNA topoisomerase 1 and tyrosyl-DNA phosphodiesterase 1 inhibition for improved anticancer activity. Med. Res. Rev. 2019;39:1427–1441. doi: 10.1002/med.21587. [DOI] [PubMed] [Google Scholar]
- 214.Wu X., Xue X., Wang L., Wang W., Han J., Sun X., Zhang H., Liu Y., Che X., Yang J., et al. Suppressing autophagy enhances disulfiram/copper-induced apoptosis in non-small cell lung cancer. Eur. J. Pharm. 2018;827:1–12. doi: 10.1016/j.ejphar.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 215.Marinello J., Delcuratolo M., Capranico G. Anthracyclines as topoisomerase II poisons: From early studies to new perspectives. Int. J. Mol. Sci. 2018;19:3480. doi: 10.3390/ijms19113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brown J.S., Sundar R., Lopez J. Combining DNA damaging therapeutics with immunotherapy: More haste, less speed. Br. J. Cancer. 2018;118:312–324. doi: 10.1038/bjc.2017.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Heinhuis K.M., Ros W., Kok M., Steeghs N., Beijnen J.H., Schellens J.H.M. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019;30:219–235. doi: 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 218.Kim N., Jinks-Robertson S. The Top1 paradox: Friend and foe of the eukaryotic genome. DNA Repair. 2017;56:33–41. doi: 10.1016/j.dnarep.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L., Liu J.F., Garber J.E., Chowdhury D., Catherine J., Andrea A.D.D., et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumorinfiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:1–12. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li A., Yi M., Qin S., Song Y., Chu Q., Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol. 2019;12:35–47. doi: 10.1186/s13045-019-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhao B., Liu P., Fukumoto T., Fatkhutdinov N., Wu S., Lin J., Aird K.M., Tang H.Y., Liu Q., Speicher D.W., et al. Topoisomerase 1 cleavage complex enables pattern recognition and inflammation during senescence. Nat. Commun. 2020;11:908–919. doi: 10.1038/s41467-020-14652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang Z., Chen J., Hu J., Zhang H., Xu F., He W., Wang X., Li M., Lu W., Zeng G., et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J. Clin. Investig. 2019;130:4850–4862. doi: 10.1172/JCI127471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wilkinson R.D.A., McCabe N., Parkes E.E., Barros E.M., Johnston D.I., Ali R.M.M., Lappin K., Greenberg R.A., Harkin D.P., McIntosh S.A., et al. Topoisomerase II inhibitors induce cGAS-STING dependent inflammation resulting in cytokine induction and immune checkpoint activation. bioRxiv. 2019 doi: 10.1101/764662. [DOI] [Google Scholar]
- 224.Srinivas U.S., Tan B., Vellayappan B.A., Jeyasekharan A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084–101093. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Corces M.R., Granja J.M., Shams S., Louie B.H., Seoane J.A., Zhou W., Silva T.C., Groeneveld C., Wong C.K., Cho S.W., et al. The chromatin accessibility landscape of primary human cancers. Science. 2018;362:eaav1898. doi: 10.1126/science.aav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Temko D., Tomlinson I.P.M., Severini S., Schuster-Böckler B., Graham T.A. The effects of mutational processes and selection on driver mutations across cancer types. Nat. Commun. 2018;9:1857–1867. doi: 10.1038/s41467-018-04208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Alexandrov L.B., Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Helleday T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis. 2010;31:955–960. doi: 10.1093/carcin/bgq064. [DOI] [PubMed] [Google Scholar]
- 229.Palla V.V., Karaolanis G., Katafigiotis I., Anastasiou I., Patapis P., Dimitroulis D., Perrea D. gamma-H2AX: Can it be established as a classical cancer prognostic factor? Tumour Biol. 2017;39:1010428317695931. doi: 10.1177/1010428317695931. [DOI] [PubMed] [Google Scholar]
- 230.Murai J., Thomas A., Miettinen M., Pommier Y. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA-targeting anti-cancer therapies. Pharmacol. Ther. 2019;201:94–102. doi: 10.1016/j.pharmthera.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Infante Lara L., Fenner S., Ratcliffe S., Isidro-Llobet A., Hann M., Bax B., Osheroff N. Coupling the core of the anticancer drug etoposide to an oligonucleotide induces topoisomerase II-mediated cleavage at specific DNA sequences. Nucleic Acids Res. 2018;46:2218–2233. doi: 10.1093/nar/gky072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ke X., Lin W., Li X., Wang H., Xiao X., Guo Z. Synergistic dual-modified liposome improves targeting and therapeutic efficacy of bone metastasis from breast cancer. Drug Deliv. 2017;24:1680–1689. doi: 10.1080/10717544.2017.1396384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Asakura T., Yokoyama M., Shiraishi K., Aoki K., Ohkawa K. Chemotherapeutic effect of CD147antibody-labeled micelles encapsulating doxorubicin conjugate targeting cd147-expressing carcinoma cells. Anticancer Res. 2018;38:1311–1316. doi: 10.21873/anticanres.12353. [DOI] [PubMed] [Google Scholar]
- 234.Shi J., Su Y., Liu W., Chang J., Zhang Z. A nanoliposome-based photoactivable drug delivery system for enhanced cancer therapy and overcoming treatment resistance. Int. J. Nanomed. 2017;12:8257–8275. doi: 10.2147/IJN.S143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Goffart S., Hangas A., Pohjoismäki J.L.O. Twist and Turn-Topoisomerase Functions in Mitochondrial DNA Maintenance. Int. J. Mol. Sci. 2019;20:2041. doi: 10.3390/ijms20082041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Baechler S.A., Factor V.M., Dalla Rosa I., Ravji A., Becker D., Khiati S., Miller Jenkins L.M., Lang M., Sourbier C., Michaels S.A., et al. The mitochondrial type IB topoisomerase drives mitochondrial translation and carcinogenesis. Nat. Commun. 2019;10:83–96. doi: 10.1038/s41467-018-07922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]