Abstract
Background:
Adrenal tumors are commonly encountered in clinical practice, but current epidemiological data mainly originate from referral centers. We aimed to determine incidence rates (SIR), prevalence, and rates of malignancy and hormone excess in a standardized setting.
Methods:
Population-based study of all patients diagnosed with adrenal tumors in Olmsted County, Minnesota, 1/1/1995–12/31/2017 (average population 141,522). SIR and prevalence were sex- and age-standardized to the 2010 US Population.
Findings:
An adrenal tumor was diagnosed in 1,287 patients (median age 62 years; 713 (55·4%) were women, and 13 (1·0%) were children). SIR increased from 4·4 (95%CI 0·3–8·6) per 100,000 person-years in 1995 to 47·8 (95%CI 36·9–58·7) in 2017, mainly due to incidental discovery of adenomas <40mm in >40-year-olds. Prevalence of adrenal tumors in 2017 was 532/100,000 inhabitants, ranging from 13/100,000 in children to 1,900/100,000 among >65-year-olds. Of 1,287 patients, 111 (8·6%) were diagnosed with malignancy (96 (7·5%) metastases), 14 (1·1%) with pheochromocytoma, and 53 (4·1%) with overt steroid hormone excess. Malignancy was more common in children (62% vs 8% in >18-year-olds, P<·0001), tumors discovered non-incidentally (32% vs 3% in incidentalomas, P<·0001), tumors >4 cm (34% vs 7% in ≤4 cm, P<·0001), tumors with unenhanced CT attenuation >20 Hounsfield units (15% vs 1% for ≤20 Hounsfield units, P<·0001), and bilateral masses (16% vs 7% for unilateral, P=·0004).
Interpretation:
Adrenal tumor SIR increased 10 times from 1995 to 2017. Population-based data revealed lower rates of malignancy, pheochromocytoma, and overt steroid hormone excess than previously reported.
Funding:
National Institutes of Health, Maryland, USA.
Keywords: adrenal incidentaloma, adrenal mass, incidence, prevalence, diagnosis, malignancy
Background
In 1982, Geelhoed and Druy coined the term “adrenal incidentaloma” to describe an incidentally found adrenal mass in a patient without apparent clinical signs of adrenal hormone excess or malignancy.1 Before the advent and widespread availability of cross-sectional imaging, adrenal tumors were considered a rare entity that largely went undetected unless reaching a large size and compressing adjacent organs or manifesting with clinically overt hormone excess.1 Nowadays, patients with incidentally discovered adrenal masses represent a common diagnostic dilemma for all clinical specialties. Prevalence of adrenal tumors varies depending on age and studied population. Radiological series report a prevalence of 4–7% among people older than 40 years, increasing to as high as 5–10% in individuals aged 70 years or above.2–4 Autopsy studies report an overall prevalence of 1·7–3·6%, with a higher prevalence in patients above 50 years of age (5–7%).5–7 By contrast, the prevalence of adrenal tumors in children is low according to autopsy series (0·15–0·36%).5,7
In any patient with an adrenal mass, there are two critical questions that need to be answered: first, whether the adrenal mass is malignant?; and, second, whether it is hormonally active.8 Current estimates of malignancy and hormone excess are based on convenience samples, originating mainly from specialized endocrine practice and surgical series.9–11 Reported frequencies for malignancy range widely between 3% and 30% depending on age, mode of discovery, tumor size, image modality, and, importantly, history of extra-adrenal malignancy, reflecting a significant selection bias in the current literature.9–14 Similarly, the frequency of overt adrenal hormone excess is unclear, reported to affect 11–25% patients in selected cohorts.9–12
Epidemiological data on the prevalence of malignant and hormonally active adrenal tumors are essential for designing a rational diagnostic approach to adrenal tumors. However, to date, no population-based studies on overall and age-specific incidence and prevalence of adrenal tumors have been performed. In this population-based study, we aimed to describe the overall and age-specific incidence, prevalence, and types of incidental and non-incidental adrenal tumors diagnosed in a large population-based setting, and to determine the relationship between patient- and tumor-specific characteristics and risk of malignancy.
Methods
Study Design
We conducted a population-based retrospective cohort study of all patients diagnosed with adrenal masses from 01/01/1995 to 12/31/2017 living in Olmsted County, Minnesota, USA. The population of Olmsted County was 119,857 in 1995 and 160,089 in 2017 (average population 141,522, total of 375,969 unique persons during study period). Details about the Olmsted County population are available in other publications.15–17 We used resources available within the Rochester Epidemiology Project (REP) medical records-linkage system to identify all potential patients, and confirmed the diagnosis of an adrenal mass by reviewing medical records. We followed the RECORD statement on how to report observational studies that utilize routinely collected health data.18
Setting
The Rochester Epidemiology Project infrastructure links medical records across all health care providers for the entire population of Olmsted County, Minnesota, since 1966.15–17,19 This allows researchers to identify persons with specific diagnoses, surgical interventions, and other procedures, and to locate their medical records.19
Using REP, we identified all Olmsted residents who had received an International Classification of Diseases 9th or 10th edition (ICD-9 and ICD-10) diagnosistic code for an adrenal neoplasm (Appendix Table 1) from 1/1/1995 to 12/31/2017. We reviewed medical records to confirm the presence of an adrenal mass and extract clinical data.
Tumor Type and Mode of Discovery
We categorized all adrenal tumor types as either: 1) malignant masses; 2) adrenocortical adenoma and nodular hyperplasia (collectively referred to as “adenomas”, further subdivided to with and without overt steroid hormone secretion); 3) other benign tumors; and 4) pheochromocytoma. We applied the diagnostic criteria for hormone excess and malignancy based on recent guidelines and review by clinical experts (Appendix Table 2).
We defined the biochemical workup as sufficient when tests were performed to diagnose 1) primary aldosteronism (hypertensive patients only), 2) cortisol excess, and 3) catecholamine excess.
We categorized the mode of discovery as: 1) adrenal incidentaloma; 2) cancer staging or follow-up (of known extra-adrenal cancer); 3) symptoms of hormone excess; and 4) other (abdominal pain/palpable mass, systemic B symptoms, etc) (Appendix Table 2).
Use of Cross-sectional Imaging
We assessed the annual number of Olmsted County residents who underwent computed tomography (CT) or magnetic resonance imaging (MRI) of abdomen from 1995 to 2017 (Appendix Table 1).
Statistical Methods
We calculated overall and age-specific incidence rates of new patients diagnosed with adrenal masses per 100,000 person-years considering the entire Olmsted County population to be at risk. Only patients diagnosed with their first adrenal mass in 1/1/1995–12/31/2017 while living in Olmsted County were included. We estimated 95% confidence intervals (95%CI), assuming that new cases followed a Poisson distribution. Incidence was presented as standardized incidence rates (SIR) based on the age and sex distribution of the 2010 US Census population, to allow for comparison over time and with other populations.
Point prevalence of patients with known adrenal masses per 100,000 persons living in Olmsted County was estimated as of 1/1/2017. We considered subjects prevalent from time of diagnosis until death or migration out of the county, and also included patients diagnosed before 1/1/1995 and patients diagnosed prior to moving to Olmsted County. Since patients generally need to be monitored after surgery, we included patients treated with adrenalectomy when calculating prevalence.
We examined the probability of an adrenal mass being malignant within predefined categories of age, sex, tumor size, laterality, and mode of discovery using univariate and multivariate logistic regression. The model was sufficiently powered to examine the included variables (Appendix Table 2). We added unenhanced CT attenuation to the model in a secondary analysis (Appendix Table 2). The chi-squared test was used to compare other categorical variables between groups. Statistical analysis were conducted in SAS, version 9.4 (SAS Institute, Cary, North Carolina) and in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).20 Data were collected in a REDCap database.21,22 The significance level used for interpretating the results was set at 0·05.
Ethical Considerations
The project was approved by the institutional review boards of Mayo Clinic and the Olmsted Medical Center. As per Minnesota law, the patients registered in REP do not need to provide written informed consent for a specific study but must authorize the use of their data for research before researchers can review their health record. According to previous studies, 97% of patients provide authorization to use their data for research.17
Role of Funding Source
The sponsors had no role in designing the study, in collecting, analyzing or interpreting the data, or in writing the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Findings
Study Cohort
We identified 1,881 inhabitants of Olmsted County with an adrenal mass diagnosis code evaluated between 1/1/1995 and 12/31/2017 (Appendix Figure 1). We found medical records for 1,859 (98·8%) persons. After reviewing the records, we excluded 572 (30·8%) persons. In total, we confirmed 1,287 Olmsted County residents diagnosed with an adrenal mass between 1/1/1995 and 12/31/2017.
Incidence
The overall mean sex- and age-standardized incidence rates (SIR) of adrenal tumors diagnosed from 1995 to 2017 was 47 (95%CI 45–50) per 100,000 person-years with similar rates in men and women (Appendix Table 3). SIR was highest among individuals older than 65 years (142 per 100,000, 95%CI 130–154). SIR per 100,000 was lower for individuals aged 40–64 years (66 per 100,000, 95CI% 61–71) and individuals aged 18–39 years (9 per 100,000, 95%CI 7–11), and lowest in children (1·6 per 100,000, 95%CI 0·8–2·7) (Appendix Table 3).
The incidence of diagnosed adrenal tumors increased 10 times during the study period: from a SIR of 4·4 (95%CI 0·3–8·6) per 100,000 person-years in 1995 to 47·8 (95%CI 36·9–58·7) per 100,000 person-years in 2017 (Figure 1A). Incidence did not increase linearly but rather in a bell-shaped curve with the highest incidence observed in 2008 with a SIR of 72·6 (95%CI 57·9–87·2) per 100,000 person-years. The increase in incidence of adrenal tumors paralleled the increase in cross-sectional abdominal imaging studies used in Olmsted County, especially from 1995 to 2010 (Figure 1A). Incidence increased mostly in adults older than 40 years, with relatively minor increases of SIR in younger adults and children (Figure 1B). No sex differences in SIRs were observed (Figure 1C).
Figure 1.
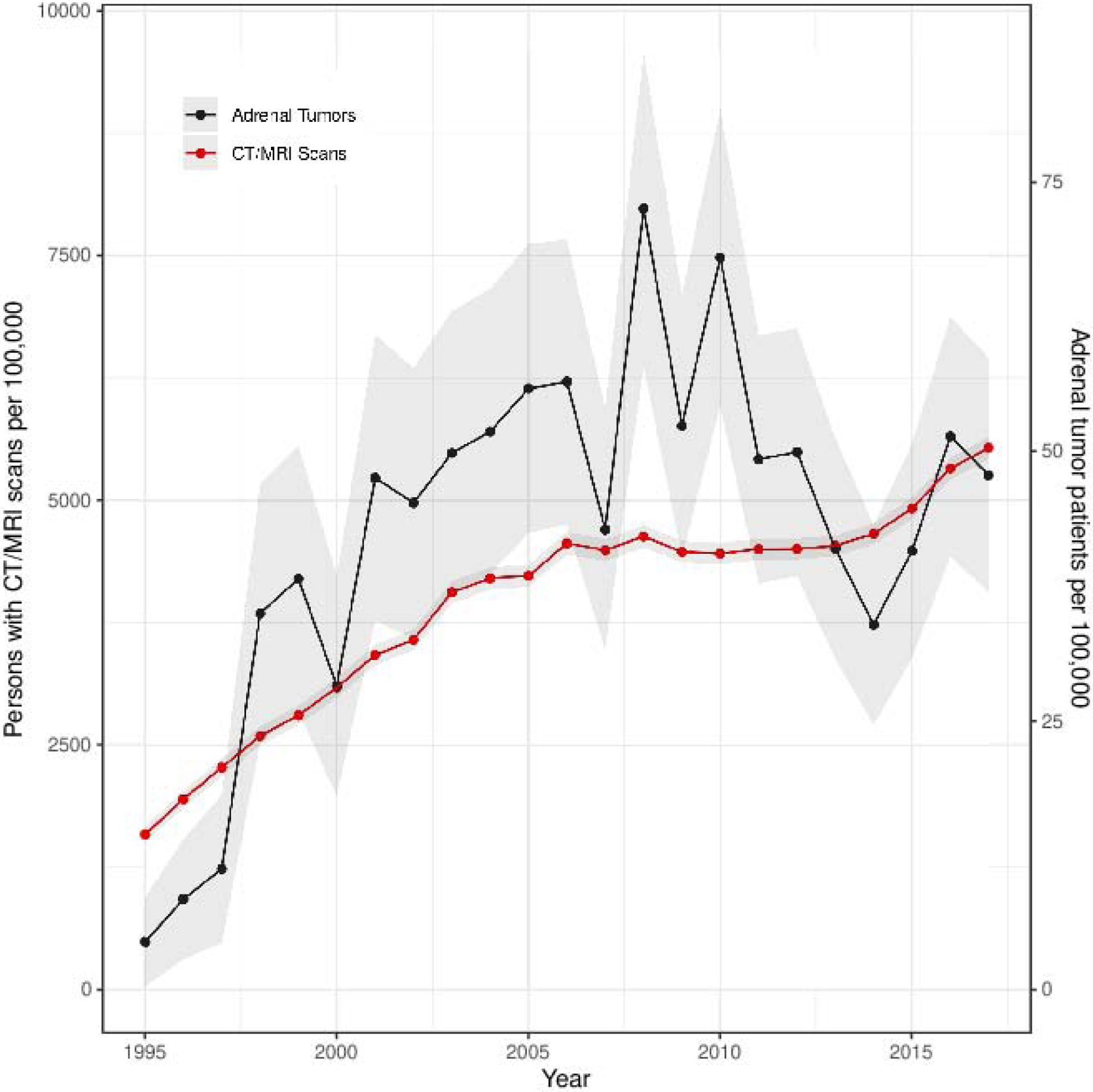

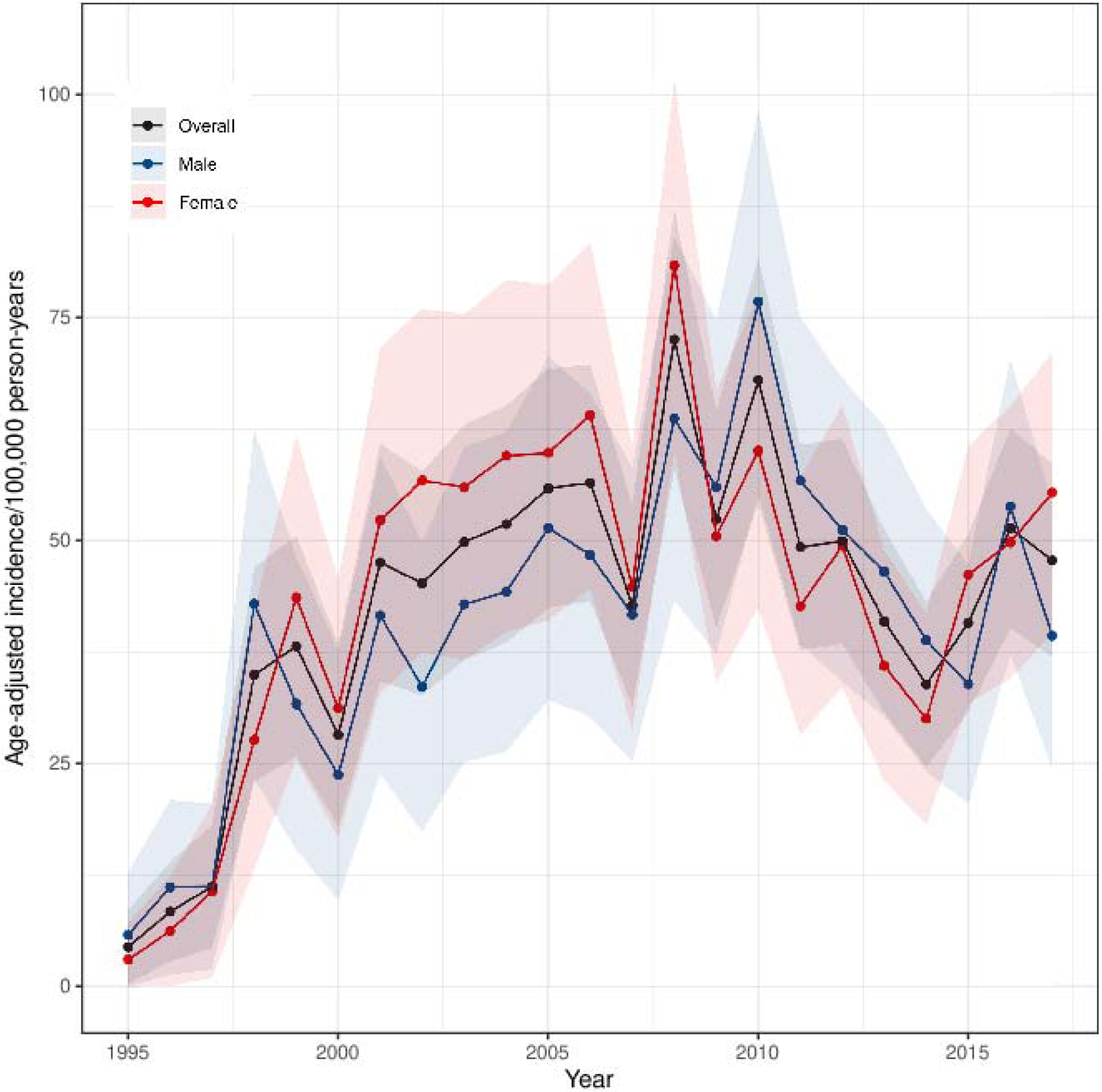


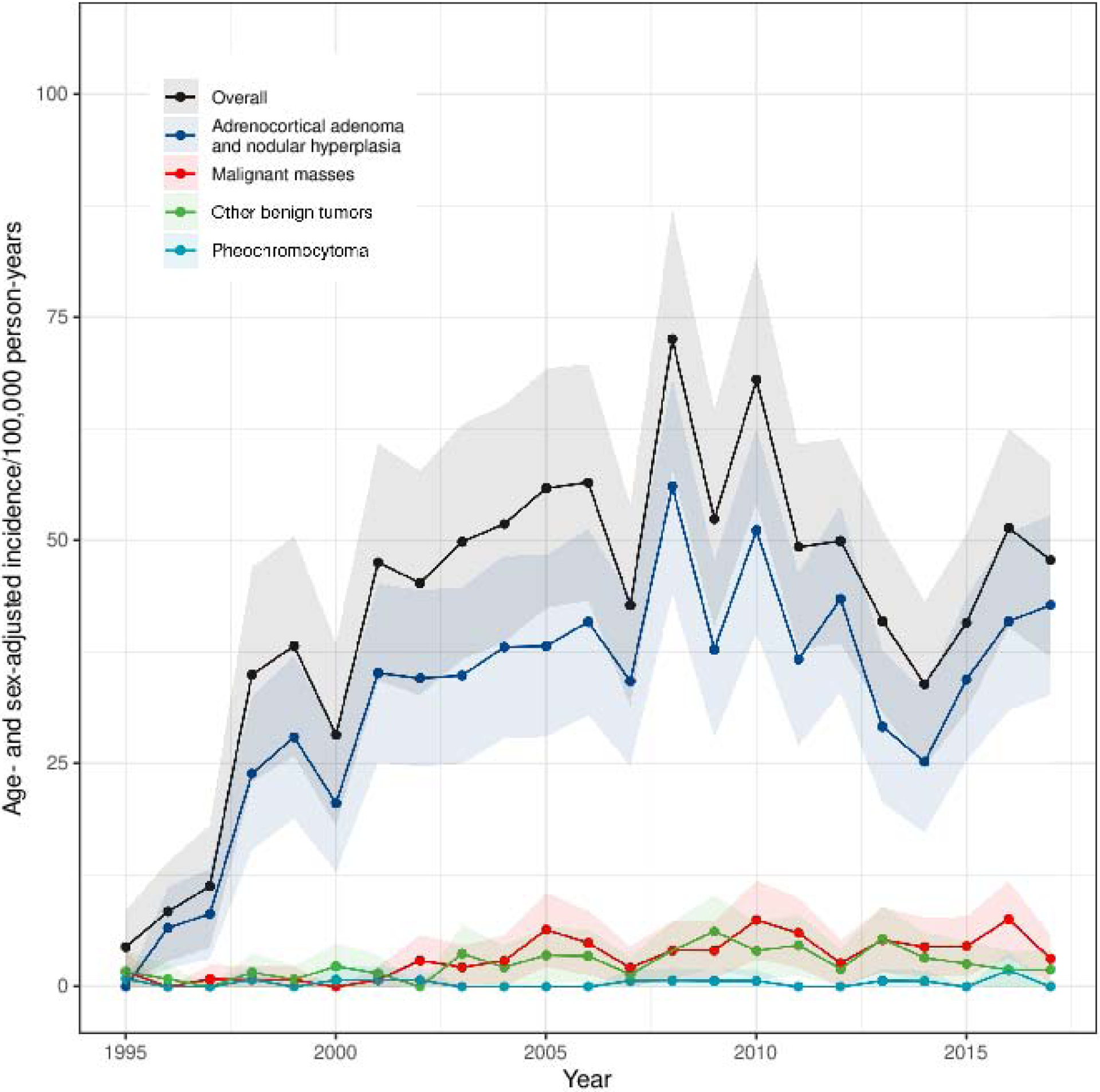
Standardized Incidence Rate of Adrenal Tumor Patients Diagnosed per 100,000 Person-years
1A. Adrenal Tumors and Abdominal CT and MRI Scans
1B. Adrenal Tumors by Age
1C. Adrenal Tumors by Sex
1D. Adrenal Tumors by Mode of Discovery
1E. Adrenal Tumors by Size
1F. Adrenal Tumors by Tumor Type
Abbreviations: SIR, standardized incidence rates. Notes: Figure 1A shows annual SIR of new patients diagnosed with adrenal tumors per 100,000 person-years (right y-axis) and annual SIR of persons undergoing CT or MRI of the abdomen (left y-axis) in Olmsted County 1995–2017. Each adrenal patient is only counted once at the year of diagnosis. Each resident with abdominal imaging is only counted once per year but can be included in multiple different years. Note different scales on y-axes. Figure 1B–F show annual SIR of adrenal tumors by age at diagnosis (1B), sex (1C), mode of discovery (1D), largest tumor diameter on imaging (1E), and tumor type (1F). Faded area represents 95% CI. Incidence rates in figure 1A and 1D–F are sex- and age-standardized to the 2010 US census population, while Figure 1B is only sex-standardized, and figure 1C is only age-standardized.
The increase in incidence of adrenal tumors was mainly observed among incidentally discovered tumors, smaller tumors, and benign adenomas without overt hormone excess (Figures 1D–1F). Comparing the earlier study period (1995–2002) to the later study period (2011–2017), the proportion of patients diagnosed with malignant adrenal masses increased from 4·3% (n=10) to 10·8% (n=52), while the proportion of patients diagnosed with overt hormone excess decreased from 14·3% (n=33) to 2·1% (n=10), and the proportion of pheochromocytoma decreased slightly from 2·2% (n=5) to 1·0% (n=5) (Appendix Table 4).
Prevalence
The sex- and age-standardized prevalence of patients with adrenal tumors at 1/1/2017 was 532 per 100,000 inhabitants (Appendix Table 5). Prevalence per 100,000 inhabitants was 425 (95%CI 393–457) for adenomas, 34 (95%CI 25–44) for other benign tumors, 12 (95%CI 7–17) for malignant tumors, and 8 (95%CI 3–12) for pheochromocytoma. Prevalence of adrenal tumors was highest among persons older than 65 years (1,900 per 100,000 inhabitants, 95%CI 1,727–2,086) and lowest among children (13 per 100,000 inhabitants, 95%CI 4–30), with minor sex differences.
During the study period, 83,559 Olmsted County residents underwent a total of 178,588 CT or MRI scans of the abdomen, corresponding to 1·54 (CI95% 1·46–1·63) patients diagnosed with an adrenal tumor per 100 residents with at least one abdominal CT or MRI.
Patient Characteristics and Tumor Types
Patients were diagnosed with adrenal tumors at a median age of 62 years (range, 0–96) and 713 (55·4%) were women. Only 13 (1·0%) patients were younger than 18 years at the time of diagnosis (Table 1). Most adrenal tumors were diagnosed incidentally by imaging performed for reasons unrelated to suspected adrenal pathology (1,050, 81·6%) (Table 2).
Table 1 -.
Patient and Adrenal Tumor Characteristics
| Total | Malignant Masses | Adrenocortical Adenoma and Nodular Hyperplasia | Other Benign Tumors | Pheochromocytoma | |
|---|---|---|---|---|---|
| Number of patients, n | 1,287 | 111 | 1,077 | 85 | 14 |
| Age at diagnosis | |||||
| Median, years (range) | 62 (0–96) | 67 (0–91) | 62 (16–96) | 59 (4–86) | 46 (26–70) |
| <18 years, n(%) | 13 (1·0) | 8 (7·2) | 3 (0·3) | 2 (2·4) | 0 |
| 18–39 years, n(%) | 92 (7·1) | 4 (3·6) | 69 (6·4) | 14 (16·5) | 5 (35·7) |
| 40–64 years, n (%) | 639 (49·7) | 40 (36·0) | 548 (50·9) | 44 (51·8) | 7 (50·0) |
| >65 years, n (%) | 543 (42·2) | 59 (53·2) | 457 (42·4) | 25 (29·4) | 2 (14·3) |
| Sex | |||||
| Female, n (%) | 713 (55·4) | 54 (48·6) | 615 (57·1) | 35 (41·2) | 9 (64·3) |
| Male, n (%) | 574 (44·6) | 57 (51·4) | 462 (42·9) | 50 (58·8) | 5 (35·7) |
| Race | |||||
| Caucasian, n (%) | 1,209 (93·9) | 102 (91·9) | 1,015 (94·2) | 80 (94·1) | 12 (85·7) |
| Asian, n (%) | 17 (1·3) | 1 (0·9) | 14 (1·3) | 1 (1·2) | 1 (7·1) |
| African-American, n (%) | 34 (2·6) | 5 (4·5) | 27 (2·5) | 2 (2·4) | 0 |
| Native American, n (%) | 6 (0·5) | 0 | 3 (0·3) | 2 (2·4) | 1 (7·1) |
| Hawaiian/Pacific Islander, n (%) | 2 (0·2) | 0 | 2 (0·2) | 0 | 0 |
| Other/Mixed, n (%) | 19 (1·5) | 3 (2·7) | 16 (1·5) | 0 | 0 |
| Ethinicity | |||||
| Non-hispanic, n (%) | 1,262 (98·1) | 105 (94·6) | 1,059 (98·3) | 84 (98·8) | 14 (1000) |
| Hispanic, n (%) | 25 (1·9) | 6 (5·4) | 18 (1·7) | 1 (1·2) | 0 |
| Location of tumor | |||||
| Left, n (%) | 709 (55·1) | 48 (43·2) | 617 (57·3) | 36 (42·4) | 8 (57·1) |
| Right, n (%) | 392 (30·5) | 34 (30·6) | 306 (28·4) | 46 (54·1) | 6 (42·9) |
| Bilateral, n (%) * | 186 (14·5) | 29 (26·1) | 154 (14·3) | 3 (3·5) | 0 |
| Tumor size # | |||||
| Median, mm (range) | 15 (5–255) | 22 (5–255) | 15 (5–85) | 25 (5–110) | 35 (9–66) |
| Size missing, n (%) | 24 (1·9) | 4 (3·6) | 17 (1·6) | 3 (3·5) | 0 |
| <20 mm, n (%) † | 784 (62·1) | 46 (43·0) | 703 (66·3) | 31 (37·8) | 4 (28·6) |
| 20–40 mm, n (%) † | 406 (32·1) | 36 (33·6) | 334 (31·5) | 31 (37·8) | 5 (35·7) |
| >40 mm, n (%)† | 73 (5·8) | 25 (23·4) | 23 (2·2) | 20 (24·4) | 5 (35·7) |
| Unenhanced CT attenuation ‡ | |||||
| Unenhanced CT available, n (%) | 660 (51·3) | 25 (22·5) | 591 (54·9) | 37 (43·5) | 7 (50·0) |
| Median, HU (range) | 8 (−110·485) | 32 (16–47) | 8 (−50–89) | 5 (−110–485) | 33 (23–40) |
| <10HU,n (%) † | 361 (54·7) | 0 | 341 (57·7) | 20 (54·1) | 0 |
| 10–19 HU,n (%) † | 157 (23·8) | 3 (12·0) | 148 (25·0) | 6 (16·2) | 0 |
| 20–29 HU, n (%) † | 71 (10·8) | 8 (32·0) | 57 (9·6) | 5 (13·5) | 1 (14·3) |
| >30 HU, n (%) † | 71 (10·8) | 14 (56·0) | 45 (7·6) | 6 (16·2) | 6 (85·7) |
Abbreviations: HU, Hounsfield units. Notes:
Bilateral defined as concomitant right and left adrenal tumors at diagnosis.
Largest diameter shown (if more than one tumor).
Percentages calculated based on non-missing data only.
Measured in homogeneous tumors only. Highest HU value shown (if more than one tumor).
Table 2 -.
Adrenal Tumor Types and Mode of Discovery
| Diagnosis | Total n (%) | Adrenal Incidentaloma n (%) | Cancer Staging or Follow·up n (%) | Symptoms of Hormone Excess n (%) | Other * n (%) |
|---|---|---|---|---|---|
| Malignant masses | 111 (8·6) | 35 (3·3) | 67 (42·9) | 2 (3·2) | 7 (36·8) |
| Adrenocortical carcinoma | 4 (0·3) | 1 (0·1) | 0 | 2 (3·2) | 1 (5·3) |
| Lymphoma | 4 (0·3) | 1 (0·1) | 3 (1·9) | 0 | 0 |
| Metastasis # | 96 (7·5) | 30 (2·9) | 62 (39·7) | 0 | 4 (21·1) |
| Neuroblastoma | 7 (0·5) | 3 (0·3) | 2 (1·3) | 0 | 2 (10·5) |
| Adrenocortical adenoma and nodular hyperplasia | 1,077 (83·7) | 925 (88·1) | 86 (55·1) | 55 (88·7) | 11 (57·9) |
| Overt hormone secretion | 53 (4·1) | 12 (1·1) | 0 | 41 (66·1) | 0 |
| Androgen excess | 1 (0·1) | 0 | 0 | 1 (1·6) | 0 |
| Overt Cushing syndrome † | 5 (0·4) | 2 (0·2) | 0 | 3 (4·8) | 0 |
| Primary aldosteronism ‡ | 47 (3·7) | 10 (1·0) | 0 | 37 (59·7) | 0 |
| Without overt hormone secretion | 1,024 (79·6) | 913 (87·0) | 86 (55·1) | 14 (22·6) | 11 (57·9) |
| Adenoma with uncertain hormone secretion | 796 (61·8) | 697 (66·4) | 81 (519) | 9 (145) | 9 (47·4) |
| Non–functioning adenoma | 139 (10·8) | 130 (12·4) | 4 (2·6) | 4 (6·5) | 1 (5·3) |
| Mild autonomous cortisol secretion § | 89 (6·9) | 86 (8·2) | 1 (0·6) | 1 (1·6) | 1 (5·3) |
| Other benign tumors | 85 (6·6) | 82 (7·8) | 2 (1·3) | 0 | 1 (5·3) |
| Calcification | 6 (0·5) | 6 (0·6) | 0 | 0 | 0 |
| Cyst ǁ | 14 (1·1) | 14 (1·3) | 0 | 0 | 0 |
| Ganglioneuroma | 2 (0·2) | 2 (0·2) | 0 | 0 | 0 |
| Hemangioma | 1 (0·1) | 1 (0·1) | 0 | 0 | 0 |
| Hematoma | 16 (1·2) | 15 (1·4) | 0 | 0 | 1 (5·3) |
| Lymphangioma | 1 (0·1) | 1 (0·1) | 0 | 0 | 0 |
| Myelolipoma | 43 (3·3) | 41 (3·9) | 2 (1·3) | 0 | 0 |
| Schwannoma | 2 (0·2) | 2 (0·2) | 0 | 0 | 0 |
| Pheo chromocytoma | 14 (1·1) | 8 (0·8) | 1 (0·6) | 5 (8·1) | 0 |
| Total | 1,287 | 1,050 | 156 | 62 | 19 |
Notes: For patients with more than one type of adrenal tumor, we reported the most clinically important tumor: adrenocortical carcinoma > pheochromocytoma > other malignant > adenoma and nodular hyperplasia with overt hormone secretion > adenoma and nodular hyperplasia without overt hormone secretion > other benign tumors. Diagnostic criteria are described in Appendix Table 2.
Include tumors found at autopsy, during surgery on other indication, and in patients evaluated for B-symptoms, palpable abdominal mass or mass-symptoms.
Metastases from lung cancer (n=49), urogenital cancer (n=15), gastrointestinal cancer (n=12), and other cancers (n=20).
Overt Cushing caused by adenoma (n=5).
Unilateral (n=25) or bilateral (n=22) primary aldosteronism. Two patients with unilateral primary aldosteronism were also diagnosed with contralateral mild autonomous cortisol secretion and one patient with contralateral non-functioning adenoma.
Mild autonomous cortisol secretion caused by adenoma (n=86), macronodular hyperplasia (n=2), or micronodular hyperplasia (n=1).
Three patients with cysts were also diagnosed with adrenal hematomas.
A malignant adrenal mass was diagnosed in 111 (8·6%) patients: 4 (0·3%) patients with ACCs and 107 (8·3%) patients with other malignant masses (adrenal metastasis, neuroblastoma, and lymphoma) (Table 2). Benign adrenal tumors were diagnosed in 1,162 (90·3%) patients. Of these, 1,077 (83·7%) were adrenocortical adenomas (N=1,074) or adrenocortical nodular hyperplasia (N=3), and 85 (6·6%) had other benign masses (including myelolipomas, cysts, and hematomas). Among the 1,050 patients with adrenal incidentalomas, 35 (3·3%) were diagnosed with malignant tumors and 12 (1·1%) with overt hormone secretion (Table 2). Clear age-predilection was observed for several diagnoses (Appendix Table 4). For example, 7 out of 8 children with malignant tumors had neuroblastoma, and 1 had ACC, whereas out of 59 patients older than 65 years with malignant tumors, 56 had metastases and 3 had primary adrenal lymphoma.
Of 53 (4·1%) patients diagnosed with overt steroid hormone excess, 47 (3·7%) had primary aldosteronism, 5 (0·4%) had overt hypercortisolism , and 1 (0·1%) had androgen excess (Table 2). Only 14 (1·1%) patients were diagnosed with pheochromocytoma and none of them had metastases at diagnosis.
Of the 1,077 patients with adenomas, only 154 (14·3%) had a DST within six months from the initial diagnosis (Appendix Table 6B), of whom 58 (37·6%) patients without symptoms of overt hormone excess failed to suppress cortisol. Considering workup performed at any time during longitudinal followup, 53 (4·9%) of the 1,077 patients with adenomas were diagnosed with overt hormone excess, 89 had mild autonomous cortisol excess (8·3% of adenomas, 36·5% of 244 adenomas with available DST), 139 were non-functioning adenomas (12·9% of adenomas, 57·0 of 244 adenomas with available DST), and 796 (73·9%) of adenomas had uncertain hormone secretion due to incomplete workup (Table 2,Appendix Table 6E).
Median adrenal tumor size was 15 mm (range 5–255). Bilateral tumors were diagnosed in 186 patients (14·5%), mainly in those with malignant tumors and adenomas (Table 1). Unenhanced CT attenuation measured in Hounsfield units (HU) was available for 660 (51·3%) patients. None of the malignant tumors and pheochromocytomas demonstrated unenhanced CT attenuation <10 HU. Of 591 patients with adenomas and available unenhanced CT attenuation data, 250 (42·3%) demonstrated indeterminate imaging characteristics (148 (25·0%) with HU 10–19, and 102 (17·3%) with HU ≥20) (Table 1).
Predictors of Malignancy
Risk of adrenal malignancy was associated with age at diagnosis, tumor size, tumor laterality, unenhanced CT attenuation value, and mode of discovery (Table 3).
Table 3 -.
Predictors of Malignancy
| n Malignant / n Total | % (95%CI) | Crude OR (95%CI) | P | Adjusted OR (95%CI) * | P | |
|---|---|---|---|---|---|---|
| Age at diagnosis | ||||||
| <18 years | 8 / 13 | 62 (36–82) | 35·2·(8·4–178·0) | <·0001 | 40·9 (7·0–265·4) | <· 0001 |
| 18–39 years | 4 / 92 | 4 (2–11) | 1 | 1 | ||
| 40–64 years | 40 / 639 | 6 (5–8) | 1·5 (0·6–5·0) | ·47 | 1·2 (0·4–4·5) | ·78 |
| 65+ years | 59 / 543 | 11 (9–14) | 2·7 (1·1–9·0) | ·062 | 1·7 (0·6–6·5) | ·35 |
| Sex | ||||||
| Women | 54 / 713 | 8 (6–10) | 1 | 1 | ||
| Men | 57 / 574 | 10 (8–13) | 1·3 (0·9–2·0) | ·14 | 0·9 (0·6–1·5) | ·79 |
| Tumor size † | ||||||
| <20 mm | 46 / 784 | 6 (4–8) | 1 | 1 | ||
| 20–40 mm | 36 / 406 | 9 (6–12) | 1·6 (1·0–2·5) | ·054 | 1.8 (1·1–3·1) | ·028 |
| >40 mm | 25 / 73 | 34 (24–46) | 8·4 (4·7–14·7) | <·0001 | 9·5 (4·3–20·8) | <·0001 |
| Unenhanced CT attenuation ‡ | ||||||
| <20 HU | 3 / 518 | 1 (0–2) | 1 | 1 # | ||
| 20–29 HU | 8 / 71 | 11 (6–21) | 21·8 (6·1–101·5) | <·0001 | 16·5 (3·3–105·2) # | ·0007 |
| 30+ HU | 14 / 71 | 20 (12–30) | 42·2 (13·3–186·9) | <·0001 | 518 (12·3–323·9) # | <·0001 |
| Laterality | ||||||
| Unilateral | 82 /1101 | 7 (6–9) | 1 | 1 | ||
| Bilateral | 29 / 186 | 16 (11–21) | 2·3 (1·4–3·6) | 0004 | 2·2 (1·1–4·0) | ·015 |
| Mode of discovery | ||||||
| Adrenal incidentaloma | 35 / 1,050 | 3 (2–5) | 1 | 1 | ||
| Cancer staging or follow·up | 67 / 156 | 43 (35–51) | 21·8 (13·8–35·0) | < 0001 | 28·0 (16·6–48·5) | <·0001 |
| Symptoms of hormone excess | 2 / 62 | 3 (1–11) | 1·0 (0·2–3·3) | ·96 | 0·8 (0·1·3·2) | ·79 |
| Other § | 7 / 19 | 37 (19–59) | 16·9 (6·0–44·8) | <·0001 | 11·5 (3·1–38·0) | ·0001 |
Abbreviations: HU, Hounsfield units; OR, odds-ratio. Notes:
Adjusted for the other variables (except unenhanced CT attenuation because of missing values). Overall AUC of adjusted model was 0·88.
Model including unenhanced CT attenuation, age, sex, size, laterality, and mode of discovery includes only 656 patients and has an overall AUC of 0·96.
Largest diameter shown (if more than one tumor).
Highest HU value shown (if more than one tumor). The <10 HU and 10–19 HU groups were combined due to no malignant cases with HU <10.
Includes tumors found at autopsy, during surgery on other indication, and in patients evaluated for B-symptoms, palpable abdominal mass or mass-related symptoms.
Risk of malignancy was highest in children (adjusted odds-ratio (aOR) 40·9, 95%CI 7·0–265·4), followed by patients older than 65 years (aOR 1·7, 95%CI 0·6–6·5), when compared to younger adults aged 18–39 years (Table 3). Tumor size >40 mm was associated with a higher risk of malignancy (aOR 9·5, 95CI% 4·3–20·8) compared to smaller tumors <20 mm. Having bilateral adrenal tumors presented a higher malignancy risk than unilateral tumors (aOR 2·2, 95%CI 1·1–4·0). In the subset of patients with available unenhanced CT scan, when compared to tumors with an unenhanced CT attenuation value <20HU, aOR for malignancy was 16·5 (95%CI 3·3–105·2) for tumors with 20–29HU and 51·8 (95%CI 12·3–323·9) for tumors with ≥30HU. Compared to adrenal incidentalomas, malignancy was more likely when an adrenal mass was discovered during cancer staging (aOR 28·0, 95%CI 16·6–48·5) or during evaluation for other reasons such as symptoms of mass effect (aOR 11·5, 95%CI 3·1–38·0) (Table 3).
Biochemical Workup
Workup for adrenal hormone excess was inconsistently performed. Of 1,287 patients, sufficient workup was performed within six months of index date for catecholamine excess in 301 (23·4%), for primary aldosteronism in 228 (19·1%), and for cortisol excess in 201 (15·6%) (Appendix Table 6A). Patients diagnosed with adrenal mass based on overt features of hormone excess were the most likely to have workup for at least one adrenal hormone excess (92%), whereas patients diagnosed during cancer staging had the most incomplete workup (Appendix Table 6A). Patients with pheochromocytoma had the most complete hormonal workup (79% with workup for catecholamine excess), while only a quarter of patients with malignant tumors had workup for at least one hormone (Appendix Table 6B). The proportion of patients with a complete hormonal workup did not change during the study period (Appendix Table 6C). When considering all testing performed during the study period (beyond the initial 6 months after diagnosis), 604 (46·9%) patients had undergone some form of hormonal workup at the end of the study period and 195 (15·2%) had undergone a complete and sufficient workup (Appendix Tables 6D,6E).
Interpretation
In this large population-based study of adrenal tumors, we found that incidence of patients diagnosed with adrenal tumors increased 10 times during the 23-year study period—an observation that correlated with the increased use of abdominal cross sectional imaging. Most adrenal tumors were incidentally discovered adrenocortical adenomas <40 mm in patients older than 40 years. We also demonstrate much lower rates of malignant adrenal tumors, pheochromocytomas, and adrenal tumors with overt steroid hormone excess than previously reported in large convenience samples, including nation-wide surveys. The risk of malignancy was strongly associated with patient and tumor characteristics.
The observed 10 times increase in incidence of adrenal tumors paralleled the increase in persons undergoing abdominal scans during 1995–2010. After 2010, despite a steady number of residents undergoing abdominal scans, incidence of adrenal tumors decreased between 2011–2014 before SIR of adrenal tumors started to increase again. SIRs of malignant tumors, tumors presenting with overt hormone excess, and tumors >40mm did not change significantly throughout the study period.
We found that the overall prevalence of adrenal tumors in 2017 was 532/100,000 persons (0·53%), ranging from 13/100,000 (0·01%) among children to 1,900/100,000 (1·9%) among adults ≥65 years of age. Radiological series have reported prevalences of 0·4–3% in studies published in 1990s and early 2000s, and 4·4–7 % in studies published after 2010.2–4,23 Although, radiological series are generally limited by selection bias, the lower prevalence found in our study may be due to under-reporting of adrenal tumors (and resulting omission of patients with incidental asymptomatic adrenal tumors).24
We found a malignancy rate of 8·6% with the majority being adrenal metastases, and only 0·3% - adrenocortical carcinomas. Among adrenal incidentalomas, only 3·3% were diagnosed as malignant, with only one case of adrenocortical carcinoma. This finding contrasts with the malignancy rates reported in convenience cohorts of patients of 0·9–1·4% for adrenocortical carcinomas and 0·7–3·8% for adrenal metastases.9,10 This could be explained by referral bias in the reported patient series.
We found that younger age, larger tumor size, non-incidental mode of discovery, bilaterality, and unenhanced CT tumor attenuation >20 HU were associated with a higher risk of malignancy. Similar to previous studies, we found that 35% of the adrenal tumors >40 mm were malignant.11,13 However, in contrast to previously reported rates of 12–14% for adrenocortical carcinoma, we found only a small number of patients with adrenocortical carcinomas, again reflective of referral bias in the previous series.11,13 Finally, in accordance with previous studies13,14,25, we found no malignancies when unenhanced CT attenuation was <10 HU and only 1% at HU <20.
In our study, we found that 4·1% of patients were diagnosed with overt cortical hormone excess and 1·1% with pheochromocytoma. These frequencies are again lower than in previous studies that reported rates of overt hormone excess between 4% and 15·8% and of pheochromocytoma between 3% and 8·5%.9–12 Our results are similar to the results of a nationwide study from the Netherlands, which reported SIR for pheochromocytoma of 0·46/100,000, as compared to 0·4/100,000 in our study.26 Notably, the diagnostic workup for hormone excess was incomplete for the majority of patients in our study, likely due to several factors. First, a number of patients in our study were diagnosed before the first guideline on management of adrenal incidentalomas was published by NIH in 2002 (with newer guidelines in 2009, 2016, and 2019).8,27–29 Secondly, the majority of patients in our study were seen by non-specialists who might not be familiar with guidelines. Finally, it is possible that there were other factors that made the complete hormonal workup unnecessary, such as histopathologic confirmation of an adrenal metastasis, stepwise approach to hormonal workup with low yield of additional hormonal testing, or patient preference.
The strengths of our study include the population-based design, which increases the generalizability of the results, its large sample size, and longitudinal access to detailed health records, which decrease the risk of misclassifying malignant tumors as benign. Limitations include mainly the initial imaging bias, which likely resulted in underestimation of both incidence and prevalence of adrenal tumors. We may also have underestimated rates of overt hormone excess and pheochromocytoma among adrenal tumors because of the low rates of hormonal workup. Similarly, we may have underestimated rates of adrenal metastases in patients with known cancer, as radiologists might not report all metastases in patients with widespread malignant disease. Other limitations include the retrospective design and inclusion of a population of only one county. As circa 90% of Olmsted County residents are Caucasians, it would improve generalizability of our results, if our findings could be replicated in a larger and more diverse population.
In conclusion, we found that the epidemiology of adrenal tumors in a population-based study differs considerably from what has previously been reported in large convenience studies. The low rates of hormonal work-up highlights a need for improved awareness of adrenal tumor management among primary care physicians. A careful consideration of age, history of extra-adrenal malignancy, and imaging findings is needed to determine the best approach in the management of patients with adrenal tumor.
Supplementary Material
Research in Context.
Evidence before this study
We screened PubMed for studies on the epidemiology of adrenal tumors, using the terms “adrenal tumor”, “adrenal neoplasm”, “adrenal mass”, or “adrenal incidentaloma” combined with “epidemiology”, “incidence”, or “prevalence”. We considered all studies published before June, 2020, in English, German, or French. The majority of previous studies were surgical series or relatively small studies from endocrine referral centers. These very selective studies reported wide ranging rates of malignancy (3–30%) and steroid hormone excess (11–25%). A few studies, including two nationwide surveys, reported more than 1,000 patients with adrenal incidentalomas. While these larger studies were less affected by referral bias, rates of pheochromocytoma (4·2–8·5%), adrenocortical carcinoma (0·9–4·6%), and overt steroid hormone excess (1·6–7·2%) were still high, reflective of subspecialized endocrine practice. No studies on incidence of adrenal tumors have been published to date, while prevalence studies were mainly based on autopsy studies and radiological series.
Added value of this study
To our knowledge, this is the first population-based study on the epidemiology of adrenal tumors. Uniquely, we used resources available within the Rochester Epidemiology Project (REP) medical records-linkage system to identify all potential patients with adrenal mass living in Olmsted County between 1/1/1995 and 12/31/2017. This allowed us to calculate not only prevalence in a population setting, but also document increase in incidence of adrenal tumors and correlate it with increase in frequency of cross-sectional abdominal imaging. The average population of Olmsted County during the study period was 141,522 (375,969 unique persons in total). During the study period 83,559 residents underwent an abdominal CT or MRI scan for a total of 178,588 scans. The REP health record-linkage system allowed us to identify all persons treated by any health care provider in a defined geographical area. This minimized the risk of referral bias and loss to follow-up. We identified and confirmed 1,287 patients who had been diagnosed with adrenal tumors. We found that the incidence of adrenal tumors increased 10 times from 1995 to 2017, parallel to the increased use of abdominal imaging. Among patients with adrenal incidentalomas, very few had pheochromocytoma (0·8%), overt steroid hormone excess (1·1%), or adrenocortical carcinoma (0·1%), while metastases were relatively common (2·9%). We found that very few patients (15·2%) received the recommended biochemical work-up for hormone excess, likely because most patients were never seen by endocrinologists. Lastly, we identified risk factors for malignancy in a population-based setting.
Implications of all the available evidence
Our population-based study clarifies the epidemiology of adrenal tumors in a population setting with several clinical implications. We have confirmed predictors of malignancy reported in convenience samples, such as age <18 years, tumor size >4 cm, and history of extra-adrenal malignancy. In addition, we clarified the role of unenhanced computed tomography Hounsfield unit measurement and adrenal mass bilaterality in predicting adrenal malignancy. We have demonstrated that in a population setting, the majority of patients with adrenal tumors have not had an appropriate work up for hormonal excess, suggestive of a gap in knowledge about the required management of adrenal tumors that needs to be addressed. We also show that most of the increase in incidence of adrenal tumors was due to incidentally discovered smaller adrenocortical adenomas in patients >40 years, while rates of malignancy and overt hormonal excess remained similar during the study period. Nevertheless, 3% of patients with adrenal incidentalomas were diagnosed with underlying malignancy and 2% with overt hormone excess, emphasizing the need for appropriate evaluation in every patient with an adrenal mass.
Acknowledgements
We wish to thank Barbara A. Abbott and her colleagues from the Rochester Epidemiology Project (Department of Health Sciences Research, Mayo Clinic) for their expert assistance in identifying potential patients and in locating medical records for review.
This research was supported by James A. Ruppe Career Development Award in Endocrinology (IB), the Catalyst Award for Advancing in Academics from Mayo Clinic (IB), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) USA under award K23DK121888 (IB), and a PhD Fellowship from Aarhus University (AE). The research was conducted using the Rochester Epidemiology Project medical record-linkage system, which is supported by the National Institute on Aging of the NIH under award numbers R01 AG034676 and AG052425. The views expressed are those of the author(s) and not necessarily those of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Dr. Bancos reports advisory board participation with Corcept, ClinCor, and HRA Pharma outside the submitted work. Dr. Arlt is an inventor on a patent on the use of steroid profiling as a biomarker tool for the differential diagnosis of adrenal tumors outside this the submitted work (PCT/GB2010/000274).
Data Sharing Statement
Statistical code and de-identified data sets are available upon request.
References
- 1.Geelhoed GW, Druy EM. Management of the adrenal “incidentaloma”. Surgery. 1982;92(5):866–874. [PubMed] [Google Scholar]
- 2.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190(5):1163–1168. [DOI] [PubMed] [Google Scholar]
- 3.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. [DOI] [PubMed] [Google Scholar]
- 4.Reimondo G, Castellano E, Grosso M, et al. Adrenal incidentalomas are tied to increased risk of diabetes: findings from a prospective study. J Clin Endocrinol Metab. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Russell RP, Masi AT, Richter ED. Adrenal cortical adenomas and hypertension. A clinical pathologic analysis of 690 cases with matched controls and a review of the literature. Medicine (Baltimore). 1972;51(3):211–225. [PubMed] [Google Scholar]
- 6.Devenyi I Possibility of normokalaemic primary aldosteronism as reflected in the frequency of adrenal cortical adenomas. J Clin Pathol. 1967;20(1):49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spain DM, Weinsaft P. Solitary Adrenal Cortical Adenoma in Elderly Female; Frequency. Arch Pathol. 1964;78:231–233. [PubMed] [Google Scholar]
- 8.Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 9.Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Cyranska-Chyrek E, Szczepanek-Parulska E, Olejarz M, Ruchala M. Malignancy Risk and Hormonal Activity of Adrenal Incidentalomas in a Large Cohort of Patients from a Single Tertiary Reference Center. Int J Environ Res Public Health. 2019;16(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637–644. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SH, Kim JH, Baek SH, et al. Characteristics of Adrenal Incidentalomas in a Large, Prospective Computed Tomography-Based Multicenter Study: The COAR Study in Korea. Yonsei Med J. 2018;59(4):501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iniguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, Biochemical, and Radiological Characteristics of a Single-Center Retrospective Cohort of 705 Large Adrenal Tumors. Mayo Clin Proc Innov Qual Outcomes. 2018;2(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delivanis DA, Bancos I, Atwell TD, et al. Diagnostic performance of unenhanced computed tomography and (18) F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clin Endocrinol (Oxf). 2018;88(1):30–36. [DOI] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd., Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd., Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing. https://www.rproject.org/. Published 2019. Accessed.
- 21.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16(4):460–484. [DOI] [PubMed] [Google Scholar]
- 24.Hammarstedt L, Muth A, Wangberg B, et al. Adrenal lesion frequency: A prospective, crosss–sectional CT study in a defined region, including systematic re-evaluation. Acta Radiol. 2010;51(10):1149–1156. [DOI] [PubMed] [Google Scholar]
- 25.Dinnes J, Bancos I, Ferrante di Ruffano L, et al. MANAGEMENT OF ENDOCRINE DISEASE: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(2):R51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berends AMA, Buitenwerf E, de Krijger RR, et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: A nationwide study and systematic review. Eur J Intern Med. 2018;51:68–73. [DOI] [PubMed] [Google Scholar]
- 27.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements. 2002;19(2):1–25. [PubMed] [Google Scholar]
- 28.Zeiger MA, Thompson GB, Duh QY, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15 Suppl 1:1–20. [DOI] [PubMed] [Google Scholar]
- 29.Vaidya A, Hamrahian A, Bancos I, Fleseriu M, Ghayee HK. The Evaluation of Incidentally Discovered Adrenal Masses. Endocr Pract. 2019;25(2):178–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


