Abstract
Background: Bronchiectasis is a multidimensional lung disease characterized by bronchial dilation, chronic inflammation, and infection. The FACED (Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea) score and Bronchiectasis Severity Index (BSI) are used to stratify disease risk and guide clinical practice. This meta-analysis aimed to quantify the accuracy of these two systems for predicting bronchiectasis outcomes.
Methods: PubMed, Embase, and the Cochrane Database of Systematic Reviews were searched for relevant studies. Quality of included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria. Pooled summary estimates, including sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated. Summary receiver operating characteristic curves were constructed, and the area under the curve (AUC) was used to evaluate prognostic performance.
Results: We analyzed 17 unique cohorts (6525 participants) from ten studies. FACED scores with a cut-off value ≥ 5 predicted all-cause mortality better than BSI with a cut-off value ≥ 9, based on pooled sensitivity (0.34 vs 0.7), specificity (0.94 vs 0.66), PLR (4.76 vs 2.05), NLR (0.74 vs 0.48), DOR (6.67 vs 5.01), and AUC (0.87 vs 0.75). Both FACED scores with a cut-off value ≥ 5 (AUC = 0.82) and BSI scores with a cut-off value ≥ 5 or 9 (both AUC = 0.80) help to predict hospitalization.
Conclusions: At a cut-off value ≥ 5, FACED scores can reliably predict all-cause mortality and hospitalization, while BSI scores can reliably predict hospitalization with a cut-off of ≥5 or ≥9. Further studies are essential to validate the prognostic performance of these two scores.
Keywords: All-cause mortality, Bronchiectasis Severity Index, Bronchiectasis, FACED, Hospitalization, Severity score
Introduction
Bronchiectasis is a chronic inflammatory and structural lung disease characterized by chronic dilation of the bronchi; clinical symptoms of the disease include persistent cough, sputum production, and recurrent respiratory infections [1,2]. In recent years, bronchiectasis has become a major health concern due to its increasing prevalence and associated healthcare costs [1–4].
Due to the lack of effective treatment options, the current management strategies for bronchiectasis focus on controlling symptoms, reducing risk, avoiding exacerbation, and slowing disease progression [1,2]. Most recommended first-line treatments for bronchiectasis are long-term antimicrobial therapies, which are costly and can cause adverse events [1,2]. Thus, the first step in clinical decision-making should be stratifying bronchiectasis patients by risk of poor prognosis in order to target treatments to those most likely to experience a net benefit. Furthermore, clearly defined stratification of bronchiectasis patients would improve the comparability of populations analyzed in different research studies [5].
Three multidimensional severity scoring systems have been derived and validated for bronchiectasis: FACED, so named because it takes into account Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea; EFACED (with Exacerbation added to FACED); and the Bronchiectasis Severity Index (BSI), which involves a 9-item scale encompassing demographic and clinical characteristics, as well as microbiological and radiological data [6,7]. FACED and BSI scores have been more extensively researched and are more widely used [6,7]. The FACED score is an easy-to-use grading system with an excellent predictive performance regarding mortality [7,8]. The BSI was developed and validated in a large multicenter study in Europe [9]. These prognostic scoring systems can help to predict mortality, hospitalization, and disease exacerbation, as well as to evaluate quality of life in patients with bronchiectasis. Although some studies have compared the accuracy of these scoring systems [10,11], whether one is better is unclear. This is an important question to address because some medical centers may lack the clinical experience or equipment to implement all scoring systems well.
Considerable effort needs to be invested to validate the prognostic performance of these scoring systems in varied settings before they can be accepted and extensively applied in research and clinical decision-making. In this meta-analysis, we aimed to quantify and compare the accuracy of the FACED and BSI systems at predicting disease outcomes (all-cause mortality, respiratory-related mortality, or hospitalization) in bronchiectasis patients. As part of the present study, we aimed to determine optimal cut-off values for each system as a basis for standardizing prognostic prediction.
Methods
This systematic review and meta-analysis was registered with the PROSPERO database of systematic reviews (CRD42018096462).
Search strategy
Two reviewers (M.H. and M.Z.) independently searched PubMed, Embase, and the Cochrane Database of Systematic Reviews to identify studies on bronchiectasis published before June 2019. The search strings are provided in the Supplementary material (e-Appendix 1). The reference lists in the included studies and in relevant review articles were screened manually.
Study eligibility
After removing duplicate references, two reviewers (M.H. and M.Z.) independently screened the titles and abstracts of the potentially relevant studies, followed by a complete review of each relevant full text. Disagreements were resolved through discussion or consultation with the third author (C.W.). All included studies were written in English or Chinese, with no restrictions on study design. All included studies used the FACED system and/or BSI system to assess bronchiectasis (diagnosed using high-resolution chest computed tomography) and had sufficient data to directly or indirectly assess the predictive performance of the FACED system and/or BSI system regarding at least one outcome of interest (mortality and/or hospitalization).
If a study contained data from several cohorts, each cohort was treated as a separate study, consistent with established practices [12]. If research subjects and reported outcomes overlapped between studies, we combined the data from those studies and analyzed the data based on methods described in a previous study [13].
Data extraction and quality assessment
Two reviewers (M.H. and M.Z.) independently assessed the quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria [14]. Relevant data were extracted from the included studies using a standardized extraction form.
Statistical analysis
Our analyses focused on the ability of the two scoring systems to predict all-cause mortality, respiratory-related mortality, or hospitalization, and on the agreement between the two scoring systems. Heterogeneity between studies was assessed and quantified using the I2 statistic. Based on the heterogeneity observed, random-effects (I2 > 50%) or fixed-effects (I2 ≤ 50%) models were used to calculate summary estimates, including sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). We generated summary receiver operating characteristic curves (SROCs) and calculated the area under the SROC (AUC) values. Meta-regression was performed to explore the sources of statistically significant heterogeneity, followed by subgroup analyses. Publication bias was assessed using Deeks’ test. Consensus between the scoring systems was evaluated using the kappa (κ) coefficient and chi-squared test. Statistical analysis was performed using RevMan 5.3 (Cochrane Collaboration), Meta-DiSc 1.4 (XI Cochrane Colloquium, Barcelona, Spain), and Stata 12.0 (Stata, College Station, TX, U.S.A.).
Results
Literature screening and assessment
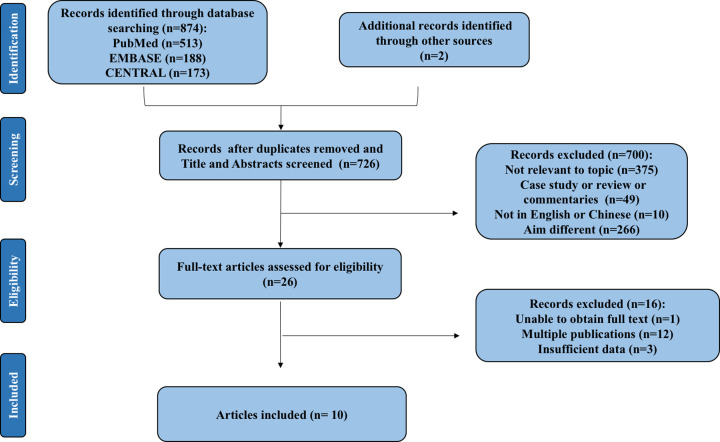
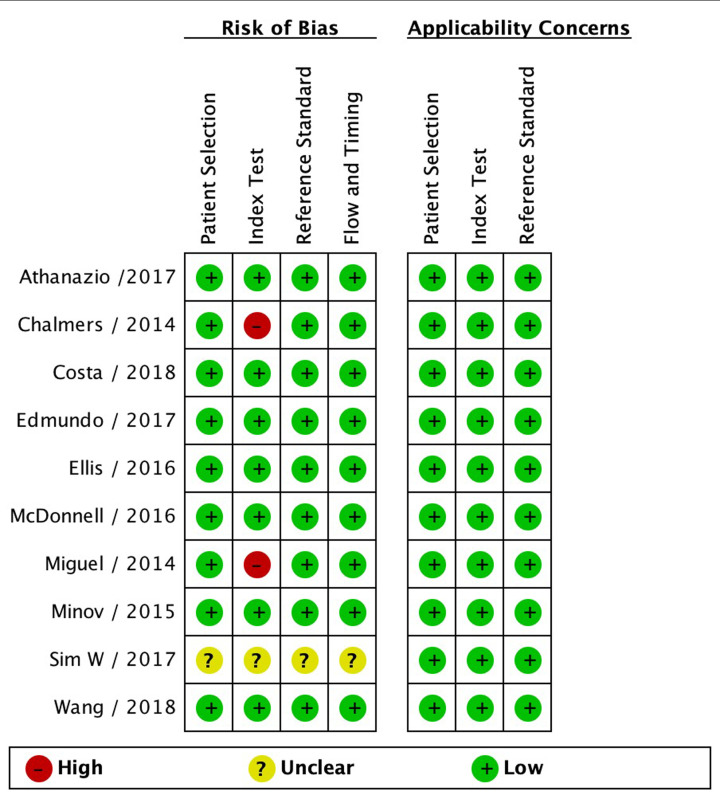
After a detailed assessment based on the eligibility criteria, the final meta-analysis included 17 unique cohorts with 6525 participants across ten publications (Figure 1) [8–11,15–20]. The characteristics of the included studies are summarized in Table 1. The QUADAS-2 assessment demonstrated that most of the included studies had a low risk of bias, indicating the reliability of the statistical results (Figure 2). For the initial analysis, we stratified the bronchiectasis patients into three groups: mild (BSI score, 0–4; FACED score, 0–2), moderate (BSI, 5–8; FACED, 3–4), or severe (BSI, 9; FACED, 5–7). Patients in these three groups were compared in terms of age, rates of all-cause or respiratory-related mortality, and rate of hospital admission (Table 2).
Figure 1. Flowchart of study selection.
Table 1. Characteristics of included studies.
| Study/year | Country | Study design | Sample size | Age (years) | Male (%) | BMI (kg/m2) | mMRC | FEV1, % predicted | Pseudomonas aeruginosa (%) | Number of affected lobes | Exacerbations (previous year) | Follow-up time (years) | Mortality (%) | Hospitalization (%) | Scales |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Martínez-García/2014a | Spain | R | 397 | 59.20 ± 17.40 | 44.30 | 26.10 ± 4.28 | 1.57 ± 1.17 | 68.50 ± 25.60 | 31.80 | 2.45 ± 1.12 | 2.47 ± 2.10 | 5 | 19.90 | NA | FACED |
| Martínez-García/2014b | Spain | R | 422 | 58.30 ± 17.70 | 42.60 | 25.35 ± 4.98 | 1.50 ± 1.15 | 68.70 (26.30) | 31.80 | 2.59 ± 1.17 | 2.57 ± 2.30 | 5 | 17.80 | NA | FACED |
| Chalmers/2014 | U.K. | P | 608 | 67 (58–75) | 39.97 | NA | 2 (1, 3) | 72.60 ± 25.00 | 11.51 | 3.0 ± 1.6 | 1.7 ± 2.0 | 4 | 10.20 | 0.08 | BSI |
| Ellis/2016 | U.K. | R | 74 | 52.50 ± 12.40 | NA | 23.40 ± 3.90 | 2.10 ± 0.90 | 68.80 ± 27.70 | 22.00 | 3.40 ± 1.50 | 4.40 ± 4.40 | 18.8 | 35 | NA | BS FACEDI |
| McDonnell/2016a | Dundee, U.K. | P | 494 | 65.30 ± 12.90 | 39.30 | 25.90 ± 5.20 | 2.30 ± 1.10 | 71.60 ± 24.7 | 12.80 | 4.40 ± 3.00 | 2.10 ± 2.60 | 4 | 8.5 | 0.05 | BSI FACED |
| McDonnell/2016b | Newcastle, England | P | 126 | 59.10 ± 14.50 | 40.5 | 26.20 ± 5.10 | 2.50 ± 1.10 | 64.00 ± 26.90 | 10.30 | 2.80 ± 1.40 | 3.40 ± 1.70 | 4 | 12.7 | 0.11 | BSI FACED |
| McDonnell/2016c | Belgium | P | 190 | 66.40 ± 16.00 | 49 | 23.90 ± 4.30 | 2.30 ± 1.20 | 69.30 ± 25.30 | 8.40 | 4.50 ± 1.30 | 1.90 ± 2.10 | 5 | 23.16 | 0.06 | BSI FACED |
| McDonnell/2016d | Monza, Italy | P | 250 | 65.10 ± 12.20 | 41.2 | 23.70 ± 4.40 | 2.00 ± 1.30 | 79.20 ± 27.50 | 21.60 | 5.50 ± 2.70 | 1.90 ± 2.00 | 4 | 5.60 | 0.09 | BSI FACED |
| McDonnell/2016e | Galway, Ireland | P | 280 | 60.50 ± 14.60 | 32.9 | 27.10 ± 5.60 | 2.00 ± 1.00 | 80.30 ± 25.90 | 13.90 | 3.40 ± 3.00 | 2.90 ± 1.30 | 5 | 15.71 | 0.03 | BSI FACED |
| McDonnell/2016f | Athens, Greece | P | 159 | 59.30 ± 16.20 | 36 | 24.60 ± 3.40 | 2.40 ± 1.50 | 70.10 ± 24.90 | 36.50 | 4.80 ± 2.50 | 2.40 ± 1.50 | 5 | 5.66 | 0.04 | BSI FACED |
| McDonnell/2016g | Vojvodina, Serbia | P | 113 | 62.00 ± 13.00 | 29.2 | 25.10 ± 4.90 | 2.50 ± 1.40 | 64.80 ± 26.20 | 1.00 | 4.70 ± 2.40 | 1.00 ± 1.25 | 5 | 17.70 | 0.02 | BSI FACED |
| Athanazio/2017 | Latin America | R | 651 | 48.20 ± 16.00 | 32.9 | 22.40 ± 11.50 | 1.52 ± 1.00 | 54.70 ± 22.10 | 39.80 | 3.30 ± 1.50 | 1.12 ± 1.40 | 5 | 14.60 | 0.30 | FACED |
| Sim/2017 | Singapore | P | 96 | 70 (59.3–77) | 37.5 | 19.20 (15.70–23.10) | NA | 47.00 (37.00–63.30) | NA | NA | NA | 5 | 42.70 | NA | BSI FACED |
| Wang/2018 | China | R | 596 | 54.81 ± 13.71 | 56.88 | NA | NA | NA | NA | NA | NA | 5 | 7.05 | 0.06 | BSI FACED |
| Rosales-Mayor/2017 | Spain | P | 182 | 68.00 ± 14.60 | 40.10 | 25.60 ± 4.60 | 1.30 ± 1.10 | 70.30 ± 21.80 | 20.90 | 3.20 ± 1.60 | 1.80 ± 1.80 | 1 | NA | 0.27 | BSI FACED |
| Costa/2018 | Portugal | R | 40 | 65.90 ± 14.10 | 45.00 | 26.20 ± 5.60 | 2 | 63.40 ± 22.10 | 12.50 | 3.60 ± 1.40 | 1.20 ± 1.50 | NA | NA | NA | BSI FACED |
| Minov/2015 | Macedonia | R | 37 | 63.40 ± 8.10 | 80.00 | 24.30 ± 3.70 | 1.83 ± 0.63 | 57.60 ± 8.70 | 8.10 | 2. 25 ± 0.78 | 2.12 ± 0.54 | NA | NA | NA | BSI FACED |
Data are presented as mean ± SD or median (interquartile range). Abbreviations: NA, not available; P, prospective; R, retrospective.
a, b, c etc means different cohorts in one study.
Figure 2. Quality assessment of included studies using the QUADAS-2 criteria.
Table 2. The distribution of bronchiectasis patients, number of all-cause and respiratory-cause deaths, and number of hospital admissions in different severity groups stratified by FACED and/or BSI.
| Study/year | Country | Scales | Mild | Moderate | Severe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | All-cause mortality | Respiratory-cause mortality | Hospitalizations | Total | All-cause mortality | Respiratory-cause mortality | Hospitalizations | Total | All-cause mortality | Respiratory-cause mortality | Hospitalizations | |||
| Martínez-García/2014a | Spain | FACED | 234 | 10 | 2 | NA | 99 | 25 | 15 | NA | 64 | 44 | 33 | NA |
| Miguel/2014b | Spain | FACED | 249 | 14 | 6 | NA | 105 | 23 | 14 | NA | 68 | 38 | 31 | NA |
| Chalmers/2014 | U.K. | BSI | 191 | 4 | NA | 13 | 224 | 13 | NA | 31 | 193 | 44 | NA | 145 |
| Ellis/2016 | U.K. | FACED | 49 | 8 | NA | NA | 19 | 13 | NA | NA | 6 | 5 | NA | NA |
| BSI | 19 | 4 | NA | NA | 32 | 9 | NA | NA | 23 | 13 | NA | NA | ||
| McDonnell/2016a | Dundee, U.K. | BSI | 136 | 1 | NA | 3 | 211 | 13 | NA | 24 | 147 | 28 | NA | 75 |
| FACED | 303 | 12 | NA | 44 | 145 | 15 | NA | 45 | 46 | 15 | NA | 13 | ||
| McDonnell/2016b | Newcastle, England | BSI | 21 | 0 | NA | 0 | 25 | 1 | NA | 5 | 80 | 15 | NA | 52 |
| FACED | 91 | 6 | NA | 37 | 27 | 7 | NA | 15 | 8 | 3 | NA | 5 | ||
| McDonnell/2016c | Leuven, Belgium | BSI | 51 | 2 | NA | 6 | 63 | 16 | NA | 18 | 76 | 26 | NA | 34 |
| FACED | 100 | 9 | NA | 23 | 65 | 19 | NA | 22 | 25 | 16 | NA | 13 | ||
| McDonnell/2016d | Monza, Italy | BSI | 67 | 0 | NA | 10 | 104 | 3 | NA | 27 | 79 | 11 | NA | 55 |
| FACED | 135 | 3 | NA | 40 | 88 | 4 | NA | 34 | 27 | 7 | NA | 18 | ||
| McDonnell/2016e | Galway, Ireland | BSI | 109 | 8 | NA | 2 | 92 | 11 | NA | 6 | 79 | 25 | NA | 30 |
| FACED | 217 | 23 | NA | 18 | 53 | 19 | NA | 15 | 10 | 2 | NA | 5 | ||
| McDonnell/2016f | Athens, Greece | BSI | 36 | 0 | NA | 1 | 43 | 0 | NA | 7 | 80 | 9 | NA | 27 |
| FACED | 104 | 0 | NA | 17 | 35 | 4 | NA | 9 | 20 | 5 | NA | 9 | ||
| McDonnell/2016g | Vojvodina, Serbia | BSI | 41 | 0 | NA | 0 | 48 | 12 | NA | 8 | 24 | 8 | NA | 12 |
| FACED | 60 | 4 | NA | 2 | 44 | 13 | NA | 3 | 9 | 3 | NA | 1 | ||
| Athanazio/2017 | Latin America | FACED | 350 | 13 | 7 | 4 | 231 | 48 | 29 | 33 | 70 | 34 | 27 | 19 |
| Sim/2017 | Singapore | BSI | 9 | 2 | NA | NA | 19 | 3 | NA | NA | 68 | 36 | NA | NA |
| FACED | 35 | 11 | NA | NA | 40 | 21 | NA | NA | 21 | 9 | NA | NA | ||
| Wang/2018 | China | BSI | 46 | 1 | NA | 15 | 244 | 5 | NA | 57 | 306 | 36 | NA | 105 |
| FACED | 441 | 17 | NA | 123 | 136 | 19 | NA | 48 | 19 | 6 | NA | 6 | ||
| Rosales-Mayor/2017 | Spain | BSI | 36 | NA | NA | NA | 47 | NA | NA | NA | 99 | NA | NA | NA |
| FACED | 108 | NA | NA | NA | 61 | NA | NA | NA | 13 | NA | NA | NA | ||
| Costa/2018 | Portugal | BSI | 13 | NA | NA | NA | 13 | NA | NA | NA | 14 | NA | NA | NA |
| FACED | 20 | NA | NA | NA | 15 | NA | NA | NA | 5 | NA | NA | NA | ||
| Minov/2015 | Macedonia | BSI | 16 | NA | NA | NA | 14 | NA | NA | NA | 7 | NA | NA | NA |
| FACED | 17 | NA | NA | NA | 14 | NA | NA | NA | 6 | NA | NA | NA | ||
Abbreviation: NA, not available.
a, b, c etc means different cohorts in one study.
Mortality prediction
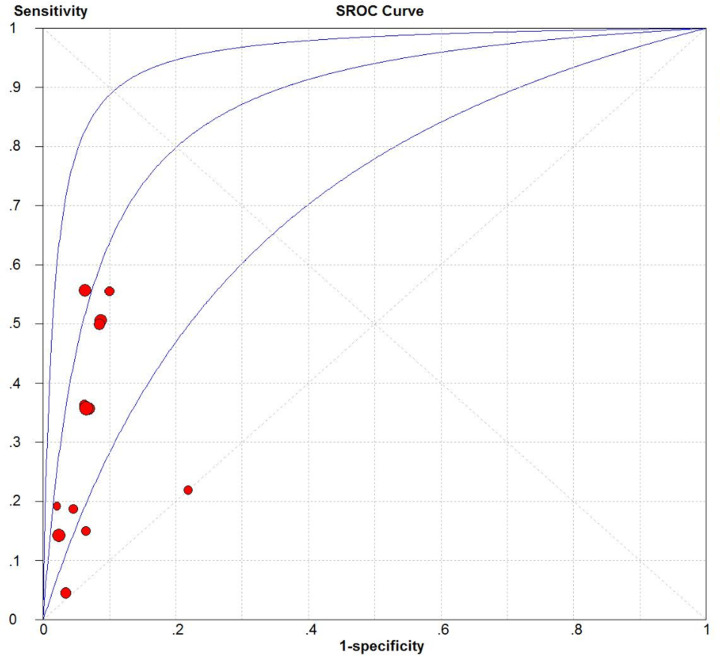
We evaluated the predictive accuracy regarding all-cause mortality of the FACED system across 13 cohorts (n=3848) [8,10,11,16,17,20], and the corresponding predictive accuracy of the BSI system across 11 cohorts (n=2986) [9,11,16,17,20]. Pooled summary estimates, including sensitivity, specificity, PLR, NLR, DOR, and AUC, were calculated using FACED and BSI scores at various cut-off values (Table 3). The FACED score at a cut-off value ≥ 5 had good predictive accuracy based on the pooled sensitivity (0.34, 95% confidence interval [CI] = 0.3–0.38), specificity (0.94, 95% CI = 0.93–0.95), PLR (4.76, 95% CI = 3.48–6.51), NLR (0.74, 95% CI = 0.62–0.88), and DOR (6.67, 95% CI = 4.25–10.45). The BSI score at a cut-off value ≥ 9 had good predictive accuracy based on the pooled sensitivity (0.70, 95% CI = 0.65–0.75), specificity (0.66, 95% CI = 0.64–0.67), PLR (2.05, 95% CI = 1.78–2.37), NLR (0.48, 95% CI = 0.38–0.61), and DOR (5.01, 95% CI = 3.85–6.53). Based on the AUC values, we found that the FACED score was better at predicting all-cause mortality than the BSI score (0.87 vs 0.75; Figures 3 and 4).
Table 3. Summary accuracy of FACED score and BSI for predicting mortality and hospitalizations at each cut-off value.
| Outcomes | Scale | Study/participants | Sensitivity (95% CI), I2 | Specificity (95% CI), I2 | PLR (95% CI), I2 | NLR (95% CI), I2 | DOR (95% CI), I2 | AUC |
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | FACED | |||||||
| ≥3 | 13/3848 | 0.76 (0.72–0.80), 72.7% | 0.68 (0.66– 0.69), 88.8% | 2.31 (2.02– 2.63), 67.5% | 0.38 (0.29– 0.49), 66.6% | 6.54 (4.71– 9.07), 47.7% | 0.77 | |
| ≥5 | 13/3848 | 0.34 (0.30–0.38), 83.0% | 0.94 (0.93– 0.95), 74.9% | 4.76 (3.48– 6.51), 58.3% | 0.74 (0.62– 0.88), 91.7% | 6.67 (4.25–10.45), 64.4% | 0.87 | |
| BSI | ||||||||
| ≥5 | 11/2986 | 0.94 (0.91– 0.96), 51.8% | 0.27 (0.25–0.29), 94.9% | 1.31 (1.18– 1.45), 88.6% | 0.29 (0.19– 0.42), 0.0% | 5.10 (3.26– 7.98), 0.0% | 0.66 | |
| ≥9 | 11/2986 | 0.70 (0.65–0.75), 77.2% | 0.66 (0.64–0.67), 93.8% | 2.05 (1.78– 2.37), 66.6% | 0.48 (0.38– 0.61), 48.1% | 5.01 (3.85– 6.53), 0.0% | 0.75 | |
| Respiratory-cause mortality | FACED | |||||||
| ≥3 | 3/1470 | 0.91 (0.85– 0.95), 24.4% | 0.63 (0.60– 0.65), 76.7% | 2.50 (2.07– 3.01), 77.4% | 0.16 (0.09– 0.27), 20.2% | 16.36 (7.97– 33.58), 37.2% | 0.64 | |
| ≥5 | 3/1470 | 0.56 (0.48– 0.63), 71.1% | 0.92 (0.90– 0.93), 8.3% | 6.42 (5.13– 8.04), 0.0% | 0.48 (0.34– 0.67), 70.4% | 13.38 (8.80– 20.34), 21.6% | 0.93 | |
| Hospitalizations | FACED | |||||||
| ≥3 | 9/2859 | 0.50 (0.46– 0.54), 90.9% | 0.67 (0.65– 0.69), 90.5% | 1.70 (1.38– 2.10), 78.6% | 0.67 (0.54– 0.84), 79.4% | 2.71 (1.79– 4.09), 71.6% | 0.69 | |
| ≥5 | 9/2859 | 0.14 (0.12– 0.17), 83.0% | 0.94 (0.92– 0.95), 72.9% | 2.60 (1.84– 3.69), 37.7% | 0.89 (0.82– 0.97), 82.3% | 2.95 (1.93– 4.49), 41.5% | 0.82 | |
| BSI | ||||||||
| ≥5 | 9/2816 | 0.94 (0.92– 0.95), 57.4% | 0.32 (0.30– 0.34), 95.9% | 1.43 (1.21– 1.69), 95.4% | 0.20 (0.09– 0.41), 80.4% | 7.61 (3.16 –18.32), 83.2% | 0.80 | |
| ≥ 9 | 9/2816 | 0.70 (0.66– 0.73), 80.1% | 0.74 (0.72– 0.76), 96.2% | 2.93 (1.90– 4.51), 95.3% | 0.39 (0.28– 0.55), 87.9% | 7.85 (3.60– 17.08), 93.1% | 0.80 |
Figure 3. SROC curve of the FACED score for predicting all-cause mortality.
Figure 4. SROC curve of the BSI score for predicting all-cause mortality.
Meta-regression and subgroup analyses of the cohorts were performed based on study design, age, follow-up time, and mortality. Regarding the FACED score with a cut-off value ≥ 3, study design and age were identified as sources of heterogeneity. In the subgroup analysis, heterogeneity decreased significantly when the analyses were restricted to older patients, especially those ≥ 65 years (I2 = 0, AUC = 0.72). However, significant heterogeneity associated with study design persisted. Regarding the BSI score with a cut-off value ≥ 5, study design had a significant influence on heterogeneity: a prospective design was associated with slightly lower heterogeneity (I2 = 84.54%). The results of the corresponding subgroup analysis are provided in Supplementary Table S1.
The results of Deeks’ test showed no significant publication bias across the included studies, based on FACED (P=0.531 for a cut-off value ≥ 3; P=0.315 for a cut-off value ≥ 5) or BSI (P=0.871 for a cut-off value ≥ 5; P=0.375 for a cut-off value ≥ 9).
Respiratory-related mortality was evaluated using data from three cohorts (n=1470) that used the FACED system [8,10]. We calculated an AUC of 0.93 for respiratory-related mortality prediction using the FACED score at a cut-off value ≥ 5.
Hospitalization prediction
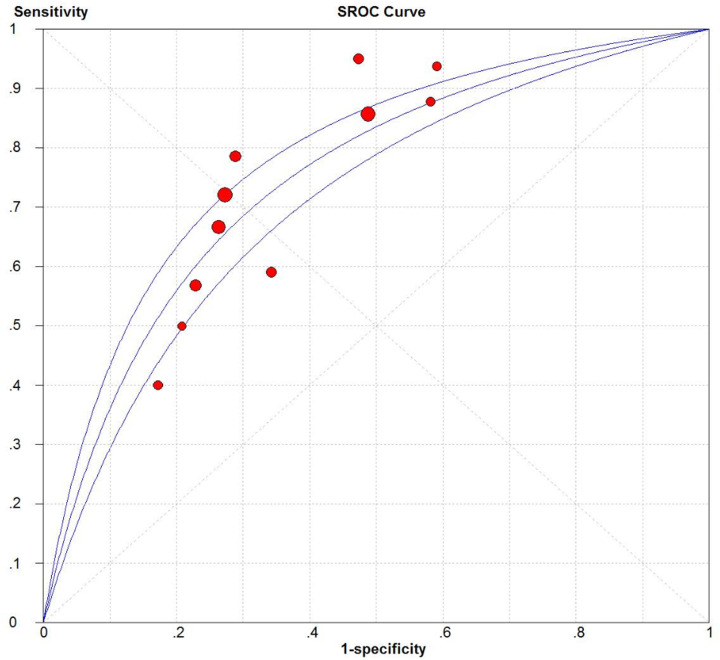
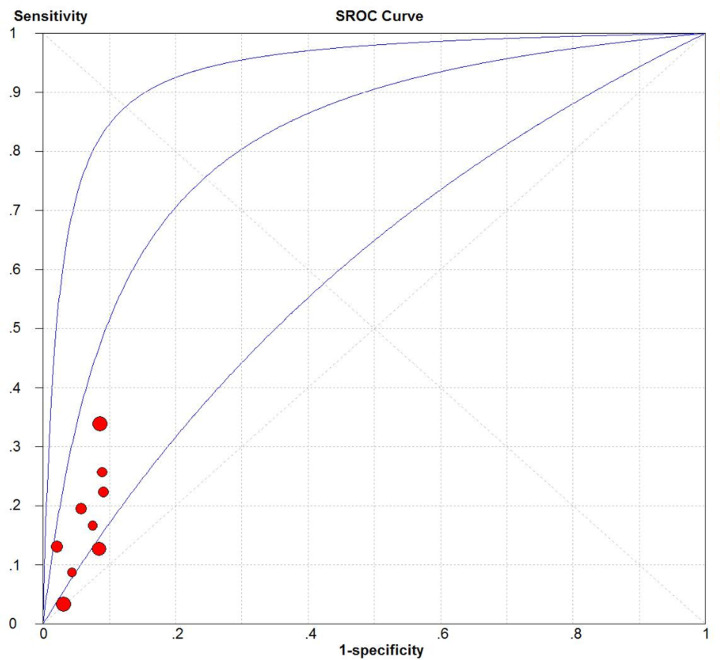
The accuracy of the two systems for predicting hospitalization of patients with bronchiectasis was evaluated using data from nine cohorts (n=2859) in the case of FACED [10,16,17] and nine cohorts (n=2859) in the case of BSI [9,16,17] (Table 3). AUC values indicated that FACED scores at a cut-off value ≥ 5 could predict hospitalization (AUC = 0.82; Figure 5), as could BSI scores at cut-off values ≥ 5 or ≥ 9 (AUC = 0.80 for both cut-off values).
Figure 5. SROC curve of the FACED score for predicting hospitalization.
Meta-regression and subgroup analyses were not performed due to the limited number of included cohorts. Deeks’ test showed no significant publication bias (P=0.497 for a cut-off value ≥ 3; P=0.129 for a cut-off value ≥ 5) or BSI (P=0.153 for a cut-off value ≥ 5; P=0.896 for a cut-off value ≥ 9).
Agreement between FACED and BSI scores
By analyzing the paired FACED and BSI data available for 333 bronchiectasis patients (Table 4), we found that the FACED and BSI systems stratified 155 patients (46.54%) into the same group (κ = 0.25, P<0.001). However, the FACED score assigned 194 patients (58.26%) to the mild group, compared to the 84 (25.22%) classified as mild based on the BSI score (P<0.001). Additionally, the BSI score classified nearly five times more patients as severe (143 [42.94%] vs 30 [9%], P<0.001). In contrast, 58 of the 333 (17.42%) bronchiectasis patients stratified into the mild group based on the FACED score were stratified into the severe group based on the BSI score.
Table 4. Agreements analysis between FACED score and BSI.
| BSI | FACED | Total | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Mild | 81 | 2 | 1 | 84 |
| Moderate | 55 | 48 | 3 | 106 |
| Severe | 58 | 59 | 26 | 143 |
| Total | 194 | 109 | 30 | 333 |
Discussion
Early identification of bronchiectasis patients with poor prognosis, leading to their close monitoring and intensive treatment, can enhance the efficiency of clinical practice, improve resource allocation, and help to optimize therapeutic outcomes. This meta-analysis summarized the prognostic performance of the FACED and BSI systems in patients with bronchiectasis for the first time. Our results show that, when appropriate cut-offs are used, the FACED score can play an important role in predicting all-cause mortality, while both the FACED and BSI scores can predict hospitalization in patients with bronchiectasis. Further research is essential to gain a better understanding of the potential prognostic roles of FACED and BSI scores in bronchiectasis.
The multidimensional and heterogeneous nature of bronchiectasis makes predicting prognoses challenging [6]. For example, risk factors associated with mortality in bronchiectasis patients include age, sex, body mass index, smoking habits, Medical Research Council dyspnea score, radiographic extent, bacterial colonization, spirometric parameters, and comorbidities (restrictive and obstructive diseases) [21–24]. FACED takes into account five of these risk factors, while BSI takes into account the same five plus two more. Therefore, both systems may be useful for the prediction of bronchiectasis outcomes and stratification by severity. However, different studies have reported different results [10,11], highlighting the need for accurate comparisons of the two systems based on current available evidence.
The accuracy of FACED and BSI scores depends on the cut-off values used. The FACED score seems to predict all-cause mortality more accurately than the BSI score. This, coupled with its simplicity, may make FACED particularly powerful. It can be used to identify patients who do not need intensive therapy. However, it can also delay needed treatment if a patient is incorrectly classified as low risk. We found that the predictive performance of the BSI score regarding all-cause mortality was inadequate. Further research may lead to an improved system being developed.
Based on our systematic review, only the FACED score has been used in research studies to predict respiratory-related mortality, for which it showed an excellent prognostic performance at a cut-off value ≥ 5 (AUC = 0.93). Thus, it can be used for the reliable identification of high-risk bronchiectasis patients, although it may incorrectly classify low-risk bronchiectasis patients as having severe disease, leading to unnecessary treatment. Therefore, it should be used with caution when predicting respiratory-related mortality.
In addition to mortality, a substantial proportion of patients with bronchiectasis experience exacerbation in terms of frequency and severity [25], leading to hospitalization in severe cases. This hospitalization is associated with rapidly growing healthcare costs [26,27]. Accurate prediction of hospitalization may help clinicians and patients to weigh the potential benefits and costs of treatment more accurately. The results of our meta-analysis indicate that both FACED and BSI scores are useful for predicting hospitalization due to bronchiectasis. However, as the FACED system does not account for previous instances of exacerbation, which is a valuable predictor of future exacerbation [28], it should be used with caution to predict hospitalization, and it may require further improvement. Indeed, the EFACED system, which accounts for exacerbations, may predict hospitalization better, and it is recommended by the Spanish guidelines [7,29]. Future research should compare EFACED and BSI in terms of hospitalization prediction.
Regardless of the cut-off values tested, neither FACED nor BSI achieved ‘perfect’ prediction, defined as PLR > 10 and NLR < 0.1 [30], regarding predicting all-cause mortality or hospitalization. This highlights the need for improvement. Bronchiectasis is associated with various etiologies and comorbidities that influence disease outcomes. The risk of death, exacerbation, and hospitalization is significantly higher in bronchiectasis patients with comorbidities than in those without [30,31]. Understanding the underlying etiologies and comorbidities may allow more comprehensive evaluation, leading to personalized treatment and better prediction of prognosis. However, neither FACED nor BSI takes comorbidities into consideration. To address this problem, the Bronchiectasis Aetiology Comorbidity Index (BACI) was developed to account for 13 comorbidities associated with high risk [32]. Future research should explore whether adding comorbidities to the FACED and BSI systems improves their performance.
Pseudomonas aeruginosa is associated with bronchiectasis and poor clinical outcomes [33]. Chronic colonization by P. aeruginosa is scored with 1 point in the FACED system and 3 points in the BSI system. P. aeruginosa has been reported to be significantly more abundant in patients with moderate or severe bronchiectasis, based on FACED scores [34]. Nevertheless, a large multinational study found that P. aeruginosa infection had no independent impact on mortality, and instead suggested that the association between P. aeruginosa and high mortality risk depends on exacerbation of the disease [35]. We hypothesize that reducing the points assigned to P. aeruginosa colonization may improve the ability of the BSI system to predict mortality.
Our conclusions should be interpreted carefully in light of the limitations of our systematic review and meta-analysis. Only 10 studies were included even after a comprehensive literature search, and some studies contained overlapping data. The evidence base comes primarily from Europe and to a lesser degree from Asia, yet the disease prevalence and hospital treatment and management practices differ by geographic region and healthcare setting. Therefore, the performance of FACED and BSI scores should be assessed in a greater diversity of settings. We excluded studies not published in English or Chinese, which may have led to bias. Additionally, the studies in our review did not adjust for the fact that during follow-up, patients may have received treatments that influenced disease outcomes.
Beyond these research limitations, the intrinsic limitations of the FACED and BSI systems should be taken into account when using them to stratify bronchiectasis patients. The prevalence of bronchiectasis and health resources in different countries should be considered when interpreting and applying the results of the FACED and BSI systems in clinical settings. For instance, the FACED score is easy to calculate owing to its simplicity, while an online calculator may be needed to determine the BSI score, and the BACI score is more complicated to calculate. Neither the FACED nor the BSI system includes all relevant factors, such as biological activity or impact of bronchiectasis on the patient's quality of life. Using a “clinical fingerprint” and “control model” approach may improve clinicians’ ability to take into account the complexity and heterogeneity of bronchiectasis, ultimately improving the quality of patient care [36].
Conclusions
The available evidence suggests that for patients with bronchiectasis, the FACED score can play an important role in predicting mortality, while both the FACED and BSI scores may be useful for predicting hospitalization. Further studies in diverse populations and healthcare settings are needed to validate our findings.
Supplementary Material
Acknowledgements
We thank Prof. Dong Tao Lin from Sichuan University for copyediting this manuscript.
Abbreviations
- AUC
area under the curve
- BACI
Bronchiectasis Aetiology Comorbidity Index
- BSI
Bronchiectasis Severity Index
- CI
confidence interval
- DOR
diagnostic odds ratio
- EFACED
exacerbation added to FACED
- FACED
forced expiratory volume in 1 s, Age, Chronic colonization, Extension, and Dyspnea
- NLR
negative likelihood ratio
- PLR
positive likelihood ratio
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies-2
- SROC
summary receiver operating characteristic
Contributor Information
Deyun Cheng, Email: huaxicdy@163.com.
Yulin Ji, Email: jiyulin@wchscu.cn.
Data Availability
The datasets supporting the conclusions of this article are included within the article and in the supplementary files.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 31671189]; and the Science and Technology Department of Sichuan Province [grant number 18ZDYF2039]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contribution
M.H. and M.Z. designed the study, screened the literature, performed the quality assessment, extracted and analyzed the data, and drafted the manuscript. C.W., Z.W., X.X., and H.W. extracted, analyzed, and interpreted the data, and revised the manuscript. D.C. and Y.J. designed, supervised the study, and revised the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Polverino E., Goeminne P.C., McDonnell M.J., Aliberti S., Marshall S.E. and Loebinger M.R. (2017) European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 50, pii: 1700629 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell A.E. (2018) Medical management of bronchiectasis. J. Thorac. Dis. 10, S3428–S3435 10.21037/jtd.2018.09.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chotirmall S.H. and Chalmers J.D. (2018) Bronchiectasis: an emerging global epidemic. BMC Pulm. Med. 18, 76 10.1186/s12890-018-0629-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McShane P.J. and Tino G. (2019) Bronchiectasis. Chest 155, 825–833 10.1016/j.chest.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 5.Poppelwell L. and Chalmers J.D. (2014) Defining severity in non-cystic fibrosis bronchiectasis. Exp. Rev. Respir. Med. 8, 249–262 10.1586/17476348.2014.896204 [DOI] [PubMed] [Google Scholar]
- 6.Martínez-García M.A., Olveira C., Máiz L., Girón R.M., Prados C., de la Rosa D. et al. (2019) Bronchiectasis: a complex, heterogeneous disease. Arch. Bronconeumol. 55, 427–433 10.1016/j.arbres.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 7.Martínez-García M.Á., Máiz L., Olveira C., Girón R.M., de la Rosa D., Blanco M. et al. (2018) Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch. Bronconeumol. 54, 79–87 10.1016/j.arbres.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Garcia M.A., De Gracia J., Relat M.V., Giron R.M., Carro L.M., De La Rosa Carrillo D. et al. (2014) Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. 43, 1357–1367 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 9.Chalmers J.D., Goeminne P., Aliberti S., McDonnell M.J., Lonni S. and Davidson J. (2014) The bronchiectasis severity index. An international derivation and validation study. Am. J. Respir. Crit. Care Med. 189, 576–585 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athanazio R., Pereira M.C., Gramblicka G., Cavalcanti-Lundgren F., de Figueiredo M.F., Arancibia F. et al. (2017) Latin America validation of FACED score in patients with bronchiectasis: an analysis of six cohorts. BMC Pulm. Med. 17, 73 10.1186/s12890-017-0417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis H.C., Cowman S., Fernandes M., Wilson R. and Loebinger M.R. (2016) Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: a 19-year cohort study. Eur. Respir. J. 47, 482–489 10.1183/13993003.01312-2015 [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Guo S., Wan C., Yang T., Zeng N., Wu Y. et al. (2017) Tumor necrosis factor-α-308 G/A polymorphism and risk of sepsis, septic shock, and mortality: an updated meta-analysis. Oncotarget 8, 94910–94919 10.18632/oncotarget.20862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh G.C., Khoo H.E., Wong M.L. and Koh D. (2008) The effects of problem-based learning during medical school on physician competency: a systematic review. CMAJ 178, 34–41 10.1503/cmaj.070565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B. et al. (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15.Rosales-Mayor E., Polverino E., Raguer L., Alcaraz V., Gabarrus A., Ranzani O. et al. (2017) Comparison of two prognostic scores (BSI and FACED) in a Spanish cohort of adult patients with bronchiectasis and improvement of the FACED predictive capacity for exacerbations. PLoS ONE 12, e0175171 10.1371/journal.pone.0175171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Ji X.B., Li C.W., Lu H.W., Mao B., Liang S. et al. (2018) Clinical characteristics and validation of bronchiectasis severity score systems for post-tuberculosis bronchiectasis. Clin. Respir. J. 12, 2346–2353 10.1111/crj.12911 [DOI] [PubMed] [Google Scholar]
- 17.McDonnell M.J., Aliberti S., Goeminne P.C., Dimakou K., Zucchetti S.C. and Davidson J. (2016) Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 71, 1110–1118 10.1136/thoraxjnl-2016-208481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minov J., Karadzinska-Bislimovska J., Vasilevska K., Stoleski S. and Mijakoski D. (2015) Assessment of the non-cystic fibrosis bronchiectasis severity: The FACED Score vs the Bronchiectasis Severity Index. Open Respir. Med. J. 9, 46–51 10.2174/1874306401509010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa J.C., Machado J.N., Ferreira C., Gama J. and Rodrigues C. (2018) The Bronchiectasis Severity Index and FACED score for assessment of the severity of bronchiectasis. Pulmonology 24, 149–154 10.1016/j.rppnen.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 20.Sim W., Siow W., Puah S., Verma A., Lee Y., Abisheganaden J. et al. (2017) The role of the bronchiectasis severity index (BSI) and faced score in the prediction of clinical outcomes in patients with bronchiectasis in singapore. Am. J. Respir. Crit. Care Med. Conf. 195, A4725 [Google Scholar]
- 21.Goeminne P.C., Nawrot T.S., Ruttens D., Seys S. and Dupont L.J. (2014) Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir. Med. 108, 287–296 10.1016/j.rmed.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 22.Loebinger M.R., Wells A.U., Hansell D.M., Chinyanganya N., Devaraj A., Meister M. et al. (2009) Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur. Respir. J. 34, 843–849 10.1183/09031936.00003709 [DOI] [PubMed] [Google Scholar]
- 23.Onen Z.P., Gulbay B.E., Sen E., Yildiz O.A., Saryal S., Acican T. et al. (2007) Analysis of the factors related to mortality in patients with bronchiectasis. Respir. Med. 101, 1390–1397 10.1016/j.rmed.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 24.Goeminne P.C., Scheers H., Decraene A., Seys S. and Dupont L.J. (2012) Risk factors for morbidity and death in non-cystic fibrosis bronchiectasis: a retrospective cross-sectional analysis of CT diagnosed bronchiectatic patients. Respir. Res. 13, 21 10.1186/1465-9921-13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Garcia M.Á., Athanazio R., Gramblicka G., Corso M., Cavalcanti Lundgren F., Fernandes de Figueiredo M. et al. (2019) Prognostic value of frequent exacerbations in bronchiectasis: the relationship with disease severity. Arch. Bronconeumol. 55, 81–87 10.1016/j.arbres.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 26.Seitz A.E., Olivier K.N., Steiner C.A., Montes de Oca R., Holland S.M. and Prevots D.R. (2010) Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 138, 944–949 10.1378/chest.10-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Rosa Carrillo D., Navarro Rolon A., Giron Moreno R.M., Montull Veiga B., Olveira Fuster C. and Padilla Galo A.. Cost of hospitalizations due to exacerbation in patients with non-cystic fibrosis bronchiectasis. Respiration 2018, 1–11, 10.1159/000489935 [DOI] [PubMed] [Google Scholar]
- 28.Menendez R., Mendez R., Polverino E., Rosales-Mayor E., Amara-Elori I., Reyes S. et al. (2017) Factors associated with hospitalization in bronchiectasis exacerbations: a one-year follow-up study. Respir. Res. 18, 176 10.1186/s12931-017-0659-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Garcia M.A., Athanazio R.A., Giron R., Maiz-Carro L., de la Rosa D. and Olveira C. (2017) Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int. J. Chron. Obstruct Pulmon. Dis. 12, 275–284 10.2147/COPD.S121943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeks J.J. and Altman D.G. (2004) Diagnostic tests 4: likelihood ratios. BMJ 329, 168–169 10.1136/bmj.329.7458.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung W.S. and Lin C.L. (2018) Acute respiratory events in patients with bronchiectasis-COPD overlap syndrome: a population-based cohort study. Respir. Med. 140, 6–10 10.1016/j.rmed.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 32.McDonnell M.J., Aliberti S., Goeminne P.C., Restrepo M.I., Finch S. and Pesci A. (2016) Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir. Med. 4, 969–979 10.1016/S2213-2600(16)30320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finch S., McDonnell M.J., Abo-Leyah H., Aliberti S. and Chalmers J.D. (2015) A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann. Am. Thorac. Soc. 12, 1602–1611 [DOI] [PubMed] [Google Scholar]
- 34.Lee S., Lee Y., Park J., Cho Y.-J., Yoon H., Lee C.-T. et al. (2018) Characterization of microbiota in bronchiectasis patients with different disease severities. J. Clin. Med. 7, 429 10.3390/jcm7110429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araujo D., Shteinberg M., Aliberti S., Goeminne P.C., Hill A.T. and Fardon T.C. (2018) The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur. Respir. J. 51, pii: 1701953 10.1183/13993003.01953-2017 [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Garcia M.A., Aksamit T.R. and Agusti A. (2020) Clinical fingerprinting: a way to address the complexity and heterogeneity of bronchiectasis in practice. Am. J. Respir. Crit. Care Med. 201, 14–19 10.1164/rccm.201903-0604PP [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and in the supplementary files.