Abstract
Broomrape parasitism on faba bean (Vicia faba L.) is the most destructive factor for this crop in Egypt. Pot experiments were conducted during the two successive seasons 2017/2018 and 2018/2019 to study the mitigation of broomrape stress on faba bean using a ten-fold dilution of 10% (w/v) spent mushroom substrate extract (SMSE) of Pleurotus ostreatus and the same dilution of culture filtrate of mushroom (MCF) grown in potato dextrose broth (PDB) at a rate of 48 l hectare−1 compared with the commercial herbicide Roundup (Glyphosate 48% emulsifiable concentrate) at a rate of 144 cm3 ha−1 on the two varieties (Misr3 and Sakha3) cultivated in broomrape-infested soil. The treatments include the use of mushroom products as foliar spray and/or soil amendment in addition to Roundup spraying as a recommended treatment. Using Gas Chromatography-Mass Spectrometry (GC-MS) spectroscopy, our results indicate that the major components of the two mushroom products were bioactive compounds such as polyphenol and high molecular weight aliphatic and aromatic hydrocarbons that may interfere with parasite and host metabolism. These results indicated that SMSE of P. ostreatus and MCF of the same mushroom grown in potato dextrose broth (PDB) gave the best control of broomrape, and increased plant height, root length, leaf area, chlorophyll concentration, relative water content and seed yield (g plant−1), as well as anatomical characters of leaves in the two faba bean varieties (Misr3 and Sakha3), such as upper and lower epidermis, palisade tissue, spongy tissue and vascular bundles. Additionally, electrolyte leakage was decreased in the treated plants compared to control plants and the plants treated with Roundup (glyphosate) because of the important role of SMSE and MCF in the improvement of faba bean water status.
Keywords: broomrape, faba bean, gas chromatography-mass spectrometry, chlorophyll concentration, anatomical characters, natural products, spent mushroom substrate extract, Roundup
1. Introduction
Faba bean is one of the most important food crops worldwide, especially in Egypt, although the area of cultivation has shrunk in the last two decades from 178,531 hectares in 1991 to 32,532 hectares in 2017. There are many biotic and abiotic stress factors affect its growth and production, such as drought stress [1] and broomrape stress [2]. Broomrapes (Orobanche sp.) are a root hollow-parasitic plant lacking chlorophyll and exclusively depending on the specific host plant for nutrition [3]. They parasitize a wide range of plant species, including leguminous, solanaceous, oil plants, cruciferous in addition to medicinal plants [3]. They cause extensive yield losses in the host crops, especially in warmer and drier regions of Africa, Europe, and Asia, mainly in faba bean fields in Egypt [2,4,5]. Broomrape infestation led to decreased anatomical characters of faba bean leaf and stem, such as the thickness of lamina leaf, diameter of vascular bundles and thickness of phloem tissue in leaves. Anatomical characters of stems, such as stem diameter and vascular cylinder, were also decreased in infested faba bean plants compared with un-infested control plants. These negative effects of broomrape parasitization threaten the income of the farmers [3]. Broomrape seeds can be easily transported to other fields by means of agricultural tools, man, propagules such as crop seeds, and animals through ingestion and excretion of Orobanche seeds [3]. Since the primary stages of infection take place underground, injury to the host plant occurs before the emergence of the parasite shoots, which hinders the discovery of infection and the development of effective control strategies. In addition, a single Orobanche plant can produce more than 500,000 seeds, which can maintain its viability for about 20 years in the soil. This offers the parasite a wide diverse genetic pool which enables broomrape to withstand environmental changes and control strategies [6]. For all the previous reasons, the available control strategies against broomrapes have not introduced applicable, effective and economical solutions as predicted [7,8].
Broomrape control mainly depends on the use of resistant cultivars of the host plants and when they are not available, control depends on the avoidance of susceptible plant species in the crop rotation [9]. Glyphosate application reduced dodder weed (Cuscta spp.) and improved plant growth of infested Egyptian clover plants [10]. However, excessive use led to the emergence of herbicide-resistant weed, with Duke and Powles [11] reporting the negative effect of glyphosate-based herbicides on human and environment health. Glyphosate has been evaluated regularly by national and international agencies [12,13], and although glyphosate has relatively low toxicity in human and animals, the International Agency for Research on Cancer (IARC) reported that glyphosate and its based formulations are probably carcinogenic in humans [14,15,16]. The use of other chemical herbicides has shown limited success for the management of broomrape parasitism. This is because of the tight physical and metabolic relation between host plant and broomrape through the attachment to the host root, hence success in chemical treatment of attached parasites is restricted to few host-broomrape species pairs where herbicides are able to selectively hinder broomrapes without injuring the host crop [6,7,17]. Biological control may introduce an efficient alternative strategy in the broomrapes control under field conditions depending on the effectiveness of natural enemies as bioagents.
The potential of myco-herbicidal microorganisms for parasitic weeds management have been studied [18]. The application of Mycorrhizal fungi combined with bacterial strains positively affected nodule numbers and total dry matter of faba bean plants under broomrape infestation [19]. Moreover, the application of bio-control agents such as Trichoderma spp. And rhizobacteria species improved plant growth characters and play pivotal role in controlling broomrape in faba bean plants [20].
Spent mushroom substrate extract (SMSE) is a legnocellulolytic substrate residue of edible mushroom cultivation [21]. It contains many bioactive compounds such as phenolic compounds, which can activate defense mechanisms of plants under biotic stress conditions. The application of SMSE led to activate the defense system of rice plants against Pyricularia oryzae infection, associated with phytoalexin accumulation and the expression of defense-related genes. Therefore, the aim of our research was to study the efficacy of SMSE containing the metabolites of mushroom grown under solid state fermentation on lignocellulitic biomass and MCF containing the metabolites of mushroom grown under submerged fermentation conditions as a natural product and mycoherbicidal agent against broomrapes parasitism and improve the growth and yield characters in stressed faba bean plants.
2. Results
2.1. GC–MS Analysis of the Ethyl acetate Extract of Both SMSE and MCF
Gas Chromatography–Mass Spectrometry (GC–MS) analysis of the ethyl acetate extract of both spent mushroom substrate extract (SMSE) and mushroom culture filtrate (MCF), shows that phenol, 2, 4-bis (1,1-dimethylethyl) was the most dominant compound in the SMSE where it represented approximately 58% of total measured compounds (Table 1), followed by 1,2-benzenedicarboxylic acid (phthalic acid), which represented about 11%, then 1-hexadecanol, 2-methyl-, which represented about 5% of total estimated compounds. Other compounds, such as thieno [3,4-c] pyridine, 1,3,4,7-tetraphenyl-, 1-hexadecanol, 2-methyl- (cetyl alcohol), 1-dodecanol, 3,7,11-trimethyl (lauryl alcohol) and 2-hexadecanol, represented about 4% for each (Table 1). Table 2 shows that the most dominant compound in the MCF was 2 (1h)-naphthalenone, octahydro-1-methyl-1-(2-p ropenyl)-, (1a,4Aa,8Aa) accounting for nearly 58% of total estimated compounds, followed by 1,2-benzenedicarboxylic acid with about 16% of the total compounds (Table 2).
Table 1.
Gas chromatography (GC) analysis of ethyl acetate extract of spent mushroom substrate extracts (SMSE).
| Compound Name | Molecular Formula | Molecular Weight | CAS Number | Area |
|---|---|---|---|---|
| THIENO[3,4-C]PYRIDINE, 1,3,4,7-TETRAPHENYL- | C31H21NS | 439 | 87636-67-7 | 3.53 |
| 1-HEXADECANOL, 2-METHYL- (Cetyl alcohol) | C17H36O | 256 | 2490-48-4 | 3.96 |
| PHENOL, 2,4-BIS(1,1-DIMETHYLETHYL) | C14H22O | 206 | 96-76-4 | 58.11 |
| 1-HEXADECANOL, 2-METHYL- | C17H36O | 256 | 2490-48-4 | 5.41 |
| Cyclopentane-1-carboxylic acid, 3-acetyl-2-acetonyl-4,4-dimethyl-, methyl ester | C14H22O4 | 254 | 78092-00-9 | 0.92 |
| 1-DODECANOL, (lauryl alcohol 3,7,11-TRIMETHYL | C15H32O | 228 | 6750-34-1 | 4.52 |
| OXIRANEPENTANOIC ACID, 3-UNDECYL-, METHYL ESTER, TRANS- | C19H36O3 | 312 | 6175-11-7 | 2.36 |
| b,4a-Epoxy-2H-cyclopenta[3,4]cyclopropa[8,9]cycloundec[1,2-b]oxiren-5(1aH)-one,2,7,9,10-tetrakis(acetyloxy)decahydro-3,6,8,8,10a-pentamethyl- | C28H38O11 | 550 | 51906-06-0 | 0.92 |
| 4,8,13-CYCLOTETRADECATRIENE-1,3-DIOL,1,5,9-TRIMETHYL-12-(1-METHYL ETHYL)- | C20H34O2 | 306 | 7220-78-2 | 0.76 |
| 1-PHENANTHRENECARBOXYLIC ACID,1,2,3,4,4A,4B,5,6,10,10A- DECAHYDRO-1,4A DIMETHYL-7-(1-METH YLETHYL)-, METHYL ESTER | C21H32O2 | 316 | 127-25-3 | 1.38 |
| 2-HEXADECANOL | C16H34O | 242 | 14852-31-4 | 3.02 |
| DOCOSANOIC ACID Behenic acid | C23H46O5 | 402 | 56554-25-7 | 0.82 |
| PODOCARP-7-EN-3a-OL (Totarol) | C20H32O | 288 | 4752-56-1 | 1.30 |
| 1,2-BENZENEDICARBOXYLIC ACID Phthalic acid | C24H38O4 | 390 | 117-81-7 | 10.91 |
| Tetracosa-2,6,14,18,22-pentaene-10,11-diol,2,6,10,15,19,23-hexamethyl- | C30H52O2 | 444 | 153650-82-9 | 0.84 |
| SPIROSOLAN-3-OL, 28-ACETYL-, ACETATE (ESTER) | C31H49NO4 | 499 | 1181-86-8 | 1.24 |
Table 2.
GC chromatographic analyses of ethyl acetate extract of mushroom culture filtrate (MCF) of P. ostreatus.
| Compound Name | Molecular Formula | Molecular Weight | CAS Number | Area |
|---|---|---|---|---|
| 2-Tridecanol | C13H28O | 200 | 1653-31-2 | 4.04 |
| 2(1H)-NAPHTHALENONE,OCTAHYDRO-1-METHYL-1-(2-PROPENYL) | C14H22O | 206 | 97571-39-6 | 57.62 |
| 1-HEXADECANOL | C16H34O | 242 | 36653-82-4 | 5.79 |
| Propanoic acid, 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl) | C27H42O4 | 430 | NA | 0.83 |
| 1-EICOSANOL | C20H42O | 298 | 629-96-9 | 4.09 |
| Undec-10-ynoic acid, heptadecyl ester | C28H52O2 | 420 | NA | 1.01 |
| 9-OCTADECENOIC ACID (Z)- 2-[(TRIMETHYLSILYL)OXY]-1-[(TRIMETHYLSILYL)OXY]METHY L]ETHYL ESTER | C27H56O4Si2 | 500 | 54284-48-9 | 0.71 |
| 2-PENTENOIC ACID,5-(DECAHYDRO-5,5,8A-TRIMETHYL-2- METHYLENE-1-NAPHTHALENYL)-3-METHYL-,[1S-[1à(E),4Aá,8Aà]- | C20H32O2 | 304 | 24470-48-2 | 0.82 |
| 1H-PURIN-6-AMINE, [(2-FLUOROPHENYL)METHYL]- | C12H10FN5 | 243 | 74421-44-6 | 0.67 |
| NONACOSANOL | C29H60O | 424 | 25154-56-7 | 2.72 |
| Phthalic acid, butyl undecyl ester | C23H36O4 | 376 | NA | 2.36 |
| ISOCHIAPIN B | C19H22O6 | 346 | NA | 0.87 |
| 4H-1-BENZOPYRAN-4-ONE,2-(3,4-DIHYDROXYPHENYL)-6,8-DI-á-D-GLUCOPYRANOSYL 5,7- DIHYDROXY- | C27H30O16 | 610 | 6068-80-0 | 0.68 |
| 2a,9a-DIHYDROXYVERRUCOSANE | C20H34O2 | 306 | NA | 1.37 |
| 1,2-BENZENEDICARBOXYLIC ACID | C24H38O4 | 390 | 117-81-7 | 16.43 |
Other compounds in the MCF existed with values ranging between 0.7–6%, like 2-Tridecanol, 1-hexadecanol and isochiapin B (Table 2). All compounds detected by GC–MS contain carbon atoms ranging between 14–31 in both extracts. The molecular weight of detected compounds ranged between 206–550 in case of SMSE and 200–610 in case of MCF. Compounds detected in both extracts included variable types of functional groups like phenols, acids, alcohols, esters and different types of carbon structures as aliphatic, aromatic and heteroaromatic structures (Table 1 and Table 2).
2.2. Effect of SMSE, MCF and Roundup on Nutrient Elements and Morphological Characters of Faba Bean Plants Under Broomrape Infestation
In general, the vegetative parameters and nutrient elements analysis of faba bean plant at 60 days from sowing varied greatly with treatment and the nutritive potential of mushroom extracts represented by SMSE or MCF was clearly observed. The highest nitrogen, phosphorous and potassium (NPK) concentrations in plant tissue were recorded for the plants treated with MCF (M4, M5, M6, S4, S5, S6), followed by the plants treated with SMSE (M1, M2, M3, S1, S2, S3), in Table 3, while it was observed that plants treated with herbicide (M7, S7) recorded the lowest values after the control. Moreover, the treatments represented by spraying plus soil amendment (M2, M5, S2 and S5) of both extracts were the most effective, followed by spraying treatment (M1, M4, S1 and S4).
Table 3.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup treatments to control broomrape infestation, on percent (%) content of nitrogen (N), potassium (K) and phosphorous (P) in faba bean plants at 60 days from sowing, during two seasons.
| Treatments 1 | N % in Plant | K % in Plant | P % in Plant | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| MC | 1.13 | 1.05 | 1.16 | 1.18 | 0.21 | 0.20 |
| M1 | 1.36 | 1.34 | 1.26 | 1.27 | 0.25 | 0.25 |
| M2 | 1.42 | 1.42 | 1.36 | 1.34 | 0.31 | 0.31 |
| M3 | 1.23 | 1.21 | 1.27 | 1.25 | 0.26 | 0.25 |
| M4 | 1.65 | 1.58 | 1.44 | 1.40 | 0.31 | 0.29 |
| M5 | 1.70 | 1.72 | 1.72 | 1.67 | 0.32 | 0.28 |
| M6 | 1.51 | 1.52 | 1.54 | 1.49 | 0.26 | 0.26 |
| M7 | 1.33 | 1.31 | 1.28 | 1.27 | 0.24 | 0.24 |
| Sc | 1.09 | 1.06 | 1.08 | 1.05 | 0.20 | 0.19 |
| S1 | 1.31 | 1.29 | 1.19 | 1.16 | 0.24 | 0.23 |
| S2 | 1.35 | 1.37 | 1.25 | 1.22 | 0.31 | 0.31 |
| S3 | 1.22 | 1.22 | 1.19 | 1.23 | 0.26 | 0.24 |
| S4 | 1.53 | 1.50 | 1.31 | 1.34 | 0.30 | 0.30 |
| S5 | 1.63 | 1.62 | 1.42 | 1.41 | 0.31 | 0.31 |
| S6 | 1.43 | 1.41 | 1.37 | 1.34 | 0.27 | 0.26 |
| S7 | 1.32 | 1.30 | 1.16 | 1.17 | 0.23 | 0.22 |
| L.S.D 2. 0.05 | 0.036 | 0.062 ** | 0.086 ** | 0.059 ** | 0.029 ** | 0.034 ** |
1 Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1); 2 Least Significant Difference, determined at 5% probability. The presence of asterisks indicates that there were statistically significant differences between treatments. ** p < 0.01.
The results obtained in Table 4 indicate that plant height, plant dry weight and root length were negatively affected with broomrape infestation. However, the application of MCF (M4, M5, M6, S4, S5, S6), followed by the plants treated with SMSE (M1, M2, M3, S1, S2, S3), improved the plant height, plant dry weight and root length. Additionally, SMSE and MCF of P. ostreatus led to a significant increase in plant height, plant dry weight and root length of faba bean plants under broomrape infestation compared to Roundup and control treatments (Table 4). The best results were recorded in the plants treated with 10% solution of spent mushroom extract + soil amendment with 10% MCF (M5), followed by the plants treated with soil amendment with 10% MCF (M6).
Table 4.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup® treatments to control broomrape infestation on plant dry weight (g), plant height and root length in faba bean plants at 60 days from sowing during two seasons.
| Treatments 1 | Plant Dry Weight (g) | Plant Height (cm) | Root Length (cm) | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| MC | 14.33 | 14.57 | 66.00 | 66.00 | 23.00 | 23.00 |
| M1 | 15.25 | 17.33 | 66.00 | 65.33 | 37.00 | 35.00 |
| M2 | 17.20 | 16.93 | 69.00 | 66.00 | 33.00 | 31.00 |
| M3 | 16.07 | 16.27 | 80.00 | 80.67 | 33.00 | 34.33 |
| M4 | 21.07 | 21.27 | 74.00 | 76.00 | 31.00 | 32.00 |
| M5 | 22.63 | 22.93 | 82.67 | 85.00 | 39.33 | 40.00 |
| M6 | 19.20 | 19.52 | 88.33 | 87.67 | 36.67 | 37.33 |
| M7 | 15.70 | 15.27 | 77.00 | 77.00 | 27.67 | 28.00 |
| SC | 14.22 | 14.23 | 64.33 | 70.67 | 24.00 | 22.67 |
| S1 | 17.35 | 17.13 | 71.33 | 77.33 | 35.00 | 34.33 |
| S2 | 18.77 | 19.40 | 78.33 | 80.33 | 34.33 | 34.67 |
| S3 | 19.13 | 19.50 | 81.00 | 84.33 | 32.00 | 32.00 |
| S4 | 17.43 | 17.03 | 81.33 | 79.33 | 31.33 | 34.33 |
| S5 | 20.00 | 19.28 | 89.67 | 87.67 | 40.00 | 37.00 |
| S6 | 17.63 | 17.23 | 81.33 | 83.33 | 38.00 | 38.00 |
| S7 | 15.33 | 15.07 | 70.67 | 74.67 | 20.00 | 21.33 |
| L.S.D 2. 0.05 | 3.13 ** | 3.01 ** | 4.36 ** | 5.17 ** | 3.73 ** | 2.92 ** |
1 Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1); 2 Least Significant Difference, determined at 5% probability. The presence of asterisks indicates that there were statistically significant differences between treatments. ** p < 0.01.
2.3. Effect of SMSE, MCF and Roundup on Phosiological and Yield Characters of Faba Bean Plants Under Broomrape Infestation
Table 5 showed that broomrape infestation significantly decreased leaf area (cm2), chlorophyll a and chlorophyll b concentrations of faba bean plants in both seasons. Conversely, the application of SMSE of P. ostreatus and MCF as well as Roundup led to a significant increase in leaf area (cm2), chlorophyll a and chlorophyll b concentrations. The best results were recorded with SMSE and MCF compared with other treatments in the tolerant cultivar. The best results were recorded in the plants treated with 10% solution of MCF + soil amendment with 10% MCF (M5) followed by the plants treated with soil amendment with 10% MCF (M6) in the tolerant cultivar. On the other hand, the best results of the previous studied characters in sensitive cultivar (Sakha3) were observed in the treatment M5 (Spraying + soil amendment with 10% MCF).
Table 5.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup® treatments to control broomrape infestation on leaf area (cm2), chlorophyll a and chlorophyll b of faba bean plants at 60 days from sowing during two seasons.
| Treatments 1 | Leaf Area (cm2) | Chlorophyll a | Chlorophyll b | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| MC | 62.72 | 63.27 | 13.43 | 13.48 | 10.52 | 9.96 |
| M1 | 74.34 | 72.65 | 14.16 | 14.01 | 12.25 | 12.47 |
| M2 | 106.31 | 105.81 | 15.00 | 14.93 | 13.09 | 12.59 |
| M3 | 130.73 | 135.26 | 14.36 | 14.34 | 12.53 | 12.75 |
| M4 | 110.16 | 107.99 | 16.97 | 16.84 | 13.59 | 13.70 |
| M5 | 141.24 | 138.54 | 25.80 | 17.14 | 20.14 | 19.07 |
| M6 | 135.77 | 137.45 | 16.21 | 16.28 | 14.04 | 14.43 |
| M7 | 129.81 | 123.26 | 10.82 | 10.80 | 7.57 | 7.44 |
| SC | 60.54 | 60.98 | 12.55 | 12.57 | 11.43 | 10.80 |
| S1 | 76.58 | 81.59 | 16.03 | 16.23 | 11.56 | 11.30 |
| S2 | 91.56 | 93.92 | 16.34 | 16.49 | 12.47 | 12.36 |
| S3 | 111.74 | 111.27 | 16.02 | 15.99 | 13.91 | 13.65 |
| S4 | 95.20 | 93.81 | 20.05 | 19.53 | 15.29 | 15.16 |
| S5 | 119.05 | 112.36 | 22.42 | 22.30 | 16.46 | 16.00 |
| S6 | 74.65 | 61.63 | 19.78 | 19.47 | 15.78 | 15.44 |
| S7 | 72.01 | 66.54 | 9.50 | 9.29 | 6.71 | 6.66 |
| L.S.D 2. 0.05 | 14.64 ** | 14.80 ** | 0.51 ** | 0.67 ** | 0.58 ** | 1.06 ** |
1 Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1); 2 Least Significant Difference, determined at 5% probability. The presence of asterisks indicates that there were statistically significant differences between treatments. ** p < 0.01.
The data in Table 6 demonstrate that the broomrape number was higher in the sensitive cultivar (Sakha3). At this stage (60 days from sowing), broomrape would not appear on the soil surface, but could be counted as clusters attached to root vessels. The most potent treatment for reducing broomrape in the sensitive cultivar was the application of herbicide (S7), followed by the treatments where MCF was applied. In case of rhizobia nodules, the application of herbicide caused a decline in nodulation in both cultivars, while other treatments based on mushroom metabolites enhanced nodulation and it was reflected in both the nodule number and nodule dry weight in the two seasons.
Table 6.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup® treatments to control broomrape infestation on broomrape number (No.), No. of Rhizobia nodules/plant, and Nodules dry weight (g/plant) at 60 days from sowing.
| Treatments 1 | No. Broomrape | No. Nodules/Plant | Nodules Dry we./Plant (g) | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| MC | 1.33 | 1.67 | 21.00 | 19.00 | 0.16 | 0.15 |
| M1 | 0.67 | 0.33 | 64.33 | 63.00 | 0.21 | 0.22 |
| M2 | 0.33 | 0.33 | 75.67 | 72.33 | 0.27 | 0.25 |
| M3 | 0.67 | 0.67 | 42.33 | 37.33 | 0.31 | 0.31 |
| M4 | 0.33 | 0.67 | 70.33 | 72.67 | 0.31 | 0.31 |
| M5 | 0.00 | 0.00 | 71.67 | 68.33 | 0.37 | 0.39 |
| M6 | 0.00 | 0.00 | 45.67 | 53.00 | 0.28 | 0.29 |
| M7 | 0.33 | 0.67 | 20.00 | 21.33 | 0.16 | 0.14 |
| SC | 3.33 | 3.67 | 40.67 | 39.67 | 0.14 | 0.14 |
| S1 | 3.67 | 3.33 | 56.67 | 59.67 | 0.23 | 0.24 |
| S2 | 2.00 | 2.33 | 50.00 | 53.33 | 0.24 | 0.26 |
| S3 | 3.33 | 3.67 | 46.67 | 48.33 | 0.23 | 0.23 |
| S4 | 2.33 | 2.67 | 44.33 | 49.67 | 0.23 | 0.26 |
| S5 | 1.33 | 1.67 | 68.00 | 66.67 | 0.28 | 0.29 |
| S6 | 1.67 | 1.33 | 43.67 | 43.67 | 0.20 | 0.20 |
| S7 | 1.00 | 0.67 | 20.33 | 24.33 | 0.15 | 0.15 |
| L.S.D 2. 0.05 | 0.93 ** | 0.98 ** | 8.36 ** | 6.54 ** | 0.06 ** | 0.041 ** |
1 Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1); 2 Least Significant Difference, determined at 5% probability. The presence of asterisks indicates that there were statistically significant differences between treatments. ** p < 0.01.
The results presented in Table 7 show that plant dry weight, plant height and pod numbers of faba bean plants at harvesting stage were significantly decreased under broomrape infestation. However, using MCF caused a significant increase in plant dry weight, plant height and pod numbers of faba bean plants in the two seasons (M4, M5, M6, S4, S5, S6), followed by the plants treated with SMSE (M1, M2, M3, S1, S2, S3). Additionally, the maximum values of these characters were obtained with the M5 treatment (spraying + soil amendment with 10% MCF), followed by M6 treatment (soil amendment with 10% MCF) in the Misr3 cultivar. Moreover, the best results in sensitive cultivar (Sakha3) were shown in treatment S5 (Spraying + soil amendment with 10% MCF), followed by the S6 treatment (soil amendment with 10% MCF) compared with Roundup and other treatments in both seasons.
Table 7.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup® treatments to control broomrape infestation on plant dry weight, plant height and pod numbers of faba bean plants at harvesting stage during two seasons.
| Treatments 1 | Dry Weight of Plant (g) | Plant Height (cm) | Pod Number/Plant | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| MC | 15.98 | 15.23 | 84.77 | 77.30 | 1.67 | 1.67 |
| M1 | 23.03 | 22.39 | 88.88 | 88.38 | 3.33 | 3.67 |
| M2 | 26.57 | 25.99 | 107.37 | 104.70 | 5.67 | 5.33 |
| M3 | 25.73 | 25.49 | 104.83 | 104.07 | 5.33 | 5.33 |
| M4 | 24.35 | 23.79 | 107.03 | 105.70 | 6.00 | 6.33 |
| M5 | 35.94 | 35.31 | 119.57 | 117.60 | 8.67 | 8.00 |
| M6 | 30.37 | 30.05 | 107.67 | 103.60 | 8.33 | 8.00 |
| M7 | 21.03 | 20.17 | 85.10 | 85.03 | 2.33 | 2.00 |
| SC | 14.20 | 14.37 | 82.37 | 83.14 | 1.33 | 1.00 |
| S1 | 22.48 | 22.04 | 94.33 | 89.57 | 3.33 | 3.33 |
| S2 | 26.09 | 25.34 | 103.67 | 102.23 | 6.00 | 5.33 |
| S3 | 25.32 | 24.52 | 99.93 | 91.97 | 5.00 | 5.00 |
| S4 | 25.05 | 23.97 | 101.93 | 92.07 | 5.67 | 6.00 |
| S5 | 34.97 | 34.21 | 117.43 | 113.77 | 7.67 | 7.33 |
| S6 | 28.03 | 27.49 | 109.00 | 105.90 | 7.00 | 6.67 |
| S7 | 17.07 | 16.57 | 80.70 | 74.07 | 1.67 | 1.33 |
| L.S.D 2. 0.05 | 2.04 ** | 2.10 ** | 6.61 ** | 9.69 ** | 1.44 ** | 1.27 ** |
1 Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1); 2 Least Significant Difference, determined at 5% probability. The presence of asterisks indicates that there were statistically significant differences between treatments. ** p < 0.01.
Infestation with broomrape led to a significant decrease in faba bean yield parameters at harvest (Table 8). Our results indicated that seed yield (g/plant), straw yield (g/plant) and 100-seed (g) significantly decreased in infested untreated faba bean plants. On the other hand, the application of SMSE and MCF caused a significant increase in seed yield (g/plant), straw yield (g/plant) and 100-seed (g) of faba bean plants in the two seasons. Furthermore, M5 treatment (Spraying + soil amendment with 10% MCF) gave the best results of the studied characters followed by M6 treatment (soil amendment with 10% MCF) in the tolerant cultivar (Misr3). Likewise, the best results in the sensitive cultivar (Sakha3) were shown in treatment S5, followed by S6 treatment, compared with Roundup and other treatments in both seasons.
Table 8.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup® treatments to control broomrape infestation on faba bean yield parameters at harvesting stage during two seasons.
| Treatments 1 | Seed Yield (g/Plant) | Straw Yield (g/Plant) | 100-Seed (g) | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| MC | 6.86 | 6.64 | 7.34 | 7.17 | 39.53 | 40.64 |
| M1 | 8.93 | 8.71 | 8.67 | 8.92 | 42.05 | 41.34 |
| M2 | 9.24 | 8.83 | 10.11 | 10.51 | 52.98 | 52.44 |
| M3 | 8.35 | 8.11 | 9.37 | 9.50 | 48.42 | 47.59 |
| M4 | 9.87 | 9.45 | 11.05 | 11.32 | 47.66 | 45.87 |
| M5 | 13.00 | 12.72 | 15.42 | 15.14 | 61.47 | 60.47 |
| M6 | 12.23 | 11.69 | 12.15 | 12.42 | 57.78 | 56.76 |
| M7 | 8.72 | 8.48 | 8.63 | 8.69 | 40.36 | 39.76 |
| Sc | 5.51 | 5.89 | 6.14 | 6.02 | 38.75 | 37.32 |
| S1 | 6.47 | 6.28 | 7.60 | 7.52 | 41.87 | 41.02 |
| S2 | 7.76 | 7.91 | 8.54 | 8.80 | 52.13 | 51.48 |
| S3 | 7.64 | 7.50 | 7.94 | 7.65 | 47.42 | 46.73 |
| S4 | 8.36 | 7.91 | 9.64 | 10.03 | 45.37 | 44.70 |
| S5 | 10.00 | 9.86 | 10.62 | 10.33 | 59.43 | 58.77 |
| S6 | 9.34 | 9.01 | 10.36 | 10.62 | 56.18 | 55.47 |
| S7 | 6.15 | 6.17 | 7.83 | 7.85 | 39.12 | 38.48 |
| L.S.D 2. 0.05 | 1.04 ** | 0.95 ** | 0.89 ** | 0.99 ** | 2.42 ** | 1.87 ** |
1 Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1); 2 Least Significant Difference, determined at 5% probability. The presence of asterisks indicates that there were statistically significant differences between treatments. ** p < 0.01.
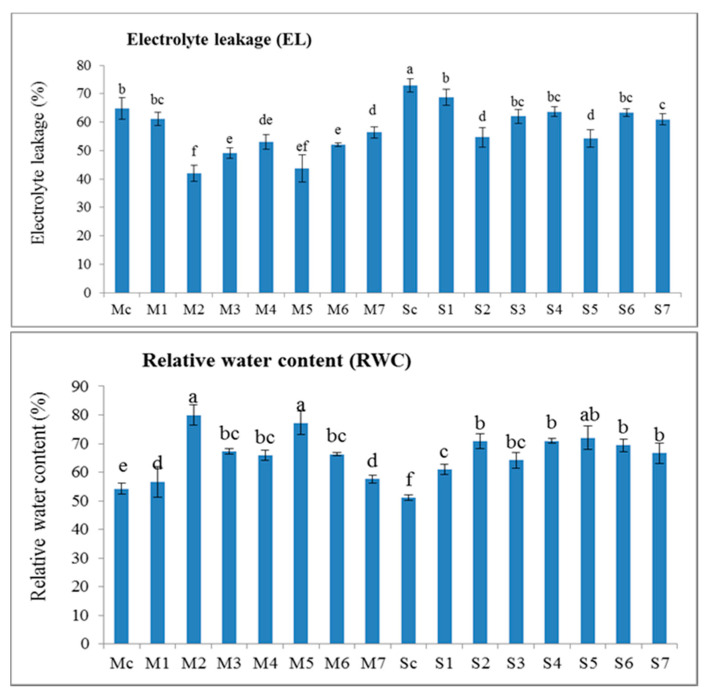
Electrolyte leakage (EL) significantly increased in infested faba bean plants with broomrape (Figure 1). This increase was significant in the infested untreated plants in the sensitive cultivar (Sakha3) compared with the tolerant one (Misr3). The application of SMSE and MCF compared with the commercial herbicide Roundup (Glyphosate 48% EC) at rate 144 cm3 ha-1, caused a significant decrease in electrolyte leakage in the two cultivars especially in the tolerant one (Misr3). The seven treatments reduced electrolyte leakage in both cultivars and the best treatments were spraying with 10% solution of SMSE + soil amendment with 10% solution of SMSE (M2), and spraying with 10% solution of MCF + soil amendment with 10% MCF (M5), followed by other treatments (Figure 1).
Figure 1.
Electrolyte leakage and relative water content in leaves of the two varieties (Misr3 and Sakha3) faba bean plants under infestation with broomrape and treated with different treatments compared with control plants (infested untreated plants) during the second season (2018/2019). Bars indicate standard errors, means with the same letter over the bar are not significantly different according to Duncan’s test at 0.05 probability level. Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1).
Broomrape parasitism also led to a significant decrease in relative water content in faba bean leaves; this decrease was greater in the sensitive cultivar (Sakha3) than in the tolerant cultivar (Misr3) (Figure 1). Additionally, our results indicate that SMSE and MCF as well as the commercial herbicide Roundup led to a significant increase in relative water content in the two cultivars, mainly in the tolerant one (Misr3). The maximum values of relative water content were recorded in the plants, which were sprayed with 10% solution of SMSE + soil amendment with 10% solution of SMSE (M2) and spraying with MCF + soil amendment with 10% MCF (M5), followed by soil amendment with 10% solution of MCF and spraying with MCF (S5).
2.4. Effect of Spent Mushroom Substrate Extract, Mushroom Culture Filtrate and Roundup on Leaves Anatomical Structure of Faba Bean Plants Under Broomrape Infestation
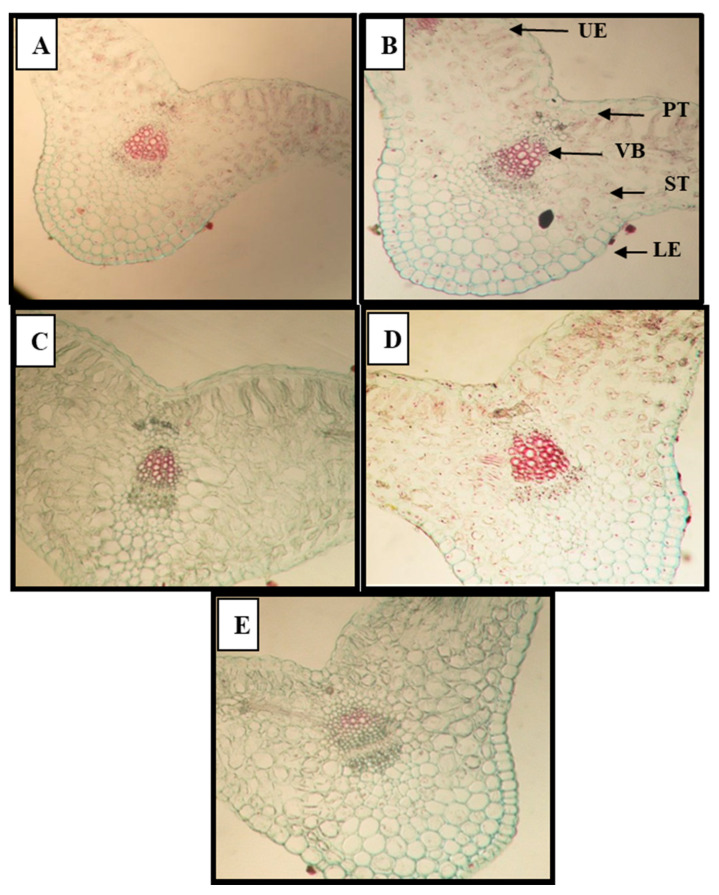
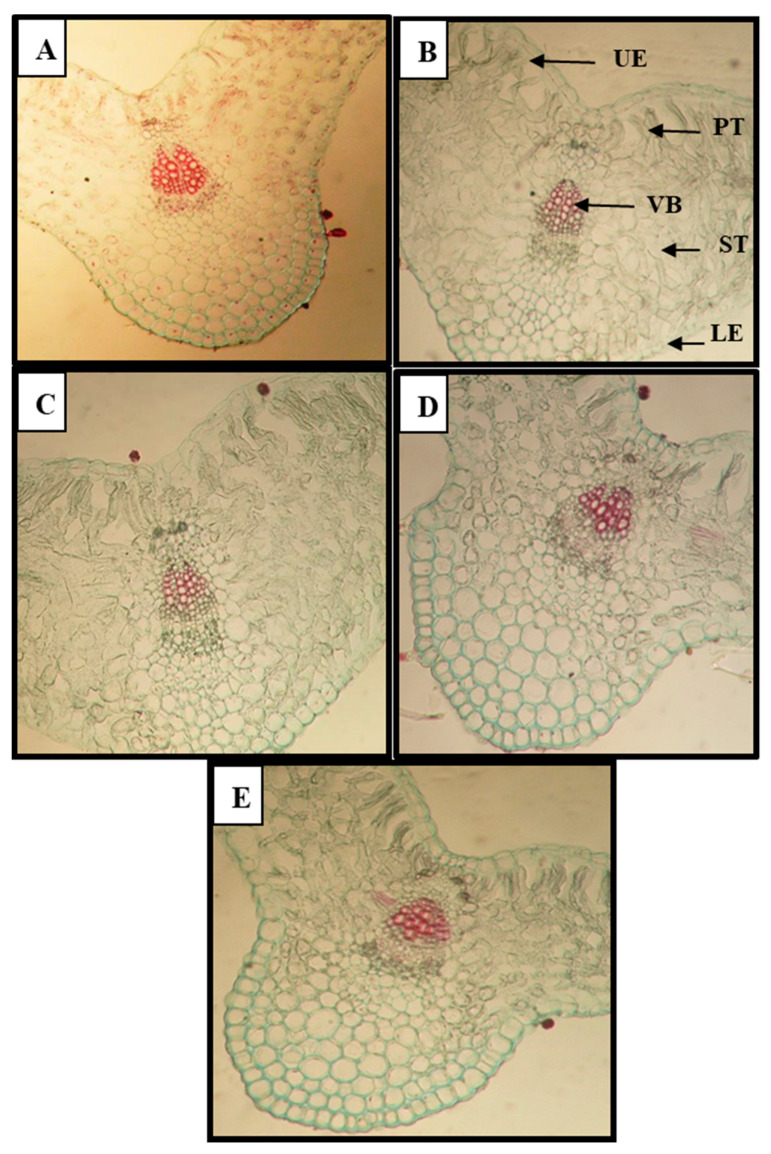
Broomrape parasitism had adverse effects on anatomical characters of infested faba bean plants. The results obtained in Table 9 and the cross sections demonstrated in Figure 2 and Figure 3 showed that anatomical characters of faba bean leaves such as leaf lamina, palisade tissue and spongy tissue, as well as number of vessels/mid vein, were harmfully affected under broomrape infestation, compared to the infested untreated plants (control) in the two cultivars. The reduction in the studied anatomical characters of leaves in the sensitive cultivar was more than in the tolerant one (Table 9). Nevertheless, the application of SMSE and MCF as well as the commercial herbicide Roundup improved the anatomical structure of infested faba bean plants in the two cultivars, mainly in the tolerant one (Misr3), versus infested untreated plants. The best results of leaf lamina, palisade tissue and spongy tissue, number of vessels/midvein in infested treated faba bean plants were recorded with spraying with 10% solution of MCF + soil amendment with 10% MCF (M5), followed by spraying with 10% solution of SMSE + soil amendment with 10% solution of SMSE (M2), followed by the other treatments (Figure 2). However, the best results in the sensitive cultivar (Sakha3) were recorded with the S5 treatment (Figure 3).
Table 9.
Effect of spent mushroom substrate extract (SMSE), mushroom culture filtrate (MCF), and Roundup® treatments to control broomrape infestation on anatomical characters of infested faba bean leaves (leaflet) with broomrape.
| Treatments | Thickness of Lamina (µm) | Thickness of Spongy Tissue (µm) | Thickness of Palisade Tissue (µm) | Number of Vessels/Midvein |
|---|---|---|---|---|
| MC | 353.35 | 221.45 | 87.56 | 27.6 |
| M1 | 362.24 | 227.67 | 91.42 | 31.5 |
| M2 | 396.04 | 238.95 | 103.19 | 35 |
| M3 | 375.05 | 221.72 | 96.15 | 30.4 |
| M4 | 381.72 | 224.45 | 98.23 | 33 |
| M5 | 403.42 | 243.02 | 106.28 | 34.5 |
| M6 | 374.76 | 222.83 | 93.54 | 32.3 |
| M7 | 378.32 | 230.44 | 97.35 | 33.2 |
| Sc | 349.91 | 221.66 | 89.54 | 25.7 |
| S1 | 357.78 | 226.18 | 95.75 | 28.5 |
| S2 | 387.51 | 235.05 | 99.92 | 32.1 |
| S3 | 368.65 | 226.70 | 92.66 | 30.2 |
| S4 | 372.29 | 231.09 | 94.14 | 31.5 |
| S5 | 389.98 | 238.14 | 97.05 | 32.9 |
| S6 | 364.23 | 229.18 | 89.23 | 31.2 |
| S7 | 354.54 | 225.42 | 87.57 | 31.7 |
Treatment codes are as follows: M = Misr3 faba bean cultivar (tolerant to broomrape parasitism), S = Sakha3 faba bean cultivar (sensitive to broomrape parasitism C -Control (without treatments), 1-Spraying with 10% solution of SMSE, 2-Spraying + soil amendment with 10% solution of SMSE, 3-Soil amendment with 10% solution of SMSE, 4-Spraying with 10% solution of MCF, 5-Spraying + soil amendment with 10% MCF, 6-Soil amendment with 10% MCF, 7-Spraying with Roundup (144 cm3 ha−1).
Figure 2.
Effect of spent mushroom substrate extract (SMSE), and mushroom culture filtrate (MCF) and Roundup on anatomical characters of infested faba bean leaves (leaflet) with broomrape in the tolerant cultivar (Misr 3) during the second season (2018/2019). A. Control of tolerant cultivar (MC) B. Spraying + soil amendment with 10% solution of SMSE (M2). C. Spraying + soil amendment with 10% MCF (M5). D. Soil amendment with 10% MCF (M6). E. Spraying with Roundup (144 cm3 ha−1) (M7). UE—Upper epidermis. PT—Palisade tissue. ST—Spongy tissue. VB—Vascular bundles. LE—Lower epidermis.
Figure 3.
Effect of spent mushroom substrate extract (SMSE), and mushroom culture filtrate (MCF) and Roundup on anatomical characters of infested faba bean leaves (leaflet) with broomrape in the sensitive cultivar (Sakha3) during the second season (2018/2019). A. Control of sensitive cultivar Sakha3 (without any treatment) (SC). B. Spraying + soil amendment with 10% solution of SMSE (S2). C. Spraying + soil amendment with 10% MCF (S5). D. soil amendment with 10% MCF (S6). E. Spraying with Roundup (144 cm3 ha−1) (S7). UE—Upper epidermis. PT—Palisade tissue. ST—Spongy tissue. VB—Vascular bundles. LE—Lower epidermis.
3. Discussion
In the current research, ecofriendly mushroom products, spent mushroom substrate extract (SMSE) and mushroom culture filtrate (MCF) are proved to be good substitutes for the chemical herbicide Roundup. The wide use of synthetic chemicals with low specificity and low biodegradability encouraged the discovery of bio-products as templates to develop biopesticides with new chemical formulas and modes of action. Glyphosate is classified as the most widely used herbicide worldwide [10]. However, the International Agency for Research on Cancer (IARC) established in March 2015 that may cause cancer in both human and animals [22].
The application of resistant varieties only to control broomrape will reduce the diversity of faba bean strains and deprive the farmer of the advantages of other varieties, such as high productivity and quality of grains. Therefore, the induction of plant defense mechanisms through the application of products such as MCF and SMSE is a promising strategy to help the plant to complete its life cycle in the presence of pathogen without a marked reduction in yield and the application of such a strategy is reflected on whole plant health [23,24,25]. Phenolic compounds are among plant secondary metabolites that play vital roles in plant defense against biotic and a biotic stresses. Phenol,2,4-bis (1,1-dimethylethyl) and 2 (1h)-naphthalenone, octahydro-1-methyl-1-(2-p ropenyl) are the most dominant phenolic compounds in the used mushroom products SMSE and MCF, respectively. These phenolic compounds participate in the regulation of stages development, and also contribute to the defense responses during exposure to pathogen infection, extreme sunlight, heavy metal stress and injuries [26]. Such compounds often possess antioxidant activity, which is attributed to their unique chemical structure [26]. Plant defense mechanisms based on phenolic compounds include physical changes, such as increasing lignification and suberization of the plant cell walls [27], as well as metabolic changes such as the de novo synthesis of pathogenesis-related (PR) proteins [28], and biosynthesis and accumulation of phenyl propanoid secondary metabolites [29].
Besides the resistance based on physical barriers, resistance could be based on the chemical response, such as the production and secretion of the toxic phenolic compounds [30]. The sunflower–broomrape interaction is combined with increasing of phenolic level, peroxidase activity [31] and accumulation of compounds on the inner walls of host-plant xylem vessels [32,33]. Coumarins such as scopoletin, scopolin and ajapin (phenolic compounds) in sunflower inhibit the germination and attachment of broomrape [34]. Fungal metabolites were reported to affect broomrape parasitism in different plants. Aybeke [35] investigated the effects of Fusarium oxysporum infection on Orobanche spp. (broomrape) with references to change in plant hormones and secondary plant constituents. The levels of plant hormones such as indole acetic acid and gibbrilic acid in the experimental group were significantly lower than those in the control group. Moreover, infection caused the accumulation of phenolic-based compounds such as syringic acid and p-coumaric acid, which later affected broomrape development. Additionally, Tadayyon et al. [36] indicated that the application of arbuscular mycorrhizal fungi (AMF) decreased broomrape germination, the number of nodules and the dry weight of the broomrape and increased dry weight of the tomato plant as well as tomato yield under broomrape infestation.
The reduction in the growth characters of infested faba bean plants, such as plant height, root length, leaf area, and fresh and dry weights, may be due to the absorption by parasitic broomrape of key nutrients that the host plant requires for growth, such as NPK. This in turn results in many harmful effects on the host faba bean plant, such as a reduction in morphological characters and nitrogen, phosphorus and potassium contents. These results are in agreement with the results of Zayed et al. [2,4,5]. The destructive effects of broomrape on physiological characters of the faba bean plant were also recorded in our experiment. Chlorophyll a and chlorophyll b concentrations as well as relative water content were significantly reduced and electrolyte leakage was increased under broomrape parasitism. These results might be due to the negative role of broomrape on growth characters of faba bean plants and the photosynthetic process, consequently decreasing chlorophyll concentration and relative water content. These detrimental effects of broomrape (as biotic stress factor) on the physiological parameters are similar to the effects of many other stress factors on various plants: drought stress [37,38,39,40], salinity stress [41,42,43,44,45] and biotic stress factors [46,47]. Briache et al. [48] reported that Misr1 and Misr3 faba bean genotypes displayed resistance levels greater than susceptible ones; moreover, Misr1 and Misr3 gave the highest yield.
Consistent with the microscopic measurements from Figure 2 and Figure 3 and Table 10, the infested faba bean plants with broomrape showed decreased anatomical features of leaves. This reduction could be due to the deleterious effect of broomrape on growth characters and nutrient uptake. Similar results were obtained with dodder parasitism on Egyptian clover [11]. The damaging effect of stress factors on anatomical characters of plants were recorded [5,49].
The application of SMSE, MCF and Roundup improved growth and anatomical characters of faba bean plants. The use of SMSE and MCF has a qualitative advantage that distinguishes it from herbicides of chemical origin, as it works to improve vegetative characteristics of faba bean in addition to its ability to resist broomrape, in contrast to the chemical pesticide that has a bad effect on the environment, which may negatively affect the host under certain conditions.
The suppression effect of phenolic compounds such as phenolic compounds such as epicatechin, procyanidin dimer, and epigallocatechin were also observed by Moshalenko and Dementev [49] in dodder plants. Glyphosate improved growth and anatomical characters of faba bean plants because of the effective role of glyphosate in preventing the enzyme 5-enolpyruvyl-shikimate-3-phosphate synthase which reduces growth of broomrape plants [50].
Advanced separation, purification and characterization of mushroom metabolites are needed for more efficiency, in addition to search in different species of mushroom and optimization the production process through development of production media and conditions.
4. Materials and Methods
4.1. Green House Experiment
Two certified cultivars of faba bean were cultivated, one sensitive to Orobanche crenata (Sakha3) and the other tolerant to Orobanche crenata (misr 3) during the successive winter seasons in 2017/2018 and 2018/2019. The experiments were carried out in Agricultural Research Station, Sakha and in EPECRS Excellence Center, Kafrelsheikh University, Egypt. These experiments were conducted in clay soils with Orobanche crenata infection in pots (30 cm in diameter) with four replicates in wire greenhouses. Sowing was done on 1 November in both seasons, each pot has two seeds of faba bean. All agricultural practices for faba bean production have been done as recommended (http://www.sharkia.gov.eg/modiriat/Agriculture/Madasel.aspx?ID=15), the treatments were presented in Table 10.
Table 10.
Treatments of greenhouse experiments during the two seasons.
| Treatments | |
|---|---|
| Mc | Control of tolerant cultivar Misr3 (without any treatment) |
| M1 | Spraying with 10% solution of spent mushroom substrate extract ( Misr3) |
| M2 | Spraying + soil amendment with 10% solution of spent mushroom substrate extract (Misr3) |
| M3 | soil amendment with 10% solution of spent mushroom substrate extract (Misr3 ) |
| M4 | Spraying with 10% solution culture filtrate (Misr3 ) |
| M5 | Spraying + soil amendment with 10% mushroom culture filtrate (Misr3) |
| M6 | soil amendment with 10% mushroom culture filtrate (Misr3) |
| M7 | Spraying with Roundup (144 cm3 ha−1) (Misr3) |
| Sc | Control of sensitive cultivar Sakha3 (without any treatment) |
| S1 | Spraying with 10% solution of spent mushroom substrate extract (Sakha3) |
| S2 | Spraying + soil amendment with 10% solution of spent mushroom substrate extract (Sakha3) |
| S3 | soil amendment with 10% solution of spent mushroom substrate extract (Sakha3) |
| S4 | Spraying with 10% solution of mushroom culture filtrate (Sakha3) |
| S5 | Spraying + soil amendment with 10% mushroom culture filtrate (Sakha3) |
| S6 | soil amendment with 10% mushroom culture filtrate (Sakha3) |
| S7 | Spraying with Roundup (144 cm3 ha−1) (Sakha3) |
4.2. Physical and Chemical Analysis of the Experimental Soil
The experimental soil was assayed according to Jackson [51] and it was clay in texture. The initial soil chemical properties were: electrical conductivity (EC) 2.4, pH 8.14, organic matter (OM) 0.55%, sand 19.83, silt 31.93, clay 49.24, total nitrogen (N) 17.35 mg kg−1, available (P) 6.83 mg kg−1, and available (K) 259.36 mg kg−1.
4.3. Spent Mushroom Source and Extraction
Spent mushroom substrate extract (SMSE) of P. ostreatus grown on rice straw was obtained after the harvesting cycles at microbiology department, soil, water and environment research institute, Sakha Agriculture Research Station, Kafr elshikh, Egypt. The obtained spent mushroom substrate (SMS) was air dried in shade for 3 days, then dried in an oven at 70 °C until reaching a constant weight.
4.4. Extraction of SMS
Dried SMS was finely grinded and suspended in 0.5 N NaOH at a ratio (1:10) with intermittent mixing during 48 h then filtered through cheesecloth and used in subsequent work.
4.5. Preparation of Mushroom Culture Filtrate
Potato Dextrose broth medium was inoculated with three agar discs of 10 mm diameter obtained from the edge of a ten days old growing P. ostreatus on potato dextrose agar plate. The inoculated culture was incubated at 28 °C on a rotary shaker at 150 rpm for 7 days and then centrifuged at 5000 rpm for 15 min. The obtained supernatant was used in subsequent work.
4.6. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
The two mushroom products, SMSE and MCF, were extracted using ethyl acetate, then the chemical compositions of the samples were measured using a Trace GC-TSQ Quantum mass spectrometer (Thermo Scientific, Austin, TX, USA), with a direct capillary column TG–5MS (30 m × 0.25 mm × 0.25 µm film thickness). The column oven temperature was initially held at 50 °C and then increased by 5 °C/min to 200 °C then held for 2 min. It was then increased to the final temperature of 290 °C by 30 °C/min and held for 2 min. The injector and MS transfer line temperatures were kept at 270 and 260 °C, respectively; helium was used as a carrier gas at a constant flow rate of 1 ml/min. The solvent delay was 3 min and diluted samples of 1 µl were injected automatically using Autosampler AS1300 coupled with GC in the split mode. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 50–500 in full scan mode. The ion source temperature was set at 200 °C. The components were identified by comparison of their retention times and mass spectra with those of the WILEY 09 and NIST 11 mass spectral databases.
4.7. Morpho-Physiological Growth Characters of Faba Bean Plants and Broomrape Number
Four samples were collected from each treatment then Nitrogen (N), Phosphorus (P) and Potassium (K) % were determined in plants at 60 days from sowing. Moreover, plant height (cm), root length (cm), leaf area, chlorophyll (a and b) and plant dry weight (g), number of broomrape, number of nodules/plant and nodules dry weight (g/plant) were determined at 60 days from sowing.
4.8. Physiological Characters
At 90 days from sowing date, the four samples of faba bean were randomly taken from each replicate to determine chlorophyll concentrations, relative water content (RWC) and electrolyte leakage (EL). The concentrations of chlorophyll a and chlorophyll b were determined according to Lichtenthaler [52] as mg g-1 fresh leaves using spectrophotometer at 663 and 648 nm. Electrolyte leakage was measured according to Szalai et al. [53], while relative water content was determined in fresh leaves as % according to Sanchez et al. [54] as follows:
| RWC = (FW − DW)/ (TW − DW) × 100. |
| Fresh weight (FW) − dry weight (DW) − turgid weight (TW) |
4.9. Morphological and Yield Characters at Harvesting Stage
At the harvesting stage, the samples of faba bean were randomly taken to determine plant height (cm), pod number/plant, seed yield g/plant, straw yield g/plant, weight of 100-seed (g).
4.10. Anatomical Studies
The samples of leaves (5 mm length) from the fifth leaf (terminal leaflet) were taken at the age of 60 days from the sowing date during the second season 2018/2019. The samples were killed and fixed in formalin acetic acid alcohol (FAA), washed in ethyl alcohol 50% and dehydrated in series of normal butyl alcohol according to the protocol which described by Nassar and El-Sahhar, [55]. Slides were examined and photomicrographed by light microscope (Leica Microscope Camera for Fluorescence Microscopy, Wetzlar, Germany).
4.11. Statistical Analysis
The experiments were arranged in a split plot design, with four replicates. The data were statistically analyzed using the analysis of variance method as described by Gomez and Gomez [56]. The mean values of the tested treatments were compared by the least significant range (L.S.R.) according to Duncan’s Multiple Range Test [57] at p = 0.05 level of probability.
5. Conclusions
The study highlights the advantage of using eco-friendly compounds from microbial origin (i.e., mushroom (P. ostreatus) metabolites) to control broomrape as a substitute for commonly used herbicides, which represent environmental pollutants and can negatively affect both human and animal health. Beside their effect on the prevention of broomrape development, mushroom metabolites possessed nutritional value and their addition enhanced plant growth, chlorophyll a and chlorophyll b concentrations, relative water content and faba bean yield. Moreover, the application of mushroom metabolites improved the anatomical characters of faba bean and increased the yield component even in broomrape-tolerant varieties of faba bean.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for funding this research through the Fast-track Research Funding Program.
Author Contributions
Conceptualization, T.E., A.A.H.S., K.A.A.A. and Y.M.H.; methodology, T.E., A.A.H.S., K.A.A.A. and Y.M.H.; software, T.E., A.A.H.S., K.A.A.A., Y.M.H., K.A.A. and M.D.F.A.; formal analysis, T.E., A.A.H.S., K.A.A.A., Y.M.H., K.A.A. and M.D.F.A.; investigation, T.E., A.A.H.S., K.A.A.A. and Y.M.H.; resources, T.E., A.A.H.S., K.A.A.A. and Y.M.H.; data curation, T.E., A.A.H.S., K.A.A.A. and Y.M.H.; writing-original draft preparation, T.E., A.A.H.S., and K.A.A.A.; writing-review and editing, T.E., A.A.H.S., K.A.A.A., Y.M.H., K.A.A. and M.D.F.A.; visualization, T.E., A.A.H.S., K.A.A.A. and Y.M.H.; funding acquisition, K.A.A.A.,Y.M.H., K.A.A. and M.D.F.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Abdelaal K.A.A. Effect of Salicylic acid and Abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. Mansoura Univ. 2015;6:1771–1788. doi: 10.21608/jpp.2015.52096. [DOI] [Google Scholar]
- 2.Zayed E., El-Afry M., Eissa S., Abdelaal K.A.A. Effect of Morphactin, TIBA and Ethephon on growth and yield of infected faba bean plants with Orobanche crenata. J. Agric. Res. Tanta Univ. 2005;31:520–535. [Google Scholar]
- 3.Habimana S., Nduwumuremyi A., Chinama R. Management of Orobanche in field crops-A review. J. Soil Sci. Plant Nutr. 2014;14:43–62. [Google Scholar]
- 4.Zayed E., El-Afry M., Eissa S., Abdelaal K.A.A. Effect of Morphactin, TIBA and Ethephon on anatomical characteristics of infected faba bean plants with Orobanche crenata. J. Agric. Res. Tanta Univ. 2005;31:536–547. [Google Scholar]
- 5.Zayed E., El-Afry M., Eissa S., Abdelaal K.A.A. Effect of Morphactin, TIBA and Ethephon on physiological characteristics of infected faba bean plants with broomrape. J. Agric. Res. Tanta Univ. 2005;31:725–738. [Google Scholar]
- 6.Joel D.M., Hershenhorn J., Eizenberg H., Aly R., Ejeta G., Rich P.J., Ransom J.K., Sauerborn J., Rubiales D. Biology and management of weedy root parasites. Hort. Rev. 2007;33:267–349. [Google Scholar]
- 7.Pérez-De-Luque A., Eizenberg H., Grenz J.H., Sillero J.C., Avila C., Sauerborn J., Rubiales D. Broomrape management in faba bean. Field Crops Res. 2010;115:319–328. doi: 10.1016/j.fcr.2009.02.013. [DOI] [Google Scholar]
- 8.Goldwasser Y., Yoneyama K., Xie X., Yoneyama K. Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul. 2008;55:21–28. doi: 10.1007/s10725-008-9253-z. [DOI] [Google Scholar]
- 9.López-Bellido R.J., Benítez-Vega J., López-Bellido L. No-tillage improves broomrape control with glyphosate in faba-bean. Agron. J. 2009;101:1394–1399. [Google Scholar]
- 10.Abdelaal K.A.A., El Menofy E.M., Nessem A.A., Elhaak M.A. The allelopathy potential and glyphosate influence on anatomical features of Egyptian clover plant (Trifolium alexandrinum L.) infested with dodder weed (Cuscuta campestris L.) Fresenius Environ. Bull. 2019;28:1262–1269. [Google Scholar]
- 11.Duke S.O., Powles S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008;64:319–325. doi: 10.1002/ps.1518. [DOI] [PubMed] [Google Scholar]
- 12.JMPR Pesticide Residues in Food—2004; Proceedings of the Joint Fao/Who Meeting on Pesticide Residues Evaluations 2004 Part ii—Toxicological, Who/pcs/06.1; Rome, Italy. 20–29 September 2004. [Google Scholar]
- 13.Williams G.M., Kroes R., Munro I.C. Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000;31:117–165. doi: 10.1006/rtph.1999.1371. [DOI] [PubMed] [Google Scholar]
- 14.Guyton K.Z., El Ghissassi F., Benbrahim-Tallaa L., Grosse Y., Loomis D., Straif K. Recent progress in mechanistic data evaluation: The iarc monographs perspective. Environ. Mol. Mutagen. 2015;56:S84. [Google Scholar]
- 15.Guyton K.Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16:490–491. doi: 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 16.IARC . Monographs, Some Organophosphate Insecticides and Herbicides: Tetrachlorvinphos, Parathion, Malathion, Diazinon and Glyphosate. Iarc Working Group; Lyon, France: 2015. [Google Scholar]
- 17.Hershenhorn J., Eizenberg H., Dor E., Kapulnik Y., Goldwasser Y. Phelipanche aegyptiaca management in tomato. Weed Res. 2009;49:34–47. doi: 10.1111/j.1365-3180.2009.00739.x. [DOI] [Google Scholar]
- 18.Murasheva V.N. Biological peculiarities and identification of the broomrape fusariosis agent. Mikolog. Fitopatol. 1995;29:53–85. [Google Scholar]
- 19.Hassan M.M., Abakeer R.A. Effects of Arbuscular Mycorrhizal Fungi (AMF) and Bacterial Strains on Orobanche crenata Forsk, on Faba Bean. Univers. J. Appl. Sci. 2013;1:27–32. doi: 10.13189/ujas.2013.010105. [DOI] [Google Scholar]
- 20.El-Dabaa M.A.T., Abd-El-Khair H. Applications of plant growth promoting bacteria and Trichodermaspp. For controlling Orobanche crenata in faba bean. Bull. Natl. Res. Cent. 2020;44 doi: 10.1186/s42269-019-0263-y. [DOI] [Google Scholar]
- 21.Nakatsuka H., Oda M., Hayashi Y., Tamura K. Effects of fresh spent mushroom substrate of Pleurotus ostreatus on soil micromorphology in Brazil. Geoderma. 2016;269:54–60. doi: 10.1016/j.geoderma.2016.01.023. [DOI] [Google Scholar]
- 22.IARC 2015. [(accessed on 20 March 2015)]; Available online: https://www.iarc.fr/wp-content/uploads/2018/11/QA_Glyphosate.pdf.
- 23.Choi D., Ward B.L., Bostock R.M. Differential induction and suppression of potato 3- hydroxy-3-methylglutaryl coenzyme A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid. Plant Cell. 1992;4:1333–1344. doi: 10.1105/tpc.4.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen Y., Gisi U., Niderman T. Local and systemic protection against Phytophthora infestants induced in potato and tomato plants by jasmonic acid and jasmonic-methyl-ester. Phytopathology. 1993;83:1054–1062. doi: 10.1094/Phyto-83-1054. [DOI] [Google Scholar]
- 25.Arfaoui A., El Hadrami A., Mabrouk Y. Treatment of chickpea with Rhizobium isolates enhances the expression of phenylpropanoid defense-related genes in response to infection by Fusarium oxysporum f. sp. ciceris. Plant Physiol. Biochem. 2007;45:470–479. doi: 10.1016/j.plaphy.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Kulbat K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016;80:97–108. [Google Scholar]
- 27.Stein B.D., Klomparens K.L., Hammerschmidt R. Histochemistry and ultrastructure of the induced resistance response of cucumber plants to Colletotrichum lagenarium. J. Phytopathol. 1993;137:177–188. doi: 10.1111/j.1439-0434.1993.tb01337.x. [DOI] [Google Scholar]
- 28.Carr J.P., Klessig D.F. The pathogenesis-related proteins of plants. In: Setlow J.K., editor. Genetic Engineering, Principles and Methods. Plenum Press; New York, NY, USA: 1989. pp. 65–89. [Google Scholar]
- 29.Bennett R.N., Wallsgrove R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994;127:617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 30.Echevarría-Zomeño S., Pérez-de-Luque A., Jorrín J., Maldonado A.M. Pre-haustorial resistance to broomrape (Orobanche cumana) in sunflower (Helianthus annuus): Cytochemical studies. J. Exp. Bot. 2006;57:4189. doi: 10.1093/jxb/erl195. [DOI] [PubMed] [Google Scholar]
- 31.Antonova T.S., Ter Borg S.J. The role of peroxidase in the resistance of sunflower against Orobanche cumana in Russia. Weed Res. 1996;36:113. doi: 10.1111/j.1365-3180.1996.tb01807.x. [DOI] [Google Scholar]
- 32.Dörr I., Staack A., Kollmann R. Resistance of Helianthus to Orobanche histological and cytological studies; Proceedings of the 3rd International Workshop on Orobanche and Related Striga Research; Amsterdam, The Netherlands. 8–12 November 1994; p. 276. [Google Scholar]
- 33.Pérez-de-Luque A., Moreno M.T., Rubiales D. Host plant resistance against broomrapes (Orobanche spp.): Defence reactions and mechanisms of resistance. Ann. Appl. Biol. 2008;152:131. doi: 10.1111/j.1744-7348.2007.00212.x. [DOI] [Google Scholar]
- 34.Serghini K., de Luque A.P.M., Castejón-Munoz L. Garcıa-Torres, and JV Jorrın, “Sunflower (Helianthus annuus L.) response to broomrape (Orobanche cernua Loefl.) parasitism: Induced synthesis and excretion of 7-hydroxylated simple coumarins. J. Exp. Bot. 2001;52:2227–2234. doi: 10.1093/jexbot/52.364.2227. [DOI] [PubMed] [Google Scholar]
- 35.Aybeke M. Fusarium infection causes phenolic accumulations and hormonal disorders in Orobanche spp. Indian J. Microbiol. 2017;57:416–421. doi: 10.1007/s12088-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadayyon A., Zafaran M., Fallah S., Bazouband M. Effects of arbuscular mycorrhizal fungal symbiosis for control of Egyptian broomrape (Orobanche aegyptiaca Pers.) in tomato (Lycopersicon esculentum L.) cultivation. Weed Biol. Manag. 2018;18:118–126. doi: 10.1111/wbm.12155. [DOI] [Google Scholar]
- 37.Rashwan E.A.A., Abdelaal K.A.A. Effect of Nano Zink-oxide foliar application on some flax cultivars under different irrigation treatments. Egypt J. Plant Breed. 2019;23:119–145. [Google Scholar]
- 38.Abdelaal K.A.A., Hafez Y.M., EL Sabagh A., Saneoka H. Ameliorative effects of Abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under drought conditions. Fresenius Environ. Bull. 2017;26:7372–7383. [Google Scholar]
- 39.Abdelaal K.A.A., Hafez Y.M., El-Afry M., Tantawy D.S., Alshaal T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018;25:30199–30211. doi: 10.1007/s11356-018-3023-x. [DOI] [PubMed] [Google Scholar]
- 40.Abdelaal K.A.A., Attia K.A., Alamery S.F., El-Afry M.M., Ghazy A.I., Tantawy D.S., Al-Doss A.A., El-Shawy E.-S.E., M. Abu-Elsaoud A., Hafez Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability. 2020;12:1736. doi: 10.3390/su12051736. [DOI] [Google Scholar]
- 41.EL Sabagh A., Abdelaal K.A.A., Barutcular C. Impact of antioxidants supplementation on growth, yield and quality traits of canola (Brassica napus L.) under irrigation intervals in North Nile Delta of Egypt. J. Exp. Biol. Agric. Sci. 2017;5:163–172. doi: 10.18006/2017.5(2).163.172. [DOI] [Google Scholar]
- 42.Helaly M.N., Mohammed Z., El-Shaeery N.I., Abdelaal K.A.A., Nofal I.E. Cucumber grafting onto pumpkin can represent an interesting tool to minimize salinity stress. Physiological and anatomical studies. Middle East J. Agric. Res. 2017;6:953–975. [Google Scholar]
- 43.El-Banna M.F., Abdelaal K.A.A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J. Plant Prod. Mansoura Univ. 2018;9:989–1001. doi: 10.21608/jpp.2018.36617. [DOI] [Google Scholar]
- 44.Hasan M.K., El Sabagh A., Sikdar M.S., Alam M.J., Ratnasekera D., Barutcular C., Abdelaal K.A., Islam M.S. Comparative adaptable agronomic traits of blackgram and mungbean for saline lands. Plant Archives. 2017;17:589–593. [Google Scholar]
- 45.Abdelaal K.A.A., EL-Maghraby L.M., Elansary H., Hafez Y.M., Ibrahim E.I., El-Banna M., El-Esawi M., Elkelish A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy. 2020;10:26. doi: 10.3390/agronomy10010026. [DOI] [Google Scholar]
- 46.El-Nashaar F., Hafez Y.M., Abdelaal K.A.A., Abdelfatah A., Badr M., El-Kady S., Yousef A. Assessment of host reaction and yield losses of commercial barley cultivars to Drechslera teres the causal agent of net blotch disease in Egypt. Fresenius Environ. Bull. 2020;29:2371–2377. [Google Scholar]
- 47.Hafez Y.M., Abdelaal K.A.A. Investigation of susceptibility and resistance mechanisms of some Egyptian wheat cultivars (Triticum aestivum L.) inoculated with Blumeria graminis f.sp. tritici using certain biochemical, molecular characterization and SEM. J. Plant Prot. Pathol. Mansoura Univ. 2015;6:443–454. doi: 10.21608/jppp.2015.53305. [DOI] [Google Scholar]
- 48.Briache F.Z., Ennami M., Mbasani-Mansi J., Gaboun F., Abdelwahd R., Fatemi Z., El-Rodeny W., Amri M., Triqui Z., Mentag R. Field and controlled conditions screenings of some faba bean (Vicia faba L.) genotypes for resistance to the parasitic plant Orobanche crenata Forsk. and investigation of involved resistance mechanisms. J. Plant Dis. Prot. 2019;126:211–224. doi: 10.1007/s41348-019-00207-x. [DOI] [Google Scholar]
- 49.Moshalenko G.P., Dementev P.E. Control of Cuscuta species. Zashchita Ras-tenii. 1991;4:48–49. (In Russian) [Google Scholar]
- 50.Duke S.O. Glyphosate. In: Kearney P.C., Kaufman D.D., editors. Herbicides: Chemistry, Degradation, and Mode of Action. Volume 3. Marcel Dekker; New York, NY, USA: 1988. pp. 1–70. [Google Scholar]
- 51.Jackson M.L. Soil Chemical Analysis. Prentice Hall Private Ltd.; New York, NY, USA: 1973. [Google Scholar]
- 52.Lichtenthaler H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- 53.Szalai G., Janda T., Padi E., Szigeti Z. Role of light in post-chilling symptoms in maize. J. Plant Physiol. 1996;148:378–383. doi: 10.1016/S0176-1617(96)80269-0. [DOI] [Google Scholar]
- 54.Sanchez F.J., de Andrés E.F., Tenorio J.L., Ayerbe L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crops Res. 2004;86:81–90. doi: 10.1016/S0378-4290(03)00121-7. [DOI] [Google Scholar]
- 55.Nassar M.A., El-Sahhar K.F. Botanical Preparations and Microscopy (Microtechnique) Volume 219 Academic Bookshop; Giza, Egypt: 1998. [Google Scholar]
- 56.Gomez K.A., Gomez A.A. Statistical Procedures for Agricultural Research. 2nd ed. John Wiley and Sons; New York, NY, USA: 1984. p. 180. [Google Scholar]
- 57.Duncan B.D. Multiple ranges and multiple F-test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]